
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 02 December 2020
Sec. Cardiovascular Therapeutics
Volume 7 - 2020 | https://doi.org/10.3389/fcvm.2020.613271
This article is part of the Research TopicImmunomodulatory Approaches in Cardiovascular DiseasesView all 13 articles
 Hana A. Itani1,2
Hana A. Itani1,2 Miran A. Jaffa3*
Miran A. Jaffa3* Joseph Elias4
Joseph Elias4 Mohammad Sabra4
Mohammad Sabra4 Patrick Zakka5
Patrick Zakka5 Jad Ballout4
Jad Ballout4 Amira Bekdash1
Amira Bekdash1 Rand Ibrahim4
Rand Ibrahim4 Moustafa Al Hariri6
Moustafa Al Hariri6 Mirna Ghemrawi7
Mirna Ghemrawi7 Bernard Abi-Saleh4
Bernard Abi-Saleh4 Maurice Khoury4
Maurice Khoury4 Samir Alam4
Samir Alam4 Rami Mahfouz7
Rami Mahfouz7 Ayad A. Jaffa8
Ayad A. Jaffa8 Sami T. Azar9
Sami T. Azar9 Marwan M. Refaat4,8*
Marwan M. Refaat4,8*Atrial fibrillation (AF) and cardiometabolic syndrome (CMS) have been linked to inflammation and fibrosis. However, it is still unknown which inflammatory cytokines contribute to the pathogenesis of AF. Furthermore, cardiometabolic syndrome (CMS) risk factors such as obesity, hypertension, insulin resistance/glucose intolerance are also associated with inflammation and increased level of cytokines and adipokines. We hypothesized that the inflammatory immune response is exacerbated in patients with both AF and CMS compared to either AF or CMS alone. We investigated inflammatory cytokines and fibrotic markers as well as cytokine genetic profiles in patients with lone AF and CMS. CMS, lone AF patients, patients with both lone AF and CMS, and control patients were recruited. Genetic polymorphisms in inflammatory and fibrotic markers were assessed. Serum levels of connective tissue growth factor (CTGF) were tested along with other inflammatory markers including platelet-to-lymphocyte ratio (PLR), monocyte-to-HDL ratio (MHR) in three groups of AF+CMS, AF, and CMS patients. There was a trend in the CTGF levels for statistical significance between the AF and AF+CMS group (P = 0.084). Genotyping showed high percentages of patients in all groups with high secretor genotypes of Interleukin-6 (IL-6) (P = 0.037). Genotyping of IFN-γ and IL-10 at high level showed an increase in expression in the AF + CMS group compared to AF and CMS alone suggesting an imbalance between the inflammatory and anti-inflammatory cytokines which is exacerbated by AF. Serum cytokine inflammatory cytokine levels showed that IL-4, IL-5, IL-10, IL-17F, and IL-22 were significant between the AF, AF+CMS, and CMS patients. Combination of both CMS and AF may be associated with a higher degree of inflammation than what is seen in either CMS or AF alone. Thus, the identification of a biomarker capable of identifying metabolic syndrome associated with disease will help in identification of a therapeutic target in treating this devastating disease.
Cardiometabolic syndrome (CMS) is a substantial cause of worldwide morbidity and mortality and represents a cluster of metabolic abnormalities and significant cardiovascular disease risk factors that can lead to non-communicable diseases (NCD) (1, 2). It has been recently reported that 25% of adults in the US have metabolic syndrome which is attributed to a higher risk for developing atherosclerotic CVD and premature cardiovascular mortality (2–4). Metabolic disorders in metabolic syndrome are associated with insulin resistance, visceral adiposity, obesity, dyslipidemia, endothelial dysfunction, and hypertension with prominent end-organ damages in the cardiovascular system, pancreas, and liver (4, 5). Mitochondrial dysfunction, chronic inflammation, gut microbiome, genetic variation, and environmental contaminants are factors that contribute to the pathogenesis of metabolic syndrome and its transition to CVD (2, 4). Genome-wide association studies (GWAS) studies performed on metabolic syndrome identified genetic variants that are involved in glucose and lipid metabolism (6). Recent studies revealed the importance of the pro-inflammatory state in metabolic syndrome through mediating vascular dysfunction (5). In addition, high levels of serum TNF-α and IL-6 have shown to be linked to obesity and insulin resistance which are key players in metabolic syndrome (2). Thus, far, there is no single biomarker capable of identifying metabolic syndrome, yet a promising panel of biomarkers were shown to be associated with disease (7). Elevated levels of pro-inflammatory cytokines (IL-6, TNF-α), adipokines (Leptin, adiponectin, ghrelin), LAR, prothrombotic factors (PAI-1), uric acid, and pro-oxidant (oxidized LDL) coupled with lower levels of anti-inflammatory cytokines (IL-10) and antioxidant factors (PON-1) are noted in metabolic syndrome (7).
Atrial fibrillation (AF), on the other hand, is a major arrhythmia defined as fast and disorganized electrical excitation of the atria affecting cardiac function (8, 9). AF is considered as a risk factor for stroke, as more than 15% of strokes in the US are due to AF (8). AF together with hypertension could lead to thromboembolic complications and congestive heart failure (10). As a consequence, AF is correlated with increased morbidity and mortality (8). Current treatments of AF target stroke prevention, rate and rhythm control (8, 9). Many research studies proposed gene therapy as a method to reduce the expression of abnormal genes related to the pathogenesis of AF such as ion channels, gap junctions, parasympathetic nervous system, and fibrosis (11). In addition, the impact of gender differences on AF is found to be prominent. Significant differences between women and men with AF are related to AF mechanisms, therapy response stroke risk reduction strategies, as well as other outcomes such as quality of life (8).
