- 1Department of Cardiology, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou, China
- 2Fujian Yirong Information Technology Corporation, Fuzhou, China
- 3Department of Epidemiology and Health Statistics, School of Public Health, Fujian Medical University, Fuzhou, China
- 4Fujian Provincial Key Laboratory of Cardiovascular Disease, Fujian Provincial Center for Geriatrics, Fujian Clinical Medical Research Center for Cardiovascular Diseases, Fuzhou, China
- 5Fujian Heart Failure Center Alliance, Fuzhou, China
Aim: Accumulating evidence suggests that MELD-XI score holds the ability to predict the prognosis of congestive heart failure. However, most of the evidence is based on the end-stage heart failure population; thus, we aim to explore the association between the MELD-XI score and the prognosis in heart failure with preserved ejection fraction (HFpEF).
Methods: A total of 30,096 patients hospitalized for HFpEF in Fujian Provincial Hospital between January 1, 2014 and July 17, 2020 with available measures of creatinine and liver function were enrolled. The primary endpoint was 60-day in-hospital all-cause mortality. Secondary endpoints were 60-day in-hospital cardiovascular mortality and 30-day rehospitalization for heart failure.
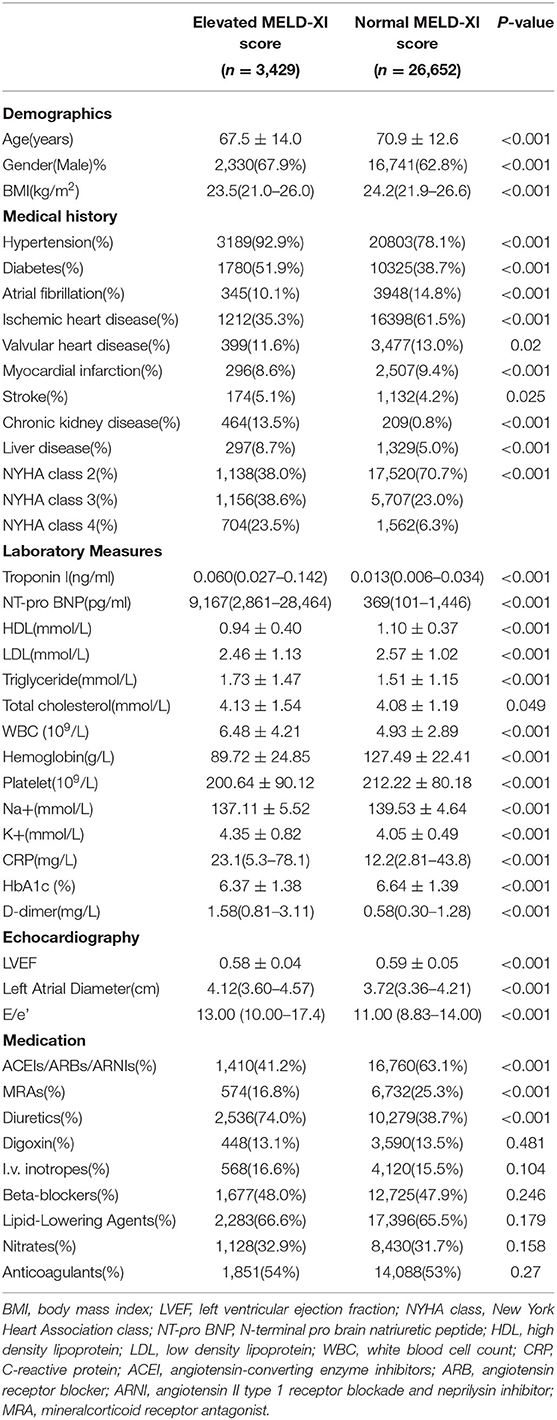
Results: A total of 222 patients died within 60 days after admission, among which 75 deaths were considered cardiogenic. And 73 patients were readmitted for heart failure within 30 days after discharge. Generally, patients with an elevated MELD-XI score tended to have more comorbidities, higher NYHA class, and higher inflammatory biomarkers levels. Meanwhile, the MELD-XI score was positively correlated with NT-pro BNP, left atrial diameter, E/e' and negatively correlated with LVEF. After adjusting for conventional risk factors, the MELD-XI score was independently associated with 60-day in-hospital all-cause mortality [hazard ratio(HR) = 1.052, 95% confidential interval (CI) 1.022–1.083, P = 0.001], 60-day in-hospital cardiovascular mortality (HR = 1.064, 95% CI 1.013–1.118, P = 0.014), and 30-day readmission for heart failure (HR = 1.061, 95% CI 1.015–1.108, P = 0.009). Furthermore, the MELD-XI score added an incremental discriminatory capacity to risk stratification models developed based on this cohort.
