- 1IRCCS San Raffaele Scientific Institute, Milan, Italy
- 2Department of Cardiology, IRCCS Policlinico San Donato, Milan, Italy
Transcatheter aortic valve implantation (TAVI) is a worldwide accepted alternative for treating patients at intermediate or high risk for surgery. In recent years, the rate of complications has markedly decreased except for new-onset atrioventricular and intraventricular conduction block that remains the most common complication after TAVI. Although procedural, clinical, and electrocardiographic predisposing factors have been identified as predictors of conduction disturbances, new strategies are needed to avoid such complications, particularly in the current TAVI era that is moving quickly toward the percutaneous treatment of low-risk patients. In this article, we will review the incidence, predictive factors, and clinical implications of conduction disturbances after TAVI.
Introduction
As transcatheter aortic valve implantation (TAVI) evolves toward treating patients with lower surgical risk and greater life expectancy (1), a significant effort should be directed at a better understanding of common complications following this procedure.
New-onset conduction disturbances are common after TAVI, occurring in as much as 34.8% of patients at hospital discharge (2), and with left bundle branch block (LBBB) being the most common significant conduction disturbance after TAVI (10.5%) (2, 3). Although many studies investigated this topic, indications for permanent pacemaker implantation (PPI) are still unclear, often resulting in overtreatment.
The aims of the present review are to elucidate the anatomical and pathophysiological basis of these complications, to systematically illustrate currently available data, and to highlight unclear areas that clinical research still need to unveil.
Anatomy and Pathophysiology
A high incidence of conduction disturbances occurs not only following TAVI, but also after surgical aortic valve replacement (4), mainly because of the close anatomical relationship between the aortic valve and fundamental structures of the heart conduction system. The atrioventricular (AV) node lies within the apex of the triangle of Koch, at the convergence of the tendon of Todaro and of the attachment of the tricuspid septal leaflet in the right atrium. It continues as the bundle of His, piercing the membranous septum and penetrating through the central fibrous body to the left. Three major variants of AV nodes have been described, with 50% of individuals exhibiting a relatively right-sided AV bundle and 30% with a left-sided AV bundle, whereas in about 20% of patients the bundle courses under the membranous septum just below the endocardium (5). The last 2 above-described variants may expose patients to a higher risk of TAVI-induced conduction disturbances, especially in patients with a short membranous septum (5).
The left bundle branch emerges immediately beneath the membranous septum and is positioned superficially on the crest of the interventricular septum, and is intimately related to the base of the interleaflet triangle separating the non-coronary and right coronary leaflets of the aortic valve (3) (Figure 1). Consequently, when operating on the aortic valve, the risk exists to mechanically damage the nearby conductive system. TAVI may acutely expose the conduction system to an ischemic and inflammatory damage, in conjunction with a subacute process of healing (6), which may account for later and overall rarer conduction disturbances. Technical aspects of TAVI procedures, especially self- vs. balloon-expandable valve deployment system (7) and depth of implantation (8), are major factors in directly determining this acute mechanical damage to the conduction system. Furthermore, especially when treating intermediate-risk patients with greater life expectancy, a balance might exist between higher pre- and post-dilation pressures, needed to reduce paravalvular leak and the risk of a direct mechanical damage to the conduction system.
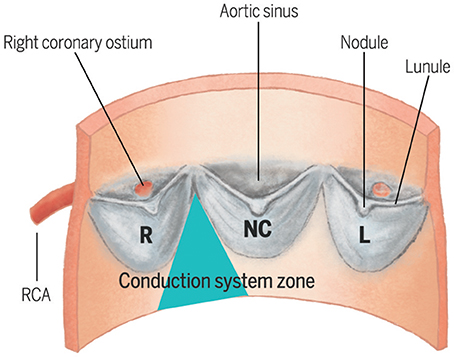
Figure 1. Spatial relationship between the three cusps of the aortic valve and the zone where the left bundle branch emerges beneath the membranous septum. L, Left cusp; NC, Non-Coronary cusp; RCA, Right Coronary Artery.
Finally, the close anatomical relationship with the aortic valve could also account for a certain degree of senile calcium deposition on the conduction system, which has been associated with the occurrence of LBBB and advanced atrioventricular block (AVB) in patients with aortic stenosis (9).
Left Bundle Branch Block
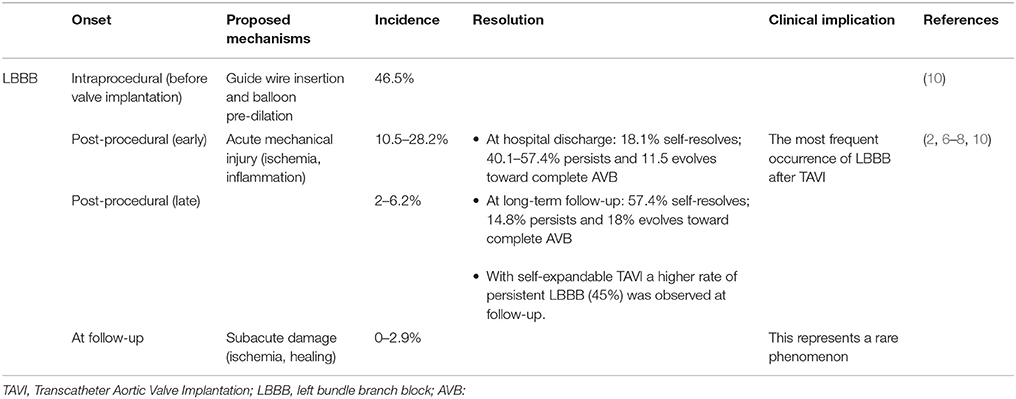
Overall the most common conduction alteration post-TAVI is new-onset LBBB (8), whose timing varies consistently and reflects different entities and reversibility of damage to the conduction system (Table 1); results of studies are summarized in Table 2. For the sake of clarity, we will refer to new onset LBBB as all LBBB which developed after TAVI, to persistent LBBB as all those who did not resolve at the time of discharge, while those patients who did not present LBBB will be referred to as LBBB-free.
