- 1Department of Critical Care Medicine, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Hematology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Emergency, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
Background: Severe thrombocytopenia is a common complication of extracorporeal membrane oxygenation (ECMO). Oseltamivir can be used to treat infection-associated thrombocytopenia.
Objective: To evaluate the effect of oseltamivir on attenuating severe thrombocytopenia during ECMO.
Methods: This was a single-center real-world study in critically ill patients supported with venous-arterial extracorporeal membrane oxygenation (VA-ECMO). Patients suspected or confirmed with influenza received oseltamivir according to the Chinese guidelines. Thrombocytopenia and survival were compared between the oseltamivir-treated and untreated group. The factors associated with survival were analyzed by multivariable Cox analysis.
Results: A total of 82 patients were included. All patients developed thrombocytopenia after initiating VA-ECMO. Twenty-three patients received oseltamivir (O+ group), and 59 did not use oseltamivir (O− group). During the first 8 days after VA-ECMO initiation, the platelet count in the O+ group was higher than that in the O− group (all P < 0.05). The patients in the O+ group had a higher median nadir platelet count (77,000/μl, 6,000–169,000/μl) compared with the O− group (49,000/μl, 2,000–168,000/μl; P = 0.04). A nadir platelet count of <50,000/μl was seen in 26% of the patients in the O+ group, compared with 53% in the O− group (P = 0.031). No significant difference in survival from cardiac failure was seen between the O+ and O− group (48 vs. 56%, P = 0.508). The Sequential Organ Failure Assessment (SOFA) score on initiation of VA-ECMO were independently associated with survival (OR = 1.12, 95% confidence interval (95% CI): 1.02–1.22, P = 0.015).
Conclusions: Oseltamivir could ameliorate VA-ECMO-related thrombocytopenia. These findings suggested the prophylactic potential of oseltamivir on severe thrombocytopenia associated with the initiation of VA-ECMO.
Background
ECMO is increasingly used as a rescue therapy for patients with refractory cardiac/respiratory failure to provide temporary support or a bridge in the management decision-making for adult and pediatric patients (1–4). There were limited clinical trial data supporting ECMO use in adults, but it was shown that ECMO might reduce mortality compared to conventional ventilation (1–4).
Platelets are activated and even impaired due to biological incompatibility and high shear stress within 15 min after starting ECMO. The activation or damage process continues throughout ECMO until it is discontinued (5). Severe thrombocytopenia is a common complication in patients supported with ECMO. Persistent severe thrombocytopenia can independently predict 90-day mortality (6). The recovery of platelet count over time will discriminate between survivors and non-survivors. Thus, the correction of thrombocytopenia is a key issue for clinicians during ECMO. Except for emergent platelet transfusion, there is currently no effective method to prevent severe thrombocytopenia when necessary.
Nevertheless, a better understanding of platelet function and biology could yield some hints to improve platelet levels during ECMO. Indeed, recent studies have highlighted the role of platelet desialylation in platelet clearance. Platelet desialylation exposes its β-galactose residues, which can be recognized by Ashwell-Morell receptors (AMRs) on hepatocytes, leading to platelet phagocytosis in the liver (7). Furthermore, platelet desialylation can be induced by exogenous neuraminidases from pathogens (8) and by intracellular neuraminidase (Neu1) translocation to the platelet surface during platelet activation (9). Therefore, desialylation could be responsible, at least in part, for the pathogenesis of thrombocytopenia in many diseases and the clearance of transfused platelets remaining in the circulation (10). It has been shown that inhibition of platelet desialylation can extend the lifespan of circulating platelets and increase their overall number (9, 11).
Oseltamivir is an antiviral agent commonly used to prevent and treat influenza A and B. It is a viral sialidase inhibitor that prevents the release of progeny virions (12). Oseltamivir can treat infection-associated thrombocytopenia by inhibiting platelet desialylation (13, 14). Therefore, this study was designed to evaluate the effect of oseltamivir on attenuating severe thrombocytopenia during ECMO. The results could provide a novel method to prevent ECMO-related thrombocytopenia in patients who were already in critical condition.
Methods
Study Design and Patients
This was a single-center real-world study conducted in critically ill patients receiving VA-ECMO support to evaluate whether oseltamivir administration would ameliorate VA-ECMO-related thrombocytopenia. This study was conducted in accordance with the principles of the Declaration of Helsinki, and was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University before the study began. The informed consent form was signed by the patients or their legal representatives.
Adults (≥18 years) who received VA- ECMO for more than 24 h in the intensive care unit (ICU) of Qilu Hospital of Shandong University from May 2016 to July 2020 were included. The patients were diagnosed with cardiac failure due to cardiogenic shock (CS), cardiac arrest (CA), or acute right ventricular dysfunction. Day (D) 0 was defined as the day when VA-ECMO was initiated. None of the enrolled patients showed thrombocytopenia on D0 just before cannulation. Before and during VA-ECMO support, the patients with suspected or confirmed influenza infection were given oseltamivir phosphate (Kewei, Yichang East Sunshine Changjiang Pharmaceutical, China, H20065415) orally or through a feeding tube, once every 12 h, 75 mg each time for 10 days.
