- 1Guilin Medical College, Guilin, China
- 2Shanghai Key Laboratory of Bioactive Small Molecules, Department of Pharmacology, School of Pharmacy, Fudan University, Shanghai, China
- 3State Key Laboratory of Quality Research in Chinese Medicine and School of Pharmacy, Macau University of Science and Technology, Macau, China
Myocardial ischemic injury is among the top 10 leading causes of death from cardiovascular diseases worldwide. Myocardial ischemia is caused mainly by coronary artery occlusion or obstruction. It usually occurs when the heart is insufficiently perfused, oxygen supply to the myocardium is reduced, and energy metabolism in the myocardium is abnormal. Pathologically, myocardial ischemic injury generates a large number of inflammatory cells, thus inducing a state of oxidative stress. This sharp reduction in the number of normal cells as a result of apoptosis leads to organ and tissue damage, which can be life-threatening. Therefore, effective methods for the treatment of myocardial ischemic injury and clarification of the underlying mechanisms are urgently required. Gaseous signaling molecules, such as NO, H2S, H2, and combined gas donors, have gradually become a focus of research. Gaseous signaling molecules have shown anti-apoptotic, anti-oxidative and anti-inflammatory effects as potential therapeutic agents for myocardial ischemic injury in a large number of studies. In this review, we summarize and discuss the mechanism underlying the protective effect of gaseous signaling molecules on myocardial ischemic injury.
Introduction
Ischemic injury is caused mainly by anerobic cell death and reperfusion (1). Endothelial dysfunction, microvascular collapse, and blood flow defects are preconditions for phenotypic expression of ischemic injury (2), which is mediated by a variety of cytokines, chemokines, and adhesion molecules, as well as extracellular matrix compounds (3). Ischemic injury causes damage to a variety of organs and tissues, such as the brain (4, 5), liver (6), intestines (7), limbs (8), and heart (9). Myocardial ischemic injury is one of the most common and serious diseases that endangers human health. It is usually caused by coronary artery stenosis or occlusion caused by coronary atherosclerosis. Acute and temporary myocardial ischemia and hypoxia can cause angina pectoris. Persistent and severe myocardial ischemic injury can cause myocardial necrosis or myocardial infarction (MI) and even heart failure (HF).
In this review, we introduce gaseous signaling molecules and summarize the mechanism of their protective effects against myocardial ischemic injury. The gaseous signaling molecules include NO, H2S, H2, the gas joint donor, such as ZYZ-803, and other gas donor molecules. The aim of this review is to provide a more comprehensive understanding of gaseous signaling molecules and to promote further research to clarify their potential clinical application for the prevention and treatment of myocardial ischemic injury.
Treatment of Myocardial Ischemic Injury
The current, treatments for myocardial ischemic injury mainly include surgical, and drug approaches. Surgical treatments include percutaneous coronary intervention (PCI) (10) and coronary artery bypass graft (CABG) (11). PCI is a procedure used to improve myocardial perfusion by cardiac catheterization to open a narrow or even occluded coronary artery lumen (12). CABG refers to the establishment of a vascular pathway between the root of the ascending aorta and the distal obstruction of the diseased coronary artery using transplanted blood vessels, to achieve blood flow recanalization by bypassing the lesion site in the coronary artery (13). Drug therapies include conventional Western drugs and conventional Chinese medicines. The main Western drugs such as the anti-platelet drug aspirin, and nitroglycerin, used to treat myocardial ischemic injury are listed in Table 1. Traditional Chinese medicines, include heart-protecting musk pill, which has a role in preventing ventricular remodeling in patients with acute MI (22) and in protecting against arteries atherosclerosis (23), and qishen yiqi drop pill (QSDP), which has a role in improving cardiac function after myocardial ischemic (24). At present, however, traditional Chinese drugs are generally not recommended as the main treatment for myocardial ischemic, although they can be used as an adjunct to Western medicine therapy. Although conventional drugs have been used, the plethora of side effects and contraindications, as well as the limited availability of raw materials, have led researchers to focus on new drug candidates.
Source of Gaseous Signaling Molecules
Current studies have shown that gas signaling molecules mediate certain inhibitory effects on oxidative stress (25), apoptosis (26), inflammation (27), and autophagy (28), and have protective effects on many organs, including the heart (29). Compared with conventional drugs, gas signaling molecules have smaller molecular weights that facilitate entry of the biofilm. Their effects are independent of the corresponding membrane receptors and cytological effects, and may or may not depend on mediation by a second messenger. Gas signaling molecules are also derived from a wide variety of sources in nature, such as garlic and onion, and they play an important role in humans. Pan et al. (30) reported that the sulfide S-ally-L-cysteine (SAC) contained in garlic can be used as an H2S donor drug for cardiac protection. Specifically, garlic has been shown to mediate cardiac protective effects such as reduction of the blood lipids and blood pressure, and is a source of antioxidants (which scavenge free radicals and inhibit lipid peroxidation) (31). Furthermore, it has been reported that the antioxidant effect of garlic may be associated with “nucleophilic tone,” which is defined as “the capacity to remove electrophiles through enzyme catalyzed, the dynamic flow of reducing equivalents from NADPH, glutathione (GSH) and reduced thioredoxin (32).” Zhao et al. (33) showed that allicin in garlic alleviated myocardial ischemic injury by promoting autophagy. In 1990, Makheja et al. (34) identified adenosine, allicin and paraffin-based polysulfide as three main anti-platelet components of onion. Among them, adenosine acts as a trigger and regulator of cardioprotection (35). Moreover, Park et al. (36) found that a methanol extract of onion alleviated myocardial ischemic injury by reducing the ROS content of hypoxic cardiomyocytes. Thus, gaseous signaling molecules like NO, H2S, and H2 have strong potential in the protection of the heart against myocardial ischemic injury. NO, H2S, and H2 are gases at room temperature and exhibit unique properties and functions in nature and living organisms including organs such as the heart.
Mechanism of Myocardial Ischemic Injury
Free-Radical Action
The Mechanism Underlying the Increase in Oxygen Free-Radicals in Myocardial Ischemia
Xanthine oxidase (XO) and xanthine dehydrogenase (XD) are present in cardiomyocytes. XO is present in only 10% of normal cardiomyocytes, while XD is present in 90%. During coronary atherosclerosis or coronary artery embolization, mitochondrial permeability changes in the human cells lead to matrix swelling, rupture of the outer membrane, release of apoptotic signaling molecules, and irreversible damage to mitochondria (37). Under conditions of myocardial ischemia, on the one hand, dysfunction of the Ca2+ pump and changes in the oxidation state of thiols due to the decrease in ATP, XD is transformed into large amounts of XO in a reaction catalyzed by a Ca2+-dependent proteolytic enzyme. On the other hand, due to the decrease in oxygen partial pressure, ATP is degraded to ADP, AMP, and hypoxanthine, which accumulates in ischemic tissues. During treatment of myocardial ischemia, the process is often accompanied by reperfusion. During reperfusion, a large amount of molecular oxygen enters into the ischemic tissues along with the blood. XO once again catalyzes the conversion of hypoxanthine to xanthine and further catalyzes the conversion of xanthine to uric acid, thus producing large amounts of and H2 Furthermore, the reaction of .OH formed with the participation of metal ions is more intense with the diffusion control of most molecules (38) (Figure 1). In addition, hypoxia leads to decreased oxygen partial pressure and ATP production in cells, increased entry of calcium ions into the mitochondria, dysfunction of mitochondrial oxidative phosphorylation, electron transport chain damage, increased entry of oxygen free-radicals into cells, and reduced Mn-SOD, leading to reduced free-radical scavenging capacity, and therefore, increasing the local level of free-radicals. In addition, free radicals are produced by increased NADPH oxidase and peroxidase activity and catechol-amine autoxidation.
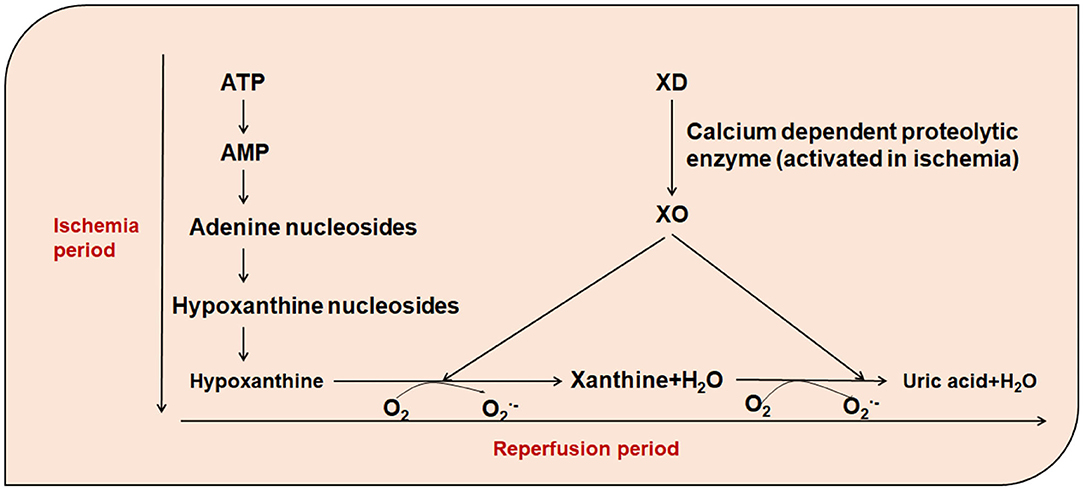
Figure 1. The role of xanthine oxidase in the presence of increased free-radicals. ATP, Adenosine triphosphate; AMP, Adenosine monophosphate; XO, Xanthine oxidase; XD, xanthine dehydrogenase; O2, oxygen; , Superoxide Anion.
The Mechanism by Which Free-Radicals Cause Myocardial Ischemic Injury
Dysfunction in free-radical removal systems, such as SOD enzymes, catalase, and ascorbic acid, results in excessive free-radical production that causes damage to biological macromolecules, such as nucleic acids, proteins and lipids, affecting their normal physiological functions. Cellular lipids, proteins and DNA also react directly with free-radicals, causing damage to the cell structure and dysfunction, accompanied by activation of the NF-κB signaling pathway (39).
Intracellular Calcium Ion Overload
Under normal conditions, the extracellular calcium ion concentration is 10,000 times higher than that inside the cell; a schematic diagram of the process of the transport of calcium ion transport is shown in Figure 2. Under conditions of myocardial ischemia, changes in intracellular and extracellular Ca2+ regulation lead to Ca2+ overload in the cytoplasm and mitochondrial matrix. The myocardial structure is damaged, function is reduced or electrophysiological disorders are caused by excessive contraction of energy-dependent muscle fibers. In addition, cell proteolysis is mediated by calpain, mitochondrial permeability transition pores are opened, inducing cell apoptosis and closure of gap junction channels, rendering cell activity out of synchronization with other pathways. Abnormal Na+-Ca2+ exchange (40), protein kinase C activation (41), and increased intracellular calcium ion levels during biofilm injury can lead to calcium ion overload in cardiac myocytes. Furthermore, the aggregation of intracellular calcium ions results in phospholipase activation and degradation, further increasing the permeability of cell membrane to calcium ions, and promoting membrane damage. In addition, during myocardial ischemia-reperfusion, intracellular calcium ion overload may cause excessive contraction of myocardial fibers, leading to arrhythmias and exacerbating the symptoms of myocardial injury.
