- 1The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 2Key Laboratory of Integrative Chinese and Western Medicine for the Diagnosis and Treatment of Circulatory Diseases of Zhejiang Province, Hangzhou, China
With the continuous improvement of living standards but the lack of exercise, aging-associated metabolic diseases such as obesity, type 2 diabetes mellitus (T2DM), and non-alcoholic fatty liver disease (NAFLD) are becoming a lingering dark cloud over society. Studies have found that metabolic disorders are near related to glucose, lipid metabolism, and cellular aging. Fibroblast growth factor 21 (FGF21), a member of the FGFs family, efficiently regulates the homeostasis of metabolism and cellular aging. By activating autophagy genes and improving inflammation, FGF21 indirectly delays cellular aging and directly exerts anti-aging effects by regulating aging genes. FGF21 can also regulate glucose and lipid metabolism by controlling metabolism-related genes, such as adipose triglyceride lipase (ATGL) and acetyl-CoA carboxylase (ACC1). Because FGF21 can regulate metabolism and cellular aging simultaneously, FGF21 analogs and FGF21 receptor agonists are gradually being valued and could become a treatment approach for aging-associated metabolic diseases. However, the mechanism by which FGF21 achieves curative effects is still not known. This review aims to interpret the interactive influence between FGF21, aging, and metabolic diseases and delineate the pharmacology of FGF21, providing theoretical support for further research on FGF21.
Introduction
Energy metabolism generally refers to the release, transfer, and utilization of energy in the process of biomass metabolism. Energy metabolism is mainly related to glucose, lipids, and proteins, among which lipometabolism and glycometabolism are particularly important. Lipid metabolism refers to digestion, absorption, synthesis, and decomposition of lipids with various related enzymes. Glucose metabolism is responsible for the formation and storage of glucose with the help of insulin (1). There is an interactive influence between glucose and lipid metabolism (2). Insulin resistance causes hyperinsulinemia, which inhibits lipolysis, increases lipid synthesis, and causes excessive lipid accumulation. Abnormal lipid metabolism, especially the accumulation of heterotopic lipids, promotes decreased insulin sensitivity in adipose tissue. The balance of energy storage and release, also known as energy homeostasis, is crucial for overall health and even survival (3). A long-term metabolic imbalance will lead to excess lipid accumulation and further contribute to obesity, non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM), known as aging-related metabolic diseases (4, 5). Adipose tissue dysfunction and excessive lipid accumulation are the basis of the pathogenesis of metabolic disease (6).
Aging is characterized by a deterioration in homeostatic process maintenance over time, leading to functional decline and increased risk for diseases. Thus, energy homeostasis is gradually disrupted with aging, and the risk of energy metabolism-related diseases increases (7). Some studies have revealed compelling evidence that energy metabolism has a crucial interaction with anti-aging regulation (8, 9). Selective elimination of aging cells can ameliorate several aging-dependent energy metabolic diseases (10, 11).
Fibroblast growth factor 21 (FGF21) is a peptide hormone synthesized by multiple organs and regulates energy homeostasis (12, 13). As a FGFs family member, FGF21 is crucial because it can directly improve lipid and glucose metabolism in cells and delay cellular aging (14). Thus, the association between FGF21, energy metabolic diseases, and aging has recently attracted increasing attention. We illustrate this systematically in this review.
Connection Between Aging and Metabolism
Cellular aging is an irreversible state of cell cycle arrest induced by various stressors, including telomere dysfunction, genotoxicity, and oxidative stress (15). Telomere shortening, which occurs after cell divisions, is a common cause of internal cellular aging. After several divisions, cells may activate p53, p21, and pRb pathways due to telomere shortening, promoting growth arrest and cell aging. Cellular aging is a complex process with dual functions, which are both beneficial and harmful to health. Aging helps clear damaged cells and is involved in tissue recovery during injury or acute stress. Senescence-associated secretory phenotype (SASP) secretes chemokines and cytokines (such as IL-1B and MCP-1) to attract immune cells and clear aging cells (16). However, consistently, excessive SASP induced by cellular aging will accumulate too many aging cells, causing insufficient tissue regeneration (17). Aging affects multiple organs, mainly those with high metabolic demands such as liver, heart, and brain (18). Therefore, aging may be a major risk factor for many metabolism-related diseases (19) and closely related to metabolism.
Metabolic dysregulation (including mitochondrial dysfunction) is one of the aging hallmarks (7, 20). Interestingly, aging-associated pathways (such as AMPK and mTOR), which are significant targets of anti-aging interventions, either directly regulate or intersect with metabolic pathways (21). With the development of technology, metabolomics has received increasing attention and quantitatively analyzes all metabolites in organisms (22, 23). Through metabolomic analysis, it was found that the existence of specific metabolic intermediates is an anti-aging intervention target, and even more directly a biomarker of aging (24). These hub metabolites represent nodes in the metabolism and aging network that play a crucial role in regulating information flow between metabolism and signaling pathways to control aging.
Nicotinamide adenine dinucleotide (NAD+), a common hub metabolite, is a crucial center connecting metabolism and aging. As an essential cofactor, NAD+ plays a central role in regulating energy metabolism, including glycolysis, fatty acid oxidation, and tricarboxylic acid (TCA) cycle, and it can also mediate DNA repair and gene expression (25). Recent studies have highlighted the various roles of NAD+ in aging. A metabolomics study quantified the plasma level of NAD+ in people aged 20 to 87, showing that the levels of NAD+ decreased significantly with age (26). Reduced levels of NAD+ are associated with several aging-related diseases, including metabolic diseases, cancer, and neurodegenerative diseases (27). Additionally, the dietary administration of NAD+ precursors has been shown to increase NAD+ levels in aging tissues, thereby improving aging and aging-related diseases (28, 29). Research has directly proven that NAD+ can directly control SASP and regulate cellular aging (30). The above studies have confirmed that the metabolite NAD+ can directly regulate cellular aging. Moreover, not only NAD+, nicotinamide adenine dinucleotide phosphate (NADP), and αKG can regulate cellular aging (31–33).
Scholars have also directly confirmed that aging is affected by metabolism through animal experiments. Mlekusch et al. found that controlling the exercise of mice, leading to a decrease in metabolic levels, will cause mice to age and shorten their life span (34). Even as early as over a century ago, it was discovered that the metabolic rate is related to aging: Rubner discovered that smaller animals have a higher resting metabolic rate. Based on this, a “rate of living hypothesis” was created, which believed that exhausting a limited number of metabolic events would lead to death (35, 36). This theory is not only applicable to mammals. The metabolic rate of birds is twice that of mammals of the same size, but their average life span is approximately three times that of mammals that match their body mass (37).
Therefore, there is no doubt that metabolism and aging are closely related, but whether FGF21 is related to it or whether it can be used as a connection point between metabolism and aging is still unknown. We will elaborate below.