According to the guidelines for the diagnosis and treatment of influenza developed by the Ministry of Health of the People's Republic of China (2011 edition), the course of oseltamivir treatment for severely ill patients could be extended to 10 days. Influenza was diagnosed with a rapid serum antigen detection method.
Data Collection
For each enrolled patient, the following variables were collected: (1) general characteristics including age, sex, and comorbidities; (2) severity of illness assessed by Acute Physiology and Chronic Health Evaluation (APACHE) II (15) and Sequential Organ Failure Assessment (SOFA) (16) scores; (3) laboratory examination data; (4) interventions including continuous renal replacement therapy (CRRT) and oxygenator circuit changes; (5) comorbidities such as disseminated intravascular coagulation (DIC), heparin-induced thrombocytopenia (HIT), sepsis, hepatic dysfunction [defined as a ≥2-fold increase in alanine aminotransferase (17)], acute kidney injury (defined as a ≥1.5-fold increase in creatinine) (18), bleeding and thrombotic events; and (6) doses of platelet transfusions (in units of platelets).
ECMO
Based on the patient's blood vessel diameter and estimated cardiac output, the ECMO cannula size was 21–23 Fr for venous drainage and 15–17 Fr for arterial reinfusion. No bicaval dual-lumen cannulas were adopted for ECMO. The Rotaflow centrifugal pump (Maquet, Rastatt, Germany) and Quadrox D or I oxygenator (Maquet, Rastatt, Germany) were used. Unfractionated heparin (100 units/kg) was given at the time of cannulation, followed by a continuous intravenous infusion of unfractionated heparin for a target activated partial thromboplastin time (aPTT) of 50–70 s, unless there was an indication for a higher level of anticoagulation.
Platelet Transfusions
During ECMO, prophylactic platelet transfusion was given when the platelet count was <50,000/μl. Patients presenting with active hemorrhage needed platelet transfusion when the platelet count was lower than 100,000/μl.
Statistical Analysis
