
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 15 January 2021
Sec. General Cardiovascular Medicine
Volume 7 - 2020 | https://doi.org/10.3389/fcvm.2020.625579
This article is part of the Research TopicThe Relationship Between Cardiovascular Disease and Other Chronic ConditionsView all 39 articles
Atherosclerotic cardiovascular disease (ACVD) is an inflammatory disease of the coronary arteries associated with atheroma formation, which can cause disability and often death. Periodontitis is ranked as the sixth most prevalent disease affecting humans affecting 740 million people worldwide. In the last few decades, researchers have focused on the effect of periodontal disease (PD) on cardiovascular disease. The aim of this review was to investigate the association between these two diseases. PD is a potential risk factor that may initiate the development, maturation, and instability of atheroma in the arteries. Two mechanisms were proposed to explain such association, either periodontal pathogens directly invade bloodstream or indirectly by increasing systemic level of inflammatory mediators. Interestingly, it has been suggested that improvement in the condition of one disease positively impact the condition of the other one. Highlighting the association between these two diseases, the importance of early diagnosis and treatment of PD and its impact on cardiovascular status may be of great value in reducing the complications associated with ACVDs. Further in vitro and in vivo studies with longer follow up are necessary to confirm the causal relationship between PD and ACVDs.
Periodontal disease (PD) is an inflammatory disease primarily initiated in response to a specific group of bacteria and characterized by a complex host-biofilm interaction (1). According to the World Health Organization, the severe form of periodontitis causes tooth loss in about 5–15% of the population worldwide, and it is considered the sixth most common disease affecting humans (2). Aberrant immune–inflammatory responses determine a patient's susceptibility to developing periodontitis, which may be modified by a range of risk factors (3). The transition from gingivitis to periodontitis initiates when the population and activity of a specific group of periodontal pathogens, predominantly Gram-negative anaerobic bacteria such as Porphyromonas gingivalis (P. ginigvalis), Aggregatibacter actinomycetemcomitans (A. a), Tannerella forsythia (T. forsythia), Treponema denticola (T. denticola) and spirochetes, increase in the subgingival biofilm (4). These quantitative and qualitative alterations in the bacterial composition of the biofilm are responsible for disturbing the normal symbiotic relationship between the host and its resident microbiota, leading to an alteration in the hosts immune response. This response can be a “double-edged sword,” in that it is an integral defense mechanism but is also simultaneously responsible for periodontal tissue breakdown proportional with the severity of the disease (5).
The inflammatory response in periodontal tissues is characterized by the local production of various pro-inflammatory mediators and enzymes such as C-reactive protein (CRP), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and matrix metalloproteinases (MMP) (6). Consequently, the rate of periodontal tissue destruction is accelerated with an increase in such mediators. Deep periodontal pockets represent a micro-environment for increased levels of inflammatory cytokines either directly or indirectly. Given the cumulative increase in inflammatory cytokines, and its potential influence on systemic disease processes, this in turn acts as a possible risk factor for several systemic diseases including atherosclerotic cardiovascular disease (ACVD) (7).
ACVDs are a group of disorders affecting the heart and blood vessels, including coronary heart disease, cerebrovascular and other peripheral artery diseases, congestive heart failure, carotid heart disease, and aneurysms. Some ACVDs include two major conditions: ischemic heart disease and cerebrovascular disease which are considered as the first and third cause of death, respectively (7). In Europe, ACVD is responsible for approximately 3.9 million deaths (45% of deaths) annually (8). According to a global survey, there were an estimated 422.7 million subjects with ACVD and 17.9 million deaths due to ACVD in 2015 (9). The pathogenic process causing ACVD is very complex. It is recognized that elevated level of low density lipoprotein cholesterol is the principle element in the pathogenesis of ACVD that change cellular permeability and has impact on arterial walls. Inflammatory cells and cytokines induce plaque formation in the walls of blood vessels, and are also responsible for propagation and rupture of the established plaque along with the resultant thrombotic complications (10).
Mechanisms that have been proposed to explain the link between PD and ACVD include the inflammatory pathways common to both diseases, such as increased levels of white blood cells (WBC), CRP, fibrinogen, intercellular adhesion molecule-1 (ICAM-1), and proinflammatory cytokines (11). Additionally, both diseases share similar risk factors such as smoking, poor oral hygiene, diabetes mellitus (DM), obesity, stress and reduced physical activities. Despite these common features, it is difficult to conclude that periodontitis is a primary causal factor of ACVD, as a result of the complexity in the confounders that correlate PD to ACVD (12).
Although stronger and more suggestive evidence has emerged to highlight a causal relationship between the two pathologies, but it was still insufficient for PD to be classified as a causal risk factor (13). Therefore, further studies are required to provide a more robust, consistent link in order to confirm PD as an independent and potentially adjustable risk factor for ACVD (12). Therefore, the current review attempts to review and update the current evidence and provide further insight into the relationship between PD and ACVD.
Atherosclerotic disease is a focal thickening of vascular intima residing between the endothelial lining and smooth muscle cell (SMC) layers of blood vessels in response to an immune response (14). Endothelial dysfunction is the earliest change in atherosclerotic formation. The primary etiological factor of atherosclerosis is un known (15). However, other risk factors significantly contribute to the development and progression of this pathology, such as aberrant profile of plasma cholesterol, smoking, hypertension, DM, and increased levels of inflammatory mediators including CRP and cytokines (15).
Atherosclerosis starts with accumulation of low density lipoprotein (LDL) within the intima layer where it is oxidized. This in turn activates increased expression in nearby endothelial cells of cell surface proteins such as ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and selectins (15). Adhesion of circulating inflammatory cells (monocytes, lymphocytes) to these adhesion molecules is increased by their diapedesis into the inflamed intima site (15). The initial development of the atherosclerotic lesion occurs through differentiation of monocytes to macrophages that scavenge on LDL, thus forming foam cells and subsequently fatty streaks (15, 16). Later, a T-leukocyte induced-cell-mediated immune response with increased level of inflammatory cytokines such as INF-γ, TNF-α, and IL-1β further accelerate atherogenesis (17). T-cell-associated mediators stimulate migration and mitosis of SMC to form a fibrous pseudo-capsule around the lesion (17). Macrophages loaded with lipids undergo apoptosis leading to formation of a necrotic core underneath the fibrous capsule, which renders it susceptible to rupture, thus leading to formation of fatal thrombosis (14).
Cumulative evidence from literature over the last decades have supported the role of PD as an independent risk factor for ACVD (18). The presence of certain periodontal pathogens, Gram-negative anaerobes in particular, in subgingival biofilm has been associated with increased risk of MI; the odds ranging between 2.52 and 2.99 with the presence of T. forsythia and P. gingivalis, respectively, in comparison to controls (19). The hallmark of periodontitis is elevation in the levels of Gram-negative bacteria that are characterized by their ability to trigger an intense immune response via their mechanism of pathogenicity, such as lipopolysaccharide (LPS) (20). Moreover, some of these bacterial species possess the potential to invade deeper tissues, reaching the circulation and inducing a systemic immune response away from their original habitat (21). Results from several in vivo and in vitro studies have suggested that periodontal bacteria associated with chronic inflammation may compromise the epithelial-barrier function by epithelial-mesenchymal transition (22–24). Epithelial-mesenchymal transition comprises cellular events starting with loss of polarity, cytoskeletal, and adhesion proteins, ending with loss of epithelial-phenotype and acquisition of mesenchymal-like characteristics (25). This results in loss of epithelial sheet coherence and formation of microulceration; thus, facilitating the penetration of motile periodontal pathogens/virulence factors to the underlying connective tissue and exposed blood vessels. On the other hand, periodontal bacteria can invade host cells as part of their defensive strategy to evade host immune responses (26). This intracellular localization provides not only protection from the body's defensive mechanisms but also a shelter from action of antimicrobials (26). Periodontopathogens such as P. gingivalis residing within the cells either stay dormant or multiply by modulating cellular machinery (27). Once multiplied, P. gingivalis leaves the epithelial cells via the endocytic recycling pathway to infect other cells or gain access to the circulatory system (28). The trafficking of P. gingivalis into endothelial cells is positively influenced by bacterial load, and certain virulent proteins such as gingipains, fimbriae, and hemagglutinin A (29). Further, invasion of gingival epithelial and endothelial cells by P. gingivalis could be synergized by Fusobacterium nucleatum (30) and T. forsythia (31).
Two mechanisms have been proposed to explain how PD influences ACVD. First, a direct mechanism by which periodontal pathogens directly invade endothelial cells. This notion is supported by polymerease chain reaction assays for atherosclerotic plaques (32). Analysis of cardiovascular specimens containing thrombus tissues demonstrated that Streptococcus mutans was the most prevalent bacteria (78%), followed by A. a (33). Atherosclerotic lesions formed in coronary arteries also exhibited the presence of other bacteria such as P. gingivalis, Prevotella intermedia, and T. forsythia (34). It is not clear how the presence of bacteria intracellularly influences atherosclerosis but some pathogens, e.g., P. gingivalis, could trigger foam cell formation or their persistence within the cells, and thereby provoke a state of secondary inflammation that leads to endothelial dysfunction (35).