The Relationship Between FGF21, Metabolism, and Aging
FGF21 and Its Family
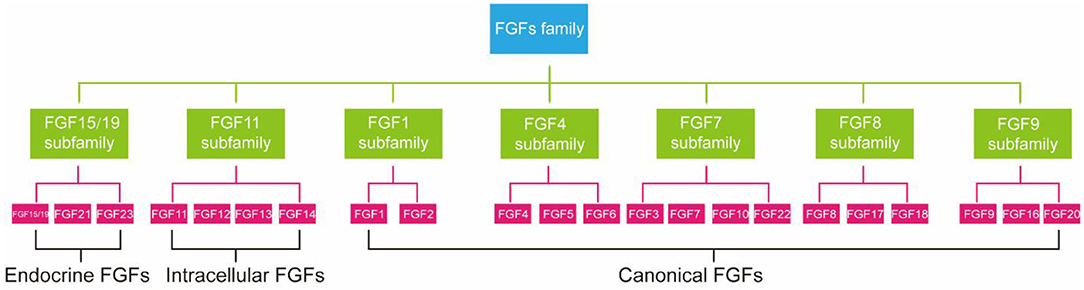
FGFs family consists of 23 members but only 18 FGFR ligands. Four family members (FGF11, FGF12, FGF13, and FGF14) cannot bind to FGFR are more correctly referred to as FGF homologous factors (38). FGFs are effective regulators of cellular aging. Not only that, mutations in FGFs have been linked to many metabolic diseases, including atherosclerosis, NAFLD, and diabetes (39). According to phylogenetic analysis, FGFs family members can be further divided into seven subfamilies (Figure 1) (40). FGF21 is a member of an endocrine FGF subfamily, which includes FGF15/19 and FGF23. FGF21 can circulate and diffuse freely in tissues as an endocrine factor because of the lack of heparin-binding domain (41). FGF21 is expressed in many tissues, including liver (42), adipocytes (43), brown adipose tissue (BAT), pancreas (44), gastrointestinal tract, brain, skeletal muscle, and heart, and it directly regulates the metabolism and aging of peripheral tissues (12). Next, we will introduce the relationship between FGF21 metabolism and aging.
FGF21, the Hub Linking Metabolism and Aging?
In 2005, FGF21 was first used as a novel metabolic regulator (45). As a coordinator of energy metabolism in multiple organs (especially liver and fat), FGF21 can regulate adipogenesis, glucose uptake, and cellular insulin sensitivity (12, 46–48). The level of FGF21 is elevated to promote the oxidation of free fatty acids (FFAs) and inhibit lipogenesis in the liver to supply energy when glucose levels are low or caloric restricted (49). In an animal experiment, Inagaki et al. found that FGF21 expression increased 28-fold in the livers of mice after 12 h of fasting. Increased FGF21 expression will stimulate ketogenesis in the liver and promote lipolysis in white adipose tissue to provide energy for activities (50). In addition to mice, in the fasting state, the level of FGF21 in human serum will also increase rapidly within a few hours to promote lipolysis (51). Proper administration of FGF21 also effectively improved insulin sensitivity and hepatic glucose uptake in obese mice (52). Studies have found that long-term administration of FGF21 to genetically obese mice will ameliorate fasting hyperglycemia via increased glucose uptake and improved hepatic insulin sensitivity (53). Lack of FGF21 in mice evokes insulin resistance and promotes gluconeogenesis and liver glucose production (54). In addition to liver and adipose tissue, the expression of FGF21 in skeletal muscle also has important metabolic functions. FGF21 could improve muscular dystrophy and atrophy through metabolic pathways (55, 56).
In addition to acting as a metabolic regulator, FGF21 can also improve aging. There are clear indications that the effect of FGF21 in preventing aging may be related to the thymus; overexpression of FGF21 can prevent aging-related changes, such as retarding thymus degeneration to prevent thymus weakness, improving immune system, and hopefully extending human life expectancy in the future (57). With aging, tissue autophagy is reduced, disrupting tissue ability to maintain protein homeostasis, thus accelerating the aging process (57). FGF21 can stimulate adiponectin secretion in fatty tissues, thus improving autophagy in target tissues to play an anti-aging role (58). Compared with normal mice, animal experiments show that fasting-induced FGF21 overexpression in mice and slowed aging (59). Transgenic overexpression of FGF21 significantly extended the life span of mice without reducing food intake or affecting NAD+ metabolism (60).
In summary, FGF21 mainly regulates aging by metabolism. It is crucial to clarify the mechanism or pathways of FGF21 in regulating metabolism and aging.
The Mechanism by Which FGF21 Regulates Aging Through Metabolism
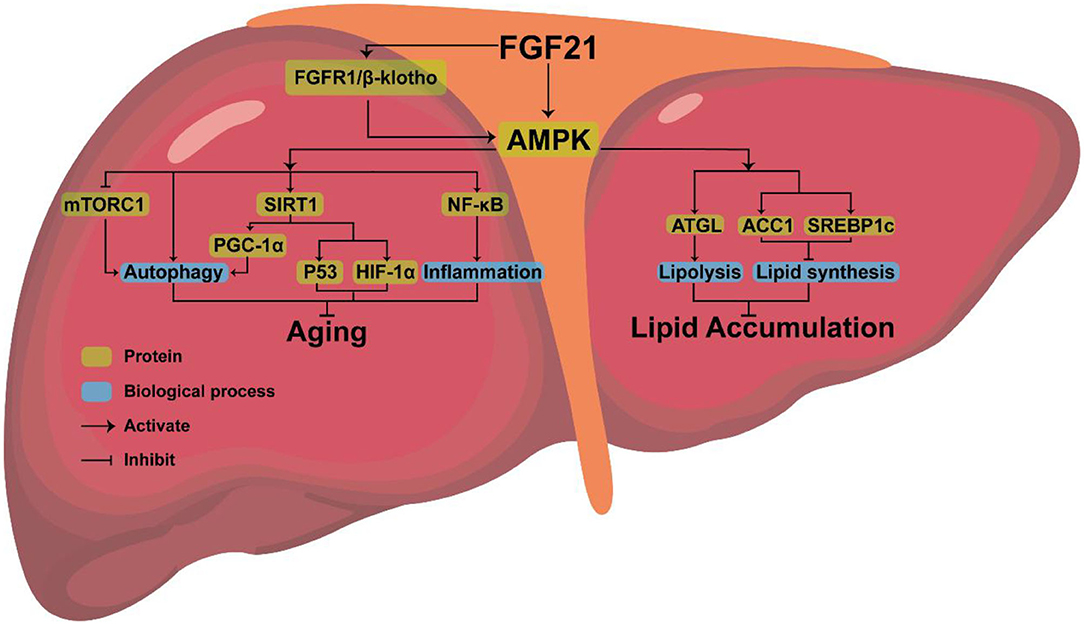
FGF21, a new type of endocrine hormone, is primarily produced by liver (61). Klotho proteins include α-klotho and β-klotho, of which β-klotho is an essential part of the FGF21 receptor complex and necessary for promoting high-affinity binding to its homologous FGF receptor (62). FGF21 signals through a receptor complex composed of fibroblast growth factor receptor 1 (FGFR1) and the coreceptor β-klotho, both required for FGF21 signaling and then activates downstream genes, to exerts its effect (63, 64). Studies have found that, as an endocrine messenger, FGF21 could induce hormonal responses in other tissues, such as the secretion of adiponectin from fat tissue and corticotropin-releasing hormone (CRH) from the hypothalamus, to maintain metabolic homeostasis (65). In addition, endocrine FGF21 can stimulate the secretion of digestive enzymes from pancreatic acinar cells, which require signaling through a tyrosine kinase receptor complex composed of an FGF receptor and β-Klotho to enhance the digestion of food in stomach (66). FGF21 can also indirectly maintain metabolic homeostasis by activating downstream pathways (67). In cultured adipocytes, FGF21 could regulate metabolism by activating MAPK and downstream ERK1/2, which triggers the activation of GLUT1 and glucose uptake (68). In liver, FGF21 positively controls the PI3K/AKT, insulin-like growth factor 1 (IGF-1), and mTOR pathways to maintain metabolic homeostasis (69).
