
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 29 April 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.636947
 Yixi Zhao1†
Yixi Zhao1† Longtao Liu2†
Longtao Liu2† Shengjie Yang1
Shengjie Yang1 Guijian Liu3
Guijian Liu3 Limin Pan1
Limin Pan1 Chun Gu3
Chun Gu3 Yang Wang1
Yang Wang1 Dan Li1
Dan Li1 Ran Zhao4
Ran Zhao4 Min Wu1*
Min Wu1*Postprandial lipemia plays an important role in the formation, occurrence, and development of atherosclerosis, and it is closely related to coronary heart disease and other diseases involving endothelial dysfunction, oxidative stress, inflammation, and other mechanisms. Therefore, it has become a focus area for further research. The studies on postprandial lipemia mainly include TG, TRL, VLDL, CM, and remnant cholesterol. Diurnal triglyceride patterns and postprandial hyperlipidemia are very relevant and are now insufficiently covered. The possible mechanisms between postprandial lipemia and cardiovascular disease have been reviewed in this article by referring to relevant literature in recent years. The research progress on the effects of postprandial lipemia on endothelial function, oxidative stress, and inflammation is highlighted. The intervention of postprandial lipemia is discussed. Non-medicinal intervention such as diet and exercise improves postprandial lipemia. As medicinal intervention, statin, fibrate, ezetimibe, omega-3 fatty acids, and niacin have been found to improve postprandial lipid levels. Novel medications such as pemafibrate, PCSK9, and apoCIII inhibitors have been the focus of research in recent years. Gut microbiota is closely related to lipid metabolism, and some studies have indicated that intestinal microorganisms may affect lipid metabolism as environmental factors. Whether intervention of gut microbiota can reduce postprandial lipemia, and therefore against AS, may be worthy of further study.
Hyperlipidemia clinical diagnosis requires fasting lipemia, but individuals are in the postprandial state for most of the 24 h. Therefore, fasting lipemia cannot comprehensively and accurately reflect lipid metabolism (1, 2). The viewpoint that atherosclerosis (AS) is a postprandial phenomenon was proposed by Zilversmit as early as 1979 (3). With the growing awareness of postprandial lipemia, its dynamic change is thought to be closely related to AS. Long-term disorders of lipid metabolism, endothelial dysfunction, and hypercoagulability can occur through postprandial hyperlipidemia, and it participates in AS-related processes (4). As an independent predictor of cardiovascular disease (CVD), postprandial triglyceride (TG), which has vital clinical implications, is a worthwhile contender as a potential therapeutic target (5, 6). The regulation of exogenous chylomicrons (CMs) is closely related to the production and secretion of endogenous very low-density lipoprotein-TG (VLDL-TG) (4). Therefore, improvement of postprandial status by intervening postprandial lipid metabolism could be a measure to reduce the risk of CVD. Our objectives were to (1) review existing literature and study the causes of postprandial hyperlipidemia, (2) review the influence of postprandial lipid metabolism on the formation of AS and study the relationship between postprandial lipemia and AS, and (3) review the effects of postprandial lipemia on endothelial function, oxidative stress, inflammation, apoptosis, endoplasmic reticulum stress, and mitochondrial dynamics, as well as analyze their mechanisms and explore potential interventions.
Dyslipidemia generally refers to all kinds of serum dyslipidemia, including total cholesterol (TC), TG, and low-density lipoprotein-cholesterol (LDL-C), and as initial factors leading to AS (7). Postprandial lipemia refers to the fluctuation of blood lipid levels between the period after eating and the premeal level and is characterized by rising levels of TG-rich lipoproteins (TRLs) (8). TG mainly comes from the decomposition of fat in the diet and can be generated by metabolic activities in the body. VLDL and CM are the main forms of plasma TG, which are collectively termed TRLs (9). The roles of TG and TRL in the pathogenesis of CVD were reviewed by the European Atherosclerosis Society Consensus Panel, which considered that the number of TRLs plays an important role in the relationship between TG and CVD, and apoB100-containing TRLs are a pathological factor promoting the formation of hyperlipidemia (10). High TG level is an important residual risk factor for coronary heart disease (CHD), and postprandial hyperlipidemia refers to the increase of TG-rich CM remnants and persistent hypertriglyceridemia (11). The study of postprandial lipemia parameters in patients with diabetes and prediabetes found that postprandial TG and TG/HDL-C were better than the corresponding fasting parameters in reflecting dyslipidemia and suggesting cardiovascular status (12). Progressive postprandial hyperlipemia is related to obesity, atherosclerosis, and other diseases (13, 14).
The modern diet consists of three to five feeding times a day, and individuals spend a significant amount of time in a non-fasting and postprandial state, meaning the levels of CM, VLDL, and other TRLs and their remnants are enhanced, which leads a continually fluctuating level of blood lipid (15). The postprandial metabolic status can be better reflected by postprandial TG concentration, and with the combination of fasting TG concentration, the degree of exposure of total TG in the arterial wall within 24 h can be described more comprehensively and accurately (16). TG reflects the burden of remnant cholesterol (RC) and can be used as its marker. A Mendelian randomized design examining the relationship between non-fasting RC and ischemic heart disease found that when non-fasting RC increased by 1 mmol/L (39 mg/dl), the causal risk of ischemic heart disease increased by 2.8 times and was not associated with decreased HDL cholesterol (17). This indicates that the increase of cholesterol content in TRL particles accelerates the development of ischemic heart disease. Teno et al. (18) observed serum lipid levels and carotid artery media thickness in 61 patients with type 2 diabetes (T2D) and found that the minimum value of postprandial TG-induced AS was 2.27 mmol/L, suggesting that this concentration level could be diagnosed as postprandial hyperlipidemia. A few aspects of non-fasting and postprandial TG were discussed, and some recommendations were proposed by a panel of clinicians and scientists. It was pointed out that TG levels <2.5 mmol/L at any time after a meal can be regarded as the ideal level of postprandial TG (19).
Circadian rhythm is essential for maintaining life cycle. The diurnal pattern of TG and gut microbiota changes after a high-fat diet, which affects physiological homeostasis (20). Glucocorticoid receptors (GC) regulate circulating TG differently during non-fasting and fasting. Animal experiments showed that the daytime TG level of mice decreased after hepatocyte-specific GC was knocked out, suggesting that circadian rhythm and daytime TG mode affect metabolism (21). The relationship between diurnal TG pattern and postprandial hyperlipidemia has not been reported enough, which is worthy of further study.
Plasma contains hepatogenic atherogenic lipoprotein in the fasting state and enterogenic atherogenic lipoprotein in the non-fasting state. Individuals spend more than 18 h a day in a non-fasting state (22). Thus, lipid metabolism cannot be accurately reflected by fasting lipemia. Postprandial lipemia independently predicts the risk of CVD and likely better reflects the plasma atherogenic lipoprotein levels (9, 23). Non-fasting TG has been shown to be superior to fasting TG in predicting cardiovascular risk (24). A study comparing the composition and size of lipoprotein showed that postprandial VLDL particles measured directly in healthy individuals were significantly increased, which may promote arteriosclerosis of postprandial hyperlipidemia (25). Recognition of non-fasting lipid profiles has been expressed by relevant associations, guidelines, and statements in the United States, Canada, the United Kingdom, and other countries (26–29).
A positive association between postprandial TG levels and CHD was proposed in 1992 (30). Case–control studies have also shown that elevated TG levels in postprandial lipemia are associated with AS and CVD (31). Studies based on postmeal status have shown that abnormal lipoproteins increase the risk of AS, of which TG metabolic capacity is a key factor (32).
In a study of 30 patients with stable CHD who underwent catheter intervention, thin-cap fibroatheroma (TCFA) detected by multivessel examination using optical coherence tomography was found independently associated with postprandial apoB48 (33). ApoB48 is the major apolipoprotein in chylomicron, and postprandial delayed hyperchylomicronemia is considered to be related to the destruction of the TCFA composed of the large lipid core under the fibrous cap in the pathogenesis of acute coronary syndrome (ACS), consequently (34).
Lipoproteins with high atherogenic effects, such as TRLs, are closely related to CHD. Therefore, it is considered that the determination of postprandial lipemia is more sensitive and prominent than fasting lipids in the risk assessment of CHD (35, 36). The clinical research protocols for postprandial lipemia have not been unified, and the evaluation of related animal models is still being explored, so the management guidelines for postprandial lipemia still need to be improved (37).
Endothelial dysfunction is considered to be an early and reversible predictor of AS and is induced by elevated TG in circulating lipids of postprandial hyperlipidemia (38). The endothelium plays a role in preventing the formation and development of AS through platelet aggregation, vascular tension, coagulation, and fibrinolysis (39). The activation of white blood cells and oxidative stress produced by monodietary fat meal may lead to endothelial dysfunction and form the early stages of AS (40, 41).
A large number of studies have shown that flow-mediated vasodilation (FMD) is affected by postprandial lipemia. It was found that TG increased but FMD decreased postprandially. The increment area under the curve (iAUC) of postprandial TG was negatively correlated with the decrement area under the curve of postprandial FMD, indicating that FMD had transient and significant injury in the postprandial phase and was closely related to postprandial TG (42–44). The imbalance between the vasodilator and vasoconstrictor is one of the characteristics of endothelial dysfunction, and the decrease of the vasodilator is mainly caused by the postprandial decrease of NO and the increase of oxidative stress (45). Robinson Ramirez-Velez recruited 14 healthy men, who were between 18 and 25 years of age, to participate in the high-fat meal (HFM) test. The TC and TG levels were detected, and endothelial function was assessed by measuring serum nitrite/nitrate levels (NO2/NO3). It was found that 2 h after the meal, the TG levels increased (p = 0.04). Endothelial function decreased to 3.3 ± 0.5% (p = 0.03) compared with 5.9 ± 1.1% at baseline, and FMD reduced from baseline level of 5.9 ± 1.1 to 3.3 ± 0.4% (p = 0.04), manifesting that postprandial lipemia leads to endothelial dysfunction by changing circulating blood lipids (46).
