
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med., 19 March 2021
Sec. Cardio-Oncology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.655808
This article is part of the Research TopicAnti-Cancer Drug-Induced CardiotoxicityView all 15 articles
 Moë Kondo1
Moë Kondo1 Megumi Kisanuki1
Megumi Kisanuki1 Yosuke Kokawa1
Yosuke Kokawa1 Seiichiro Gohara1
Seiichiro Gohara1 Osamu Kawano1
Osamu Kawano1 Shuntaro Kagiyama1
Shuntaro Kagiyama1 Toru Maruyama2*
Toru Maruyama2* Keita Odashiro1
Keita Odashiro1 Yoshihiko Maehara1
Yoshihiko Maehara1Cardiac arrest occurred in an 85-year-old female administered osimertinib for advanced lung cancer expressing epidermal growth factor receptor (EGFR) mutations. Electrocardiogram (ECG) recorded at recurrence of spontaneous circulation showed sinus rhythm associated with mild QT prolongation (QTc = 455 ms) to which silent myocardial ischemia and coadministration of itraconazole and herbal drug causing hypokalemia (2.1 mEq/L) may have contributed. Discontinuation of osimertinib, itraconazole and herbal drug, potassium supplementation and percutaneous coronary intervention alleviated QT prolongation (QTc = 432 ms). Osimertinib is the third-generation tyrosine kinase inhibitor lengthening QT interval, and careful monitoring of ECG, serum potassium and drugs coadministered during chemotherapy including osimertinib are highly required.
Cardiovascular complications of anticancer drugs are the main interest within the field of cardiooncology (1). Osimertinib, a third-generation tyrosine kinase inhibitor (TKI), is a first-line therapy for patients with cancer expressing epidermal growth factor receptor (EGFR) mutations. This anticancer drug has adverse effects including cardiotoxicity such as coronary tonus elevation and QT prolongation in electrocardiogram (ECG), because vascular and cardiac potassium channels are regulated largely by EGFR tyrosine kinase (2). However, osimertinib-induced QT prolongation leading to the possible development of Torsades de Pointes (TdP) has received less attention (3). We herein describe a case of 85-year-old female patient with EGFR driver mutant lung cancer treated with osimertinib, which lengthened QT interval causing abortive sudden cardiac death (SCD).
An 85-year-old female was conveyed by ambulance to the emergency room of our hospital due to cardiac arrest. She had lost her consciousness at 15:15 after she visited the outpatient clinic of another hospital for the treatment with advanced lung cancer (adenocarcinoma: cT2aN1M1b, Stage IVB). Bystander resuscitation was started immediately after the cardiac arrest, and emergency call was requested at 15:18. Ambulance crews confirmed cardiac arrest at 15:28, and she restored spontaneous circulation at 15:34 after the first discharge of public automated external defibrillator (AED). When she admitted to our hospital by ambulance at 15:40, her body temperature was 36.2°C, consciousness level in Glasgow Coma Scale was E1V1M1, and reflex to light was prompt. Blood pressure was 154–103 mmHg, pulse was regular, and its rate was 81 bpm. No abnormal physical findings were found. She had been started administration of oral osimertinib (40 mg once daily) as a first-line therapy of lung cancer after confirming EGFR mutation (T790M), oral itraconazole (200 mg once daily) for prevention of opportunistic fungal infection and crude herbal anticancer drug used in Chinese traditional medicine (TJ-48). She had no complaint of chest oppression or syncope nor family history of unexpected or abortive SCD in her relatives.