Onset and Self-Resolution
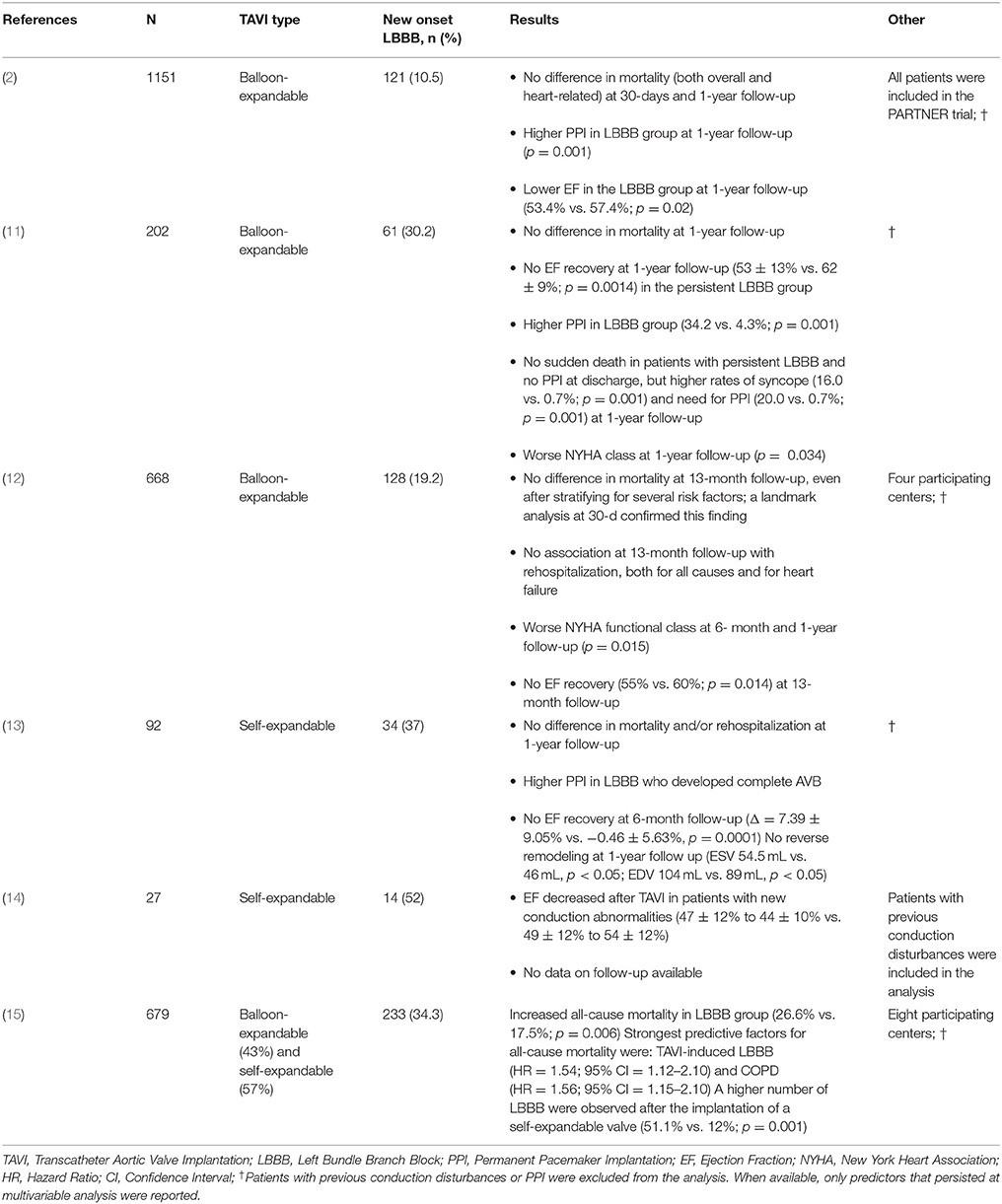
Urena et al. analyzed a cohort of 202 patients undergoing TAVI with a balloon-expandable valve and with no previous conduction disturbance or PPI, and showed that of the 61 (30.2%) who developed LBBB during hospital stay, 85.2% recovered normal conductive function (59% at 7-day median discharge and 26.2% at long-term follow-up) (8); these findings were in concordance with other studies (6, 16, 17) and demonstrates that most of the new-onset LBBB are transient and do not require PPI implantation. In a cohort of 91 patients undergoing TAVI with self-expandable valve and with no exclusion of patients with previous conduction disturbances and/or PPI, Piazza et al. observed a higher incidence of 54% new-onset LBBB and of 45% at 6-month follow-up (7). These findings, corroborated by other studies (10), further suggest that self-expandable valves may cause a more severe mechanical injury to the conduction system as compared to balloon-expandable valves. Moreover, it was suggested that by not excluding patients with previous conduction disturbances and/or PPI, a higher rate of persistent LBBB might be observed (8).
Impaired Function Recovery and Reverse Remodeling
Historically, the unfavorable effect of LBBB on systolic function is attributed to alterations in global and regional contraction and was proven both in otherwise normal subjects (18) and in hypertensive patients (19); furthermore, an adverse effect on diastolic function (19) and worse prognosis in comorbid patients (20) were also observed. Consequently, concerns were raised that in patients undergoing TAVI who develop persistent LBBB, a reduced EF recovery and therefore reduced benefits from the procedure might be observed.
Nazif et al. showed that in such cases a detrimental effect exists, with less or no EF recovery as compared to LBBB-free patients (58.1% vs. 52.8% at follow-up; p = 0.001) (2), independently of baseline EF. Carrabba et al. further elucidated that patients with new-onset LBBB lacked not only EF improvement, but also left ventricular remodeling (13). Urena et al. showed a decreased EF in patients with persistent LBBB at 1-year follow-up (Δ = 4.75 ± 8.02%, p = 0.031) (8), and Tzikas et al. reported similar findings also in patients treated with self-expandable valves (14). In another study by Urena et al., the only predictors of a lack of EF recovery were higher baseline EF and new onset LBBB (6).
Impact on Survival and Functional Class
There was no evidence of an impact of new-onset LBBB on patients survival after-TAVI (2, 6, 8, 13, 21) in all but one study by Houthuizen et al., which included patients with high logistic EuroSCORE (21%), therefore more prone to higher mortality rate (28.3%), regardless of whether the new-onset LBBB resolved spontaneously or not. No impact on rehospitalization was observed at 1-year follow-up (6, 8, 21) and no sudden death was reported in patients with new-onset LBBB and no PPI (8). The lack of increased mortality persisted also after a landmark analysis at 30-days (6).
Nonetheless, a poorer New York Heart Association class was observed at follow-up (18% vs. 7% in class II or higher, p = 0.015) (6, 8). Testa et al. failed to prove such a difference, although, when considering the high PPI rate in LBBB-free group (17 vs. 18%), it might be attributable to a worse-than-normal mechanical function also in the LBBB-free group (21). Therefore, in patients with persistent LBBB after TAVI, a strategy of early resynchronization seems reasonable, especially in patients with reduced LVEF.
Finally, new-onset persistent LBBB was also associated with an increased risk of AVB and need of PPI at follow-up (13.9 vs. 3.0%, p = 0.001, median time to PPI: 12 months) (6, 8). Although further studies are needed in order to confirm these findings, in this setting it might be reasonable to implement a strategy of close (24–48 h) ECG monitoring during the first months after TAVI or after systematic electrophysiology study (8).
Predictors of Left-Bundle Branch Block After TAVI
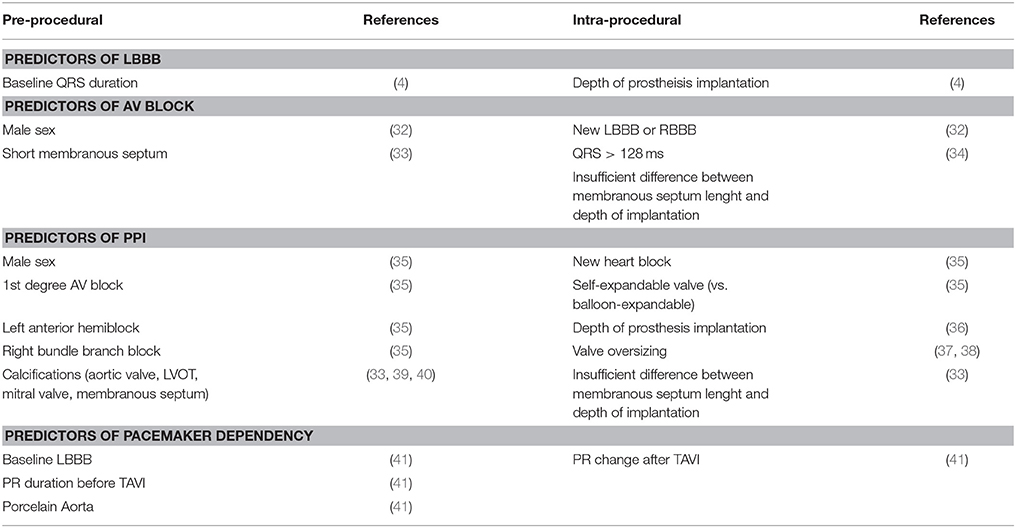
Common limitations of studies investigating this topic are the inclusion of patients with pre-TAVI conduction disturbances and not taking in due consideration of the role of self- vs. balloon-expandable valves (3, 7), which led to controversial results in the past (6) (Table 3). When all these factors were taken into account, predictors of new-onset persistent LBBB were ventricular depth of the prosthesis (odds ratio [OR] = 1.37 for each increase of 1 mm) and baseline QRS duration (OR = 1.24 for each increase of 4 ms) (8); no predictors of transient LBBB were found (8) (Table 4).
