
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 03 February 2021
Sec. General Cardiovascular Medicine
Volume 7 - 2020 | https://doi.org/10.3389/fcvm.2020.617277
The incidence of myocardial infarction (MI) increases every year worldwide. Better diagnostic and prognostic biomarkers for clinical applications are the consistent pursuit of MI research. In addition to electrocardiogram, echocardiography, coronary angiography, etc., circulating biomarkers are essential for the diagnosis, prognosis, and treatment effect monitoring of MI patients. In this review, we assessed both strength and weakness of MI circulating biomarkers including: (1) originated from damaged myocardial tissues including current golden standard cardiac troponin, (2) released from non-myocardial tissues due to MI-induced systems reactions, and (3) preexisted in blood circulation before the occurrence of MI event. We also summarized newly reported MI biomarkers. We proposed that the biomarkers preexisting in blood circulation before MI incidents should be emphasized in research and development for MI prevention in near future.
Cardiovascular diseases are a leading cause of mortality in humans, and nearly 20 million individuals worldwide die from acute cardiovascular events every year. Myocardial infarction (MI), also known as a heart attack, is a myocardial injury caused by myocardial ischemia (1). In 2018, the fourth Universal Definition of Myocardial Infarction emphasized the difference between acute myocardial infarction (AMI) and myocardial injury and divided MI into five types (2, 3) (Figures 1, 2).
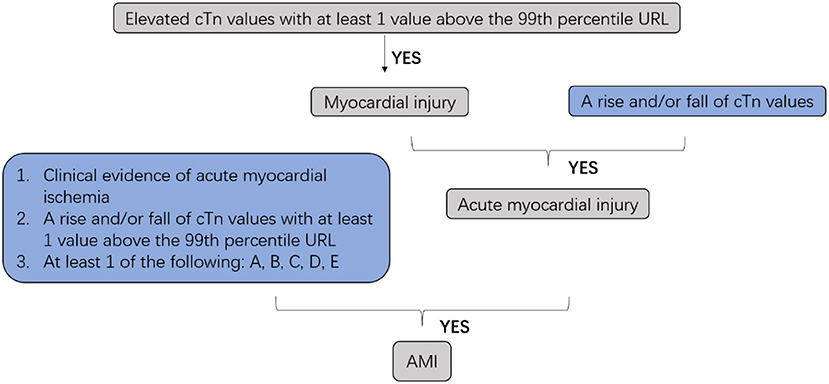
Figure 1. Clinic defined acute myocardial infarction (AMI). cTn, cardiac troponin; URL, upper reference limit; AMI, acute myocardial infarction; A: Symptoms of myocardial ischemia; B: New ischemic ECG changes; C: Development of pathological Q waves; D: Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology; E: Identification of a coronary thrombus by angiography or autopsy (not for types 2 or 3 MIs).
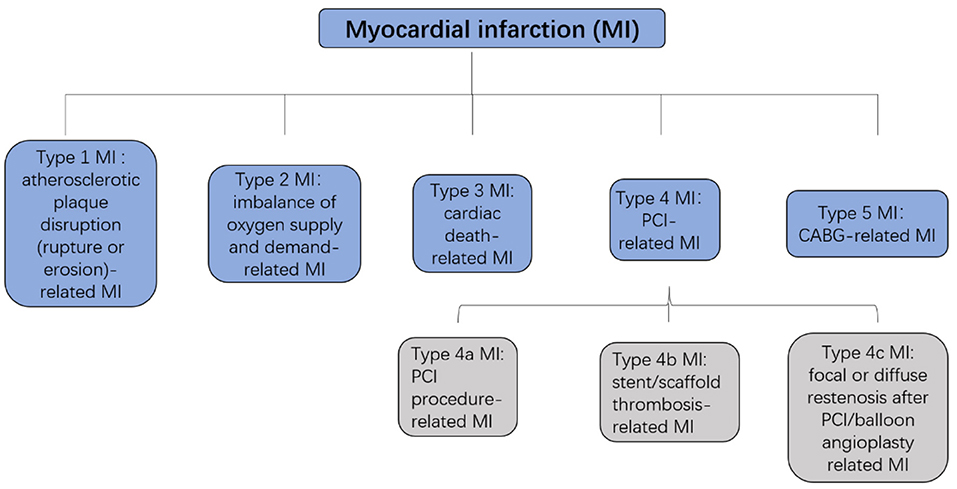
Figure 2. Five types of MI. MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
Approximately 1.5 million individuals in the United States suffer from MI every year (4). We have followed the information of MI patients admitted to the Affiliated Hospital of Qingdao University in China from January 2015 to August 2020. The total incidents, patient age, gender, symptoms, and companion diagnosis are summarized in Figure 3, which are consistent with published knowledge that the MI patients are largely male ranging from 60 to 75 years old with typical symptoms, such as chest pain, chest stuffiness, and dizziness.
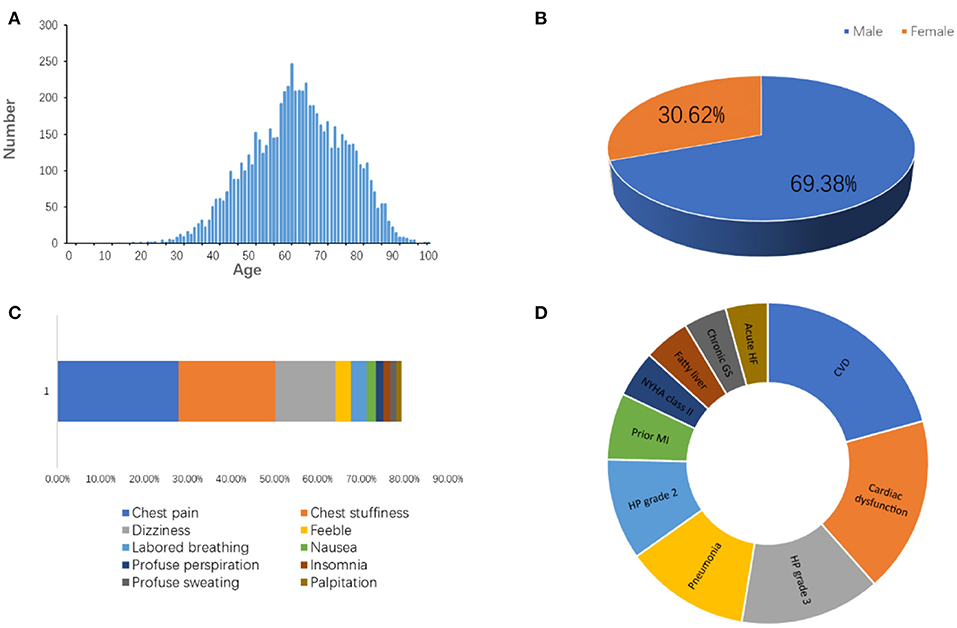
Figure 3. The information of patients diagnosed as MI admitted to the Affiliated Hospital of Qingdao University from 2015 to 2020. (A) Age distribution. (B) Gender distribution. (C) Symptoms. (D) Companion diagnosis. CVD, cardiovascular disease; HP, high blood pressure; MI, myocardial infarction; GS, gastritis; HF, heart failure.
The high incidence of MI results in financial burdens to both families and society and affects the life quality of MI patients.
In addition to ECG, echocardiography, coronary angiography, etc., circulating biomarkers are essential for MI diagnosis. The MI circulating biomarkers have gone through a long process from discoveries to clinical applications. Table 1 summarizes the milestone of the MI biomarker research and development during the past 70 years.
The AMI circulating biomarkers can be divided into three categories: (1) the biomarkers originated from damaged myocardial tissues and released into blood circulation (Table 2); (2) biomarkers with increased levels in blood circulation due to systems reactions after the MI events (Table 3); and (3) biomarkers with abnormal serum levels before the occurrence of MI event (Table 4). Both the strength and weakness of these MI circulating biomarkers will be discussed in the following sections. We also discussed the strengths and weaknesses of newly reported MI biomarkers. We proposed that the biomarkers preexisted in blood circulation before MI incidents should be emphasized in research and development for MI prevention in near future.
Lactate dehydrogenase (LDH) was considered as a useful biomarker in diagnosing AMI (51, 83). LDH has five isoenzymes. LDH-1 is expressed in the heart but it is not heart-specific (83). Circulating LDH-1 increases within 6–12 h from onset of chest pain. It peaks at 1–3 days and returns to normal within 8–14 days. Due to its low sensitivity and specificity, LDH is only used to distinguish acute from subacute MI in patients with positive troponins while CK and CK-MB are negative (51). Moreover, a LDH-1:LDH-2 ratio >1 is reported to be specific for diagnosing AMI (51).
