
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 06 April 2021
Sec. Thrombosis and Haemostasis
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.655226
This article is part of the Research TopicHighlights in Thrombosis: 2021View all 9 articles
Background: Previous prediction models for recurrent thromboembolism (VTE) are often complicated to apply and have not been implemented widely.
Aim: To develop and internally validate a potential new prediction model for recurrent VTE that can be used without stopping anticoagulant treatment for D-dimer measurements in patients with provoked and unprovoked DVT.
Methods: Cohort data of 479 patients treated in a clinical care pathway at Maastricht University Medical Center were used. Predictors for the Cox proportional hazards model (unprovoked DVT, male gender, factor VIII levels) were derived from literature and using forward selection procedure. The scoring rule was internally validated using bootstrapping techniques and the predictive ability was compared to existing prediction models.
Results: Patients were followed for a median of 3.12 years after stopping anticoagulation treatment (IQR 0.78, 3.90). Sixty-four of 479 patients developed recurrent VTE (13%). The scoring rule consisted of unprovoked DVT (yes: 2 points), male sex (yes: 1 point), and factor VIII > 213 % (yes: 2 points) and was categorized into three groups [i.e., low risk (score 0), medium risk (scores 1, 2, or 3) and high risk (scores 4 and 5)]. The concordance statistic was 0.68 (95% CI: 0.61, 0.75).
Conclusion: The discriminative ability of the new Continu-8 score was adequate. Future studies shall verify this score in an independent setting without stopping anticoagulation treatment.
Secondary prevention of venous thromboembolism (VTE) is important to improve care in patients with deep vein thrombosis (DVT) or pulmonary embolism (PE) (1–4). VTE is the third most common cardiovascular disease, and it contributes relevantly to the global disease burden (1–3, 5, 6). The estimated incidence is between 1 and 2 per 1,000 person-years and it is associated with a significant morbidity and mortality, both in short-term and long-term perspectives (5, 7, 8). In addition, VTE is associated with substantial healthcare costs (9–11). Though anticoagulation treatment is very effective in treatment and prevention of VTE, it is associated with a significant risk of bleeding complications (12–14); the corresponding case-fatality rate is estimated to be 6% (7). To guide treatment decisions in secondary prevention of VTE, it is important to discriminate those 25% of patients who will recur within 5 years from the 75% of patients who will not (2).
A number of predictors for VTE recurrence have been identified, the presence of reversible risk factors and active cancer being the most relevant (14–16). Consistently, all large cohort studies found an association between the absence of reversible risk factors such as recent surgery, pregnancy and estrogen treatment, and recurrent VTE (2, 14, 17). Men bear a 2-fold risk of VTE recurrence compared to women (18). In addition, elevated D-dimers 1 months after stopping anticoagulation are associated with a high risk of VTE recurrence (19–21). The drawback of a management according to D-dimer levels is that anticoagulation must be stopped at least for 1 month. Factor VIII was studied as another surrogate for an increased coagulation activity by several authors (22–24). More predictors have been suggested in patients without cancer, the applicability in clinical practice is however limited (2). How to combine the predictors optimally is still elusive.
Several prediction models for recurrence of VTE have been developed, yet none of them is strongly recommended so far (14, 25, 26). Rodger and colleagues followed 600 patients with a first, unprovoked proximal VTE for a median of 18 months after stopping anticoagulant treatment and studied 69 predictors using a logistic regression model in males and females (“HERDOO2”) (27, 28). D-dimer, age, body mass index, and post-thrombotic signs were included in the model. However, the risk of overfitting was high (2.5 events per predictor) and the application is limited to women with an unprovoked VTE (25). The prediction rule was validated in a prospective cohort management study (28). Eichinger and colleagues included 929 patients with a first, unprovoked proximal or distal VTE, and followed the patients for a median of 43.3 months after stopping anticoagulation treatment (29). Eight prespecified predictors were studied in 176 recurrent events (22 events per predictor) in a Cox proportional hazards model (the “Vienna prediction model”). Finally, quantitative D-dimer measurements, sex and site of index event were included in the model. The Vienna prediction model was updated (30) and externally validated in two other cohorts with varying results (31, 32). The “DASH” score was derived using individual patient-data from seven prospective studies including patients with a first episode of proximal VTE (33). Six variables obtained from univariate analysis and theoretical considerations were included in a Cox regression analysis and the model was derived from backward selection (40 events per predictor). The final model comprised D-dimer, age, sex, and hormone therapy. The DASH score was externally validated in a retrospective cohort (34).
