- 1School of Human Nutrition, McGill University, Montreal, QC, Canada
- 2Faculté des Sciences Administratives, Université Laval, Québec, QC, Canada
- 3Australian Centre for Precision Health, University of South Australia, Adelaide, SA, Australia
- 4McGill Center for the Convergence of Health and Economics, Desautels Faculty of Management, Montreal, QC, Canada
Background: Cardiovascular disease (CVD) is a complex disease resulting from multiscale risk factors including genetics, age, and psychosocial factors (PSFs) such as depression and social isolation. However, previous research has lacked in operationalizing multiscale risk factors to determine individual and interactive associations over the life course. Therefore, this study aimed to evaluate individual and interactive associations of multiscale risk factors for CVD outcomes including genetics and PSFs at middle and older-aged stages of the life course.
Methods: Baseline data from the Canadian Longitudinal Study on Aging (CLSA; n = 9,892 with genome-wide genotyping data) was used for this investigation. A 39 single nucleotide polymorphism polygenic risk score (PRS) for CVD was constructed. PSFs consisted of: (1) Depressive symptoms categorized into: “none” (Group 1, reference), “current” (Group 2), “clinical depression with no current symptoms” (Group 3), and “potential, recurrent depression” (Group 4); and (2) Social isolation index as a binary variable comprised of marital status, living arrangements, retirement status, contacts, and social participation. Heart-related disorders (HRD: myocardial infarction, angina and heart disease) was the primary outcome of interest and peripheral/vascular-related disorders (PVRD: stroke, peripheral vascular disease and hypertension) was the secondary outcome. Multivariable logistic regression models adjusted for socio-demographic factors were conducted stratified by age group (middle-aged: 45–69 years, older-aged: ≥70 years).
Results: PRS was associated with HRD among middle- and older-aged participants [OR (95% confidence interval)] [1.06 (1.03–1.08), 1.06 (1.03–1.08), respectively]. Most depressive symptoms groups compared to the reference associated with HRD and PVRD, but only Group 4 associated with PVRD among older-aged [1.69 (1.08–2.64)]. Social isolation was associated with only PVRD among middle-aged [1.84 (1.04–3.26)]; however, socially isolated CLSA participants were underrepresented in the genotyped cohort (1.2%). No significant PRS*PSFs interactions were observed.
Conclusions: Genetics and PSFs are independently associated with CVD. Varying observations across age groups underscores the need to advance research on multiscale risk factors operating both at a given point in time and over the life course. Future cohort studies may benefit from use of mobile assessment units to enable better reach to socially isolated participants for collection of biospecimens.
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, accounting for nearly 30% of deaths globally, and the second leading cause of death in Canada (1, 2). CVD can be categorized into heart-related disorders (HRD) including myocardial infarction, angina and heart disease and peripheral/vascular-related disorders (PVRD) including cerebrovascular accident (CVA), hypertension and peripheral vascular disease (3). Multiscale risk factors consider combinations of factors that are involved along the pathway of disease pathogenesis and represent a more all encompassing approach to studying disease risk (4). This investigation assessed the associations between CVD and multiscale risk factors of genetics, psychosocial factors (PSFs) and life course stage.
Genome-wide association studies (GWAS) have identified several single nucleotide polymorphisms (SNPs) that are associated with an increased risk of coronary artery disease (CAD), the most common form of heart disease (5). From these, a polygenic risk score (PRS) has been developed to quantify genetic risk according to the number of SNP alleles carried by an individual (6). GWAS have primarily focused on SNPs associating with HRD (6) and to a lesser extent with PVRD (7), and the genetic predisposition may differ between types of CVD (8). While GWAS with CVD outcomes have predominantly been conducted among cohorts of middle-aged individuals, emerging evidence suggests that effects of genetic variation on health outcomes may dampen as individuals approach the later stages of the life course (9). Non-genetic risk factors may be increasingly relevant for chronic disease risk or co-morbidities at the later stages of life as experience and environmental exposure cumulate over a person's life course (9, 10), underscoring the importance of assessing the performance of PRS in older-aged groups. Moreover, recent gene-environment interaction studies provide supportive evidence that non-genetic factors (healthy lifestyle) may counteract polygenic risk of CAD and may be particularly beneficial for subgroups at highest polygenic risk (6). While lifestyle is an important modifiable risk factor, no previous study has examined psychosocial factors (PSFs) as possible moderators of polygenic risk of CAD despite robust evidence for individual associations between PSFs and CVD outcomes. In particular, depression has been consistently associated with CVD (3, 11), while social isolation is a growing problem among older-aged individuals and has been more recently linked with CVD (12, 13). While they can be related constructs, social isolation does not consistently correlate with depression (14) as it more often reflects a circumstantial rather than emotional state (such as loneliness, a strong risk factor for depression) (15). Poor PSFs can negatively influence lifestyle, including being linked with a less healthful diet and reduced physical activity (16–18). Given the growing recognition of the role of psychosocial well-being in chronic disease risk (3), PSFs warrant consideration as possible moderators of the associations between genetic susceptibility to CVD and CVD outcomes, as they represent environmental contributors to CVD that are upstream of lifestyle which has been reported to be a modifier of the association between genetic susceptibility to CVD and CVD outcomes (6). Therefore, the objective of the present study was to assess individual and interactive associations of PSFs (depression and social isolation) and polygenic risk of CAD with CVD outcomes among middle-aged and older adults. Since depression and social isolation do not necessarily manifest together, we evaluated each PSF as a separate exposure variable.
