
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Cardiovasc. Med. , 09 October 2017
Sec. Atherosclerosis and Vascular Medicine
Volume 4 - 2017 | https://doi.org/10.3389/fcvm.2017.00063
This article is part of the Research Topic Extracellular Vesicle-Mediated Processes in Cardiovascular Diseases View all 11 articles
Exosomes are defined as submicron (30–150 nm), lipid bilayer-enclosed extracellular vesicles (EVs), specifically generated by the late endosomal compartment through fusion of multivesicular bodies with the plasma membrane. Produced by almost all cells, exosomes were originally considered to represent just a mechanism for jettisoning unwanted cellular moieties. Although this may be a major function in most cells, evolution has recruited the endosomal membrane-sorting pathway to duties beyond mere garbage disposal, one of the most notable examples being its cooption by retroviruses for the generation of Trojan virions. It is, therefore, tempting to speculate that certain cell types have evolved an exosome subclass active in intracellular communication. We term this EV subclass “signalosomes” and define them as exosomes that are produced by the “signaling” cells upon specific physiological or environmental cues and harbor cargo capable of modulating the programming of recipient cells. Our recent studies have established that signalosomes released by mesenchymal stem/stromal cells (MSCs) represent the main vector of MSC immunomodulation and therapeutic action in animal models of lung disease. The efficacy of MSC-exosome treatments in a number of preclinical models of cardiovascular and pulmonary disease supports the promise of application of exosome-based therapeutics across a wide range of pathologies within the near future. However, the full realization of exosome therapeutic potential has been hampered by the absence of standardization in EV isolation, and procedures for purification of signalosomes from the main exosome population. This is mainly due to immature methodologies for exosome isolation and characterization and our incomplete understanding of the specific characteristics and molecular composition of signalosomes. In addition, difficulties in defining metrics for potency of exosome preparations and the challenges of industrial scale-up and good manufacturing practice compliance have complicated smooth and timely transition to clinical development. In this manuscript, we focus on cell culture conditions, exosome harvesting, dosage, and exosome potency, providing some empirical guidance and perspectives on the challenges in bringing exosome-based therapies to clinic.
The intracellular transfer of diverse moieties via extracellular vesicles (EVs) has been proposed to be a widespread process. Cells release diverse EVs that include exosomes, microvesicles (MVs) and apoptotic bodies (1, 2). The classification of such EV subtypes is mainly based on their biogenesis and resultant biophysical properties, such as size, density, and predominant protein markers. Originally, the class of EVs generated through the endosomal pathway (exosomes) was assumed to represent a mere mechanism for the cell to jettison unwanted moieties (3, 4). We now understand that exosome biogenesis is a process governed by the endosomal-sorting complex machinery and involves the formation of intraluminal vesicles within multivesicular bodies (MVBs). Mature MVBs fuse with the plasma membrane and subsequently secrete the enclosed exosomes into the extracellular environment. During their biogenesis, exosomes associate with an array of bioactive cargo from their parental cell. Such cargo has been reported to include genetic information in the form of small noncoding RNAs, free fatty acids, surface receptors, and proteins (Figure 1) (5, 6). It is considered that the biophysical properties of EVs, including their cargo, reflect the stimulus triggering their formation (7), implying specific packaging of “message” prior to export from the parent cell. In turn, the secretion of these biologically loaded signaling vectors to the extracellular environment represents an important method of cell-to-cell communication, dubbed the “new endocrinology”.
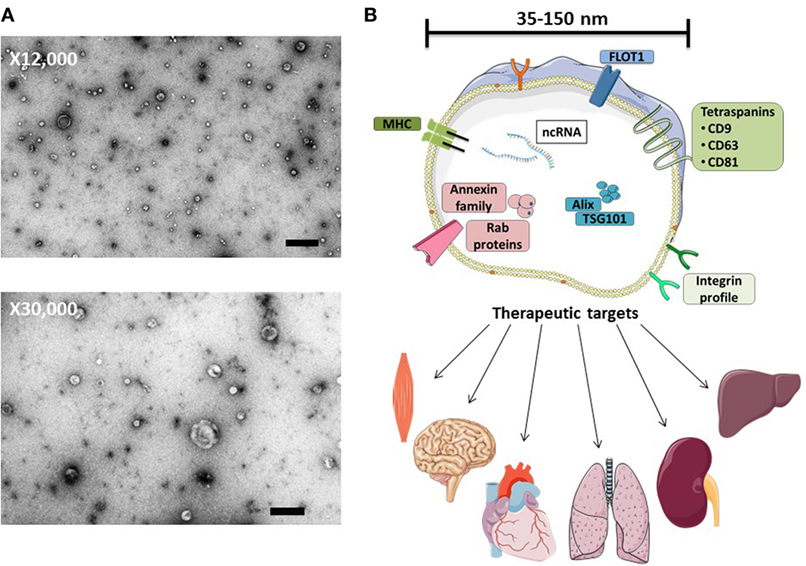
Figure 1. MSC-exosome morphology and composition. (A) Transmission electron microscopy (TEM) images of human bone marrow-derived MSC-exosomes (low magnification, 12,000×, scale bar = 500 nm, and high magnification, 30,000×, scale bar = 100 nm) representative TEM images adapted from Ref. (8). (B) MSC-exosomes are surrounded by a phospholipid bilayer and may contain proteins, such as annexins (these are important for transport); tetraspanins such as CD9, CD81, and CD63; and other proteins, such as Alix and TSG101, that are involved in exosomal biogenesis from endosomes. MSC-exosome therapy has shown beneficial effects in numerous preclinical models, demonstrating histological and functional benefits in multiple organs. Abbreviations: FLOT1, flotillin-1; MHC, major histocompatibility complex; TSG101, tumor susceptibility gene 101.
Exosomes have been shown to play important roles in a broad range of pathological conditions, such as cancer (9), liver and kidney disease (10), neurodegenerative disorders (11), and numerous cardiopulmonary disorders (12, 13). More recently, in addition to their prognostic and diagnostic value, exosomes have also been reported to represent novel therapeutic reagents across multiple disciplines.
The therapeutic capacity of exosomes generated by mesenchymal stem/stromal cells (MSCs) that have been derived from different organs, such as bone marrow, umbilical cord, adipose tissue, or placenta has been tested in various disease models. In the cases where cells and their respective exosomes were studied in parallel, exosome treatment has demonstrated a similar or even superior therapeutic capacity to MSC treatment (14). MSC-exosomes have provided beneficial effects in numerous disease models promoting functional recovery and neurovascular plasticity following traumatic brain injury (15), reducing myocardial infarction size (16, 17), ameliorating hypoxia-induced pulmonary hypertension (18), aiding repair of kidney injury (19, 20), and orchestrating neurological protection by the transfer of microRNA (21, 22). MSC-exosome-based approaches for the treatment of different disease models are highlighted in Table 1.
While the functional roles of exosomes have been extensively reported [reviewed in Ref. (43–46)], few reviews have addressed the challenges underlying the transition of exosome-based therapies from animal models to clinical development. Furthermore, the full realization of their therapeutic potential has been hampered by a lack of standardization in exosome isolation and characterization. Herein, we will focus on the therapeutic application of MSC-exosomes and outline topics relevant to the facilitation of their development as a pharmaceutical preparation, focusing on exosome harvesting, dosing and potency, and providing guidance on the current challenges in bringing exosome-based therapies to clinic.
