
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 04 June 2021
Sec. Cardiovascular Epidemiology and Prevention
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.584108
This article is part of the Research Topic What do we know about COVID-19 implications for cardiovascular disease? View all 109 articles
 Alvaro Petersen-Uribe1†
Alvaro Petersen-Uribe1† Alban Avdiu1†
Alban Avdiu1† Peter Martus2
Peter Martus2 Katja Witzel1
Katja Witzel1 Philippa Jaeger1
Philippa Jaeger1 Monika Zdanyte1
Monika Zdanyte1 David Heinzmann1
David Heinzmann1 Elli Tavlaki1
Elli Tavlaki1 Verena Warm1
Verena Warm1 Tobias Geisler1
Tobias Geisler1 Karin Müller1
Karin Müller1 Meinrad Gawaz1
Meinrad Gawaz1 Dominik Rath1*
Dominik Rath1*COVID-19 may lead to severe acute respiratory distress syndrome (ARDS) resulting in increased morbidity and mortality. Heart failure and/or pre-existing cardiovascular disease may correlate with poor outcomes and thus require special attention from treating physicians. The present study sought to investigate a possible impact of impaired myocardial function as well as myocardial distress markers on mortality or ARDS with need for mechanical ventilation in 157 consecutive patients with confirmed SARS-CoV-2 infection. All patients were admitted and treated at the University Hospital of Tübingen, Germany, during the first wave of the pandemic. Electrocardiography, echocardiography, and routine blood sampling were performed at hospital admission. Impaired left-ventricular and right-ventricular function, tricuspid regurgitation > grade 1, and elevated RV-pressure as well as thrombotic and myocardial distress markers (D-dimers, NT-pro-BNP, and troponin-I) were associated with mechanical ventilation and/or all-cause mortality. Impaired cardiac function is more frequent amidst ARDS, leading to subsequent need for mechanical ventilation, and thus denotes a poor outcome in COVID-19. Since a causal treatment for SARS-CoV-2 infection is still lacking, guideline-compliant cardiovascular evaluation and treatment remains the best approach to improve outcomes in COVID-19 patients with cardiovascular comorbidities.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an emerging cause of acute respiratory distress syndrome (ARDS) (1). Depending on the severity of ARDS, mechanical ventilation is the cornerstone for treatment of these critically ill patients (2). Patients in need for mechanical ventilation endure prolonged intra-hospital stay, neurological dysfunctions associated with concomitant anesthesia, and increased incidence of thrombosis and thromboembolism due to pro-thrombotic effects of SARS-CoV-2 and immobilization (3). Most importantly, severe respiratory failure is strongly associated with increased mortality in COVID-19 patients (4).
COVID-19 may cause severe acute myocardial injury or exacerbate an underlying chronic cardiovascular disease. Elevated levels of myocardial distress markers NT-pro-BNP and troponin are common findings in these patients (5). Moreover, pre-existing cardiovascular disease and compromised myocardial function have been associated with worse outcomes (6). Electrocardiography (ECG), echocardiography, and blood sampling for specific myocardial distress markers, e.g., troponin I and NT-pro-BNP, are essential for identifying COVID-19 patients with cardiovascular risk in order to improve management and consequently course of the disease. Since we currently lack a specific treatment for COVID-19, management of pre-existing or developing cardiac impairment is critical for improving outcomes in severely affected patients. Effects of impaired myocardial function on development of progressive respiratory failure and subsequent need for mechanical ventilation are unknown so far. Here, we report that markers of myocardial distress and impaired myocardial function are associated with progressive respiratory failure and increased mortality.
In March and April 2020, this prospective study enrolled 157 consecutive patients diagnosed with severe COVID-19-associated respiratory failure, including the first wave of COVID-19 infections at the University Hospital of Tübingen, Germany. The aim of the current study was to enroll all COVID-19-positive patients requiring hospital admission. Hence, a confirmed SARS-CoV-2 infection requiring hospital admission represented the only selection criterion. According to our official hospital database, 187 patients with confirmed SARS-CoV-2 infection were treated in our university hospital in March and April 2020. We managed to include 84.0% of these COVID-19 patients into the current study. Within 24 h after hospital admission, an extensive cardiovascular assessment including ECG, transthoracic echocardiography (TTE), and testing for myocardial distress biomarkers (e.g., pro-NT-BNP and troponin I) was performed. Written informed consent was obtained wherever possible (n = 128, 81.5%). We strongly assume that the remaining patients would not have refused to participate in the study since the cardiologic assessment was performed routinely and not purely study associated. In mechanically ventilated patients, the patient consent was obtained once invasive ventilation was discontinued or after discharge. In these patients, no study-associated measurements were performed but already existing clinical data was analyzed in accordance with the local ethics committee. The study was approved by the institutional ethics committee (238/2018BO2) and complies with the Declaration of Helsinki and good clinical practice guidelines (7–9).
SARS-CoV-2 was detected from nasopharyngeal secretions using real-time reverse transcriptase polymerase chain reaction. Severe respiratory failure was defined according to the Berlin Definition of Acute Respiratory Distress Syndrome (10).