Statistical analyses were conducted using SPSS 17.0 (IBM, Armonk, NY, USA) and GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA). The continuous variables were presented as medians [interquartile range (IQR)] or means ± standard deviations (SD), based on data normality distribution analyzed using the Kolmogorov-Smirnov test. The continuous variables were analyzed using the Mann-Whitney U-test. The categorical data are presented as n (%) and were analyzed using the chi-square test. Survival analysis was performed using the Kaplan-Meier method, and the curves were compared using the log-rank test. A multivariable Cox proportional hazards regression model was used to evaluate the predictors of survival from cardiac failure. All tests were two-sided. A P-value of < 0.05 was considered statistically significant.
Results
Characteristics of the Patients
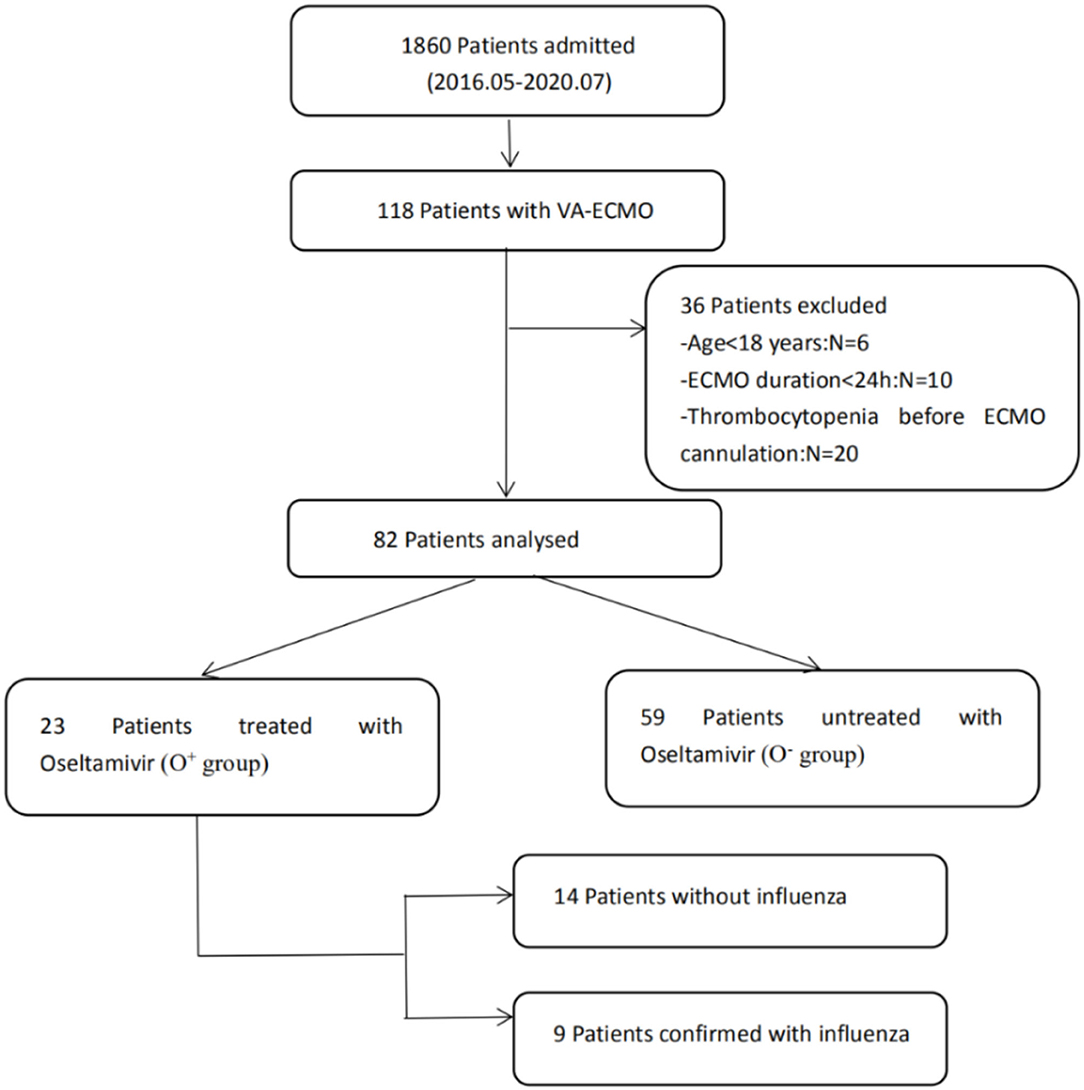
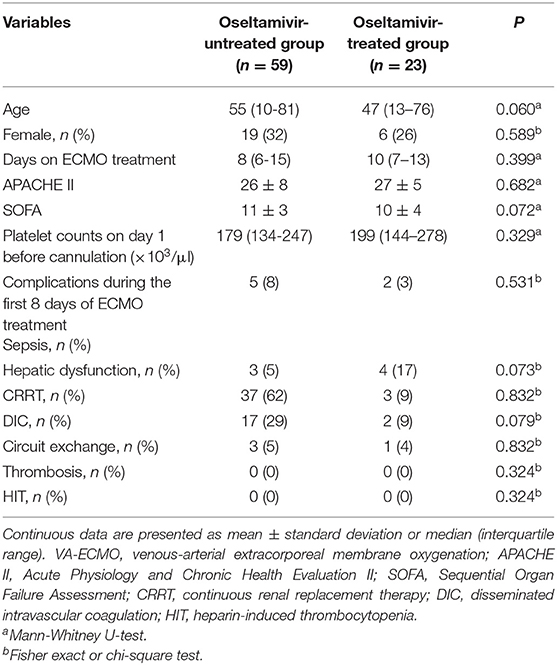
A total of 82 patients without thrombocytopenia before cannulation were included (Figure 1). They all received VA-ECMO support due to refractory cardiac failure, including 59 patients with CS, 13 patients with CA, and 10 patients with acute right ventricular dysfunction. Among them, 23 patients were suspected of influenza infection with flu-like symptoms, including cough, fatigue, sore throat, headache, and fever. Despite the confirmation with influenza infection in only nine patients by rapid serum antigen detection, all patients received a full course of oseltamivir anti-influenza treatment empirically, from 2 to 3 days before VA-ECMO until 7–8 days after VA-ECMO, for a total of 10 days, because they were admitted during the seasonal influenza pandemic. The clinical characteristics of the oseltamivir-treated patients (O+ group) and untreated patients (O− group) were described in Table 1. No differences in any demographic variables were found between the two groups. The APACHE II score, SOFA score, and baseline platelet count at VA-ECMO initiation were not statistically different between the two groups. One patient in the O+ group developed HIT on day 12 and was successfully weaned from VA-ECMO on day 14.
Clinical Outcomes
Overall, 85% of the patients had thrombocytopenia and 44% developed severe thrombocytopenia (platelet count <50,000/μl). As shown in Figure 2, a decrease in platelet count was observed within <24 h after initiating VA-ECMO. The nadir platelet count was observed on day 4, and the platelet started to increase on day 8. The biphasic temporal pattern was seen in both groups (Figure 2).