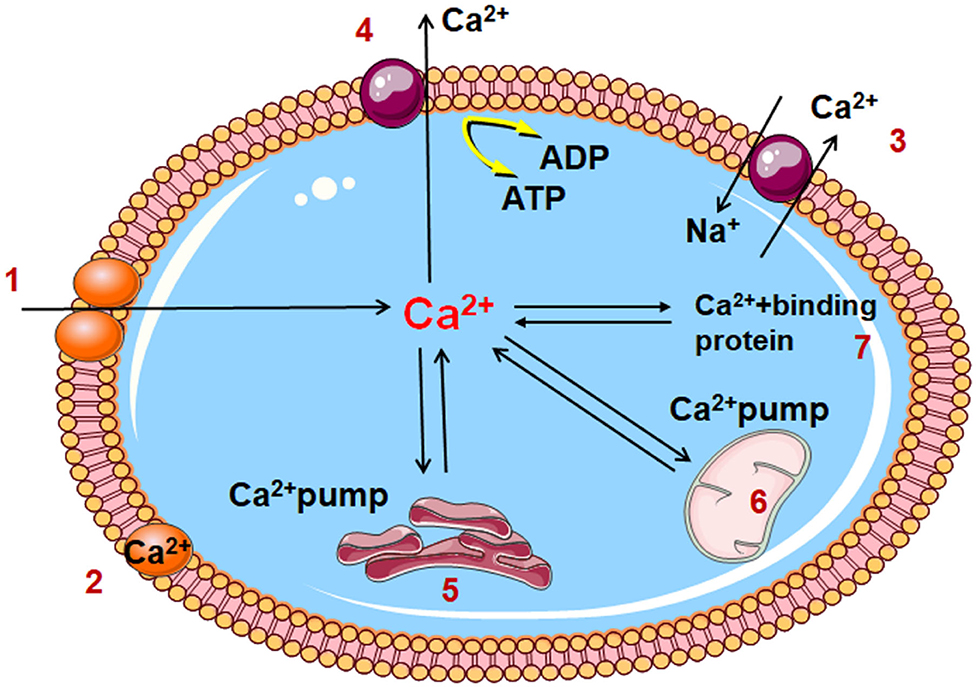
Figure 2. Diagram of cell calcium transport pattern 1. Voltage dependent calcium channel; 2. Cell membrane binding calcium; 3. Na+/ Ca2+ exchange; 4. Calcium channel of cell membrane 5. Sarcoplasmic reticulum; 6. Mitochondria; 7. Cytoplasmic binding calcium; A TP, Adenosine triphosphate; ADP, Adenosine diphosphate.
Endothelial Cells Appear to Have Intercellular Gaps Through Which White Blood Cells Migrate
Inflammation, which is the response of the body to pathogenic factors, comprises injury and anti-injury processes. Inflammatory mediators, such as histamine and bradykinin, adhesion of leukocytes, release of proteolytic enzymes and active oxygen metabolites, can cause endothelial cell mediating contraction/dilation of underlying smooth muscle cells, and movement between cells, resulting in increased osmotic pressures of cell crystals and colloids. At this point, the white blood cells migrate through the intercellular gaps. In experimental myocardial ischemic injury, pro-inflammatory cytokines, including TNF-α, IL-6, IL-1, participate in post-ischemic responses (42), although some inflammatory cytokines, such as COX1, mPGEs1, also protect against post-ischemic response (43).
Mechanism Underlying the Role of no in Myocardial Ischemic Injury
NO and the Myocardium
NO is a gas at room temperature, although it acts as a first messenger due to its fat-solubility. NO is also known as endothelium-derived vasodilator factor (EDRF). As a gaseous signaling molecule that is known for its vasodilatation effects (44). NO is a free-radical, which is relatively stable compared to most species, and exhibits a select spectrum of rapid and life-limiting reactions in biological systems. In vivo, NO synthesis requires L-arginine, oxygen, and NOS, in addition to the cofactors tetrahydrofolate (BH4), NADPH, flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), heme and calmodulin (45). Thus, NO synthesis from L-arginine and dioxygen is a multistep process catalyzed by the mammalian NOS isoenzymes in a unique active site constructed around heme and BH4 cofactors. NO is otherwise unable to dissociate from ferrous heme. The shift in the potential of the heme on NO binding from −300 to 0 mV in the presence of substrate indicates that the dissociation constant for NO binding to ferrous nNOSoxy is 0.17 nM. A similar interaction between substrate and heme-bound dioxygen increases the kinetic stability of the oxyferrous complex, slowing the rate of decay via electron transfer from BH4 or via superoxide release (46). There are three subtypes of NOS: endothelial (e-NOS), neuronal (n-NOS), and inducible NOS (i-NOS) (47). e-NOS is highly expressed in coronary vessels and endocardial endothelial cells (48). e-NOS/NO signaling can reduce the area of myocardial ischemia to alleviate adverse cardiac remodeling (49) and inhibit ROS and angiotensin II (ANG II)-induced endothelial cell apoptosis (50). NO also serves as an effective regulator of blood pressure and blood flow (51). The protective effect of NO on endothelial cells has also been confirmed in mice with e-NOS damage. The changes of NO are closely related to hypertension (52) and coronary heart disease (53). However, in the presence of e-NOS/NO, DNA synthesis in smooth muscle cells is inhibited (54, 55).Similar to e-NOS, n-NOS is also expressed in the cardiovascular system, primarily in the sarcoplasmic reticulum (56), although a small fraction is expressed in the mitochondria (57), Golgi bodies (58), and myofilms (57). n-NOS plays a key role in protecting the myocardium from oxidative stress, systolic/diastolic dysfunction, poor structural remodeling, and arrhythmias in failing hearts (59, 60). Furthermore, n-NOS/NO regulates cardiac electrophysiology and intracellular Ca2+ protein homeostasis, targeting myosin through S-nitrosylation and phosphorylation, dynamic regulation of mitochondrial activity and biological activity (61). i-NOS is expressed in cardiomyocytes in response to specific cytokines. The i-NOS/NO signaling pathway can reduce the occurrence of obvious HF, but excessive expression often causes cardiomyopathy, arrhythmias and even sudden cardiac death (62). In addition, NO metabolites include nitrite, which is an important repository of NO in blood and tissue, and nitroso mercaptan (63, 64). NO and nitroso mercaptan levels are reduced in ischemia or hypoxia (65). Circulating levels of these metabolites directly regulate their tissue storage (66), and increasing levels of nitrite and nitrosothiol in the heart is an effective cardiac protection strategy (67, 68). Currently, common exogenous NO donors include NaNO2 and furoxan, which releases NO in the presence of mercaptan.
Oxidative Stress and NO
The updated definition of “oxidative stress” is “an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage (69).” In general, NO has a protective effect on the damage caused by myocardial ischemia when appropriate amounts of are in equilibrium with antioxidants and oxygen free radicals (70). Tan et al. (71) demonstrated that NO is an effective antioxidant experimentally by evaluating the cardiac protective function of baicalein in rats with acute myocardial infarction (AMI). Furthermore, the possible molecular mechanism was explored by administration of baicalein and/or e-NOS inhibitor L-NAME before inducing AMI. Analysis of the corresponding indicators, such as creatine kinase, creatine kinase MB isoenzyme, lactate dehydrogenase and cardiac troponin T revealed that baicalin activated the e-NOS signaling pathway and inhibited oxidative stress in rats with AMI through e-NOS signal transduction. Xiao et al. (72) reported the same protective effect of NO in another study using luteolin as the experimental drug, in an experimental model of myocardial ischemic injury induced by diabetes. It was found that after activation by luteolin, e-NOS inhibited the binding of Nrf2 to Keap1, Nrf2 was then transferred to the nucleus, where it combined with antioxidant genes, such as ARE, to produce an antioxidant effect (72).
However, excessive NO can have harmful effects on the heart muscle. For example, Tao et al. (73) simulated myocardial ischemia-reperfusion in vivo by ligating the coronary arteries of mice for 30 min followed by reperfusion for 3 or 24 h. Comparison of the myocardial infarct size in mice, production of peroxynitrite, NO and superoxide as well as i-NOS and gp91phox protein expression, it was found that the adiponectin globular domain structure reduced the myocardial ischemia/reperfusion induced i-NOS/gp91phox protein expression, reduced the generation of NO/peroxide, blocked oxygen nitrite formation, and reversed adiponectin−/− mice to expand the infarction effect. Thus, it is clear that the balance of NO content is a very important factor for regulating ischemic injury.
Anti-apoptotic Effects and NO
Accumulating evidence shows that the NO system plays a key role in the regulation of myocardial cell apoptosis (74, 75). For example, Wang et al. (76) found that ginsenoside Rg3 (GSRg3) mediated myocardial protection and inhibited MI/R-induced apoptosis by up-regulating the Akt/e-NOS/NO signaling pathway. Liu et al. (77) found that hydromorphine administered post-treatment protected the isolated rat heart from reperfusion injury by activating P13K/Akt/e-NOS signal transduction. Moreover, downregulation of MIR-134 was also shown to activate the P13K/Akt/e-NOS signaling pathway to protect muscle I/R injury (78). Increased intracellular calcium production is the main mediator of myocardial cell apoptosis induced by ischemia (79). Therefore, the restoration of intracellular calcium inflow can effectively prevent ischemic myocardial cell apoptosis. Li et al. (79) showed that KMUP-1 [7-[2-[4-(2-chlorophenyl) piperazinyl] ethyl]−1] activated e-NOS expression and restored the intracellular calcium flow by upregulating the NO/cGMP/MAPK signaling pathway, which inhibited the apoptosis induced by myocardial ischemia. Similarly, phosphorylation and expression of e-NOS were increased.
The Anti-inflammatory Effects of NO
Inhibition of myocardial inflammation and pathological remodeling after MI injury is very important for the treatment of ischemic cardiomyopathy. The inflammatory factor COX, which is a rate-limiting enzyme of prostaglandin (PG), catalyzes the conversion of arachidonic acid to PGH2 (80). COX exists as COX1 in large amounts of normal cells and COX2, which is closely related to NO associated signaling pathways, is induced by stress (80). Shinmura et al. (81) showed that NO protects the ischemic myocardium by stimulating COX2 to produce cell-protective prostaglandins, such as PGE2 and PGI. In the later pretreatment, it was found that inhibition of i-NOS eliminated prostaglandin synthesis, while inhibition of COX2 had no effect on i-NOS activity (81), but resulted in loss of the protective effect, indicating that the activity of COX2 activity was driven by i-NOS. Pang et al. (82) found that COX2 inhibited AKT formation and promoted the production of NO in i-NOS, thereby preventing H9C2 cells harm due to hypoxia/reoxygenation. In addition to i-NOS, e-NOS also plays an important role in the anti-inflammatory pathway. For example, O-nitrophenyl ethyl caffeate (CAPE-oNO2) inhibits inflammation following myocardial ischemia-reperfusion injury via the e-NOS/NF-kB pathway (83).
In summary, a large amount of evidence (82–85) indicates that NO exerts an anti-inflammatory effect on MI by regulating the activity of inflammatory cytokines or being regulated by inflammatory cytokines.