Twelve-channel ECG was registered according to standard procedure. Peripheral venous blood was drawn for routine laboratory parameters.
TTE was performed by our Cardio-COVID-19 team. Left ventricular ejection fraction (LVEF) was assessed visually and measured using Simpson's method (11). Impaired LVEF was defined as an EF <50% (12). Impaired right ventricular function (RV-function) was evaluated combining visual assessment and measuring of tricuspid annular plane systolic excursion (TAPSE). TAPSE was assessed by placing an M-mode cursor through the lateral tricuspid valve annulus in the apical four-chamber view. Then, the total systolic excursion distance of the tricuspid annulus was measured. Impaired RV-function was defined by TAPSE <20 mm (13). Mitral regurgitation was assessed based on regurgitant orifice area and width of vena contracta (14). Severity of aortic stenosis was defined based on valve area measured by continuity equation and planimetry (15). Jet/left ventricular outflow tract width ratio, pressure half time, as well as diastolic flow reversal in proximal descending aorta were used to quantify severity of aortic regurgitation (16). Central jet area and width of vena contracta were applied to determine tricuspid regurgitation (14). Right ventricular pressure was estimated using the simplified Bernoulli equitation [RVPsys = 4 × (Vmax)2] (17) when tricuspid regurgitation was present (18). High probability of pulmonary hypertension was defined as RV-pressure > 35 mmHg (19).
Finally, the presence of pericardial effusion (PE) was visually assessed (20).
All patients were followed up for 30 days after study inclusion for the primary combined endpoint (poor outcome): mechanical ventilation and/or mortality. Secondary endpoint included all-cause mortality and mechanical ventilation.
SPSS version 26.0 (SPSS Inc., Chicago, IL) and GraphPad Prism8.4.0 (GraphPad Software, San Diego, CA) were used for all statistical analyses. Student's t-test was applied for normally distributed data, whereas Mann–Whitney U-test served for analysis of non-normally distributed data. Accordingly, mean values are presented as mean ± standard deviation and median values are presented as median and 25th/75th percentiles. Categorical endpoints were analyzed via cross-tabulations and Chi-square tests. Correlations of non-normally distributed were assessed using Spearman's rank correlation coefficient (rho). Kaplan–Meier curves with log rank tests were applied to compare survival between groups, whereas multiple Cox regression analyses were used to analyse independent associations between myocardial distress markers and the combined endpoint after adjustment for epidemiological factors. Regarding Cox regression analyses, LVEF, RV-pressure, and age were included as continuous variables, whereas RV-function (normal vs. impaired), significant TR, arterial hypertension, coronary artery disease, as well as diabetes mellitus (no vs. yes) were coded as binary variables. Discriminatory performance of myocardial distress markers and other clinical factors was evaluated using receiver operator curves (ROC) and expressed as c-statistics with 95% CI. Depending on the area under the curve (AUC), ROC 0.5 suggests no discrimination, ≥0.7– <0.8 acceptable, ≥0.8– <0.9 excellent, and ≥0.9 outstanding discrimination (21). ROC analyses using combinations of predictors were based on multiple logistic regression analysis with leaving one out correction. Ninety-five percent CIs of these areas are included in the figures. Course of biomarkers and respective associations with poor outcome were analyzed via linear mixed-models with random intercept.
A total of 157 patients were included, and their baseline characteristics are shown in Table 1. Stratification according to incidence of the combined endpoint is presented in Table 2. Routine blood sampling was performed in the whole collective; ECG and echocardiography were performed in 136 (86.6%) and 133 (84.7%) patients, respectively. Rate of mechanical ventilation within 30 days after hospital admission was 44.6% (n = 70). Twenty (12.7%) patients developed severe ARDS in the course of the hospital stay. Twenty-two (14.0%) patients were already mechanically ventilated at admission. Twenty-eight (17.8%) patients were intubated due to rapidly increasing respiratory failure and for airway protection. A total of 25 patients died (15.9%); two patients died without being mechanically ventilated (1.3%).
Patients with poor outcome displayed a significantly lower LVEF, worse RV-function, more severe tricuspid regurgitation, and increased RV pressure when compared to those with milder course of COVID-19 (Table 2 and Figure 1).
