
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 28 June 2021
Sec. Cardiovascular Epidemiology and Prevention
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.653405
This article is part of the Research Topic Non-Invasive Measures of Cardiovascular Function and Health: Special considerations for assessing lifestyle behaviours View all 15 articles
 Chin-Feng Tsai1
Chin-Feng Tsai1 Pang-Shuo Huang2
Pang-Shuo Huang2 Jien-Jiun Chen2
Jien-Jiun Chen2 Sheng-Nan Chang2
Sheng-Nan Chang2 Fu-Chun Chiu2
Fu-Chun Chiu2 Ting-Tse Lin3
Ting-Tse Lin3 Ling-Ping Lai4
Ling-Ping Lai4 Juey-Jen Hwang4
Juey-Jen Hwang4 Chia-Ti Tsai4*
Chia-Ti Tsai4*Background: Left atrial (LA) size represents atrial fibrillation (AF) burden and has been shown to be a predictor for AF stroke. The CHA2DS2-VASc score is also a well-established predictor of AF stroke. It is unknown to cardiologists whether these two risk scores are correlated, whether both are independent prognostic predictors and complimentary to each other, or whether one of them is a major determinant of stroke risk for AF patients.
Method: A total of 708 patients from the National Taiwan University Atrial Fibrillation Registry were longitudinally followed up for more than 15 years. Left atrial size was measured by M mode of echocardiography. Adverse thromboembolic endpoints during follow-up were defined as ischemic stroke or transient ischemic attack.
Results: The mean age was 72.1 ± 12.9 years, with 53% men. Both LA size and CHA2DS2-VASc score were associated with the risk of stroke in univariate analyses. There was a weak but significant positive correlation between LA size and CHA2DS2-VASc score (r = 0.17, P < 0.0001). Patients with higher CHA2DS2-VASc scores had a higher mean LA size (P < 0.01 for trend). When combining LA size and CHA2DS2-VASc score in the multivariable Cox model, only CHA2DS2-VASc score remained statistically significant [HR 1.39 (1.20–1.63); P < 0.001].
Conclusion: LA size is not an independent predictor of AF stroke, and calculation of CHA2DS2-VASc score may be an alternative to measurement of echocardiographic LA size when evaluating the risk of stroke for AF patients.
The left atrium (LA) of human heart functions to act as a contractile pump that delivers 15 to 30% of the left ventricular (LV) filling, as a reservoir that collects pulmonary venous return during LV systole, and as a conduit for the passage of stored blood from the LA to the LV during early LV diastole (1). LA enlargement has been proven to be associated with increased cardiovascular events, such as stroke, congestive heart failure, cardiovascular death, and atrial fibrillation (AF) (2). Patients with enlarged LA are more prone to AF, and an enlarged LA is more likely to maintain AF. On the other hand, AF has also been known to affect LA remodeling and geometry. Regardless of whether LA enlargement is a cause for or a consequence of AF, LA structure, and function represent AF burden and duration, conferring a risk for stroke and systemic embolization (3).
Several studies have investigated the relationship between the LA size and stroke in general populations, showing increased risk of stroke when LA was enlarged (4–11). Given that LA enlargement represents increased burden and duration of AF, it is proposed as an independent predictor of risk for stroke among AF populations (11). Therefore, the more the AF burden is, the higher the risk of cardiac embolic stroke is. However, performing echocardiography is an expensive modality and is less feasible in large population screening. Instead, the CHA2DS2-VASc score has been demonstrated to be another good scoring scheme to predict stroke risk in patients with AF and could be easily calculated and used in routine clinical practice (12). The CHA2DS2-VASc score is based on a point system in which two points are assigned for a history of stroke or transient ischemic attack (TIA) and age more than 75 years, and one point is assigned for presence of hypertension, diabetes, congestive heart failure, and vascular diseases or a female gender.
Therefore, both LA size and CHA2DS2-VASc score have been proposed as the predictors of AF-related stroke. However, echocardiography may not be immediately available in all clinics. Calculation of CHA2DS2-VASc score is an easier tool to use in clinics to predict stroke risk for patients with AF. The aims of this study were to assess whether LA size and the CHA2DS2-VASc score were correlated and to determine which one was the independent predictor of AF-related stroke in a long prospective AF follow-up cohort in Taiwan.