Methods
Study Design and Cohort
This cross-sectional analysis used baseline data from the Canadian Longitudinal Study on Aging (CLSA). Data from 50,000 participants between the ages of 45–85 years old (forming the CLSA Tracking and Comprehensive Cohorts) upon recruitment was collected via telephone survey on demographic, behavioral, physical, economic, psychological, and social aspects of their lives (19). The Comprehensive Cohort is a subset of 30,000 CLSA participants that had in-depth information collected at data collection sites (DCS) including further interviews, in-person physical examinations and biospecimen sample collections. Out of the CLSA Comprehensive Cohort, 9,896 randomly sampled participants had genome-wide genotyping performed on DNA samples (19).
Polygenic Risk Score
Genome-wide genotyping was performed using the Affymetrix UK Biobank Axiom array (20). Out of 50 SNPs used to create a PRS by Khera et al. (6), 39 were present in the CLSA genotyping data. The PRS was made by multiplying the number of CAD risk alleles by their weighted risk estimate (natural log of published odds ratio) for each of the SNPs seen in Supplementary Table 1 (6). These values were then summed across all SNPs and multiplied by the total number of SNPs divided by the sum of the weighted risk estimates. The resulting PRS used in the present analysis could range from 0 (no SNPs) to 78 (all 39 SNPs).
Depressive Symptoms
Depressive symptoms were assessed using the short form of the Center for Epidemiological Studies—Depression (CES-D-10) Scale. Scores ranged from 0 to 30, where a score of >10 indicated current depressive symptoms (positive screen for depression) and a score of <10 indicating no evidence of current depressive symptoms (negative screen) (21). Additionally, participants were assessed for presence of clinical depression by being asked “Has a doctor ever told you that you suffer from clinical depression?” (21). Based on these two assessments of depressive symptoms, participants were categorized into four groups as previously described by Liu et al. (3). Group 1 had no evidence of any depressive symptoms with a negative screen for depression and a negative response to the question on clinical depression. Group 2 are considered to have current depressive symptoms with a positive screen for depression but a negative response to the question on clinical depression. Group 3 are considered clinically depressed without any current depressive symptoms with a negative screen for depression but a positive response to the question on clinical depression. Group 4 have potential, recurrent depression with both a positive screen for depression and a positive response to the question on clinical depression.
Social Isolation Index
Social Isolation Index (SII) was created according to methods developed by Menec et al. (22) using responses to five sets of questions in regard to marital status, living arrangements, social contacts, retirement status and social participation. The index was scored between 0 and 5 with higher scores indicating higher levels of social isolation (22). A social isolation score from 0 to 2 was classified as not socially isolated (coded as 0) and a score from 3 to 5 was classified as socially isolated (coded as 1) (22).
Outcome Measures
The outcome measures were defined in the same manner as Liu et al. for consistency of methods (3). The six CLSA questions for physician-diagnosed CVD were subdivided into two groups: heart-related disorders (HRD) and peripheral/vascular-related disorders (PVRD). HRD was the primary outcome of interest, composed of heart disease, myocardial infarction and angina. These were each assessed with the following questions: “Has a doctor ever told you that you have… (i) heart disease (including congestive heart failure or chronic heart failure)?; (ii) a heart attack or myocardial infarction?”; (iii) angina (or chest pain due to heart disease)?” PVRD was the secondary outcome of interest and was composed of hypertension, cerebrovascular accident and peripheral vascular disease. These were each assessed with the follow questions: “Has a doctor ever told you that you have… (i) high blood pressure or hypertension?”; (ii) experienced a stroke or cerebrovascular accident (CVA)?”; (iii) peripheral vascular disease or poor circulation in your limbs?” These CVD subgroups share common risk factors and pathophysiology with the major point of convergence being anatomical location (23).
