
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 12 July 2021
Sec. Structural Interventional Cardiology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.600356
This article is part of the Research TopicTranscatheter Aortic Valve Implantation: Current challenges and future directionsView all 8 articles
 Jie Li1
Jie Li1 Yinghao Sun1
Yinghao Sun1 Shengneng Zheng1
Shengneng Zheng1 Guang Li1
Guang Li1 Haojian Dong1
Haojian Dong1 Ming Fu1
Ming Fu1 Yujing Mo1
Yujing Mo1 Yi Li2
Yi Li2 Huadong Liu3
Huadong Liu3 Zhaoyan Xu4
Zhaoyan Xu4 Liting Zhang5
Liting Zhang5 Yong Cao6
Yong Cao6 Ruixin Fan1
Ruixin Fan1 D. Scott Lim7*
D. Scott Lim7* Jianfang Luo1*
Jianfang Luo1*Background: The consequence of valve malposition (VM) during transcatheter aortic valve replacement (TAVR) can be severe, but the determinants of VM with self-expandable TAVR have not been thoroughly evaluated. We aimed to investigate the anatomical predictors of VM during self-expandable TAVR.
Methods: In this multicenter retrospective study, TAVR was performed using the Venus A-Valve. The baseline, computed tomography, and procedural characteristics along with clinical outcomes were collected. Multivariate logistic regression model and receiver operating characteristic (ROC) curve analyses were performed.
Results: A total of 84 consecutive patients (23 with VM) were included. Stepwise regression showed that annulus perimeter/left ventricular outflow tract perimeter (AL ratio) and sinotubular junction (STJ) height were predictors of VM. The ROC curve indicated a moderate strength of AL ratio [area under the curve (AUC) 0.71, cutoff 0.96] and a weak strength of STJ height (AUC 0.69, cutoff 23.8 mm) to predict VM. The combination of both predictors revealed a higher predictive value of VM (AUC 0.77). In multivariate analysis, AL ratio <0.96 [odds ratio (OR) 3.98, p = 0.015] and STJ height ≥23.8 mm (OR 4.63, p = 0.008) were strong independent predictors of VM. The presence of both predictors was associated with a very high risk of VM (OR 10.67, p = 0.002). The rate of moderate-to-severe paravalvular regurgitation was higher in patients with VM at 30 days (26.1 vs. 4.9%, p = 0.011).
Conclusions: A conical left ventricular outflow tract and tall aortic sinuses were strong anatomical predictors of VM during self-expandable TAVR.
Transcatheter aortic valve replacement (TAVR) has become an alternative to surgical aortic valve replacement in patients with symptomatic severe aortic stenosis (1–4). However, unlike standard open surgical aortic valve replacement in which the surgical valve is sewn into place, implantation of the transcatheter heart valve can incur the risk of malposition during TAVR, which if significant may lead to severe aortic regurgitation or valve embolization and potentially necessitate implantation of a second prosthesis. According to the Valve Academic Research Consortium-2 (VARC-2) definitions, valve malposition (VM) can be categorized as migration, embolization, or ectopic deployment (5). Despite a low incidence, VM has been related to a higher mortality after TAVR (6, 7). Several predictors of VM in patients undergoing TAVR have been reported, including highly calcified aortic valves and the use of self-expandable (as compared with balloon-expandable) prostheses (6, 7), but the anatomical features associated with VM in self-expandable TAVR have not been thoroughly evaluated and specifically not evaluated in the Chinese population, which tends to have a significantly higher calcium burden (8). Therefore, we aimed to further investigate the anatomical predictors of VM during self-expandable TAVR with the Venus A-Valve.
In this multicenter retrospective study, TAVR was performed in patients with symptomatic severe aortic stenosis using the self-expandable Venus A-Valve (Venus MedTech, Hangzhou, China). The Venus A-Valve has a similar supra-annular design as the Medtronic CoreValve but stronger radial force at the inflow end, which may be advantageous in bicuspid anatomy and severe calcification (9). The access routes and size of the prostheses were determined by the individual heart teams based on multidetector computed tomography (MDCT). Standardized measurements on MDCT and on angiography were both performed by two independent investigators. Patients with optimal position and malposition of the prostheses were included, while those with suboptimal position of the prostheses were excluded. The baseline, MDCT, and procedural characteristics along with clinical outcomes were collected. This study was approved by the Research Ethics Committee of Guangdong Provincial People's Hospital (No. GDREC2019384H), with a waiver of informed consent.