Mechanism of H2S Protection Against Myocardial Ischemic Injury
Induction of H2S
Hydrogen sulfide (H2S) was the second gaseous signaling molecule discovered after NO. As with NO, the role of H2S in organs or tissues such as the kidney (86, 87), brain (88, 89), and heart (90, 91) has been documented and has been shown to play an important role in the regulation of cardiovascular activities. H2S is a colorless gas with the odor of rotten eggs (92). In vivo, the main enzymes responsible for H2S synthesis are cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST) (93). Yang et al. (62) confirmed that CSE is mainly expressed in the cardiovascular system by preparing CSE gene-deficient mice and measuring the H2S content. H2S levels in the aorta and arterioles were decreased by ~50 and 80%, respectively, in CSE-deficient mice compared with those in the wild-type mice and the serum H2S levels were also decreased by 50%, indicating that CSE is the main source of H2S in the cardiovascular system. Numerous studies (94–99) have shown that H2S preconditioning can significantly antagonize MI injury, reduce the MI area, reduce troponin I levels and the lever of oxidative stress, apoptosis and inflammation. Calvert et al. (99) showed that H2S increased the nuclear translocation of Nrf2 and upregulated the phosphorylation of PKCε and STAT3 in the early stage of pre-treatment. In the later stage of H2S pre-treatment stage, the expression of HO-1, thioredoxin 1 and heat shock protein 90 (HSP90) increased, and the activity of pro-apoptotic factors decreased (99). However, H2S does not usually play a direct antioxidant role. H2S is first dissociated as HS−, then HS− and then S2− in solution. The percentages of HS− and H2S in solution are 81 and 19%, respectively, while the concentration of S2− is almost negligible, indicating that most of the influence of H2S is mediated by thiols (100).
In vitro, commonly used H2S donors include NaHS, morpholine-4-methoxyphenylmorpholine-morpholine-phosphodisulfate (GYY4137), DATS-MSN and s-propargyl-cystine (SPRC), all of which have protective effects against MI. NaHS is the most commonly used H2S donor in vitro. For example, Li et al. (94) used NaHS as the H2S donor to demonstrate that H2S pre-treatment reduced ER/SR stress in the hypoxia/reoxygenation model in H9C2 rat cardiomyocytes and inhibited cardiomyocyte apoptosis. Similarly, Wang et al. (101) used NaHS as a donor to study the protective effect of H2S in rats with heart failure (HF). GYY4137 is a water-soluble H2S donor (102), which at physiological pH and temperature, releases low concentrations of H2S into in aqueous solution for several hours, conditions which simulate the time course of H2S release in vivo (103). In this way, GYY4137 protected the myocardium from I/R injury by reducing oxidative stress and apoptosis (104). DATS-MSN is a long-term sustained release H2S donor, which can prevent myocardial I/R injury (105). Compared with NaHS and GYY4137, Sun et al. (105) demonstrated that DATS-MSN provides superior cardiovascular protection, which may be related to the its capacity for long-term slow release of H2S. SPRC, which is a novel endogenous H2S water-soluble regulator synthesized by Zhu et al., promotes angiogenesis by activating signal transduction factors and transcriptional activators (106) (Figure 3).
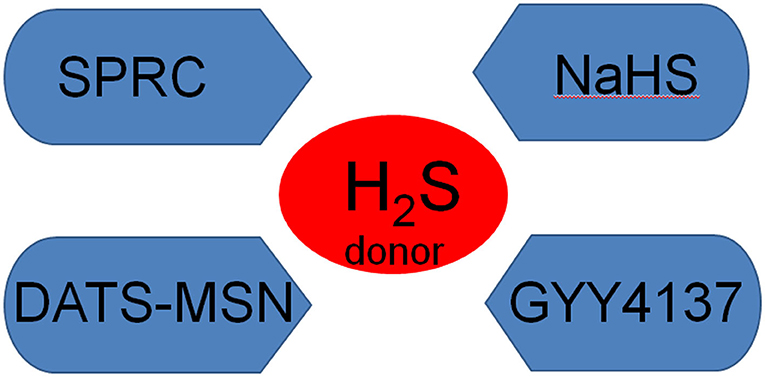
Figure 3. The main H2S donors NaHS, sodium hydrosulfide; GYY4137, morpholine-4-methoxyphenylmorpholine-morpholine-phosphodisulfate; SPRC, s-propargyl-cystine.
Antioxidant Effect of H2S
Studies have shown that ROS and Reactive nitrogen species (RNS), such as peroxides (·O2), can be eliminated by H2S at any time, and that the antioxidant activity of H2S is higher than that of cysteine, GSH and other antioxidants (107). However, the physiological concentration of H2S is much lower than that of other typical antioxidants (108). Nevertheless, exogenous H2S has been shown to have a strong antioxidant capacity and protect cells from damage in physiological systems exposed to ROS and RNS. In addition, despite the differences in concentration of gaseous signaling molecules, H2S is a small molecule with properties that allow it to pass freely through the cell membrane, thus enhancing its antioxidant activities compared with macromolecule antioxidants in the microenvironment (109). H2S exerts its antioxidant effects through extensive indirect signal conduction rather than direct effects on ROS/RNS. Thus, H2S treatment can provide far-reaching and long-lasting antioxidant protection in cells.
H2S exerts its antioxidant effects by regulating the expression and activity of classic antioxidants such as GSH and Thioredoxin (Trx). GSH, a tripeptide composed of glycine, glutamate, and cysteine, is one of the major antioxidants in cells. Furthermore, cysteine is synthesized from methionine via a thiogenic pathway to produce the key enzymes required for H2S synthesis (CSE and CBS) in each step of the catalytic reaction. Studies have shown that H2S promotes GSH production, thereby protecting the heart from oxidative stress (110). Trx is a small molecule (~12 kDa) containing a cysteine-glycine-proline-cysteine motif at its catalytic site. The two cysteine residues are the main sites for Trx oxidation and promote ROS reduction through thiol-disulfide exchange. Oxidized Trx is reduced by the Trx reductase and then reduced by NADPH. Trx activity has been shown to perform intracellular and extracellular functions in ROS elimination to protect against oxidative stress (111). A recent study showed that H2S treatment not only increased Trx expression in ischemic HF models, but also attenuated high-fat-induced left ventricular remodeling (112). Mice expressing dominant Trx-negative mutations did not respond to H2S treatment, indicating that the cardioprotective effect of H2S in this HF model is Trx-dependent. Finally, H2S has also been shown to regulate the expression of Trx interaction protein (TXNIP), which binds to and inhibits Trx activity, in endothelial cells (113).
As an important antioxidant stress transcription factor, Nrf2 regulates the expression of many antioxidant genes and cell protective genes. In a mouse model of myocardial ischemia, H2S preconditioning activated Nrf2 signaling, up-regulated the expression of antioxidant proteins HO-1 and thioredoxin 1, and reduced myocardial ischemic injury (99). It has been reported that myocardial mitochondria are the main sites of oxidative stress induction during myocardial ischemia-reperfusion (MI/R), with different responses to H2S regulation in suborganelles. Furthermore, MI/R damage to the submusculoskeletal mitochondria in rat heart plays an important role in reducing H2S mediated oxidative stress (114). The antioxidant function of H2S is due, in part, to the direct removal of ROS and/or inhibition of ROS production. Geng et al. (115) showed that H2S reduces lipid peroxidation by removing O2.− and H2O2 in isoproterenol-induced damaged myocardium. In hypoxic/reoxygenation rat cardiomyocytes, H2S reduces ROS levels and protects the myocardium by inhibiting the activity of mitochondrial complex IV, enhancing the activity of superoxide dismutase (SOD) enzymes, such as Mn-SOD and CuZnSOD (116).
OS plays an important role in the pathogenesis of HF. Oxidative stress leads to apoptosis, which may cause damage to cardiomyocytes. H2S is an effective ROS scavenger and has a protective effect on HF. Sirtuin-1 (SIRT1) is a highly conservative nicotinamide adenine dinucleotide (NAD)-dependent histone acetyl enzyme that plays a key role in promoting cell survival under conditions of oxidative stress. Using NaHS as the H2S donor, Wu et al. (117), showed that NaHS increased SIRT1 expression under conditions of oxidative stress, and reduced H9C2 cardiomyocyte apoptosis via the SIRT1 pathway.
Anti-apoptotic Effect and H2S
H2S has anti-apoptotic properties. In vivo studies have demonstrated that H2S activates pro-growth kinases, such as PKC/ERK1/2 and PI3K/Akt, and activation of PKC/STAT3 signals, leading to increased expression of anti-apoptotic molecules, such as Hsp90, Hsp70, and Bcl-2 (118, 119). Numerous studies (106, 120–125) have shown that H2S plays a protective role in ischemic myocardium through apoptosis-related signaling pathways. For example, Ning et al. (126) demonstrated that the H2S donor NaHS resisted AMI-induced apoptosis by upregulating the GSK-3β/β-catenin signaling pathway. NaHS reduced the apoptosis of myocardial cells after myocardial ischemia-reperfusion injury in rats by down-regulating the JNK signaling pathway (127). The CSE/H2S pathway mediates the protective effect of trimetazidine against hypoxia/reoxygenation induced apoptosis in H9C2 cells (128). Meng et al. found that pre-administration of GYY4137 as a H2S donor increased Bcl-2 expression in ischemic myocardium, while decreasing the expression of Bax, expression and caspase-3 activity, indicating that GYY4137 prevents myocardial ischemic injury (104) TUNEL assays showed that SPRC treatment for 30 min before hypoxia significantly reduced the apoptosis of isolated papillary muscle cells caused by hypoxia/reoxygenation injury and protected muscle morphology (129). Subsequent studies in HF rats showed that the expression of Bax induced by ischemia caused by ligation of the left coronary artery decreased the expression of Bcl-2, thereby triggering the activities of caspase 9 and caspase 3. The sustained release preparation of SPRC (CR-SPRC) increased Bcl-2 levels and reduced the levels of Bax, caspase 3 and caspase 9, thereby protecting myocardial cells (130). SPRC, which is also used as a H2S donor, enhanced cell activity, restored downstream gene expression regulated by GP130/STAT3, inhibited cell apoptosis, and antagonized mitochondrial dysfunction and intracellular Ca2+ overload in adriamycin-induced cardiac toxicity (130).
Moreover, genes involved in apoptosis regulation, such as microRNAs (miRNAs), can also act as important mediators that protect the ischemic myocardium. It was found that myocardial cell hypoxia/reoxygenation (HR) injury increased apoptosis, upregulated the expression of miRNA-1, and down-regulated the expression of Bcl-2 (131). However, H2S pretreatment reduced myocardial cells apoptosis after HR injury. Furthermore, this approach also down-regulated miRNA-1 expression and up-regulate Bcl-2 expression (131). MiRNA-133a is involved in the protective effect of H2S against myocardial cell apoptosis induced by ischemia/reperfusion (132). In addition, miRNA-208B-3P, miRNA-128-3P, and miRNA-320 are all related to myocardial ischemia and apoptosis, with down-regulation of miRNA-208B-3P inhibiting of myocardial injury caused by ischemia/reperfusion in rats (133), while inhibition of miRNA-128-3P protects myocardial cells from ischemia/reperfusion injury by upregulation of P70S6k1/P-P70S6k1 (134), and down-regulation of miRNA-320 inhibits apoptosis of myocardial cells and provides protection against myocardial ischemia and reperfusion injury by targeting IGF-1 (135). However, it is not known whether these miRNAs are related to H2S, and further research is needed to clarify this point.