Conclusion: The MELD-XI score was associated with short-term adverse events and provided additional discriminatory capacity to risk stratification models in patients hospitalized for HFpEF.
Introduction
Patients with heart failure with preserved ejection fraction(HFpEF) account for 22–73% of the total population of heart failure (1). Compared with patients with heart failure with reduced ejection fraction(HFrEF), the prognosis of patients with HFpEF appears to be better, but there have not been many improvements throughout the years (2). Previous studies underscored that HFpEF is often accompanied by liver dysfunction and renal dysfunction, which are independent predictors for a poor prognosis (3–7). The mutual pathophysiological mechanism shared by HFpEF, impaired liver, and renal function is speculated to be the combination of insufficient perfusion, hormonal imbalance, and inflammation (3, 6, 8).
As an indicator of liver dysfunction and renal dysfunction, model of end-stage liver dysfunction (MELD-XI), has been shown to be negatively correlated with the survival rate in end-stage heart failure patients undergoing cardiac transplantation. It is used as a simple tool to assess appropriate candidates (9, 10). Furthermore, recent studies also suggested that the MELD-XI score is associated with poor prognosis in congestive and acute heart failure (11, 12).
To our knowledge, there is sparse evidence addressing the predictive value of the MELD-XI score in HfpEF, and this study may represent the largest real-world cohort to evaluate the utility of the MELD-XI score calculated at admission as a predictor for short-term prognoses in patients hospitalized for HFpEF.
Methods
Data Source and Study Definition
Study Population
A total of 30,096 patients hospitalized for HFpEF in Fujian Provincial Hospital between January 1, 2014 and July 17, 2020 with available measures of liver function were retrospectively enrolled. HFpEF was defined based on the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure, which is characterized by a left ventricular ejection fraction (LVEF) ≥50% (1). Patients with acute coronary syndrome, acute phase of stroke, advanced cancer, pregnancy, active rheumatic disease, advanced liver cirrhosis, and undergoing dialysis were excluded.
Endpoints
The primary endpoint was 60-day in-hospital all-cause mortality. Secondary endpoints were 60-day in-hospital cardiovascular mortality and 30-day rehospitalization for heart failure. The 30-day readmission for heart failure was defined as hospital admission for decompensated heart failure from 24 h to 30 days after discharge. In-hospital cardiovascular mortality was death, consistent with a ventricular tachyarrhythmia, and occurred in the absence of a known non-cardiac condition as the proximate cause of the death (13).
Biochemical Measurements
Liver function tests and renal function tests were quantified in a core laboratory (Roche, Modular-P chemical analyzer). Based on routine laboratory standards in Fujian Provincial Hospital, the upper limit was 23 μmol/L (1.35 mg/dl) for total bilirubin, similar to the standard published by previously studies (7).
The MELD-XI score was calculated as follows: 5.11 × Ln (total bilirubin as mg/dl)+11.76 × Ln (creatinine as mg/dl) + 9.44 (14). A MELD-XI score > 9.44 was considered elevated (12). eGFR was calculated using the simplified modification of diet in renal disease (MDRD) formula described by the National Kidney Foundation as follows: eGFR (ml/min/1.73 m2) = 175×(Scr)−1.154 × (Age)−0.203 × (0.742 if female) (15).
