
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 13 May 2021
Sec. Heart Failure and Transplantation
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.632318
Background: Anemia is a commonly occurring comorbidity in patients with heart failure (HF). Although there are a few reports of a higher prevalence of mortality and hospitalization-related outcomes due to accompanying anemia, other studies suggest that anemia does not have an adverse impact on the prognostic outcomes of HF. Two meta-analyses in the past decade had reported the adverse impact of anemia on both mortality and hospitalization- related outcomes. However, only one of these studies had evaluated the outcome while using multivariable adjusted hazard ratios. Moreover, several studies since then reported the prognostic influence of anemia in HF. In this present study, we evaluate the prognostic impact of anemia on mortality and hospitalization outcomes in patients with HF.
Methods: We carried out a systematic search of the academic literature in the scientific databases EMBASE, CENTRAL, Scopus, PubMed, Cochrane, ISI Web of Science, clinicaltrial.gov, and MEDLINE based on the PRISMA guidelines. Meta-analysis was then performed to evaluate the effect (presented as risk ratio) of anemia on the overall mortality and hospitalization outcome in patients with HF.
Results: Out of 1,397 studies, 11 eligible studies were included with a total of 53,502 (20,615 Female, 32,887 Male) HF patients (mean age: 71.6 ± 8.3-years, Hemoglobin: 11.9 ± 1.5 g/dL). Among them, 19,794 patients suffered from anemia (Hb: 10.5 ± 1.6), and 33,708 patients did not have anemia (Hb: 13.2 ± 1.7 g/dL). A meta-analysis revealed a high-odds ratio (OR) for the overall mortality in patients with anemia (OR: 1.43, 95% CI: 1.29–1.84). A high-risk ratio was also reported for hospitalization as the outcome in patients with anemia (1.22, 1.0–1.58).
Conclusion: This systematic review and meta-analysis provide evidence of the high risk of mortality and hospitalization-related outcomes in patients with HF and anemia. The study confirms the findings of previously published meta-analyses suggesting anemia as an important and independent risk factor delineating the prognostic outcome of chronic HF.
Heart failure (HF) is one of the most common types of cardiovascular disorders in the world (1, 2). According to the American Heart Association, HF is a complex cardiac syndrome that occurs as a result of a structural or functional dysfunction resulting in an impaired blood ejection across the body (3). Mentz and O'Connor (4) suggested a range of dysfunctions in the endothelial, renal system, and venous pathways that could eventually result in the remodeling of the myocardial structure leading to HF. Based on the statistics from the Global Burden of Disease Studies, HF is categorized as a rising global epidemic that accounts for more than 17 million deaths worldwide each year (5–7).
The patients with HF also exhibit a range of comorbidities that have a substantial impact on the prognostic outcome of the disease (8, 9), and the patient's quality of life (10). Studies have suggested that anemia is one of the most common comorbidities associated with HF (11–13). Recent studies have reported a high prevalence rate of anemia (28 to 58%) in patients with HF (14–16). The inception of anemia in patients with HF is largely associated with multifactorial reasons including deficits in iron metabolism, renal function, bone marrow function, and synthesis/response of erythropoietin (14, 15). Anand (17) suggested that the dysfunctional blunted response of erythropoietin due to the disruption in renal function as a result of HF could be one of the most critical factors contributing to the development of anemia. A substantial reduction in the renal flow of blood, in combination with dysfunctions in changes in the glomerular filtration rate, could influence the PO2 levels which ultimately has a detrimental effect on the structure of erythropoietin eventually leading to anemia.
Evidence also suggests that the adjunct medication-induced iron deficiency could explain the prevalence of anemia in patients with HF (18–20). Sirbu et al. (20) suggested that conventional drugs administered to patients with HF i.e., beta blockers, calcium channel blockers, Angiotensin converting enzyme inhibitor, and aspirin can substantially impair iron metabolism resulting in anemia.
In retrospect, anemia, accompanying HF, has an overall adverse impact on the prognostic outcome of patients. Nagatomo et al. (18) reported that anemia leads to an elevated hyper-hemodynamic in patients with HF. Moreover, increased cardiac workload by the means of stroke volume could also influence the sympathetic nervous activity which eventually can contribute to cardiac remodeling especially in the left ventricle, eventually promoting morbidity and mortality in HF patients (21, 22). Despite having an overall influence on the morbidity and mortality in the adult population, there is still no consensus regarding the prognostic influence of anemia on the mortality and hospitalization-related outcomes in patients with HF. Only a few high-quality studies have addressed the predominant impact of anemia on the prognostic outcome of HF patients in terms of mortality (23–26). However, only a few studies also report that anemia has no prognostic effect on the mortality and hospitalization-related outcomes in patients with HF (27, 28).
To date, only two systematic reviews and meta-analyses have reported the prognostic impact of anemia on the mortality and hospitalization-related outcomes in patients with HF (12, 29). Both of these studies reported a high relative risk ratio of mortality, hospitalization- related outcome in patients with HF, and anemia. However, several high-quality randomized controlled trials (23, 24), observation studies (25, 26), and retrospective cohort studies (30–32), that evaluate the prognostic influence of anemia on mortality and hospitalization outcomes in patients with HF, have been published recently. Therefore, there is a need for an updated systematic review and meta-analysis of the data.
This systematic review and meta-analysis provide an update on the current state of evidence regarding the prognostic influence of anemia in patients with HF, and risk ratios associated with the mortality and hospitalization-related outcomes in HF patients with anemia. The findings from the present study may provide cardiologists with a better understanding of the prognostic influence of anemia in patients with HF.
