- 1Department of Obstetrics and Gynecology, Spirito Santo Hospital of Pescara, Pescara, Italy
- 2Fetal Medicine Unit, St George's University Hospitals NHS Foundation Trust, University of London, London, United Kingdom
- 3Vascular Biology Research Centre, Molecular and Clinical Sciences Research Institute, St George's University of London, London, United Kingdom
Hypertensive disorders of pregnancy (HDP) occur in almost 10% of gestations. These women are known to have higher cardiovascular morbidity and mortality later in life in comparison with parous controls who had normotensive pregnancies. Several studies have demonstrated that women with preeclampsia present in a state of segmental impaired myocardial function, biventricular chamber dysfunction, adverse biventricular remodeling, and hypertrophy, a compromised hemodynamic state and indirect echocardiographic signs of localized myocardial ischemia and fibrosis. These cardiac functional and geometric changes are known to have strong predictive value for cardiovascular disease in non-pregnant subjects. A “dose effect” response seems to regulate this relationship with severe HDP, early-onset HDP, coexistence of fetal growth disorders, and recurrence of HDP resulting in poorer cardiovascular measures. The mechanism underlying the relationship between HDP in younger women and cardiovascular disease later in life is unclear but could be explained by sharing of pre-pregnancy cardiovascular risk factors or due to a direct impact of HDP on the maternal cardiovascular system conferring a state of increased susceptibility to future metabolic or hemodynamic insults. If so, the prevention of HDP itself would become all the more urgent. Shortly after delivery, women who experienced HDP express an increased risk of classic cardiovascular risk factors such as essential hypertension, renal disease, abnormal lipid profile, and diabetes with higher frequency than controls. Within one or two decades after delivery, this group of women are more likely to experience premature cardiovascular events, such as symptomatic heart failure, myocardial ischemia, and cerebral vascular disease. Although there is general agreement that women who suffered from HDP should undertake early screening for cardiovascular risk factors in order to allow for appropriate prevention, the exact timing and modality of screening has not been standardized yet. Our findings suggest that prevention should start as early as possible after delivery by making the women aware of their increased cardiovascular risk and encouraging weight control, stop smoking, healthy diet, and daily exercise which are well-established and cost-effective prevention strategies.
Introduction
Numerous epidemiological studies have proven the relationship between hypertensive disorders in pregnancy (HDP) and increased cardiovascular risk factors and diseases (CVD) later in life (1, 2). This is not surprising as several studies already demonstrated that women with preeclampsia (PE) present in pregnancy a state of impaired myocardial contractility and relaxation, biventricular systo-diastolic chamber dysfunction, adverse biventricular remodeling and hypertrophy, an impaired hemodynamic state, and indirect echocardiographic signs of localized myocardial ischemia and fibrosis (3–5). The above described structural and functional cardiovascular changes do not completely reverse at 1 year postpartum notwithstanding the unloading conditions and are known to be highly predictive for future cardiovascular adverse outcome (3, 6). Furthermore, clinical data has shown that the cardiopulmonary complications already occur in 6% of severe PE and are associated with increased maternal mortality (7). The American Heart Association has already recognized PE as independent risk factor for CVD and introduced this complication of pregnancy in the algorithms for the evaluation of future cardiovascular risk score (8). The physiopathology for this additional CVD risk is still unknown with the existing literature hypothesizing the possibility of pregnancy-induced CVD risk vs. pre-conceptional susceptibility toward an increased risk of CVD or both. The aim of this review is to elucidate the association between HDP and future cardiovascular risk factors and CVD. HDP include gestational hypertension (GH), PE or eclampsia, chronic hypertension, and PE superimposed on top of chronic hypertension. This review will focus on HDP involving new-onset hypertension after 20 weeks' gestation, but other complications of pregnancy such as normotensive small for gestational age or fetal growth restriction as well as normotensive preterm birth, placental abruption, and stillbirth will not be included. This review will focus on the future cardiovascular health of these women, but other outcomes, such as renal failure, diabetes, or other dysmetabolic conditions will not be examined.
Search Strategy and Selection of Articles
A literature examination was accomplished to identify all reports in the English language literature (Medline, National Library of Medicine) published after 2000 until now as previous meta-analysis showed that there were not suitable papers on this topic from 1946 to 1999 (9). The search terms used were “pre-eclampsia,” “gestational hypertension,” “hypertension during pregnancy,” “hypertensive disorders of pregnancy,” “long term outcomes,” “cardiovascular risk,” “cardiovascular health,” “cardiovascular disease,” “essential hypertension,” “peripheral artery disease,” “asymptomatic atherosclerosis,” “diastolic dysfunction,” “heart failure,” “coronary artery disease,” “cerebrovascular disease.” We excluded abstracts of oral communications and posters of congresses even if available on Medline. Furthermore, the bibliography of the selected papers were dissected to further identify pertinent studies. The combined set encompassed 230 articles which were reviewed, and a total of 87 articles were considered appropriate for this review. The relevant data were extracted from the full text of the published papers.
Results
The research yielded 59 prospective (3, 6, 10–66) and 28 retrospective studies (67–93) which were synthetically described in Table 1. The number of women analyzed in each study ranged from 58 to 1,072,330 in prospective studies and from 71 to 1,452,926 in retrospective ones. The year of pregnancy included into the studies ranged from 1952 to 2017 in prospective studies and from 1939 to 2016 in retrospective articles. The following complications of pregnancy were included into the studies: HDP, placental infarction and abruption, preterm delivery, low birth weight offspring, small-for-gestational-age fetuses, fetal growth restriction, stillbirth, gestational diabetes mellitus, and pre-gestational diabetes mellitus. The interval between delivery and cardiovascular risk assessment or between delivery and cardiovascular event occurrence were assessed in the majority of the studies, the median of follow up ranging from at least 7 weeks post-partum to more than 30 years after delivery. The median age of the women at the assessment of CV risk or at the occurrence of CV event ranged from 25 to 71 years in the different studies. The following cardiovascular risks or events were differently assessed in the numerous included studies: essential hypertension, heart failure, coronary, cerebro-vascular and peripheral artery disease, asymptomatic atherosclerosis, dyslipidemia, metabolic syndrome, type II diabetes, and renal dysfunction.
The following outcomes will be analyzed in this review:
• Essential hypertension
• Peripheral artery disease
• Asymptomatic atherosclerosis
• Asymptomatic heart failure
• Heart failure
• Coronary artery disease
• Cerebrovascular disease.
