
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Cardiovasc. Med., 10 February 2021
Sec. Hypertension
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.607998
This article is part of the Research TopicDiabetes, Hypertension and Cardiovascular DiseasesView all 14 articles
 Fedor Simko1,2,3*
Fedor Simko1,2,3* Tomas Baka1
Tomas Baka1Elevated heart rate (HR) is a well-recognized but somewhat neglected risk factor among the healthy population and various cardiovascular pathologies (1). High HR is fraught with a spate of detrimental cardiovascular consequences including immense myocardial oxygen demand in reduced diastolic perfusion time (2) and low, oscillatory vascular shear stress with high tensile stress triggering endothelial dysfunction (3). Although beta-blockers (BBs) are considered to be the cornerstone treatment of elevated HR in various cardiovascular pathologies, they are associated with negative inotropy, a number of side effects, and undesirable metabolic actions limiting their usage (4, 5). Thus, new approaches to HR reduction are being continuously sought out.
The inhibition of the If current in the sinoatrial node (SAN) seems to offer a promising approach to the reduction of elevated HR. Indeed, the SAN's pacemaker cells are inherently capable of cyclic variations of the resting membrane potential necessary for spontaneous depolarization. The SAN's spontaneous slow diastolic depolarization is administered by a mixed sodium/potassium inward current, known as an If current, through the “funny” (f)-channel (6). Structurally, the f-channel belongs to hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels and is activated by both hyperpolarization in the diastolic voltage range and intracellular cyclic adenosine monophosphate (7). Ivabradine selectively inhibits the If current, thus reducing the steepness of SAN's diastolic depolarization, ensuing diastole prolongation without affecting action potential duration or inducing negative inotropy (7, 8).
Several studies have assessed ivabradine's efficacy in clinical settings. In the SHIFT study, the investigation of 6,558 patients with systolic heart failure (HF) during a median 22.9 month follow-up period revealed that the addition of ivabradine to an established HF therapy significantly reduced the primary composite endpoint of hospital admission for worsening HF or cardiovascular death. Considering the results of the SHIFT study, ivabradine is recommended for patients with systolic HF and HR above 70 bpm despite an evidence-based optimal medical therapy (with or without BB) to reduce the composite endpoint of hospitalization and mortality (9, 10). The BEAUTIFUL study comprised 10,917 systolic HF patients with HR above 70 bpm suffering from stable coronary artery disease (CAD), and the primary endpoint was a composite of cardiovascular death and hospital admission for acute myocardial infarction or HF. Although neither the primary endpoint nor the cardiovascular death rate improved, ivabradine reduced the secondary endpoints of hospital admissions for myocardial infarction and coronary revascularization (11). However, in the SIGNIFY study involving 19,102 patients with stable CAD but without HF, ivabradine did not reduce the compound primary endpoint of cardiovascular death and myocardial infarction (12).
Hypertension, however, is a substantially different condition, and data regarding ivabradine's effect on peripheral blood pressure (BP) in a hypertensive population are scanty. Yet ivabradine's interference with central BP (CBP) was indicated in several studies. Lopatin and Vitale (13) reviewed five studies analyzing ivabradine's effect on CBP in patients with CAD: two studies reported a neutral effect, while in two other studies and in one study, ivabradine decreased and increased CBP, respectively. In 12 normotensive patients with stable CAD and HR ≥ 70 bmp, a 3 week ivabradine treatment reduced brachial systolic and diastolic BP, while the HR reduction did not increase central aortic BP (14). Moreover, in patients with arterial hypertension and CAD treated with ivabradine, the increase in HR between resting conditions and early recovery post exercise showed a trend toward correlation with the radial augmentation index (15).
Besides ivabradine's HR-reducing action, which is considered to be a principal mechanism of its benefit, ivabradine exerts a number of pleiotropic effects, some of which may partly be HR independent (16, 17) and some of which are still emerging.
BBs have been a well-established means for HR reduction and the improvement of the energetic state of the myocardium in various cardiovascular diseases (18). The important advantage of ivabradine over BB seems to be its apparent independence from the sympathetic nervous system, thus avoiding negative inotropy or alpha-adrenoceptor-mediated coronary vasoconstriction (17).
According to generally accepted assumptions, ivabradine exerts a neutral effect on arterial BP in both experimental and clinical settings (8–12). However, based on several recent pieces of evidence, ivabradine could reduce BP under certain conditions:
• In an experiment with NG-nitro-L-arginine methyl ester (L-NAME)-induced nitric oxide-deficient hypertension in rats, ivabradine (10 mg/kg/day) reduced HR and systolic BP measured by non-invasive tail-cuff plethysmography during a period of 4 weeks. Systolic BP was reduced from the first week by ivabradine treatment and continued to decrease each week. In the fourth week of the experiment, ivabradine reduced systolic BP by 15%, and the 4 week average systolic BP was decreased by 8% via ivabradine compared to that in the L-NAME group (19). In another study with L-NAME-induced hypertension, ivabradine reduced systolic BP not only in the L-NAME group (by 21%) but even in the control group (by 26%) (20).
