
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Cardiovasc. Med., 15 March 2021
Sec. Hypertension
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.602086
This article is part of the Research TopicHighlights in Hypertension: 2021View all 8 articles
 Anouk Tanghe1,2
Anouk Tanghe1,2 Bert Celie1,3
Bert Celie1,3 Samyah Shadid4
Samyah Shadid4 Ernst Rietzschel5,6
Ernst Rietzschel5,6 Jos Op ‘t Roodt7
Jos Op ‘t Roodt7 Koen D. Reesink7
Koen D. Reesink7 Elsa Heyman2†
Elsa Heyman2† Patrick Calders1*†
Patrick Calders1*†Introduction: Patients with type 2 diabetes mellitus are at high risk to develop vascular complications resulting in high morbidity and mortality. Cocoa flavanols are promising nutraceuticals with possible beneficial vascular effects in humans. However, limited research is currently available on the vascular effects in a diabetic population with inconsistent results. Possible reasons for this inconsistency might be heterogeneity in the given intervention (dose per time and day, single dose vs. split-dose, placebo formula) and the studied population (blood pressure at baseline, duration of diabetes, use of vasoactive antihypertensive and antidiabetic drugs, sex). Therefore, we aimed to develop a randomized, double-blinded, placebo-controlled cross-over trial to investigate whether cocoa flavanols have an acute impact on blood pressure and vascular reactivity in patients with type 2 diabetes with and without arterial hypertension.
Methods and Analysis: We will include participants in four groups: (i) patients with type 2 diabetes without arterial hypertension, (ii) patients with type 2 diabetes with arterial hypertension and 1 antihypertensive drug, (iii) non-diabetic participants with essential hypertension and 1 antihypertensive drug, and (iv) healthy controls. All participants will complete the same protocol on both testing days, consuming high-flavanol cocoa extract (790 mg flavanols) or placebo. Macrovascular endothelial function (flow-mediated dilation) and blood pressure will be measured before and after capsule ingestion. Forearm muscle vasoreactivity (near-infrared spectroscopy) and brachial artery blood flow (echo-doppler) will be assessed in response to a dynamic handgrip exercise test after capsule ingestion. Data will be analyzed with a random intercept model in mixed models.
Clinical Trial Registration: www.Clinicaltrials.gov, identifier: NCT03722199.
Nitric Oxide, produced by the endothelial cells, is of crucial importance for general vascular health. It triggers relaxation of the vascular smooth muscle cells through accumulation of intracellular cyclic guanosine monophosphate (cGMP) (1–5).
Type 2 diabetes (T2DM) is the most prevalent type [90% (6, 7)] of diabetes mellitus, a highly prevalent disorder [estimated at 425 million people worldwide in 2017 and is expected to be 629 million in 2045 (8)] and poses a challenge to global health. It is characterized by chronic hyperglycemia, which increases oxidative stress. Free radicals easily bind with and simultaneously deactivate nitric oxide to form peroxynitrite. Both high amounts of oxidative stress and nitric oxide depletion increase the risk for developing micro- (retinopathy, nephropathy, and neuropathy) and macrovascular (cardiovascular, cerebrovascular, and peripheral artery diseases) complications (9, 10). These complications decrease quality of life and increase the global burden of T2DM in terms of health care costs, morbidity [hypertension is present in >60% of all patients with T2DM (11)], and even mortality (12–15). The past years researchers have been investigating products to limit or delay the onset of diabetic vascular complications with special attention for non-pharmacological approaches to counter polypharmacy.
Flavanols are promising nutraceuticals from the flavonoid family, a class of polyphenols (16), which can be found in several fruits, beans, teas, red wine, but predominantly in cocoa products (16, 17). Especial interest goes to flavanols derived from the seeds of the cocoa bean (Theobroma cacao), cocoa flavanols (CF), as they have higher antioxidant activity and more phenolic compounds (18). The increased attention for the effects of cocoa originates from research on the Kuna Indians. In contrast to migrated Kuna Indians to urban areas, Kuna Indians living on the San Blas Islands off the coast of Panama show low blood pressure (BP), even with increasing age, and have lower frequency of diabetes mellitus, cancer, and cardiovascular diseases (19). Causal research for this cardiovascular protection focused on environmental factors including nutrition and revealed that island-dwelling Kuna drink daily more than five cups of cocoa with high concentrations of flavanols and procyanidins (19–21). Starting from evidence based on further research on the vascular effects of CF, the European Food Safety Authority (EFSA) stated that CF help to preserve endothelium-dependent vasodilation in healthy populations, if taken in quantities exceeding 200 mg CF daily. This equals 10 g high-flavanol dark chocolate or 2.5 g high-flavanol cocoa powder (22).
The mechanisms of action of these CF are still debated. It is believed that they improve endothelial function (23), decrease BP (24), ameliorate insulin sensitivity (25–27), influence various inflammatory processes (28), and prevent platelet aggregation (29, 30) via antioxidant properties (31, 32), increasing nitric oxide bioavailability (33, 34), and inhibition of the angiotensin-converting-enzyme activity (35, 36). These effects seem to be induced, at least in part, by epicatechin, a highly active monomeric form of CF (37, 38).
It could be presumed that populations with T2DM could benefit from the intake of CF as it potentially reduces cardiovascular risk. CF would indeed improve both endothelial function and insulin sensitivity and so influence cardiovascular as well as metabolic disorders (39). Nonetheless, limited research with a high degree of inconsistency due to large heterogeneity (dose per time or day, acute or chronic intake, single dose vs. split-dose, placebo formula, and characteristics of population e.g., sex, BP at baseline, and use of vasoactive drugs like insulin and antihypertensives) is reported.