Statistical Analysis
Continuous variables were presented as mean ± SD when normally distributed, as median and interquartile range (IQR) when skewed. Categorical variables were presented as frequencies and percentages. Differences between groups were evaluated by Student t, Mann-Whitney U test, or chi-square test as appropriate. The Spearman correlation test was applied to explore the association between the MELD-XI score and NT-pro BNP. A total of nine cox regression models were developed to estimate the association between the baseline MELD-XI score and the three study endpoints. Covariates in model 1 included age and gender. Covariates in model 2 included age, gender, elevated blood pressure (BP) at admission (>140/90mmHg), history of diabetes, history of atrial fibrillation, history of stroke, history of myocardial infarction, New York Heart Association (NYHA) class, LVEF, white blood cell count (WBC), triglyceride. Covariates in model 3 included age, gender, elevated BP at admission, history of diabetes, history of atrial fibrillation, history of stroke, history of myocardial infarction, NYHA class, NT-pro BNP, LVEF, WBC, triglyceride, use of digoxin, use of intravenous inotropes, use of beta-blocker, use of lipid-lowering agents. Models 1, 2, and 3 were used to estimate the risk of the three endpoints separately. Logistic regression and area under the receiver operating characteristic (ROC) curve were applied to evaluate the discriminatory capacity of the models for predicting 60-day all-cause in-hospital mortality. After adjusting for covariates stated above, age, LVEF, NYHA class, WBC, triglyceride remained independent risk factors, and thus were included in model 4.
These analyses were conducted using a statistical software package (SPSS version 25.0, IBM, Armonk, NY, USA). A P-value < 0.05 was considered statistically significant.
Results
Study Population
Participants had a mean age of 70.7 ± 12.8, 64.1% were male, 19,048(57.6%) had an ischemic etiology of heart failure, 1,627(5.4%) had history of liver disease, 2,299(7.6%) had elevated total bilirubin(>1.35 mg/dl), 673(2.2%) had a previous diagnosis of chronic kidney disease(CKD), and 9,715(32.3%)had an eGFR < 60 ml/min/1.73 m2.