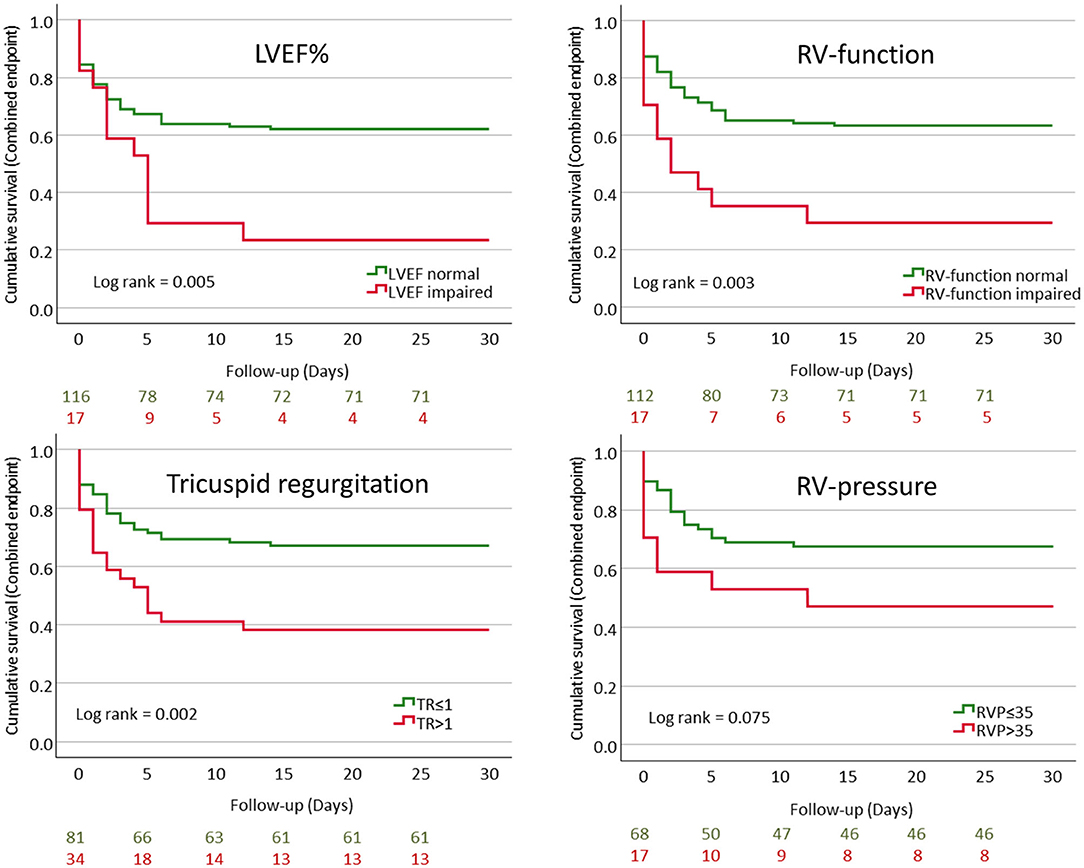
Figure 1. Kaplan–Meier curves showing cumulative event-free survival for the combined endpoint (mechanical ventilation and/or mortality) stratified according to LVEF%, RV-function, tricuspid regurgitation, and RV-pressure.
Multivariable Cox-regression analysis revealed that impaired LVEF and RV-function as well as tricuspid regurgitation >1 and increased RV-pressure were independently associated with poor outcome (Table 3).
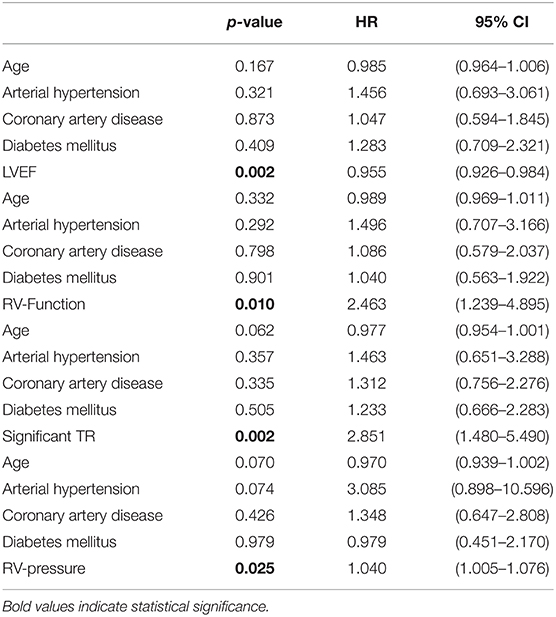
Table 3. Cox regression with markers of myocardial function as well as epidemiological factors as independent variables and the combined endpoint as dependent variables.
Amidst patients with poor outcome, leucocyte count, D-dimers, C-reactive protein, procalcitonin, troponin I, NT-pro-BNP, CK, AST, and LDH levels were significantly higher when compared to COVID-19 patients with a more favorable course of disease (Table 2).
Increased QTc interval and a higher heart rate, just as a larger proportion of T wave inversion, were more frequently observed in patients requiring ventilation in the course of disease (Table 2). The locations of inverted T waves were distributed as follows: Lead I: n = 6 (42.9%), lead II: n = 2 (14.3%), lead III: n = 5 (35.7%), lead aVR: n = 10 (71.4%), lead aVL: n = 6 (42.9%), lead aVF: n = 5 (35.7%), lead V1: n = 6 (42.9%), lead V2: n = 5 (35.7%), lead V3: n = 7 (50.0%), lead V4: n = 6 (42.9%), lead V5: n = 4 (28.6%), and lead V6: n = 3 (21.4%), respectively.
Mechanically ventilated patients showed significantly progressive D-dimer levels when compared to the remaining subjects (p = 0.043). Furthermore, non-survivors showed significantly progressive NT-pro-BNP and troponin-I levels when compared to survivors (p = 0.002 and p <0.001, respectively) (Table 4, Figure 2).