As presented in Table 1, at the moment, six studies investigated the effect of chronic CF intake (8 weeks−1 year) on vascular function (40–45) in patients with T2DM. Only two showed a statistically and clinically relevant decrease in BP (40, 45) and only two indicated a statistically improvement of endothelial function (41, 42) (Table 1). The heterogeneity of results about mid- to long-term effects of CF in patients with diabetes had been recently approached through a systematic review and meta-analysis (48). This paper indeed shows low quality of evidence of slight improvements in BP after mid/long-term CF ingestion. However, risk of bias, imprecision of the publications, and inconsistency and heterogeneity among the reports are reported and could be cause for the lack of a definite conclusion. This meta-analysis ultimately highlights the need for further research with a robust methodology taking into account possible confounding factors like hypertension at baseline and intake of BP lowering medication. Antihypertensive drugs, which were never considered in these papers on chronic effects of CF in patients with T2DM, have indeed a great impact on vasoreactivity (49) and may hence interfere with the effects of CF.
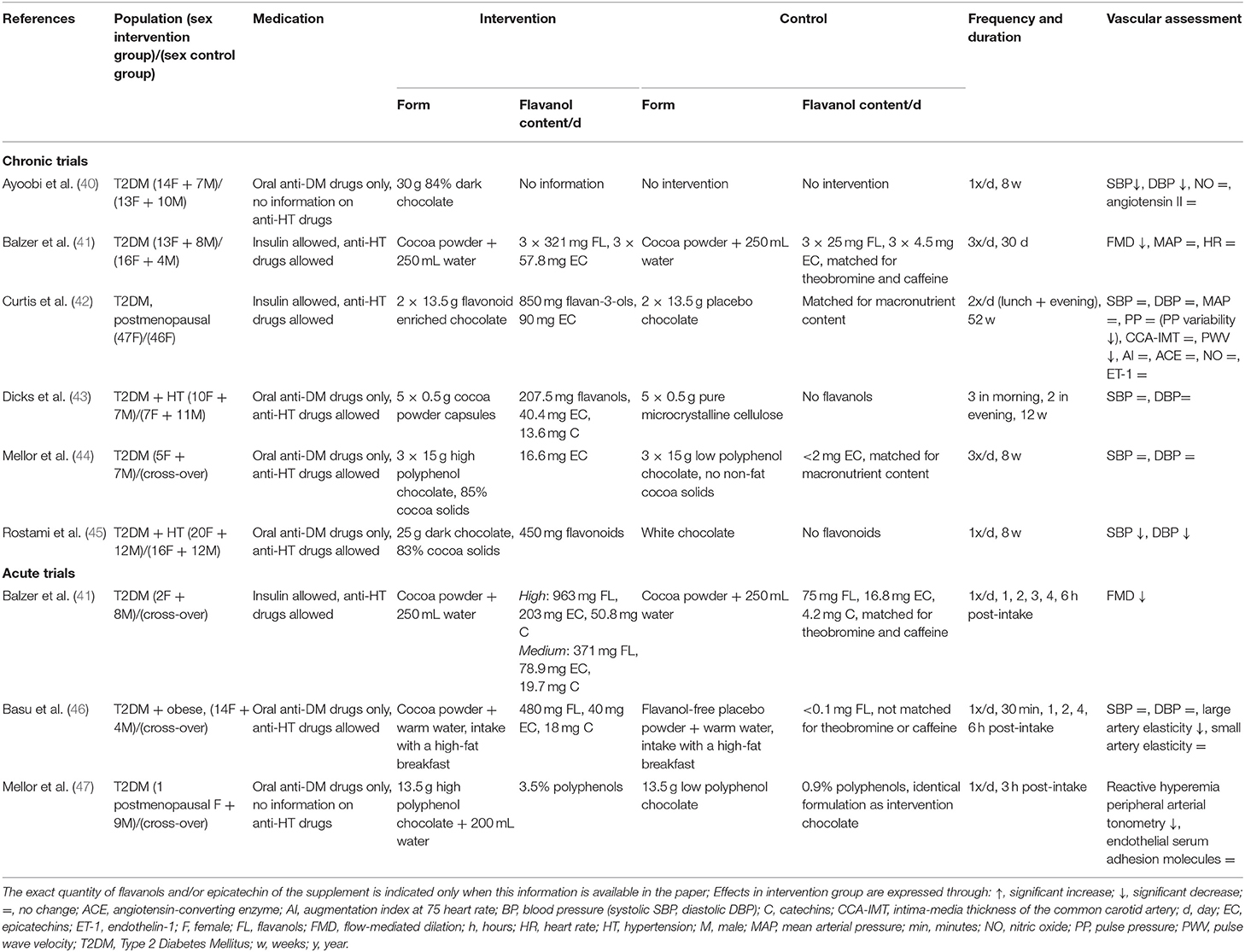
Table 1. Characteristics of chronic and acute trials examining the effect of cocoa flavanols in patients with type 2 diabetes.
The first step for profoundly testing these possible confounding factors would lie in the design of acute protocols applied to several well-characterized (particularly hypertension and its medications) groups of patients with T2DM. Up to now, three studies evaluated the vascular effects of acute CF supplementation in patients with T2DM (41, 46, 47). Two detected a significant improvement of endothelial function (41, 47) and one indicated a decrease in large artery elasticity (46) (Table 1). However, the same way as in the chronic studies, the three acute studies either gave no information about antihypertensive drugs or allowed the use of antihypertensive treatment (different types are reported) without considering this treatment as a possible confounding factor in the analyses (Table 1). To our knowledge, only four studies investigated the effect of CF when combined with antihypertensive drugs (different types are reported) in non-diabetic hypertensive adults (50, 51), in non-diabetic heart transplant recipients (52), and in non-diabetic adults with congestive heart failure (53). These showed a supplementary effect of cocoa intake on BP (50, 51) and/or endothelial function (50, 52, 53).
In addition, together with the heart, the micro- and macrocirculation determine the hemodynamics of the circulating system (54). It is important to study both systems simultaneously, but apart from one report (46) none of these studies in patients with T2DM investigated the effect of CF simultaneously in both of these vascular beds (i.e., micro and macrovascular beds) (Table 1).