Generally, patients with elevated MELD-XI score tended to be male, had worse cardiac function, and higher inflammatory biomarker levels. Furthermore, patients with elevated MELD-XI score were more likely to have previous diagnosis of hypertension and diabetes mellitus. However, patients with an elevated MELD-XI score seemed to receive less prescription of angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB), angiotensin II type 1 receptor blockade and neprilysin inhibitor (ARNI), and mineralcorticoid receptor antagonist (MRA) (Table 1).
Association Between Cardiac Function and MELD-XI Score
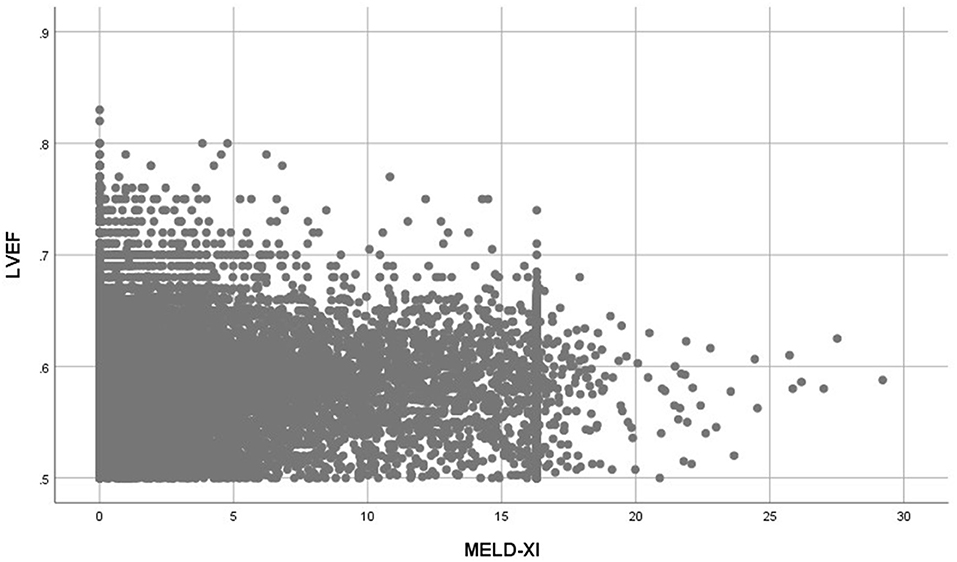
Patients with elevated MELD-XI scores had significantly higher NT-pro BNP levels (Median = 9,167 pg/ml vs. 369 pg/ml, P < 0.001), higher left atrial diameter (Median = 4.12 cm vs. 3.72 cm, P < 0.001), higher E/e' (Median = 13.00 vs. 11.00, P < 0.001) and lower LVEF (0.58 ± 0.04 vs. 0.59 ± 0.05, P < 0.001) (Table 1). The MELD-XI score had a moderate correlation with NT-pro BNP (Spearman rho = 0.497, P < 0.001) and a weak correlation with left atrial diameter (Spearman rho = 0.209, P < 0.001) and E/e' (Spearman rho = 0.090, P < 0.001). A weak and inverse correlation was also determined between the MELD-XI score and LVEF (Spearman rho = −0.084, P < 0.001) (Figure 3).
MELD-XI Score and Short-Term Adverse Events
A total of 222 patients died within 60 days after admission, among which 75 deaths were considered cardiogenic. And among 29,844 living patients within 30 days after discharge, 73 were readmitted for heart failure within 30 days after discharge. Patients with elevated MELD-XI scores suffered a significantly higher risk of mortality, cardiovascular mortality, and readmission for heart failure compared with those with normal MELD-XI (Figure 1). After adjusting for conventional risk factors, the MELD-XI score remained a predominant predictor for 60-day in-hospital all-cause mortality (HR = 1.052, 95%CI 1.022–1.083, P = 0.001), 60-day in-hospital cardiovascular mortality (HR = 1.064, 95% CI 1.013–1.118, P = 0.014), and 30-day readmission (HR = 1.061, 95% CI 11.015–1.108, P = 0.009) (Table 2).
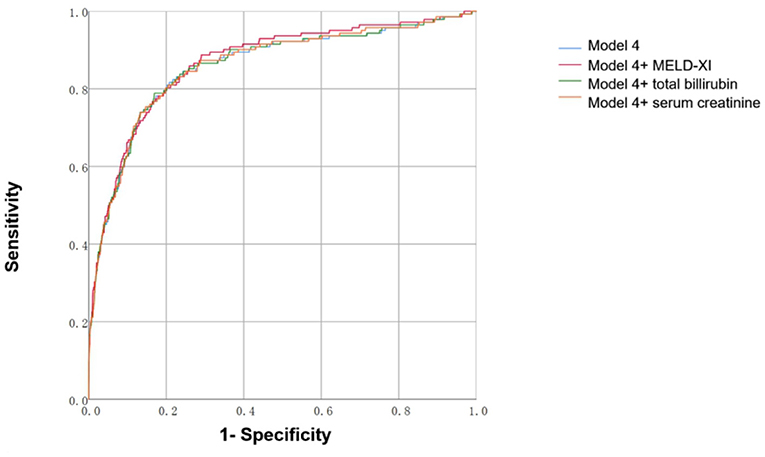
Figure 1. Kaplan–Meier estimates of short-term clinical outcomes of according to baseline MELD-XI score.