Creatine kinase (CK) activity was considered a better predictor of myocardial injury and an independent indicator of AMI for 20 years (8). CK is a dimeric enzyme, consisting of two subunits, M and B, and has three isoenzymes, CK-BB (CK1), CK-MB (CK2), and CK-MM (CK3) (52). Among them, only CK-MB is found in the heart, but CK-MB is also detected in other organs, such as uterus, tongue, etc. (84). When released into the blood, CK-MB can be divided into two groups, MB1 and MB2. When AMI occurs, MB2 passes into blood companying with significant change in the MB2:MB1 ratio. An MB2:MB1 ratio ≥1.5 is considered as an indicator of AMI (5). CK-MB is an excellent biomarker in diagnosis of AMI during the first 6 h, and at the same time, the negative predictive value during the first 6 h is 97% (5). Furthermore, it was reported that the CK-MB relative index (CK-MB/total CK × 100) could be used for diagnosis of MI. If this index is 2.5% or above, CK-MB has a great possibility released from heart (53). Finally, the total CK and CK-MB levels are correlated with infarct size and provide possibility to predict prognosis. CK-MB, however, cannot detect minor myocardial damage (54).
Myoglobin is a biomarker for early detection and/or exclusion of cardiac injury because the serum level of myoglobin rises in the first 30 min after the onset of an acute event (9). Negative values are more meaningful in the clinic than positive values due to its low-specificity (55).
Increased serum cardiac troponin (cTn) level is the gold standard for AMI diagnosis. Combined changes of cTn with clinical manifestations and ECG could initially identify AMI in the early stage after the onset of chest pain, which can decrease mortality significantly.
Cardiac troponin I (cTnI) is presented in cardiac muscle tissue. Cardiac troponin T (cTnT) is expressed in both skeletal and cardiac myocytes. There is no report that cTnI increases after non-cardiac tissues are damaged while cTnT is more complicated because elevated cTnT may be derived from skeletal muscles. Thus, cTnI is more specific in diagnosing MI (2, 85, 86). High sensitive (hs)-cTn assays measure cTn concentrations 5- to 100-fold lower than conventional assays. Anda et al. suggested that use of risk stratification thresholds for hs-cTnl could identify patients with suspected acute coronary syndrome and at least 2 h of symptoms as low risk at presentation irrespective of age and sex (87). Due to higher sensitivity and specificity compared with others biomarkers, cTnI plays an important role in diagnosis of MI and high-sensitivity (hs)-cTn assays are routinely used in clinic (46).
Fatty acid-binding proteins (FABPs) belong to a family of proteins that are responsible for the transportation of fatty acids and lipophilic materials into or out of cells (88). There are several types of tissue-specific FABPs, including heart-type, liver-type, intestinal-type, epidermal-type, brain-type, ileal-type, myelin-type, adipocyte-type, and testis-type FABPs (88). Heart type fatty acid-binding protein (H-FABP) is a small (15 kDa) soluble protein. Despite not being cardiac-specific, H-FABP plays an essential part in metabolism of fatty acid (FA) inside cardiomyocytes and present at high concentrations in cardiomyocytes cytoplasm (89). Other tissues such as skeletal muscle, brain, and kidney also produce it, although at a lower concentration than in myocardium (90). Watanabe indicated that a “decreased immunoreactivity for H-FABP may be a good histological biomarker of damaged cardiomyocytes” (22). Serum concentration of H-FABP increases in the first 1–2 h after symptom onset, which provides possibility for early diagnosis of AMI, and peak concentration is achieved in approximately 5–10 h, after which it returns to its reference range within 24–36 h (91). Notably, H-FABP demonstrates different sensitivity and specificity at different cutoff, and the highest sensitivity of H-FABP is measured at 4 h (88%) (56). However, its sensitivity and specificity are lower than hs-cTn and thereby it cannot be used as a standalone biomarker for AMI diagnosis. Further studies may help to determine the most precise cutoff point of H-FABP for AMI diagnosis and its additional usage in cardiovascular emergencies (57).
Recently, more attention has been paid on H-FABP as a biomarker for immediate myocardial injury and even for relatively long-term post-ischemic prognosis. Although H-FABP has relatively poor effects on AMI diagnosis, there is evidence that H-FABP plays an important role in evaluating in-stent restenosis and achievement of heart reperfusion after ischemic attack (57, 58, 92). Huang et al. (59) also found H-FABP to be a more sensitive biomarker than cTnI and CK-MB for sensing post-ischemic myocardial reperfusion injury. Additional analysis showed that H-FABP could be a biomarker that can independently predict adverse cardiac events on different levels and provide information for a risk evaluation for clinicians (60, 93, 94). Furthermore, a high negative predictive value of H-FABP test can help to rule out AMI earlier (61), which can reduce hospitalizations and expenses. It is worth mentioning that in 2018, Jo et al. proved that H-FABP would be a more useful biomarker to detect myocardial ischemic injury than CK-MB and cTnT (95). Based on the properties of H-FABP, the combination of H-FABP with other biomarkers, for instance, H-FABP at the early stage and cTnI at the late stage of AMI, may achieve better diagnostic and prognostic significance.
The myosin-binding protein C family consists of three isoforms (96). Notably, the cardiac myosin-binding protein C (cMyC) is expressed in the heart specifically (24, 25). cMyC is more abundant in myocardial tissue and in blood circulation than cTn. cMyC is essential in assembly and function of cardiac sarcomere (26, 27, 63). Due to the delayed appearance of cTn, patients who demonstrate acute chest pain need to test repeatedly to determine AMI while cMyC appears earlier and rises faster in AMI patients (97). Thus, cMyC has an advantage over cTn for early diagnosis of AMI.
It is reported that cMyC rises and falls more rapidly after AMI in patients with vascular risk factors and/or underlying chronic heart disease (98). The ability to distinguish patients suffering MI or acute chest pain by cMyC is similar to that of hs-cTnT and hs-cTnI and superior to s-cTnI (99). In patients presenting <3 h of chest pain onset, cMyC is superior to hs-cTnT (99). In short, cMyC is a promising AMI biomarker with the strengths of ruling in/out AMI more effectively at early onset.
Interleukins (ILs) are a group of cytokines that are expressed by leukocytes. ILs can be divided into four major groups based on distinguishing structural features. The human genome encodes more than 50 interleukins and related proteins.
IL-1 family is a group of 11 cytokines, which induces a complex network of proinflammatory cytokines via expression of integrins on leukocytes and endothelial cells, and regulates and initiates inflammatory responses. In 2004, Patti et al. evaluated that interleukin-1 receptor antagonist (IL-1Ra) increased early in patients with AMI, especially in those with premonitory infarction and symptom onset ≤3 h, and preceded other biomarkers of necrosis (15). IL-1Ra may be an important early adjuvant toward diagnosis of AMI in the emergency department.
IL-32 is a newly discovered inflammatory cytokine with eight isoforms in most mammals. IL-32 is found to be highly expressed in human atherosclerotic plaques and significantly increased in patients with heart failure after MI (16, 17). Furthermore, the soluble IL-1 receptor 2 (sIL-1R2), IL-1, IL-6 plays an important role in myocardial remodeling after MI (64, 65, 100). For the time being, the IL-related therapy of MI is effective, indicating that further research on ILs is needed for MI diagnosis and therapy.
Insulin-like growth factor 1 (IGF-1) is an anabolic hormone that controls growth and metabolism of many cell types. In 1997, Scheinowitz indicated that IGF may protect cardiac function after AMI (23). The majority of circulating IGF-1 molecules combine with IGF binding proteins (IGFBPs), which can modulate IGF-1 binding to the IGF-1 receptor (IGF1R) (101). Evidence shows that IGF-1 levels are correlated with occurrence of coronary heart disease, which functions possibly by affecting atherosclerosis progression (102).
Free IGF-1 is inferior to CK-MB as an indicator of myocardial damage (103). However, IGF-1 plays an important role in some other aspects of MI. IGF-1 can affect vascular function and atherosclerosis by anti-inflammatory and anti-apoptotic actions as well as by stimulating angiogenesis (67–69, 104). At the same time, there is also evidence indicating that IGF-1 has indirect effects on the cardiovascular system by increasing insulin sensitivity (69–71). On the one hand, IGF-1 prevents recruitment of monocytes/macrophages from atherosclerotic plaques, production of proinflammatory cytokines, conversion of macrophages into lipid-laden foam cells, and extracellular matrix degradation. IGF-1 also promotes smooth muscle cell (SMC) migration, proliferation, and SMC-dependent matrix deposition, all of which may contribute to IGF-1-induced reduction in plaque burden and increase in plaque stability (72). By the mechanism described above, IGF-1 stabilizes plaque so as to decrease MI events. Furthermore, IGF-1 treatment is effective to reduce adverse cardiac remodeling after cardiac ischemia/reperfusion injury, when IGF-1 is administered systemically (105). Finally, Yao et al. found that combination of hepatocyte growth factor (HGF) and IGF-1 promote connexin 43 expression and improve ventricular arrhythmia after MI in a rat model (106), which may exhibit therapeutic potential for ventricular arrhythmias after MI.