Discussion
Essential Hypertension
All authors who addressed the issue of the relationship between HDP and future development of essential hypertension unanimously found that women who developed HDP have an increased risk of having high blood pressure later in life (Table 1). There seems to be a “dose-dependent” effect of HDP and future risk of developing chronic hypertension depending on the severity of the hypertension in pregnancy, the onset of the complication in pregnancy, the need of iatrogenic preterm delivery, the association to fetal growth disorders and the numbers of pregnancies complicated by HDP. In particular Hauspurg et al. found that “HDP were associated with an aOR of 1.86 (95%CI 1.37–2.52) of development hypertension at one year with PE having an aOR of 2.35 (95%CI 1.63–3.41) whereas GH having an aOR of 1.61 (95%CI 1.09–2.39)” (53). Moreover, “women who had PE showed an aOR of 3.23 (95%CI 1.56–6.68) of hypertension with abnormal biomarkers later in life” (53). Other authors found that early vs. late onset PE had a higher risk of developing chronic hypertension late in life (3, 6). Furthermore, Tooher et al. found that “the severity of hypertension in pregnancy tracked with increased risk of future hypertension” (82), while subsequent pregnancies did not seem to confound a first episode of HDP and later CVD (82). Moreover, in a recent meta-analysis. Brouwers et al. have demonstrated that subjects with recurrent PE had a higher risk of future essential hypertension than formerly PE women with a successive unaffected gestation (RR 2.3, 95% CI 1.9 to 2.9) (94). The relation between HDP, future occurrence of essential hypertension and other pre-pregnancy CV risk has also been assessed to try and isolate the effect of pregnancy on this outcome. On this subject, Cho et al. found that the development of high blood pressure in pre-eclamptic women was related to pre-pregnancy factors such as family history, obesity and high blood pressure (86). This latter result is only partially confirmed by Mito et al. who found that women who had HDP presented an higher risk of developing high blood pressure 5 years after the index pregnancy “even after adjusting for confounding factors such as age, body mass index, family history of hypertension and salt intake (odds ratio 7.1, 95% CI, 2.0–25.6, P < 0.003)” (44). Bergen et al. also found that although adjustment for BMI attenuate the relationship between HDP in childbirth age and future chronic hypertension by 65%, however it remained significant (46). In contrast, Wang et al. (83) found that “history of HDP had a four-fold multivariable-adjusted risk (95% CI 2.29–6.24) of hypertension,” while no influence was established by pre-conceptional anthropometric indices on the incidence of high blood pressure after delivery (83). A stratification of the risk of hypertension depending on the year post-partum after a gestation affected by HDP has also been addressed in several studies. Interestingly, Behrens et al. found that the risk of hypertension associated with HDP was high closely after a complicated gestation and continued for more than two decades (40). About 30% of subjects who suffered from a HDP may develop high blood pressure within 10 years after a complicated gestation; “this risk was 12- to 25-fold higher in the first year, up to 10-fold in 10 year, 2-fold after 20 year” (40). In term of number of women to be screened to detect one case of essential hypertension, Groenhof et al. found that at the age of 35, 9 women with HDP needed to be screened to detect 1 clinically relevant hypertension (64). The risk of developing essential high blood pressure after a gestation affected by HDP has also been correlated to the presence of specific echocardiographic findings (6). Specifically, Melchiorre et al. found that formerly pre-eclamptic normotensive women with moderate-severe echocardiographic left ventricle (LV) anomalies identified at 1 year after delivery were more likely to develop high blood pressure at 2 years after delivery (50%) in comparison to those with normal LV function/geometry or mild LV dysfunction/remodeling (3.5%) with a relative risk of chronic high blood pressure in women with LV moderate-severe abnormalities of 14.5 (95% CI 5.14 to 40.89, P < 0.001) (6). In the above mentioned study the severity of LV dysfunction and hypertrophy was graded according to the European Association and American Society of Echocardiography guidelines (EAE/ASE) (95, 96). Specifically: LV remodeling-hypertrophy was defined mild, moderate or severe if LV mass/body surface area (g/m2) was between 96 and 108, 109 and 121 or ≥122, respectively; LV diastolic dysfunction was defined as mild in the case of impaired myocardial relaxation pattern (grade I), moderate in the case of pseudo-normal filling pattern (grade II) or severe if restrictive filling was seen (grade III) accordingly to the EAA/ASE diagnostic algorithms for the diagnosis and grading of diastolic dysfunction (96). LV systolic function was graded based on the ejection fraction value (EF) as mildly, moderately or severely abnormal if EF (%) was between 45 and 54, 30 and 44 or <30. This finding has been confirmed by the subsequent echocardiographic studies (97).
Peripheral Artery Disease
HDP have also been associated with increased risk of developing peripheral artery disease (98).
“Peripheral artery disease” (PAD) refers to an abnormal narrowing of arteries other than those that supply the heart or brain, commonly caused by atherosclerosis. PAD most commonly affects the lower extremities vessels (99). “Depending on the degree of narrowing at each vascular site, a range of severity of symptoms may occur, while many patients will remain asymptomatic throughout their life. Occasionally acute events occur, often associated with thrombosis and/or embolism and/or occlusion of a major artery” (ESC task force PAD) (99).
Ray et al. in their population-based retrospective cohort study on more than one million women, assessed the association between HDP, placental abruption and infarction (defined as maternal placental syndromes) and the occurrence of hospital admission or revascularization for PAD at least 90 days after the delivery discharge date (67). They found that the risk of PAD in women who suffered from maternal placental syndromes was 3.8 (2.4–5.9) higher than that of women who had an uneventful pregnancy (67). The future risk of PAD remained significant after adjusting for traditional risk factors for CVD, including maternal smoking and metabolic syndrome (adjusted risk 3: 1.9–4.8) and was highest in women who had HDP in combination with fetal compromise, compared to women who had not (67). Lykke et al. also found that severe PE increased the risk of subsequent thromboembolism of 1.9-fold (range 1.35–2.70) and that the relationship was “dose-dependent” (16). Subsequent studies confirmed the increased risk of PAD in women who had HDP.
