
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 03 September 2019
Sec. Atherosclerosis and Vascular Medicine
Volume 6 - 2019 | https://doi.org/10.3389/fcvm.2019.00126
This article is part of the Research Topic Established and Novel Roles of Platelets in Health and Disease View all 20 articles
Platelet concentrates (PCs) are biological autologous products derived from the patient's whole blood and consist mainly of supraphysiologic concentration of platelets and growth factors (GFs). These GFs have anti-inflammatory and healing enhancing properties. Overall, PCs seem to enhance bone and soft tissue healing in alveolar ridge augmentation, periodontal surgery, socket preservation, implant surgery, endodontic regeneration, sinus augmentation, bisphosphonate related osteonecrosis of the jaw (BRONJ), osteoradionecrosis, closure of oroantral communication (OAC), and oral ulcers. On the other hand, no effect was reported for gingival recession and guided tissue regeneration (GTR) procedures. Also, PCs could reduce pain and inflammatory complications in temporomandibular disorders (TMDs), oral ulcers, and extraction sockets. However, these effects have been clinically inconsistent across the literature. Differences in study designs and types of PCs used with variable concentration of platelets, GFs, and leucocytes, as well as different application forms and techniques could explain these contradictory results. This study aims to review the clinical applications of PCs in oral and craniofacial tissue regeneration and the role of their molecular components in tissue healing.
Platelet concentrates (PCs) are biological autologous products derived from the patient's whole blood that consist mainly of supraphysiological concentrations of platelets and growth factors (GFs). There are two main types of PCs: platelet-rich plasma (PRP) and platelet rich fibrin (PRF), which can be pure (i.e., P-PRP, P-PRF) or rich in leucocytes (i.e., L-PRP, L-PRF) (1, 2). PCs can also be prepared with or without red blood cells (3). PRP and P-PRF are prepared from anti-coagulated blood, whereas L-PRF is prepared from non-anti-coagulated blood. PC is prepared using gravitational centrifugation techniques, standard cell separators, or autologous selective filtration technique (plateletpheresis) (3). PCs contain high concentrations of growth factors (GFs) and cytokines that play a vital role in the healing of various tissues. PCs have been used alone or as an adjunctive treatment to enhance soft and hard tissue regeneration in dentoalveolar, and maxillofacial surgeries (4–6).
There are four phases of tissue healing: hemostasis phase, inflammatory phase, reparative phase, and remodeling phase. Blood clot serves as a matrix that allows cell movement and proliferation. Platelets are essential for blood clot formation (7). The inflammatory phase starts from the first day to the seventh day after injury (8). In this phase, platelet releases different growth factors that attract inflammatory cells: neutrophils, macrophages and lymphocytes to the injured site. These cells secrete pro-inflammatory cytokines: Tumor necrosis factor alpha (TNF-α), interleukins (IL-1, IL-6, IL-18) that enhance angiogenesis and tissue healing (9, 10). In the reparative phase, mesenchymal stem cells (MSCs) are recruited from the adjacent soft tissue or blood vessels (11). Different growth factors such as bone morphogenic proteins (BMPs) and TGF-β play a role in MSCs recruitment. They enhance bone regeneration as they induce osteoblast differentiation (12). The remodeling phase is characterized by replacement of woven bone with lamellar bone. GFs are also involved in this phase (10, 12).
This study aims to review the clinical applications of PCs in oral and maxillofacial surgery procedures including maxillary sinus augmentation, alveolar ridge augmentation, implant surgery, jaw cysts, periodontal surgery, socket preservation, endodontic surgery, alveolar clefts, cleft lip and palate, oroantral communication (OAC), oral ulcers, osteoradionecrosis, bisphosphonate related osteonecrosis of the jaw (BRONJ), and temporomandibular joint disorders. In addition, the main cellular and molecular components of PCs were discussed with an emphasis on their regenerative properties.
PRP is the first generation of PCs. It can be prepared using centrifugation or plasmapheresis. The centrifugation can be done in one or two steps (i.e., single or double spin) using various forces (g) and centrifugation times. The centrifugation time ranges from 8 to 30 min (13).The most commonly used centrifugation forces (g) range from 100 to 1,000 g (14, 15), however, some protocols used forces (g) as high as 3,731 g (16).The most common described one step centrifugation protocol is plasma rich in growth factors (Anitua's PRGF). In this protocol, plasma is divided into two fractions, the top fraction (platelet poor plasma) and the bottom fraction, including the buffy coat, contains higher concentration of platelets. For the two-step centrifugation protocol, first spin separates the blood in the centrifugation tubes into three layers: red blood cells (RBCs) in the bottom, a buffy coat (BC) rich in platelets and leukocytes in the middle, and platelet poor plasma (PPP) in the top. To produce P-PRP, the PPP layer, and the upper part of the BC layer are transferred to another tube and exposed to a second centrifugation. Then, most of the PPP layer is removed and the platelet pellet concentration in the bottom of the centrifugation tube is resuspended in a small volume of plasma to produce P-PRP. In this protocol, most of leukocytes are discarded. To produce L-PRP, the PPP, the whole BC, and part of RBCs layer are transferred to another tube and centrifuged. The L-PRP consists of the platelet and leukocyte concentration and some RBCs suspended in a small fraction of plasma (1). The increase in platelet count achieved with different PRP methods can range from 2.6 to 7.3 folds, but the two spin protocols the ones that yield higher platelet concentration (13).
PRP can be applied without activation to the recipient site as the exposed collagen or thrombin produced as a result of tissue injury activates platelets (17). However, an activator such as calcium chloride (CaCl2), thrombin, collagen, calcium gluconate, lysis by freezing, photoactivation, or a mixture of CaCl2 and thrombin is commonlly added before the application. Calcium chloride is the most commonly used activator to counteract the effect of the anticoagulants. The purpose of platelet activation is to enhance the release of GFs from α-granules and to form the gel (17–19) (Table 1).
PRF is the second generation of PCs. L-PRF is more commonly used than P-PRF. It can be prepared with a simple inexpensive process without the addition of anticoagulants by using a single spin protocol [3,000 rpm (~700 g) for 10 min] that results in 1–2 folds increase in platelet count (20). The slow polymerization during PRF preparation generates a fibrin network that enhances cell migration and proliferation. In addition, this network acts as a reservoir of platelets, leukocytes, and GFs (21). L-PRF forms a gel that can be applied directly to the surgical site or can be compressed with a special kit to form a membrane that used to cover bone grafts in different augmentation procedures (22).
P-PRF can be prepared only by one method (Fibrinet PRFM kit) using two spin protocol. Blood is collected in specified tubes containing tri-sodium citrate and a separator gel, and then centrifuged at 1,100 rpm for 6 min. This separates the blood into three layers: RBCs, BC, and PPP. The BC and the PPP layers are collected and activated with CaCl2, followed by second centrifugation at 4,500 rpm for 15 min. Then, platelet rich fibrin matrix (PRFM) clot can be collected and applied to the surgical site (23) (Table 1).
PCs consist of different concentration of platelets, white blood cells, plasma proteins, and GFs. Each component may affect tissue regeneration.
Platelets are ~2.5 μm long cell fragments derived from bone marrow megakaryocytes (24). The normal blood platelet count in healthy individuals ranges from 150,000 to 450,000 platelets/μl. Although platelets lack nuclei, they contain other organelles including mitochondria, microtubules, α-granules, dense granules, and lysosomes (25).