Increased systemic levels of inflammatory cytokines due to PD is the second suggested mechanism (indirect pathway). PD stimulates a systemic inflammatory response which results in chronically elevated levels of different cytokines, also related to atherosclerotic vascular disease, such as IL-1β, IL-6, IL-8, TNF-α, and monocyte chemoattractant protein-1. Some can enhance rapid hepatic synthesis and secretion of intravascular plasma proteins such as CRP protein and fibrinogen (36, 37). Additionally, bacterial products such as LPS could enter the circulation and induce a potent immune response. These aforementioned factors could initiate atherosclerosis by their action on endothelial cells, modulating lipid metabolism, and increasing oxidative stress (38). This was supported by results from a previous study that indicated endothelial dysfunction in patients with periodontitis (39).
Despite robust evidence drawn from many studies linking PD to the initiation and progression of ACVD (which is discussed in section Effects of Periodontal Disease on the Incidence of Atherosclerotic Cardiovascular Diseases), these results require further support to pinpoint the exact pathological mechanism(s) between PD and ACVD. The presence of atherosclerotic diseases and their hypothesized relation with PD are illustrated in Figure 1.
As previously discussed, the available literature has provided ample evidence in relation to the existence of a relationship between PD and ACVD. However, this link is not easily comprehended and it could be further complicated by the presence of other systemic diseases, genetic factors, and lifestyle-related habits. These factors could simultaneously influence the progression of PD and ACVD.
Chronic stress is a response associated with stimulation of a sympathetic nervous system which induces the adrenal glands to increase secretion of adrenalin and cortisol in order to cope with the stress (40). Furthermore, stress activates the hypothalamus-pituitary-adrenal axis, which together with a trigger from the sympathetic nervous system causes upregulation of catecholamines, glucocorticoids and inflammatory cytokines (41). Limited studies are available on the influence of stress on the progression of PD in humans; however, it is well-documented that the systemic level of inflammatory cytokines is significantly increased in response to prolonged stress (41). These cytokines are common to destructive events of PD. Experimental in vitro, and in vivo animal studies have provided potential mechanisms by which stress can contribute to periodontal tissue breakdown (42). Concomitantly, several types of stress are involved in the development of cardiovascular disease, including oxidative stress, mental stress, hemodynamic stress and social stress. The relationship of stress and ACVD has been thoroughly investigated and the results suggest that individuals suffering from ACVD and under psychological stress are more prone to transient myocardial ischemia, risk of recurrent ACVD and increased mortality (41).
Smoking is a well-recognized risk factor for PD and ACVD. Among toxic products generated during smoking, nicotine is one of the most harmful (43). This substance is responsible for vasoconstriction that compromises delivery of nutrients to the periodontium. In addition, nicotine significantly suppresses cellular/humoral immune responses and causes neutrophil dysfunction (43). Similarly, toxic products produced during burning of tobacco induce the atherogenic mechanism by increasing oxidation of LDL, causing chronic inflammation in the intima layer and subsequent endothelial dysfunction (44, 45). Smoking increase platelet aggregation, blood viscosity and shifts the pro- and antithrombotic balance toward increased coagulation. The contribution of smoking to the pathophysiology of PD and ACVD has been demonstrated by a significant reduction in the strength of association between these two diseases after adjustment of smoking (46). A systematic review showed that 11 out of 15 cross-sectional studies had suggested a modest relationship between ACVD and PD after adjusting for several risk factors including smoking (47).
Diabetes mellitus (DM) is a metabolic disease that adversely affects the body through different mechanisms including periodontal and cardiovascular health. Persistent increase in glucose level is expressed as microvascular changes leading to endothelial cells dysfunction (48), mainly via increased level of TNF-α, interleukins and proteinases that enhances apoptosis of endothelial cells (49). DM-associated complications also affect the bone healing capacity, mineral density, and turn-over rate (50). These DM-associated systemic events significantly increase progression rate of PD. The odds of periodontitis are increased by almost three- to four-fold in diabetic patients compared to healthy controls (51). This is supported by several studies that demonstrated an increase in the prevalence and severity of PD regardless of age, gender, and ethnic group (52). Undoubtedly, DM is a profound risk factor for initiation and progression of periodontitis and ACVD that should be considered when studying the association between these two diseases.
Both periodontitis and ACVD are multifactorial diseases whose development and progression require interaction between several factors, among which is genetic predisposition. A study conducted on twins, utilizing quantitative genetic analyses, showed evidence of an association between ACVD and PD (53). Interestingly, three of the loci among four genes significantly associated with PD, namely ANRIL/CDKN2B-AS1, PLG, and CAMTA1/VAMP3, showed association with ACVD (54). Furthermore, results from a candidate-gene association study also concluded that periodontitis and ACVD are genetically related through at least one susceptibility locus (55). Despite the fact that these studies highlighted a novel shared pathologic pathway between the two conditions, larger scale genetic studies are highly recommended.
Any potential contribution of periodontitis to the pathology of ACVD should be carefully interpreted as many confounding factors could affect both conditions and result in overestimation of this relationship. Thus, adjustment of these risk factors need to be taken into consideration during statistical analysis.
Endothelial dysfunction is the earliest stage of atherosclerosis and a possible link between PD and ACVD (56). Several studies have linked periodontitis to endothelial dysfunction and this relationship is sustained by several shared biomarkers of periodontitis, ACVD and endothelial dysfunction (47). Despite the potential for these biomarkers to identify the strength of this correlation, they are still not considered as “gold standard” diagnostic markers (47). Upon initiation of periodontitis, expression of inflammatory cytokines markedly increases together with alteration in the lipid profile which could contribute to the development and aggravation of thrombogenesis and thromboembolic events (57). It has been reported that PD is significantly associated with upregulation of biomarkers responsible for endothelial dysfunction and dyslipidemia such as CRP, tissue plasminogen activator (t-PA), and LDL-cholesterol (C), TNF-α (58). Additionally, periodontitis is associated with higher levels of other inflammatory serum biomarkers including von Willebrand factor (vWF), fibrinogen, and endothelial progenitors' cells (58). Interestingly, levels of these serum biomarkers are reduced following periodontal therapy (59, 60).
A systematic review investigated the serum level of a group of mutual biomarkers in order to define the strength of evidence relating PD, CVD, and endothelial dysfunction. The analysis of results indicated that the levels of different inflammatory markers, IL-6 and CRP in particular, were elevated. These outcomes of this systematic review suggested that endothelial dysfunction may be the link between PD and ACVD (61). Furthermore, it was found that ACVD is associated with more severe periodontitis and this was marked by higher serum level of high sensitivity (hs)-CRP (62). Elevated level of hs-CRP due to periodontitis exerts stress additional to the previously existing inflammatory activity of atherosclerotic lesion; consequently, increasing the risk of ACVD (63). Recently, periodontitis was found to be associated with high levels of IL-6, PTX3, and sTWEAK in patients with cerebral small vessel disease, increasing by almost 3 times the likelihood of having this type of ACVD (64). This was supported by results from an in vivo study that indicated changes in vascular inflammatory biomarkers, IL-6, PTX3, and sTWEAK, in systemic circulation after injection of LPS from P. gingivalis in rats (65).
Indeed, the current literature has provided valuable information about shared biomarkers between PD and ACVD which may offer predictive and diagnostic potential to significantly reduce the risk of developing undesirable cardiac events at earlier stages (Table 1). However, further studies are required in this regard as the exact signaling downstream of ACVD and PD biomarkers has not yet been fully elucidated.
The joint workshop between the European Federation of Periodontology (EFP) and the American Academy of Periodontology (AAP) in 2012 presented evidence linking PD and ACVD (75). The evidence included the role of periodontopathogenic bacteria in ACVD and clinical (epidemiological and intervention) studies that support the association between these two diseases (76) which will be highlighted in this section.
Clinically, it is very difficult to find the causative agents of atherosclerosis. Firstly, the endothelial injury usually develops and progresses without symptoms, potentially masking the initiating agent. Secondly, multiple factors can lead to a common inflammatory response such as an atherosclerotic lesion, and these factors could be co-existent, which further complicates identifying the causative factor (as discussed in section Confounders Between Periodontal and Atherosclerotic Cardiovascular Diseases). Additionally, studies relating to interventions performed in this respect have reported mixed results, such as no change, temporary worsening of signs after periodontal treatment or improvement in signs (75, 77). Nevertheless, any reported evidence on the potential role of periodontal pathogens in promoting atherosclerosis has to fulfill the following seven proofs (76).
Proof 1: Periodontal bacteria can reach systemic vascular tissues Undoubtedly, many studies have shown that oral bacteria in general and periodontopathogenic in particular can enter the systemic circulation and cause bacteremia (12, 14, 76, 78–80). A previous systematic review has shown that following periodontal procedures the incidence of bacteremia can be as high as 49.4% (81). The prevalences of periodontopathogenic bacteria in systemic vascular tissue following periodontal procedures and in atheromatous lesions without periodontal procedure in subjects with chronic periodontitis are summarized in Table 2. It can be concluded that periodontal pathogens could potentially invade the systemic vascular tissue following periodontal procedures as well as contribute to atheromatous lesions. As Koch's postulate cannot be applied in humans, the direct cause-effect of these periodontopathogenic bacteria in development of atherosclerosis still needs to be confirmed.
