
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 22 February 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.624313
Background: Vascular calcification (VC) is a subclinical manifestation of vascular disease burden among older adults, conferring an elevated mortality risk. Biomarkers capable of detecting and risk-stratifying VC associated with advanced age remains unavailable, impeding our effort to provide optimal care to geriatric patients.
Objectives: In this study, we aimed to investigate whether circulating miR-125b served as a potential indicator for VC in relatively healthy older adults.
Methods: Community-dwelling older adults (age ≥65) were prospectively recruited during 2017, followed by clinical features documentation and VC rating based on aortic arch calcification (AAC) and abdominal aortic calcification (AbAC). Multiple logistic regression was done to evaluate the relationship between circulating miR-125b levels, VC presence and severity, followed by selecting the optimal cutoff point for VC diagnosis.
Results: A total of 343 relatively healthy older adults (median age, 73.8 years; 40% male; 59.8% having AAC) were enrolled, with a median circulating miR-125b level of 0.012 (interquartile range, 0.003–0.037). Those with more severe AAC had progressively decreasing miR-125b levels (p<0.001). Multiple regression analyses showed that having higher miR-125b levels based on the median value were associated with a substantially lower risk of AAC [odds ratio (OR) 0.022, 95% confidence interval (CI) 0.011–0.044] compared to those having lower ones. An optimal cutoff of miR-125b for identifying AAC in older adults was 0.008, with a sensitivity and specificity of 0.86 and 0.80, respectively. Similar findings were obtained when using AbAC as the endpoint.
Conclusions: We found that miR-125b serves as an independent indicator for VC in relatively healthy older adults, and may potentially be linked with VC pathophysiology.
Traditional cardiovascular risk factors include several morbidities [hypertension, diabetes mellitus (DM), and hypercholesterolemia] and a higher age. An increased age per se contributes to unfavorable morphological and functional changes involving cardiovascular tissues including the myocardium and the major vessels (1), culminating in a significantly elevated risk of major adverse cardiac events (MACEs) compared to their younger counterparts (2). Age also plays an integral role in the estimation of 10-year atherosclerotic cardiovascular disease (ASCVD) risk as endorsed by major professional societies (3). Importantly, chronological aging places affected individuals at an increased risk of vascular calcification (VC), which involves the ectopic deposition of calcium apatite in the vascular wall resembling osteogenesis. Furthermore, the presence of VC has been found to accelerate the progress of vascular inflammation as patients get older (4), and the combination of VC and vascular aging/inflammation increases the future risk of cardiovascular mortality. From this perspective, an in-depth understanding of the pathogenesis of aging-related VC and more instrumentally, how to identify those at risk of developing this vascular morbidity assumes importance in this era of population aging.
The pathophysiology of VC comprises of passive calcium deposition surrounding a mineralization core and the active osteoid-like substance secretion from phenotypically switched resident cells within vascular wall. Epigenetic processes, especially non-coding RNAs, have been implicated as vital players during the course of VC, shaping its initiation and propagation process (5). Among the purview of non-coding RNAs, microRNAs (miRNAs) are important members that influence the risk of VC through modulating the osteoblastic differentiation tendency of vascular smooth muscle cells (VSMCs) (5). Specifically, miR-125b has been the prototype with comprehensive evidence supporting its antagonistic effect against VC; Goettsch and colleagues first disclosed that miR-125b repressed VSMC calcification in vitro nearly a decade ago (6). Subsequent studies further unveil the versatile role of miR-125b in vascular pathologies involving endothelial cells, VSMCs, and infiltrating macrophages (7). Judging from the pathophysiological importance of miR-125b in VC and the feasibility of detecting miRNAs in biological fluids, it is tempting to speculate whether miR-125b can assist in diagnosing and risk stratifying VC in the clinical setting. We previously showed that among 223 patients without and with uremic VC, circulating miR-125b inversely correlated with VC severity and its baseline levels could effectively predict the risk of VC worsening in the future (8). Moreover, VC related to chronic kidney disease (CKD) and to aging may share similarities in risk factors and pathologies (9). Consequently, our previous results inspire us to hypothesize that circulating miR-125b levels may exhibit similar associations with the severity of aging-related VC in older adults. We harnessed a prospectively enrolled cohort of community-dwelling older adults to examine this hypothesis.
The study protocol has been approved by the institutional review board of National Taiwan University Hospital (No. 201601091RIND). The protocol adhered to the Declaration of Helsinki, and all participants provided written informed consent.
The study protocol has been published previously (10). In brief, community-dwelling older adults (age ≥65) were prospectively recruited from health examination programs and clinics of National Taiwan University Hospital BeiHu Branch as well as Taipei municipal long-term care service centers during 2017. Exclusion criteria consisted of those who could not communicate in a conscious state. After enrollment, participants were instructed to complete a dedicated questionnaire documenting their sociodemographic profile, self-report comorbidities, and current medication regimens. Following the recording of clinical features, we measured their anthropometric parameters and physical indices [blood pressure (BP) and heart rate (HR)]. Under the fasting status, participants received 10 mL of blood drawing and dipstick urinalysis. We assayed their complete hemogram and serum biochemistry (nutrition, lipid profile, glucose, renal function, and inflammation-related parameters). Participants' estimated glomerular filtration rate (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease (MDRD) formula.