Anti-inflammatory Effects and H2S
The anti-inflammatory action of H2S results from the inhibition of leukocyte rolling, adhesion and flipping. In addition, it inhibits NF-κB and reduces the production of the inflammatory cytokines IL-1 and TNF-α (136). The levels of TNF-α, IL-1, and IL-6 in the serum of rats were shown to be increased after AMI, and the levels of ICAM-1 mRNA and NF-κB in the myocardial tissues were significantly increased. However, the levels of these factors decreased after NaHS, which inhibited the synthesis of inflammatory factors, such as IL-6 and nuclear transcription factors after MI in rats, thereby reducing myocardial injury and protecting myocardial tissue (137).
Mechanism of H2 Protection Against Myocardial Ischemic Injury
Induction of H2
H2 is widely distributed in nature. As a colorless and odorless reducing gas, it is not only small in size, but also freely crosses the blood-brain barrier, with no residue as a result of metabolism (138). Compared with NO and H2S, H2 has a smaller molecular weight and is more likely to enter the biofilm. H2 is less cytotoxic than other medical gases, and its low reactivity with other gases allows it to be mixed with other therapeutic gases, including inhaled anesthetics (139). Therefore, H2 is expected to become the fourth most important gaseous signaling molecule after NO and H2S (140). The synthetic sources of H2 are endogenous and exogenous. H2 is not normally produced in human cells due to the absence of enzymes with hydrogenase activity. However, under normal human physiological conditions, more than 12 L of H2 is produced daily, mainly by the fermentation of undigested carbohydrates produced by the microbiome (141). Studies have shown that, to play an antioxidant role, the content of endogenous hydrogen must be significantly higher than the minimum concentration of exogenous hydrogen (142). H2 is excreted in three main ways: through breathing, flatulence and metabolization by microorganisms in the colon (143). The human body can obtain exogenous H2 through inhalation and drinking or injection of hydrogen-rich water (144). Studies have shown that drinking hydrogen-rich water in daily life is beneficial for some chronic diseases (145). Nagatani et al. (146) found that intravenous hydrogen salt was safe and effective in 38 patients with acute ischemic stroke. In addition, eye drops or external products that produce H2 can be absorbed into the blood through the skin, thus representing a potential strategy for the use H2 to treat diseases (147). Intake of exogenous H2 has been shown to have a great effect on the body (1) in the studies of multiple organs or systems including the central nervous system (145, 148, 149), cardiovascular system (122, 150), lung (151, 152), renal system (145), liver (153), pancreas (154, 155), intestinal (156, 157). In the study of the cardiovascular system, H2 has been shown to H2 play an important role in myocardial ischemic injury (158).
Antioxidant Effect of H2
Oxidative stress is the main cause of myocardial ischemic injury (159). Ohsawa et al. (158) found that H2 selectively reduced the levels of hydroxyl radicals and cytotoxic ROS to effectively protect cells, although H2 cannot reduce free-radicals by interacting with excessive ROS. This discovery provides a new strategy for the treatment of myocardial ischemic injury. In recent years, Nrf2 has been identified as a transcription factor closely related to the oxidative stress caused by H2. Xie et al. (160) evaluated the role of the Nrf2/HO-1 signaling pathway in ischemia induced in H9C2 myocardial cells in vitro through serum and glucose (SGD) deprivation. The results showed that SGD caused myocardial cell damage and down-regulated the Nrf2/HO-1 signaling pathway. In contrast, a H2-rich gas alleviated the cell damage caused by cell exposure to SGD and up-regulated Nrf2/HO-1. In addition, RNA interference mediated silencing of the Nrf2 gene, the influence of H2 on HO-1 induction and cardiac protection was significantly reduced.
In summary, H2 gas protects myocardial cells from myocardial damage caused by ischemia by eliminating ·OH free-radicals and activating the Nrf2/HO-1 signaling pathway. In addition, the Nrf2/ARE pathway also plays an important role in selective oxidation. Studies have shown that H2 can protect the myocardium by activating Nrf2-ARE signaling pathway (161, 162).
Anti-apoptotic Effects of H2
During MI/R, oxygen free-radicals, calcium overload and MPTP opening lead to mitochondrial swelling and rupture, releasing apoptosis-inducing factors and apoptosis-related proteins, and further initiating the caspase cascade to induce programmed apoptosis (163). Recent studies have shown that the PI3K/AKT pathway is crucial for cardiomyocyte apoptosis (164). Chen et al. (165) found that high concentrations of H2 protected mouse hearts from ischemia-reperfusion injury by activating the PI3K/AKT1 pathway. Forkhead box protein O (FoxO) is downstream of PI3K/AKT and is inhibited by PI3K/AKT. Generally speaking, after FoxO is activated, the cells cycle is blocked in the G1/S phase and apoptosis is promoted. Therefore, hydrogen may regulate FoxO expression via the PI3K/AKT signaling pathway, thus playing an anti-apoptotic role (1). In addition, hydrogen-enriched saline also showed protective and antiapoptotic effects on MI/R injury by down-regulating the AKT/GSK3 signaling pathway (166) and upregulating the JAK/STAT signaling pathway (167).
Anti-inflammatory Effects of H2
Zhang et al. (168) demonstrated that ischemia/reperfusion (I/R) induced elevated levels of the pro-inflammatory cytokines TNF-α and IL-1β in myocardial cells, which were attenuated by hydrogen-rich saline. Hydrogen-rich saline has an anti-inflammatory effect on the local MI/R injury in the heart. There are few reports on the protective effects of H2 against myocardial ischemia injury and inflammation, which requires further investigation.
Study on the Mechanism of Myocardial Ischemic Injury by Combining no and H2S
Accumulating evidence indicates the potential coupling of NO and H2S at different levels. ZYZ-803, which is a novel synthetic H2S and NO co-donor, was developed by combining SPRC with furoxan. However, ZYZ-803 releases H2S and NO more slowly and in a more prolonged manner than SPRC and/or furoxan (169). ZYZ-803 has fewer side-effects and lower concentrations than SPRC and furoxan alone. The cardioprotective effect of ZYZ-803 was significantly stronger than that of the H2S and/or NO donors alone. Furthermore, ZYZ-803 releases H2S and NO by stimulating CSE and endothelial NO synthase (e-NOS), respectively, to produce physiological activity. Currently, there are few reports on ZYZ-803, most of which focus on the mechanism of angiogenesis (170) and vasodilation (171) and the protective effects against HF (172).
Chang et al. (120) used ZYZ-803 as the combined gas donor to study its anti-apoptotic effects on MI injury. By releasing H2S and NO, ZYZ-803 down-regulated the RIP3-CaMKII signaling pathway and alleviated ERS-related necrotic apoptosis after AMI. DL-propargylglycine (PAG) and e-NOS inhibitors of N(G)-nitro-L-arginine methyl ester (L-NAME), which inhibit of CSE and L-NAME, respectively, significantly inhibited the cardioprotective effect of ZYZ-803, while the inhibitory effect of PAG+L-NAME was more obvious. In addition, Wu et al. (171) found that blocking CSE and/or e-NOS inhibited the generation of H2S and NO produced by ZYZ-803 and reversed its cardiovascular protective effects. It has been reported that NO promotes CSE expression in vascular tissues and increases H2S levels, while L-NAME inhibits the vasodilatory effect of H2S (171, 173). Furthermore, H2S enhances the production of NO through calcium-dependent activation of e-NOS in endothelial cells (174). The generation of NO is obviously inhibited by CSE knockout, while CSE overexpression promotes the generation of NO (175). These data suggest that PAG not only inhibits CSE and reduces H2S, but also inhibits e-NOS activity and NO concentrations in HF. L-NAME not only inhibits e-NOS and reduces NO, but also reduces CSE expression and H2S levels. Thus, H2S and NO have synergistic effects, while H2S regulates the biological function of NO, and vice versa.
Discussion
In the past few years, substantial progress has been made in the field of gaseous signaling molecule donors. In this review, we have summarized the mechanisms by which gaseous signaling molecules (NO, H2S, H2) protect myocardial ischemia (Figure 4), including the gas co-donors that regulate gas molecules that protect against myocardial ischemic injury. Since most of the experimental articles listed in the review included normal and model groups, and the gaseous signaling molecule donor did almost no damage to normal cardiomyocytes, the off-target effect was rarely seen. However, when the concentration of gaseous signaling molecules such as H2S is too high, poisoning and other phenomena will occur. Therefore, targeting and other approaches can be adopted to increase the concentration of gas signaling molecules locally within the lesion to achieve the therapeutic effect of gaseous signaling molecules.
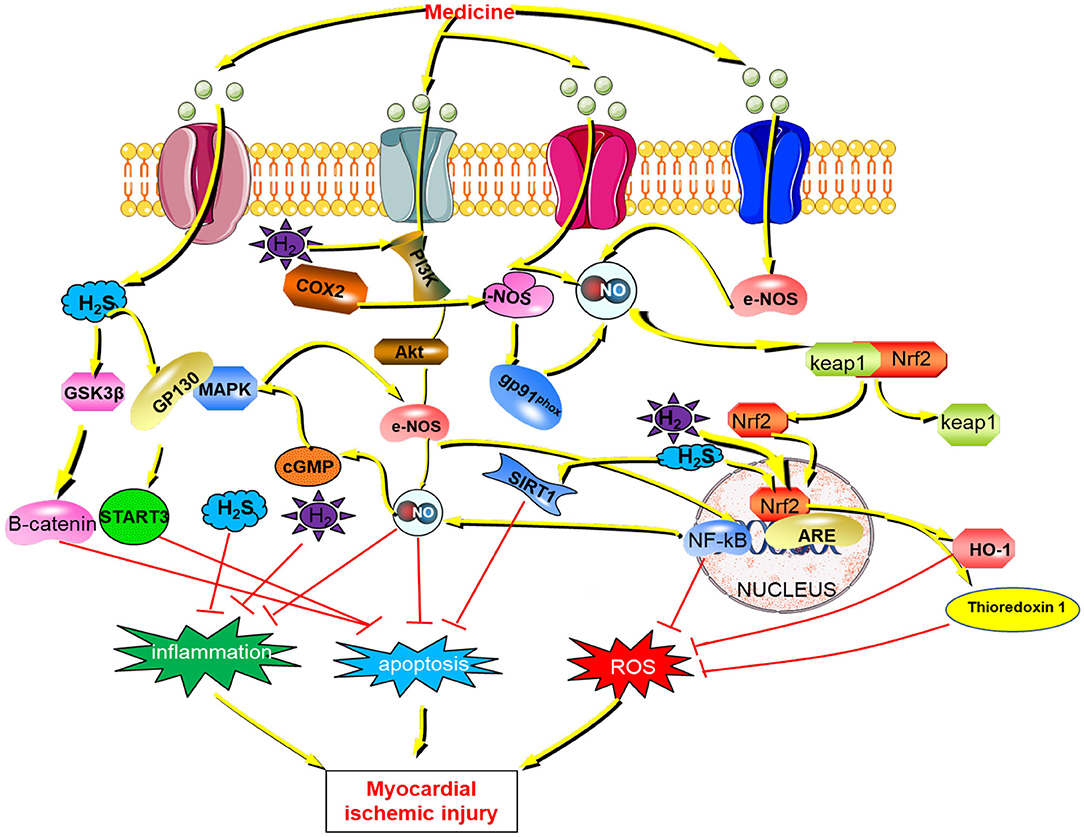
Figure 4. The mechanism of gaseous signaling molecules (NO, H2, H2S) against myocardial ischemic injury H2S, hydrogen sulfide; GSK3β, Glycogen synthase kinase-3β; GP130, Glycoprotein 130;STAT3, Signal transducers and activators of transcription 3; PI3K, class I phosphatidylinositol 3-kinase; COX2, Cyclooxygenase 2; AKT, Serine/threonine protein kinase; MAPK, mitogen-activated protein kinase; cGMP, cyclic guanosinc monophosphate; NO, nitric oxide; i-NOS, Inducible nitric oxide synthase; e-NOS, endothelial nitric oxide synthase; Keap1, Kelch Like ECH Associated Protein 1; Nrf2, Nuclear factor erythroid 2 p45-related factor 2; NF-kB, Nuclear factor-kappa B; HO-1, Hemeoxygenase-1; SIRT1, Sirtuin1; ROS, Reactive oxygen species.