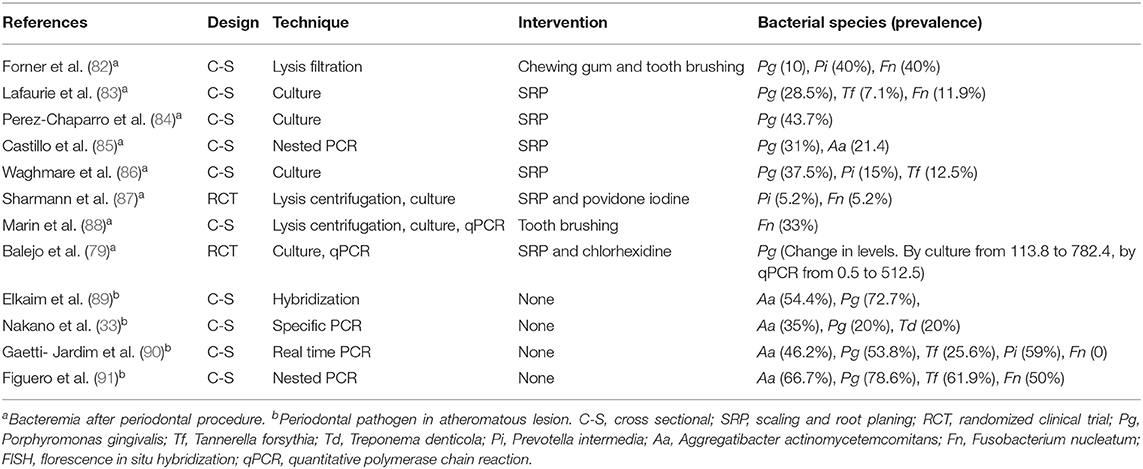
Table 2. Selected studies on bacteremia of periodontal pathogens and periodontal pathogens identified in atheromatous lesions in subjects with chronic periodontitis.
Proof 2. Periodontal bacteria can be found in the affected tissues There is sufficient evidence from several studies that different oral bacterial species can be identified in atheromatous lesions using DNA, RNA, antigen and passive sequencing (91–93). Analyses of samples have shown that periodontitis subjects are at high risk for development of atherosclerosis (94).
Proof 3. Evidence of live periodontal bacteria at the affected site Detection of live periodontopathogenic bacteria is essential to fulfill this proof. Live P. gingivalis and A. a have been isolated from atheromatous lesions by at least two studies (95, 96).
Proof 4. In vitro evidence of invasion of affected cell types A number of in vitro studies showed that periodontopathogenic bacteria can invade different types of host cells. Studies have demonstrated invasion of endothelial cells by P. gingivalis (97, 98) and the mechanism as well as the importance of the particular strain type have been evaluated in a further study (99).
Proof 5. Demonstration that periodontal bacteria can promote atherosclerosis in animal models of disease The review provided by EFP and AAP in 2012 demonstrated evidence that periodontopathogenic bacteria can induce and promote atherosclerosis (75). P. gingivalis has been shown to enhance atherosclerosis in murine (100), rabbit (101), and pig (102) models. Furthermore, when mice with hyperlipidemic conditions were infected orally with P. gingivalis, T. forsythia, T. denticola and F. nucleatum, viable bacteria of these species were detected in oral epithelium, aorta and atherosclerotic plaque (21, 103).
Proof 6. In vitro and in vivo evidence that non-invasive mutants cause significantly reduced pathology (animal model) The importance of the strain of bacterial species in respect of invasion of vascular tissue and cells has been examined. The non-invasive fimbrillinA deficient mutant of P. gingivalis was not found to promote atherosclerosis and resulted in less pro-inflammatory mediators than the invasive wild type strain of P. gingivalis (100).
Proof 7. Fulfill modified Koch's postulate to demonstrate that a human atheroma isolate causes disease in animal models To achieve this proof, the periodontopathogen has to be isolated from human atheroma and lead to atheroma formation in an animal model after inoculation. A strain of P. gingivalis has been isolated from human atherosclerotic plaque (95). Furthermore, evidence is available that inoculation of periodontopathogenic bacteria has the ability to induce atherosclerosis in animal models (21, 103, 104). However, the strains used were not obtained from human atherosclerotic plaque; therefore, this proof has not been entirely fulfilled yet.
Overall, numerous studies are available to support proofs 1 to 6, but not yet for proof 7. Nevertheless, the evidence from the first six proofs supports that periodontopathogenic bacteria are associated with atherosclerosis.
The association between PD and atherosclerosis has been intensively examined in cohort and case control studies. In general, studies on subjects with periodontitis as defined by probing pocket depth (PPD), clinical attachment loss (CAL) and alveolar bone loss have a higher prevalence of subclinical ACVD (18). Those subjects were also suggested to exhibit higher prevalence of ACVD and risk of stroke or MI (105), increased prevalence or incidence of peripheral artery disease (106, 107), and higher prevalence of arterial fibrillation (108). Taking all the evidence from observational studies (Table 3) into account, it can be concluded that the odds ratio of atherosclerotic disease is greater in patients with PD in comparison to non-PD individuals. Furthermore, The results of some interventional studies have suggested that some preventive oral hygiene measures such as regular toothbrushing and oral health interventions including self-performed oral hygiene habits (124), dental prophylaxis (103), increased self-reported visits to the dental office (123) and periodontal treatment (125, 126) can reduced the incidence of ACVD events.
The effect of periodontal therapy on primary prevention of ACVDs such as ischemic heart disease and cardiovascular death has not been examined to date due to methodological, financial and, most importantly, ethical considerations (76). Therefore, surrogate markers of cardiovascular events have been examined rigorously and periodontal therapy has shown significant influence on these markers as summarized in Table 4. However, evidence regarding the long-term effect of periodontal therapy on these surrogate markers is scarce. Further, the effect of periodontal therapy on clinical outcomes of cardiovascular events has not been examined yet (76).
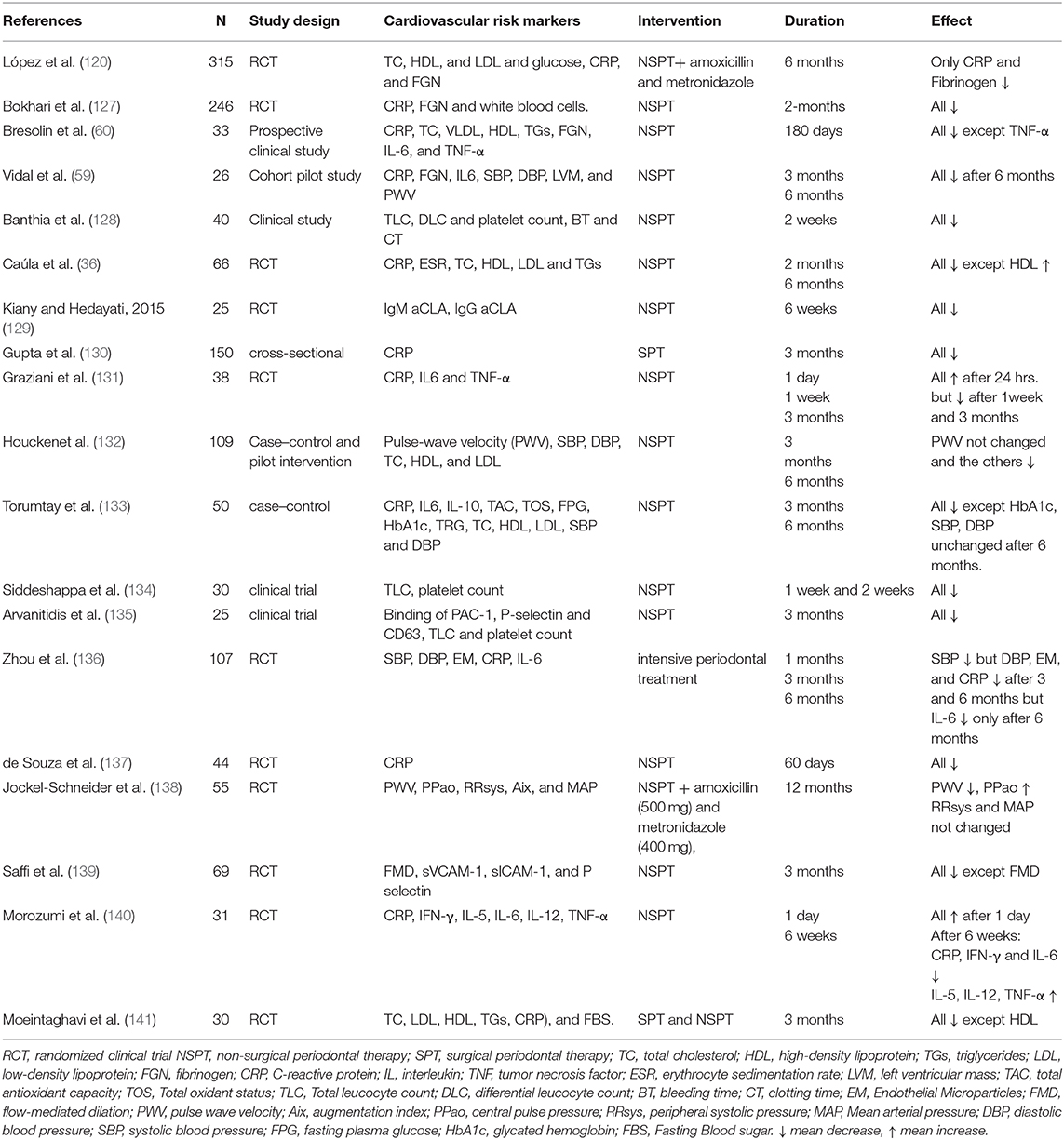
Table 4. Selected studies that have demonstrated effects of periodontal therapy on cardiovascular risk markers.