The Venus A-Valve has three radiopaque markers at half a cell (5–6 mm) above the inflow end, indicating the optimal landing zone (Appendix 1). The default initial position of the markers was aligned with the annular plane. Optimal position on angiography was defined as the markers being within the range from 4 mm above to 8 mm below the annulus, such that the covered cells of the prosthesis remain in contact with the annulus. Suboptimal position denoted markers being outside the range of optimal position but with acceptable hemodynamics (less than moderate paravalvular regurgitation evaluated by angiography and transesophageal echocardiography). Malposition indicated severe migration of the first valve, resulting in unacceptable paravalvular regurgitation and implantation of a second valve. These definitions were modified according to VARC-2 criteria (Figure 1).
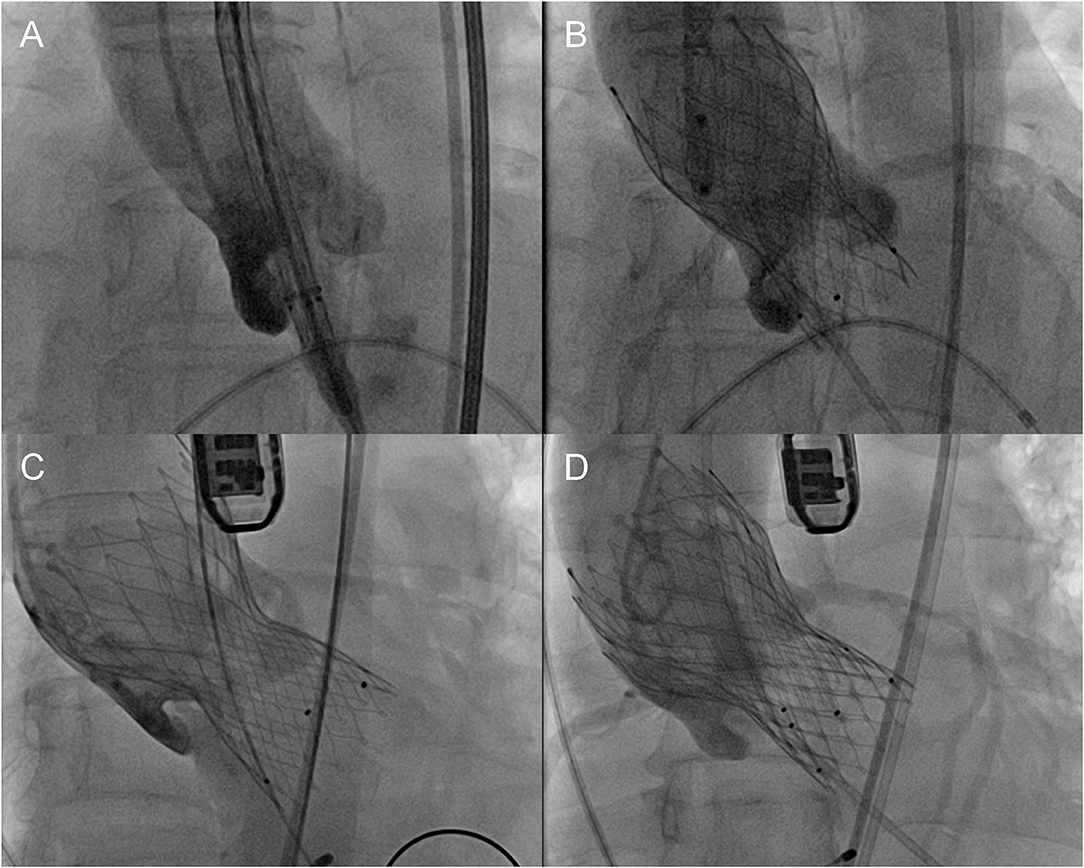
Figure 1. Definition of different positions of the prosthesis. (A) Initial position, (B) optimal position, (C) malposition, and (D) implantation of a second prosthesis.