• In a study that sought to improve non-dipping HR in a rat model of L-NAME-induced hypertension, daytime and nighttime systolic BP and HR were measured weekly after administration of the daily dose of ivabradine (10 mg/kg/day) at either daytime or nighttime during a period of 4 weeks. Interestingly, both daytime- and nighttime-dosed ivabradine decreased both daytime and nighttime systolic BP in hypertensive rats each week, reaching the largest 14% systolic BP decline during the last week of the experiment (21).
• In the three rat models of acute stress induced by handling (mild stress), restraint (moderate stress), or immobilization (severe stress), ivabradine (5 mg/kg) was administered intraperitoneally 30 min before stress exposure. In the groups pretreated with ivabradine, lower values of HR and mean arterial BP were observed in the baseline period, during exposure to stressors, as well as during the rest period following stress exposure in all three types of stressors applied and all intervals investigated (22).
• Two studies assessed the effect of acute or chronic ivabradine on HR and BP in spontaneously hypertensive rats and Wistar–Kyoto controls as measured by carotid catheterization under pentobarbital anesthesia. The acute administration of four consecutive ivabradine doses (1 mg/kg, i.v.) decreased systolic, diastolic, and mean BP in hypertensive rats and in controls (except for systolic BP, which remained unchanged) and increased pulse pressure in both rat strains (23). The chronic, 28 day administration of ivabradine (8.4 mg/kg/day via subcutaneous osmotic minipump) decreased systolic, diastolic, and mean BP and increased pulse pressure in both rat strains (24).
• In healthy volunteers treated with ivabradine (30 mg), propranolol (40 mg), or a placebo, hemodynamic parameters were investigated at rest and before and during tilt and exercise tests 2 and 5 h after drug intake. Ivabradine significantly reduced systolic BP at rest. However, during tilt and exercise tests, only propranolol but not ivabradine reduced systolic BP (25).
The mechanisms underlying the ambiguity of ivabradine's effect on BP in different conditions remain elusive. Yet the following two factors might be considered determining: (i) the pathophysiology of the ivabradine-treated disease, as in a rat model of isoproterenol-induced HF, ivabradine prevented detrimental systolic BP decline indicative of improved cardiac function (26), while in a rat model of L-NAME-induced hypertension, ivabradine decreased systolic BP by exerting antihypertensive properties (19); and (ii) concomitant therapy, as in pivotal clinical studies, e.g., SHIFT, BEAUTIFUL, or SIGNIFY, ivabradine was administered on top of the evidence-based optimal medical therapy, often including drugs modulating the sympathetic nervous system and/or renin–angiotensin–aldosterone system (8–12), thus presumably giving minimal space for ivabradine to exert an effect on BP. In clinical studies with HF patients, HR reduction without affecting BP was considered to be desirable, since the BP-reducing effect of well-established HF therapeutics such as BB, angiotensin-converting enzyme inhibitors (ACEis), angiotensin II type 1 receptor blockers (ARBs), or mineralocorticoid receptor antagonists (MRAs) can limit the achievement of the target doses. Interestingly, it has been shown that the efficacy and safety of ivabradine in HF patients were independent of systolic BP (27).
Although experimental data on ivabradine's BP-reducing effect are scarce and large prospective clinical studies featuring ivabradine and a hypertensive population are lacking, numerous potential mechanisms contributing to the BP reduction by ivabradine in experiments demonstrated in this study could be considered. Indeed, several pleiotropic effects of ivabradine, such as anti-inflammatory and antioxidant actions, the improvement of endothelium-dependent and endothelium-independent vascular relaxation, anti-atherosclerotic effects, and the attenuation of the neurohumoral activation, might individually or in concert contribute to BP reduction (Figure 1).
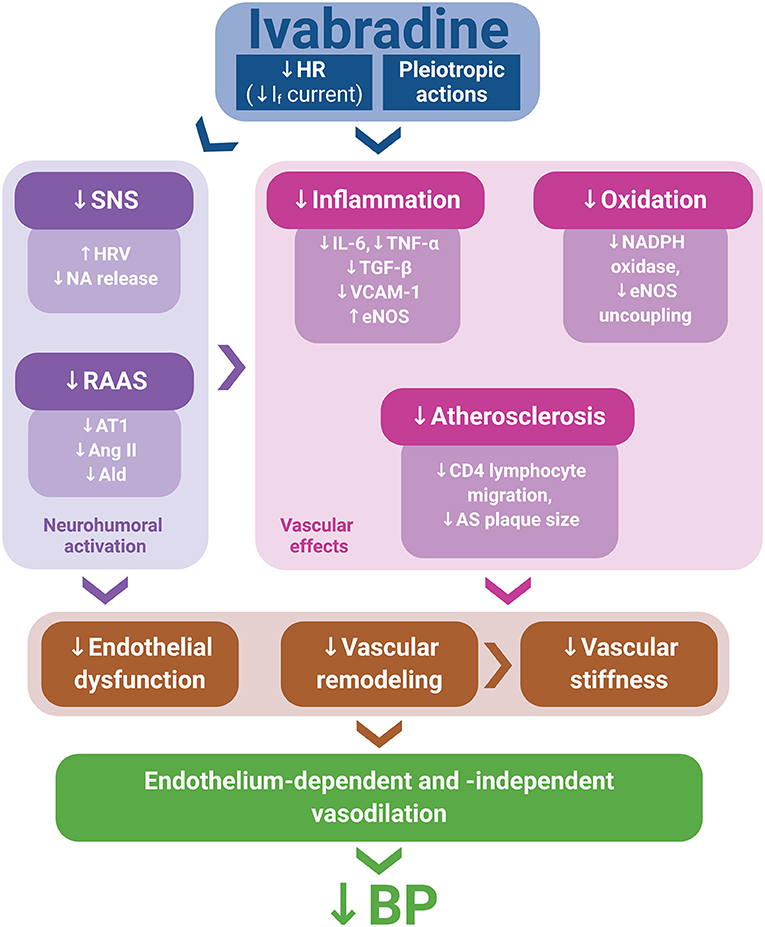
Figure 1. Ivabradine's pleiotropic actions, along with heart rate (HR) reduction via If current inhibition, might mitigate neurohumoral activation, inflammation, oxidative stress, and atherosclerosis development, thus improving endothelial function and attenuating vascular remodeling and stiffness. These effects may contribute to endothelium-dependent and endothelium-independent vasodilation, potentially resulting in blood pressure (BP) reduction. Ald, aldosterone; Ang II, angiotensin II; AS, atherosclerotic; AT1, angiotensin II type 1 receptor; eNOS, endothelial nitric oxide synthase; HRV, heart rate variability; NA, noradrenaline; RAAS, renin–angiotensin–aldosterone system; SNS, sympathetic nervous system.