Platelet ultrastructure can be divided into four zones: the peripheral zone, the sole-gel zone, the organelle zone, and the membrane system. The peripheral zone contains the plasma membrane, a smooth membrane rich in glycocalyx [glycoprotein (GP)], lipid bilayer area, and actin filaments. The glycocalyx is essential for platelet adhesion to subendothelial cells, platelet activation, and aggregation (25). The sole gel zone consists of actin microfilaments, a matrix in which all the platelet organelles are suspended, and microtubules (25). The organelle zone contains the secretory organelles: α-granules, dense-granules and lysosomes. The α-granules are the most abundant platelet organelles, each platelet has 50–80 α-granules. The α-granules are round or oval in shape, and 200 to 500 nm in diameter. They contain adhesive proteins (von Willebrand factor (VWF), fibrinogen, and thrombospondin), GFs, microbicidal proteins (thymosin-β4, thrombocidins 1 and 2), immune mediators (complement C3 and C4 precursors), as well as coagulant and fibrinolytic proteins (Factors V, IX, XIII, antithrombin, and plasminogen) (26). The dense granules are smaller than the α-granules. Each platelet contains 3–5 dense-granules. Dense granules contain adenosine triphosphate (ATP), adenosine diphosphate (ADP), serotonin, histamine, polyphosphate, pyrophosphate, calcium, magnesium and potassium. Lysosomes are spherical and each platelet contains <3 lysosomes that contain degrading enzymes (26). The membrane system, besides the plasma membrane, contains the Golgi apparatus, the canalicular system, dense tubules, and the endoplasmic reticulum (27).
The primary function of platelet is to enhance hemostasis through four steps: platelet adhesion, activation, secretion and aggregation. Also, platelets play a vital role in inflammation, tissue healing, and antimicrobial host defense (27).
Platelet α-granules store GFs, adhesion proteins, coagulation and fibrinolytic proteins that are secreted upon activation. These bioactive molecules have bone regenerative properties as they enhance osteogenesis and angiogenesis (28–30). GFs include transforming growth factor-beta (TGF-β), fibroblast growth factors (FGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), platelet derived growth factors AB (PDGF-AB, PDGF-BB), and brain-derived neurotrophic factor (BDNF) (31). GFs play a role in chemotaxis, proliferation, cell differentiation, the formation of extracellular matrix, osteogenesis, and prevention of bone resorption (29, 30, 32) (Table 2). The clinical applications of GFs are limited due to protein instability, short duration of action, high costs, and they required high doses. This is why PCs are very practical alternatives to deliver GFs (55).
Platelet dense granules contain ADP, ATP, serotonin, histamine, polyphosphate, pyrophosphate, calcium, magnesium, and potassium. ATP was found to increase bone mineralization at low concentration but decrease bone mineralization at high concentration (41). On the other hand, polyphosphate at low concentration (10 μM) inhibits matrix mineralization in osteoblast cell culutres (44), whereas at higher concentration (100 μM), it enhances osteoblast fucntion and mineralization (45). Serotonin is the fastest-released molecule from platelet after activation, regardless of the type of activation material (56). It reduces bone mineral density and inhibits osteoblast differentiation and proliferation (42). Also, it inhibits FXIII-A, which mediates the assembly of plasma fibronectin in cell cultures (43).
Plasma proteins are one of main components of blood plasma. They include albumin, globulins (i.e., fibronectin), coagulation proteins (i.e., fibrinogen, prothrombin, thrombin, III, IV, V, VI, VII, VIII, IX, X, IX, XII, XIII), and the complement system. Albumin was reported to enhance bone healing when used as adjunctive to freeze-dried cancellous bone grafts compared to bone graft alone (46). Fibronectin is an adhesive protein that plays a key role in wound healing: enhancing re-epithelization and extracellular matrix formation (ECM) (47). Fibronectin can interact with different types of cells and cytokines to form the ECM through its specific function domains and binding sites (47). In addition, fibronectin with beta-tricalcium phosphate (β-TCP) improve bone regeneration in rat calvarial bone defects (48). Thrombin was found to control osteoblast function and fracture healing in mice (49). Fibrinogen 3D scaffolds improve bone regeneration by increasing transforming growth factor-beta (TGF-β) (52). In osteoblast cell cultures, the inhibition of FXIII-A transglutaminase reduces fibronectin, collagen matrix assembly and mineralization (43). The complement system maintains cell proliferation, cell turnover, and enhances angiogenesis and tissue regeneration (53) (Table 2).
PCs may contain leukocytes depending on the preparation protocol. The leukocyte count in whole blood ranges from 4.5 to 11.0 × 109/L. In PCs, leukocyte counts range from 0/L in platelet poor plasma (PPP) to 35.8 × 109/L in L-PRP (57, 58). Leukocytes could enhance or impair tissue healing depending on their environment (58). Neutrophils are the primary white blood cells that migrate to the injured tissue and promote phagocytosis of dead tissue and microbes (59). Monocytes enhance host defense and promote arterogenesis (60). Lymphocytes are not required at the early stages of wound tissue healing, but an innate cellular immune response is required for tissue repair (61). Some studies proposed that leukocytes stimulate the healing process in damaged tissue as they secrete GFs, and simultaneously suppress the growth of bacteria, thus reducing the infection (62–65). However, there is no evidence support the antimicrobial effects of leucocyte- rich PCs (66). Furthermore, other reports showed a positive correlation between the total number of leukocytes in PRP and increased levels of pro-inflammatory cytokines and reactive oxygen species (ROS) released by neutrophils in damaged tissue indicating that high concentration of leukocytes in PRP may inhibit the healing process, and thus the presence of leukocytes should be controlled (67).
Erythrocytes are the most common cell type of blood cells that carry oxygen to all body tissues. PCs can be prepared with or without erythrocytes. However, many researchers do not pay attention to erythrocyte found in PC and their possible role on tissue regeneration and repair. In addition, there is a lack of clinical and in vivo studies assessing the effect of RBCs in tissue regeneration. Thus, the role of erythrocytes on tissue regeneration is unknown. Only an in vitro study done by Braun et al. (68) showed that RBC rich-PRP resulted in synoviocyte cell death and thus RBCs may have deleterious effect on cartridge regeneration (68).
PCs have been used alone or as an adjunctive treatment to enhance soft and hard tissue regeneration in several oral and maxillofacial interventions such as sinus augmentation, implant surgery, alveolar ridge augmentation, socket preservation, periodontal surgery, endodontic surgery, jaw cysts, oral ulcers, alveolar clefts, jaw osteonecrosis, Oroantral communication, and temporomandibular disorders. Underneath we discuss each one of those interventions in details (Table 3). Figures 1–6 showing some clinical applications of PCs in oral and craniofacial tissue regeneration.
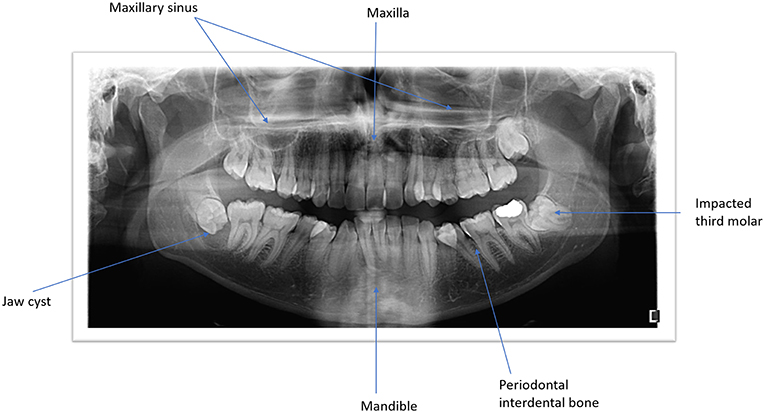
Figure 1. Panoramic view of both jaws illustrating the anatomic structures and the pathological conditions.