Several important limitations appear with regard to the existing prediction rules: (1) anticoagulation treatment shall be stopped for measuring D-dimers, (2) the application is difficult, and (3) the application is limited to patients with unprovoked VTE in case of the DASH and HERDOO2 score (25).
With the present investigation, we aimed to develop and internally validate a potential new prediction model for recurrent VTE that can be used in patients with provoked and unprovoked proximal DVT without stopping anticoagulant treatment.
Data of a prospective cohort study observing patients with a proximal DVT were used, the details of which have been published (15). All consecutive patients treated within a clinical care pathway (CCP) at Maastricht University Medical Center (MUMC) were included. Inclusion criteria were (a) objectively confirmed first proximal lower extremity DVT (popliteal vein, femoral vein, common femoral vein, iliac vein), (b) diagnosed at MUMC between 1st of June 2003 and 30th of June 2013, and (c) aged 18 or older. Patients were managed in a specialized outpatient unit for 2 years as part of the CCP (Figure 1). Details of the CCP including risk assessment and treatment decisions are described elsewhere (15). Some patient groups were usually not treated within the CCP: distal DVT, pulmonary embolism, and patients with cancer. MUMC is the only tertiary hospital in the province of Limburg, the Netherlands. The study protocol was approved by the appropriate ethical committee (METC 15-4-256) and it was carried out in accordance with the Declaration of Helsinki.
All data were collected prospectively in-line with clinical routine and were recorded in a structured database. Regular visits were scheduled until 24 months. At the first visit of the CCP, structured history was taken as well as physical examination. Clinical risk factors were assessed. Laboratory tests were performed 1 months after stop anticoagulant treatment (e.g., D-dimer, factor VIII). Clinical outcomes were assessed until the last visit. Patients were additionally followed over the course of further outpatient visits and accessing MUMC and general practitioner records. Observer were not aware about outcomes while assessing predictors and predictors while assessing outcomes, respectively. Laboratory tests were conducted at pre-specified time points as previously described (35). A protocol was implemented to ensure adequate pre-analytical conditions (15). D-dimer levels were determined using the Vidas assay (bioMérieux Clinical Diagnostics, Marcy-l'Etoile, France) or Innovance, respectively (Siemens Healthcare, Marburg, Germany). Factor VIII was analyzed using a one-stage assay (Actin FS, Siemens Healthcare, Marburg, Germany) on a Sysmex CA7000 (distributed by Siemens Healthcare, Marburg, Germany).
We defined recurrent VTE as symptomatic, objectively confirmed proximal or distal DVT, PE, or other venous thrombosis, whereas confirmation was done using compression ultrasound, spiral computed tomography, or ventilation-perfusion lung scanning. A diagnostic work-up was conducted in case of signs and symptoms suggesting VTE as done in routine clinical practice. Recurrence of DVT was defined as (a) a new non-compressible vein in the contralateral leg, (b) a new non-compressible vein of the same leg as the first event (previously unaffected), (c) a clear proximal extension of the known thrombus, or (d) a new non-compressible site of a vein that was effected but previously re-canalized (15, 36–38). D-dimer were defined as positive if above or equal 500 ng/ml. Factor VIII was considered positive if above or equal 213% (80th percentile of the study population). “Unprovoked DVT” was defined as DVT without the presence of a reversible risk factor (20). All other variables investigated in the cohort study were defined previously (20).
The selection of predictor variables was based on five principles: (a) firmly established risk factors for recurrence, (b) easy to determine in clinical practice, (c) must be applicable in a broad range of patients. (d) the predictive value must be high in our own cohort, (e) maximum four predictors to avoid overfitting.
The following risk factors were considered because of previous publications: (1) Unprovoked DVT of the index event is considered to be the most important risk factor for recurrence. This was confirmed in many cohort studies in different settings and populations (14). In contrast to previous prediction models, we included this factor in the prediction model making it applicable to patients with provoked DVT as well. (2) Male sex is an established risk factor for VTE recurrence. A higher risk of recurrence in men was observed in a number of cohort studies and an individual-patient meta-analysis summarizing this evidence estimated a 2.2-fold higher risk in men compared to women (18). This variable was already implemented in two previous prediction models (Vienna prediction model; DASH score). (3) Elevated D-dimer measured 1 month after stopping anticoagulation is associated with an increased risk of recurrent VTE. A systematic review and meta-analysis summarizing the data estimated an 8.9% annual risk in patients with elevated D-dimer compared to 3.5% annual risk in patients without (19). We decided however not to include D-dimer in the prediction model in order to avoid the requirement of stopping the anticoagulation for 1 month. (4) Different cohort studies observed a higher risk of recurrence in patients with a high factor VIII compared to patients without (23, 24, 39). Presence of inflammation was defined as an active systematic inflammatory disorder such as inflammatory bowel disease or inflammatory rheumatologic disease (39).