Vascular endothelial growth factor (VEGF) is a highly specific growth factor for vascular endothelial cells, which can promote vascular permeability, extracellular matrix denaturation, vascular endothelial cell migration, proliferation, and angiogenesis. VEGF family include VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta growth factor (PGF).
VEGF-A, which is actively produced in damaged myocardium to foster angiogenesis and tissue repair, is a major effector of endothelial junction disruption and vascular leakage (42, 107, 108). Clinical studies on VEGF-A in patients with AMI have demonstrated that low levels of circulating VEGF-A are an independent risk factor for adverse clinical outcomes after AMI (43, 44). VEGF-A 165b is the main anti-angiogenic isoform of VEGF-A and associates with infarct size in patients with AMI and dysregulated VEGF-A 165b in aging endothelial cells contribute to the risk of coronary heart disease (109–111). Study shows that the assessment of VEGF-A 165b combined with VEGF-A may predict main adverse cardiovascular events (MACEs) in clinical practice (112) and VEGF-A 165b might play a negative regulatory role in AMI as an inhibitor of angiogenesis in myocardium. According to that, therapy aimed at VEGF-A 165b might be significant to reperfusion of myocardium after STEMI (109). In addition, a low VEGF-C value may independently predict all-cause mortality in patients with suspected or known CHD (113).
In summary, VEGF has little effect on diagnosis of MI while it has significant effect on prognosis and MI treatment.
Matrix metalloproteinases (MMPs) are a group of zinc ion-dependent proteases that degrade collagen and proteoglycans and play an important role in the development of atherosclerosis (114). MMPs play a pivotal role in post-myocardial infarction cardiac remodeling as well as in the development of adverse outcomes (115). Circulating MMP-28, a new member in the family of MMPs, could be considered a predictor for short-term prognosis in patients with myocardial infarction (73).
A recent study reported that in a well-treated contemporary population of AMI patients, 42% of patients without diabetes have elevated admission plasma glucose levels and AMI event rates are increased with the elevated admission plasma glucose levels (116). In addition, fasting blood glucose levels are significantly increased in patients suffering AMI (117). These findings highlight the importance to research and develop the circulating biomarkers usable for MI prevention.
Aspartate Aminotransferase (AST) catalyzes the reversible transfer of an α-amino group between aspartate and glutamate. AST plays an important role in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, and red blood cells. In 1954, AST is established as a biomarker in assisting AMI diagnosis (5). However, it was abandoned because increased serum AST levels are associated with various diseases and also present in both pre- and after AMI events.
A whole new understanding of miRNA began in 2003 when Ambros showed that miRNA participates in regulating development process in worms (118). MicroRNAs (miRNAs/miRs) are a class of tissue-specific or cell-specific small (19–25 nucleotides) non-coding RNAs, which have effects on various biological processes including cell growth, proliferation, differentiation, and apoptosis (119, 120). MicroRNAs are circulating in plasma/serum and have been tested as biomarkers for cardiovascular diseases (30). Among them, microRNA-499 has been shown to be expressed in myocardium and skeletal muscle in mammals, and the blood samples from patients have high levels of microRNA-499 before the AMI events (31, 32). Circulating miR-499 has good sensitivity and specificity for differentiating AMI from non-AMI (0.84 and 0.97, respectively) and it is considered for early diagnosis of AMI (33).
By detecting plasma concentration of microRNA-145 in patients, Zhang et al. indicated that low microRNA-145 levels correlate inversely with the severity of AMI (34). They also speculated that circulating microRNA-145 might not only be of use in diagnosing MI but could also potentially be helpful in prognosticating cardiac function and the risk of developing heart failure. In 2015, Jia et al. indicated that miR-125b-5p and miR-30d-5p have a value for early diagnosis of AMI, and miR-30d-5p might37 have a higher diagnostic value than cTnI (35). MicroRNA-208b (34), microRNA-133a (121), miR-486 as well as miR-150 (36), and microRNA-21 (37) may be novel biomarkers used for the diagnosis of AMI.
Zhong et al. indicated that differential expression of long non-coding RNAs (LncRNAs) would be helpful to understand molecular mechanism of AMI and might be useful biomarkers for non-invasive diagnostic application (122). LncRNAs are a set of RNA transcripts containing more than 200 nucleotides, which cannot transcript protein but have same effects with miRNAs (123). It has been reported that lncRNAs are involved in regulation of cardiac development, pathogenesis of heart failure, and the role of cardiovascular aging (38, 39). Previous studies demonstrated that the serum levels of three lncRNAs, namely H19, MIAT, and MALAT1, are significantly increased in AMI patients when compared with healthy volunteers, which indicates that these lncRNAs are promising biomarkers for the diagnosis of AMI (124).
S100 belongs to the family of EF-hand proteins and is a calcium-binding protein with a low molecular weight of 10–12 kD. Its amino acid sequence is highly conserved in vertebrates and it has high homology with calmodulin and other EF-hand type calcium-binding proteins. At present, there are at least 21 different types of S100 proteins. They are also named as damage-associated molecular pattern molecules (DAMPs). S100 protein consists of two isomeric subunits(α/β) with αα, αβ, and ββ combinations (125).
S100 proteins are normally present in cells derived from the neural crest, chondrocytes, adipocytes, myoepithelial cells, macrophages, Langerhans cells, dendritic cells, and keratinocytes. It has been shown that heart and skeletal muscle are rich in S100A while most S100B is found in the brain (10, 126). S100 proteins have been implicated in a variety of intracellular and extracellular functions. S100 proteins are involved in regulation of protein phosphorylation, transcription factors, Ca2+ homeostasis, the dynamics of cytoskeleton constituents, enzyme activities, cell growth and differentiation, and the inflammatory response.
Several members of the S100 protein family are useful as biomarkers for certain cancers (127). Further, S100 proteins are biomarkers for inflammatory diseases and can mediate inflammation and act as antimicrobials (128). Serum S100A0 is a useful biomarker for diagnosing AMI, which is also better than CK-MB for differentiating AMI from angina pectoris (11). S100A1 is abundant in the heart, especially ventricular cardiomyocytes (12), which can be used to detect the postmortem diagnosis of AMI at an early stage (13). S100A4 expression protects cardiac myocytes against myocardial ischemia and is required for stabilization of cardiac function after MI (14). In 2011, serum levels of S100B, S100A6, S100P, and soluble receptor for advanced glycation endproduct (sRAGE) were analyzed in 882 patients. It was found that serum levels of S100B, S100A6, and S100P are associated with ischemic myocardial injury in acute coronary syndrome (ACS), and expression of these S100 proteins is related to myocardial infarct size (129). As for S100B, it plays an important role in down-regulating cardiac myocyte hypertrophy and is an attractive therapeutic target for treatment of cardiovascular disease (74). In addition, S100B expression may affect cardiac metabolism in diabetic post-MI remodeling and function (75).
More attention should be directed toward S100 as early diagnostic biomarker for MI prevention.
Heparanase (HPA) is an endo-b-D-glucuronidase capable of degrading heparan sulfate (HS) and heparin side chains (130), whose expression is particularly in placenta, platelet, keratinocyte, and active cells of the immune system. HPA plays a role in tumor growth, angiogenesis, cell invasion, and activation of the coagulation system (131, 132). Here we mainly discuss its effects on atherosclerosis, stenosis, and thrombosis, which are all associated with arterial plaque development and rupture (133).
Blich et al. found that HPA levels increase nearly 9-fold in patients with AMI while 3-fold in patients with stable angina (SA) compared to healthy individuals (47). They found that high levels of HPA promotes plaque toward vulnerability, which is pathological basis for MI. Furthermore, HPA functions as a mediator to enhance expression of tissue factor and generation of factor Xa, which are two critical components in blood coagulation (77, 134). Thrombosis caused by rapid blood clotting might lead to disruption of coronary blood blow thereby onset of AMI. In addition, HPA is a predictive biomarker for high thrombus burden in patients with STMI (135). HPA levels are associated with plaque vulnerability and progression and may thus be considered as a pre-diagnostic biomarker and potentially therapeutic target for prevention of acute heart diseases.
