
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 20 April 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.598046
Background and Objectives: The second-generation drug-eluting stents have been used to treat chronic total occlusion lesion. However, there is limited evidence of the clinical outcomes that whether the second-generation drug-eluting stents is superior to first-generation ones in patients with chronic total occlusion lesion undergoing percutaneous coronary intervention. The study aimed to compare the differences in clinical outcomes between the two generations drug-eluting stents in patients with those by a meta-analysis.
Methods: PubMed, Embase, the Cochrane library and Web of science databases were systemically searched before March, 2021. Randomized controlled trials and observational studies were included to compare the second-generation drug-eluting stents with the first-generation ones in patients with chronic total occlusion lesion undergoing percutaneous coronary intervention. The clinical outcomes were major adverse cardiac events (MACE), target vessel revascularization, myocardial infarction, all-cause death. Fixed effects models were used to calculate the odds ratio (OR) and 95% confidence interval (CI) of each clinical outcome. Sensitivity analysis was performed to detect potential sources of heterogeneity. Subgroup analyses were used to assess the differential effects.
Results: The meta-analysis included eight studies involving 4,583 patients with chronic total occlusion lesion undergoing percutaneous coronary intervention. Pooled analysis showed that the incidence of MACE (OR = 0.68, 95%CI 0.54–0.85, P = 0.0008), target vessel revascularization (OR = 0.70, 95%CI 0.54–0.91, P = 0.007), and myocardial infarction (OR = 0.58, 95%CI 0.37–0.93, P = 0.02) were lower in the second-generation drug-eluting stents compared with the first-generation ones. However, there was not difference in all-cause deaths between two drug-eluting stents (OR = 0.67, 95%CI 0.45–1.01, P = 0.05).
Conclusions: The second-generation drug-eluting stents are associated with lower MACE, target vessel revascularization, and myocardial infarction compared with the first-generation ones in patients with chronic total occlusion lesion undergoing percutaneous coronary intervention. The results of this study can provide a reference for the selection of stents in patients with chronic total occlusion lesion. Further randomized controlled trials are needed to verify that the second-generation drug-eluting stents is superior to the first-generation ones in patients with chronic total occlusion (Registered by PROSPERO, CRD42020158406).
Chronic total occlusion (CTO) lesion is characterized by the complete or near complete occlusion of coronary artery with or without minimal downstream flow (TIMI flow grade 0 or 1) for more than 3 months (1). In patients undergoing coronary angiography, CTO lesion accounted for 25%, and about 50% of CTO lesion were located in the right coronary artery (2, 3). Meanwhile, the CTO patients had a high incidence of comorbidity, such as 34% of CTO patients with diabetes, 75% with hypertension and 82% with hyperlipidemia (3). In addition, long-term CTO lesion is prone to cause myocardial ischemia and hypoxia, cardiomyopathy, leading to the decline of pump blood function, seriously affecting the health of patients (4). Infract-related artery-CTO increased the risk of ventricular tachycardia/ventricular fibrillation by more than three times (5).
Kereiakes first reported the treatment of CTO lesion with antegrade technique in 1985. Since then, percutaneous coronary intervention (PCI) has been one of the most common treatments for CTO lesion (6). With the rapid development of CTO-PCI technology and the wide application of coronary stents, the problem of in-stent restenosis is gradually exposed. The in-stent restenosis after CTO-PCI with bare mental stents was as high as 50%, which undoubtedly hindered its application in this situation (7). The first-generation drug-eluting stent (DES) was introduced to solve in-stent restenosis in 2002, and its anti-proliferative drugs can inhibit the proliferation of vascular endothelial cells and eliminate neointimal hyperplasia, the incidence of in-stent restenosis after CTO-PCI was reduced to 7–8.2% (8–10). Nevertheless, the risk of very late stent thrombosis was increased compared with bare metal stent (11). Against this background, the second-generation DES was introduced to overcome the risk of very late stent thrombosis in the first-generation DES. Compared with first-generation DES, the second-generation DES reduced the risk of very late stent thrombosis by 67–76%, and was recommended by the European Society of Cardiology guidelines (class I, level of evidence A) (12, 13). However, the evidence of the CTO-PCI used second-generation DES was limited. Randomized trials and observation studies showed no difference between the two generations stents for patients with CTO (14–19). In addition, several observation studies appear to reveal potential benefit of second-generation DES (20, 21).