The National Taiwan University Atrial Fibrillation Registry (NTUAFR) was first established in Jan 1998, and so far the patients had been followed up for more than 15 years. The NTUAFR includes data on inpatients and outpatients with incident AF. The enrollment was staggered, and details of selection of AF patients have been described previously and according with current guidelines (13–16). The presence of AF was determined by taking the patient's history, serial ECG, and/or ambulatory ECG monitoring. A total of 1,233 patients were recruited. Patients with palpitations without ECG documentation were excluded from the registry (16). Patients with hyperthyroidism were excluded because AF in these patients might be curable. Patients who refused to join the study or were lost to follow-up were also excluded. Finally, the study population consisted of 708 consecutive adult AF patients from the NTUAFR with detailed echocardiographic data. The mean age was 72.1 ± 12.9 years, and 53% (375/708) were men. The study protocols were reviewed and approved by the institutional review committee, and all patients agreed to participate in the study.
At enrollment, the medical history was recorded, and transthoracic echocardiographic data of left atrial and left ventricular dimensions, left ventricular ejection fraction, and severity of valvular heart disease were also recorded. The LA size was determined as the M-mode measurement of the anteroposterior diameter of LA in parasternal long-axis view. We defined the cutoff value of LA enlargement as LA diameter more than 50 mm, as LA diameter more than 50 mm had been previously reported to be associated with a higher risk of stroke and systemic embolization in patients with AF (11). Besides, in our study the LA size was not controlled for body size because it did not significantly affect the results and it has been reported that LA size not controlled for body size was associated with an increased risk of stroke in patients with AF (17).
All the baseline characteristics were collected as previously described (13–15). The medical diagnoses of hypertension (blood pressure consistently above 140/90 mmHg or treated with hypertension medication), heart failure (admission for congestive heart failure), diabetes mellitus (DM) (fasting glucose >125 mg/dL or treatment with oral hypoglycemic agent and/or insulin), and vascular disease (peripheral arterial disease, coronary artery disease, and myocardial infarction) are also searched in the medical records (12).
The CHA2DS2-VASc scores were calculated and recorded as measures of stroke risk. The CHA2DS2-VASc score is based on a point system in which two points are assigned for a history of stroke or TIA and age more than 75 years, and one point is assigned for presence of hypertension, diabetes, congestive heart failure, and vascular diseases or a female gender (12). We categorized the patients into 3 groups (CHA2DS2-VASc score ≦2, 3–5 and ≥6), because CHA2DS2-VASc score ≦2 is associated with a lower risk of stroke but CHA2DS2-VASc score ≥6 is associated with a significantly higher risk of stroke (16, 18, 19).
Adverse thromboembolic endpoints during follow-up were defined as incident episodes of ischemic stroke or TIA. Ischemic stroke was defined as sudden-onset and focal or global neurological deficits that were not explained by other origins, with supporting evidence from imaging studies. Hemorrhagic stroke was excluded from the study because it was generally a complication of use of anticoagulant and not related to AF per se.
Continuous variables are presented as means ± standard deviation and categorical variables as percentages. The comparison of data was performed by using the chi-squared test (categorical variables) or the Student's t-test (continuous variables). The time to first episode of stroke event were depicted with the Kaplan–Meier estimate of the survival function. Difference between the survival curves was tested by the log-rank statistics. The independent effect of variables to predict thromboembolic events was calculated using a Cox proportional hazards regression model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated accordingly. To test the accuracy of different risk factors in predicting the occurrence of thromboembolic events, the receiver operator characteristic curves (ROC) were constructed.
The ROC analyses gave an estimate of the overall discriminate ability of risk predictor by the area under the curve (AUC) statistic or c statistic. We calculated sensitivity and specificity across the range of possible cutoff values of risk predictor scores. AUCs and associated 95% CIs were calculated. The correlation between CHA2DS2-VASc score and LA size was determined by calculating the Pearson's correlation coefficient (r). The Cochran–Armitage trend test was used for the trend of increase of LA size with different groups with increasing mean CHA2DS2-VASc score. P < 0.05 was considered statistically significant. Statistical analysis was performed using STATA version 7.0 for Windows (STATA, Inc., Texas, USA).