While a longer QRS duration may be related to baseline conduction system damage and increased vulnerability (8), increased risk of new onset LBBB with lower valve implantation might reflect a more permanent damage to the conduction system with a more ventricular positioning (6). Moreover, this risk factor is consistent also when self-expandable valves are considered (42–44), suggesting that it might be intrinsic of the TAVI procedure.
Advanced Conduction Disturbances After TAVI and PPI
A high rate of new AV and intraventricular conduction delays is observed within the first 48 h of TAVI, with a significant resolution by 30 days. About 22% of patients undergoing TAVI develop a post-operative new-onset AV block after balloon valvuloplasty or after valve deployment. These patients have a 5-fold higher risk of permanent AV block requiring a PPI (45). However, most of the complete AV block as well as the new-onset LBBB and AV blocks tends to disappear within the first days after TAVI: in a cohort of patients implanted with CoreValve, 19.7% had an absolute indication to PPI secondary to the development of advanced II degree AV-block and/or III degree AV block; however half of the advanced conduction delays resolved beyond the periprocedural period, waiting for more than 24 h following TAVI (46).
Incidence of PPI After TAVI
The overall rate of PPI after TAVI ranges from 2 to 51% in a meta-analysis including 41 studies. The rate of PPI implant was 5 times more frequent in patients receiving a self-expandable Medtronic CoreValve (25–28%) compared to those who received a balloon-expandable Edwards Sapien/Sapien XT valve (5–7%) (47).
This increased risk of PPI with the CoreValve system was confirmed in the CHOICE randomized trial (Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis), in which the rate of new PPI in the CoreValve group was 38% while in the Sapien XT group was 23.4% (p = 0.001) (48). The SURTAVI trial also confirmed high rates of PPI with both old generation CoreValve (25.5%) and new generation Evolut R (26.7%), despite the inclusion of intermediate-risk patients (49).
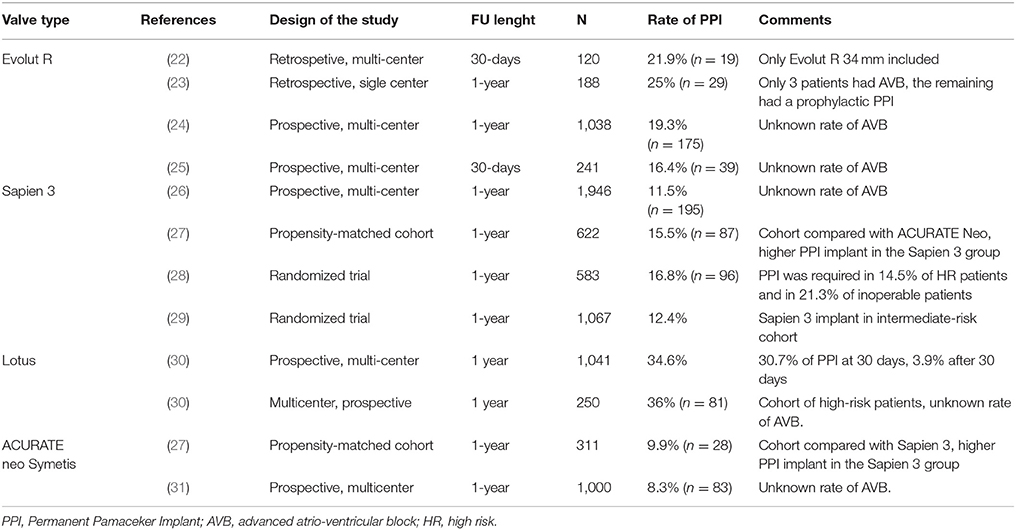
Focusing only on the latest-generation transcatheter heart valves, the incidence of PPI ranged between 2.3 and 36.1%. For balloon-expandable prostheses, the PPI rate was between 4.0 and 24.0% when using the new-generation Sapien 3 device, and a similar figure was observed with the previous generation Sapien XT device (ranging between 2.3 and 28.2%). For self-expandable prostheses, the PPI rates were higher with the early generation CoreValve device (16.3–37.7%), and despite a reduction in PPI rates with the new Evolut R, the rates remained relatively higher (14.7–26.7%) (50). These data are confirmed also in the latest experience with the new Evolut R device: among 1,038 patients, the rate of PPI was 17.8%. Similarly, the experience with the latest-generation Evolut PRO valve reports a rate of PPI of 11.8%; however, these results are limited by the low number of patients included in this early feasibility trial (n = 60) (51). A low incidence of PPI has been reported in case of Acurate neo implantation: in a recent large experience collected in 1,000 patients, the overall incidence of PPI was 8.3% (26); these data are confirmed in a recent propensity matched analysis comparing the Acurate neo and the Sapien 3: a high success rates was achieved for both valves, and the clinical and procedural results were comparable. However, Acurate neo required less frequently a PPI (9.9% vs. 15.5%; p = 0.02). Finally, the Lotus valve has been associated with higher rates of PPI than other devices (31.9–41.0%) (28, 52–54); this could partially be attributable to its peculiar design, including Adaptive Seal technology, which guarantees less paravalvular leak, but might poses a major risk toward the conduction system. Recently introduced strategies for higher implants (including the Lotus Edge Depth Guard Technology) might reduce the aforementioned stress on the conduction system and lead to lower PPI rates.
The prevalence of PPI among the most widely commercially available valves is reported in Figure 2. Although a clear trend can be observed, a huge variability in PPI was observed amongst different registries, even when the same valve was involved (Figure 2) (1, 22–25, 27, 29–31, 49, 51–75).
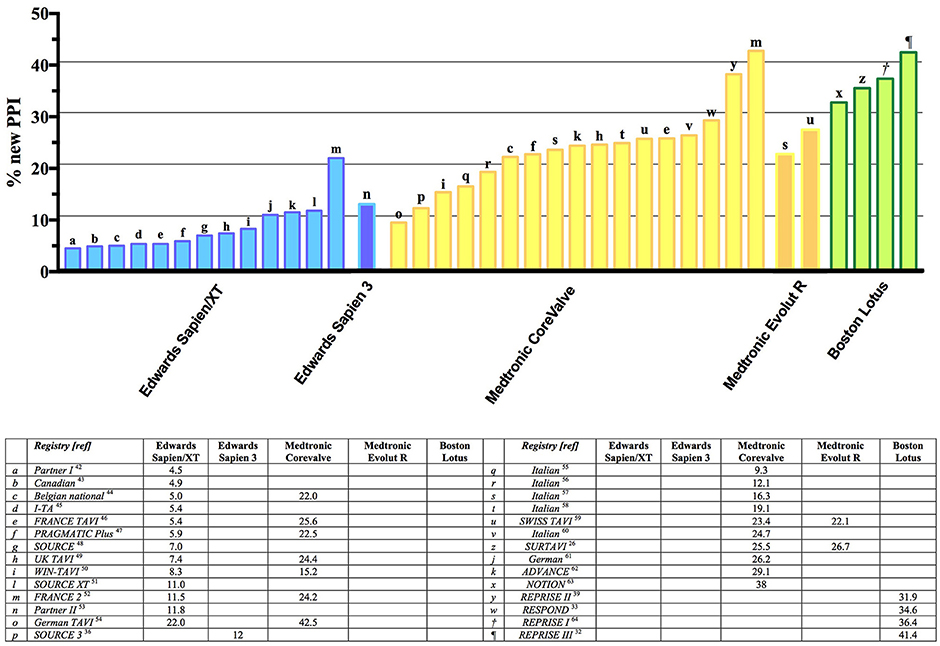
Figure 2. Summary of major trials and registries involving different types (both self-expandable and balloon-expandable) of valve and reporting incidence of new PPI. PPI, Permanent Pacemaker Implantation.