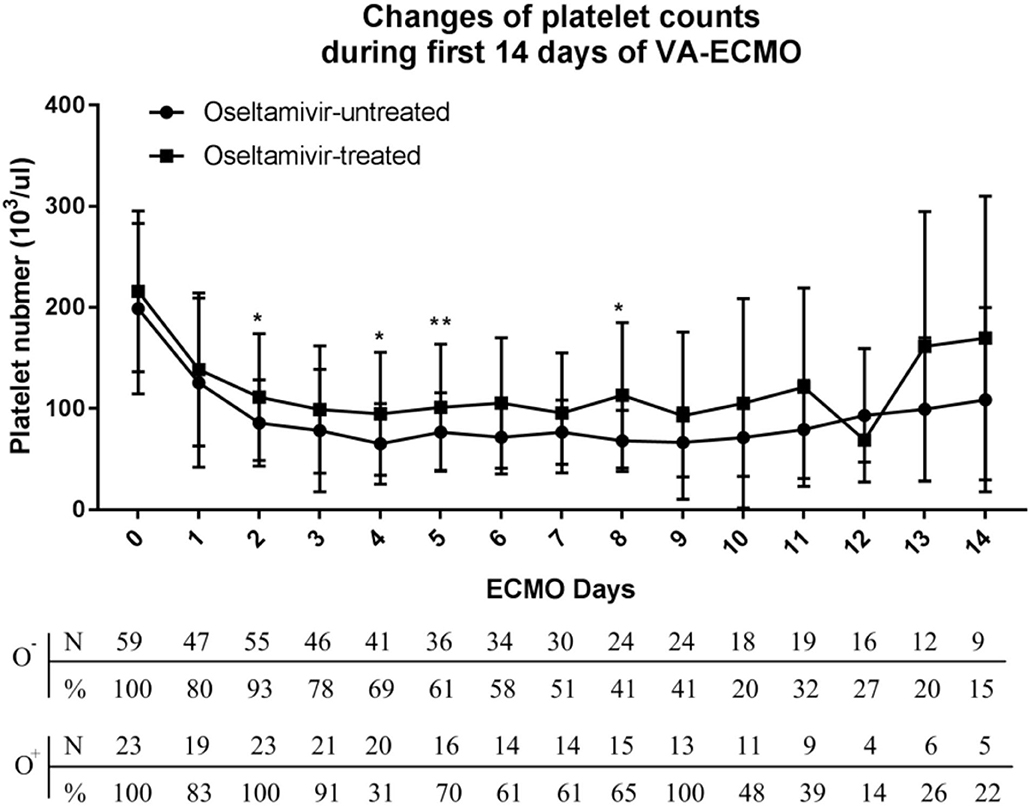
Figure 2. Variation of platelet counts in the oseltamivir-treated and oseltamivir–untreated groups during the first 14 days of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support.
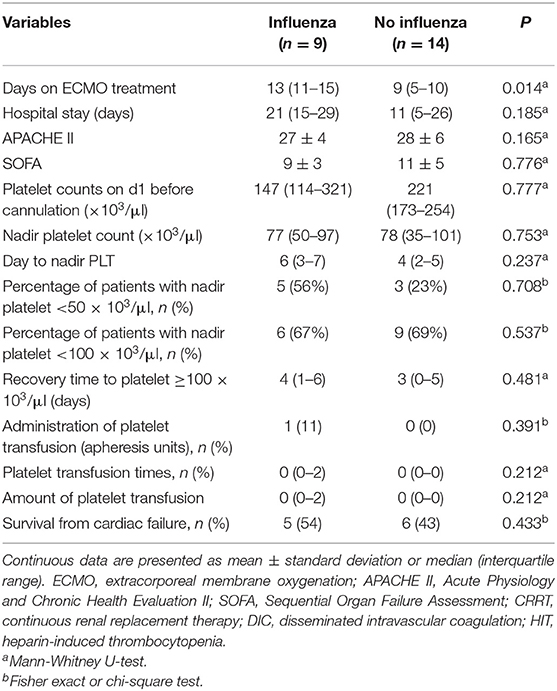
During the first 8 days after VA-ECMO initiation, the platelet count in the O+ group was higher than that in the O− group on day 2 (113,000 ± 62,700 vs. 85,700 ± 42,800/μl, P = 0.04), day 4 (94,800 ± 60,800 vs. 65,000 ± 39,800/μl, P = 0.02), day 6 (105,500 ± 64,600 vs. 71,500 ± 36,100/μl, P < 0.001), and day 8 (113,000 ± 71,800 vs. 68,000 ± 30,400/μl, P = 0.01). The patients in the O+ group had a higher median nadir platelet count (77,000/μl, 6,000–169,000/μl), compared with the O− group (49,000/μl, 2,000–168,000/μl; P = 0.04). A nadir platelet count <50,000/μl was seen in 26% of the patients in the O+ group, compared with 53% in the O− group (P = 0.031). There was no difference in platelet count recovery time between the two groups. Platelet transfusions were administered in 4% of the patients in the O+ group, compared with 32% in the O− group (P = 0.022), and the O+ group received a smaller amount of platelets (0.09 ± 0.42 vs. 0.95 ± 2.41 apheresis units; P = 0.007). There was a tendency toward fewer bleeding events in the O+ group compared with the O− group (9 vs. 29%, P = 0.052) (Table 2). In the O+ group, there was no difference in platelet count changes regardless of influenza infection status (P > 0.05) (Table 3).