The primary outcome was all-cause mortality at 30 days. The secondary outcomes were VARC-2 defined complications at 30 days.
All MDCT images were analyzed by two independent investigators using 3 mensio software (Pie Medical, Bilthoven, The Netherlands). First, we needed to fine-tune the default central line of the aortic root. Second, the nadirs of all coronary sinuses, which determined the annular plane, were marked manually. Then we depicted the inner contour at the level of annulus, left ventricular outflow tract (LVOT; 4 mm below the annulus), and sinotubular junction (STJ); and then the area, perimeter, maximum diameter, and minimum diameter could be calculated automatically. At the level of the ascending aorta, the maximum diameter and minimum diameter were measured manually. Mean diameter was calculated as (maximum diameter + minimum diameter)/2. The eccentricity index was calculated as (1 – minimum diameter/maximum diameter) × 100%. STJ height was measured on the central line between STJ and annulus automatically. The type of native aortic valve was identified visually in the short-axis view of the aortic root. The volume of calcification was automatically calculated for the aortic root and each coronary cusp. The inferior margins of both coronary ostia were marked manually in the long-axis view, and then the coronary heights would be generated automatically. The aortic root angle was also measured automatically once the annular plane was determined. AL ratio was defined as annulus perimeter/LVOT perimeter.
Continuous variables were presented as mean with standard deviation or median with interquartile range and were compared using Students t-test or the Mann–Whitney U test, respectively. Categorical variables were presented as percentages and were compared using chi-squared or Fisher's exact test. The baseline, MDCT, and procedural variables with significant difference between two groups were analyzed by Spearman's correlation and then selected in a stepwise forward conditional regression model. The variables generated by stepwise regression were introduced in the final binary multivariate logistic regression model along with anatomical variables of interest (bicuspid aortic valve and aortic root calcification). The receiver operating characteristic (ROC) curve analysis was performed to estimate the discriminative power and to identify the cutoff value of independent predictors for VM. All tests were two-tailed, and p < 0.05 was considered significant. All statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA).
From April 2016 to February 2020, 203 consecutive patients underwent TAVR with Venus A-Valve in five medical centers across Guangdong, China; 23 (11.3%) had VM, and all of them were valve migration toward the left ventricle. The incidence of VM was lower in patients with tricuspid aortic valve as compared with those with bicuspid aortic valve (7.1 vs. 16.7%, p = 0.032, Appendix 2). A total of 61 patients (30.0%) met the criteria of optimal position of the prostheses. Thus, a total of 84 patients were included in this analysis.
The baseline characteristics are shown in Table 1. The percentage of male was higher (p = 0.039), and the left ventricular ejection fraction was lower (p = 0.033) in patients with VM. The MDCT characteristics were shown in Table 2. In patients with VM, the dimensions of the annulus, LVOT, and STJ were generally larger; the left main coronary artery was higher (p = 0.031); and AL ratio (p = 0.003) was smaller. The procedural characteristics are shown in Table 3. The sizes of prostheses were significantly different between two groups (p = 0.009), and the prosthesis perimeter/LOVT perimeter ratio was lower (p = 0.026) in the malposition group. Correlation analysis showed that all the variables with significant difference between two groups were strongly correlated to either AL ratio or STJ height. Thus, only these two anatomical variables were selected in the stepwise regression, and both of them were found to be independent predictors of VM.
The ROC curve indicated a moderate strength of AL ratio to predict VM [area under the curve (AUC) 0.71, 95% confidence interval (CI) 0.59–0.84, p = 0.003, Figure 2]. The cutoff value of AL ratio was 0.96 with a sensitivity of 70% and a specificity of 71%. The ROC curve showed a weak strength of STJ height to predict VM (AUC 0.69, 95% CI 0.55–0.83, p = 0.011, Figure 2). The cutoff value of STJ height was 23.8 mm with a sensitivity of 52% and a specificity of 85%. The combination of both predictors revealed a higher predictive value of VM (AUC 0.77, 95% CI 0.64–0.89, p < 0.001, Figure 2).