As reported by Auffret et al. (76), 2 main factors should be taken into account when evaluating the real incidence of PPI among different studies: first of all, indications to PPI are not uniform and do not always follow the canonic indication reported in the guidelines. As an example, some teams undertook prophylactic PPI in patients with new-onset LBBB after TAVR, which in turn resulted in an increased rate of PPI after TAVI. Moreover, the shorter period of observation after TAVI can underestimate the real incidence of PPI after the procedure. As demonstrated, a reduction in PPI rates has been observed with a strict adherence to Class I and II indications as recommended by clinical guidelines (12). Moreover, as experience, confidence and knowledge grows, a trend toward less PPI in single center registries has been observed (77).
As already mentioned, many of the newly developed advanced AV block resolves spontaneously, therefore according to the European Society of Cardiology guidelines, a prophylactic implantation of PPI after TAVI should be avoided and reserved only to those patients with recurrent AVB after an appropriate period of clinical observation with ECG monitoring (Class I, Level of Evidence C). Table 3 reports currently available data about the rate of advanced conduction disturbances requiring PPI.
Finally, the real incidence of PPI can be altered in some studies where patients with prior implant of PPI were included in the denominator, although not being exposed to the risk of new PPI implant.
Although guidelines remain vague and clear indications for PPI are still missing, many multicenter and literature-based decisional algorithms exist. In a recent state-of-the-art review, Auffret et al. proposed (76):
- ECG continuous monitoring until discharge for all patients who undergo TAVI;
- Same day PPI in all patients with a class I/II indication for PPI before TAVI;
- Temporary pacemaker for 24 h if new-onset LBBB and up to 48 h if new advanced AVB;
- PPI if new-onset LBBB persists 48 h after TAVI and QRS duration > 160 msec; consider loop recorder and/or electrophysiological studies and/or 30 days ECG monitoring in all other cases;
- PPI if advanced AV block persists 48 h after TAVI or recur before discharge (28, 54, 55, 57, 58, 78–82).
Predictors of PPI After TAVI
In a recent meta-analysis of 41 studies including 11,210 TAVI recipients, male sex, first-degree AV block, left anterior hemiblock, and right bundle-branch block (RBBB) were identified as pre-procedural predictors of PPI, whereas the presence of intraoperative heart block and the use of a self-expandable prosthesis were the procedural predictors (35). In that study, the implantation of a CoreValve system was associated with a 2.5-fold higher risk of PPI, which was confirmed in another systematic review and in the recent report of the Society of Thoracic Surgeons Transcatheter Valve Therapy registry. Baseline RBBB is probably the strongest, most consistent clinical predictor of PPI; it has been identified in more than half of the studies evaluating multivariable predictors of PPI. Calcifications of the aortic valve (39), LVOT, and mitral annulus (40) and depth of prosthesis implantation (36) have been associated with PPI after TAVI. Proposed cut-off values for valve implantation depth predicting new-onset LBBB or PPI were 7 mm or 25% of the stent frame in the LVOT with the Sapien valve (37) and ranged from 6 to 7.8 mm with the CoreValve system (83) and from 5 to 6.7 mm with the Lotus valve (37). Values of 10 to 15% of valve oversizing have been associated with an increased risk of PPI with first-generation devices (37, 38). Concerning the post-procedural management of TAVI recipients, of particular interest are the predictors of delayed AVB after TAVI. In a larger series of 1,064 patients (45% with self-expandable valves), of whom 71 (6.7%) presented with delayed AVB (occurring 24 h after TAVI), Toggweiller et al. identified male sex and the presence of LBBB or RBBB after TAVI as independent predictors of delayed AVB (32). Mouillet et al. also proposed a post-TAVI QRS duration cutoff of >128 ms as a predictor of the evolution to AVB 24-h after TAVI (34). Baseline RBBB, PR interval duration before and after TAVI, PR interval change (>28 ms) within 3 days of TAVI, and porcelain aorta have been highlighted as independent predictors of pacemaker dependency at 1 year after TAVI (41). Finally, the membranous septum length, a surrogate for the distance between the aortic ring and the piercing bundle of His, has been proven as a major pre-intervention predictors of advanced AV block and PPI (33). In fact, mechanical compression of the emerging conduction tissue is easier if the membranous septum is too short and insufficient difference between this measure and the depth of implantation is achieved during TAVI.
Prognostic Impact of PPI After TAVI
Right ventricular apical pacing results in a left ventricular electrical activation sequence resembling left bundle-branch block. The resulting electrical asynchrony is manifest in a prolonged QRS duration due to slow myocardial conduction. Consequently, left ventricular contraction is altered, and significant interventricular and intraventricular dyssynchrony may occur (84) as result of a non-physiological activation. Ventricular desynchronization imposed by right ventricular apical pacing causes chronic left ventricular remodeling (85), including asymmetric hypertrophy and redistribution of cardiac mass, mitral regurgitation (86), increased left atrial diameter and reduced ejection fraction (87).
These adverse effects on ventricular structure and function likely explain the association of right ventricular pacing with increased risks of atrial fibrillation and heart failure in randomized clinical trials of pacemaker therapy. The MOST (Mode Selection Trial) demonstrated that heart failure during conventional cardiac pacing can be explained by complex interactions between substrate and promoters (11). Substrate is represented by clinical variables including atrial rhythm, AV conduction, ventricular conduction, ventricular function, symptomatic heart failure, and myocardial infarction. The promoters of heart failure are specific to the implementation of cardiac pacing and contain 2 constituents: ventricular desynchronization and AV desynchronization. Based on this model, patients with a very high-risk substrate (low ejection fraction, history of heart failure) are more likely to receive a negative impact from chronic right ventricular pacing (88).