Therefore, the hypothesis that the second-generation DES is superior to the first-generation DES in the treating CTO lesion was proposed. The purpose of this meta-analysis was to verify this hypothesis by comparing the differences in clinical outcomes between the two DES in patients with CTO, to seek an optimal treatment for CTO lesion and to provide evidence of clinical treatment.
PubMed, Embase, the Cochrane library and Web of science databases were systemically searched before March, 2021. Search for the following keywords: “drug-eluting stents” AND “percutaneous coronary intervention” AND “chronic total occlusion” OR “chronic total coronary occlusion” OR “coronary chronic total occlusion” OR “CTO” without language restrictions. Set update reminder on PubMed to follow up on the latest research. The inclusion criteria for study selection were as follows: (1) patients with at least one coronary CTO; (2) comparisons of the second-generation DES (Everolimus eluting stent or Zotarolimus eluting stent or Biolimus-eluting stent) vs. the first-generation DES (Sirolimus-eluting stent or Paclitaxel-eluting stent); (3) original articles reporting at least one of these outcomes: MACE, target vessel revascularization, myocardial infarction, all-cause death, cardiac death. Exclusion criteria were: (1) duplicate publication; (2) review, conference abstract, letter, case reports; (3) study with incomplete or inaccurate data; (3) animal experiment.
After removal of duplicates, the titles, abstracts and full-text articles of all articles were reviewed by two investigators (Qiao X and Zhang WJ) independently to determine the study according to the eligibility criteria. Discrepancy was solved to reduce bias through negotiation with the third party (Liang XY and Li Y). Any disagreement was resolved with third party by discussion (Wang Zhl). The quality of each randomized controlled trial (RCT) was evaluated using the Cochrane tool of Collaboration for assessing risk of bias (22). All components with low risk in a trial were considered as having a low risk of bias, trial with >one unclear risk was considered to have a moderate risk of bias and trial with >one high-risk components was considered as having a high risk of bias. The nonrandomized studies were evaluated according to the Newcastle-Ottawa scale checklist (23). This scale checklist assessed the selection, comparability and outcome of the experiment and control groups in the original study, and containing 8 items with full marks of 9 scores, 0–5 scores for low-quality literature, and 6–9 scores for high-quality literature. The present meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses consensus statement for randomized trials and Meta-analysis of Observational Studies in Epidemiology consensus statement for non-randomized studies (24). All clinical protocols included in the study were approved by local ethics and patient informed consent. The study protocol was registered in PROSPERO (CRD42020158406).
Data on participants and procedural characteristics, follow-up duration, and the clinical outcomes were extracted independently by two researchers (Qiao X and Zhang WJ) from the original publications, and negotiate divergences with the two independent authors (Liang XY and Li Y). All data extracted were checked by the last author (Wang Zhl). The clinical outcomes were MACE, target vessel revascularization, myocardial infarction, all-cause death. The MACE was defined as the original report. The target vessel revascularization was defined as any repetitive revascularization of the target vessel. The all-cause death was defined as death attributed to various causes.
Outcomes were analyzed by an intention-treat analysis. Continuous variables were expressed as averages or medians, and categorical variables were described using absolute numbers and proportions (%). Statistical software such as Review Manager Version 5.3 software (The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata version 12.0 software (Statacorp LP, College Station, Texas, USA) were used for statistical analysis. The Mantel-Haenszel method was applied to calculate the odds ratio (OR) and 95% confidence interval (CI) of each outcome. Heterogeneity was assessed by the Cochrane Q statistic with Pearson chi-square test and the Higgins I2 test. In case of less heterogeneity (I2 < 50%, fixed effect model was utilized, otherwise random effect model was performed for calculate the pooled OR. In addition, sensitivity analysis was performed to determine whether the omittance of a single study result affected the stability of the overall results. Subgroup analysis was utilized to assess the differential effects. Two-tailed P-values were exploited for all results, and statistical significance set at P < 0.05. Visual estimation of funnel plot and the Begg's and Egger's tests was used to detect the possibility of publication bias.
Trial Sequential Analysis version 0.9.5.10 software (Copenhagen Trial Unit, CTU) was used to evaluate the random errors and the required information size of clinical outcomes, which based on an α of 0.05 and a power of 0.8 for RCTs.