As a ubiquitous gas, NO also mediates and hemostasis and homeostasis, which enhances the protective effect on the heart. Studies have shown that vascular endothelial cells are the main cellular source of synthesized NO in vivo, and the synergistic effect of endothelial cells and platelet NOS is conducive to regulating platelet activation and inhibiting platelet adhesion and aggregation (176). NO also plays an important role in maintaining vascular tone and blood pressure homeostasis.
H2S inhibition of oxidative stress to protect cardiomyocytes is dependent on Nrf2-mediated induction of cellular protection genes. John et al. found that H2S induced upregulation of Nrf2 inactivates Keap1 through modification of C226 and C613 while Nrf2 controls CBS, CSE, and Sqrdl (sulfide: quinone reductase-like), which suggests the existence of a feedback loop between Nrf2 and H2S. H2S also protects the heart from oxidative stress through S-acidification.
Anti-inflammatory strategies are an important aspect of drug therapy for myocardial ischemic injury. On the one hand, inflammatory responses fundamentally affect the long-term and short-term performance of solid organ allografts; on the other hand, the transplantation process including the surgical trauma itself, in addition to the associated ischemia-reperfusion injury may lead to acute and chronic inflammatory responses that affect allograft function in the long-term (177). If the inflammation is resolved, the tissue can heal without sequelae. Otherwise, acute inflammation may become chronic, stimulating tissue remodeling and ultimately leading to fibrosis and loss of organ or tissue function (177). Therefore, drugs that effectively control inflammation and acomprehensive understanding of the mechanism of its action are urgently required. However, the pathways by which the gaseous signaling molecules (NO, H2S and H2) exert their anti-inflammatory effects in myocardial ischemic injury remain to be elucidated.
NO has long been recognized as a gaseous signaling molecule that acts independently; however, recent studies have shown that H2S is an important enhancer of NO in blood vessels. Specifically, H2S may act as a system enhancer for e-NOS/sGC/cGMP/PKG, mainly by stimulating NO release in stable and semi-stable pools, and by stimulating calcium mobilization to stimulate e-NOS activity. Similarly, when the biological effect of vascular NO system is weakened, the stimulatory effect of H2S on the e-NOS/sGC/cGMP/PKG system is weakened (178). These findings indicate that there is crosstalk between the gaseous signaling molecules, and that they do not function independently. Although there are few studies on the interaction between NO/H2 and H2S/H2 at present, advances in science and technology will facilitate relevant studies on these aspects.
To data, many donors of gaseous signaling molecules have been identified, although the exact concentration of gaseous signaling molecules in various samples is unknown, which hinders progress in this field of research. Therefore, an appropriate and accurate method is urgently required. In addition, gaseous signaling molecules such as H2S, specific inhibitors and stable donors are lacking. Due to the wide range of current inhibitor molecules and the instability of donor molecules, the actual changes in H2S concentrations conflict with the expected results.
Experimental studies have shown that gaseous signaling molecules with co-donor drugs, such as ZYZ-803, have a much better effect on the treatment of diseases than in individual drugs administered with gas signaling molecules. Therefore, gaseous co-donor drugs and their targets will be an important focus of research. Moreover, gaseous signaling molecules have shown certain therapeutic effects in many animal experiments related to myocardial ischemic injury. Clarification of the specific targets of gaseous signaling molecules in myocardial ischemic injury and evaluation in clinical applications is of great significance. It is believed that with advanced in scientific research technology and the continuous efforts of researchers, gaseous signaling drugs will soon enter clinical research.
Summary
The aim of myocardial ischemic treatment is to relieve symptoms, reduce the incidence of angina pectoris and MI, and delay the development of coronary atherosclerosis. In recent years, myocardial ischemic injury has attracted increasing attention due to its high risk of death. The protective effect of gaseous signaling molecules on myocardial ischemic injury is self-evident, and clarification of the specific targets of gaseous signaling molecules in myocardial ischemia is of great and urgent significance for their clinical application. Hopefully, this review will serve as a reference for guidance of future research into the effects of gaseous signaling molecules on myocardial ischemic injury.
Author Contributions
WW and TG wrote the manuscript. YM revised the manuscript. XC and YZ proved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work has been supported by grants from National Natural Science Foundation of China (No. 81973320), Shanghai Committee of Science and Technology of China Rising-Star Program (No. 19QA1401500), and Macau Science and Technology Development Fund (FDCT) (067/2018/A2 and 033/2017/AMJ, 0007/2019/AKP, 0052/2020/A).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AMI, Acute myocardial ischemia; ANG II, Angiotensin II; BH4, tetrahydrobiopterin; CABG, Coronary artery bypass graft; CAPE-oNO2, o-nitrophenyl ethyl caffeate; CBS, Cystathionine β-synthase; COX1, Cyclooxygenase-1; CSE, Cystathionine γ-lyase; e-NOS, Endothelial NO synthase; ER, Endoplasmic reticulum; FAD, Flavin adenine dinucleotide; FMN, Flavin mononucleotide; FoxO, Forkhead box protein O; GSH, Glutathione; GSRg3, Ginsenoside Rg3; GYY4137, morpholine-4-methoxyphenylmorpholine-morpholine-phosphodisulfate; HF, Heart failure; H2, Hydrogen; H2S, Hydrogen sulfide; HSP90, Heat shock protein 90; IL-1, Interleukin 1; IL-6, Interleukin 6; Keap1, Kelch-like ECH-associated protein 1; KMUP-1, 7-[2-[4-(2-chlorophenyl) piperazinyl] ethyl]−1; L-NAME, N(G)-nitro-L-arginine methyl ester; i-NOS, Inducible NO synthase; I/R, Ischemia-reperfusion; MI, Myocardial infarction; mPGEs1, Microsomal PGE synthase-1; 3-MST, 3-mercaptopyruvate sulfurtransferase; NF-κB, Nuclear transcription factor-kappa B; n-NOS, Neuronal NO synthase; NO, Nitric Oxide; Nrf2, Nuclear factor erythroid 2 p45-related factor 2; OS, Oxidative stress; PAG, DL-propargylglycine; PCI, Percutaneous coronary intervention; PG, Prostaglandin; PI3K, Phosphoinositide 3-kinase; QSQP, Qishen yiqi drop pill; RNS, Reactive nitrogen species; ROS, Reactive oxygen species; SAC, S-ally-l-cysteine; SGD, Serum and glucose; SOD, Superoxide dismutase; SPRC, S-propargyl-cystine; SR, Sarcoplasmic reticulum; TNF-α, Tumor necrosis factor-α; Trx, Thioredoxin; TUNEL, Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling; TXNIP, Trx interaction protein; XD, Xanthine dehydrogenase; XO, Xanthine oxidase.
References
1. Li L, Li X, Zhang Z, Liu L, Zhou Y, Liu F. Protective mechanism and clinical application of hydrogen in myocardial ischemia-reperfusion injury. Pak J Biol Sci. (2020) 23:103–12. doi: 10.3923/pjbs.2020.103.112
2. Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. (1999) 43:860–78. doi: 10.1016/S0008-6363(99)00187-X
3. Lutz J, Thürmel K, Heemann U. Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. J Inflamm. (2010) 7:27. doi: 10.1186/1476-9255-7-27
4. Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. (2010) 26:5–13. doi: 10.3233/NRE-2010-0531
5. Gaire BP, Song MR, Choi JW. Sphingosine 1-phosphate receptor subtype 3 (S1P3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their M1 polarization. J Neuroinflammation. (2018) 15:284. doi: 10.1186/s12974-018-1323-1
6. Nastos C, Kalimeris K, Papoutsidakis N, Tasoulis MK, Lykoudis PM, Theodoraki K, et al. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. (2014) 2014:906965. doi: 10.1155/2014/906965
7. Montero MF, Saurim R, Bonservizi WG, Koike MK, Taha MO. Heart injury following intestinal ischemia reperfusion in rats is attenuated by association of ischemic preconditioning and adenosine. Acta Cir Bras. (2014) 29 (Suppl. 2):67–71. doi: 10.1590/S0102-8650201400140013
8. Pederson WC. Acute ischemia of the upper extremity. Orthop Clin North Am. (2016) 47:589–97. doi: 10.1016/j.ocl.2016.03.004
9. Lejay A, Fang F, John R, Van JA, Barr M, Thaveau F, et al. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol. (2016) 91:11–22. doi: 10.1016/j.yjmcc.2015.12.020
10. Finch W, Lee MS. Percutaneous coronary intervention for coronary bifurcation lesions. Rev Cardiovasc Med. (2017) 18:59–66. doi: 10.3909/ricm0868
11. Modolo R, Chichareon P, Kogame N, Dressler O, Crowley A, Ben-Yehuda O, et al. contemporary outcomes following coronary artery bypass graft surgery for left main disease. J Am Coll Cardiol. (2019) 73:1877–86. doi: 10.1016/j.jacc.2018.12.090
12. Grines CL, Harjai KJ, Schreiber TL. Percutaneous coronary intervention: 2015 in review. J Interv Cardiol. (2016) 29:11–26. doi: 10.1111/joic.12272
13. Godoy LC, Ko DT, Rao V, Farkouh ME. The role of coronary artery bypass surgery versus percutaneous intervention in patients with diabetes and coronary artery disease. Prog Cardiovasc Dis. (2019) 62:358–63. doi: 10.1016/j.pcad.2019.07.004
14. Mangieri A, Gallo F, Sticchi A, Khokhar AA, Laricchia A, Giannini F, et al. Dual antiplatelet therapy in coronary artery disease: from the past to the future prospective. Cardiovasc Interv Ther. (2020) 35:117–29. doi: 10.1007/s12928-020-00642-w
15. Ahn JH, Ahn Y, Jeong MH, Kim JH, Hong YJ, Sim DS, et al. Ticagrelor versus clopidogrel in acute myocardial infarction patients with multivessel disease; from korea acute myocardial infarction registry-national institute of health. J Cardiol. (2020) 75:478–84. doi: 10.1016/j.jjcc.2019.11.003
16. Kouretas PC, Myers AK, Kim YD, Cahill PA, Myers JL, Wang YN, et al. Heparin and nonanticoagulant heparin preserve regional myocardial contractility after ischemia-reperfusion injury: role of nitric oxide. J Thorac Cardiovasc Surg. (1998) 115:440–8; discussion 8–9. doi: 10.1016/S0022-5223(98)70288-0