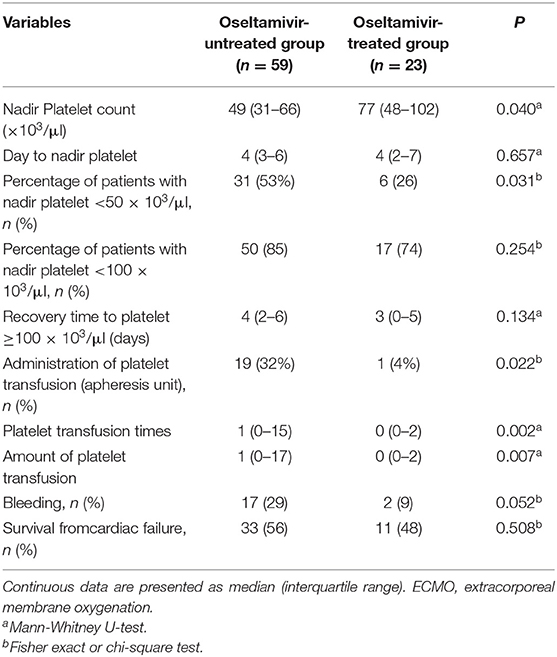
Table 2. Changes in platelet count and treatment outcomes in patients with refractory cardiac failure during the first 8 days of ECMO.
Prognosis Analysis
No significant difference in survival from cardiac failure was seen between the O+ and O− group (48 vs. 56%, P = 0.508) (Figure 3). As shown in Table 4, among the variables possibly associated with survival, only the SOFA scores at VA-ECMO initiation were independently associated with survival (OR = 1.12, 95% CI: 1.02–1.22, P = 0.015).
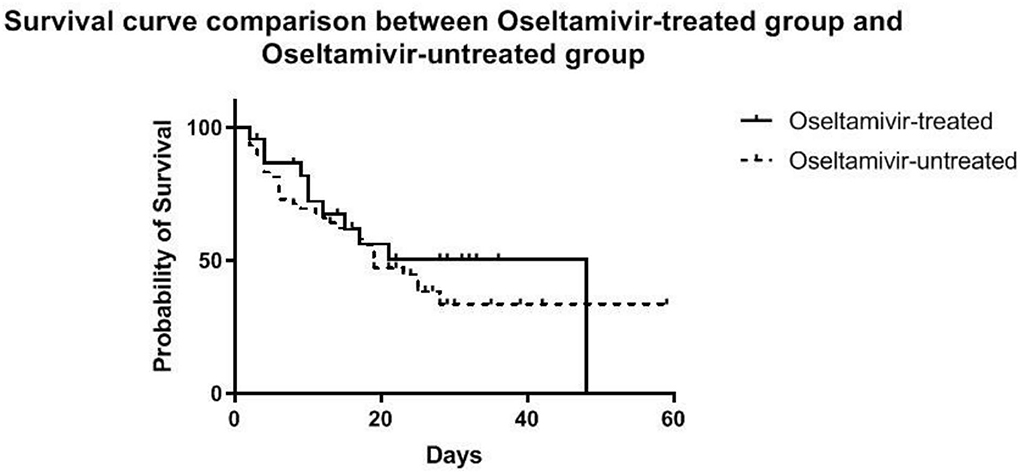
Figure 3. Estimated survival of patients during VA-ECMO support until the time of discharge, stratified into the oseltamivir -treated and -untreated group.
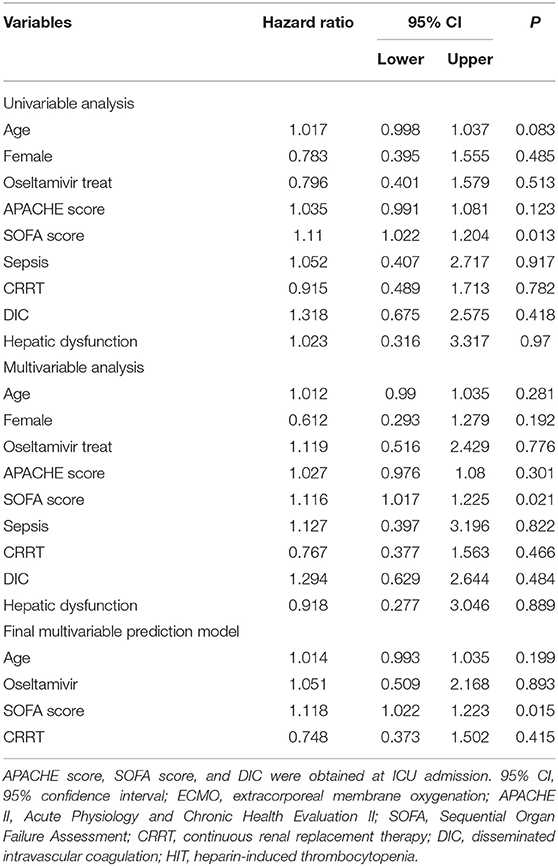
Table 4. Multivariate Cox proportional hazard analysis of factors influencing survival in all patients who received ECMO.