A systematic review and meta-analysis was carried out based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (33).
Scientific databases (EMBASE, MEDLINE, CENTRAL, PubMed, Cochrane, ISI Web of Science, and clinicaltrial.gov, and Scopus) were searched from inception until September 2020. The following MeSH keywords: “heart failure,” “HF,” “Chronic heart failure,” “anemia,” “Hemoglobin,” and “Hb” were used in different combinations during the search across the academic databases. Manual screening of the bibliography section of the included studies were performed to identify further relevant studies. The inclusion criteria for the study were as follows:
• Studies that evaluated the prevalence of HF patients with anemia.
• Studies performed in the human population.
• Studies that evaluated the influence of anemia on the outcomes of short-, long-term mortality, and hospitalization outcomes.
• Studies published after 2008.
• Studies reporting the outcomes of mortality and hospitalization with the adjusted hazard ratio.
• Randomized-quasi-randomized and controlled clinical trials, observational prospective or retrospective studies.
• Studies published in peer-reviewed scientific journals or presented at conferences.
• English language studies.
The screening of the studies was independently performed by two reviewers. Cases of disagreements were resolved by discussions with a third independent reviewer. The following data were extracted from the included studies: author information, descriptive data, sample distribution, hemoglobin values, events of mortality, and events of hospitalizations. In cases of missing quantitative data, attempts were made to contact the corresponding authors of the publication.
Quality (risk of bias) of each study was assessed by ROBINS-I, a Cochrane risk of bias assessment tool for RCTs randomized controlled trials and non-RCTs randomized controlled trials i.e., ROBINS-I (34, 35) and Cochrane risk of bias assessment tool for the randomized controlled trials (36). The ROBINS-I tool considers inadequate randomization, selective reporting, concealed allocation, classification, and missing data as major threats for instigating bias. The Cochrane tool for assessing randomized controlled trials assesses biases such as concealment of allocation, generation of random sequence, selective reporting, and blinding of outcome. The evaluation of methodological quality was done independently by two reviewers. Cases of disagreements were resolved by discussion with the third reviewer.
Meta-analysis of the included studies was done using the Comprehensive Meta-analysis software version 2.0 (37). The within group meta-analysis was performed using a random effects model (38). We estimated the pooled odds ratio from the included studies. Heterogeneity among the studies was assessed by I2 statistics, with the threshold for interpreting heterogeneity as follows: I2 statistics between 0 and 25%, negligible heterogeneity; 25–75%, moderate heterogeneity; and ≥75%, substantial heterogeneity (39). We distributed the data and performed the analysis for the overall mortality and hospitalization outcome, reporting odds ratio, confidence intervals (CI) of 95% level of significance, and heterogeneity. We also carried out separated sub-group analyses to evaluate the comparative influence of mortality between HF patients with a reduced and preserved ejection fraction. Publication bias was estimated using the Duval and Tweedy's trim and fill procedure (40). This non-parametric method tests for publication bias and adjusts the estimated overall effect size. Briefly an iterative method is used in which some of the extreme values remaining are removed, and a new mean effect is calculated. The alpha level of significance was set at 95%.
A systematic search resulted in 1,370 studies. Additional 27 studies were identified after screening the bibliography of articles (Figure 1). A total of 11 studies fulfilled the inclusion criteria. Three of the included studies were retrospective cohort studies (30–32), three were prospective cohort studies (41–43), two were randomized controlled trials (23, 24), two were observation studies (25, 26), and one was a retrospective observational study (44). The characteristics of the included studies are summarized in Table 1 and the clinical features of the patients are summarized in Table 2.
A total of 57,386 patients with HF were included in the 11 studies, among them, 20,615 were females and 35,944 were males. In the included studies, three studies defined that their patients suffered from acute HF (31, 32, 44) and one had reported that they included cases with both acute and chronic HF (26). The rest of the seven studies did not provide details concerning the nature of the HF cases they included (23–26, 30, 41, 42). Besides, five of the included studies had also reported the ejection fraction values for their sample (23, 30, 32, 41, 42). The average ejection fraction for all the included studies was found to be 47.5 ± 15.2% for the HF patients with anemia, and 44.9 ± 15.6% for the HF patients without anemia. Moreover, the HF was defined by seven of the included studies with echocardiography (23, 24, 30, 32, 41, 42, 44), one study used the NT-proB-type Natriuretic Peptide blood test (25), and three studies did not define their measures for identifying HF (26, 31, 43).
Furthermore, the average age of the patients was 70.5 ± 8.6-years. All the included 11 studies had followed the World Health Organization classification to define anemia i.e., hemoglobin levels <13.0 g/dl for males and <12.0 g/dl for females. Moreover, among the anemic patients, mild anemia was defined as Hb ≥ 9.1 g/dl, moderate anemia was defined as 6.1 g/dl ≤ Hb <9 g/dl, and severe anemia was defined as Hb <6 g/dl. In our study, the average hemoglobin level was 12 ± 1.5 g/dL. In the subgroup distribution for the patients with/without anemia, a total of 21,400 patients have anemia, whereas 35,986 patients do not have anemia. Two studies did not report the gender distribution for this sub-sample of the patients with/without anemia (26, 31). From the studies that reported the gender distribution, a total of 2,931 females and 17,933 males have anemia, whereas 13,690 females and 21,728 males do not have anemia. The average age of the sub sample with and without anemia was 70.4 ± 5.4 and 65.9 ± 5.7-years, respectively. The average hemoglobin levels in the sub sample with and without anemia was reported to be 10.5 ± 1.5 and 13.4 ± 1.6 g/dL, respectively.
