- 1Wildlife Conservation and Science, Zoos Victoria, Elliott Avenue, Parkville, VIC, Australia
- 2School of Earth, Atmospheric and Life Sciences, University of Wollongong, Wollongong, NSW, Australia
- 3Melbourne Zoo, Zoos Victoria, Elliot Avenue, Parkville, VIC, Australia
Conservation breeding programs (CBPs) are often the lifeline between extinction and survival for many imperilled amphibian species. With the goal of recovering wild populations, CBP success is reliant on their ability to successfully manage ex situ populations over time, breed viable offspring, and maintain genetic diversity and adaptive potential. Reproductive technologies have emerged as an important tool in the conservation toolkit to allow managers to improve reproductive output and genetic management, and their use in amphibian conservation is expanding. To date, studies investigating the efficacy of hormone therapies in amphibians typically only report spawning and fertility rates and do not monitor offspring to later stages of development. For the first time, here we assess the effect of hormone therapies on captive breeding outcomes beyond oviposition, to the point of metamorphosis, in the critically endangered Baw Baw frog, Philoria frosti. To determine the effect of hormone therapy on spawning success and offspring viability, male-female pairs were administered either 0 µg/g gonadotropin-releasing hormone agonist (GnRHa), 0.5 µg/g GnRHa, or 0.5 µg/g GnRHa + 10 µg/g metoclopramide (MET) (n = 12 pairs/treatment), and the number of pairs ovipositing, time to oviposition, clutch size, metamorph mass, and the proportion and number (mean and total) of offspring to metamorphosis were quantified. Overall, the percentage of pairs that oviposited was high across all treatment groups (92-100%). The percentage of fertile clutches was highest in the GnRHa group (92%) and lowest in the GnRHa + MET group (82%), though differences were not statistically significant. Both hormone treatment groups took significantly less time to oviposit than the control pairs. Notably, the proportion of eggs developing to metamorphosis was significantly higher in the GnRHa group, resulting in 74% (total eggs=539) metamorphosing compared to approximately 50% in the control and GnRHa+MET treatments (total eggs= 273 and 264, respectively). Interestingly, weight at metamorphosis was statistically similar across all groups, and results are consistent with previous studies in this species that show a narrow range in size at metamorphosis. The continued application of GnRHa is recommended to improve conservation outcomes for the critically endangered Baw Baw frog. The outcomes of this research advance our understanding of the impact of hormone therapies on reproductive outcomes and will inform amphibian conservation breeding programs globally.
1 Introduction
Conservation breeding programs (CBPs) are increasingly vital in mitigating biodiversity loss by safeguarding threatened species (Stuart et al., 2004). These programs serve as a crucial measure to prevent species extinction and provide a buffer period for effectively managing key threatening processes. Typically, CBPs engage in addressing multiple objectives, including preserving genetic diversity, breeding for population recovery (supplementation, introduction, or reintroduction), conducting targeted research on threats, or a combination of these (Mcfadden et al., 2018).
While the decline of vertebrate taxa has reached unprecedented levels, amphibians are in the most perilous position. An estimated 41% of the 8,020 assessed amphibian species are currently threatened with extinction (IUCN, 2023), making them a focal taxon for the development of CBPs. Compared with many other taxa, amphibians are typically smaller in body size, meaning that their long term management under ex situ CBPs demands less infrastructure, resources, and financial investment (Stuart et al., 2004). However, effective establishment of amphibian CBPs is often marred as a result of delayed initiation of programs until population sizes become critically low, and/or limited knowledge of a species’ captive requirements (Harding et al., 2016). Couple this with the fact that many species display complex life-histories and require specific environmental cues to stimulate breeding, research to refine conservation breeding approaches is critically needed. In particular, protocols to optimize reproduction, growth, and development are urgently needed to ensure the retention of genetic diversity and facilitate long-term program success.
