- Division of Cardiology, Department of Medicine, the Lillehei Heart Institute, University of Minnesota at Twin Cities, Minneapolis, MN, United States
Atrial fibrillation (AF) is one of the most common types of arrhythmias and increases cardiovascular morbidity and mortality. Current therapeutic approaches to AF that focus on rhythm control have high recurrence rates and no life prolongation value. While possible explanations include toxicity of current therapies, another likely explanation may be that current therapies do not address fundamental mechanisms of AF initiation and maintenance. Inflammation has been shown to affect signaling pathways that lead to the development of AF. This paper reviews the roles of inflammation in the occurrence, development, and mechanisms of AF and reviews the therapeutic implications of the correlation of inflammation and AF.
Introduction
Atrial fibrillation (AF) is one of the most common abnormal heart rhythms. AF influences more than 33.5 million people worldwide (1, 2). AF is associated with clinical consequences that reduce the quality of life and increase mortality from cardiovascular disease (3). The onset and maintenance of AF may have different mechanisms, but it is clear that structural and electrical remodeling perpetuate AF. Structural remodeling includes atrial fibrosis, a process closely related to inflammation (4). Inflammatory infiltration has been observed in atria of AF patients (5), and inflammation is known to affect signaling pathways for AF development (4). In this review article, the relationship between inflammation and AF, possible novel mechanistic understandings, and therapeutic approaches arising from this association will be discussed.
The Relationship Between Inflammation and the Pathogenesis of AF
Inflammation can alter atrial electrophysiology and structure to increase the vulnerability to AF. These two effects are known as atrial electrical and structural remodeling, respectively. AF initiation, by triggers, and maintenance, by a change in substrate, are likely by distinct but overlapping mechanisms.
A major substrate for chronic AF is thought to be atrial fibrosis and the associated slowing and disarray of conduction (6, 7). Evidence for the relationship of AF and fibrosis includes the degree of atrial fibrosis positively associated with AF persistence (8) and the occurrence and recurrence of postoperative AF in patients undergoing open heart surgery (9, 10). The observed changes in atrial structure during AF include atrial dilatation, atrial cardiomyocyte hypertrophy, dedifferentiation, fibrosis, apoptosis, and myolysis (11). Fibrosis is a hallmark of structural remodeling and is an important AF substrate (11). Overexpressing TGFβ1, a profibrotic cytokine, increases atrial fibrosis and vulnerability of AF (12). TNF-α, discussed below, may contribute to AF by activating the TGF-β/Smad2/3 signaling pathway to induce atrial fibrosis (13). In addition, Galectin-3 is thought to act as a marker of fibrosis (14), and elevated levels of circulating galectin-3 predict the prevalence and incidence of AF (15). These examples point out a plausible link between inflammation and AF through structural remodeling.
AF is a hypercoagulable state, and hypercoagulability is associated with systemic inflammation and can promote fibrosis. In adult atrial fibroblasts, thrombin has been shown to cause fibrotic and inflammatory responses. In transgenic mice, enhanced thrombin increased the episodes of AF. In AF goats, decreased thrombin generation reduced AF complexity and AF-related fibrosis. These results suggest that activated coagulation plays a potential role in atrial remodeling (16).
AF electrical remodeling is classically thought to include action potential shortening, reducing electrical connections between cells, and alterations in Ca2+ handling. Connexins form gap junctions linking atrial myocytes electrically, and alterations of the distribution and amount of atrial connexin 40 and connexin 43 are associated with inflammation (17). Furthermore, NF-κB may alter the expression of the sodium channel, which is the main channel generating current for conduction (18). Therefore, there are plausible ways in which inflammation may contribute to electrical remodeling and the risk for AF.
Evidence for an Association Between Local Inflammation and AF
Local inflammatory conditions, including pericarditis and myocarditis, are associated with a high incidence of AF (19). Consistent with local inflammation contributing to the arrhythmia, AF patients have immune cell infiltrates in the atria (20), and activation of leukocytes is increased in patients with perioperative AF (21). This suggests that immune cell infiltration in the atria may be a link between inflammation and AF. For example, AF patients have higher CD45+ lymphocytes (22) and CD68+ macrophages counts in the atria than do controls.
Suggesting a role for innate immune responses, cardiac MCP-1, a cytokine that can recruit monocytes, dendritic cells and memory T cells, is increased in AF patients (23) and is also associated with circulating fibrosis biomarkers (24). The level of MCP-1-Induced Protein is increased in age-related AF patients compared with the other groups (24). Toll-like receptors (TLRs) are involved in innate immunity, and TLR 2 and 4 have been shown to be potential novel biomarkers for new-onset AF after acute myocardial infarction (25).
The NLRP3 (NACHT, LRR, and PYD domain containing protein 3) inflammasome is an important inflammatory signaling complex and central to innate immunity. The components of NLRP3 inflammasome have been found in both cardiomyocytes (CMs) and cardiac fibroblasts (7). NLRP3 inflammasome activation contributes to formation of caspase-1 to produce the active forms of interleukins-18 (IL-18) and IL-1β, two pro-inflammatory cytokines (26). The activity of NLRP3 inflammasome is elevated in the atrial CMs from paroxysmal and chronic AF patients (27), and expressions of NLRP3, ASC, and active caspase-1 (Casp1-p20) are increased in persistent AF patients (28). In a mouse with constitutive expression of active NLRP3, spontaneous atrial ectopy and inducible AF occur (27). Furthermore, in AF dogs and spontaneous AF mice, the activity of the NLRP3 inflammasome is enhanced in atrium (27). Therefore, the activation of the NLRP3 inflammasome leads to electric and structural remodeling in atrium to contribute to AF (28). Proposed mechanisms linking NLRP3 activation to AF include abnormal sarcoplasmic reticulum Ca2+ release (7), action potential shortening, and atrial hypertrophy.