The Duval and Tweedy's trim and fill method was used to identify any missing studies based on the random effect models on both sides of the funnel plot. The overall random effect models determined the point estimates and the 95% confidence intervals for all the combined studies as 1.42 (1.23–1.64). The results of the trim and fill method indicated that one study was missing on the left side of the funnel plot. The trim and fill method reported the imputed estimate of 1.37 (1.18–1.60). The publication bias is reported in Figure 2.
We analyzed the risk of bias in the methodology of the non-randomized controlled trials with the ROBINS-I tool (summarized in Table 3). The overall risk was found to be low in the included studies. We observed that the methodological risk of bias was highest for the selection of reported results and deviation from intended intervention. The overall risk of bias is summarized in Figure 3.
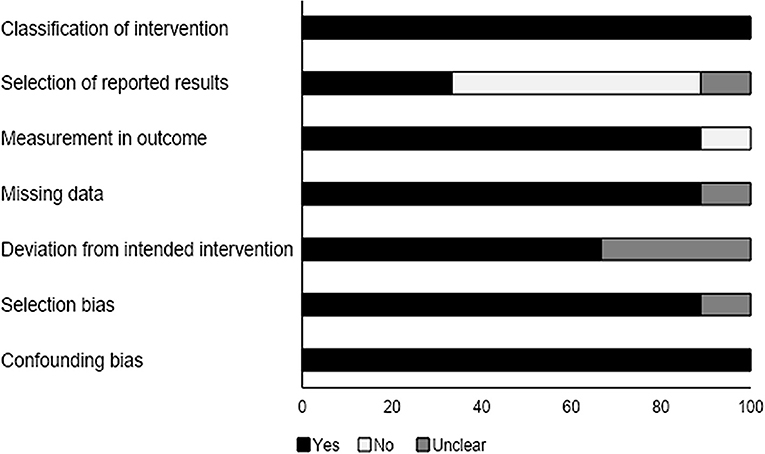
Figure 3. Risk of bias according to the Cochrane risk of bias assessment for the non-randomized controlled trials.
We analyzed the risk of bias for the randomized controlled studies using the Cochrane risk of bias assessment tool. Results are summarized in Table 4. The overall risk was low in the included studies. We observed an unclear risk of bias for the selective reporting section (Figure 4).
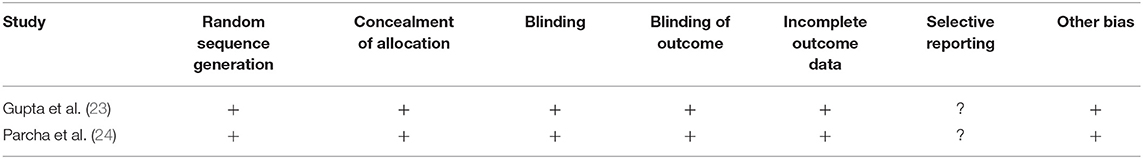
Table 4. Risk of bias within studies according to the Cochrane risk of bias assessment tool for randomized controlled trials.
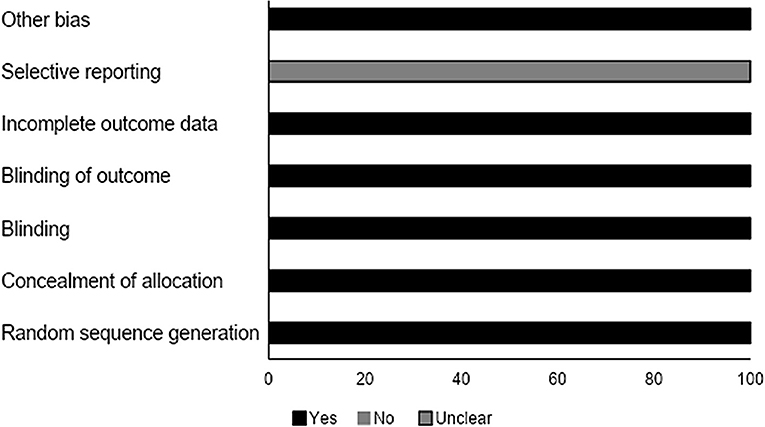
Figure 4. Risk of bias according to the Cochrane risk of bias assessment for the randomized controlled trials.
The overall mortality rate was reported by 10 studies (25, 26, 31, 41–47). The outcome was reported by all the studies for a 1-year duration. The estimated pooled odds ratio was 1.43 (95% CI: 1.25–1.63, p < 0.001) (Figure 5), with moderate heterogeneity (I2: 56.1%).