We adopted two approaches for semi-quantitatively examining the presence and the extent of VC, aortic arch calcification (AAC) and abdominal aorta calcification (AbAC), based on our prior work (8, 11, 12) and the existing literature (13, 14). A majority of the participants received posteroanterior chest radiography after documenting their baseline clinical features. These films were identified with results rated semi-quantitatively as having no AAC, and having category 1, 2, and 3 AAC. Some of the participants received lateral lumbar spine radiography, with AbAC rated based on the well-validated Kauppila scores (range 0–24, higher scores meaning more severe calcification) (15, 16). Image interpretation was performed by two researchers (CTC and JWH) with an excellent consistency, while controversies were resolved by another physician.
Part of the collected blood was centrifuged with plasma cryopreserved. Existing reports suggest that circulating miRNAs remain stable in stored sera/plasma without repetitive freeze-thaw cycles (17). First-time thawed sera were subject to cell-free small RNA extraction using the miRNeasy Serum/Plasma kit (QIAGEN, Netherland), which has been credited for enriching low abundant levels of miRNAs especially in biological fluids (18), according to the manufacturer's instruction. The procedure of reverse transcription (RT) and quantitative polymerase chain reaction (qPCR) was briefly described below. A maximal of 2 μg extracted RNA was mixed with miScript Reverse Transcriptase Mix, HiSpec Buffer, and miScript Nucleics Mix (all from miScript PCR System; QIAGEN, Netherland) in a fixed ratio. Mixtures were centrifuged and subsequently incubated at 37°C for 60 min, at 95°C for 5 min to obtain cDNAs. A maximal of 3 ng cDNA per reaction were then diluted in RNase-free water, mixed with miScript Universal Primer, QuantiTect SYBR Green PCR master mix, and the primers of miR-125b (Cat No. MS00006629; QIAGEN, Netherland) or those of Caenorhabditis elegans miR-39. This was followed by a gentle mix of the mixture, which was dispensed into plates and subjected to real-time PCR cycler (7200 HT thermal cycler, Applied Biosystems, Foster City, CA).
Since studies have shown that U6 is not an appropriate control for quantifying circulating miRNAs (19), we used the spiked-in control, Caenorhabditis elegans miR-39, for normalization purpose (18). Results based on this approach reportedly reduce fluctuations in data and permit cross-study comparisons. Data were then averaged with miR-125b levels calculated using the ΔΔCt method (10). If the participants' miR-125b expression levels were undetectable upon PCR cycling despite the presence of measurable Caenorhabditis elegans miR-39, we substituted their miR-125b cycle number with 40, which was the lowest detectable value specified by the cycler, followed by calculation.
We first used the Kolmogorov-Smirnov test to determine whether the collected continuous variables and circulating miR-125b levels were parametric. For parametric and non-parametric ones, we used means ± standard deviations and medians with interquartile ranges for expression, respectively, while for categorical variables, we used numbers with percentages in parentheses for expression. Comparisons between 2 groups of parametric or non-parametric variables were done by the Student's t-test or Mann-Whitney U-test, respectively, while comparisons between categorical ones were made by the chi-square test. We used one-way analysis of variance and Kruskal-Wallis test to compare parametric and non-parametric variables of >2 groups, respectively.
We first compared participants' clinical features and laboratory profiles between those with and without AAC and between those with different AAC severities. We also examined whether circulating miR-125b levels differed depending on VC. This was followed by multiple regression analyses with stepwise backward variable selection, with AAC presence as the dependent variable, incorporating variables with a p < 0.1 in univariate analysis, and miR-125b. MiR-125b levels were accounted for in the regression models in different styles, as a continuous variable or binarily divided based on the mean or median value. We further used the receiver-operating characteristics (ROC) curves to evaluate the performance of each regression model, followed by the calculation of the area under ROC curves (AUROCs). Youden's index was utilized to capture the optimal cutoff of circulating miR-125b to identify AAC, followed by the comparison of clinical features between those with ≥ and < the cutoff value and a repeated regression analysis.
Several sensitivity analyses were planned beforehand. First, we incorporated miR-125b levels in tertiles or quartiles into the multiple regression models with VC status as the dependent variables, incorporating the same set of variables as described above, with AUROC obtained. In addition, another set of multiple logistic regression analyses with AbAC as the dependent variable was performed, incorporating variables with a p < 0.1 between those with and without AbAC.