Covariates
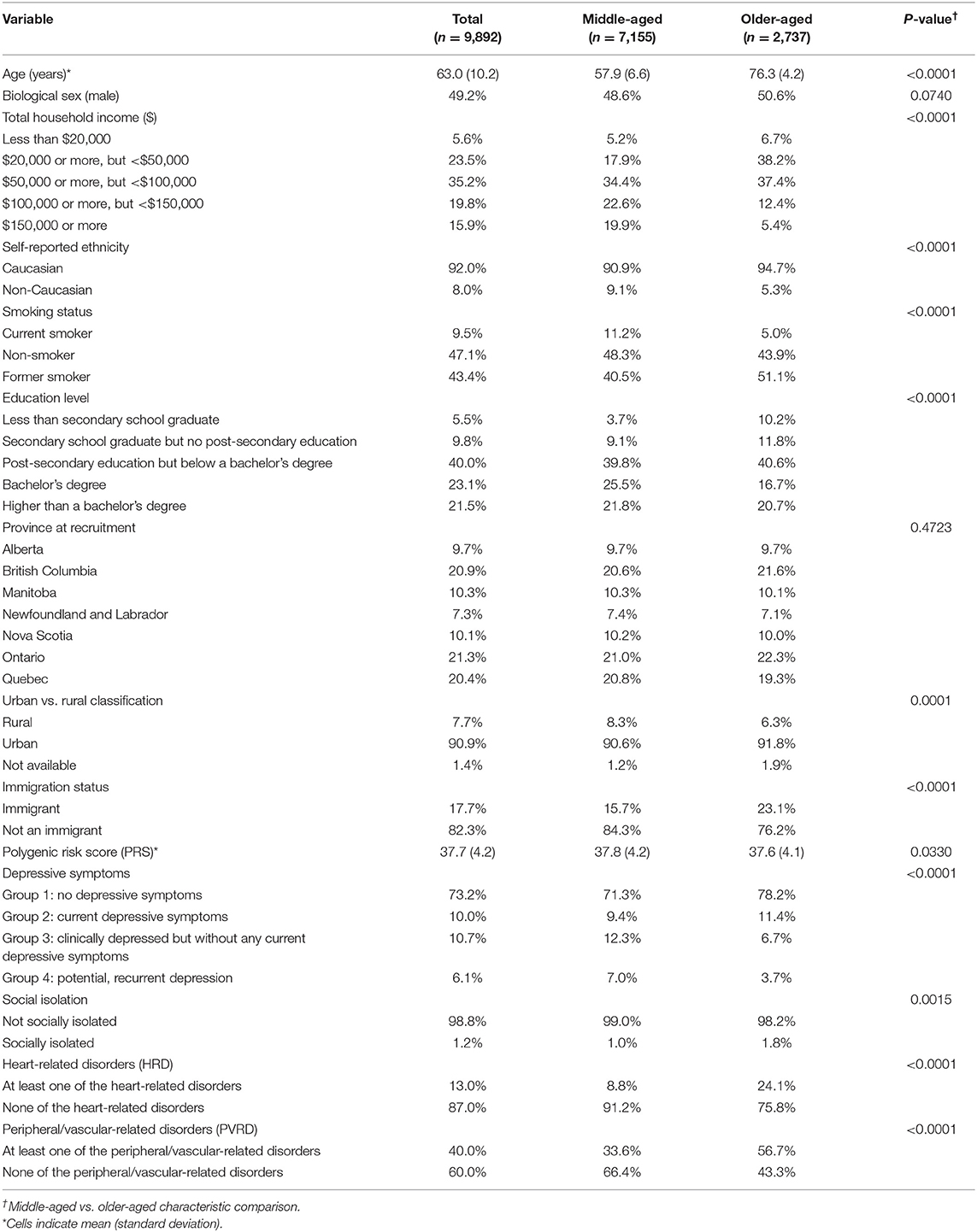
Age (years) and the first five principal components of ancestry were included in the regression model as continuous co-variates. The following categorical co-variates were also included: biological sex (male vs. female), education level (<secondary school; secondary school graduate but no post-secondary education; post-secondary education but below bachelor's degree; bachelor's degree; and > bachelor's degree), province at recruitment (Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island, Quebec, Saskatchewan), total household income ($20,000 or more, but <$50,000; $50,000 or more, but <$100,000; $100,000 or more, but <$150,000; $150,000 or more), smoking status (current smoker; former smoker; and never smoked), urban/rural classification (urban; rural; postal code link to dissemination area) and immigration status (immigrant vs. not an immigrant). A statistical model with more conservative covariate adjustment was also conducted and results are presented in the Supplementary Material.
Multiple Imputation
A statistical multiple imputation (MI) was performed using SAS 9.4 to minimize bias due to missing data. Missing data was not substantial with the exception of one variable used to construct the SII, “social contacts with children,” which had n = 1,488 observations missing (15%). The fully conditional method (FCS) was used to impute missing variables since the outcome variables (HRD and PVRD) are binary (24). MI was performed using 20 imputation datasets. Results reported herein were generated with the use of MI. Corresponding results generated from a complete case analysis (CCA) are included as Supplementary Material.