Numbers/ frequencies or median/ inter-quartile ranges was reported to describe patient characteristics. The subgroups “provoked by surgery,” “non-surgical transient risk factor,” “unprovoked VTE,” as well as “active cancer” are mutually exclusive groups. Incidence rates per 100 patient-years were reported.
A commonly used rule-of-thumb states that at least 10 events should be recorded for each predictor included in the analysis. We allowed a maximum of 4 predictors, corresponding to over 15 events per predictor variable. Incomplete predictor values were imputed using stochastic regression imputation to prevent a loss of statistical precision and to reduce the likelihood of selection bias. A cox proportional hazards model was used to determine associations between predictor variables and recurrent VTE. The analysis was adjusted for periods of anticoagulation by including this variable as a time-varying co-variable. We used stepwise forward selection to arrive at a model containing only predictors that contributed significantly to the model, using a relatively liberal p-value for selection of 0.10 to make sure potentially important predictors would not be omitted. Using the regression coefficients (i.e., the natural logarithm of the hazard ratios) we simplified the model into a score based on integers only by selecting the smallest whole numbers that would still preserve the relative differences in importance between predictors. To adjust for potential overfitting, standard bootstrapping techniques were used to internally validate the model. Using the 1,000 bootstrap samples, we corrected the optimism-corrected C-statistic, which is an estimation of the C-statistic when the model is applied to future patients. The C-statistic, or concordance statistic, is a measure of discrimination (i.e., the ability of the model to separate outcomes). Patients were ranked according to their risk score and we created three groups of roughly similar size. As long as a risk score that has no unit rather than a prediction model was build, we did not create calibration plots and did not calculate the agreement between predicted and observed outcomes. A Kaplan-Meier curve stratified by risk category was used instead to assess differences in time-to-event between risk groups. A sensitivity analysis was conducted after excluding cancer patients.
The R statistical package was used for analysis [R Development Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.].
Four-hundred and seventy-nine patients were included in the study cohort and followed for a median of 3.12 years (IQR 0.78, 3.90) after stopping anticoagulation treatment. Patient characteristics are reported in Table 1. Five patients were lost to follow-up within the 2 years of CCP (1%; moved abroad), and 17 patients were lost during the extended follow-up (3.6%; Figure 1). Median age was 58.0 years (inter-quartile range, IQR 46.1, 71.1), and 242 were female (50.5%). All patients received vitamin K antagonists. Unprovoked DVT was present in 265 cases (55.3%). Sixty-four recurrent VTE were observed (25% of the patients), comprising 39 patients with DVT (60.9%), 20 patients with PE (31.3%), and 5 patients with other VTE (7.8%).
The full list of predictors analyzed are reported in a previous publication (15). Five predictors were statistically significant in univariate analysis: (1) unprovoked VTE, (2) male sex, (3) elevated D-dimer, (4) high factor VIII, and (5) presence of inflammation. The corresponding number of events, incidence rates per 100 patient-years, and unadjusted hazard ratios are reported in Table 2.
Using the predictors mentioned above, we added “unprovoked VTE,” “male sex,” “high factor VIII,” and “presence of inflammation” in a stepwise manner to the multivariable Cox proportional hazards model. We skipped D-dimer for practicability reasons (discussed above). The variable “presence of inflammation” and was omitted from the final model beause it did not improve the discrimitative ability. The adjusted hazard ratios (HR's) for unprovoked VTE, male sex, and high factor VIII were 2.19 [95% confidence interval (CI): 1.22–3.91, p = 0.008], 1.62 (95% CI: 0.95–2.77, p = 0.077), and 2.07 (95% CI: 1.23–3.47, p = 0.006), respectively. A sensitivity analysis after excluding cancer patients (n = 12) did not change these HR. After converting to integers, the score ranged from 0 to 5 and was categorized into three groups [i.e., low risk (score 0), medium risk (scores 1, 2, or 3) and high risk (scores 4 and 5)]. The 5-years probability of recurrence for the low, medium, and high-risk groups was 7.7% (95% CI: 2.9–12.2%), 12.1% (95% CI: 4.2–19.3%), and 29.1% (95% CI: 20.9–36.5%). Figure 2 illustrates the cumulative recurrence according to risk categories of the Continu-8 score. The optimism-corrected C-statistic of the Continu-8 score was 0.68 (95% CI 0.61, 0.75).