Several plausible explanations for the potential BP-reducing effect of ivabradine are emerging. Besides HR reduction, ivabradine was shown to exert manifold pleiotropic effects within the vasculature in terms of inflammation and oxidative stress reduction and improvement of endothelial function and vascular elasticity (28). Indeed, in low-shear-stress-damaged isolated endothelial cells, ivabradine prevented inflammation and oxidative stress via the TOR/eNOS pathway (29). Ivabradine reduced reactive oxygen species levels in atherogenic diet-induced hypercholesterolemic rabbits (30). In apolipoprotein E-knockout mice, ivabradine reduced NADPH oxidase activity and prevented eNOS uncoupling (31); decreased monocyte chemotactic protein mRNA, markers of superoxide production and lipid peroxidation, and atherosclerotic plaque size (32); reduced the aortic mRNA expression of IL-6, TNF-α, and TGF-β (33); and downregulated pro-apoptotic and pro-inflammatory genes (23, 34). In hypercholesterolemic mice, ivabradine reduced the expression of pro-inflammatory VCAM-1 and enhanced the expression of anti-inflammatory eNOS on the inner surface of the aorta (35). These potentially protective effects of ivabradine could result in the improvement of aortic elasticity and endothelium-dependent relaxation (30, 33, 36). Indeed, ivabradine reduced neointimal hyperplasia and intima–media ratio in hypercholesterolemic rabbits (30) and attenuated aortic fibrosis and stiffness in diabetic mice (37). Ivabradine also inhibited the chemokine-induced migration of CD4-positive lymphocytes, thus potentially curbing atherosclerosis development (38). In clinical settings, ivabradine improved aortic elasticity and endothelial function in chronic systolic HF (36) and fostered the flow- and nitroglycerin-mediated dilation of the brachial artery in patients with CAD (39). Similarly, in patients with chronic stable CAD, HR reduction by ivabradine improved flow-mediated vasodilation and reduced the arterial stiffness of the brachial artery (40).
Increasing the magnitude of wall shear stress via HR reduction seems to be the underlying mechanism of ivabradine's arterial protection (35). Moreover, ivabradine increased the brain capillary density in mice with chronic mental stress (41), enhanced capillary formation in mice with myocardial infarction (42), and improved coronary reserve in rats afflicted with myocardial infarction potentially by the reduction of periarteriolar collagen (43). Taken together, these findings suggest that ivabradine may improve endothelium-dependent and endothelium-independent vascular relaxation, resulting in vasodilation along with improvement of microcirculation, thus contributing to BP reduction and improved organ perfusion.
Ivabradine was also shown to exert cardioprotection by the attenuation of both apoptosis and matrix metalloproteinase expression (44), to improve mitochondrial respiration, and to enhance ATP production and calcium retention capacity independent of HR reduction (16). Moreover, ivabradine showed a positive inotropic action induced by enhanced sarcoplasmic/endoplasmic reticulum calcium ATPase 2a (SERCA2a) activity (45). Thus, the vascular and cardiac protective pathways, along with HR reduction, may underlie ivabradine's effects of cardiovascular benefit.
The potential relation of ivabradine to neurohumoral systems should be taken into account. Although ivabradine is considered to exert its principal protection as a direct and selective HR reducer via inhibition of the If current in the SAN, its potential interaction with the sympathetic nervous system cannot be excluded. In the above-mentioned experiment with three acute stress rat models, the reduced BP in ivabradine-pretreated rats exposed to handling stress was associated with reduced adrenaline and noradrenaline release into the blood stream compared to placebo treatment (22). Furthermore, in Dahl salt-sensitive rats, chronic ivabradine treatment reduced mortality along with the reduction of urinary noradrenaline excretion (46). In a rat model of doxorubicin-induced HF, the measuring of HR variability indicated an ivabradine-induced improvement of the autonomic imbalance (47). In substudies of large clinical trials, the HR variability analysis has shown an ivabradine-mediated shift toward a parasympathetic tone (48, 49); and in hypertensive patients with metabolic syndrome, ivabradine reduced sympathetic activation (50).