Statistical Analysis
Characteristics between the middle-aged and older-aged participants were compared using Chi square tests and independent t-tests. Multi-variable logistic regression models were used to test the main and interactive associations of PRS and PSFs with HRD and PVRD. Separate models were run for depression and SII. For depressive symptoms, the reference category was Group 1 (no depressive symptoms). The reference category for the social isolation variable was not socially isolated (coded as 0). These logistic regression models were performed for all participants, and also for a predefined subgroup analysis stratified by age: middle-aged participants between 45 and 69 years old (n = 7,155) and older participants aged 70 years and above (n = 2,737). This age stratification was chosen because the mean age of individuals in GWAS of CVD is most commonly 69 or younger (6), thus, age 70 represented a target cut-off to evaluate the performance of a PRS for CAD amongst an older-aged sample. All analyses were conducted using SAS version 9.4 (SAS Institute, North Carolina) and all p-values were two-sided with alpha set at 0.05.
As a sensitivity analysis, the continuous PRS was separated into quintiles to test the interaction association between extreme categories of PRS and the PSFs. Quintile 1 (lowest PRS scores) was compared to the reference quintile 5 (highest PRS scores) since categorizing a PRS may enable detection of associations between the extremes of genetic risk (6).
Results
Population Characteristics
Our analytical sample included n = 9,892 of the 9,896 participants who had data available for the exposure (PRS and PSFs) and outcomes variables (HRD and PVRD) upon MI. Four participants had genetic data missing for principal components analysis of ancestry. Upon PRS calculation with genotyping data for the analytical sample, the range of PRS was between 22.2–54.5. Table 1 shows the characteristics of the analytical sample based on the MI analysis. Middle-aged adults comprised 72% of the sample and 28% were older-aged adults. Compared to middle-aged groups, the older-aged group had a lower prevalence of the following characteristics: high income bracket households, non-Caucasian participants, current smokers, post-secondary education graduates, rural-residing participants, and group 3 and 4 depressive symptoms. The older-aged group had a higher prevalence of immigrants and socially isolated participants, and the mean PRS was slightly lower compared to the middle-aged group. Overall, the majority of all participants fell within Group 1 of depressive symptoms (no depressive symptoms) and were considered not socially isolated. As expected, a greater proportion of older-aged participants reported at least one CVD outcome compared to the middle-aged subgroup. These descriptive statistics were similar in the CCA (see Supplementary Table 2).
Main Effect Associations Between PRS and CVD Outcomes
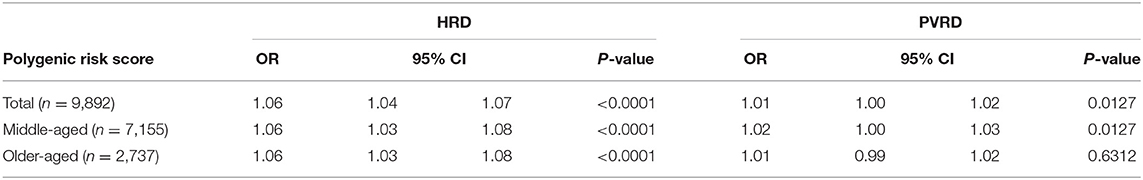
The PRS was significantly associated with HRD in the total sample as well as in the middle-aged and older-aged subgroups. The association between the PRS and PVRD was significant among the total sample and middle-aged subgroups, but was not significant in the older-aged subgroup (Table 2).