Laboratory examination on admission included serum chemistry showing total protein 5.8 g/dL, albumin 3.2 g/dL, CK 215 IU/L (CK-MB 19 IU/L), CRP 0.3 mg/dL, BNP 348.5 pg/mL, troponin T (TnT) 0.01 ng/mL, Na 139 mEq/L, Ca 9.2 mEq/L, and K 2.1 mEq/L. Hematology indicated mild anemia (hemoglobin of 10.3 g/dl) associated with white blood cell and platelet counts of 8,500/μL and 25.1 × 104/μL, respectively. ECG showed sinus rhythm, left axis deviation, flat or inverted T waves observed in II, III, aVF, V5−6, and QT prolongation (grade I), i.e., QT interval corrected by heart rate (QTc = QT/RR0.5) was 455 ms (Figure 1A). Chest X-ray showed cardiomegaly and chest computed tomography demonstrated bilateral pleural effusion (Figures 2A,B). Ejection fraction was 64% on echocardiogram. Chemotherapeutic regimen including osimertinib, oral itraconazole and herbal medicine (TJ-48) were discontinued, and supplementation of potassium was started. Considering possible ischemic T wave changes (Figure 1A), coronary angiogram was performed. Stenotic lesion (99%) was found in the right coronary artery (segment 1) and collateral circulation supplied from left anterior descending artery was observed (Figures 3A,B). Percutaneous coronary intervention (PCI) was completed successfully by stent implantation at the culprit lesion (Figures 3C,D). QTc interval after the successful PCI and restoration of hypokalemia (K of 4.0 mEq/L) was 432 ms (Figure 1B), and she discharged on foot 33 days after admission without any neurological sequelae of cardiac arrest. Administration of osimertinib (40 mg once daily) was resumed in the hospital where she had been started chemotherapy under the monitoring of ECG and serum potassium concentration while coadministration of itraconazole and herbal drug was interrupted.
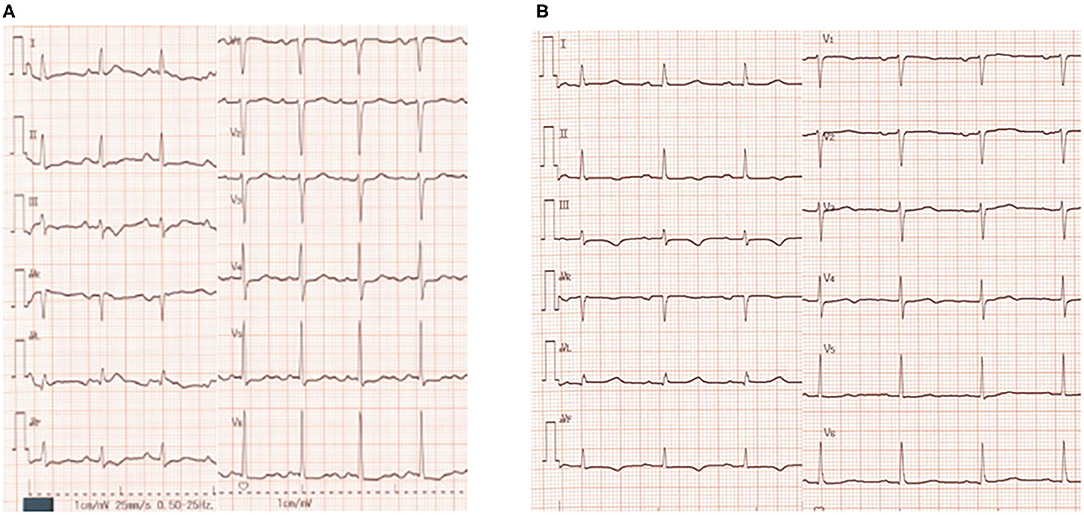
Figure 1. Twelve-lead electrocardiogram (ECG) on admission (A) and before discharge (B). QTc interval was 455 ms (A) and 432 ms (B), respectively. Herat rate correction of QT interval was performed by Bazett's formula.