Type II diabetes, a major component of CMS, is considered a strong risk factor for AF, and metabolic syndrome has been associated with AF recently (12). Most metabolic syndrome components are found to have an additive effect on the risk of AF (13, 14). Oxidative stress and inflammation are common components related to the pathogenesis of both metabolic syndrome and AF (13). Other components such as hypertension, dyslipidemia, and abdominal adiposity increase the risk of new-onset AF (14, 15). Given the circumstances, studies determined the strong association of low HDL cholesterol, elevated fasting glucose levels in increasing the risk of AF (14, 15). Furthermore, elevated levels of inflammatory mediators were encountered in atrial biopsies from patients with AF (16). The synergistic effect of CMS components is capable of exacerbating the outcome of AF and coronary heart diseases leading to cardiovascular mortality (17). Regardless, the exact mechanism behind the association between CMS and AF is still insufficient and further studies are required to understand the etiology and relation between CMS in AF (15). Increasing evidence supports the role of inflammation-associated cytokines and chemokines in the pathogenesis of atrial fibrillation (AF). Several pharmacological interventions with established anti-inflammatory effects and corticosteroids are associated with AF. Many inflammatory pathways lead to electrical and structural remodeling of the atria that predispose to AF. The Infiltration of immune cells including macrophages and T lymphocytes releasing inflammatory cytokines and inflammatory mediators such as C-reactive protein (CRP) enhance the inflammatory response in cardiac tissue are major players in the development of AF. In the current study, we investigated the inflammatory profile of fibrotic markers as well as cytokine genetic profiles in patients with lone AF, CMS, and combination of both AF and CMS. We further compared the plasma levels of inflammatory cytokines in those patients. The understanding of the inflammatory pathophysiology in AF and CMS patients will help us to identify specific and potential therapeutic strategies for the prevention of AF.
This prospective study included four study groups: patients with CMS and lone AF, patients with CMS without lone AF, patients with lone AF without CMS, and a control group with neither CMS nor lone AF (previously healthy). According to the modified NCEP ATP III criteria, the following are cardiometabolic risk factors and the presence of any three of these factors is required for a diagnosis of CMS: Abdominal Obesity [>40”/102 cm in men and >35”/88 cm in women], hypertriglyceridemia (≥150 mg/dL or ≥1.7 mmol/L), reduced HDL-C [<40 mg/dL (1.03 mmol/L) in men and <50 mg/dL (1.29 mmol/L) in women], elevated blood pressure [≥130/85 mm Hg or use of medication for hypertension], impaired Fasting Glucose [≥100 mg/dL (5.6 mmol/L) or use of medication for hyperglycemia]. Subjects were excluded from this study if they have a history of moderate or severe mitral regurgitation or stenosis, severe aortic stenosis, hyperthyroidism, acute infection, chronic inflammatory diseases, active malignancy, chronic liver disease, cardiomyopathy, or heart failure (EF < 40%) or history of cardiac surgery including CABG or valve replacements.
CMS patients (n = 11), lone AF patients (n = 27), and patients with both lone AF with CMS (n = 33) and Controls (n = 4) were recruited for this study from the American University of Beirut Medical Center (AUBMC). All patients provided an informed consent form approved by the Institutional Review Board (IRB) at the AUBMC. Patients were asked to complete a standardized questionnaires to collect baseline information.
The cytokine genotyping is based on PCR-SSP methodology which provides sequence-specific oligonucleotide primers for amplification of selected TNF-α, TGF-β1, IFN-γ, IL-6, and IL-10 alleles and the human β-globin gene by the polymerase chain reaction (PCR). These alleles are known to be associated with the expression level of these factors. The primer pairs are designed to have perfect matches only with a single allele or group of alleles. Under strictly controlled PCR conditions, perfectly matched primer pairs result in the amplification of target sequences (i.e., a positive result), while mismatched primer pairs do not result in amplification (i.e., a negative result).
Pre-optimized primers are presented (dried) in different wells of a 96-well 0.2 ml thin-walled tube tray for PCR and are ready for the addition of DNA samples, recombinant Taq polymerase, and specially formulated dNTP-buffer mix (D-mix). Each tray includes a negative control reaction tube that detects the presence of the internal control product generated by the tray. After the PCR process, the amplified DNA fragments are separated by agarose gel electrophoresis and are visualized by staining with ethidium bromide and exposure to ultraviolet light. Interpretation of PCR-SSP results is based on the presence or absence of a specific amplified DNA fragment. Since amplification during the PCR reaction may be adversely affected by various factors (pipetting errors, poor DNA quality, presence of inhibitors, etc.) an internal control primer pair is included in every PCR reaction. The control primer pair amplifies a conserved region of the human β-globin gene, which is present in all DNA samples and is used to verify the integrity of the PCR reaction. In the presence of a positive typing band (specific amplification of a cytokine allele), the product of the internal control primer may be weak or absent due to the difference in concentration and melting temperatures between the specific primer pairs and the internal control primer pair. The amplified DNA fragments of the specific cytokine primer pairs are smaller than the product of the internal control primer pair, but larger than the diffuse, unincorporated primer band. Thus, a positive reaction for a specific cytokine allele or allele group is visualized on the gel as an amplified DNA fragment between the internal control product band and the unincorporated primer band.
Serum samples were tested for 13 human inflammatory cytokines using the LEGENDplexTM Human Th cytokine panel for interleukins (ILs, pg/mL) IL-2, 4, 5, 6, 9, 10, 13, 17A, 17F, 21, 22, IFN-γ and TNF-α, which are collectively secreted by Th1, Th2, Th9, Th17, Th22, and T follicular cells (Biolegend). Samples were treated following the manufacturer's instructions and measured with a BD FACS Aria™SORP cell sorter (BD Biosciences). Analysis was done using Data Analysis V8.0 software. Serum levels of IL-18, connective tissue growth factor (CTGF) were tested along with other inflammatory markers including platelet-to-lymphocyte ratio (PLR) and monocyte-to-HDL ratio (MHR).
Our analysis was initiated by carrying out descriptive analysis to provide a summary statistics to all parameters. Continuous parameters were summarized using mean and standard deviation, and categorical parameters were presented using count and percent. To assess the crude unadjusted associations between the IL levels and the groups (AF, AF+CMS, and CMS), we employed the conservative non-parametric tests Mann-Whitney U test for the comparisons between the two groups, and Kruskal Wallis for comparisons between multiple groups. Our adjusted analysis was carried out to model the ILs as a function of the groups (AF, AF+CMS, and CMS), and selected clinical and demographic characteristics. In this respect, multiple linear regression was carried out using the logarithmic scale of the ILs employed to achieve symmetry in the outcomes. The AF group was chosen as the reference group. Significance level was chosen to be 0.05, and ILs found to be significantly associated with the groups (AF, AF+CMS, and CMS) at the level of unadjusted analysis were further considered in the multivariable linear regression. To assess the association between categorical variables as in the groups (AF, AF+CMS, CMS) and the stratified genetic polymorphisms (High, Intermediate, Low), we employed the Fisher's Exact test to be on the conservative side when reporting the p-values, given that some of the expected cells were <5 (Table 2). In Table 3, the mean of ILs are presented across the different groups along with the corresponding P-values obtained using the Kruskal-Wallis test, and in Table 4 results of the multivariable adjusted analysis for the clinical and demographic characteristics are displayed In Appendix 1-Table a, a detailed summary statistics of the ILs are presented across the different groups. In Appendix 1-Table b, the P-values corresponding to the Mann-Whitney U tests unadjusted and adjusted for multiple comparisons are presented between the groups as well as the P-values for the Kruskal Wallis tests for multiple group comparisons. Box-plots pertaining to the ILs that were significantly associated with the groups (AF, AF+CMS, and CMS) were included in Appendix 2 – Figure 1(a–e). Our data analysis was conducted using SPSS 23, and STATA 14.