In this study, we will take into account these results, assumptions, and points of heterogeneity as will be explained in this paper. In addition, this study will investigate the impact of CF on both micro- and macrovascular functions. As CF would increase nitric oxide, we hypothesize more effect on macrovessels as microvessels are also dependent on other vasodilators like prostaglandins. However, as little is known so far, our research will provide novel insights on this matter. Moreover, acute protocols will help to be sure of the efficacy of the dose chosen, to know which of the vascular (either micro or macrovascular) beds would be more impacted, and to identify whether some patients would be non-responders because of their medications. Hence, since our study has a robust methodology, it may act as a sort of “pilot” for the setup of long-term trials.
This acute, randomized, double-blinded, placebo-controlled cross-over study aims to investigate whether a single intake of a high dose of CF (790 mg flavanols) induces an improvement in endothelial function (primary outcome), a reduction in BP, and an enhancement in muscle vasoreactivity and oxygenation in patients with T2DM compared to healthy controls.
Because hypertension is a common comorbidity in patients with T2DM and as little is known so far concerning possible interferences between antihypertensive drugs and CF actions especially in patients with diabetes mellitus, this study will additionally investigate possible influence of beta-blockers (BB), angiotensin converting enzyme inhibitor (ACEi), or angiotensin receptor blocker (ARB) in diabetic persons with arterial hypertension and non-diabetic persons with essential hypertension treated with these drugs.
The trial is a single center study and will be executed at the Ghent University Hospital (Belgium). All participants will complete the same protocol on both test days, however consuming a different type of capsules in a randomized order: high-flavanol cocoa extract or placebo. The flavanols used in this trial were extracted from the cocoa bean. A wash-out period of minimal 3 days and maximal 2 weeks is provided. The first measurement was conducted on October 5th, 2018 and since the COVID-19 pandemic forced to cancel all measurements for about 6 months, the end date of the study was postponed and is estimated in June 2021.
All subjects are divided in four groups: (i) patients with T2DM with arterial hypertension (with BB, n = 5/ACEi, n = 5/ARB, n = 5) (n = 15), (ii) patients with T2DM without arterial hypertension (n = 10), (iii) non-diabetic patients with essential arterial hypertension (BB, n = 10/ACEi, n = 10/ARB, n = 10) (n = 30), and (iv) healthy participants (control group; n = 20).
Inclusion and exclusion criteria are summarized in Table 2.
Subjects are recruited by endocrinologists and other medical specialists, general practitioners, dieticians and investigator's acquaintances. Flyers are distributed in the Ghent University Hospital and by pharmacists and physiotherapists. In addition, the essential information for possible participants of our study is disseminated on media and social media.
No patient or patient advisor was/is involved with study design, recruitment or conduct.
As depicted in Figure 1, patients will arrive at the lab (Ghent University Hospital) at 8 o'clock after an overnight fast of minimal 8 h. Blood samples will be collected and participants will be evaluated for body composition [weight, height, skinfolds, and fat% (bio-electrical impedance analyzer)] and BP, followed by a standardized breakfast with negligible flavonoid and nitrate/nitrite amounts, in accordance to guidelines of a dietician (Table 3), and consumed within 15 min. Participants have to choose 1 formula of breakfast which will be consumed at both visits. After 15 min rest period, a baseline Flow-Mediated Dilation (FMD) measurement will be performed, followed by the ingestion of CF-enriched capsules (2.5 g of cocoa extract which contains 790 mg flavanols of which 150 mg epicatechin, Naturex©, France) or placebo (maltodextrin and an equivalent dose of theobromine and caffeine compared to the CF-enriched capsules) (Table 4). The dose of CF given in this study is based on the research of Balzer et al. (41) who investigated the effect of different doses of CF (high dose = 963 mg CF of which 203 mg epicatechin, medium dose = 371 mg CF of which 78.9 mg epicatechin, and low dose (control) = 75 mg CF of which 16.8 mg epicatechin), closely matched for theobromine and caffeine, on FMD in 10 patients with T2DM. The equilibration of theobromine and caffeine, both vasoactive compounds of cocoa (38, 56, 57), in the different interventions is a very important quality of this study and will ensure to test isolated vascular effects of CF.
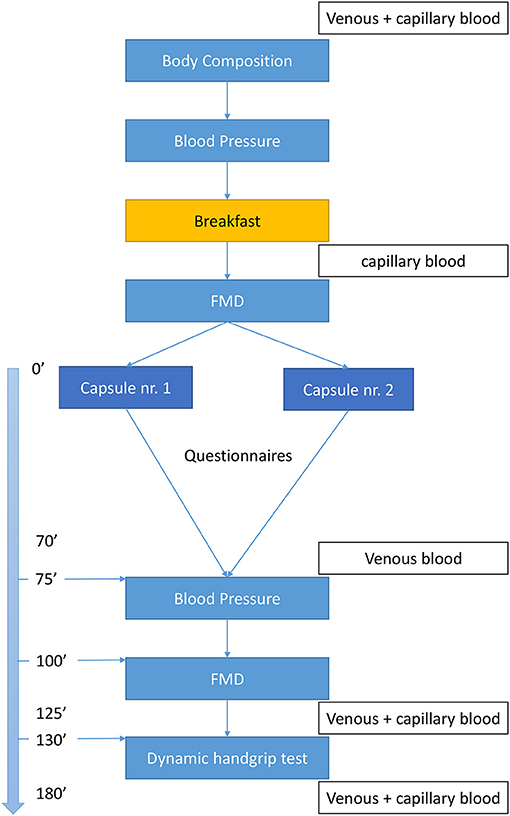
Figure 1. Flowchart. FMD, flow-mediated dilation test; capillary blood, finger prick to measure capillary glycaemia (only patients with T2DM).
The investigator will ensure all capsules are taken in properly. After ingestion, the participants will remain seated for 70 min. Thereafter, blood samples will be collected and a second BP and FMD assessment followed by a dynamic handgrip exercise (dHGE) test with simultaneous near-infrared-spectroscopy (NIRS) monitoring and blood flow measurements will be executed. Venous blood samples will be drawn before and after the dHGE test.