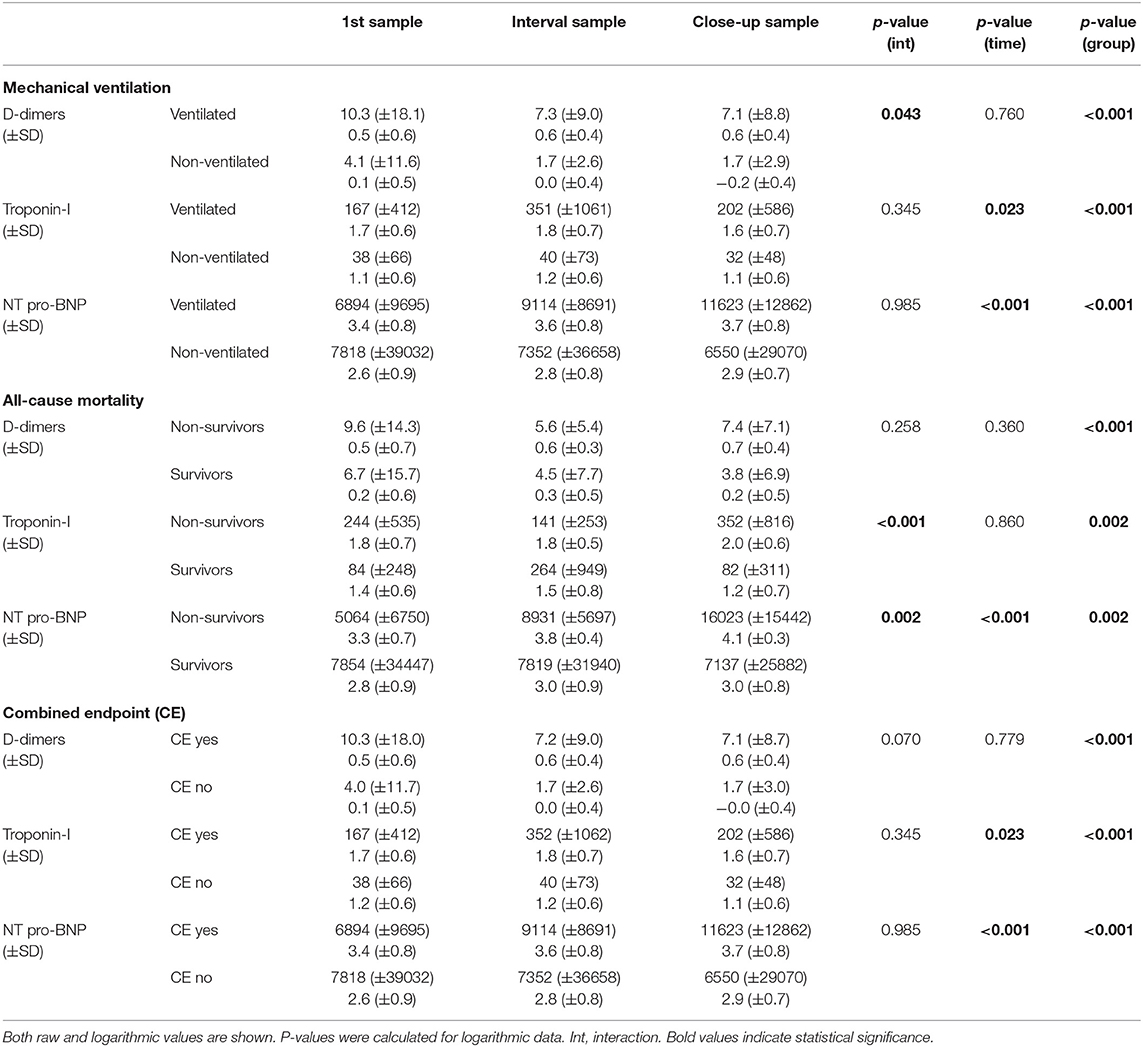
Table 4. D-dimer, troponin-I, and NT-pro-BNP levels at admission (1st sample), median of hospital stay (interval sample), and discharge/death (close-up sample) stratified according to mechanical ventilation, all-cause mortality, and the combined endpoint.
