
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci. , 03 February 2025
Sec. Human-Wildlife Interactions
Volume 6 - 2025 | https://doi.org/10.3389/fcosc.2025.1470223
This article is part of the Research Topic Mapping Human-Wildlife Conflicts: Understanding and Predicting Spatial Patterns View all articles
 Malyasri Bhattacharya1
Malyasri Bhattacharya1 Debanjan Sarkar1
Debanjan Sarkar1 Sneha Pandey1
Sneha Pandey1 Indranil Mondal2
Indranil Mondal2 Sambandam Sathyakumar3
Sambandam Sathyakumar3 R. Suresh Kumar3
R. Suresh Kumar3 Gautam Talukdar1*
Gautam Talukdar1*The Asiatic black bear (Ursus thibetanus), classified as a vulnerable species on the IUCN Red List, is an important mammal species found in the state of Sikkim, India. Studies carried out in Khangchendzonga National Park have documented the presence of these bears, highlighting their crucial conservation importance in the region. The population of Black bears are restricted to small habitat patches, which over the years have become fragmented by road networks and urban settlements. In such fragmented landscapes, connecting corridors play a crucial role in maintaining wildlife movement and genetic diversity. We assessed connectivity between eight protected areas in Sikkim using MaxENT and Circuitscape. 65 black bear presence locations (collected through Camera traps and sign surveys) and 24 environmental variables were used to model the corridors. Habitat suitability map was generated through MaxENT modelling approach. Our analysis suggests that there are multiple options to maintain connectivity for black bears in Sikkim. We mapped seven corridors and five pinch points (bottlenecks in connectivity), and calculated metrics to estimate their quality and importance. Our model output was supported by high AUC value (0.921) and field validation by questionnaire surveys and sign surveys to assess black bear presence and habitat use. Our results showed that 300 km² of the suitable regions are within the protected areas in Sikkim. The highest quality linkages as measured by the ratio of cost-weighted distance to Euclidean distance (CWD:EucD) and cost-weighted distance to least-cost path (CWD:LCP) were Khangchendzonga and Barsey, suggesting that these protected areas (National Parks and Wildlife Sanctuaries) and the developed corridors play important role in maintaining connectivity. We mapped pinch-points which are habitat where black bear movement is restricted due to unfavorable environments, linear infrastructures, built up/settlements or a combination of factors and our model predicted pinch points near few settlement areas; Mangan, Dikchu, Pangthang, Kabi, Yuksum and Lachen. Ground truthing confirmed that these areas also coincide with Black bear conflict zones in Sikkim.
Protected areas (PAs) are crucial for conservation but individual PAs may be too small to support stable populations of large wide-ranging mammals (Mohammadi et al., 2021; Rezaei et al., 2022; Dutta et al., 2005). The rapid conversion of natural habitats outside protected areas is leading to habitat fragmentation and isolation of PAs (DeFries et al., 2005). Connectivity corridors are crucial for the long-term viability of a species as they facilitate the species movements from one habitat to another to maintain gene flow. Some animals exhibit a one-time movement such as ‘dispersal from natal habitat’ (e.g., tiger). Also, some animals exhibit regular (seasonal) movements (to and like migration (e.g., elephant, several species of birds, etc.) (Nayeri et al., 2022). Fragmented and altered habitats have modified and decreased connectivity for species, thereby restricting the species’ movement. Globally, many connectivity studies have focused on a single species or groups of closely related taxa (Haddad et al., 2003; Ersoy et al., 2019; Brennan et al., 2020; Lookingbill et al., 2022).In Iran and Iraq studies have been focused on Brown bear and Persian leopard habitat connectivity (Ashrafzadeh et al., 2020; Kaszta et al., 2021). Corridors have been identified for ‘flagship species’ such as the Giant panda (Ailuropoda melanoleuca) (Wang et al., 2014; Hou et al., 2014), Red panda (Ailurus fulgens) (Tobgay and Mahavik, 2020), and Bengal tiger (Panthera tigris) (Yumnam et al., 2014; Mondal et al., 2016). Various approaches are being advanced to model connectivity for different species, each offering unique insights into how animals move across landscapes. One common method involves habitat suitability models, which identify areas most favorable for a species based on environmental factors such as food availability, cover, and terrain. These models are often paired with least-cost path analysis, a technique that predicts the most efficient movement corridors by estimating the easiest or least ‘costly’ routes for animals to traverse between key habitats, avoiding barriers like roads or developed areas. On the other hand, landscape connectivity models such as Circuitscape take a more comprehensive approach by simulating multiple potential movement paths. Unlike least-cost path analysis, Circuitscape ensures redundancy in corridor identification by accounting for various pathways an animal might use, including those that may not be the most direct but are still critical for long-term connectivity. This multi-path simulation is particularly important for maintaining ecological resilience, as it helps safeguard against the disruption of a single corridor due to environmental changes or human activities. Genetic studies assess gene flow between fragmented populations, revealing how habitat connectivity affects genetic diversity. The creation of wildlife corridors, including natural habitat linkages and artificial structures like overpasses and underpasses, allows safe passage across human-altered landscapes, particularly around roads. Remote sensing and satellite imagery further enhance the understanding of land-use changes and their impact on corridor connectivity, while community-based conflict mitigation strategies in agricultural and livestock-dominated areas help minimize human-wildlife conflicts (Koen et al., 2014; Brodie et al., 2015; Choe et al., 2017). In Sikkim, over 47.08% of the total geographical area is forested (India State of Forest Report, 2021), yet there is limited information on the connectivity between habitat patches outside protected areas, where anthropogenic activities have a substantial effect. This is particularly concerning given that four of the eight bear species found globally are native to the Indian subcontinent. Among these, the Asiatic black bear (Ursus thibetanus) has been prioritized for conflict mitigation efforts due to its frequent interactions with humans (Can et al., 2014). Understanding the connectivity between habitats, especially outside protected areas, is essential for effective conservation and conflict reduction for this species. The species is listed globally as Vulnerable in the IUCN (A2CD) Redlist, Appendix I in CITES, and Schedule II of Indian Wildlife (Protection) Act, 1972. A significant level of variation in distribution (Figure 1) exists for Asiatic black bears, much of which is attributed to differences in habitat types, climate, food availability, topography, and other geographical differences (Sathyakumar and Choudhary, 2007; Bashir et al., 2018). Most of the suitable habitats for U. thibetanus are in forested mountain habitats in the Indian Himalayan region and hills of northeast of India up to treeline (4300 m in eastern Himalaya) characterized by inaccessible terrains, thick understory vegetation, abundant food resources, and good denning sites (Sathyakumar, 2001; Sathyakumar and Choudhary, 2007; Sathyakumar et al., 2012; Bista and Aryal, 2013). The unavailability of food resources often compels Black bears to move into anthropogenic areas, leading to increased human-wildlife conflicts (Sharma et al., 2010; Bashir et al., 2018). Asiatic black bear requires expansive areas to sustain viable populations, with home range estimates of 107.23 km² for males and 49.53 km² for females in the Kashmir Himalaya, India (Sharma et al., 2010). Telemetry studies, such as those conducted in China (Reid et al., 1991), Japan (Ohsako, 1995), and India (Ashraf, 2008), have been instrumental in providing detailed insights into the home range, habitat use, and movement patterns of black bears (Charoo et al., 2011). These studies help in understanding how black bears utilize large landscapes, which is crucial for their conservation, as they highlight the spatial requirements necessary for population viability and inform strategies for habitat protection and connectivity (Marifatul Haq et al., 2022).