Totally 384 community-dwelling older people were recruited during the study period, among whom 41 (10.7%) did not receive chest radiography, leaving 343 (89.3%) in the analysis. No significant difference in demographic distribution was noted between included and excluded ones. The median age of these 343 older adults were 73.8 (68.6–78.6) years, with 40% male. Most participants were morbidity-free, with less than half having hypertension (43%), coronary artery disease (18%), and DM (11%) (Table 1). Among them, 205 (59.8%) had AAC. Those with AAC had a significantly higher age (p = 0.008), more likely to have DM (p = 0.004) and received anti-diabetic medications (p = 0.003), and had significantly lower hemoglobin (p = 0.022) but higher globulin (p = 0.032) and glucose (p = 0.01) than those without (Table 1). Specifically, a greater severity of AAC was paralleled by an increasing age (p < 0.001), a higher prevalence of DM (p = 0.002) and using anti-diabetics (p = 0.001), and a higher fasting glucose (p = 0.033) (Table 1).
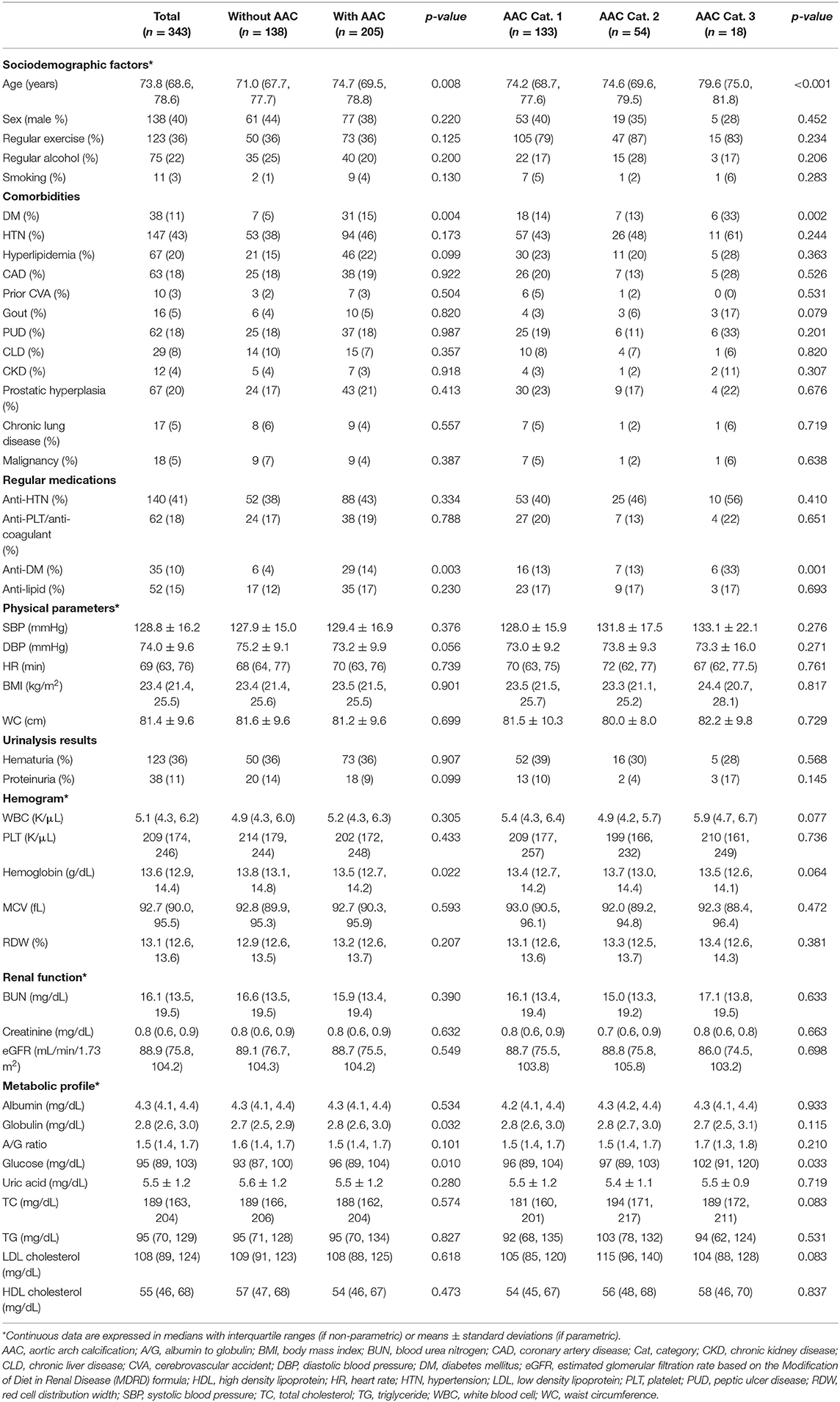
Table 1. Comparison of features between community-dwelling older adults with and without different AAC severities.