Similarly, data regarding ivabradine's interaction with the renin–angiotensin–aldosterone system are emerging. In ApoE-deficient mice, ivabradine reduced the serum level of angiotensin II (Ang II) (51), reduced the mRNA expression and protein of the Ang II type 1 receptor (AT1 receptor) (33), and downregulated Ang II-regulated pro-inflammatory genes (34). Moreover, in rats with myocardial infarction, ivabradine reduced the myocardial protein expression of the AT1 receptor (43, 52) and tissue angiotensin-converting enzyme (52). In L-NAME-induced hypertension, along with BP reduction, ivabradine reduced the serum concentration of aldosterone and the aldosterone/Ang II ratio (19). Blunting the sympathetic nervous system or the renin–angiotensin–aldosterone system may contribute to the potential BP-reducing effect of ivabradine via the reduction of peripheral artery resistance or circulating volume.
In the SHIFT study, ivabradine created hope for the treatment of HF patients with elevated HR. However, some studies with ivabradine were neutral and did not meet expectations. Thus, the indication for ivabradine should be considered carefully (53). On the other hand, the unique nature of ivabradine could be considered in several off-label indications (54), such as inappropriate sinus tachycardia (55) or postural orthostatic tachycardia syndrome (56). Ivabradine was recently shown to improve hypertensive heart function in rats with L-NAME-induced hypertension (19).
Hypertension with elevated HR might be another indication for ivabradine (57). Increased HR in hypertension is an undesirable condition that worsens the prognosis; thus, the decision regarding the optimal treatment of elevated HR in hypertension is an issue at the crossroads and has attracted professional attention for decades (1, 58). Ivabradine could become a candidate for this indication, considering a number of its pleiotropic effects:
• Based on several examples presented in this work, ivabradine might be able to reduce BP and could contribute to the reduction of the hemodynamic burden in hypertension. According to a scientific statement from the American Heart Association on the detection, evaluation, and management of resistant hypertension, the number of patients with resistant hypertension is expected to significantly increase (59, 60); therefore, seeking new approaches to BP control will be of utmost importance (61, 62).
• It has been previously shown that besides the increase in daily HR mean, insufficient HR decline during bedtime, i.e., non-dipping HR, increases cardiovascular risk (63–65). Moreover, non-dipping HR seems to be more frequent in hypertensive patients with chronic kidney disease than in the hypertensive population without kidney affliction (66). HR reduction with ivabradine reaches its peak effect in 3 to 4 h and lasts 8 to 12 h after ingestion (67), thus subjecting ivabradine to a flexible dosing scheme for targeting mean HR or nighttime HR. A well-tailored dosing of ivabradine might reverse non-dipping HR to a desirable HR dipping pattern (21, 57), thus presumably further reducing cardiovascular risk in hypertension.
• As opposed to BB (4, 5), ivabradine has not been observed to have negative metabolic effects (68, 69), which would be beneficial for hypertensive patients with metabolic syndrome prone to dyslipidemia, hyperuricemia, or diabetes mellitus.
• Ivabradine does not induce anxiety or other behavioral disorders in rats (70, 71), whereas BB therapy was shown to be associated with psychological disorders, such as nightmares (72, 73).
• Ivabradine was found to exert hypertensive heart protection. Indeed, in L-NAME-induced hypertension, ivabradine improved the systolic and diastolic dysfunctions of the remodeled left ventricle (LV) (19); and in a transverse aortic constriction mouse model, ivabradine reduced LV hypertrophy, fibrosis, and cardiomyocyte apoptosis and improved LV function (74). In a pig model of chronic Ang II infusion-induced hypertension and diastolic LV dysfunction, the acute administration of ivabradine improved LV filling parameters by an HR-independent mechanism (75). Moreover, ivabradine exerted an antihypertrophic effect on the aorta in spontaneously hypertensive rats (24) and renoprotection in rats with L-NAME-induced hypertension (76).
Taking into account ivabradine's HR- and (potential) BP-reducing effects associated with target organ protection and the lack of undesirable metabolic and behavioral consequences (often seen with BB), it appears reasonable to suggest the consideration of ivabradine for hypertensive patients with elevated HR, especially for those co-afflicted with metabolic disorders.
FS conceived and drafted the manuscript. TB revised the manuscript. Both authors participated in data analysis and interpretation and approved the submitted version.