Black bears in Himalaya rely on Climatic information to use the habitat connectivity as they inhabit a wide range of elevations, from subtropical forests to alpine regions, where temperature, precipitation, and seasonal variability greatly influence habitat availability and food resources. Climatic factors such as temperature and moisture levels directly affect vegetation types and abundance, which are critical for the bear’s diet and shelter. Black bears exhibit seasonal migrations in response to climate-driven changes in food availability. For instance, in higher elevations, bears often move to lower altitudes during winter months to escape harsh climatic conditions. These seasonal shifts highlight the importance of climate in determining the timing and routes of their movements, and thus habitat connectivity. Thus, climate plays a significant role in determining the bear’s movement patterns and habitat use.
With this background information, the objectives of this study were: 1) to delineate connectivity corridors for Asiatic black bears in Sikkim, identifying areas that ensure long-term connectivity between protected areas, and 2) to identify pinch points where human-black bear interactions are more frequent. The research aimed to address two key questions: (1) What are the critical habitat corridors that facilitate connectivity between protected areas for Asiatic black bears in Sikkim? and (2) Which areas in Sikkim experience the highest frequency of human-black bear conflicts? The underlying hypothesis is that Asiatic black bears use specific natural corridors, such as riparian zones or forest patches, to move between protected areas, and that these corridors are disrupted by human activities like deforestation and infrastructure development.
Sikkim, a Himalayan state spanning 7,096 km² in north-eastern India (Figure 1), has 47.08% forest cover spanning for 3,341.03 km² (India State of Forest Report, 2021). Sikkim is within the Global 200 Ecoregions (Olson et al., 2001) and the Eastern Himalaya biodiversity hotspot (Myers et al., 2000). In terms of climate- the state experiences an annual rainfall ranging from 2000 mm to 4000 mm, peaking in June-August. Its elevation varies from 270m to 8596m, the highest point being Mt. Khangchendzonga. The state is home to eight protected areas (PAs), including one National Park (NP) and seven Wildlife Sanctuaries (WLS), covering a combined area of 3330.28 km², which constitutes 46.93% of the state’s landmass (Figure 1; Table 1). The state’s forest cover includes subtropical forests, located at lower elevations (below 1500m), which are characterized by a mix of broadleaf species. Moving higher, temperate forests dominate between 1500m and 3500m, where oak, rhododendron, and coniferous species are prevalent. At the highest altitudes, alpine forests and meadows, found above 4000m, are sparse and adapted to harsh conditions, providing crucial seasonal foraging grounds for species such as the black bear during summer months. These habitat types are vital for maintaining the ecological corridors that allow species to migrate between seasonal ranges.
Land use in Sikkim, primarily centered on agriculture, tourism, and livestock rearing, plays a significant role in shaping these wildlife corridors. Shifting cultivation and terrace farming are common, especially in subtropical regions, potentially leading to habitat fragmentation and corridor disruption. The growing tourism sector, concentrated around key protected areas, adds further pressure through the expansion of infrastructure such as hotels and recreational areas, which encroach on wildlife habitats. Livestock grazing, especially in higher-elevation temperate and alpine regions, can lead to competition for resources between domestic animals and wildlife, increasing the risk of human-wildlife conflict (Basnett et al., 2021).
Moreover, human settlements and infrastructure, present significant challenges for corridor connectivity (Chanchani et al., 2010; Das et al., 2013). Roads like NH10, which connects Sikkim to the rest of India, and other smaller roads through forested areas, create barriers to wildlife movement and increase the likelihood of roadkill. The planned expansion of such infrastructure in the state, driven by tourism and economic growth, threatens to further fragment wildlife corridors unless mitigated by wildlife-friendly design solutions, such as underpasses or overpasses, are implemented.
The primary livelihoods of the local population are majorly tourism, agriculture, and livestock rearing. The Lepcha, Bhutia, and Nepalese communities rely on the forests for various resources such as fuelwood, ferns, timber, medicinal plants, and fodder (Basnett et al., 2020).
This study used MaxENT (through kuenm package), and Circuitscape software to map corridor connectivity and bottlenecks among eight protected areas (PAs) for black bears in Sikkim. Circuitscape applies circuit theory, modeling the landscape as a continuous resistance surface where movement mimics current flow. Low-resistance areas facilitate movement (e.g., suitable habitats), while high-resistance areas hinder it (e.g., urban areas or barriers) (Dickson et al., 2019). While a large portion of Sikkim offers suitable habitats for black bears, the PAs were selected as nodes for the species due to their suitability as habitats and the legal protection they offer to the species and its habitat.
Field surveys and interaction with local communities across Sikkim helped gather data on black bear presence and conflict. Using 65 black bear occurrence points and 24 environmental and bioclimatic variables, a habitat permeability layer was generated in R studio with the kuenm package (Cobos et al., 2019). The Habitat permeability layer highlights the ease of species movement through the landscape, focusing on habitat features influencing dispersal, unlike species distribution maps that show geographic range.