Table 1 shows the baseline characteristics or parameters for the study population (AF, AF+CMS, CMS) and are described in terms of mean for continuous data, and percentages for categorical data. These parameters included HDL-C, LDL-C, total cholesterol, systolic blood pressure (SBP), diastolic Blood pressure (DBP), age, gender and smoking status. At baseline, the study population included relatively equal distribution of women (58.3, 54.8, and 36.36%) and men (41.67, 45.16, and 63.64%) among the AF, AF+CMS, and CMS groups.
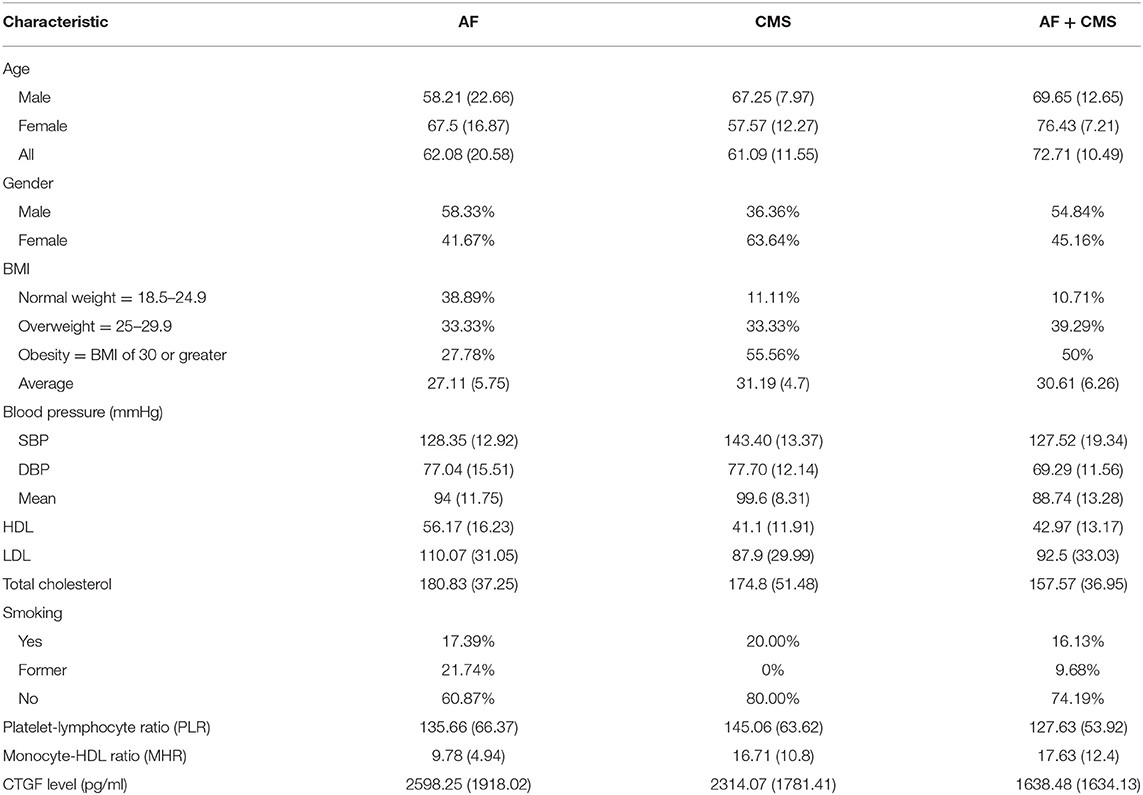
Table 1. Baseline characteristics of the study population described in terms of mean (standard deviation) for continuous data, and percentages for categorical data.
To assess genetic polymorphisms in inflammatory markers such as tumor necrosis factor alpha (TNF-α), transforming growth factor beta 1 (TGF-β1), interleukin 10 (IL-10), interleukin 6 (IL-6), interferon gamma (IFN-γ), DNA extraction was performed from lone AF, CMS, AF + CMS, and control patients whose clinical data is described in Table 1. The levels of these genetic polymorphisms were stratified to high, intermediate and low (Table 2) and the associations between these classes and the groups (AF, AF+CMS, and CMS) were determined using Fisher's Exact tests to be on the conservative side when reporting significant associations especially in the presence of small cell count and expected cells that are <5. Genotyping showed high percentages of patients in the AF, AF+CMS, CMS groups with high secretor genotypes of TGF-β1 (81.48, 70, 72.73%, Fisher's exact test P = 0.19) but it did not reach statistical significance. Genotyping showed high percentages of patients in the AF, AF+CMS, CMS groups with high secretor genotypes of IL-6 (95.65, 60, 86.67%, Fisher's exact test P = 0.037), respectively as shown in Table 2. IL-10 stratified levels were associated with the AF, AF+CMS, CMS groups with Fisher's exact test P = 0.05 (Table 2). Genotyping of IFN-γ and IL-10 at high level showed an increase in expression in the AF + CMS group compared to AF and CMS alone suggesting an imbalance between the inflammatory and anti-inflammatory cytokines which is exacerbated by AF.
