
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cardiovasc. Med. , 04 December 2020
Sec. Cardio-Oncology
Volume 7 - 2020 | https://doi.org/10.3389/fcvm.2020.568720
This article is part of the Research Topic What do we know about COVID-19 implications for cardiovascular disease? View all 109 articles
 Sherry-Ann Brown1*
Sherry-Ann Brown1* Svetlana Zaharova1
Svetlana Zaharova1 Peter Mason2
Peter Mason2 Jonathan Thompson3
Jonathan Thompson3 Bicky Thapa3
Bicky Thapa3 David Ishizawar2
David Ishizawar2 Erin Wilkes4
Erin Wilkes4 Gulrayz Ahmed3
Gulrayz Ahmed3 Jason Rubenstein2
Jason Rubenstein2 Joyce Sanchez5
Joyce Sanchez5 David Joyce6
David Joyce6 Balaraman Kalyanaraman7
Balaraman Kalyanaraman7 Michael Widlansky2
Michael Widlansky2Overlapping commonalities between coronavirus disease of 2019 (COVID-19) and cardio-oncology regarding cardiovascular toxicities (CVT), pathophysiology, and pharmacology are special topics emerging during the pandemic. In this perspective, we consider an array of CVT common to both COVID-19 and cardio-oncology, including cardiomyopathy, ischemia, conduction abnormalities, myopericarditis, and right ventricular (RV) failure. We also emphasize the higher risk of severe COVID-19 illness in patients with cardiovascular disease (CVD) or its risk factors or cancer. We explore commonalities in the underlying pathophysiology observed in COVID-19 and cardio-oncology, including inflammation, cytokine release, the renin-angiotensin-aldosterone-system, coagulopathy, microthrombosis, and endothelial dysfunction. In addition, we examine common pharmacologic management strategies that have been elucidated for CVT from COVID-19 and various cancer therapies. The use of corticosteroids, as well as antibodies and inhibitors of various molecules mediating inflammation and cytokine release syndrome, are discussed. The impact of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) is also addressed, since these drugs are used in cardio-oncology and have received considerable attention during the COVID-19 pandemic, since the culprit virus enters human cells via the angiotensin converting enzyme 2 (ACE2) receptor. There are therefore several areas of overlap, similarity, and interaction in the toxicity, pathophysiology, and pharmacology profiles in COVID-19 and cardio-oncology syndromes. Learning more about either will likely provide some level of insight into both. We discuss each of these topics in this viewpoint, as well as what we foresee as evolving future directions to consider in cardio-oncology during the pandemic and beyond. Finally, we highlight commonalities in health disparities in COVID-19 and cardio-oncology and encourage continued development and implementation of innovative solutions to improve equity in health and healing.
In early 2020, the World Health Organization (WHO) designated the new, highly contagious, and unnervingly fatal disease COVID-19 caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a global pandemic. By June 1, 2020, the WHO reported more than 6 million confirmed cases and 370,000 deaths across nearly 220 countries and territories, with the US having the highest number of confirmed cases (1.7 million) and deaths (100,000) (1).
Although initially thought to be primarily a lung disease, COVID-19 also involves marked toxicity to the cardiovascular system. As data has emerged, it has become clear to our cardio-oncology group (2–7) that much of the cardiovascular toxicity reported in COVID-19 is also observed in cardio-oncology, with overlap in underlying pathophysiology. Additionally, pharmacologic options frequently used or currently being studied in cardio-oncology are also proving beneficial in COVID-19. This begs the question of whether evaluating commonalities in the toxicities, pathophysiology, and pharmacology of COVID-19 and cardio-oncology would be informative for advancing understanding and avenues for research in cardio-oncology, as well as COVID-19. Cardio-oncology is an emerging field in medicine focused on the prevention, surveillance, detection, and management of injury to the cardiovascular system from cancer therapies or from cancer itself. The cardiovascular injuries are inflicted by an exogenous source, primarily pharmacologic or radiologic cancer therapy. In COVID-19, the cardiovascular injuries are also incited by an exogenous source, primarily SARS-CoV-2. Due to the exogenous nature of the original source of injury, in addition to pathophysiology mediating the injury, some authors refer to these cardiovascular injuries in COVID-19 as “toxicities” (8–10), which is also the term conventionally used in cardio-oncology (11–13). While cancer therapies and SARS-CoV-2 are two very different entities, the havoc they both wreak on the cardiovascular system is thought-provoking.
In this perspective, we share the overarching viewpoint that these commonalities exist and are intriguing, and consequently, the dynamic research efforts surrounding COVID-19 may be able to inform new understanding and avenues for investigation in cardio-oncology. A clear understanding of the mechanisms of various forms of CVT in cardio-oncology remains elusive. Development of novel concepts, paradigms, and drug utilization trends based on observations identified in CVT related to COVID-19 may help advance research and clinical practice in cardio-oncology. To this end, we first present cardiovascular toxicities common to COVID-19 and cardio-oncology, then we expound on underlying pathophysiology. This is followed by description of pharmacologic options being pursued in both COVID-19 and cardio-oncology. Finally, we discuss ramifications of these commonalities in the context of Cardio-Oncologic care and research in the pandemic and beyond (Table 1).
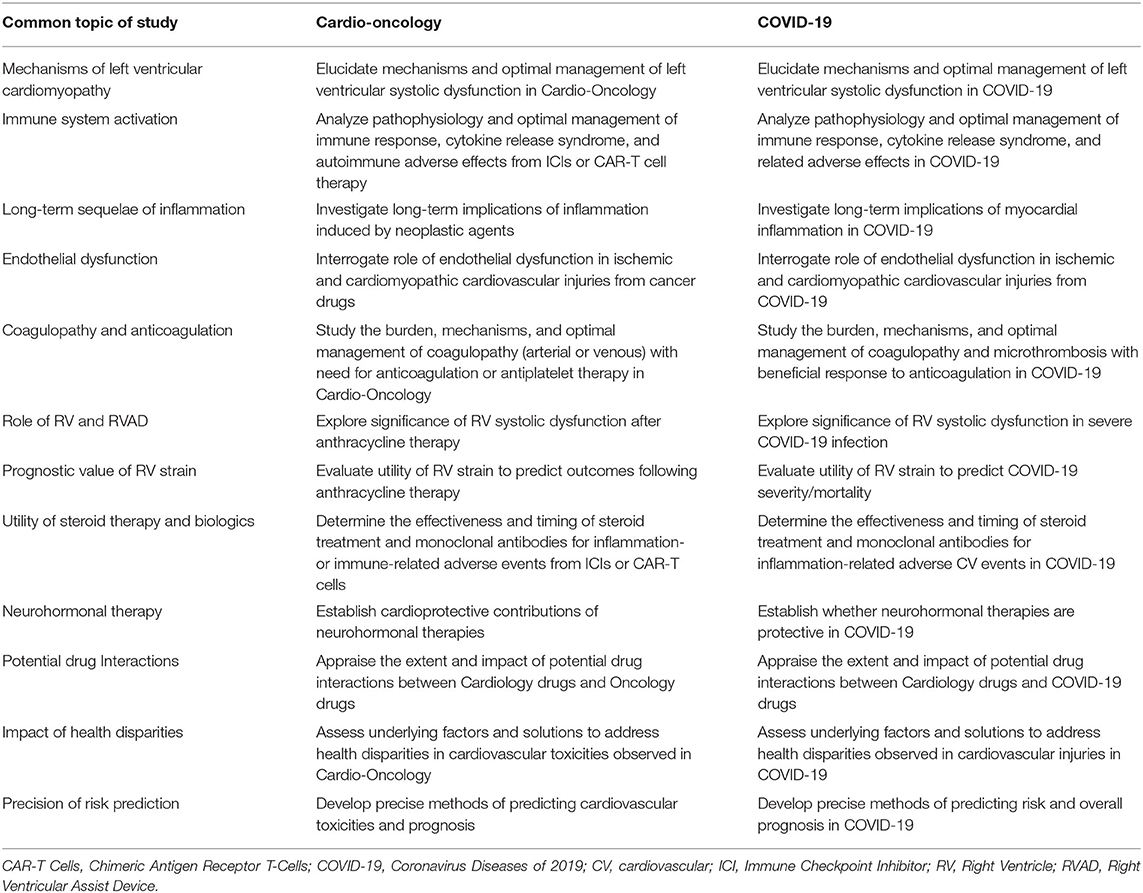
Table 1. Mechanisms, concepts, and paradigms: commonalities in toxicity, pathophysiology, and pharmacology of cardio-oncology and COVID-19.
In COVID-19, SAR-CoV-2 causes direct and indirect cardiovascular injury, which typically manifests as cardiomyopathy, myopericarditis, ischemia, or arrythmia (14–25). SARS-CoV-2 has been discovered in cardiac tissue (15, 25), similar to SARS-CoV-1 infection in which 35% of patients had viral RNA expressed in cardiac tissue (26). Patients with pre-existing CVD and cancer or CVD risk factors (e.g., diabetes mellitus, chronic kidney disease, obesity, and advanced age) are among those at highest risk of poor outcomes, i.e., increased morbidity and mortality from COVID-19 (10, 22, 27–30). According to a retrospective analysis of 72,314 cases in China, patients with pre-existing CVD morbidities had a five-fold increase in mortality, and a COVID-19-related death rate of 10.5% (22). Indirectly, patients with CVD morbidities are inherently more susceptible to the adverse effects of viral infection and the body's adaptive response. The systemic effects of COVID-19 causing fever, hypoxia, hypotension, and tachycardia may not be well-tolerated in patients with underlying cardiomyopathy or obstructive coronary artery disease, and this may manifest as further myocardial injury, and increased incidence of decompensated heart failure and type II myocardial infarction (20). Evidence of myocardial injury (e.g., elevated troponin), is common in patients hospitalized with COVID-19 (10). When present, elevated cardiac biomarkers such as brain natriuretic peptide and serum troponin have been associated with increased mortality in patients with COVID-19 (18).