All measurements post intake will be performed within 2 h as highest circulating concentrations of flavanols are found between 90 and 120 min after consumption (58, 59).
Participants will fill in questionnaires (once) to evaluate factors which may impact vascular reactivity [i.e., daily physical activity [International Physical Activity Questionnaire—long version (60)], flavanols-intake [a self-designed questionnaire about the frequency of flavanol-enriched food intake (16, 17, 61)], sleep behavior (Epworth Sleepiness Scale, STOP-BANG), and autonomic function (Autonomic Symptom Profile-COMPASS). We will also assess quality of life and general health status (36-Item Short Form Health Survey, World Health Organization Quality of Life questionnaire-BREF, and only for patients with T2DM Diabetes Quality Of Life questionnaire). In addition to the questionnaires, each participant's physical activity levels (62) and glycemic excursions and variability (63) will be objectively measured during 1 usual week using accelerometry and a Continuous Glucose Monitoring System, respectively.
Subjects will be asked not to participate in other trials from 3 weeks before start of this study to 1 week after the second study day. Supplement or vitamin consumption that could interfere with the mechanisms of action of flavanols have to be suspended for at least 4 weeks prior start of the study. In addition, to minimize flavanol intake before each study day, participants will be asked to follow guidelines (16, 17, 61):
➣ 3 days before each study day, participants will be asked to drink maximal two cups of tea per day, to avoid red wine or cider, to refrain from eating chocolate (in any form), beans, and rhubarb and to consume maximal two small portions of fruits (piece, juice, or jam) or one portion in combination with 10 g nuts.
➣ Twenty-four hours before each study day, participants will be asked to refrain from vigorous physical activity (apart from daily movements as climbing stairs, biking to the train station, walking to the car etc.), alcohol or caffeine containing drinks (e.g., coffee, cola, and tea). They have to consume the same meal the evening before both study days.
➣ Eight hours before the actual start of the study, participants will be asked to fasten (no food or drink intake, apart a small amount of water) and, importantly, to take in their medication as usual (identical dose as prescribed by their physician). Antihypertensive medication need to be taken in exactly 2 h before actual start of the study. Antidiabetics must be taken in at home or at the lab during breakfast depending on type of the drug. Metformin, SGLT-2 inhibitors, and GLP-1 analoga may be taken in at home or at the lab and all sulfonylurea drugs must be taken in at the lab during breakfast. For insulin, the long-acting insulin must be taken in at home and the short-acting insulin must be taken in at the lab during breakfast.
In this cross-over study, every participant will receive CF-enriched and placebo capsules. Randomization of capsules will be done by sealed envelopes and type of capsules will be indicated by number 1 or number 2. Each participant may choose 1 envelope at the first study day. Both types of capsules have an identical look and taste. Hence, participants will be blinded to their group allocation. Furthermore, since type of capsules will be identified by numbers, outcome assessors and personnel involved in data collection and data analysis will be blinded to participants' group allocation throughout the entire trial.
All researchers, outcome assessors, data collector, data manager, data entry personnel, and statistician will receive special training regarding the standard procedure and data management. During the recruitment period, our data collector will record the baseline characteristics of participants in case report forms and all data will be checked by the data manager.
Study data will be pseudonymized, collected, and managed using REDCap, a secure, GDPR-proofed, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for data integration and interoperability with external sources (64, 65).
Solely two main investigators (endocrinologist and study responsible) will have the authority to decode the pseudonymized data. Concerning obtained data of FMD measurements, all clips will be saved in dicom-format and stored at a central server (University Ghent).
Adverse events after intake will be described as it is monitored by oral interrogation during the study day and by e-mail if participants experience some side-effects. So far, no adverse effects were defined in literature. However, in the article of Monagas et al. (66) 1 out of 42 included subjects reported constipation during the intake of cocoa concomitant with 250 mL skim milk for 4 weeks, which was solved by increasing fiber intake.
FMD is a non-invasive technique and the gold standard to measure endothelial function. The FMD measurement will be performed at the brachial artery, at the non-dominant side, following a standardized protocol described by Thijssen et al. (67). An ultrasound system (GE, Vivid 7) with a linear probe (12L, 7–10 MHz) will be used to assess the brachial artery diameter based upon longitudinal images with the lumen–intima interface visualized on both (anterior and posterior) walls. The arterial occlusion will be performed via a sphygmomanometer cuff (Hokanson, SC5™, 6 × 83 cm), placed 5–10 cm below the elbow, inflated and held between 220 and 240 mmHg (68, 69) for 5 min. For stability, the probe will be hold tight by a stand-off probe support. To assess the effect of the reactive hyperemia, the brachial artery will be visualized pre-occlusion for 90 s (mean of three individual clips comprising each 30 s) and post-occlusion for 4 min with additional 15 s before cuff release. Although it is recommended to perform an FMD measurement in fasting state (67), this is not feasible since the entire protocol lasts around 5 h. However, the measurements will be done 15 and 150 min after food intake (standardized breakfast at each visit). Boundaries for diameter measurement will be identified automatically by means of a boundary tracking software (Quipu, diastolic phase) and optically controlled by a single, independent and blinded investigator. When tracking is impaired, the investigator will restore this tracking with maintenance of region of interest. Data obtained from false tracking or altered region of interest will be removed from analysis. FMD measurement is a challenging technique and has a significant learning curve. At least 100 scans supervised by a specialist were done prior to the start of this study.
BP measurements will be carried out at the dominant side, in sitting position, after 3 min of rest by an automatic device (Tango+, SunTech Medical). Heart rate and systolic and diastolic BP will be assessed for 21 min with an interval of 3 min, pre- and post-capsules intake. During the recording, participants will be asked to remain calm and silent and refrain from drinking. Systolic and diastolic BP, as well as mean arterial pressure, pulse pressure, and heart rate will be used in analysis.