PIK3C2A belongs to phosphoinositide 3-kinases (PI3Ks), which is a family of enzymes that phosphorylate the 3′-OH position of the inositol ring of phosphatidylinositol (PI), and regulate a broad range of signaling pathways (136). A retrospective study showed that the level of PIK3C2A gene expression in patients with AMI is significantly lower than that of healthy individuals. Low expression of PIK3C2A is an independent risk factor and could serve as a potential biomarker to predict the risk of AMI (78).
Copeptin, a neuropeptide, has attracted interest for its use as part of a dual marker strategy in combination with cTn for the early rule-out of chest pain patients with suspected NSTEMI (137). A large pooled cohort showed that copeptin below cut-off in combination with hs-cTn below the upper limit of normal range may be used in more than 2.4-times more patients presenting with suspected acute coronary syndrome than a single biomarker strategy based on very low hs-cTn (79). Furthermore, men with suspected NSTEMI have higher copeptin levels, and certain predictors of copeptin elevation are gender-specific (80).
Mitsugumin 53 (MG53), a muscle-specific protein belonging to the tripartite motif family, has been demonstrated to protect the heart against oxidative injury. A study demonstrated that elevated serum MG53 levels have a significant adverse outcome after a 3-year follow-up among patients with STEMI. The measurement of MG53 could be used as a novel biomarker to improve the current means of risk stratification of AMI (81).
The urine albumin-to-creatinine ratio (uACR) has been verified to be independently associated with increased long-term risks of cardiovascular and total mortality in survivors of MI (28). The serum albumin-to-creatinine ratio (sACR) was found to be an independent prognostic biomarker in patients with AMI on admission to the emergency department and a useful biomarker for early risk stratification of patients with AMI (82).
Except for MI diagnosis, the biomarkers mentioned above are also involved in atherosclerosis progression, evaluation of cardiac function, prognosis, etc. (Table 5).
Many circulatory factors are associated with function and prognosis in patients with cardiovascular diseases (142, 143). Many other functions for potential serum MI biomarkers have been reported. For example, the levels of protooncogene tyrosine-protein kinase (SRC), C-C motif chemokine ligand 17 (CCL17), and chymotrypsin C (CTRC) are all significantly decreased in extracellular vesicles (EVs) lysates from MI patients but remain unaltered in the normal control plasma samples (144). The EVs isolated from plasmas provide additional diagnostic value and improve pathophysiological understanding compared to plasma alone in the context of MI, but further study on EVs is required. Moreover, by using proteomic analysis, elevated serum levels of Pregnancy Zone Protein (PZP) and Leucine-Rich Alpha-2-Glycoprotein (LRG) are shown to be independent risk factors for early-onset MI. Therefore, inflammation-associated LRG and PZP may be novel MI biomarkers (145). Furthermore, there is evidence indicating that galectin-3 may be a promising biomarker for evaluation of severity and prognosis of AMI (146). Recently, it was demonstrated that the levels of microparticles (MPs), especially CD31+CD42−EMPs and CD144+EMPs, have the order of normal subjects < stable angina < unstable angina < MI, indicating that MPs have the potential capacity to distinguish stable angina, unstable angina, and MI (147).
MI is an aging-related systems disease caused by multiple genetic and environmental factors in addition to lifestyles (148, 149). It is understandable that high-sensitivity cardiac troponin (hs-cTn) is the current golden standard for AMI diagnosis since it is the specific heart tissue damage product. However, other circulating biomarkers are needed for prevention, prognosis, and treatment effect monitoring purposes.
Both glucose and HPA have pre-diagnostic properties. It has been proved that fasting blood glucose is an independent risk factor for Gensini score in AMI patients (150). In addition, a cohort study has shown that MI with diabetes mellitus tend to develop cardiogenic shock and have worse outcomes (151). A 10-year cohort study demonstrates that relatively high but clinically normal serum glycated hemoglobin A1c (HbA1c) and thyrotropin (TSH) may increase risk of coronary heart disease (CHD) (152). Most cancer biomarkers, such as CA199 and carcinoembryonic antigen (CEA), are either specific glycan structures or heavily glycosylated proteins (153). Among them, elevated carbohydrate antigen 125 (CA125) can be used to predict mortality risk at 6 months following AMI (154), which is consistent with our previous findings that the circulating CA125 levels are higher in fibrosis-associated diseases than in most types of cancers (155).
We classified the MI biomarkers for their diagnostic, prognostic, and preventive purposes and also discussed other biological functions of the biomarkers in current review. However, certain biomarkers belong to more than one category listed in Tables 1–5. Since the development of MI is a long process accompanied with multiple changes of the systems, biomarkers preexisting in blood circulation before MI incidents should be emphasized in research and development for MI prevention in the near future.
YW drafted the manuscript and drew the figures. NP, YA, MX, LT, and LZ revised the manuscript for important intellectual content. YW and LZ were responsible for manuscript concept and design and edited the final manuscript. All authors approved the final version of the manuscript.
This research was supported by the Natural Science Foundation of China (Grants 81672585) and the Taishan Scholar Fellowship to LZ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Alexander Lu of St. Louis University in the United States for English editing of the manuscript.
1. Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1545–602. doi: 10.1016/S0140-6736(16)31678-6
2. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617
3. Chapman AR, Adamson PD, Shah ASV, Anand A, Strachan FE, Ferry AV, et al. High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation. (2020) 141:161–71. doi: 10.1161/CIRCULATIONAHA.120.047269
4. Xu SS, Jiang JJ, Zhang Y, Chen TT, Zhu M, Fang CF, et al. Discovery of potential plasma protein biomarkers for acute myocardial infarction via proteomics. J Thorac Dis. (2019) 11:3962–72. doi: 10.21037/jtd.2019.08.100
5. Aydin S, Ugur K, Aydin S, Sahin I, Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag. (2019) 15:1–10. doi: 10.2147/VHRM.S166157
6. Ladue JS, Wroblewski F, Karmen A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science. (1954) 120:497–9. doi: 10.1126/science.120.3117.497
7. Wroblewski F, Ladue JS. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. (1955) 90:210–3. doi: 10.3181/00379727-90-21985
8. Knudsen J, Steenstrup B, Byrjalsen I, Hildebrandt P, Sorensen S. At what level of serum total creatine kinase activity can measurement of serum creatine kinase MB isoenzyme activity be omitted in suspected myocardial infarction? Scand J Clin Lab Invest. (1989) 49:661–5. doi: 10.3109/00365518909091542
9. Klocke FJ, Copley DP, Krawczyk JA, Reichlin M. Rapid renal clearance of immunoreactive canine plasma myoglobin. Circulation. (1982) 65:1522–8. doi: 10.1161/01.CIR.65.7.1522
10. Suzuki F, Nakajima T, Kato K. Peripheral distribution of nervous system-specific S-100 protein in rat. J Biochem. (1982) 92:835–8. doi: 10.1093/oxfordjournals.jbchem.a133996
11. Usui A, Kato K, Sasa H, Minaguchi K, Abe T, Murase M, et al. S-100ao protein in serum during acute myocardial infarction. Clin Chem. (1990) 36:639–41. doi: 10.1093/clinchem/36.4.639
12. Remppis A, Greten T, Schafer BW, Hunziker P, Erne P, Katus HA, et al. Altered expression of the Ca2+-binding protein S100A1 in human cardiomyopathy. BBA Mol Cell Res. (1996) 1313:253–7. doi: 10.1016/0167-4889(96)00097-3
13. Bi HT, Yang Y, Huang JY, Li YM, Ma CL, Cong B. Immunohistochemical detection of S100A1 in the postmortem diagnosis of acute myocardial infarction. Diagn Pathol. (2013) 8:84. doi: 10.1186/1746-1596-8-84
14. Doroudgar S, Quijada P, Konstandin M, Ilves K, Broughton K, Khalafalla FG, et al. S100A4 protects the myocardium against ischemic stress. J Mol Cell Cardiol. (2016) 100:54–63. doi: 10.1016/j.yjmcc.2016.10.001
15. Patti G, D'Ambrosio A, Mega S, Giorgi G, Zardi EM, Zardi DM, et al. Early interleukin-1 receptor antagonist elevation in patients with acute myocardial infarction. J Am Coll Cardiol. (2004) 43:35–8. doi: 10.1016/j.jacc.2003.07.032
16. Heinhuis B, Popa CD, van Tits BL, Kim SH, Zeeuwen PL, van den Berg WB, et al. Towards a role of interleukin-32 in atherosclerosis. Cytokine. (2013) 64:433–40. doi: 10.1016/j.cyto.2013.05.002
17. Damen MSMA, Popa CD, Netea MG, Dinarello CA, Joosten LAB. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis. (2017) 264:83–91. doi: 10.1016/j.atherosclerosis.2017.07.005
18. Fischer E, Van Zee KJ, Marano MA, Rock CS, Kenney JS, Poutsiaka DD, et al. Interleukin-1 receptor antagonist circulates in experimental inflammation and in human disease. Blood. (1992) 79:2196–200. doi: 10.1182/blood.V79.9.2196.bloodjournal7992196
19. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. (1996) 87:2095–147. doi: 10.1182/blood.V87.6.2095.bloodjournal8762095
20. Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest. (1997) 100:2648–52. doi: 10.1172/JCI119808
21. Loppnow H, Werdan K, Reuter G, Flad HD. The interleukin-1 and interleukin-1 converting enzyme families in the cardiovascular system. Eur Cytokine Netw. (1998) 9:675–80.