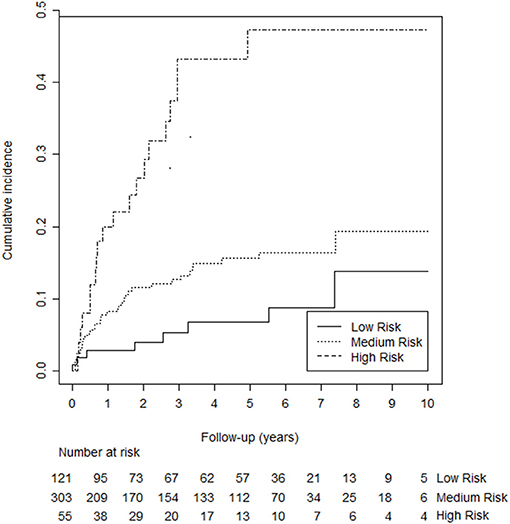
Figure 2. Kaplan-Meier curves illustrating cumulative recurrence according to risk categories of the Continu-8 score. The 5-years probability was 7.7% in the low-risk group (95% CI: 2.9–12.2%), 12.1% in the medium-risk group (95% CI: 4.2–19.3%), and 29.1% in the high-risk group (95% CI: 20.9–36.5%).
Using data from a prospective cohort study following patients with a proximal DVT, we developed and internally validated a potential new prediction model for recurrent VTE that can be used in patients with provoked and unprovoked proximal DVT without stopping anticoagulant treatment. The performance of the prediction model was adequate.
Our findings are essentially in-line with previous studies. The four most important predictors were already confirmed in other observational studies: (a) unprovoked DVT (2, 14, 17, 40, 41), (b) male sex (18), (c) elevated D-dimer (19), and (d) high factor VIII (22–24, 42, 43). In addition, inflammatory conditions were associated with recurrent VTE (44); this effect was however not significant anymore in multivariate analysis. In contrast to older studies, the risk of recurrence was very low in patients with pregnancy-related DVT or contraceptive use (45). In fact, the number of recurrent events is very low, leading to wide confidence intervals, and impeding the implementation in the prediction model. Our interpretation is that the awareness on the pregnancy and estrogen-related risk is much higher nowadays, leading to strict avoidance or medical prophylaxis in such patients.
Previous prediction models were developed in patients with unprovoked VTE only (25, 27, 29, 33). In contrast, we incorporated this variable in order to apply the model to all patients with proximal DVT, extending the prediction model to a more broader range of patients. Male sex was already included in the Vienna prediction model as well as the DASH score (29, 33). In contrast to HERDOO2 (27), Vienna prediction model (29, 30), and DASH (33), we did not include D-dimer in order to facilitate risk assessment without stopping anticoagulation treatment. High factor VIII was added to the DASH score in a sub-analysis of the MEGA follow-up study investigating the predictive value of factor VIII for recurrent VTE, what improved the c-statistics of the DASH score (22). We did also not include age (27, 33), body mass index (27), presence of post-thrombotic syndrome (27), and site of index event (29) in the model.