Asymptomatic Atherosclerosis
Several studies have addressed the issue of the relationship between HDP and asymptomatic/pre-clinical atherosclerosis (Table 1). “Asymptomatic atherosclerosis” refers to the coronary/carotid artery inflammatory disease while the condition is still in a subclinical stage but the presence of atherosclerosis can be well-identified and quantified by several invasive and non-invasive techniques, including coronary angiography, ultrasonography, computed tomography, and magnetic resonance imaging (100). In a prospective cohort study, “a history of PE was associated with an increased risk of coronary artery calcifications (CAC) >30 years after the index pregnancy”, even after controlling individually for traditional risk factors although this association was not more significant when corrected for current hypertension (39). Specifically, the authors found that the odds of presenting a higher CAC score was 3.54 (CI: 1.39–9.02) times greater in formerly pre-eclamptic women compared to women who did not develop PE without adjustments and it was 2.61 (CI: 0.9–7.14) times greater after correction for current high blood pressure (39). On the contrary, the association between CAC score and history of PE remained significant after adjusting for body mass index alone (odds: 3.20; CI: 1.21–8.49) (39). It has been also shown that one third of subjects who had PE express features of coronary atherosclerosis on vascular computed tomography imaging as compared to one fifth of women from the reference group imaging and this result is manifest as early as at age 45–55 years when women are on average 16 ± 6 years postpartum (59). In contrast, a prospective case-control study showed that the “average maximum carotid intima media thickness (CIMT) was similar among women with vs. without PE (0.831 mm vs. 0.817, p = 0.38), and PE was not a significant predictor of CIMT in a multiple linear regression model (p = 0.63), despite more electrocardiograms compatible with coronary disease” (74). Specifically, the authors of the above mentioned prospective study defined “abnormal electrocardiograms” the ones with at least one of the following: the presence of Q waves/isolated infarct, new left bundle branch block, ST elevation, ST depression, T wave inversion (74). The conventional measurement of common carotid artery intima-media thickness (CCA-IMT) does not represent this (60) as opposed to measurement of the individual CCA intima and media thicknesses which visibly reflect augmented vascular risk (60).
Asymptomatic Heart Failure
Several studies assessed the relationship between HDP and the subsequent development of asymptomatic (stage B) or symptomatic (stage C) heart failure (97, 101). The American Heart Association and American College of Cardiologists define asymptomatic stage B heart failure as any subject being affected by LV hypertrophy or dysfunction (102). At this regard, it should be taken into account that several echocardiographic studies in pregnancy already showed a state biventricular dysfunction and hypertrophy, low cardiac output and high total vascular resistancein pre-eclamptic women in pregnancy vs. normotensive matched pregnant controls (3–5) A subsequent prospective longitudinal follow-up study of PE vs. controls assessed at 1 and 2 years post-partum showed, integrating conventional echocardiography, color and pulsed wave tissue Doppler, strain and strain rate techniques, that the prevalence of asymptomatic stage B heart failure (HF-B) at one year postpartum follow-up was significantly higher in preterm vs. term preeclampsia and controls (70 vs. 25 vs. 10%, respectively; p < 0.001), but not in term preeclampsia vs. controls (6). Similarly, moderate-severe left ventricular (LV) dysfunction and remodeling (according to the EAE/ASE diagnosis and grading of abnormal LV structure and function) were significantly more prevalent in formerly preterm PE women compared with those who had term preeclampsia and controls (3, 6). Similarly, Soma-Pillay et al. showed that women with early onset PE had an increased risk of diastolic dysfunction at 1 year post-partum (RR 3.41, 95% CI: 1.11–10.5, p = 0.04) and regardless of the presence of chronic hypertension (56). Breetveld et al. in their prospective longitudinal cohort study confirmed that the prevalence of HF-B was consistently high (1 in 4) amongst formerly PE women at 1 and 4 years postpartum (41). Moreover, in a subsequent observational case-control study, he confirmed that the prevalence of HF-B in formerly PE women was three-fold higher than that detected for healthy parous controls (25 vs. 8%, P < 0.05) at more than 4 years post-partum (47). The increased risk of asymptomatic HF in formerly PE women was further confirmed by Ghossein-Doha et al. who also found that prehypertension increased this risk significantly, while metabolic syndrome elements did not (42). Orabona et al. conducted a prospective study on hemolysis, elevated liver enzymes, and a low platelet count syndrome (HELLP) and PE women and also documented in both HELLP and PE groups a higher prevalence of LV concentric remodeling, diastolic dysfunction and reduced LV ejection fraction at 6 months to 4 years post-partum vs. matched healthy controls (81). Bokslag et.al. also found that history of PE predisposes in middle age, 9–16 years after pregnancy, to worse LV diastolic function, which could increase the likelihood of later heart failure with preserved ejection fraction (84).
Heart Failure
Several large registry-based studies addressed the issue of the relationship between HDP and symptomatic heart failure. Specifically, in a retrospective registry-cohort study on short term cardiovascular outcome in women who had HDP, Jarvie et al. scrutinized all hospital-based deliveries in Florida from 2004 to 2010 and following cardiovascular (CV), non-CV and any second hospitalization to any Florida hospital within 36 months of index delivery excluding subsequent deliveries (89). They found that “women with HDP had twice the risk of CV readmission within 3 years of delivery (6.4 vs. 2.5/1,000 deliveries; P < 0.001), with higher rates among African American women. Heart failure was the most common reason for CV readmission accounting for 78.6% of all CV readmissions and 84.4% of CV readmissions in women with HDP” (89). Furthermore, in a retrospective longitudinal registry study on long term outcome in women who had HDP, Kuo et. al. using National Health Insurance Research Database, found that formerly PE and eclamptic women were at increased risks of congestive heart failure (hazard ratio 9.1, and 7.4, respectively) with a drastic increase of congestive heart failure occurrence at 3 and 10 years since the index pregnancy (90). Similarly, Chen et al. in a retrospective cohort study found that heart failure incidence was greater in the HDP group than controls (9.83 vs. 1.67 per 10,000 person-years), was more likely to develop within 5 years and that severe or recurrent HDP had a greater risk (85).
Coronary Heart Disease
Numerous studies have assessed the relationship between HDP and subsequent coronary heart disease (Table 1). “Coronary artery disease” refers to the clinical manifestations of atherosclerosis and comprehends a range of diseases that result from atheromatous change in coronary vessels. Specifically, it includes the following: (stable and unstable) angina pectoris, myocardial infarction, sudden cardiac death (100).