Baseline characteristics of study patients are summarized in Table 1. The mean age was 72.1 ± 12.9 years, and 53% (375/708) were men. Among the study subjects, 44.9% (318/708) aged over 75 years, 59.9% (424/708) had history of hypertension, 25.8% (183/708) had history of DM, 10.6% (75/708) had history of congestive heart failure, and 32.6% (231/708) had vascular diseases. The mean CHADS2-VASc score was 3.02 ± 1.53.
Regarding the echocardiographic data, 21.3% of the study subjects had LA dimension more than 50 mm. The mean left ventricular ejection fraction was 64.1 ± 12.7 %. The mean LV end-systolic dimension (LVESD) was 58.9 ± 16.5 mm. The mean LV end-diastolic dimension (LVEDD) was 33.7 ± 11.9 mm.
At the end of the follow-up, 70 AF patients developed ischemic stroke or TIA. We first investigate whether CHA2DS2-VASc score was associated with risk of stroke in our cohort (8, 9). As expected, CHA2DS2-VASc score was incrementally associated with the risk of stroke. Using the univariate Cox model, we found that there was a 42% increase of risk of stroke with a one-point increase of CHA2DS2-VASc score in this very long follow-up cohort (HR 1.42, 95% CI 1.22–1.66, P < 0.001).
The event-free survival from stroke comparing patients with different levels of CHA2DS2-VASc score is shown in Figure 1. The cut-point CHA2DS2-VASc score for each category was defined to make the case number similar in each group. Patients with a higher CHA2DS2-VASc score were more likely to develop ischemic stroke or TIA than those with lower CHA2DS2-VASC score (log-rank P < 0.0001).
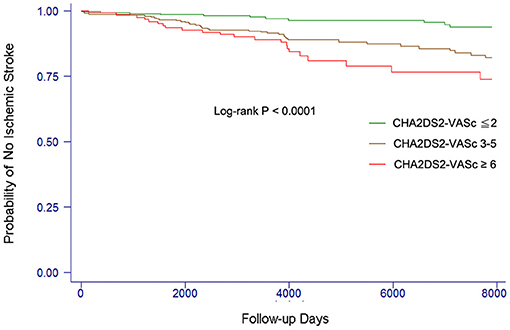
Figure 1. Kaplan–Meier curves showing the development of ischemic stroke among patients with different levels of CHA2DS2-VASc score. The log-rank analysis showed significant difference (P < 0.001).
In the present study, we also investigated whether the risk of ischemic stroke or TIA was increased when the LA size increased (3, 8, 9, 11). We found that LA size was incrementally associated with the risk of stroke. Using the univariate Cox model, there was a 30% increase of risk of stroke with a 1-mm increase of LA size (HR 1.30, 95% CI 1.04–1.62, P = 0.019). The event-free survival from stroke comparing patients with different LA size is shown in Figure 2. Patients with LA size >50 mm were more likely to develop ischemic stroke or TIA than those with LA size ≦50 mm (log-rank P = 0.0005).
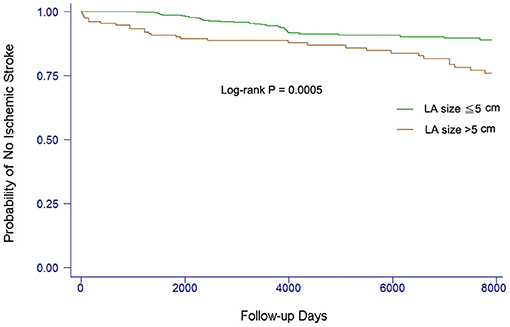
Figure 2. Kaplan–Meier curves showing the development of ischemic stroke among patients with different LA sizes. The log-rank analysis showed significant difference (P < 0.001).
Since both CHA2DS2-VASC score and LA size were associated with the risk of stroke for patients with AF, we also use ROC and c statistic (AUC statistic) to test the accuracy of CHA2DS2-VASC score and LA size in predicting the occurrence of stroke or TIA (Figure 3). For the CHA2DS2-VASC score, the AUC or c statistic for stroke events was 0.662 (95% CI, 0.601–0.723). For the LA size, the AUC or c statistic for stroke events was 0.595 (95% CI, 0.516–0.674). The c statistic of CHA2DS2-VASC score was larger than that of LA size, but not statistically significant (P = 0.187; Figure 3).