The p-value of Kolmogorov-Smirnov test of circulating miR-125b levels was <0.001, indicating its non-parametric feature. The mean and median miR-125b values among 343 participants were 0.075 ± 0.3 and 0.012 (0.003, 0.037), respectively. Those with AAC had significantly lower circulating miR-125b than those without [the former vs. the latter, 0.004 (0.002, 0.01) vs. 0.041 (0.02, 0.125); p < 0.001]. Participants with a greater AAC severity also had progressively decreased miR-125b levels [without vs. category 1 vs. 2 vs. 3, 0.041 (0.02, 0.125) vs. 0.006 (0.002, 0.013) vs. 0.004 (0.002, 0.007) vs. 0.001 (0.0006, 0.003); p < 0.001]. Participants with higher than median miR-125b levels had significantly lower prevalence of DM (p = 0.004), less likely to receive anti-diabetics (p = 0.005), and less AAC (high vs. low, 24 vs. 93%; p < 0.001) compared to those with lower-than median levels.
We subsequently conducted multiple regression analyses to examine risk factors for AAC, incorporating miR-125b in different styles. Regression analyses showed that higher miR-125b levels were significantly associated with a lower probability of having AAC [odds ratio (OR) <0.001 per one unit of miR-125b value, p < 0.001], while higher age increased the probability [OR 1.075 per year, 95% confidence interval (CI) 1.023–1.130] (model 1; Table 2). Having higher miR-125b levels based on the mean and median values similarly were associated with a substantially lower risk of having AAC (division based on mean, OR 0.032, 95% CI 0.011–0.094; based on median, OR 0.022, 95% CI 0.011–0.044) (models 2 and 3; Table 2). The AUROCs of models 1, 2, and 3 were 0.893 (95% CI 0.859–0.927), 0.786 (95% CI 0.736–0.835), and 0.890 (95% CI 0.853–0.926), respectively (Figure 1A), supporting the superiority of using the median-based categorization of miR-125b. Youden's index identified that the optimal cutoff of miR-125b level for identifying AAC presence was 0.008, with a sensitivity and specificity of 0.86 and 0.80, respectively (Figure 1A).
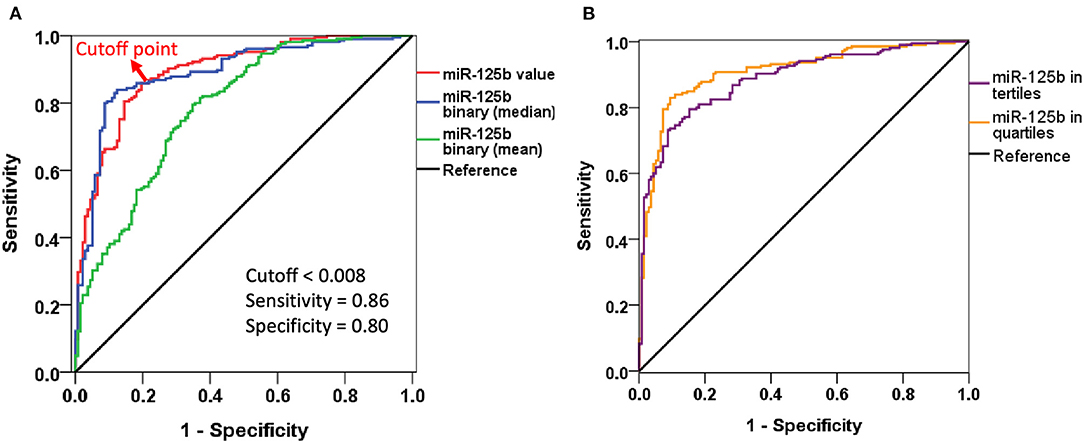
Figure 1. Receiver-operating characteristics curves of different logistic regression models incorporating miR-125b levels. (A) Including miR-125b value or in binary division. (B) Including miR-125b in tertiles or quartiles.
We further divided participants based on the cutoff value 0.008, yielding 195 (56.7%) with high circulating miR-125b levels. Those with a higher-than-cutoff miR-125b had significantly lower prevalence of DM (p = 0.003), less likely to receive anti-diabetics (p < 0.001), and a lower red cell distribution width (RDW) (p = 0.008) than those with lower-than-cutoff values (Supplementary Table 1). Participants with a cutoff-based high miR-125b level were less likely to have AAC and less severe AAC than those without (p < 0.001) (Supplementary Table 1). Another multiple logistic regression showed that having a higher-than-cutoff miR-125b was associated with a lower probability of AAC (OR 0.014, 95% CI 0.006–0.038).
After dividing participants based on miR-125b tertiles, we found that participants with an increasing miR-125b tertile also had a progressively lower probability of exhibiting AAC (for tertile 2 and 3 vs. 1, OR 0.062 and 0.007, 95% CI 0.021–0.182 and 0.002–0.021, respectively) than those within the lowest tertile (model S1; Table 3). Similar findings were obtained when we analyzed the risk based on miR-125b quartiles (model S2; Table 3). The AUROCs for models S1 and S2 were 0.887 (95% CI 0.853–0.922) and 0.908 (95% CI 0.876–0.941), respectively (Figure 1B).