Shafieesabet et al. explored the acute effect of HFM on the changes of peripheral vascular endothelial function in patients with heart failure with reduced ejection fraction (HFrEF). The peripheral vascular endothelial function of patients was evaluated by EndoPAT 2000 technology at baseline and 1, 2, 3, and 4 h after oral TG load using reactive hyperemia index and pulse wave amplitude. Compared with obviously postprandial vascular dilation in healthy individuals, HFrEF patients did not show the same changes. It is suggested that peripheral vascular endothelium function was damaged postprandially in HFrEF patients (47). Postprandial TRL was considered to be the cause of AS. Postprandial TRL is thought to be a contributing factor to AS; Whisner et al. assessed the effects of meal with different dietary components on endothelial function in 10 adolescents aged 10–17 years. TG and FMD were measured at baseline and 4 h postprandially. The results showed that TG at 4 h was negatively correlated with FMD after high-fat and low-fiber meal (β = −0.087; 95% CI = −0.138~-0.037; p = 0.001), meaning the importance of the reverse relationship between TRL and FMD in AS (48) (Figure 1).
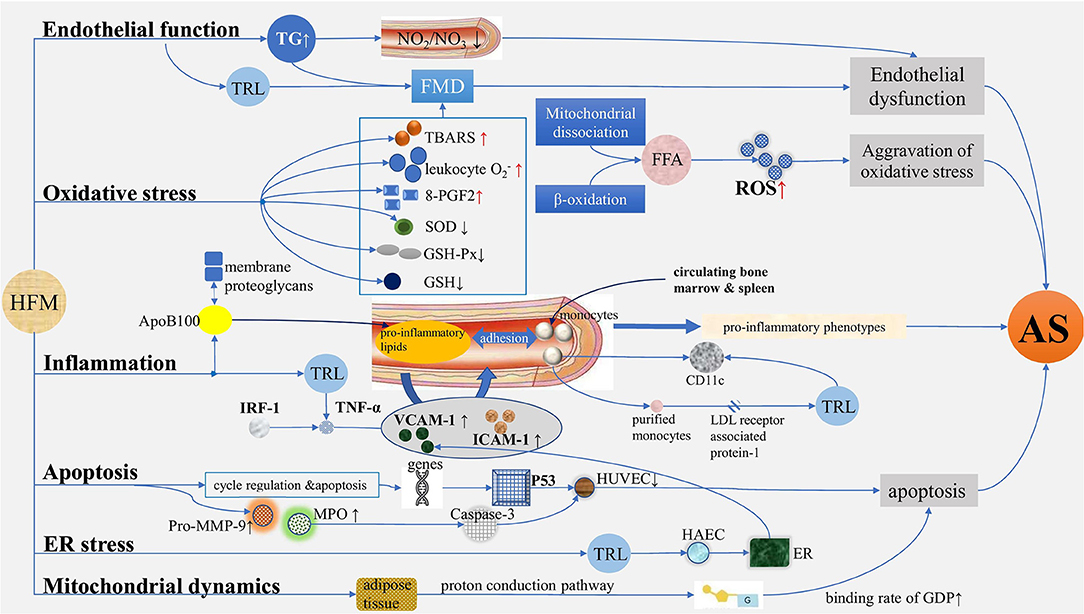
Figure 1. Mechanisms of atherosclerosis induced by postprandial lipemia. (1) Endothelial function: TG increased after HFM, while serum nitrite/nitrate levels and FMD decreased, suggesting endothelium dysfunction. (2) Oxidative stress: In terms of oxidative stress, TBARS, leukocyte , and 8-PGF2 increased, and SOD, GSH-PX, and GSH decreased after HFM. ROS are produced in large quantities due to the increased levels of free fatty acids mediated by mitochondrial dissociation and β-oxidation. (3) Inflammation: ApoB100-containing lipoproteins enter into the arterial subendothelium, thus generating proinflammatory lipids, promoting the expression of adhesion factors such as, VCAM-1 and ICAM-1, which induce the augmentation of adhesion interaction with monocytes in turn, generating proinflammatory phenotypes and AS; TRL isolated from human plasma after HFM regulated TNF-α-induced VCAM-1 expression by regulating IRF-1-dependent transcription mechanism. The expression of CD11c on monocytes increased after a meal, and the purified monocytes internalized TRL isolated from postprandial blood through LDL receptor associated protein-1, which also caused the upregulation of CD11c. (4) Apoptosis: With the relation of cell cycle regulation and apoptosis, a group of genes of HUVEC differentially expressed in serum of postprandial hyperlipidemia after a meal, causing proliferation of HUVEC, significantly decreased and may be achieved by activating p53 network; the activities of pro-MMP-9, MPO, and caspase-3 increased significantly after a meal, showing apoptosis effect. (5) ER stress: The expansion of ER was significantly increased by HAEC treated with postprandial TRL, and the production of TRL increased the adhesion of VCAM-1-dependent monocytes to inflammatory endothelium, indicating ER stress plays a role in the regulation of VCAM-1 transcription in endothelial cells during the process of AS. (6) Mitochondrial dynamics: The binding rate of GDP increased by 85% after HFM, and the increase was the largest in early postprandial period. TG, triglyceride; HFM, high-fat meal; FMD, flow-mediated vasodilation; TBARS, thiobarbiturate reactants; 8-PGF2, 8-external prostaglandin F2; SOD, superoxide dismutase; GSH-PX, glutathione peroxidase; ROS, reactive oxygen species; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; AS, atherosclerosis; TRL, TG-rich lipoprotein; LDL, low-density lipoprotein; HUVEC, human umbilical vein endothelial cell; ER, endoplasmic reticulum; HAEC, human aortic endothelial cell.
Modern lifestyles lead to an exaggerated and prolonged postprandial metabolism, oxidation, and immune imbalance for most of the day, known as postprandial oxidative stress, which promotes the development of CVD (49).
There is a strong relationship between the pro-oxidative load caused by absorption of postprandial lipid hydroperoxides and the impairment of endothelium-dependent vasodilation induced by postprandial lipemia (50). Reactive oxygen species (ROS) are produced in large quantities under physiological and pathological conditions such as inflammation, ionizing radiation, and ischemia, due to the increased levels of free fatty acids mediated by mitochondrial dissociation and β-oxidation after meals (51). Postprandial TRL lipolysis products were found to activate mitochondrial ROS, damage human brain microvascular endothelial cells in vitro, and promote AS (52, 53). Control of oxidative stress is important for the stability of endothelial function (54).
Tsai et al. conducted a clinical trial evaluating the effect of postprandial lipemia on oxidative stress in 16 healthy subjects without coronary risk factors. Serum TG, plasma glutathione peroxidase (GSH-PX) levels, and urine excretion of 8-external prostaglandin F2 (8-PGF2) were monitored, and brachial artery FMD was evaluated by high-resolution ultrasound before and 2, 4, and 6 h after the standard HFM. The results showed that after HFM, TG increased significantly; GSH-PX level decreased, declaring increased antioxidant enzyme consumption. The excretion of 8-PGF2 increased, indicating the increase of oxidative modification products. FMD decreased, suggesting that oxidative stress was enhanced after meal and endothelial dysfunction occurred in healthy individuals (55). Bae et al. (56) carried out HFM tests in 11 healthy people. Serum TG, PMA-activated leukocyte production, and FMD were detected at baseline and 2 h after meal. Postprandial serum TG and PMA-activated leukocyte production were significantly higher than before meal, whereas the postprandial FMD level was lower than before meal, which suggested that an acute increase in postprandial TG may cause endothelial dysfunction through elevated oxidative stress. Neri et al. conducted an HFM test in 40 patients with T2D, 40 patients with impaired glucose tolerance (IGT), and 40 healthy subjects. Serum TG, markers of systemic oxidative stress, and endothelial function were measured before and 2, 4, and 8 h after a meal. The results showed that the postprandial TG in each group was higher than the baseline, and the postprandial TG level of T2D patients was significantly higher than that of the IGT group, which was higher than that of the control group. Postprandial TG was significantly enriched in T2D patients and IGT patients at 4 h, while TG was enriched at 2 and 4 h in healthy subjects. The urinary level of free 8-iso-prostaglandin F2α (8-iso-PGF2α; F2-isoprostanes) in each group was higher than that in the fasting state. FMD in T2D and IGT patients decreased significantly at 4 h, demonstrating that the changes of postprandial lipemia were closely related to the systematic markers of oxidative stress and led to the impairment of vasodilation function (57). It was found that thiobarbituric acid reactive substances (TBARS) increased and red blood cell reducing glutathione (GSH) and superoxide dismutase (SOD) decreased after HFM in patients with T2D with macrovascular complications, illustrating that damage to antioxidant capacity might lead to endothelial dysfunction (58). Fat load leads to aggravation of oxidative stress and augment of postprandial lipemia, both of which promote the development of CVD (59).
This evidence suggests that oxidative stress induced by postprandial lipemia may have detrimental effects on multiple stages of AS, in addition to direct damage to endothelial function (Figure 1).
The instability of lipid metabolism and chronic postprandial inflammation are the underlying pathogenesis of AS-induced CVD (41). ApoB100-containing lipoproteins enter the arterial subendothelium and are retained by interaction with membrane proteoglycans through charge-based interactions and sphingolipases. The lipoproteins then become proinflammatory lipids by modification of aggregation, saccharification, and oxidation, promoting the expression of adhesion factors such as, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), inducing the augmentation of adhesion interaction of monocytes from circulating bone marrow and spleen, generating proinflammatory phenotypes, and producing the early stages of AS (60).
Inflammation plays a key role in the activation of endogenous and adaptive immune pathways in the pathophysiological process of AS. Vascular inflammation is caused by the accumulation and modification of atherosclerotic lipoprotein in the subendothelium, which leads to the changes in vascular structure, the development of AS, and the pathological formation of CVD (60). Postprandial lipemia may be the main factor leading to inflammation and it affects endothelial function by changing the state of inflammation (61, 62). A single feeding is thought to have the ability to produce a transient and low-intensity inflammatory response (63). The augment of postprandial TG is related to the upregulation of proinflammatory genes in endothelial cells, the increased expression of leukocyte activation markers, and the involvement of the complement system. These processes together constitute part of postprandial inflammation and are considered to be the potential mechanism of promoting AS (64–66).