The search results are shown (Figure 1). The initial search retrieved 1,522 articles from medical databases and other sources, of which 554 were duplicates. After browsing titles, abstract and reading the full text, two randomized controlled trials and six nonrandomized controlled studies containing 4,583 patients with CTO lesion undergoing PCI were determined. Among them, three studies included 637 patients of Caucasian and five studies contained 3,946 patients of Asian, 1,801 (39%) patients used second-generation DES and 2,782 (61%) received first-generation DES. The baseline characteristics of the studies included are presented (Table 1). The duration of follow-up in the study included was from 9 months to 60 months. The majority of participants were males, which accounted for 78%, 61% of patients had multi-vessel diseases. The average age of the patients used the second -generation DES was from 58 to 60 years, while that of patients undergoing the first -generation DES was from 61 to 68 years. CTO patients with hypertension accounted for 63%, diabetes 35% and hyperlipidemia 32% in this studies. The mean LVEF of patients was from 43 to 58%. The baseline characteristics and procedure of the participants included are summarized (Table 2).
The Cochrane quality assessment shows that there was one unclear risk of bias in two randomized trials, which had a moderate risk of bias (Supplementary Table 1). The quality assessment of nonrandomized studies evaluated by the Newcastle-Ottawa scale checklist present that five studies had scores ≥6 points, which were high quality literature, and one study had scores < 6 points, which were low quality literature (Supplementary Table 2).
Since one of two RCTs did not have data on the clinical outcomes of MACE or all-cause death, only target vessel revascularization and myocardial infarction were assessed for random errors and the required information size. The cumulative Z-curve does not cross either the conventional boundary or the trial sequential monitoring boundary, which suggested there was no significant difference between the second-generation DES and the first-generation DES in treatment of target vessel revascularization and myocardial infarction (Supplementary Figure 1). The trial sequential analysis of these two RCTs presented that the comparison of the second-generation DES and the first-generation DES in the treatment of target vessel revascularization and myocardial infarction were far from sufficient in terms of the required information size, and further RCTs are needed to determine whether the second-generation DES is superior to the first-generation DES in the treatment of target vessel revascularization and myocardial infarction.
The MACE is appeared in seven studies, the second-generation DES provided a significant advantage over the first-generation DES in reducing the incidence of MACE in Figure 2A (OR = 0.68, 95%CI 0.54–0.85, P = 0.0008), and without significant heterogeneity among studies (I2 = 42%, P heterogeneity = 0.11). According to visual estimation, the funnel plot was asymmetrical and publication bias was found in Egger's and Begg's tests (P = 0.042 and P = 0.019, respectively) (Supplementary Figure 2A). Similarly, Seven of the eight studies had a data on the target vessel revascularization, whose rate are significantly reduced in patients with second-generation DES compared with the patients with first-generation DES in Figure 2B (OR = 0.70, 95% CI 0.54–0.91, P = 0.007), there was no significant heterogeneity across the enrolled trials (I2 = 10%, P heterogeneity = 0.35). The funnel plot showed slightly asymmetrical but Egger's and Begg's tests did not show publication bias (P = 0.368 and P = 0.089, respectively) (Supplementary Figure 2B). Meanwhile, compared with the first-generation DES group, the second-generation DES group has a trend to reduce the incidence of myocardial infarction in all eight studies in Figure 2C (OR = 0.58, 95% CI 0.37–0.93, P = 0.02), the analysis revealed no significant heterogeneity among studies (I2 = 0%, P heterogeneity = 0.77). Although the funnel plot presented asymmetry according to visual estimation, there was no publication bias according to Egger's and Begg's tests (P = 0.063 and P = 0.053, respectively) (Supplementary Figure 2C). Conversely, seven of the eight studies show that the all-cause death of the second-generation DES was not significantly lower than that of the first-generation DES in Figure 2D (OR = 0.67, 95% CI 0.45–1.01, P = 0.05) and there was no significant heterogeneity among studies (I2 = 0%, P heterogeneity = 0.93). The funnel plot exhibited symmetry and there was no publication bias in Egger's and Begg's tests (P = 0.764 and P = 0.560, respectively) (Supplementary Figure 2D).