The permeability layer was then used as an input in Circuitscape and Linkagemapper in ArcGIS software to model corridors and delineate pinch points among the eight PAs in Sikkim. Ground surveys were conducted to validate the quality of these corridors and pinch points, while also identifying potential causes of conflict.
We have used the ‘Kuenm’ package in R version 3.6.3 to calibrate, evaluate and build the habitat permeability layer for black bears in the Sikkim landscape. Figure 2 shows the methodological flowchart followed during modelling.
We used 48 camera trap locations (deployed in different PAs and data available for the years 2009, 2016, and 2017) (Bashir et al., 2018) and 17 indirect evidences (claw or rake marks on tree barks, locations of conflicts, and validated bear interface locations collected from the field in 2019, Supplementary Figure 1) as presence points for running the models. Details of the camera trapping methods can be found in WII-DST-NMSHE (2020). To account for potential spatial bias, we generated a spatial bias surface using background sampling that reflects the sampling effort and accessibility across the study area. This bias surface is derived from the density of presence records and corrected for areas with over-representation, ensuring that spatial bias does not unduly influence the predictions.
A total of 65 species occurrence points (represented in Figure 1) are used to model a habitat permeability layer. The presence points were split into training (70%) and testing (30%) for building the model using random splitting to ensure that the training and test datasets were representative of the overall distribution of presence points across the study area.
Initially, we downloaded and processed 38 variables (Supplementary Table 1)-19 Bioclim layers (Near present), 12 NDVI (Normalized Difference Vegetation Index) layers (2017), elevation, aspect, distance from forests, nightlight (as a surrogate of human presence), distance from PAs, distance from rivers. Using the Pearson correlation test in SDMToolbox (Brown et al., 2017) we identified and removed the correlated layers (R> 0.70) (Supplementary Figure 2) giving us 24 layers to prepare the habitat permeability layer (Table 2). All layers were resampled into 90 meters, similar extent, projection and converted in ASCII format using SDMtoolbox (Brown et al., 2017).
We executed model calibration with ten replicates, bootstrap replicate type and clog log output for the final model building. In total,186 candidate models were created by combining six values of the regularization multiplier (0.5, 1, 2, 3, 4, 5) and 29 possible combinations of feature classes (linear = l, quadratic = q, product = p, threshold = t, and hinge = h). The ‘regularization multiplier’ addresses the issue of model overfitting by limiting the complexity of the model and generating a less localized prediction (Phillips and Dudík, 2008). Different feature classes in MaxENT impose different constraints upon estimated species distribution (Elith et al., 2011). The best model was statistically significant with low omission rates and low Akaike information criterion (AICc).
We evaluated the results of the MaxENT model using the AUC value (Area under Receiver Operating Characteristic Curve). A high AUC value reflects that the model prediction is non-random and can accurately map locations where the species is present or absent. The final output was then converted to a friction layer using the SDM toolbox in ArcGIS and used as a resistance surface for Circuitscape.
Circuitscape uses circuit theory to predict animal movement patterns between fragmented or heterogeneous landscapes (McRae et al., 2008). In circuit theory, the landscape is conceptualized as an interconnected network of habitat patches, with each patch representing a node in the circuit. The flow of current through the circuit represents the movement of organisms across the landscape, with the resistance of each habitat patch influencing the ease or difficulty of movement between patches. We used the National Parks and Wildlife Sanctuaries of Sikkim as our focal node locations, which represent larger landscape features that encompass multiple habitat patches, ensuring they are significantly larger than the black bears’ estimated home range and daily movement patterns. One-to-all modelling method was used in Circuitscape V4.0 (McRae et al., 2008) to model the corridors for black bears in Sikkim Landscape. The friction layer created using MaxENT output was used as a resistance surface for delineating the pinch points. We modified the source strength values of the focal nodes (Default value: 1) based on field data, PAs with higher incidents of black-bear interface were given higher source strength value (closer to 1) and lower source strength signifies less black-bear interface in and around the PA (Table 1).
To map connectivity between PAs in the landscape, we used tools that integrate least-cost path (LCP) approaches with circuit theory. We employed the program Linkage Mapper (McRae and Kavanagh, 2011) to map corridors and LCPs between pairs of adjacent PAs. Linkage Mapper identifies adjacent core areas, creates a network of these core areas using adjacency and distance data, calculates cost-weighted distances and least-cost paths, and combines least-cost corridors into a single map. The least-cost path is the route with the minimum cost-weighted distance between a source and a destination (Adriaensen et al., 2003).
We calculated two metrics to evaluate the quality of each linkage. The first metric is the ratio of cost-weighted distance (CWD) and the Euclidean distance (EucD) between each pair of PAs. For the highest quality linkage, the cost-weighted distance equals the Euclidean distance, resulting in a ratio of 1. This ratio reflects the difficulty of moving between PAs relative to their proximity. The second metric is the ratio of the cost-weighted distance to the length of the least-cost path (CWD: LCP), indicating the average resistance encountered along the optimal path between the PAs.
Once corridors were mapped, we used the ‘Pinchpoint Mapper’ tool in Linkagemapper 2.0 to map the pinch points or corridor bottlenecks where movement would be funneled, and thus, it may be essential to keep them intact. Even a slight area loss in these pinch points would disproportionately compromise connectivity (Castilho et al., 2015). We used a cut-off width of 1 km to create the Pinch points.
The model performance was evaluated based on statistical significance of AUC value, omission rates (OR), and the AICc values. At first, multiple iterations were done and more than 20 models were generated using various combinations of covariates and occurrence points. Candidate models with the lowest omission rate (at 5%) were selected and the final model was the one with the lowest AICc value out of these models. Our final selected model had an AICc value of 1627.332 (ΔAIC=0) with an omission rate of 5%= 0.056, regularization multiplier = 1, and selected feature class = threshold. We used Area Under the ROC Curve (AUC) values as the measure of our model performance, AUC value >0.7 is considered an indicator of good performance in MaxENT models (Ancillotto et al., 2020; Araújo and New, 2007). The final model’s AUC value was 0.921 (Supplementary Figure 5), indicating an excellent output (Su et al., 2021; Bai et al., 2018).
Jackknife tests of variable importance were used to identify those with significant individual effects. Major contributing variables of the model were Bio12-Annual precipitation (19.2%), Bio07- Annual temperature range (14.4%), and slope (11.7%). The contribution of the remaining 21 variables was <10% each (Table 2). The Jackknife test results and response curve of the final models are given in (Supplementary Figures 3, 4). The values are averages over 10 replicate runs.