A comprehensive characterization of the tissue/cellular source of exosomes is imperative for exosome-based therapeutics. Detailed methods for obtaining human MSCs from several tissues, including bone marrow (BMSCs), Wharton’s jelly (WJMSCs), umbilical cord blood, and adipose tissue are well reported (47, 48). By definition, MSCs must adhere to plastic, demonstrate a baseline differentiation potential to osteocytes, chondrocytes, and adipocytes in vitro, and express the presence of widely accepted surface markers (Table 2) (49). However, donor-to-donor variability remains a prominent challenge. Studies have found that BMSCs obtained from older donors have slower proliferation and reduced differentiation potential in vitro. Furthermore, discrepancies in the differentiation capacity and transcriptome profiles are reported to be tissue and species dependent (50–52). To what extent do these uncertainties affect the therapeutic capacity of MSCs and their resultant exosomes remains unclear. Thus, in addition to validated MSC isolation procedures, investigators should adhere to carefully selected donor eligibility criteria in accordance with the appropriate ethical and regulatory approval, employing strict control measures to prevent risk of relevant communicable disease agents or diseases (RCDADs), such as human immunodeficiency virus (HIV), hepatitis C virus, and cytomegalovirus. Moreover, donor screening should include a comprehensive medical record review, physical assessment, and medical history interview, with records documented in compliance with appropriate regulatory frame. The International Society of Extracellular Vesicles (ISEV), the Food and drug Administration (FDA), the International Council for Harmonization (ICH) of Technical Requirements for Pharmaceuticals for Human Use, and the European Medicines Agency (EMA) provide extensive guidance for the development and generation of novel biological medicines with regard to donor/patient care, product safety, and quality (53–56). The demonstration that exosomes generated by MSCs isolated from WJMSCs are as effective as BMSC-exosomes in treating rodent disease models (8) may facilitate standardization and consistency of MSC lines for exosome harvesting. Moreover, the umbilical cord may possess several advantages over bone marrow. First, the umbilical cord represents a more readily available source than bone marrow. Second, it is often viewed as discarded medical waste that does not require any invasive procedures or cadaver procurement.
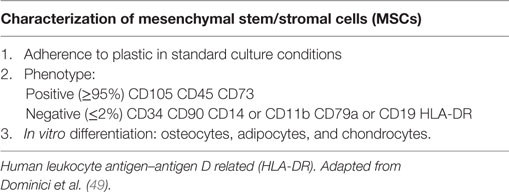
Table 2. Minimal criteria for defining MSCs, as put forth by The International Society for Cellular Therapy.
Previous studies have reported that the protein and RNA profile of exosomes reflect the cell culture conditions and microenvironmental stimuli that triggered their release. With this in mind, it begs to question can “stimulating” and/or preconditioning cells be used as a means to generate a more homogenous or efficacious exosome population? Interestingly, in an experimental model of hyperoxia-induced bronchopulmonary dysplasia (BPD), Waszak and coworkers found that the conditioned media (CM) derived from hyperoxia-preconditioned rat BMSCs (95% O2, for 24 h) provided greater protection in vivo compared to CM collected from cells grown under control conditions (57). Clearly, in the absence of further characterization, one can only speculate where the observed augmentation of activity resides. Other studies have found that MSCs cultured in hypoxia conditions (<5% O2) exhibited an altered protein expression pattern compared to MSCs cultured in the so-called “normoxia” (58). Furthermore, in a murine hind limb ischemia model, they showed that intra-arterial injection of MSCs cultured in both “normoxic” or hypoxic conditions enhanced revascularization compared with saline controls; however, the functional recovery of mice that received hypoxia preconditioned MSCs was faster (59). These reports suggest that preconditioning MSCs in different oxygen environments may improve their tissue regenerative potential.
Indeed, “hypoxia” (1% O2) has been shown to increase exosome production in numerous cell types in vitro. Previous studies have shown that the hypoxia-induced elevation in exosome secretion is chiefly governed by hypoxia-inducible factor-1 alpha (HIF-1α) and is independent of apoptosis (60). Often, laboratory cell culture conditions are at atmospheric oxygen levels (21% O2, corresponding to a PO2 of ~159 mmHg); following adjustment to 5% CO2, this equates to ~19.95% O2 (~150 mmHg). Although, it is challenging to accurately measure tissue oxygen concentrations in vivo, it is well recognized that most of the human body tissue is normally exposed to much lower O2 levels. This can range from 160 to 100 mmHg in the alveoli, >35 mmHg in the brain, and ~25 mmHg in skeletal muscle (61–63). Notably, in the bone marrow, arguably the most common origin of MSCs, the PO2 is reported to be ~40 mmHg, while the umbilical cord vasculature PO2 is reported to be between 10 and 30 mmHg (63). Organ oxygen levels have been extensively reviewed (63). However, it is important to remember that the different techniques used to measure oxygen concentration in vivo are subject to their own advantages and limitations.
In vivo, the oxygen concentration of an organ is an indication of its physiological state and reflects the balance between oxygen delivery and its metabolic consumption. Consequently, in a physiological condition, organs are subject their own unique “physioxia” status. On balance, the routine laboratory cell culture conditions expose MSCs to oxygen levels higher than those in their physiologic niches, and this departure from “physioxia” may precipitate a “perceived hyperoxia” response. Thus, it is important to recognize this factor when interpreting results of experiments performed in atmospheric “normoxia,” and also to realize that the impact of this factor may vary, depending on the particular study and the metrics assessed.
In turn, several questions remain unanswered. The optimal oxygen concentration for in vitro MSC culture and the effect that it may have on subsequent exosome production remains undefined at this point. Existing reports indicate that optimization is likely to be both MSC origin and disease model specific. Thus, additional studies assessing the effect of oxygen levels on MSCs and their resultant exosomes are much needed.
Cells generate three major EV classes: apoptotic bodies, MVs, and exosomes. Arguably, it is often assumed each subtype represents a homogenous vesicle population that can be distinguished based on biophysical properties such as size or density. However, it has become obvious that even within such subtypes, there is heterogeneity (2, 64). Although, the field lacks tools to distinguish vesicles from different routes of biogenesis, recent evidence has demonstrated that MSCs release distinct EV subpopulations that differ in biophysical, proteomic, and RNA repertoires. Specifically, Kowal and coworkers found that large-, medium-, and small-sized EVs can be isolated by sequential low-, intermediate-, and high-speed centrifugation, respectively. Among the small-EVs (exosomes), four subcategories were defined by their degree of enrichment in CD63, CD9, and/or CD81 tetraspanins (64). In accordance, Lai et al. found that MSCs secrete many distinct subtypes of vesicles, which differ in RNA and protein composition (65). It is relevant to note that the study involved an immortalized, iPS-derived MSC cell line that will likely secrete a more restricted range of exosome subtypes than those generated by primary cells.