The majority of researches relating periodontal treatment to ACVD have focused on assessing and quantifying systemic inflammatory biomarkers and endothelial function, as these atherosclerotic risk factors allow the estimation of treatment outcomes over a shorter period. Previous studies have shown that intensive periodontal treatment temporarily increases the blood levels of inflammatory markers, and worsens endothelial function, possibly through the release of bacteria or inflammatory cytokines into the blood stream. However, after several weeks, local and systemic inflammatory markers as well as PD parameters are lower, with considerably better endothelial function than before treatment (142–145). Moreover, carotid intimal-medial thickness is decreased 6 months after periodontal treatment (146). Several randomized controlled clinical trials, case control studies and systematic reviews with meta-analyses have been published in the last decade (Table 4) and they support the notion that periodontal treatment has an effect on cardiovascular events by reducing many cardiovascular risk factors.
Future intervention researches are needed to further clarify the relationship between PD and ACVD, particularly in terms of the biological impacts of PD on the atherogenic cascade by influencing the vascular endothelium. At the same time, there is still a need for more long-term interventional studies, preferably using more homogeneous methodologies for evaluating ACVD events, to determine whether the stated advantages of periodontal treatment translate into a reduction of ACVD occurrence.
Among the different medications used for treatment and prevention of ACVD, statins have demonstrated therapeutic potential in treating PD, which was further confirmed when used via a local delivery system (147, 148). Statins are inhibitors of 3-hydroxy-methylglutaryl coenzyme A reductase (HMG-CoA reductase). These medications have different ring structures and they are known to reduce the level of LDL and cholesterol in the blood for the prevention of ACVD (149, 150). Apart from their main action of lowering lipid levels, statins have several pleiotropic effects including anti-inflammatory, antioxidative, antibacterial and immunoregulatory functions (151, 152).
The anti-inflammatory effect of statins is due to their capability to inhibit pro-inflammatory cytokines and upregulate anti-inflammatory and/or proresolution molecules. This effect is primarily attributed to the activation of extracellular signal-regulated protein kinases (ERK), mitogen activated protein kinase (MAPK), protein kinase (PI3-Akt) signaling pathway, and suppression of NF-κB activation pathways. Furthermore, statins are able to modulate host response to bacterial challenges; thereby preventing inflammation-mediated bone resorption and stimulating new bone formation (153). Local delivery of statins using experimental animal models contributed in preventing alveolar bone resorption as a result of their anti-inflammatory, anti-microbial and bone remodeling properties besides their metalloproteinases inhibitory effect (154).
A 5-year population based competitive follow-up study investigated the effect of systemically administrated statins on the tooth loss rate as compared to participants not on statin medications. The study reported a reduction in the incidence of tooth loss in patients on statin therapy compared to controls (155). In addition, significant improvement in clinical signs of periodontal inflammation was recorded compared to those not taking statins (148).
The effect of statins on periodontitis in otherwise healthy patients was assessed in a review by Petit et al. (153). The primary outcomes included CAL, PPD, and gingival index as well as level of inflammatory biomarkers in serum and gingival crevicular fluid. For clinical parameters, contradictory results were reported on using statins as local adjunct to non-surgical therapy. However, most of these studies consistently reported significant reduction in proinflammatory mediators such as IL-8 and IL-6 associated with upregulation of anti-inflammatory cytokines such as IL-10 (156–158). To the contrary, local application of statins with different periodontal surgeries resulted in significant improvement in PPD, CAL, and bony defects (159–162). The systemic impact of statins on the outcomes of non-surgical periodontal treatment have not been fully elucidated. There are discrepancies in the results of the available studies that are mainly due to variations in the follow-up period and/or their design (153, 163). Cellular and molecular effects of statins on periodontal tissues and their clinical impact are illustrated in Figure 2.
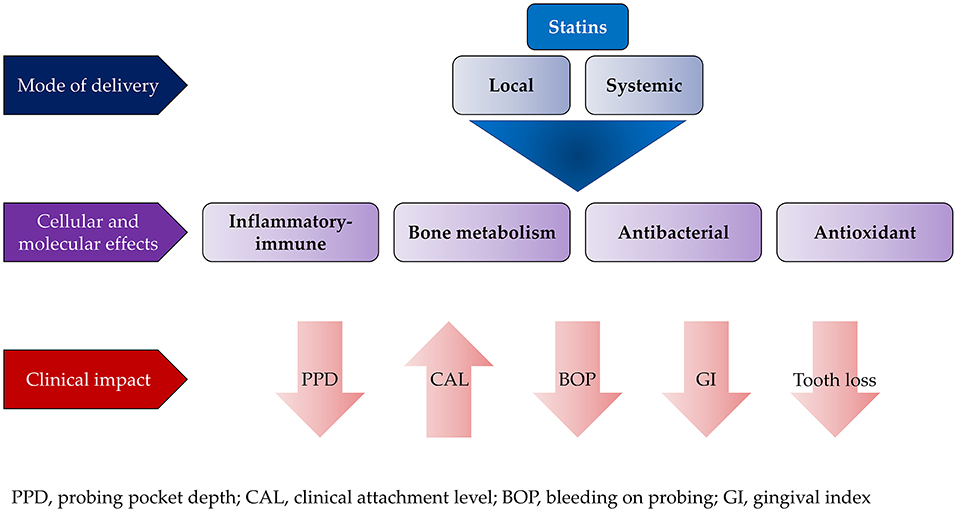
Figure 2. Effect of local and systemic use of statins on the outcome of conventional periodontal treatment.
In general, local application of statins was found to achieve better treatment outcome than systemic application when used as an adjunct to periodontal therapies. Despite the promising results of statins, their effects on different aspects of soft and hard tissue healing need further exploration, especially on wound healing and regeneration.
As detailed previously, a substantial body of evidence supports the relationship between PD and ACVD. Although many studies have reported that periodontal therapy significantly increases surrogate markers of ACVD within a short time, followed by improvement in systemic inflammation and endothelial function (76, 164), invasive dental procedures including periodontal treatment have not been associated with increased risk of MI (165). Furthermore, hemoglobin A1c (Hb A1c) has been found to decrease after periodontal therapy, which is of clinical relevance (143).
Dental practitioners have to be aware of the association between these two diseases. Patients with severe periodontitis should be advised to see a physician to check for signs of ACVD. Those patients should be informed that PD is associated with increased risk of cardiovascular complications and therefore their periodontal condition requires treatment. Furthermore, subjects with ACVD have to adhere to proper oral hygiene measures and regular check-ups with a dental practitioner (18, 76).
Although there is lack in evidence of a direct cause-effect relationship between PD and ACVD, evidence from published studies have confirmed the reduction in the systemic burden of inflammation following periodontal therapy. Thus, cardiologists should notify patients with atherosclerosis about the importance of good oral and dental health. Patients should be advised of the need to have regular home and professional dental care. Furthermore, the physician can recommend referral to a dentist or periodontist for oral and periodontal examination, assessment and treatment when necessary (76). Cooperation between the dentist and the cardiologist is of paramount importance for patients on anticoagulant/antiplatelet medication prior to any oral or periodontal surgeries to avoid any complications such as excessive bleeding and ischemic events.
Evidence from the studies detailed in this review supports the notion that there is a link between PD and ACVD. These two diseases share several systemic inflammatory mechanisms including increases in levels of inflammatory mediators, lipids, and hemostatic and thrombotic factors. Furthermore, they share several risk factors such as smoking and genetics. However, the extent of the impact of PD on the initiation and progression of ACVD is not clear yet and needs to be further examined. Microbiological studies have shown that periodontal pathogens can cause bacteremia and invasion of distant tissues. Evidence from epidemiological studies shows that the odds ratio of atherosclerotic disease is greater in patients with PD in comparison to non-PD individuals. Interventional studies could not examine the effect of periodontal therapy on primary prevention of ACVD such as ischemic heart disease and cardiovascular death due to methodological, financial and, most importantly, ethical considerations. Therefore, surrogate markers of cardiovascular events have been examined rigorously and periodontal therapy has shown significant influence on these markers in the short term. On the other hand, amongst medications used for treatment and prevention of ACVD, statins have shown positive impact on periodontal treatment outcome. Several mechanisms have been proposed regarding the effect of statins on PD outcome, but again this needs to be further investigated. Therefore, it is too early to mark PD as a causal factor with direct relation to the etiology and incidence of ACVD. Further studies should be conducted in vivo and in vitro to determine the cause-effect relationship between PD and ACVD, besides longitudinal studies and with longer follow up are advised to provide solid confirmation to support this relationship and clarify the link between PD and ACVD.