DeVerse et al. examined IRF-1 and TNF-α-induced VCAM-1 in human aortic endothelial cells (HAECs) stimulated by TRL through immunofluorescence in a microfluidic device. It was found that TRL isolated from human plasma after HFM regulated TNF-α-induced VCAM-1 expression by regulating the IRF-1-dependent transcription mechanism, which indicated that postprandial TRL plays a direct role in regulating the expression of IRF-1 and downstream inflammatory reaction, affecting endothelial function and producing an atherogenic effect (67). The adhesion of monocytes in the arterial wall is associated with AS. Gower et al. detected the blood of healthy subjects with standardized HFM by flow cytometry and found that the expression of CD11c on monocytes increased at 3.5 h after a meal, and the degree of upregulation was related to postprandial TG. The purified monocytes internalized TRL isolated from postprandial blood through LDL receptor associated protein-1, which also caused the upregulation of CD11c. These results suggest that mononuclear cells in the postprandial state can internalize lipids, upregulate CD11c, increase adhesion to VCAM-1, and increase the risk of atherosclerosis (68). Gorzelak-Pabis et al. studied the effects of a single high-fat diet on the barrier function and inflammatory status of human umbilical vein endothelial cells (HUVECs). Serum from healthy volunteers was extracted before and 3 h after a meal to induce HUVECs. The integrity of HUVECs was measured in the RTCA-DP-xCELLigence system. Fasting and postprandial TG were measured. The expression of proinflammatory cytokine mRNA was detected by RT-PCR. Postprandial TG level increased, and postprandial serum decreased the integrity of HUVECs. The mRNA expression of IL-33, IL-32, MCP-1, and CX3C chemokines was significantly increased, which revealed that a single HFM could aggravate the inflammatory process and destroy the endothelial barrier (69). Herieka et al. reviewed 57 studies on HFM-induced postprandial inflammation and found that the increase in systemic inflammation promoting the development of AS can be explained, at least in part, by frequent consumption of excess dietary fat. The results of postprandial inflammation markers were compared with the highly consistent low-grade human endotoxemia model, which showed that plasma-borne inflammatory markers such as, cytokines and soluble adhesion factors did not continuously increase after HFM, while the level of proinflammatory leukocyte surface markers increased in almost all measured studies. It was proposed that the study of inflammation induced by postprandial lipemia could be carried out by focusing on leukocytes in the future (70). Recently, a study has shown that the formation of proatherogenic endothelial phenotype is promoted by postprandial metabolism changes in TRL characterized with oxylipin signature in dyslipidemic subjects (71).
The mechanism of atherosclerotic formation by postprandial lipids and lipoproteins is directly or indirectly involved through partial induction of the inflammatory state. Therefore, anti-inflammatory therapy may further reduce the risk of atherosclerotic CVD in addition to reducing cardiovascular risk factors and improving lipid-lowering therapy (72) Figure 1.
Dejeans et al. cultured HUVECs in a medium containing 10% serum extracted from healthy subjects before and after HFM to explore the effect of postprandial lipemia on vascular endothelial cells. It was found that the proliferation of HUVECs in postprandial hyperlipidemia was significantly lower than that before meal. The transcriptomic profiles of endothelial cells were changed, among which, a group of genes differentially expressed before and after meal was related to cell cycle regulation and apoptosis, and may be achieved by activating the p53 network. It was indicated that the transcription of genes related to apoptosis in vascular endothelial cells after exposure to postprandial hyperlipidemia may promote vascular dysfunction (73). Spallarossa et al. carried out an HFM test in 15 people between 20 and 45 years of age. Blood samples were collected before and 1, 2, and 4 h after a meal. Plasma TG, myeloperoxidase (MPO), and matrix metalloprotein-9 (MMP-9) activities were measured. HUVECs were cultured in human serum and annexin PI staining and caspase-3 activity were detected by flow cytometry. The TG, activities of MPO, and pro-MMP-9 increased significantly at 4 h after meal. Postprandial serum significantly increased the percentage of annexin-positive HUVECs and the activity of caspase-3. These results indicate that HFM can promote the apoptosis of endothelial cells, and the apoptosis rate is closely related to the increase in MPO and pro-MMP-9 activity, suggesting a possible mechanism of endothelial injury induced by postprandial lipemia (74) (Figure 1).
As previously reported, HAEC can induce an atherosclerotic state by TRL isolated from human blood after HFM. Wang et al. explored the role of endoplasmic reticulum (ER) stress in TRL metabolism and VCAM-1 regulation. Blood samples were collected before and 3.5 h after HFM from healthy subjects and hypertriglyceridemia patients. TRL was separated from postprandial blood samples by ultracentrifugation. HAECs were cultured in Petri dishes for 4 h. The HAEC endoplasmic reticulum morphology was imaged by confocal microscopy and immunofluorescence staining of calreticulin. It was found that the expansion of ER was significantly increased by HAEC treated with postprandial TRL, and the production of TRL after meals increased the adhesion of VCAM-1-dependent monocytes to the inflammatory endothelium. It was indicated that ER stress plays a role in the regulation of VCAM-1 transcription in endothelial cells during the process of atherosclerosis induced by postprandial lipemia (75) (Figure 1).
The mitochondria are thought to be the cell's power source because they produce the metabolic fuel ATP (76). Inflammation, apoptosis, and oxidative stress can promote the development of atherosclerotic plaques, and dysfunction caused by mitochondrial injury can promote these processes (77). Animal experiments showed that the binding rate of mitochondrial guanosine diphosphate (GDP) in brown adipose tissue was measured at one or more time points after the dietary test to indicate the uncoupling respiration rate, and Na+-K+-ATPase activity represented the coupled rate. It was found that the binding rate of GDP increased by 85% after the test, and the increase was the largest in the early postprandial period. It significantly decreased at 10 h after meal. This indicates that the proton conduction pathway in brown adipose tissue can be activated after a single meal (78) (Figure 1).
Postprandial lipemia is closely related to dietary habits and meal composition (99). The generation of CM and the change in postprandial lipemia were highly affected by the single meal nutrition structure (100). The release of fatty acids from solid food was lower than that from liquid or semisolid food, and the postprandial TG elevation caused by solid food was lower than that of liquid food (101). A range of metabolic, physiological, or functional disorders may occur transiently after ingestion of a high-energy diet rich in fat, and the process is strongly associated with postprandial TG (102). Postprandial CM-TG response increased with an increase in dietary fat content in normal weight and obese individuals (79). The remnant lipoprotein (RLP) particles containing apoB100 and apoB48 promote the formation and development of AS, and the intervention of postprandial RLP interferes with this (103).
A systematic review and meta-analysis of the effects of fat content on postprandial TG response after an oral fat tolerance test showed that there was no difference in response to saturated fatty acids (SFA) and unsaturated fatty acids within 4 h; a lower response to polyunsaturated fatty acids occurred within 8 h, and only a trend was observed for monounsaturated fatty acids, indicating that the postprandial TG response stimulated by different fatty acids is not enough to distinguish in fat tolerance tests shorter than 8 h (104). Studies in patients with type 2 diabetes showed that TG, TG-AUC, and TRL-TG levels improved after a fat tolerance test in patients receiving a Mediterranean diet rich in olive oil for 3 years (80). A research on the chain length of SFA showed that a diet rich in medium-chain SFA like coconut oil had the advantage in reducing postprandial TG and had the potential to improve postprandial lipemia (81). A study of patients with metabolic syndrome found that a diet rich in polyphenols reduced postprandial VLDL content and increased intermediate-density lipoprotein-cholesterol content, and the composition of modified LDL particles changed (82). A high monounsaturated fatty acid diet may have cardioprotective effects in patients with metabolic syndrome by improving postprandial oxidative stress (83).
Postprandial lipid response varies with different dietary types. Hansson et al. studied the effects of different dairy products with similar fat content on postprandial TG of healthy adults. It was found that TG increment AUC of sour cream was 61% (p < 0.01), 53% (p < 0.01), and 23% (p = 0.05) higher than that of whipped cream, butter, and cheese, respectively, indicating that the change of dietary structure may reduce postprandial lipemia (84). In addition, tart cherry has the ability to increase antioxidant activity and reduce TG level after HFM (85) (Table 1).
Numerous studies have shown that exercise can have a beneficial effect on postprandial lipemia (86–88, 105). Postprandial lipemia can be reduced by a single exercise (89). Exercise intensity and duration and other factors affect the improvement of postprandial lipemia (90). Exercise intensity is important for the protection of vascular function in adolescent boys between 12 and 15 years of age (91). Studies have demonstrated that adolescent boys between 12.5 and 14.1 years of age who repeatedly sprint for a short time have lower postprandial TG and FMD levels than those who do not exercise (92). Acute high-intensity interval running also reduced postprandial TG in adolescent boys age 11.3–12.9 years (93). Adults who regularly exercise had lower postprandial TG and lower oxidative stress levels such as TBARS than inactive adults, which reduces the negative postprandial changes affecting vascular health (51). Long-term exercise may alleviate postprandial lipid or oxidative stress in older adults with chronic diseases or those with elevated fasting label values (106). In addition, moderate- to high-intensity brief exercise can reduce postprandial TG, while high-intensity interval exercise reduces postprandial free fatty acid, thiobarbituric acid reactive substances, and protein carbonyl oxidative stress markers (94–96). Furthermore, high-intensity training may be more effective than moderate continuous training in reducing vascular injury (97).
There are many mechanisms involved in exercise intervention of postprandial lipemia. Regular aerobic exercise protects endothelial function after a high-fat mixed meal (98). A study to evaluate the effect of 4 h interrupted sitting with 5 min stair climbing after an HFM on vascular found that FMD decreased from 9.41 ± 2.61 to 10.34 ± 3.30% after exercise, indicating that the improvement of lifestyle can prevent postprandial vascular dysfunction. Exercise increases fat oxidation after meals (107, 108). McAllister et al. (109) explored the effect of acute moderate resistance exercise on alleviating postprandial oxidative stress and found that the AUC of advanced oxidation protein products at 4 h after meal was significantly lower than that at rest, and total nitrate/nitrite increased, illustrating that acute resistance exercise can reduce postprandial oxidative stress (Table 1).