22. Watanabe K, Wakabayashi H, Veerkamp JH, Ono T, Suzuki T. Immunohistochemical distribution of heart-type fatty acid-binding protein immunoreactivity in normal human tissues and in acute myocardial infarct. J Pathol. (1993) 170:59–65. doi: 10.1002/path.1711700110
23. Scheinowitz M, Abramov D, Eldar M. The role of insulin-like and basic fibroblast growth factors on ischemic and infarcted myocardium: a mini review. Int J Cardiol. (1997) 59:1–5. doi: 10.1016/S0167-5273(96)02902-6
24. Fougerousse F, Deleozide AL, Fiszman MY, Schwartz K, Beckmann JS, Carrier L. Cardiac myosin binding protein C gene is specifically expressed in heart during murine and human development. Circ Res. (1998) 82:130–3. doi: 10.1161/01.RES.82.1.130
25. Kuster DWD, Barefield D, Govindan S, Sadayappan S. A sensitive and specific quantitation method for determination of serum cardiac myosin binding protein-C by electrochemiluminescence immunoassay. Jove J Vis Exp. (2013) 50786:1–8. doi: 10.3791/50786
26. Aye TT, Scholten A, Taouatas N, Varro A, Van Veen TAB, Vos MA, et al. Proteome-wide protein concentrations in the human heart. Mol Biosyst. (2010) 6:1917–27. doi: 10.1039/c004495d
27. Marjot J, Kaier TE, Martin ED, Reji SS, Copeland O, Iqbal M, et al. Quantifying the release of biomarkers of myocardial necrosis from cardiac myocytes and intact myocardium. Clin Chem. (2017) 63:990–6. doi: 10.1373/clinchem.2016.264648
28. Berton G, Cordiano R, Palmieri R, Cucchini F, De Toni R, Palatini P. Microalbuminuria during acute myocardial infarction - a strong predictor for 1-year mortality. Eur Heart J. (2001) 22:1466–75. doi: 10.1053/euhj.2000.2582
29. Plutzky J. The vascular biology of atherosclerosis. Am J Med. (2003) 115:55–61. doi: 10.1016/j.amjmed.2003.09.010
30. Wang C, Jing Q. Non-coding RNAs as biomarkers for acute myocardial infarction. Acta Pharmacol Sin. (2018) 39:1110–9. doi: 10.1038/aps.2017.205
31. Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, et al. Plasma MicroRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. (2010) 56:1183–5. doi: 10.1373/clinchem.2010.144121
32. Chen X, Zhang LZ, Su T, Li H, Huang Q, Wu D, et al. Kinetics of plasma microRNA-499 expression in acute myocardial infarction. J Thorac Dis. (2015) 7:890–6. doi: 10.3978/j.issn.2072-1439.2014.11.32
33. Zhao JY, Yu HR, Yan P, Zhou XH, Wang Y, Yao YH. Circulating microRNA-499 as a diagnostic biomarker for acute myocardial infarction: a meta-analysis. Dis Markers. (2019) 2019:6121696. doi: 10.1155/2019/6121696
34. Zhang WQ, Xie BQ. A meta-analysis of the relations between blood microRNA-208b detection and acute myocardial infarction. Eur Rev Med Pharmaco. (2017) 21:848–54.
35. Jia KG, Shi P, Han XJ, Chen TN, Tang HX, Wang J. Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction. Mol Med Rep. (2016) 14:184–94. doi: 10.3892/mmr.2016.5246
36. Zhang R, Lan C, Pei H, Duan GY, Huang L, Li L. Expression of circulating miR-486 and miR-150 in patients with acute myocardial infarction. BMC Cardiovasc Disor. (2015) 15:51. doi: 10.1186/s12872-015-0042-0
37. Zhang Y, Liu YJ, Liu T, Zhang H, Yang SJ. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur Rev Med Pharmaco. (2016) 20:323–9.
38. Vausort M, Wagner DR, Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ Res. (2014) 115:668–77. doi: 10.1161/CIRCRESAHA.115.303836
39. Ma YJ, Huang DS, Yang FF, Tian M, Wang YM, Shen D, et al. Long noncoding RNA highly upregulated in liver cancer regulates the tumor necrosis factor-alpha-induced apoptosis in human vascular endothelial cells. DNA Cell Biol. (2016) 35:296–300. doi: 10.1089/dna.2015.3203
40. Zhang, Cheng YJ, Sara JDS, Liu LJ, Liu LP, Zhao X, et al. Circulating MicroRNA-145 is associated with acute myocardial infarction and heart failure. Chin Med J Peking. (2017) 130:51–6. doi: 10.4103/0366-6999.196573
41. Zhao Z, Du S, Shen S, Wang L. MicroRNA-132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL-1beta. J Cell Physiol. (2020) 235:2710–21. doi: 10.1002/jcp.29175
42. Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, et al. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol. (2019) 234:17690–703. doi: 10.1002/jcp.28395
43. Matsudaira K, Maeda K, Okumura N, Yoshikawa D, Morita Y, Mitsuhashi H, et al. Impact of low levels of vascular endothelial growth factor after myocardial infarction on 6-month clinical outcome - results from the nagoya acute myocardial infarction study. Circ J. (2012) 76:1509–16. doi: 10.1253/circj.CJ-11-1127
44. Niu JM, Han X, Qi HX, Yin J, Zhang ZQ, Zhang ZT. Correlation between vascular endothelial growth factor and long-term prognosis in patients with acute myocardial infarction. Exp Ther Med. (2016) 12:475–9. doi: 10.3892/etm.2016.3286
45. Garcia R, Bouleti C, Sirol M, Logeart D, Monnot C, Ardidie-Robouant C, et al. VEGF-A plasma levels are associated with microvascular obstruction in patients with ST-segment elevation myocardial infarction. Int J Cardiol. (2019) 291:19–24. doi: 10.1016/j.ijcard.2019.02.067
46. Ladenson JH. A personal history of markers of myocyte injury [myocardial infarction]. Clin Chim Acta. (2007) 381:3–8. doi: 10.1016/j.cca.2007.02.039
47. Blich M, Golan A, Arvatz G, Sebbag A, Shafat I, Sabo E, et al. Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscl Throm Vas. (2013) 33:E56. doi: 10.1161/ATVBAHA.112.254961
48. Morawiec B, Kawecki D. Copeptin: a new marker in cardiology. J Cardiovasc Med. (2013) 14:19–25. doi: 10.2459/JCM.0b013e3283590d59
49. Kohr MJ, Evangelista AM, Ferlito M, Steenbergen C, Murphy E. S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. J Mol Cell Cardiol. (2014) 69:67–74. doi: 10.1016/j.yjmcc.2014.01.010
50. Tang Y, Zhang Y, Chen Y, Xiang Y, Xie Y. Role of the microRNA, miR-206, and its target PIK3C2alpha in endothelial progenitor cell function - potential link with coronary artery disease. FEBS J. (2015) 282:3758–72. doi: 10.1111/febs.13372
51. Schmiechen NJ, Han C, Milzman DP. ED use of rapid lactate to evaluate patients with acute chest pain. Ann Emerg Med. (1997) 30:571–7. doi: 10.1016/S0196-0644(97)70071-4
52. Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. BBA Mol Basis Dis. (2006) 1762:164–80. doi: 10.1016/j.bbadis.2005.09.004
53. Keffer JH. Myocardial markers of injury - evolution and insights. Am J Clin Pathol. (1996) 105:305–20. doi: 10.1093/ajcp/105.3.305
54. Hawkins RC, Tan HL. Comparison of the diagnostic utility of CK, CK-MB (activity and mass), troponin T and troponin I in patients with suspected acute myocardial infarction. Singapore Med J. (1999) 40:680–4.