FZ: conceptualization and first draft of sections Introduction, Biomarkers Shared by Periodontal and Atherosclerotic Cardiovascular Diseases, and Clinical Significance of the Link Between PD and ACVD for Dental Practitioners and Cardiologists. SG: conceptualization and first draft of sections Effects of Periodontal Disease on the Incidence of Atherosclerotic Cardiovascular Diseases and Conclusions and Future Research. AA: drawing the figures and first draft of sections Pathogenesis of Atherosclerotic Cardiovascular Diseases Including the Role of Periodontal Disease, Confounders Between Periodontal and Atherosclerotic Cardiovascular Diseases, and Biomarkers Shared by Periodontal and Atherosclerotic Cardiovascular Diseases. AS: first draft of sections Effects of Periodontal Disease on the Incidence of Atherosclerotic Cardiovascular Diseases and Effects of Statins as Medication for ACVD on PD. JY, SG, and AA: critically reviewed the manuscript and final version editing. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Sanz M, D'Aiuto F, Deanfield J, Fernandez-Avilés F. European workshop in periodontal health and cardiovascular disease-scientific evidence on the association between periodontal and cardiovascular diseases: a review of the literature. Eur Heart J Suppl. (2010) 12:B3–12. doi: 10.1093/eurheartj/suq003
2. Dye BA. Global periodontal disease epidemiology. Periodontology 2000. (2012) 58:10–25. doi: 10.1111/j.1600-0757.2011.00413.x
3. Seymour GJ, Gemmell E. Cytokines in periodontal disease: where to from here? Acta Odontol Scand. (2001) 59:167–73. doi: 10.1080/000163501750266765
4. Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. (1994) 5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x
5. Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci. (2015) 23:329–55. doi: 10.1590/1678-775720140259
6. Atilla G, Sorsa T, Rönka H, Emingil G. Matrix metalloproteinases (MMP-8 and−9) and neutrophil elastase in gingival crevicular fluid of cyclosporin-treated patients. J Periodontol. (2001) 72:354–60. doi: 10.1902/jop.2001.72.3.354
7. Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, et al. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J Immunol. (2012) 189:3178–87. doi: 10.4049/jimmunol.1201053
8. Mehta RH, Rathore SS, Radford MJ, Wang Y, Wang Y, Krumholz HM. Acute myocardial infarction in the elderly: differences by age. J Am Coll Cardiol. (2001) 38:736–41. doi: 10.1016/S0735-1097(01)01432-2
9. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. (2017) 70:1–25. doi: 10.1016/j.jacc.2017.04.052
10. Pedrigi RM, de Silva R, Bovens SM, Mehta VV, Petretto E, Krams R. Thin-cap fibro atheroma rupture is associated with a fine interplay of shear and wall stress. Arterioscleros Thromb Vasc Biol. (2014) 34:2224–31. doi: 10.1161/ATVBAHA.114.303426
11. Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol. (2007) 78:2289–302. doi: 10.1902/jop.2007.070140
12. Joshipura K, Zevallos JC, Ritchie CS. Strength of evidence relating periodontal disease and atherosclerotic disease. Compend Contin Educ Dent. (2009) 30:430–9.
13. Beukers NG, van der Heijden GJ, van Wijk AJ, Loos BG. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J Epidemiol Commun Health. (2017) 71:37–42. doi: 10.1136/jech-2015-206745
14. Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. (1999) 138:S419–20. doi: 10.1016/S0002-8703(99)70266-8
16. Leishman SJ, Do HL, Ford PJ. Cardiovascular disease and the role of oral bacteria. J Oral Microbiol. (2010) 2:1–13. doi: 10.3402/jom.v2i0.5781
17. Jawień J. New insights into immunological aspects of atherosclerosis. Polskie Archiwum Medycyny Wewnetrznej. (2008) 118:127–31. doi: 10.20452/pamw.332
18. Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol. (2020) 47:268–88. doi: 10.1111/jcpe.13189
19. Genco R, Wu T, Grossi S, Falkner K, Zambon J, Trevisan M. Periodontal microflora related to the risk for myocardial infarction: a case control study. J Dent Res. (1999) 78:20.
20. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. (2014) 64:57–80. doi: 10.1111/prd.12002
21. Velsko IM, Chukkapalli SS, Rivera MF, Lee JY, Chen H, Zheng D, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS ONE. (2014) 9:e97811. doi: 10.1371/journal.pone.0097811
22. Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz Ö. Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic pathogen, Porphyromonas gingivalis. Front Cell Infect Microbiol. (2017) 7:493. doi: 10.3389/fcimb.2017.00493
23. Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. J Periodontal Res. (2018) 53:565–74. doi: 10.1111/jre.12546
24. Yamada M, Takahashi N, Matsuda Y, Sato K, Yokoji M, Sulijaya B, et al. A bacterial metabolite ameliorates periodontal pathogen-induced gingival epithelial barrier disruption via GPR40 signaling. Sci Rep. (2018) 8:9008. doi: 10.1038/s41598-018-27408-y
25. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. (2009) 119:1420–8. doi: 10.1172/JCI39104
26. Deniset JF, Pierce GN. Possibilities for therapeutic interventions in disrupting Chlamydophila pneumoniae involvement in atherosclerosis. Fundament Clin Pharmacol. (2010) 24:607–17. doi: 10.1111/j.1472-8206.2010.00863.x
27. Hertzén E, Johansson L, Wallin R, Schmidt H, Kroll M, Rehn AP, et al. M1 protein-dependent intracellular trafficking promotes persistence and replication of Streptococcus pyogenes in macrophages. J Innate Immun. (2010) 2:534–45. doi: 10.1159/000317635
28. Takeuchi H, Furuta N, Morisaki I, Amano A. Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cell Microbiol. (2011) 13:677–91. doi: 10.1111/j.1462-5822.2010.01564.x
29. Hajishengallis G, Wang M, Harokopakis E, Triantafilou M, Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate beta2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect Immun. (2006) 74:5658–66. doi: 10.1128/IAI.00784-06
30. Saito A, Inagaki S, Kimizuka R, Okuda K, Hosaka Y, Nakagawa T, et al. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol Med Microbiol. (2008) 54:349–55. doi: 10.1111/j.1574-695X.2008.00481.x
31. Inagaki S, Onishi S, Kuramitsu HK, Sharma A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia”. Infect Immun. (2006) 74:5023–8. doi: 10.1128/IAI.00062-06
32. Ford PJ, Gemmell E, Chan A, Carter CL, Walker PJ, Bird PS, et al. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol. (2006) 21:206–11. doi: 10.1111/j.1399-302X.2006.00276.x
33. Nakano K, Nemoto H, Nomura R, Inaba H, Yoshioka H, Taniguchi K, et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol. (2009) 24:64–8. doi: 10.1111/j.1399-302X.2008.00479.x
34. Pucar A, Milasin J, Lekovic V, Vukadinovic M, Ristic M, Putnik S, et al. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J Periodontol. (2007) 78:677–82. doi: 10.1902/jop.2007.060062
35. Roth GA, Moser B, Huang SJ, Brandt JS, Huang Y, Papapanou PN, et al. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J Thromb Haemostasis. (2006) 4:2256–61. doi: 10.1111/j.1538-7836.2006.02128.x
36. Caúla AL, Lira-Junior R, Tinoco EM, Fischer RG. The effect of periodontal therapy on cardiovascular risk markers: a 6-month randomized clinical trial. J Clin Periodontol. (2014) 41:875–2. doi: 10.1111/jcpe.12290
37. Zeigler CC, Wondimu B, Marcus C, Modéer T. Pathological periodontal pockets are associated with raised diastolic blood pressure in obese adolescents. BMC Oral Health. (2015) 15:41. doi: 10.1186/s12903-015-0026-6
38. Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscleros Thromb Vasc Biol. (2004) 24:2227–36. doi: 10.1161/01.ATV.0000147534.69062.dc
39. Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscleros Thromb Vasc Biol. (2003) 23:1245–9. doi: 10.1161/01.ATV.0000078603.90302.4A
40. Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol. (2016) 14:665–73. doi: 10.2174/1570159X14666151208113006
41. Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. (2009) 12:1–21. doi: 10.1080/10253890802046281
42. Lu H, Xu M, Wang F, Liu S, Gu J, Lin S. Chronic stress enhances progression of periodontitis via α1-adrenergic signaling: a potential target for periodontal disease therapy. Exp Mol Med. (2014) 46:e118. doi: 10.1038/emm.2014.65