We also examined whether circulating miR-125b levels were associated with AbAC presence and severity. Fifty (14.6%) of participants had lumbar spine films available for evaluation, among whom 19 (38%) and 31 (62%) did not have and had AbAC, respectively. Those with AbAC had significantly lower miR-125b levels than those without [with vs. without, 0.007 (0.002, 0.025) vs. 0.023 (0.01, 0.044); p = 0.016]. A multiple logistic regression with stepwise backward variable selection, using AbAC as the dependent variable and incorporating age, sex, DM, globulin, and cutoff-based high miR-125b, showed that having a high circulating miR-125b was similarly associated with a lower risk of AbAC than those without (OR 0.213, 95% CI 0.053–0.852).
In the current study, we prospectively enrolled a moderate sized group of older adults with few morbidities, and tested whether their circulating miR-125b levels exhibited associations with their aortic calcification status and severities. Regression analyses showed that having a high circulating miR-125b level were associated with a lower probability of carrying AAC and AbAC, independent of demographic profiles, morbidities, physical features, and multiple laboratory parameters. Importantly, we identified the cutoff point of miR-125b levels for differentiating between older adults with and without VC, which can be used in subsequent researches. Our findings thus support the notion that miRNAs participate in aging-related VC as well and can serve as a biomarker for diagnosis and even risk stratification.
Prior studies focusing on VC mostly involved patients with DM or CKD, both of which are significant risk enhancers for VC in experimental and clinical reports (20); few specifically address VC predominantly related to aging, while available ones mostly examine coronary artery calcification (CAC). Yano et al. reported that CAC severity predicted incident coronary heart disease and stroke among older adults (21), while Everson-Rose and colleagues identified CAC as a risk factor for impaired walking speed (22). On the other hand, risk factors for aging-related VC in older adults remain elusive and are rarely recognized. An increased waist circumference and age-related loss of lean body mass are independently associated with a higher risk of having renal artery calcification and AbAC, respectively, among community-dwelling older adults (23, 24), while a low bone mineral density and hyperphosphatemia increase the risk of AbAC in the elderly as well (25). However, nearly all available findings harness clinical features or body composition data for risk factor analysis regarding aging-related VC; pathophysiology-based risk factors are rarely examined. In this sense, results from this study substantially extend the existing knowledge by elucidating a novel miRNA-based risk factor for this vascular morbidity; furthermore, we also affirm the clinical utility of this miRNA as a circulating biomarker for estimating the probability of aging-related VC.
The cutoff value of circulating miR-125b we uncovered for recognizing aging-related VC (0.008) differs from that reported previously for uremic VC (0.06–0.07) (8). Prior reports suggested that circulating miRNAs levels decreased as eGFR declined (26), and factors that contribute to the development and worsening of VC frequently intertwine with each other and complicate the pathogenesis, as the degree of renal dysfunction progresses. From this perspective and based on the fact that miR-125b is a negative predictor of VC, the threshold value of miR-125b required for differentiating between the absence and presence of uremic VC may have to be larger than it should be among the general population, in order to better capture those without such illness. Nonetheless, more evidence is needed to clarify the pathophysiological nature of this change in cutoff values.
Mechanisms responsible for altering circulating miR-125b levels in those with aging-related VC remain unclear. It has been summarized previously that miR-125b played a pivotal role in attenuating the probability of VSMC trans-differentiation into osteoblast-like cells, thereby decreasing VC tendency (7). Results from in vitro experiments affirm that miR-125b participates early during the course of osteogenesis by directly targeting Cbfβ and indirectly suppressing the effect of RUNX2, a vital osteoblast differentiation marker (27). It is thus likely that miR-125b serves as a negative indicator of VC regardless of VC origin. However, we believe that miR-125b participates more deeply in the process of aging-related VC. Biological aging of tissue stem cells has recently been found to down-regulate several competence-regulating miRNAs, one of which is miR-125b (28). The decreased expression of miR-125b following cellular senescence potentially alters tissue responses to environmental signals, leading to abnormal phenotype generation, such as VC. In addition, microRNA levels are known to be affected by medications such as antiplatelet medications (29), and they may also be surrogates of platelet reactivities (30) that potentially influence future cardiovascular risk (31). MiR-125b has been implicated in the pathogenesis of aortic valve calcification (32), a potential surrogate co-existing with AAC (33). Finally, aging is frequently accompanied by the emergence of subclinical chronic inflammation, or “inflammaging,” due to cellular senescence with rising oxidative stress from worn-off mitochondria, inflammasome activation, and immune-dysregulation (34). Lower miR-125b expressions correlate with an age-associated increase in CCL4 levels (35), both of which are involved in the pathogenesis of cardiovascular calcification (32). Based on these findings, the strong association between circulating miR-125b levels and the status and severity of aging-related VC appears reasonable; this relationship has also been affirmed previously for uremic VC and potentially applicable to diabetic VC as well. A pictorial summary of our findings and results from prior literature is provided in Figure 2.