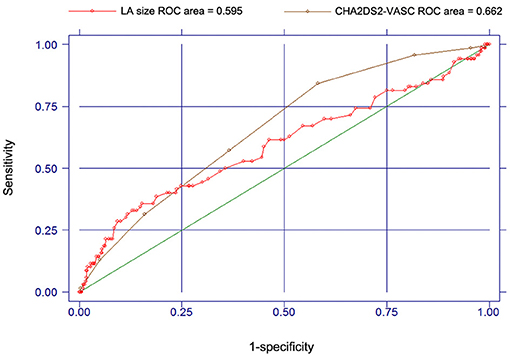
Figure 3. Receiver operating characteristic curves and c-statistics for CHA2DS2-VASC score and LA size in predicting the occurrence of stroke or TIA.
In the present study we showed both CHA2DS2-VASc score and LA size could predict the risk of stroke for patients with AF (20, 21). We further hypothesized that the CHA2DS2-VASc score predicted the risk of stroke through its association with LA size. To address this issue, we first investigated whether CHA2DS2-VASc score correlated with LA size, which had never been addressed before our study.
Interestingly, we found a positive correlation between LA size and CHA2DS2-VASc score (r = 0.17, P < 0.0001). There is an increase of mean LA size when the mean CHA2DS2-VASc score increases (P < 0.01 for trend; Figure 4). It indicates that AF patients with a higher CHA2DS2-VASc score may have a larger LA size.
We have shown a positive correlation between the CHA2DS2-VASc score and LA size, and both CHA2DS2-VASc score and LA size are predictors of stroke risk for patients with AF. It is logical to speculate that the CHA2DS2-VASc score predicts the risk of stroke through its association with LA size since LA size represents AF burden. To determine if CHA2DS2-VASc score predicted the risk of stroke through its association with LA size or if CHA2DS2-VASc score and LA size are independent predictors, we ran a multivariable Cox model.
Unexpectedly, when both CHA2DS2-VASc score and LA size were incorporated simultaneously into the Cox model, only CHA2DS2-VASc score remained statistically significant (HR 1.39; 95% CI 1.20–1.63; P < 0.001; Table 2).
The present study showed that LA enlargement was significantly associated with an increased risk of stroke in a very long-term AF follow-up cohort, but the association disappeared if the CHA2DS2-VASc score was also included in the multivariable model. The LA size was positively correlated with the CHA2DS2-VASc score. These results indicate that the association of LA size with incident stroke in patients with AF is through its correlation with the CHA2DS2-VASc score and in evaluating the risk of stroke for patients with AF, calculating the CHA2DS2-VASc score is enough without the need for LA size measurement.
A recent large-scale, prospective study with 2-year follow-up has shown that LA enlargement is independently associated with an increased risk of stroke and systemic embolization (11). However, another substudy of Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study with a 3.5-year follow-up has shown that larger LA size is not associated with increased risk of stroke (22). Finally, sub-analyses of three clinical trials with a 1.6-year follow-up have also shown that left ventricular dysfunction is an independent predictor of stroke while LA size is not (23). In these studies, the follow-up periods were short, and the relationship between CHA2DS2-VASc score and LA size was not addressed. In the present study, with the longest follow-up of AF patients, we showed that LA size was positively and linearly correlated with CHA2DS2-VASc score but did not provide additional information in the risk stratification of stroke.
LA enlargement could promote blood stasis, which in turn predisposes thrombus formation, especially in the left atrial appendage (24). Many studies have shown that LA dimension could reflect the AF burden, which has been reported to be associated with the risk of stroke in several device-detected AF trials (25–27). Therefore, since AF burden is difficult to measure in patients without cardiac implanted electronic devices, it has been proposed that LA dimension could be used as a good surrogate marker of AF burden and duration. However, while measurement of LA dimension may add additional information in AF patient care, echocardiography is a less feasible modality and not recommended for broad screening of unselected populations in the current guidelines and trials (28, 29). Our study first showed the positive correlation between LA size and the CHA2DS2-VASc score. This result further increases the simplicity of quantification of AF burden and duration for screening a large number of AF patients. Application of the CHA2DS2-VASc score seems useful in assessing the AF burden and duration in large AF population screen without the need to perform echocardiography or implantation of cardiac electronic device.