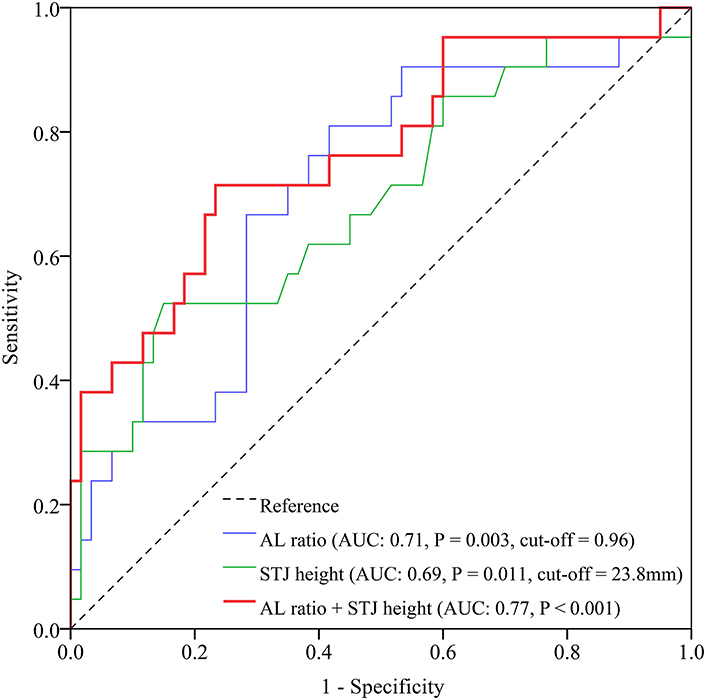
Figure 2. ROC curves of AL ratio, STJ height, and the combination of both for prediction of valve malposition. AL, annulus perimeter/left ventricular outflow tract perimeter; AUC, area under the curve; ROC, receiver operating characteristic; STJ, sinotubular junction.
In multivariate analysis (Table 4), AL ratio <0.96 [odds ratio (OR) 3.98, 95% CI 1.31–12.14, p = 0.015] and STJ height ≥23.8 mm (OR 4.63, 95% CI 1.48–14.46, p = 0.008) were both strong independent anatomical predictors of VM. The presence of both predictors was associated with a very high risk of VM (OR 10.67, 95% CI 2.45–46.57, p = 0.002).
There was no mortality at 30 days after TAVR in the included population. The rate of moderate-to-severe paravalvular regurgitation was higher in patients with VM (26.1 vs. 4.9%, p = 0.011) than in those with optimal position of the prostheses. No myocardial infarction was observed. The incidences of disabling stroke, major bleeding, major vascular complications, acute kidney injury (stage 2 or 3), permanent pacemaker implantation, and new-onset atrial fibrillation were all comparable between the two groups (Table 5).
In this multicenter retrospective study of TAVR, we utilized the modified definition of optimal position and malposition of the self-expandable Venus A-Valve; and we compared the baseline, MDCT, and procedural characteristics between those two groups. We determined that AL ratio <0.96 and STJ height ≥23.8 mm were both strong independent anatomical predictors of VM during self-expandable TAVR (Figure 3).
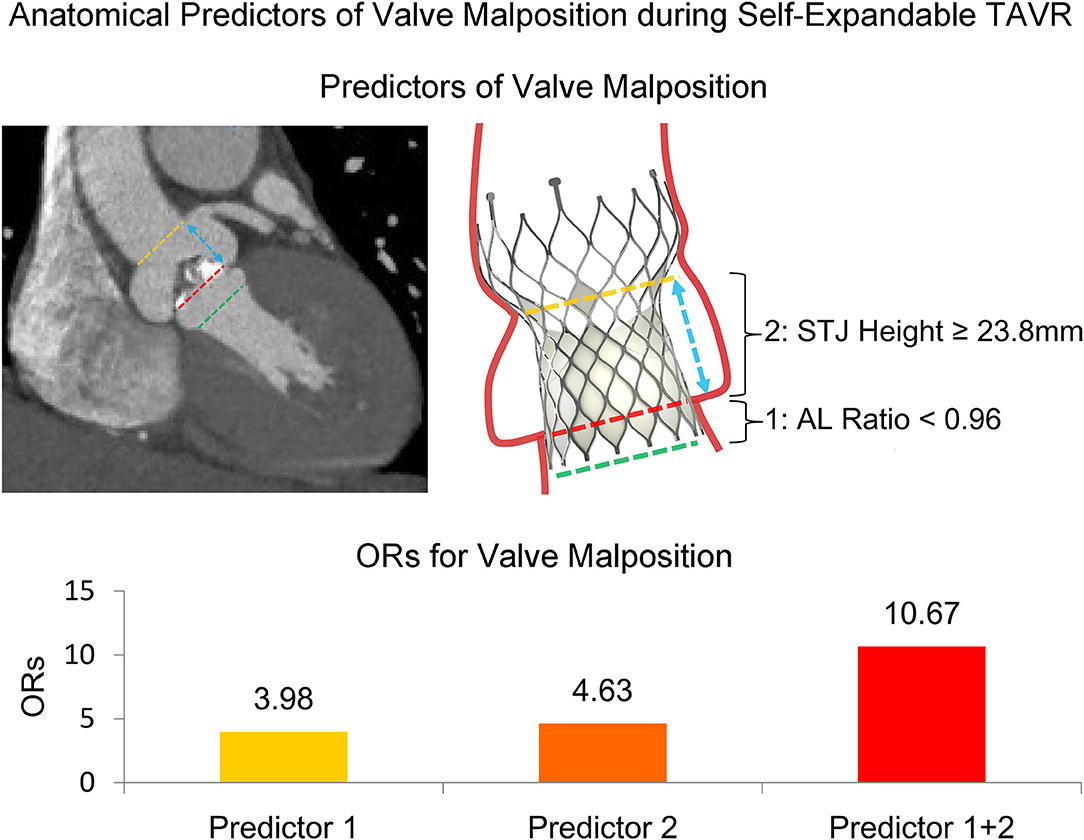
Figure 3. Anatomical predictors of valve malposition during self-expandable transcatheter aortic valve replacement. The dash lines indicate the planes of STJ, annulus, and LVOT. The arrow indicates STJ height. AL, annulus perimeter/LVOT perimeter; LVOT, left ventricular outflow tract; OR, odds ratio; STJ, sinotubular junction; TAVR, transcatheter aortic valve replacement.