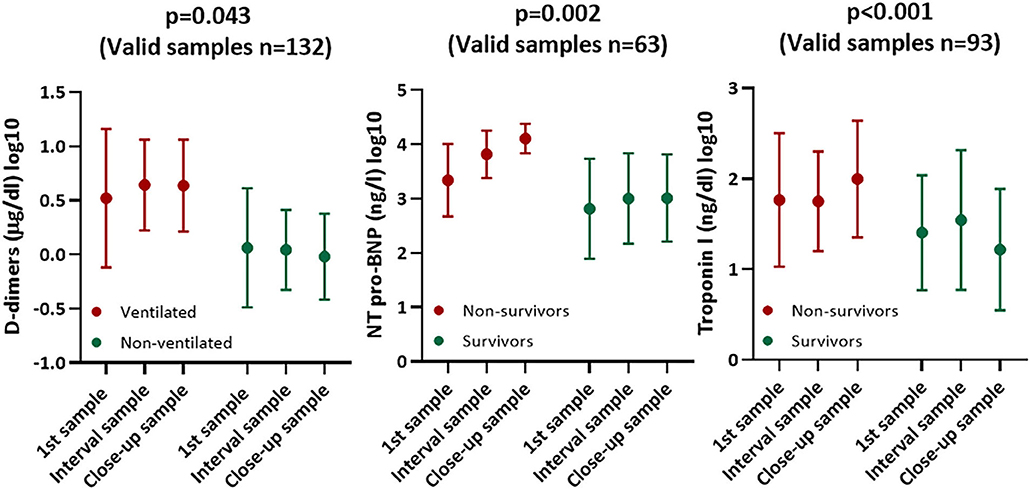
Figure 2. Diagrams (mean ± SD) showing course of cardiac and thrombotic biomarkers stratified according to survival and mechanical ventilation.
LVEF correlated significantly with troponin I and NT-pro-BNP at admission (rho = −0.310, p <0.001 and rho = −0.456, p <0.001, respectively). TAPSE correlated significantly with troponin I (rho = −0.293, p = 0.003). Finally, RV-pressure was significantly associated with troponin I and NT-pro-BNP (rho = 0.310, p = 0.005 and rho = 0.511, p <0.001, respectively) (Figure 3).
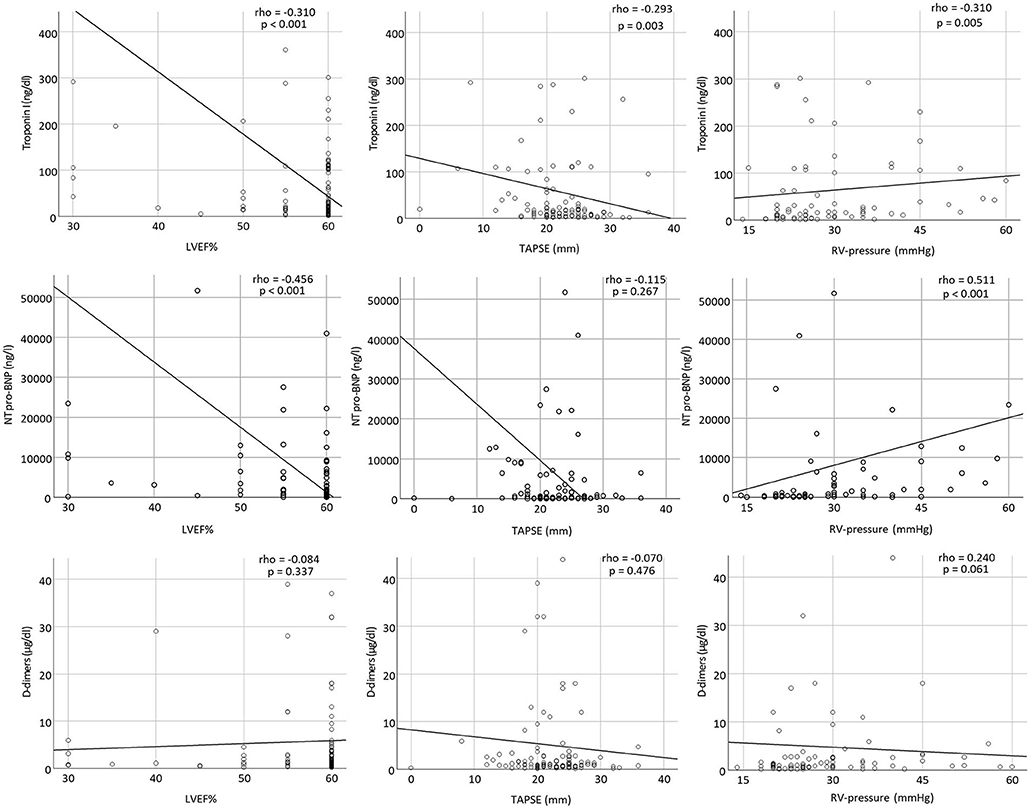
Figure 3. Scatter plots showing correlations between troponin I, NT-pro-BNP, and D-dimers with LVEF%, TAPSE, and RV-pressure at admission.
ROC analyses (combined endpoint) revealed an AUC of 0.588 for a multivariable model containing age, arterial hypertension, coronary artery disease, diabetes mellitus type II, and LVEF, 0.475 for a combination of age, arterial hypertension, coronary artery disease, diabetes mellitus type II, and RV-function, 0.520 for age, arterial hypertension, coronary artery disease, diabetes mellitus type II, and significant TR, and 0.590 for age, arterial hypertension, coronary artery disease, diabetes mellitus type II, and elevated RV-pressure. Cardiac biomarkers and D-dimer showed significantly better predictive performance (AUC 0.737 for D-dimers, 0.764 for NT-pro-BNP, and 0.735 for troponin-I). The best discrimination performance was achieved by a model including D-dimers, NT-pro-BNP, and troponin-I (AUC 0.788), whereas a combined model including age, arterial hypertension, coronary artery disease, diabetes mellitus type II, LVEF, RV-function, significant TR, and elevated RV-pressure performed poorly in predicting the combined endpoint (AUC 0.603) (Figure 4).