Predictive Value of Models Includes MELD-XI
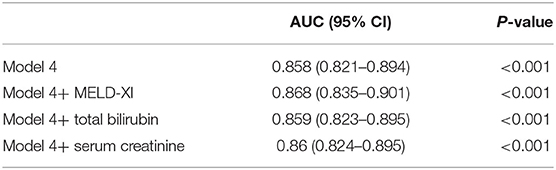
We constructed risk stratification models for prediction of in-hospital all-cause mortality. As shown in Figure 2 and Table 3, adding the MELD-XI score slightly increased the discriminatory capacity of model 4 (AUC = 0.868 vs. 0.858, P = 0.0162), which was better than models including total bilirubin or serum creatinine (AUC = 0.868 vs. 0.859, P = 0.0296; AUC = 0.868 vs. 0.860, P = 0.0161).
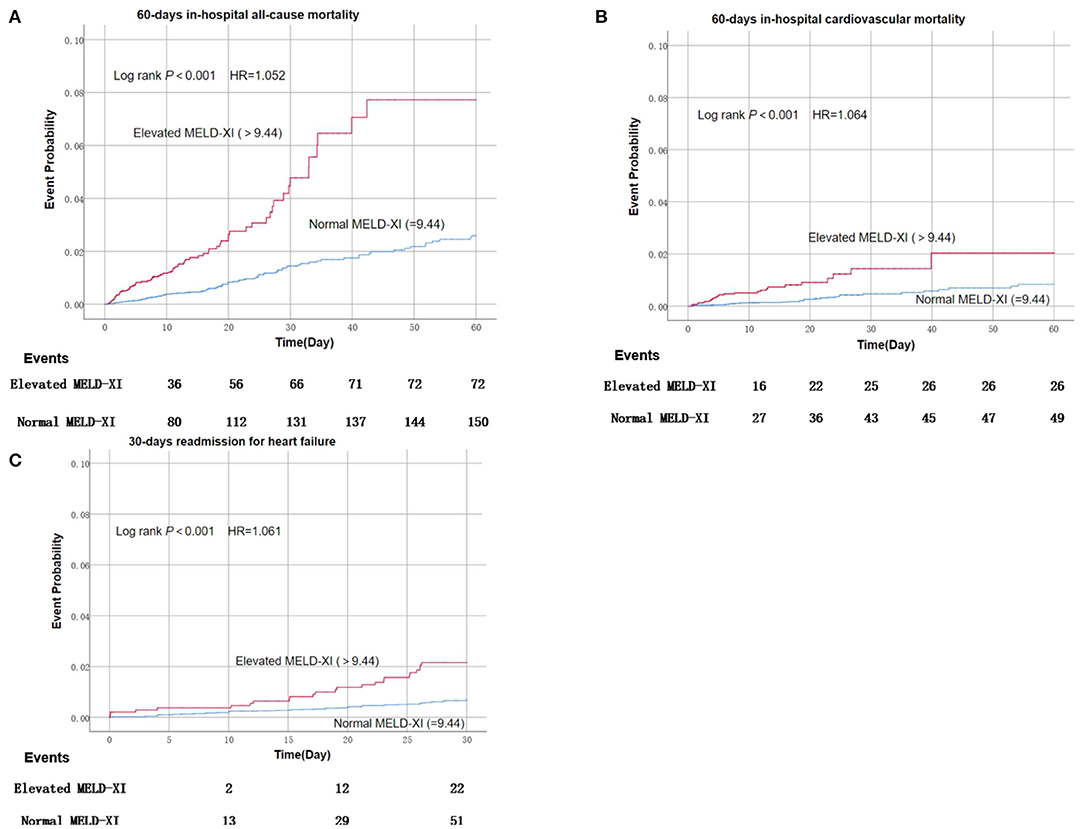
Figure 2. (A-C) Area under the receiver operating characteristic curve of models predicting 60-day in-hospital all-cause mortality and cumulative events. Model 4: Adjusted for age, LVEF, NYHA class, WBC, triglyceride. Calculated as: −0.015 + 0.019 × age −14.604 × LVEF + 1.293 × NYHA class III (1) [OR 2.871 × NYHA Class IV (1)] + 0.046 × WBC(109/L) + 0.118 × triglyceride(mmol/L). Model 4+MELD-XI: −0.420 + 0.024 × age −14.488 × LVEF + 1.135 × NYHA class III (1) [OR 2.567 × NYHA Class IV (1)] + 0.043 × WBC(109/L) + 0.109 × triglyceride(mmol/L) + 0.053 × MELD-XI. Model 4+serum creatinine: −0.163 + 0.021 × age −14.593 × LVEF + 1.267 × NYHA class III (1) [OR 2.830 × NYHA Class IV (1)] + 0.046 × WBC(109/L) + 0.116 × triglyceride(mmol/L) + 0.031 × serum creatinine(mg/dl). Model 4+total bilirubin: −0.128 + 0.020 × age −14.639 × LVEF + 1.282 × NYHA class III (1) [OR 2.842 × NYHA Class IV (1)] + 0.044 × WBC(109/L) + 0.118 × triglyceride(mmol/L) + 0.083 × total bilirubin (mg/dl).