Initially, we expected that echocardiographic LA size was a good surrogate measurement of AF burden and LA size was the ultimate independent predictor of stroke risk for patients with AF. However, echocardiography may not be immediately available in all clinics. Calculation of CHA2DS2-VASc score is an easier tool to use in clinics to predict stroke risk for patients with AF, and we hypothesized that CHA2DS2-VASc score was effective to predict stroke risk through its correlation with LA size. However, unexpectedly, we found that CHA2DS2-VASc score, but not LA size, was the only independent predictor of AF stroke risk.
LA size is a surrogate measurement of AF burden, but whether AF burden is directly related to AF stroke risk remains debated (30–32). A significant proportion of cause of stroke in AF patients may be atheroemboli or atherosclerotic occlusion from carotid arteries, aorta, or other sources. This atheroembolic burden is related to atherosclerosis burden. Interestingly, all of the components of CHA2DS2-VASc score are risk factors of systemic atherosclerosis. Our results imply the possibility that a large proportion of cause of stroke in AF patients is atheroemboli or atherosclerotic occlusion from the arterial circulating system. To sum up, application of the CHA2DS2-VASc score still remain the mainstream to assess the stroke risk in large AF populations.
In addition to the possibility that the cause of stroke in AF patients is atheroemboli or atherosclerotic occlusion from the arterial circulating system, another possibility is that the cause of stroke is the thrombus in the left atrial appendage and the mechanism of thrombus formation in the left atrial appendage is a pathological process in the appendage endocardium, which is similar to vascular endothelial dysfunction or an atherosclerosis process, but not enlarged LA or left atrial appendage. It is logical to speculate that risk factors of endothelial dysfunction or atherosclerosis, such as the components of CHA2DS2-VASc score, are also risk factors of appendage endocardial pathology.
Our study first shows a positive correlation between CHA2DS2-VASc score and LA size. Regarding the plausible explanation of this correlation, all of the components of CHA2DS2-VASc score are predisposing factors of AF attack and LA remodeling. Patients with a higher CHA2DS2-VASc score may have more predisposing factors of AF attack and suffer from more AF episodes, which may increase their LA size.
The major limitation of the present study is no measurement of the LA volume. Because this is a large cohort with standard cares for AF patients, LA volume is not a routine measurement in our cohort. LA diameter measurement is more widely employed in daily practice. Furthermore, it has been shown that LA volume can be well-estimated from LA diameter using a non-linear equation with an elliptical model (33). Finally, a recent large-scale AF follow-up study also used echocardiographic M-mode measurement of the anteroposterior diameter of LA in parasternal long-axis view but not LA volume as one of their study parameters (11).
In conclusion, the present study shows a positive correlation between echocardiographic LA size and CHA2DS2-VASc score. LA size is not an independent predictor of AF-related stroke but provides a diagnostic value to predict AF-related stroke risk through its association with CHA2DS2-VASc score. Calculation of the CHA2DS2-VASc score may be an alternative to measure echocardiographic LA size when evaluating the risk of stroke for patients with AF.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s. The corresponding author has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The studies involving human participants were reviewed and approved by National Taiwan University Hospital. The patients/participants provided their written informed consent to participate in this study.
The content of this manuscript has been presented at the ESC Congress 2019.
C-FT and P-SH: study design, analyses, conducting, and manuscript preparation. J-JC, S-NC, and L-PL: study design. F-CC: analyses. T-TL: study design and analyse. J-JH: study design and planning. C-TT: study design, analyses, and manuscript preparation. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AF, atrial fibrillation; AUC, area under curve; DM, diabetes mellitus; ECG, electrocardiography; LA, left atrium; LV, left ventricle; NTUAFR, National Taiwan University Atrial Fibrillation Registry; ROC, receiver operator characteristics; TIA, transient ischemic attack.
1. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. (2002) 90:1284–9. doi: 10.1016/S0002-9149(02)02864-3
2. Di Tullio MR, Qian M, Thompson JLP, Labovitz AJ, Mann DL, Sacco RL, et al. Left atrial volume and cardiovascular outcomes in systolic heart failure: effect of antithrombotic treatment. ESC Heart Fail. (2018) 5:800–8. doi: 10.1002/ehf2.12331
3. Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. (2014) 35:1457–65. doi: 10.1093/eurheartj/eht500
4. Caplan LR, D'Cruz I, Hier DB, Reddy H, Shah S. Atrial size, atrial fibrillation, and stroke. Ann Neurol. (1986) 19:158–61. doi: 10.1002/ana.410190208
5. Aronow WS, Gutstein H, Hsieh FY. Risk factors for thromboembolic stroke in elderly patients with chronic atrial fibrillation. Am J Cardiol. (1989) 63:366–7. doi: 10.1016/0002-9149(89)90349-4
6. Cabin HS, Clubb KS, Hall C, Perlmutter RA, Feinstein AR. Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol. (1990) 65:1112–6. doi: 10.1016/0002-9149(90)90323-S
7. Corbalan R, Arriagada D, Braun S, Tapia J, Huete I, Kramer A, et al. Risk factors for systemic embolism in patients with paroxysmal atrial fibrillation. Am Heart J. (1992) 124:149–53. doi: 10.1016/0002-8703(92)90933-M
8. Nagarajarao HS, Penman AD, Taylor HA, Mosley TH, Butler K, Skelton TN, et al. The predictive value of left atrial size for incident ischemic stroke and all-cause mortality in African Americans: the atherosclerosis risk in communities (aric) study. Stroke. (2008) 39:2701–6. doi: 10.1161/STROKEAHA.108.515221
9. Bouzas-Mosquera A, Broullon FJ, Alvarez-Garcia N, Mendez E, Peteiro J, Gandara-Sambade T, et al. Left atrial size and risk for all-cause mortality and ischemic stroke. CMAJ. (2011) 183:E657–64. doi: 10.1503/cmaj.091688
10. Lai CL, Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Left atrial dimension and risk of stroke in women without atrial fibrillation: the Chin-Shan community cardiovascular cohort study. Echocardiography. (2011) 28:1054–60. doi: 10.1111/j.1540-8175.2011.01489.x
11. Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep. (2016) 6:31042. doi: 10.1038/srep31042
12. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. doi: 10.1378/chest.09-1584
13. Tsai CT, Lai LP, Lin JL, Chiang FT, Hwang JJ, Ritchie MD, et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. (2004) 109:1640–6. doi: 10.1161/01.CIR.0000124487.36586.26
14. Tsai CT, Hsieh CS, Chang SN, Chuang EY, Ueng KC, Tsai CF, et al. Genome-wide screening identifies a KCNIP1 copy number variant as a genetic predictor for atrial fibrillation. Nat Commun. (2016) 7:10190. doi: 10.1038/ncomms10190
15. Chang SNC, Lai LP, Chiang FT, Lin JL, Hwang JJ, Tsai CT. The CRP gene polymorphism predicts the risk of thromboembolic stroke in atrial fibrillation: a more than 10-year prospective follow-up study. J Thromb Haemost. (2017) 15:1541–6. doi: 10.1111/jth.13735
16. Chiang CE, Wu TJ, Ueng KC, Chao TF, Chang KC, Wang CC, et al. 2016 Guidelines of the Taiwan heart rhythm society and the taiwan society of cardiology for the management of atrial fibrillation. J Formos Med Assoc. (2016) 115:893–952. doi: 10.1016/j.jfma.2016.10.005
17. Xu Y, Zhao L, Zhang L, Han Y, Wang P, Yu S. Left atrial enlargement and the risk of stroke: a meta-analysis of prospective cohort studies. Front Neurol. (2020) 11:26. doi: 10.3389/fneur.2020.00026
18. Tomasdottir M, Friberg L, Hijazi Z, Lindbäck J, Oldgren J. Risk of ischemic stroke and utility of CHA(2) DS(2) -VASc score in women and men with atrial fibrillation. Clin Cardiol. (2019) 42:1003–9. doi: 10.1002/clc.23257
19. Chen LY, Norby FL, Chamberlain AM, MacLehose RF, Bengtson LGS, Lutsey PL, et al. CHA(2)DS(2)-VASc score and stroke prediction in atrial fibrillation in whites, blacks, and hispanics. Stroke. (2019) 50:28–33. doi: 10.1161/STROKEAHA.118.021453
20. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. (2014) 130:e199–267. doi: 10.1161/CIR.0000000000000040
21. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.5603/KP.2016.0172
22. Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. J Am Coll Cardiol. (2005) 45:2026–33. doi: 10.1016/j.jacc.2005.03.020
23. Atrial Fibrillation Investigators: Atrial Fibrillation Aspirin Anticoagulation Study; European Atrial Fibrillation Study; Stroke Prevention in Atrial Fibrillation Study; Boston Area Anticoagulation Trial for Atrial Fibrillation Study; Canadian Atrial Fibrillation Study; Veterans Affairs Prevention in Atrial Fibrillation Study. Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. (1998). 158:1316–20. doi: 10.1001/archinte.158.12.1316
24. Shirani J, Alaeddini J. Structural remodeling of the left atrial appendage in patients with chronic non-valvular atrial fibrillation: implications for thrombus formation, systemic embolism, and assessment by transesophageal echocardiography. Cardiovasc Pathol. (2000) 9:95–101. doi: 10.1016/S1054-8807(00)00030-2
25. Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. (1990) 82:792–7. doi: 10.1161/01.CIR.82.3.792
26. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn strategies based on atrial fibrillation information from implanted devices). Eur Heart J. (2014) 35:508–16. doi: 10.1093/eurheartj/eht491
27. Chen-Scarabelli C, Scarabelli TM, Ellenbogen KA, Halperin JL. Device-detected atrial fibrillation: what to do with asymptomatic patients? J Am Coll Cardiol. (2015) 65:281–94. doi: 10.1016/j.jacc.2014.10.045
28. Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. a report of the American college of cardiology foundation appropriate use criteria task force, American society of echocardiography, American heart association, American society of nuclear cardiology, heart failure society of America, heart rhythm society, society for cardiovascular angiography and interventions, society of critical care medicine, society of cardiovascular computed tomography, and society for cardiovascular magnetic resonance endorsed by the American college of chest physicians. J Am Coll Cardiol. (2011) 57:1126–66. doi: 10.1016/j.echo.2010.12.008
29. Lindekleiv H, Lochen ML, Mathiesen EB, Njolstad I, Wilsgaard T, Schirmer H. Echocardiographic screening of the general population and long-term survival: a randomized clinical study. JAMA Intern Med. (2013) 173:1592–8. doi: 10.1001/jamainternmed.2013.8412
30. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. (2009) 2:474–80. doi: 10.1161/CIRCEP.109.849638
31. Viles-Gonzalez JF, Halperin JL. Everything counts in large amounts: device-detected atrial high-rate arrhythmias. Circ Arrhythm Electrophysiol. (2009) 2:471–3. doi: 10.1161/CIRCEP.109.909101
32. Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. (2015) 8:1040–7. doi: 10.1161/CIRCEP.114.003057
Keywords: left atrial size, CHA2DS2-VASc score, stroke, atrial fibrillation, Asia
Citation: Tsai C-F, Huang P-S, Chen J-J, Chang S-N, Chiu F-C, Lin T-T, Lai L-P, Hwang J-J and Tsai C-T (2021) Correlation Between CHA2DS2-VASc Score and Left Atrial Size in Patients With Atrial Fibrillation: A More Than 15-Year Prospective Follow-Up Study. Front. Cardiovasc. Med. 8:653405. doi: 10.3389/fcvm.2021.653405
Received: 14 January 2021; Accepted: 22 April 2021;
Published: 28 June 2021.
Edited by:
Lee Stoner, University of North Carolina at Chapel Hill, United StatesReviewed by:
Gabriel Zieff, University of North Carolina at Chapel Hill, United StatesCopyright © 2021 Tsai, Huang, Chen, Chang, Chiu, Lin, Lai, Hwang and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chia-Ti Tsai, Y3R0c2FpMTk5OUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.