
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 02 February 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.636073
This article is part of the Research Topic What do we know about COVID-19 implications for cardiovascular disease? View all 109 articles
 Ehud Chorin
Ehud Chorin Matthew Dai
Matthew Dai Edward Kogan
Edward Kogan Lalit Wadhwani
Lalit Wadhwani Eric Shulman
Eric Shulman Charles Nadeau-Routhier
Charles Nadeau-Routhier Robert Knotts
Robert Knotts Roi Bar-Cohen
Roi Bar-Cohen Chirag Barbhaiya
Chirag Barbhaiya Anthony Aizer
Anthony Aizer Douglas Holmes
Douglas Holmes Scott Bernstein
Scott Bernstein Michael Spinelli
Michael Spinelli David Park
David Park Larry Chinitz
Larry Chinitz Lior Jankelson*
Lior Jankelson*Background: The COVID-19 pandemic has resulted in worldwide morbidity at unprecedented scale. Troponin elevation is a frequent laboratory finding in hospitalized patients with the disease, and may reflect direct vascular injury or non-specific supply-demand imbalance. In this work, we assessed the correlation between different ranges of Troponin elevation, Electrocardiographic (ECG) abnormalities, and mortality.
Methods: We retrospectively studied 204 consecutive patients hospitalized at NYU Langone Health with COVID-19. Serial ECG tracings were evaluated in conjunction with laboratory data including Troponin. Mortality was analyzed in respect to the degree of Troponin elevation and the presence of ECG changes including ST elevation, ST depression or T wave inversion.
Results: Mortality increased in parallel with increase in Troponin elevation groups and reached 60% when Troponin was >1 ng/ml. In patients with mild Troponin rise (0.05–1.00 ng/ml) the presence of ECG abnormality and particularly T wave inversions resulted in significantly greater mortality.
Conclusion: ECG repolarization abnormalities may represent a marker of clinical severity in patients with mild elevation in Troponin values. This finding can be used to enhance risk stratification in patients hospitalized with COVID-19.
Coronavirus Disease (COVID19) pandemic, induced by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is reaching now historical magnitude as one of the deadliest outbreaks in modern history (1). As of December 30 2020, over 80 million individuals were reported to be infected by SARS-CoV-2, with more than 1.8 million deaths (2). Recent reports (3, 4) revealed that cardiac complications are common (≈20–25%) in COVID19 infection and are associated with increased mortality. However, in those reports, “cardiac complications” were defined according to clinical and laboratory parameters (troponin levels) without systematic electrocardiographic (ECG) evaluation. It is unknown if elevations in troponin levels are reflective of a primary myocardial infarction, supply-demand inequity, or non-ischemic direct myocardial injury. The ECG is an attractive diagnostic tool as it is widely available and can be rapidly performed without inducing significant exposure of caregivers to SARS-CoV-2. ECG has been demonstrated to aid with prognostication in population-based studies (5, 6) and thus offers a particularly appealing modality during the current pandemic. We thus sought to determine whether findings on the first presenting ECG provide prognostic information and provide insights on myocardial injury. We reviewed ECGs of consecutive patients with COVID19 infection requiring hospitalization. We examined our findings stratified by troponin levels and clinical condition.
This is a retrospective study performed at NYU Langone Medical Center, New York, USA. We included 204 consecutive adult patients hospitalized at NYU Langone Medical Center with COVID19 disease. Medical records were reviewed to obtain baseline characteristics, laboratory data, and ECGs. Troponin I concentrations were assessed via the Abbott Architect method (Abbott, Abbott Park, Illinois) wherein the 99th percentile for a normal population is 0.05 ng/mL and the maximal Troponin level was recorded. Descriptive analyses were performed by troponin levels stratified into normal (0.00–0.05 ng/mL), mildly elevated (0.05–1 ng/mL), and significantly elevated (>1 ng/mL). The first, presenting ECGs were reviewed and interpreted by five senior cardiologists who were blinded to the clinical status of the patients. Data reviewed from each ECG included heart rate, rhythm categorized as normal sinus rhythm or atrial fibrillation/flutter (AF), atrioventricular block (AVB), right bundle branch block (RBBB), left bundle branch block (LBBB), a non-specific intraventricular conduction block (QRS duration >120 ms), the presence of ST segment or T-wave changes (localized ST elevation, localized T-wave inversion, or other non-specific repolarization abnormalities). The closing date of follow-up was April 15th 2020. Collected data on the closing date included arrhythmic events and mortality. The study was reviewed and approved by the NYU Institutional Review Board and Quality Improvement initiative in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, with a waiver of informed consent.