Interestingly, a previous study on cardiac function and structure in pre-eclamptic women showed the presence of echocardiographic findings suggestive of segmental myocardial ischemia and fibrosis (4–6). Specifically, significantly higher prevalence of basal septal post-systolic shortening, identified by Color Tissue Doppler-derived strain analysis, associated to septal bugling and segmental abnormal myocardial strain and strain rate indices were found in a minority of pre-eclamptic women with severe disease vs. normotensive pregnant controls and these echocardiographic findings have been associated in human autopsy studies and in animal in vitro studies to regional myocardial ischemia (4–6). Not surprising the prevalence of ischemic heart disease in formerly PE women is significantly higher than in parous controls with an uneventful pregnancy (1, 101) (Figure 1). As well as it has been demonstrated in chronic hypertension, this higher risk of developing coronary artery disease in women who had HDP show a “dose dependent effect,”, being higher in the presence of a more severe disease in pregnancy, in the case of associated fetal growth abnormalities and iatrogenic preterm birth and if PE recurred in subsequent pregnancies (55, 94) (Figures 2, 3). Moreover, the association was not explained by adjustment for confounding variables, although it was attenuated by the presence of other CV risk factors (55, 94). In particular, Tooher et. al. directed a retrospective cohort study on all subjects who delivered at a tertiary hospital in Sydney between the years 1980 and 1989 (n = 31,656) of whom 4,387 had HDP (82). The whole cohort were studied for linkage analysis to future CVDs and he found that the formerly HDP women were at increased risk of admission for future CVD vs. normotensive parous controls (OR 2.1; 95% CI, 1.7–2.6) (82). The median time from the index gestation to the occurrence of CVD was 20 years with a range of 3–29 years (82). Another study demonstrated that among a cohort of subjects with acute coronary syndrome, positive history of pregnancy adverse outcome was related with more severe disease and worse outcome (103). In particular, at presentation with acute coronary syndrome, women who had PE were younger and had more conventional CV risk factors such as essential high blood pressure and an increased soluble fms-like tyrosine kinase:placental growth factor ratio compared to women who had uncomplicated gestation (103). There was also an increased risk of recurrence of acute coronary syndrome at 1 year in women with previous PE (hazard ratio, 6.8) (103). Moreover, women with ≥2 complicated gestation had an increased cardiac mortality risk as shown by Theilen et al. (aHR = 3.3, 95% CI 2–5.4) (91). A large registry-study in Norway also revealed that GH was associated with increased risk of subsequent cardiovascular disease and the highest risk was noticed when GH was combined with fetal growth abnormalities infants and/or preterm delivery (65). Subsequent studies confirmed this association between HDP and increased risk of myocardial infarction at 40–70y with an HR of 2.08 for formerly PE women and 1.56 or formerly GH women (62). Furthermore, an interesting study on the relationship between coronary flow velocity reserve (CFVR) by Doppler echocardiography and previous HDP found that the mean coronary flow velocity reserve was significantly impaired in the early-onset than in the late-onset PE and in the control group with a positive relation between gestational age at PE diagnosis and coronary flow velocity reserve which notable persisted significant after adjustment for conventional cardiac risk factors such as body mass index, blood pressure, and glycated hemoglobin (61).
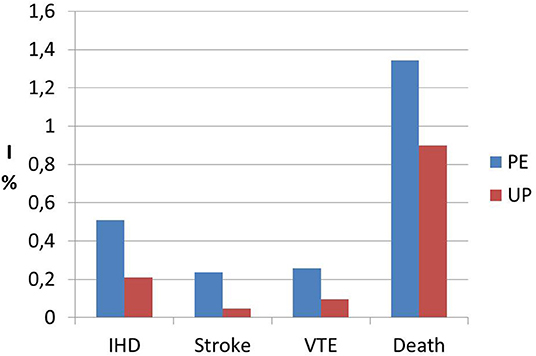
Figure 1. Incidence data (I) of ischemic heart disease (IHD), stroke, venous thromboembolism (VTE) and death from any cause in formerly PE women (PE) vs. women who had an uneventful pregnancy (UP). Modified from Bellamy et al. (1).
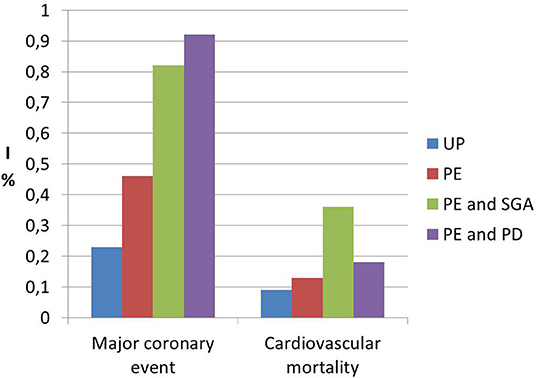
Figure 2. Incidence data (I) of future risk of major coronary events and cardiovascular mortality in formerly PE women (PE) vs. women who had an uneventful pregnancy (UP), showing the dose effect response of specific characteristics of pregnancy with preeclamspia such as small for gestational age (SGA) and preterm delivery (PD). Modified from Riise et al. (55).
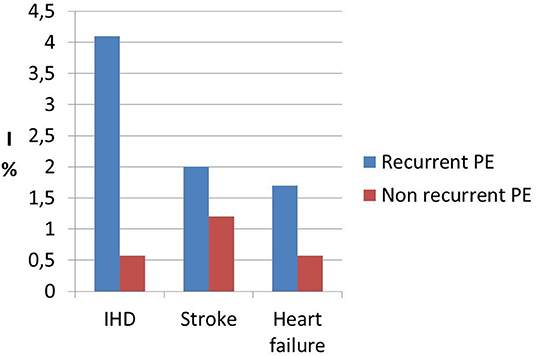
Figure 3. Incidence data (I) of ischemic heart disease (IHD), stroke and heart failure in recurrent preeclamspia women (recurrent PE) vs. women affected by a single pregnancy with PE and subsequent normal pregnancy (not recurrent PE) showing the “dose effect” response of recurrent preeclampsia. Modified from Brouwers et al. (94).
Cerebro-Vascular Disease
There is general agreement on the link between HDP and subsequent risk of stroke (Table 1). It has been demonstrated that not only PE/Eclampsia increase the risk of cerebrovascular events with an HR of 10.7 (p < 0.0001) (90), but Haug et al. (62) and Tooher et al. (82) is also associated with increased risk of stroke. A large population-based study found that formerly GH women had a 1.3-fold (95% CI 0.9–1.7) higher risk of suffering from cerebrovascular disease compared with parous controls with uncomplicated gestation (55). In general, HDP women seem to have an increased risk of cerebrovascular events (HR 1.47, 1.15–1.87) at 40–70 years (62). Moreover, no significant difference was found in relation to the use of antihypertensive drugs or the duration of HDP and subsequent hospitalization for stroke (82). Again, there seems to be a “dose dependent” effect of HPD on cerebrovascular mortality, with women who had more than two complicated gestation showing an increased mortality from stroke (aHR = 5.10), as well as the other causes of cardiovascular mortality, compared to parous women who had only 1 or 0 pregnancies complicated by HDP (1, 91, 94) (Figures 1, 3).