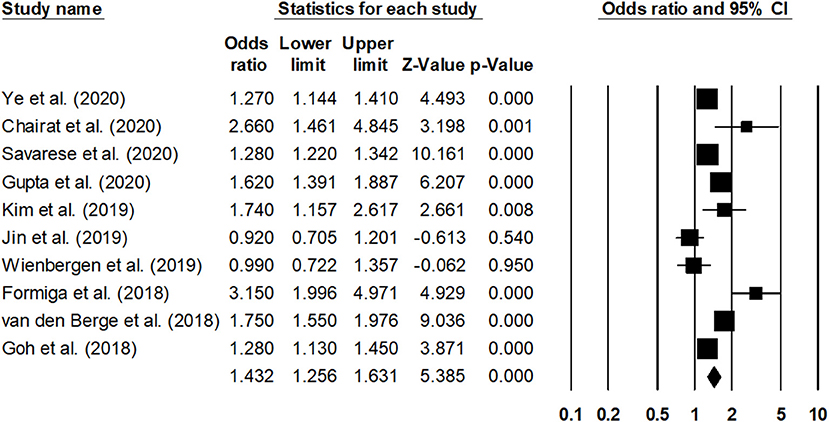
Figure 5. Forest plot for studies evaluating the overall 1-year overall mortality outcome. The adjusted odds ratios are presented as black boxes, whereas 95% confidence intervals are presented as whiskers. A negative odds ratio represents a reduction in the risk of mortality in heart failure patients with anemia, whereas the positive odds ratio represents an increase in the risk of mortality in heart failure patients with anemia.
Sub-group analysis for the overall mortality rate with a reduced ejection fraction was reported by two studies (30, 41). The estimated pooled odds ratio was 1.25 (95% CI: 1.09–1.45, p < 0.01) (Figure 6), with no heterogeneity (I2: 0%). Likewise, sub-group analysis for overall mortality rate with a preserved ejection fraction was reported by four studies (23, 30, 32, 42). The estimated pooled odds ratio was 1.38 (95% CI: 1.06–1.79, p < 0.02) (Figure 7), with moderate heterogeneity (I2: 48.5%).
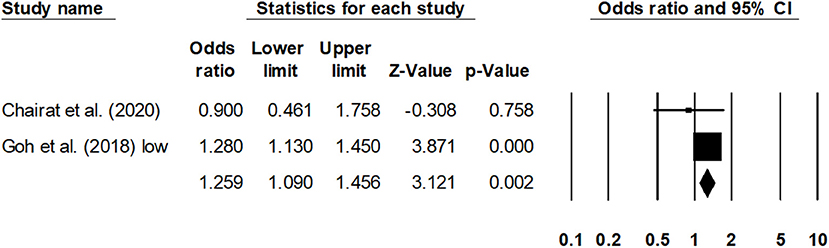
Figure 6. Forest plot for studies evaluating the overall 1-year overall mortality outcome for HF patients with reduced ejection fraction. The adjusted odds ratios are presented as black boxes, whereas 95% confidence intervals are presented as whiskers. A negative odds ratio represents a reduction in the risk of mortality in heart failure patients with anemia, whereas the positive odds ratio represents an increase in the risk of mortality in heart failure patients with anemia.
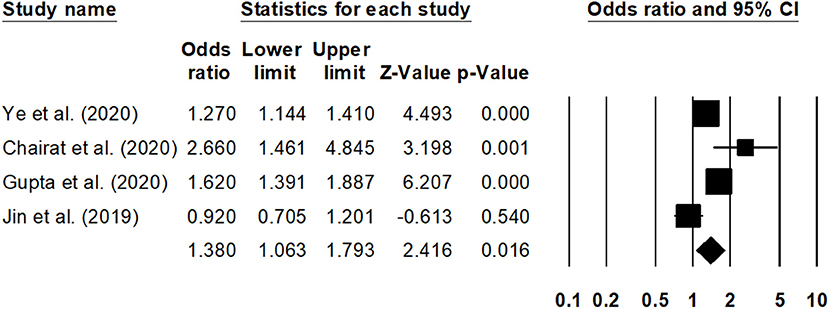
Figure 7. Forest plot for studies evaluating the overall 1-year overall mortality outcome for HF patients with preserved ejection fraction. The adjusted odds ratios are presented as black boxes, whereas 95% confidence intervals are presented as whiskers. A negative odds ratio represents a reduction in the risk of mortality in heart failure patients with anemia, whereas the positive odds ratio represents an increase in the risk of mortality in heart failure patients with anemia.
The overall hospitalization outcome was reported by four studies (23, 24, 42, 44), with two studies reporting the outcome at a 1-year follow up (23, 42), one at a 1.5-year follow up (44), and one after 24-weeks (24). The pooled rate ratio was 1.22 (95% CI: 1.0–1.58, p: 0.04) (Figure 8), with no heterogeneity (I2: 0%).
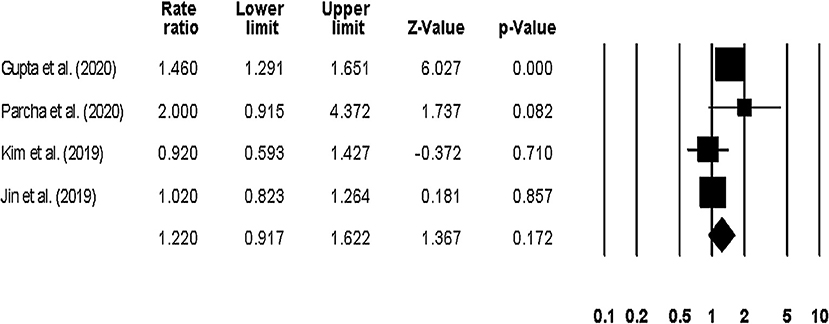
Figure 8. Forest plot for studies evaluating the overall hospitalization outcome. The adjusted odds ratios are presented as black boxes, whereas 95% confidence intervals are presented as whiskers. A negative odds ratio represents a reduction in the risk of mortality in heart failure patients with anemia, whereas the positive odds ratio represents an increase in the risk of mortality in heart failure patients with anemia.