Within the conservation management tools available for CBPs, reproductive technologies such as hormone therapies, assisted fertilization, and genome resource banking, are increasingly being employed to assist with reproductive output and genetic management (Browne et al., 2019; Clulow et al., 2019; Silla et al., 2023; Silla and Kouba, 2022; Della Togna et al., 2020). These technologies have been applied widely across mammalian taxa (Holt, 2003) but are now increasingly being developed and applied to amphibians. Reproductive technologies allow conservation managers to improve propagation (breeding participation, spawning success, clutch size), facilitate pair-wise breeding (control genetic couplings), improve fertilization rates, and enhance the genetic diversity of offspring (Silla and Byrne, 2019). While the utilization of reproductive technologies will no doubt provide valuable management strategies for optimizing amphibian CBPs and assisting threatened species recovery, adoption of these methods remains limited to only a small proportion of total threatened species. This is in part because the successful application of reproductive technologies requires species-specific optimization and refinement to produce effective results (Silla and Byrne, 2019). Such species specificity makes the development of broad treatment protocols challenging. Equally challenging for conservation managers is a current gap in knowledge regarding the assessment of fitness determining traits of offspring generated through the application of reproductive technologies (Silla and Byrne, 2024). Specifically, often studies reporting on the development of reproductive technologies only assess the number of clutches oviposited or fertility rates and typically do not monitor offspring throughout development, particularly to critical life stages such as metamorphosis.
The critically endangered Baw Baw frog, Philoria frosti, is endemic to Mount Baw Baw plateau in Victoria, Australia (Hollis, 2011). Once abundant, the species has suffered catastrophic wild population decline and is now perilously close to extinction in the wild (D.J. Gilbert, pers. comm.). While factors contributing to the decline are not completely understood, most evidence suggests that the declines are linked to lethal effects of the chytrid fungus (Batrachochytrium dendrobatidis) (Burns, 2021; Hunter et al., 2018). Baw Baw frog survival currently relies on a dedicated CBP that broadly aims to; i) optimize growth and development across all life stages (Gilbert et al., 2020), ii) optimize breeding and translocation protocols, and iii) develop effective biobanking tools to preserve genetic diversity (Silla et al., 2023). Here we aimed to build on reproductive technologies previously developed for this species (see Silla et al., 2023) by testing the efficacy of hormone therapy on spawning success in pair-wise (male-female) treatment groups. Male-female pairs were allocated to one of three treatments: 1) a control treatment with no hormone therapy, 2) an experimental treatment whereby frogs were treated with gonadotropin releasing-hormone analogue (GnRHa), and 3) an experimental treatment whereby frogs were treated with GnRHa plus metoclopramide. For the first time we follow developing offspring through to metamorphosis, providing results of the effect of hormone therapies on the proportion and total eggs developing to metamorphosis and the size of offspring at the time of metamorphosis.
2 Materials and methods
2.1 Ethics statement
The protocols and procedures described herein were conducted following review and approval by the Zoos Victoria Animal Ethics Committee (ZV22009) in accordance with the National Health and Medical Research Council Australian code for the care and use of animals for scientific purposes.
2.2 Study species
The Baw Baw frog is a medium-sized (46-55mm snout-vent length) terrestrial species with a large parotoid gland positioned on each shoulder (Figure 1A). Having disappeared from much of its former range, the species is now restricted to a small area of protected montane gully habitat (1000-1300m altitude), on the Mount Baw Baw Plateau in the Central Highlands of Victoria, Australia (Gilbert et al., 2020). The Baw Baw frog is a long-lived species with an estimated lifespan of 17+ years, males are known to reach sexual maturity at 3.5 years and females at 4.5-5.5 years (Hollis, 2011). Commencement of the annual breeding season corresponds with an increase in ambient temperature during austral spring (Hollis, 2011). Male calling behavior occurs from September to March, with a peak in October and November which coincides with peak breeding activity (Hollis, 2011). Breeding typically occurs along shallow seepage lines, with oviposition taking place below the surface within natural cavities formed from vegetation, fallen logs, and embedded rocks (Clemann and Swan, 2023). Terrestrial nest-sites vary in their depth below ground, and while wet, typically retain little free water (Hollis, 2011; Gilbert et al., 2020). Amplexus in this species is inguinal and relatively large, unpigmented eggs are deposited into a foam nest (Hollis, 2011) (Figures 1B, C). Hatching has been observed to occur between 10-15 days following oviposition (D. Gilbert unpublished data). Tadpoles hatch and develop within the nest site, or may be free-swimming if washed into shallow waterbodies nearby (Clemann and Swan, 2023; Gilbert et al., 2020). Under natural field conditions, tadpole development to metamorphosis is completed within 10-18 weeks (Malone, 1985).