Conclusions
HDP is associated with increased risk of cardiovascular diseases later in life. This is not surprising as several studies have demonstrated that women with PE present in a state of cardiac dysfunction, ventricular hypertrophy and indirect echocardiographic signs of localized myocardial ischemia and fibrosis (103, 104). Moreover, the structural and functional cardiovascular changes do not completely reverse at 1 year post-partum and are known to have strong prognostic value for future cardiovascular morbidity and mortality in non-pregnant subjects (104). The relationship is stronger in the case of severe or early-onset HDP, concomitant fetal growth disorders, need for iatrogenic preterm delivery and recurrent HDP. Adjustments for confounders (such as family history of cardiovascular diseases, high BMI, hypertension, diabetes, and dyslipidemia) do not eliminate, but attenuate this relationship. The underlying mechanisms have not been fully elucidated, but a concomitance of pre-pregnancy predisposition to increased risk of cardiovascular disease and a direct effect of pregnancy on the cardiovascular system may play a role in determining this excess of cardiovascular morbidity and mortality in women who experienced PE and GH in pregnancy. Guidelines regarding timing and extent of cardiovascular follow-up as well as strategies of prevention after HDP are lacking, but it is reasonable to recommend that screening should be started as early as 1 year after delivery and should primarily include awareness of the women of their future increased CV risk and lifestyle modifications with weight control, smoking cessation, healthy diet, and daily exercise. Future studies should address the issue of a structured screening for cardiovascular disease and the impact of timely preventive intervention in improving cardiovascular health in this group of young women.
Author Contributions
AK, BT, and KM conceived the study. Articles were examined by AM, VG, AR, and KM. KM drafted the paper. All authors contributed in writing and revising the paper.
Funding
AR and VG were part of the iPLACENTA project, which has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 765274.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. (2007) 335:974. doi: 10.1136/bmj.39335.385301.BE
2. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. (2013) 28:1–19. doi: 10.1007/s10654-013-9762-6
3. Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. (2008) 52:873–80. doi: 10.1161/HYPERTENSIONAHA.108.117358
4. Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. (2011) 57:85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321
5. Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. (2012) 31:454–71. doi: 10.3109/10641955.2012.697951
6. Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. (2011) 58:709–15. doi: 10.1161/HYPERTENSIONAHA.111.176537
7. Bauer ST, Cleary KL. Cardiopulmonary complications of pre-eclampsia. Semin Perinatol. (2009) 33:158–65. doi: 10.1053/j.semperi.2009.02.008
8. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−2011 update: a guideline from the American heart association. Circulation. (2011) 123:1243–62. doi: 10.1161/CIR.0b013e31820faaf8
9. Gamble DT, Brikinns B, Myint PK, Bhattacharya S. Hypertensive disorders of pregnancy and subsequent cardiovascular disease: current national and international guidelines and the need for future research. Front Cardiovasc Med. (2019) 6:55. doi: 10.3389/fcvm.2019.00055
10. Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman-Breen CO, et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. (2003) 42:982–9. doi: 10.1016/j.ajkd.2003.07.001
11. Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. (2003) 42:39–42. doi: 10.1161/01.HYP.0000074428.11168.EE
12. Manten GT, Sikkema MJ, Voorbij HA, Visser GH, Bruinse HW, Franx A. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy. (2007) 26:39–50. doi: 10.1080/10641950601146574
13. Berends AL, de Groot CJ, Sijbrands EJ, Sie MP, Benneheij SH, Pal R, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. (2008) 51:1034–41. doi: 10.1161/HYPERTENSIONAHA.107.101873
14. Edlow AG, Srinivas SK, Elovitz MA. Investigating the risk of hypertension shortly after pregnancies complicated by preeclampsia. Am J Obstet Gynecol. (2009) 200:e60. doi: 10.1016/j.ajog.2008.10.012
15. Haukkamaa L, Moilanen L, Kattainen A, Luoto R, Kahonen M, Leinonen M, et al. Pre-eclampsia is a risk factor of carotid artery atherosclerosis. Cerebrovasc Dis. (2009) 27:599–607. doi: 10.1159/000216834
16. Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. (2009) 53:944 −51. doi: 10.1161/HYPERTENSIONAHA.109.130765
17. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. (2009) 114:961–70. doi: 10.1097/AOG.0b013e3181bb0dfc
18. Smith GN, Walker MC, Liu A, Wen SW, Swansburg M, Ramshaw H, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. (2009) 200:58. doi: 10.1016/j.ajog.2008.06.035
19. Canti IC, Komlos M, Martins-Costa SH, Ramos JG, Capp E, Corleta H. Risk factors for cardiovascular disease ten years after preeclampsia. São Paulo Med J. (2010) 128:10–3. doi: 10.1590/S1516-31802010000100003
20. Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. (2010) 56:166–71. doi: 10.1161/HYPERTENSIONAHA.110.150078
21. Callaway LK, David McIntyre H, Williams GM, Najman JM, Lawlor DA, Mamun A. Diagnosis and treatment of hypertension 21 years after a hypertensive disorder of pregnancy. Aust N Z J Obstet Gynaecol. (2011) 51:437–40. doi: 10.1111/j.1479-828X.2011.01345.x
22. Andersgaard AB, Acharya G, Mathiesen EB, Johnsen SH, Straume B, Øian P. Recurrence and long-term maternal health risks of hypertensive disorders of pregnancy: a population-based study. Am J Obstet Gynecol. (2012) 206:143.e1–8. doi: 10.1016/j.ajog.2011.09.032