17. Paciaroni E, Luca C, Paciaroni E, Luca C. Discontinuous transdermal nitroglycerin as treatment for stable angina in the elderly: a double-blind multicentre study. The transdermal tng trial group of istituto nazionale ricovero e cura anziani (I.N.R.C.A.). Eur Heart J. (1991) 12:1076–80. doi: 10.1093/oxfordjournals.eurheartj.a059840
18. Hansson NH, Sorensen J, Harms HJ, Kim WY, Nielsen R, Tolbod LP, et al. Metoprolol reduces hemodynamic and metabolic overload in asymptomatic aortic valve stenosis patients: a randomized trial. Circ Cardiovasc Imaging. (2017) 10:e006557. doi: 10.1161/CIRCIMAGING.117.006557
19. Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. the save investigators. N Engl J Med. (1992) 327:669–77. doi: 10.1056/NEJM199209033271001
20. Tani S, Nagao K, Anazawa T, Kawamata H, Furuya S, Takahashi H, et al. Effects of enalapril and losartan in left ventricular remodeling after acute myocardial infarction: a possible mechanism of prevention of cardiac events by angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high-risk myocardial infarction. Intern Med. (2009) 48:877–2. doi: 10.2169/internalmedicine.48.1948
21. Pioruńska-Stolzmann M, Pioruńska-Mikołajczak A. The influence of simvastatin on lipase and cholesterol esterase activity in the serum of men with coronary heart disease. Pharmacol Res. (2001) 43:359–62. doi: 10.1006/phrs.2000.0787
22. Cen W, Chen Z, Gu N, Hoppe R. Prevention of ami induced ventricular remodeling: inhibitory effects of heart-protecting musk pill on IL-6 and TNF-Alpha. Evid Based Complement Alternat Med. (2017) 2017:3217395. doi: 10.1155/2017/3217395
23. Lu L, Qin Y, Chen C, Zhang X, Xu X, Lv C, et al. The atheroprotective roles of heart-protecting musk pills against atherosclerosis development in apolipoprotein E-deficient mice. Ann Transl Med. (2019) 7:714. doi: 10.21037/atm.2019.12.22
24. JianXin C, Xue X, ZhongFeng L, Kuo G, FeiLong Z, ZhiHong L, et al. Qishen Yiqi Drop Pill improves cardiac function after myocardial ischemia. Sci Rep. (2016) 6:24383. doi: 10.1038/srep24383
25. Zhang HX, Liu SJ, Tang XL, Duan GL, Ni X, Zhu XY, et al. H2S Attenuates LPS-Induced acute lung injury by reducing oxidative/nitrative stress and inflammation. Cell Physiol Biochem. (2016) 40:1603–12. doi: 10.1159/000453210
26. Yang G, Yang W, Wu L, Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem. (2007) 282:16567–76. doi: 10.1074/jbc.M700605200
27. Ali FF, Abdel-Hamid HA, Toni ND. H2S attenuates acute lung inflammation induced by administration of lipopolysaccharide in adult male rats. Gen Physiol Biophys. (2018) 37:421–31. doi: 10.4149/gpb_2018002
28. Chen Q, Yu S, Zhang K, Zhang Z, Li C, Gao B, et al. Exogenous h2s inhibits autophagy in unilateral ureteral obstruction mouse renal tubule cells by regulating the ros-ampk signaling pathway. Cell Physiol Biochem. (2018) 49:2200–13. doi: 10.1159/000493824
29. Sonobe T, Haouzi P. H2S concentrations in the heart after acute H2S administration: methodological and physiological considerations. Am J Physiol Heart Circ Physiol. (2016) 311:H1445–58. doi: 10.1152/ajpheart.00464.2016
30. Pan LL, Liu XH, Gong QH, Yang HB, Zhu YZ. Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxid Redox Signal. (2012) 17:106–18. doi: 10.1089/ars.2011.4349
31. Sharma AK, Munajjam A, Vaishnav B, Sharma R, Sharma A, Kishore K, et al. Involvement of adenosine and standardization of aqueous extract of garlic (Allium sativum Linn.) on cardioprotective and cardiodepressant properties in ischemic preconditioning and myocardial ischemia-reperfusion induced cardiac injury. J Biomed Res. (2012) 26:24–36. doi: 10.1016/S1674-8301(12)60004-9
32. Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. (2014) 66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045
33. Zhao R, Xie E, Yang X, Gong B. Alliin alleviates myocardial ischemia-reperfusion injury by promoting autophagy. Biochem Biophys Res Commun. (2019) 512:236–43. doi: 10.1016/j.bbrc.2019.03.046
34. Makheja AN, Bailey JM. Antiplatelet constituents of garlic and onion. Agents Actions. (1990) 29:360–3. doi: 10.1007/BF01966468
35. Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. (2008) 103:203–15. doi: 10.1007/s00395-007-0687-7
36. Park S, Kim MY, Lee DH, Lee SH, Baik EJ, Moon CH, et al. Methanolic extract of onion (Allium cepa) attenuates ischemia/hypoxia-induced apoptosis in cardiomyocytes via antioxidant effect. Eur J Nutr. (2009) 48:235–42. doi: 10.1007/s00394-009-0007-0
37. Moris D, Spartalis M, Tzatzaki E, Spartalis E, Karachaliou GS, Triantafyllis AS, et al. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann Transl Med. (2017) 5:324. doi: 10.21037/atm.2017.06.17
38. Lee BE, Toledo AH, Anaya-Prado R, Roach RR, Toledo-Pereyra LH. Allopurinol, xanthine oxidase, and cardiac ischemia. J Investig Med. (2009) 57:902–9. doi: 10.2310/JIM.0b013e3181bca50c
39. Siomek A. NF-kappaB signaling pathway and free radical impact. Acta Biochim Pol. (2012) 59:323–31. doi: 10.18388/abp.2012_2116
40. Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, et al. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature. (2017) 545:93–7. doi: 10.1038/nature22082
41. Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol. (2008) 294:C169–77. doi: 10.1152/ajpcell.00282.2007
42. Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol. (2017) 8:467. doi: 10.3389/fneur.2017.00467
43. Rodrigo R, Libuy M, Feliú F, Hasson D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis Markers. (2013) 35:773–90. doi: 10.1155/2013/974358
44. Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, et al. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci. (2018) 19:2605. doi: 10.3390/ijms19092605
45. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. (1994) 298:249–58. doi: 10.1042/bj2980249
46. Ost TW, Daff S. Thermodynamic and kinetic analysis of the nitrosyl, carbonyl, and dioxy heme complexes of neuronal nitric-oxide synthase. The roles of substrate and tetrahydrobiopterin in oxygen activation. J Biol Chem. (2005) 280:965–73. doi: 10.1074/jbc.M411191200
47. Llorens S, Jordan J, Nava E. The nitric oxide pathway in the cardiovascular system. J Physiol Biochem. (2002) 58:179–88. doi: 10.1007/BF03179855
48. Balligand JL, Kobzik L, Han X, Kaye DM, Belhassen L, et al. Nitric oxide-dependent parasympathetic signaling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. J Biol Chem. (1995) 270:14582–6. doi: 10.1074/jbc.270.24.14582
49. Li F, Zong J, Zhang H, Zhang P, Xu L, Liang K, et al. Orientin reduces myocardial infarction size via enos/no signaling and thus mitigates adverse cardiac remodeling. Front Pharmacol. (2017) 8:926. doi: 10.3389/fphar.2017.00926
50. Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. (1999) 6:964–8. doi: 10.1038/sj.cdd.4400581
51. Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. (1989) 2:997–1000. doi: 10.1016/S0140-6736(89)91013-1
52. Miyamoto Y, Saito Y, Kajiyama N, Yoshimura M, Shimasaki Y, Nakayama M, et al. Endothelial nitric oxide synthase gene is positively associated with essential hypertension. Hypertension. (1998) 32:3–8. doi: 10.1161/01.HYP.32.1.3
53. Onat A, Sari I, Hergenç G, Yazici M, Uyarel H, Can G, et al. Predictors of abdominal obesity and high susceptibility of cardiometabolic risk to its increments among Turkish women: a prospective population-based study. Metabolism. (2007) 56:348–56. doi: 10.1016/j.metabol.2006.10.016
54. Nakaki T, Nakayama M, Kato R, Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. (1990) 189:347–53. doi: 10.1016/0922-4106(90)90031-R
55. Nunokawa Y, Tanaka S. Interferon-gamma inhibits proliferation of rat vascular smooth muscle cells by nitric oxide generation. Biochem Biophys Res Commun. (1992) 188:409–15. doi: 10.1016/0006-291X(92)92400-R
56. Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci USA. (1999) 96:657–62. doi: 10.1073/pnas.96.2.657
57. Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, et al. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. (2007) 115:483–92. doi: 10.1161/CIRCULATIONAHA.106.643791
58. Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. (2010) 120:816–26. doi: 10.1172/JCI40736
59. Zhang YH, Jin CZ, Jang JH, Wang Y. Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. J Physiol. (2014) 592:3189–200. doi: 10.1113/jphysiol.2013.270306
60. Zhang YH, Casadei B. Sub-cellular targeting of constitutive NOS in health and disease. J Mol Cell Cardiol. (2012) 52:341–50. doi: 10.1016/j.yjmcc.2011.09.006
61. Zhang YH. Nitric oxide signalling and neuronal nitric oxide synthase in the heart under stress. F1000Res. (2017) 6:742. doi: 10.12688/f1000research.10128.1
62. Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. (2002) 109:735–43. doi: 10.1172/JCI0213265
63. Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. (2005) 569:913–24. doi: 10.1113/jphysiol.2005.095729
64. Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Zhang Y, Lee TS, Kolb EM, et al. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. (2006) 26:1281–7. doi: 10.1161/01.ATV.0000221230.08596.98
65. Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A, Webb A, Bond R, McLean P, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA. (2004) 101:13683–8. doi: 10.1073/pnas.0402927101
66. Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. (2005) 1:290–7. doi: 10.1038/nchembio734
67. Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. (2007) 104:19144–9. doi: 10.1073/pnas.0706579104
68. Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, Novalija E, Hogg N, Broniowska KA, Novalija J, et al. Role of S-nitrosothiol transport in the cardioprotective effects of S-nitrosocysteine in rat hearts. Free Radic Biol Med. (2007) 43:1086–94. doi: 10.1016/j.freeradbiomed.2007.06.016
69. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
70. Berges A, Van Nassauw L, Bosmans J, Timmermans JP, Vrints C. Role of nitric oxide and oxidative stress in ischaemic myocardial injury and preconditioning. Acta Cardiol. (2003) 58:119–32. doi: 10.2143/AC.58.2.2005264
71. Baicalein pretreatment confers cardioprotection against acute myocardial infarction by activating the endothelial nitric oxide synthase signaling pathway and inhibiting oxidative stress. Mol Med Rep. (2014) 9:2429–34. doi: 10.3892/mmr.2014.2091
72. Xiao C, Xia ML, Wang J, Zhou XR, Lou YY, Tang LH, et al. Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxid Med Cell Longev. (2019) 2019:2719252. doi: 10.1155/2019/2719252
73. Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. (2007) 115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941
74. Yeh JL, Hsu JH, Wu PJ, Liou SF, Liu CP, Chen IJ, et al. KMUP-1 attenuates isoprenaline-induced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br J Pharmacol. (2010) 159:1151–60. doi: 10.1111/j.1476-5381.2009.00587.x
75. Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Guo Y, Jones WK, Xuan YT, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. (1999) 96:11507–12. doi: 10.1073/pnas.96.20.11507
76. Wang Y, Hu Z, Sun B, Xu J, Jiang J, Luo M. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the Bcell lymphoma/Bcell lymphomaassociated X protein pathway. Mol Med Rep. (2015) 11:4518–24. doi: 10.3892/mmr.2015.3336
77. Liu Q, Li Z, Liu Y, Xiao Q, Peng X, Chen Q, et al. Hydromorphine postconditioning protects isolated rat heart against ischemia-reperfusion injury via activating P13K/Akt/eNOS signaling. Cardiovasc Ther. (2018) 36:e12481. doi: 10.1111/1755-5922.12481
78. Xiao JM, Wang JJ, Sun LL. Effect of miR-134 against myocardial hypoxia/reoxygenation injury by directly targeting NOS3 and regulating PI3K/Akt pathway. Acta Cir Bras. (2019) 34:e201900802. doi: 10.1590/s0102-865020190080000002
79. Lee ML, Sulistyowati E, Hsu JH, Huang BY, Dai ZK, Wu BN, et al. KMUP-1 ameliorates ischemia-induced cardiomyocyte apoptosis through the NO(-)cGMP(-)MAPK signaling pathways. Molecules. (2019) 24:1376. doi: 10.3390/molecules24071376
80. Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and−2. J Biol Chem. (1996) 271:33157–60. doi: 10.1074/jbc.271.52.33157
81. Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, et al. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. (2002) 90:602–8. doi: 10.1161/01.RES.0000012202.52809.40
82. Pang L, Cai Y, Tang EH, Yan D, Kosuru R, Li H, et al. Cox-2 inhibition protects against hypoxia/reoxygenation-induced cardiomyocyte apoptosis via akt-dependent enhancement of inos expression. Oxid Med Cell Longev. (2016) 2016:3453059. doi: 10.1155/2016/3453059
83. Li D, Wang X, Huang Q, Li S, Zhou Y, Li Z. Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-kappaB pathway in vivo and in vitro. Redox Biol. (2018) 15:62–73. doi: 10.1016/j.redox.2017.11.023
84. Nitric oxide mediates cardiac protection of tissue kallikrein by reducing inflammation and ventricular remodeling after myocardial ischemia/reperfusion. Life Sci. (2008) 82:156–65. doi: 10.1016/j.lfs.2007.10.021
85. Yuan X, Xiang Y, Zhu N, Zhao X, Ye S, Zhong P, et al. Salvianolic acid A protects against myocardial ischemia/reperfusion injury by reducing platelet activation and inflammation. Exp Ther Med. (2017) 14:961–6. doi: 10.3892/etm.2017.4619
86. Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, et al. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. (2018) 40:163–76. doi: 10.1007/s11357-018-0018-y
87. Wu D, Wang J, Li H, Xue M, Ji A, Li Y. Role of Hydrogen Sulfide in Ischemia-Reperfusion Injury. Oxid Med Cell Longev. (2015) 2015:186908. doi: 10.1155/2015/186908
88. Kumar M, Sandhir R. Hydrogen sulfide in physiological and pathological mechanisms in brain. CNS Neurol Disord Drug Targets. (2018) 17:654–70. doi: 10.2174/1871527317666180605072018
89. McCarty MF, O'Keefe JH, DiNicolantonio JJ. A diet rich in taurine, cysteine, folate, B12 and betaine may lessen risk for Alzheimer's disease by boosting brain synthesis of hydrogen sulfide. Med Hypotheses. (2019) 132:109356. doi: 10.1016/j.mehy.2019.109356
90. Donnarumma E Trivedi RK Lefer DJProtective actions of H2S in acute myocardial infarction and heart failure. Compr Physiol. (2017) 7:583–602. doi: 10.1002/cphy.c160023
91. Yang R, Jia Q, Ma SF, Wang Y, Mehmood S, Chen Y. Exogenous H2S mitigates myocardial fibrosis in diabetic rats through suppression of the canonical Wnt pathway. Int J Mol Med. (2019) 44:549–58. doi: 10.3892/ijmm.2019.4237
92. di Masi A, Ascenzi P. H2S: a “double face” molecule in health and disease. Biofactors. (2013) 39:186–96. doi: 10.1002/biof.1061
93. Corsello T, Komaravelli N, Casola A. Role of hydrogen sulfide in nrf2- and sirtuin-dependent maintenance of cellular redox balance. Antioxidants. (2018) 7:129. doi: 10.3390/antiox7100129
94. Li C, Hu M, Wang Y, Lu H, Deng J, Yan X. Hydrogen sulfide preconditioning protects against myocardial ischemia/reperfusion injury in rats through inhibition of endo/sarcoplasmic reticulum stress. Int J Clin Exp Pathol. (2015) 8:7740–51.
95. Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. (2006) 26:154–61. doi: 10.1097/01.shk.0000225722.56681.64
96. Jin Hf, Wang Y, Wang Xb, Sun Y, Tang Cs, Du Jb. Sulfur dioxide preconditioning increases antioxidative capacity in rat with myocardial ischemia reperfusion (I/R) injury. Nitric Oxide. (2013) 32:56–61. doi: 10.1016/j.niox.2013.04.008
97. Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. (2015) 172:1587–606. doi: 10.1111/bph.12811
98. Pan TT, Chen YQ, Bian JS. All in the timing: a comparison between the cardioprotection induced by H2S preconditioning and post-infarction treatment. Eur J Pharmacol. (2009) 616:160–5. doi: 10.1016/j.ejphar.2009.05.023
99. Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. (2009) 105:365–74. doi: 10.1161/CIRCRESAHA.109.199919
100. Filipovic MR, Zivanovic J, Alvarez B, Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem Rev. (2018) 118:1253–337. doi: 10.1021/acs.chemrev.7b00205
101. Wang X, Wang Q, Guo W, Zhu YZ. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: a mechanism through cardiac mitochondrial protection. Biosci Rep. (2011) 31:87–98. doi: 10.1042/BSR20100003
102. Rose P, Dymock BW, Moore PK. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol. (2015) 554:143–67. doi: 10.1016/bs.mie.2014.11.014
103. Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. (2008) 117:2351–60. doi: 10.1161/CIRCULATIONAHA.107.753467
104. Meng G, Wang J, Xiao Y, Bai W, Xie L, Shan L, et al. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res. (2015) 29:203–13. doi: 10.7555/JBR.28.20140037
105. Sun X, Wang W, Dai J, Jin S, Huang J, Guo C, et al. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci Rep. (2017) 7:3541. doi: 10.1038/s41598-017-03941-0
106. Kan J, Guo W, Huang C, Bao G, Zhu Y, Zhu YZ. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid Redox Signal. (2014) 20:2303–16. doi: 10.1089/ars.2013.5449
107. Wedmann R, Bertlein S, Macinkovic I, Böltz S, Miljkovic JLj, Muñoz LE, et al. Working with “H2S”: facts and apparent artifacts. Nitric Oxide. (2014) 41:85–96. doi: 10.1016/j.niox.2014.06.003
108. Filipovic MR. Persulfidation (S-sulfhydration) and H2S. Handb Exp Pharmacol. (2015) 230:29–59. doi: 10.1007/978-3-319-18144-8_2
109. Filipovic MR, Miljkovic J, Allgäuer A, Chaurio R, Shubina T, Herrmann M, et al. Biochemical insight into physiological effects of H(2)S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem J. (2012) 441:609–21. doi: 10.1042/BJ20111389
110. Xie ZZ, Liu Y, Bian JS. Hydrogen sulfide and cellular redox homeostasis. Oxid Med Cell Longev. (2016) 2016:6043038. doi: 10.1155/2016/6043038
111. Matsuzawa A. Thioredoxin and redox signaling: roles of the thioredoxin system in control of cell fate. Arch Biochem Biophys. (2017) 617:101–5. doi: 10.1016/j.abb.2016.09.011
112. Nicholson CK, Lambert JP, Molkentin JD, Sadoshima J, Calvert JW. Thioredoxin 1 is essential for sodium sulfide-mediated cardioprotection in the setting of heart failure. Arterioscler Thromb Vasc Biol. (2013) 33:744–51. doi: 10.1161/ATVBAHA.112.300484
113. Spindel ON World C Berk BC. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxid Redox Signal. (2012) 16:587–96. doi: 10.1089/ars.2011.4137
114. Ansari SB, Kurian GA. Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chem Biol Interact. (2016) 252:28–35. doi: 10.1016/j.cbi.2016.03.036
115. Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. (2004) 318:756–63. doi: 10.1016/j.bbrc.2004.04.094
116. Sun WH, Liu F, Chen Y, Zhu YC. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem Biophys Res Commun. (2012) 421:164–9. doi: 10.1016/j.bbrc.2012.03.121
117. Wu D, Hu Q, Liu X, Pan L, Xiong Q, Zhu YZ. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide. (2015) 46:204–12. doi: 10.1016/j.niox.2014.11.006
118. Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. (2011) 51:169–87. doi: 10.1146/annurev-pharmtox-010510-100505
119. Hu J, Xue Y, Tang K, Fan J, Du J, Li W, et al. The protective effects of hydrogen sulfide on the myocardial ischemia via regulating Bmal1. Biomed Pharmacother. (2019) 120:109540. doi: 10.1016/j.biopha.2019.109540
120. Chang L, Wang Z, Ma F, Tran B, Zhong R, Xiong Y, et al. ZYZ-803 mitigates endoplasmic reticulum stress-related necroptosis after acute myocardial infarction through downregulating the RIP3-CaMKII signaling pathway. Oxid Med Cell Longev. (2019) 2019:6173685. doi: 10.1155/2019/6173685
121. Citi V, Piragine E, Testai L, Breschi MC, Calderone V, Martelli A, Citi V, Piragine E, Testai L, et al. The role of hydrogen sulfide and H2S-donors in myocardial protection against ischemia/reperfusion injury. Curr Med Chem. (2018) 25:4380–401. doi: 10.2174/0929867325666180212120504
122. Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, et al. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. (2009) 31:267–74. doi: 10.1097/SHK.0b013e318180ff89
123. Lilyanna S, Peh MT, Liew OW, Wang P, Moore PK, Richards AM, et al. GYY4137 attenuates remodeling, preserves cardiac function and modulates the natriuretic peptide response to ischemia. J Mol Cell Cardiol. (2015) 87:27–37. doi: 10.1016/j.yjmcc.2015.07.028
124. Snijder PM, de Boer RA, Bos EM, van den Born JC, Ruifrok WP, Vreeswijk-Baudoin I, et al. Gaseous hydrogen sulfide protects against myocardial ischemia-reperfusion injury in mice partially independent from hypometabolism. PLoS ONE. (2013) 8:e63291. doi: 10.1371/journal.pone.0063291
125. Zhang Y, Li H, Zhao G, Sun A, Zong NC, Li Z, et al. Hydrogen sulfide attenuates the recruitment of CD11b(+)Gr-1(+) myeloid cells and regulates Bax/Bcl-2 signaling in myocardial ischemia injury. Sci Rep. (2014) 4:4774. doi: 10.1038/srep04774
126. Ge N, Liu C, Li G, Xie L, Zhang Q, Li L, et al. Hydrosulfide attenuates acute myocardial ischemic injury through the glycogen synthase kinase-3beta/beta-catenin signaling pathway. Int J Mol Med. (2016) 37:1281–9. doi: 10.3892/ijmm.2016.2538