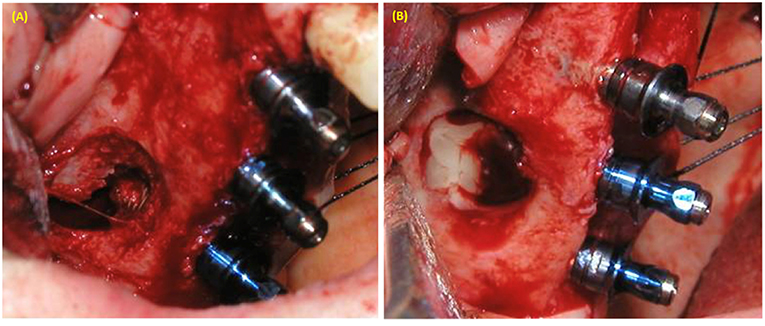
Figure 2. Maxillary sinus augmentation using lateral window approach. (A) Perforation of sinus membrane during lateral window approach in sinus lifting with simultaneous implant placement. (B) PRP was used to cover the perforated sinus membrane.
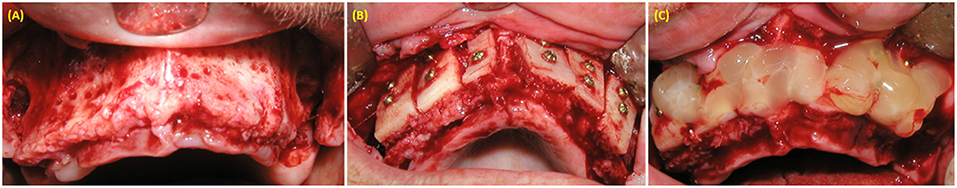
Figure 3. Alveolar maxillary width reconstruction using allograft cortico-cancellous block. (A) exposure of alveolar ridge. (B) Fixation of bone graft with fixation screws. (C) PRP covering the bone graft material.
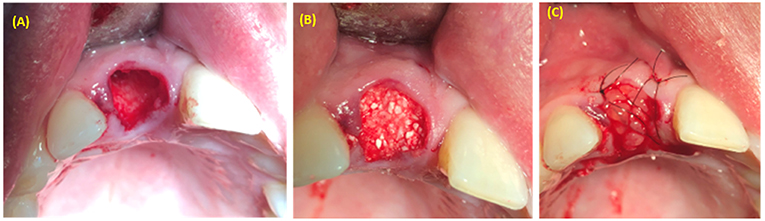
Figure 4. Alveolar socket preservation using bone graft covered with PRP. (A) Extraction socket. (B) Bone graft applied inside the extraction socket. (C) PRP covered bone graft material.
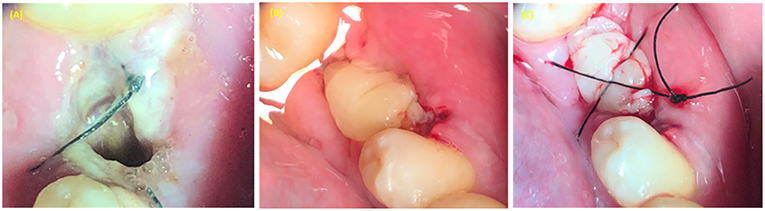
Figure 5. PRP use in treatment of alveolar osteitis (inflammation of alveolar socket). (A) Alveolar socket. (B) PRP applied in the extraction socket. (C) Figure 8 suture type used to stabilize the PRP in the alveolar socket.
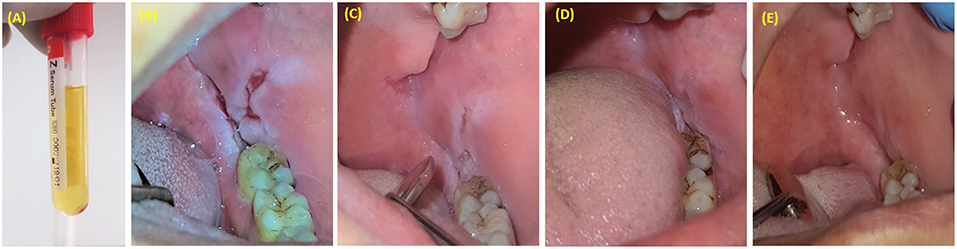
Figure 6. PRP injection as an adjunctive treatment with corticosteroids for resistant oral pemphigus vulgaris. (A) 1.5 ml of PRP was injected once a week for 3 weeks. (B) Pemphigus vulgaris lesion located posterior to mandibular third molar. (C) One week after last injection. (D) Six weeks after last injection. (E) Six months after last injection.
Sinus augmentation is a surgical procedure aims to restore the resorbed bone in the posterior maxilla caused by tooth loss and is indicated in cases with no enough bone height to accommodate dental implants. PRF has been used as sole filling material or combined with bone graft materials in sinus augmentation. The use of PRF as a sole filling material with simultaneous implant placement showed successful results, although there was no control group and these findings could be similar to the use of implant as a tent in which blood clot filled the gap around the dental implant. Furthermore, PRF reduced the healing time when combined with demineralized freeze-dried bone allograft. However, it does not affect the maturation of deproteinized bovine bone (69). In addition, PRF can be used as a membrane to cover the lateral window approach in sinus augmentation. The use of PRF or collagen as membranes in sinus augmentation with xenografts showed similar results in terms of percentages of new bone formation and the amount residual bone (70). The addition of PRP to anorganic bovine bone (ABB) increased the volume of newly formed bone and improved the osteoconductive properties of ABB, although it did not affect the implant success compared to ABB alone (71). However, in a systematic review, assessed the effect of PRP combined with bone grafts in sinus augmentation, showed no differences in clinical outcomes including implant success and complications (72). Also, the use of PCs as an adjunctive treatment for maxillary sinus elevation was not supported by other studies (73). Therefore, further standardized randomized clinical trials (RCTs) are requried.
Different augmentation techniques such as ridge augmentation, guided bone regeneration (GBR), and ridge splitting and expansion can be used to manage alveolar ridge resorption. Different bone grafts (autografts, allograft, xenografts, alloplasts) with or without biological materials such as PCs are used for alveolar ridge augmentation. In alveolar ridge augmentation with the titanium mesh (Ti-mesh), it was found that covering the mesh with PRP prevents the mesh exposure and bone resorption (74). PRP was found to significantly increase alveolar ridge width and the percentage of vital bone achieved with cancellous allograft (75). In addition, covering the autogenous bone blocks in anterior maxillary augmentation with PRF increased bone width and decreased bone resorption (110). On the other hand, limited evidence was found regarding the effect of PRGF on new bone formation when combined with other bone substitutes, but it also shown some benefits on soft tissue healing, pain, and swelling (76). However, due to limited studies, further research is required.
PCs have been used alone or as an adjunctive material in the treatment of intra-bony periodontal defects. Overall, PRP as an adjunctive material significantly enhanced periodontal bone healing (77). PRP or PRF can reduce pocket depth and enhance clinical attachment level (77). Moreover, the use of PRP combined with bone grafts was found to enhance periodontal bone healing, whereas, no beneficial effect was found when used with guided tissue regeneration (GTR) (78). PRF was effective when used alone or combined with open flap debridement, although, there was a lack of evidence regarding its role when combined with bone grafts or GTR (78).
Regarding the efficacy of PCs in periodontal plastic surgery, studies showed that PRF membrane did not regenerate gingival recession, clinical attachment level, or the width of keratinized mucosa compared to other biomaterials such as connective tissue grafts (111).
Platelet concentrates have been used to accelerate healing and regenerate bone in socket preservation procedures. L-PRF reduced the healing time and bone resorption when applied on the extracted tooth socket (80). Moreover, L-PRF was reported to enhance alveolar socket preservation in height and width compared to PRP (81). In addition, PCs reduce alveolar osteitis compared to empty socket after third molar extraction (82, 83). However, PCs have a beneficial effect in accelerating wound healing and reducing postoperative symptoms; some studies showed conflicting results in hard tissue regeneration (4, 5). Overall, there was a moderate evidence to support the use of PCs in socket preservation (84).