23. Bhattacharya S, Prescott GJ, Iversen L, Campbell DM, Smith WC, Hannaford PC. Hypertensive disorders of pregnancy and future health and mortality: a record linkage study. Pregnancy Hypertens. (2012) 2:1–7. doi: 10.1016/j.preghy.2011.08.116
24. Drost JT, van der Schouw YT, Maas AH, Verschuren WM. Longitudinal analysis of cardiovascular risk parameters in women with a history of hypertensive pregnancy disorders: the doetinchem cohort study. BJOG. (2013) 120:1333–9. doi: 10.1111/1471-0528.12254
25. Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the avon longitudinal study of parents and children. Circulation. (2012) 125:1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784
26. Skjaerven R, Wilcox AJ, Klungsøyr K, Irgens LM, Vikse BE, Vatten LJ, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. (2012) 345:e7677. doi: 10.1136/bmj.e7677
27. Smith GN, Pudwell J, Walker M, Wen SW. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. (2012) 34:830–5. doi: 10.1016/S1701-2163(16)35381-6
28. Spaan JJ, Sep SJS, van Balen VL, Spaanderman MEA, Peeters LLH. Metabolic syndrome as a risk factor for hypertension after preeclampsia. Obstet Gynecol. (2012) 120:311–7. doi: 10.1097/AOG.0b013e31825f21ff
29. Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens. (2012) 30:351–8. doi: 10.1097/HJH.0b013e32834e5ac7
30. Drost JT, Arpaci G, Ottervanger JP, de Boer MJ, van Eyck J, van der Schouw YT, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: the preeclampsia risk evaluation in females study (PREVFEM). Euro. J. Prev. Cardiol. (2012) 19:1138–44. doi: 10.1177/1741826711421079
31. Hermes W, Tamsma JT, Grootendorst DC, Franx A, van der Post J, van Pampus MG, et al. Cardiovascular risk estimation in women with a history of hypertensive pregnancy disorders at term: a longitudinal follow-up study. BMC Pregnancy Childbirth. (2013) 13:126. doi: 10.1186/1471-2393-13-126
32. Scholten RR, Hopman MT, Sweep FC, Van de Vlugt MJ, Van Dijk AP, Oyen WJ, et al. Co-occurrence of cardiovascular and prothrombotic risk factors in women with a history of preeclampsia. Obstet Gynecol. (2013) 121:97–105. doi: 10.1097/AOG.0b013e318273764b
33. van Rijn BB, Nijdam ME, Bruinse HW, Roest M, Uiterwaal CS, Grobbee DE, et al. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol. (2013) 121:1040–8. doi: 10.1097/AOG.0b013e31828ea3b5
34. Kurabayashi T, Mizunuma H, Kubota T, Kiyohara Y, Nagai K, Hayashi K. Pregnancy-induced hypertension is associated with maternal history and a risk of cardiovascular disease in later life: a Japanese cross-sectional study. Maturitas. (2013) 75:227–31. doi: 10.1016/j.maturitas.2013.04.002
35. Nakimuli A, Elliott AM, Kaleebu P, Moffett A, Mirembe F. Hypertension persisting after pre-eclampsia: a prospective cohort study at mulago hospital, uganda. PLoS ONE. (2013) 8:e85273. doi: 10.1371/journal.pone.0085273
36. Tooher J, Chiu CL, Yeung K, Lupton SJ, Thornton C, Makris A, et al. High blood pressure during pregnancy is associated with future cardiovascular disease: an observational cohort study. BMJ Open. (2013) 3:e002964. doi: 10.1136/bmjopen-2013-002964
37. Hosaka M, Asayama K, Staessen JA, Tatsuta N, Satoh M, Kikuya M, et al. Relationship between maternal gestational hypertension and home blood pressure in 7-year-old children and their mothers: tohoku study of child development. Hypertens Res. (2015) 38:776–82. doi: 10.1038/hr.2015.63
38. Black MH, Zhou H, Sacks DA, Dublin S, Lawrence JM, Harrison TN, et al. Hypertensive disorders first identified in pregnancy increase risk for incident prehypertension and hypertension in the year after delivery. J Hypertens. (2016) 34:728–35. doi: 10.1097/HJH.0000000000000855
39. White WM, Mielke MM, Araoz PA, Lahr BD, Bailey KR, Jayachandran M, et al. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obstet Gynecol. (2016) 214:519.e1–519.e8. doi: 10.1016/j.ajog.2016.02.003
40. Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. (2017) 12:358. doi: 10.1136/bmj.j3078
41. Breetveld NM, Ghossein-Doha C, Van Kuijk S, Van Dijk AP, Van Der Vlugt MJ, Heidemas WM, et al. Prevalence of asymptomatic heart failure in formerly pre-eclamptic women: a cohort study. Ultrasound Obstet Gynecol. (2017) 49:134–42. doi: 10.1002/uog.16014
42. Ghossein-Doha C, Van Neer J, Wissink B, Breetveld M, De Windt LJ, Van Dijk APJ, et al. Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound Obstet Gynecol. (2017) 49:143–149. doi: 10.1002/uog.17343
43. Grandi SM, Reynier P, Platt RW, Basso O, Filion KB. The timing of onset of hypertensive disorders in pregnancy and the risk of incident hypertension and cardiovascular disease. Int J Cardiol. (2018) 270:273–5. doi: 10.1016/j.ijcard.2018.06.059
44. Mito A, Arata N, Qiu D, Sakamoto N, Murashima A, Ichihara A, et al. Hypertensive disorders of pregnancy: a strong risk factor for subsequent hypertension 5 years after delivery. Hypertens Res. (2018) 41:141–6. doi: 10.1038/hr.2017.100
45. Benschop L, Duvekot JJ, Versmissen J, van Broekhoven V, Steegers EAP, Roeters van Lennep JE. Blood pressure profile 1 year after severe preeclampsia. Hypertension. (2018) 71:491–8. doi: 10.1161/HYPERTENSIONAHA.117.10338
46. Bergen NE, Schalekamp-Timmermans S, Roos-Hesselink J, Roeters van Lennep JE, Jaddoe VVW, Steegers EAP. Hypertensive disorders of pregnancy and subsequent maternal cardiovascular health. Eur J Epidemiol. (2018) 33:763–71. doi: 10.1007/s10654-018-0400-1
47. Breetveld NM, Ghossein-Doha C, van Neer J, Sengers MJJM, Geerts L, van Kuijk SMJ, et al. Decreased endothelial function and increased subclinical heart failure in women several years after pre-eclampsia. Ultrasound Obstet Gynecol. (2018) 52:196–204. doi: 10.1002/uog.17534
48. Clemmensen TS, Christensen M, Løgstrup BB, Kronborg CJS, Knudsen UB. Reduced coronary flow velocity reserve in women with previous pre-eclampsia: the link to increased cardiovascular disease risk? Ultrasound Obstet Gynecol. (2019). doi: 10.1002/uog.20407. [Epub ahead of print].