Discussion
Thrombocytopenia is a common complication of ECMO (6). Oseltamivir can be used to treat infection-associated thrombocytopenia (13, 14). This study aimed to evaluate the effect of oseltamivir on attenuating severe thrombocytopenia during ECMO. The results suggested that oseltamivir could ameliorate VA-ECMO-related thrombocytopenia. These findings indicated the potential prophylactical effect of oseltamivir on severe thrombocytopenia associated with the initiation of VA-ECMO. This was the first study to provide evidence that oseltamivir could prevent severe thrombocytopenia induced by VA-ECMO.
The prevailing view has been that platelets are activated by the artificial surface of ECMO and impaired by high shear stress in the extracorporeal circuit, leading to an early decline in platelet count (19–21). It could be hypothesized that platelet desialylation participated in this process through intracellular Neu1 translocation to the platelet surface, which contributed to increased clearance of the platelets. As observed in this study, the platelet count dropped dramatically after the start of VA-ECMO, and the declining trend continued until days 8–10, except for one patient in the O+ group who developed HIT on day 12.
Oseltamivir, as a viral neuraminidase inhibitor, has been shown to significantly increase platelet counts in a mouse model of anti-GPIba-mediated ITP by inhibiting platelet desialylation (22). Oseltamivir was also successfully used to treat an adult ITP patient who responded poorly to conventional therapies, including corticosteroids, intravenous immunoglobulin (IVIG), recombinant human thrombopoietin, rituximab, danazol, and vindesine (23). A multi-center and randomized controlled trial showed that platelet desialylation levels were increased significantly in septic patients, and the administration of oseltamivir attenuated thrombocytopenia associated with sepsis (14). Recently, a retrospective analysis showed that oseltamivir use increased the platelet counts regardless of influenza status (13), supporting the findings in the present study.
Therefore, we speculated that oseltamivir might improve ECMO-induced thrombocytopenia by inhibiting platelet desialylation. Meanwhile, platelet activation might be attenuated through suppression of desialylation, as desialylation and platelet activation were previously shown to exist in a positive feedback loop (22). In addition to the platelet-reducing effect exerted by extracorporeal circuit, critical illness severity and comorbid conditions are associated with the development of thrombocytopenia in critically ill patients (24). In this study, none of the examined complications (hepatic dysfunction, renal dysfunction, sepsis, HIT, thrombosis, and DIC) were associated with the patient's prognosis, only the SOFA score was associated with survival.
Thrombocytopenia increases the bleeding risk during ECMO, especially because of the need for anticoagulants to avoid circuit clogging, which leads to a higher threshold for platelet transfusion in patients receiving ECMO compared with other critically ill patients (25). The Extracorporeal Life Support Organization (ELSO) has recommended that the platelet count should be maintained above 100,000/μL (26). So far, platelet transfusion is the only readily available treatment for thrombocytopenia. However, platelet transfusion is extremely costly with limited resources. Moreover, the benefits of multiple platelet transfusions for patients receiving ECMO remains unknown. Platelet transfusion was shown to be associated with increased risks of thrombosis and in-hospital mortality in large retrospective studies involving patients with platelet consumptive disorders (27). Recent studies have suggested that a more restrictive platelet transfusion practice might be applicable to critically ill neonates (28, 29). In this study, oseltamivir use attenuated thrombocytopenia during VA-ECMO and reduced the requirement for platelet transfusion. A trend of reduced bleeding events was also seen in the treatment O+ group.
In this study, oseltamivir treatment did not affect the survival from cardiac failure, although, by day 8 after starting ECMO, significantly higher platelet counts that was considered as a predictor of good prognosis for ECMO patients after cardiac surgery (6). The reason for this contradiction might be that only a few patients received oseltamivir treatment.
This study had some limitations and the results must be interpreted with caution. First, the retrospective and observational nature that relied on the availability of recorded data made the studies prone to selection bias. Second, the relatively small number of ECMO patients treated with oseltamivir limited the statistical power of the analysis. Since oseltamivir has been shown to attenuate severe thrombocytopenia regardless of influenza status, it might be widely used in ECMO patients in future prospective clinical trials. We only evaluated the effect of oseltamivir on the prevention of severe thrombocytopenia in patients receiving VA-ECMO, and the findings might not be generalized to VV- ECMO. Third, we should be aware that there were both known and unknown confounders affecting the platelet counts. Influenza itself could cause thrombocytopenia through desialylation. Still, we could not differentiate whether oseltamivir acted on platelet neuraminidase or viral neuraminidase. Infections can induce inflammatory responses including elevated IL6, which has been shown to up-regulate hepatic thrombopoietin and increase platelet production (30). Further, research on the mechanism of oseltamivir on reducing thrombocytopenia induced by ECMO was needed.
Last, flow cytometry detection of the expression of platelet activation markers, which was considered as an alternative indicator of platelet function (31–34), was not performed in this study since platelet aggregation and adherence results needed to be interpreted with caution during thrombocytopenia (35).