Main Effect Associations Between PSFs and CVD Outcomes
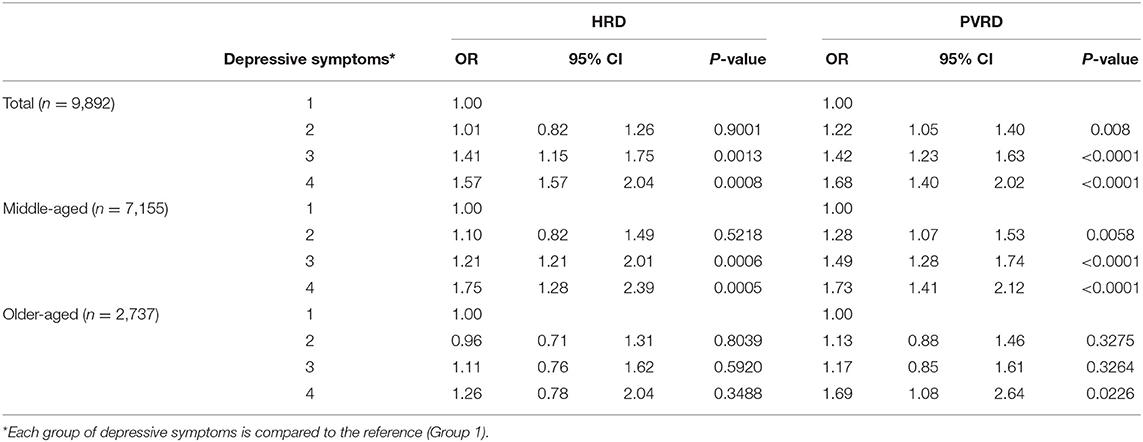
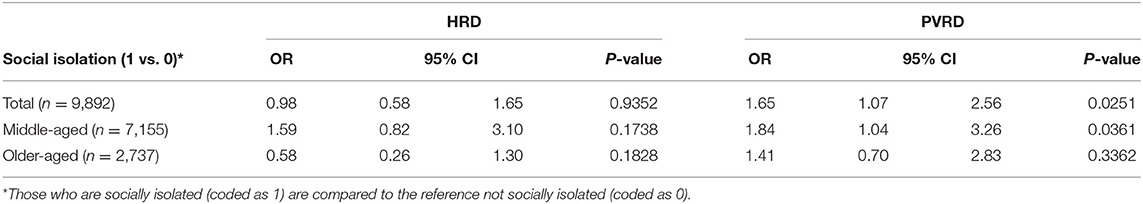
Most depressive symptoms compared to the reference (Group 1, no depressive symptoms) significantly associated with both HRD and PVRD among the total sample and middle-aged, however, only Group 4 (potential, recurrent depression) compared to the reference was significantly associated with PVRD among older-aged (Table 3). Being socially isolated compared to not socially isolated was not significantly associated with HRD among the total sample as well as the middle-aged and older-aged subgroups. Being socially isolated was significantly associated with PVRD among the total sample and middle-aged participants, but not among the older-aged subgroup (Table 4).
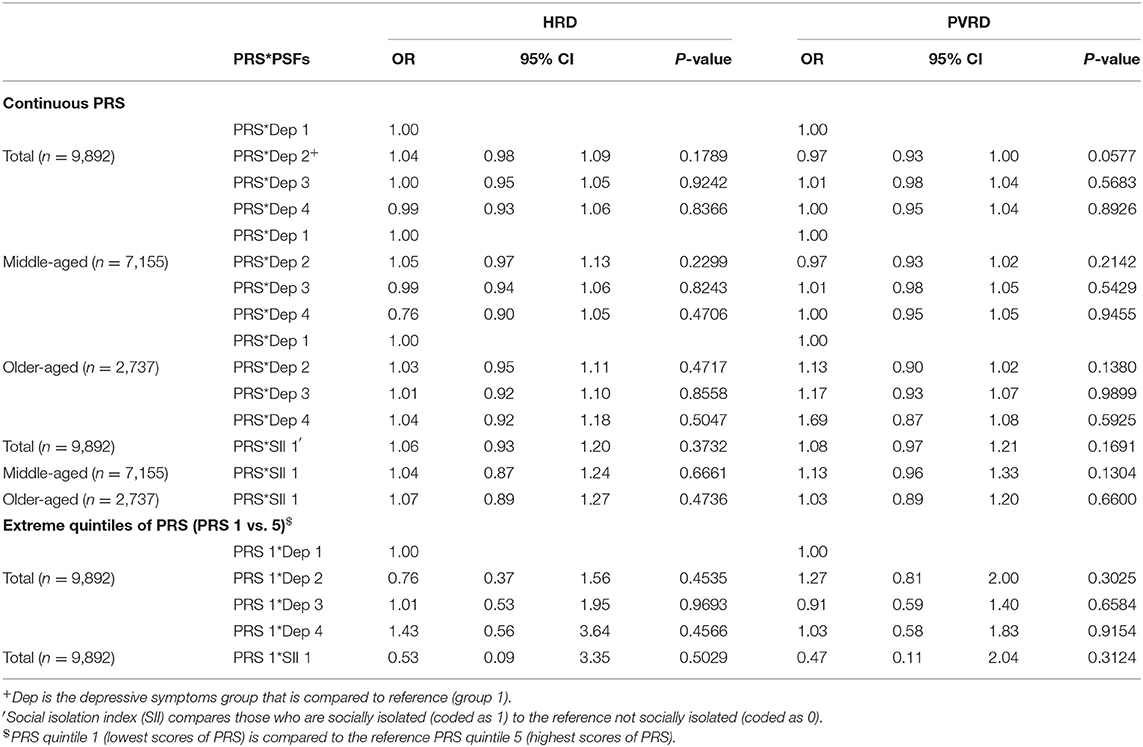
PRS*PSF Interaction Associations With CVD Outcomes
When PRS was modeled as a continuous variable, there were no significant interactions between the PRS and either PSF (depressive symptoms and social isolation) among the total sample or age subgroups. Additionally, no significant interactions or trends were observed between the extreme quintiles of PRS for either PRS*depressive symptoms or PRS*social isolation among the total analytical sample (Table 5), middle-aged or older-aged participants (results not shown). Corresponding results in the CCA were very similar to the analysis using MI (Supplementary Tables 3–7).