AMPK, the downstream protein of FGF21, consists of three subunits (AMPKα, AMPKβ, AMPKγ), each with multiple phosphorylation sites, which can regulate lipid metabolism (70). AMPK phosphorylates sterol regulatory element binding protein-1c (SREBP1c) at Ser372, inhibiting the proteolytic cleavage of the precursor SREBP1c to mature SREBP1c, thereby inhibiting steatosis in diet-induced hepatic insulin-resistant mice (71). AMPK can also indirectly inhibit the expression of SREBP1c by reducing mTORC activity, thus decreasing liver lipid content (72) or phosphorylating adipose triglyceride lipase (ATGL), to stimulate TG lipase activity and activate lipolysis (73).
Amazingly, AMPK is highly conserved in eukaryotes, giving them the ability to expand their lifespan (74). By activating AMPK, FGF21 may delay aging and extend mammals' lifespan (75). Increased longevity has been observed in transgenic worms expressing the modified AMPK-γ subunit (76, 77). Overexpression of a single AMPK-α subunit in the fat body also extended the life span of fruit flies (78).
As a pro-longevity kinase, AMPK can also prevent cellular aging by activating downstream pathways (79–81). AMPK is associated with some downstream pathways involved in controlling aging, such as rapamycin complex 1 (mTORC1), nuclear factor kappa-B (NF-κB), and sirtuin-1(SIRT1). Low levels of inflammation promote aging (82). AMPK may extend longevity by inhibiting the NF-κB pathway and NF-κB-mediated inflammatory response (83). Activation of mTORC1 will inhibit autophagy and accelerate aging, while FGF21 exposure can inhibit the activation of mTORC1 in the liver resulting in anti-aging effects (84). AMPK can directly activate SIRT1 (75, 85, 86). After being triggered, SITR3 will mediate autophagy to anti-aging (87). FGF21 regulates mitochondrial biogenesis by activating PGC-1α through the FGF21-AMPK-SIRT1 pathway (88). FGF21 also stimulated the expression of PGC-1α in mouse liver (89) and human dopaminergic neurons (90, 91). Improved mitochondrial function and activation of PGC-1α play a crucial anti-aging role (92). AMPK-SIRT1 axis is also connected to several other aging-linked targets, such as p53 and HIF1α (93). Therefore, it is clear that anti-aging effects can be achieved by activating AMPK, but whether FGF21 can inhibit aging by upregulating the expression of AMPK requires further experimental proof.
Combined with the above analysis, we believe that AMPK, the downstream protein of FGF21, may be the key to FGF21 simultaneous aging and metabolism regulation (Figure 2).
The Actions of FGF21 in Aging-Related Metabolic Diseases
The possible mechanism by which FGF21 simultaneously improves metabolism and cellular aging has been described above. However, the relationship between FGF21 and various aging-related metabolic diseases has not yet been clarified; thus, in the following, we will focus on the relationship between FGF21 and obesity, T2DM, and NAFLD.
FGF21 With Obesity
Obesity is a global epidemic metabolic disease that affects infants, children, and adults (94). Over the past three decades, the global prevalence of obesity has nearly doubled; the number of obese and overweight people has exceeded that of underweight people globally (95). Obesity is associated with several complications, such as cardiovascular disease, hypertension, dyslipidemia, NAFLD, insulin resistance, hyperglycemia, T2DM, and is related to neurodegenerative diseases, and cancer (96, 97). Thus, it is urgent to pay more attention to obesity.
Studies have found that FGF21 is nearly negatively related to obesity. FGF21 transgenic mice fed high-fat, high-carbohydrate (HFHC) food are resistant to weight gain and obesity (45). Activation of FGF21 will cause weight loss in obese patients. For example, recombinant FGF21 treatment can reduce weight without changing the food intake of diet-induced obese (DIO) mice (98). After FGF21 intervention for 6 weeks, the weight and fat content of DIO mice were significantly lower than those of mice that did not receive FGF21 treatment. Lipid homeostasis and hepatic steatosis in DIO mice in the FGF21 treatment group were significantly improved. An animal experiment also found that chronic treatment with recombinant FGF21 reduced serum and liver TGs levels in diet-induced obese mice by inhibiting sterol regulatory element-binding protein-1 (SREBP-1), a transcription factor critical for fat formation, to achieve the goal of weight loss (98). Potential application of FGF21 as an anti-obesity molecule has even been approved in a study (99), in which exogenous FGF21 was administered.
Interestingly, FGF21 can promote weight loss, and FGF21 analogs also have the same effect. The FGF21 analog Fc-FGF21 protein (RG), results in weight gain inhibition in diabetic mice (100). FGF21, an FGF21 mutant used in combination with other weight-reducing drugs or exercise, could effectively cause weight loss in db/db mice (101). Recombinant murine FGF21 and leptin coadministration can also reduce the weight of mice (102). As a result, FGF21 is currently considered a potential target for the treatment of obesity.
FGF21 With T2DM
Diabetes mellitus (DM) is defined as a metabolic disease characterized by hyperglycemia due to insulin secretion, insulin action, or a combination of both. T2DM, one subtype of DM, accounts for 90–95% of diabetic cases (103). World Health Organization (WHO) estimates that 347 million people worldwide have DM, of which 90% are T2DM (104). T2DM is characterized by insulin resistance, a relative lack of insulin, and hyperglycemia. Complications of T2DM include heart disease, stroke, diabetic retinopathy, and kidney failure (105). Because of its pervasiveness and dangerous effects, T2DM deserves more attention.
Some studies have found that FGF21 is an emerging T2DM treatment target. FGF21 can increase the therapeutic benefits of antidiabetic compounds such as metformin, glucagon/glucagon-like peptide 1 (GLP1) analogs, and thiazolidinedione (TZD) (106). The direct use of FGF21 can also reduce plasma glucose and TGs to near-normal levels in diabetic patients and animals. The experiment found that administering human recombinant FGF21 for 6 weeks can significantly improve fasting plasma glucose, TGs, insulin, and glucagon in diabetic rhesus monkeys (107). Interestingly, although human recombinant FGF21 has an excellent effect on lowering plasma glucose, it will not cause a hypoglycemic crisis. Human recombinant FGF21 can also improve the lipoprotein profile, including increasing high-density lipoprotein cholesterol, lowering low-density lipoprotein cholesterol, and significantly decreasing weight. Studies have also found that FGF21 can protect islets from glycolipid toxicity and cytokine-induced apoptosis and increase the insulin content of pancreatic β cells, which helps maintain glucose homeostasis in T2DM mice (108). Taken together, these results all support the idea that FGF21 is feasible for treating T2DM.
FGF21 With NAFLD
NAFLD is one of the most common liver diseases globally, and it affects 25% of the population globally and 8% of children (109, 110). The disease is characterized by accumulating TGs in hepatocytes with little or no alcohol consumption (111). The NAFLD spectrum includes non-alcoholic fatty liver (NAFL) in the early stage, non-alcoholic steatohepatitis (NASH) in the middle stage, fibrosis in the late stage, and liver fibrosis associated with cirrhosis, liver failure, and even hepatocellular carcinoma (HCC) (112). Moreover, NAFLD is predicted to be the most frequent indication for liver transplantation in Western countries by 2030 (113).