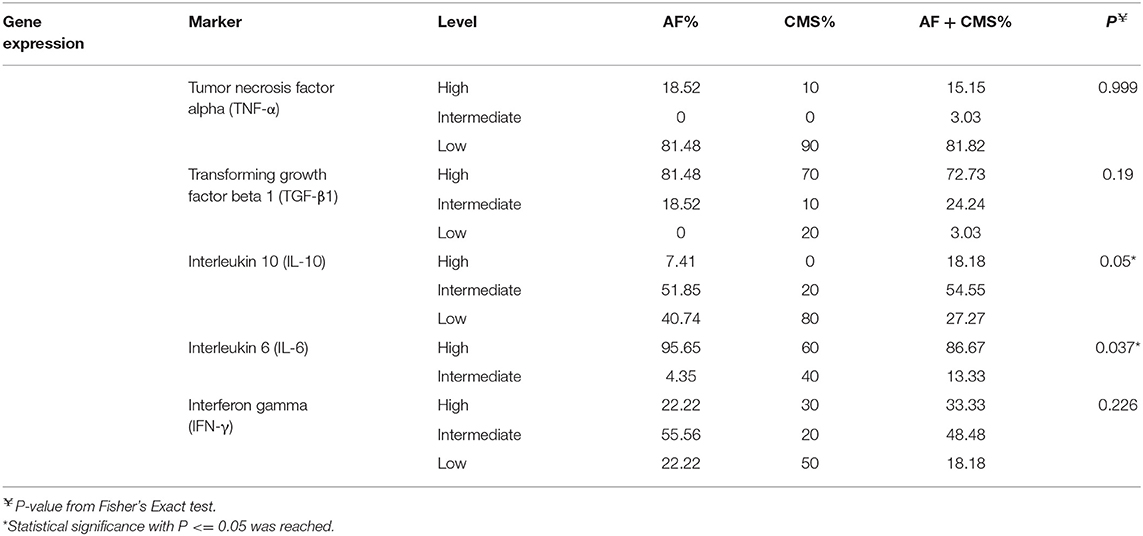
Table 2. Genetic polymorphisms in inflammatory (TNFα, TGF-β, IL-10, IL-6, and IFN-γ) stratified to high, intermediate and low levels in patients with AF, CMS, and AF +CMS.
Serum levels of connective tissue growth factor were tested along with other inflammatory fibrotic markers including connective tissue growth factor (CTGF), platelet-to-lymphocyte ratio (PLR) and monocyte-to-HDL ratio (MHR). CTGF levels were not statistically significant among the groups (Kruskal-Wallis test P = 0.227); there was a trend in the CTGF levels between the AF and AF+CMS group (Mann-Whitney test P = 0.084). PLR was highest in groups with CMS and lowest in the AF group but was not statistically significant (Kruskal-Wallis test P = 0.75, and Mann-Whitney test P > 0.05) for all group comparisons. The monocyte-to-HDL ratio (MHR) was significantly different between groups (Krukal-Wallis test P = 0.005). MHR was significantly different in particular between AF and AF+CMS groups (unadjusted Mann-Whitney test P = 0.002 and adjusted P = 0.006 for multiple comparisons). Significant difference in MHR was also detected between AF and CMS groups with Mann-Whitney test P = 0.029 unadjusted for multiple comparisons; however, the Mann-Whitney test adjusted for multiple comparisons was not significant with P = 0.087. No significant difference in MHR was detected between the AF+CMS and the CMS group (unadjusted Mann-Whitney test, P = 0.816).
To assess the level of inflammatory cytokines in the above cohort, human serum cytokine levels were quantified in patients with lone AF, CMS, and AF + CMS. Serum interleukin levels of IL-4, IL-5, IL-10, and IL-17F and IL-22 were significantly different in all groups (Table 3 and Appendix 1-Tables a,b, Appendix 2 Figures 1a–e). The remaining ILs did not exhibit a significant difference across the groups.
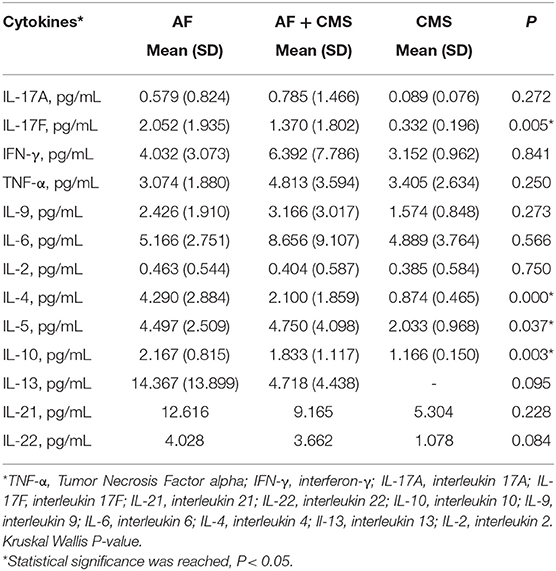
Table 3. Inflammatory cytokine mean levels in AF, CMS, and AF + CMS patients along with corresponding standard deviations (SD).
The summary statistics for IL-4 across the three groups: AF, AF+CMS, and CMS depicted in Appendix1-Table A, showed that IL-4 was highest in AF group compared to AF+CMS and CMS group (4.290±2. vs. 2.100±1.859 and 0.874± 0.465 pg/mL, respectively). Kruskal Wallis test was carried out in order to determine if there was an overall group effect on IL-4 levels between the three different groups (AF, AF+CMS, CMS). Two of the groups had significant difference in IL-4 (P < 0.0001, Kruskal Wallis; Table 3 and Appendix 1-Table b). To compare the level of IL-4 between all the groups in a pairwise manner (Appendix 1-Table b), we performed Mann-Whitney U tests and presented unadjusted and adjusted P-values for multiple comparisons. Our results indicated that there were unadjusted significance in two pairwise comparisons (AF and AF+CMS; AF and CMS (P < 0.05), and one insignificant pairwise comparison between AF+CMS and CMS (P > 0.05). This indicated that there was a difference in IL-4 between AF and the remaining two groups which are AF+CMS and CMS. IL-4 was higher in AF compared to that of AF+CMS group (4.290 vs. 2.100, unadjusted P = 0.001; adjusted P = 0.006) as shown in Appendix 1 Tables a,b. In addition, IL-4 was higher in AF compared to the CMS group (4.290 vs. 0.874 pg/mL, unadjusted P = 0.0001; adjusted P = 0.0001). The remaining comparison, between AF+CMS and CMS were insignificant with Mann-Whitney test unadjusted P = 0.06 and adjusted P = 0.256, respectively.