Sub-group analysis on only long-term (1–1.5-years) hospitalization outcomes showed a pooled risk ratio of 1.15 (95% CI: 0.84–1.56, p: 0.36) (Figure 9), with no heterogeneity (I2: 0%).
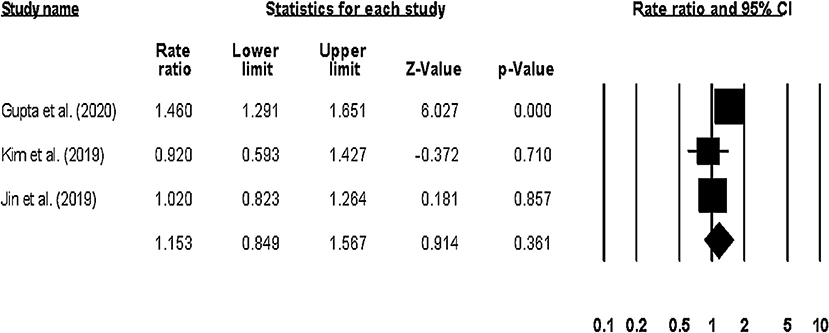
Figure 9. Forest plot for studies evaluating the overall long-term hospitalization outcome. The adjusted odds ratios are presented as black boxes whereas 95% confidence intervals are presented as whiskers. A negative odds ratio represents a reduction in the risk of mortality in heart failure patients with anemia, whereas the positive odds ratio represents an increase in the risk of mortality in heart failure patients with anemia.
This systematic review and meta-analysis provide a comprehensive state of evidence regarding the prognostic influence of anemia in patients with HF. We observed high overall risk ratios associated with the overall mortality and hospitalization-related outcomes in HF patients with anemia.
The management of HF is one of the most challenging aspects for cardiologists worldwide due to its atypical pathophysiological mechanism, coexisting morbidities, and manifestations (48–50). Presence of comorbidities, such as anemia, makes it even more challenging for clinicians to evaluate the cardiovascular condition of the patient, thus potentially contributing to an increased morbidity and mortality-related outcomes (12, 51, 52). Recent literature has increasingly recognized the rising prevalence of anemia in patients with HF (53–55). Several mechanisms are linked with the aggravation of anemia in patients with HF (14, 17–19). The study by Anand and Gupta (14) suggests that the absolute/functional nutritional deficiency of iron, response/synthesis of erythropoietin, and adverse effects of medications are the most significant factors aggravating the onset of anemia. Moreover, the increase in pro-inflammatory markers in patients with HF could also contribute to anemia by activating the GATA-binding proteins and nuclear factor kappa light chain enhancer, eventually inhibiting the production of renal erythropoietin or resulting in a blunted-erythropoietin response (56–58). In addition to these inflammatory changes, deficiency and/or mutation of genes that regulate hematopoiesis have also been reported to eventually worsen cardiac remodeling i.e., hypertrophy in the left ventricle (59, 60). These changes eventually promote the increased risks of morbidities that can lead to re-hospitalizations and eventually to an increased mortality (12).
In our present review, we analyzed a range of studies that reported a predominant influence of anemia on all-cause 1-year mortality related outcomes in patients with HF. Ye et al. (32) evaluated the overall all-cause mortality in 3,279 patients and reported a significant (p: 0.01) increase in the mortality-related events in HF patients with anemia as compared to patients without anemia. This relationship between anemia and mortality was proportional to the severity of the anemia i.e., the more severe the anemia, the higher the mortality rates. Similarly, Jin et al. (42) reported higher mortality related outcomes for the anemic group (14.6%) as compared to the non-anemic group (8.7%) in a Chinese cohort of 1,604 patients. The authors further showed that the mortality rates in anemic patients further increased in patients with renal dysfunction (21%). This increase in mortality-related events in HF patients with anemia may be due to several mechanisms, such as enhancement in oxidative stress, fluid-retention, cardiac-hypoxia, sympathetic nervous activity, and renin-angiotensin-aldosterone activity (61, 62). Sanderson (63) further suggested that a decrease in the neuro-hormonal activation as a result of a reduced vascular-resistance and decreased blood viscosity could further reduce the glomerular filtration rate and enhance the overall retention of water and salt content leading to an expanded extracellular plasma volume, eventually increasing the morbidity and mortality related outcomes in patients with HF. In our present meta-analysis, we confirm these findings and report a high-odds ratio (OR: 1.43, 95% CI: 1.25–1.63) associated with the overall mortality-related factors in patients with HF. Besides, in a further subgroup comparative analysis of mortality between HF patients with a reduced and preserved ejection fraction, we observed differences in terms of mortality between the two groups.
We also assessed the risk of hospitalization-related outcomes associated with anemia in patients with HF. We observed that all the studies included in our systematic review had reported an overall increase in the hospitalization-related outcomes for HF patients with anemia. Kim et al. (44) showed a high rate of re-hospitalization in anemic patients (Hazard ratio: 0.92, 0.59–1.42) as compared to non-anemic patients with HF. The authors also suggest that the anemia diagnosis at discharge could serve as a predictor not only for the morbidity-but also for the mortality-related outcomes, thus emphasizing the importance of managing anemia during hospitalization to reduce the adverse events in patients with HF. RELAX randomized controlled trial by Parcha et al. (24) reported a higher incidence of hospitalization in anemic patients primarily due to cardiac or renal problems even at the end of a 24-week period (24). The authors suggest that the timely recognition and management of anemia could be crucial for stemming the deterioration of the symptomatic manifestations in patients with HF.