Similarly, a wide spectrum of cancer therapies has been associated with CVT, such as cardiomyopathy, myopericarditis, ischemia, and arrhythmias (11, 31). Radiation therapy can lead to all of these toxicities in the absence of chemotherapy. Various chemotherapy and targeted cancer therapy regimens can also result in CVT. Anthracyclines most commonly associate with cardiomyopathy, and can also bring about conduction abnormalities, myocarditis, or pericardial disease. Tyrosine kinase inhibitors commonly associate with hypertension, and less commonly with cardiomyopathy or ischemia. Immune checkpoint inhibitors are most notorious for myocarditis, and can also prompt pericarditis, cardiomyopathy, conduction abnormalities, and ischemia. Many other CVT are noted in cardio-oncology, with a variety of drug classes. In addition, tachycardia and elevated biomarkers may also portend poor prognosis in cardio-oncology (32, 33). There is therefore much overlap of CVT and prognostic factors in cardio-oncology with CVT and prognostic biomarkers in COVID-19. Furthermore, some types of cancers and cancer treatments weaken patients' immune systems and increase risk of any infection. Cancer patients' immunosuppression often also associates with blunted or delayed symptoms, which could in turn delay urgent therapy and increase mortality in COVID-19.
Interesting to consider is any potential synergistic CVT in patients on cancer therapies in COVID-19. A prospective cohort of 800 cancer patients with COVID-19 analyzed in late April 2020 linked COVID-19 mortality with older age, male gender and comorbidities such as hypertension and cardiovascular disease (14). There was no association between receipt of cytotoxic chemotherapy, targeted therapies, radiation therapy, or other cancer therapies and COVID-19 mortality in this cohort. Similarly, a retrospective cohort of 928 cancer patients from the USA, Canada and Spain associated advanced age, smoking, progressive malignancy and increased comorbidities COVID-19 mortality, but failed to show associations with cancer type and type of anticancer therapy with COVID-19 mortality (34).Thus, the role of chemotherapy and other cancer systemic therapies in COVID-19 mortality remains uncertain.
Recent studies emerging in parallel in the pandemic and in cardio-oncology indicate that the RV may play an important role in the prognosis of patients with COVID-19 or CVT from cancer therapy; RV failure generally associates with worse outcomes in a variety of populations, and patients with COVID-19 or CVT from cancer therapies may be no different (35–38). While the mechanisms of insult to the RV in COVID-19 are different from those in cardio-oncology, similar changes are noted in the ventricle and these may have prognostic value. Importantly, RV longitudinal strain (RVLS) has emerged as a key player in the prediction of RV failure in both COVID-19 and cardio-oncology (36, 38).
In COVID-19, RVLS inversely associates with myocardial injury, mechanical ventilation, acute respiratory distress syndrome (ARDS), and mortality, as well as signs of systemic inflammation such as heart rate, D-dimer, and C-reactive protein, as well as thromboembolism (38). In addition to RVLS, RV dilation and systolic dysfunction also predict mortality in COVID19 (39). Abnormalities in RV strain, size, and systolic function in COVID-19 may result from ARDS, pulmonary hypertension with increased pulmonary vascular resistance due to acute lung injury or thromboembolism, in addition to CO2 retention, positive pressure ventilation, or other causes of acute myocardial injury (19, 40–46). In one COVID-19 study, of 10 patients with RV dilation, 50% had PE noted on CTA; and of 21 total deaths in that COVID-19 cohort, 62% had RV dilation (39). Some patients with apparent ARDS do not respond as expected to low pressure ventilation strategies per ARDSNet ventilation protocols (47). Prone positioning in COVID-19 improves oxygenation and reduces the risk and need for mechanical ventilation or extracorporeal membrane oxygenation (ECMO) in patients on mechanical ventilation, but the maneuver appears to also reduce the risk of RV failure in ARDS including COVID-19 (48–50). Anecdotally, we have observed that the typical progression from hypoxemic respiratory failure to multi-system organ failure with escalating pressor requirements can be blunted with insertion of a percutaneous right ventricular assist device (RVAD) connected to an oxygenator. In all cases, the pressor requirement has been eliminated upon initiation of RVAD flows. These observations are consistent with our experiences in using these devices to treat other forms of RV failure which are frequently misdiagnosed as distributive shock.
In cardio-oncology, changes are also noted in RV strain, structure, function, and size in patients with breast cancer and lymphoma who receive anthracycline chemotherapy (36, 51). Although the left ventricle is more commonly studied, the RV also shows impairment in contractility, with temporal changes of decreased RVLS and increased right ventricular end systolic volume (RVESV) preceding reduction in right ventricular ejection fraction (RVEF) (36). Additionally, patients with end-stage heart failure as a result of cardiomyopathy from anthracycline therapy benefit from RV assist device support (52). The underlying pathophysiology of RV dysfunction in anthracycline CVT is likely similar to LV dysfunction. LV dysfunction results from release of cytokines and inflammatory markers, related to generation of reactive oxygen species, disruption of mitochondrial biogenesis, and activation of apoptosis, and double-stranded DNA breaks (53–55). This is a recent and novel area of inquiry in cardio-oncology. Additional studies are needed to determine whether RV size, function, and longitudinal strain can predict CVT and mortality in cardio-oncology (36), as has been found in COVID-19.
A multi-ethnic study of more than 3,500 individuals with COVID-19 was published in the New England Journal of Medicine (NEJM) (56). While <40% of patients in the study were hospitalized, African Americans composed almost 80% of inpatients admitted with COVID-19 and associated CVT. A higher rate of comorbidities associated with the risk for hospitalization, and African Americans had higher rates of comorbidities. This is similar to general trends in health disparities, in which African Americans have higher rates of CVD, obesity, hypertension, and diabetes than Caucasians (57), and are therefore at higher risk for CVT related to COVID-19. These disparities were found to associate with inequities in socioeconomic demographics in the NEJM report, as in prior studies (56, 57). Notably, ACE (I/D) polymorphisms have been implicated in COVID-19 and related CVT, and vary across racial groups (58). However, this alone does not explain the disparities observed in COVID-19. The D/D polymorphism that associates with the development and severity of sarcoidosis (59), which is more prevalent, complex, and mortal in African-Americans (60), is the same polymorphism that is suggested to associate with protection in COVID-19 (61–63). Nevertheless, African Americans have had the highest proportions of severe and fatal illness from COVID-19 and consequent CVT. In the same way, CVT in cardio-oncology has been reported at higher rates in African Americans, with similar underlying reasons (64–68).
It is worth continuing to study shared toxicities in COVID-19 and cardio-oncology. For example, increased attention to emerging special topics such as RV strain, function, and predictive value in COVID-19 may help elucidate sequelae of commonalities to optimize care and survival of our patients in COVID-19 and also in cardio-oncology (Figure 1). Perhaps studying the pathophysiology and host characteristics in patients with abnormal RV size, function, and longitudinal strain in COVID-19 could also help us better understand the pathophysiology of abnormal RV size, function, and longitudinal strain in some patients after anthracycline therapy. Further, it will be important to address the disproportionate percentages of African-Americans with severe and fatal CVT related to both COVID-19 (69–73) and cancer therapies in cardio-oncology (64–68).
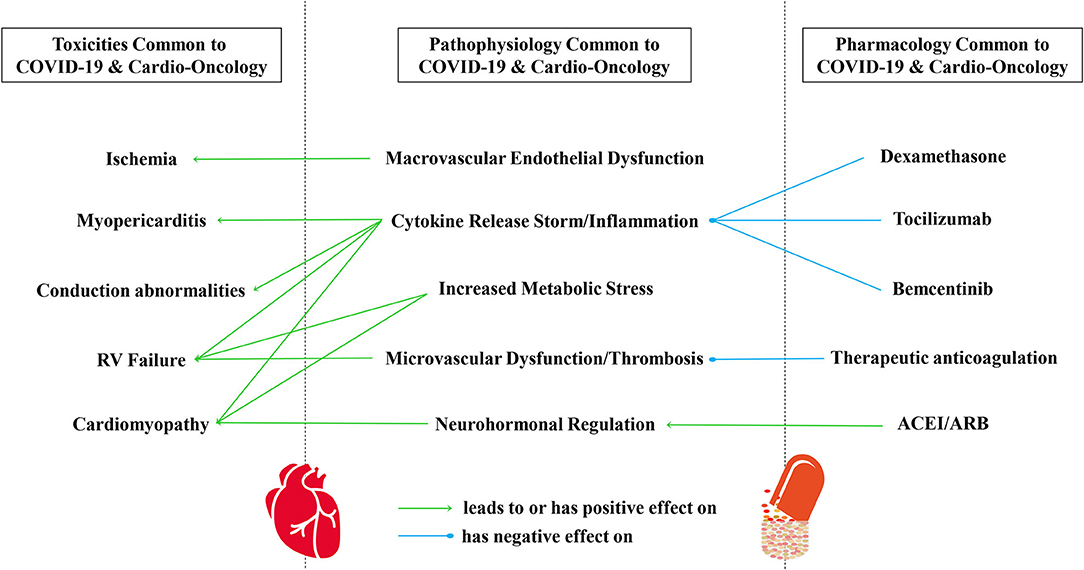
Figure 1. A conceptual framework of commonalities in CV toxicities, pathophysiology, and pharmacology common to COVID-19 and cardio-oncology. CV toxicities common to COVID-19 and cardio-oncology include myocardial injury, cardiomyopathy, myopericarditis, ischemia, conduction abnormalities, and RV failure, in part mediated by immune system activation, cytokine release syndrome, and arterial and venous coagulopathy. All of these are also examples of oncologic CV toxicities that can result from pharmacologic or radiation therapies. Indeed, the pathophysiology and modulation of SARS-CoV-2 infection remains under investigation, with components of viral infection/invasion, macrovascular endothelial dysfunction, cytokine release syndrome/inflammation, microvascular dysfunction/thrombosis, neurohormonal regulation, coagulopathy, and increased metabolic stress. Several pharmacologic considerations have risen to the surface during the pandemic, involving steroids, cancer immunotherapy, biologic antibodies and inhibitors, drug repurposing, the role of cyp450 and drug transporters in drug-drug interactions, anticoagulation, and neurohormonal regulation. ARB, Angiotensin Receptor Blocker; ACEI, Angiotensin Converting Enzyme Inhibitor; CV, Cardiovascular; COVID-19, Coronavirus Disease of 2019; RV, Right Ventricle; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus.