There is growing evidence that aging is a vital risk factor for the occurrence of NAFLD (114, 115). Studies have found that cellular aging can induce mitochondrial dysfunction and reduce fat metabolism, resulting in excessive accumulation of hepatocytes and fatty degeneration (116). Mitochondrial dysfunction contributes to increased reactive oxygen species (ROS) (117, 118). Excessive ROS will lead to abnormal inflammation of the liver, leading to the deterioration of NASH. In aged mice fed a high-fat diet, age-related mitochondrial dysfunction further promotes oxidative stress, leading to worsening of NAFLD (119). In an animal experiment, rats were divided into obesity-prone and obesity-resistant groups. The former group showed more severe steatosis and significantly increased mRNA levels of p16 and p21, cellular aging-related genes, in the liver (120). In vitro studies found that the lack of cellular aging-related gene p53 in primary cultured hepatocytes could reduce the level of apoptosis and steatosis (121). Liver biopsies from patients with NAFLD also showed a significant increase in p53 expression in the liver compared to normal controls (122). These experimental results all confirmed that the overexpression of aging-related genes could promote hepatocyte steatosis, suggesting a crucial relationship between aging and NAFLD.
NAFLD can be improved by taking FGF21 as a therapeutic target, which is crucial for regulating cellular aging and energy metabolism. Some studies found that administration of FGF21, FGF21 analogs, or adenoviral delivery of FGF21 will reduce hepatic steatosis in diverse rodent models of NAFLD (123–125). In diet-induced obese mice, FGF21 or FGF21 analogs also decreased the expression of lipogenic genes, including stearoyl-CoA desaturase-1(SCD1), fatty acid synthase (FASN), and sterol regulatory element binding transcription factor 1 (SREBF1) (126). Inhibition of the expression of the lipogenic genes can effectively inhibit the synthesis of lipids and promote lipolysis. Acting as an autocrine agent, FGF21 can also activate genes that protect against oxidative stress to treat NAFLD. Many animal experiments support this conclusion. FGF21 also protects mice from acetaminophen-induced hepatic oxidative damage (127). In obese diabetic mice, FGF21 treatment reduces hepatic oxidative damage and lipid peroxidation (128). Moreover, in wild-type mice, FGF21 increases transcription of the oxidative stress response and antioxidant genes, including superoxide dismutase 2 (Sod2), glutathione peroxidase 1 (Gpx1), sirtuin (Sirt1), and forkhead box transcription factor 3 (Foxo3) (129).
Potential Therapeutic Pharmacology of FGF21
FGF21 has beneficial pharmacological effects on T2DM, obesity, and NAFLD, and a large number of preclinical studies have been reported (99, 130). A single injection of FGF21 in obese (ob/ob) mice and DIO mice, can cause a rapid reduction in blood glucose and plasma insulin levels. At the same time, glucose tolerance and insulin sensitivity improve (131). Nevertheless, the application of natural FGF21 as a drug has encountered some obstacles. Because it requires intravenous administration and the circulation half-life (0.5–2 h) is too short, it may be due to rapid renal clearance and proteolysis. Thus, this has led to the development of FGF21 analogs and FGF21-receptor agonists.
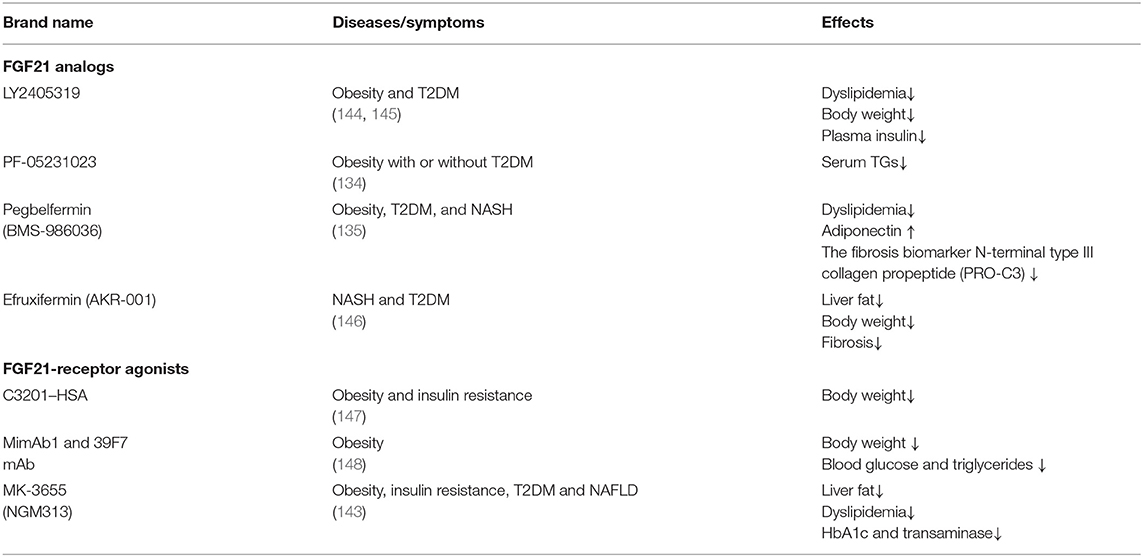
LY2405319 (Eli Lilly and Co.), an FGF21 analog, was the first analog to be applied in obese patients with T2DM (132). It can improve dyslipidemia, reduce plasma insulin and body weight, and increase adiponectin levels. Nevertheless, only a tendency to decrease glucose was observed. This study shows that the treatment of LY2405319 is generally well-tolerated. PF-05231023 (Pfizer Co.) consists of two recombinant FGF21 molecules fused into the antibody fragment, which is a long-acting FGF21 analog that is can be administered once a week. For T2DM patients with hypertriglyceridemia who received a single dose of PF-05231023 (0.5–200 mg), dose-dependent decreases in triglycerides were observed. In addition, total cholesterol and low-density lipoprotein cholesterol decreased, while high-density lipoprotein cholesterol increased in the high-dose group (133). Another study with PF-05231023 reported a direct effect of FGF21 in the absence of weight loss (134). Recently, two clinical trials showned that pegbelfermin (BMS-986036), a PEGylated long-acting FGF21 analog (Bristol-Myers Squibb Co.), can be administered once a week. Subcutaneous injection of pegbelfermin for 12 weeks in obese and T2D patients can improve dyslipidemia, increase adiponectin and reduce the N-terminal level of fibrosis biomarker type III collagen propeptide (PRO-C3) without causing changes in HbA1c (135). A phase IIa clinical trial confirmed the efficacy of pegbelfermin in the treatment of NASH. Patients in this trial received subcutaneous pegbelfermin once a week for 16 weeks (136). The results showed that liver fat content in patients with NASH was significantly reduced and well-tolerated. Efruxifermin (AKR-001) is an FGF21-fc analog that has a sustained effect on insulin sensitivity and lipid metabolism in patients with T2DM. Short-term adverse reactions are limited, but more research is needed to study potential long-term safety issues (137).