The summary statistics for IL-5 across the three groups: AF, AF+CMS, and CMS displayed in Appendix1-Table a showed that IL_5 was highest in AF+CMS group compared to lone AF and CMS group (4.497±2.509 vs. 4.750±4.098 and 2.033± 0.968 pg/mL, respectively). To determine if there was an overall group effect on IL-5 between the three different groups (AF, AF+CMS, CMS), we performed a Kruskal Wallis test which indicated that at least two groups had significant difference in IL-5 (P = 0.037, Table 3 and Appendix 1-Table b). To compare the IL-5 between all the groups in pairwise manner (Appendix 1-Table b), we performed Mann-Whitney U tests and presented unadjusted and adjusted P-values for multiple comparisons. Our results showed unadjusted Significance (P < 0.05) in one pairwise comparison among AF and CMS groups, and two insignificant in two pairwise comparisons between AF and AF+CMS and between AF+CMS and CMS group (P > 0.05). This indicated that IL-5 was higher in AF compared to CMS group (4.497 vs. 2.033 pg/mL, unadjusted P = 0.004). However, after we adjusted for the multiple comparisons, AF and CMS groups continued to show significant difference in IL-5 values with Adjusted P = 0.034.
The summary statistics for IL-10 across the three groups AF, AF+CMS, and CMS were depicted in Appendix 1-Table a showed that IL-10 was highest in AF group compared to AF+CMS and the CMS group(2.167±0.815 vs. 1.833±1.117 and 1.166± 0.150 pg/mL, respectively). To determine if there was an overall group effect on IL-10, Kruskal Wallis test (Table 3 and Appendix 1-Table b) was performed that indicating that at least two groups had significant difference in IL-10 (P = 0.003). Mann- Whitney U test was carried out to compare the IL-10 between all the groups (Appendix 1-Table b) and unadjusted and adjusted P for multiple comparisons were reported. Our results indicate significance in all three pairwise comparisons (unadjusted P < 0.05). This indicates that IL-10 was highest in the AF groups compared to the AF+CMS group (2.167 vs. 1.833, unadjusted P = 0.035) and highest in AF compared to the CMS group (2.167 vs. 1.166 pg/mL, unadjusted P = 0.002). In addition, our results indicated that IL-10 was highest in AF+CMS compared to the CMS group (1.833 vs. 1.166 pg/mL, unadjusted P = 0.039). However, after we adjusted for the multiple comparison, only AF and CMS groups had significant difference in the IL-10 (adjusted P = 0.003). The remaining comparisons between AF and AF+CMS, and AF+CMS and CMS lost the significance difference that was caught before the adjustment for multiple comparisons. These groups exhibited non-significant difference in the IL-10 after adjustment for multiple comparisons (AF and AF+CMS; AF+CMS and CMS, adjusted P = 0.16) (Appendix 1-Table b).
The summary statistics for IL-17F across the three groups AF, AF+CMS, and CMS (Appendix 1-Table a) showed that IL_17F was highest in AF group compared to AF+CMS and the CMS group (2.052±1.935 vs. 1.370±1.802 and 0.332± 0.196 pg/mL, respectively). To determine if there was an overall group effect on IL-17F, Kruskal Wallis test (Table 3 and Appendix 1-Table b) was carried out between the three different groups AF, AF+CMS, and CMS indicating that at least two groups had significant difference in IL-17F (P = 0.005). Mann- Whitney U test was carried out to compare the IL-17F values between all the groups (Appendix 1-Table b), and unadjusted and adjusted P for multiple comparisons were presented. Our results indicate significance in two pairwise comparisons (AF and AF+CMS; AF and CMS, Unadjusted P < 0.05) and insignificant pairwise comparison (AF+CMS and CMS, unadjusted P = 0.05). This indicated that IL-17F was higher in AF compared to the AF+CMS group (2.052 vs. 1.370 pg/mL, unadjusted P = 0.039); and higher in AF compared to the CMS group (2.052 vs. 0.332, unadjusted P = 0.001). However, after we adjusted for multiple comparisons, only AF and CMS groups had significant difference in the IL-17F (adjusted P = 0.005). The remaining comparisons between AF and AF+CMS groups, and the CMS and AF+CMS groups lost the significant differences that were caught before the adjustment for multiple comparisons and showed non-significant difference in the IL-17F after adjustment for multiple comparisons (multiple comparison, adjusted P = 0.102 for the comparisons between AF and AF+CMS, and P = 0.326 for the comparisons between AF+CMS and CMS).
IL-18 level was assessed by ELISA and was not significant across the groups AF (262 pg/mL), AF+CMS (234 pg/mL), CMS (191 pg/mL) and control (224 pg/mL).
The summary statistics for IL-22 across the three groups AF, AF+CMS, and CMS displayed in Appendix 1-Table a, showed that IL_22 was highest in AF group compared to AF+CMS and the CMS group (4.028±4.508 vs. 3.662±4.639 and 1.078± 1.475 pg/mL, respectively). Kruskal Wallis test carried out in order to determine if there was an overall group effect on the IL-22 between the three different groups (Table 3 and Appendix 1-Table b). Our results indicated that none of the groups had significant difference in IL-22 after adjusting for multiple comparisons (P = 0.084). IL-22 levels were insignificant between all the groups in pairwise manner (Appendix 1-Table b) P > 0.05 in two comparisons (AF and AF+CMS, unadjusted P = 0.992 for multiple comparisons; AF and CMS, P = 0.076). However, IL-22 was significantly higher in the AF+CMS compared to the CMS group (3.662 vs. 1.078 pg/mL, unadjusted P = 0.021 for multiple comparisons). However, this significant difference in IL-22 between the AF+CMS and CMS group was no longer present after adjusting for multiple comparisons.
In line with gene expression data, the cytokine serum levels revealed a trend of high levels of IL-6, IFNγ, TNFα, and IL-17A in blood samples drawn from patients with AF CMS compared to patients with CMS or AF alone (Table 3). These findings suggest that CMS promotes further the inflammatory process in AF patients. To assess the imbalance of inflammation in those patients, we measured cytokines contributing to the anti-inflammatory responses and protective pathways involved in tissue repair. Furthermore, we found that IL-4, IL-10, IL-13, IL-17F were significantly higher in patients with AF compared to AF CMS patients (Table 3). There was a trend in higher levels of IL-21, IL-22 in AF patients alone but didn't reach significance as shown in Table 3. In summary our results showed that when a group effect was present on any IL, this effect was mainly triggered by AF since it was the group that exhibited the difference in ILs when compared to AF+CMS and the CMS groups.