Discussion
This observational study investigated main and interactive associations of polygenic risk and PSFs on CVD outcomes by age group using baseline data from a Canadian cohort. This represented a multiscale approach that assessed combinations of risk factors with CVD outcomes, and to our knowledge is the first such investigation evaluating genetic, psychosocial, and biological (age) risk factors with CVD.
The findings suggest that polygenic risk and PSFs are independently associated with CVD outcomes, but that these relationships may differ according to stage of the life course. GWAS have increasingly implicated common SNPs as risk factors for CVD, however, GWAS cohorts that evaluated CVD outcomes have been predominantly restricted to middle-aged adults (5). In order for our investigation to extend upon previous work on depression and CVD using CLSA data, CVD outcomes were grouped into HRD and PVRD. While our results suggest that a PRS for CAD is associated with HRD to a similar extent among both middle-aged and older-aged individuals, the association was attenuated for PVRD among the older-aged group. This may be due to the fact that the PRS is predictive of CAD and some participants with HRD were present in the PVRD group due to the nature of the construction of CVD groupings. Thus, the results for the PRS and PVRD associations should be interpreted cautiously, particularly because the associations among the total and middle-aged group were weakly significant and survival bias may have affected the outcome amongst the older-aged group. Nevertheless, future studies are warranted to evaluate the performance of PRS among different age groups, because a growing body of evidence suggests that the influence of genetic variation may differ across the life course (9). No significant interactions were observed between polygenic risk and PSFs, however, further research is required to confirm this finding, due to the limited number of participants who were socially isolated in our sample. In addition, future research should consider characterizing depression with more precision, particularly typical vs. atypical depression rather than solely detecting its presence (i.e., symptoms and clinical history) (25).
Among PSFs, various degrees of depressive symptoms were significantly associated with HRD and PVRD among middle-aged adults, but most depressive groups were not associated with CVD outcomes among older-aged adults. These findings align with previous results for depression and CVD in the larger CLSA Comprehensive Cohort (3) and suggest that depressive symptoms may play a greater role at the mid-stage of the life course. Indeed, considering stage of the life course is important because older-aged adults have been described to exhibit differential stability of emotional experience such that positive states are maintained longer, and negative states are more quickly dismissed (26). This aging-related motivational shift may explain why depressive symptoms were not associated with CVD outcomes among the older-aged subgroup to the same degree that we observed among the middle-aged subgroup.
Social isolation was significantly associated with increased risk of PVRD among the middle-aged subgroup, but not the older-aged subgroup. This finding was unexpected since elderly adults have been identified as more vulnerable to social isolation (12). However, a likely explanation for our finding is an under-representation of socially isolated CLSA participants in the subset that had genome-wide genotyping performed, which may have impacted statistical power particularly among the elderly subgroup of our analysis that had a very low prevalence of social isolation. Menec et al. recently reported the prevalence of social isolation in the overall CLSA (n = 47,752) to be 5.1% (22). The prevalence in our analytical sample, comprised of the participants who had genotyping data available, was remarkably lower at 1.2%. The Comprehensive Cohort was chosen based on proximity and ability to be present at a CLSA data collection site (DCS) where blood samples were collected. Participants who were unable or unwilling to go to a DCS were excluded (27). CLSA participants who are socially isolated likely could not visit a DCS due to access barriers (e.g., living outside of proximity buffer or not being able to access transportation). CLSA follow-up efforts may benefit from incorporating mobile examination centers so that physical assessments and biospecimen collection can be obtained from a greater proportion of socially isolated participants, particularly because social isolation may be an emerging risk factor for CVD (12). Indeed, mobile examination centers have been used successfully in other population cohort studies to better reach participants who are unable or unwilling to go to a DCS (28).
Strengths of this investigation include the use of a national cohort with detailed data on participant genetics, health and lifestyle, and psychosocial factors. Additionally, we applied methods that were used in previous related studies for consistency, including our construction of a PRS for CAD (6) and our use of the same CVD outcomes (3), depressive symptoms (3), and SII (22) groupings that were utilized by separate investigations conducted with CLSA data. Since CVD affects men and women differently, we performed a supplementary sex-stratified analysis (data not shown). Our results were largely unaltered, with the exception that the main effect association between social isolation and PVRD was only statistically significant among men. However, given the low prevalence of socially isolated participants, this observation should be interpreted with caution.