The strength of our investigation is that a relatively high number of events were available per predictor (64 events for 4 predictors studies), resulting in a considerable precision of the estimates. In addition, it was conducted in a reasonable number of patients, conducting a long-term follow-up, combined with a low number of patients lost. Of course, we are faced with limitations as well. First, only patients with (proximal) DVT were studied. At the present moment, the results of our study cannot be applied to patients with PE. We believe however that future external validations will confirm our results because previous prediction models using similar sets of predictors were generated in populations including PE (22, 27, 29, 30, 33). Secondly, a very low number of patients with cancer were included, preventing the application of the prediction model to this special group of patients. Thirdly, factor VIII was measured after stopping anticoagulation. Even though the impact of anticoagulation treatment on factor VIII measurements is assumed to be low, we cannot fully exclude that the results would be different while continuing anticoagulation treatment. Fourthly, due to the specific characteristics of the study population (proximal DVT as index event only, few cancer patients) and the predictor variables implemented in the model (unprovoked DVT, sex, increased coagulation activity), we were not able to conduct sensitivity analyses in meaningful subgroups of patients to assess the internal validity. However, our results are essentially in-line with previous studies suggesting external validity. Fifthly, the exact number of patients with distal DVT as the recurrent event type was not recorded. However, there were few patients only and we do not believe that this might have introduced any bias. Sixthly, even though bootstrapping techniques were used to adjust for potential overfitting, we cannot fully exclude that such effects might have affected the results. Seventhly, we did not discuss a risk-benefit trade-off to decide on the cut-off to be considered for prolonged anticoagulation. Given the apparent limitations of the study, this was beyond the focus of the current manuscript. Eighthly, we did not include age-adjusted D-Dimer cut-offs in the prediction model because it was beyond this manuscript's focus. Ninthly, our data were obtained with vitamin K-antagonists used in most patients and we cannot fully exclude that this might have affected the results.
What do the results of the study mean for scientific inquiry and clinical practice? First, our study confirms that prediction models or clinical prediction rules, respectively, can be applied to patients with DVT. Even though a c-value of 0.68 is not a high number, it is similar to previous prediction models (27, 29, 30, 33). Thus, the predictive ability of our score appears to be adequate and supports further investigation. Besides, it was possible to apply the prediction rule to all patients with proximal DVT, not only unprovoked DVT. Secondly, the set of predictors resembles previous prediction rules, verifying these results as well (27, 29, 30, 33). Third, the set of predictors incorporated in prediction models for recurrent VTE can be simplified to few variables that can be easily scored in clinical practice. Fourth, factor VIII measurements might replace D-dimer in order to prevent stopping anticoagulation. However, these results must be verified in patients with different settings and populations. In particular, future studies might confirm these results in patients without stopping anticoagulation while measuring factor VIII.
With the present investigation, we were able to develop and validate a new prediction model to be used in patients with both, provoked and unprovoked DVT, potentially without stopping anticoagulation treatment. Awaiting external validation in patients with PE and other populations and settings without stopping anticoagulation treatment, the prediction model has the potential to improve care in patients with VTE.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medisch-ethische toetsingscommissie azM/UM (METC), number 15-4-256. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MN and AT developed the study design, collected the data, and wrote the manuscript. MN and SV developed the analysis plan. SV conducted the statistical analysis. AT developed and implemented the clinical care pathway and acted as principal investigator. MN, AT, SV, MP, and HT reviewed the study design and statistical analysis and wrote the manuscript. All authors contributed to the article and approved the submitted version.