In addition to FGF21 analogs, the FGFR1/β-klotho complex (FGF21 receptor agonists, FGF21RAs) opened a new door for aging-associated metabolic diseases, which have been tested in non-human primates (NHPs) and humans (138, 139). Thus far, it is unclear whether FGF21 receptor agonists have therapeutic advantages over FGF21 analogs. The first FGF21RA is C3201, an 18 kDa bispecific avimer peptide with high affinity and specificity for FGFR1 and β-klotho (140). The avimer showed FGF2-like activity, which was more potent than FGF21. It had a terminal half-life of 50 h after fusion with human serum albumin (C3201-HSA), and simulated the effects of FGF21 on obese monkeys, which lowered body weight. Due to the excellent pharmacokinetics and targeting specificity of monoclonal antibodies (mAbs), many research methods have been used to develop agonist mAbs for FGF21 receptor complexes. Two fully humanized FGF21-mimetic mAbs (mimAb1 and 39F7 mAb) bind to distinct conformational epitopes of β-klotho with high affinity and specifically activate cellular signaling via the FGFR1c–β-klotho complex. Injection of mimab1 in obese monkeys, resulted in FGF21 like metabolic effects, including body weight, plasma insulin, plasma triglyceride and glucose levels (141). In vitro characterization demonstrated that, 39F7 mAb is specific for β-Klotho/FGFR1c activation, but it does not compete with FGF21. Furthermore, the agonistic activity of 39F7 mAB required the full IgG molecule to be bivalent, suggesting that 39F7 functions by promoting β-Klotho/FGFR1c dimerization (142). Recently, Merck Sharp & Dohme is developed a monthly antibody MK-3655 (previous name NGM313) that activates the β-klotho-FGFR1c complex. A single-dose of MK-3665 can reduce liver fat content, improve dyslipidemia, and reduce HbA1c and transaminase in patients with obesity, insulin resistance and NAFLD (143) (Table 1).
FGF21 analogs and FGF21RA tested in clinical trials are generally well-tolerated. However, PF-05231023 increased heart rate and blood pressure and caused moderate changes in bone resorption and resorption markers, which is consistent with the effect of FGF21-induced bone loss (139). Therefore, PF-05231023 improved the safety of FGF21 induced bone loss, which was observed in mice. Another side effect is the production of anti-fgf21 antibody caused by the immunogenicity of engineered FGF21, which was detected in more than 50% of patients treated with pegbelfermin and those treated with ly2405319(135). Of note, the duration of all the above clinical trials for FGF21 treatment is quite short (several weeks). Since FGF21 based treatment is targeted for chronic metabolic diseases, which often require lifelong medication, the safety issues related to long-term treatment need to be thoroughly examined in the future.
Summary
Aging and metabolism are inextricably linked. With aging, metabolic function decreases, and metabolic homeostasis becomes unbalanced. Long-term metabolic imbalance will also accelerate cellular aging. Studies have found that steatotic hepatocytes always display severe DNA damage and cell cycle arrest, indicating that they have entered an aging state (149). Inducing aging cells in vivo and in vitro will disrupt the metabolic balance and promote excessive lipid deposition.
As a metabolism regulator, FGF21 can regulate the homeostasis of lipid and glucose metabolism and improve cellular aging (150, 151). AMPK, the downstream protein of FGF21, may be the key to FGF21's effect. AMPK plays a pivotal role in lipid metabolism. By upregulating the expression of ATGL, AMPK promotes lipolysis. In contrast, by regulating the expression of ACC1 and SREBP1c, AMPK can inhibit lipid synthesis. After being activated by FGF21, AMPK can also regulate the autophagy-related gene mTORC1 and the inflammation gene NF-κB to achieve anti-aging effects. AMPK can control the autophagy-related pathways SIRT1/PGC-1α, SIRT1, and its downstream aging-related genes P53, HIF-1α to prevent cellular aging. At present, some FGF21 analogs (LY2405319, PF-05231023, pegbelfermin, efruxifermin, etc.) and FGF21 receptor agonists (C3201–HSA, mimAb1, and 39F7 mAb, NGM313, etc.) have been confirmed by animal or clinical experiments to have an excellent ability to reduce weight and improve glucose and lipid metabolism. However, no clinical studies have clarified the efficacy of FGF21 analogs and receptor agonists to improve cell aging. This requires further in-depth research.
Overall, the above preclinical and clinical studies indicate that FGF21 has a vital role in alleviating dyslipidemia, lipid metabolism and glucose metabolism through different molecular pathways and/or target organs. FGF may soon become a key target for the treatment of aging-related metabolic diseases. Although FGF21 has received much attention and is frequently used in treating aging-related diseases, it is almost entirely involved in improving metabolism. There are few or no studies involving FGF21 for the treatment of aging-related metabolic diseases by improving aging. I hope this review, which summarizes many previous studies, can help promote more research on FGF21 in aging-related metabolic disorders.
Author Contributions
JY, YN, and BH participated in drafting the manuscript. JC, ML, and MY provided technical assistance. ZC and BH revised the manuscript. All of the authors read and approved the final manuscript.
Funding
This study was supported by the Zhejiang Provincial Natural ScienceFoundation of China (No. LY17H290007), the Fund of State Administration of Traditional Chinese Medicine of Zhejiang Province (No. 2020ZB081), the Fund of Health Commission of Zhejiang Province (No. 2018KY550), Research fund project of Zhejiang Chinese Medical University (No. 2019ZG03).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest. (1998) 101:1203–9. doi: 10.1172/JCI579
2. Ding HR, Wang JL, Ren HZ, Shi XL. Lipometabolism and glycometabolism in liver diseases. Biomed Res Int. (2018) 2018:1287127. doi: 10.1155/2018/1287127
3. Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. (1998) 280:1378–83. doi: 10.1126/science.280.5368.1378
4. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
5. Nowicki EM, Billington CJ, Levine AS, Hoover H, Must A, Naumova E. Overweight, obesity, and associated disease burden in the Veterans Affairs ambulatory care population. Mil Med. (2003) 168:252–6. doi: 10.1093/milmed/168.3.252
6. O'Rourke RW. Adipose tissue and the physiologic underpinnings of metabolic disease. Surg Obes Relat Dis. (2018) 14:1755–63. doi: 10.1016/j.soard.2018.07.032
7. Azzu V, Valencak TG. Energy metabolism and ageing in the mouse: a mini-review. Gerontology. (2017) 63:327–36. doi: 10.1159/000454924
8. Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. (2012) 61:1315–22. doi: 10.2337/db11-1300
9. Riera CE, Dillin A. Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol. (2015) 17:196–203. doi: 10.1038/ncb3107
10. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. (2011) 479:232–6. doi: 10.1038/nature10600
11. Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. (2016) 15:973–7. doi: 10.1111/acel.12458
12. Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. (2016) 78:223–41. doi: 10.1146/annurev-physiol-021115-105339
13. Cuevas-Ramos D, Aguilar-Salinas CA. Modulation of energy balance by fibroblast growth factor 21. Horm Mol Biol Clin Investig. (2016) 30:20160023. doi: 10.1515/hmbci-2016-0023
14. Salminen A, Kaarniranta K, Kauppinen A. Integrated stress response stimulates FGF21 expression: systemic enhancer of longevity. Cell Signal. (2017) 40:10–21. doi: 10.1016/j.cellsig.2017.08.009
15. Passos JF, Saretzki G, von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. (2007) 35:7505–13. doi: 10.1093/nar/gkm893
16. Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. (2018) 9:5435. doi: 10.1038/s41467-018-07825-3