Platelet concentrates have been used to enhance bone regeneration prior to implant placement or to treat bone defects in cases of peri-implantitis. Overall, there is moderate evidence to support the use of PCs in the early phases of osseointegration (84). Both PRF and PRP have been found to enhance bone regeneration and reduce marginal bone loss around dental implants (80, 112). However, the application of PRF during immediate implantation does not seem to improve implant stability or bone healing (86). In cases of peri-implantitis, PRF application after surgical debridement was found to reduce peri-implant pocket depth and to increase keratinized mucosa compared to conventional flap surgery only (87).
PCs have been used in different root canal procedures including apexification, apexogenesis, pulpotomy, and endodontic apical surgery. PCs have been found to enhance peri apical bone regeneration, root development, and pulp vitality (88). In addition, a comprehensive systematic review of clinical evidence, showed that application of PCs are successful procedures in the treatment of immature teeth, although the level of evidence was weak (only 5 RCTs and 21 case reports were included), and thus further well-designed RCTs with longer follow-up are required (89).
Jaw cysts are pathological cavities formed within the jaw bones or soft tissues, that are usually lined by epithelial layer. Radicular cysts are the most common inflammatory cysts that are usually located around the apexes of necrotic teeth. The standard treatment for inflammatory jaw cysts is enucleation (113). Fewer reports assessed PCs application in bone regeneration after cyst enucleation. Filling the cyst cavity with bone graft and PRP was found to accelerate bone healing compared to bone graft only (90). In addition in a pilot study consisted of 10 cystic lesions, PRP was found to significantly accelerate bone healing after 6 months of follow-up (91). However, in another study, PRP did not show any significant acceleration in bone healing after 6 months of treatment compared to the control group (empty defect) (92). Thus, due to limited evidence and absence of RCTs, further standardized studies are required.
Cleft lip and plate are congenital defects in maxillary and nasal processes that resulted in cleft lip and/or palate. Surgical closure is the treatment of choice in such patients. PRP was found to enhance soft tissue closure of complete cleft palate and to reduce the incidence of oronasal fistula compared to surgical closure alone. Therefore, it could reduce the need for additional surgical procedures (93). In addition, mixing the PRGF with bone grafts resulted in complete closure of 90.9% cases with recurrent cleft palate fistulas (94). However, due to limited evidence, further standardized studies are required.
Alveolar clefts are congenital bone defects in the alveolar bone that affect 75% of cleft lip or cleft lip and palate patients (114). The combination of iliac graft and PRP reduce bone resorption compared to iliac graft alone in patients requiring alveolar cleft augmentation (95, 96). On the other hand, adding PRF to iliac bone grafts, did not benefit maxillary alveolar bone thickness, height, and density or the percentage of newly formed bone (97, 98). However, due to limited evidence, further studies are required.
BRONJ is commonly manifested by exposed necrotic bone that persists for 8 weeks or more in patients with metastasis or osteoporosis under antiresorptive medications such as alendronate. PRF has been found to enhance soft tissue healing and reduce pain of surgically debrided BRONJ cases (99). In addition, Erbium Chromium: Yttrium Scandium Gallium Garnet laser (Er,Cr:YSGG) assessed surgery combined with PRP application enhance healing of BRONJ cases (100). The combination of bone morphogenetic protein 2 (BMP-2) and L-PRF in treating BRONJ cases was found to accelerate healing compared to L-PRF alone (101). However, in a systematic review summarizing the combination protocol (surgery and PCs), concluded that there is no sufficient data to support this protocol and thus further studies are required (115).
Osteoradionecrosis of the jaws is defined as bone death caused by radiation therapy of head and neck cancer. PCs have been found to be effective in treating patient with osteoradionecrosis (103). The application of L-PRF combined with surgical debridement using piezosurgery in managing cases of osteoradionecrosis, resulted in complete healing of 67% of cases within 1-year follow-up (102). This promising application could reduce the need for bone resection in such cases, although due to the limited evidence, further research is required.
Oroantral communication (OAC) is an abnormal communication between the oral cavity and the maxillary sinus that could occur due to pathological conditions or during extraction of maxillary posterior teeth. The treatment of OAC depends on the size of the communication and it is usually achieved by buccal advancement flaps. PRF clots enhance wound closure and reduce pain and swelling compared to buccal advancement flap (104). In addition, PRF membrane could be used to manage OAC of size ≤ 5 mm (105). However, further standardized studies are required to shed the light in such promised application.
Pemphigus vulgaris is an autoimmune disease that affects the skin and mucous membranes. It manifests in oral cavity as painful erosive lesions (ulcers) that are usually treated with corticosteroids (116). In such cases, the repeated injections of PRP accelerate tissue healing and reduce pain and discomfort during mastication (106). Oral ulcers can also occur after bone marrow allogeneic transplants resulting in graft vs. host disease (GvHD). Application of PCs gel in those patients reduces pain, enhances mastication, and accelerates healing (107). In addition, PRF membrane could improve tissue healing after excision of oral mucosal lesions such as leukoplakia and lichen planus (108). PCs seem to be promising materials for managing oral ulcers, however, due to limited studies, further research is required.
TMDs are diseases of multifactorial origin that affect the temporomandibular joint articular surfaces as well as the surrounding masticatory muscles (117). PRP injection has been used to reduce pain and to improve mouth opening in patients with TMDs. In a systematic review, PRP injection was found to reduce pain and improve jaw movements in 4 out of 6 studies compared to hyaluronic acid or saline injections (109).
PCs have been used extensively in oral and craniofacial interventions for hard tissue regeneration. Overall, PCs seem to have a beneficial role on bone regeneration in different clinical procedures, such as socket preservation, implant surgery, sinus augmentation, periodontal surgery, osteonecrosis, and alveolar ridge augmentation (71, 75, 78, 80, 90, 95, 96, 100, 112), although, there was some inconsistent findings. The regenerative potential of PCs is attributed to their contents of bioactive molecules, specifically GFs known to enhance osteogenesis, angiogenesis and tissue regeneration (28–30). However, due to limited studies, further research is required.
PCs seem to improve soft tissue healing in socket preservation (81), periodontal surgery (77), oral ulcers (106), oroantral communication (105), osteonecrosis (100), and alveolar ridge augmentation (74, 75). The improvement on soft tissue closure obtained by PCs could indirectly enhance bone regeneration in different surgical procedures.
In addition, PCs could reduce inflammatory complications such as pain and swelling and could improve mouth opening and masticatory function in patients with in temporomandibular disorders (TMDs) (109, 118), oral ulcers (106), jaw osteonecrosis, and extraction sockets (4). This beneficial role could be attributed to their content of GFs that have an essential role in inflammation, cell movement and metabolism (119). In addition, PCs may have immunomodulatory effects; PC can induce considerable changes in the level of proinflammatory mediators such as an increased level of lipoxin A4 (LXA4) and thus suggests that PCs could prohibit cytokine secretion, reduce inflammation and promote tissue healing (120).
However, the effect of PCs on bone regeneration has been clinically inconsistent across the literature (4, 76). Differences in study designs and types of PCs used with variable concentration of platelets, growth factors, and leucocytes, as well as different application forms and techniques could explain these contradictory results (6). Also, the centrifuge characteristics and different centrifugation protocols could affect the cells, growth factors and fibrin architecture of PCs and thus any modification of the original protocols should be investigated separately in order to avoid confusion and inaccurate results (121). In addition, a recent study suggested the possible negative effect of platelet dense granule contents including serotonin in bone regeneration (23). Serotonin inhibits osteogenic activity and osteoblastic differentiation and proliferation (42, 122, 123). Therefore, PCs preparation protocols require standardization.