43. Haber J. Smoking is a major risk factor for periodontitis. Current opinion in periodontology. (1994) 1:12–18.
44. Salahuddin S, Prabhakaran D, Roy A. Pathophysiological mechanisms of tobacco-related CVD. Global Heart. (2012) 7:113–20. doi: 10.1016/j.gheart.2012.05.003
45. Li H, Fagerberg B, Sallsten G, Borné Y, Hedblad B, Engström G, et al. Smoking-induced risk of future cardiovascular disease is partly mediated by cadmium in tobacco: Malmö Diet and Cancer Study. Environ Epidemiol. (2019) 3:22. doi: 10.1097/01.EE9.0000605856.29855.fb
46. Rydén L, Buhlin K, Ekstrand E, de Faire U, Gustafsson A, Holmer J, et al. Periodontitis increases the risk of a first myocardial infarction: a report from the PAROKRANK study. Circulation. (2016) 133:576–83. doi: 10.1161/CIRCULATIONAHA.115.020324
47. Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. (2003) 8:38–53. doi: 10.1902/annals.2003.8.1.38
48. Roy S, Sato T, Paryani G, Kao R. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes. (2003) 52:1229–34. doi: 10.2337/diabetes.52.5.1229
49. Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. (2008) 172:1411–8. doi: 10.2353/ajpath.2008.071070
50. Brown ML, Yukata K, Farnsworth CW, Chen DG, Awad H, Hilton MJ, et al. Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE. (2014) 9:e99656. doi: 10.1371/journal.pone.0099656
51. Apoorva SM, Sridhar N, Suchetha A. Prevalence and severity of periodontal disease in type 2 diabetes mellitus (non-insulin-dependent diabetes mellitus) patients in Bangalore city: an epidemiological study. J Indian Soc Periodontol. (2013) 17:25–9. doi: 10.4103/0972-124X.107470
52. Xavier AC, Silva IN, Costa Fde O, Corrêa DS. [Periodontal status in children and adolescents with type 1 diabetes mellitus]. Arquivos brasileiros de endocrinologia e metabologia. (2009) 53:348–54. doi: 10.1590/S0004-27302009000300009
53. Mucci LA, Hsieh CC, Williams PL, Arora M, Adami HO, de Faire U, et al. Do genetic factors explain the association between poor oral health and cardiovascular disease? A prospective study among Swedish twins. Am J Epidemiol. (2009) 170:615–21. doi: 10.1093/aje/kwp177
54. Aarabi G, Zeller T, Seedorf H, Reissmann DR, Heydecke G, Schaefer AS, et al. Genetic susceptibility contributing to periodontal and cardiovascular disease. J Dent Res. (2017) 96:610–7. doi: 10.1177/0022034517699786
55. Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, El Mokhtari N-E, et al. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. (2009) 5:e1000378. doi: 10.1371/journal.pgen.1000378
56. Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. (2010) 9:830–4. doi: 10.1016/j.autrev.2010.07.016
57. Emingil G, Buduneli E, Aliyev A, Akilli A, Atilla G. Association between periodontal disease and acute myocardial infarction. J Periodontol. (2000) 71:1882–6. doi: 10.1902/jop.2000.71.12.1882
58. Joshipura KJ, Wand HC, Merchant AT, Rimm EB. Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res. (2004) 83:151–5. doi: 10.1177/154405910408300213
59. Vidal F, Cordovil I, Figueredo CM, Fischer RG. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J Clin Periodontol. (2013) 40:681–7. doi: 10.1111/jcpe.12110
60. Bresolin AC, Pronsatti MM, Pasqualotto LN, Nassar PO, Jorge AS, da Silva EA, et al. Lipid profiles and inflammatory markers after periodontal treatment in children with congenital heart disease and at risk for atherosclerosis. Vasc Health Risk Manage. (2013) 9:703–9. doi: 10.2147/VHRM.S52187
61. Almeida A, Fagundes NCF, Maia LC, Lima RR. Is there an association between periodontitis and atherosclerosis in adults? A systematic review. Curr Vasc Pharmacol. (2018) 16:569–82. doi: 10.2174/1570161115666170830141852
62. Gupta S, Suri P, Patil PB, Rajguru JP, Gupta P, Patel N. Comparative evaluation of role of hs C-reactive protein as a diagnostic marker in chronic periodontitis patients. J Family Med Prim Care. (2020) 9:1340. doi: 10.4103/jfmpc.jfmpc_1063_19
63. Deepa D, Gupta C, Gupta A. Assessment of high-sensitivity C-reactive protein values in chronic periodontitis patients with and without cardiovascular disease: a cross-sectional study. J Clin Prev Cardiol. (2016) 5:108. doi: 10.4103/2250-3528.192677
64. Leira Y, Rodríguez-Yáñez M, Arias S, López-Dequidt I, Campos F, Sobrino T, et al. Periodontitis is associated with systemic inflammation and vascular endothelial dysfunction in patients with lacunar infarct. J Periodontol. (2019) 90:465–74. doi: 10.1002/JPER.18-0560
65. Leira Y, Iglesias-Rey R, Gómez-Lado N, Aguiar P, Sobrino T, D'Aiuto F, et al. Periodontitis and vascular inflammatory biomarkers: an experimental in vivo study in rats. Odontology. (2020) 108:202–12. doi: 10.1007/s10266-019-00461-3
66. Gita B, Sajja C, Padmanabhan P. Are lipid profiles true surrogate biomarkers of coronary heart disease in periodontitis patients? A case-control study in a south Indian population. J Indian Soc Periodontol. (2012) 16:32–6. doi: 10.4103/0972-124X.94601
67. Domingues JEG, Vettore MV, Lima ES. Association between markers of cardiovascular risk and clinical parameters of periodontitis. Rev Odontol UNESP. (2013) 42:336–43. doi: 10.1590/S1807-25772013000500004
68. Kalburgi V, Sravya L, Warad S, Vijayalaxmi K, Sejal P, Hazeil D. Role of systemic markers in periodontal diseases: a possible inflammatory burden and risk factor for cardiovascular diseases? Ann Med Health Sci Res. (2014) 4:388–92. doi: 10.4103/2141-9248.133465
69. Ramírez JH, Parra B, Gutierrez S, Arce RM, Jaramillo A, Ariza Y, et al. Biomarkers of cardiovascular disease are increased in untreated chronic periodontitis: a case control study. Austral Dental J. (2014) 59:29–36. doi: 10.1111/adj.12139
70. Gupta M, Chaturvedi R, Jain A. Role of cardiovascular disease markers in periodontal infection: understanding the risk. Indian J Dental Res. (2015) 26:231–6. doi: 10.4103/0970-9290.162873
71. Cotič J, Ferran M, Karišik J, Jerin A, Pussinen PJ, Nemec A, et al. Oral health and systemic inflammatory, cardiac and nitroxid biomarkers in hemodialysis patients. Med Oral Patol Oral Cir Bucal. (2017) 22:e432–9. doi: 10.4317/medoral.21629
72. Pedroso JdF, Lotfollahi Z, Albattarni G, Arrruda Schulz M, Monteiro A, Sehnem AL, et al. Influence of Periodontal Disease on cardiovascular markers in Diabetes Mellitus patients. Sci Rep. (2019) 9:16138. doi: 10.1038/s41598-019-52498-7
73. Ameen M, Attia AM, Felimban A, Al-Dweghri T, Fattni A, Azab E, et al. Evaluation of cardiac biomarkers in smokers and non-smokers with chronic periodontitis. Int J Health Sci. (2020) 14:26–32.
74. Boyapati R, Vudathaneni V, Nadella SB, Ramachandran R, Dhulipalla R, Adurty C. Mapping the link between cardiac biomarkers and chronic periodontitis: a clinico-biochemical study. J Indian Soc Periodontol. (2020) 24:309–15. doi: 10.4103/jisp.jisp_417_19
75. Reyes L, Herrera D, Kozarov E, Roldán S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol. (2013) 40(Suppl.) 14:S30–50. doi: 10.1111/jcpe.12079
76. Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontology 2000. (2020) 83:66–89. doi: 10.1111/prd.12302
77. Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!”–epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dental Res. (2010) 89:879–902. doi: 10.1177/0022034510375281
78. Ratto-Tespestini A, Chaparro PJ, Romito G, Figueiredo L, Faveri M, Carillo H, et al. Comparison of independent and dependent culture methods for the detection of transient bacteremia in diabetic subjects with chronic periodontitis. Biomédica Revista del Instituto Nacional de Salud. (2016) 36:156–61. doi: 10.7705/biomedica.v36i1.2674
79. Balejo RDP, Cortelli JR, Costa FO, Cyrino RM, Aquino DR, Cogo-Müller K, et al. Effects of chlorhexidine preprocedural rinse on bacteremia in periodontal patients: a randomized clinical trial. J Appl Oral Sci. (2017) 25:586–95. doi: 10.1590/1678-7757-2017-0112
80. Dhotre S, Jahagirdar V, Suryawanshi N, Davane M, Patil R, Nagoba B. Assessment of periodontitis and its role in viridans streptococcal bacteremia and infective endocarditis. Indian Heart J. (2018) 70:225–32. doi: 10.1016/j.ihj.2017.06.019
81. Horliana ACRT, Chambrone L, Foz AM, Artese HPC, Rabelo MdS, Pannuti CM, et al. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS ONE. (2014) 9:e98271. doi: 10.1371/journal.pone.0098271
82. Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. (2006) 33:401–7. doi: 10.1111/j.1600-051X.2006.00924.x
83. Lafaurie GI, Mayorga-Fayad I, Torres MF, Castillo DM, Aya MR, Barón A, et al. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J Clin Periodontol. (2007) 34:873–9. doi: 10.1111/j.1600-051X.2007.01125.x
84. Pérez-Chaparro PJ, Gracieux P, Lafaurie GI, Donnio P-Y, Bonnaure-Mallet M. Genotypic characterization of Porphyromonas gingivalis isolated from subgingival plaque and blood sample in positive bacteremia subjects with periodontitis. J Clin Periodontol. (2008) 35:748–53. doi: 10.1111/j.1600-051X.2008.01296.x