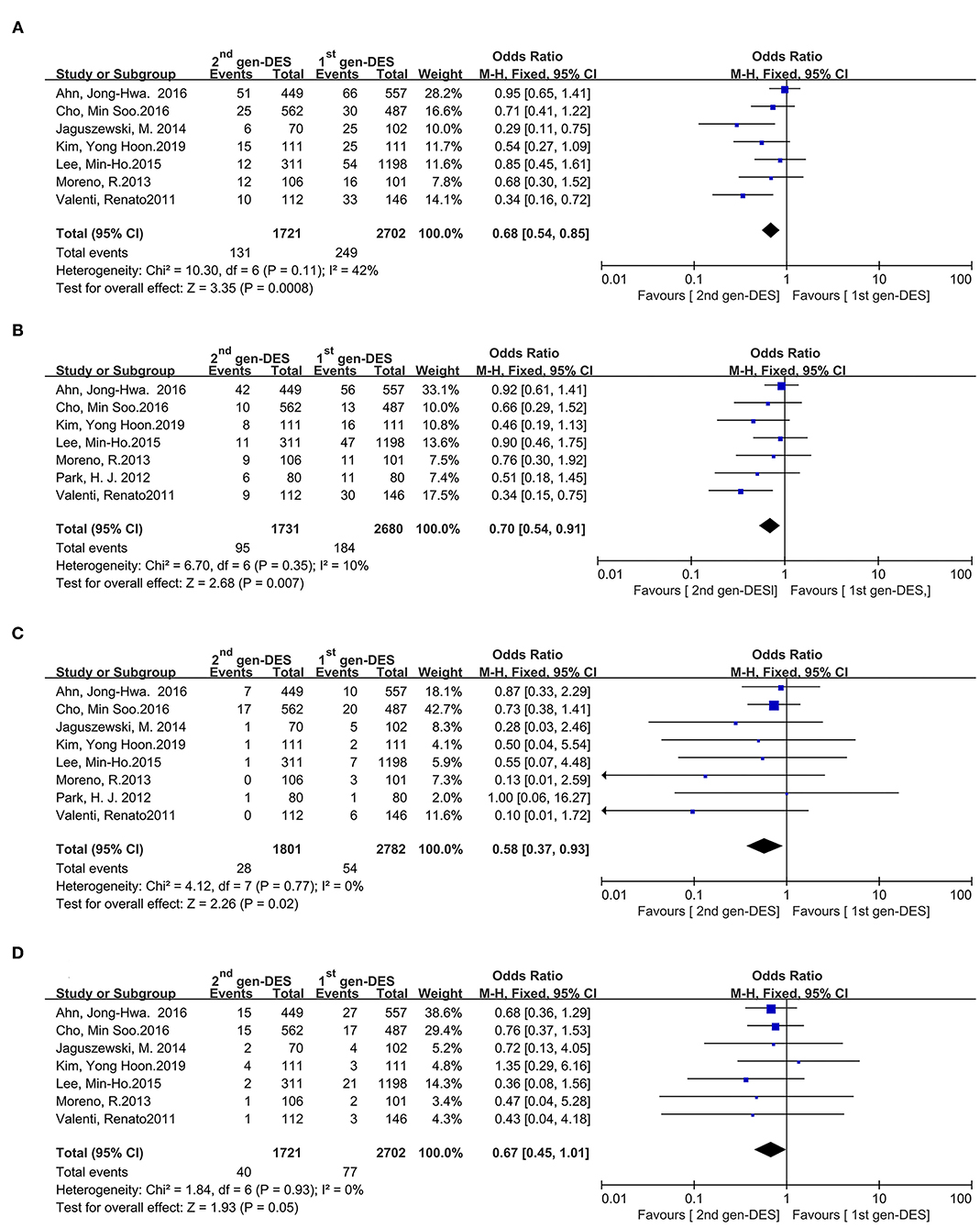
Figure 2. Comparison of clinical outcomes between the second- and first-generation DES groups. (A) major adverse cardiac events, (B) target vessel revascularization, (C) myocardial infarction, (D) all-cause death. 2nd gen-DES, the second- generation DES; 1st gen -DES, the first- generation DES.