Discussion
It has been noticed that both liver dysfunction and renal dysfunction are common comorbidities during congestive heart failure as well as associated with ominous prognosis (3–7). As a combined index of liver function and renal function, it has been shown that the MELD-XI score holds the ability to predict adverse events in patients with acute heart failure and end-stage heart failure (11, 12). To our knowledge, this study is the first to find the MELD-XI score to be a risk factor for short-term adverse events, improving risk stratification models for patients hospitalized with HFpEF.
The predictive ability of liver dysfunction and renal dysfunction have been confirmed in heart failure. A study by Allen et al. (16) demonstrated that abnormal liver function tests are prevalent in patients with heart failure, and total bilirubin could be one of the most powerful predictors for poor prognosis among all liver function tests. Furthermore, Prenner et al. (5) conducted a post-hoc study of TOP-CAT trial and indicated that albumin was also a strong predictor of adverse events in patients with HFpEF. Data from a most recent post-hoc study of TOP-CAT suggested that not only kidney function tests but also their variability between visits are independently correlated with clinical outcomes (17).
As a modification of the MELD score, the MELD-XI score excludes international normalized ratio and consists of only total bilirubin and serum creatinine, thus could be applied to heart failure patients receiving anticoagulants. Previous investigators confirmed that higher MELD-XI value is associated with mortality in patients with advanced heart failure undergoing heart transplantation, thus could serve as a simple quantitative tool for screening appropriate candidates receiving heart transplantation (11, 18). Afterward, the MELD-XI score was also validated in patients with acute heart failure as an innovative prognosticator (12, 19). Recently Abe et al. (20) retrospectively analyzed 562 patients with decompensated heart failure and concluded that MELD-XI was an independent predictor of mortality in general heart failure patients. In our cohort, 53% patients received anticoagulants; hence, MELD-XI seems an appropriate approach to estimate liver and renal function compared to original MELD score and MELD-Na score. And to extend this conclusion from previous study, we analyzed short-term events separately and confirmed the prognostic role of MELD-XI in the setting of HFpEF.
Analyzing each possible risk factor is the foundation of accurate risk stratification, which ultimately contributes to the management strategy of patients with HFpEF (1). A Seattle Heart Failure Model was derived from the population of general heart failure then validated in 9,942 patients, which provided an overall AUC of 0.729 (21). The MUSIK risk score, which contains 10 variables (eGFR), was developed in a cohort of 992 ambulatory heart failure patients and demonstrated good performance of predicting mortality (22). Risk stratification models developed in our study contained only six variables and yielded good discriminatory capacity for short-term adverse events, which was improved slightly by adding the MELD-XI score.