In clinical practice, VM could account for a majority of acute paravalvular regurgitation during TAVR and may necessitate implantation of a second prosthesis (10). However, the anatomical feature associated with VM has not been extensively investigated, particularly in a population with high calcium burden (6, 11). The recent TRAVEL registry has shed some new light on this topic and reported that the self-expandable valve was more susceptible to VM than the balloon-expandable valve in TAVR (7). Importantly, the study of new-generation devices also found a higher rate of implantation of a second valve with the self-expandable Evolut R than with the balloon-expandable Sapien 3 (2.7 vs. 0.5%) (12). The higher risk of VM during self-expandable TAVR advocated a further investigation of the anatomical predictors of VM in this setting.
To our knowledge, this is the first report that the AL ratio and STJ height are related to VM. The presence of both AL ratio <0.96 and STJ height ≥23.8 mm can increase the risk of VM at ~10-fold. Therefore, we propose a concept of the “conical LVOT,” which indicates a larger LVOT than the annulus. It is reasonable that a “conical LVOT” may not provide enough upward support force for the prosthesis during the course of release, which could prevent malposition of the prosthesis to the ventricle. It is also explainable that a higher STJ usually accompanies a larger sinus of Valsalva and therefore has a weaker radial force for the outflow of the prosthesis after release. While only the Venus A-Valve was included in this study, the AL ratio and STJ height might be helpful in evaluating the risk of VM when using other self-expandable valves with similar geometric design and releasing process (e.g., Medtronic CoreValve).
Some potentially important risk factors discovered by previous studies were not reproduced in our study, which could be the results of limited statistical power. On the one hand, bicuspid aortic valve was found to be an independent predictor (OR 3.43, p < 0.001) of VM in the TRAVEL registry (7). Similarly, the rate of bicuspid aortic valve was higher in the malposition group than in the optimal position group in our study, but without significant difference (65.2 vs. 54.1%, p = 0.358). On the other hand, in a sub-study of the PARTNER trial, heavy leaflet calcification was identified as an important cause of valve embolization or valve-in-valve procedure (6), which was not found in our study. As the problem of bicuspid anatomy and severe calcification was prominent in Chinese patients (8), both of these two variables were introduced in the final multivariate regression model to minimize the underlying bias.