A maximal dHGE will be performed with simultaneous NIRS (OxiplexTS, ISS, Champaign, IL, USA) monitoring to assess the oxygenation and vasoreactivity at the level of arterioles and capillaries of Musculus flexor digitorum superficialis, Musculus flexor carpi ulnaris, and Musculus flexor carpi radialis. A reliable handgrip exercise protocol, specifically designed for patient populations using NIRS measurements, will be carried out according to Celie et al. (70). In order to minimize bias and to work with relative values, an arterial occlusion of the ipsilateral upper arm will be performed by a pneumatic cuff (Hokanson, SC5™, 6 × 83 cm) inflated and held between 220 and 240 mmHg for 5 to maximal 7 min, until maximal deoxygenation (hemoglobin and myoglobin) values are reached (71). In this study, the maximal voluntary contraction will be calculated by means of two attempts with 10 s in between. The exercise test, consisting of 2-min periods of an incremental cyclic contractions protocol (1 s contraction, 1 s relaxation) followed by 1-min rest periods, will start at 20% of the maximal voluntary contraction and increase by 10% of the maximal voluntary contraction each step until exhaustion. Total hemoglobin [sum of deoxy- and oxyhemogblin, which reflects the change in regional blood volume (72)], deoxyhemoglobin and –myoglobin, and saturation will be assessed according to Celie et al. (70).
Blood flow through the brachial artery via echo-doppler will be measured according to Celie et al. (71) (same transducer and linear probe as used for the FMD measurement) at baseline (before cuff occlusion), immediately after cuff occlusion, and in the periods of rest after each increasing step.
Levels of uric acid (photometry, Architect c16000, Abbott), vitamin C (colorimetric method, R-biopharm/Roche reagent, Indiko Plus, Thermo Fisher), and glucose (photometry, Architect c16000, Abbott) will be measured at each study day in whole blood (anticoagulant-free tubes). Plasma and serum derived from EDTA and anticoagulant-free tubes will be centrifuged (3,500 g for 10 min at 10°C) and thereafter stored at −80°C together with a cryovial whole blood in the medical biobank of the Ghent University Hospital (Bioresource center Ghent, Gent, Belgium, ID: BE 71067049) (73). At the end of the study, cholesterol (photometry, Architect c16000, Abbott), high density lipoproteins (photometry, Architect c1600, Abbott), triglycerides (photometry, Architect c16000, Abbott), high sensitive C-reactive protein (particle-enhanced immunonephelometry, BN II, Siemens), vitamin E (liquid/liquid extraction followed by detection via UPLC-DAD), free fatty acids (enzymatic colorimetry, Cobas 8000 e801, Roche Diagnostics), and free insulin (Cobas e801 Roche, ECLIA) will be determined in serum, while hemoglobin A1c (exchange chromatography—Tosoh HLV-723 G8) and haptoglobin (Behring Nephelometer analyzer II), a marker of iron metabolism (74), will be determined in whole blood. All samples will be analyzed at the lab of clinical biology of Ghent University Hospital.
In each participant, physical activity [steps per day and moderate-to-vigorous physical activity (MVPA)] will be evaluated with an accelerometer [Actigraph wGT3X-BT, dominant side, hip (75)] and glycemic excursions (percentages of time in hyper- or hypoglycemic ranges and time in range), risk for hypo- and hyperglycemia (Low and High Blood Glucose Indexes, LBGI and HBGI), and glycemic variability [standard deviation, coefficient of variation, and mean amplitude of glucose excursion (MAGE)] (76) by continuous interstitial glucose measurement (blind mode, IPro2, Medtronic, abdomen) for 1 week, immediately after the second study day. During this week, the above-mentioned restrictions concerning food intake and physical activity are not applicable and participants are encouraged to maintain their normal daily habits. Additionally, participants are asked to fill in a diary concerning their food intake and their physical activities for each day.
Sample size calculations are based on previous research focusing on interventional studies with FMD measurements (77, 78) who established that in cross-over trials at least 20–30 participants are required for a minimal detectable change of 1.5–2% in FMD (80% power, alpha-level = 0.05). Sample size calculation was done by SAS power and sample size, indicating a minimum of nine participants in each group (41). Data will be analyzed taking into account volunteer- and methodology-related factors (79).
Effects of CF ingestion on primary and secondary outcome measures will be evaluated within and between groups. Data will be analyzed using a random intercept model in mixed models. Fixed effects will be the group, the supplementation (cocoa flavanols vs. placebo), the time (pre- vs. post-intake of capsules) during each visit for repeated measurements, as well as group × supplementation interaction and time × supplementation interactions. We will also consider the order of both visits in a first intent and this factor will be kept in analyses only if significant. As all groups are matched by age, sex, and BMI, only medication intake, level of physical activity, glycemic excursions, risk for hypo- or hyperglycemia, and glycemic variability will be considered as covariates when analyzing data. In case of drop-outs or missing data, the participant is still included in data analysis providing one out of two visits is completed.
Results will be presented as mean ± standard deviation with its corresponding 95% confidence interval. Level of significance will be set at p < 0.05. Data analysis will be conducted using IBM SPSS statistics version 26.
In recent years, there has been an increased attention to polyphenols and their beneficial effects on vascular health. Several studies have been carried out in healthy participants to assess this. However, the studies, acute as well as chronic, on patients with T2DM with a high risk for vascular complications are scarce and show inconsistent results. Possible reasons for this inconsistency might be heterogeneity in the given intervention (dose, duration, source), the studied population (duration of diabetes, severity of comorbidities, BP at baseline, sex), and possibly used medication (vasoactive antihypertensive and antidiabetic drugs). Hence, there is a high need for more acute and chronic research concerning this topic in this population.
This work will provide novel data helpful for the development of strategies in the nutritional education of particularly vulnerable populations, given their high risk for developing cardiovascular disease, including non-pharmacological therapies and strategies that employ lifestyle modification. This intervention might also have implications for the preparation of recommendations in clinical practice guidelines and quality improvement programs aimed at the care of patients with T2DM.