Conclusions
Oseltamivir increased the median nadir platelet count, decreased the prevalence of severe thrombocytopenia, and reduced platelet transfusions. Those results supported the clinical effect of oseltamivir in preventing VA-ECMO-induced severe thrombocytopenia. Future studies were warranted to confirm this benefit.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Qilu Hospital of Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YL: designed the study and drafted the manuscript. LW: participated in the design of the study helped to draft the manuscript. JZ: collected the medical data. HH: performed the statistical analysis. HL: participated in coordination and helped to collect the medical data. CL: participated in the statistical analysis and data curation. HG: embellished the manuscript. YC: conceived of the study and helped to draft the manuscript. XC: proofread the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China [Grant No. 81100348] and Project funded by China Postdoctoral Science Foundation [Grant Nos. 2020T130072ZX and 2014M551921].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ECMO, extracorporeal membrane oxygenation; VA-ECMO, venous-arterial extracorporeal membrane oxygenation; SOFA, Sequential Organ Failure Assessment; VV, veno-venous; VA, veno-arterial; AMRs, Ashwell-Morell receptors; Neu1, neuraminidase; CS, cardiogenic shock; CA, cardiac arrest; ICU, intensive care unit; D, day; APACHE, Acute Physiology and Chronic Health Evaluation; CRRT, continuous renal replacement therapy; DIC, disseminated intravascular coagulation; HIT, heparin-induced thrombocytopenia; aPTT, activated partial thromboplastin time; IQR, interquartile range; VV- ECMO, veno-venous ECMO; APACHE II, Acute Physiology and Chronic Health Evaluation II; 95% CI, 95% confidence interval.
References
1. MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. (2012) 38:210–20. doi: 10.1007/s00134-011-2439-2
2. Allen S, Holena D, McCunn M, Kohl B, Sarani B. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med. (2011) 26:13–26. doi: 10.1177/0885066610384061
3. Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. (2012) 27:741.e9–18. doi: 10.1016/j.jcrc.2012.02.013
4. Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. (2008) 17(Suppl. 4):S41–7. doi: 10.1016/j.hlc.2008.08.009
5. Robinson TM, Kickler TS, Walker LK, Ness P, Bell W. Effect of extracorporeal membrane oxygenation on platelets in newborns. Crit Care Med. (1993) 21:1029–34. doi: 10.1097/00003246-199307000-00018
6. Opfermann P, Bevilacqua M, Felli A, Mouhieddine M, Bachleda T, Pichler T, et al. Prognostic impact of persistent thrombocytopenia during extracorporeal membrane oxygenation: a retrospective analysis of prospectively collected data from a cohort of patients with left ventricular dysfunction after cardiac surgery. Crit Care Med. (2016) 44:e1208–18. doi: 10.1097/CCM.0000000000001964
7. Hoffmeister KM, Falet H. Platelet clearance by the hepatic Ashwell-Morrell receptor: mechanisms and biological significance. Thromb Res. (2016) 141 (Suppl. 2):S68–72. doi: 10.1016/S0049-3848(16)30370-X
8. Grewal PK, Aziz PV, Uchiyama S, Rubio GR, Lardone RD, Le D, et al. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell-Morell receptor. Proc Natl Acad Sci U S A. (2013) 110:20218–23. doi: 10.1073/pnas.1313905110
9. Jansen AJ, Josefsson EC, Rumjantseva V, Liu QP, Falet H, Bergmeier W, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbα metalloproteinase-mediated cleavage in mice. Blood. (2012) 119:1263–73. doi: 10.1182/blood-2011-05-355628
10. Hoffmeister KM, Josefsson EC, Isaac NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of chilled blood platelets. Science. (2003) 301:1531–4. doi: 10.1126/science.1085322
11. Jansen AJ, Peng J, Zhao HG, Hou M, Ni H. Sialidase inhibition to increase platelet counts: a new treatment option for thrombocytopenia. Am J Hematol. (2015) 90:E94–5. doi: 10.1002/ajh.23953
12. Kamali A, Holodniy M. Influenza treatment and prophylaxis with neuraminidase inhibitors: a review. Infect Drug Resist. (2013) 6:187–98. doi: 10.2147/IDR.S36601
13. Shaim H, McCaffrey P, Trieu JA, DeAnda A, Yates SG. Evaluating the effects of oseltamivir phosphate on platelet counts: a retrospective review. Platelets. (2020) 31:1080–4. doi: 10.1080/09537104.2020.1714576
14. Li MF, Li XL, Fan KL, Yu YY, Gong J, Geng SY, et al. Platelet desialylation is a novel mechanism and a therapeutic target in thrombocytopenia during sepsis: an open-label, multicenter, randomized controlled trial. J Hematol Oncol. (2017) 10:104. doi: 10.1186/s13045-017-0476-1
15. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29. doi: 10.1097/00003246-198510000-00009
16. Moreno R, Vincent JL, Matos R, Mendonça A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. (1999) 25:686–96. doi: 10.1007/s001340050931
17. Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. (2013) 39:1704–13. doi: 10.1007/s00134-013-3037-2
18. Meyer AD, Wiles AA, Rivera O, Wong EC, Freishtat RJ, Rais-Bahrami K, et al. Hemolytic and thrombocytopathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med. (2012) 13:e255–61. doi: 10.1097/PCC.0b013e31823c98ef
19. Jiritano F, Serraino GF, Ten Cate H, Fina D, Matteucci M, Mastroroberto P, et al. Platelets and extracorporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med. (2020) 46:1154–69. doi: 10.1007/s00134-020-06031-4
20. Abrams D, Baldwin MR, Champion M, Agerstrand C, Eisenberger A, Bacchetta M, et al. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive Care Med. (2016) 42:844–52. doi: 10.1007/s00134-016-4312-9
21. Laine A, Niemi T, Suojaranta-Ylinen R, Raivio P, Soininen L, Lemström K, et al. Decreased maximum clot firmness in rotational thromboelastometry (ROTEM®) is associated with bleeding during extracorporeal mechanical circulatory support. Perfusion. (2016) 31:625–33. doi: 10.1177/0267659116647473
22. Li J, van der Wal DE, Zhu G, Xu M, Yougbare I, Ma L, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. (2015) 6:7737. doi: 10.1038/ncomms8737
23. Shao L, Wu Y, Zhou H, Qin P, Ni H, Peng J, et al. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets. (2015) 26:495–7. doi: 10.3109/09537104.2014.948838
24. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. (2011) 139:271–8. doi: 10.1378/chest.10-2243
25. Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. (2015) 29:90–101. doi: 10.1016/j.tmrv.2014.12.001
26. Winkler AM. Transfusion management during extracorporeal support. In: Brogan TV, Lequier L, Lorusso R, editors. Extracorporeal Life Support: The ELSO Red Book. 5th ed. Ann Arbor: Extracorporeal Life Support Organization (2017).
27. Goel R, Ness PM, Takemoto CM, Krishnamurti L, King KE, Tobian AA. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood. (2015) 125:1470–6. doi: 10.1182/blood-2014-10-605493
28. Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med. (2019) 380:242–51. doi: 10.1056/NEJMoa1807320
29. Keene SD, Patel RM, Stansfield BK, Davis J, Josephson CD, Winkler AM. Blood product transfusion and mortality in neonatal extracorporeal membrane oxygenation. Transfusion. (2020) 60:262–8. doi: 10.1111/trf.15626
30. Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. (2001) 98:2720–5. doi: 10.1182/blood.V98.9.2720
31. Balle CM, Jeppesen AN, Christensen S, Hvas AM. Platelet function during extracorporeal membrane oxygenation in adult patients: a systematic review. Front Cardiovasc Med. (2018) 5:157. doi: 10.3389/fcvm.2018.00157
32. Lukito P, Wong A, Jing J, Arthur JF, Marasco SF, Murphy DA, et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J Thromb Haemost. (2016) 14:2253–60. doi: 10.1111/jth.13497
33. Chung JH, Yeo HJ, Kim D, Lee SM, Han J, Kim M, et al. Changes in the levels of beta-thromboglobulin and inflammatory mediators during extracorporeal membrane oxygenation support. Int J Artif Organs. (2017) 40:575–80. doi: 10.5301/ijao.5000617
34. Kalbhenn J, Schlagenhauf A, Rosenfelder S, Schmutz A, Zieger B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J Heart Lung Transplant. (2018) 37:985–91. doi: 10.1016/j.healun.2018.03.013
Keywords: extracorporeal membrane oxygenation, veno-arterial, thrombocytopenia, platelets, cardiac failure, oseltamivir, desialylation
Citation: Li Y, Wang L, Zhang J, Han H, Liu H, Li C, Guo H, Chen Y and Chen X (2021) Oseltamivir Improved Thrombocytopenia During Veno-Arterial Extracorporeal Membrane Oxygenation in Adults With Refractory Cardiac Failure: A Single-Center Retrospective Real-World Study. Front. Cardiovasc. Med. 8:645867. doi: 10.3389/fcvm.2021.645867
Received: 24 December 2020; Accepted: 05 July 2021;
Published: 26 July 2021.
Edited by:
Warwick Wolf Butt, Royal Children's Hospital, AustraliaReviewed by:
Graeme MacLaren, National University Hospital, SingaporeJune Li, University of Toronto, Canada
Copyright © 2021 Li, Wang, Zhang, Han, Liu, Li, Guo, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomei Chen, Y2hlbnhtMDA4QDE2My5jb20=
†These authors have contributed equally to this work
 Yuan Li1†
Yuan Li1† Lin Wang
Lin Wang Hui Han
Hui Han Han Liu
Han Liu Chaoyang Li
Chaoyang Li Haipeng Guo
Haipeng Guo Yuguo Chen
Yuguo Chen Xiaomei Chen
Xiaomei Chen