Our meta-analysis is in agreement with these reports. We showed high risk ratios of re-hospitalizations in anemic patients with HF (1.22, 1.27–1.78). Subgroup analysis of long term (>1-year) hospitalization-related outcome also revealed a high-risk hospitalization ratio in anemic patients with HF (1.15, 0.84–1.56).
Our systematic review and meta-analysis have a few limitations. First and foremost, this systematic review and meta-analysis was not registered in a review repository such as PROSPERO. Although the lack of registration might raise concerns regarding the validity of this present review (64), we would like to assure our reader that we made attempts to register our review at these repositories, but because of the current pandemic crisis, the waiting time at the PROSPERO repository was >1-year. Secondly, we did not evaluate the gender differences in terms of the risks of hospitalization and mortality associated with anemia in patients with heart failure. We understand the importance of evaluating the gender differences in terms of the prognostic significance it possesses for clinicians. Therefore, we strongly recommend future studies to address this limitation by conducting gender sub-group analysis to outline the gender differences in terms of the prognostic outcome of anemia in HF. Thirdly, our understanding of the short- and long-term hospitalization related outcomes in HF patients with anemia may be biased due to an insufficient data in the eligible included studies. In our included pool of four studies that had reported the hospitalization related outcomes in HF patients with anemia, only one study had evaluated the outcome during a 24-week follow-up (24), whereas the other three studies had evaluated the long-term (1–1.5-years) outcomes (23, 42, 44). Therefore, the outcomes of risk ratio overall hospitalization could be biased, and we would suggest our reader to evaluate this statistic with caution. Additionally, short- and long-term prognostic influences of anemia on mortality were not evaluated in this review and meta-analysis, since all the included studies had reported a 1-year mortality outcome. Previous studies have stressed upon the high risks of mortality-related outcomes especially during the short-term periods (43). Therefore, future studies are needed to address these limitations by conducting more high-quality studies and sharing their descriptive data in open access data repositories. The evaluation of these outcomes would be highly beneficial for medical practitioners to predict the prognostic outcomes regarding mortality and re-hospitalization in HF patients with anemia. Lastly, we would like to mention that usually an important limitation in the literature involving patients with heart failure is that the researchers do not report the definitions of anemia they adopted. In this present study, however, we found that all the included studies had adopted the World Health Organization's classification to define anemia i.e., Hb < 13.0 g/dl for males and Hb < 12.0 g/dl for females.
In conclusion, in this present systematic review and meta-analysis, we provide a confirmatory evidence regarding the high prognostic influence of anemia in patients with HF. We provide statistical evidence of the high mortality and hospitalization-related risks associated with anemia in patients with HF. The findings from the present study can further contribute in developing clinical awareness of the widespread prevalence of anemia in patients with chronic HF. This may help cardiologists to develop the best practice guidelines for determining the appropriate treatment approach for controlling adverse outcomes in HF patients with anemia.
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding author/s.
HX conceived and designed the study and was involved in the writing of the manuscript. HS, WC, and QL collected the data and performed the literature search. All authors have read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Cardiovascular Diseases (CVDs). Available online at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed November 14, 2020).
2. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. (2016) 13:368–78. doi: 10.1038/nrcardio.2016.25
3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 62:e147–239. doi: 10.1161/CIR.0b013e31829e8776
4. Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. (2016) 13:28–35. doi: 10.1038/nrcardio.2015.134
5. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2015) 385:117–71. doi: 10.1016/S0140-6736(14)61682-2
6. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. (2017) 3:7–11. doi: 10.15420/cfr.2016:25:2
7. Zannad F. Rising incidence of heart failure demands action. Lancet. (2018) 391:518–9. doi: 10.1016/S0140-6736(17)32873-8
8. Sharma M, Nayanisri K, Jain R, Ranjan R. Predictive Value of Fasting Plasma Glucose on First Antenatal Visit before 20 Weeks of Gestation to Diagnose Gestational Diabetes Mellitus. JCDR (2018). Available online at: http://jcdr.net/article_fulltext.asp?issn = 0973-709x&year = 2018&volume = 12&issue = 2&page = QC01&issn = 0973-709x&id = 11177 (accessed March 11, 2020).
9. Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. (2011) 124:136–43. doi: 10.1016/j.amjmed.2010.08.017
10. Macabasco-O'Connell A, DeWalt DA, Broucksou KA, Hawk V, Baker DW, Schillinger D, et al. Relationship between literacy, knowledge, self-care behaviors, and heart failure-related quality of life among patients with heart failure. J Gen Intern Med. (2011) 26:979–86. doi: 10.1007/s11606-011-1668-y
11. Grote Beverborg N, van Veldhuisen DJ, van der Meer P. Anemia in heart failure: still relevant? JACC Heart Fail. (2018) 6:201–8. doi: 10.1016/j.jchf.2017.08.023
12. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. (2008) 52:818–27. doi: 10.1016/j.jacc.2008.04.061
13. Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, et al. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. (2006) 48:2485–9. doi: 10.1016/j.jacc.2006.08.034
14. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. (2018) 138:80–98. doi: 10.1161/CIRCULATIONAHA.118.030099
15. Cleland JGF, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, et al. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol. (2016) 1:539–47. doi: 10.1001/jamacardio.2016.1161
16. von Haehling S, Gremmler U, Krumm M, Mibach F, Schön N, Taggeselle J, et al. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP Registry. Clin Res Cardiol. (2017) 106:436–43. doi: 10.1007/s00392-016-1073-y
17. Anand IS. Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol. (2008) 52:501–11. doi: 10.1016/j.jacc.2008.04.044
18. Nagatomo Y, Yoshikawa T, Okamoto H, Kitabatake A, Hori M, J-CHF Investigators. Anemia is associated with blunted response to β-blocker therapy using carvedilol - insights from Japanese Chronic Heart Failure (J-CHF) study. Circ J. (2018) 82:691–8. doi: 10.1253/circj.CJ-17-0442
19. Okonko DO, Anker SD. Anemia in chronic heart failure: pathogenetic mechanisms. J Card Fail. (2004) 10(Suppl. 1):S5–9. doi: 10.1016/j.cardfail.2004.01.004
20. Sirbu O, Sorodoc V, Jaba IM, Floria M, Stoica A, Profire L, et al. The influence of cardiovascular medications on iron metabolism in patients with heart failure. Medicina. (2019) 55:329. doi: 10.3390/medicina55070329
21. Vaisman N, Silverberg DS, Wexler D, Niv E, Blum M, Keren G, et al. Correction of anemia in patients with congestive heart failure increases resting energy expenditure. Clin Nutr. (2004) 23:355–61. doi: 10.1016/j.clnu.2003.08.005
22. Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J. (1972) 83:415–26. doi: 10.1016/0002-8703(72)90445-0
23. Gupta K, Kalra R, Rajapreyar I, Joly JM, Pate M, Cribbs MG, et al. Anemia, mortality, and hospitalizations in heart failure with a preserved ejection fraction (from the TOPCAT Trial). Am J Cardiol. (2020) 125:1347–54. doi: 10.1016/j.amjcard.2020.01.046
24. Parcha V, Patel N, Kalra R, Bhargava A, Prabhu SD, Arora G, et al. Clinical, demographic, and imaging correlates of anemia in heart failure with preserved ejection fraction (from the RELAX Trial). Am J Cardiol. (2020) 125:1870–8. doi: 10.1016/j.amjcard.2020.03.006
25. Savarese G, Jonsson Å, Hallberg A-C, Dahlström U, Edner M, Lund LH. Prevalence of, associations with, and prognostic role of anemia in heart failure across the ejection fraction spectrum. Int J Cardiol. (2020) 298:59–65. doi: 10.1016/j.ijcard.2019.08.049
26. Wienbergen H, Pfister O, Hochadel M, Fach A, Backhaus T, Bruder O, et al. Long-term effects of iron deficiency in patients with heart failure with or without anemia: the RAID-HF follow-up study. Clin Res Cardiol. (2019) 108:93–100. doi: 10.1007/s00392-018-1327-y
27. Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, et al. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med. (2005) 165:2237–44. doi: 10.1001/archinte.165.19.2237
28. Tymińska A, Kapłon-Cieślicka A, Ozierański K, Peller M, Balsam P, Marchel M, et al. Anemia at hospital admission and its relation to outcomes in patients with heart failure (from the Polish Cohort of 2 European Society of Cardiology Heart Failure Registries). Am J Cardiol. (2017) 119:2021–9. doi: 10.1016/j.amjcard.2017.03.035
29. He S-W, Wang L-X. The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review. Congest Heart Fail. (2009) 15:123–30. doi: 10.1111/j.1751-7133.2008.00030.x
30. Chairat K, Rattanavipanon W, Tanyasaensook K, Chindavijak B, Chulavatnatol S, Nathisuwan S. Relationship of anemia and clinical outcome in heart failure patients with preserved versus reduced ejection fraction in a rural area of Thailand. Int J Cardiol Heart Vasc. (2020) 30:100597. doi: 10.1016/j.ijcha.2020.100597
31. Formiga F, Chivite D, Ariza-Solé A, Corbella X. Mild anemia and mortality in very old adults hospitalized for acute heart failure. J Am Geriatr Soc. (2018) 66:2432–4. doi: 10.1111/jgs.15562
32. Ye S-D, Wang S-J, Wang G-G, Li L, Huang Z-W, Qin J, et al. Association between anemia and outcome in patients hospitalized for acute heart failure syndromes: findings from Beijing Acute Heart Failure Registry (Beijing AHF Registry). Intern Emerg Med. (2020) 16:183–92. doi: 10.1007/s11739-020-02343-x
33. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
34. Jørgensen L, Paludan-Müller AS, Laursen DRT, Savović J, Boutron I, Sterne JAC, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. (2016) 5:80. doi: 10.1186/s13643-016-0259-8
35. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
36. Savović J, Weeks L, Sterne JAC, Turner L, Altman DG, Moher D, et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. (2014) 3:37. doi: 10.1186/2046-4053-3-37