This study was supported by research grants VEGA 1/0035/19 and VEGA 2/0112/19.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Simko F, Baka T, Paulis L, Reiter RJ. Elevated heart rate and nondipping heart rate as potential targets for melatonin: a review. J Pineal Res. (2016) 61:127–37. doi: 10.1111/jpi.12348
2. Böhm M, Reil JC, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med. (2015) 128:219–28. doi: 10.1016/j.amjmed.2014.09.016
3. Giannoglou GD, Chatzizisis YS, Zamboulis C, Parcharidis GE, Mikhailidis DP, Louridas GE. Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol. (2008) 126:302–12. doi: 10.1016/j.ijcard.2007.08.077
4. Carella AM, Antonucci G, Conte M, Di Pumpo M, Giancola A, Antonucci E. Antihypertensive treatment with beta-blockers in the metabolic syndrome: a review. Curr Diabetes Rev. (2010) 6:215–21. doi: 10.2174/157339910791658844
5. Marketou M, Gupta Y, Jain S, Vardas P. Differential metabolic effects of beta-blockers: an updated systematic review of nebivolol. Curr Hypertens Rep. (2017) 19:22. doi: 10.1007/s11906-017-0716-3
6. Ide T, Ohtani K, Higo T, Tanaka M, Kawasaki Y, Tsutsui H. Ivabradine for the treatment of cardiovascular diseases. Circ J. (2019) 83:252–60. doi: 10.1253/circj.CJ-18-1184
7. DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs. (2007) 67(Suppl. 2):15–24. doi: 10.2165/00003495-200767002-00003
8. Koruth JS, Lala A, Pinney S, Reddy VY, Dukkipati SR. The clinical use of ivabradine. J Am Coll Cardiol. (2017) 70:1777–84. doi: 10.1016/j.jacc.2017.08.038
9. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. (2010) 376:875–85. doi: 10.1016/S0140-6736(10)61198-1
10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis treatment of acute chronic heart failure: the Task Force for the diagnosis treatment of acute chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
11. Fox K, Ford I, Steg PG, Tendera M, Ferrari R, BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. (2008) 372:807–16. doi: 10.1016/S0140-6736(08)61170-8
12. Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. (2014) 371:1091–9. doi: 10.1056/NEJMoa1406430
13. Lopatin YM, Vitale C. Effect of ivabradine on central aortic blood pressure in patients with stable coronary artery disease: what do we know? Int J Cardiol. (2016) 224:145–8. doi: 10.1016/j.ijcard.2016.09.054
14. Dillinger JG, Maher V, Vitale C, Henry P, Logeart D, Manzo Silberman S, et al. Impact of ivabradine on central aortic blood pressure and myocardial perfusion in patients with stable coronary artery disease. Hypertension. (2015) 66:1138–44. doi: 10.1161/HYPERTENSIONAHA.115.06091
15. Fischer-Rasokat U, Honold J, Lochmann D, Liebetrau C, Leick J, Hamm C, et al. Ivabradine therapy to unmask heart rate-independent effects of β-blockers on pulse wave reflections. Clin Res Cardiol. (2014) 103:487–94. doi: 10.1007/s00392-014-0679-1
16. Kleinbongard P, Gedik N, Witting P, Freedman B, Klöcker N, Heusch G. Pleiotropic, heart rate-independent cardioprotection by ivabradine. Br J Pharmacol. (2015) 172:4380–90. doi: 10.1111/bph.13220
17. Heusch G. Pleiotropic action(s) of the bradycardic agent ivabradine: cardiovascular protection beyond heart rate reduction. Br J Pharmacol. (2008) 155:970–1. doi: 10.1038/bjp.2008.347
18. Bangalore S, Messerli FH, Kostis JB, Pepine CJ. Cardiovascular protection using beta-blockers: a critical review of the evidence. J Am Coll Cardiol. (2007) 50:563–72. doi: 10.1016/j.jacc.2007.04.060
19. Simko F, Baka T, Poglitsch M, Repova K, Aziriova S, Krajcirovicova K, et al. Effect of ivabradine on a hypertensive heart and the renin-angiotensin-aldosterone system in L-NAME-induced hypertension. Int J Mol Sci. (2018) 19:3017. doi: 10.3390/ijms19103017
20. Simko F, Repova K, Krajcirovicova K, Aziriova S, Paulis L, Baka T. Remodelling of the aorta and kidney in L-NAME-induced hypertension in rats: comparison of the protective effect of ivabradine with captopril and melatonin. Diabetologia. (2015) 58(Suppl. 1):S547.
21. Baka T, Simko F. Ivabradine reversed nondipping heart rate in rats with l-NAME-induced hypertension. Clin Exp Pharmacol Physiol. (2019) 46:607–10. doi: 10.1111/1440-1681.13075
22. Ondicova K, Hegedusova N, Tibensky M, Mravec B. Ivabradine reduces baseline and stress-induced increase of heart rate and blood pressure and modulates neuroendocrine stress response in rats depending on stressor intensity. Gen Physiol Biophys. (2019) 38:165–73. doi: 10.4149/gpb_2018046
23. Albaladejo P, Challande P, Kakou A, Benetos A, Labat C, Louis H, et al. Selective reduction of heart rate by ivabradine: effect on the visco-elastic arterial properties in rats. J Hypertens. (2004) 22:1739–45. doi: 10.1097/00004872-200409000-00018
24. Albaladejo P, Carusi A, Apartian A, Lacolley P, Safar ME, Bénétos A. Effect of chronic heart rate reduction with ivabradine on carotid and aortic structure and function in normotensive and hypertensive rats. J Vasc Res. (2003) 40:320–8. doi: 10.1159/000072696
25. Joannides R, Moore N, Iacob M, Compagnon P, Lerebours G, Menard JF, et al. Comparative effects of ivabradine, a selective heart rate-lowering agent, and propranolol on systemic and cardiac haemodynamics at rest and during exercise. Br J Clin Pharmacol. (2006) 61:127–37. doi: 10.1111/j.1365-2125.2005.02544.x
26. Simko F, Baka T, Repova K, Aziriova S, Krajcirovicova K, Paulis L, et al. Ivabradine improves survival and attenuates cardiac remodeling in isoproterenol-induced myocardial injury. Fundam Clin Pharmacol. (2020). doi: 10.1111/fcp.12620. [Epub ahead of print].