Potential mechanisms of cardiovascular injury in COVID-19 include hemodynamic derangement or hypoxemia, increased metabolic stress, demand ischemia, microvascular dysfunction or thrombosis due to hypercoagulability, or systemic inflammation and cytokine storm, which may also destabilize existing coronary artery plaques (16, 74–76). Although not yet demonstrated with SARS-CoV-2, an autopsy study of people who died from SARS-CoV-1 infection demonstrated that 35% of patients had viral RNA expressed in cardiac tissue (26). Further, a recent study illustrated in vitro direct infection of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) by SARS-CoV-2 (77). Microscopy and RNA-sequencing provided evidence that SARS-CoV-2 enters hiPSC-CMs via the cell surface receptor ACE2. The study also demonstrated that in response to SARS-CoV-2 infection, the hiPSC-CMs upregulated the innate immune response and antiviral clearance gene pathways, in addition to downregulating ACE2 expression.
ACE2 receptors are the SARS-CoV-2 entry point into human cells (10, 78). Patients with pre-existing CVD or CV risk factors, which associate with heightened systemic inflammation, have higher levels of ACE2 receptor expression than the general population (10, 79, 80). In normal physiology, ACE2 is counter-regulatory and anti-inflammatory (79, 80). Interestingly, a particular angiotensin converting enzyme (ACE) genetic polymorphism (D/D), although not a ACE2 polymorphism, associates with decreased ACE2 levels and has been suggested to be protective in patients with COVID-19 (61–63). The physiologic effects of ACE and ACE2 are typically in some degree of homeostatic equilibrium, with ACE mediating inflammation, oxidative stress, and vasoconstriction, and ACE2 also being vasodilatory (81). SARS-CoV-2 may remove ACE2 from this homeostatic pathway due to both the virus and the receptor being internalized from the cell surface in COVID-19 (81).
The inflammatory response elicited by SARS-CoV-2 is implicated in direct suppression of cardiac contractility (75). Evidence of new contractile dysfunction was reported in ~30% of patients with critical illness related to COVID-19, and cardiac or circulatory shock is a common pathway to fatal outcomes (82, 83). This is reminiscent of CVT in cardio-oncology, in which increased metabolic stress, cytokine release, inflammation, macrovascular endothelial dysfunction, microvascular dysfunction, thrombosis, and neurohormonal dysregulation can all result in impairment of cardiac contractility underlying cardiomyopathy.
Two recent studies evaluating immunologic characteristics of peripheral blood samples from COVID-19 patients have emerged from China (84, 85). In these studies, severe cases of COVID-19 were associated with depletion of CD8+ T-cells, suggesting that upregulation of immune checkpoint molecules that downregulate T-cells may play an important role in impairing the immune response to the virus. These early studies should be interpreted with caution given the small sample sizes, and continued investigation will shed light on the mechanisms of immune dysregulation induced by COVID-19.
Immune checkpoint inhibitors (ICIs) are drugs that target immune checkpoint molecules such as programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). These drugs have dramatically improved overall survival for patients with a wide range of malignancies (86). Inflammatory cytokines, such as interferon-γ and type I interferons, induce PD-L1 expression on immune and tumor cells (87). Interaction of the PD-L1 and PD-1 proteins leads to T-cell exhaustion, and blockade of this interaction with PD-1/PD-L1 inhibitors restores effector function to CD8+ T-cells, allowing for destruction of malignant cells. Chief among concerns with ICIs during the pandemic is whether ICIs can increase COVID-19-related complications, particularly CVT. A retrospective study found patients receiving ICIs to be at higher risk of hospitalization and severe outcomes from COVID-19 (88). Strong conclusions are difficult to draw from this small, retrospective, single-center study in which only 31 patients received ICIs. A prospective observational study from the UK Coronavirus Center Monitoring Project found no association between COVID-19 mortality and ICI treatment in the 44 patients who received ICIs (89). Ongoing large-scale prospective data may shed further light on this interaction.
Many cancer patients receiving ICIs possess comorbidities that enhance risk for poor outcomes related to COVID-19. ICIs and COVID-19 can cause overlapping organ toxicities, particularly pulmonary and cardiac, which inform risk-benefit decisions on ICI use during the pandemic. ICIs can induce immune-mediated cardiotoxicity, including myocarditis, pericarditis, heart failure, arrhythmias, and MI. These events are uncommon, occurring in <3% of patients who receive ICIs, but carry high risk of mortality (90).
The pathophysiology of the immunologic mechanisms of cardiotoxicity with ICIs and COVID-19 likely differ, but macrophages may play roles in both pathways, which could contribute to anecdotal response to glucocorticoid responsiveness for ICI and COVID-19 toxicities (Table 2). The renin-angiotensin system has been implicated in the pathophysiology of both COVID-19 and tumorigenesis, with data suggesting the RAAS pathway promotes an immunosuppressive tumor microenvironment (91–93). However, much like COVID-19, the impact of ACE inhibitors on survival outcomes with ICIs is currently unclear (94). Given the prevalence of ICI use, it is essential to exert a coordinated effort to track COVID-19 incidence in patients receiving ICIs, as well as rates of pulmonary and cardiac sequelae and mortality to truly understand the long-term impact of the virus on this large population.
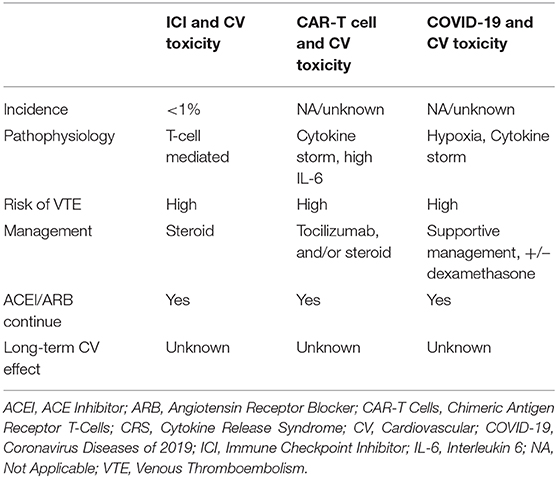
Table 2. Clinical characteristics of similar CV toxicity in ICI therapy, CAR T-cell therapy, and COVID-19.
In COVID-19, the inflammatory cytokine IL-6 has also been shown to play a role in critically ill patients, in whom “cytokine release storm” or “cytokine release syndrome” (CRS) pathophysiology leads to cardiopulmonary complications and multisystem failure (95). Clinical manifestations of CRS include fever, chills, fatigue, myalgias, arthralgias, nausea, vomiting, and diarrhea (96). In the patient with CRS, cardiovascular manifestations include tachycardia, hypotension, elevated troponin, heart failure, and in severe cases, cardiogenic shock (96, 97). IL-6 could possibly mediate cardiac dysfunction and hemodynamic instability (98). In general, IL-6 elevation has associated with cardiovascular complications such as atherosclerosis, MI, and heart failure.
IL-6 and other cytokines are key components of the human body host defense system against infection, yet high levels of these cytokines in a hyperinflammatory response can lead to CRS (99, 100). Cytokine release syndrome can be a fatal complication due to exaggerated inflammatory response in COVID-19, partially mediated by immune cells fighting the viral infection by increasing inflammatory cytokines via activation of intracellular NF-κB (101), but also in large part mediated by the ACE2 and AT1 receptors, which are generally highly expressed on epithelial cells in the lung and endothelium (20, 102–104). A main function of ACE2 is to convert angiotensin II (Ang II) into angiotensin-(1-7), a counter-regulatory peptide that dampens the inflammatory effects of Ang II via AT1 (101, 105). After the SARS-CoV-2 S-protein attaches to ACE2 on respiratory epithelium, ACE2 is down-regulated (77). The resulting SARS-CoV-2-mediated imbalance of serum Ang II/angiotensin-(1-7) drives net activation of AT1 signaling [which is dependent on serum Ang II/angiotensin-(1-7)] in pulmonary epithelial cells. It is well-established that COVID-19 infection causes hyperactivation of the angiotensin 1 receptor (AT1), which leads to disproportionate activation of nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome in lung epithelial cells and endothelium (106, 107), as well as activation of STAT3 and the NF-κB pathway, producing potent pro-inflammatory cytokines IL-6, IL-1β, and IL-18 (101, 108–110). The detrimental pathophysiological consequence of the hyperinflammatory response includes enhanced activation of reactive oxygen species (ROS) release, fibrosis, vasoconstriction, and programmed cell death, that contribute to the CRS pathophysiology. Interestingly, ACE2 and AT1 are known to be expressed at extremely low levels on hematopoietic stem cells (HSC) and endothelial progenitor cells (EPC) (111). A recent study demonstrated for the first time that ACE2 is expressed on very small embryonic-like stem cells (VSELs) (112). Pre-clinical data demonstrated that interaction of ACE2 receptor with the COVID-19 spike protein activated the NLRP3 inflammasome in VSELs and HSC leading to programmed cell death (112); the contribution of this to CRS is yet unclear.
Unlike traditional chemotherapy, CAR-T cell therapy is a novel form of immunotherapy in cardio-oncology to treat individuals with refractory hematologic malignancies, and is commonly associated with toxicity related to CRS. CAR-T cell therapy utilizes genetically engineered T-cells to attack cancer cells (113). The activation of CAR-T cells when engaged with antigen in a malignant cell leads to its CAR-T cell proliferation, which further activates monocytes and macrophages, leading to release of proinflammatory cytokines and chemokines such as IL-6, IL-8, IL-10, interferon-gamma (INF-y), monocyte chemoattractant protein-1b, and granulocyte-macrophage colony-stimulating factor (114, 115). These proinflammatory cytokines are potential mediators for CRS in patients with cancer, with a similar cascade in patients with COVID-19.
Arterial and venous coagulopathy has emerged as an important factor in COVID-19 pathophysiology and cardio-oncology, especially in critically ill patients (116–120), in part related to underlying endothelial cell dysfunction and inflammation in patients with COVID-19 or cancer (117, 121, 122).