Evidence of a Relationship Between Systemic Inflammation and AF
Inflammation is a necessary biological process for the defense of organisms against pathogens (29, 30). Nevertheless, uncontrolled inflammation constitutes a positive feedback loop that can lead to numerous unintended diseases (20). There is considerable evidence to support a direct relationship between inflammation and AF (31). For example, new-onset AF has been observed frequently in the critically ill or in patients with sepsis (32, 33). Pneumococcal pneumonia has also been shown to be associated with AF (34).
The systemic inflammatory response after coronary artery bypass grafting is correlated with AF occurrence (35). In the FIBRO-RISK study, biomarkers of inflammation and myocardial fibrosis were validated as predictors for AF recurrence (36). Elevated inflammatory biomarkers levels in serum are correlated with prevalence and prognosis of AF (37, 38). In a prospective pilot study including patients with paroxysmal AF undergoing catheter ablation, patients with up-regulation of inflammatory biomarkers had more frequent early recurrence of AF in the first post-ablation week (39).
The relationship of AF with systemic inflammation is suggested by an increased prevalence in patients with autoimmune diseases. Rheumatoid arthritis (RA) is correlated with the development and maintenance of AF (40–42). In many clinical studies, the prevalence of AF is shown to be increased in RA patients (43). In a collagen-induced arthritis rat model, RA can cause AF and increase AF duration (44), suggesting roles in the initiation and maintenance of the arrhythmia (41).
Other systemic inflammatory conditions are associated with AF. Psoriasis is correlated with increase of AF risk in a nationwide cohort of these patients (45). In large population-based studies and in meta-analyses, psoriatic arthritis patients have a higher AF risk than in the general population (45–47). Furthermore, the psoriasis severity tracks with AF risk, suggesting a cause and effect relationship (45). In inflammatory bowel disease (IBD) patients, P-wave dispersion, a risk factor for AF development, is higher than that in healthy individuals (48). Furthermore, atrial electromechanical delay is higher in active IBD patients (49, 50), again suggesting a cause and effect relationship between the two conditions. In patients with systemic sclerosis and systemic lupus erythematosus, AF and atrial ectopic beats are frequent, and the rate of transient AF occurrence may be 20–30% (51). In ankylosing spondylitis patients, the risk of AF has been shown higher than in the general population in large population-based studies and meta-analyses (52–54). In summary, local and systemic inflammation are associated with AF. Nevertheless, the relationships vary depending on the type of inflammation. This suggests the possibilities that inflammation may be a stronger causative agent in some types of AF than in others and that different types of inflammation may be more or less proarrhythmic.
Inflammatory Biomarkers are Correlated With AF
Biomarker profiles can predict AF risk and the prognosis after AF ablation (55, 56). Nevertheless, the initiating and sustaining factors for AF may be different, and inflammation has been associated with both. In a study on patients with new onset AF, the early recurrence of AF was related to inflammatory markers, and inflammatory markers were associated with development of permanent AF (57). A list of possible inflammatory factors correlated with AF is presented in (Table 1), and data supporting the associations are discussed below.
C reactive protein (CRP), an acute-phase protein whose circulating concentrations rise in response to inflammation, is increased in patients with AF (58). Moreover, there is a dose dependence of CRP and AF risk (59), and elevated CRP predicts the incidence of AF after cardioversion, catheter ablation or cardiac surgery (60–62). The AF-CRP relationship seems to hold for the high sensitivity test. High-sensitivity CRP is associated with AF development and persistence (63), and predicts increased mortality in AF patients (64). Nevertheless, CRP and AF were not correlated when measured before cardioversion (65), and CRP has not been helpful when used to predict postoperative AF (66, 67). These findings indicate that CRP may not be pathogenic in AF and that there may be different relationships between AF and inflammation depending on the inciting cause or longevity of the AF. For example, it is conceivable that inflammation has different relationships to the initiation and maintenance of AF. Alternatively, different types of inflammation may have varying degrees of effect on AF.