In our hands, ongoing studies aim to address the relationships between MSC-exosome subtypes and therapeutic efficacy and to explore the hypothesis that a discrete subtype is responsible for the therapeutic activity in our established experimental models of BPD, a chronic lung disorder of infants (8). Here, we isolated exosomes from either human BMSCs or WJMSCs by differential centrifugation, followed by tangential flow filtration and iodixanol density floatation before separating exosome subtypes by size-exclusion chromatography (SEC) (described in Figure 2). This approach separates MSC-exosome subtypes based on their size, and in accordance with previous reports, we demonstrate a shift in protein markers associated with different exosome subtypes. Specifically, we found that CD63 and flotillin-1 (FLOT1) is associated with “large”-exosomes (>80 nm), while Alix and TSG101 is enriched in “small”-exosomes (<80 nm). Few studies assess the ratio of such markers; however, further investigation is warranted as it may provide a tool for distinguishing exosome subtypes. Ongoing in vivo studies are testing whether the different MSC-exosome subtypes exhibit differential therapeutic efficacy in a number of animal models currently utilized by our group and collaborators.
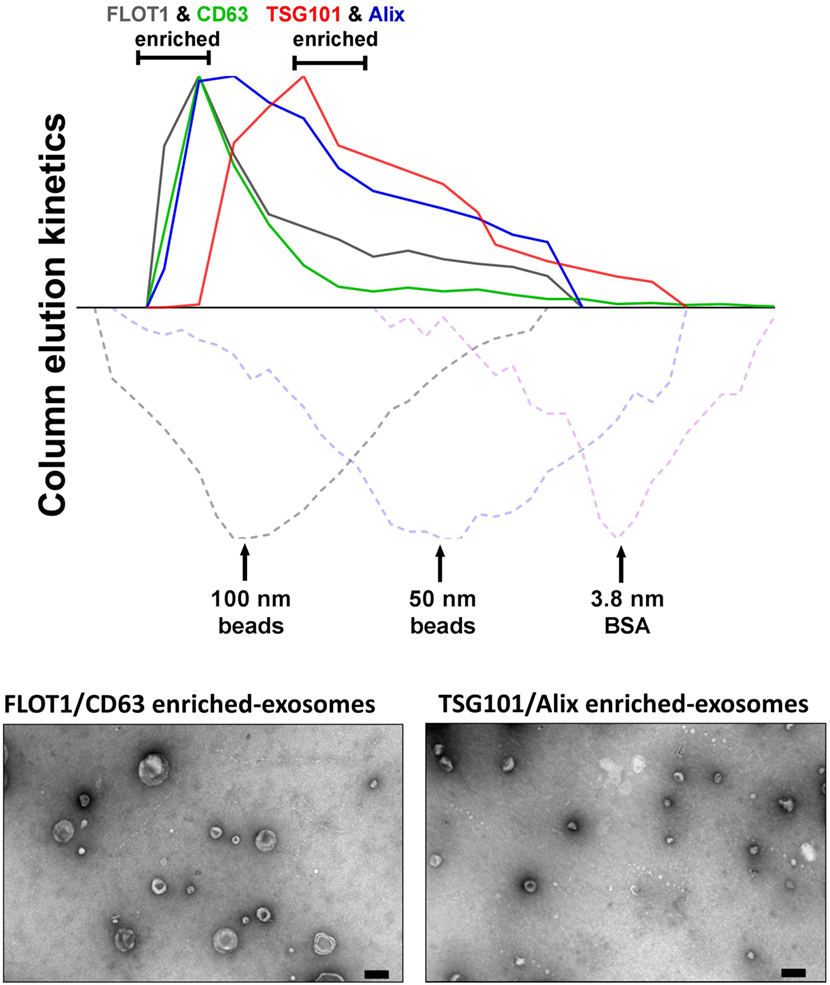
Figure 2. Isolation of MSC-exosomes subtypes by size-exclusion chromatography (SEC). Briefly, exosomes were isolated directly from cell culture supernatants following a 36 h harvest period in serum-free-media. Cell culture media were subjected to differential centrifugation, 300 × g for 10 min, followed by 3,000 × g for 10 min, and 13,000 × g for 30 min to remove any cells, cell debris, and large apoptotic bodies in suspension, respectively. Conditioned media (CM) was concentrated 50-fold by tangential flow filtration (TFF) and the exosomes were purified using OptiPrep™ (iodixanol) cushion density flotation (3.5 h at 100,000 × g, 4°C), as previously described (8). Heterogeneous exosomes were further purified by size using size-exclusion chromatography (SEC). Here, sepharose CL-2B (80 mL) was washed with 1 × SSPE buffer [containing 1 mM EDTA and 149 mM NaCl in 0.20 mM phosphate buffer (pH 7.4)]. The column was packed with washed sepharose CL-2B to create a column with an internal diameter of 1.6 cm and height of 40 cm. Exosomes (1 ml, corresponding to 60 × 106 MSC equivalents) were added to column with a flow rate of 1 ml/min. Fractions (1 ml) were collected and assessed by dot plots and electron microscopy. The elution kinetics of 100 nm, 50 nm, and bovine serum albumin (BSA) were used to estimate exosome elution kinetics. TSG101, Alix, CD63, and FLOT1 levels were assessed by dot plots and are reported as relative intensity. Here, we identify two distinct MSC-exosome subtypes. The larger exosomes (>80 nm) have a greater flotillin-1 (FLOT1) and CD63 enrichment, while smaller exosomes (<80 nm) have a greater TSG101 and Alix ratio.
Regulatory frameworks often require a mechanism-of-action that details the identity, quantification, and characterization of such bioactive substances responsible for the therapeutic effect(s). Moreover, disclosure of non-active components (“excipients”) in drug preparations should be acknowledged (56, 66). Knowing exosome subtypes harbor different protein and genetic cargo, it is fair to speculate that they likely mediate different effects on targets cells. Thus, improved separation techniques that distinguish between “non-active” and therapeutic exosome subtypes may help focus the search for the bioactive substance(s) responsible for such beneficial effects. The “one size fits all” hypothesis may not work for exosome-based therapeutics. Although a specific exosome subset may induce beneficial therapeutic effects in a specific disease model, it is important to recognize that a different exosome subpopulation may afford the beneficial effects in a different disease model.
Isolation methods impact exosome integrity, in vivo biodistribution and metabolic fate (56). Exosome isolation techniques from various biological fluids and cell culture medium have been extensively reviewed (67–69). It is well established that widely applied exosome isolation techniques, such as differential ultracentrifugation (UC), promote vesicle aggregation and often co-isolate soluble factors and protein (70). Thus, a consensus in the field has shifted toward more “gentle” isolation techniques to ultimately reduce contaminants (non-EV material), maintain integrity, and isolate “bioactive” vesicles from heterogeneous EV populations. To date, popular avenues of investigation include gradient density isolation and SEC, with the latter being more suited to enclosed tissues culture systems. Variations of such approaches have been shown to effectively separate exosomes from proteins and soluble factors in different biological fluids (71, 72). However, layered density-based procedures may achieve enrichment rather than true exosome isolation, where the influence of UC parameters coupled with high-sucrose concentrations may change the osmotic environment (69). Furthermore, UC methods are impracticable for large-scale bioprocessing.