MN reports receiving grants from the Swiss National Science Foundation (SNSF), during the conduct of the study; and research grants from Bayer, Stago, Roche diagnostics, outside of the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.655226/full#supplementary-material
1. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. (2014) 12:1580–90. doi: 10.1111/jth.12698
2. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. (2010) 376:2032–9. doi: 10.1016/S0140-6736(10)60962-2
3. Scheres LJJ, Lijfering WM, Cannegieter SC. Current and future burden of venous thrombosis: not simply predictable. Res Pract Thromb Haemost. (2018) 2:199–8. doi: 10.1002/rth2.12101
4. Mai V, Bertoletti L, Cucherat M, Jardel S, Grange C, Provencher S, et al. Extended anticoagulation for the secondary prevention of venous thromboembolic events: an updated network meta-analysis. PLoS ONE. (2019) 14:e0214134. doi: 10.1371/journal.pone.0214134
5. Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. (2013) 126:832 e13–21. doi: 10.1016/j.amjmed.2013.02.024
6. Secemsky EA, Rosenfield K, Kennedy KF, Jaff M, Yeh RW. High burden of 30-day readmissions after acute venous thromboembolism in the United States. J Am Heart Assoc. (2018) 7:e009047. doi: 10.1161/JAHA.118.009047
7. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. (2007) 5:692–9. doi: 10.1111/j.1538-7836.2007.02450.x
8. Siegal DM, Eikelboom JW, Lee SF, Rangarajan S, Bosch J, Zhu J, et al. Venous thromboembolism, variations in incidence of venous thromboembolism in low-, middle-, high-income countries. Cardiovasc Res. (2021) 117:576–84. doi: 10.1093/cvr/cvaa044
9. Shahi A, Chen AF, Tan TL, Maltenfort MG, Kucukdurmaz F, Parvizi J. The incidence and economic burden of in-hospital venous thromboembolism in the United States. J Arthroplasty. (2017) 32:1063–6. doi: 10.1016/j.arth.2016.10.020
10. Mahan CE, Borrego ME, Woersching AL, Federici R, Downey R, Tiongson J, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. (2012) 108:291–302. doi: 10.1160/TH12-03-0162
11. Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE. European Union-28: an annualised cost-of-illness model for venous thromboembolism. Thromb Haemost. (2016) 115:800–8. doi: 10.1160/TH15-08-0670
12. van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. (2014) 124:1968–75. doi: 10.1182/blood-2014-04-571232
13. Nagler M, Bachmann LM, Schmid P, Raddatz Muller P, Wuillemin WA. Patient self-management of oral anticoagulation with vitamin K antagonists in everyday practice: efficacy and safety in a nationwide long-term prospective cohort study. PLoS ONE. (2014) 9:e95761. doi: 10.1371/journal.pone.0095761
14. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149:315–52. doi: 10.1016/j.chest.2015.11.026
15. Nagler M, Ten Cate H, Prins MH, Ten Cate-Hoek AJ. Risk factors for recurrence in deep vein thrombosis patients following a tailored anticoagulant treatment incorporating residual vein obstruction. Res Pract Thromb Haemost. (2018) 2:299–309. doi: 10.1002/rth2.12079
16. Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, et al. Emerging risk factors, cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. (2019) 4:163–73. doi: 10.1001/jamacardio.2018.4537
17. Iorio A, Kearon C, Filippucci E, Marcucci M, Macura A, Pengo V, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. (2010) 170:1710–6. doi: 10.1001/archinternmed.2010.367
18. Douketis J, Tosetto A, Marcucci M, Baglin T, Cosmi B, Cushman M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. (2011) 342:d813. doi: 10.1136/bmj.d813
19. Verhovsek M, Douketis JD, Yi Q, Shrivastava S, Tait RC, Baglin T, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med. (2008) 149:481–90, W94. doi: 10.7326/0003-4819-149-7-200810070-00008
20. Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. (2006) 355:1780–9. doi: 10.1056/NEJMoa054444
21. Bruinstroop E, Klok FA, Van De Ree MA, Oosterwijk FL, Huisman MV. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost. (2009) 7:611–8. doi: 10.1111/j.1538-7836.2009.03293.x
22. Timp JF, Lijfering WM, Flinterman LE, van Hylckama Vlieg A, le Cessie S, Rosendaal FR, et al. Predictive value of factor VIII levels for recurrent venous thrombosis: results from the MEGA follow-up study. J Thromb Haemost. (2015) 13:1823–32. doi: 10.1111/jth.13113
23. Eischer L, Gartner V, Schulman S, Kyrle PA, Eichinger S A.-F. Investigators. 6 versus 30 months anticoagulation for recurrent venous thrombosis in patients with high factor VIII. Ann Hematol. (2009) 88:485–90. doi: 10.1007/s00277-008-0626-1
24. Cosmi B, Legnani C, Cini M, Favaretto E, Palareti G. D-dimer and factor VIII are independent risk factors for recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Res. (2008) 122:610–7. doi: 10.1016/j.thromres.2007.12.024