127. Li HW, Xiao FY. Effect of hydrogen sulfide on cardiomyocyte apoptosis in rats with myocardial ischemia-reperfusion injury via the JNK signaling pathway. Eur Rev Med Pharmacol Sci. (2020) 24:2054–61. doi: 10.26355/eurrev_202002_20383
128. Zheng W, Liu C. The cystathionine gamma-lyase/hydrogen sulfide pathway mediates the trimetazidine-induced protection of H9c2 cells against hypoxia/reoxygenation-induced apoptosis and oxidative stress. Anatol J Cardiol. (2019) 22:102–11. doi: 10.14744/AnatolJCardiol.2019.83648
129. Liang YH, Shen YQ, Guo W, Zhu YZ. SPRC protects hypoxia and re-oxygenation injury by improving rat cardiac contractile function and intracellular calcium handling. Nitric Oxide. (2014) 41:113–9. doi: 10.1016/j.niox.2014.05.010
130. Huang C, Kan J, Liu X, Ma F, Tran BH, Zou Y, et al. Cardioprotective effects of a novel hydrogen sulfide agent-controlled release formulation of S-propargyl-cysteine on heart failure rats and molecular mechanisms. PLoS ONE. (2013) 8:e69205. doi: 10.1371/journal.pone.0069205
131. Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang Y, et al. Involvement of miR-1 in the protective effect of hydrogen sulfide against cardiomyocyte apoptosis induced by ischemia/reperfusion. Mol Biol Rep. (2014) 41:6845–53. doi: 10.1007/s11033-014-3570-2
132. Ren L, Wang Q, Chen Y, Ma Y, Wang D. Involvement of MicroRNA-133a in the protective effect of hydrogen sulfide against ischemia/reperfusion-induced endoplasmic reticulum stress and cardiomyocyte apoptosis. Pharmacology. (2019) 103:1–9. doi: 10.1159/000492969
133. Bian C, Xu T, Zhu H, Pan D, Liu Y, Luo Y, et al. Luteolin inhibits ischemia/reperfusion-induced myocardial injury in rats via downregulation of microRNA-208b-3p. PLoS ONE. (2015) 10:e0144877. doi: 10.1371/journal.pone.0144877
134. Chen GH, Xu CS, Zhang J, Li Q, Cui HH, Li XD, et al. Inhibition of miR-128-3p by tongxinluo protects human cardiomyocytes from ischemia/reperfusion injury via upregulation of p70s6k1/p-p70s6k1. Front Pharmacol. (2017) 8:775. doi: 10.3389/fphar.2017.00775
135. Song CL, Liu B, Diao HY, Shi YF, Zhang JC, Li YX, et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget. (2016) 7:39740–57. doi: 10.18632/oncotarget.9240
136. Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. (2007) 6:917–35. doi: 10.1038/nrd2425
137. Liu F, Liu GJ, Liu N, Zhang G, Zhang JX, Li LF. Effect of hydrogen sulfide on inflammatory cytokines in acute myocardial ischemia injury in rats. Exp Ther Med. (2015) 9:1068–74. doi: 10.3892/etm.2015.2218
138. Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. (2015) 36:273–9. doi: 10.1055/s-0034-1395509
139. Nakao A, Kaczorowski DJ, Wang Y, Cardinal JS, Buchholz BM, Sugimoto R, et al. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. J Heart Lung Transplant. (2010) 29:544–53. doi: 10.1016/j.healun.2009.10.011
140. Zhang JY, Liu C, Zhou L, Qu K, Wang R, Tai MH, et al. A review of hydrogen as a new medical therapy. Hepatogastroenterology. (2012) 59:1026–32. doi: 10.5754/hge11883
141. Strocchi A, Levitt MD. Maintaining intestinal H2 balance: credit the colonic bacteria. Gastroenterology. (1992) 102:1424–6. doi: 10.1016/0016-5085(92)90790-6
142. Kajiya M, Sato K, Silva MJ, Ouhara K, Do PM, Shanmugam KT, et al. Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochem Biophys Res Commun. (2009) 386:316–21. doi: 10.1016/j.bbrc.2009.06.024
143. Sahakian AB, Jee SR, Pimentel M. Methane and the gastrointestinal tract. Dig Dis Sci. (2010) 55:2135–43. doi: 10.1007/s10620-009-1012-0
144. Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. (2010) 44:971–82. doi: 10.3109/10715762.2010.500328
145. Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology. (2009) 34:501–8. doi: 10.1038/npp.2008.95
146. Nagatani K, Nawashiro H, Takeuchi S, Tomura S, Otani N, Osada H, et al. Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: initial clinical studies. Med Gas Res. (2013) 3:13. doi: 10.1186/2045-9912-3-13
147. Oharazawa H, Igarashi T, Yokota T, Fujii H, Suzuki H, Machide M, et al. Protection of the retina by rapid diffusion of hydrogen: administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. (2010) 51:487–92. doi: 10.1167/iovs.09-4089
148. Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. (2007) 361:670–4. doi: 10.1016/j.bbrc.2007.07.088
149. Abraini JH, Gardette-Chauffour MC, Martinez E, Rostain JC, Lemaire C. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J Appl Physiol. (1994). 76:1113-8. doi: 10.1152/jappl.1994.76.3.1113
150. Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. (2008) 373:30–5. doi: 10.1016/j.bbrc.2008.05.165
151. Nakamura T, Henson PM, Murphy RC. Occurrence of oxidized metabolites of arachidonic acid esterified to phospholipids in murine lung tissue. Anal Biochem. (1998) 262:23–32. doi: 10.1006/abio.1998.2749
152. Yoshimi N, Ikura Y, Sugama Y, Kayo S, Ohsawa M, Yamamoto S, et al. Oxidized phosphatidylcholine in alveolar macrophages in idiopathic interstitial pneumonias. Lung. (2005) 183:109–21. doi: 10.1007/s00408-004-2525-0
153. Liu Q, Shen WF, Sun HY, Fan DF, Nakao A, Cai JM, et al. Hydrogen-rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. (2010) 30:958–68. doi: 10.1111/j.1478-3231.2010.02254.x
154. Tani S, Itoh H, Okabayashi Y, Nakamura T, Fujii M, Fujisawa T, et al. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci. (1990) 35:367–74. doi: 10.1007/BF01537416
155. Chen H, Sun YP, Li Y, Liu WW, Xiang HG, Fan LY, et al. Hydrogen-rich saline ameliorates the severity of l-arginine-induced acute pancreatitis in rats. Biochem Biophys Res Commun. (2010) 393:308–13. doi: 10.1016/j.bbrc.2010.02.005
156. Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. (2007) 28:1450–9. doi: 10.1111/j.1745-7254.2007.00695.x
157. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. (1993) 69:238–49.
158. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. (2007) 13:688–94. doi: 10.1038/nm1577
159. LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules. (2019) 24:2076. doi: 10.3390/molecules24112076
160. Xie Q, Li XX, Zhang P, Li JC, Cheng Y, Feng YL, Xie Q, Li XX, Zhang P, et al. Hydrogen gas protects against serum and glucose deprivationinduced myocardial injury in H9c2 cells through activation of the NFE2related factor 2/heme oxygenase 1 signaling pathway. Mol Med Rep. (2014) 10:1143–9. doi: 10.3892/mmr.2014.2283
161. Miyata T, Takizawa S, van Ypersele de Strihou C. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: novel therapeutic targets. Am J Physiol Cell Physiol. (2011) 300:C226-31. doi: 10.1152/ajpcell.00430.2010
162. Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando). (2012) 26:103–14. doi: 10.1016/j.trre.2011.10.006
163. Chen-Scarabelli C, Agrawal PR, Saravolatz L, Abuniat C, Scarabelli G, Stephanou A, et al. The role and modulation of autophagy in experimental models of myocardial ischemia-reperfusion injury. J Geriatr Cardiol. (2014) 11:338–48. doi: 10.11909/j.issn.1671-5411.2014.01.009
164. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. (2004) 61:448–60. doi: 10.1016/j.cardiores.2003.09.024
165. Chen O, Cao Z, Li H, Ye Z, Zhang R, Zhang N, et al. High-concentration hydrogen protects mouse heart against ischemia/reperfusion injury through activation of thePI3K/Akt1 pathway. Sci Rep. (2017) 7:14871. doi: 10.1038/s41598-017-14072-x
166. Yue L, Li H, Zhao Y, Li J, Wang B. Effects of hydrogen-rich saline on Akt/GSK3beta signaling pathways and cardiac function during myocardial ischemia-reperfusion in rats. Zhonghua Yi Xue Za Zhi. (2015) 95:1483–7. doi: 10.3760/cma.j.issn.0376-2491.2015.19.011
167. Groner B, von Manstein V. Jak Stat signaling and cancer: opportunities, benefits and side effects of targeted inhibition. Mol Cell Endocrinol. (2017) 451:1–14. doi: 10.1016/j.mce.2017.05.033
168. Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. (2011) 148:91–5. doi: 10.1016/j.ijcard.2010.08.058
169. Hu Q, Wu D, Ma F, Yang S, Tan B, Xin H, et al. novel angiogenic activity and molecular mechanisms of ZYZ-803, a slow-releasing hydrogen sulfide-nitric oxide hybrid molecule. Antioxid Redox Signal. (2016) 25:498–514. doi: 10.1089/ars.2015.6607
170. Xiong Y, Chang LL, Tran B, Dai T, Zhong R, Mao YC, et al. ZYZ-803, a novel hydrogen sulfide-nitric oxide conjugated donor, promotes angiogenesis via cross-talk between STAT3 and CaMKII. Acta Pharmacol Sin. (2020) 41:218–28. doi: 10.1038/s41401-019-0255-3
171. Wu D, Hu Q, Ma F, Zhu YZ, Wu D, Hu Q, Ma F, et al. vasorelaxant effect of a new hydrogen sulfide-nitric oxide conjugated donor in isolated rat aortic rings through cGMP Pathway. Oxid Med Cell Longev. (2016) 2016:7075682. doi: 10.1155/2016/7075682
172. Wu D, Hu Q, Xiong Y, Zhu D, Mao Y, Zhu YZ. Novel H2S-NO hybrid molecule (ZYZ-803) promoted synergistic effects against heart failure. Redox Biol. (2018) 15:243–52. doi: 10.1016/j.redox.2017.11.020
173. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. (2001) 20:6008–16. doi: 10.1093/emboj/20.21.6008
174. Kida M, Sugiyama T, Yoshimoto T, Ogawa Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci. (2013) 48:211–5. doi: 10.1016/j.ejps.2012.11.001
175. Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med. (2013) 17:879–88. doi: 10.1111/jcmm.12077
176. Radomski MW, Moncada S. Regulation of vascular homeostasis by nitric oxide. Thromb Haemost. (1993) 70:36–41. doi: 10.1055/s-0038-1646156
177. Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. (2004) 66:523–7. doi: 10.1111/j.1523-1755.2004.761_11.x
Keywords: myocardial ischemia, NO, H2S, H2, gas co-dornor, protecting mechanisms
Citation: Wang W-l, Ge T-y, Chen X, Mao Y and Zhu Y-z (2020) Advances in the Protective Mechanism of NO, H2S, and H2 in Myocardial Ischemic Injury. Front. Cardiovasc. Med. 7:588206. doi: 10.3389/fcvm.2020.588206
Received: 28 July 2020; Accepted: 28 September 2020;
Published: 30 October 2020.
Edited by:
Ian Megson, University of the Highlands and Islands, United KingdomReviewed by:
James Cobley, University of the Highlands and Islands, United KingdomSuowen Xu, University of Science and Technology of China, China
Copyright © 2020 Wang, Ge, Chen, Mao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Chen, Y2hlbnh1QGdsbWMuZWR1LmNu; Yicheng Mao, bWFveWNAZnVkYW4uZWR1LmNu; Yi-zhun Zhu, eXp6aHVAbXVzdC5lZHUubW8=
 Wei-lu Wang
Wei-lu Wang Tian-yu Ge
Tian-yu Ge Xu Chen
Xu Chen Yicheng Mao
Yicheng Mao Yi-zhun Zhu
Yi-zhun Zhu