The incidence of VM requiring a second prosthesis was around 11% in a Chinese population with Venus A-Valve in this study, which is much higher than that (3~4%) in western population with CoreValve (13, 14). This may be attributed to the more complex anatomy and higher degree of calcification of the aortic root in Chinese population (8) as well as a higher radial force at the inflow section of Venus A-Valve, which could enhance the pushing force downward during releasing of the prosthesis and cause VM in complicated cases (9). It has been reported that the new-generation repositionable self-expandable Evolut R and Evolut PRO have lower rates of valve migration, moderate-to-severe paravalvular regurgitation, and implantation of a second prosthesis than the earlier generation CoreValve (15–17). Similarly, it is anticipated that the second-generation repositionable Venus A-Plus Valve will likely have a better performance in terms of VM (18).
As VM relates to clinical outcome, while there was no mortality at 30 days after TAVR in our study, the rate of moderate-to-severe paravalvular regurgitation was higher in patients with VM, which was in accordance with the TRAVEL registry (7). Although the implantation of a second valve can be a safe and effective therapeutic option (13, 14), VM has been related to increased morbidity and mortality (6, 7). Therefore, we hope that the new anatomical predictors of VM could further improve the outcomes after TAVR.
There were several limitations in this study. First, there can be inherent bias as a retrospective observational study with a relatively small sample size. Second, the MDCT data were not adjudicated by a core laboratory. Finally, the ORs for predictors of VM might be overestimated to some extent because the patients with suboptimal position of the prostheses were excluded (a preliminary analysis of all patients failed to reveal any predictors, while the results of analyses in all patients using different grouping and in patients with tricuspid aortic valve were basically consistent to our finding, see Appendix 2). However, these results should be valued since the MDCT and angiography were both evaluated by two independent investigators, and multivariate regression model was applied. Future study should focus on the next-generation self-expandable valves to further corroborate our findings.
In this multicenter retrospective study, we found that AL ratio <0.96 and STJ height ≥23.8 mm were strong independent anatomical predictors of VM during self-expandable TAVR in a Chinese population with high risk of VM (because of high percentage of bicuspid anatomy and severe calcification of the aortic valves). These results needed further corroboration in the next-generation self-expandable valves.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Research Ethics Committee of Guangdong Provincial People's Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JLi, YS, and SZ contributed equally to study design, data acquisition, statistical analysis, and drafting of the manuscript. JLu approved the submission of the final version. GL, HD, MF, YM, YL, HL, ZX, LZ, YC, RF, and DL contributed greatly to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by High-level Hospital Construction Project from Guangdong Provincial People's Hospital (DFJH201807) and Guangzhou Science and Technology Project (202102020041).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.600356/full#supplementary-material
AL, annulus perimeter/left ventricular outflow tract perimeter; AUC, area under the curve; CI, confidence interval; LVOT, left ventricular outflow tract; MDCT, multidetector computed tomography; OR, odds ratio; ROC, receiver operating characteristic; STJ, sinotubular junction; TAVR, transcatheter aortic valve replacement; VM, valve malposition.
1. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1093/eurheartj/ehx391
2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. (2017) 70:252–89. doi: 10.1016/j.jacc.2017.03.011
3. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
4. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380:1706–15. doi: 10.1056/NEJMoa1816885
5. Kappetein AP, Head SJ, Genereux P, Piazza N, Van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. (2012) 60:1438–54. doi: 10.1016/j.jacc.2012.09.001
6. Makkar RR, Jilaihawi H, Chakravarty T, Fontana GP, Kapadia S, Babaliaros V, et al. Determinants and outcomes of acute transcatheter valve-in-valve therapy or embolization: a study of multiple valve implants in the U.S. PARTNER trial (Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve). J Am Coll Cardiol. (2013) 62:418–30. doi: 10.1016/j.jacc.2013.04.037
7. Kim WK, Schafer U, Tchetche D, Nef H, Arnold M, Avanzas P, et al. Incidence and outcome of peri-procedural transcatheter heart valve embolization and migration: the TRAVEL registry (TranscatheteR HeArt Valve EmboLization and Migration). Eur Heart J. (2019) 40:3156–65. doi: 10.1093/eurheartj/ehz429
8. Jilaihawi H, Wu Y, Yang Y, Xu L, Chen M, Wang J, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc. (2015) 1:752–61. doi: 10.1002/ccd.25863
9. Liao YB, Zhao ZG, Wei X, Xu YN, Zuo ZL, Li YJ, et al. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: preliminary experiences in China. Catheter Cardiovasc Interv. (2017) 89:528–33. doi: 10.1002/ccd.26912
10. Witkowski A, Jastrzebski J, Dabrowski M, Chmielak Z. Second transcatheter aortic valve implantation for treatment of suboptimal function of previously implanted prosthesis: review of the literature. J Interv Cardiol. (2014) 27:300–7. doi: 10.1111/joic.12120
11. Ibebuogu UN, Giri S, Bolorunduro O, Tartara P, Kar S, Holmes D, et al. Review of reported causes of device embolization following trans-catheter aortic valve implantation. Am J Cardiol. (2015) 115:1767–72. doi: 10.1016/j.amjcard.2015.03.024
12. Finkelstein A, Steinvil A, Rozenbaum Z, Halkin A, Banai S, Barbash I, et al. Efficacy and safety of new-generation transcatheter aortic valves: insights from the Israeli transcatheter aortic valve replacement registry. Clin Res Cardiol. (2019) 108:430–7. doi: 10.1007/s00392-018-1372-6
13. Gerckens U, Latsios G, Mueller R, Buellesfeld L, John D, Yuecel S, et al. Procedural and mid-term results in patients with aortic stenosis treated with implantation of 2 (in-series) CoreValve prostheses in 1 procedure. JACC Cardiovasc Interv. (2010) 3:244–50. doi: 10.1016/j.jcin.2009.11.010
14. Ussia GP, Barbanti M, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. The valve-in-valve technique for treatment of aortic bioprosthesis malposition an analysis of incidence and 1-year clinical outcomes from the italian CoreValve registry. J Am Coll Cardiol. (2011) 57:1062–8. doi: 10.1016/j.jacc.2010.11.019
15. Sorajja P, Kodali S, Reardon MJ, Szeto WY, Chetcuti SJ, Hermiller Jr, et al. Outcomes for the commercial use of self-expanding Prostheses in Transcatheter Aortic Valve Replacement: a report from the STS/ACC TVT registry. JACC Cardiovasc Interv. (2017) 10:2090–8. doi: 10.1016/j.jcin.2017.07.027
16. Forrest JK, Kaple RK, Tang GHL, Yakubov SJ, Nazif TM, Williams MR, et al. Three generations of self-expanding transcatheter aortic valves: a report from the STS/ACC TVT registry. JACC Cardiovasc Interv. (2020) 13:170–9. doi: 10.1016/j.jcin.2019.08.035
17. Sun Y, Li J, Fan R, Li G, Fu M, Luo S, et al. Outcomes of Evolut R versus corevalve after transcatheter aortic valve implantation: a meta-analysis. Heart Lung Circ. (2020) 29:288–94. doi: 10.1016/j.hlc.2018.12.013
Keywords: computed tomography, malposition, predictor, self-expandable, transcatheter aortic valve replacement
Citation: Li J, Sun Y, Zheng S, Li G, Dong H, Fu M, Mo Y, Li Y, Liu H, Xu Z, Zhang L, Cao Y, Fan R, Lim DS and Luo J (2021) Anatomical Predictors of Valve Malposition During Self-Expandable Transcatheter Aortic Valve Replacement. Front. Cardiovasc. Med. 8:600356. doi: 10.3389/fcvm.2021.600356
Received: 29 August 2020; Accepted: 04 June 2021;
Published: 12 July 2021.
Edited by:
Richard Jabbour, Imperial College London, United KingdomReviewed by:
Paolo Denti, San Raffaele Hospital (IRCCS), ItalyCopyright © 2021 Li, Sun, Zheng, Li, Dong, Fu, Mo, Li, Liu, Xu, Zhang, Cao, Fan, Lim and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Scott Lim, U0w5UENAdmlyZ2luaWEuZWR1; Jianfang Luo, amlhbmZhbmdsdW9Ac2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.