27. Komajda M, Böhm M, Borer JS, Ford I, Robertson M, Manolis AJ, et al. Efficacy and safety of ivabradine in patients with chronic systolic heart failure according to blood pressure level in SHIFT. Eur J Heart Fail. (2014) 16:810–6. doi: 10.1002/ejhf.114
28. Dominguez-Rodriguez A, Abreu-Gonzalez P. Ivabradine and the anti-inflammatory effects in patients with ischemic heart disease. Int J Cardiol. (2016) 221:627–8. doi: 10.1016/j.ijcard.2016.07.096
29. Li B, Zhang J, Wang Z, Chen S. Ivabradine prevents low shear stress induced endothelial inflammation and oxidative stress via mTOR/eNOS pathway. PLoS ONE. (2016) 11:e0149694. doi: 10.1371/journal.pone.0149694
30. Koniari I, Mavrilas D, Apostolakis E, Papadimitriou E, Papadaki H, Papalois A, et al. Inhibition of atherosclerosis progression, intimal hyperplasia, and oxidative stress by simvastatin and ivabradine may reduce thoracic aorta's stiffness in hypercholesterolemic rabbits. J Cardiovasc Pharmacol Ther. (2016) 21:412–22. doi: 10.1177/1074248415617289
31. Kröller-Schön S, Schulz E, Wenzel P, Kleschyov AL, Hortmann M, Torzewski M, et al. Differential effects of heart rate reduction with ivabradine in two models of endothelial dysfunction and oxidative stress. Basic Res Cardiol. (2011) 106:1147–58. doi: 10.1007/s00395-011-0227-3
32. Custodis F, Baumhäkel M, Schlimmer N, List F, Gensch C, Böhm M, et al. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation. (2008) 117:2377–87. doi: 10.1161/CIRCULATIONAHA.107.746537
33. Custodis F, Fries P, Müller A, Stamm C, Grube M, Kroemer HK, et al. Heart rate reduction by ivabradine improves aortic compliance in apolipoprotein E-deficient mice. J Vasc Res. (2012) 49:432–40. doi: 10.1159/000339547
34. Aquila G, Morelli MB, Vieceli Dalla Sega F, Fortini F, Nigro P, Caliceti C, et al. Heart rate reduction with ivabradine in the early phase of atherosclerosis is protective in the endothelium of ApoE-deficient mice. J Physiol Pharmacol. (2018) 69:35–52. doi: 10.26402/jpp.2018.1.04
35. Luong L, Duckles H, Schenkel T, Mahmoud M, Tremoleda JL, Wylezinska-Arridge M, et al. Heart rate reduction with ivabradine promotes shear stress-dependent anti-inflammatory mechanisms in arteries. Thromb Haemost. (2016) 116:181–90. doi: 10.1160/TH16-03-0214
36. Bonadei I, Sciatti E, Vizzardi E, Fabbricatore D, Pagnoni M, Rossi L, et al. Effects of ivabradine on endothelial function, aortic properties and ventricular-arterial coupling in chronic systolic heart failure patients. Cardiovasc Ther. (2018) 36:e12323. doi: 10.1111/1755-5922.12323
37. Reil JC, Hohl M, Reil GH, Granzier HL, Kratz MT, Kazakov A, et al. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. (2013) 34:2839–49. doi: 10.1093/eurheartj/ehs218
38. Walcher T, Bernhardt P, Vasic D, Bach H, Durst R, Rottbauer W, et al. Ivabradine reduces chemokine-induced CD4-positive lymphocyte migration. Mediators Inflamm. (2010) 2010:751313. doi: 10.1155/2010/751313
39. Mangiacapra F, Colaiori I, Ricottini E, Balducci F, Creta A, Demartini C, et al. Heart Rate reduction by IVabradine for improvement of ENDothELial function in patients with coronary artery disease: the RIVENDEL study. Clin Res Cardiol. (2017) 106:69–75. doi: 10.1007/s00392-016-1024-7
40. Hohneck AL, Fries P, Ströder J, Schneider G, Wagenpfeil S, Schirmer SH, et al. Effects of heart rate reduction with ivabradine on vascular stiffness and endothelial function in chronic stable coronary artery disease. J Hypertens. (2019) 37:1023–31. doi: 10.1097/HJH.0000000000001984
41. Custodis F, Gertz K, Balkaya M, Prinz V, Mathar I, Stamm C, et al. Heart rate contributes to the vascular effects of chronic mental stress: effects on endothelial function and ischemic brain injury in mice. Stroke. (2011) 42:1742–9. doi: 10.1161/STROKEAHA.110.598607
42. Wu X, You W, Wu Z, Ye F, Chen S. Ivabradine promotes angiogenesis and reduces cardiac hypertrophy in mice with myocardial infarction. Anatol J Cardiol. (2018) 20:266–72. doi: 10.14744/AnatolJCardiol.2018.46338
43. Dedkov EI, Zheng W, Christensen LP, Weiss RM, Mahlberg-Gaudin F, Tomanek RJ. Preservation of coronary reserve by ivabradine-induced reduction in heart rate in infarcted rats is associated with decrease in perivascular collagen. Am J Physiol Heart Circ Physiol. (2007) 293:H590–8. doi: 10.1152/ajpheart.00047.2007