To determine whether serum levels of these cytokines correlated with atrial remodeling, we studied correlations between cytokine levels and parameters in the selected cohort of patients. The inflammatory markers that were significantly associated with the groups (AF, AF+CMS, and CMS) were considered in our multivariable analyses that were adjusted for clinical and demographic characteristics. These parameters included HDL, LDL, total cholesterol, SBP, DBP, age, gender and ever smoked. AF was taken as our reference group. The IL were all transformed to logarithmic scale to achieve symmetry and multivariable linear regressions were carried out whereby the ILs were modelled as functions of the aforementioned clinical and demographic patients' characteristics. Significant ILs at the univariable level of analysis which were considered in the multivariable analyses included IL-4, IL-5, IL-10, and IL-17-F. Results of the multivariable analysis were displayed in Table 4.
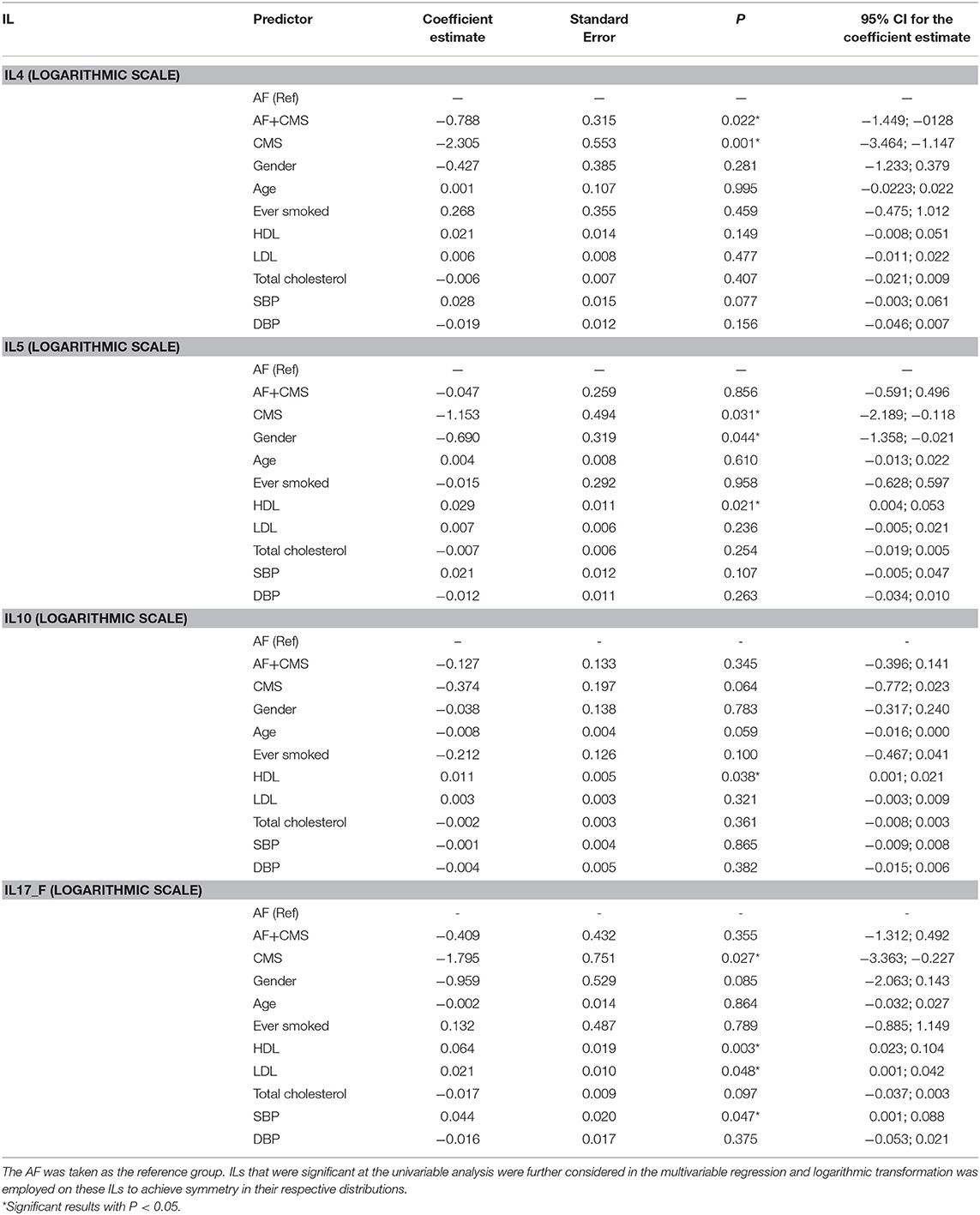
Table 4. Multivariable analysis with IL being the outcome of interest and group (AF, AF +CMS, and CMS) as the main predictor, and clinical and demographic characteristics as covariates to factor for in the analysis.
Our results showed that when adjusting for age, gender, ever smoked, HDL, LDL, total Cholesterol, SBP and DBP, a significant difference in IL-4 level was detected between AF group and AF+CMS, and between AF and CMS group, the AF+CMS had reduced levels of IL4 by 2.19 units compared to AF group (P = 0.022) as shown in Table 4. Moreover, the CMS group also exhibited a decrease in IL-4 by 9.97 pg/mL compared to AF (P = 0.001). The clinical and demographic characteristics were not significantly associated with IL-4. With respect to IL-5 the adjusted analysis showed that IL-5 exhibited a significant difference between AF and CMS groups. The CMS group had a decrease in the mean IL-5 by 3.15 pg/mL (P = 0.031) compared to AF. Gender was shown to be significantly associated with IL-5 with females having a decrease in IL-5 by 1.99 units compared to males (P = 0.044). HDL was shown to have an incremental association with IL-5 whereby IL-5 increases by 3% when HDL increases by 1 pg/mL (P = 0.021). IL-10 was not significantly different between the different groups. Only HDL showed a significant incremental association with IL-10 whereby IL-10 increased by 1% when HDL increased by 1 mg/dL (P = 0.038). Age had a borderline significant inverse association with IL-10 (P = 0.059) whereby IL-10 was shown to decrease by about 1% when age increased by 1 year. IL-17F was shown to be significantly different between the AF and CMS group (P = 0.027). CMS exhibited a decrease in the mean IL-17F by 5.98 pg/mL compared to AF. SBP, HDL and LDL were also significantly associated with IL-17F (P-values were, respectively 0.047, 0.003, and 0.048) as shown in Table 3.