85. Castillo DM, Sánchez-Beltrán MC, Castellanos JE, Sanz I, Mayorga-Fayad I, Sanz M, et al. Detection of specific periodontal microorganisms from bacteraemia samples after periodontal therapy using molecular-based diagnostics. J Clin Periodontol. (2011) 38:418–27. doi: 10.1111/j.1600-051X.2011.01717.x
86. Waghmare AS, Vhanmane PB, Savitha B, Chawla RL, Bagde HS. Bacteremia following scaling and root planing: a clinico-microbiological study. J Indian Soc Periodontol. (2013) 17:725–30. doi: 10.4103/0972-124X.124480
87. Sahrmann P, Manz A, Attin T, Zbinden R, Schmidlin PR. Effect of application of a PVP-iodine solution before and during subgingival ultrasonic instrumentation on post-treatment bacteraemia: a randomized single-centre placebo-controlled clinical trial. J Clin Periodontol. (2015) 42:632–9. doi: 10.1111/jcpe.12416
88. Marín MJ, Figuero E, González I, O'Connor A, Diz P, Álvarez M, et al. Comparison of the detection of periodontal pathogens in bacteraemia after tooth brushing by culture and molecular techniques. Med Oral Patol Oral Cir Bucal. (2016) 21:e276–84. doi: 10.4317/medoral.20842
89. Elkaïm R, Dahan M, Kocgozlu L, Werner S, Kanter D, Kretz JG, et al. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Periodontal Res. (2008) 43:224–31. doi: 10.1111/j.1600-0765.2007.01018.x
90. Gaetti-Jardim E, Marcelino SL, Feitosa ACR, Romito GA, Avila-Campos MJ. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol. (2009) 58:1568–75. doi: 10.1099/jmm.0.013383-0
91. Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, Tejerina JM, del Castro JA, Gutiérrez JM, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. (2011) 82:1469–77. doi: 10.1902/jop.2011.100719
92. Figuero E, Lindahl C, Marín MJ, Renvert S, Herrera D, Ohlsson O, et al. Quantification of periodontal pathogens in vascular, blood, and subgingival samples from patients with peripheral arterial disease or abdominal aortic aneurysms. J Periodontol. (2014) 85:1182–93. doi: 10.1902/jop.2014.130604
93. Serra e Silva Filho W, Casarin RC, Nicolela EL Jr, Passos HM, Sallum AW, et al. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS ONE. (2014) 9:e109761. doi: 10.1371/journal.pone.0109761
94. Armingohar Z, Jørgensen JJ, Kristoffersen AK, Abesha-Belay E, Olsen I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol. (2014) 6:1–13. doi: 10.3402/jom.v6.23408
95. Rafferty B, Jönsson D, Kalachikov S, Demmer RT, Nowygrod R, Elkind MS, et al. Impact of monocytic cells on recovery of uncultivable bacteria from atherosclerotic lesions. J Internal Med. (2011) 270:273–80. doi: 10.1111/j.1365-2796.2011.02373.x
96. Kozarov EV, Dorn BR, Shelburne CE, Dunn WA Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscleros Thromb Vasc Biol. (2005) 25:e17–8. doi: 10.1161/01.ATV.0000155018.67835.1a
97. Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. (1998) 66:5337–43. doi: 10.1128/IAI.66.11.5337-5343.1998
98. Dorn BR, Dunn WA Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. (1999) 67:5792–8. doi: 10.1128/IAI.67.11.5792-5798.1999
99. Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol. (2015) 7:28788. doi: 10.3402/jom.v7.28788
100. Gibson FC III, Hong C, Chou HH, Yumoto H, Chen J, Lien E, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. (2004) 109:2801–6. doi: 10.1161/01.CIR.0000129769.17895.F0
101. Jain A, Batista EL Jr, Serhan C, Stahl GL, Van Dyke TE. Role for periodontitis in the progression of lipid deposition in an animal model. Infect Immun. (2003) 71:6012–8. doi: 10.1128/IAI.71.10.6012-6018.2003
102. Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscleros Thromb Vasc Biol. (2005) 25:1446–51. doi: 10.1161/01.ATV.0000167525.69400.9c
103. Chukkapalli SS, Velsko IM, Rivera-Kweh MF, Zheng D, Lucas AR, Kesavalu L. Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in ApoE null Mice. PLoS ONE. (2015) 10:e0143291. doi: 10.1371/journal.pone.0143291
104. Velsko IM, Chukkapalli SS, Rivera-Kweh MF, Zheng D, Aukhil I, Lucas AR, et al. Periodontal pathogens invade gingiva and aortic adventitia and elicit inflammasome activation in αvβ6 integrin-deficient mice. Infect Immun. (2015) 83:4582–93. doi: 10.1128/IAI.01077-15
105. Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol. (2013) 40(Suppl 14):S70–84. doi: 10.1111/jcpe.12062
106. Ahn YB, Shin MS, Han DH, Sukhbaatar M, Kim MS, Shin HS, et al. Periodontitis is associated with the risk of subclinical atherosclerosis and peripheral arterial disease in Korean adults. Atherosclerosis. (2016) 251:311–8. doi: 10.1016/j.atherosclerosis.2016.07.898
107. Mendez MV, Scott T, LaMorte W, Vokonas P, Menzoian JO, Garcia R. An association between periodontal disease and peripheral vascular disease. Am J Surg. (1998) 176:153–7. doi: 10.1016/S0002-9610(98)00158-5
108. Chen DY, Lin CH, Chen YM, Chen HH. Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population-based, cohort study. PLoS ONE. (2016) 11:e0165601. doi: 10.1371/journal.pone.0165601
109. Ajwani S, Mattila KJ, Tilvis RS, Ainamo A. Periodontal disease and mortality in an aged population. Special Care Dentist. (2003) 23:125–30. doi: 10.1111/j.1754-4505.2003.tb00297.x
110. Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, et al. Periodontal disease as a risk factor for ischemic stroke. Stroke. (2004) 35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D
111. Sim SJ, Kim HD, Moon JY, Zavras AI, Zdanowicz J, Jang SJ, et al. Periodontitis and the risk for non-fatal stroke in Korean adults. J Periodontol. (2008) 79:1652–8. doi: 10.1902/jop.2008.080015
112. Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. (2008) 117:1668–74. doi: 10.1161/CIRCULATIONAHA.107.711507
113. Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol. (2009) 66:505–12. doi: 10.1002/ana.21742
114. Xu F, Lu B. Prospective association of periodontal disease with cardiovascular and all-cause mortality: NHANES III follow-up study. Atherosclerosis. (2011) 218:536–42. doi: 10.1016/j.atherosclerosis.2011.07.091
115. Hayashida H, Saito T, Kawasaki K, Kitamura M, Furugen R, Iwasaki T, et al. Association of periodontitis with carotid artery intima-media thickness and arterial stiffness in community-dwelling people in Japan: the Nagasaki Islands study. Atherosclerosis. (2013) 229:186–91. doi: 10.1016/j.atherosclerosis.2013.04.002
116. Heaton B, Applebaum KM, Rothman KJ, Brooks DR, Heeren T, Dietrich T, et al. The influence of prevalent cohort bias in the association between periodontal disease progression and incident coronary heart disease. Ann Epidemiol. (2014) 24:741–6. doi: 10.1016/j.annepidem.2014.07.006
117. Vedin O, Hagström E, Gallup D, Neely ML, Stewart R, Koenig W, et al. Periodontal disease in patients with chronic coronary heart disease: prevalence and association with cardiovascular risk factors. Eur J Prevent Cardiol. (2015) 22:771–8. doi: 10.1177/2047487314530660
118. Górski B, Nargiełło E, Opolski G, Ganowicz E, Górska R. The association between dental status and systemic lipid profile and inflammatory mediators in patients after myocardial infarction. Adv Clin Exp Med. (2016) 25:625–30. doi: 10.17219/acem/62937
119. Hansen GM, Egeberg A, Holmstrup P, Hansen PR. Relation of periodontitis to risk of cardiovascular and all-cause mortality (from a Danish Nationwide Cohort Study). Am J Cardiol. (2016) 118:489–93. doi: 10.1016/j.amjcard.2016.05.036
120. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. (2012) 33:2551–67. doi: 10.1093/eurheartj/ehs184
121. Bengtsson VW, Persson GR, Berglund J, Renvert S. A cross-sectional study of the associations between periodontitis and carotid arterial calcifications in an elderly population. Acta Odontol Scand. (2016) 74:115–20. doi: 10.3109/00016357.2015.1050603
122. Nordendahl E, Gustafsson A, Norhammar A, Näsman P, Rydén L, Kjellström B. Severe periodontitis is associated with myocardial infarction in females. J Dent Res. (2018) 97:1114–21. doi: 10.1177/0022034518765735
123. Sen S, Giamberardino LD, Moss K, Morelli T, Rosamond WD, Gottesman RF, et al. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. (2018) 49:355–62. doi: 10.1161/STROKEAHA.117.018990
124. Park SY, Kim SH, Kang SH, Yoon CH, Lee HJ, Yun PY, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J. (2019) 40:1138–45. doi: 10.1093/eurheartj/ehy836
125. Holmlund A, Lampa E, Lind L. Poor response to periodontal treatment may predict future cardiovascular disease. J Dent Res. (2017) 96:768–73. doi: 10.1177/0022034517701901
126. Lee YL, Hu HY, Chou P, Chu D. Dental prophylaxis decreases the risk of acute myocardial infarction: a nationwide population-based study in Taiwan. Clin Intervent Aging. (2015) 10:175–82. doi: 10.2147/CIA.S67854
127. Bokhari SA, Khan AA, Butt AK, Azhar M, Hanif M, Izhar M, et al. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. (2012) 39:1065–74. doi: 10.1111/j.1600-051X.2012.01942.x
128. Banthia R, Jain P, Banthia P, Belludi S, Parwani S, Jain A. Effect of phase I periodontal therapy on pro-coagulant state in chronic periodontitis patients–a clinical and haematological study. J Irish Dental Assoc. (2013) 59:183–8.