37. Bax L, Yu L-M, Ikeda N, Moons KG. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. (2007) 7:40. doi: 10.1186/1471-2288-7-40
38. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
39. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
40. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
41. Goh VJ, Tromp J, Teng T-HK, Tay WT, Van Der Meer P, Ling LH, et al. Prevalence, clinical correlates, and outcomes of anaemia in multi-ethnic Asian patients with heart failure with reduced ejection fraction. ESC Heart Fail. (2018) 5:570–8. doi: 10.1002/ehf2.12279
42. Jin X, Cao J, Zhou J, Wang Y, Han X, Song Y, et al. Outcomes of patients with anemia and renal dysfunction in hospitalized heart failure with preserved ejection fraction (from the CN-HF registry). Int J Cardiol Heart Vasc. (2019) 25:100415. doi: 10.1016/j.ijcha.2019.100415
43. van den Berge JC, Constantinescu AA, van Domburg RT, Brankovic M, Deckers JW, Akkerhuis KM. Renal function and anemia in relation to short- and long-term prognosis of patients with acute heart failure in the period 1985-2008: a clinical cohort study. PLoS ONE. (2018) 13:e0201714. doi: 10.1371/journal.pone.0201714
44. Kim MC, Kim KH, Cho JY, Lee KH, Sim DS, Yoon HJ, et al. Pre-discharge anemia as a predictor of adverse clinical outcomes in patients with acute decompensated heart failure. Korean J Intern Med. (2019) 34:549–58. doi: 10.3904/kjim.2017.337
45. Negi PC, Dev M, Paul P, Pal Singh D, Rathoure S, Kumar R, et al. Prevalence, risk factors, and significance of iron deficiency and anemia in nonischemic heart failure patients with reduced ejection fraction from a Himachal Pradesh heart failure registry. Indian Heart J. (2018) 70(Suppl. 3):S182–8. doi: 10.1016/j.ihj.2018.10.032
46. Parrish JB, Weinstock-Guttman B, Smerbeck A, Benedict RHB, Yeh EA. Fatigue and depression in children with demyelinating disorders. J Child Neurol. (2013) 28:713–8. doi: 10.1177/0883073812450750
47. Simone M, Viterbo RG, Margari L, Iaffaldano P. Computer-assisted rehabilitation of attention in pediatric multiple sclerosis and ADHD patients: a pilot trial. BMC Neurol. (2018) 18:82. doi: 10.1186/s12883-018-1087-3
48. Braunwald E. The war against heart failure: the Lancet lecture. Lancet. (2015) 385:812–24. doi: 10.1016/S0140-6736(14)61889-4
49. Gutierrez C, Blanchard DG. Diastolic heart failure: challenges of diagnosis and treatment. Am Fam Phys. (2004) 69:2609–16. Available online at: https://www.aafp.org/afp/2004/0601/p2609.html
50. McLean RC, Jessup M. The challenge of treating heart failure: a diverse disease affecting diverse populations. JAMA. (2013) 310:2033–4. doi: 10.1001/jama.2013.282773
51. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Open Heart. (2019) 6:e001012. doi: 10.1136/openhrt-2019-001012
52. Jacob C, Altevers J, Barck I, Hardt T, Braun S, Greiner W. Retrospective analysis into differences in heart failure patients with and without iron deficiency or anaemia. ESC Heart Fail. (2019) 6:840–55. doi: 10.1002/ehf2.12485
53. Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. (2003) 107:223–5. doi: 10.1161/01.CIR.0000052622.51963.FC
54. Ikama MS, Nsitou BM, Kocko I, Mongo NS, Kimbally-Kaky G, Nkoua JL. Prevalence of anaemia among patients with heart failure at the Brazzaville University Hospital. Cardiovasc J Afr. (2015) 26:140–2. doi: 10.5830/CVJA-2015-021
55. Tang Y-D, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. (2006) 113:2454–61. doi: 10.1161/CIRCULATIONAHA.105.583666
56. Dabek J, Kułach A, Gasior Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): a new potential therapeutic target in atherosclerosis? Pharmacol Rep. (2010) 62:778–83. doi: 10.1016/S1734-1140(10)70338-8
57. Dec GW. Anemia and iron deficiency–new therapeutic targets in heart failure? N Engl J Med. (2009) 361:2475–7. doi: 10.1056/NEJMe0910313
58. van der Meer P, Voors AA, Lipsic E, Smilde TDJ, van Gilst WH, van Veldhuisen DJ. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol. (2004) 44:63–7. doi: 10.1016/j.jacc.2004.03.052
59. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. (2017) 377:111–21. doi: 10.1056/NEJMoa1701719
60. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. (2018) 71:875–86. doi: 10.1016/j.jacc.2017.12.037
61. Silverberg D, Wexler D, Blum M, Wollman Y, Iaina A. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. (2003) 18(Suppl. 8):viii7–12. doi: 10.1093/ndt/gfg1084
62. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. (2010) 121:2592–600. doi: 10.1161/CIRCULATIONAHA.109.886473
63. Sanderson JE. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables and plasma hormones. Br Heart J. (1994) 71:490. doi: 10.1136/hrt.71.5.490-b
Keywords: anemia, hemoglobin, heart failure, mortality, hospitalization
Citation: Xia H, Shen H, Cha W and Lu Q (2021) The Prognostic Significance of Anemia in Patients With Heart Failure: A Meta-Analysis of Studies From the Last Decade. Front. Cardiovasc. Med. 8:632318. doi: 10.3389/fcvm.2021.632318
Received: 23 November 2020; Accepted: 29 March 2021;
Published: 13 May 2021.
Edited by:
Gaetano Ruocco, Regina Montis Regalis Hospital, ItalyReviewed by:
Stefania Paolillo, University of Naples Federico II, ItalyCopyright © 2021 Xia, Shen, Cha and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiaoli Lu, bHFsX2RyQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.