Recently, many studies emphasize a pathogenic link of inflammation to the development of AF and CMS. Here, we show that activated immune mediators play a key role in the pathogenesis of AF that is aggravated in combination with CMS due to an imbalance in pro-inflammatory profile and anti-inflammatory cytokine response. Th-17 cells, a unique subset of CD4+ T cells, are associated with an increase in IL-17A, IL-17F, IL-21, IL-22 and IFNγ production, and that these cytokines play important roles inflammation, autoimmunity, host defense, and tissue repair (Figure 1). IL-21 is a pleiotropic cytokine with effects on innate and adaptive immune cells and has been shown to promote Th-17 and Th-1 cells and inhibit Treg cells and has synergistic effects with IL-17A or IL-17F. IL-17A a pleiotropic pro-inflammatory cytokine, has been implicated in promoting a pro-inflammatory response and fibrosis and among signature Th-17 derived effector cytokines. Pro-inflammatory cytokines produced by immune cells such as IL-6 not only induce platelet activation but are also associated with adverse outcomes in AF patients. CRP has been considered a downstream marker of the inflammatory cascade, specific cytokines such as IL-6 and TNF-α have also been linked to AF. IL-6 is a pleotropic cytokine that mediates a variety of biological activities including pro-inflammatory responses and stimulates the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway. IL-6 is produced by immune cells such as leukocytes and fibroblasts as well as non-immune cells such as endothelial cells, vascular smooth muscle cells and ischemic cardiomyocytes. Previous studies are consistent with our results showing an increase in IL-6 plasma levels in AF. TNF-α secreted by macrophages including those in fat and leukocytes induces activation of the transcription factor nuclear factor (NF)-kB. Thus, both cytokines exhibit a pleiotropic pro-inflammatory response, including myocyte and fibroblast differentiation, proliferation, and migration. Among other “adipokines,” IL-6 and TNF-α are also shown to promote CMS, thus, dysregulation of adipokine synthesis and release plays a critical role in insulin resistance. On the other hand, IL-4 is known to suppress the production of some inflammatory cytokines from immune cells and has been shown to promote tissue repair. The elevated levels of IL-4 in blood samples from AF patients compared to combined CMS AF patients highlights IL-4 role in anti-inflammatory responses; thus, less contribution to the inflammation in CMS AF patients. The balance between anti-inflammatory and inflammatory cytokines, such as IL-10 and TNF-α (tumor necrosis factor α), is also associated with AF recurrence. Inducing inhibitory markers or activating pathways that suppress inflammation including IL-10 may be protective and a potential therapeutic strategy for AF that could attenuate adverse cardiac effects.
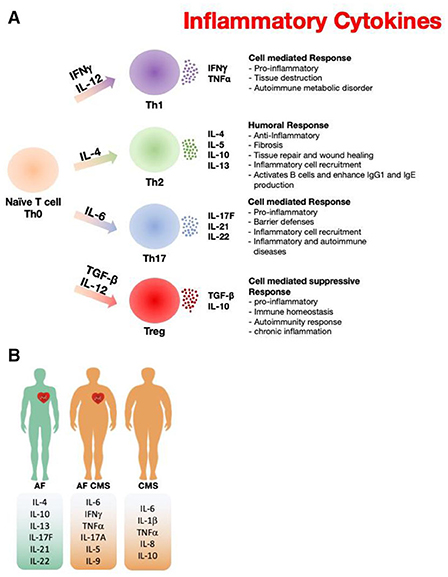
Figure 1. Paradigm illustrating the role of inflammatory markers in atrial fibrillation and cardiometabolic syndrome. (A) The inflammatory cytokine released from different types of T cells that have been reported in AF and CMS. (B) Differences of the inflammatory cytokine profile among AF, CMS, and AF+CMS recruited patients.
Despite that both the electrophysiology and structural properties of the atria are critically affected by inflammatory processes, up till today, the anti-inflammatory drugs remain unsatisfactory. Various inflammatory cascades underlying AF may also differ between patients due to genetic polymorphisms such as IL-1, IL-6, and IL-10. For instance, AF patients with high levels of IL-6 owing to its gene polymorphism altering its gene expressions levels as we have shown int his study, did contributes to the pathogenesis of AF. Thus, novel therapies targeting IL-6 in addition to modification of other factors may prove to be beneficial for this disease.
In parallel, an accumulating body of evidence indicates that inflammatory pathways not only interfere with ion channel function of myocytes, but also regulate extracellular homogeneity of atrial tissue and fibrosis. Inflammation is a crucial indicator of fibrosis because of inflammatory signals such as NADPH oxidase, ROS production, cytokines, growth factors, angiotensin II as well as mechanical stretch provoke fibroblast proliferation, migration and differentiation into myofibroblasts. The latter are the principle subtypes in the diseased atrial myocardium to produce cytokines, TGF and MMPs. In this study, genotyping showed high percentages of patients with high secretor genotypes of IL-6 and TGF-β1.
The platelet-to-lymphocyte ratio (PLR) is considered as a new biomarker for predicting inflammation (18). Elevated platelets trigger the infiltration of neutrophils, monocytes, and lymphocytes to the vasculature and hence it is correlated with bad prognosis in CVD (19, 20). On the other hand, monocyte to high-density lipoprotein (HDL) ratio (MHR) is also an inflammatory biomarker predictive for many CVD (21). Monocyte-to-high density lipoprotein ratio (MHR) has been proposed as a novel prognostic indicator of cardiovascular diseases based on the pro-inflammatory effect of monocyte and anti-inflammatory effect of HDL. It has been reported to be related to cardiovascular outcomes in patients with chronic kidney disease and the recurrence of atrial fibrillation. In our present study, interestingly CMS in addition to AF (CMS +AF) displayed a higher MHR ratio suggesting a higher inflammatory response compared to CMS and AF alone. Red blood cell distribution width (RDW) measures the volume range of variation of red blood cells (RBC) (22). The pro-inflammatory state is associated with high RDW (23). As a consequence, RDW was found to be a significant predictor for the development of atrial fibrillation (24).