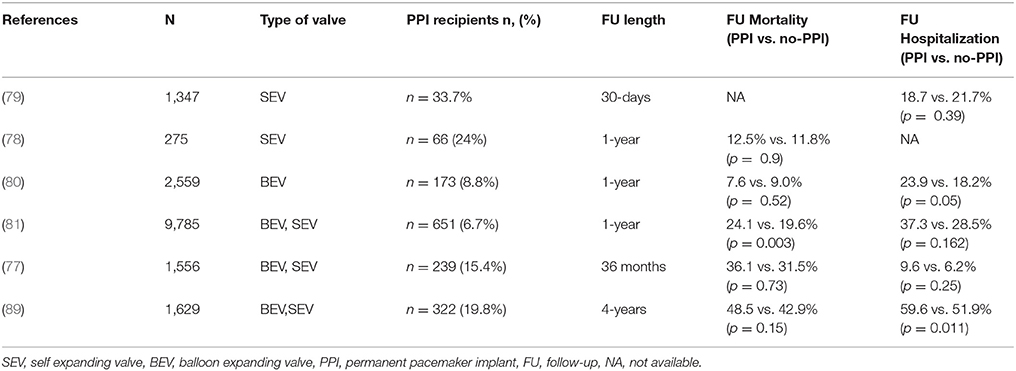
The negative impact of PPI in TAVI patients has been largely explored in observational and retrospective studies (6). PPI after TAVI has been linked to and increased risk of recurrent hospitalizations for cardiovascular reasons and less recovery of left ventricular EF among patients with baseline impaired left ventricular function (89). In a meta-analysis published by Regueiro et al., the authors demonstrated a trend trough a reduction of cardiovascular deaths associated with the implantation of the PPI. The reason could be linked to the protective effect of pacing against the progression toward complete AV block and sudden death after TAVI. Conversely, the negative impact of PPI implant on mortality after TAVI was showed in a large patient population of 9,785 subjects. After multivariate adjustment, the authors found that PPI in TAVI patients was associated with a 31% increased risk for 1-year mortality and a 33% increased risk for a composite of mortality or heart failure admission at 1-year. Moreover, PPI was found to be associated with a prolonged length of stay in hospital (7 days vs. 6 days; p < 0.001) and in the intensive care unit (56.7 vs. 45.0 h; p < 0.001) (90). A smaller recent study of 1,973 patients from the PARTNER trial (91) and an international multicentre registry noted a trend toward increased 1-year mortality in patients with new PPI, but it did not reach statistical significance (92). Similarly, in a small study conducted on a cohort of patients treated with first-generation CoreValve, PPI was not associated with increased mortality at 1-year follow-up (93). Actually, only the large experience from the Society of Thoracic Surgeons/American College of Cardiology TVT registry demonstrated a negative influence of PPI on clinical outcome (90). Notably, PPI after TAVI has also been found to be protective against sudden death (92). The results of the most important studies on PPI and outcomes in TAVI patients are reported in Table 5 (89–94).
The heterogeneity of data regarding PPI after TAVI can be interpreted in the light of the following points:
1) The negative effects of chronic right ventricular pacing may be difficult to demonstrate in the sicker TAVI population with a reduced life expectancy. A longer follow-up period is necessary to demonstrate the detrimental effect of chronic pacing.
2) The negative impact of chronic pacing could have a prognostic importance mainly in patients with reduced left ventricular EF.
3) The impact of right apical pacing on left ventricular EF is dependent both on the percentage of pacing and on pacing modality (i.e., DDD vs. VVI). Only few patients after TAVI have evidence of pacemaker-dependency, so that the negative impact of PPI implant becomes hard to be demonstrated.
4) The negative effect of chronic pacing is counterbalanced by the protective effect that PPI has at follow-up after TAVI. Patients with baseline RBBB and those with long LBBB (QRS length >160 ms) are at higher risk of death after discharge probably due to the development of AVB (92, 95). In this setting, PPI should be protective against the risk of suddendeath.
Future Perspectives
As TAVI becomes a widespread technology, it is becoming a safe and valid alternative for the treatment of aortic stenosis also in patients at intermediate surgical risk. The development of new transcatheter valves has led to a reduction in significant perivalvular leaks, but with a milder impact in the rate of PPI after TAVI. One of the main challenges in the TAVI field will be the reduction of advanced conduction disturbances needing PPI. This goal could be achieved through a better understanding of the clinical and procedural factors implicated in the development of conduction disturbances after TAVI and through a careful monitoring of patients developing conduction delays in order to avoid futile PPI. In this context, further studies should investigate the optimal timing for PPI after TAVI and evaluate factors associated with the development and recovery of conduction disturbances. Moreover, considering the aforementioned difference in PPI amongst different devices, it is reasonable to expect advancements in technology that could minimize the need of PPI especially when TAVI will be expanded to low-risk patients.
Author Contributions
All the authors contributed to the manuscript production and in the final revision. AM, CM, MP, and OD structured the manuscript giving contribute to table, figures and text editing. AC, LT, and AL revisited the article implementing the final manuscript form.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AV, Atrioventricular; AVB, Advanced Atrioventricular Block; EF, Ejection Fraction; LBBB, Left Bundle Branch Block; OR, Odds Ratio; PPI, Permanent Pacemaker Implantation; TAVI, Transcatheter Aortic Valve Implantation.
References
1. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374:1609–20. doi: 10.1056/NEJMoa1514616
2. Nazif TM, Williams MR, Hahn RT, Kapadia S, Babaliaros V, Rodés-Cabau J, et al. Clinical implications of new-onset left bundle branch block after transcatheter aortic valve replacement: analysis of the PARTNER experience. Eur Heart J. (2014) 35:1599–607. doi: 10.1093/eurheartj/eht376
3. van der Boon RM, Nuis R-J, Van Mieghem NM, Jordaens L, Rodés-Cabau J, van Domburg RT, et al. New conduction abnormalities after TAVI—frequency and causes. Nat Rev Cardiol. (2012) 9:454–63. doi: 10.1038/nrcardio.2012.58
4. Poels TT, Houthuizen P, Van Garsse LAFM, Soliman Hamad MA, Maessen JG, Prinzen FW, et al. Frequency and prognosis of new bundle branch block induced by surgical aortic valve replacement. Eur J Cardio-thoracic Surg. (2014) 47:e47–53. doi: 10.1093/ejcts/ezu435
5. Kawashima T, Sato F. Visualizing anatomical evidences on atrioventricular conduction system for TAVI. Int J Cardiol. (2014) 174:1–6. doi: 10.1016/j.ijcard.2014.04.003
6. Urena M, Webb JG, Cheema A, Serra V, Toggweiler S, Barbanti M, et al. Impact of new-onset persistent left bundle branch block on late clinical outcomes in patients undergoing transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv. (2014) 7:128–36. doi: 10.1016/j.jcin.2013.08.015
7. Piazza N, Nuis RJ, Tzikas A, Otten A, Onuma Y, Garcia Garcia H, et al. Persistent conduction abnormalities and requirements for pacemaking six months after transcatheter aortic valve implantation. EuroIntervention (2010) 6:475–84. doi: 10.4244/EIJ30V6I4A80
8. Urena M, Mok M, Serra V, Dumont E, Nombela-Franco L, DeLarochellière R, et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon-expandable valve. J Am Coll Cardiol. (2012) 60:1743–52. doi: 10.1016/j.jacc.2012.07.035
9. MacMillan RM, Demorizi NM, Gessman LJ, Maranhao V. Correlates of prolonged HV conduction in aortic stenosis. Am Heart J. (1985) 110:56–60. doi: 10.1016/0002-8703(85)90514-9
10. Nuis RJ, Van Mieghem NM, Schultz CJ, Tzikas A, Van Der Boon RM, Maugenest AM, et al. Timing and potential mechanisms of new conduction abnormalities during the implantation of the Medtronic CoreValve System in patients with aortic stenosis. Eur Heart J. (2011) 32:2067–74. doi: 10.1093/eurheartj/ehr110
11. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation (2003) 107:2932–7. doi: 10.1161/01.CIR.0000072769.17295.B1
12. Petronio AS, Sinning JM, Van Mieghem N, Zucchelli G, Nickenig G, Bekeredjian R, et al. Optimal implantation depth and adherence to guidelines on permanent pacing to improve the results of transcatheter aortic valve replacement with the medtronic corevalve system: the CoreValve prospective, international, post-market ADVANCE-II study. JACC Cardiovasc Interv. (2015) 8:837–46. doi: 10.1016/j.jcin.2015.02.005
13. Carrabba N, Valenti R, Migliorini A, Marrani M, Cantini G, Parodi G, et al. Impact on left ventricular function and remodeling and on 1-year outcome in patients with left bundle branch block after transcatheter aortic valve implantation. Am J Cardiol. (2015) 116:125–31. doi: 10.1016/j.amjcard.2015.03.054