Figure 1. Baw Baw frog, Philoria frosti. Images shown are (A) an adult male Baw Baw frog, (B) male-female pair in amplexus, and (C) amplectant pair spawning unpigmented embryos into a foam nest. Photographs courtesy of Damian Goodall- Zoos Victoria.
2.3 Study animals and adult husbandry
A total of 36 adult male and 36 adult female Baw Baw frogs were involved in the study, representing the current adult breeding stock of the conservation breeding and reintroduction program for the species. All frogs were founding animals either collected from the wild as adult individuals, or reared in captivity from wild collected clutches, obtained from natural populations located at Mount Baw Baw, Victoria. Ages of both the male and female frogs ranged between approximately nine and sixteen years old. Baw Baw frogs were maintained in two isolated biosecurity facilities located at Zoos Victoria’s Melbourne Zoo (Parkville, VIC, Australia). Internal lighting within the facilities were controlled using a photocell light-sensitive sensor set to replicate local photoperiod. During the experimental period, lighting was provided using LED plant spectrum tubes (Fluval 3.0 Plant Spectrum) suspended above each shelf and programmed to provide varying color spectrum and intensity throughout the day. Ambient temperature within the facilities is cycled annually to reflect seasonal changes in the average climatic conditions experienced in the alpine areas on Mount Baw Baw where the species naturally occurs. Annual temperatures range from 5 to 15°C, including a 6-week overwintering period (21st July- 31st August). Frogs entering the breeding tanks at the commencement of the study were maintained on a 10°C/15°C night/day temperature cycle, corresponding with the natural conditions experienced during peak breeding activity in the field. The frogs were fed twice per week alternating between medium sized crickets (Acheta domestica; ~3-5 crickets per individual) and pill bugs (Armadillidium vulgare; ~3-5 pill bugs per individual). Crickets were gut-loaded 48 hours prior to feeding with insect booster (Womberoo) and dusted with a multivitamin supplement (Multical dust, Vetafarm) prior to feeding. Pill bugs were fed without supplementation due to their naturally high calcium levels.
2.4 Hormone-induced spawning
To determine the effect of hormone therapy on spawning success, 36 male-female pairs were allocated to one of three treatments (n = 12 pairs per treatment): 1) 0 μg/g GnRHa (control group), 2) 0.5 μg/g GnRHa, and 3) 0.5 μg/g GnRHa + 10 μg/g metoclopramide (MET; Sigma-Aldrich). The hormones selected and their formulations were chosen based on a preliminary study in this species (Silla et al., 2023), in addition to previous research in other anuran species (Trudeau et al., 2013, Trudeau et al., 2010). In brief, GnRHa is one of the most commonly employed exogenous hormones to induce spawning and gamete-release in amphibians, with recent research into the use of GnRHa in combination with MET (or other dopamine antagonist) as a possible way of potentiating the effect of GnRHa (reviewed in Silla and Langhorne, 2022). The GnRH analogue (leuprolide acetate salt; Sigma-Aldrich) was suspended in bacteriostatic saline (Bacteriostatic Water Australia) to generate a 5mg/ml stock suspension prior to further dilution to the required dose. Hormones were diluted in 100 μL of simplified amphibian ringer (SAR; composition (in mM): NaCl 113; KCl 2; CaCl2 1.35; NaHCO3 1.2) and administered via subcutaneous injection into the dorsal lymph sac using ultra-fine 31-guage needles, following hormone injection protocols used previously (Silla et al., 2023, Silla et al., 2019). Frogs in the control treatment received 100 μL of simplified amphibian ringer only.