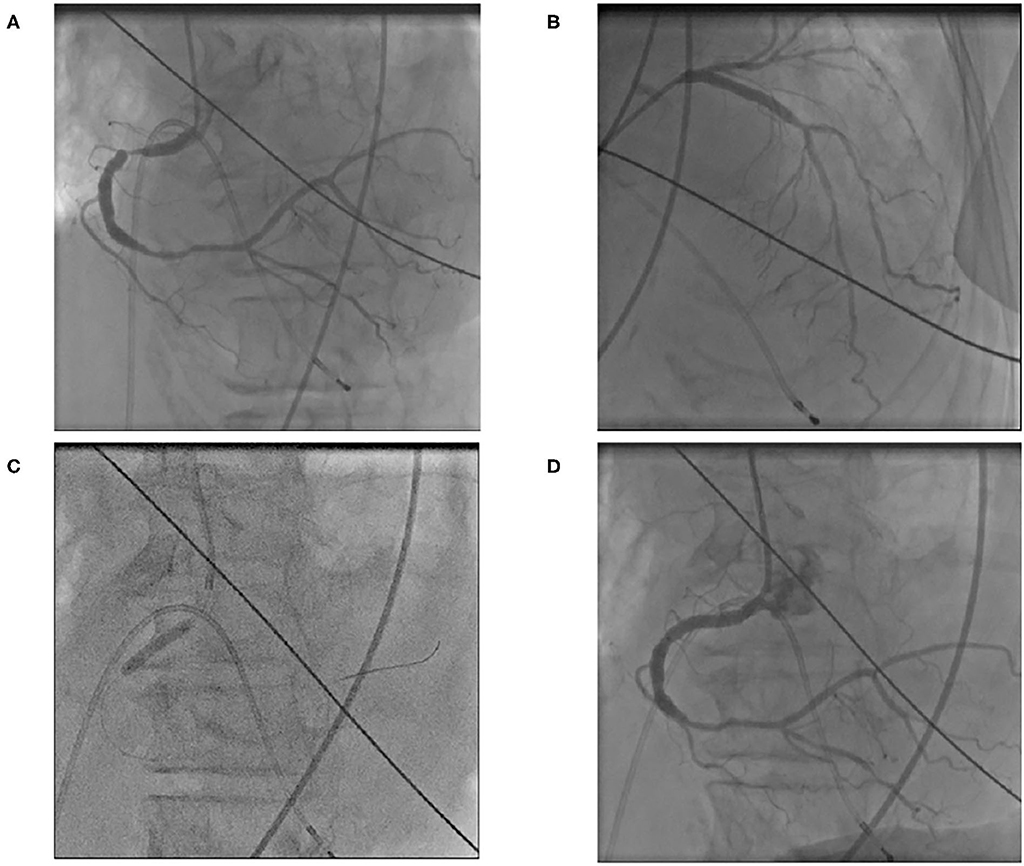
Figure 3. Right coronary angiogram showed severe (99%) stenotic lesion in segment 1 (A). Collateral circulation from left anterior descending artery was visualized (B). Stent implantation was performed (C) and right coronary angioplasty was completed (D).
Osimertinib is an oral, third-generation TKI as an emerging therapeutic modality in various cancers with EGFR T790M mutation (2). One of the obstacles for wide use of this promising anticancer agent is QT prolongation. QT interval is lengthened as a result of ventricular potassium current reduction and late sodium current augmentation (4). Bian et al. (5) reported a first case of 85-year-old male patient with advanced non-small cell lung cancer associated with TdP induced by oral osimertinib (80 mg once daily) and concomitant intravenous moxifloxacin, a broad-spectrum fluoroquinolone antibiotics known to lengthen the QT interval (QTc of 484 ms). Hypokalemia (2.94 mEq/L), impaired left ventricular ejection fraction (41%) and coadministration of moxifloxacin were concluded to underlie the development of TdP. They lost this case and hence cautioned cardio-oncologists to identify cancer patients at high risk of QT prolongation leading to the development of TdP (5). Cancer development and apoptosis is governed by driver mutation of signal transduction process mediated by several growth factor receptor including EGFR which is linked to many potassium channels, i.e., Kv1.3 (6), Kv10.1 (7), Kv4.3 (8), and Kir2.3 (9) are modulated by EGFR kinase via phosphorylation of tyrosine in their specific residues. These potassium channels regulate vascular tonus and QT interval in ECG. Therefore, EGFR-TKI shows variable cardiotoxicity including vascular constriction and QT prolongation. Although the serum concentration of osimertinib was not monitored, and genetic testing of hereditary long QT syndrome was not performed in this case, careful ECG monitoring is essential for cancer patients under the chemotherapy including osimertinib (10).