14. Tzikas A, Van Dalen BM, Van Mieghem NM, Gutierrez-Chico JL, Nuis RJ, Kauer F, et al. Frequency of conduction abnormalities after transcatheter aortic valve implantation with the medtronic-corevalve and the effect on left ventricular ejection fraction. Am J Cardiol. (2011) 107:285–9. doi: 10.1016/j.amjcard.2010.09.015
15. Houthuizen P, Van Garsse LAFM, Poels TT, de Jaegere P, van der Boon RMA, Swinkels BM, et al. Left bundle-branch block induced by transcatheter aortic valve implantation increases risk of death. Circulation (2012) 126:720–28. doi: 10.1161/CIRCULATIONAHA.112.101055
16. Gutiérrez M, Rodés-Cabau J, Bagur R, Doyle D, DeLarochellière R, Bergeron S, et al. Electrocardiographic changes and clinical outcomes after transapical aortic valve implantation. Am Heart J. (2009) 158:302–8. doi: 10.1016/j.ahj.2009.05.029
17. Godin M, Eltchaninoff H, Furuta A, Tron C, Anselme F, Bejar K, et al. Frequency of conduction disturbances after transcatheter implantation of an Edwards Sapien aortic valve prosthesis. Am J Cardiol. (2010) 106:707–12. doi: 10.1016/j.amjcard.2010.04.029
18. Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, Wooley CF. Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation (1989) 79:845–53. doi: 10.1161/01.CIR.79.4.845
19. Li ZB, Wachtell K, Okin PM, Gerdts E, Liu JE, Nieminen MS, et al. Association of left bundle branch block with left ventricular structure and function in hypertensive patients with left ventricular hypertrophy: the LIFE study. J Hum Hypertens. (2004) 18:397–402. doi: 10.1038/sj.jhh.1001709
20. Francia P, Balla C, Paneni F, Volpe M. Left bundle-branch block - pathophysiology, prognosis and clinical management. Clin Cardiol. (2007) 30:326–30. doi: 10.1002/clc.20034
21. Testa L, Latib A, De Marco F, De Carlo M, Agnifili M, Latini RA, et al. Clinical impact of persistent left bundle-branch block after transcatheter aortic valve implantation with CoreValve revalving system. Circulation (2013) 127:1300–7. doi: 10.1161/CIRCULATIONAHA.112.001099
22. Wenaweser P, Stortecky S, Schütz T, Praz F, Gloekler S, Windecker S, et al. Transcatheter aortic valve implantation with the NVT Allegra transcatheter heart valve system: first-in-human experience with a novel self-expanding transcatheter heart valve. EuroIntervention (2016) 12:71–7. doi: 10.4244/EIJV12I1A13
23. Silaschi M, Conradi L, Wendler O, Schlingloff F, Kappert U, Rastan AJ, et al. The JUPITER registry: one-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv. (2017) 91:1345–51. doi: 10.1002/ccd.27370
24. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363:1597–607. doi: 10.1056/NEJMoa1008232
25. Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk. Acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. (2010) 55:1080–90. doi: 10.1016/j.jacc.2009.12.014
26. Möllmann H, Hengstenberg C, Hilker M, Kerber S, Schäfer U, Rudolph T, et al. Real-world experience using the ACURATE neoTM prosthesis: 30-day outcomes of 1000 patients enrolled in the SAVI-TF registry. EuroIntervention (2017) 13:e1764–70. doi: 10.4244/EIJ-D-17-00628
27. Bosmans JM, Kefer J, De Bruyne B, Herijgers P, Dubois C, Legrand V, et al. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. (2011) 12:762–67. doi: 10.1510/icvts.2010.253773
28. Meredith I, Dumonteil N, Blackman D, Tchétché D, Walters D, Hildick-Smith D, et al. Repositionable percutaneous aortic valve implantation with the LOTUS valve: 30-day and 1-year outcomes in 250 high-risk surgical patients. EuroIntervention (2017) 13:788–95. doi: 10.4244/EIJ-D-16-01024
29. D'Onofrio A, Rubino P, Fusari M, Salvador L, Musumeci F, Rinaldi M, et al. Clinical and hemodynamic outcomes of “all-comers” undergoing transapical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation (I-TA). J Thorac Cardiovasc Surg. (2011) 142:768–75. doi: 10.1016/j.jtcvs.2011.06.026
30. Auffret V, Lefevre T, Van Belle E, Eltchaninoff H, Iung B, Koning R, et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to FRANCE TAVI. J Am Coll Cardiol. (2017) 70:42–55. doi: 10.1016/j.jacc.2017.04.053
31. Chieffo A, Buchanan GL, Van Mieghem NM, Tchetche D, Dumonteil N, Latib A, et al. Transcatheter aortic valve implantation with the Edwards SAPIEN versus the medtronic corevalve revalving system devices: a multicenter collaborative study: The PRAGMATIC plus initiative (Pooled-RotterdAm-Milano-Toulouse in Collaboration). J Am Coll Cardiol. (2013) 61:830–6. doi: 10.1016/j.jacc.2012.11.050
32. Toggweiler S, Stortecky S, Holy E, Zuk K, Cuculi F, Nietlispach F, et al. The electrocardiogram after transcatheter aortic valve replacement determines the risk for post-procedural high-degree AV block and the need for telemetry monitoring. JACC Cardiovasc Interv. (2016) 9:1269–76. doi: 10.1016/j.jcin.2016.03.024
33. Hamdan A, Guetta V, Klempfner R, Konen E, Raanani E, Glikson M, et al. Inverse relationship between membranous septal length and the risk of atrioventricular block in patients undergoing transcatheter aortic valve implantation. JACC Cardiovasc Interv. (2015) 8:1218–28. doi: 10.1016/j.jcin.2015.05.010
34. Mouillet G, Lellouche N, Lim P, Meguro K, Yamamoto M, Deux JF, et al. Patients without prolonged QRS after TAVI with corevalve device do not experience high-degree atrio-ventricular block. Catheter Cardiovasc Interv. (2013) 81:882–7. doi: 10.1002/ccd.24657
35. Siontis GCM, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J Am Coll Cardiol. (2014) 64:129–40. doi: 10.1016/j.jacc.2014.04.033
36. Fraccaro C, Buja G, Tarantini G, Gasparetto V, Leoni L, Razzolini R, et al. Incidence, predictors, and outcome of conduction disorders after transcatheter self-expandable aortic valve implantation. Am J Cardiol. (2011) 107:747–54. doi: 10.1016/j.amjcard.2010.10.054
37. Rodríguez-Olivares R, Van Gils L, El Faquir N, Rahhab Z, Di Martino LFM, et al. Importance of the left ventricular outflow tract in the need for pacemaker implantation after transcatheter aortic valve replacement. Int J Cardiol. (2016) 216:9–15. doi: 10.1016/j.ijcard.2016.04.023
38. Katsanos S, Van Rosendael P, Kamperidis V, Van Der Kley F, Joyce E, Debonnaire P, et al. Insights into new-onset rhythm conduction disorders detected by multi-detector row computed tomography after transcatheter aortic valve implantation. Am J Cardiol. (2014) 114:1556–61. doi: 10.1016/j.amjcard.2014.08.020
39. Latsios G, Gerckens U, Buellesfeld L, Mueller R, John D, Yuecel S, et al. “Device landing zone” calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter Cardiovasc Interv. (2010) 76:431–39. doi: 10.1002/ccd.22563
40. Abramowitz Y, Kazuno Y, Chakravarty T, Kawamori H, Maeno Y, Anderson D, et al. Concomitant mitral annular calcification and severe aortic stenosis: prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur Heart J. (2017) 38:1194–203. doi: 10.1093/eurheartj/ehw594
41. Naveh S, Perlman GY, Elitsur Y, Planer D, Gilon D, Leibowitz D, et al. Electrocardiographic predictors of long-term cardiac pacing dependency following transcatheter aortic valve implantation. J Cardiovasc Electrophysiol. (2017) 28:216–23. doi: 10.1111/jce.13147
42. Piazza N, Onuma Y, Jesserun E, Kint PP, Maugenest AM, Anderson RH, et al. Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc Interv. (2008) 1:310–6. doi: 10.1016/j.jcin.2008.04.007
43. Baan J, Yong ZY, Koch KT, Henriques JPS, Bouma BJ, Vis MM, et al. Factors associated with cardiac conduction disorders and permanent pacemaker implantation after percutaneous aortic valve implantation with the CoreValve prosthesis. Am Heart J. (2010) 159:497–503. doi: 10.1016/j.ahj.2009.12.009
44. Aktug Ö, Dohmen G, Brehmer K, Koos R, Altiok E, Deserno V, et al. Incidence and predictors of left bundle branch block after transcatheter aortic valve implantation. Int J Cardiol. (2012) 160:26–30. doi: 10.1016/j.ijcard.2011.03.004
45. Bleiziffer S, Ruge H, Hörer J, Hutter A, Geisbüsch S, Brockmann G, et al. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. (2010) 3:524–30. doi: 10.1016/j.jcin.2010.01.017
46. Bjerre Thygesen J, Loh PH, Cholteesupachai J, Franzen O, Søndergaard L. Reevaluation of the indications for permanent pacemaker implantation after transcatheter aortic valve implantation. J Invasive Cardiol. (2014) 26:94–9.