44. Chen SL, Hu ZY, Zuo GF, Li MH, Li B. I(f) current channel inhibitor (ivabradine) deserves cardioprotective effect via down-regulating the expression of matrix metalloproteinase (MMP)-2 and attenuating apoptosis in diabetic mice. BMC Cardiovasc Disord. (2014) 14:150. doi: 10.1186/1471-2261-14-150
45. Xie M, Huang HL, Zhang WH, Gao L, Wang YW, Zhu XJ, et al. Increased sarcoplasmic/endoplasmic reticulum calcium ATPase 2a activity underlies the mechanism of the positive inotropic effect of ivabradine. Exp Physiol. (2020) 105:477–88. doi: 10.1113/EP087964
46. Kakehi K, Iwanaga Y, Watanabe H, Sonobe T, Akiyama T, Shimizu S, et al. Modulation of sympathetic activity and innervation with chronic ivabradine and β-blocker therapies: analysis of hypertensive rats with heart failure. J Cardiovasc Pharmacol Ther. (2019) 24:387–96. doi: 10.1177/1074248419829168
47. El-Naggar AE, El-Gowilly SM, Sharabi FM. Possible ameliorative effect of ivabradine on the autonomic and left ventricular dysfunction induced by doxorubicin in male rats. J Cardiovasc Pharmacol. (2018) 72:22–31. doi: 10.1097/FJC.0000000000000586
48. Böhm M, Borer JS, Camm J, Ford I, Lloyd SM, Komajda M, et al. Twenty-four-hour heart rate lowering with ivabradine in chronic heart failure: insights from the SHIFT Holter substudy. Eur J Heart Fail. (2015) 17:518–26. doi: 10.1002/ejhf.258
49. Kurtoglu E, Balta S, Karakus Y, Yasar E, Cuglan B, Kaplan O, et al. Ivabradine improves heart rate variability in patients with nonischemic dilated cardiomyopathy. Arq Bras Cardiol. (2014) 103:308–14. doi: 10.5935/abc.20140109
50. Tsioufis KP, Dimitriadis K, Koutra E, Kalos T, Fragoulis C, Nikolopoulou L, et al. Effects of ivabradine on sympathetic overdrive and arterial stiffening in hypertensive patients with metabolic syndrome: a 6 month follow-up study. J Am Coll Cardiol. (2018) 71:1807. doi: 10.1016/S0735-1097(18)32348-9
51. Busseuil D, Shi Y, Mecteau M, Brand G, Gillis MA, Thorin E, et al. Heart rate reduction by ivabradine reduces diastolic dysfunction and cardiac fibrosis. Cardiology. (2010) 117:234–42. doi: 10.1159/000322905
52. Milliez P, Messaoudi S, Nehme J, Rodriguez C, Samuel JL, Delcayre C. Beneficial effects of delayed ivabradine treatment on cardiac anatomical and electrical remodeling in rat severe chronic heart failure. Am J Physiol Heart Circ Physiol. (2009) 296:H435–41. doi: 10.1152/ajpheart.00591.2008
53. McMurray JJ. It is BEAUTIFUL we should be concerned about, not SIGNIFY: is ivabradine less effective in ischaemic compared with non-ischaemic LVSD?. Eur Heart J. (2015) 36:2047–9. doi: 10.1093/eurheartj/ehv190
54. Oliphant CS, Owens RE, Bolorunduro OB, Jha SK. Ivabradine: a review of labeled and off-label uses. Am J Cardiovasc Drugs. (2016) 16:337–47. doi: 10.1007/s40256-016-0178-z
55. Cappato R, Castelvecchio S, Ricci C, Bianco E, Vitali-Serdoz L, Gnecchi-Ruscone T, et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: a prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J Am Coll Cardiol. (2012) 60:1323–9. doi: 10.1016/j.jacc.2012.06.031
56. McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace. (2011) 13:427–30. doi: 10.1093/europace/euq390
57. Simko F, Baka T. Chronotherapy as a potential approach to hypertensive patients with elevated heart rate? Br J Clin Pharmacol. (2019) 85:1861–2. doi: 10.1111/bcp.14020
58. Palatini P, Rosei EA, Casiglia E, Chalmers J, Ferrari R, Grassi G, et al. Management of the hypertensive patient with elevated heart rate: statement of the Second Consensus Conference endorsed by the European Society of Hypertension. J Hypertens. (2016) 34:813–21. doi: 10.1097/HJH.0000000000000865
59. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. (2018) 72:e53–90. doi: 10.1161/HYP.0000000000000084
60. Ruilope LM, Rodríguez-Sánchez E, Navarro-García JA, Segura J, Órtiz A, Lucia A, Ruiz-Hurtado G. Resistant hypertension: new insights and therapeutic perspectives. Eur Heart J Cardiovasc Pharmacother. (2020) 6:188–93. doi: 10.1093/ehjcvp/pvz057