25. Ensor J, Riley RD, Moore D, Snell KI, Bayliss S, Fitzmaurice D. Systematic review of prognostic models for recurrent venous thromboembolism (VTE) post-treatment of first unprovoked VTE. BMJ Open. (2016) 6:e011190. doi: 10.1136/bmjopen-2016-011190
26. Albertsen IE, Sogaard M, Goldhaber SZ, Piazza G, Skjoth F, Overvad TF, et al. Development of sex-stratified prediction models for recurrent venous thromboembolism: a Danish Nationwide Cohort Study. Thromb Haemost. (2020) 120:805–14. doi: 10.1055/s-0040-1708877
27. Rodger MA, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. (2008) 179:417–26. doi: 10.1503/cmaj.080493
28. Rodger MA, Le Gal G, Anderson DR, Schmidt J, Pernod G, Kahn SR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. (2017) 356:j1065. doi: 10.1136/bmj.j1065
29. Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. (2010) 121:1630–6. doi: 10.1161/CIRCULATIONAHA.109.925214
30. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc. (2014) 3:e000467. doi: 10.1161/JAHA.113.000467
31. Tritschler T, Mean M, Limacher A, Rodondi N, Aujesky D. Predicting recurrence after unprovoked venous thromboembolism: prospective validation of the updated Vienna Prediction Model. Blood. (2015) 126:1949–51. doi: 10.1182/blood-2015-04-641225
32. Marcucci M, Iorio A, Douketis JD, Eichinger S, Tosetto A, Baglin T, et al. Risk of recurrence after a first unprovoked venous thromboembolism: external validation of the Vienna Prediction Model with pooled individual patient data. J Thromb Haemost. (2015) 13:775–81. doi: 10.1111/jth.12871
33. Tosetto A, Iorio A, Marcucci M, Baglin T, Cushman M, Eichinger S, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost. (2012) 10:1019–25. doi: 10.1111/j.1538-7836.2012.04735.x
34. Tosetto A, Testa S, Martinelli I, Poli D, Cosmi B, Lodigiani C, et al. External validation of the DASH prediction rule: a retrospective cohort study. J Thromb Haemost. (2017) 15:1963–70. doi: 10.1111/jth.13781
35. Bouman AC, Smits JJ, Ten Cate H, Ten Cate-Hoek AJ. Markers of coagulation, fibrinolysis and inflammation in relation to post-thrombotic syndrome. J Thromb Haemost. (2012) 10:1532–8. doi: 10.1111/j.1538-7836.2012.04798.x
36. Carrier M, Rodger MA, Wells PS, Righini M, Leg G. Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost. (2011) 9:1119–25. doi: 10.1111/j.1538-7836.2011.04254.x
37. Prandoni P, Lensing AW, Bernardi E, Villalta S, Bagatella P, Girolami A, et al. The diagnostic value of compression ultrasonography in patients with suspected recurrent deep vein thrombosis. Thromb Haemost. (2002) 88:402–6. doi: 10.1055/s-0037-1613229
38. Kyrle PA, Kammer M, Eischer L, Weltermann A, Minar E, Hirschl M, et al. The long-term recurrence risk of patients with unprovoked venous thromboembolism: an observational cohort study. J Thromb Haemost. (2016) 14:2402–9. doi: 10.1111/jth.13524
39. Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. (2018) 6:142. doi: 10.3389/fped.2018.00142
40. Baglin T, Palmer CR, Luddington R, Baglin C. Unprovoked recurrent venous thrombosis: prediction by D-dimer and clinical risk factors. J Thromb Haemost. (2008) 6:577–82. doi: 10.1111/j.1538-7836.2008.02889.x
41. Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. (2007) 92:199–205. doi: 10.3324/haematol.10516
42. Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. (2000) 343:457–62. doi: 10.1056/NEJM200008173430702
43. Cristina L, Benilde C, Michela C, Mirella F, Giuliana G, Gualtiero P. High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. Br J Haematol. (2004) 124:504–10. doi: 10.1046/j.1365-2141.2003.04795.x
44. Horvei LD, Grimnes G, Hindberg K, Mathiesen EB, Njolstad I, Wilsgaard T, et al. C-reactive protein, obesity, and the risk of arterial and venous thrombosis. J Thromb Haemost. (2016) 14:1561–71. doi: 10.1111/jth.13369
Keywords: venous thrombosis/epidemiology, venous thrombosis/therapy, venous thrombosis/mortality, risk factors, clinical decision making, health services research
Citation: Nagler M, Van Kuijk SMJ, Ten Cate H, Prins MH and Ten Cate-Hoek AJ (2021) Predicting Recurrent Venous Thromboembolism in Patients With Deep-Vein Thrombosis: Development and Internal Validation of a Potential New Prediction Model (Continu-8). Front. Cardiovasc. Med. 8:655226. doi: 10.3389/fcvm.2021.655226
Received: 18 January 2021; Accepted: 11 March 2021;
Published: 06 April 2021.
Edited by:
Yugo Yamashita, Kyoto University, JapanReviewed by:
Yuji Nishimoto, Hyogo Prefectural Amagasaki Hospital, JapanCopyright © 2021 Nagler, Van Kuijk, Ten Cate, Prins and Ten Cate-Hoek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Nagler, bWljaGFlbC5uYWdsZXJAaW5zZWwuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.