49. Ditisheim A, Wuerzner G, Ponte B, Vial Y, Irion O, Burnier M, et al. Prevalence of hypertensive phenotypes after preeclampsia: a prospective cohort study. Hypertension. (2018) 71:103–9. doi: 10.1161/HYPERTENSIONAHA.117.09799
50. Egeland GM, Skurtveit S, Staff AC, Eide GE, Daltveit AK, Klungsøyr K, et al. Pregnancy-related risk factors are associated with a significant burden of treated hypertension within 10 years of delivery: findings from a population-based norwegian cohort. J Am Heart Assoc. (2018) 7:1. doi: 10.1161/JAHA.117.008318
51. Escouto DC, Green A, Kurlak L, Walker K, Loughna P, Chappell L, et al. Postpartum evaluation of cardiovascular disease risk for women with pregnancies complicated by hypertension. Pregnancy Hypertens. (2018) 13:218–24. doi: 10.1016/j.preghy.2018.06.019
52. Grandi SM, Vallée-Pouliot K, Reynier P, Eberg M, Platt RW, Arel R, et al. Hypertensive disorders in pregnancy and the risk of subsequent cardiovascular disease. Paediatr Perinat Epidemiol. (2017) 31:412–21. doi: 10.1111/ppe.12388
53. Hauspurg A, Countouris ME, Jeyabalan A, Hubel CA, Roberts JM, Schwarz EB, et al. Risk of hypertension and abnormal biomarkers in the first year postpartum associated with hypertensive disorders of pregnancy among overweight and obese women. Pregnancy Hypertens. (2019) 15:1–6. doi: 10.1016/j.preghy.2018.10.009
54. Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. (2019) 40:1113–20. doi: 10.1093/eurheartj/ehy863
55. Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, Nygard O, et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol. (2019) 282:81–7. doi: 10.1016/j.ijcard.2019.01.097
56. Soma-Pillay P, Louw MC, Adeyemo AO, Makin J, Pattinson RC. Cardiac diastolic function after recovery from pre-eclampsia. Cardiovasc J Afr. (2018) 29:26–31. doi: 10.5830/CVJA-2017-031
57. Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. (2018) 169:224–32. doi: 10.7326/M17-2740
58. Timpka S, Fraser A, Schyman T, Stuart JJ, Åsvold BO, Mogren I, et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol. (2018) 33:1003–10. doi: 10.1007/s10654-018-0429-1
59. Zoet GA, Benschop L, Boersma E, Budde RPJ, Fauser BCJM, van der Graaf Y, et al. Prevalence of subclinical coronary artery disease assessed by coronary computed tomography angiography in 45- to 55-year-old women with a history of preeclampsia. Circulation. (2018) 137:877–9. doi: 10.1161/CIRCULATIONAHA.117.032695
60. Akhter T, Larsson A, Larsson M, Naessen T. Sub-clinical atherosclerosis in the common carotid artery in women with/without previous pre-eclampsia: a seven-year follow-up. Atherosclerosis. (2019) 290:206–13. doi: 10.1016/j.preghy.2019.08.090
61. Clemmensen TS, Christensen M, Kronborg CJS, Knudsen UB, Løgstrup BB. Long-term follow-up of women with early onset pre-eclampsia shows subclinical impairment of the left ventricular function by two-dimensional speckle tracking echocardiography. Pregnancy Hypertens. (2018) 14:9–14. doi: 10.1016/j.preghy.2018.07.001
62. Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, et al. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the nord-trøndelag health study. JAMA Cardiol. (2019) 4:628–35. doi: 10.1001/jamacardio.2019.1746
63. Hromadnikova I, Kotlabova K, Dvorakova L, Krofta L. Maternal cardiovascular risk assessment 3-to-11 years postpartum in relation to previous occurrence of pregnancy-related complications. J Clin Med. (2019) 8:544. doi: 10.3390/jcm8040544
64. Groenhof TKJ, Zoet GA, Franx A, Gansevoort RT, Bots ML, Groen H, et al. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. (2019) 73:171–8. doi: 10.1161/HYPERTENSIONAHA.118.11726
65. Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Iversen AC, et al. Association between gestational hypertension and risk of cardiovascular disease among 617 589 Norwegian women. J Am Heart Assoc. (2018) 7:e008337. doi: 10.1161/JAHA.117.008337
66. Timokhina E, Kuzmina T, Strizhakov A, Pitskhelauri E, Ignatko I, Belousova V. Maternal cardiac function after normal delivery, preeclampsia, and eclampsia: a prospective study. J Pregnancy. (2019) 2019:9795765 doi: 10.1155/2019/9795765
67. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. (2005) :366:1797–803. doi: 10.1016/S0140-6736(05)67726-4
68. Nijdam ME, Timmerman MR, Franx A, Bruinse HW, Numans ME, Grobbee DE, et al. Cardiovascular risk factor assessment after pre-eclampsia in primary care. BMC Fam Pract. (2009) 10:77. doi: 10.1186/1471-2296-10-77
69. Ben-Ami S, Oron G, Ben-Haroush A, Blickstein D, Hod M, Bar J. Primary atherothrombotic occlusive vascular events in premenopausal women with history of adverse pregnancy outcome. Thromb Res. (2010) 125:124–7. doi: 10.1016/j.thromres.2009.05.017
70. Lykke JA, Langhoff-Roos j, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol. (2010) 24:323–33. doi: 10.1111/j.1365-3016.2010.01120.x
71. Borna S, Neamatipoor E, Radman N. Risk of coronary artery disease in women with history of pregnancies complicated by preeclampsia and LBW. J. Matern Fetal Neonatal Med. (2012) 25:1114–16. doi: 10.3109/14767058.2011.624218
72. Gastrich MD, Gandhi SK, Pantazopoulos J, Zang EA, Cosgrove NM, Cabrera J, et al. Cardiovascular outcomes after preeclampsia or eclampsia complicated by myocardial infarction or stroke. Obstet Gynecol. (2012) 120:823–31. doi: 10.1097/AOG.0b013e31826ae78a
73. Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS study. Heart. (2012) 98:1136e1141. doi: 10.1136/heartjnl-2011-301548
74. McDonald SD1, Ray J, Teo K, Jung H, Salehian O, Yusuf S, et al. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis. (2013) 229:234–9. doi: 10.1016/j.atherosclerosis.2013.04.020
75. Watanabe K, Kimura C, Iwasaki A, Mori T, Matsushita H, Shinohara K, et al. Pregnancy-induced hypertension is associated with an increase in the prevalence of cardiovascular disease risk factors in Japanese women. Menopause. (2015) 22:656–9. doi: 10.1097/GME.0000000000000361
76. Barry DR, Utzschneider KM, Tong J, Gaba K, Leotta DF, Brunzell JD, et al. Intraabdominal fat, insulin sensitivity, and cardiovascular risk factors in postpartum women with a history of preeclampsia. Am J Obstet Gynecol. (2015) 213:104.e1–104.e11. doi: 10.1016/j.ajog.2015.05.040
77. Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol. (2016) 215:484.e1–484.e14. doi: 10.1016/j.ajog.2016.05.047
78. Ray JG, Booth GL, Alter DA, Vermeulen MJ. Prognosis after maternal placental events and revascularization: PAMPER study. Am J Obstet Gynecol. (2016) 214:106.e1–106.e14. doi: 10.1016/j.ajog.2015.08.021
79. Facca TA, Mastroianni-Kirsztajn G, Sabino ARP, Passos MT, Dos Santos LF, Famá EAB, et al. Pregnancy as an early stress test for cardiovascular and kidney disease diagnosis. Pregnancy Hypertens. (2018) 12:169–73. doi: 10.1016/j.preghy.2017.11.008
80. Fatma J, Karoli R, Siddiqui Z, Gupta HP, Chandra A, Pandey M. Cardio-metabolic risk profile in women with previous history of pre-eclampsia. J Assoc Physicians India. (2017) 65:23–7.