129. Kiany F, Hedayati A. Evaluation of serum anti-cardiolipin antibodies after non-surgical periodontal treatment in chronic periodontitis patients. Odontology. (2015) 103:203–9. doi: 10.1007/s10266-014-0149-2
130. Gupta B, Sawhney A, Patil N, Yadav M, Tripathi S, Sinha S, et al. Effect of surgical periodontal therapy on serum C-reactive protein levels using ELISA in both chronic and aggressive periodontitis patient. J Clin Diagnost Res. (2015) 9:Zc01-5. doi: 10.7860/JCDR/2015/14680.6558
131. Graziani F, Cei S, Orlandi M, Gennai S, Gabriele M, Filice N, et al. Acute-phase response following full-mouth versus quadrant non-surgical periodontal treatment: a randomized clinical trial. J Clin Periodontol. (2015) 42:843–52. doi: 10.1111/jcpe.12451
132. Houcken W, Teeuw WJ, Bizzarro S, Alvarez Rodriguez E, Mulders TA, van den Born BJ, et al. Arterial stiffness in periodontitis patients and controls. A case-control and pilot intervention study. J Hum Hypertens. (2016) 30:24–9. doi: 10.1038/jhh.2015.41
133. Torumtay G, Kirzioglu FY, Öztürk Tonguç M, Kale B, Calapoglu M, Orhan H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Periodontal Res. (2016) 51:489–98. doi: 10.1111/jre.12328
134. Siddeshappa ST, Nagdeve S, Yeltiwar RK, Parvez H, Deonani S, Diwan V. Evaluation of various hematological parameters in patients with periodontitis after nonsurgical therapy at different intervals. J Indian Soc Periodontol. (2016) 20:180–3. doi: 10.4103/0972-124X.175172
135. Arvanitidis E, Bizzarro S, Alvarez Rodriguez E, Loos BG, Nicu EA. Reduced platelet hyper-reactivity and platelet-leukocyte aggregation after periodontal therapy. Thromb J. (2017) 15:5–. doi: 10.1186/s12959-016-0125-x
136. Zhou QB, Xia WH, Ren J, Yu BB, Tong XZ, Chen YB, et al. Effect of intensive periodontal therapy on blood pressure and endothelial microparticles in patients with prehypertension and periodontitis: a randomized controlled trial. J Periodontol. (2017) 88:711–22. doi: 10.1902/jop.2017.160447
137. de Souza AB, Okawa RT, Silva CO, Araújo MG. Short-term changes on C-reactive protein (CRP) levels after non-surgical periodontal treatment in systemically healthy individuals. Clin Oral Investig. (2017) 21:477–84. doi: 10.1007/s00784-016-1817-0
138. Jockel-Schneider Y, Bechtold M, Haubitz I, Störk S, Fickl S, Harks I, et al. Impact of anti-infective periodontal therapy on parameters of vascular health. J Clin Periodontol. (2018) 45:354–63. doi: 10.1111/jcpe.12849
139. Saffi MAL, Rabelo-Silva ER, Polanczyk CA, Furtado MV, Montenegro MM, Ribeiro IWJ, et al. Periodontal therapy and endothelial function in coronary artery disease: a randomized controlled trial. Oral Dis. (2018) 24:1349–57. doi: 10.1111/odi.12909
140. Morozumi T, Yashima A, Gomi K, Ujiie Y, Izumi Y, Akizuki T, et al. Increased systemic levels of inflammatory mediators following one-stage full-mouth scaling and root planing. J Periodontal Res. (2018) 53:536–44. doi: 10.1111/jre.12543
141. Moeintaghavi A, Arab HR, Moghaddam MA, Shahmohammadi R, Bardan BY, Soroush Z. Evaluation of effect of surgical and nonsurgical periodontal therapy on serum C-reactive protein, triglyceride, cholesterol, serum lipoproteins and fasting blood sugar in patients with severe chronic periodontitis. Open Dent J. (2019) 13:15–21. doi: 10.2174/1874210601913010015
142. Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. (2007) 356:911–20. doi: 10.1056/NEJMoa063186
143. Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D'Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. (2014) 41:70–9. doi: 10.1111/jcpe.12171
144. D'Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. (2005) 84:269–73. doi: 10.1177/154405910508400312
145. D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J. (2006) 151:977–84. doi: 10.1016/j.ahj.2005.06.018
146. Piconi S, Trabattoni D, Luraghi C, Perilli E, Borelli M, Pacei M, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. (2009) 23:1196–204. doi: 10.1096/fj.08-119578
147. Estanislau IM, Terceiro IR, Lisboa MR, Teles Pde B, Carvalho RdS, Martins RS, et al. Pleiotropic effects of statins on the treatment of chronic periodontitis–a systematic review. Brit J Clin Pharmacol. (2015) 79:877–85. doi: 10.1111/bcp.12564
148. Lindy O, Suomalainen K, Mäkelä M, Lindy S. Statin use is associated with fewer periodontal lesions: a retrospective study. BMC Oral Health. (2008) 8:16. doi: 10.1186/1472-6831-8-16
149. Martin-Ruiz E, Olry-de-Labry-Lima A, Ocaña-Riola R, Epstein D. Systematic review of the effect of adherence to statin treatment on critical cardiovascular events and mortality in primary prevention. J Cardiovasc Pharmacol Therapeut. (2018) 23:200–15. doi: 10.1177/1074248417745357
150. Pasin L, Landoni G, Castro ML, Cabrini L, Belletti A, Feltracco P, et al. The effect of statins on mortality in septic patients: a meta-analysis of randomized controlled trials. PLoS ONE. (2014) 8:e82775. doi: 10.1371/journal.pone.0082775
151. Margaritis M, Sanna F, Antoniades C. Statins and oxidative stress in the cardiovascular system. Curr Pharmaceut Design. (2017) 23:7040–7. doi: 10.2174/1381612823666170926130338
152. Zeiser R. Immune modulatory effects of statins. Immunology. (2018) 154:69–75. doi: 10.1111/imm.12902
153. Petit C, Batool F, Bugueno IM, Schwinté P, Benkirane-Jessel N, Huck O. Contribution of statins towards periodontal treatment: a review. Mediat Inflammat. (2019) 2019:6367402. doi: 10.1155/2019/6367402
154. Bertl K, Steiner I, Pandis N, Buhlin K, Klinge B, Stavropoulos A. Statins in nonsurgical and surgical periodontal therapy. A systematic review and meta-analysis of preclinical in vivo trials. J Periodontal Res. (2018) 53:267–87. doi: 10.1111/jre.12514
155. Meisel P, Kroemer HK, Nauck M, Holtfreter B, Kocher T. Tooth loss, periodontitis, and statins in a population-based follow-up study. J Periodontol. (2014) 85:e160. doi: 10.1902/jop.2013.130456
156. Gunjiganur Vemanaradhya G, Emani S, Mehta DS, Bhandari S. Effect of 1.2% of simvastatin gel as a local drug delivery system on Gingival Crevicular Fluid interleukin-6 & interleukin-8 levels in non surgical treatment of chronic periodontitis patients. Arch Oral Biol. (2017) 82:55–61. doi: 10.1016/j.archoralbio.2017.05.022
157. Surve SM, Acharya AB, Thakur SL. Efficacy of subgingivally delivered atorvastatin and simvastatin as an adjunct to scaling and root planing. Drug Metab Personal Therapy. (2015) 30:263–9. doi: 10.1515/dmpt-2015-0024
158. Grover HS, Kapoor S, Singh A. Effect of topical simvastatin (1.2 mg) on gingival crevicular fluid interleukin-6, interleukin-8 and interleukin-10 levels in chronic periodontitis - a clinicobiochemical study. J Oral Biol Craniofacial Res. (2016) 6:85–92. doi: 10.1016/j.jobcr.2015.11.003
159. Kinra P, Gupta H, Khan S, Ahmad MS. Evaluation of the relative efficacy of an allograft used alone and that in combination with simvastatin in the treatment of human periodontal infrabony defects - a clinical and radiological study. J Taibah Univ Med Sci. (2010) 5:75–88. doi: 10.1016/S1658-3612(10)70136-0
160. Martande SS, Kumari M, Pradeep AR, Singh SP, Suke DK, Guruprasad CN. Platelet-rich fibrin combined with 1.2% atorvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. (2016) 87:1039–46. doi: 10.1902/jop.2016.150306
161. Pradeep AR, Garg V, Kanoriya D, Singhal S. Platelet-rich fibrin with 1.2% rosuvastatin for treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. (2016) 87:1468–73. doi: 10.1902/jop.2016.160015
162. Ranjan R, Patil SR, Veena HR. Effect of in-situ application of simvastatin gel in surgical management of osseous defects in chronic periodontitis-A randomized clinical trial. J Oral Biol Craniofacial Res. (2017) 7:113–8. doi: 10.1016/j.jobcr.2017.05.005
163. Fentoglu O, Sözen T, Oz SG, Kale B, Sönmez Y, Tonguç MO, et al. Short-term effects of periodontal therapy as an adjunct to anti-lipemic treatment. Oral Dis. (2010) 16:648–54. doi: 10.1111/j.1601-0825.2010.01668.x
164. D'Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. (2013) 40(Suppl. 14):S85–105. doi: 10.1111/jcpe.12061
Keywords: periodontal therapy, relation, periodontal disease, cardiovascular diseases, atherosclerosis
Citation: Zardawi F, Gul S, Abdulkareem A, Sha A and Yates J (2021) Association Between Periodontal Disease and Atherosclerotic Cardiovascular Diseases: Revisited. Front. Cardiovasc. Med. 7:625579. doi: 10.3389/fcvm.2020.625579
Received: 03 November 2020; Accepted: 17 December 2020;
Published: 15 January 2021.
Edited by:
Cristina Vassalle, Gabriele Monasterio Tuscany Foundation (CNR), ItalyReviewed by:
Ismail Dogu Kilic, Pamukkale University, TurkeyCopyright © 2021 Zardawi, Gul, Abdulkareem, Sha and Yates. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarhang Gul, c2FyaGFuZy5oYW1hQHVuaXZzdWwuZWR1Lmlx
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.