Interleukins (ILs) are a group of cytokines involved in the inflammatory response. IL-1 is a key factor regulating innate immune and inflammatory responses. IL-1β is secreted by activated macrophages. There is evidence that IL-1β activation is involved in pressure overload-induced AF (71). IL-2 directly affects T cell activation. IL-2 is associated with shortening of the action potential duration (72), and increased blood levels of IL-2 are correlated with AF risk in the early period after coronary bypass surgery (73). In addition, IL-2 can predict AF after cardioversion (74). IL-6 acts as a pro-inflammatory cytokine and initiates activations of Janus Kinase and Ras-mediated signaling. IL-6 is upstream of CRP and TNF-α production (75, 76). Therefore, it stands to reason from the above discussion that high levels of serum IL-6 are linked to the recurrence of AF after electrical cardioversion and catheter ablation (61). Elevated circulating IL-6 levels have also been correlated with increased incidence of AF (77) and development of AF in postoperative bypass surgery patients (78). Nevertheless, IL-6 does not differ between new-onset AF and chronic AF, suggesting that IL-6 may be better associated with initiation than maintenance of AF (79). IL-8 induces migration of leukocytes and leads to phagocytosis (5). IL-8 levels are increased in the right atrium and coronary sinus in permanent AF patients, compared with paroxysmal AF patients, and elevated IL-8 levels have been shown also in those with longer AF durations (80). After cardioversion, the IL-18 level is elevated in patients with AF recurrence (74, 80). Despite the relationship of some inflammatory cytokines to AF risk, the direct pathogenic role of these cytokines remains to be established.
TNF-α, a cytokine present in systemic inflammation, is associated with fibrosis (68). Higher TNF-α levels are linked to greater risk of AF (61) and with AF presence in the setting of valvular disease (69). The levels of TNF-α are also increased in patients with permanent AF, compared with patients with paroxysmal AF (23). Furthermore, soluble TNF-α predicts exercise-induced AF vulnerability (70). Finally, myeloperoxidase (MPO) produces hypohalous acid in response to infection. Circulated MPO predicts AF recurrence after AF ablation (81).
Correlation of Inflammatory Cell Populations and AF
White blood cells (WBC) possibly play a role in AF pathogenesis. In the Framingham Heart Study, WBC count was associated with the incidence of AF (82) and with AF recurrence in a recent meta-analysis (83). The neutrophil/lymphocyte ratio (NLR), a routinely available marker of the systemic inflammatory response, predicts AF after bypass surgery (84). The NLR also predicts AF occurrence and recurrence in patients undergoing ablation or surgery (85–87). In a retrospective study in patients with acute AF who were successfully converted to sinus rhythm by amiodarone, NLR was shown to predict AF recurrence (88). Nevertheless, in another prospective cohort study in patients undergoing a successful electrical cardioversion in non-valvular AF, NLR was not useful to predict AF recurrence (89).
In summary, systemic inflammatory diseases are associated with AF, and a worsening inflammatory state is related to a higher AF risk. These relationships suggest a cause and effect conjunction. Given the wide range and systemic nature of the inflammatory diseases associated with AF, the implication is that the immune effect on the heart can be transmitted by blood and that a wide range of systemic inflammatory mediators may trigger AF.
Inflammation Suppression And AF
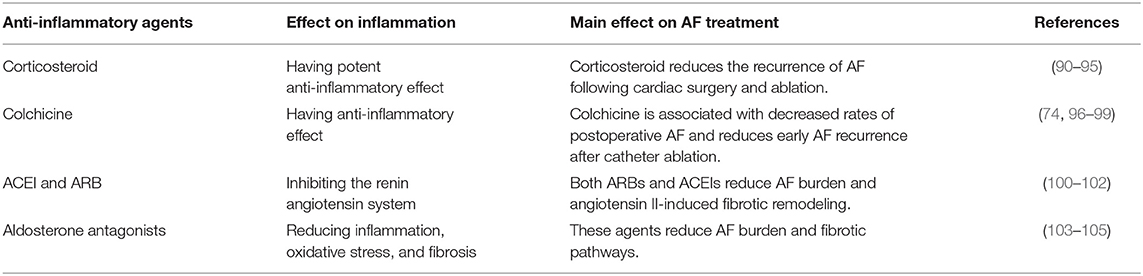
Another line of evidence suggesting a relationship between inflammation and AF comes from the antiarrhythmic effects of suppression of inflammation. Possible anti-inflammatory treatments for AF are shown in (Table 2).
Corticosteroids have a potent anti-inflammatory effect on reducing recurrence of AF after ablation therapy (90, 91) and post-operative AF after cardiac surgery (91, 92). Moreover, low-dose corticosteroids prevent the recurrence of AF (93). Just as the relationship of inflammatory markers and AF was not uniform, high dose steroids are associated with increase of arrhythmia risk (94) and, in one trial, steroids did not impact the clinical outcomes of AF ablation (95). Since steroids have multiple side effects and a complex effect on AF, they may not be an ideal therapy. Nevertheless, steroids affecting AF is another line of evidence supporting the relationship of AF and inflammation.
Colchicine has an anti-inflammatory effect (96), and short-term use of colchicine has been linked to decreasing the rates of postoperative AF (97) and also reducing early recurrence of AF after catheter ablation (98). In a meta-analysis, colchicine decreases AF after cardiac surgery or radiofrequency ablation (99). This beneficial effect of colchicine on preventing AF has been attributed to the decreased level of CPR and IL-6 (74).
Inflammation is closely related to the activation of the renin angiotensin system (RAS). Both angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) can reduce new-onset AF in patients with hypertension. In a study of 82 patients with paroxysmal AF, an ACEI and an ARB reduced AF burden and inflammation (100). In a mouse model, AF can be inhibited by suppressing angiotensin II-induced fibrotic remodeling (101). Suggesting complexity of the relationship between RAS and AF, the administration of an ACEI and an ARB increased the risk of AF after cardiac bypass in a retrospective study (102). Aldosterone is part of the same RAS system, and aldosterone antagonists might be beneficial for AF via decreasing inflammation, oxidative stress, and fibrosis (103). Spironolactone treatment significantly prevented the alterations of atrial structure and function and reduced fibrotic pathways in a canine model with persistent AF. Spironolactone therapy also reduced the burden of AF in human studies (104). Moreover, mineralocorticoid receptor antagonists attenuate postoperative and heart failure related AF (105), and eplerenone can reduce AF burden (105).