PRP and PRF are the main types of PCs that applied in different clinical procedures. PRP is the only PCs that can be injected inside the temporomandibular joint (TMJ) spaces in treating patients with TMDs (109, 118). PRF membrane is used for closure of oroantral communication (105). Both materials could be used for other clinical interventions. Their main challenges are the stability in the application site and the need for an optimized delivery system that allow gradual release of growth factors (124).
Platelet concentrate (PC) are biological blood-derived products that are rich in platelets and GFs. Overall, PCs seem to enhance bone and soft tissue healing, reduce pain and swelling in alveolar ridge augmentation, periodontal surgery, sinus augmentation, socket preservation, implant surgery, endodontic regeneration, BRONJ, osteoradionecrosis, closure of oroantral communication (OAC), oral ulcers, and temporomandibular disorders. On the other hand, no effect was reported in gingival recession and guided tissue regeneration (GTR) procedures. However, due to the limited available evidence and the heterogeneity among different studies, further research is required to shed light on these promising biological materials.
FA-H wrote the review and participated in design the review. MM participated in writing part of the clinical applications. HA-W revised the manuscript. JT revised the manuscript. ZB and FT designed and revised the manuscript.
This work was supported by grants from NSERC RGPIN-2019-04340, Faculty of Dentistry, McGill University, International Team for Implantology (ITI) grant (ITI 1362-2018 and ITI 1320-2018), Dr. Florence Johnston grant, CRC 241487, FRQ-S/RSBO/UdeMtl 5213, CFI/IOF 31705, and MEDTEQ (FT). FA-H was supported by scholarships from Al Awn Foundation for Development, Yemen, Ph.D training award from Funds de Recherche Québec - Santé (FRQS code: 257709), Alpha Omega Foundation of Canada grant and he received the Graduate Excellence Award from Faculty of Dentistry, McGill University. MM was supported by scholarships from the College of Dentistry, Jazan University through Saudi Arabian Cultural Bureau, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Dr. Abdel-Hameed Hijazi from Cairo University for sharing photos for pemphigus vulgaris case.
1. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. (2009) 27:158–67. doi: 10.1016/j.tibtech.2008.11.009
2. Dohan Ehrenfest DM, Bielecki T, Mishra A, Borzini P, Inchingolo F, Sammartino G, et al. In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr Pharm Biotechnol. (2012) 13:1131–7. doi: 10.2174/138920112800624328
3. Harrison P. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thromb Haemost. (2018) 16:1895–900. doi: 10.1111/jth.14223
4. Al-Hamed FS, Tawfik MA, Abdelfadil E, Al-Saleh MAQ. Efficacy of platelet-rich fibrin after mandibular third molar extraction: a systematic review and meta-analysis. J Oral Maxillofacial Surg. (2017) 75:1124–35. doi: 10.1016/j.joms.2017.01.022
5. Annunziata M, Guida L, Nastri L, Piccirillo A, Sommese L, Napoli C. The role of autologous platelet concentrates in alveolar socket preservation: a systematic review. Transfusion Med Hemother. (2018) 45:195–203. doi: 10.1159/000488061
6. Del Corso M, Vervelle A, Simonpieri A, Jimbo R, Inchingolo F, Sammartino G, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: periodontal and dentoalveolar surgery. Curr Pharmaceut Biotechnol. (2012) 13:1207–30. doi: 10.2174/138920112800624391
7. Sela JJ, Bab IA. Healing of Bone Fracture: general concepts, principles of bone regeneration. Principles Bone Regenerat. (2012) 2012:1–8. doi: 10.1007/978-1-4614-2059-0_1
8. Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. (2002) 17:513–20. doi: 10.1359/jbmr.2002.17.3.513
9. Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. (2003) 88:873–84. doi: 10.1002/jcb.10435
10. Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. (2001) 16:1004–14. doi: 10.1359/jbmr.2001.16.6.1004
11. Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. (2009) 27:1887–98. doi: 10.1002/stem.103
12. Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. (2000) 20:8783–92. doi: 10.1128/MCB.20.23.8783-8792.2000
13. Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutaneous Aesthetic Surg. (2014) 7:189–97. doi: 10.4103/0974-2077.150734
14. Perez AG, Lana JF, Rodrigues AA, Luzo AC, Belangero WD, Santana MH. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. (2014) 2014:176060. doi: 10.1155/2014/176060
15. Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Br J Haematol. (1976) 34:395–402. doi: 10.1111/j.1365-2141.1976.tb03586.x
16. Kahn RA, Cossette I, Friedman LI. Optimum centrifugation conditions for the preparation of platelet and plasma products. Transfusion. (1976) 16:162–5. doi: 10.1046/j.1537-2995.1976.16276155111.x
17. Harrison S, Vavken P, Kevy S, Jacobson M, Zurakowski D, Murray MM. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med. (2011) 39:729–34. doi: 10.1177/0363546511401576
18. Cavallo C, Roffi A, Grigolo B, Mariani E, Pratelli L, Merli G, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. (2016) 2016:6591717. doi: 10.1155/2016/6591717
19. Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord. (2016) 17:67. doi: 10.1186/s12891-016-0920-3
20. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. (2006) 101:e45–e50. doi: 10.1016/j.tripleo.2005.07.009
21. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. (2006) 101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008
22. Gassling V, Douglas T, Warnke PH, Acil Y, Wiltfang J, Becker ST. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. (2010) 21:543–9. doi: 10.1111/j.1600-0501.2009.01900.x
23. Badran Z, Abdallah MN, Torres J, Tamimi F. Platelet concentrates for bone regeneration: current evidence and future challenges. Platelets. (2018) 29:105–12. doi: 10.1080/09537104.2017.1327656
24. Thon JN, Italiano JE. Platelet formation. Semin Hematol. (2010) 47:220–6. doi: 10.1053/j.seminhematol.2010.03.005
25. White JG. Platelet structure. In: Michelson AD, editor. Platelets. San Diego, CA: Elsevier/Academic Press (2013). p. 117–44. doi: 10.1016/B978-0-12-387837-3.00007-9
26. White JG. Platelet secretion. In: Michelson AD, editor. Platelets. San Diego, CA: Elsevier/Academic Press (2013). p. 343–66. doi: 10.1016/B978-0-12-387837-3.00018-3
27. Gremmel T, Frelinger AL III, Michelson AD. Platelet Physiology. Seminars in thrombosis and hemostasis. Semin Thromb Hemost. (2016) 42:191–204. doi: 10.1055/s-0035-1564835
28. Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. (2002) 110:771–81. doi: 10.1172/JCI15463
29. Wu M, Chen G, Li YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. (2016) 4:16009. doi: 10.1038/boneres.2016.9
30. Shakir S, MacIsaac ZM, Naran S, Smith DM, Bykowski MR, Cray JJ, et al. Transforming growth factor beta 1 augments calvarial defect healing and promotes suture regeneration. Tissue Eng Part A. (2015) 21:939–47. doi: 10.1089/ten.tea.2014.0189
31. Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dentistry. (2016) 2:19. doi: 10.1186/s40729-016-0052-4
32. Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. (2015) 29:1463–86. doi: 10.1101/gad.266551.115
33. Zhou WL, Li LL, Qiu XR, An Q, Li MH. Effects of combining insulin-like growth factor 1 and platelet-derived growth factor on osteogenesis around dental implants. Chin J Dent Res. (2017) 20:105–9. doi: 10.3290/j.cjdr.a38275
34. Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. (2001) 106:148–56. doi: 10.1159/000046610
35. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. (1999) 5:623–8. doi: 10.1038/9467
36. Kilian O, Hartmann S, Dongowski N, Karnati S, Baumgart-Vogt E, Hartel FV, et al. BDNF and its TrkB receptor in human fracture healing. Ann Anatomy. (2014) 196:286–95. doi: 10.1016/j.aanat.2014.06.001
37. Lee YM, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol. (2010) 22:805–16. doi: 10.1093/intimm/dxq431
38. Franchimont N, Wertz S, Malaise M. Interleukin-6: an osteotropic factor influencing bone formation? Bone. (2005) 37:601–6. doi: 10.1016/j.bone.2005.06.002
39. Yang A, Lu Y, Xing J, Li Z, Yin X, Dou C, et al. IL-8 enhances therapeutic effects of BMSCs on bone regeneration via CXCR2-mediated PI3k/Akt signaling pathway. Cell Physiol Biochem. (2018) 48:361–70. doi: 10.1159/000491742
40. Adams CS, Mansfield K, Perlot RL, Shapiro IM. Matrix regulation of skeletal cell apoptosis. Role of calcium and phosphate ions. J Biol Chem. (2001) 276:20316–22. doi: 10.1074/jbc.M006492200
41. Cutarelli A, Marini M, Tancredi V, D'Arcangelo G, Murdocca M, Frank C, et al. Adenosine Triphosphate stimulates differentiation and mineralization in human osteoblast-like Saos-2 cells. Dev Growth Differ. (2016) 58:400–8. doi: 10.1111/dgd.12288
42. Nam SS, Lee JC, Kim HJ, Park JW, Lee JM, Suh JY, et al. Serotonin inhibits osteoblast differentiation and bone regeneration in rats. J Periodontol. (2016) 87:461–9. doi: 10.1902/jop.2015.150302
43. Cui C, Kaartinen MT. Serotonin (5-HT) inhibits Factor XIII-A-mediated plasma fibronectin matrix assembly and crosslinking in osteoblast cultures via direct competition with transamidation. Bone. (2015) 72:43–52. doi: 10.1016/j.bone.2014.11.008
44. Hoac B, Kiffer-Moreira T, Millan JL, McKee MD. Polyphosphates inhibit extracellular matrix mineralization in MC3T3-E1 osteoblast cultures. Bone. (2013) 53:478–86. doi: 10.1016/j.bone.2013.01.020
45. Muller WE, Wang X, Diehl-Seifert B, Kropf K, Schlossmacher U, Lieberwirth I, et al. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. (2011) 7:2661–71. doi: 10.1016/j.actbio.2011.03.007
46. Skaliczki G, Schandl K, Weszl M, Major T, Kovacs M, Skaliczki J, et al. Serum albumin enhances bone healing in a nonunion femoral defect model in rats: a computer tomography micromorphometry study. Int Orthopaedics. (2013) 37:741–5. doi: 10.1007/s00264-012-1770-8
47. Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. (2015) 12:313–6. doi: 10.1111/iwj.12109
48. Escoda-Francoli J, Sanchez-Garces MA, Gimeno-Sandig A, Munoz-Guzon F, Barbany-Cairo JR, Badiella-Busquets L, et al. Guided bone regeneration using beta-tricalcium phosphate with and without fibronectin—An experimental study in rats. Clin Oral Implants Res. (2018) 29:1038–49. doi: 10.1111/clr.13370
49. Sato N, Ichikawa J, Wako M, Ohba T, Saito M, Sato H, et al. Thrombin induced by the extrinsic pathway and PAR-1 regulated inflammation at the site of fracture repair. Bone. (2016) 83:23–34. doi: 10.1016/j.bone.2015.10.005
50. Aronovich A, Nur Y, Shezen E, Rosen C, Zlotnikov Klionsky Y, Milman I, et al. A novel role for factor VIII and thrombin/PAR1 in regulating hematopoiesis and its interplay with the bone structure. Blood. (2013) 122:2562–71. doi: 10.1182/blood-2012-08-447458
51. Kawao N, Tamura Y, Okumoto K, Yano M, Okada K, Matsuo O, et al. Plasminogen plays a crucial role in bone repair. J Bone Miner Res. (2013) 28:1561–74. doi: 10.1002/jbmr.1921
52. Vasconcelos DM, Goncalves RM, Almeida CR, Pereira IO, Oliveira MI, Neves N, et al. Fibrinogen scaffolds with immunomodulatory properties promote in vivo bone regeneration. Biomaterials. (2016) 111:163–78. doi: 10.1016/j.biomaterials.2016.10.004
53. Rutkowski MJ, Sughrue ME, Kane AJ, Ahn BJ, Fang S, Parsa AT. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res. (2010) 59:897–905. doi: 10.1007/s00011-010-0220-6
54. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. (2017) 13:208–19. doi: 10.1038/nrendo.2016.153
55. Aravamudhan A, Ramos DM, Nip J, Subramanian A, James R, Harmon MD, et al. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr Pharmaceut Des. (2013) 19:3420–8. doi: 10.2174/1381612811319190008
56. Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. (2012) 120:5209–16. doi: 10.1182/blood-2012-07-445080
57. Fitzpatrick J, Bulsara MK, McCrory PR, Richardson MD, Zheng MH. Analysis of platelet-rich plasma extraction: variations in platelet and blood components between 4 common commercial kits. Orthopaedic J Sports Med. (2017) 5:2325967116675272. doi: 10.1177/2325967116675272
58. King W, Toler K, Woodell-May J. Role of white blood cells in blood- and bone marrow-based autologous therapies. Biomed Res Int. (2018) 2018:6510842. doi: 10.1155/2018/6510842
59. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology. (2013) 13:159–75. doi: 10.1038/nri3399
60. Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, et al. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukocyte Biol. (2006) 80:59–65. doi: 10.1189/jlb.0206087
61. Schaffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg. (1998) 85:444–60. doi: 10.1046/j.1365-2168.1998.00734.x
62. Badade PS, Mahale SA, Panjwani AA, Vaidya PD, Warang AD. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J Dent Res. (2016) 27:300–4. doi: 10.4103/0970-9290.186231
63. Kasten P, Vogel J, Luginbuhl R, Niemeyer P, Weiss S, Schneider S, et al. Influence of platelet-rich plasma on osteogenic differentiation of mesenchymal stem cells and ectopic bone formation in calcium phosphate ceramics. Cells Tissues Organs. (2006) 183:68–79. doi: 10.1159/000095511
64. Pavlovic V, Ciric M, Jovanovic V, Stojanovic P. Platelet rich plasma: a short overview of certain bioactive components. Open Med. (2016) 11:242–7. doi: 10.1515/med-2016-0048
65. Barrick B, Campbell EJ, Owen CA. Leukocyte proteinases in wound healing: roles in physiologic and pathologic processes. Wound Repair Regener. (1999) 7:410–22. doi: 10.1046/j.1524-475X.1999.00410.x
66. D'Asta F, Halstead F, Harrison P, Zecchi Orlandini S, Moiemen N, Lord J. The contribution of leucocytes to the antimicrobial activity of platelet-rich plasma preparations: a systematic review. Platelets. (2018) 29:9–20. doi: 10.1080/09537104.2017.1317731
67. Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS ONE. (2015) 10:e0121713. doi: 10.1371/journal.pone.0121713
68. Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med. (2014) 42:1204–10. doi: 10.1177/0363546514525593
69. Ali S, Bakry SA, Abd-Elhakam H. Platelet-rich fibrin in maxillary sinus augmentation: a systematic review. J Oral Implantol. (2015) 41:746–53. doi: 10.1563/AAID-JOI-D-14-00167
70. Gassling V, Purcz N, Braesen JH, Will M, Gierloff M, Behrens E, et al. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: a preliminary study. J Cranio-Maxillo-Facial Surg. (2013) 41:76–82. doi: 10.1016/j.jcms.2012.10.015
71. Torres J, Tamimi F, Martinez PP, Alkhraisat MH, Linares R, Hernandez G, et al. Effect of platelet-rich plasma on sinus lifting: a randomized-controlled clinical trial. J Clin Periodontol. (2009) 36:677–87. doi: 10.1111/j.1600-051X.2009.01437.x
72. Esposito M, Grusovin MG, Rees J, Karasoulos D, Felice P, Alissa R, et al. Effectiveness of sinus lift procedures for dental implant rehabilitation: a Cochrane systematic review. Eur J Oral Implantol. (2010) 3:7–26.