The eight PAs in Sikkim vary significantly in their area (Table 1) and our MaxENT output (Figure 3) showed that most of the suitable areas for the species lie within and around the PA boundaries in Sikkim, 300 km² of the suitable regions are within the protected areas in Sikkim. There are four districts in Sikkim and based on the output, Black bears’ suitable habitats are located in distinct patches across West, East, and North districts. The modelled species’ presence was between 1400m to 3000m elevation. The output was converted into a resistance layer in ArcGIS using SDMtoolbox (Brown et al., 2017) for modelling corridors and Pinchpoint.
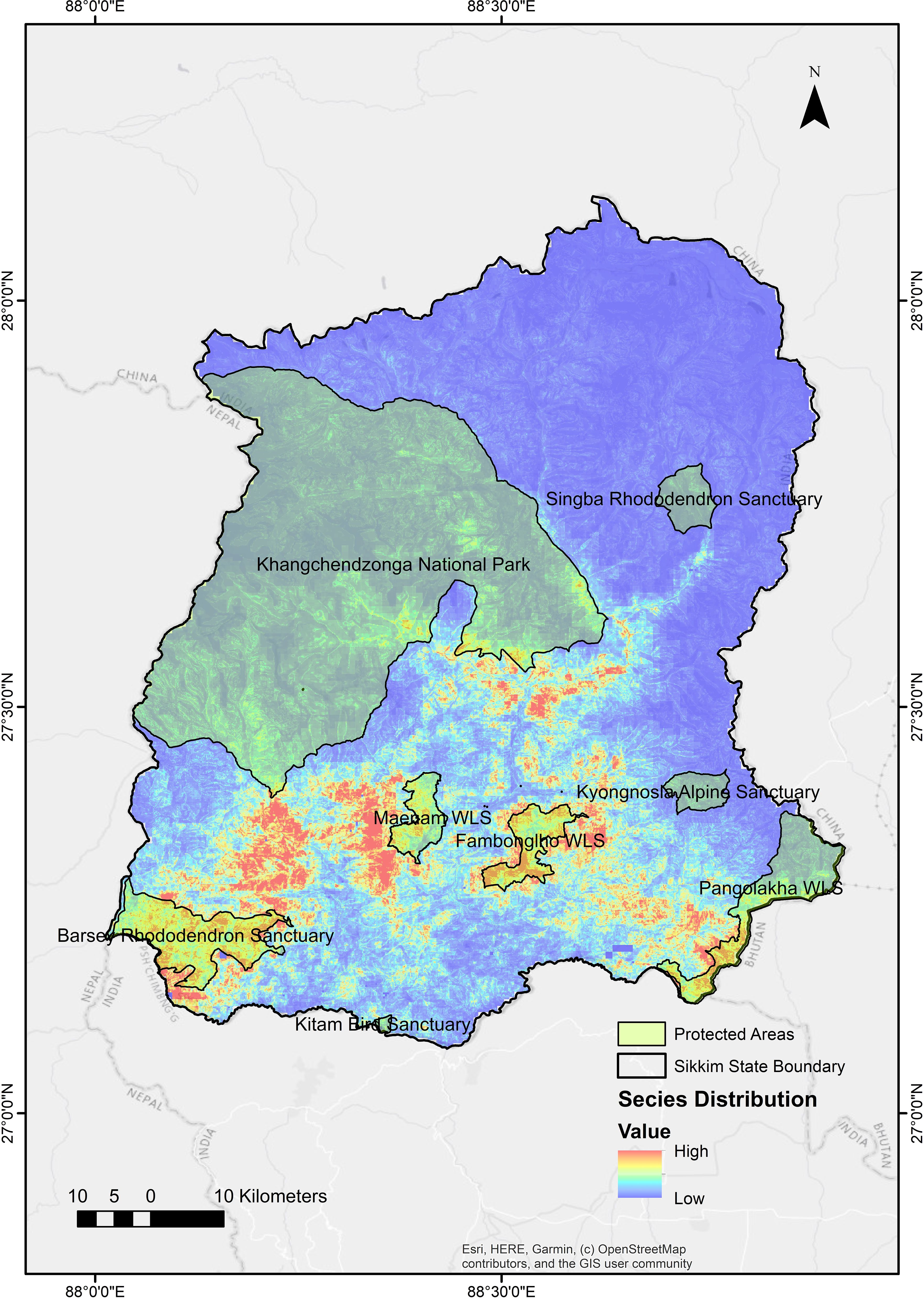
Figure 3. Suitable areas for the species within and around the protected areas boundaries in Sikkim.
This study provides the first account of the potential connectivity corridors and pinch points between different PAs for black bears within Sikkim. We have delineated eight corridors across PAs through different landscapes. The corridors (Figure 4) between Singba Rhododendron Sanctuary and Khangchendzonga NP; Fambong Lho WLS and Kyongnosla Alpine Sanctuary; and Khangchendzonga NP, Barsey Rhododendron Sanctuary, and Maenam WLS have the potentially high current flow based on the model, indicating critical pathways for movement of the species.
The model exhibited the presence of pinch points between each pair of PAs (focal nodes), illustrating crucial areas for keeping the habitat connected. We identified fifteen pinch points in the corridors we mapped (Figure 5). Analyzing the corridors between individual pairs of PAs highlights areas with the highest pairwise current flow, indicating constricted movement pathways between two PAs.
Fifteen linkages were identified (Table 3) and the pinch points within reserve forest areas receive protection from the forest department. These pinch points are: 1. Kyongnosla WLS- Pangolakha WLS; 2. Khangchendzonga NP and Kyongnosla WLS; 3. Maenam WLS and Khangchendzonga NP.