Statistical analysis was performed using IBM SPSS Statistics 26, and figures were constructed using GraphPad Prism 8. Continuous variables are expressed as mean ± standard deviation or median (25th−75th percentile), and categorical variables are expressed as count (percentages). Normality of data samples was assessed using Shapiro-Wilk test. Two sample hypothesis testing for continuous variables was performed using Student's t-test if samples had normal distributions and Mann-Whitney U test if samples did not have normal distributions. Hypothesis testing for categorical variables was performed using Fisher's exact test. Significance testing for Kaplan-Meier curves was performed using log-rank test. For predictors of mortality, univariate analysis was performed using Cox proportional hazards regression, and significant univariate predictors were included in the multivariate analysis.
We included 204 patients in our cohort with a mean follow up time of 24.2 ± 7.4 days. The clinical and epidemiological characteristics stratified by ECG abnormalities are presented in Table 1. The mean age was 64 ± 13 years and 76% were male. Comorbidities were common: 30% of patients had diabetes mellitus, 56% had hypertension, 12% had coronary artery disease, 3% had heart failure, and 6% had chronic obstructive pulmonary disease (COPD). Baseline electrocardiographic characteristics revealed mean HR of 89 ± 16 bpm and mean Bazett-corrected QT interval of 444 ± 26 ms. The vast majority were in normal sinus rhythm (95%), while 5% of patients had AF. Atrioventricular block was rare: 9 (4%) patients had a first degree AV block and no patients had second or third degree AV block. Abnormal intraventricular conduction was found in 11% (with RBBB in 8%, LBBB in 3%). Repolarization abnormalities (ST elevation, ST depression, or T wave inversion) were common (36 patients, 17.6%): one patient (0.5%) had localized ST elevation, 12 (5.9%) had ST depression, and 28 (13.7%) had localized T-wave inversion. Patients with repolarization abnormalities demonstrated higher troponin levels and a trend toward higher mortality (Table 1). One patient presented with a fever of 103.1 F which unmasked a previously unknown type I Brugada pattern (Figure 1). Fifty (25%) patients died of respiratory or multi-organ failure. In univariate and multivariate Cox regression analyses, clinical predictors of death included age and elevated Troponin (Table 2), but did not include gender, race or cardiovascular comorbidities (CAD, CHF, HTN). The mortality rate increased with incrementally higher troponin group: 14/120 [11.7%] for patients with negative troponin, 24/64 [37.5%] for patients with mildly elevated troponin, and 12/20 [60%], for patients with significantly elevated troponin (p < 0.01; Figure 2). The presence of an abnormal ECG finding resulted in significantly lower survival in the intermediate Troponin elevation group (0.05–1 ng/ml) but not in the low (<0.05 ng/ml) or high (> 1 ng/ml) Troponin elevation groups (Figure 3). In multivariate regression analysis, T wave inversion but not ST depression remained a significant predictor of mortality (HR 2.71, 95% CI 1.01–7.25, p = 0.04) in the intermediate Troponin group.