Severe COVID-19 infection requiring critical care admission has been associated with increased incidence of venous thromboembolism (VTE) (117, 123), due to hyperinflammation and a hypercoagulable state (124, 125). The incidence has been reported to be 3 to 4-fold greater than in the general population (117, 123). In critically ill patients in the general population, the cumulative incidence of VTE is around 9.6% (126, 127), while in COVID-19 patients it is reported to be between 31 and 42% (117, 123). Thrombotic events in COVID-19 mostly categorize VTE, but in some patients, a significant number of arterial thrombosis are also being reported. In one study, 3.7% of the 31% reported cases had ischemic strokes, while in another study population two ischemic strokes and one limb ischemia were reported (117, 123). Endotheliitis with underlying hyperinflammation, along with hypoxia leading to increased blood viscosity, are suspected to cause increased coagulopathy in severe COVID-19 infection (128, 129). Excess cytokine release also results in macroscopic or microscopic endothelial injury, leading to a prothrombotic state (130). Elevation of D-dimer above normal values on admission or over time during the disease process has been associated with poor outcomes in patients with severe COVID-19 (125). Close monitoring of D-dimer, aPTT/PT, fibrinogen, and platelet count in hospitalized COVID-19 patients is recommended as derangement of these coagulation parameters can be an early sign of disseminated intravascular coagulation (DIC) (125).
A similar phenomenon is observed in cancer patients (131). Similar factors are associated with thrombosis, with circulating microparticles, procoagulants, and endothelial dysfunction contributing to disruption of normal blood flow and hyperviscosity (120, 132, 133). Cancer also poses a 4 times increased risk of VTE as compared to general population while chemotherapy increases the risk to 6.5 times (134). Patients who receive CAR-T cell therapy are also at increased risk for venous thromboembolism, potentially mediated by CRS and high levels of IL-6 (135, 136), in the setting of underlying hypercoagulability due to the presence of the cancer itself. Other pharmacologic cancer therapies can also associate with thrombosis. Cisplatin and tyrosine kinases often lead to coronary or peripheral arterial thrombosis related to endothelial injury, thromboxane synthesis, and platelet activation and aggregation, placing patients at 1.5- to 1.7-fold or as high as 6-fold increased risk of acute coronary syndromes [see review in expert consensus statement (137)].
Furthermore, many chemotherapeutics associate with endothelial dysfunction and consequent ischemia in the absence of thrombosis. In these cases, ischemia is due to vasospasm. This phenomenon can be caused by 5-fluorouracil (5-FU), capecitabine (5-FU pro-drug), paclitaxel, docetaxel, cisplatin (especially when combined with bleomycin or vincristine), cyclophosphamide, and tyrosine kinase inhibitors (e.g., sorafenib and sunitinib) [see review in expert consensus statement (137)]. Undiagnosed underlying coronary artery disease is thought to be a likely pre-disposing condition. Likewise, endothelial dysfunction and consequent ischemia in the absence of thrombosis are also suspected in some patients with COVID-19 who present with ACS and non-obstructed coronary arteries; severe hypoxia, CRS, plaque rupture, vasospasm, and microthromboembolism are also on the differential in these patients (25, 138, 139).
Shared pathophysiology in COVID-19 and cardio-oncology also have important implications. For example, ICIs and CAR-T cells used as cancer therapy can lead to excessive activation of the immune system and inflammation and subsequently autoimmune and inflammatory adverse CV effects. Despite the favorable responses from CAR-T cell and ICI therapy in cardio-oncology, we still have limited evidence and understanding of CVT from these immunotherapies or their long-term impact. We can potentially fill our knowledge gap on CVT due to CRS related to CAR-T cell therapy, or supranormal activation of the immune system related to ICIs, in cardio-oncology by pursuing a better understanding of the inflammatory pathophysiology from the ongoing COVID-19 pandemic. The reverse is also true, and long-term sequelae of CRS on the cardiovascular system should be investigated and addressed in patients who have had COVID-19 or in cancer patients who have received CAR-T cell therapy. Similarly, coagulopathy in COVID-19 is an emerging topic with the majority of evidence stemming from observational studies and autopsies (128). The hypercoagulability of cancer, which is often treated by cardio-oncologists, can be informative for COVID-19, given a role for anticoagulation to address thromboembolism in these hypercoagulable states. Additionally, the role of endothelial dysfunction can be further elucidated in both COVID-19 and cardio-oncology with anticipated shared vascular pathophysiology, albeit with different mechanisms of endothelial injury.
Given the robust inflammatory response induced by COVID-19, corticosteroids are under investigation and have been demonstrating promising efficacy for treating the disease. Dexamethasone has recently garnered significant international attention for the treatment of COVID-19 with the pre-print publication (not yet peer reviewed) of the phase 3 RECOVERY trial. Patients were randomized to dexamethasone at 6 mg daily for up to 10 days vs. standard care. Dexamethasone significantly reduced deaths in patients who required supplemental oxygen or mechanical ventilation (140). Notably, the pre-print manuscript does not quantify the number of patients with cancer included in the analysis and may be difficult to generalize to an oncology population with COVID-19.
Corticosteroids have been mainstays of treatment for immune-related adverse events (irAEs) induced by ICIs and CAR-T cells in cancer patients, owing to their ability to rapidly dampen inflammation and quickly reverse irAEs (141, 142). In the widely utilized, evidence-based irAE management guidelines published by the American Society of Clinical Oncology (ASCO), high-dose corticosteroids are recommended as first-line management of most grade 2 or higher irAEs (143). For cardiovascular irAEs, including myocarditis, pericarditis, heart failure, and vasculitis, high-dose corticosteroids are recommended for any grade of toxicity (141, 142, 144). Thus, steroids may be helpful to quell activated immune responses leading to CVT due to various endogenous sources, whether cancer therapy or COVID-19.
Tocilizumab is an IL-6 receptor antagonist and is indicated as the first-line agent for the management of CRS in cancer patients (145–148). The use of tocilizumab in COVID-19 is an extrapolation based on the evidence of promising outcomes from using the drug to treat CRS from CAR-T cell therapy in cancer patients. Off-label use of tocilizumab is an option used in the management of severe cases of COVID-19 on compassionate grounds, supported by a case series from China (149) and a pilot open, single-arm multicenter study from Italy (150), particularly if tocilizumab is administered within 6 days of admission (HR 2.2, 95% CI 1.3–6.7, p < 0.05) (150). Additionally, a large retrospective cohort study demonstrated that tocilizumab decreased risk of death or minimized risk for invasive mechanical ventilation in patients with severe COVID-19 (adjusted HR 0.61, 95% CI 0.40–0.92; p = 0.020) (151). A smaller, retrospective cohort study demonstrated a significantly shorter need for vasopressor support in severely ill COVID-19 patients who received tocilizumab (152).
Cardiac dysfunction due to CRS is largely reversible, and in severe cases mitigated by tocilizumab (153). In some severe cases not responding to tocilizumab, the corticosteroid is added. In rare cases, when the patient does not respond to tocilizumab or steroid, other agents such as anakinra (IL-1R inhibitor) and etanercept (anti-TNFα) are potential options to hinder inflammatory pathways (114, 154). Siltuximab is a chimeric monoclonal antibody that also binds IL-6; however, no studies have been published on its use in the management of CRS in cancer patients to date (96).
Next generation novel immunotherapeutics could also affect COVID-19-related incidence and outcomes. For instance, AXL is a receptor tyrosine kinase which mediates tumor invasion, metastasis, and epithelial-mesenchymal transition. AXL also negatively modulates cancer immune responses through signaling pathways involving dendritic cells, natural killer cells and macrophages (155). Given its role in cancer metastasis and immune function, numerous AXL inhibitors are being used in clinical trials to treat advanced malignancies. AXL mediates viral entry into cells and modulates inflammatory responses induced by viral infections (156, 157). AXL is also overexpressed on myocardial cells in patients with heart failure and in patients who experience LV remodeling after STEMI (158). It is conceivable that through immune and cardiovascular impacts, investigational drugs that target AXL may impact outcomes of cancer patients with COVID-19 infection, and clinical trial sponsors and investigators should be encouraged to track and study COVID-19-infected trial patients to better understand these complex interactions. To this end, bemcentinib, an oral AXL inhibitor under investigation as a cancer immunotherapeutic, has recently been repurposed to combat COVID-19 as part of the Accelerating COVID-19 Research & Development (ACCORD) platform in the United Kingdom.
Interestingly, human antibodies have been isolated from the convalescent serum of COVID-19 survivors and when coupled have been shown to be protective. Perhaps the use of these emerging dual antibodies may be as efficacious for COVID-19 patients as the dual antibodies trastuzumab and pertuzumab have been for breast cancer patients. Developing therapeutics from antibodies such as these may help provide safer effective options for COVID-19 patients and facilitate avoiding potential or proven drug-drug interactions.
The antiviral drug remdesivir is an investigational drug being used to treat COVID-19, and concomitant use with drugs that are strong CYP3A4 inducers is not recommended (159). The CYP450 enzyme system (which includes CYP3A4) forms the backbone for metabolism of multiple drugs and plays a vital role in metabolism of numerous cardio-oncologic drugs including beta blockers, calcium channel blockers, statins, cyclophosphamide, docetaxel, cisplatin, and tyrosine kinase inhibitors (160). In particular, the antiandrogen drugs apalutamide and enzalutamide used to treat prostate cancer are strong CYP3A4 inducers (161–163). Both agents can associate with CVT, such as atrial fibrillation, hypertension, and ischemic heart disease, especially in individuals with pre-existing cardiovascular diseases (161–164). Concurrent use of remdesivir with these drugs should be avoided at the interface of COVID-19 and cardio-oncology. Of note, in cardio-oncology, the calcium channel blockers diltiazem and verapamil are moderate inhibitors of CYP3A4, which also metabolizes cancer pharmacologic drugs such as doxorubicin, imatinib, and ibrutinib (160). In vitro, remdesivir is a substrate for CYP2C8, CYP2D6, and CYP3A4, and an inhibitor of CYP3A4, as well as a substrate for p-glycoprotein and organic anion transporting polypeptides 1B1 (OATP1B1) and an inhibitor of OATP1B1 (165). P-glycoprotein and OATP1B1 are membrane transporters known to help mediate drug-drug interactions. However, remdesivir generally has a low potential for clinically significant drug-drug interactions mediated by the CYP450 system or drug transporters (165–167), since remdesivir functions as a prodrug that is rapidly metabolized to the active bioavailable form (165, 168).
Empiric therapeutic anticoagulation associates with better prognosis in severe COVID-19 cases, with improved in-hospital mortality in retrospective analyses (129, 169). While practice has varied across centers during the pandemic, anticoagulation should be considered based on COVID-19 patient factors and risk stratification (119).