QT interval is regulated strictly by many factors including basic heart rate, electrolytes, body temperature, sex hormones, and so on. Therefore, key factor of QT prolongation in this case is not determined easily. This case is an 85-year-old female patient, and old woman per se is a risk factor of QT prolongation (1). Oral itraconazole, a representative antifungal agent, is known to elevate the serum concentration of osimertinib due to competitive metabolism mediated by cytochrome P450 3A4 (CYP3A4). Chronic myocardial ischemia requiring PCI and hypokalemia (2.1 mEq/L) caused partly by Chinese herbal drug may have also contributed to the QT prolongation. Osimertinib causes coronary tonus elevation induced by vascular constriction mediated by inhibition of vascular EGFR (10), which may have aggravated silent myocardial ischemia in this case. Chinese traditional medicine of TJ-48 is used widely in Japan under the adjuvant anticancer therapeutic strategy as in this case. This herbal medicine is reported to enhance the efficacy of chemotherapy and to attenuate the adverse effects and complications of anticancer treatment (11). However, long-term use of Chinese herbal medicines is sometimes associated with hypokalemia caused by pseudoaldosteronism that is evoked by licorice contained in many herbal medicines. Although the incidence of hypokalemia induced by the administration of TJ-48 is not reported, such incidence by other Chinese medicine ranges from 6.6% (TJ-54) to 33% (TJ-9 and TJ-68) according to the Japanese Adverse Drug Report Database (12).
Considering the serum chemistry (normal CK-MB isozyme and negative TnT test) and echocardiographic left ventricular wall motion, cardiac arrest caused by acute coronary syndrome was unlikely. Silent myocardial ischemia is associated with QT prolongation caused by electrical instability and sympathovagal imbalance (13), and QT prolongation per se is responsible for SCD whatever the causes to prolong QT intervals are (14). Well-developed collateral circulation may have made coronary artery disease silent in this case (Figures 3A,B). This case description includes limitations, i.e., ECG was not retrieved from AED, serum TnT was assayed only once, and serum concentration of osimertinib was not assayed. In spite of these limitations, cardiac arrest in this case was supposed to be due to possible development of TdP based on several predisposing factors lengthening QT interval. Because defibrillation by means of public AED restored her spontaneous circulation, and recurrence of cardiac arrest was prevented after normalization of QT interval by elective PCI, discontinuation of causative drugs and potassium supplementation. Although QT prolongation in this case was grade I, this interval was measured at rest and supine position. QT interval dynamicity is accentuated by daily physical activity (15), and the relation between sudden increase in QT interval and the development of TdP is open to debate. This case taught us the necessity of collaboration between oncologists and cardiologists in the earlier clinical course of cancer patients.
We have described an 85-year-old female patient with EGFR-mutant advanced lung cancer treated with standard regimen of osimertinib, which underlay in part grade I QT prolongation causing abortive SCD. Reversible factors promoting QT prolongation were removed after admission and this patient was discharged safely. Although osimertinib-induced QT prolongation is mild to moderate (grade I or II) and not frequent (3–10%) in AURA study and FLAURA trial to verify the safety and efficacy of osimertinib (16, 17), TdP may be induced by standard dose of osimertinib, when multiple risk factors to lengthen QT interval are incidentally overlapped. Careful ECG monitoring and appropriate management of risk factors lengthening QT interval such as interacting drugs, electrolyte imbalance and cardiac ischemia during chemotherapy including osimertinib are highly required.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
This case report article was carried out in collaboration between all coauthors. MKo was taking main care of this case. MKi had an equal contribution to this case report. YK was supervising MKo and MKi. SG was performing PCI in this case. OK was investigating the novelty and the rarity of this case. SK was exploring the literature relating to this case. TM performed writing of the main part of this manuscript. KO had the initial concept of this manuscript preparation and provided this manuscript with deep insight. YM supervised collaboration of the cardiology team. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank ward staffs of Kyushu Central Hospital for clinical assistance.