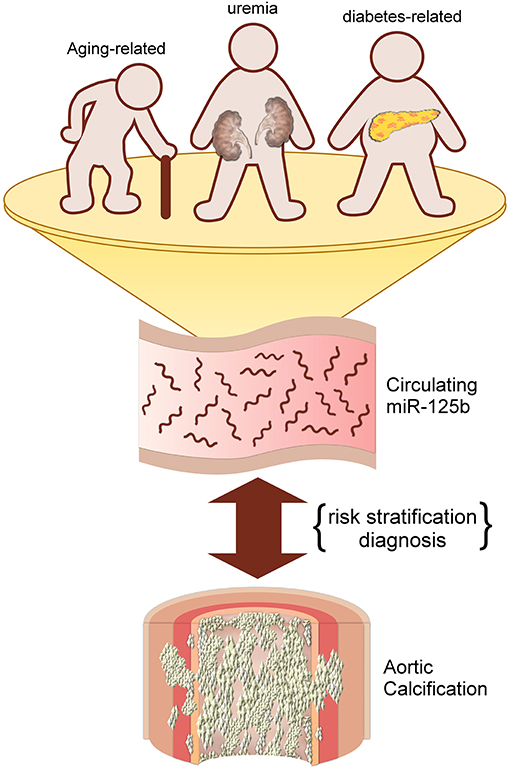
Figure 2. An illustrative diagram depicting the potential utility of circulating miR-125b for predicting aortic calcification/vascular calcification of different origins.
Our study has its strength and limitations. Risk factors and diagnostic biomarkers for aging-related VC are rarely reported in the literature, and our findings greatly extend the utility of circulating miRNAs in triaging VC with regard to the associated risk. Our sample size is adequate, with robust results obtained. However, several issues should be bore in mind before interpreting these data. First, a comprehensive evaluation of aortic calcification severity was not undertaken, and we used semi-quantitative rating schemes to gauge AAC and AbAC extent only. However, the approach we adopted has been repeatedly tested in the existing literature, with results validated in different populations (13–16, 36, 37). Therefore, we believe that our results remain valid. Second, this study did not address the temporal relationship between miR-125b levels and VC progression, so we could not be certain whether miR-125b could foretell the course of aging-related VC in the future. Third, we examined only one microRNA as a marker for VC in this study, whose sensitivity could be limited compared to microRNA combinatorial panels. However, this marker has been validated previously in other population. We are currently in the process of identifying other circulating microRNA candidates for detecting VC. In addition, we did not examine the incidence of aortic valve calcification in our cohort. Finally, there may be other interfering factors that we did not collect or adjust for, such as bone mineral density, body adiposity, and biochemical parameter (serum calcium or phosphate). Since these older adults are relatively health with few morbidities, we believe that these factors are unlikely to influence our findings.
In conclusion, we prospectively enrolled a group of healthy community-dwelling older adults for analyzing the association between circulating miR-125b and aging-related VC. The close relationship between this miRNA biomarker and the risk/severity of VC serves to inform us that miRNA-based diagnostics may be useful as a non-invasive and radiation-free approach for identifying VC, an important age-related morbidity that increases the risk of adverse outcomes among this ever-rising population.
The raw data supporting the conclusions of this article will be made available by the authors, subjected to administrative regulations.
The studies involving human participants were reviewed and approved by the institutional review board of the National Taiwan University Hospital (No. 201601091RIND). The patients/participants provided their written informed consent to participate in this study.
C-TC and D-SH: study design. C-TC and J-WH: data analysis. C-TC, D-SH, and J-WH: article drafting. All authors approved the final version of the manuscript.
This study was financially sponsored by National Taiwan University Hospital BeiHu Branch (11001) and Ministry of Science and Technology, Taiwan (MOST 109-2314-B-002-193-MY3).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the staff of Second Core Laboratory, Department of Medical Research of National Taiwan University Hospital and the National Taiwan University Center of Genomic Research and Precision Medicine for their technical input.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.624313/full#supplementary-material
AAC, aortic arch calcification; AbAC, abdominal aortic calcification; ASCVD, atherosclerotic cardiovascular disease; AUROC, area under receiver-operating characteristics curve; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HR, heart rate; MACE, major adverse cardiac events; MDRD, Modification of Diet in Renal Disease; miRNA, microRNA; OR, odds ratio; ROC, receiver-operating characteristics; VC, vascular calcification; VSMC, vascular smooth muscle cell.