Study procedures were approved by the ethical committee of Ghent University Hospital on April 5th, 2018 (STUDY B670201835660). To enquire all participants and to ensure making an informed decision to participate, each subject will receive an informed consent that first is explained orally by an investigator and thereafter sent by e-mail providing the opportunity to reread this form and to ask questions afterwards. The informed consent contains all details of the research (background, aims, and possible risks or advantageous) and is written in Dutch for good understanding. In case of important protocol modifications, all participants will be informed by e-mail. The results will be submitted to an international peer-reviewed journal and presented at scientific conferences. They will be disseminated through digital science communication platforms, including academic social media, to extend its outreach and usefulness.
AT, BC, SS, ER, JO, KR, EH, and PC were involved in the methodological design and drafting of the trial protocol. AT, SS, ER, PC, and EH have overall responsibility for the design, conduct, and decision to submit for publication. BC, JO, and KR are co-researchers. BC designed the dynamic handgrip exercise test and set up the plan for analysis. JO and KR designed the flow-mediated dilation measurement and set up the plan for analysis. AT, ER, SS, PC, and EH recruit participants into the study. AT, PC, and EH drafted the manuscript. All authors will contribute to data interpretation, conclusions, and dissemination and read and approved the final manuscript.
This research was funded by the Special Research Fund/Bijzonder Onderzoeksfonds (BOF) at Ghent University, Grant Number BOF17/DOC/050. This funding source was not involved in the setup of this trial protocol; in the writing of the report; and in the decision to submit the article for publication and will not be involved in the collection, analysis, and interpretation of data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.602086/full#supplementary-material
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-blockers; BP, blood pressure (SBP, systolic blood pressure; DBP, diastolic blood pressure); CF, cocoa-derived flavanols; dHGE, dynamic handgrip exercise; FMD, flow-mediated dilation; NIRS, Near-infrared spectroscopy; T2DM, type 2 diabetes mellitus.
1. Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation. (2009) 119:1433–41. doi: 10.1161/CIRCULATIONAHA.108.827022
2. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric-oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in-vivo. Circulation. (1995) 91:1314–9. doi: 10.1161/01.CIR.91.5.1314
3. Joannides R, Richard V, Haefeli WE, Linder L, Luscher TF, Thuillez C. Role of basal and stimulated release of nitric oxide in the regulation of radial artery caliber in humans. Hypertension. (1995) 26:327–31. doi: 10.1161/01.HYP.26.2.327
4. Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci USA. (1996) 93:9114–9. doi: 10.1073/pnas.93.17.9114
5. Takahashi M, Ikeda U, Masuyama J, Funayama H, Kano S, Shimada K. Nitric oxide attenuates adhesion molecule expression in human endothelial cells. Cytokine. (1996) 8:817–21. doi: 10.1006/cyto.1996.0109
6. Holman N, Young B, Gadsby R. Current prevalence of type 1 and type 2 diabetes in adults and children in the UK. Diabetic Med. (2015) 32:1119–20. doi: 10.1111/dme.12791
7. Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, Pinach S, et al. Incidence of type 1 and type 2 diabetes in adults aged 30–49 years: the population-based registry in the province of Turin, Italy. Diabetes Care. (2005) 28:2613–9. doi: 10.2337/diacare.28.11.2613
8. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Prac. (2014) 103:137–49. doi: 10.1016/j.diabres.2013.11.002
9. Honing ML, Morrison PJ, Banga JD, Stroes ES, Rabelink TJ. Nitric oxide availability in diabetes mellitus. Diabetes Metab Rev. (1998) 14:241–9. doi: 10.1002/(SICI)1099-0895(1998090)14:3<241::AID-DMR216>3.0.CO;2-R
10. Giacco F, Brownlee M, Schmidt AM. Oxidative stress and diabetic complications. Circ Res. (2010) 107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545
11. Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. (2012) 380:601–10. doi: 10.1016/S0140-6736(12)60987-8
12. Koopmanschap M. Coping with type II diabetes: the patient's perspective. Diabetologia. (2002) 45:S21–2. doi: 10.1007/s00125-002-0861-2
13. Bahia LR, Araujo DV, Schaan BD, Dib SA, Negrato CA, Leao MP, et al. The costs of type 2 diabetes mellitus outpatient care in the Brazilian public health system. Value Health. (2011) 14(5 Suppl. 1):S137–40. doi: 10.1016/j.jval.2011.05.009
14. Massi-Benedetti M. The cost of diabetes in Europe-type II: the CODE-2 study. Diabetologia. (2002) 45:S1–4. doi: 10.1007/s00125-002-0860-3
15. Hays NP, Galassetti PR, Coker RH. Prevention and treatment of type 2 diabetes: current role of lifestyle, natural product, and pharmacological interventions. Pharmacol Ther. (2008) 118:181–91. doi: 10.1016/j.pharmthera.2008.02.003
16. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. (2004) 79:727–47. doi: 10.1093/ajcn/79.5.727