The pathophysiological connection between MELD-XI and HFpEF might consist of several aspects. First, decompensated cardiac function provides insufficient perfusion to both the kidneys and liver, thus leading to decreased glomerular filtration rate as well as tissue damage of renal and liver (6, 8, 23). Second, elevated right atrial pressure due to heart failure, especially HFpEF, usually results in passive hepatic venous congestion, lipid metabolism disorder, and even cirrhosis (4, 23–25). Third, neurohumoral disorder results in vascular contractility disorder in HfpEF, which further reduces renal blood flow and liver perfusion (25, 26). And in turn, impaired liver and renal function contribute to hemodynamic changes and inflammatory activity, eventually resulting in cardiac stiffness (3, 6, 24). Data from our study have confirmed the association between MELD-XI and NT-pro BNP, which reinforced that volume overload is the reflexion of the vicious cycle formed by HFpEF and impaired liver and renal function. And we also found that MELD-XI was correlated with cardiac function indexes derived from echocardiography, which supports that impaired liver and renal function is responsible for the deteriorated cardiac stiffness during HFpEF.
Our study demonstrated that the MELD-XI score not only was associated with short-term adverse events but also offered increased discriminatory ability for risk stratification models in patients hospitalized for HFpEF.
Limitation
First, as a retrospective study based on the data of a single center, the conclusion should be further validated in different regions and different populations. Second, we failed to analyze the association between long-term prognosis and the MELD-XI score since we have not been able to complete the follow-up procedure due to the large scale of this study, although it is still crucial to focus on the risk stratification of long-term events. Third, considering the extendibility of the conclusion, we only excluded patients with advanced liver cirrhosis and those undergoing dialysis, not all patients with previously diagnosed liver disease and renal disease.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Fujian Provincial Hospital Ethics Committee (福绺省立猻癢缦理秔瑘缚). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SW and YW built this large scale database containing the clinical information of heart failure patients in Fujian Provincial Hospital. SW and XiaoX conducted the statistical analysis. YG is the overseer of this study. Rest of the team contributed to the idea gathering. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China General Program (No. 81873495), Natural Science Foundation of Fujian Province (No. 2018J01242), and Fujian Provincial Health Commission Youth Key Talents Project (Key Category) (No. 2014-ZQN-ZD-2).
Conflict of Interest
YW is a software engineer employed by Fujian Yirong Information Technology Corporation and she provided her information technological support for free.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland J, Coats A, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
2. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355:251–9. doi: 10.1056/NEJMoa052256
3. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. (2016) 18:588–98. doi: 10.1002/ejhf.497
4. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. (2018) 5:262–70. doi: 10.1002/ehf2.12222
5. Prenner SB, Kumar A, Zhao L, Cvijic ME, Basso M, Spires T, et al. Effect of Serum albumin levels in patients with heart failure with preserved ejection fraction (from the TOPCAT Trial). Am J Cardiol. (2020) 125:575–82. doi: 10.1016/j.amjcard.2019.11.006
6. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. (2016) 12:610–23. doi: 10.1038/nrneph.2016.113
7. Ambrosy AP, Vaduganathan M, Huffman MD, Khan S, Kwasny MJ, Fought AJ, et al. EVEREST trial investigators, Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail. (2012) 14:302–11. doi: 10.1093/eurjhf/hfs007
8. Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation. (2016) 133:1696–703. doi: 10.1161/CIRCULATIONAHA.115.020894
9. Chokshi A, Cheema FH, Schaefle KJ, Jiang J, Collado E, Shahzad K, et al. Hepatic dysfunction and survival after orthotopic heart transplantation: application of the MELD scoring system for outcome prediction. J Heart Lung Transplant. (2012) 31:591–600. doi: 10.1016/j.healun.2012.02.008