Other treatments suggesting a relationship between AF and inflammation include TNF-α gene ablation preventing structural remodeling of the atrium and decreased vulnerability of AF in response to exercise in exercised mice (70). Moreover, renal denervation can decrease inducibility of AF via reduction of sympathetic RAS activity and inhibition of inflammation and fibrosis in a model of renal impairment (106).
Summary
AF is a complex disease with a multifactorial etiology. Data reviewed in this article supports the idea that inflammation may be one driver of AF. Mechanisms of inflammation-related AF include inflammation-induced alteration of electrophysiological properties, initiation of early and late afterdepolarizations, remodeling of cardiac structure, and enhanced fibrosis. These inflammatory factors induce the occurrence of ectopic activity and re-entry which contribute to the initiation and maintenance of AF. Inflammatory diseases are associated with AF, AF is characterized by increased inflammatory markers, and anti-inflammatory treatments decrease AF risk. Both systemic and cardiac localized inflammation are associated with AF risk.
Nevertheless, inflammation is multifaceted, and different types of inflammation appear to affect AF risk differently. Moreover, inflammation seems to have variable effects on different types and durations of AF. Therefore, there is much left to sort out about the relationship between AF and inflammation.
Prospects and Limitations
Current AF therapies are limited by high rates of recurrence and complications. Generally, they fail to address the underlying pathology and exacerbate ion channel dysregulation and structural inhomogeneities that are linked to disease progression. Along with the unchanged mortality rates with a rhythm management strategy, this situation implies that we have a good deal more room for better therapeutic options. Moreover, it suggests that the current strategies may not be addressing fully the underlying pathophysiological drivers of AF. One such driver appears to be inflammation.
While a clear causal link between AF and inflammation has yet to be determined, anti-inflammatory therapeutic strategies may be a next logical step in AF care, one not burdened by proarrhythmic risk.
Author Contributions
XZ wrote the content of the manuscript. SD provided critical review.
Funding
This work is supported by National Institutes of Health grant R01HL104025 and R01HL134791 (to SD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. (2014) 114:1453–68. doi: 10.1161/CIRCRESAHA.114.303211
2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. (2014) 129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119
3. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.1093/eurheartj/ehw210
4. Ciconte G, Conti M, Evangelista M, Pappone C. Atrial fibrillation in autoimmune rheumatic diseases: from pathogenesis to treatment. Rev Recent Clin Trials. (2018) 13:170–5. doi: 10.2174/1574887113666180418110721
5. Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. (2015) 79:495–502. doi: 10.1253/circj.CJ-15-0138
6. Abe I, Teshima Y, Kondo H, Kaku H, Kira S, Ikebe Y, et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. (2018) 15:1717–27. doi: 10.1016/j.hrthm.2018.06.025
7. Chen G, Chelu MG, Dobrev D, Li N. Cardiomyocyte inflammasome signaling in cardiomyopathies and atrial fibrillation: mechanisms and potential therapeutic implications. Front Physiol. (2018) 9:1115. doi: 10.3389/fphys.2018.01115
8. Gramley F, Lorenzen J, Plisiene J, Rakauskas M, Benetis R, Schmid M, et al. Decreased plasminogen activator inhibitor and tissue metalloproteinase inhibitor expression may promote increased metalloproteinase activity with increasing duration of human atrial fibrillation. J Cardiovasc Electrophysiol. (2007) 18:1076–82. doi: 10.1111/j.1540-8167.2007.00906.x
9. Saito T, Tamura K, Uchida D, Saito T, Togashi M, Nitta T, et al. Histopathological features of the resected left atrial appendage as predictors of recurrence after surgery for atrial fibrillation in valvular heart disease. Circ J. (2007) 71:70–8. doi: 10.1253/circj.71.70
10. Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. (2002) 54:390–6. doi: 10.1016/S0008-6363(02)00251-1
11. De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. (2011) 89:754–65. doi: 10.1093/cvr/cvq357
12. Verheule S, Sato T, Everett Tt, Engle SK, Otten D, Rubart-von der Lohe M, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-β1. Circ Res. (2004) 94:1458–65. doi: 10.1161/01.RES.0000129579.59664.9d
13. Liew R, Khairunnisa K, Gu Y, Tee N, Yin NO, Naylynn TM, et al. Role of tumor necrosis factor-α in the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circ J. (2013) 77:1171–9. doi: 10.1253/circj.CJ-12-1155