Several emerging technology platforms have shown promise in isolating exosomes from various sample matrices, with each method exploiting a particular biophysical trait of exosomes such as their size, density, shape, or surface receptors (Figure 3) (72). The final goal is an isolation method that is label free, distinguishes between exosome subtypes and interfering components, and can facilitate a large-scale production of exosomes.
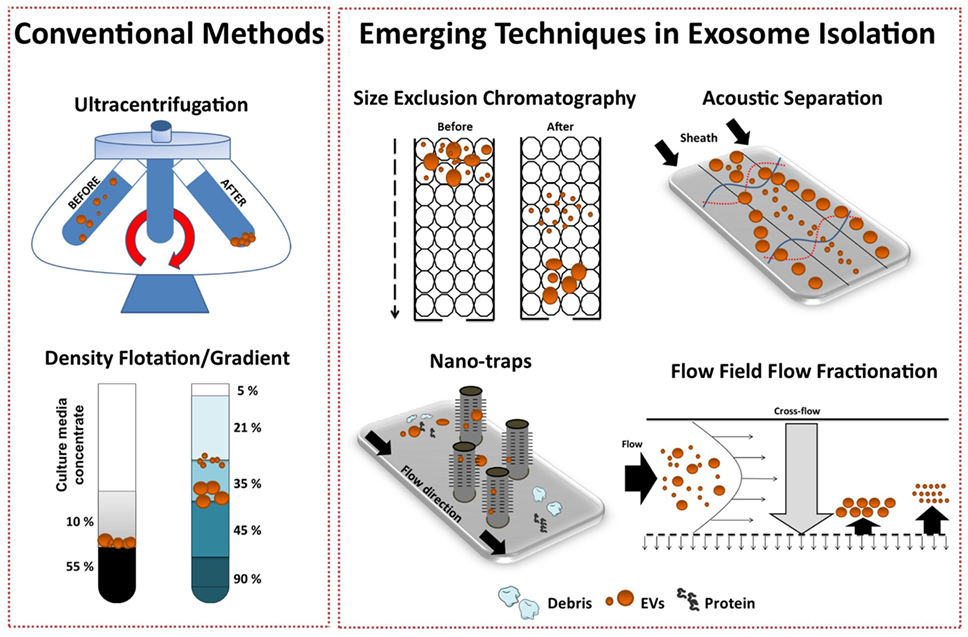
Figure 3. Conventional and emerging exosome isolation techniques. Ultracentrifugation (UC) is the most common exosome isolation method. Here, sedimentation of solutes including vesicles is governed by their size/density. Variations of UC such as layered or cushion based-density gradient UC are also widely employed. New methods are required to facilitate large-scale, high-yield production of exosomes for clinical applications. Several emerging technology platforms have shown promise in isolating exosomes from various sample matrices. Techniques, such as size-exclusion chromatography, ciliated micropillars nano-traps, acoustic wave separation technology, and flow field-flow fraction (F4), exploit unique biophysical traits of exosomes.
Recently, Lee and colleagues employed a label-free acoustic nano-filter system to isolate exosomes based on their size (73). Specifically, using ultrasound standing waves exert differential acoustic force, they isolated exosomes from both cell culture media and stored red blood cell products. They effectively separated exosomes (149 nm) and MVs (410 nm). Although, its application for high-throughput exosome preparations is yet to be established, previous studies have used label-free acoustic wave systems to isolate circulating tumor cells (74).
Exosomes can also be separated by size with 10 nm accuracy using variations of flow field-flow fractionation (F4) (75). Asymmetrical F4 (AF4) is a one-phase chromatography method that uses parabolic-flow to drive exosomes across a flow channel. A crossflow intercepts the parabolic-flow perpendicular to the channel and distributes particulate components against the flow chamber wall. Subsequently, exosomes are separated based on differences in diffusivity. Smaller particles diffuse further from the accumulation wall and are eluted earlier than larger ones. Successful attempts have been made using AF4 to isolate exosomes from human neural stem cell culture (76) and melanoma cell lines (77). AF4 approaches provide promise for “label-free” isolation of large-scale exosome production.
To effectively utilize the size difference between exosomes, other EV subtypes and cellular debris, Wang and colleagues fabricated a porous silicon nanowire-on-micropillar “nano-trap” made from ciliated micropillars (78). This fabricated microfluidic device preferentially traps exosomes with a diameter of 40–100 nm, while filtering out proteins, larger EVs, and cellular debris. Moreover, trapped exosomes can be recovered by dissolving the porous silicon nanowires in PBS buffer. However, in this proof-of-concept study, the authors noted poor vesicle retention (~60%) and only assessed small sample volume (30 μl), thus scalability is yet to be demonstrated.
Overall, to support the development of exosome-based therapeutics, research efforts should focus on the development of “label-free” exosome isolation techniques that can support high-throughput systems/scale-up requirements and are capable of distinguishing exosome subtypes. Although a number of highly sophisticated technologies for EV isolation have emerged recently, their application is mainly in the biomarker field, as tools for exosome-based diagnostics. Although such emerging technologies hold great promise, the large-scale preparation of isolated exosome subtypes to be used as the basis of exosome-based pharmaceutical products will probably depend, at least in the near future, on modifications of classic industrial processes such as SEC.
Currently, investigators use several different methods to quantify exosome dosage, making inter-study comparison troublesome. Common quantitative practices include reporting cell equivalents, protein concentration, and/or specialized quantitative analytical measurements by instruments, such as tunable resistive pulse sensing (TRPS) and nanoparticle tracking analysis (NTA), with each method harboring its own advantages and limitations [for recent reviews (79–82)]. The need to standardize exosome dosing is imperative. Of interest, methods such as TRPS are currently used to verify particle size and characterization for liposome-encapsulated forms of doxorubicin and are accepted within the definition of bioequivalence as set forth by the FDA and EMA. Although enumeration of analytical criteria is beyond the scope of this review, we acknowledge that the field is limited by current technology and lacks the ability to accurately assess exosomes at a single vesicle level. Thus, to aid inter-study comparison, we recommend that in addition to providing extensive detail of standardized cell culture conditions and pre-analytical protocols, investigators should measure exosome concentration using multiple quantification tools, where possible. A summary of the advantages and limitations of common methods used to determine exosome dose are highlighted in Table 3. Establishing an exosome potency assay is a novel approach which holds great promise in standardizing exosome dosing.