17. Wei W, Ji S. Cellular senescence: molecular mechanisms and pathogenicity. J Cell Physiol. (2018) 233:9121–35. doi: 10.1002/jcp.26956
18. Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. (2008) 454:1065–71. doi: 10.1038/nature07216
19. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. (1999) 282:1523–9. doi: 10.1001/jama.282.16.1523
20. Verdin E. NAD? in aging, metabolism, and neurodegeneration. Science. (2015) 350:1208–13. doi: 10.1126/science.aac4854
21. Sharma R, Ramanathan A. The aging metabolome-biomarkers to hub metabolites. Proteomics. (2020) 20:e1800407. doi: 10.1002/pmic.201800407
22. Jang C, Chen L, Rabinowitz JD. Metabolomics and isotope tracing. Cell. (2018) 173:822–37. doi: 10.1016/j.cell.2018.03.055
23. Liu X, Locasale JW. Metabolomics: a Primer. Trends Biochem Sci. (2017) 42:274–84. doi: 10.1016/j.tibs.2017.01.004
24. Hoffman JM, Lyu Y, Pletcher SD, Promislow DEL. Proteomics and metabolomics in ageing research: from biomarkers to systems biology. Essays Biochem. (2017) 61:379–88. doi: 10.1042/EBC20160083
25. Cantó C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. (2015) 22:31–53. doi: 10.1016/j.cmet.2015.05.023
26. Clement J, Wong M, Poljak A, Sachdev P, Braidy N. The plasma NAD(+) metabolome is dysregulated in “normal” aging. Rejuvenation Res. (2019) 22:121–30. doi: 10.1089/rej.2018.2077
27. Yoshino J, Baur JA, Imai SI. NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. (2018) 27:513–28. doi: 10.1016/j.cmet.2017.11.002
28. Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, et al. NAD(+) in aging: molecular mechanisms and translational implications. Trends Mol Med. (2017) 23:899–916. doi: 10.1016/j.molmed.2017.08.001
29. Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. (2018) 27:529–47. doi: 10.1016/j.cmet.2018.02.011
30. Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, et al. NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol. (2019) 21:397–407. doi: 10.1038/s41556-019-0287-4
31. Wang L, Davis SS, Borch Jensen M, Rodriguez-Fernandez IA, Apaydin C, Juhasz G, et al. JNK modifies neuronal metabolism to promote proteostasis and longevity. Aging Cell. (2019) 18:e12849. doi: 10.1111/acel.12849
32. Nóbrega-Pereira S, Fernandez-Marcos PJ, Brioche T, Gomez-Cabrera MC, Salvador-Pascual A, Flores JM, et al. G6PD protects from oxidative damage and improves healthspan in mice. Nat Commun. (2016) 7:10894. doi: 10.1038/ncomms10894
33. Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. (2014) 510:397–401. doi: 10.1038/nature13264
34. Mlekusch W, Tillian H, Lamprecht M, Trutnovsky H, Horejsi R, Reibnegger G. The effect of reduced physical activity on longevity of mice. Mech Ageing Dev. (1996) 88:159–68. doi: 10.1016/0047-6374(96)01734-4
35. Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. (2005) 208:1717–30. doi: 10.1242/jeb.01556
36. Brys K, Vanfleteren JR, Braeckman BP. Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp Gerontol. (2007) 42:845–51. doi: 10.1016/j.exger.2007.02.004
37. Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. (2004) 3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x
38. Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. (2015) 4:215–66. doi: 10.1002/wdev.176
39. Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. (2010) 2010:218142. doi: 10.4061/2010/218142
40. Itoh N. The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull. (2007) 30:1819–25. doi: 10.1248/bpb.30.1819
41. Itoh N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. (2010) 342:1–11. doi: 10.1007/s00441-010-1024-2
42. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. (2000) 1492:203–6. doi: 10.1016/S0167-4781(00)00067-1
43. Zhang X, Yeung DCY, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. (2008) 57:1246. doi: 10.2337/db07-1476
44. Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. (2009) 137:1795–804. doi: 10.1053/j.gastro.2009.07.064
45. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. (2005) 115:1627–35. doi: 10.1172/JCI23606
46. Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. (2014) 124:515–27. doi: 10.1172/JCI67353
48. Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. (2020) 16:654–67. doi: 10.1038/s41574-020-0386-0
49. Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol (Oxf). (2013) 78:489–96. doi: 10.1111/cen.12095
50. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. (2007) 5:415–25. doi: 10.1016/j.cmet.2007.05.003
51. Holmes D. Metabolism: fasting induces FGF21 in humans. Nat Rev Endocrinol. (2016) 12:3. doi: 10.1038/nrendo.2015.202
52. Zhu S, Wu Y, Ye X, Ma L, Qi J, Yu D, et al. FGF21 ameliorates nonalcoholic fatty liver disease by inducing autophagy. Mol Cell Biochem. (2016) 420:107–19. doi: 10.1007/s11010-016-2774-2
53. Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. (2009) 150:4084–93. doi: 10.1210/en.2009-0221
54. Wang C, Dai J, Yang M, Deng G, Xu S, Jia Y, et al. Silencing of FGF-21 expression promotes hepatic gluconeogenesis and glycogenolysis by regulation of the STAT3-SOCS3 signal. Febs j. (2014) 281:2136–47. doi: 10.1111/febs.12767
55. Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, et al. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol. (2012) 181:583–92. doi: 10.1016/j.ajpath.2012.04.004
56. Kim CS, Joe Y, Choi HS, Back SH, Park JW, Chung HT, et al. Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle. J Inflamm (Lond). (2019) 16:17. doi: 10.1186/s12950-019-0221-3
57. Youm YH, Horvath TL, Mangelsdorf DJ, Kliewer SA, Dixit VD. Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc Natl Acad Sci USA. (2016) 113:1026–31. doi: 10.1073/pnas.1514511113
58. Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. (2008) 24:604–12. doi: 10.1016/j.tig.2008.10.002
59. Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. (2008) 8:77–83. doi: 10.1016/j.cmet.2008.05.006
60. Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. (2012) 1:e00065. doi: 10.7554/eLife.00065
61. Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. (2014) 63:4057–63. doi: 10.2337/db14-0595
62. Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. (2007) 104:7432–7. doi: 10.1073/pnas.0701600104
63. Patel V, Adya R, Chen J, Ramanjaneya M, Bari MF, Bhudia SK, et al. Novel insights into the cardio-protective effects of FGF21 in lean and obese rat hearts. PLoS ONE. (2014) 9:e87102. doi: 10.1371/journal.pone.0087102
64. Zhang C, Huang Z, Gu J, Yan X, Lu X, Zhou S, et al. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia. (2015) 58:1937–48. doi: 10.1007/s00125-015-3630-8
65. Salminen A, Kaarniranta K, Kauppinen A. Regulation of longevity by FGF21: interaction between energy metabolism and stress responses. Ageing Res Rev. (2017) 37:79–93. doi: 10.1016/j.arr.2017.05.004
66. Coate KC, Hernandez G, Thorne CA, Sun S, Le TDV, Vale K, et al. FGF21 is an exocrine pancreas secretagogue. Cell Metab. (2017) 25:472–80. doi: 10.1016/j.cmet.2016.12.004
67. Grahame Hardie D. Regulation of AMP-activated protein kinase by natural and synthetic activators. Acta Pharm Sin B. (2016) 6:1–19. doi: 10.1016/j.apsb.2015.06.002
68. Ge X, Chen C, Hui X, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J Biol Chem. (2011) 286:34533–41. doi: 10.1074/jbc.M111.248591
69. Minard AY, Tan SX, Yang P, Fazakerley DJ, Domanova W, Parker BL, et al. mTORC1 is a major regulatory node in the FGF21 signaling network in adipocytes. Cell Rep. (2016) 17:29–36. doi: 10.1016/j.celrep.2016.08.086
70. Wang Q, Liu S, Zhai A, Zhang B, Tian G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol Pharm Bull. (2018) 41:985–93. doi: 10.1248/bpb.b17-00724
71. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. (2011) 13:376–88. doi: 10.1016/j.cmet.2011.03.009
72. Jo HK, Kim GW, Jeong KJ, Kim DY, Chung SH. Eugenol ameliorates hepatic steatosis and fibrosis by down-regulating SREBP1 gene expression via AMPK-mTOR-p70S6K signaling pathway. Biol Pharm Bull. (2014) 37:1341–51. doi: 10.1248/bpb.b14-00281
73. Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. (2011) 13:739–48. doi: 10.1016/j.cmet.2011.05.002
74. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. (2007) 8:774–85. doi: 10.1038/nrm2249
75. Salminen A, Kauppinen A, Kaarniranta K. FGF21 activates AMPK signaling: impact on metabolic regulation and the aging process. J Mol Med (Berl). (2017) 95:123–31. doi: 10.1007/s00109-016-1477-1
76. Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. (2007) 17:1646–56. doi: 10.1016/j.cub.2007.08.047
77. Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. (2002) 109:357–62. doi: 10.1172/JCI0214571
78. Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, et al. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. (2013) 17:101–12. doi: 10.1016/j.cmet.2012.12.006
79. Curtis R, O'Connor G, DiStefano PS. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell. (2006) 5:119–26. doi: 10.1111/j.1474-9726.2006.00205.x
80. Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. (2011) 470:404–8. doi: 10.1038/nature09706
81. Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. (2014) 20:10–25. doi: 10.1016/j.cmet.2014.03.002
82. Salminen A, Kaarniranta K. Genetics vs. entropy: longevity factors suppress the NF-kappaB-driven entropic aging process. Ageing Res Rev. (2010) 9:298–314. doi: 10.1016/j.arr.2009.11.001
83. Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl). (2011) 89:667–76. doi: 10.1007/s00109-011-0748-0
84. Gong Q, Hu Z, Zhang F, Cui A, Chen X, Jiang H, et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. (2016) 64:425–38. doi: 10.1002/hep.28523
85. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. (2008) 105:3374–9. doi: 10.1073/pnas.0712145105
86. Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. (2009) 20:325–31. doi: 10.1016/j.tem.2009.03.008
87. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy. (2010) 6:186–8. doi: 10.4161/auto.6.1.10817
88. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci USA. (2010) 107:12553–8. doi: 10.1073/pnas.1006962107
89. Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. (2009) 106:10853–8. doi: 10.1073/pnas.0904187106
90. Mäkelä J, Tselykh TV, Maiorana F, Eriksson O, Do HT, Mudò G, et al. Fibroblast growth factor-21 enhances mitochondrial functions and increases the activity of PGC-1α in human dopaminergic neurons via Sirtuin-1. Springerplus. (2014) 3:2. doi: 10.1186/2193-1801-3-2
91. Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho CY, et al. Role of mitochondria in diabetic peripheral neuropathy: influencing the NAD(+)-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. (2019) 145:177–209. doi: 10.1016/bs.irn.2019.04.002
92. Austin S, St-Pierre J. PGC1α and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. (2012) 125:4963–71. doi: 10.1242/jcs.113662
93. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. (2012) 13:225–38. doi: 10.1038/nrm3293
94. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. (2017). 288:1–8.
95. Speakman JR, O'Rahilly S. Fat: an evolving issue. Dis Model Mech. (2012) 5:569–73. doi: 10.1242/dmm.010553
96. Legler J, Fletcher T, Govarts E, Porta M, Blumberg B, Heindel JJ, et al. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. (2015) 100:1278–88. doi: 10.1210/jc.2014-4326
97. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. (2017) 122:1–7. doi: 10.1016/j.phrs.2017.05.013
98. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. (2009) 58:250–9. doi: 10.2337/db08-0392
99. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. (2008) 149:6018–27. doi: 10.1210/en.2008-0816
100. Hecht R, Li YS, Sun J, Belouski E, Hall M, Hager T, et al. Rationale-based engineering of a potent long-acting FGF21 analog for the treatment of type 2 diabetes. PLoS ONE. (2012) 7:e49345. doi: 10.1371/journal.pone.0049345
101. Ye XL, Gao HS, Wang WF, Ren GP, Liu MY, He K, et al. Optimization and characterization of a novel FGF21 mutant. Yao Xue Xue Bao. (2012) 47:897–903. doi: 10.1134/S1021443711040170
102. Véniant MM, Hale C, Helmering J, Chen MM, Stanislaus S, Busby J, et al. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS ONE. (2012) 7:e40164. doi: 10.1371/journal.pone.0040164
103. Zimmet P, Shi Z, El-Osta A, Ji L. Epidemic T2DM, early development and epigenetics: implications of the Chinese Famine. Nat Rev Endocrinol. (2018) 14:738–46. doi: 10.1038/s41574-018-0106-1
104. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. (2004) 27:1047–53. doi: 10.2337/diacare.27.5.1047
105. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
106. Kim KH, Lee MS. FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti-diabetic drugs. J Endocrinol. (2015) 226:R1–16. doi: 10.1530/JOE-15-0160
107. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. (2007) 148:774–81. doi: 10.1210/en.2006-1168
108. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. (2006) 55:2470–8. doi: 10.2337/db05-1435
109. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
110. Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0140908. doi: 10.1371/journal.pone.0140908
111. Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. (2017) 49:197–211. doi: 10.1080/03602532.2017.1293683
112. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
113. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. (2015) 62:S47–64. doi: 10.1016/j.jhep.2014.12.012
114. Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. (2004) 89:3864–71. doi: 10.1210/jc.2003-031986
115. Gong Z, Tas E, Yakar S, Muzumdar R. Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Mol Cell Endocrinol. (2017) 455:115–30. doi: 10.1016/j.mce.2016.12.022
116. Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. (2017) 8:15691. doi: 10.1038/ncomms15691
117. Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. (2010) 6:347. doi: 10.1038/msb.2010.5
118. Miwa S, Jow H, Baty K, Johnson A, Czapiewski R, Saretzki G, et al. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat Commun. (2014) 5:3837. doi: 10.1038/ncomms4837
119. Lohr K, Pachl F, Moghaddas Gholami A, Geillinger KE, Daniel H, Kuster B, et al. Reduced mitochondrial mass and function add to age-related susceptibility toward diet-induced fatty liver in C57BL/6J mice. Physiol Rep. (2016) 4:e12988. doi: 10.14814/phy2.12988
120. Zhang X, Zhou D, Strakovsky R, Zhang Y, Pan YX. Hepatic cellular senescence pathway genes are induced through histone modifications in a diet-induced obese rat model. Am J Physiol Gastrointest Liver Physiol. (2012) 302:G558–64. doi: 10.1152/ajpgi.00032.2011
121. Elston R, Inman GJ. Crosstalk between p53 and TGF-β Signalling. J Signal Transduct. (2012) 2012:294097. doi: 10.1155/2012/294097
122. Tomita K, Teratani T, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, et al. p53/p66Shc-mediated signaling contributes to the progression of non-alcoholic steatohepatitis in humans and mice. J Hepatol. (2012) 57:837–43. doi: 10.1016/j.jhep.2012.05.013
123. Tanaka N, Takahashi S, Zhang Y, Krausz KW, Smith PB, Patterson AD, et al. Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochim Biophys Acta. (2015) 1852:1242–52. doi: 10.1016/j.bbadis.2015.02.012
124. Zarei M, Barroso E, Palomer X, Dai J, Rada P, Quesada-López T, et al. Hepatic regulation of VLDL receptor by PPARβ/δ and FGF21 modulates non-alcoholic fatty liver disease. Mol Metab. (2018) 8:117–31. doi: 10.1016/j.molmet.2017.12.008
125. Bao L, Yin J, Gao W, Wang Q, Yao W, Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. (2018) 175:3379–93. doi: 10.1111/bph.14383
126. Keinicke H, Sun G, Mentzel CMJ, Fredholm M, John LM, Andersen B, et al. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect. (2020) 9:755–68. doi: 10.1530/EC-20-0152
127. Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology. (2014) 60:977–89. doi: 10.1002/hep.27060
128. Lee JH, Kang YE, Chang JY, Park KC, Kim HW, Kim JT, et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res. (2016) 8:4750–63.