In corridor analysis, Euclidean distance represents the straight-line distance between two points, commonly used as a baseline for spatial comparison (Seegmiller and Shirabe, 2023). The Euclidean distance (EucD) between various protected areas (PAs) or focal nodes ranged from 6.56 km to 33 km (Table 3). Cost-weighted distance (CWD), on the other hand, accounts for the resistance or cost associated with traversing different terrain types, such as land cover, elevation, and road density, to identify the most efficient path between locations (Seegmiller et al., 2021). In our model, the CWD ranged from 52.34 km to 339.3 km. The Least Cost Path (LCP), ranging from 7.39 km to 36.28 km, identifies the path with the lowest cumulative cost between two points, factoring in terrain and resistance values. LCP is a critical tool for designing wildlife corridors, promoting species movement and survival in urban landscapes (Cohen et al., 2009; Balbi et al., 2021). These concepts are essential in corridor analysis, helping to determine optimal connectivity by balancing spatial distance, terrain features, and resistance values to establish effective pathways for wildlife and ecosystems.
The linkages metric varied between different pairs of PAs. The ratio of CWD: EucD is the lowest (3.74) (Table 3) between Khangchendzonga NP and Barsey Rhododendron WLS, indicating the highest quality along the shortest path for this pair. This ratio is highest (11.35) for Kyongnosla Alpine Sanctuary and Pangalokha WLS, meaning movement is difficult between different PAs after accounting for Euclidean distance. For example- For example, although Kyongnosla-Pangalokha and Fambonglho-Maenam are similar in terms of Euclidean distance (6.75 and 6.56 respectively), the cost of moving between the former is much higher than the latter (CWD: EucD ratio 11.35 and 7.97, respectively).
The ratio of CWD: LCP is the lowest between Khangchendzonga NP and Barsey Rhododendron WLS (CWD: LCP = 3.3) indicating low resistance to movement along the path of lowest resistance, and it is highest between Kyongnosla and Pangalokha (CWD: LCP = 10.37), indicating high resistance along the path of least resistance.
Species survival depends on their ability to move, adapt to changing conditions, and fulfill seasonal needs, which often extend beyond protected areas (PAs). According to some recent studies habitat loss and fragmentation has accelerated the global extinction crisis (Marifatul Haq et al., 2022), and conservation resources remain limited, therefore it is crucial to identify and prioritize the most critical areas for conservation efforts (Kaszta et al., 2020). Black bears, for example, move across different elevational gradients in search of resources (Izumiyama and Shiraishi, 2004; Sathyakumar and Choudhary, 2007). The Asiatic black bear can navigate human-modified landscapes, such as orchards and tea gardens, for feeding and moving between areas. These areas can serve as corridors, used both day and night, underscoring the importance of PAs and nearby habitats like Reserved Forests (Sathyakumar and Choudhary, 2007). Thus, a well-connected network of PAs is crucial for species survival. Recent research at global level supports the role of corridors in maintaining species viability (Ashrafzadeh et al., 2020; Mohammadi and Almasieh 2022). Connectivity corridors are vital for preserving gene flow through interpopulation dispersal between isolated habitat patches (Hanski and Ovaskainen, 2000), which positively affects demographic factors and metapopulation dynamics (Hanski, 1998).
Three delineated corridors (i.e., 1. Kyongnosla WLS- Pangolakha WLS; 2. Khangchendzonga NP and Kyongnosla WLS; 3. Maenam WLS and Khangchendzonga NP) fall within reserve forests and have legal protection. The remaining corridors and pinch points (Figures 4, 5) are outside PAs and reserve forests mentioned below.
Fambhonglho WLS and Khangchendzonga NP (Figures 4, 5A): Our model identified Pinchpoint near two significant human habitats, Mangan and Dikchu. Ground surveys in the two areas confirmed indirect black bear sightings and incidents of human-black bear interface. The number of bear attacks is also high in Singtam, Kazor, and Singhik.
Fambong Lho WLS – Kyongnosla Alpine WLS (Figures 4, 5B): This corridor is close to the state capital, Gangtok. Expansion of urban areas, tourist pressure, and road networks potentially threaten this corridor. The pinchpoint in this corridor is near Pangthang and Kabi. Locals of the area confirmed black bear interface incidents, mainly near agricultural lands. Another Pinchpoint is modelled near Golitar (Near Gangtok). Through ground survey and camera traps, we found direct and indirect evidence of black bear and conflict incidents (with humans and livestock) in the Golitar area.
Khangchendzonga NP and Barsey Rhododendron WLS (Figures 4, 5C): This corridor is in West Sikkim and is close to Yuksum and Pelling. The model corridor pinch point is near Yuksum. We found major human-animal interface incidents reported near the southern buffer range of Khangchendzonga NP. The corridors and pinch points are adjacent to Gerethang reserve forests in west Sikkim. High human-bear interface incidences are reported near Meli, Geyzing, and Singyan. Tourism pressure is a significant threat to this corridor, particularly in areas near Geyzing and Pelling.
Khangchendzonga NP and Shingba WLS (Figure 4A): This corridor lies in the North Sikkim near Chungthang and Lachung, connecting Singhba with Eastern parts of Khangchendzonga NP. Locals of Lachung and Chungthang reported interface incidents that happened mainly near maize fields. Maximum number of bear attacks were reported in Lachung, Khedum, Beechu, Bop, Bheemnala, and Lingtem.
Urban expansion poses a significant challenge to conserving corridors, as road networks increasingly fragment the modeled corridors outside protected areas (PAs) and reserved forests (RFs) due to ongoing development activities. Millet and barley fields often serve as black bear interface zones, where local villagers are vulnerable to attacks while protecting their crops and livestock (Abbas et al., 2015; Srivastava and Tyagi, 2016; Jamtsho and Wangchuk, 2016).
The black bears in this landscape frequently encounters humans outside protected areas (PAs) (Bashir et al., 2018). Although 46.93% of Sikkim’s land falls within PAs, human-black bear interactions have increased over the past 10-15 years (Basnett et al., 2020). This rise in conflicts can be attributed to several factors: 1. Failure in mast production within PAs and reserved forests, likely due to climate change. For example, 2009, a drought year with the lowest rainfall in over 40 years, also saw a surge in human-bear conflicts (Rahman et al., 2012). Most conflict zones are in agricultural areas and settlements. There have also been reports of human-bear encounters near major cities in Sikkim (Jamwal, 2018), likely due to large garbage dumps near human settlements (Ghosh, 2018); 2. Increasing urbanization and tourism; and 3. Shortened hibernation periods for black bears at higher elevations, influenced by climate change (Sharma et al., 2010).