73. Pocaterra A, Caruso S, Bernardi S, Scagnoli L, Continenza MA, Gatto R. Effectiveness of platelet-rich plasma as an adjunctive material to bone graft: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Oral Maxillofac Surg. (2016) 45:1027–34. doi: 10.1016/j.ijom.2016.02.012
74. Torres J, Tamimi F, Alkhraisat MH, Manchon A, Linares R, Prados-Frutos JC, et al. Platelet-rich plasma may prevent titanium-mesh exposure in alveolar ridge augmentation with anorganic bovine bone. J Clin Periodontol. (2010) 37:943–51. doi: 10.1111/j.1600-051X.2010.01615.x
75. Eskan MA, Greenwell H, Hill M, Morton D, Vidal R, Shumway B, et al. Platelet-rich plasma-assisted guided bone regeneration for ridge augmentation: a randomized, controlled clinical trial. J Periodontol. (2014) 85:661–8. doi: 10.1902/jop.2013.130260
76. Dragonas P, Schiavo JH, Avila-Ortiz G, Palaiologou A, Katsaros T. Plasma rich in growth factors (PRGF) in intraoral bone grafting procedures: a systematic review. J Cranio-Maxillo-Facial Surg. (2019) 47:443–53. doi: 10.1016/j.jcms.2019.01.012
77. Zhou S, Sun C, Huang S, Wu X, Zhao Y, Pan C, et al. Efficacy of adjunctive bioactive materials in the treatment of periodontal intrabony defects: a systematic review and meta-analysis. Biomed Res Int. (2018) 2018:8670832. doi: 10.1155/2018/8670832
78. Panda S, Doraiswamy J, Malaiappan S, Varghese SS, Del Fabbro M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: a systematic review and meta-analysis. J Invest Clin Dentistry. (2016) 7:13–26. doi: 10.1111/jicd.12117
79. Schliephake H. Clinical efficacy of growth factors to enhance tissue repair in oral and maxillofacial reconstruction: a systematic review. Clin Implant Dent Relat Res. (2015) 17:247–73. doi: 10.1111/cid.12114
80. Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, et al. Regenerative potential of leucocyte-and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J Clin Periodontol. (2017) 44:225–34. doi: 10.1111/jcpe.12658
81. Niu W, Wang P, Ge S, Ji P. Effects of platelet concentrates used in alveolar ridge preservation: a systematic review. Implant Dent. (2018) 27:498–506. doi: 10.1097/ID.0000000000000797
82. Unsal H, Erbasar HGN. Evaluation of the effect of platelet-rich fibrin on the alveolar osteitis incidence and periodontal probing depth after extracting partially erupted mandibular third molars extraction. Niger J Clin Pract. (2018) 21:201–5. doi: 10.4103/njcp.njcp_1_17
83. Prataap N, Sunil PM, Sudeep CB, Ninan VS, Tom A, Arjun MR. Platelet-rich plasma and incidence of alveolar osteitis in high-risk patients undergoing extractions of mandibular molars: a case-control study. J Pharm Bioallied Sci. (2017) 9:S173–9. doi: 10.4103/jpbs.JPBS_151_17
84. Strauss FJ, Stahli A, Gruber R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: a systematic review. Clin Oral Implants Res. 29 (Suppl. 1) (2018) 18:6–19. doi: 10.1111/clr.13275
85. Kotsovilis S, Markou N, Pepelassi E, Nikolidakis D. The adjunctive use of platelet-rich plasma in the therapy of periodontal intraosseous defects: a systematic review. J Periodontal Res. (2010) 45:428–43. doi: 10.1111/j.1600-0765.2009.01236.x
86. Diana C, Mohanty S, Chaudhary Z, Kumari S, Dabas J, Bodh R. Does platelet-rich fibrin have a role in osseointegration of immediate implants? A randomized, single-blind, controlled clinical trial. Int J Oral Maxillofac Surg. (2018) 47:1178–88. doi: 10.1016/j.ijom.2018.01.001
87. Hamzacebi B, Oduncuoglu B, Alaaddinoglu EE. Treatment of peri-implant bone defects with platelet-rich fibrin. Int J Periodontics Restorat Dentistry. (2015) 35:415–22. doi: 10.11607/prd.1861
88. Meschi N, Castro AB, Vandamme K, Quirynen M, Lambrechts P. The impact of autologous platelet concentrates on endodontic healing: a systematic review. Platelets. (2016) 27:613–33. doi: 10.1080/09537104.2016.1226497
89. Metlerska J, Fagogeni I, Nowicka A. Efficacy of autologous platelet concentrates in regenerative endodontic treatment: a systematic review of human studies. J Endodontics. (2019) 45:20–30.e1. doi: 10.1016/j.joen.2018.09.003
90. Nagaveni NB, Praveen RB, Umashankar KV, Pranav B, Sreedevi R, Radhika NB. Efficacy of platelet-rich-plasma (PRP) in bone regeneration after cyst enucleation in pediatric patients–a clinical study. J Clin Pediatr Dentistry. (2010) 35:81–7. doi: 10.17796/jcpd.35.1.q69168v5268234k9
91. Meshram VS, Lambade PN, Meshram PV, Kadu A, Tiwari MS. The autologous platelet rich fibrin: a novel approach in osseous regeneration after cystic enucleation: a pilot study. Indian J Dent Res. (2015) 26:560–4. doi: 10.4103/0970-9290.176915
92. Ramanathan A, Cariappa KM. Effect of platelet-rich plasma on bone regeneration after removal of cysts and benign tumours of the jaws. Oral Maxillofac Surg. (2014) 18:445–52. doi: 10.1007/s10006-013-0435-0
93. El-Anwar MW, Nofal AA, Khalifa M, Quriba AS. Use of autologous platelet-rich plasma in complete cleft palate repair. Laryngoscope. (2016) 126:1524–8. doi: 10.1002/lary.25868
94. Gonzalez-Sanchez JG, Jimenez-Barragan K. [Closure of recurrent cleft palate fistulas with plasma rich in growth factors]. Acta Otorrinolaringol Esp. (2011) 62:448–53. doi: 10.1016/j.otoeng.2011.06.001
95. Marukawa E, Oshina H, Iino G, Morita K, Omura K. Reduction of bone resorption by the application of platelet-rich plasma (PRP) in bone grafting of the alveolar cleft. J Cranio-Maxillofac Surg. (2011) 39:278–83. doi: 10.1016/j.jcms.2010.04.017
96. Gupta C, Mehrotra D, Mohammad S, Khanna V, Kumar Singh G, Singh G, et al. Alveolar bone graft with platelet rich plasma in cleft alveolus. J Oral Biol Craniofac Res. (2013) 3:3–8. doi: 10.1016/j.jobcr.2013.02.002
97. Omidkhoda M, Jahnabin A, Khoshandam F, Eslami F, Hosseini Zarch SH, Tavakol Afshari J, et al. Efficacy of platelet-rich fibrin combined with autogenous bone graft in the quality and quantity of maxillary alveolar cleft reconstruction. Iran J Otorhinolaryngol. (2018) 30:329–34. doi: 10.4103/njcp.njcp_1_17
98. Saruhan N, Ertas U. Evaluating of platelet-rich fibrin in the treatment of alveolar cleft with iliac bone graft by means of volumetric analysis. J Craniofac Surg. (2018) 29:322–6. doi: 10.1097/SCS.0000000000004125
99. Fernando de Almeida Barros Mourao C, Calasans-Maia MD, Del Fabbro M, Le Drapper Vieira F, Coutinho de Mello Machado R, Capella R, et al. The use of Platelet-rich Fibrin in the management of medication-related osteonecrosis of the jaw: a case series. J Stomatol Oral Maxillofac Surg. (in press). doi: 10.1016/j.jormas.2019.02.011
100. Mauceri R, Panzarella V, Maniscalco L, Bedogni A, Licata ME, Albanese A, et al. Conservative surgical treatment of bisphosphonate-related osteonecrosis of the jaw with Er,Cr:YSGG laser and platelet-rich plasma: a longitudinal study. Biomed Res Int. (2018) 2018:3982540. doi: 10.1155/2018/3982540
101. Park JH, Kim JW, Kim SJ. Does the addition of bone morphogenetic protein 2 to platelet-rich fibrin improve healing after treatment for medication-related osteonecrosis of the jaw? J Oral Maxillofac Surg. (2017) 75:1176–84. doi: 10.1016/j.joms.2016.12.005
102. Bilimoria R, Young H, Patel D, Kwok J. The role of piezoelectric surgery and platelet-rich fibrin in treatment of ORN and MRONJ: a clinical case series. Oral Surg. (2018) 11:136–43. doi: 10.1111/ors.12318
103. Scala M, Gipponi M, Mereu P, Strada P, Corvo R, Muraglia A, et al. Regeneration of mandibular osteoradionecrosis defect with platelet rich plasma gel. in vivo. (2010) 24:889–93.