1. Letnes JM, Nes B, Vaardal-Lunde K, Slette Martine B, Mølmen-Hansen Harald E, Aspenes Stian T, et al. Left atrial volume, cardiorespiratory fitness, and diastolic function in healthy individuals: the HUNT study, Norway. J Am Heart Assoc. (2020) 9:e014682. doi: 10.1161/JAHA.119.014682
2. Helber I, Alves CMR, Grespan SM, Veiga ECA, Moraes PIM, Souza JM, et al. The impact of advanced age on major cardiovascular events and mortality in patients with ST-elevation myocardial infarction undergoing a pharmaco-invasive strategy. Clin Interv Aging. (2020) 15:715–22. doi: 10.2147/CIA.S218827
3. Goff DC, Lloyd-Jones Donald M, Bennett G, Coady S, D'Agostino Ralph B, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. (2014) 129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98
4. Joshi FR, Rajani NK, Abt M, Woodward M, Bucerius J, Mani V, et al. Does vascular calcification accelerate inflammation?: a substudy of the dal-PLAQUE trial. J Am Coll Cardiol. (2016) 67:69–78. doi: 10.1016/j.jacc.2015.10.050
5. Hou Y-C, Lu C-L, Yuan T-H, Liao M-T, Chao C-T, Lu K-C. The epigenetic landscape of vascular calcification: an integrative perspective. Int J Mol Sci. (2020) 21:980. doi: 10.3390/ijms21030980
6. Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC. miR-125b regulates calcification of vascular smooth muscle cells. Am J Pathol. (2011) 179:1594–600. doi: 10.1016/j.ajpath.2011.06.016
7. Chao C-T, Yeh H-Y, Yuan T-H, Chiang C-K, Chen H-W. MicroRNA-125b in vascular diseases: an updated systematic review of pathogenetic implications and clinical applications. J Cell Mol Med. (2019) 23:5884–94. doi: 10.1111/jcmm.14535
8. Chao C-T, Liu Y-P, Su S-F, Yeh H-Y, Chen H-Y, Lee P-J, et al. Circulating MicroRNA-125b predicts the presence and progression of uremic vascular calcification. Arteriosc Thromb Vasc Biol. (2017) 37:1402–14. doi: 10.1161/ATVBAHA.117.309566
9. Kooman JP, Dekker MJ, Usvyat LA, Kotanko P, van der Sande FM, Schalkwijk CG, et al. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol. (2017) 313:F938–50. doi: 10.1152/ajprenal.00256.2017
10. Chao C-T, Yeh H-Y, Han D-S, Huang J-W, Huang K-C. Determinants of circulating microRNA-125b, a risk predictor of vascular calcification, among community-dwelling older adults. Clin Transl Med. (2020) 10:e145. doi: 10.1002/ctm2.145
11. Chao C-T, Yuan T-H, Yeh H-Y, Chen H-Y, Huang J-W, Chen H-W. Risk factors associated with altered circulating micro RNA−125b and their influences on uremic vascular calcification among patients with end-stage renal disease. J Am Heart Assoc. (2019) 8:e010805. doi: 10.1161/JAHA.118.010805
12. Chen S-I, Chiang C-L, Chao C-T, Chiang C-K, Huang J-W. Gustatory function and the uremic toxin, phosphate, are modulators of the risk of vascular calcification among patients with chronic kidney disease: a pilot study. Toxins. (2020) 12:420. doi: 10.3390/toxins12060420
13. Hashimoto H, Iijima K, Hashimoto M, Son B-K, Ota H, Ogawa S, et al. Validity and usefulness of aortic arch calcification in chest X-ray. J Atherosc Thromb. (2009) 16:256–64. doi: 10.5551/jat.E570
14. Bannas P, Jung C, Blanke P, Treszl A, Derlin T, Adam G, et al. Severe aortic arch calcification depicted on chest radiography strongly suggests coronary artery calcification. Eur Radiol. (2013) 23:2652–7. doi: 10.1007/s00330-013-2877-z
15. Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PWF. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham heart study. Calcif Tissue Int. (2001) 68:271–6. doi: 10.1007/BF02390833
16. Honkanen E, Kauppila L, Wikström B, Rensma PL, Krzesinski J-M, Aasarod K, et al. Abdominal aortic calcification in dialysis patients: results of the CORD study. Nephrol Dial Transplant. (2008) 23:4009–15. doi: 10.1093/ndt/gfn403
17. Glinge C, Clauss S, Boddum K, Jabbari R, Jabbari J, Risgaard B, et al. Stability of circulating blood-based microRNAs - pre-analytic methodological considerations. PLoS ONE. (2017) 12:e0167969. doi: 10.1371/journal.pone.0167969
18. Farina NH, Wood ME, Perrapato SD, Francklyn CS, Stein GS, Stein JL, et al. Standardizing analysis of circulating microRNA: clinical and biological relevance. J Cell Biochem. (2014) 115:805–11. doi: 10.1002/jcb.24745
19. Xiang M, Zeng Y, Yang R, Xu H, Chen Z, Zhong J, et al. U6 is not a suitable endogenous control for the quantification of circulating microRNAs. Biochem Biophys Res Commun. (2014) 454:210–4. doi: 10.1016/j.bbrc.2014.10.064