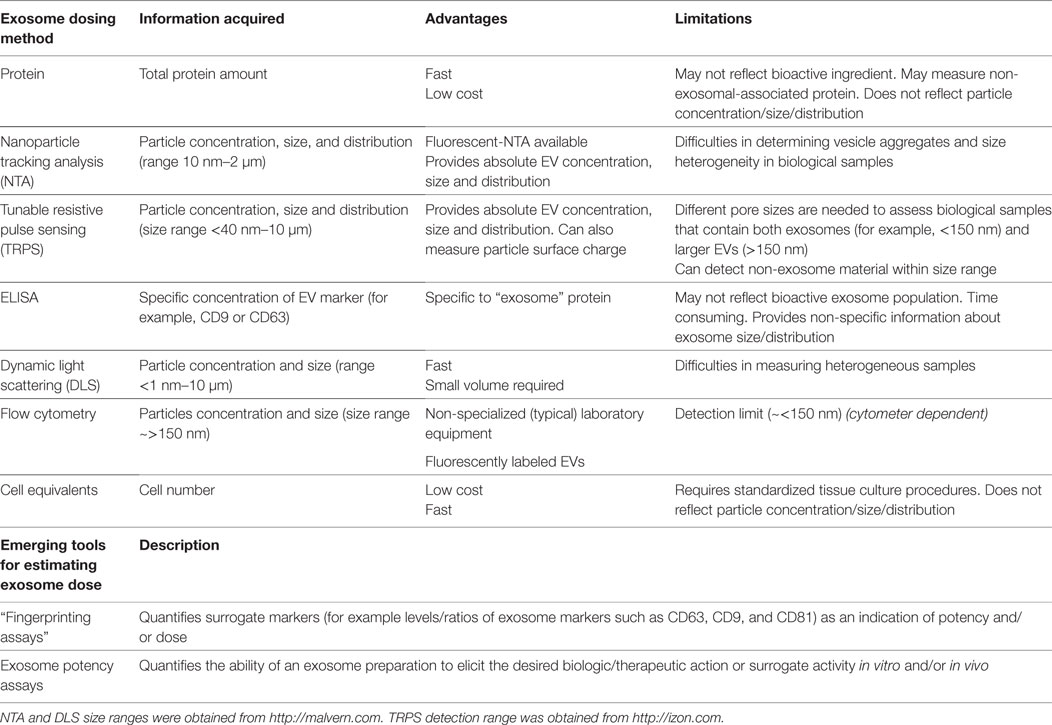
Table 3. A summary of the advantages and limitations of common methods used to determine exosome dose.
The definition of the bioactive substance(s) will remain a crucial question in the preclinical development of exosome-based therapeutics. With an orchestra of bioactive cargo and diverse physiological effects (Figure 1B), identification of “one” bioactive substance or a singular mechanism-of-action appears improbable. By FDA standards, potency is defined as the products specific ability or capacity to affect a given result (66). With no “gold-standard” technique for their quantification, assessment of exosome potency would be a valuable tool in overcoming the inconsistencies in preparations and batch-to-batch variation. For example, exosomes obtained from two separate donors may be normalized via a given quantitative method; however, the “bioactive” load may differ, subsequently the potency and degree of efficacy will not be the same. Thus, investigators should consider employing a unique exosome potency unit (EPU) to standardize practices and minimize variation between different samples. Presently, attempts to define an exosome potency metric utilized the immunomodulatory properties of MSC-exosomes. For example, Jiao et al. described an in vitro potency assay for MSC-exosomes based on the release of IL-10 from mononuclear cells following incubation with exosome preparations, and other studies have shown that T-cell proliferation assays may be modified to provide the basis for assays on exosome immunomodulatory potency (29, 83). Growing evidence also suggests that MSC-exosomes can modulate macrophage phenotypes (8, 35). Macrophages play a pivotal part in regulating immune responses. They assume both phagocytic “defensive” roles and exhibit regulatory “anti-inflammatory” actions, facilitating both the initiation and the resolution of inflammation (84). With this consideration in mind, our ongoing studies are exploring an in vitro macrophage polarization assay as a means of assessing MSC-exosome potency. Briefly, the potency assay involves adding MSC-exosomes to murine bone marrow-derived macrophages (BMDMs) that are polarized to the classically activated (proinflammatory) M1-phenotype. The functional endpoint is the capacity of MSC-exosomes to suppress the mRNA induction of TNFα. The half maximal effective concentration (EC50 value, 50% inhibition in TNFα mRNA levels, relative to M1 control) is transformed to an arbitrary EPU (described in Figure 4) (8). In turn, an EPU could potentially be applied to standardize dosing between different exosome preparations. In all cases, potency assays need to be disease specific, fit-for-purpose, and employ relevant functional end-points.
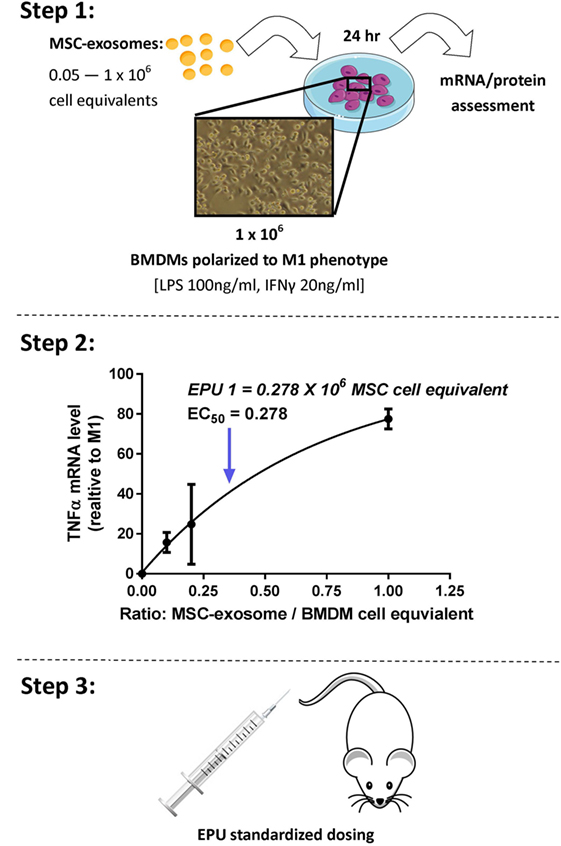
Figure 4. Stepwise approach to developing an MSC-exosome potency assay. Considering limitations in current exosome quantification techniques, assessment of exosome potency would be a valuable tool to standardize exosome dosing. Step 1: primary murine bone marrow-derived macrophages (BMDMs) were obtained by flushing the femur and tibia of 6- to 8-week-old FVB mice. M1 polarization was initiated by lipopolysaccharide (LPS) 100 ng/ml and interferon-γ (IFNγ) 20 ng/ml stimulation. Macrophage polarization (1 × 106 BMDMs) was initiated with/without the presence of MSC-exosome preparations (0.05–1 × 106 cell equivalents), providing several ratios of MSC-exosome cell equivalents-to-BMDMs. After 24 h, total RNA was isolated and TNFα mRNA levels were assessed by RT-qPCR. The functional endpoint is the capacity of MSC-exosomes to suppress the mRNA induction of TNFα. Step 2: an EC50 value (50% reduction in TNFα mRNA, relative to M1 control) is transformed to an arbitrary exosome potency unit (EPU). Data were adapted from our recent work (8), where we demonstrated purified human umbilical cord MSC-exosomes, dose dependently suppressed mRNA levels of TNFα in alveolar macrophages in vitro. Step 3: an EPU can be applied to standardize dosing between different exosome preparations or as a means of correlating potency to exosome quantity.