Empiric prophylactic anticoagulation also associates with better outcomes in cancer patients who are hospitalized and have reduced mobility, or are ambulatory and have (170, 171):
• advanced or metastatic pancreatic cancer,
• intermediate-high VTE risk based on cancer type or Khorana score,
• or treatment with immunomodulatory drugs and steroids or other systemic antineoplastic therapies.
It has been suggested that ACE inhibitors may counteract resulting unopposed ACE-mediated effects in COVID-19 (81). Thus, the influence of these vasoactive and cardiovascular remodeling drugs on the risk and severity of COVID-19 has been under investigation, with some studies suggesting benefit, juxtaposed with initial speculations about harm (28, 172–181). It is unknown whether polymorphisms in ACE, or polymorphisms in ACE2 that may contribute to COVID-19 prognosis [see pre-prints (182, 183)], also determine the prognosis of patients in cardio-oncology treated with RAAS regulators, such as ACE inhibitors and ARBs.
It is important to note that ACEIs and ARBs have established benefits in protecting the myocardium. They are among first-line therapy for various CVT (e.g., cardiomyopathy, hypertension, and myocardial infarction) in cancer patients and survivors, along with beta blockers, to mitigate symptoms and prolong survival (184). Withdrawal of these agents instigates clinical decompensation in high-risk patients, such as rapid relapse of dilated cardiomyopathy in cancer patients with CVT due to neoplastic agents (28, 172, 185). Consequently, patients receiving ACEIs or ARBs should continue ACEI/ARB therapy during the COVID-19 pandemic (172, 186, 187). Taken together, these findings suggest overlapping utility of these drugs in both cardio-oncology and COVID-19.
It is also important to study shared pharmacologic management opportunities in COVID-19 and cardio-oncology. Corticosteroids and immunomodulatory drugs such as tocilizumab and bemcentinib and other analogous therapies are being used or studied in cardio-oncology and have also been repurposed during the pandemic to temper the inflammation milieu initiated by SARS-CoV-2. Further, thousands of cancer patients are currently enrolled in clinical trials combining ICIs with investigational novel therapeutics across the world, accounting for another special risk population in the COVID-19 pandemic. Ongoing clinical trials on anti-interleukins in COVID-19 patients (188, 189) (NCT04330638, NCT04317092) will also help us to elucidate benefits and outcomes. Accordingly, an algorithm has been proposed to incorporate anti-inflammatory agents such as tocilizumab, canakinumab (IL-1β monoclonal antibody), anakinra, etanercept, and infliximab (TNFα monoclonal antibody) to curb CRS in acute COVID-19 infection (190). The antiviral remdesivir carries a low risk of modulating the membrane drug transporter p-glycoprotein and the cytochrome protein 450 family of enzymes and potentially interacting with CV and cardio-oncology drugs. Nevertheless, caution is recommended with the combined use of any drugs that modulate p-glycoprotein or CYP450, due to their potential for drug-drug interactions and resultant effects on the CV system in cardio-oncology and COVID-19. Therapeutic anticoagulants are another class of medications found to be useful in both COVID-19 and cardio-oncology, due to their beneficial effects on thromboembolism. Clinical studies are being pursued to determine the impact of direct oral anticoagulants or aspirin and statin to limit arterial or venous thrombotic risk in cancer (120). Regulators of the RAAS (primarily ACEI/ARB) have also taken centerstage, as many patients are on these medications to treat hypertension or other common comorbidities that increase the risk of a more severe course in those with COVID-19. There has been debate about whether these RAAS modulator drugs augment the risk of COVID-19 infection, given the virus' use of the ACE receptor to enter host cells. ACE receptor gene polymorphisms have also been implicated in the prediction of disease severity, with questionable regulation by the ACEI/ARB drug class. Pursuit of observational studies and clinical trials continues to help elucidate the impact of ACEIs and ARBs in the pandemic (27). Further study should also define the interplay between SARS-CoV-2 and RAAS and explore any differential effects of ACEI vs. ARB therapy and tumor specific responses (91, 93, 94, 185). Future collaboration among basic and clinical scientists should focus on the biological rationale for the treatment of COVID-19 patients as well as limited understanding with respect to the interaction of RAAS inhibitors, ACE2 levels and SARS CoV-2 infectivity in humans (27).
As we recover from the COVID-19 pandemic, we should not let opportunities for learning surreptitiously slip from our grasp. The myriad of overlap of CV toxicities, pathophysiology, and innovative management in COVID-19 and cardio-oncology provide multiple paths for exploration that could lead to greater understanding of both COVID-19 and CVT noted in cardio-oncology (Figure 1). It would behoove us in cardio-oncology to continue to closely study these toxicities, pathophysiology, and pharmacologic options in COVID-19 to help update our understanding in cardio-oncology. Cardio-oncology continues to expand as a relatively new medical subspecialty. Knowledge gaps in CVT toxicity, pathophysiology, and pharmacology in cardio-oncology may benefit from the application of novel concepts, paradigms, and drug use from overlapping forms of CVT in COVID-19 (Table 1).
In addition to short-term morbidity and mortality, patients who recover from COVID-19 infection may be at increased risk of future incident CVD and CVD-related complications (191, 192). Severe acute respiratory syndrome coronavirus (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV) infection have been implicated in causing diabetes, hypertension and altered lipid metabolism (193–195). The increase in CVD risk profile combined with the possibility of viral-mediated impairment in cardiac and/or pulmonary function may combine to further increase the risk and complexity of future CVD events. Aggressive risk factor modification and prophylactic therapy may prove important in mitigating long-term CVT. Optimal prevention and management of CVT will require a multidisciplinary approach with close collaborations among various medical specialties and researchers.
As our current practices change and new questions arise, future studies in cardio-oncology should focus on studying the emerging cardiovascular epidemiology of COVID-19, as well as the impact of changed practices on the health of patients with cancer and CVD. To reduce the rate of transmission while providing safe and timely care for patients with cancer and CVD, temporary recommendations favored telehealth visits in telecardio-oncology and deferral of non-urgent procedures, similar to the rest of the population (10, 196). Cardiac imaging surveillance was limited to patients who were more likely to have abnormal testing or at higher risk for cancer-related CVT, particularly if test results would guide initiation of cardioprotective medications or impact cancer therapy delivery (197).
Of utmost importance is ensuring equity in our distribution of hope, health, and healing in the midst of and beyond the pandemic, as we extend lessons learned from COVID-19 to cardio-oncology. Ethnic health disparities during the pandemic have amplified a pre-existing broken healthcare structure, with disproportionate percentages of African-Americans severely and fatally affected by COVID-19 (69–73). The pandemic has been set on a backdrop of inequity, in which African-American cancer patients are known to be more susceptible to CVT following cancer therapies (64–68). The higher risk for African Americans in both COVID-19 and cardio-oncology is of multifactorial etiology, including higher rates of CVD and CVD risk factors, which are often also underdiagnosed and undertreated (57, 69, 198–206). Underlying causes of the plethora of inequalities in healthcare are largely structural and socioeconomic and reflect our imperfections as a society, with socioeconomic status being a risk factor for CVD, CVT, and COVID-19 (198, 207, 208). We must recognize the imbalance of comorbidities and sociodemographics in ethnic populations, in order to make equitable progress in the post-pandemic era. The lasting impact of COVID-19 in cardio-oncology need not be the challenges we faced while caring for our patients during the pandemic. The long-term sequelae should be steps we have taken to optimize quality and quantity of life for all.
Thus, there are several areas of overlap, similarity, and interaction in the toxicity, pathophysiology, and pharmacology profiles in COVID-19 and cardio-oncology syndromes. Learning more about either will likely provide some level of insight into both, with further illumination of CVT mechanisms and new paradigms of drug utilization to help guide research and clinical practice in both COVID-19 and cardio-oncology. Such an approach can be informative peri-pandemic, and should perhaps be pursued long after the pandemic, to assess for evidence of long-term independent or synergistic CV effects in survivors of COVID-19 and cancer, with equity at the forefront of our efforts.
The original contributions generated in the study are included in the article, further inquiries can be directed to the corresponding author.
S-AB conceived, designed, and helped draft the manuscript. SZ, PM, JT, BT, DI, EW, GA, JR, JS, and DJ helped draft the manuscript. BK and MW helped design and draft the manuscript. All authors revised and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. WHO. WHO Coronavirus Disease (COVID-19) Dashboard. (2020). Available online at: https://covid19.who.int/ (accessed October 01, 2020).