The modelled corridors, especially the corridors and pinch points beyond PAs and reserve forests, need to be extensively surveyed to develop a strategic conservation plan to ensure the species’ dispersal and survivability and minimize the human-black bear conflict. Furthermore, a study on the movement ecology of black bears using satellite telemetry is required to understand the functionality of these modelled corridors, identify the spatial and habitat requirements, and detect the hibernation pattern of the species.
The survival of Asiatic black bear is linked to their ability to move across landscapes that extend beyond protected areas (PAs), highlighting the importance of connectivity corridors for facilitating movement, resource access, and gene flow. Habitat loss and fragmentation, driven by urbanization, agriculture, and tourism, threaten these corridors and increase human-wildlife conflicts, particularly between black bears and local communities. Our study identifies critical corridors and pinch points, many of which are outside PAs and reserved forests, emphasizing the need for strategic conservation planning in these areas. Ground surveys and local reports confirm the high frequency of human-black bear conflicts, underlining the urgency of incorporating community-based conservation efforts. Protection of corridors for a large mammal will aid in dispersing other flora and fauna. There are multiple incidents of Human-bear conflict (i.e., crop damage, livestock damage, casualty) outside PAs, especially in the bottleneck corridors identified in this study. We recommend extensive surveys (i.e., camera trapping, sign surveys, scat analysis (for population estimation as well as diet study), interaction with local people for prevention and management of the conflicts) in these bottleneck areas to create habitat management plans to ensure safe dispersal of the species among different PAs. Black bear food plants could be planted in a planned way outside the PA boundaries and along the corridors to reduce human-bear interface to ensure possible movement of black bears inside and outside the PAs. Use of traditional and modern crop protection measures, and sensitization workshops for the locals to be accorded high priority. Informing the potential conflict locations, ways to improve habitats outside PA and available solutions that are essential strategies in the overall restoration effort. Further studies can be carried out to check the transboundary connectivity through satellite telemetry and corridor modelling. As Sikkim shares international boundaries with Bhutan, China, and Nepal, transboundary landscape connectivity will enable genetic dispersal across geopolitical boundaries and maintain Asiatic black bears’ continuous movement in the event of habitat shift or expansion owing to future climate change. The integration of ecological corridors into conservation planning, coupled with efforts to mitigate human-wildlife conflicts, will be crucial for ensuring the long-term survival of black bears in the region.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because this research work focuses solely on spatial mapping and ground validation of connectivity between different PAs for Black bears and does not involve any clinical research on animals or any form of medical research on humans. Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
MB: Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. DS: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. SP: Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. IM: Writing – original draft. SS: Writing – review & editing. RSK: Writing – original draft. GT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the funding agency United Nations Development Programme (UNDP) and Swiss Agency for Development and Cooperation (SDC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2025.1470223/full#supplementary-material
Abbas F. I., Bhatti Z. I., Haider J., Mian A. (2015). Bears in Pakistan: distribution, population biology and human conflicts. J. Bioresource Manage. 2, 1. doi: 10.35691/JBM.5102.0015
Adriaensen F., Chardon J. P., De Blust G., Swinnen E., Villalba S., Gulinck H., et al. (2003). The application of ‘least-cost’modelling as a functional landscape model. Landscape urban Plann. 64, 233–247. doi: 10.1016/S0169-2046(02)00242-6
Ancillotto L., Bosso L., Smeraldo S., Mori E., Mazza G., Herkt M., et al. (2020). An African bat in Europe, Plecotus gaisleri: Biogeographic and ecological insights from molecular taxonomy and Species Distribution Models. Ecol. Evol. 10, 5785–5800. doi: 10.1002/ece3.v10.12
Araújo M. B., New M. (2007). Ensemble forecasting of species distributions. Trends Ecol. Evol. 22, 42–47. doi: 10.1016/j.tree.2006.09.010
Ashraf N. V. K. (2008). Walking the bears: rehabilitation of Asiatic black bears in Arunachal Pradesh (Wildlife Trust of India), 126.
Ashrafzadeh M. R., Khosravi R., Adibi M. A., Taktehrani A., Wan H. Y., Cushman S. A. (2020). A multi-scale, multi-species approach for assessing effectiveness of habitat and connectivity conservation for endangered felids. Biol. Conserv. 245, 108523. doi: 10.1016/j.biocon.2020.108523
Bai W., Connor T., Zhang J., Yang H., Dong X., Gu X., et al. (2018). Long-term distribution and habitat changes of protected wildlife: giant pandas in Wolong Nature Reserve, China. Environ. Sci. pollut. Res. 25, 11400–11408. doi: 10.1007/s11356-018-1407-6
Balbi M., Croci S., Petit E. J., Butet A., Georges R., Madec L., et al. (2021). Least-cost path analysis for urban greenways planning: A test with moths and birds across two habitats and two cities. J. Appl. Ecol. 58, 632–643. doi: 10.1111/1365-2664.13800
Bashir T., Bhattacharya T., Poudyal K., Qureshi Q., Sathyakumar S. (2018). Understanding patterns of distribution and space-use by Ursus thibetanus in Khangchendzonga, India: Initiative towards conservation. Mamm. Biol. 92, 11–20. doi: 10.1016/j.mambio.2018.04.004
Basnett T., Timalsina N., Thapa R. B., Bista D., Pant B., Karky B. S., et al. (2020). Forest carbon stock assessment in selected red panda habitats in ilam and panchthar districts, nepal.