In 2002, Lamparski et al. described a method for the production and characterization of clinical grade exosomes derived from dendritic cells for their application in cancer vaccine clinical trials (85). Using ultrafiltration coupled with a sucrose/deuterium oxide UC cushion, they isolated vesicles (50–90 nm in diameter) containing major histocompatibility complex (MHC) class-I, -II, and CD1, and tetraspanin molecules (CD9, CD63, and CD81) In addition, in 2005, Navabi and coworkers detailed the development of a method for the preparation and characterization of good manufacturing practice (GMP)-grade exosomes from the ascites fluid of ovarian cancer patients (86). Since then our understanding of exosome biology has improved and the development of specialized isolation and characterization methods has allowed investigators to more accurately isolate and characterize exosome populations.
Considering the development of exosome-based therapeutics, lessons could be learned from cell therapy. The in vitro expansion of cells (such as MSCs) is required to deliver an effective therapeutic dose, with the absence of having a detrimental impact on the quality of the cell. Upon scaling-up, process analytical technology (PAT), a system proposed by the FDA, may be implemented to monitor the manufacturing process through continuous measurement of cell parameters (87, 88). Monitoring bioprocess parameters, such as population doubling time, temperature, metabolite concentrations, pH, pO2 and pCO2, may help ensure optimal exosome quality and quantity, as previous reports have shown that subtle acidic pH shifts may impair exosome aggregation inhibiting forces and, in turn, promote aggregation and reduce functionality (56).
Recently, the inevitable shift to using tissue culture bioreactors has been used to generate large-scale EV preparations. Indeed, Watson and colleagues demonstrated that hollow-fiber bioreactors promotes enhanced exosome production (~40-fold greater EVs/ml of CM) when compared to conventional 2D tissue culture preparations (89). However, it remains unclear if such methods enhance generalized EV production or simply reflect a reduction in exosome re-uptake. Under the considerations discussed above, relating to EV diversity and the possibility that only specific exosome subtypes may represent the therapeutic agent, it is premature to assume that production of higher EV numbers will necessarily yield a higher efficacy final product. Optimization of MSC culture conditions will, therefore, require the parallel development of a dependable and easily adoptable potency assay.
With evidence to suggest that exosomes can be stored at −20°C for up to 6 months with no loss to their biochemical activity, “off-the-shelf” exosome-based products represent an attractive pharmaceutical formulation (56, 66, 90). Although standardized storage procedures remain to be defined, current storage protocols use isotonic buffers to prevent pH shifts during storage, avoid freeze–thaw cycles and are absent of dimethyl sulfoxide (DMSO) and glycerol as previous reports have shown these agents may impact exosome integrity (91). With a lack of data addressing the impact of storage time and excipients on exosome structural stability and functional efficacy, more studies are warranted to help define a provisional “shelf-life” for exosome-based products and facilitate the manufacturing and distribution process.
Promising preclinical data that demonstrated dendritic cell-derived EVs containing MHC–peptide complexes could alter tumor growth in immune competent mice led to a phase I anti-melanoma clinical trial conducted in France (92) and a phase I anti-non-small cell lung cancer clinical trial in the United States (93) (clinical trial applications highlighted in Table 4). Both clinical trials administered autologous dendritic cell-derived EVs that met their respective current GMP standards. Such clinical trials to date are important for their demonstration of both the feasibility and the short-term safety of autologous EV administration, but safety considerations for therapies based on exogenous exosome-based products will arguably be more stringent. Nevertheless, it is very encouraging to note that preclinical studies have established immunomodulation as the main therapeutic mechanism of MSC-exosomes action. Immunomodulation is clearly involved in the autologous exosome clinical trials mentioned above, and this may provide guidelines and precedent for clinical trials using exogenous exosomes. In this context, a recent clinical case involving treatment of a steroid-refractory graft-vs-host disease patient with MSC-EVs derived from unrelated bone marrow donors produced encouraging results (94).
Ultimately, issues raised in this review aim to provide a basic guidance for investigators on key issues to consider for the smooth transition of exosome-based therapies from the preclinical model into clinical development (Figure 5). Among them, determining the optimal dose, the appropriate time window for exosome administration, the number of doses, and route of administration that achieves maximal efficacy without adverse effects are the most important issues to resolve. Such issues will be disease/model specific and clearly beyond the scope of this work.
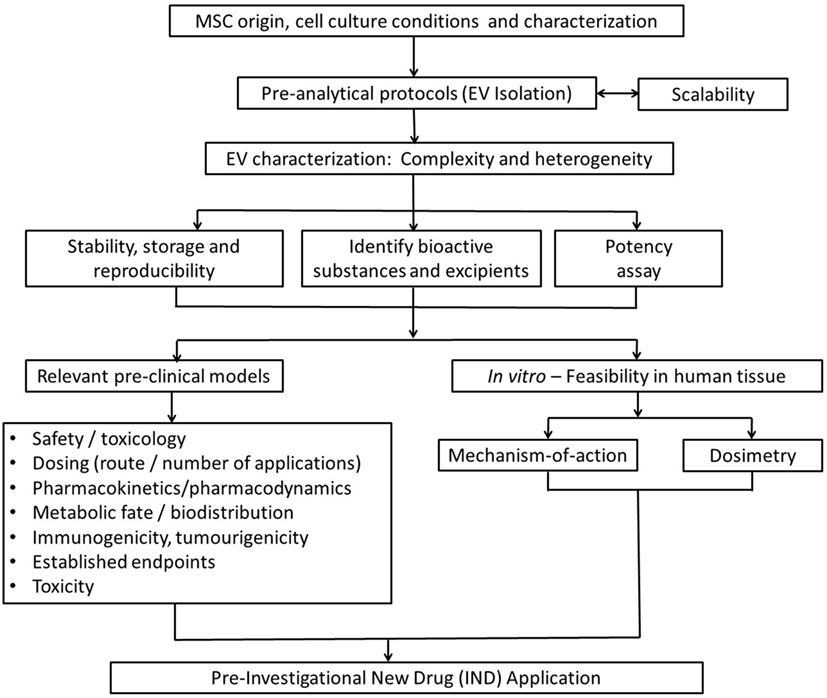
Figure 5. Strategic flowchart for the preclinical testing of exosome-based therapeutics. EV, extracellular vesicle. Adapted from Ref. (97).
Exosome-based therapeutics represent a most promising next generation approach for treating a diverse number of diseases, particularly diseases the pathogenesis of which involves a primary (or major) inflammatory component. The efficacy of MSC-exosome treatments has been robustly established in numerous preclinical models, but development of large-scale GMP-grade exosome-based pharmaceuticals and subsequent clinical trials demand the resolution of several technological and mechanistic issues, reflecting the cautious navigation in unknown seas for this relatively novel field. Among the major issues to be resolved are the definition of an EPU, the standardization of MSC culture conditions and protocols for exosome harvest and storage. Although safety considerations need also to be addressed, it is expected that safety concerns for cell-free, exosome-based clinical trials will be arguably milder than those relevant to live cell MSC trials currently in progress, as mutagenicity and oncogenicity concerns will be null.
GW participated in data collection, analysis, and manuscript writing. SK and SAM contributed to final article editing and approval.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported in part by NIH grants RO1 HL085446 and RO1 HL055454 (SK) and a United Therapeutics Corp. Sponsored Research Grant (SK and SAM).
1. Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles (2013) 2:20389. doi:10.3402/jev.v2i0.20389
2. Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep (2016) 6:22519. doi:10.1038/srep22519
3. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem (1987) 262:9412–20.
4. Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep (2011) 3:15. doi:10.3410/B3-15
5. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev (2012) 64:676–705. doi:10.1124/pr.112.005983
6. Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol (2014) 29:116–25. doi:10.1016/j.ceb.2014.05.004
7. de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles (2012) 1:18396. doi:10.3402/jev.v1i0.18396
8. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med (2017). doi:10.1164/rccm.201705-0925OC
9. Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis and drug resistance: a comprehensive review. Cancer Metastasis Rev (2013) 32:623–42. doi:10.1007/s10555-10013-19441-10559
10. Krause M, Samoylenko A, Vainio SJ. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front Cell Dev Biol (2015) 3:65. doi:10.3389/fcell.2015.00065
11. Howitt J, Hill AF. Exosomes in the pathology of neurodegenerative diseases. J Biol Chem (2016) 23:26589–97. doi:10.1074/jbc.R116.757955
12. Hugel B, Martínez MC, Kunzelmann C, Freyssinet J-M. Membrane microparticles: two sides of the coin. Physiology (2005) 20:22. doi:10.1152/physiol.00029.2004
13. Ailawadi S, Wang X, Gu H, Fan G-C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta (2015) 1852:1–11. doi:10.1016/j.bbadis.2014.10.008
14. Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med (2009) 180:1122–30. doi:10.1164/rccm.200902-0242OC
15. Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg (2015) 122:856–67. doi:10.3171/2014.11.JNS14770
16. Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (2010) 4:214–22. doi:10.1016/j.scr.2009.12.003
17. Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res (2013) 10:301–12. doi:10.1016/j.scr.2013.01.002
18. Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation (2012) 126:2601–11. doi:10.1161/CIRCULATIONAHA.112.114173
19. Dorronsoro A, Robbins PD. Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther (2013) 4:39–39. doi:10.1186/scrt187
20. Zhang G, Wang D, Miao S, Zou X, Liu G, Zhu Y. Extracellular vesicles derived from mesenchymal stromal cells may possess increased therapeutic potential for acute kidney injury compared with conditioned medium in rodent models: a meta-analysis. Exp Ther Med (2016) 11:1519–25. doi:10.3892/etm.2016.3076
21. Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of mir-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells (2012) 30:1556–64. doi:10.1002/stem.1129
22. Kalani A, Tyagi N. Exosomes in neurological disease, neuroprotection, repair and therapeutics: problems and perspectives. Neural Regen Res (2015) 10:1565–7. doi:10.4103/1673-5374.165305
23. Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res (2016) 110:319–30. doi:10.1093/cvr/cvw054
24. Zhu Y-G, Feng X-M, Abbott J, Fang X-H, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of E. coli endotoxin-induced acute lung injury in mice. Stem Cells (2014) 32:116–25. doi:10.1002/stem.1504
25. Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun (2015) 6:8472. doi:10.1038/ncomms9472
26. Monsel A, Zhu Y-G, Gennai S, Hao Q, Hu S, Rouby J-J, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med (2015) 192:324–36. doi:10.1164/rccm.201410-1765OC
27. Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol (2015) 182:349–60. doi:10.1016/j.ijcard.2014.12.043
28. Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (2014) 92:387–97. doi:10.1007/s00109-013-1110-5
29. Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem (2015) 37:2415–24. doi:10.1159/000438594
30. Kim D-K, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A (2016) 113:170–5. doi:10.1073/pnas.1522297113
31. Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, et al. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep (2016) 6:34562. doi:10.1038/srep34562
32. Mead B, Tomarev S. BMSC-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med (2017) 6:1273–85. doi:10.1002/sctm.16-0428
33. Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig A-K, Radtke S, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med (2015) 4:1131–43. doi:10.5966/sctm.2015-0078
34. Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab (2013) 33:1711–5. doi:10.1038/jcbfm.2013.152
35. Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med (2017) 6:1018–28. doi:10.1002/sctm.16-0363
36. Tan CY, Lai RC, Wong W, Dan YY, Lim S-K, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther (2014) 5:76. doi:10.1186/scrt465
37. Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev (2013) 22:845–54. doi:10.1089/scd.2012.0395
38. Yang J, Liu X-X, Fan H, Tang Q, Shou Z-X, Zuo D-M, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One (2015) 10:e0140551. doi:10.1371/journal.pone.0140551
39. Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal micrornas suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med (2016) 5:1425–39. doi:10.5966/sctm.2015-0367
40. Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med (2015) 13:49. doi:10.1186/s12967-015-0417-0
41. Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther (2014) 5:40. doi:10.1186/scrt428
42. Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One (2012) 7:e33115. doi:10.1371/journal.pone.0033115
43. Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med (2011) 6:481–92. doi:10.2217/rme.11.35
44. Kourembanas S. Stem cell-based therapy for newborn lung and brain injury: feasible, safe, and the next therapeutic breakthrough? J Pediatr (2014) 164:954–6. doi:10.1016/j.jpeds.2014.01.064
45. Mitsialis SA, Kourembanas S. Stem cell-based therapies for the newborn lung and brain: possibilities and challenges. Semin Perinatol (2016) 40:138–51. doi:10.1053/j.semperi.2015.12.002
46. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells (2017) 35:851–8. doi:10.1002/stem.2575
47. Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med (2005) 140:138–43. doi:10.1007/s10517-005-0430-z
48. Hendijani F. Explant culture: an advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif (2017) 50:e12334. doi:10.1111/cpr.12334
49. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy (2006) 8:315–7. doi:10.1080/14653240600855905
50. Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int (2012) 36:747–53. doi:10.1042/CBI20110183
51. Choudhery MS, Badowski M, Muise A, Harris DT. Comparison of human adipose and cord tissue derived mesenchymal stem cells. Cytotherapy (2013) 15:330–43. doi:10.1016/j.jcyt.2012.11.010
52. Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med (2014) 12:8. doi:10.1186/1479-5876-12-8
53. Halme DG, Kessler DA. FDA regulation of stem-cell–based therapies. N Engl J Med (2006) 355:1730–5. doi:10.1056/NEJMhpr063086
54. Harron DWG. Technical requirements for registration of pharmaceuticals for human use: the ICH process. In: Griffin JP, editor. The Textbook of Pharmaceutical Medicine. Oxford: Blackwell Publishing Ltd (2007). p. 552–64.
55. Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol (2012) 3:253. doi:10.3389/fimmu.2012.00253
56. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles (2015) 4:30087. doi:10.3402/jev.v4.30087
57. Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thebaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev (2012) 21:2789–97. doi:10.1089/scd.2010.0566
58. Riis S, Stensballe A, Emmersen J, Pennisi CP, Birkelund S, Zachar V, et al. Mass spectrometry analysis of adipose-derived stem cells reveals a significant effect of hypoxia on pathways regulating extracellular matrix. Stem Cell Res Ther (2016) 7:52. doi:10.1186/s13287-016-0310-7
59. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells (2008) 26:2173–82. doi:10.1634/stemcells.2007-1104
60. Burnley-Hall N, Willis G, Davis J, Rees DA, James PE. Nitrite-derived nitric oxide reduces hypoxia-inducible factor 1α-mediated extracellular vesicle production by endothelial cells. Nitric Oxide (2017) 63:1–12. doi:10.1016/j.niox.2016.12.005
61. Boekstegers P, Riessen R, Seyde W. Oxygen partial pressure distribution within skeletal muscle: indicator of whole body oxygen delivery in patients? Adv Exp Med Biol (1990) 277:507–14. doi:10.1007/978-1-4684-8181-5_57
62. Le Q-T, Chen E, Salim A, Cao H, Kong CS, Whyte R, et al. An Evaluation of tumor oxygenation and gene expression in patients with early stage non–small cell lung cancers. Clin Cancer Res (2006) 12:1507–14. doi:10.1158/1078-0432.CCR-05-2049
63. Carreau A, Hafny-Rahbi BE, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med (2011) 15:1239–53. doi:10.1111/j.1582-4934.2011.01258.x
64. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A (2016) 113:E968–77. doi:10.1073/pnas.1521230113
65. Lai RC, Tan SS, Yeo RWY, Choo ABH, Reiner AT, Su Y, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles (2016) 5:29828. doi:10.3402/jev.v5.29828
66. Batrakova EV, Kim MS. Development and regulation of exosome-based therapy products. Wiley Interdiscip Rev Nanomed Nanobiotechnol (2016) 8:744–57. doi:10.1002/wnan.1395
67. Théry C, Amigorena S, Raposo G, Clayton A. Chapter 3: Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology. John Wiley & Sons, Inc (2006). Unit 3.22 p. doi:10.1002/0471143030.cb0322s30
68. Momen-Heravi F, Balaj L, Alian S, Mantel P-Y, Halleck Allison E, Trachtenberg Alexander J, et al. Current methods for the isolation of extracellular vesicles. Biol Chem (2013) 394:1253–6. doi:10.1515/hsz-2013-0141
69. Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles (2013) 2:20360. doi:10.3402/jev.v2i0.20360
70. Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles (2015) 4:29509. doi:10.3402/jev.v4.29509
71. Böing AN, Van Der Pol E, Grootemaat AE, Coumans FAW, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles (2014) 3:23430. doi:10.3402/jev.v3.23430
72. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics (2017) 7:789–804. doi:10.7150/thno.18133
73. Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano (2015) 9:2321–7. doi:10.1021/nn506538f
74. Li P, Mao Z, Peng Z, Zhou L, Chen Y, Huang P-H, et al. Acoustic separation of circulating tumor cells. Proc Natl Acad Sci U S A (2015) 112:4970–5. doi:10.1073/pnas.1504484112
75. Sitar S, Kejžar A, Pahovnik D, Kogej K, Tušek-Žnidarič M, Lenassi M, et al. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal Chem (2015) 87:9225–33. doi:10.1021/acs.analchem.5b01636
76. Kang D, Oh S, Ahn S-M, Lee B-H, Moon MH. Proteomic analysis of exosomes from human neural stem cells by flow field-flow fractionation and nanoflow liquid chromatography−tandem mass spectrometry. J Proteome Res (2008) 7:3475–80. doi:10.1021/pr800225z
77. Petersen KE, Manangon E, Hood JL, Wickline SA, Fernandez DP, Johnson WP, et al. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem (2014) 406:7855–66. doi:10.1007/s00216-014-8040-0
78. Wang Z, Wu H-J, Fine D, Schmulen J, Hu Y, Godin B, et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip (2013) 13:2879–82. doi:10.1039/c3lc41343h
79. Akers JC, Ramakrishnan V, Nolan JP, Duggan E, Fu C-C, Hochberg FH, et al. Comparative analysis of technologies for quantifying extracellular vesicles (evs) in clinical cerebrospinal fluids (CSF). PLoS One (2016) 11:e0149866. doi:10.1371/journal.pone.0149866
80. Chia BS, Low YP, Wang Q, Li P, Gao Z. Advances in exosome quantification techniques. Trends Anal Chem (2017) 86:93–106. doi:10.1016/j.trac.2016.10.012
81. Koritzinsky EH, Street JM, Star RA, Yuen PST. Quantification of exosomes. J Cell Physiol (2017) 232:1587–90. doi:10.1002/jcp.25387
82. Rupert DLM, Claudio V, Lässer C, Bally M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim Biophys Acta (2017) 1861:3164–79. doi:10.1016/j.bbagen.2016.07.028
83. Jiao J, Milwid JM, Yarmush ML, Parekkadan B. A mesenchymal stem cell potency assay. Methods Mol Biol (2011) 677:221–31. doi:10.1007/978-1-60761-869-0_16
84. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity (2016) 44:450–62. doi:10.1016/j.immuni.2016.02.015
85. Lamparski HG, Metha-Damani A, Yao J-Y, Patel S, Hsu D-H, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods (2002) 270:211–26. doi:10.1016/S0022-1759(02)00330-7
86. Navabi H, Croston D, Hobot J, Clayton A, Zitvogel L, Jasani B, et al. Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol Dis (2005) 35:149–52. doi:10.1016/j.bcmd.2005.06.008
87. Placzek MR, Chung I-M, Macedo HM, Ismail S, Mortera Blanco T, Lim M, et al. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface (2009) 6:209–32. doi:10.1098/rsif.2008.0442
88. Heathman TRJ, Nienow AW, Mccall MJ, Coopman K, Kara B, Hewitt CJ. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med (2015) 10:49–64. doi:10.2217/rme.14.73
89. Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials (2016) 105:195–205. doi:10.1016/j.biomaterials.2016.07.003
90. Vishnubhatla I, Corteling R, Stevanato L, Hicks C, Sinden J. The development of stem cell-derived exosomes as a cell-free regenerative medicine. J Circ Biomark (2016) 3:2. doi:10.5772/58597
91. Lőrincz ÁM, Timár CI, Marosvári KA, Veres DS, Otrokocsi L, Kittel Á, et al. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J Extracell Vesicles (2014) 3:25465. doi:10.3402/jev.v3.25465
92. Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med (2005) 3:10–10. doi:10.1186/1479-5876-3-10
93. Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of exosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med (2005) 3:9. doi:10.1186/1479-5876-3-9
94. Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia (2014) 28:970–3. doi:10.1038/leu.2014.41
95. Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther (2008) 16:782–90. doi:10.1038/mt.2008.1
96. Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology (2016) 5:e1071008. doi:10.1080/2162402X.2015.1071008
Keywords: exosomes, extracellular vesicles, exosome-based therapeutics, mesenchymal stem cells, preclinical
Citation: Willis GR, Kourembanas S and Mitsialis SA (2017) Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency. Front. Cardiovasc. Med. 4:63. doi: 10.3389/fcvm.2017.00063
Received: 10 July 2017; Accepted: 25 September 2017;
Published: 09 October 2017
Edited by:
Elena Aikawa, Brigham and Women’s Hospital, United StatesReviewed by:
Rajeev Malhotra, Massachusetts General Hospital, United StatesCopyright: © 2017 Willis, Kourembanas and Mitsialis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Alex Mitsialis, YWxleC5taXRzaWFsaXNAY2hpbGRyZW5zLmhhcnZhcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.