2. Brown SA. Preventive cardio-oncology: the time has come. Front Cardiovasc Med. (2019) 6:187. doi: 10.3389/fcvm.2019.00187
3. Brown SA, Rhee JW, Guha A, Rao VU. Innovation in precision cardio-oncology during the coronavirus pandemic and into a post-pandemic world. Front Cardiovasc Med. (2020) 7:145. doi: 10.3389/fcvm.2020.00145
4. Brown SA, Sandhu N, Herrmann J. Systems biology approaches to adverse drug effects: the example of cardio-oncology. Nat Rev Clin Oncol. (2015) 12:718–31. doi: 10.1038/nrclinonc.2015.168
5. Brown SA, Nhola L, Herrmann J. Cardiovascular toxicities of small molecule tyrosine kinase inhibitors: an opportunity for systems-based approaches. Clin Pharmacol Ther. (2017) 101:65–80. doi: 10.1002/cpt.552
6. Brown SA, Ray JC, Herrmann J. Precision cardio-oncology: a systems-based perspective on cardiotoxicity of tyrosine kinase inhibitors and immune checkpoint inhibitors. J Cardiovasc Transl Res. (2020) 13:402–16. doi: 10.1007/s12265-020-09992-5
7. Brown SA, Okwuosa TM, Barac A, Volgman AS. The role of angiotensin-converting enzyme inhibitors and β-blockers in primary prevention of cardiac dysfunction in breast cancer patients. J Am Heart Assoc. (2020) 9:e015327. doi: 10.1161/JAHA.119.015327
8. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. (2020) 116:1666–87. doi: 10.1093/cvr/cvaa106
9. Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA, Bohula EA. A current review of COVID-19 for the cardiovascular specialist. Am Heart J. (2020) 226:29–44. doi: 10.1016/j.ahj.2020.04.025
10. Ganatra S, Hammond SP, Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncology. (2020) 2:350–5. doi: 10.1016/j.jaccao.2020.03.001
11. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. (2017) 70:2536–51. doi: 10.1016/j.jacc.2017.09.1096
12. Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, et al. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J Am Coll Cardiol. (2015) 65:2739–46. doi: 10.1016/j.jacc.2015.04.059
13. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. (2019) 140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497
14. Cheng P, Zhu H, Witteles RM, Wu JC, Quertermous T, Wu SM, et al. Cardiovascular risks in patients with COVID-19: potential mechanisms and areas of uncertainty. Curr Cardiol Rep. (2020) 22:34. doi: 10.1007/s11886-020-01293-2
15. Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvêa M, Viu Degaspare N, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. (2020) 4:790–4. doi: 10.1016/S2352-4642(20)30257-1
16. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:819–24. doi: 10.1001/jamacardio.2020.1096
17. Kochav SM, Coromilas E, Nalbandian A, Ranard LS, Gupta A, Chung MK, et al. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. (2020) 13:e008719. doi: 10.1161/CIRCEP.120.008719
18. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
19. Zeng J-H, Liu Y-X, Yuan J, Wang F-X, Wu W-B, Li J-X, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. (2020) 48:773–7. doi: 10.1007/s15010-020-01424-5
20. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. doi: 10.1038/s41569-020-0360-5
21. Zhu H, Rhee JW, Cheng P, Waliany S, Chang A, Witteles RM, et al. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr Cardiol Rep. (2020) 22:32. doi: 10.1007/s11886-020-01292-3
22. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
23. Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. (2020) 2:1326–30. doi: 10.1016/j.jaccas.2020.04.009
24. Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. (2020) 395:1516. doi: 10.1016/S0140-6736(20)30912-0
25. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. (2020) 22:911–5. doi: 10.1002/ejhf.1828
26. Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. (2009) 39:618–25. doi: 10.1111/j.1365-2362.2009.02153.x
27. Ky B, Mann DL. COVID-19 clinical trials: a primer for the cardiovascular and cardio-oncology communities. JACC CardioOncol. (2020) 5:501–17. doi: 10.1016/j.jacbts.2020.04.003
28. Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. (2020) 323:1769–70. doi: 10.1001/jama.2020.4812
29. Asokan I, Rabadia SV, Yang EH. The COVID-19 pandemic and its impact on the cardio-oncology population. Curr Oncol Rep. (2020) 22:60. doi: 10.1007/s11912-020-00945-4
30. Lee LYW, Cazier JB, Starkey T, Turnbull CD, Kerr R, Middleton G, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. (2020) 395:1919–26.
31. Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol. (2017) 70:2552–65. doi: 10.1016/j.jacc.2017.09.1095
32. Singh D, Thakur A, Tang WH. Utilizing cardiac biomarkers to detect and prevent chemotherapy-induced cardiomyopathy. Curr Heart Fail Rep. (2015) 12:255–62. doi: 10.1007/s11897-015-0258-4
33. Meinardi MT, Van Der Graaf WT, Gietema JA, Van Den Berg MP, Sleijfer DT, De Vries EG, et al. Evaluation of long-term cardiotoxicity after epirubicin containing adjuvant chemotherapy and locoregional radiotherapy for breast cancer using various detection techniques. Heart. (2002) 88:81–2. doi: 10.1136/heart.88.1.81
34. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. (2020) 395:1907–18. doi: 10.1016/S0140-6736(20)31187-9
35. Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:1463–82. doi: 10.1016/j.jacc.2018.12.076
36. Zhao R, Shu F, Zhang C, Song F, Xu Y, Guo Y, et al. Early detection and prediction of anthracycline-induced right ventricular cardiotoxicity by 3-dimensional echocardiography. JACC CardioOncol. (2020) 2:13–22. doi: 10.1016/j.jaccao.2020.01.007
37. Nagata Y, Wu VC, Kado Y, Otani K, Lin FC, Otsuji Y, et al. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3D echocardiography. Circ Cardiovasc Imaging. (2017) 10:e005384. doi: 10.1161/CIRCIMAGING.116.005384
38. Li Y, Li H, Zhu S, Xie Y, Wang B, He L, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. (2020) 13:2287–99. doi: 10.1016/j.jcmg.2020.04.014
39. Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. (2020) 13:2459–61. doi: 10.1016/j.jcmg.2020.05.010
40. Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. (2016) 42:862–70. doi: 10.1007/s00134-015-4141-2
41. Bull TM, Clark B, McFann K, Moss M. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. (2010) 182:1123–8. doi: 10.1164/rccm.201002-0250OC
42. Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. (2009) 35:1850–8. doi: 10.1007/s00134-009-1569-2
43. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
44. Xie Y, Wang X, Yang P, Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothoracic Imaging. (2020) 2:e200067. doi: 10.1148/ryct.2020200067
45. Rotzinger D, Beigelman-Aubry C, von Garnier C, Qanadli S. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res. (2020) 190:58–9. doi: 10.1016/j.thromres.2020.04.011
46. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. (2020) 142:184–6. doi: 10.1161/CIRCULATIONAHA.120.047430
47. Network ARDS. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. (2000) 342:1301–8. doi: 10.1056/NEJM200005043421801
48. Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest. (2007) 132:1440–6. doi: 10.1378/chest.07-1013
49. Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. (2013) 368:2159–68. doi: 10.1056/NEJMoa1214103
50. Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. (2020) 8:e48.
51. Boczar KE, Aseyev O, Sulpher J, Johnson C, Burwash IG, Turek M, et al. Right heart function deteriorates in breast cancer patients undergoing anthracycline-based chemotherapy. Echo Res Pract. (2016) 3:79–84. doi: 10.1530/ERP-16-0020
52. Oliveira GH, Dupont M, Naftel D, Myers SL, Yuan Y, Tang WH, et al. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: outcomes from the INTERMACS Registry (interagency registry for mechanically assisted circulatory support). J Am Coll Cardiol. (2014) 63:240–8. doi: 10.1016/j.jacc.2013.09.040
53. Vejpongsa P, Yeh ET. Topoisomerase 2β: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther. (2013) 95:45–52. doi: 10.1038/clpt.2013.201
54. Nebigil CG, Désaubry L. Updates in anthracycline-mediated cardiotoxicity. Front Pharmacol. (2018) 9:1262. doi: 10.3389/fphar.2018.01262
55. Moazeni S, Cadeiras M, Yang EH, Deng MC, Nguyen KL. Anthracycline induced cardiotoxicity: biomarkers and “Omics” technology in the era of patient specific care. Clin Transl Med. (2017) 6:17. doi: 10.1186/s40169-017-0148-3
56. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with covid-19. N Engl J Med. (2020) 382:2534–43. doi: 10.1056/NEJMsa2011686
57. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. (2017) 136:e393–423. doi: 10.1161/CIR.0000000000000534
58. Ned RM, Yesupriya A, Imperatore G, Smelser DT, Moonesinghe R, Chang MH, et al. The ACE I/D polymorphism in US adults: limited evidence of association with hypertension-related traits and sex-specific effects by race/ethnicity. Am J Hypertens. (2012) 25:209–15. doi: 10.1038/ajh.2011.182
59. Fløe A, Hoffmann HJ, Nissen PH, Møller HJ, Hilberg O. Genotyping increases the yield of angiotensin-converting enzyme in sarcoidosis–a systematic review. Dan Med J. (2014) 61:A4815.
60. Mirsaeidi M, Machado RF, Schraufnagel D, Sweiss NJ, Baughman RP. Racial difference in sarcoidosis mortality in the United States. Chest. (2015) 147:438–49. doi: 10.1378/chest.14-1120
61. Trojanowicz B, Ulrich C, Fiedler R, Martus P, Storr M, Boehler T, et al. Modulation of leucocytic angiotensin-converting enzymes expression in patients maintained on high-permeable haemodialysis. Nephrol Dial Transplant. (2018) 33:34–43. doi: 10.1093/ndt/gfx206
62. Delanghe JR, Speeckaert MM, De Buyzere ML. The host's angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. (2020) 505:192–3. doi: 10.1016/j.cca.2020.03.031
63. Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. (2020) 6:11. doi: 10.1038/s41421-020-0147-1
64. Hasan S, Dinh K, Lombardo F, Kark J. Doxorubicin cardiotoxicity in African Americans. J Natl Med Assoc. (2004) 96:196–9.
65. Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D'Ascenzo F, Malavasi V, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. (2013) 112:1980–4. doi: 10.1016/j.amjcard.2013.08.026
66. Finkelman BS, Putt M, Wang T, Wang L, Narayan H, Domchek S, et al. Arginine-nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. J Am Coll Cardiol. (2017) 70:152–62. doi: 10.1016/j.jacc.2017.05.019
67. Litvak A, Batukbhai B, Russell SD, Tsai HL, Rosner GL, Jeter SC, et al. Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer. (2018) 124:1904–11. doi: 10.1002/cncr.31260
68. Baron KB, Brown JR, Heiss BL, Marshall J, Tait N, Tkaczuk KH, et al. Trastuzumab-induced cardiomyopathy: incidence and associated risk factors in an inner-city population. J Card Fail. (2014) 20:555–9. doi: 10.1016/j.cardfail.2014.05.012
70. Haynes N, Cooper LA, Albert MA, Cardiologists AoB. At the heart of the matter: unmasking and addressing COVID-19's toll on diverse populations. Circulation. (2020) 142:105–7. doi: 10.1161/CIRCULATIONAHA.120.048126
71. Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet. (2020) 395:1243–4. doi: 10.1016/S0140-6736(20)30893-X
72. Douglas M, Katikireddi SV, Taulbut M, McKee M, McCartney G. Mitigating the wider health effects of covid-19 pandemic response. BMJ. (2020) 369:m1557. doi: 10.1136/bmj.m1557
73. Chung RY, Dong D, Li MM. Socioeconomic gradient in health and the covid-19 outbreak. BMJ. (2020) 369:m1329. doi: 10.1136/bmj.m1329
74. Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. Reply. N Engl J Med. (2019) 380:e21. doi: 10.1056/NEJMc1901647
75. Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. (2018) 72:2071–81. doi: 10.1016/j.jacc.2018.08.1043
76. Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. (2020) 173:350–61. doi: 10.7326/M20-2566
77. Sharma A, Garcia G, Arumugaswami V, Svendsen CN. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. bioRxiv. (2020). doi: 10.1101/2020.04.21.051912
78. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
79. Perlot T, Penninger JM. ACE2—from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. (2013) 15:866–73. doi: 10.1016/j.micinf.2013.08.003
80. Anguiano L, Riera M, Pascual J, Valdivielso JM, Barrios C, Betriu A, et al. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transpl. (2015) 30:1176–85. doi: 10.1093/ndt/gfv025
81. South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. (2020) 318:H1084–H90. doi: 10.1152/ajpheart.00217.2020
82. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. (2020) 323:1612–4. doi: 10.1001/jama.2020.4326
83. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. (2020) 46:1294–7. doi: 10.1007/s00134-020-06028-z
84. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. (2020) 17:533–5. doi: 10.1038/s41423-020-0402-2
85. Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. (2020) 17:541–3. doi: 10.1038/s41423-020-0401-3
86. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. (2019) 2:e192535. doi: 10.1001/jamanetworkopen.2019.2535
87. Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. (2019) 29:3766. doi: 10.1016/j.celrep.2019.11.113
88. Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. (2020) 26:1218–23. doi: 10.1038/s41591-020-0979-0
89. Lee LYW, Cazier JB, Starkey T, Turnbull CD, Kerr R, Middleton G, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. (2020) 395:1919–26. doi: 10.1016/S0140-6736(20)31173-9
90. Oren O, Yang EH, Molina JR, Bailey KR, Blumenthal RS, Kopecky SL. Cardiovascular health and outcomes in cancer patients receiving immune checkpoint inhibitors. Am J Cardiol. (2020) 125:1920–6. doi: 10.1016/j.amjcard.2020.02.016
91. Xie G, Cheng T, Lin J, Zhang L, Zheng J, Liu Y, et al. Local angiotensin II contributes to tumor resistance to checkpoint immunotherapy. J Immunother Cancer. (2018) 6:88. doi: 10.1186/s40425-018-0401-3
92. Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci Transl Med. (2017) 9:eaan5616. doi: 10.1126/scitranslmed.aan5616
93. Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ. (2018) 363:k4209. doi: 10.1136/bmj.k4209
94. Medjebar S, Richard C, Fumet J-D, Malo J, Elkrief A, Blais N, et al. Angiotensin-converting enzyme inhibitor prescription is associated with decreased progression-free survival (PFS) and overall survival (OS) in patients with lung cancers treated with PD-1/PD-L1 immune checkpoint blockers. Am Soc Clin Oncol. (2019) 37:e20512. doi: 10.1200/JCO.2019.37.15_suppl.e20512
95. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). Treasure Island, FL: StatPearls Publishing Copyright, LLC (2020).
96. Yáñez L, Sánchez-Escamilla M, Perales MA. CAR T cell toxicity: current management and future directions. Hemasphere. (2019) 3:e186. doi: 10.1097/HS9.0000000000000186
97. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. (2018) 15:47–62. doi: 10.1038/nrclinonc.2017.148
98. Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. (2004) 363:203–9. doi: 10.1016/S0140-6736(03)15326-3
99. Huang L, Zhao X, Qi Y, Li H, Ye G, Liu Y, et al. Sepsis-associated severe interleukin-6 storm in critical coronavirus disease 2019. Cell Mol Immunol. (2020) 17:1092–4. doi: 10.1038/s41423-020-00522-6
100. Liu Z, Li J, Chen D, Gao R, Zeng W, Chen S, et al. Dynamic interleukin-6 level changes as a prognostic indicator in patients with COVID-19. Front Pharmacol. (2020) 11:1093. doi: 10.3389/fphar.2020.01093
101. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. (2020) 20:355–62. doi: 10.1038/s41577-020-0331-4
102. Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. (2020) 92:595–601. doi: 10.1002/jmv.25726
103. Ratajczak MZ, Kucia M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia. (2020) 34:1726–9. doi: 10.1038/s41375-020-0887-9
104. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. (2005) 11:875–9. doi: 10.1038/nm1267
105. Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. (2020) 46:1105–8. doi: 10.1007/s00134-020-06059-6
106. Ren XS, Tong Y, Ling L, Chen D, Sun HJ, Zhou H, et al. NLRP3 gene deletion attenuates angiotensin II-induced phenotypic transformation of vascular smooth muscle cells and vascular remodeling. Cell Physiol Biochem. (2017) 44:2269–80. doi: 10.1159/000486061
107. Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. (2019) 10:50. doi: 10.3389/fmicb.2019.00050
108. Schieffer B, Bünte C, Witte J, Hoeper K, Böger RH, Schwedhelm E, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. (2004) 44:362–8. doi: 10.1016/j.jacc.2004.03.065
109. Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. (2006) 48:1050–7. doi: 10.1161/01.HYP.0000248135.97380.76
110. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. (2019) 19:477–89. doi: 10.1038/s41577-019-0165-0
111. Park TS, Zambidis ET. A role for the renin-angiotensin system in hematopoiesis. Haematologica. (2009) 94:745–7. doi: 10.3324/haematol.2009.006965
112. Ratajczak MZ, Bujko K, Ciechanowicz A, Sielatycka K, Cymer M, Marlicz W, et al. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45. Stem Cell Rev Rep. (2020) 20:1–12. doi: 10.1007/s12015-020-10010-z
113. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
114. Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. (2018) 24:731–8. doi: 10.1038/s41591-018-0041-7
115. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. (2018) 6:56. doi: 10.1186/s40425-018-0343-9
116. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. (2020) 18:844–7. doi: 10.1111/jth.14768
117. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) 191:145–7. doi: 10.1016/j.thromres.2020.04.013
118. Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. (2011) 364:1305–14. doi: 10.1056/NEJMoa1014475
119. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. (2020) 75:2950–73. doi: 10.1016/j.jacc.2020.04.031
120. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. (2017) 70:926–38. doi: 10.1016/j.jacc.2017.06.047
121. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
122. Bick RL. Cancer-associated thrombosis. N Engl J Med. (2003) 349:109–11. doi: 10.1056/NEJMp030086
123. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. (2020) 46:1089–98. doi: 10.1007/s00134-020-06062-x
124. Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. (2020) 507:167–73. doi: 10.1016/j.cca.2020.04.027
125. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. (2020) 135:2033–40. doi: 10.1182/blood.2020006000
126. Cook D, Crowther M, Meade M, Rabbat C, Griffith L, Schiff D, et al. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. (2005) 33:1565–71. doi: 10.1097/01.CCM.0000171207.95319.B2
127. Zhang C, Zhang Z, Mi J, Wang X, Zou Y, Chen X, et al. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore). (2019) 98:e15833. doi: 10.1097/MD.0000000000015833
128. Becker RC. COVID-19 update: covid-19-associated coagulopathy. J Thromb Thrombolysis. (2020) 50:54–67. doi: 10.1007/s11239-020-02134-3
129. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. (2020) 18:1094–9. doi: 10.1111/jth.14817
130. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. (2018) 16:231–41. doi: 10.1111/jth.13911
131. Thachil J, Falanga A, Levi M, Liebman H, Di Nisio M, Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Management of cancer-associated disseminated intravascular coagulation: guidance from the SSC of the ISTH. J Thromb Haemost. (2015) 13:671–5. doi: 10.1111/jth.12838
132. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. (2008) 111:4902–7. doi: 10.1182/blood-2007-10-116327
133. Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. (2020) 38:496–520. doi: 10.1200/JCO.19.01461
134. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. (2003) 107(Suppl. 1):I17-21. doi: 10.1161/01.CIR.0000078466.72504.AC
135. Liu H, Yang Y, Jiang J, Wang X, Zhang C, Jiang Y, et al. Coexistence of a huge venous thromboembolism and bleeding tendency in cytokine release syndrome during CAR-T therapy. Onco Targets Ther. (2019) 12:8955–60. doi: 10.2147/OTT.S223697
136. Hashmi H, Mirza A-S, Darwin A, Logothetis C, Garcia F, Kommalapati A, et al. Incidence and management of venous thrombo-embolism (VTE) associated with CD19-directed chimeric antigen receptor (CAR) T-cell therapy: a single institution experience. Biol Blood Marrow Transplant. (2020) 26:S265. doi: 10.1016/j.bbmt.2019.12.430
137. Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologia intervencionista). Catheter Cardiovasc Interv. (2016) 87:E202–23. doi: 10.1002/ccd.26379
138. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. (2020) 382:2478–80. doi: 10.1056/NEJMc2009020
139. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. (2020) 191:9–14. doi: 10.1016/j.thromres.2020.04.024
140. Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. (2020) NEJMoa2021436. doi: 10.1101/2020.06.22.20137273
141. Ghosh AK, Chen DH, Guha A, Mackenzie S, Walker JM, Roddie C. CAR T cell therapy–related cardiovascular outcomes and management. Systemic disease or direct cardiotoxicity? JACC CardioOncol. (2020) 2:97–109. doi: 10.1016/j.jaccao.2020.02.011
142. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
143. Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline summary. J Oncol Pract. (2018) 14:247–9. doi: 10.1200/JOP.18.00005
144. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. (2019) 115:854–68. doi: 10.1093/cvr/cvz026
145. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. (2014) 6:224ra25. doi: 10.1126/scitranslmed.3008226
146. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. (2014) 371:1507–17. doi: 10.1056/NEJMoa1407222
147. Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. (2018) 23:943–7. doi: 10.1634/theoncologist.2018-0028
148. Abboud R, Keller J, Slade M, DiPersio JF, Westervelt P, Rettig MP, et al. Severe cytokine-release syndrome after t cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant. (2016) 22:1851–60. doi: 10.1016/j.bbmt.2016.06.010
149. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. (2020) 92:814–8. doi: 10.1002/jmv.25801
150. Sciascia S, Aprà F, Baffa A, Baldovino S, Boaro D, Boero R, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. (2020) 38:529–32.
151. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. (2020) 2:e474–84. doi: 10.1016/S2665-9913(20)30173-9
152. Kewan T, Covut F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine. (2020) 24:100418. doi: 10.1016/j.eclinm.2020.100418
153. Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. (2019) 74:3099–108. doi: 10.1016/j.jacc.2019.10.038
154. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. (2014) 20:119–22. doi: 10.1097/PPO.0000000000000035
155. Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. (2019) 18:153. doi: 10.1186/s12943-019-1090-3
156. Morizono K, Xie Y, Olafsen T, Lee B, Dasgupta A, Wu AM, et al. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. (2011) 9:286–98. doi: 10.1016/j.chom.2011.03.012
157. Fujimori T, Grabiec AM, Kaur M, Bell TJ, Fujino N, Cook PC, et al. The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal Immunol. (2015) 8:1021–30. doi: 10.1038/mi.2014.129
158. Caldentey G, García De Frutos P, Cristóbal H, Garabito M, Berruezo A, Bosch X, et al. Serum levels of growth arrest-specific 6 protein and soluble AXL in patients with ST-segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. (2019) 8:708–16. doi: 10.1177/2048872617740833
159. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. (2020). Available online at: https://www.covid19treatmentguidelines.nih.gov/ (accessed October 01, 2020).