20. Krishnan P, Moreno PR, Turnbull IC, Purushothaman M, Zafar U, Tarricone A, et al. Incremental effects of diabetes mellitus and chronic kidney disease in medial arterial calcification: synergistic pathways for peripheral artery disease progression. Vasc Med. (2019) 24:383–94. doi: 10.1177/1358863X19842276
21. Yano Y, O'Donnell CJ, Kuller L, Kavousi M, Erbel R, Ning H, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. (2017) 2:986–94. doi: 10.1001/jamacardio.2017.2498
22. Everson-Rose SA, Mendes de Leon CF, Roetker NS, Lutsey PL, Alonso A. Subclinical cardiovascular disease and changes in self-reported mobility: multi-ethnic study of atherosclerosis. J Gerontol Biol Sci Med Sci. (2018) 73:218–24. doi: 10.1093/gerona/glx103
23. Ricalde A, Allison M, Rifkin D, Shaw R. Anthropometric measures of obesity and renal artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. (2018) 271:142–7. doi: 10.1016/j.atherosclerosis.2018.02.031
24. Rodríguez AJ, Scott D, Khan B, Khan N, Hodge A, English DR, et al. Low relative lean mass is associated with increased likelihood of abdominal aortic calcification in community-dwelling older Australians. Calcif Tissue Int. (2016) 99:340–9. doi: 10.1007/s00223-016-0157-z
25. Figueiredo CP, Rajamannan NM, Lopes JB, Caparbo VF, Takayama L, Kuroishi ME, et al. Serum phosphate and hip bone mineral density as additional factors for high vascular calcification scores in a community-dwelling: The São Paulo Ageing & Health Study (SPAH). Bone. (2013) 52:354–9. doi: 10.1016/j.bone.2012.10.019
26. Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JYZ, Gleadle JM. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transplant. (2011) 26:3794–802. doi: 10.1093/ndt/gfr485
27. Huang K, Fu J, Zhou W, Li W, Dong S, Yu S, et al. MicroRNA-125b regulates osteogenic differentiation of mesenchymal stem cells by targeting Cbfβ in vitro. Biochimie. (2014) 102:47–55. doi: 10.1016/j.biochi.2014.02.005
28. Watanabe K, Ikuno Y, Kakeya Y, Kito H, Matsubara A, Kaneda M, et al. Functional similarities of microRNAs across different types of tissue stem cells in aging. Inflamm Regen. (2018) 38:9. doi: 10.1186/s41232-018-0066-9
29. Carino A, De Rosa S, Sorrentino S, Polimeni A, Sabatino J, Caiazzo G, et al. Modulation of circulating microRNAs levels during the switch from clopidogrel to ticagrelor. Biomed Res Int. (2016) 2016:3968206. doi: 10.1155/2016/3968206
30. Pordzik J, Jakubik D, Jarosz-Popek J, Wicik Z, Eyileten C, De Rosa S, et al. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: bioinformatic analysis and review. Cardiovasc Diabetol. (2019) 18:113. doi: 10.1186/s12933-019-0918-x
31. Pordzik J, Pisarz K, De Rosa S, Dyve Jones A, Eyileten C, Indolfi C, et al. The potential role of platelet-related microRNAs in the development of cardiovascular events in high-risk populations, including diabetic patients: a review. Front Endocrinol. (2018) 9:74. doi: 10.3389/fendo.2018.00074
32. Ohukainen P, Syväranta S, Näpänkangas J, Rajamäki K, Taskinen P, Peltonen T, et al. MicroRNA-125b and chemokine CCL4 expression are associated with calcific aortic valve disease. Ann Med. (2015) 47:423–9. doi: 10.3109/07853890.2015.1059955
33. Sabatino J, Wicik Z, De Rosa S, Eyileten C, Jakubik D, Spaccarotella C, et al. MicroRNAs fingerprint of bicuspid aortic vale. J Mol Cell Cardiol. (2019) 134:98–106. doi: 10.1016/j.yjmcc.2019.07.001
34. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
35. Cheng N-L, Chen X, Kim J, Shi AH, Nguyen C, Wersto R, et al. MicroRNA-125b modulates inflammatory chemokine CCL4 expression in immune cells and its reduction causes CCL4 increase with age. Aging Cell. (2015) 14:200–8. doi: 10.1111/acel.12294
36. Lee ST, Chao CT, Huang JW, Huang LC. Vascular calcification as an underrecognized risk factor for frailty in 1783 community-dwelling elderly individuals. J Am Heart Assoc. (2020) 9:e017308. doi: 10.1161/JAHA.120.017308
Keywords: aortic calcification, biomarker, chronic kidney disease, epigenetics, geriatrics, microRNA, miR-125b, vascular calcification
Citation: Chao C-T, Han D-S and Huang J-W (2021) Circulating microRNA-125b Levels Are Associated With the Risk of Vascular Calcification in Healthy Community-Dwelling Older Adults. Front. Cardiovasc. Med. 8:624313. doi: 10.3389/fcvm.2021.624313
Received: 31 October 2020; Accepted: 28 January 2021;
Published: 22 February 2021.
Edited by:
Yun Fang, University of Chicago, United StatesReviewed by:
Salvatore De Rosa, University of Catanzaro, ItalyCopyright © 2021 Chao, Han and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jenq-Wen Huang, MDA3Mzc4QG50dWguZ292LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.