55. Mair J, Artner-Dworzak E, Lechleitner P, Morass B, Smidt J, Wagner I, et al. Early diagnosis of acute myocardial infarction by a newly developed rapid immunoturbidimetric assay for myoglobin. Br Heart J. (1992) 68:462–8. doi: 10.1136/hrt.68.11.462
56. Kalay N, Yarlioglues M, Ardic I, Kaya MG, Vardar A, Inanc T, et al. The role of heart-type fatty acid-binding protein in predicting properties of coronary atherosclerosis in patients with acute coronary syndrome. Coronary Artery Dis. (2010) 21:435–40. doi: 10.1097/MCA.0b013e32833db539
57. Chen K, Chen QJ, Wang LJ, Liu ZH, Zhang Q, Yang K, et al. Increment of HFABP level in coronary artery in-stent restenosis segments in diabetic and nondiabetic minipigs: HFABP overexpression promotes multiple pathway-related inflammation, growth and migration in human vascular smooth muscle cells. J Vasc Res. (2016) 53:27–38. doi: 10.1159/000446652
58. de Lemos JA, Antman EM, Morrow DA, Llevadot J, Giugliano RP, Coulter SA, et al. Heart-type fatty acid binding protein as a marker of reperfusion after thrombolytic therapy. Clin Chim Acta. (2000) 298:85–97. doi: 10.1016/S0009-8981(00)00259-X
59. Huang ZY, Zhong XW, Irwin MG, Ji SY, Wong GT, Liu YA, et al. Synergy of isoflurane preconditioning and propofol postconditioning reduces myocardial reperfusion injury in patients. Clin Sci. (2011) 121:57–69. doi: 10.1042/CS20100435
60. Suzuki M, Hori S, Noma S, Kobayashi KJ. Prognostic value of a qualitative test for heart-type fatty acid-binding protein in patients with acute coronary syndrome. Int Heart J. (2005) 46:601–6. doi: 10.1536/ihj.46.601
61. Bivona G, Agnello L, Bellia C, Lo Sasso B, Ciaccio M. Diagnostic and prognostic value of H-FABP in acute coronary syndrome: still evidence to bring. Clin Biochem. (2018) 58:1–4. doi: 10.1016/j.clinbiochem.2018.04.021
62. Body R, Carlton E, Sperrin M, Lewis PS, Burrows G, Carley S, et al. Troponin-only manchester acute coronary syndromes (T-MACS) decision aid: single biomarker re-derivation and external validation in three cohorts. Emerg Med J. (2017) 34:349. doi: 10.1136/emermed-2016-205983
63. Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. (2010) 48:866–75. doi: 10.1016/j.yjmcc.2009.11.014
64. Jing R, Long TY, Pan W, Li F, Xie QY. IL-6 knockout ameliorates myocardial remodeling after myocardial infarction by regulating activation of M2 macrophages and fibroblast cells. Eur Rev Med Pharmaco. (2019) 23:6283–91. doi: 10.26355/eurrev_201907_18450
65. Orrem HL, Shetelig C, Ueland T, Limalanathan S, Nilsson PH, Husebye T, et al. Soluble IL-1 receptor 2 is associated with left ventricular remodelling in patients with ST-elevation myocardial infarction. Int J Cardiol. (2018) 268:187–92. doi: 10.1016/j.ijcard.2018.05.032
66. Yang ZC, Shi L, Xue Y, Zeng T, Shi Y, Lin YZ, et al. Interleukin-32 increases in coronary arteries and plasma from patients with coronary artery disease. Clin Chim Acta. (2019) 497:104–9. doi: 10.1016/j.cca.2019.07.019
67. Li YX, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscl Throm Vas. (2003) 23:2178–84. doi: 10.1161/01.ATV.0000099788.31333.DB
68. Hutter R, Sauter BV, Reis ED, Roque M, Vorchheimer D, Carrick FE, et al. Decreased reendothelialization and increased neointima formation with endostatin overexpression in a mouse model of arterial injury. Circulation. (2003) 107:1658–63. doi: 10.1161/01.CIR.0000058169.21850.CE
69. Nakao-Hayashi J, Ito H, Kanayasu T, Morita I, Murota S. Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells. Atherosclerosis. (1992) 92:141–9. doi: 10.1016/0021-9150(92)90273-J
70. Sesti G, Sciacqua A, Cardellini M, Marini MA, Maio R, Vatrano M, et al. Plasma concentration of IGE-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care. (2005) 28:120–5. doi: 10.2337/diacare.28.1.120
71. Yakar S, Liu JL, Fernandez AM, Wu YP, Schally AV, Frystyk J, et al. Liver-specific IGF-1 gene deletion leads to muscle insulin insensitivity. Diabetes. (2001) 50:1110–8. doi: 10.2337/diabetes.50.5.1110
72. Higashi Y, Gautam S, Delafontaine P, Sukhanov S. IGF-1 and cardiovascular disease. Growth Horm IGF Res. (2019) 45:6–16. doi: 10.1016/j.ghir.2019.01.002
73. Zhou K, Li YM, Xu YW, Guo R. Circulating matrix metalloproteinase-28 levels are related to GRACE scores and short-term outcomes in patients with acute myocardial infarction. Biomed Res Int. (2020) 1–8. doi: 10.1155/2020/9206703
74. Tsoporis JN, Mohammadzadeh F, Parker TG. S100B: a multifunctional role in cardiovascular pathophysiology. Amino Acids. (2011) 41:843–7. doi: 10.1007/s00726-010-0527-1
75. Mohammadzadeh F, Desjardins JF, Tsoporis JN, Proteau G, Leong-Poi H, Parker TG. S100B: role in cardiac remodeling and function following myocardial infarction in diabetes. Life Sci. (2013) 92:639–47. doi: 10.1016/j.lfs.2012.09.011
76. Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the pravastatin or atorvastatin evaluation and infection therapy: thrombolysis in myocardial infarction (PROVE IT-TIMI 22) trial. Am Heart J. (2008) 155:49–55. doi: 10.1016/j.ahj.2007.08.018
77. Nadir Y, Brenner B, Gingis-Velitski S, Levy-Adam F, Ilan N, Zcharia E, et al. Heparanase induces tissue factor pathway inhibitor expression and extracellular accumulation in endothelial and tumor cells. Thromb Haemostasis. (2008) 99:133–41. doi: 10.1055/s-0037-1608919
78. Tan BC, Liu M, Yang YS, Liu L, Meng FB. Low expression of PIK3C2A gene A potential biomarker to predict the risk of acute myocardial infarction. Medicine. (2019) 98:e15061. doi: 10.1097/MD.0000000000015061
79. Giannitsis E, Slagman A, Hamm CW, Gehrig S, Vollert JO, Huber K. Copeptin combined with either non-high sensitivity or high sensitivity cardiac troponin for instant rule-out of suspected non-ST segment elevation myocardial infarction. Biomarkers. (2020) 25:649–58. doi: 10.1080/1354750X.2020.1833084
80. Vargas KG, Tajsic M, Latsuzbaia A, Bastian S, Andric T, Kassem M, et al. Gender-based differences of copeptin alone or combined with troponin for early rule-out of non-ST-elevation myocardial infarction. Am J Emerg Med. (2020). doi: 10.1016/j.ajem.2020.08.053
81. Xie HY, Yan ZJ, Feng S, Zhu TQ, Zhu ZB, Ni JW, et al. Prognostic value of circulating MG53 Levels in acute myocardial infarction. Front Cardiovasc Med. (2020) 7:596107. doi: 10.3389/fcvm.2020.596107
82. Liu H, Zhang JN, Yu J, Li DZ, Jia Y, Cheng YS, et al. Prognostic value of serum albumin-to-creatinine ratio in patients with acute myocardial infarction Results from the retrospective evaluation of acute chest pain study. Medicine. (2020) 99:e22049. doi: 10.1097/MD.0000000000022049
83. Heinova D, Rosival I, Avidar Y, Bogin E. Lactate dehydrogenase isoenzyme distribution and patterns in chicken organs. Res Vet Sci. (1999) 67:309–12. doi: 10.1053/rvsc.1999.0317
84. Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, et al. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. (1985) 313:1050–4. doi: 10.1056/NEJM198510243131704
85. Rittoo D, Jones A, Lecky B, Neithercut D. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases. J Am Coll Cardiol. (2014) 63:2411–20. doi: 10.1016/j.jacc.2014.03.027
86. Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. (2011) 58:1819–24. doi: 10.1016/j.jacc.2011.08.026