Drugs related to lipid metabolism also affect postprandial blood lipids (110). Statins are considered an effective method to prevent and treat CVD because they can significantly reduce the level of TG (11). In obese and diabetic patients, postprandial FMD was improved by statins (111–113). Changes in postprandial lipoprotein metabolism after statins have been studied (114), and atorvastatin significantly reduced postprandial TG, TRL apoB48, VLDL, and IDL apoB100 levels in T2D patients with high TG levels (115). However, it is still controversial whether statins can reduce the level of apoB48 in patients with hypertriglyceridemia (110).
Fibrates are agonists of peroxisome proliferator-activated receptor α (PPAR-α), regulating lipoprotein metabolism via transcription factors. Fibrates have advantages in reducing fasting and postprandial TG and TRL remnant particles (7). Yamashita et al. reported that treatment with pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), enhances reverse cholesterol transport and improves postprandial hyperlipidemia in patients with dyslipidemia (116). Pemafibrate was demonstrated to improve liver function test values and is unlikely to enhance serum creatinine or reduce estimated glomerular filtration rate (117). Sairyo et al. (118) reported that pemafibrate attenuates postprandial hypertriglyceridemia by suppressing the postprandial increase of CM and the accumulation of CM remnants more effectively than fenofibrate in mice. Compared with conventional fibrates, pemafibrate has a better benefit–risk balance, which can be applied to patients who are difficult to use existing fibrates such as patients taking statins or with renal insufficiency (119).
Ezetimibe is a novel drug for the treatment of dyslipidemia, which selectively inhibits cholesterol absorption by inhibiting Niemann–Pick C1-like protein (NPC1L1). Ezetimibe reduced the postprandial TG-AUC and apoB100 concentration and reduce postprandial endothelial dysfunction (120). In type IIB hyperlipidemia patients, ezetimibe significantly reduced fasting TG, LDL-C, apoB48, and apoB100 levels as well as postprandial TG and apoB48 (121). It is reported that ezetimibe can improve TG and endothelial function in patients with CHD and hypertriglyceridemia during statin treatment (122). Ezetimibe combined with statins reduced the secretion of apoB48, which may be achieved by affecting the metabolism of TRL in intestinal cells (123). In addition, ezetimibe combined with statins reduced the incidence of long-term cardiovascular end events in patients with acute coronary syndrome (124). Therefore, the study of statins combined with ezetimibe to improve postprandial blood lipid levels is worthy of attention.
Tinker et al. (125) proposed that omega-3 fatty acids may reduce the postprandial TRL apoB by inhibiting the synthesis and secretion of apoB in the liver and intestine. In middle-aged and older adults >40 years, it was found that a high-fish diet can inhibit the production of apoB100 and promote its catabolism to reduce the level of TRL apoB48 after meals (126). Omega-3 fatty acid supplementation significantly inhibited postprandial TG and improved postprandial endothelial dysfunction in healthy subjects (127). In addition, it enhanced the clearance of CM-TG by increasing LPL activity (128). Postprandial TG, apoB48 total AUC, and VLDL apoB100 total AUC were reduced by omega-3 fatty acids in patients with familial hypercholesterolemia (129).
The effect of niacin on decreasing postprandial TG may be achieved by limiting FFA and inhibiting lipoprotein synthesis (130, 131). In healthy subjects, taking 2 g of extended-release niacin 1 h before an HFM effectively reduced the incremental AUC of TG and FFA and inhibited postprandial triglyceridemia, which may be the result of a significant restriction of FFA (132). However, the negative effects of niacin were reported in HPS2THRIVE and AIM-HIGH trials, which means its clinical application needs further study (133, 134).
Recently, proprotein convertase subtilisin/kexin type 9 (PCSK9) has been regarded as an endogenous inhibitor of LDL-C, and anti-PCSK9 monoclonal antibodies have been used for treating hypercholesterolemia (135, 136). Chan et al. reported the effect of subcutaneous injection of PCSK9 inhibitor with evolocumab for 8 weeks on postprandial TRL in healthy adult men. Evolocumab reduced the total area under the curve VLDL-apoB100 (p < 0.001), but did not significantly change the kinetics of apoB48 (137).
ApoCIII regulates TG metabolism by inhibiting TG hydrolysis. It has been reported to predict postprandial hypertriglyceridemia independently (138). Evidence showed that apoCIII not only inhibited LPL activity but also restrained the removal of TRLs by LPL-independent pathways (139). ApoCIII inhibitors may be a novel option for the treatment of severe hypertriglyceridemia by effectively reducing plasma TG.
Traditional Chinese medicine is also widely used in the intervention of atherosclerosis. Zhu et al. found that Ilexgenin A (IA) extracted from Ilex hainanensis can induce endothelial cells to produce NO, reduce the production of inflammatory cytokines and reactive oxygen species, and improve endothelial dysfunction (140, 141). In a randomized controlled crossover trial with healthy subjects, the experimental group received HFM and a platycodi radix beverage (AP). The control group received HFM and a placebo. Blood samples were collected at 0, 2, 4, and 6 h after a meal, and TG and lipoprotein lipase quality were analyzed. The results showed that the plasma lipoprotein lipase quality in the AP group increased significantly at 6 h, and VLDL-TG decreased significantly. It is suggested that a platycodi radix beverage may improve postprandial TG response, reduce postprandial lipemia, and decrease the risk of AS by improving the quality of lipoprotein lipase (142).
Fasting lipemia is a diagnostic standard for hyperlipidemia; the body is in the postprandial state most of the time, so the effect of postprandial lipemia on the human body is worthy of attention. The increase in postprandial TG is the main manifestation of postprandial hyperlipidemia. The relationship between diurnal TG pattern and postprandial blood lipid is worthy of further exploration. The results show that TG has a larger diurnal organism than TC, HDL-C, LDL-C, and other lipoproteins. Therefore, postprandial lipemia detection may reflect the true level of TG. A meta-analysis of 113 studies showed that the level of TG 4 h after the fat tolerance test was the most representative (143). Research showed that compared with the detection of postprandial TG, increment and total area under the curve before and after the meal tested hourly for 6 h and subjects that had blood drawn before and 4 h after meals without restriction of activity had results similar to those of the previous test, which provided a simple method for the detection and application of postprandial lipemia (144). Maraki et al. (145) have also shown that postprandial lipemia may be accurately described by the OFTT with reduced frequency of blood sampling.
The mechanism of AS induced by TRL needs to be further studied on the basis of the postprandial TG curve, and the standardized fat load test still needs improvement (143, 146). CVD caused by postprandial dyslipidemia has been reflected in many clinical cases. The mechanism of postprandial lipemia affecting the occurrence and development of AS may be endothelial dysfunction, oxidative stress, inflammation, or others (147–151). The accumulation of hepatic apoB100 and circulating intestinal apoB48 TRL are characteristic phenomena of postprandial lipemia, and both of them are involved in the development of atherosclerotic plaque (100). It has been suggested that postprandial RLP and remnant-like particles are sensitive and important in the risk estimates of AS (152). Many uncontrollable factors [such as, genetic background (153), age, sex, and menopausal state], as well as lifestyle (diet, physical activity, smoking, and drug use), can affect postprandial lipids (9, 87, 154, 155). Non-medicinal therapy including diet adjustment and exercise has beneficial effects on CVD (156). The mechanism of anti-AS of dietary intervention on postprandial lipemia is mainly inflammatory change, while exercise shows more improvement in postprandial oxidative stress. The anti-atherosclerotic mechanism of medicinal interventions such as, statins, fibrate, ezetimibe, and omega-3 fatty acids on postprandial lipemia is mostly manifested as improvement of vascular endothelial function. As novel intervention methods, pemafibrate, PCSK9 inhibitors, and apoCIII inhibitors are the hot spots of current research.
The joint detection of fasting lipids and postprandial lipids is helpful for clinicians to collect the lipid metabolism of patients more comprehensively, evaluate the risk of cardiovascular disease more accurately, and play a complementary role in diagnosis and treatment. Therefore, postprandial lipemia detection is recommended for clinical application as a Supplementary Material. Recently, postprandial lipemia was found to be well-suited to be measured by quantile-dependent expression, which significantly increases the exposure of individual's gene phenotypes in plasma TG (140). Further research on postprandial dyslipidemia and postprandial hyperlipidemia and an in-depth exploration of its mechanism and intervention measures may provide a new direction for the regulation of public health. Gut microbiota is closely related to lipid metabolism, and some studies have indicated that intestinal microorganisms may affect lipid metabolism as environmental factors (157). There are few reports on the relationship between postprandial lipemia and gut microbiota, but animal studies have shown that gut microbiota increases the level of epoxyeicosatrienoic acid and improves CVD by inhibiting postprandial soluble epoxide hydrolase (158). Whether intervention of gut microbiota can reduce postprandial lipemia, and therefore against AS, may be worthy of further study.
YZ, LL, and MW designed the article and wrote the manuscript. SY, GL, and LP created the table. CG and YW designed the figure. DL and RZ commented on the manuscript. All authors approved the manuscript for publication.