A combination of both CMS and AF may be associated with a higher degree of inflammation in patients than what is seen in either CMS or AF alone. The discovery of a single inflammatory marker or inflammatory pathway contributing to AF may be a promising in the management of these diseases.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by AUB Institution Review Board. The patients/participants provided their written informed consent to participate in this study.
BA-S, MK, STA, SA, and MR helped in the patients' recruitment for this study. JE, MS, PZ, JB, AB, RI, MA, and MG helped in the human samples and data collection. HI, MA, AJ, and MR analyzed serum inflammatory cytokine and connective tissue growth factor levels. RM supervised the gene polymorphisms analysis of the inflammatory markers. MR, MAJ, and HI conceptualized the manuscript and the different sections included. MAJ carried out the statistical analysis of the data including the descriptive, non-parametric and multivariable analyses, prepared the tables that displayed the results of these analyses and the boxplots figures, and wrote and interpreted the statistical methods and results in the manuscript. MR supervised the study.
This study has been supported by the Lebanese National Council for Scientific Research (CNRS-L)—Grant Research Programme (2014). American Society of Nephrology (ASN), Foundation for Kidney Research (HAI) to funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.613271/full#supplementary-material
1. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
2. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. (2017) 11:215–25. doi: 10.1177/1753944717711379
3. Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, Dalleck LC. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: a pooled analysis. Prev Med Rep. (2017) 7:211–5. doi: 10.1016/j.pmedr.2017.07.004
4. Mendrick DL, Diehl AM, Topor LS, Dietert RR, Will Y, La Merrill MA, et al. Metabolic syndrome and associated diseases: from the bench to the clinic. Toxicol Sci. (2018) 162:36–42. doi: 10.1093/toxsci/kfx233
5. Grandl G, Wolfrum C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol. (2018) 40:215–24. doi: 10.1007/s00281-017-0666-5
6. Brown AE, Walker M. Genetics of insulin resistance and the metabolic syndrome. Curr Cardiol Rep. (2016) 18:75. doi: 10.1007/s11886-016-0755-4
7. Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int J Med Sci. (2016) 13:25–38. doi: 10.7150/ijms.13800
8. Westerman S, Wenger N. Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev. (2019) 15:136–44. doi: 10.2174/1573403X15666181205110624
9. Chung MK, Refaat M, Shen WK, Kutyifa V, Cha YM, Di Biase L, et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. Apr. (2020) 75:1689–713. doi: 10.1016/j.jacc.2020.02.025
10. Naccache S, Ben Kilani M, Tlili R, Ben Ameur Y, Boujnah MR. Atrial fibrillation and hypertension: State of the art. Tunis Med. (2017) 95:455–60.
11. Hucker WJ, Hanley A, Ellinor PT. Improving atrial fibrillation therapy: is there a gene for that? J Am Coll Cardiol. (2017) 69:2088–95. doi: 10.1016/j.jacc.2017.02.043
12. Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. (2005) 105:315–8. doi: 10.1016/j.ijcard.2005.02.050
13. Tadic M, Ivanovic B, Cuspidi C. What do we currently know about metabolic syndrome and atrial fibrillation? Clin Cardiol. (2013) 36:654–62. doi: 10.1002/clc.22163
14. Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. (2008) 117:1255–60. doi: 10.1161/CIRCULATIONAHA.107.744466
15. Tanner RM, Baber U, Carson AP, Voeks J, Brown TM, Soliman EZ, et al. Association of the metabolic syndrome with atrial fibrillation among United States adults (from the REasons for Geographic and Racial Differences in Stroke [REGARDS] Study). Am J Cardiol. (2011) 108:227–32. doi: 10.1016/j.amjcard.2011.03.026
16. Vural Ü, Aglar AA. What is the role of metabolic syndrome and obesity for postoperative atrial fibrillation after coronary bypass grafting? BMC Cardiovasc Disord. (2019) 19:147. doi: 10.1186/s12872-019-1130-3
17. Kumar P, Gehi AK. Atrial fibrillation and metabolic syndrome: understanding the connection. J Atr Fibrillation. (2012) 5:647. doi: 10.4022/jafib.647
18. Turkmen K, Erdur FM, Ozcicek F, Ozcicek A, Akbas EM, Ozbicer A, et al. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. (2013) 17:391–6. doi: 10.1111/hdi.12040
19. Azab B, Shah N, Akerman M, McGinn JT Jr. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. (2012) 34:326–34. doi: 10.1007/s11239-012-0718-6
20. Langer HF, Gawaz M. Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost. (2008) 99:480–6. doi: 10.1160/TH07-11-0685
21. Zhang Y, Li S, Guo YL, Wu NQ, Zhu CG, Gao Y, et al. Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med. (2016) 48:305–12. doi: 10.3109/07853890.2016.1168935
22. Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. (1991) 9(Suppl 1):71–4. doi: 10.1016/0736-4679(91)90592-4
23. Emans ME, Gaillard CA, Pfister R, Tanck MW, Boekholdt SM, Wareham NJ, et al. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int J Cardiol. (2013) 168:3550–5. doi: 10.1016/j.ijcard.2013.05.002
Keywords: cardiac arrhythmia, cardiovascular diseases, heart diseases, atrial fibrillation, inflammatory markers, metabolic syndrome, gene expression
Citation: Itani HA, Jaffa MA, Elias J, Sabra M, Zakka P, Ballout J, Bekdash A, Ibrahim R, Al Hariri M, Ghemrawi M, Abi-Saleh B, Khoury M, Alam S, Mahfouz R, Jaffa AA, Azar ST and Refaat MM (2020) Genomic and Proteomic Study of the Inflammatory Pathway in Patients With Atrial Fibrillation and Cardiometabolic Syndrome. Front. Cardiovasc. Med. 7:613271. doi: 10.3389/fcvm.2020.613271
Received: 01 October 2020; Accepted: 10 November 2020;
Published: 02 December 2020.
Edited by:
Raffaele Altara, Oslo University Hospital, NorwayReviewed by:
Rod Passman, Northwestern University, United StatesCopyright © 2020 Itani, Jaffa, Elias, Sabra, Zakka, Ballout, Bekdash, Ibrahim, Al Hariri, Ghemrawi, Abi-Saleh, Khoury, Alam, Mahfouz, Jaffa, Azar and Refaat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miran A. Jaffa, bXMxNDhAYXViLmVkdS5sYg==; Marwan M. Refaat, bXI0OEBhdWIuZWR1Lmxi
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.