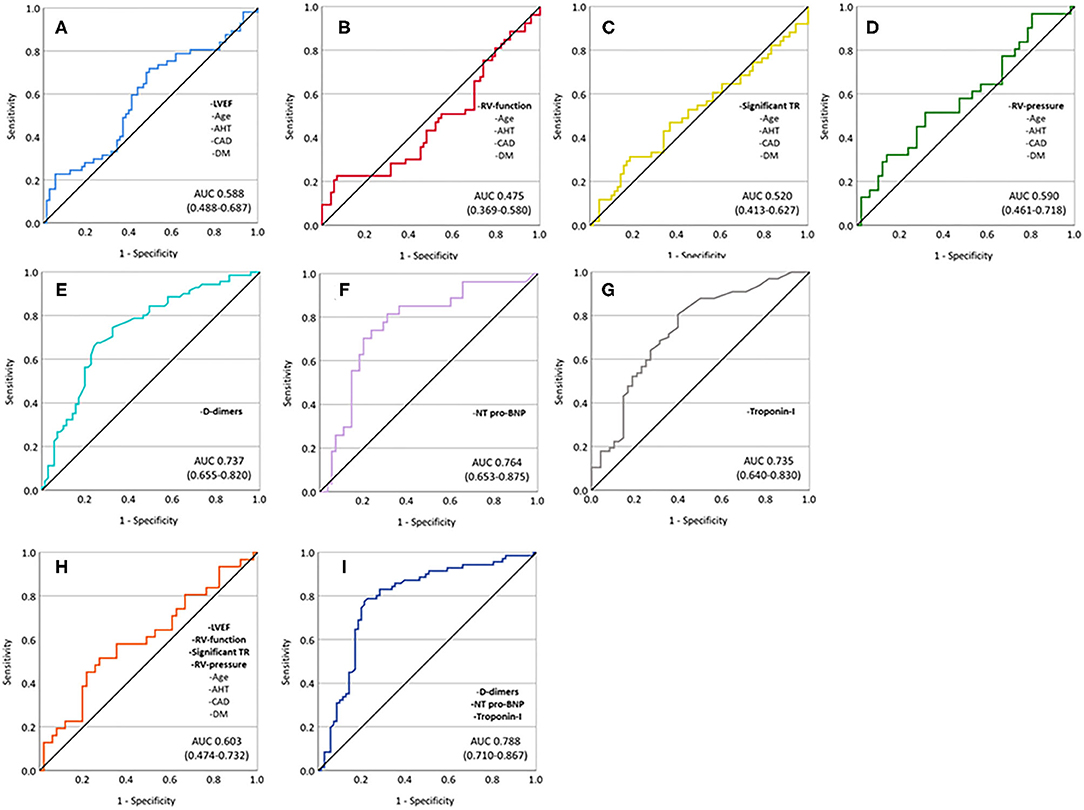
Figure 4. ROC analyses showing predictive performance of different univariate and multivariable models. (A) Age, arterial hypertension, coronary artery disease, diabetes mellitus, and impaired LVEF; (B) age, arterial hypertension, coronary artery disease, diabetes mellitus, and impaired RV-function; (C) age, arterial hypertension, coronary artery disease, diabetes mellitus, and significant TR; (D) age, arterial hypertension, coronary artery disease, diabetes mellitus, and elevated RV-pressure; (E) D-dimers; (F) NT-pro-BNP; (G) troponin-I; (H) age, arterial hypertension, coronary artery disease, diabetes mellitus, impaired LVEF, impaired RV-function, significant TR, and elevated RV-pressure; and (I) D-dimers, NT-pro-BNP, and troponin-I.
The major findings of this study are as follows: (1) Early impaired left and right ventricular systolic function, higher degree tricuspid regurgitation, and higher RV-pressure are more prevalent among COVID-19-positive patients with poor outcome. (2) The course of the myocardial distress markers NT-pro-BNP and troponin-I may predict outcome in COVID-19 patients. (3) Troponin I and NT-pro-BNP correlate with LVEF, RV-function, and RV pressure at admission. (4) A combined model including D-dimers, troponin-I, and NT-pro-BNP may facilitate risk assessment in COVID-19 patients.
The current findings provide further evidence that an extensive cardiologic assessment of patients suffering from COVID-19 is required at the earliest time point before severe respiratory symptoms are evident.
Our current data and previous reports emphasize that myocardial injury represents a prevalent finding in COVID-19 patients with respiratory insufficiency. Severe respiratory failure and ARDS are currently considered as the main cause of COVID-19-associated morbidity and mortality (22). Recently, Richardson and collaborators reported that 12.2% of hospitalized COVID-19 patients require mechanical ventilation (23). Among those, up to 20% developed cardiac injury, defined as an increase in troponin I (24). Interestingly, in our consecutive collective, ~45% of patients required mechanical ventilation, with 24% showing significant troponin I elevation. Susceptibility to SARS-CoV-2 infection seems to be higher in patients with pre-existing cardiovascular disease (25). Furthermore, these patients suffer from increased morbidity and mortality (26).