Since the total length of stent implanted in most studies was ≥ 40 mm, the 40 mm was used as the cut-off point of stent length for subgroup analysis, and subjects were CTO patients with long lesions and multiple stents implanted (20). Meanwhile, the race (Asian vs. Caucasian) and study designs (RCT vs. Non-RCT) were analyzed as subgroup. The occurrence of MACE, target vessel revascularization and myocardial infarction in CTO patients with second-generation DES are lower than that in those patients with first-generation DES when the total length of stent implanted ≥ 40 mm (Supplementary Figures 3A–C). Similarity, when the study designs of non-RCT and the race of Caucasian, the second-generation DES perform better than the first-generation DES in incidence of MACE, target vessel revascularization and myocardial infarction in CTO patients (Supplementary Figures 3A–C). However, the incidence of all-cause death seems to be no significant difference caused by the study design, race and total length of stent implanted (Supplementary Figure 3D). In addition, the original study analyzed clinical outcome by intention-treat analysis and no separate data were available, subgroup analysis also did not apply to differences in stent types.
There is a moderate statistical heterogeneity in the MACE (I2 = 42%) (Figure 2A). Sensitivity analysis revealed that the study by Ahn, Jong-Hwa in 2016 is the source of statistical heterogeneity for the MACE in the meta-analysis (Supplementary Figure 4) (16). After excluding the results of this study, the statistical significance of the MACE pooled analysis remains unchanged (OR = 0.57, 95%CI 0.43–0.76, P < 0.0001, while heterogeneity among the remaining studies is reduced (I2 = 19%) (Supplementary Figure 5).
This meta-analysis enrolled two RCTs and six nonrandomized controlled studies, which compared the clinical outcomes associated with the second-generation DES and the first-generation DES in patients with CTO lesion undergoing PCI. The principal finding is that the second-generation DES was superior to reduce the incidence of MACE, target vessel revascularization and myocardial infarction in patients with CTO lesion compared with the first-generation DES. In contrast, the use of the second-generation DES did not appear a lower incidence of all-cause death than that of the first-generation DES.
In 1985, the success rate of antegrade wire technique in treating CTO lesion was only 53% (4). In recent years, with the application of antegrade wire technique, retrograde wire technique, antegrade dissection re-entry and hybrid strategy, success rates of CTO-PCI have significantly increased while maintaining low risk of complications (25). The first-generation DES significantly reduced the rates of restenosis in the treatment of CTO lesion compared with bare-metal stent, but the stent coated with permanent materials increased the risk of very late stent thrombosis, which defined as more than 360 days after PCI according to the Academic Research Consortium (11, 26). Although the incidence of very late thrombosis after PCI is as low as 0.4–2% under dual antiplatelet therapy, the occurrence of stent thrombosis is devastating, usually presenting as major myocardial infarction that often requires reintervention (27, 28). The second-generation DES as a novel therapeutic material, which have thinner scaffolds and more biocompatible polymers, seems to demonstrate potential benefits, which were widely used to improve safety, reduce the dose of antiproliferative drugs and ameliorate the release kinetics. The EXPERT CTO Multicenter trial published in 2015 showed that it is beneficial to use the everolimus-eluting stent in patients with CTO lesion (29). However, the use of the first-generation DES and the second-generation DES is still controversial. In particular, whether the second-generation DES is better than the first-generation DES in the treatment of CTO lesion, there is no basis for guidance and consensus. Therefore, it is necessary to explore the clinical outcome of two generations of stent in the treatment of CTO lesion.
This meta-analysis showed that the second-generation DES had a low incidence tendency of MACE, target vessel revascularization, and myocardial infarction compared with the first-generation one. However, there was no difference in all-cause deaths between the two generation DES, which was similar to the results of a meta-analysis by Lanka et al. in 2014 (30). That meta-analysis enrolling 1,174 patients showed that the second-generation DES were associated with a lower incidence of death, target vessel revascularization compared with the first-generation DES, but the incidence of myocardial infarction and stent thrombosis was similar (30), while most participants in this study had multi-vessel diseases (61%) and the total length of stent implantation was more than 40 mm. Further analysis showed that the second-generation DES was better than the first-generation DES in these patients with multi-vessel lesion, long occlusive lesion requiring multiple stent implantation (total stent length ≥ 40 mm). Similarly, the incidence of MACE, target vessel revascularization and myocardial infarction in Caucasian in the second-generation DES might be lower than that in the first-generation DES. It suggests that patients with CTO lesion in Caucasians are more suitable for the second- generation DES than those in Asians.