A number of limitations to our approach must be acknowledged. We did not assess other relevant PSFs, such as anxiety (which was not assessed in the CLSA) and social support. In addition, the non-specific format of CLSA questions about participant CVD diagnosis history could have introduced response bias (e.g., only providing a response option for heart disease rather than specifying coronary or congenital heart disease). To account for this measurement error, CVD variables were categorized into broad groupings rather than individual CVD outcomes (3). In addition, the use of reported physician diagnosed CVD, rather than direct confirmation of disease, is a limitation. Potential survival bias in the CLSA cohort may have prevented certain associations from being detected. For example, if mortality from CVD is truly higher among older individuals with less favorable genetic or psychosocial status, elderly participants who have been diagnosed with CVD and have high polygenic risk, or are socially isolated or present with depression, would be underrepresented in CLSA (29). We did not correct for multiple testing; however, a Bonferroni correction may not be appropriate owing to the possible interrelationship between PSFs. Moreover, due to the cross-sectional nature of the study the directionality of associations between PSFs [particularly depression (30)] and CVD outcomes cannot be determined, and no data on further incidence of CVD according to PSFs or PRS was available at the time of this analysis. Lastly, lack of genetic data for a larger sample of socially isolated participants may have also affected statistical power to detect certain main-effect or interactive associations.
This study provides evidence that polygenic risk of CAD and PSFs are associated with CVD outcomes, but that stage of the life course and type of CVD are important considerations underscoring the importance of a multiscale risk factor approach. Future studies that explore the bidirectional nature of depression and CVD and identification of mediators of associations between PSFs and CVD, potentially lifestyle, are warranted in order to provide insight into multiscale strategies to prevent CVD.
Data Availability Statement
The datasets presented in this article are not readily available because data are available from the Canadian Longitudinal Study on Aging (www.clsa-elcv.ca) for researchers who meet the criteria for access to de-identified CLSA data. Requests to access the datasets should be directed to www.clsa-elcv.ca.
Ethics Statement
The studies involving human participants were reviewed and approved by McGill University Faculty of Agricultural and Environmental Sciences Research Ethics Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GM conducted the statistical analysis, wrote the original draft of the manuscript, and prepared all figures and tables. CP and LD provided feedback on statistical analysis and methodology and helped review and edit the manuscript. HH assisted with statistical analyses and methodology and helped review and edit the manuscript. DN obtained ethics approval, provided access to the data, designed the statistical approach, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by a CIHR Catalyst Grant (19CA001) and FRQSC Team Grant (2015-SE-179342), GM was a recipient of a CIHR Canada Graduate Scholarship—Master's. Funding for the CLSA is provided by the Government of Canada through the Canadian Institutes of Health Research under grant reference: LSA 94473 and the Canada Foundation for Innovation.
Disclaimer
The opinions expressed in this manuscript are the author's own and do not reflect the views of the Canadian Longitudinal Study on Aging.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was made possible with data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). This research has been conducted using the CLSA's Baseline Comprehensive Dataset version 4.0, under Application Number 19CA001. The CLSA is led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.599671/full#supplementary-material
References
1. World Health Organization. Cardiovascular Disease Fact Sheet (2017). Available online at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed April 30, 2020).
2. Public Health Agency of Canada. Heart Disease in Canada Fact Sheet. (2016). Available online at: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/heart-disease-maladies-coeur-eng.pdf (accessed April 30, 2020).
3. Liu J, Son S, Mcintyre J, Narushima M. Depression and cardiovascular diseases among Canadian older adults: a cross-sectional analysis of baseline data from the CLSA Comprehensive Cohort. J Geriatr Cardiol. (2019) 16:847–54. doi: 10.11909/j.issn.1671-5411.2019.12.001
4. Xia CH, Ma Z, Cui Z, Bzdok D, Thirion B, Bassett DS, et al. Multi-scale network regression for brain-phenotype associations. Hum Brain Mapp. (2020) 41:2553–66. doi: 10.1101/628651
5. Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. (2007) 357:443–53. doi: 10.1056/NEJMoa072366
6. Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. (2016) 375:2349–58. doi: 10.1056/NEJMoa1605086