As the precise mechanisms leading to myocardial damage in COVID-19 await a thorough investigation, current research suggests that myocardial damage may result from direct viral or inflammatory myocardial injury and may be augmented by systemic inflammatory response, which further promotes microcirculatory impairment or arrest (22). Diagnosing COVID-19-induced direct myocardial damage is a challenging process requiring myocardial biopsy as a gold standard, although SARS-CoV-2 genome could not be identified within the myocardium in biopsy and autopsy findings so far (27). Furthermore, cardiac MRI may help identify myocarditis as a cause of impaired LV-function. These two diagnostic modalities were, however, not applied in our department during the first COVID-19 wave due to patient overload and protection of clinic personnel.
According to available echocardiography findings prior to the diagnosis of SARS-CoV-2 infection, impaired systolic LV-function was commonly a chronic condition, whereas impaired RV-function tended to be a new finding in the current patient cohort. This suggests that elevated RV-pressure and RV-dysfunction is an acute process caused by COVID-19-induced ARDS. Significantly elevated BNP and troponin-I levels found in mechanically ventilated COVID-19 patients in our cohort support the development of acute right ventricular failure, which is consistent with recent findings (28, 29). Furthermore, pulmonary distress could fittingly account for QRS prolongation and higher amount of abnormal T waves seen in our collective (30). Impaired LV-function at hospital admission may fasten this process by congestion caused by elevated LVEDP and thus raising pulmonary artery pressure. Our hypothesis of COVID-19-induced right ventricular failure as a result of major hemodynamic stress is presented in Figure 5.
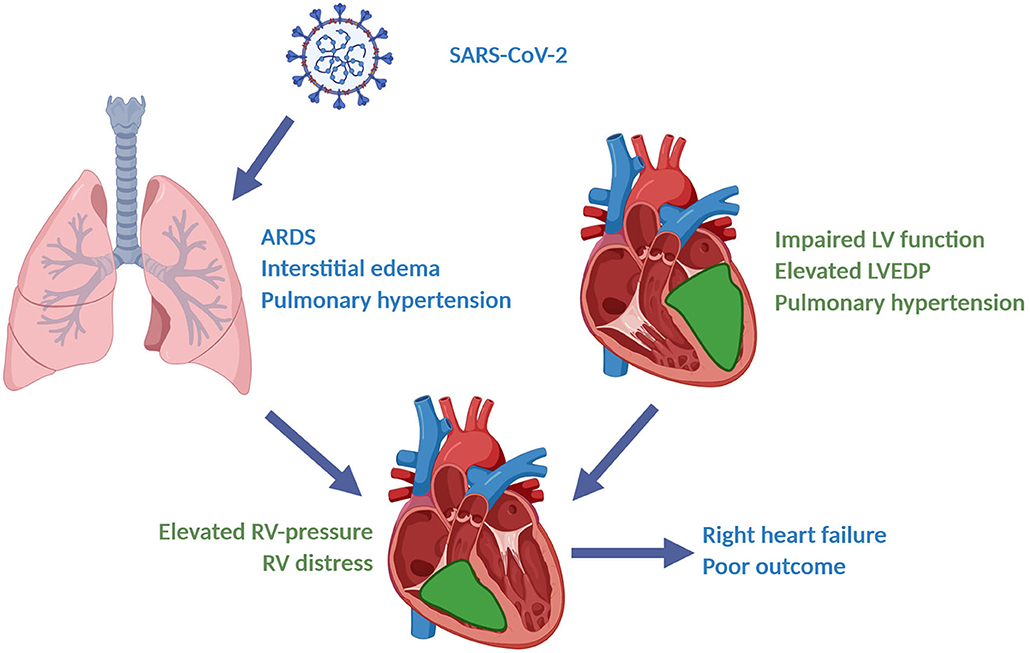
Figure 5. Right heart failure caused by COVID-19-induced severe ARDS and preexisting left heart failure: A hypothesis. (Figure created with BioRender®).
As cardiovascular comorbidities and myocardial injury significantly contribute to mortality in COVID-19, early cardiologic assessment and identification of high-risk patients is of critical importance to optimize the management and improve prognosis of COVID-19 patients.
The current study offers several major limitations. First, we could not differentiate between COVID-19-induced and non-COVID-19-induced impairment of myocardial function, which may have affected outcome to an unknown degree. Second, the number of patients enrolled was low, rendering generation of risk prediction models difficult. Third, we were not able to include all COVID-19-positive patients admitted to our hospital during the first wave of the disease. Finally, biomarker levels were not available for all patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the institutional ethics committee (238/2018BO2) and complies with the declaration of Helsinki and good clinical practice guidelines. The patients/participants provided their written informed consent to participate in this study.