17. Arts IC, van de Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J Agric Food Chem. (2000) 48:1746–51. doi: 10.1021/jf000025h
18. Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. (2003) 51:7292–5. doi: 10.1021/jf0344385
19. Hollenberg NK, Fisher ND, McCullough ML. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J Am Soc Hypertens. (2009) 3:105–12. doi: 10.1016/j.jash.2008.11.001
20. McCullough ML, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz HH, et al. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol. (2006) 47:S103–9. doi: 10.1097/00005344-200606001-00003
21. Chevaux KA, Jackson L, Villar ME, Mundt JA, Commisso JF, Adamson GE, et al. Proximate, mineral and procyanidin content of certain foods and beverages consumed by the Kuna Amerinds of Panama. J Food Composition Anal. (2001) 14:553–63. doi: 10.1006/jfca.2001.1027
22. EFSA Panel on Dietetic Products Nutrition Allergies. Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. (2012) 10:2809. doi: 10.2903/j.efsa.2012.2809
23. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. (2008) 88:38–50. doi: 10.1093/ajcn/88.1.38
24. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. (2017) 4:CD008893. doi: 10.1002/14651858.CD008893.pub3
25. Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. (2005) 46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70
26. Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. (2005) 81:611–4. doi: 10.1093/ajcn/81.3.611
27. Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. (2008) 138:1671–6. doi: 10.1093/jn/138.9.1671
28. Goya L, Martin MA, Sarriá B, Ramos S, Mateos R, Bravo L. Effect of cocoa and its flavonoids on biomarkers of inflammation: studies of cell culture, animals and humans. Nutrients. (2016) 8:212. doi: 10.3390/nu8040212
29. Bordeaux B, Yanek LR, Moy TF, White LW, Becker LC, Faraday N, et al. Casual chocolate consumption and inhibition of platelet function. Prev Cardiol. (2007) 10:175–80. doi: 10.1111/j.1520-037X.2007.06693.x
30. Hermann F, Spieker LE, Ruschitzka F, Sudano I, Hermann M, Binggeli C, et al. Dark chocolate improves endothelial and platelet function. Heart. (2006) 92:119–20. doi: 10.1136/hrt.2005.063362
31. Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. (2000) 130(8S Suppl.):2109S–14S. doi: 10.1093/jn/130.8.2109S
32. Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, et al. Flavanol-rich cocoa drink lowers plasma F2-isoprostane concentrations in humans. Free Radical Biol Med. (2004) 37:411–21. doi: 10.1016/j.freeradbiomed.2004.05.013
33. Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. (2003) 290:1030–1. doi: 10.1001/jama.290.8.1030
34. Heiss C, Kleinbongard P, Dejam A, Perré S, Schroeter H, Sies H, et al. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am College Cardiol. (2005) 46:1276–83. doi: 10.1016/j.jacc.2005.06.055
35. Actis-Goretta L, Ottaviani JI, Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem. (2006) 54:229–34. doi: 10.1021/jf052263o
36. Persson IAL, Persson K, Hägg S, Andersson RGG. Effects of cocoa extract and dark chocolate on angiotensin-converting enzyme and nitric oxide in human endothelial cells and healthy volunteers-A nutrigenomics perspective. J Cardiovasc Pharmacol. (2011) 57:44–50. doi: 10.1097/FJC.0b013e3181fe62e3
37. Schroeter H, Heiss C, Spencer JP, Keen CL, Lupton JR, Schmitz HH. Recommending flavanols and procyanidins for cardiovascular health: current knowledge and future needs. Mol Aspects Med. (2010) 31:546–57. doi: 10.1016/j.mam.2010.09.008
38. Aprotosoaie AC, Miron A, Trifan A, Luca VS, Costache I-I. The cardiovascular effects of cocoa polyphenols-an overview. Diseases. (2016) 4:39. doi: 10.3390/diseases4040039
39. Kim J-a, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. (2006) 113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213
40. Ayoobi N, Jafarirad S, Haghighizadeh MH, Jahanshahi A. Protective effect of dark chocolate on cardiovascular disease factors and body composition in type 2 diabetes: a parallel, randomized, clinical trial. Iranian Red Crescent Med J. (2017) 19:e21644. doi: 10.5812/ircmj.21644
41. Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients: a double-masked, randomized, controlled trial. J Am College Cardiol. (2008) 51:2141–9. doi: 10.1016/j.jacc.2008.01.059
42. Curtis PJ, Potter J, Kroon PA, Wilson P, Dhatariya K, Sampson M, et al. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: a double-blind randomized controlled trial. Am J Clin Nutr. (2013) 97:936–42. doi: 10.3945/ajcn.112.043745
43. Dicks L, Kirch N, Gronwald D, Wernken K, Zimmermann BF, Helfrich HP, et al. Regular intake of a usual serving size of flavanol-rich cocoa powder does not affect cardiometabolic parameters in stably treated patients with type 2 diabetes and hypertension-a double-blinded, randomized, placebo-controlled trial. Nutrients. (2018) 10:1435. doi: 10.3390/nu10101435
44. Mellor DD, Sathyapalan T, Kilpatrick ES, Beckett S, Atkin SL. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabetic Med. (2010) 27:1318–21. doi: 10.1111/j.1464-5491.2010.03108.x
45. Rostami A, Khalili M, Haghighat N, Eghtesadi S, Shidfar F, Heidari I, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscl. (2015) 11:21–9.