81. Orabona R, Vizzardi E, Sciatti E, Prefumo F, Bonadei I, Valcamonico A, et al. Maternal cardiac function after HELLP syndrome: an echocardiography study. Ultrasound Obstet Gynecol. (2017) 50:507–13. doi: 10.1002/uog.17358
82. Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. (2017) 70:798–803. doi: 10.1161/HYPERTENSIONAHA.117.09246
83. Wang L, Leng J, Liu H, Zhang S, Wang J, Li W, et al. Association between hypertensive disorders of pregnancy and the risk of postpartum hypertension: a cohort study in women with gestational diabetes. J Hum Hypertens. (2017) 31:725–30. doi: 10.1038/jhh.2017.46
84. Bokslag A, Franssen C, Alma LJ, Kovacevic I, Kesteren FV, Teunissen PW, et al. Early-onset preeclampsia predisposes to preclinical diastolic left ventricular dysfunction in the fifth decade of life: an observational study. PLoS ONE. (2018) 13:e0198908. doi: 10.1371/journal.pone.0198908
85. Chen SN, Cheng CC, Tsui KH, Tang PL, Chern CU, Huang WC, et al. Hypertensive disorders of pregnancy and future heart failure risk: a nationwide population-based retrospective cohort study. Pregnancy Hypertens. (2018) 3:110–5. doi: 10.1016/j.preghy.2018.05.010
86. Cho GJ, Kim HY, Park JH, Ahn KH, Hong SC, Kim HJ„, et al. Prepregnancy factors are associated with development of hypertension later in life in women with pre-eclampsia. J Womens Health. (2019) 28:984–9. doi: 10.1089/jwh.2018.7165
87. Dunietz GL, Strutz KL, Holzman C, Tian Y, Todem D, Bullen B L, et al. Moderately elevated blood pressure during pregnancy and odds of hypertension later in life: the POUCHmoms longitudinal study. BJOG. (2017) 124:1606–13. doi: 10.1111/1471-0528.14556
88. Fossum S, Halvorsen S, Vikanes ÅV, Roseboom TJ, Ariansen I, Næss Ø. Cardiovascular risk profile at the age of 40-45 in women with previous hyperemesis gravidarum or hypertensive disorders in pregnancy: a population-based study. Pregnancy Hypertens. (2018) 12:129–35. doi: 10.1016/j.preghy.2018.04.013
89. Jarvie JL, Metz TD, Davis MB, Ehrig JC, Kao DP. Short-term risk of cardiovascular readmission following a hypertensive disorder of pregnancy. Heart. (2018) 104:1187–94. doi: 10.1136/heartjnl-2017-312299
90. Kuo YL, Chan TF, Wu CY, Ker CR, Tu HP. Preeclampsia-eclampsia and future cardiovascular risk among women in Taiwan. Taiwan J Obstet Gynecol. (2018) 57:364–9. doi: 10.1016/j.tjog.2018.04.035
91. Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. (2018) 219:107.e1–107.e6. doi: 10.1016/j.ajog.2018.04.002
92. Orabona R, Sciatti E, Vizzardi E, Prefumo F, Bonadei I, Valcamonico A, et al. Inappropriate left ventricular mass after preeclampsia: another piece of the puzzle inappropriate LVM and PE. Hypertens Res. (2019) 42:522–9. doi: 10.1038/s41440-018-0163-9
93. Sia WW, Pertman SM, Yan RM, Tsuyuki RT. Are preeclampsia and adverse obstetrical outcomes predictors of cardiovascular disease? a case-control study of women with heart disease. J Obstet Gynaecol Can. (2019) 41:1760–7. doi: 10.1016/j.jogc.2019.03.023
94. Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. (2018) 125:1642–54. doi: 10.1111/1471-0528.15394
95. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. (2006) 7:79–108. doi: 10.1016/j.euje.2005.12.014
96. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. (2009) 10:165–93. doi: 10.1093/ejechocard/jep007
97. Castleman JS, Ganapathy R, Taki F, Lip GY, Steeds RP, Kotecha D. Echocardiographic structure and function in hypertensive disorders of pregnancy: a systematic review. Circ Cardiovasc Imaging. (2016) 9:e004888. doi: 10.1161/CIRCIMAGING.116.004888
98. McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. (2010) 55:1026–39. doi: 10.1053/j.ajkd.2009.12.036
99. Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesendorsed by: the European Stroke Organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. (2018). 39:763–816. doi: 10.1093/eurheartj/ehx095
100. Toth PP. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract. (2008) 62:1246–54. doi: 10.1111/j.1742-1241.2008.01804.x
101. Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. (2019) 105:1273–78. doi: 10.1136/heartjnl-2018-313453
102. Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults; a report of the American college of cardiology foundation/American heart association task force on practice guidelines: developed in collaboration with the international society for heart and lung transplantation. Circulation. (2009). 119:1977–2016. doi: 10.1016/j.jacc.2008.11.009
103. Grand'Maison S, Pilote L, Schlosser K, Stewart DJ, Okano M, Dayan N. Clinical features and outcomes of acute coronary syndrome in women with previous pregnancy complications. Can J Cardiol. (2017) 33:1683–92. doi: 10.1016/j.cjca.2017.08.025
Keywords: preeclampsia, gestational hypertension, hypertensive disorders of pregnancy, essential hypertension, cardiovascular disease, heart failure, coronary artery disease
Citation: Melchiorre K, Thilaganathan B, Giorgione V, Ridder A, Memmo A and Khalil A (2020) Hypertensive Disorders of Pregnancy and Future Cardiovascular Health. Front. Cardiovasc. Med. 7:59. doi: 10.3389/fcvm.2020.00059
Received: 08 November 2019; Accepted: 24 March 2020;
Published: 15 April 2020.
Edited by:
Dexter Canoy, University of Oxford, United KingdomReviewed by:
Maria Nelander, Uppsala University, SwedenChristoph Sinning, Universitäres Herzzentrum Hamburg GmbH (UHZ), Germany
Malamo Countouris, University of Pittsburgh, United States
Copyright © 2020 Melchiorre, Thilaganathan, Giorgione, Ridder, Memmo and Khalil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asma Khalil, YXNtYWtoYWxpbDc5QGdvb2dsZW1haWwuY29t
 Karen Melchiorre
Karen Melchiorre Basky Thilaganathan2,3
Basky Thilaganathan2,3 Veronica Giorgione
Veronica Giorgione Anna Ridder
Anna Ridder