129. Boparai RK, Arum O, Miquet JG, Masternak MM, Bartke A, Khardori RK. Resistance to the beneficial metabolic effects and hepatic antioxidant defense actions of fibroblast growth factor 21 treatment in growth hormone-overexpressing transgenic mice. Int J Endocrinol. (2015) 2015:282375. doi: 10.1155/2015/282375
130. Adams AC, Halstead CA, Hansen BC, Irizarry AR, Martin JA, Myers SR, et al. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS ONE. (2013) 8:e65763. doi: 10.1371/journal.pone.0065763
131. Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models–association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. (2009) 297:E1105–14. doi: 10.1152/ajpendo.00348.2009
132. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. (2013) 18:333–40. doi: 10.1016/j.cmet.2013.08.005
133. Dong JQ, Rossulek M, Somayaji VR, Baltrukonis D, Liang Y, Hudson K, et al. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br J Clin Pharmacol. (2015) 80:1051–63. doi: 10.1111/bcp.12676
134. Kim AM, Somayaji VR, Dong JQ, Rolph TP, Weng Y, Chabot JR, et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metab. (2017) 19:1762–72. doi: 10.1111/dom.13023
135. Charles ED, Neuschwander-Tetri BA, Pablo Frias J, Kundu S, Luo Y, Tirucherai GS, et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity (Silver Spring). (2019) 27:41–9. doi: 10.1002/oby.22344
136. Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. (2019) 392:2705–17. doi: 10.1016/S0140-6736(18)31785-9
137. Verzijl CRC, Van De Peppel IP, Struik D, Jonker JW. Pegbelfermin (BMS-986036): an investigational PEGylated fibroblast growth factor 21 analogue for the treatment of nonalcoholic steatohepatitis. Expert Opin Investig Drugs. (2020) 29:125–33. doi: 10.1080/13543784.2020.1708898
138. Kolumam G, Chen MZ, Tong R, Zavala-Solorio J, Kates L, van Bruggen N, et al. Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βklotho complex. EBioMedicine. (2015) 2:730–43. doi: 10.1016/j.ebiom.2015.05.028
139. Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. (2016) 23:427–40. doi: 10.1016/j.cmet.2016.02.001
140. Smith R, Duguay A, Bakker A, Li P, Weiszmann J, Thomas MR, et al. FGF21 can be mimicked in vitro and in vivo by a novel anti-FGFR1c/β-Klotho bispecific protein. PLoS ONE. (2013) 8:e61432. doi: 10.1371/journal.pone.0061432
141. Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, et al. Treating diabetes and obesity with an FGF21-mimetic antibody activating the βKlotho/FGFR1c receptor complex. Sci Transl Med. (2012) 4:162ra153. doi: 10.1126/scitranslmed.3004690
142. Min X, Weiszmann J, Johnstone S, Wang W, Yu X, Romanow W, et al. Agonistic β-Klotho antibody mimics fibroblast growth factor 21 (FGF21) functions. J Biol Chem. (2018) 293:14678–14688. doi: 10.1074/jbc.RA118.004343
143. Depaoli A, Phung VAN, Bashir MR, Morrow L, Beysen C, Yan A, et al. 140-LB: NGM313, a novel activator of b-Klotho/FGFR1c, improves insulin resistance and reduces hepatic fat in obese, nondiabetic subjects. Diabetes. (2019) 68:140–LB. doi: 10.2337/db19-140-LB
144. Kharitonenkov A, Adams AC. Inventing new medicines: The FGF21 story. Mol Metab. (2014) 3:221–9. doi: 10.1016/j.molmet.2013.12.003
145. Struik D, Dommerholt MB, Jonker JW. Fibroblast growth factors in control of lipid metabolism: from biological function to clinical application. Curr Opin Lipidol. (2019) 30:235–43. doi: 10.1097/MOL.0000000000000599
146. Kaufman A, Abuqayyas L, Denney WS, Tillman EJ, Rolph T. AKR-001, an Fc-FGF21 analog, showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type 2 diabetes patients. Cell Rep Med. (2020) 1:100057. doi: 10.1016/j.xcrm.2020.100057
147. Sánchez-Garrido MA, Habegger KM, Clemmensen C, Holleman C, Müller TD, Perez-Tilve D, et al. Fibroblast activation protein (FAP) as a novel metabolic target. Mol Metab. (2016) 5:1015–24. doi: 10.1016/j.molmet.2016.07.003
148. Zarei M, Barroso E, Leiva R, Barniol-Xicota M, Pujol E, Escolano C, et al. Heme-regulated eIF2α kinase modulates hepatic FGF21 and is activated by PPARβ/δ deficiency. Diabetes. (2016) 65:3185–99. doi: 10.2337/db16-0155
149. Aravinthan A, Scarpini C, Tachtatzis P, Verma S, Penrhyn-Lowe S, Harvey R, et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol. (2013) 58:549–56. doi: 10.1016/j.jhep.2012.10.031
150. Lewis JE, Ebling FJP, Samms RJ, Tsintzas K. Going back to the biology of FGF21: new insights. Trends Endocrinol Metab. (2019) 30:491–504. doi: 10.1016/j.tem.2019.05.007
Keywords: fibroblast growth factor 21, aging, metabolic disease, glycometabolism, pharmacology, lipometabolism
Citation: Yan J, Nie Y, Cao J, Luo M, Yan M, Chen Z and He B (2021) The Roles and Pharmacological Effects of FGF21 in Preventing Aging-Associated Metabolic Diseases. Front. Cardiovasc. Med. 8:655575. doi: 10.3389/fcvm.2021.655575
Received: 21 January 2021; Accepted: 05 March 2021;
Published: 31 March 2021.
Edited by:
Zhouguang Wang, Albert Einstein College of Medicine, United StatesReviewed by:
Xinghui Zhao, University of Kentucky, United StatesDedong Li, University of Pittsburgh, United States
Copyright © 2021 Yan, Nie, Cao, Luo, Yan, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyun Chen, emhpeXVuY2hlbjYzQDE2My5jb20=; Beihui He, Z3JhZjMwM0BzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Junbin Yan
Junbin Yan Yunmeng Nie1†
Yunmeng Nie1† Minmin Luo
Minmin Luo Zhiyun Chen
Zhiyun Chen Beihui He
Beihui He