46. Basu A, Betts NM, Leyva MJ, Fu D, Aston CE, Lyons TJ. Acute cocoa supplementation increases postprandial HDL cholesterol and insulin in obese adults with type 2 diabetes after consumption of a high-fat breakfast. J Nutr. (2015) 145:2325–32. doi: 10.3945/jn.115.215772
47. Mellor DD, Madden LA, Smith KA, Kilpatrick ES, Atkin SL. High-polyphenol chocolate reduces endothelial dysfunction and oxidative stress during acute transient hyperglycaemia in type 2 diabetes: a pilot randomized controlled trial. Diabetic Med. (2013) 30:478–83. doi: 10.1111/dme.12030
48. Tanghe A, Heyman E, Wyngaert KV, Van Ginckel A, Celie B, Rietzschel E, et al. Evaluation of blood pressure lowering effects of cocoa flavanols in diabetes mellitus: a systematic review and meta-analysis. J Functional Foods. (2021) 79:104399. doi: 10.1016/j.jff.2021.104399
49. Sudano I, Spieker LE, Hermann F, Flammer A, Corti R, Noll G, et al. Protection of endothelial function: targets for nutritional and pharmacological interventions. J Cardiovasc Pharmacol. (2006) 47:S136–50. doi: 10.1097/00005344-200606001-00008
50. d'El-Rei J, Cunha AR, Burlá A, Burlá M, Oigman W, Neves MF, et al. Characterisation of hypertensive patients with improved endothelial function after dark chocolate consumption. Int J Hypertens. (2013) 2013:985087. doi: 10.1155/2013/985087
51. de Jesus Romero-Prado MM, Curiel-Beltran JA, Miramontes-Espino MV, Cardona-Munoz EG, Rios-Arellano A, Balam-Salazar LB. Dietary flavonoids added to pharmacological antihypertensive therapy are effective in improving blood pressure. Basic Clin Pharmacol Toxicol. (2015) 117:57–64. doi: 10.1111/bcpt.12360
52. Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. (2007) 116:2376–82. doi: 10.1161/CIRCULATIONAHA.107.713867
53. Flammer AJ, Sudano I, Wolfrum M, Thomas R, Enseleit F, Periat D, et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J. (2012) 33:2172–80. doi: 10.1093/eurheartj/ehr448
54. Struijker-Boudier HA. From macrocirculation to microcirculation: benefits of preterax. Am J Hypertens. (2007) 20(S1):15S−8S. doi: 10.1016/j.amjhyper.2007.04.013
55. Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes−2019. Diabetes Care. (2019) 42(Suppl. 1):S13–28. doi: 10.2337/dc19-S002
56. Echeverri D, Montes FR, Cabrera M, Galán A, Prieto A. Caffeine's vascular mechanisms of action. Int J Vasc Med. (2010) 2010:834060. doi: 10.1155/2010/834060
57. Aprotosoaie AC, Luca SV, Miron A. Flavor chemistry of cocoa and cocoa products-an overview. Comprehens Rev Food Sci Food Safety. (2016) 15:73–91. doi: 10.1111/1541-4337.12180
58. Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr. (2008) 88:1685–96. doi: 10.3945/ajcn.2008.26457
59. Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. (2006) 103:1024–9. doi: 10.1073/pnas.0510168103
60. Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. (2006) 9:755–62. doi: 10.1079/PHN2005898
61. de Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J Agric Food Chem. (2000) 48:5331–7. doi: 10.1021/jf000549h
62. Qiu S, Cai X, Yin H, Sun Z, Zügel M, Steinacker JM, et al. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc Diabetol. (2018) 17:64. doi: 10.1186/s12933-018-0711-2
63. Buscemi S, Re A, Batsis J, Arnone M, Mattina A, Cerasola G, et al. Glycaemic variability using continuous glucose monitoring and endothelial function in the metabolic syndrome and in type 2 diabetes. Diabetic Med. (2010) 27:872–8. doi: 10.1111/j.1464-5491.2010.03059.x
64. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Informatics. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
65. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
66. Monagas M, Khan N, Andres-Lacueva C, Casas R, Urpí-Sardà M, Llorach R, et al. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr. (2009) 90:1144–50. doi: 10.3945/ajcn.2009.27716
67. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circul Physiol. (2010) 300:H2–H12. doi: 10.1152/ajpheart.00471.2010
68. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci. (2001) 101:629–35. doi: 10.1042/cs1010629
69. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am College Cardiol. (2002) 39:257–65. doi: 10.1016/S0735-1097(01)01746-6
70. Celie B, Boone J, Van Coster R, Bourgois J. Reliability of near infrared spectroscopy (NIRS) for measuring forearm oxygenation during incremental handgrip exercise. Eur J Appl Physiol. (2012) 112:2369–74. doi: 10.1007/s00421-011-2183-x
71. Celie BM, Boone J, Dumortier J, Derave W, De Backer T, Bourgois JG. Possible influences on the interpretation of functional domain (FD) near-infrared spectroscopy (NIRS): An explorative study. Appl Spectrosc. (2016) 70:363–71. doi: 10.1177/0003702815620562
72. Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, et al. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol. (2003) 95:149–58. doi: 10.1152/japplphysiol.00695.2002
73. T'Joen V, Phlypo S, Bekaert S. Bimetra biobank: a high quality biobank facility to stimulate translational biomedical research. Open J Bioresour. (2018) 5:1–8. doi: 10.5334/ojb.37
74. Langlois MR, Delanghe JR, De Buyzere ML, Bernard DR, Ouyang J. Effect of haptoglobin on the metabolism of vitamin C. Am J Clin Nutr. (1997) 66:606–10. doi: 10.1093/ajcn/66.3.606
75. Migueles JH, Cadenas-Sanchez C, Ekelund U, Nyström CD, Mora-Gonzalez J, Löf M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. (2017) 47:1821–45. doi: 10.1007/s40279-017-0716-0
76. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. (2017) 40:1631–40. doi: 10.2337/dc17-1600
77. Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am College Cardiol. (2008) 51:1959–64. doi: 10.1016/j.jacc.2008.02.044
78. Sorensen KE, Celermajer DS, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, et al. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Heart. (1995) 74:247–53. doi: 10.1136/hrt.74.3.247
79. van Mil AC, Greyling A, Zock PL, Geleijnse JM, Hopman MT, Mensink RP, et al. Impact of volunteer-related and methodology-related factors on the reproducibility of brachial artery flow-mediated vasodilation: analysis of 672 individual repeated measurements. J Hypertens. (2016) 34:1738–45. doi: 10.1097/HJH.0000000000001012
Keywords: type 2 diabetes, cocoa flavanols, vascular reactivity, blood pressure, muscular oxygenation, antihypertensive drugs
Citation: Tanghe A, Celie B, Shadid S, Rietzschel E, Op ‘t Roodt J, Reesink KD, Heyman E and Calders P (2021) Acute Effects of Cocoa Flavanols on Blood Pressure and Peripheral Vascular Reactivity in Type 2 Diabetes Mellitus and Essential Hypertension: A Protocol for an Acute, Randomized, Double-Blinded, Placebo-Controlled Cross-Over Trial. Front. Cardiovasc. Med. 8:602086. doi: 10.3389/fcvm.2021.602086
Received: 02 December 2020; Accepted: 16 February 2021;
Published: 15 March 2021.
Edited by:
Isabella Sudano, University Hospital Zürich, SwitzerlandReviewed by:
Rosa Maria Bruno, University of Pisa, ItalyCopyright © 2021 Tanghe, Celie, Shadid, Rietzschel, Op ‘t Roodt, Reesink, Heyman and Calders. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick Calders, cGF0cmljay5jYWxkZXJzQHVnZW50LmJl
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.