14. Kang Q, Li X, Yang M, Fernando T, Wan Z. Galectin-3 in patients with coronary heart disease and atrial fibrillation. Clin Chim Acta. (2018) 478:166–70. doi: 10.1016/j.cca.2017.12.041
15. Fashanu OE, Norby FL, Aguilar D, Ballantyne CM, Hoogeveen RC, Chen LY, et al. Galectin-3 and incidence of atrial fibrillation: the Atherosclerosis risk in communities (ARIC) study. Am Heart J. (2017) 192:19–25. doi: 10.1016/j.ahj.2017.07.001
16. Spronk HM, De Jong AM, Verheule S, De Boer HC, Maass AH, Lau DH, et al. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur Heart J. (2017) 38:38–50. doi: 10.1093/eurheartj/ehw119
17. Ryu K, Li L, Khrestian CM, Matsumoto N, Sahadevan J, Ruehr ML, et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am J Physiol Heart Circ Physiol. (2007) 293:H1231–41. doi: 10.1152/ajpheart.00607.2006
18. Shang LL, Sanyal S, Pfahnl AE, Jiao Z, Allen J, Liu H, et al. NF-κB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. (2008) 294:C372–9. doi: 10.1152/ajpcell.00186.2007
19. Morgera T, Di Lenarda A, Dreas L, Pinamonti B, Humar F, Bussani R, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. (1992) 124:455–67. doi: 10.1016/0002-8703(92)90613-Z
20. Scott L J, Li N, Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int J Cardiol. (2019) 287:195–200. doi: 10.1016/j.ijcard.2018.10.020
21. Fontes ML, Mathew JP, Rinder HM, Zelterman D, Smith BR, Rinder CS. Multicenter study of perioperative ischemia research: atrial fibrillation after cardiac surgery/cardiopulmonary bypass is associated with monocyte activation. Anesth Analg. (2005) 101:17–23. doi: 10.1213/01.ANE.0000155260.93406.29
22. Chen MC, Chang JP, Liu WH, Yang CH, Chen YL, Tsai TH, et al. Increased inflammatory cell infiltration in the atrial myocardium of patients with atrial fibrillation. Am J Cardiol. (2008) 102:861–5. doi: 10.1016/j.amjcard.2008.05.038
23. Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. (2010) 7:438–44. doi: 10.1016/j.hrthm.2009.12.009
24. Zhang G, Abuduoufu A, Zhou X, Li Y, Zhang L, Lu Y, et al. Monocyte chemoattractant protein-1-induced protein in age-related atrial fibrillation and its association with circulating fibrosis biomarkers. Cardiology. (2019) 142:244–9. doi: 10.1159/000499932
25. Zhang P, Shao L, Ma J. Toll-like receptors 2 and 4 predict new-onset atrial fibrillation in acute myocardial infarction patients. Int Heart J. (2018) 59:64–70. doi: 10.1536/ihj.17-084
26. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. (2019) 19:477–89. doi: 10.1038/s41577-019-0165-0
27. Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. (2018) 138:2227–42. doi: 10.1161/CIRCULATIONAHA.118.035202
28. Van Wagoner DR, Chung MK. Inflammation, inflammasome activation, and atrial fibrillation. Circulation. (2018) 138:2243–6. doi: 10.1161/CIRCULATIONAHA.118.036143
29. Hunter P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. (2012) 13:968–70. doi: 10.1038/embor.2012.142
30. Kao YH, Chen YC, Cheng CC, Lee TI, Chen YJ, Chen SA. Tumor necrosis factor-α decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit Care Med. (2010) 38:217–22. doi: 10.1097/CCM.0b013e3181b4a854
31. Zhang H, Li J, Chen X, Wu N, Xie W, Tang H. Association of systemic inflammation score with atrial fibrillation: a case-control study with propensity score matching. Heart Lung Circ. 27:489–96 (2018). doi: 10.1016/j.hlc.2017.04.007
32. Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. (2014) 18:688. doi: 10.1186/s13054-014-0688-5
33. Walkey AJ, Hogarth DHG. Lip GYH. Optimizing atrial fibrillation management: from ICU and beyond. Chest. (2015) 148:859–64. doi: 10.1378/chest.15-0358
34. Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias LA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine. (2000) 79:210–21. doi: 10.1097/00005792-200007000-00002
35. Galea R, Cardillo MT, Caroli A, Marini MG, Sonnino C, Narducci ML, et al. Inflammation and C-reactive protein in atrial fibrillation: cause or effect? Tex Heart Inst J. (2014) 41:461–8. doi: 10.14503/THIJ-13-3466
36. Korodi S, Toganel R, Benedek T, Hodas R, Chitu M, Ratiu M, et al. Impact of inflammation-mediated myocardial fibrosis on the risk of recurrence after successful ablation of atrial fibrillation - the FIBRO-RISK study: protocol for a non-randomized clinical trial. Medicine. (2019) 98:e14504. doi: 10.1097/MD.0000000000014504
37. Guo Y, Lip GY. Apostolakis S:Inflammation in atrial fibrillation. J Am Coll Cardiol. (2012) 60:2263–70. doi: 10.1016/j.jacc.2012.04.063
38. Vílchez JA, Roldán V, Hernández-Romero D, Valdés M, Lip GY, Marín F. Biomarkers in atrial fibrillation: an overview. Int J Clin Pract. (2014) 68:434–43. doi: 10.1111/ijcp.12304