Basnett R., Kumar A., Vishwakarma A., Boro B. K. (2021). Seasonal diets of Asiatic black bear (Ursus thibetanus) in the Khangchendzonga National Park, Eastern Himalaya India. J. Natural History 55, 163–175. doi: 10.1080/00222933.2021.1899324
Bista R., Aryal A. (2013). Status of the Asiatic black bear Ursus thibetanus in the southeastern region of the Annapurna Conservation Area, Nepal. Zoology Ecol. 23, 83–87. doi: 10.1080/21658005.2013.774813
Brennan A., Beytell P., Aschenborn O., Du Preez P., Funston P. J., Hanssen L., et al. (2020). Characterizing multispecies connectivity across a transfrontier conservation landscape. J. Appl. Ecol. 57, 1700–1710. doi: 10.1111/1365-2664.13716
Brodie J. F., Giordano A. J., Dickson B., Hebblewhite M., Bernard H., Mohd-Azlan J., et al. (2015). Evaluating multispecies landscape connectivity in a threatened tropical mammal community. Conserv. Biol. 29, 122–132. doi: 10.1111/cobi.2015.29.issue-1
Brown J. L., Bennett J. R., French C. M. (2017). SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 5, e4095. doi: 10.7717/peerj.4095
Can Ö.E., D'Cruze N., Garshelis D. L., Beecham J., Macdonald D. W. (2014). Resolving human-bear conflict: A global survey of countries, experts, and key factors. Conserv. Lett. 7, 501–513. doi: 10.1111/conl.2014.7.issue-6
Castilho C. S., Hackbart V. C., Pivello V. R., dos Santos R. F. (2015). Evaluating landscape connectivity for Puma concolor and Panthera onca among Atlantic forest protected areas. Environ. Manage. 55, 1377–1389. doi: 10.1007/s00267-015-0463-7
Chanchani P., Rawat G. S., Goyal S. P. (2010). Unveiling a wildlife haven: status and distribution of four Trans-Himalayan ungulates in Sikkim, India. Oryx 44, 366–375. doi: 10.1017/S0030605310000293
Charoo S. A., Sharma L. K., Sathyakumar S. (2011). Asiatic black bear–human interactions around Dachigam National Park, Kashmir, India. Ursus 22 (2), 106–113. doi: 10.2192/URSUS-D-10-00021.1
Choe H., Thorne J. H., Hijmans R., Kim J., Kwon H., Seo C. (2017). Meta-corridor solutions for climate-vulnerable plant species groups in South Korea. J. Appl. Ecol. 54, 1742–1754. doi: 10.1111/jpe.2017.54.issue-6
Cobos M. E., Peterson A. T., Barve N., Osorio-Olvera L. (2019). kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ 7, e6281. doi: 10.7717/peerj.6281
Cohen Y., Amit-Cohen I., Cohen A., Shoshani M. (2009). Least cost path for green corridors delineation in metropolitan margins: The distance weighting effects. J. Spatial Sci. 54, 63–78. doi: 10.1080/14498596.2009.9635167
Das N., Chatterjee S., Roy U. (2013). Habitat-based ecological analysis of urban space in mountain environment: An appraisal for Gangtak town, Sikkim, India. Ecol. Environ. Conserv. 19, 707–715.
DeFries R., Hansen A., Newton A. C., Hansen M. C. (2005). Increasing isolation of protected areas in tropical forests over the past twenty years. Ecol. Appl. 15, pp.19–pp.26. doi: 10.1890/03-5258
Dickson B. G., Albano C. M., Anantharaman R., Beier P., Fargione J., Graves T. A., et al. (2019). Circuit-theory applications to connectivity science and conservation. Conserv. Biol. 33, 239–249. doi: 10.1111/cobi.13230
Dutta T., Sharma S., McRae B. H., Roy P. S., DeFries R. (2016). Connecting the dots: mapping habitat connectivity for tigers in central India. Regional Environ. Change 16, 53–67. doi: 10.1007/s10113-015-0877-z
Elith J., Phillips S. J., Hastie T., Dudík M., Chee Y. E., Yates C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity distributions 17, 43–57. doi: 10.1111/j.1472-4642.2010.00725.x
Ersoy O., Aydar E., Şen E., Gourgaud A. (2019). Contrasting fragmentation and transportation dynamics during the emplacement of Dikkartın rhyodacitic dome; Erciyes stratovolcano, central Turkey. Mediterr. Geosci. Rev. 1, 223–242. doi: 10.1007/s42990-019-00014-4
Ghosh S. (2018). Food drives bear-human conflict in Khangchendzonga Biosphere Reserve (Mongabay). Available online: at: https://india.mongabay.com/2018/05/food-drives-bear-human-conflict-in-khangchendzonga-biosphere-reserve/ (Accessed May 5, 2021).
Haddad N. M., Bowne D. R., Cunningham A., Danielson B. J., Levey D. J., Sargent S., et al. (2003). Corridor use by diverse taxa. Ecology 84, 609–615. doi: 10.1890/0012-9658(2003)084[0609:CUBDT]2.0.CO;2
Hanski I., Ovaskainen O. (2000). The metapopulation capacity of a fragmented landscape. Nature 404, 755–758. doi: 10.1038/35008063
Hou N., Dai Q., Ran J., Jiao Y., Cheng Y., Zhao C. (2014). A corridor design for the giant panda in the Niba Mountain of China. Chin. J. Appl. Environ. Biol. 20, 1039–1045. doi: 10.3724/SP.J.1145.2014.06003
Forest Survey of India. (2021). Indian state of forest report. Available online at: http://fsi.nic.in/details.php?pgID=forest-report-2021-details (Accessed April 1, 2021).
Izumiyama S., Shiraishi T. (2004). Seasonal changes in elevation and habitat use of the Asiatic black bear (Ursus thibetanus) in the Northern Japan Alps. Mammal Study 29, 1–8. doi: 10.3106/mammalstudy.29.1
Jamtsho Y., Wangchuk S. (2016). Assessing patterns of human–Asiatic black bear interaction in and around Wangchuck Centennial National Park, Bhutan. Global Ecol. Conserv. 8, 183–189. doi: 10.1016/j.gecco.2016.09.004
Jamwal N. (2018). Climate change exacerbates human-wildlife conflicts in sikkim. Third Pole 24. Available online at: https://dialogue.earth/en/nature/human-wildlife-sikkim/ (Accessed July 24, 2022).
Kaszta Ż., Cushman S. A., Macdonald D. W. (2020). Prioritizing habitat core areas and corridors for a large carnivore across its range. Anim. Conserv. 23, 607–616. doi: 10.1111/acv.12575
Kaszta Ż., Cushman S. A., Slotow R. (2021). Temporal non-stationarity of path-selection movement models and connectivity: an example of african elephants in kruger national park. Front. Ecol. Evol. 9, 553263.