47. Erkapic D, De Rosa S, Kelava A, Lehmann R, Fichtlsscherer S, Hohloser SH. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J Cardiovasc Electrophysiol. (2012) 23:391–7. doi: 10.1111/j.1540-8167.2011.02211.x
48. Abdel-Wahab M, Neumann F-J, Mehilli J, Frerker C, Richardt D, Landt M, et al. 1-year outcomes after transcatheter aortic valve replacement with balloon-expandable versus self-expandable valves. J Am Coll Cardiol. (2015) 66:791–800. doi: 10.1016/j.jacc.2015.06.026
49. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376:1321–31. doi: 10.1056/NEJMoa1700456
50. van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: a systematic review. Eur Heart J. (2018) 39:2003–13. doi: 10.1093/eurheartj/ehx785
51. Forrest JK, Mangi AA, Popma JJ, Khabbaz K, Reardon MJ, Kleiman NS, et al. Early outcomes with the evolut pro repositionable self-expanding transcatheter aortic valve with pericardial wrap. JACC Cardiovasc Interv. (2018) 11:160–8. doi: 10.1016/j.jcin.2017.10.014
52. Meredith IT, Walters DL, Dumonteil N, Worthley SG, Tchétché D, Manoharan G, et al. 1-year outcomes with the fully repositionable and retrievable lotus transcatheter aortic replacement valve in 120 high-risk surgical patients with severe aortic stenosis: Results of the REPRISE II study. JACC Cardiovasc Interv. (2016) 9:376–84. doi: 10.1016/j.jcin.2015.10.024
53. Feldman TE, Reardon MJ, Rajagopal V, Makkar RR, Bajwa TK, Kleiman NS, et al. Effect of mechanically expanded vs self-expanding transcatheter aortic valve replacement on mortality and major adverse clinical events in high-risk patients with aortic stenosis. JAMA (2018) 319:27. doi: 10.1001/jama.2017.19132
54. Falk V, Wöhrle J, Hildick-Smith D, Bleiziffer S, Blackman DJ, Abdel-Wahab M, et al. Safety and efficacy of a repositionable and fully retrievable aortic valve used in routine clinical practice: the RESPOND Study. Eur Heart J. (2017) 38:3359–66. doi: 10.1093/eurheartj/ehx297
55. Grube E, Van Mieghem NM, Bleiziffer S, Modine T, Bosmans J, Manoharan G, et al. Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis. J Am Coll Cardiol. (2017) 70:845–53. doi: 10.1016/j.jacc.2017.06.045
56. Linke A, Holzhey D, Möllmann H, Manoharan G, Schäfer U, Frerker C, et al. Treatment of aortic stenosis with a self-expanding, resheathable transcatheter valve. Circ Cardiovasc Interv. (2018) 11:e005206. doi: 10.1161/CIRCINTERVENTIONS.117.005206
57. Wendler O, Schymik G, Treede H, Baumgartner H, Dumonteil N, Neumann F-J, et al. SOURCE 3: 1-year outcomes post-transcatheter aortic valve implantation using the latest generation of the balloon-expandable transcatheter heart valve. Eur Heart J. (2017) 38:2717–26. doi: 10.1093/eurheartj/ehx294
58. Reichenspurner H, Schaefer A, Schäfer U, Tchétché D, Linke A, Spence MS, et al. Self-expanding transcatheter aortic valve system for symptomatic high-risk patients with severe aortic stenosis. J Am Coll Cardiol. (2017) 70:3127–36. doi: 10.1016/j.jacc.2017.10.060
59. Möllmann H, Walther T, Siqueira D, Diemert P, Treede H, Grube E, Nickenig G, et al. Transfemoral TAVI using the self-expanding ACURATE neo prosthesis: one-year outcomes of the multicentre “CE-approval cohort.” EuroIntervention. (2017) 13:e1040–6. doi: 10.4244/EIJ-D-17-00187
60. Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, et al. Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry. Circulation (2010) 122:62–9. doi: 10.1161/CIRCULATIONAHA.109.907402
61. Ludman PF, Moat N, de Belder MA, Blackman DJ, Duncan A, Banya W, et al. Transcatheter aortic valve implantation in the UK: temporal trends, predictors of outcome and 6 year follow up: a report from the UK TAVI registry 2007 to 2012. Circulation (2015) 131:1181–90. doi: 10.1161/CIRCULATIONAHA.114.013947
62. Chieffo A, Petronio AS, Mehilli J, Chandrasekhar J, Sartori S, Lefèvre T, et al. 1-Year clinical outcomes in women after transcatheter aortic valve replacement: results from the first WIN-TAVI registry. JACC Cardiovasc Interv. (2018) 11:1–12. doi: 10.1016/j.jcin.2017.09.034
63. Schymik G, Lefèvre T, Bartorelli AL, Rubino P, Treede H, Walther T, et al. European experience with the second-generation Edwards SAPIEN XT transcatheter heart valve in patients with severe aortic stenosis: 1-year outcomes from the SOURCE XT Registry. JACC Cardiovasc Interv. (2015) 8:657–69. doi: 10.1016/j.jcin.2014.10.026
64. Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. Surv Anesthesiol. (2013) 57:62–3. doi: 10.1097/01.SA.0000426523.25196.4e
65. Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. (2011) 32:198–204. doi: 10.1093/eurheartj/ehq339
66. Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention (2008) 4:242–49. doi: 10.4244/EIJV4I2A43
67. Ussia GP, Barbanti M, Petronio AS, Tarantini G, Ettori F, Colombo A, et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J. (2012) 33:969–76. doi: 10.1093/eurheartj/ehr491
68. Petronio AS, De Carlo M, Bedogni F, Marzocchi A, Klugmann S, Maisano F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv. (2010) 3:359–66. doi: 10.1161/CIRCINTERVENTIONS.109.930453
69. Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation (2011) 123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533
70. Noble S, Stortecky S, Heg D, Tueller D, Jeger R, Toggweiler S, et al. Comparison of procedural and clinical outcomes with Evolut R versus Medtronic CoreValve: A Swiss TAVI registry analysis. EuroIntervention (2017) 12:e2170–76. doi: 10.4244/EIJ-D-16-00677
71. Petronio AS, De Carlo M, Bedogni F, Maisano F, Ettori F, Klugmann S, et al. 2-year results of CoreValve implantation through the subclavian access: a propensity-matched comparison with the femoral access. J Am Coll Cardiol. (2012) 60:502–7. doi: 10.1016/j.jacc.2012.04.014
72. Buellesfeld L, Gerckens U, Schuler G, Bonan R, Kovac J, Serruys PW, et al. 2-Year follow-up of patients undergoing transcatheter aortic valve implantation using a self-expanding valve prosthesis. J Am Coll Cardiol. (2011) 57:1650–57. doi: 10.1016/j.jacc.2010.11.044