39. Richter B, Gwechenberger M, Socas A, Zorn G, Albinni S, Marx M, et al. Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol. (2012) 101:217–25. doi: 10.1007/s00392-011-0383-3
40. Bandyopadhyay D, Banerjee U, Hajra A, Chakraborty S, Amgai B, Ghosh RK, et al. Trends of cardiac complications in patients with rheumatoid arthritis: analysis of the united states national inpatient sample; 2005-2014. Curr Probl Cardiol. (2019) 100455. doi: 10.1016/j.cpcardiol.2019.100455
41. Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. (2017) 38:1717–27. doi: 10.1093/eurheartj/ehw208
42. Bacani AK, Crowson CS, Roger VL, Gabriel SE, Matteson EL. Increased incidence of atrial fibrillation in patients with rheumatoid arthritis. Biomed Res Int. (2015) 2015:809514. doi: 10.1155/2015/809514
43. Ungprasert P, Srivali N, Kittanamongkolchai W. Risk of incident atrial fibrillation in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis. (2017) 20:434–41. doi: 10.1111/1756-185X.12820
44. Dai H, Wang X, Yin S, Zhang Y, Han Y, Yang N, et al. Atrial fibrillation promotion in a rat model of rheumatoid arthritis. J Am Heart Assoc. (2017) 6:e007320. doi: 10.1161/JAHA.117.007320
45. Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a danish nationwide cohort study. Eur Heart J. (2012) 33:2054–64. doi: 10.1093/eurheartj/ehr285
46. Jamnitski A, Symmons D, Peters MJ, Sattar N, McInnes I, Nurmohamed MT. Cardiovascular comorbidities in patients with psoriatic arthritis: a systematic review. Ann Rheum Dis. (2013) 72:211–6. doi: 10.1136/annrheumdis-2011-201194
47. Chiu HY, Chang WL, Huang WF, Wen YW, Tsai YW, Tsai TF. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. (2015) 73:429–38. doi: 10.1016/j.jaad.2015.06.023
48. Dogan Y, Soylu A, Eren GA, Poturoglu S, Dolapcioglu C, Sonmez K, et al. Evaluation of QT and P wave dispersion and mean platelet volume among inflammatory bowel disease patients. Int J Med Sci. (2011) 8:540–6. doi: 10.7150/ijms.8.540
49. Efe TH, Cimen T, Ertem AG, Coskun Y, Bilgin M, Sahan HF, et al. Atrial Electromechanical properties in inflammatory bowel disease. Echocardiography. (2016) 33:1309–16. doi: 10.1111/echo.13261
50. Kristensen SL, Lindhardsen J, Ahlehoff O, Erichsen R, Lamberts M, Khalid U, et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. (2014) 16:477–84. doi: 10.1093/europace/eut312
51. Seferović PM, Ristić AD, Maksimović R, Simeunović DS, Ristić GG, Radovanović G, et al. Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology. (2006) 45:iv39–42. doi: 10.1093/rheumatology/kel315
52. Szabo SM, Levy AR, Rao SR, Kirbach SE, Lacaille D, Cifaldi M, et al. Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum. (2011) 63:3294–304. doi: 10.1002/art.30581
53. Mathieu S, Gossec L, Dougados M, Soubrier M. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res. (2011) 63:557–63. doi: 10.1002/acr.20364
54. Keller JJ, Hsu JL, Lin SM, Chou CC, Wang LH, Wang J, et al. Increased risk of stroke among patients with ankylosing spondylitis: a population-based matched-cohort study. Rheumatol Int. (2014) 34:255–63. doi: 10.1007/s00296-013-2912-z
55. Jabati S, Fareed J, Liles J, Otto A, Hoppensteadt D, Bontekoe J, et al. Biomarkers of inflammation, thrombogenesis, and collagen turnover in patients with atrial fibrillation. Clin Appl Thromb Hemost. (2018) 24:718–23. doi: 10.1177/1076029618761006
56. Hijazi Z, Aulin J, Andersson U, Alexander JH, Gersh B, Granger CB, et al. Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. (2016) 102:508–17. doi: 10.1136/heartjnl-2015-308887
57. Smit MD, Maass AH, De Jong AM, Muller Kobold AC, Van Veldhuisen DJ, Van Gelder IC. Role of inflammation in early atrial fibrillation recurrence. Europace. (2012) 14:810–7. doi: 10.1093/europace/eur402
58. Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. (2001) 56:375–80. doi: 10.2143/AC.56.6.2005701
59. Marott SC, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol. (2010) 56:789–95. doi: 10.1016/j.jacc.2010.02.066
60. Watanabe E, Arakawa T, Uchiyama T, Kodama I, Hishida H. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol. (2006) 108:346–53. doi: 10.1016/j.ijcard.2005.05.021
61. Wu N, Xu B, Xiang Y, Wu L, Zhang Y, Ma X, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. (2013) 169:62–72. doi: 10.1016/j.ijcard.2013.08.078
62. Jiang Z, Dai L, Song Z, Li H, Shu M. Association between C-reactive protein and atrial fibrillation recurrence after catheter ablation: a meta-analysis. Clin Cardiol. (2013) 36:548–54. doi: 10.1002/clc.22157
63. Acampa M, Lazzerini PE, Guideri F, Tassi 3, Lo Monaco A, Martini G. Inflammation and atrial electrical remodelling in patients with embolic strokes of undetermined source. Heart Lung Circ. (2019) 28:917–22. doi: 10.1016/j.hlc.2018.04.294
64. Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. (2003) 41:37S−42S. doi: 10.1016/S0735-1097(02)02953-4
65. Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, et al. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. (2007) 53:1652–7. doi: 10.1373/clinchem.2006.083923
66. Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. (2005) 128:3421–7. doi: 10.1378/chest.128.5.3421
67. Zarauza J, Rodríguez Lera MJ, Fariñas Alvarez C, Hernando JP, Ceballos B, Gutiérrez B, et al. [Relationship between C-reactive protein level and early recurrence of atrial fibrillation after electrical cardioversion]. Rev Esp Cardiol. (2006) 59:125–9. doi: 10.1016/S1885-5857(06)60120-5
68. Zacharia E, Papageorgiou N, Ioannou A, Siasos G, Papaioannou S, Vavuranakis M, et al. Providencia and D. tousoulis: inflammatory biomarkers in atrial fibrillation. Curr Med Chem. (2019) 26:837–54. doi: 10.2174/0929867324666170727103357
69. Qu YC, Du YM, Wu SL, Chen QX, Wu HL, Zhou SF. Activated nuclear factor-κB and increased tumor necrosis factor-α in atrial tissue of atrial fibrillation. Scand Cardiovasc J. (2009) 43:292–7. doi: 10.1080/14017430802651803
70. Lakin R, Polidovitch N, Yang S, Guzman C, Gao X, Wauchop M, et al. Inhibition of soluble TNF-α prevents adverse atrial remodeling and atrial arrhythmia susceptibility induced in mice by endurance exercise. J Mol Cell Cardiol. (2019) 129:165–173. doi: 10.1016/j.yjmcc.2019.01.012
71. Matsushita N, Ishida N, Ibi M, Saito M, Takahashi M, Taniguchi S, et al. IL-1β plays an important role in pressure overload-induced atrial fibrillation in mice. Biol Pharm Bull. (2019) 42:543–6. doi: 10.1248/bpb.b18-00363
72. da Silva RM: Influence of inflammation and atherosclerosis in atrial fibrillation. Curr Atheroscler Rep. (2017) 19:2. doi: 10.1007/s11883-017-0639-0
73. Hak Ł, Myśliwska J, Wieckiewicz J, Szyndler K, Siebert J, Rogowski J. Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). J Interferon Cytokine Res. (2009) 29:327–32. doi: 10.1089/jir.2008.0082.2906
74. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12:230–43. doi: 10.1038/nrcardio.2015.2
75. Olejniczak K, Kasprzak A. Biological properties of interleukin 2 and its role in pathogenesis of selected diseases–a review. Med Sci Monit. (2008) 14:RA179–89. Retrieved from: https://www.medscimonit.com/
76. Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. (2009) 157:243–52. doi: 10.1016/j.ahj.2008.10.009
77. Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the heart and soul study. Am Heart J. (2008) 155:303–9. doi: 10.1016/j.ahj.2007.09.006
78. Kaireviciute D, Blann AD, Balakrishnan B, Lane DA, Patel JV, Uzdavinys G, et al. Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb Haemost. (2010) 104:122–7. doi: 10.1160/TH09-12-0837
79. Gedikli O, Dogan A, Altuntas I, Altinbas A, Ozaydin M, Akturk O, et al. Inflammatory markers according to types of atrial fibrillation. Int J Cardiol. (2007) 120:193–7. doi: 10.1016/j.ijcard.2006.09.015
80. Liuba I, Ahlmroth H, Jonasson L, Englund A, Jönsson A, Säfström K, et al. Source of inflammatory markers in patients with atrial fibrillation. Europace. (2008) 10:848–53. doi: 10.1093/europace/eun111
81. Li SB, Yang F, Jing L, Ma J, Jia YD, Dong SY. Myeloperoxidase and risk of recurrence of atrial fibrillation after catheter ablation. J Investig Med. (2013) 61:722–7. doi: 10.2310/JIM.0b013e3182857fa0
82. Rienstra M, Sun JX, Magnani JW, Sinner MF, Lubitz SA, Sullivan LM, et al. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. (2012) 109:533–7. doi: 10.1016/j.amjcard.2011.09.049
83. Weymann A, Sabashnikov A, Ali-Hasan-Al-Saegh S, Popov AF, Jalil Mirhosseini S, Baker WL, et al. Predictive role of coagulation, fibrinolytic, and endothelial markers in patients with atrial fibrillation, stroke, and thromboembolism: a meta-analysis, meta-regression, and systematic review. Med Sci Monit Basic Res. (2017) 23:97–140. doi: 10.12659/MSMBR.902557
84. Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. (2013) 11:55–9. doi: 10.1586/erc.12.159
85. Weymann A, Ali-Hasan-Al-Saegh S, Sabashnikov A, Popov AF, Mirhosseini SJ, Liu T, et al. Prediction of new-onset and recurrent atrial fibrillation by complete blood count tests: a comprehensive systematic review with meta-analysis. Med Sci Monit Basic Res. (2017) 23:179–222. doi: 10.12659/MSMBR.903320
86. Shao Q, Chen K, Rha SW, Lim HE, Li G, Liu T. Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Arch Med Res. (2015) 46:199–206. doi: 10.1016/j.arcmed.2015.03.011
87. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. (2013) 88:218–30. doi: 10.1016/j.critrevonc.2013.03.010