The work was supported by the National Natural Science Foundation of China (Grant Nos. 81202805, 81973689, 81573821, and 82074254) and the Beijing Natural Science Foundation (Grant Nos. 7172185 and 7202176).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. (2016) 37:1944–58. doi: 10.1093/eurheartj/ehw152
2. van Rooijen MA, Mensink RP. Palmitic acid versus stearic acid: effects of interesterification and intakes on cardiometabolic risk markers - a systematic review. Nutrients. (2020) 12:615–39. doi: 10.3390/nu12030615
3. Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. (1979) 60:473-85. doi: 10.1161/01.CIR.60.3.473
4. Plaisance EP, Fisher G. Exercise and dietary-mediated reductions in postprandial lipemia. J Nutr Metab. (2014) 2014:1–16. doi: 10.1155/2014/902065
5. Orem A, Yaman SO, Altinkaynak B, Kural BV, Yucesan FB, Altinkaynak Y, et al. Relationship between postprandial lipemia and atherogenic factors in healthy subjects by considering gender differences. Clin Chim Acta. (2018) 480:34–40. doi: 10.1016/j.cca.2018.01.038
6. Tomlinson B, Chan P, Lam CWK. Postprandial hyperlipidemia as a risk factor in patients with type 2 diabetes. Expert Rev Endocrinol Metab. (2020) 15:147–57. doi: 10.1080/17446651.2020.1750949
7. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.15829/1560-4071-2020-3826
9. Dias CB, Moughan PJ, Wood LG, Singh H, Garg ML. Postprandial lipemia: factoring in lipemic response for ranking foods for their healthiness. Lipids Health Dis. (2017) 16:178–89. doi: 10.1186/s12944-017-0568-5
10. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. European atherosclerosis society consensus, triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. (2011) 32:1345–61. doi: 10.1016/S1567-5688(11)70033-2
11. Nakamura K, Miyoshi T, Yunoki K, Ito H. Postprandial hyperlipidemia as a potential residual risk factor. J Cardiol. (2016) 67:335–9. doi: 10.1016/j.jjcc.2015.12.001
12. Chakraborty M, Singh P, Dsouza JMP, Pethusamy K, Thatkar PV. Fasting and postprandial lipid parameters: a comparative evaluation of cardiovascular risk assessment in prediabetes and diabetes. J Family Med Prim Care. (2020) 9:287–92. doi: 10.4103/jfmpc.jfmpc_769_19
13. Higgins V, Asgari S, Hamilton JK, Wolska A, Remaley AT, Hartmann B, et al. Postprandial dyslipidemia, hyperinsulinemia, and impaired gut peptides/bile acids in adolescents with obesity. J Clin Endocrinol Metab. (2020) 105:1228–41. doi: 10.1210/clinem/dgz261
14. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. (2019) 40:537–57. doi: 10.1210/er.2018-00184
15. Watkins J, Simpson A, Betts JA, Thompson D, Holliday A, Deighton K, et al. Galactose ingested with a high-fat beverage increases postprandial lipemia compared with glucose but not fructose ingestion in healthy men. J Nutr. (2020) 150:1765–72. doi: 10.1093/jn/nxaa105
16. Pozuelo-Sanchez I, Villasanta-Gonzalez A, Alcala-Diaz JF, Vals-Delgado C, Leon-Acuna A, Gonzalez-Requero A, et al. Postprandial lipemia modulates pancreatic alpha-cell function in the prediction of type 2 diabetes development: the CORDIOPREV study. J Agric Food Chem. (2020) 68:1266–75. doi: 10.1021/acs.jafc.9b06801
17. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. (2013) 61:427–36. doi: 10.1016/j.jacc.2012.08.1026
18. Teno S, Uto Y, Nagashima H, Endoh Y, Iwamoto Y, Omori Y, et al. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care. (2000) 23:1401–6. doi: 10.2337/diacare.23.9.1401
19. Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. (2011) 9:258–70. doi: 10.2174/157016111795495549
20. Guo Y, Zhu X, Zeng S, He M, Xing X, Wang C. miRNA-10a-5p alleviates insulin resistance and maintains diurnal patterns of triglycerides and gut microbiota in high-fat diet-fed mice. Mediators Inflamm. (2020) 2020:1–8. doi: 10.1155/2020/8192187
21. Quagliarini F, Mir AA, Balazs K, Wierer M, Dyar KA, Jouffe C, et al. Cistromic reprogramming of the diurnal glucocorticoid hormone response by high-fat diet. Mol Cell. (2019) 76:531–45.e5. doi: 10.1016/j.molcel.2019.10.007
22. Salinas CAA, Chapman MJ. Remnant lipoproteins: are they equal to or more atherogenic than LDL? Curr Opin Lipidol. (2020) 31:132–9. doi: 10.1097/MOL.0000000000000682
23. Nordestgaard BG. A test in context: lipid profile, fasting versus non-fasting. J Am Coll Cardiol. (2017) 70:1637–46. doi: 10.1016/j.jacc.2017.08.006
24. Langsted A, Nordestgaard BG. Non-fasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. (2019) 51:131–41. doi: 10.1016/j.pathol.2018.09.062
25. Farukhi ZM, Demler OV, Caulfield MP, Kulkarni K, Wohlgemuth J, Cobble M, et al. Comparison of non-fasting and fasting lipoprotein subfractions and size in 15,397 apparently healthy individuals: an analysis from the VITamin D and OmegA-3 TriaL. J Clin Lipidol. (2020) 14:241–51. doi: 10.1016/j.jacl.2020.02.005
26. Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. (2016) 32:1263–82. doi: 10.1016/j.cjca.2016.07.510
27. Harris KC, Benoit G, Dionne J, Feber J, Cloutier L, Zarnke KB, et al. Hypertension Canada's (2016) Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, and assessment of risk of pediatric hypertension. Can J Cardiol. (2016) 32:589–97. doi: 10.1016/j.cjca.2016.02.075
28. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2011) 123:2292–333. doi: 10.1161/CIR.0b013e3182160726
29. Downs JR, O'Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the (2014). U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med. (2015) 163:291–7. doi: 10.7326/M15-0840
30. Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, et al. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb Vasc Biol. (1992) 12:1336–45. doi: 10.1161/01.ATV.12.11.1336
31. Kats D, Sharrett AR, Ginsberg HN, Nambi V, Ballantyne CM, Hoogeveen RC, et al. Postprandial lipemia and the risk of coronary heart disease and stroke: the atherosclerosis risk in communities (ARIC) Study. BMJ Open Diabetes Res Care. (2017) 5:1–6. doi: 10.1136/bmjdrc-2016-000335
32. Verwer BJ, Scheffer PG, Vermue RP, Pouwels PJ, Diamant M, Tushuizen ME. NAFLD is related to post-prandial triglyceride-enrichment of HDL particles in association with endothelial and HDL dysfunction. Liver Int. (2020) 40:2439–44. doi: 10.1111/liv.14597
33. Kurihara O, Okajima F, Takano M, Kato K, Munakata R, Murakami D, et al. Postprandial hyperchylomicronemia and thin-cap fibroatheroma in non-culprit lesions. Arterioscler Thromb Vasc Biol. (2018) 38:1940–7. doi: 10.1161/ATVBAHA.118.311245
34. Okajima F, Kurihara O, Takano M. Acute coronary syndrome and postprandial delayed hyperchylomicronemia. Aging. (2019) 11:2549–50. doi: 10.18632/aging.101969
35. Sahade V, Franca S, Badaro R, Fernando Adan L. Obesity and postprandial lipemia in adolescents: risk factors for cardiovascular disease. Endocrinol Nutr. (2012) 59:131–9. doi: 10.1016/j.endoen.2011.08.004
36. Noda Y, Miyoshi T, Oe H, Ohno Y, Nakamura K, Toh N, et al. Alogliptin ameliorates postprandial lipemia and postprandial endothelial dysfunction in non-diabetic subjects: a preliminary report. Cardiovasc Diabetol. (2013) 12:1–8. doi: 10.1186/1475-2840-12-8
37. Ziouzenkova O, Ochiai M. Evaluating the appropriate oral lipid tolerance test model for investigating plasma triglyceride elevation in mice. Plos ONE. (2020) 15:e0235875. doi: 10.1371/journal.pone.0235875
38. Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Africa. (2012) 23:222–31. doi: 10.5830/CVJA-2011-068
39. Silveira A, Carlo A, Adam M, McLeod O, Lundman P, Boquist S, et al. VIIaAT complexes, procoagulant phospholipids, and thrombin generation during postprandial lipemia. Int J Lab Hematol. (2018) 40:251–7. doi: 10.1111/ijlh.12773
40. Thom NJ, Early AR, Hunt BE, Harris RA, Herring MP. Eating and arterial endothelial function: a meta-analysis of the acute effects of meal consumption on flow-mediated dilation. Obes Rev. (2016) 17:1080–90. doi: 10.1111/obr.12454
41. Vazquez-Madrigal C, Lopez S, Grao-Cruces E, Millan-Linares MC, Rodriguez-Martin NM, Martin ME, et al. Dietary fatty acids in postprandial triglyceride-rich lipoproteins modulate human monocyte-derived dendritic cell maturation and activation. Nutrients. (2020) 12:1–13. doi: 10.3390/nu12103139
42. Petersen KS, Rogers CJ, West SG, Proctor DN, Kris-Etherton PM. The effect of culinary doses of spices in a high-saturated fat, high-carbohydrate meal on postprandial lipemia and endothelial function: a randomized, controlled, crossover pilot trial. Food Funct. (2020) 11:3191–200. doi: 10.1039/C9FO02438G
43. Koemel NA, Sciarrillo CM, Bode KB, Dixon MD, Lucas EA, Jenkins NDM, et al. Postprandial metabolism and vascular function: impact of aging and physical activity level. Int J Sport Nutr Exerc Metab. (2020) 30:1–8. doi: 10.1123/ijsnem.2020-0063
44. Madhu S, Sinha B, Aslam M, Mehrotra G, Dwivedi S. Postprandial triglyceride responses and endothelial function in prediabetic first-degree relatives of patients with diabetes. J Clin Lipidol. (2017) 11:1415–20. doi: 10.1016/j.jacl.2017.08.001