10. Kato TS, Stevens GR, Jiang J, Schulze PC, Gukasyan N, Lippel M, et al. Risk stratification of ambulatory patients with advanced heart failure undergoing evaluation for heart transplantation. J Heart Lung Transplant. (2013) 32:333–40. doi: 10.1016/j.healun.2012.11.026
11. Kim MS, Kato TS, Farr M, Wu C, Givens RC, Collado E, et al. Hepatic dysfunction in ambulatory patients with heart failure: application of the MELD scoring system for outcome prediction. J Am Coll Cardiol. (2013) 61:2253–61. doi: 10.1016/j.jacc.2012.12.056
12. Biegus J, Zymliński R, Sokolski M, Siwołowski P, Gajewski P, Nawrocka-Millward S, et al. Impaired hepato-renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail. (2016) 18:1518–21. doi: 10.1002/ejhf.644
13. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. (2012) 366:1881–90. doi: 10.1056/NEJMoa1003833
14. Simpson J, Jhund PS, Silva Cardoso J, Martinez F, Mosterd A, Ramires F, et al. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS-HF risk scores: an analysis of mortality and morbidity in PARADIGM-HF. J Am Coll Cardiol. (2015) 66:2059–71. doi: 10.1016/j.jacc.2015.08.878
15. Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. (2001) 37(1 Suppl 2): S66–70. doi: 10.1053/ajkd.2001.20748
16. Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. (2009) 11:170–7. doi: 10.1093/eurjhf/hfn031
17. Segar MW, Patel RB, Patel KV, Fudim M, DeVore AD, Martens P, et al. Association of visit-to-visit variability in kidney function and serum electrolyte indexes with risk of adverse clinical outcomes among patients with heart failure with preserved ejection fraction. JAMA Cardiol. (2020) 6:68–77. doi: 10.1001/jamacardio.2020.5592
18. Assenza GE, Graham DA, Landzberg MJ, Valente AM, Singh MN, Bashir A, et al. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. (2013) 99:491–6. doi: 10.1136/heartjnl-2012-303347
19. Inohara T, Kohsaka S, Shiraishi Y, Goda A, Sawano M, Yagawa M, et al. Prognostic impact of renal and hepatic dysfunction based on the MELD-XI score in patients with acute heart failure. Int J Cardiol. (2014) 176:571–3. doi: 10.1016/j.ijcard.2014.08.052
20. Abe S, Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, et al. Liver dysfunction assessed by model for end-stage liver disease excluding INR (MELD-XI) scoring system predicts adverse prognosis in heart failure. PLoS ONE. (2014) 9: e100618. doi: 10.1371/journal.pone.0100618
21. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. (2006) 113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102
22. Vazquez R, Bayes-Genis A, Cygankiewicz I, Pascual-Figal D, Grigorian-Shamagian L, Pavon R, et al. The MUSIC Risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J. (2009) 30:1088–96. doi: 10.1093/eurheartj/ehp032
23. Durante-Mangoni E, Parrella A, Pafundi PC, Vitrone M, Ragone E, De Rosa I, et al. Liver histopathological findings in advanced heart failure: a reappraisal of cardiac cirrhosis concept. Intern Emerg Med. (2019) 14:931–40. doi: 10.1007/s11739-019-02033-3
24. van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. (2010) 16:84–90. doi: 10.1016/j.cardfail.2009.08.002
25. Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Kawamoto A, et al. Analysis of liver metabolism in a rat model of heart failure. Int J Cardiol. (2012) 161:130–6. doi: 10.1016/j.ijcard.2011.07.056
Keywords: heart failure with preserved ejection fraction, MELD-XI score, prognosis, short-term, risk stratification
Citation: Wang S, Wang Y, Luo M, Lin K, Xie X, Lin N, Yang Q, Zou T, Chen X, Xie X and Guo Y (2021) MELD-XI Score Is Associated With Short-Term Adverse Events in Patients With Heart Failure With Preserved Ejection. Front. Cardiovasc. Med. 8:650191. doi: 10.3389/fcvm.2021.650191
Received: 06 January 2021; Accepted: 07 April 2021;
Published: 25 May 2021.
Edited by:
Manuel Martínez-Sellés, Gregorio Marañón Hospital, SpainReviewed by:
Eisuke Amiya, The University of Tokyo Hospital, JapanKristen M. Tecson, Baylor Scott & White Research Institute (BSWRI), United States
Copyright © 2021 Wang, Wang, Luo, Lin, Xie, Lin, Yang, Zou, Chen, Xie and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yansong Guo, eXNndW8xMjM0QDE2My5jb20=
 Sunying Wang
Sunying Wang Yuwei Wang2
Yuwei Wang2 Xiaoxu Xie
Xiaoxu Xie