88. Karavelioglu Y, Karapinar H, Yüksel M, Memiç K, Sarak T, Kurt R, et al. Neutrophil to lymphocyte ratio is predictor of atrial fibrillation recurrence after cardioversion with amiodarone. Clin Appl Thromb Hemost. (2015) 21:5–9. doi: 10.1177/1076029613518368
89. Aribaş A, Akilli H, Gül EE, Kayrak M, Demir K, Duman C, et al. Can neutrophil/lymphocyte ratio predict recurrence of non-valvular atrial fibrillation after cardioversion? Anadolu Kardiyol Derg. (2013) 13:123–30. doi: 10.5152/akd.2013.036
90. Dernellis J, Panaretou M. Relationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur Heart J. (2004) 25:1100–7. doi: 10.1016/j.ehj.2004.04.025
91. Deftereos S, Giannopoulos G, Papoutsidakis N, Panagopoulou V, Kossyvakis C, Raisakis K, et al. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol. (2013) 62:1817–25. doi: 10.1016/j.jacc.2013.08.726
92. Halonen J, Halonen P, Järvinen O, Taskinen P, Auvinen T, Tarkka M, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. (2007) 297:1562–7. doi: 10.1001/jama.297.14.1562
93. Won H, Kim JY, Shim J, Uhm JS, Pak HN, Lee MH, et al. Effect of a single bolus injection of low-dose hydrocortisone for prevention of atrial fibrillation recurrence after radiofrequency catheter ablation. Circ J. (2013) 77:53–9. doi: 10.1253/circj.CJ-12-0728
94. Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, et al. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. (2011) 124:1107–17. doi: 10.1161/CIRCULATIONAHA.111.029223
95. Iskandar S, Reddy M1, Afzal MR1, Rajasingh J1, Atoui M1, Lavu M1, et al. Use of oral steroid and its effects on atrial fibrillation recurrence and inflammatory cytokines post ablation - the steroid af study. J Atr Fibrillation. (2017) 9:1604. doi: 10.4022/jafib.1604
96. Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology. (2006) 45:274–82. doi: 10.1093/rheumatology/kei140
97. Imazio M, Brucato A, Ferrazzi P, Rovere ME, Gandino A, Cemin R, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the prevention of the postpericardiotomy syndrome (COPPS) atrial fibrillation substudy. Circulation. (2011) 124:2290–5. doi: 10.1161/CIRCULATIONAHA.111.026153
98. Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Kaoukis A, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. (2012) 60:1790–6. doi: 10.1016/j.jacc.2012.07.031
99. Verma S, Eikelboom JW, Nidorf SM, Al-Omran M6, Gupta N, Teoh H, et al. Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. (2015) 15:96. doi: 10.1186/s12872-015-0068-3
100. Roşianu SH, Roşianu AN, Aldica M, Căpâlneanu R, Buzoianu AD. Inflammatory markers in paroxysmal atrial fibrillation and the protective role of renin-angiotensin-aldosterone system inhibitors. Clujul Med. (2013) 86:217–21. Retrieved from: https://medpharmareports.com/index.php/mpr/article/view/121
101. Rudolph TK, Ravekes T, Klinke A, Friedrichs K, Mollenhauer M, Pekarova M, et al. Nitrated fatty acids suppress angiotensin II-mediated fibrotic remodelling and atrial fibrillation. Cardiovasc Res. (2016) 109:174–84. doi: 10.1093/cvr/cvv254
102. Miceli A, Capoun R, Fino C, Narayan P, Bryan AJ, Angelini GD, et al. Effects of angiotensin-converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. (2009) 54:1778–84. doi: 10.1016/j.jacc.2009.07.008
103. Korantzopoulos P, Goudevenos JA. Aldosterone signaling in atrial fibrillation another piece in the puzzle of atrial remodeling. J Am Coll Cardiol. (2010) 55:771–3. doi: 10.1016/j.jacc.2009.10.032
104. Bafford R, Sui XX, Park M, Miyahara T, Newfell BG, Jaffe IZ, et al. Mineralocorticoid receptor expression in human venous smooth muscle cells: a potential role for aldosterone signaling in vein graft arterialization. Am J Physiol Heart Circ Physiol. (2011) 301:H41–7. doi: 10.1152/ajpheart.00637.2010
105. Liu T, Korantzopoulos P, Shao Q, Zhang Z, Letsas KP, Li G. Mineralocorticoid receptor antagonists and atrial fibrillation: a meta-analysis. Europace. (2016) 18:672–8. doi: 10.1093/europace/euv366
Keywords: atrial fibrillation, driver, inflammatory biomarker, atrial remodeling, anti-inflammatory treatment
Citation: Zhou X and Dudley SC Jr (2020) Evidence for Inflammation as a Driver of Atrial Fibrillation. Front. Cardiovasc. Med. 7:62. doi: 10.3389/fcvm.2020.00062
Received: 23 December 2019; Accepted: 26 March 2020;
Published: 29 April 2020.
Edited by:
Xun Ai, Rush University Medical Center, United StatesReviewed by:
Alexander Maass, University Medical Center Groningen, NetherlandsMichelle M. Monasky, IRCCS Policlinico San Donato, Italy
Copyright © 2020 Zhou and Dudley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel C. Dudley Jr., c2R1ZGxleUB1bW4uZWR1
 Xiaoxu Zhou
Xiaoxu Zhou Samuel C. Dudley Jr.
Samuel C. Dudley Jr.