160. Fatunde OA, Brown SA. The role of CYP450 drug metabolism in precision cardio-oncology. Int J Mol Sci. (2020) 21:604. doi: 10.3390/ijms21020604
161. Iacovelli R, Verri E, Cossu Rocca M, Aurilio G, Cullurà D, De Cobelli O, et al. The incidence and relative risk of cardiovascular toxicity in patients treated with new hormonal agents for castration-resistant prostate cancer. Eur J Cancer. (2015) 51:1970–7. doi: 10.1016/j.ejca.2015.06.106
162. Lu-Yao G, Nikita N, Keith SW, Nightingale G, Gandhi K, Hegarty SE, et al. Mortality and hospitalization risk following oral androgen signaling inhibitors among men with advanced prostate cancer by pre-existing cardiovascular comorbidities. Eur Urol. (2020) 77:158–66. doi: 10.1016/j.eururo.2019.07.031
163. Saltalamacchia G, Frascaroli M, Bernardo A, Quaquarini E. Renal and cardiovascular toxicities by new systemic treatments for prostate cancer. Cancers (Basel). (2020) 12:1750. doi: 10.3390/cancers12071750
164. Angiolillo DJ, Fernández-Ortiz A, Bernardo E, Barrera Ramírez C, Sabaté M, Fernandez C, et al. Platelet aggregation according to body mass index in patients undergoing coronary stenting: should clopidogrel loading-dose be weight adjusted? J Invasive Cardiol. (2004) 16:169–74.
165. Yang K. What do we know about remdesivir drug interactions? Clin Transl Sci. (2020) 13:842–4. doi: 10.1111/cts.12815
166. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of covid-19—preliminary report. N Engl J Med. (2020) 383:1813–26. doi: 10.1056/NEJMoa2007764
167. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2020) 395:1569–78. doi: 10.1016/S0140-6736(20)31022-9
168. Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J Med Chem. (2017) 60:1648–61. doi: 10.1021/acs.jmedchem.6b01594
169. Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. (2020) 76:122–4. doi: 10.1016/j.jacc.2020.05.001
170. Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. (2019) 20:e566–81. doi: 10.1016/S1470-2045(19)30750-8
171. Key NS, Bohlke K, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update summary. J Oncol Pract. (2019) 15:661–4. doi: 10.1200/JOP.19.00368
172. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N Engl J Med. (2020) 382:1653–9. doi: 10.1056/NEJMsr2005760
173. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. (2005) 436:112–6. doi: 10.1038/nature03712
174. Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. (2017) 21:234. doi: 10.1186/s13054-017-1823-x
175. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. (2020) 126:1671–81. doi: 10.1161/CIRCRESAHA.120.317242
176. Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:1020–6. doi: 10.1001/jamacardio.2020.1855
177. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of covid-19. N Engl J Med. (2020) 382:2441–8. doi: 10.1056/NEJMoa2008975
178. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N Engl J Med. (2020) 382:2431–40. doi: 10.1056/NEJMoa2006923
179. de Abajo FJ, Rodríguez-Martín S, Lerma V, Mejía-Abril G, Aguilar M, García-Luque A, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. (2020) 395:1705–14. doi: 10.1016/S0140-6736(20)31030-8
180. Alashi A, Lang R, Seballos R, Feinleib S, Sukol R, Cho L, et al. Reclassification of coronary heart disease risk in a primary prevention setting: traditional risk factor assessment. Cardiovasc Diagn Ther. (2019) 9:214–20. doi: 10.21037/cdt.2019.04.05
181. Khera R, Clark C, Lu Y, Guo Y, Ren S, Truax B, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease-19. medRxiv. (2020). doi: 10.1101/2020.05.17.20104943
182. Stawiski EW, Diwanji D, Suryamohan K, Gupta R, Fellouse FA, Sathirapongsasuti F, et al. Human ACE2 receptor polymorphisms predict SARS-CoV-2 susceptibility. bioRxiv. (2020). doi: 10.1101/2020.04.07.024752
183. Calcagnile M, Forgez P, Iannelli A, Bucci C, Alifano M, Alifano P. ACE2 polymorphisms and individual susceptibility to SARS-CoV-2 infection: insights from an in silico study. bioRxiv. (2020). doi: 10.1101/2020.04.23.057042
184. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. (2016) 37:1671–80. doi: 10.1093/eurheartj/ehw022
185. Sommerstein R, Kochen MM, Messerli FH, Grani C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. (2020) 9:e016509. doi: 10.1161/JAHA.120.016509
186. Cardiology ACo. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail. (2020) 26:370. doi: 10.1016/j.cardfail.2020.04.013
187. Simone Gd. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. European Society of Cardiology (2020).
188. National Cancer Institute N. COVID-19 and Its Implications for Thrombosis and Anticoagulation: ClinicalTrials.gov Identifier: NCT04317092. (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04317092 (accessed October 01, 2020).
189. Bart N. Lambrecht UH, Ghent. ClinicalTrials.gov. (2020). Available online at: https://www.clinicaltrials.gov/ct2/show/NCT04330638 (accessed April 24, 2020).
190. Wang L, Zhang Y, Zhang S. Cardiovascular impairment in COVID-19: learning from current options for cardiovascular anti-inflammatory therapy. Front Cardiovasc Med. (2020) 7:78. doi: 10.3389/fcvm.2020.00078
191. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. (2020) 5:831–40. doi: 10.1001/jamacardio.2020.1286
192. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. (2020) 41:1798–800. doi: 10.1093/eurheartj/ehaa231
193. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. (2010) 47:193–9. doi: 10.1007/s00592-009-0109-4
194. Zhang Z, He G, Filipowicz NA, Randall G, Belov GA, Kopek BG, et al. Host lipids in positive-strand RNA virus genome replication. Front Microbiol. (2019) 10:286. doi: 10.3389/fmicb.2019.00286
195. Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. (2010) 8:422–32. doi: 10.1016/j.chom.2010.10.006
196. Parikh A, Kumar AA, Jahangir E. Cardio-oncology care in the time of COVID-19 and the role of telehealth. JACC CardioOncol. (2020) 2:356–8. doi: 10.1016/j.jaccao.2020.04.003
197. Calvillo-Arguelles O, Abdel-Qadir H, Ky B, Liu JE, Lopez-Mattei JC, Amir E, et al. Modified routine cardiac imaging surveillance of adult cancer patients and survivors during the COVID-19 pandemic. JACC CardioOncol. (2020) 2:345–9. doi: 10.1016/j.jaccao.2020.04.001
198. Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socio-economic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Ann Epidemiol. (2010) 20:617–28. doi: 10.1016/j.annepidem.2010.05.003
199. Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, et al. Twenty-two year trends in incidence of myocardial infarction, CHD mortality, and case-fatality in four US communities, 1987 to 2008. Circulation. (2012) 125:1848–57. doi: 10.1161/CIRCULATIONAHA.111.047480
200. Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. (2014) 348:135–8. doi: 10.1097/MAJ.0000000000000308
201. Braithwaite D, Tammemagi CM, Moore DH, Ozanne EM, Hiatt RA, Belkora J, et al. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer. (2009) 124:1213–9. doi: 10.1002/ijc.24054
202. Breathett K, Liu WG, Allen LA, Daugherty SL, Blair IV, Jones J, et al. African Americans are less likely to receive care by a cardiologist during an intensive care unit admission for heart failure. JACC Heart Fail. (2018) 6:413–20. doi: 10.1016/j.jchf.2018.02.015
203. Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Ferdinand K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. (2004) 351:2049–57. doi: 10.1056/NEJMoa042934
204. Giblin EM, Adams KF Jr, Hill L, Fonarow GC, Williams FB, Sharma PP, et al. Comparison of hydralazine/nitrate and angiotensin receptor neprilysin inhibitor use among black versus nonblack americans with heart failure and reduced ejection fraction (from CHAMP-HF). Am J Cardiol. (2019) 124:1900–6. doi: 10.1016/j.amjcard.2019.09.020
205. Frierson GM, Howard EN, DeFina LE, Powell-Wiley TM, Willis BL. Effect of race and socioeconomic status on cardiovascular risk factor burden: the cooper center longitudinal study. Ethn Dis. (2013) 23:35–42.
206. Liu Q, Leisenring WM, Ness KK, Robison LL, Armstrong GT, Yasui Y, et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the childhood cancer survivor study. J Clin Oncol. (2016) 34:1634–43. doi: 10.1200/JCO.2015.66.3567
207. Caplin DA, Smith KR, Ness KK, Hanson HA, Smith SM, Nathan PC, et al. Effect of population socioeconomic and health system factors on medical care of childhood cancer survivors: a report from the childhood cancer survivor study. J Adolesc Young Adult Oncol. (2017) 6:74–82. doi: 10.1089/jayao.2016.0016
Keywords: cardio-oncology, COVID-19, pandemic, telemedicine, inflammation, cytokine release syndrome, right ventricle, health disparities
Citation: Brown S-A, Zaharova S, Mason P, Thompson J, Thapa B, Ishizawar D, Wilkes E, Ahmed G, Rubenstein J, Sanchez J, Joyce D, Kalyanaraman B and Widlansky M (2020) Pandemic Perspective: Commonalities Between COVID-19 and Cardio-Oncology. Front. Cardiovasc. Med. 7:568720. doi: 10.3389/fcvm.2020.568720
Received: 01 June 2020; Accepted: 06 November 2020;
Published: 04 December 2020.
Edited by:
Hendrik Tevaearai Stahel, Bern University Hospital, SwitzerlandReviewed by:
Abdelrahman Ibrahim Abushouk, Harvard Medical School, United StatesCopyright © 2020 Brown, Zaharova, Mason, Thompson, Thapa, Ishizawar, Wilkes, Ahmed, Rubenstein, Sanchez, Joyce, Kalyanaraman and Widlansky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sherry-Ann Brown, c2hicm93bkBtY3cuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.