In the initial pooled analysis, the second-generation DES group was better than the first-generation DES group in reducing the incidence of MACE. However, there was moderate heterogeneity. Further sensitivity analysis showed that one study with clinical heterogeneity was the source of statistical heterogeneity. This heterogeneity may be due to the fact that the total length of stent implantation was < 40 mm in most of Asian population analyzed in this study. The results showed that there was no significant difference between the second- and first-generation DES (HR = 1.00, 95%CI 0.67–1.50, P = 0.99). Therefore, it is considered that this study has an obvious clinical heterogeneity, which is the main source of MACE pooled analysis heterogeneity. It is worth noting that the results of the subgroup analysis showed that the second-generation DES was better than the first-generation DES when the total length of stent implantation ≥ 40 mm and in Caucasian. Combined with sensitivity analysis and subgroup analysis, the results demonstrate that the incidence of MACE tends to be reduced in the second-generation DES group. In addition, the second-generation DES in the non-RCT subgroup had a lower incidence of MACE, target vascular revascularization and myocardial infarction (consistent with the results of the initial pooled analysis) in the subgroup analysis, while there was no difference between the two generations DES in the RCT subgroup. Combined with this subgroup analysis and the trial sequential analysis evaluation of the RCTs, the heterogeneity may be due to intergroup one between RCT and non-RCT (differences of study design). On the one hand, although 3 months was recognized as the accepted cutoff, different studies had definitions of CTO lesion. In observational studies, the estimated occlusion time of CTO lesion was >3 months. In the RCTs of CATOS, the estimated occlusion time of CTO lesion was ≥1 month, while the estimated occlusion time of lesion was ≥2 weeks in the CIBELES trial (14, 15), which may affect the results by including partial patients with subtotal occlusion. On the other hand, clinical heterogeneity among patients included in observational studies may also be an important source of heterogeneity. The study by Valenti showed that the second-generation DES was significantly superior to the first-generation DES in reducing the incidence of MACE (22.6 vs. 8.9%, P = 0.003), target vessel revascularization (20.5 vs. 8%, P = 0.005) and in-stent restenosis or re-occlusion (31.4 vs. 11.8%, P = 0.001) in patients with long occlusions, extensive calcification, bridging collaterals and non-tapered stump (20). The study by Jaguszewsk enrolled highly selected patients with isolated CTO, and which showed that compared with first-generation DES, the second-generation DES significantly reduced the risk of MACE during 1 year follow-up (2.8 vs. 17.6%, HR = 0.15, 95%CI, 0.06–0.36, P = 0.01) (21). It is suggested that the second-generation DES is superior to the first-generation DES, indicating that the second-generation DES has excellent performance in complex CTO lesion. In conclusion, it is still believed that the second-generation DES has a significant advantage over the first-generation DES in reducing the incidence of MACE, target vascular revascularization and myocardial infarction. Other clinical outcome such as all-cause death did not show significant heterogeneity.
Although this result shows that the second-generation DES can reduce the incidence of MACE, target vessel revascularization and myocardial infarction in patients with CTO compared with the first-generation DES. However, the interpretation of this result should be cautious. First of all, since most of the patients in this study are male, it is not clear whether there are gender differences in the results of this study. Therefore, whether the second-generation DES has clinical efficacy compared with the first-generation DES needs to be further confirmed for female patients with CTO. Secondly, most of the patients in this study have been implanted the second-generation DES, while the number of patients implanted the first-generation DES is very small, which may affect this result. Thirdly, race and patients with multi-vessel lesion, long occlusive lesion requiring multiple stents should be considered, the Asian population and isolated CTO patients need to weigh the advantages and disadvantages to make a prudent decision. Fourthly, trial sequential analysis evaluations show that the sample size of two RCTs has not reached the expected information of trial sequential analysis. The pooled results are driven by observational studies. Therefore, clinicians need to make prudent decisions regarding non-highly selective individual CTO patients, such as CTO patients with acute coronary syndromes or bifurcation lesion. Finally, the success rate of coronary interventional in the treatment of CTO mainly depends on the operation of the guide wire, the characteristics of the lesion and the situation of the patients. These factors also need to be considered.