87. Bularga A, Lee KK, Stewart S, Ferry AV, Chapman AR, Marshall L, et al. High-sensitivity troponin and the application of risk stratification thresholds in patients with suspected acute coronary syndrome. Circulation. (2019) 140:1557–68. doi: 10.1161/CIRCULATIONAHA.119.042866
88. Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. (2006) 47:39–48. doi: 10.1007/BF03194597
89. Alhadi HA, Fox KAA. Do we need additional markers of myocyte necrosis: the potential value of heart fatty-acid-binding protein. QJM Int J Med. (2004) 97:187–98. doi: 10.1093/qjmed/hch037
90. Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. (2017) 14:135–50. doi: 10.11909/j.issn.1671-5411.2017.02.008
91. Otaki Y, Watanabe T, Kubota I. Heart-type fatty acid-binding protein in cardiovascular disease: a systemic review. Clin Chim Acta. (2017) 474:44–53. doi: 10.1016/j.cca.2017.09.007
92. Das DK, Barua PK, Jones RM. Release of fatty acid-binding protein from ischemic-reperfused rat heart and its prevention by mepacrine. Biochim Biophys Acta. (1991) 1073:394–401. doi: 10.1016/0304-4165(91)90148-A
93. O'Donoghue M, de Lemos JA, Morrow DA, Murphy SA, Buros JL, Cannon CP, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. (2006) 114:550–7. doi: 10.1161/CIRCULATIONAHA.106.641936
94. Ishii J, Ozaki Y, Lu JC, Kitagawa F, Kuno T, Nakano T, et al. Prognostic value of serum concentration of heart-type fatty acid-binding protein relative to cardiac troponin T on admission in the early hours of acute coronary syndrome. Clin Chem. (2005) 51:1397–404. doi: 10.1373/clinchem.2004.047662
95. Jo MS, Lee J, Kim SY, Kwon HJ, Lee HK, Park DJ, et al. Comparison between creatine kinase MB, heart-type fatty acid-binding protein, and cardiac troponin T for detecting myocardial ischemic injury after cardiac surgery. Clin Chim Acta. (2019) 488:174–8. doi: 10.1016/j.cca.2018.10.040
96. Kaier TE, Twerenbold R, Puelacher C, Marjot J, Imambaccus N, Boeddinghaus J, et al. Direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation. (2017) 136:1495–508. doi: 10.1161/CIRCULATIONAHA.117.028084
97. Kaier TE, Alaour B, Marber M. Cardiac myosin-binding protein C-from bench to improved diagnosis of acute myocardial infarction. Cardiovasc Drugs Ther. (2019) 33:221–30. doi: 10.1007/s10557-018-6845-3
98. Baker JO, Tyther R, Liebetrau C, Clark J, Howarth R, Patterson T, et al. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. (2015) 110:23. doi: 10.1007/s00395-015-0478-5
99. Jiang XW, Tian HW, Qiao SB. Letter by Jiang et al. regarding article, “direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction”. Circulation. (2018) 138:543. doi: 10.1161/CIRCULATIONAHA.117.032597
100. Bageghni SA, Hemmings KE, Yuldasheva NY, Maqbool A, Gamboa-Esteves FO, Humphreys NE, et al. Fibroblast-specific deletion of interleukin-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI Insight. (2019) 5:e125074. doi: 10.1172/jci.insight.125074
101. Allard JB, Duan CM. IGF-binding proteins: why do they exist and why are there so many? Front Endocrinol. (2018) 9:117. doi: 10.3389/fendo.2018.00117
102. Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease - a population-based case-control study. Circulation. (2002) 106:939–44. doi: 10.1161/01.CIR.0000027563.44593.CC
103. Anastasilakis AD, Koulaxis D, Upadhyay J, Pagkalidou E, Kefala N, Perakakis N, et al. Free IGF-1, Intact IGFBP-4, and PicoPAPP-A are altered in acute myocardial infarction compared to stable coronary artery disease and healthy controls. Horm Metab Res. (2019) 51:112–9. doi: 10.1055/a-0794-6163
104. Higashi Y, Sukhanov S, Shai SY, Danchuk S, Tang R, Snarski P, et al. Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation. (2016) 133:2263. doi: 10.1161/CIRCULATIONAHA.116.021805
105. Heinen A, Nederlof R, Panjwani P, Spychala A, Tschaidse T, Reffelt H, et al. IGF1 treatment improves cardiac remodeling after infarction by targeting myeloid cells. Mol Ther. (2019) 27:46–58. doi: 10.1016/j.ymthe.2018.10.020
106. Yao J, Ke J, Zhou Z, Tan G, Yin Y, Liu M, et al. Combination of HGF and IGF-1 promotes connexin 43 expression and improves ventricular arrhythmia after myocardial infarction through activating the MAPK/ERK and MAPK/p38 signaling pathways in a rat model. Cardiovasc Diagn Ther. (2019) 9:346–54. doi: 10.21037/cdt.2019.07.12
107. Pannitteri G, Petrucci E, Testa U. Coordinate release of angiogenic growth factors after acute myocardial infarction: evidence of a two-wave production. J Cardiovasc Med. (2006) 7:872–9. doi: 10.2459/01.JCM.0000253831.61974.b9
108. Kranz A, Rau C, Kochs M, Waltenberger J. Elevation of vascular endothelial growth factor-A serum levels following acute myocardial infarction. Evidence for its origin and functional significance. J Mol Cell Cardiol. (2000) 32:65–72. doi: 10.1006/jmcc.1999.1062
109. Hueso L, Rios-Navarro C, Ruiz-Sauri A, Chorro FJ, Nunez J, Sanz MJ, et al. Dynamics and implications of circulating anti-angiogenic VEGF-A(165)b isoform in patients with ST-elevation myocardial infarction. Sci Rep. (2017) 7:9962. doi: 10.1038/s41598-017-10505-9
110. Peiris-Pages M. The role of VEGF 165b in pathophysiology. Cell Adh Migr. (2012) 6:561–8. doi: 10.4161/cam.22439
111. Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF(165)b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. (2004) 64:7822–35. doi: 10.1158/0008-5472.CAN-04-0934
112. Harada K, Kikuchi R, Ishii H, Shibata Y, Suzuki S, Tanaka A, et al. Association between the ratio of anti-angiogenic isoform of VEGF-A to total VEGF-A and adverse clinical outcomes in patients after acute myocardial infarction. IJC Heart Vasc. (2018) 19:3–7. doi: 10.1016/j.ijcha.2018.03.004
113. Wada H, Suzuki M, Matsuda M, Ajiro Y, Shinozaki T, Sakagami S, et al. VEGF-C and mortality in patients with suspected or known coronary artery disease. J Am Heart Assoc. (2018) 7:e010355. doi: 10.1161/JAHA.118.010355
114. Myasoedova VA, Chistiakov DA, Grechko AV, Orekhov AN. Matrix metalloproteinases in pro-atherosclerotic arterial remodeling. J Mol Cell Cardiol. (2018) 123:159–67. doi: 10.1016/j.yjmcc.2018.08.026
115. Yabluchanskiy A, Li YJ, Chilton RJ, Lindsey ML. Matrix metalloproteinases: drug targets for myocardial infarction. Curr Drug Targets. (2013) 14:276–86. doi: 10.2174/1389450111314030002
116. Ritsinger V, Jensen J, Ohm D, Omerovic E, Koul S, Frobert O, et al. Elevated admission glucose is common and associated with high short-term complication burden after acute myocardial infarction: insights from the VALIDATE-SWEDEHEART study. Diabetes Vasc Dis Res. (2019) 16:582–4. doi: 10.1177/1479164119871540
117. Zhang QH, Zhao G, Yang NL, Zhang LJ. Fasting blood glucose levels in patients with different types of diseases. Prog Mol Biol Transl. (2019) 162:277–92. doi: 10.1016/bs.pmbts.2019.01.004
118. Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. (2003) 113:673–6. doi: 10.1016/S0092-8674(03)00428-8
119. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. (2004) 116:281–97. doi: 10.1016/S0092-8674(04)00045-5
120. Kloosterman WP, Plasterk RHA. The diverse functions of MicroRNAs in animal development and disease. Dev Cell. (2006) 11:441–50. doi: 10.1016/j.devcel.2006.09.009
121. Zhu L, Liu F, Xie H, Feng J. Diagnostic performance of microRNA-133a in acute myocardial infarction: a meta-analysis. Cardiol J. (2018) 25:260–7.
122. Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science. (2018) 362:911–7. doi: 10.1126/science.aau3879
123. Zhai H, Li XM, Liu F, Chen BD, Zheng H, Wang XM, et al. Expression pattern of genome-scale long noncoding RNA following acute myocardial infarction in Chinese Uyghur patients. Oncotarget. (2017) 8:31449–64. doi: 10.18632/oncotarget.16355
124. Wang XM, Li XM, Song N, Zhai H, Gao XM, Yang YN. Long non-coding RNAs H19, MALAT1 and MIAT as potential novel biomarkers for diagnosis of acute myocardial infarction. Biomed Pharmacother. (2019) 118:109208. doi: 10.1016/j.biopha.2019.109208
125. Isobe T, Okuyama T. The amino-acid sequence of S-100 protein (PAP I-b protein) and its relation to the calcium-binding proteins. Eur J Biochem. (1978) 89:379–88. doi: 10.1111/j.1432-1033.1978.tb12539.x
126. Kato, Kimura S, Haimoto H, Suzuki F. S100a0 (alpha alpha) protein: distribution in muscle tissues of various animals and purification from human pectoral muscle. J Neurochem. (1986) 46:1555–60. doi: 10.1111/j.1471-4159.1986.tb01776.x
127. Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. (2008) 35:1014–9. doi: 10.1111/j.1600-0560.2007.00953.x
128. Wolf R, Ruzicka T, Yuspa SH. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function. Amino Acids. (2011) 41:789–96. doi: 10.1007/s00726-010-0666-4
129. Cai XY, Lu L, Wang YN, Jin C, Zhang RY, Zhang Q, et al. Association of increased S100B, S100A6 and S100P in serum levels with acute coronary syndrome and also with the severity of myocardial infarction in cardiac tissue of rat models with ischemia-reperfusion injury. Atherosclerosis. (2011) 217:536–42. doi: 10.1016/j.atherosclerosis.2011.05.023
130. Freeman, Parish CR. Human platelet heparanase: purification, characterization and catalytic activity. Biochem J. (1998) 330:1341–50. doi: 10.1042/bj3301341
131. Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. BBA Rev Cancer. (2001) 1471:M99–M108. doi: 10.1016/S0304-419X(01)00017-8
132. Nadir Y, Brenner B. Heparanase-a link between coagulation, angiogenesis, and cancer. Rambam Maimonides Med J. (2012) 3:e0002. doi: 10.5041/RMMJ.10069
133. Vlodavsky, Blich M, Li JP, Sanderson RD, Ilan N. Involvement of heparanase in atherosclerosis and other vessel wall pathologies. Matrix Biol. (2013) 32:241–51. doi: 10.1016/j.matbio.2013.03.002
134. Baker AB, Gibson WJ, Kolachalama VB, Golomb M, Indolfi L, Spruell C, et al. Heparanase regulates thrombosis in vascular injury and stent-induced flow disturbance. J Am Coll Cardiol. (2012) 59:1551–60. doi: 10.1016/j.jacc.2011.11.057
135. Gurbuz AS, Ozturk S, Efe SC, Yilmaz MF, Yanik RE, Yaman A, et al. Heparanase is a predictive marker for high thrombus burden in patients with ST-segment elevation myocardial infarction. Biomarkers. (2019) 24:600–6. doi: 10.1080/1354750X.2019.1628809
136. Mountford JK, Petitjean C, Putra HWK, McCafferty JA, Setiabakti NM, Lee H, et al. The class II PI 3-kinase, PI3KC2 alpha, links platelet internal membrane structure to shear-dependent adhesive function. Nat Commun. (2015) 6:6535. doi: 10.1038/ncomms7535
137. Giannitsis E, Clifford P, Slagman A, Ruedelstein R, Liebetrau C, Hamm C, et al. Multicentre cross-sectional observational registry to monitor the safety of early discharge after rule-out of acute myocardial infarction by copeptin and troponin: the Pro-Core registry. BMJ Open. (2019) 9:e028311. doi: 10.1136/bmjopen-2018-028311
138. Pinchi E, Frati P, Aromatario M, Cipolloni L, Fabbri M, La Russa R, et al. miR-1, miR-499 and miR-208 are sensitive markers to diagnose sudden death due to early acute myocardial infarction. J Cell Mol Med. (2019) 23:6005–16. doi: 10.1111/jcmm.14463
139. Zhou CY, Cui QT, Su GB, Guo XL, Liu XC, Zhang J. MicroRNA-208b alleviates post-infarction myocardial fibrosis in a rat model by inhibiting GATA4. Med Sci Monitor. (2016) 22:1808–16. doi: 10.12659/MSM.896428
140. Li K, Lin T, Chen LD, Wang N. MicroRNA-93 elevation after myocardial infarction is cardiac protective. Med Hypotheses. (2017) 106:23–5. doi: 10.1016/j.mehy.2017.07.003
141. Tan H, Qi J, Fan BY, Zhang J, Su FF, Wang HT. MicroRNA-24-3p attenuates myocardial ischemia/reperfusion injury by suppressing RIPK1 expression in mice. Cell Physiol Biochem. (2018) 51:46–62. doi: 10.1159/000495161
142. Rullman E, Melin M, Mandic M, Gonon A, Fernandez-Gonzalo R, Gustafsson T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin Res Cardiol. (2020) 109:655–72. doi: 10.1007/s00392-019-01554-3
143. Ferreira JP, Pizard A, Machu JL, Bresso E, Rocca HB, Girerd N, et al. Plasma protein biomarkers and their association with mutually exclusive cardiovascular phenotypes: the FIBRO-TARGETS case-control analyses. Clin Res Cardiol. (2020) 109:22–33. doi: 10.1007/s00392-019-01480-4
144. Gidlof O, Evander M, Rezeli M, Marko-Varga G, Laurell T, Erlinge D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci Rep. (2019) 9:8991. doi: 10.1038/s41598-019-45473-9
145. Xuan, Li H, Li LL, Tian QW, Wang Q, Zhang BB, et al. Screening and identification of pregnancy zone protein and leucine-rich alpha-2-glycoprotein as potential serum biomarkers for early-onset myocardial infarction using protein profile analysis. Proteom Clin Appl. (2019) 13:e1800079. doi: 10.1002/prca.201800079
146. Li MX, Yuan Y, Guo K, Lao Y, Huang XS, Feng L. Value of galectin-3 in acute myocardial infarction. Am J Cardiovasc Drug. (2020) 20:333–42. doi: 10.1007/s40256-019-00387-9
147. Wang B, Li T, Han X, Li Y, Cheng W, Wang L, et al. The level of circulating microparticles in patients with coronary heart disease: a systematic review and meta-analysis. J Cardiovasc Transl Res. (2019) 13:702–12. doi: 10.1007/s12265-019-09945-7
148. Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. (2011) 17:320–9. doi: 10.1038/nm.2328
149. Kasper P, Martin A, Lang S, Kutting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 1–17. (2020). doi: 10.1007/s00392-020-01709-7
150. Sharda M, Sharma N, Meena SR, Meena JK, Kumar P. Effect of on admission blood glucose level and HbA1c value on short term prognosis in acute STEMI. J Assoc Physicians India. (2019) 67:84–5.
151. Echouffo-Tcheugui JB, Kolte D, Khera S, Aronow HD, Abbott JD, Bhatt DL, et al. Diabetes mellitus and cardiogenic shock complicating acute myocardial infarction. Am J Med. (2018) 131:778–86 e1. doi: 10.1016/j.amjmed.2018.03.004
152. Li H, Cui Y, Zhu Y, Yan H, Xu W. Association of high normal HbA1c and TSH levels with the risk of CHD: a 10-year cohort study and SVM analysis. Sci Rep. (2017) 7:45406. doi: 10.1038/srep45406
153. Hu M, Lan Y, Lu A, Ma X, Zhang L. Glycan-based biomarkers for diagnosis of cancers and other diseases: PAST, present, and future. Progress Mol Biol Transl Sci. (2019) 162:1–24. doi: 10.1016/bs.pmbts.2018.12.002
154. Falcao F, Oliveira F, Cantarelli F, Cantarelli R, Brito Junior P, Lemos H, et al. Carbohydrate antigen 125 for mortality risk prediction following acute myocardial infarction. Sci Rep. (2020) 10:11016. doi: 10.1038/s41598-020-67548-8
Keywords: serum biomarkers, myocardial infarction, MI diagnosis, MI prognosis, circulating biomarkers
Citation: Wu Y, Pan N, An Y, Xu M, Tan L and Zhang L (2021) Diagnostic and Prognostic Biomarkers for Myocardial Infarction. Front. Cardiovasc. Med. 7:617277. doi: 10.3389/fcvm.2020.617277
Received: 14 October 2020; Accepted: 29 December 2020;
Published: 03 February 2021.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Shinya Goto, Tokai University, JapanCopyright © 2021 Wu, Pan, An, Xu, Tan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Tan, cWR0YW5saWp1YW5AMTI2LmNvbQ==; Lijuan Zhang, emhhbmdsakBxZHVob3NwaXRhbC5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.