45. Tousoulis D, Kampoli AM, Papageorgiou CTN, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. (2012) 10:4–18. doi: 10.2174/157016112798829760
46. Ramirez-Velez R. Postprandial lipemia induces endothelial dysfunction and higher insulin resistance in healthy subjects. Endocrinol Nutr. (2011) 58:529–35. doi: 10.1016/j.endoen.2011.07.007
47. Shafieesabet A, Scherbakov N, Ebner N, Sandek A, Lokau S, von Haehling S, et al. Acute effects of oral triglyceride load on dynamic changes in peripheral endothelial function in heart failure patients with reduced ejection fraction and healthy controls. Nutr Metab Cardiovasc Dis. (2020) 30:1961–6. doi: 10.1016/j.numecd.2020.05.018
48. Whisner CM, Angadi SS, Weltman NY, Weltman A, Rodriguez J, Patrie JT, et al. Effects of low-fat and high-fat meals, with and without dietary fiber, on postprandial endothelial function, triglyceridemia, and glycemia in adolescents. Nutrients. (2019) 11:1–9. doi: 10.3390/nu11112626
49. Diekmann C, Huber H, Preuss M, Preuss P, Predel HG, Stoffel-Wagner B, et al. Moderate postmeal walking has no beneficial effects over resting on postprandial lipemia, glycemia, insulinemia, and selected oxidative and inflammatory parameters in older adults with a cardiovascular disease risk phenotype: a randomized crossover trial. J Nutr. (2019) 149:1930–41. doi: 10.1093/jn/nxz148
50. Wallace JP, Johnson B, Padilla J, Mather K. Postprandial lipaemia, oxidative stress and endothelial function: a review. Int J Clin Pract. (2010) 64:389–403. doi: 10.1111/j.1742-1241.2009.02146.x
51. Johnson BD, Padilla J, Harris RA, Wallace JP. Vascular consequences of a high-fat meal in physically active and inactive adults. Appl Physiol Nutr Metab. (2011) 36:368–75. doi: 10.1139/h11-028
52. Nyunt T, Britton M, Wanichthanarak K, Budamagunta M, Voss JC, Wilson DW, et al. Mitochondrial oxidative stress-induced transcript variants of ATF3 mediate lipotoxic brain microvascular injury. Free Radic Biol Med. (2019) 143:25–46. doi: 10.1016/j.freeradbiomed.2019.07.024
53. Aung HH, Altman R, Nyunt T, Kim J, Nuthikattu S, Budamagunta M, et al. Induction of lipotoxic brain microvascular injury is mediated by activating transcription factor 3-dependent inflammatory, and oxidative stress pathways. J Lipid Res. (2016) 57:955–68. doi: 10.1194/jlr.M061853
54. Rouyer O, Auger C, Charles AL, Talha S, Meyer A, Piquard F, et al. Effects of a high fat meal associated with water, juice, or champagne consumption on endothelial function and markers of oxidative stress and inflammation in young, healthy subjects. J Clin Med. (2019) 8:859–72. doi: 10.3390/jcm8060859
55. Tsai W-C, Li Y-H, Lin C-C, Chao T-H, Chen J-H. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci. (2004) 106:315–9. doi: 10.1042/CS20030227
56. Bae J-H, Bassenge E, Kim K-B, Kim Y-N, Kim K-S, Lee H-J, et al. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. (2001) 155:517–23. doi: 10.1016/S0021-9150(00)00601-8
57. Neri S, Calvagno S, Mauceri B, Misseri M, Tsami A, Vecchio C, et al. Effects of antioxidants on postprandial oxidative stress and endothelial dysfunction in subjects with impaired glucose tolerance and Type 2 diabetes. Eur J Nutr. (2010) 49:409–16. doi: 10.1007/s00394-010-0099-6
58. Saxena R, Madhu SV, Shukla R, Prabhu KM, Gambhir JK. Postprandial hypertriglyceridemia and oxidative stress in patients of type 2 diabetes mellitus with macrovascular complications. Clin Chim Acta. (2005) 359:101–8. doi: 10.1016/j.cccn.2005.03.036
59. Kullisaar T, Shepetova J, Zilmer K, Songisepp E, Rehema A, Mikelsaar M, et al. An antioxidant probiotic reduces postprandial lipemia and oxidative stress. Cent Eur J Biol. (2011) 6:32–40. doi: 10.2478/s11535-010-0103-4
60. Shah PK. Inflammation, infection, and atherosclerosis. Trends Cardiovasc Med. (2019) 29:468–72. doi: 10.1016/j.tcm.2019.01.004
61. Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. (2012) 220:22–33. doi: 10.1016/j.atherosclerosis.2011.08.012
62. Teng KT, Chang CY, Kanthimathi MS, Tan AT, Nesaretnam K. Effects of amount and type of dietary fats on postprandial lipemia and thrombogenic markers in individuals with metabolic syndrome. Atherosclerosis. (2015) 242:281–7. doi: 10.1016/j.atherosclerosis.2015.07.003
63. Brown M, McClean CM, Davison GW, Brown JCW, Murphy MH. Preceding exercise and postprandial hypertriglyceridemia: effects on lymphocyte cell DNA damage and vascular inflammation. Lipids Health Dis. (2019) 18:125–37. doi: 10.1186/s12944-019-1071-y
64. Schnitzler JG, Dallinga-Thie GM, Kroon J. The role of (Modified) lipoproteins in vascular function: a duet between monocytes and the endothelium. Curr Med Chem. (2019) 26:1594–609. doi: 10.2174/0929867325666180316121015
65. Masuda D, Yamashita S. Postprandial hyperlipidemia and remnant lipoproteins. J Atheroscler Thromb. (2017) 24:95–109. doi: 10.5551/jat.RV16003
66. Di Renzo L, Merra G, Botta R, Gualtieri P, Manzo A, Perrone MA, et al. Post-prandial effects of hazelnut-enriched high fat meal on LDL oxidative status, oxidative and inflammatory gene expression of healthy subjects: a randomized trial. Eur Rev Med Pharmacol Sci. (2017) 21:1610–26.
67. DeVerse JS, Sandhu AS, Mendoza N, Edwards CM, Sun C, Simon SI, et al. Shear stress modulates VCAM-1 expression in response to TNF-alpha and dietary lipids via interferon regulatory factor-1 in cultured endothelium. Am J Physiol Heart Circ Physiol. (2013) 305:1149–57. doi: 10.1152/ajpheart.00311.2013
68. Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. (2011) 31:160–6. doi: 10.1161/ATVBAHA.110.215434
69. Gorzelak-Pabis P, Wozniak E, Wojdan K, Chalubinski M, Broncel M. Single triglyceride-rich meal destabilizes barrier functions and initiates inflammatory processes of endothelial cells. J Interferon Cytokine Res. (2020) 40:43–53. doi: 10.1089/jir.2018.0173
70. Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res. (2014) 58:136–46. doi: 10.1002/mnfr.201300104
71. Rajamani A, Borkowski K, Akre S, Fernandez A, Newman JW, Simon SI, et al. Oxylipins in triglyceride-rich lipoproteins of dyslipidemic subjects promote endothelial inflammation following a high fat meal. Sci Rep. (2019) 9:8655–72. doi: 10.1038/s41598-019-45005-5
72. Meessen ECE, Warmbrunn MV, Nieuwdorp M, Soeters MR. Human postprandial nutrient metabolism and low-grade inflammation: a narrative review. Nutrients. (2019) 11:3000–21. doi: 10.3390/nu11123000
73. Dejeans N, Maier JA, Tauveron I, Milenkovic D, Mazur A. Modulation of gene expression in endothelial cells by hyperlipaemic postprandial serum from healthy volunteers. Genes Nutr. (2010) 5:263–74. doi: 10.1007/s12263-010-0166-x
74. Spallarossa P, Garibaldi S, Barisione C, Ghigliotti G, Altieri P, Tracchi I, et al. Postprandial serum induces apoptosis in endothelial cells: Role of polymorphonuclear-derived myeloperoxidase and metalloproteinase-9 activity. Atherosclerosis. (2008) 198:458–67. doi: 10.1016/j.atherosclerosis.2007.11.030
75. Wang YI, Bettaieb A, Sun C, DeVerse JS, Radecke CE, Mathew S, et al. Triglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stress. PLoS ONE. (2013) 8:e78322. doi: 10.1371/journal.pone.0078322
76. Yu EP. Bennett MR. Mitochondrial DNA damage and atherosclerosis. Trends Endocrinol Metab. (2014) 25:481–7. doi: 10.1016/j.tem.2014.06.008
77. Wang Y, Subramanian M, Yurdagul A, Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J, et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. (2017) 171:331–345. doi: 10.1016/j.cell.2017.08.041
78. Lupien JR, Click Z, Saito M, Bary GA. Guanosine diphosphate binding to brown adipose tissue mitochondria is increased after single meal. Am J Physiol. (1985) 249:R694–8. doi: 10.1152/ajpregu.1985.249.6.R694
79. Vors C, Pineau G, Drai J, Meugnier E, Pesenti S, Laville M, et al. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose-effect trial. J Clin Endocrinol Metab. (2015) 100:3427–35. doi: 10.1210/jc.2015-2518
80. Gomez-Marin B, Gomez-Delgado F, Lopez-Moreno J, Alcala-Diaz JF, Jimenez-Lucena R, Torres-Pena JD, et al. Long-term consumption of a Mediterranean diet improves postprandial lipemia in patients with type 2 diabetes: the Cordioprev randomized trial. Am J Clin Nutr. (2018) 108:963–70. doi: 10.1093/ajcn/nqy144
81. Panth N, Dias CB, Wynne K, Singh H, Garg ML. Medium-chain fatty acids lower postprandial lipemia: a randomized crossover trial. Clin Nutr. (2020) 39:90–6. doi: 10.1016/j.clnu.2019.02.008
82. Della Pepa G, Vetrani C, Vitale M, Bozzetto L, Costabile G, Cipriano P, et al. Effects of a diet naturally rich in polyphenols on lipid composition of postprandial lipoproteins in high cardiometabolic risk individuals: an ancillary analysis of a randomized controlled trial. Eur J Clin Nutr. (2020) 74:183–92. doi: 10.1038/s41430-019-0459-0