We submitted this manuscript at the end of the first COVID-19 wave at the University Hospital Hospital of Tübingen. We have previously submitted an interimanalysis of COVID-19 positive patients to a different journal, which has been accepted for publication onMay 28th (Rath et al., Clin Res Cardiol 2020 Jun 14:1-9). The endpoint differed however and the number of events was significantly lower. Fewer patients were enrolled. Finally, additional analyses were performed in the current manuscript.
AP-U and AA: data collection, data analysis, and drafting of the manuscript. PM: expert data analysis. KW, PJ, MZ, DH, ET, VW, TG, and KM: data collection and critical revision. MG: study concept and drafting of the manuscript. DR: data collection, and data analysis, drafting of the manuscript, and study concept. All authors contributed to the article and approved the submitted version.
This project was supported by the German Research Foundation (DFG) KFO-274–Project number 190538538, by the German Research Foundation (DFG)–Project number 374031971–TRR 240, and funded by the German Research Foundation (DFG)–Project number 335549539–GRK2381.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thankfully acknowledge the work of Lydia Laptev for assisting us to isolate and process the blood samples. We acknowledge support by Open Access Publishing Fund of University of Tübingen.
1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. (2020) 55:105924. doi: 10.1016/j.ijantimicag.2020.105924
2. Aoyama H, Uchida K, Aoyama K, Pechlivanoglou P, Englesakis M, Yamada Y, et al. Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: a systematic review and network meta-analysis. JAMA Netw Open. (2019) 2:e198116. doi: 10.1001/jamanetworkopen.2019.8116
3. Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Browne P, et al. More on COVID-19 coagulopathy in Caucasian patients. Br J Haematol. (2020) 189:1044–9. doi: 10.1111/bjh.16791
4. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
5. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. doi: 10.1038/s41569-020-0360-5
6. Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. (2020) 14:247–50. doi: 10.1016/j.dsx.2020.03.013
7. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res. (1997) 35:2–3.
8. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice.J Postgrad Med. (2001) 47:199–203.
9. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Med Etika Bioet. (2002) 9:12–9.
10. Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
11. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. (1989) 2:358–67. doi: 10.1016/S0894-7317(89)80014-8
12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
14. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. (2010) 11:307–32. doi: 10.1093/ejechocard/jeq031
15. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:2438–88. doi: 10.1161/CIR.0000000000000029
16. Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr. (2010) 11:223–44. doi: 10.1093/ejechocard/jeq030
17. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. (1984) 70:657–62. doi: 10.1161/01.CIR.70.4.657
18. Parasuraman S, Walker S, Loudon BL, Gollop ND, Wilson AM, Lowery C, et al. Assessment of pulmonary artery pressure by echocardiography-a comprehensive review. Int J Cardiol Heart Vasc. (2016) 12:45–51. doi: 10.1016/j.ijcha.2016.05.011
19. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. (2016) 37:67–119. doi: 10.1093/eurheartj/ehv317
20. Pepi M, Muratori M. Echocardiography in the diagnosis and management of pericardial disease. J Cardiovasc Med. (2006) 7:533–44. doi: 10.2459/01.JCM.0000234772.73454.57
21. Hosmer David W. Lemeshow S. Model-Building Strategies and Methods for Logistic Regression. Applied Logistic Regression. (2000). p. 91–142. doi: 10.1002/0471722146
22. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. (2020) 215:108427. doi: 10.1016/j.clim.2020.108427
23. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
24. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
25. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. (2020) 94:91–5. doi: 10.1016/j.ijid.2020.03.017
26. Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. (2020). doi: 10.1016/j.ajem.2020.04.052. [Epub ahead of print].
27. Ho JS, Sia CH, Chan MY, Lin W, Wong RC. Coronavirus-induced myocarditis: a meta-summary of cases. Heart Lung. (2020) 49:681–5. doi: 10.1016/j.hrtlng.2020.08.013
28. Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. (2020) 76:1244–58. doi: 10.1016/j.jacc.2020.06.068
29. Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. (2020) 311:116–21. doi: 10.1016/j.ijcard.2020.03.087
Keywords: COVID-19, mechanical ventilation, mortality, myocardial function, prognosis
Citation: Petersen-Uribe A, Avdiu A, Martus P, Witzel K, Jaeger P, Zdanyte M, Heinzmann D, Tavlaki E, Warm V, Geisler T, Müller K, Gawaz M and Rath D (2021) Impaired Myocardial Function Is Prognostic for Severe Respiratory Failure in the Course of COVID-19 Infection. Front. Cardiovasc. Med. 8:584108. doi: 10.3389/fcvm.2021.584108
Received: 16 July 2020; Accepted: 30 April 2021;
Published: 04 June 2021.
Edited by:
Mingxing Xie, Huazhong University of Science and Technology, ChinaReviewed by:
Hack-Lyoung Kim, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, South KoreaCopyright © 2021 Petersen-Uribe, Avdiu, Martus, Witzel, Jaeger, Zdanyte, Heinzmann, Tavlaki, Warm, Geisler, Müller, Gawaz and Rath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominik Rath, dominik.rath@med.uni-tuebingen.de
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.