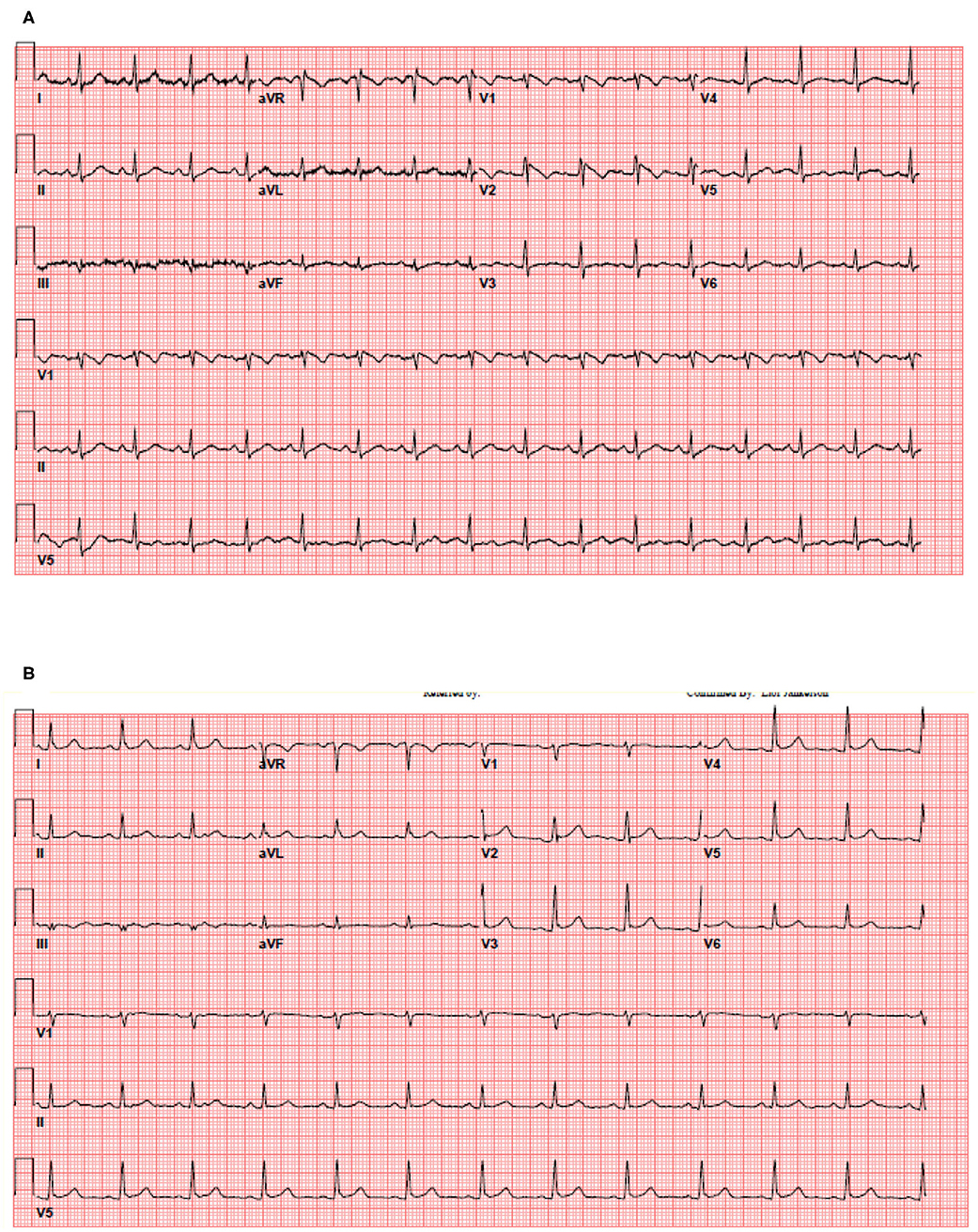
Figure 1. Thirty five year old female patient without significant medical history presented with a fever of 103.1 F. (A) The patient's initial 12-lead electrocardiogram in the emergency department. (B) The patient's repeat 12-lead electrocardiogram with resolution of fever.
Multiple mechanisms have been shown to explain the frequent COVID-19 induced cardiovascular injury. These include direct injury to the myocardium induced by a cytokine storm resulting from a hyperinflammatory state, microvascular damage resulting from abnormal activation of the coagulation cascade including disseminated intravascular coagulation and thrombosis, supply-demand mismatch resulting from respiratory induced tissue hypoxia in conjunction with increase in metabolic demand of infection and inflammation, and myocardial injury by direct entry of SARS-CoV-2 into cardiomyocytes expressing the ACE2 receptor (7–9). In this retrospective cohort study we further assess the interaction of ECG abnormalities and Troponin elevation. We demonstrate that (1) myocardial injury defined by elevated Troponin is indeed prevalent in patients hospitalized with COVID-19 but is more often mild, associated with low-level elevation in troponin concentration. (2) more significant myocardial injury, as evident by increased Troponin level may be associated with higher risk of mortality. (3) In the group of patients with mild Troponin elevation (0.05–1 ng/ml), ECG abnormalities, and particularly T wave inversions are associated with significantly increased mortality. Consistent with our findings, a recent study had demonstrated that T wave inversion is highly frequent finding in patients with COVID-19, conferring increased risk for mortality and particularly when accompanied by Troponin elevation (10).