83. Perez-Martinez P, Garcia-Quintana JM, Yubero-Serrano EM, Tasset-Cuevas I, Tunez I, Garcia-Rios A, et al. Postprandial oxidative stress is modified by dietary fat: evidence from a human intervention study. Clin Sci. (2010) 119:251–61. doi: 10.1042/CS20100015
84. Hansson P, Holven KB, Øyri LKL, Brekke HK, Biong AS, Gjevestad GO, et al. Meals with similar fat content from different dairy products induce different postprandial triglyceride responses in healthy adults: a randomized controlled cross-over trial. J Nutr. (2019) 149:422–31. doi: 10.1093/jn/nxy291
85. Polley KR, Oswell NJ, Pegg RB, Cooper JA. Tart cherry consumption with or without prior exercise increases antioxidant capacity and decreases triglyceride levels following a high-fat meal. Appl Physiol Nutr Metabol. (2019) 44:1209–18. doi: 10.1139/apnm-2018-0535
86. Farinha JB, Macedo CEO, Rodrigues-Krause J, Kruger RL, Boeno FP, Macedo RCO, et al. Effects of two combined exercise designs associated with high-fat meal consumption on postprandial lipemia, insulinemia, oxidative stress. J Strength Cond Res. (2018) 32:1422–30. doi: 10.1519/JSC.0000000000001984
87. O'Doherty AF, Jones HS, Sathyapalan T, Ingle L, Carroll S. The effects of acute interval exercise and strawberry intake on postprandial lipemia. Med Sci Sports Exerc. (2017) 49:2315–23. doi: 10.1249/MSS.0000000000001341
88. Lopes Kruger R, Costa Teixeira B, Boufleur Farinha J, Cauduro Oliveira Macedo R, Pinto Boeno F, Rech A, et al. Effect of exercise intensity on postprandial lipemia, markers of oxidative stress, and endothelial function after a high-fat meal. Appl Physiol Nutr Metab. (2016) 41:1278–84. doi: 10.1139/apnm-2016-0262
89. Chiu CH, Yang TJ, Liang HJ, Chang CK, Wu CL. A single bout of exercise reduces postprandial lipemia but has no delayed effect on hemorheological variables. Chin J Physiol. (2018) 61:181–7. doi: 10.4077/CJP.2018.BAG570
90. Teeman CS, Kurti SP, Cull BJ, Emerson SR, Haub MD, Rosenkranz SK. Postprandial lipemic and inflammatory responses to high-fat meals: a review of the roles of acute and chronic exercise. Nutr Metabol. (2016) 13:80–94. doi: 10.1186/s12986-016-0142-6
91. Bond B, Gates PE, Jackman SR, Corless LM, Williams CA, Barker AR. Exercise intensity and the protection from postprandial vascular dysfunction in adolescents. Am J Physiol Heart Circ Physiol. (2015) 308:1443–50. doi: 10.1152/ajpheart.00074.2015
92. Sedgwick MJ, Morris JG, Nevill ME, Barrett LA. Effect of repeated sprints on postprandial endothelial function and triacylglycerol concentrations in adolescent boys. J Sports Sci. (2015) 33:806–16. doi: 10.1080/02640414.2014.964749
93. Thackray AE, Barrett LA, Tolfrey K. Acute high-intensity interval running reduces postprandial lipemia in boys. Med Sci Sports Exerc. (2013) 45:1277–84. doi: 10.1249/MSS.0b013e31828452c1
94. Ferreira AP, Ferreira CB, Brito CJ, Souza VC, Cordova C, Nobrega OT, et al. The effect of aerobic exercise intensity on attenuation of postprandial lipemia is dependent on apolipoprotein E genotype. Atherosclerosis. (2013) 229:139–44. doi: 10.1016/j.atherosclerosis.2013.03.027
95. Gabriel B, Ratkevicius A, Gray P, Frenneaux MP, Gray SR. High-intensity exercise attenuates postprandial lipaemia and markers of oxidative stress. Clin Sci. (2012) 123:313–21. doi: 10.1042/CS20110600
96. Wilhelmsen A, Mallinson J, Jones R, Cooper S, Taylor T, Tsintzas K. Chronic effects of high-intensity interval training on postprandial lipemia in healthy men. J Appl Physiol (1985). (2019) 127:1763–71. doi: 10.1152/japplphysiol.00131.2019
97. Ramirez-Velez R, Correa-Rodriguez M, Tordecilla-Sanders A, Aya-Aldana V, Izquierdo M, Correa-Bautista JE, et al. Exercise and postprandial lipemia: effects on vascular health in inactive adults. Lipids Health Dis. (2018) 17:69–80. doi: 10.1186/s12944-018-0719-3
98. Das EK, Lai PY, Robinson AT, Pleuss J, Ali MM, Haus JM, et al. Regular aerobic, resistance, and cross-training exercise prevents reduced vascular function following a high sugar or high fat mixed meal in young healthy adults. Front Physiol. (2018) 9:183–96. doi: 10.3389/fphys.2018.00183
99. Lairon D, Defoort C. Effects of nutrients on postprandial lipemia. Curr Vasc Pharmacol. (2011) 9:309–12. doi: 10.2174/157016111795495576
100. Desmarchelier C, Borel P, Lairon D, Maraninchi M, Valero R. Effect of nutrient and micronutrient intake on chylomicron production and postprandial lipemia. Nutrients. (2019) 11:1299–329. doi: 10.3390/nu11061299
101. Dias CB, Zhu X, Thompson AK, Singh H, Garg ML. Effect of the food form and structure on lipid digestion and postprandial lipaemic response. Food Funct. (2019) 10:112–24. doi: 10.1039/C8FO01698D
102. Dimina L, Mariotti F. The postprandial appearance of features of cardiometabolic risk: acute induction and prevention by nutrients and other dietary substances. Nutrients. (2019) 11:1963–86. doi: 10.3390/nu11091963
103. Nakajima K, Tanaka A. Postprandial remnant lipoproteins as targets for the prevention of atherosclerosis. Curr Opin Endocrinol Diabetes Obes. (2018) 25:108–17. doi: 10.1097/MED.0000000000000393
104. Monfort-Pires M, Delgado-Lista J, Gomez-Delgado F, Lopez-Miranda J, Perez-Martinez P, Ferreira S. Impact of the content of fatty acids of oral fat tolerance tests on postprandial triglyceridemia: systematic review and meta-analysis. Nutrients. (2016) 8:580–95. doi: 10.3390/nu8090580
105. Ma SX, Zhu Z, Cao ZB. Effects of interrupting sitting with different activity bouts on postprandial lipemia: a randomized crossover trial. Scand J Med Sci Sports. (2020) 31:633–42. doi: 10.1111/sms.13886
106. Bloomer RJ, Fisher-Wellman KH, Bell HK. The effect of long-term, high-volume aerobic exercise training on postprandial lipemia and oxidative stress. Phys Sportsmed. (2010) 38:64–71. doi: 10.3810/psm.2010.04.1763
107. Chiu CH, Yang TJ, Chen CH, Zeng MJ. High fat meals increases postprandial fat oxidation rate but not postprandial lipemia. Lipids Health Dis. (2019) 18:182–9. doi: 10.1186/s12944-019-1129-x
108. Yang TJ, Wu CL, Chiu CH. High-intensity intermittent exercise increases fat oxidation rate and reduces postprandial triglyceride concentrations. Nutrients. (2018) 10:3390–401. doi: 10.3390/nu10040492
109. McAllister MJ, Steadman KS, Renteria LI, Case MJ, Butawan MB, Bloomer RJ, et al. Acute resistance exercise reduces postprandial lipemia and oxidative stress in resistance-trained men. J Strength Cond Res. (2020). doi: 10.1519/JSC.0000000000003831. [Epub ahead of print].
110. Pirillo A, Norata GD, Catapano AL. Postprandial lipemia as a cardiometabolic risk factor. Curr Med Res Opin. (2014) 30:1489–503. doi: 10.1185/03007995.2014.909394
111. Nagashima H, Endo M. Pitavastatin prevents postprandial endothelial dysfunction via reduction of the serum triglyceride level in obese male subjects. Heart Vessels. (2011) 26:428–34. doi: 10.1007/s00380-010-0071-7
112. Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. (2005) 111:2518–24. doi: 10.1161/01.CIR.0000165070.46111.9F
113. Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. (2002) 106:1211–8. doi: 10.1161/01.CIR.0000027569.76671.A8
114. Kolovou G, Anagnostopoulou K, Salpea K, Daskalopoulou S, Mikhailidis D. The effect of statins on postprandial lipemia. Current Drug Targets. (2007) 8:551–60. doi: 10.2174/138945007780362809
115. Hogue J-C, Lamarche B, Deshaies Y, Tremblay AJ, Bergeron J, Gagné C, et al. Differential effect of fenofibrate and atorvastatin on in vivo kinetics of apolipoproteins B-100 and B-48 in subjects with type 2 diabetes mellitus with marked hypertriglyceridemia. Metabolism. (2008) 57:246–54. doi: 10.1016/j.metabol.2007.09.008
116. Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S, et al. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor alpha modulator, in patients with dyslipidemia: results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol. (2018) 12:173–84. doi: 10.1016/j.jacl.2017.10.006
117. Yamashita S, Masuda D, Matsuzawa Y. Clinical applications of a novel selective PPARα modulator, pemafibrate, in dyslipidemia and metabolic diseases. J Atheroscler Thromb. (2019) 26:389–402. doi: 10.5551/jat.48918
118. Sairyo M, Kobayashi T, Masuda D, Kanno K, Zhu Y, Okada T, et al. A novel selective PPARα modulator (SPPARMα), K-877 (Pemafibrate), attenuates postprandial hypertriglyceridemia in mice. J Atheroscler Thromb. (2018) 25:142–52. doi: 10.5551/jat.39693
119. Yamashita S, Masuda D, Matsuzawa Y. Pemafibrate, a new selective PPARα modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep. (2020) 22:5–22. doi: 10.1007/s11883-020-0823-5
120. Yunoki K, Nakamura K, Miyoshi T, Enko K, Kohno K, Morita H, et al. Ezetimibe improves postprandial hyperlipemia and its induced endothelial dysfunction. Atherosclerosis. (2011) 217:486–91. doi: 10.1016/j.atherosclerosis.2011.04.019