7. Kullo IJ, Leeper NJ. The genetic basis of peripheral arterial disease: current knowledge, challenges, and future directions. Circ Res. (2015) 116:1551–60. doi: 10.1161/CIRCRESAHA.116.303518
8. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. (2018) 50:1219–24. doi: 10.1038/s41588-018-0183-z
9. Tanisawa K, Ito T, Sun X, Ise R, Oshima S, Cao ZB, et al. Strong influence of dietary intake and physical activity on body fatness in elderly Japanese men: age-associated loss of polygenic resistance against obesity. Genes Nutr. (2014) 9:416. doi: 10.1007/s12263-014-0416-4
10. Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey Smith G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: mendelian randomisation study. BMJ. (2020) 369:m1203. doi: 10.1136/bmj.m1203
11. Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, et al. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry. (2014) 14:371. doi: 10.1186/s12888-014-0371-z
12. Zhou Z, Lin C, Ma J, Towne SD, Han Y, Fang Y. The association of social isolation with the risk of stroke among middle-aged and older adults in China. Am J Epidemiol. (2019) 188:1456–65. doi: 10.1093/aje/kwz099
13. Yanguas J, Pinazo-Henandis S, Tarazona-Santabalbina FJ. The complexity of loneliness. Acta Biomed. (2018) 89:302–14. doi: 10.23750/abm.v89i2.7404
14. Taylor HO, Taylor RJ, Nguyen AW, Chatters L. Social isolation, depression, and psychological distress among older adults. J Aging Health. (2018) 30:229–46. doi: 10.1177/0898264316673511
15. Matthews T, Danese A, Wertz J, Odgers CL, Ambler A, Moffitt TE, et al. Social isolation, loneliness and depression in young adulthood: a behavioural genetic analysis. Soc Psychiatry Psychiatr Epidemiol. (2016) 51:339–48. doi: 10.1007/s00127-016-1178-7
16. Hammig O. Health risks associated with social isolation in general and in young, middle and old age. PLoS ONE. (2019) 14:e0219663. doi: 10.1371/journal.pone.0219663
17. Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. (2009) 31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002
18. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
19. About the CLSA Research Platform: Canadian Longitudinal Study on Aging. (2020). Available online at: https://www.clsa-elcv.ca/about-us (accessed April 30, 2020).
20. Forgetta V, Darmon-Zwaig C, Belisle A, Li R, Balion C, Roshandel D, et al. The Canadian Longitudinal Study on Aging: Genome-Wide Genetic Data on 9,900 Participants. (2018). Available online at: https://www.clsa-elcv.ca/doc/2748 (accessed April 30, 2020).
21. Derived Variable - Depression (DEP): Canadian Longitudinal Study on Aging. (2018). Available online at: https://www.clsa-elcv.ca/doc/2528 (accessed April 30, 2020).
22. Menec VH, Newall NE, Mackenzie CS, Shooshtari S, Nowicki S. Examining individual and geographic factors associated with social isolation and loneliness using Canadian Longitudinal Study on Aging (CLSA) data. PLoS ONE. (2019) 14:e0211143. doi: 10.1371/journal.pone.0211143
23. Atherosclerosis: Heart and Stroke Foundation (2020). Available online at: https://www.heartandstroke.ca/heart/conditions/atherosclerosis (accessed April 30, 2020).
24. Multiple Imputation in SAS Part 1. UCLA: Statistical Consulting Group (2020). Available online at: https://stats.idre.ucla.edu/sas/seminars/multiple-imputation-in-sas/mi_new_1/ (accessed April 30, 2020).
25. Penninx BW. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. (2017) 74(Pt B):277–86. doi: 10.1016/j.neubiorev.2016.07.003
26. Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. J Pers Soc Psychol. (2000) 79:644–55. doi: 10.1037/0022-3514.79.4.644
27. Raina P, Wolfson C, Kirkland S, Griffith LE, Balion C, Cossette B, et al. Cohort profile: the canadian longitudinal study on aging (CLSA). Int J Epidemiol. (2019) 48:1752–53j. doi: 10.1093/ije/dyz173
28. Center for Disease Control and Prevention. National Health and Nutrition Examination Survey. (2020). Available online at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2015 (accessed May 10, 2020).
29. Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Commun Health. (2004) 58:635–41. doi: 10.1136/jech.2003.008466
Keywords: cardiovascular disease, polygenic risk score, depression—epidemiology, social isolation, aging
Citation: Menniti G, Paquet C, Han HY, Dube L and Nielsen DE (2021) Multiscale Risk Factors of Cardiovascular Disease: CLSA Analysis of Genetic and Psychosocial Factors. Front. Cardiovasc. Med. 8:599671. doi: 10.3389/fcvm.2021.599671
Received: 27 August 2020; Accepted: 22 February 2021;
Published: 16 March 2021.
Edited by:
Ailin Barseghian, University of California, Irvine, United StatesReviewed by:
Izabella Uchmanowicz, Wroclaw Medical University, PolandGiulio Francesco Romiti, Sapienza University of Rome, Italy
Copyright © 2021 Menniti, Paquet, Han, Dube and Nielsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daiva E. Nielsen, ZGFpdmEubmllbHNlbkBtY2dpbGwuY2E=
 Gabriella Menniti
Gabriella Menniti Catherine Paquet
Catherine Paquet Hannah Yang Han
Hannah Yang Han Laurette Dube
Laurette Dube Daiva E. Nielsen
Daiva E. Nielsen