61. Simko F, Reiter RJ, Paulis L. Melatonin as a rational alternative in the conservative treatment of resistant hypertension. Hypertens Res. (2019) 42:1828–31. doi: 10.1038/s41440-019-0318-3
62. Simko F, Pechanova O. Recent trends in hypertension treatment: perspectives from animal studies. J Hypertens Suppl. (2009) 27:S1–4. doi: 10.1097/01.hjh.0000358829.87815.d4
63. Baka T, Simko F. Nondipping heart rate: a neglected cardiovascular risk factor based on autonomic imbalance? Auton Neurosci. (2018) 210:83–4. doi: 10.1016/j.autneu.2018.02.001
64. Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Blunted heart rate dip during sleep and all-cause mortality. Arch Intern Med. (2007) 167:2116–21. doi: 10.1001/archinte.167.19.2116
65. Eguchi K, Hoshide S, Ishikawa J, Pickering TG, Schwartz JE, Shimada K, et al. Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J Hypertens. (2009) 27:2265–70. doi: 10.1097/HJH.0b013e328330a938
66. Biyik Z, Yavuz YC, Altintepe L, Celik G, Guney I, Oktar SF. Nondipping heart rate and associated factors in patients with chronic kidney disease. Clin Exp Nephrol. (2019) 23:1298–305. doi: 10.1007/s10157-019-01782-x
67. Choi HY, Noh YH, Cho SH, Ghim JL, Choe S, Kim UJ, et al. Evaluation of pharmacokinetic and pharmacodynamic profiles and tolerability after single (2.5, 5, or 10 mg) and repeated (2.5, 5, or 10 mg bid for 4.5 days) oral administration of ivabradine in healthy male Korean volunteers. Clin Ther. (2013) 35:819–35. doi: 10.1016/j.clinthera.2013.04.012
68. Borer JS, Tardif JC. Efficacy of ivabradine, a selective I(f) inhibitor, in patients with chronic stable angina pectoris and diabetes mellitus. Am J Cardiol. (2010) 105:29–35. doi: 10.1016/j.amjcard.2009.08.642
69. Vaillant F, Lauzier B, Ruiz M, Shi Y, Lachance D, Rivard ME, et al. Ivabradine and metoprolol differentially affect cardiac glucose metabolism despite similar heart rate reduction in a mouse model of dyslipidemia. Am J Physiol Heart Circ Physiol. (2016) 311:H991–1003. doi: 10.1152/ajpheart.00789.2015
70. Aziriova S, Repova K, Krajcirovicova K, Baka T, Zorad S, Mojto V, et al. Effect of ivabradine, captopril and melatonin on the behaviour of rats in L-nitro-arginine methyl ester-induced hypertension. J Physiol Pharmacol. (2016) 67:895–902.
71. Krajcirovicova K, Aziriova S, Baka T, Repova K, Adamcova M, Paulis L, et al. Ivabradine does not impair anxiety-like behavior and memory in both healthy and L-NAME-induced hypertensive rats. Physiol Res. (2018) 67(Suppl. 4):S655–64. doi: 10.33549/physiolres.934048
72. Brismar K, Mogensen L, Wetterberg L. Depressed melatonin secretion in patients with nightmares due to beta-adrenoceptor blocking drugs. Acta Med Scand. (1987) 221:155–8. doi: 10.1111/j.0954-6820.1987.tb01260.x
73. Gleiter CH, Deckert J. Adverse CNS-effects of beta-adrenoceptor blockers. Pharmacopsychiatry. (1996) 29:201–11. doi: 10.1055/s-2007-979572
74. Yu Y, Hu Z, Li B, Wang Z, Chen S. Ivabradine improved left ventricular function and pressure overload-induced cardiomyocyte apoptosis in a transverse aortic constriction mouse model. Mol Cell Biochem. (2019) 450:25–34. doi: 10.1007/s11010-018-3369-x
75. Melka J, Rienzo M, Bizé A, Jozwiak M, Sambin L, Hittinger L, et al. Improvement of left ventricular filling by ivabradine during chronic hypertension: involvement of contraction-relaxation coupling. Basic Res Cardiol. (2016) 111:30. doi: 10.1007/s00395-016-0550-9
Keywords: ivabradine, blood pressure, endothelial dysfunction, vascular stiffness, neurohumoral activation
Citation: Simko F and Baka T (2021) Ivabradine and Blood Pressure Reduction: Underlying Pleiotropic Mechanisms and Clinical Implications. Front. Cardiovasc. Med. 8:607998. doi: 10.3389/fcvm.2021.607998
Received: 18 September 2020; Accepted: 07 January 2021;
Published: 10 February 2021.
Edited by:
Modar Kassan, University of Tennessee Health Science Center (UTHSC), United StatesReviewed by:
Sung Joon Kim, Seoul National University, South KoreaCopyright © 2021 Simko and Baka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fedor Simko, ZmVkb3Iuc2lta29AZm1lZC51bmliYS5zaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.