Though troponin elevation above the 99th percentile of the upper reference limit is considered the central marker of “myocardial injury,” mild elevation between 0.05 and 1 is often non-specific and associated with non-vascular etiologies such as strain, myocyte necrosis and increased cell membrane permeability (11–13). Indeed, mild Troponin elevation was a frequent finding in our cohort, present in 31% of patients with COVID-19. In this regard, our data suggests that assessment for the presence of ECG abnormalities can be used to enhance inpatient risk stratification in those patients with mild Troponin elevation, with potentially intensification of monitoring and therapy. Finally, as persistent fever is a frequent clinical feature of COVID-19, as well as potential side effect of the novel vaccines, caregivers should be familiar with the phenomena of fever induced Brugada pattern and not mistake it for ST elevation myocardial infarction. For patients who present with transient, fever induced Brugada pattern, elective challenge with sodium channel blocking agent (Procainamide, Flecainide, Ajmalin) after resolution of the acute illness can establish the diagnosis of Brugada.
Our study has several limitations. This is an observational, retrospective study. Because of its retrospective nature, the study is subject to selection bias, and its results imply association, not cause and effect. Relatively short follow-up time after was available. The study was not aimed at providing mechanistic insight for the cause of repolarization changes and Troponin elevation. We did not asses structural information from echo due to limited number of tests performed. However, this study was directed at assessing the presenting ECG as a readily available tool for risk stratification in combination with Troponin, a simple blood test.
The data analyzed in this study is subject to the following licenses/restrictions: Data sharing will be considered pending request. Requests to access these datasets should be directed to bGlvci5qYW5rZWxzb25Abnl1bWMub3Jn.
The studies involving human participants were reviewed and approved by Quality Control Act With Accordance to the NYU Langone Health IRB. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LJ and EC: concept, data collection, and manuscript. MD and EK: statistics. LW, ES, CN-R, RK, and RB-C: data collection. CB, AA, DH, SB, MS, DP, and LC: manuscript writing and review. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ECG, Electrocardiography; COPD, Chronic obstructive pulmonary disease; CAD, Coronary artery disease; CHF, Congestive heart failure; CKD, Chronic kidney disease; HTN, Hypertension; DM, Diabetes mellitus; LFTs, Liver function tests.
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. WHO. Coronavirus Disease (COVID-19) Pandemic. Geneva: World Health Organization (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
4. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:811–18. doi: 10.1001/jamacardio.2020.1017
5. Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in post-menopausal women: the Women's Health Initiative. Circulation. (2006) 113:481–9. doi: 10.1161/CIRCULATIONAHA.105.537415
6. Daviglus ML, Liao Y, Greenland P, Dyer AR, Liu K, Xie X, et al. Association of nonspecific minor ST-T abnormalities with cardiovascular mortality: the Chicago Western electric study. JAMA. (1999) 282:530–6. doi: 10.1001/jama.281.6.530
7. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. (2020) 140:1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941
8. Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. (2020) 323:1769–1770. doi: 10.1001/jama.2020.4812
9. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiology. (2020) 5:831–40. doi: 10.1001/jamacardio.2020.1286
10. Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. (2006) 92:987–93. doi: 10.1136/hrt.2005.071282
11. Romero J, Alviz I, Parides M, Diaz JC, Briceno D, Gabr M, et al. T-wave inversion as a manifestation of COVID-19 infection: a case series. J Interv Card Electrophysiol. (2020) 59:485–93. doi: 10.1007/s10840-020-00896-7
12. Januzzi JL Jr, McCarthy CP. Trivializing an elevated troponin: adding insult to injury? J Am Coll Cardiol. (2019) 73:10–2. doi: 10.1016/j.jacc.2018.10.042
Keywords: predictors, mortality, troponin, COVID−19, ECG
Citation: Chorin E, Dai M, Kogan E, Wadhwani L, Shulman E, Nadeau-Routhier C, Knotts R, Bar-Cohen R, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Spinelli M, Park D, Chinitz L and Jankelson L (2021) Electrocardiographic Risk Stratification in COVID-19 Patients. Front. Cardiovasc. Med. 8:636073. doi: 10.3389/fcvm.2021.636073
Received: 30 November 2020; Accepted: 14 January 2021;
Published: 02 February 2021.
Edited by:
Andrew F. James, University of Bristol, United KingdomReviewed by:
Richard David Walton, Université de Bordeaux, FranceCopyright © 2021 Chorin, Dai, Kogan, Wadhwani, Shulman, Nadeau-Routhier, Knotts, Bar-Cohen, Barbhaiya, Aizer, Holmes, Bernstein, Spinelli, Park, Chinitz and Jankelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lior Jankelson, bGlvci5qYW5rZWxzb25Abnl1bWMub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.