73. Gerckens U, Tamburino C, Bleiziffer S, Bosmans J, Wenaweser P, Brecker S, et al. Final 5-year clinical and echocardiographic results for treatment of severe aortic stenosis with a self-expanding bioprosthesis from the ADVANCE Study. Eur Heart J. (2017) 38:2729–38. doi: 10.1093/eurheartj/ehx295
74. Thyregod HGH, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. (2015) 65:2184–94. doi: 10.1016/j.jacc.2015.03.014
75. Meredith IT, Worthley SG, Whitbourn RJ, Antonis P, Montarello JK, Newcomb AE, et al. Transfemoral aortic valve replacement with the repositionable Lotus Valve system in high surgical risk patients: the REPRISE I study. EuroIntervention (2014) 9:1264–70. doi: 10.4244/EIJV9I11A216
76. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation (2017) 136:1049–69. doi: 10.1161/CIRCULATIONAHA.117.028352
77. Landes U, Barsheshet A, Finkelstein A, Guetta V, Assali A, Halkin A, et al. Temporal trends in transcatheter aortic valve implantation, 2008–2014: patient characteristics, procedural issues, and clinical outcome. Clin Cardiol. (2017) 40:82–8. doi: 10.1002/clc.22632
78. Harnath A, Gomes B, Herwig V, Gatto F, Watremez S, Katus HA, Bekeredjian R. First experience with the 34mm self-expanding Evolut R in a multi-center registry. EuroIntervention (2018) 14:298–300. doi: 10.4244/EIJ-D-18-00137
79. Vavuranakis M, Kariori M, Scott L, Kalogeras K, Siasos G, Vrachatis D, et al. Impact of “high” implantation on functionality of self-expandable bioprosthesis during the short- and long-term outcome of patients who undergo TAVI. Is high implantation beneficial? Cardiovasc Ther. (2018) 36: e12330. doi: 10.1111/1755-5922.12330
80. Popma JJ, Reardon MJ, Khabbaz K, Harrison JK, Hughes GC, Kodali S, et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self-expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery. JACC Cardiovasc Interv. (2017) 10:268–75. doi: 10.1016/j.jcin.2016.08.050
81. Husser O, Kim W-K, Pellegrini C, Holzamer A, Walther T, Mayr PN, et al. Multicenter comparison of novel self-expanding versus balloon-expandable transcatheter heart valves. JACC Cardiovasc Interv. (2017) 10:2078–87. doi: 10.1016/j.jcin.2017.06.026
82. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet (2016) 387:2218–25. doi: 10.1016/S0140-6736(16)30073-3
83. Guetta V, Goldenberg G, Segev A, Dvir D, Kornowski R, Finckelstein A, et al. Predictors and course of high-degree atrioventricular block after transcatheter aortic valve implantation using the CoreValve revalving system. Am J Cardiol. (2011) 108:1600–5. doi: 10.1016/j.amjcard.2011.07.020
84. Prinzen FW, Peschar M. Relation between the pacing induced sequence of activation and left ventricular pump function in animals. Pacing Clin Electrophysiol. (2002) 25:484–98. doi: 10.1046/j.1460-9592.2002.00484.x
85. Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol Circ Physiol. (1990) 259:H300–8. doi: 10.1152/ajpheart.1990.259.2.H300
86. Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J. A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol. (2004) 44:1619–25. doi: 10.1016/j.jacc.2004.07.036
87. Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. (2004) 44:1883–8. doi: 10.1016/j.jacc.2004.06.074
88. Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation (2006) 113:2082–8. doi: 10.1161/CIRCULATIONAHA.105.608356
89. Chamandi C, Barbanti M, Munoz-Garcia A, Latib A, Nombela-Franco L, Gutiérrez-Ibanez E, et al. Long-term outcomes in patients with new permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2018) 11:301–10. doi: 10.1016/j.jcin.2017.10.032
90. Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli S, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. society of thoracic surgeons/American college of cardiology TVT Registry. JACC Cardiovasc Interv. (2016) 9:2189–99. doi: 10.1016/j.jcin.2016.07.026
91. Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, et al. PARTNER publications office. predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2015) 8:60–9. doi: 10.1016/j.jcin.2014.07.022
92. Urena M, Webb JG, Tamburino C, Munoz-Garcia AJ, Cheema A, Dager AE, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation (2014) 129:1233–43. doi: 10.1161/CIRCULATIONAHA.113.005479
93. De Carlo M, Giannini C, Bedogni F, Klugmann S, Brambilla N, De Marco F, et al. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am Heart J. (2012) 163:492–9. doi: 10.1016/j.ahj.2011.12.009
94. Ledwoch J, Franke J, Gerckens U, Kuck K-H, Linke A, Nickenig G, et al. German transcatheter aortic valve interventions registry investigators. Incidence and predictors of permanent pacemaker implantation following transcatheter aortic valve implantation: analysis from the german transcatheter aortic valve interventions registry. Catheter Cardiovasc Interv. (2013) 82:E569–77. doi: 10.1002/ccd.24915
Keywords: transcatheter aortic valve implantation, pacemaker, left bundle branch block, right bundle branch block, aortic stenosis
Citation: Mangieri A, Montalto C, Pagnesi M, Lanzillo G, Demir O, Testa L, Colombo A and Latib A (2018) TAVI and Post Procedural Cardiac Conduction Abnormalities. Front. Cardiovasc. Med. 5:85. doi: 10.3389/fcvm.2018.00085
Received: 18 April 2018; Accepted: 12 June 2018;
Published: 03 July 2018.
Edited by:
Crochan John O'Sullivan, Triemli Hospital, SwitzerlandReviewed by:
Moritz Seiffert, University Heart Center Hamburg GmbH, GermanyGidon Yehuda Perlman, Hadassah Medical Center, Israel
Copyright © 2018 Mangieri, Montalto, Pagnesi, Lanzillo, Demir, Testa, Colombo and Latib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Mangieri, YW50b25pby5tYW5naWVyaUBnbWFpbC5jb20=
 Antonio Mangieri
Antonio Mangieri Claudio Montalto
Claudio Montalto Matteo Pagnesi
Matteo Pagnesi Giuseppe Lanzillo
Giuseppe Lanzillo Ozan Demir
Ozan Demir Luca Testa
Luca Testa Antonio Colombo1
Antonio Colombo1 Azeem Latib
Azeem Latib