104. Bilginaylar K. Comparison of the clinical outcomes of buccal advancement flap versus platelet-rich fibrin application for the immediate closure of acute oroantral communications. J Craniofac Surg. (2019) 30:e45–9. doi: 10.1097/SCS.0000000000004958
105. Demetoglu U, Ocak H, Bilge S. Closure of oroantral communication with plasma-rich fibrin membrane. J Craniofac Surg. (2018) 29:e367–70. doi: 10.1097/SCS.0000000000004360
106. EL-Komy MHM, Hassan AS, Abdel Raheem HM, Doss SS, EL-Kaliouby M, Saleh NA, et al. Platelet-rich plasma for resistant oral erosions of pemphigus vulgaris: a pilot study. Wound Repair Regen. (2015) 23:953–5. doi: 10.1111/wrr.12363
107. Picardi A, Ferraro AS, Miranda M, Meconi F, Lanti A, Adorno G, et al. Therapeutic efficiency of platelet gel for the treatment of oral ulcers related to chronic graft versus host disease after allogeneic haematopoietic stem cell transplantation. Oral Implantol. (2017) 10:398–405. doi: 10.11138/orl/2017.10.4.398
108. Pathak H, Mohanty S, Urs AB, Dabas J. Treatment of oral mucosal lesions by scalpel excision and platelet-rich fibrin membrane grafting: a review of 26 sites. J Oral Maxillofac Surg. (2015) 73:1865–74. doi: 10.1016/j.joms.2015.03.041
109. Bousnaki M, Bakopoulou A, Koidis P. Platelet-rich plasma for the therapeutic management of temporomandibular joint disorders: a systematic review. Int J Oral Maxillofac Surg. (2018) 47:188–98. doi: 10.1016/j.ijom.2017.09.014
110. Moussa M, El-Dahab OA, El Nahass H. Anterior maxilla augmentation using palatal bone block with platelet-rich fibrin: a controlled trial. Int J Oral Maxillofac Implants. (2016) 31:708–15. doi: 10.11607/jomi.3926
111. Moraschini V, Barboza Edos S. Use of platelet-rich fibrin membrane in the treatment of gingival recession: a systematic review and meta-analysis. J Periodontol. (2016) 87:281–90. doi: 10.1902/jop.2015.150420
112. Stahli A, Strauss FJ, Gruber R. The use of platelet-rich plasma to enhance the outcomes of implant therapy: a systematic review. Clin Oral Implants Res. 29 (Suppl. 1) (2018) 18:20–36. doi: 10.1111/clr.13296
113. Hill CM, Renton T. Oral surgery II: Part 3. Cysts of the mouth and jaws and their management. Br Dent J. (2017) 223:573–84. doi: 10.1038/sj.bdj.2017.916
114. Guo J, Li C, Zhang Q, Wu G, Deacon SA, Chen J, et al. Secondary bone grafting for alveolar cleft in children with cleft lip or cleft lip and palate. Cochrane Database Syst Rev. (2011) 2011:Cd008050. doi: 10.1002/14651858.CD008050.pub2
115. Lopez-Jornet P, Sanchez Perez A, Amaral Mendes R, Tobias A. Medication-related osteonecrosis of the jaw: is autologous platelet concentrate application effective for prevention and treatment? A systematic review. J Cranio-Maxillo-Facial Surg. (2016) 44:1067–72. doi: 10.1016/j.jcms.2016.05.004
116. Kala N, Manjeu J, Dominic N, Babu S Oral pemphigus without skin lesions treated with pulse steroid therapy. J Indian Soc Periodontol. (2018) 22:551–4. doi: 10.4103/jisp.jisp_345_18
117. Ahmad M, Schiffman EL. Temporomandibular joint disorders and orofacial pain. Dent Clin N Am. (2016) 60:105–24. doi: 10.1016/j.cden.2015.08.004
118. Fernandez-Ferro M, Fernandez-Sanroman J, Blanco-Carrion A, Costas-Lopez A, Lopez-Betancourt A, Arenaz-Bua J, et al. Comparison of intra-articular injection of plasma rich in growth factors versus hyaluronic acid following arthroscopy in the treatment of temporomandibular dysfunction: a randomised prospective study. J Cranio-Maxillo-Facial Surg. (2017) 45:449–54. doi: 10.1016/j.jcms.2017.01.010
119. Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. (2013) 9:721–30. doi: 10.1038/nrrheum.2013.141
120. El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. (2007) 78:661–9. doi: 10.1902/jop.2007.060302
121. Dohan Ehrenfest DM, Pinto NR, Pereda A, Jimenez P, Corso MD, Kang BS, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. (2017) 1–14. doi: 10.1080/09537104.2017.1293812
122. Bradaschia-Correa V, Josephson AM, Mehta D, Mizrahi M, Neibart SS, Liu C, et al. The selective serotonin reuptake inhibitor fluoxetine directly inhibits osteoblast differentiation and mineralization during fracture healing in mice. J Bone Miner Res. (2017) 32:821–33. doi: 10.1002/jbmr.3045
123. Wu X, Al-Abedalla K, Rastikerdar E, Abi Nader S, Daniel NG, Nicolau B, et al. Selective serotonin reuptake inhibitors and the risk of osseointegrated implant failure: a cohort study. J Dent Res. (2014) 93:1054–61. doi: 10.1177/0022034514549378
Keywords: platelet concentrates, clinical applications, growth factors, platelets, oral tissue regeneration
Citation: Al-Hamed FS, Mahri M, Al-Waeli H, Torres J, Badran Z and Tamimi F (2019) Regenerative Effect of Platelet Concentrates in Oral and Craniofacial Regeneration. Front. Cardiovasc. Med. 6:126. doi: 10.3389/fcvm.2019.00126
Received: 15 May 2019; Accepted: 12 August 2019;
Published: 03 September 2019.
Edited by:
Paul Jurasz, University of Alberta, CanadaReviewed by:
Aneta Radziwon-Balicka, Herlev Hospital, DenmarkCopyright © 2019 Al-Hamed, Mahri, Al-Waeli, Torres, Badran and Tamimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faleh Tamimi, ZmFsZWgudGFtaW1pbWFyaW5vQG1jZ2lsbC5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.