1. Sadasivan C, Zhabyeyev P, Labib D, White JA, Paterson DI, Oudit GY. Cardiovascular toxicity of PI3Kα inhibitors. Clin Sci. (2020) 134:2595–622. doi: 10.1042/CS20200302
2. Schiefer M, Hendriks LEL, Dinh T, Lalji U, Dingemans AC. Current perspective: osimertinib-induced QT prolongation: new drugs with new side-effects need careful patient monitoring. Eur J Cancer. (2018) 91:92–8. doi: 10.1016/j.ejca.2017.12.011
3. Porta-Sánchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies. A systematic review. J Am Heart Assoc. (2017) 6:e007724. doi: 10.1161/JAHA.117.007724
4. Zhabyeyev P, McLean B, Chen X, Vanhaesebroeck B, Oudit GY. Inhibition of PI3Kinase-α is pro-arrhythmic and associated with enhanced late Na+ current, contractility, and Ca2+ release in murine hearts. J Mol Cell Cardiol. (2019) 132:98–109. doi: 10.1016/j.yjmcc.2019.05.008
5. Bian S, Tang X, Lei W. A case of torsades de pointes induced by the third- generation EGRF-TKI, osimertinib combined with moxifloxacin. BMC Pulm Med. (2020) 20:181. doi: 10.1186/s12890-020-01217-4
6. Teisseyre A, Palko-Labuz A, Sroda-Pomianek K, Michalak K. Voltage-gated potassium channel Kv1.3 as a target in therapy of cancer. Front Oncol. (2019) 9:933. doi: 10.3389/fonc.2019.00933
7. Wu W, Dong MQ, Wu XG, Sun HY, Tse HF, Lau CP, et al. Human ether-à-go-go gene potassium channels are regulated by EGFR tyrosine kinase. Biochim Biophys Acta. (2012) 1823:282–9. doi: 10.1016/j.bbamcr.2011.10.010
8. Zhang YH, Wu W, Sun HY, Deng XL, Cheng LC, Li X, et al. Modulation of human cardiac transient outward potassium current by EGFR tyrosine kinase and Src-family kinases. Cardiovasc Res. (2012) 93:424–33. doi: 10.1093/cvr/cvr347
9. Zhang DY, Zhang YH, Sun HY, Lau CP, Li GR. Epidermal growth factor receptor tyrosine kinase regulates the human inward rectifier potassium K(IR)2.3 channel, stably expressed in HEK 293 cells. Br J Pharmacol. (2011) 164:1469–78. doi: 10.1111/j.1476-5381.2011.01424.x
10. Chaar M, Kamta J, Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther. (2018) 11:6227–37. doi: 10.2147/OTT.S170138
11. Wang Z, Qi F, Cui Y, Zhao L, Sun X, Tang W, et al. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics. Biosci Trends. (2018) 12:220–39. doi: 10.5582/bst.2018.01144
12. Ishida T, Kawada K, Morisawa S, Jobu K, Morita Y, Miyamura M. Risk factors for pseudoaldosteronism with Yokusankan use: analysis using the Japanese Adverse Drug Report (JADER) database. Biol Pharm Bull. (2020) 43:1570–6. doi: 10.1248/bpb.b20-00424
13. Tomassoni G, Pisanó E, Gardner L, Krucoff MW, Natale A. QT prolongation and dispersion in myocardial ischemia and infarction. J Electrocardiol. (1998) 30(Suppl.):187–90. doi: 10.1016/s0022-0736(98)80073-3
14. O'Neal WT, Singleton MJ, Roberts JD, Tereshchenko LG, Sotoodehnia N, Chen LY, et al. Association between QT interval components and sudden cardiac death: the ARIC study. Circ Arrhythm Electrophysiol. (2017) 10:e005485. doi: 10.1161/CIRCEP.117.005485
15. Nakaji G, Fujihara M, Fukata M, Yasuda S, Odashiro K, Maruyama T, et al. Open-loop, clockwise QT-RR hysteresis immediately before the onset of torsades de pointes in type 2 long QT syndrome. J Electrocardiol. (2010) 43:261–3. doi: 10.1016/j.jelectrocard.2009.12.005
16. Yang JCH, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. (2017) 35:1288–96. doi: 10.1200/JCO.2016.70.3223
Keywords: cancer, herbal drug, osimertinib, QT prolongation, cardiooncology
Citation: Kondo M, Kisanuki M, Kokawa Y, Gohara S, Kawano O, Kagiyama S, Maruyama T, Odashiro K and Maehara Y (2021) Case Report: QT Prolongation and Abortive Sudden Death Observed in an 85-Year-Old Female Patient With Advanced Lung Cancer Treated With Tyrosine Kinase Inhibitor Osimertinib. Front. Cardiovasc. Med. 8:655808. doi: 10.3389/fcvm.2021.655808
Received: 19 January 2021; Accepted: 22 February 2021;
Published: 19 March 2021.
Edited by:
Margaret Rose Cunningham, University of Strathclyde, United KingdomReviewed by:
Gavin Oudit, University of Alberta, CanadaCopyright © 2021 Kondo, Kisanuki, Kokawa, Gohara, Kawano, Kagiyama, Maruyama, Odashiro and Maehara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toru Maruyama, bWFydXlhbWFAYXJ0c2NpLmt5dXNodS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.