Koen E. L., Bowman J., Sadowski C., Walpole A. A. (2014). Landscape connectivity for wildlife: development and validation of multispecies linkage maps. Methods Ecol. Evol. 5, 626–633. doi: 10.1111/mee3.2014.5.issue-7
Lookingbill T. R., Minor E. S., Mullis C. S., Nunez-Mir G. C., Johnson P. (2022). Connectivity in the urban landscape (2015-2020): who? Where? What? When? Why? And how? Curr. Landscape Ecol. Rep. 7, 1–14. doi: 10.1007/s40823-021-00068-x
Marifatul Haq S., Calixto E. S., Rashid I., Hussain Malik A., Kumar M., Ahmad Khuroo A. (2022). Anthropogenic pressure and tree carbon loss in the temperate forests of Kashmir Himalaya. Bot. Lett. 169, 400–412. doi: 10.1080/23818107.2022.2073259
McRae B. H., Dickson B. G., Keitt T. H., Shah V. B. (2008). Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 89, 2712–2724. doi: 10.1890/07-1861.1
McRae B. H., Kavanagh D. M. (2011). Linkage mapper connectivity analysis software (Seattle WA: The Nature Conservancy).
Mohammadi A., Almasieh K. (2022). Human-brown bear conflict in the southernmost part of its distribution in iran (Roshan kooh no-hunting area, fars province). J. Natural Environ. 75 (4), 539–550.
Mohammadi A., Almasieh K., Nayeri D., Ataei F., Khani A., López-Bao J. V., et al. (2021). Identifying priority core habitats and corridors for effective conservation of brown bears in iran. Sci. Rep. 11 (1), 1044.
Mondal I., Habib B., Talukdar G., Nigam P. (2016). Triage of means: Options for conserving tiger corridors beyond designated protected lands in India. Front. Ecol. Evol. 4, 133. doi: 10.3389/fevo.2016.00133
Myers N., Mittermeier R. A., Mittermeier C. G., Da Fonseca G. A., Kent J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Nayeri D., Mohammadi A., Zedrosser A., Soofi M. (2022). Characteristics of natural and anthropogenic mortality of an endangered brown bear population. J. Nat. Conserv. 70, 126288.
Olson D. M., Dinerstein E., Wikramanayake E. D., Burgess N. D., Powell G. V., Underwood E. C., et al. (2001). Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51, 933–938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Phillips S. J., Dudík M. (2008). Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31, 161–175. doi: 10.1111/j.0906-7590.2008.5203.x
Rahman H., Karuppaiyan R., Senapati P. C., Ngachan S. V., Kumar A. (2012). An analysis of past three decade weather phenomenon in the mid-hills of Sikkim and strategies for mitigating possible impact of climate change on agriculture. Climate Change Sikkim: Patterns impacts initiatives, 1–18.
Reid D., Jiang M., Teng Q., Qin Z., Hu J. (1991). Ecology of the Asiatic black bear (Ursus thibetanus) in Sichuan, China. Mammalia. 55 (2), 221–238. doi: 10.1515/mamm.1991.55.2.221
Rezaei S., Mohammadi A., Bencini R., Rooney T., Naderi M. (2022). Identifying connectivity for two sympatric carnivores in human-dominated landscapes in central iran. PloS One 17 (6), e0269179.
Sathyakumar S. (2001). Status and management of Asiatic black bear and Himalayan brown bear in India. Ursus, 21–29.
Sathyakumar S., Choudhary A. (2007). Distribution and status of the Asiatic black bear Ursus thibetanus in India. J. Bombay Natural History Soc. 104, 316–323.
Sathyakumar S., Rahul Kaul N. V. K., Ashraf A. M., Menon V. (2012). National Bear Conservation and Welfare Action Plan. (India: Ministry of Environment and Forests, Wildlife Institute of India, and Wildlife Trust of India).
Seegmiller L., Shirabe T. (2023). A method for finding a least-cost corridor on an ordinal-scaled raster cost surface. Ann. GIS 29, 205–225. doi: 10.1080/19475683.2023.2166585
Seegmiller L., Shirabe T., Tomlin C. D. (2021). A method for finding least-cost corridors with reduced distortion in raster space. Int. J. Geographical Inf. Sci. 35, 1570–1591. doi: 10.1080/13658816.2020.1850734
Sharma L. K., Charoo S. A., Sathyakumar S. (2010). “Investigations on the ecology and behaviour of Asiatic black bear using satellite telemetry. A case study from the Dachigam National Park, Kashmir, India,” in ENVIS bulletin: Wildlife & Protected Areas (Wildlife Institute of India, Dehradun), 86–94.
Srivastava R., Tyagi R. (2016). Wildlife corridors in India: Viable legal tools for species conservation? Environ. Law Rev. 18, 205–223. doi: 10.1177/1461452916662114
Su H., Bista M., Li M. (2021). Mapping habitat suitability for Asiatic black bear and red panda in Makalu Barun National Park of Nepal from Maxent and GARP models. Sci. Rep. 11, 14135. doi: 10.1038/s41598-021-93540-x
Tobgay S., Mahavik N. (2020). Potential habitat distribution of Himalayan red panda and their connectivity in Sakteng Wildlife Sanctuary, Bhutan. Ecol. Evol. 10, 12929–12939. doi: 10.1002/ece3.v10.23
Wang H. E., Bénar C. G., Quilichini P. P., Friston K. J., Jirsa V. K., Bernard C. (2014). A systematic framework for functional connectivity measures. Front. Neurosci. 8, 405. doi: 10.3389/fnins.2014.00405
WII-DST-NMSHE Task Force IV Report (2020). Assessment and monitoring of climate change effects on wildlife species and ecosystems for developing adaptation and mitigation strategies in the Indian himalayan region wildlife institute of india (Dehradun 248 002, India), 359.
Keywords: Circuitscape, MaxENT, corridor, conflict, pinch point
Citation: Bhattacharya M, Sarkar D, Pandey S, Mondal I, Sathyakumar S, Kumar RS and Talukdar G (2025) Identifying corridors for Asiatic black bear (Ursus thibetanus) in a part of Eastern Himalayas, India. Front. Conserv. Sci. 6:1470223. doi: 10.3389/fcosc.2025.1470223
Received: 25 July 2024; Accepted: 13 January 2025;
Published: 03 February 2025.
Edited by:
Mayukh Chatterjee, North of England Zoological Society, United KingdomReviewed by:
Himanshu Shekhar Palei, Independent Researcher, Gangtok, IndiaCopyright © 2025 Bhattacharya, Sarkar, Pandey, Mondal, Sathyakumar, Kumar and Talukdar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gautam Talukdar, Z2F1dGFtQHdpaS5nb3YuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.