The limitations of this study should be recognized. First, although the combination of MeSH and free-words is adopted in literature retrieval and manual retrieval in this study, it is still possible to ignore the original research and affect the analysis results. Second, the data of this study are mainly from observational studies, including only two randomized studies (14, 15), which cannot exclude differences in baseline characteristics, methodology, drug therapies. In the absence of randomized observational studies, measured or unmeasured confounding factor may affect the pooled results. Third, the difference of the race populations may be an important source of heterogeneity. The second-generation DES was beneficial to Caucasian patients from three studies (15, 20, 21), which supports the applicability of this result. Fourth, despite the effects of multi-vessel lesion and long occlusive lesion. However, the subgroup analysis showed that patients with total length of stent implantation more than 40 mm may be suitable for the second-generation DES. Fifth, the visual estimation of the funnel plot and Egger's and Begg's tests detected publication bias of the MACE, which may be due to the fact that fewer than 10 studies were included and the results may be conservative. Final, the use of various crossing strategies and treated different vessels may be an important source of heterogeneity.
This meta-analysis supports that the use of second-generation DES is superior to first-generation DES in the treatment of CTO lesion, and the former has significant clinical outcomes in reducing MACE, target vessel revascularization, myocardial infarction in patients with CTO. Further large-scale, well-designed RCTs of patients with CTO are also needed to assess the benefits of the second-generation DES in certain subgroups, such as highly selective individual CTO patients or patients with Asian population, or patients using the same crossing strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
XQ: writing - original draft, methodology, and software. W-JZ: formal analysis, visualization, and software. W-FG and X-YL: validation and data curation. YL: investigation and validation. Z-LW: conceptualization and supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.598046/full#supplementary-material
1. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. (2014) 35:2541–619. doi: 10.1093/eurheartj/ehu278
2. Tsai TT, Stanislawski MA, Shunk KA, Armstrong EJ, Grunwald GK, Schob AH, et al. Contemporary incidence, management, and long-term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions: insights from the VA CART program. JACC Cardiovasc Intervent. (2017) 10:866–75. doi: 10.1016/j.jcin.2017.02.044
3. Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. (2012) 59:991–7. doi: 10.1016/j.jacc.2011.12.007
4. Mehran R, Claessen BE, Godino C, Dangas GD, Obunai K, Kanwal S, et al. Long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Cardiovasc Intervent. (2011) 4:952–61. doi: 10.1016/j.jcin.2011.03.021
5. Di Marco A, Anguera I, Teruel L, Dallaglio P, González-Costello J, León V, et al. Chronic total occlusion of an infarct-related artery: a new predictor of ventricular arrhythmias in primary prevention implantable cardioverter defibrillator patients. Europace. (2017) 19:267–74. doi: 10.1093/europace/euw009
6. Kereiakes DJ, Selmon MR, McAuley BJ, McAuley DB, Sheehan DJ, Simpson JB. Angioplasty in total coronary artery occlusion: experience in 76 consecutive patients. J Am Coll Cardiol. (1985) 6:526–33. doi: 10.1016/S0735-1097(85)80108-X
7. Ybarra LF, Cantarelli MJC, Lemke VMG, Quadros AS. Percutaneous coronary intervention in chronic total occlusion. Arq Bras Cardiol. (2018) 110:476–83. doi: 10.5935/abc.20180077
8. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. (2002) 346:1773–80. doi: 10.1056/NEJMoa012843
9. Van den Branden BJ, Rahel BM, Laarman GJ, Slagboom T, Kelder JC, Ten Berg JM, et al. Five-year clinical outcome after primary stenting of totally occluded native coronary arteries: a randomised comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (PRISON II study). EuroIntervention. (2012) 7:1189–96. doi: 10.4244/EIJV7I10A190
10. Rubartelli P, Petronio AS, Guiducci V, Sganzerla P, Bolognese L, Galli M, et al. Comparison of sirolimus-eluting and bare metal stent for treatment of patients with total coronary occlusions: results of the GISSOC II-GISE multicentre randomized trial. Eur Heart J. (2010) 31:2014–20. doi: 10.1093/eurheartj/ehq199
11. Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Intervent. (2013) 6:1267–74. doi: 10.1016/j.jcin.2013.06.015
12. Räber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. (2012) 125:1110–21. doi: 10.1161/CIRCULATIONAHA.111.058560
13. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
14. Park HJ, Kim HY, Lee JM, Choi YS, Park CS, Kim DB, et al. Randomized comparison of the efficacy and safety of zotarolimus-eluting stents vs. sirolimus-eluting stents for percutaneous coronary intervention in chronic total occlusion–CAtholic Total Occlusion Study (CATOS) trial. Circ J. (2012) 76:868–75. doi: 10.1253/circj.CJ-11-1021
15. Moreno R, García E, Teles R, Rumoroso JR, Cyrne Carvalho H, Goicolea FJ, et al. Randomized comparison of sirolimus-eluting and everolimus-eluting coronary stents in the treatment of total coronary occlusions: results from the chronic coronary occlusion treated by everolimus-eluting stent randomized trial. Circ Cardiovasc Intervent. (2013) 6:21–8. doi: 10.1161/CIRCINTERVENTIONS.112.000076
16. Ahn JH, Yang JH, Yu CW, Kim JS, Lee HJ, Choi RK, et al. First-generation versus second-generation drug-eluting stents in coronary chronic total occlusions: two-year results of a Multicenter Registry. PLoS ONE. (2016) 11:e0157549. doi: 10.1371/journal.pone.0157549
17. Cho MS, Lee PH, Lee SW, Chang M, Roh JH, Yoon SH, et al. Comparison of second- and first-generation drug eluting stent for percutaneous coronary chronic total occlusion intervention. Int J Cardiol. (2016) 206:7–11. doi: 10.1016/j.ijcard.2015.12.032
18. Kim YH, Her AY, Rha SW, Choi BG, Choi SY, Byun JK, et al. Five-year clinical outcomes of first-generation versus second-generation drug-eluting stents following coronary chronic total occlusion intervention. J Geriatr Cardiol. (2019) 16:639–47. doi: 10.11909/j.issn.1671-5411.2019.08.006
19. Lee MH, Lee JM, Kang SH, Yoon CH, Jang Y, Yu CW, et al. Comparison of outcomes after percutaneous coronary intervention for chronic total occlusion using everolimus- versus sirolimus- versus paclitaxel-eluting stents (from the Korean National Registry of Chronic Total Occlusion Intervention). Am J Cardiol. (2015) 116:195–203. doi: 10.1016/j.amjcard.2015.04.010
20. Valenti R, Vergara R, Migliorini A, Parodi G, Buonamici P, Cerisano G, et al. Comparison of everolimus-eluting stent with paclitaxel-eluting stent in long chronic total occlusions. Am J Cardiol. (2011) 107:1768–71. doi: 10.1016/j.amjcard.2011.01.063
21. Jaguszewski M, Gilis-Siek N, Ciecwierz D, Strozyk A, Fijalkowski M, Rynkiewicz A, et al. Early-generation versus new-generation drug-eluting stents in isolated chronic total occlusion: on the road to extinction? J Invasive Cardiol. (2014) 26:209–14.
22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
25. Brilakis ES, Karmpaliotis D, Vo MN, Garcia S, Michalis L, Alaswad K, et al. Advances in the management of coronary chronic total occlusions. J Cardiovasc Transl Res. (2014) 7:426–36. doi: 10.1007/s12265-014-9556-6
26. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
27. Moreno R, Fernández C, Hernández R, Alfonso F, Angiolillo DJ, Sabaté M, et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. (2005) 45:954–9. doi: 10.1016/j.jacc.2004.11.065
28. Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. (2001) 103:1967–71. doi: 10.1161/01.CIR.103.15.1967
29. Kandzari DE, Kini AS, Karmpaliotis D, Moses JW, Tummala PE, Grantham JA, et al. Safety and effectiveness of everolimus-eluting stents in chronic total coronary occlusion revascularization: results from the EXPERT CTO multicenter trial (evaluation of the XIENCE coronary stent, performance, and technique in chronic total occlusions). JACC Cardiovasc Intervent. (2015) 8:761–9. doi: 10.1016/j.jcin.2014.12.238
Keywords: coronary artery disease, chronic total occlusion, meta-analysis, percutaneous coronary intervention, second-generation drug-eluting stents
Citation: Qiao X, Zhang W-J, Guo W-F, Li Y, Liang X-Y and Wang Z-L (2021) Comparison of Clinical Outcomes Between Second-and First-Generation Drug-Eluting Stents in Patients With Chronic Total Occlusion Lesion: A Meta-Analysis. Front. Cardiovasc. Med. 8:598046. doi: 10.3389/fcvm.2021.598046
Received: 23 August 2020; Accepted: 22 March 2021;
Published: 20 April 2021.
Edited by:
Burak Pamukcu, Acibadem University, TurkeyReviewed by:
Selcuk Gormez, Acibadem University, TurkeyCopyright © 2021 Qiao, Zhang, Guo, Li, Liang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Lu Wang, d2FuZ3pobEBsenUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.