121. Masuda D, Nakagawa-Toyama Y, Nakatani K, Inagaki M, Tsubakio-Yamamoto K, Sandoval JC, et al. Ezetimibe improves postprandial hyperlipidaemia in patients with type IIb hyperlipidaemia. Eur J Clin Invest. (2009) 39:689–98. doi: 10.1111/j.1365-2362.2009.02163.x
122. Yunoki K, Nakamura K, Miyoshi T, Enko K, Kubo M, Murakami M, et al. Impact of hypertriglyceridemia on endothelial dysfunction during statin ± ezetimibe therapy in patients with coronary heart disease. Am J Cardiol. (2011) 108:333–9. doi: 10.1016/j.amjcard.2011.03.049
123. Tremblay AJ, Lamarche Bt, Hogue J-C, Couture P. Effects of ezetimibe and simvastatin on apolipoprotein B metabolism in males with mixed hyperlipidemia. J Lipid Res. (2009) 50:1463–71. doi: 10.1194/jlr.P800061-JLR200
124. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. (2015) 372:2387–97. doi: 10.1056/NEJMoa1410489
125. Tinker LF, Parks EJ, Behr SR, Schneeman BO, Davis PA. (n-3) fatty acid supplementation in moderately hypertriglyceridemic adults changes postprandial lipid and apolipoprotein B responses to a standardized test meal. J Nutr. (1999) 129:1126–34. doi: 10.1093/jn/129.6.1126
126. Ooi EMM, Lichtenstein AH, Millar JS, Diffenderfer MR, Lamon-Fava S, Rasmussen H, et al. Effects of therapeutic lifestyle change diets high and low in dietary fish-derived FAs on lipoprotein metabolism in middle-aged and elderly subjects. J Lipid Res. (2012) 53:1958–67. doi: 10.1194/jlr.P024315
127. Miyoshi T, Noda Y, Ohno Y, Sugiyama H, Oe H, Nakamura K, et al. Omega-3 fatty acids improve postprandial lipemia and associated endothelial dysfunction in healthy individuals - a randomized cross-over trial. Biomed Pharmacother. (2014) 68:1071–7. doi: 10.1016/j.biopha.2014.10.008
128. Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res. (2003) 44:455–63. doi: 10.1194/jlr.M200282-JLR200
129. Chan DC, Pang J, Barrett PH, Sullivan DR, Burnett JR, van Bockxmeer FM, et al. Omega-3 fatty acid ethyl esters diminish postprandial lipemia in familial hypercholesterolemia. J Clin Endocrinol Metab. (2016) 101:3732–9. doi: 10.1210/jc.2016-2217
130. King JM, Crouse JR, Terry JG, Morgan TM, Spray BJ, Miller NE. Evaluation of effects of unmodified niacin on fasting and postprandial plasma lipids in normolipidemic men with hypoalphalipoproteinemia. Am J Med. (1994) 97:323–31. doi: 10.1016/0002-9343(94)90298-4
131. O'Keefe JH, Harris WS, Nelson J, Windsor SL. Effects of pravastatin with niacin or magnesium on lipid levels and postprandial lipemia. Am J Cardiol. (1995) 76:480–4. doi: 10.1016/S0002-9149(99)80134-9
132. Usman MH, Qamar A, Gadi R, Lilly S, Goel H, Hampson J, et al. Extended-release niacin acutely suppresses postprandial triglyceridemia. Am J Med. (2012) 125:1026–35. doi: 10.1016/j.amjmed.2012.03.017
133. Group HTC. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. (2013) 34:1279–91. doi: 10.1093/eurheartj/eht055
134. Teo KK, Goldstein LB, Chaitman BR, Grant S, Weintraub WS, Anderson DC, et al. Extended-release niacin therapy and risk of ischemic stroke in patients with cardiovascular disease: the atherothrombosis intervention in metabolic syndrome with low HDL/high triglycerides: impact on global health outcome (AIM-HIGH) trial. Stroke. (2013) 44:2688–93. doi: 10.1161/STROKEAHA.113.001529
135. D'Ardes D, Santilli F, Guagnano MT, Bucci M, Cipollone F. From endothelium to lipids, through microRNAs and PCSK9: a fascinating travel across atherosclerosis. High Blood Press Cardiovasc Prev. (2020) 27:1–8. doi: 10.1007/s40292-019-00356-y
136. Garçon D, Moreau F, Ayer A, Dijk W, Prieur X, Arnaud L, et al. Circulating rather than intestinal PCSK9 (proprotein convertase subtilisin kexin type 9) regulates postprandial lipemia in mice. Arterioscler Thromb Vasc Biol. (2020) 40:2084–94. doi: 10.1161/ATVBAHA.120.314194
137. Chan DC, Watts GF, Somaratne R, Wasserman SM, Scott R, Barrett PHR. Comparative effects of PCSK9 (Proprotein convertase subtilisin/kexin type 9) inhibition and statins on postprandial triglyceride-rich lipoprotein metabolism. Arterioscler Thromb Vasc Biol. (2018) 38:1644–55. doi: 10.1161/ATVBAHA.118.310882
138. Zhang T, Tang X, Mao L, Chen J, Kuang J, Guo X, et al. HDL-associated apoCIII plays an independent role in predicting postprandial hypertriglyceridemia. Clin Biochem. (2020) 79:14–22. doi: 10.1016/j.clinbiochem.2020.02.004
139. Rocha NA, East C, Zhang J, McCullough PA. ApoCIII as a cardiovascular risk factor and modulation by the novel lipid-lowering agent volanesorsen. Curr Atheroscler Rep. (2017) 19:62–71. doi: 10.1007/s11883-017-0697-3
140. Williams PT. Quantile-dependent expressivity of postprandial lipemia. PLoS ONE. (2020) 15:e0229495. doi: 10.1371/journal.pone.0229495
141. Zhu Y, Li M, Lu Y, Li J, Ke Y, Yang J. Ilexgenin a inhibits mitochondrial fission and promote Drp1 degradation by Nrf2-induced PSMB5 in endothelial cells. Drug Dev Res. (2019) 80:481–9. doi: 10.1002/ddr.21521
142. Lee H, Lim Y, Park SY, Cho SM, Choe JS, Jeong S, et al. Platycodi radix beverage ameliorates postprandial lipemia response through lipid clearance of triglyceride-rich lipoprotein: a randomized controlled study in healthy subjects with a high-fat load. Nutr Res Pract. (2018) 12:371–7. doi: 10.4162/nrp.2018.12.5.371
143. Mihas C, Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, et al. Diagnostic value of postprandial triglyceride testing in healthy subjects: a meta-analysis. Curr Vasc Pharmacol. (2011) 9:271–80. doi: 10.2174/157016111795495530
144. Sciarrillo CM, Koemel NA, Kurti SP, Emerson SR. Validity of an abbreviated, clinically feasible test for postprandial lipemia in healthy adults: a randomized cross-over study. Nutrients. (2019) 11:180–7. doi: 10.3390/nu11010180
145. Maraki M, Aggelopoulou N, Christodoulou N, Katsarou C, Anapliotis P, Kavouras SA, et al. Validity of abbreviated oral fat tolerance tests for assessing postprandial lipemia. Clin Nutr. (2011) 30:852–7. doi: 10.1016/j.clnu.2011.05.003
146. Kolovou D G, Mikhailidis DP, Nordestgaard BG, Bilianou H, Panotopoulos G. Definition of postprandial lipaemia. Curr Vasc Pharmacol. (2011) 9:292–301. doi: 10.2174/157016111795495611
147. Wang YI, Schulze J, Raymond N, Tomita T, Tam K, Simon SI, et al. Endothelial inflammation correlates with subject triglycerides and waist size after a high-fat meal. Am J Physiol Heart Circ Physiol. (2011) 300:784–91. doi: 10.1152/ajpheart.01036.2010
148. Ansar S, Koska J, Reaven PD. Postprandial hyperlipidemia, endothelial dysfunction, and cardiovascular risk: focus on incretins. Cardiovasc Diabetol. (2011) 10:61–72. doi: 10.1186/1475-2840-10-61
149. Ursini F. Sevanian A. Postprandial oxidative stress. Biol Chem. (2002) 383:3–4. doi: 10.1515/BC.2002.062
150. Chan DC, Pang J, Romic G, Watts GF. Postprandial hypertriglyceridemia and cardiovascular disease: current and future therapies. Curr Atheroscler Rep. (2013) 15:309–18. doi: 10.1007/s11883-013-0309-9
151. O'Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. (2007) 100:899–904. doi: 10.1016/j.amjcard.2007.03.107
152. Tanaka A. Postprandial hyperlipidemia and atherosclerosis. Curr Atheroscler Rep. (2005) 11:322–9. doi: 10.5551/jat.11.322
153. Parnell LD, Ordovas JM, Lai CQ. Environmental and epigenetic regulation of postprandial lipemia. Curr Opin Lipidol. (2018) 29:30–5. doi: 10.1097/MOL.0000000000000469
154. Bozzetto L, Della Pepa G, Vetrani C, Rivellese AA. Dietary impact on postprandial lipemia. Front Endocrinol. (2020) 11:337–50. doi: 10.3389/fendo.2020.00337
155. Leon-Acuña A, Torres-Peña JD, Alcala-Diaz JF, Vals-Delgado C, Roncero-Ramos I, Yubero-Serrano E, et al. Lifestyle factors modulate postprandial hypertriglyceridemia: from the CORDIOPREV study. Atherosclerosis. (2019) 290:118–24. doi: 10.1016/j.atherosclerosis.2019.09.025
156. Bohl M, Bjornshave A, Rasmussen KV, Schioldan AG, Amer B, Larsen MK, et al. Dairy proteins, dairy lipids, and postprandial lipemia in persons with abdominal obesity (DairyHealth): a 12-wk, randomized, parallel-controlled, double-blinded, diet intervention study. Am J Clin Nutr. (2015) 101:870–8. doi: 10.3945/ajcn.114.097923
157. Caesar R, Fak F, Backhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. (2010) 268:320–8. doi: 10.1111/j.1365-2796.2010.02270.x
Keywords: postprandial lipemia, triglyceride, atherosclerosis, endothelial dysfunction, oxidative stress, inflammation
Citation: Zhao Y, Liu L, Yang S, Liu G, Pan L, Gu C, Wang Y, Li D, Zhao R and Wu M (2021) Mechanisms of Atherosclerosis Induced by Postprandial Lipemia. Front. Cardiovasc. Med. 8:636947. doi: 10.3389/fcvm.2021.636947
Received: 22 December 2020; Accepted: 05 March 2021;
Published: 29 April 2021.
Edited by:
Johannes A. Schmid, Medical University of Vienna, AustriaReviewed by:
Kazufumi Nakamura, Okayama University, JapanCopyright © 2021 Zhao, Liu, Yang, Liu, Pan, Gu, Wang, Li, Zhao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Wu, d3VtaW4xOTc2MjAwMEAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.