Individuals within each male-female pair were administered a single hormone injection corresponding to their experimental treatment. Hormone administration occurred directly prior to each pair entering the breeding tanks. Males and females within each pair were injected at the same time, as previous research has shown that this approach is more effective than administering hormones to male frogs prior to females (Silla et al., 2018). Immediately prior to hormone injection, frogs were weighed to the nearest 0.01 g and the dose administered was adjusted according to an individual’s body mass. Male body mass ranged from 9.29 g to 16.39 g (mean± SEM male mass = 12.05 ± 0.27 g; n=36). Female body mass ranged from 12.77 g to 23.46 g (mean± SEM male mass = 17.01 ± 0.42 g; n=36). The body mass of males and females did not differ significantly between treatment groups (one-way analysis of variance (ANOVA), male mass: F 2,35 = 0.303, P = 0.740; female mass: F 2,35 = 2.325, P = 0.114).
Following hormone administration, male-female pairs were placed into specifically designed breeding enclosures, one pair per enclosure (Figures 2A, B). Each breeding enclosure (36cm x 60cm x 24.6cm) consisted of a glass aquarium with a raised, ventilated mesh canopy (36cm x 60cm x 35cm). Each enclosure was connected to an automated irrigation system circulating carbon filtered water. To offer a variety of potential oviposition sites, each enclosure contained a layer of aquarium gravel, which varied in depth throughout the enclosure to create shallow pools, established live plants, and pieces of curved plastic (Figures 2A, B). Following hormone treatment, breeding enclosures were visually inspected daily for the presence of eggs and the date of oviposition recorded. Unfertilized eggs or those exhibiting early embryonic failure were carefully removed from the clutch to avoid decomposing eggs negatively influencing viable embryos (Silla and Byrne, 2024). Male-female pairs entered the breeding enclosures on October 10, 2023, during the peak of the species’ natural breeding season.

Figure 2. Captive breeding facility and metamorph frogs. Images shown are (A, B) Baw Baw frog breeding enclosures with established natural substrate and automated irrigation and filtration, and (C) Baw Baw frog offspring generated from the present study following release to wild habitat at Mount Baw Baw. Photographs courtesy of Damian Goodall and Deon Gilbert- Zoos Victoria.
2.5 Offspring husbandry
Eggs and tadpoles were reared in the same two isolated biosecurity facilities where the adult breeding enclosures were located. Eggs were removed from the breeding tanks approximately 2 days following oviposition to minimize disturbance from the adults and placed into tadpoles rearing tanks. Throughout egg and tadpole development, offspring were maintained on a 12°C/15°C night/day temperature cycle. Tadpole rearing tanks were constructed with purpose-built aquaria arranged in banks, each containing 10 individual tanks measuring 21cm x 22cm x 20cm. Two banks were constructed totaling 20 individual tadpole tanks, each clutch was reared individually. These tanks were divided by mesh screens to allow for air and water movement. Additionally, each bank, measuring six tanks long and two tanks wide, featured a sump area (16cm x 22cm x 20cm) at one end equipped with an aquarium water pump to facilitate water flow. An overflow system was incorporated for water change filtration. To simulate natural habitat conditions, approximately 3cm of substrate, composed of decomposing granite and organic material sourced from field breeding sites, was added to the bottom of each tadpole tank. To mitigate the risk of disease transfer, the substrate underwent heat treatment and drying (40°C for 12 hours) before being introduced into the tanks. Under natural conditions Baw Baw frog larvae develop in darkness in underground cavities and are sensitive to light during development (Hollis, 2004). To ensure minimal light disturbance in the captive facility, the sides of the tanks were covered with black vinyl contact, and a removable black plastic lid was fitted on top. Tadpoles were monitored approximately once per week to track development progress and remove any dead or rotting eggs. Water depth in each tank was maintained at approximately 2cm, and the internal substrate was graded on an angle to create a small terrestrial portion, thus preventing drowning during metamorphosis. All offspring metamorphosed between December 10-16, 2023, approximately 9-weeks after the date of oviposition. Following complete metamorphosis (full tail reabsorption), a subset of frogs were placed on a small (2.5 cm diameter) petri dish and weighed to the nearest 0.01 g using digital scales (Pesola touch screen digital pocket scale). Overall, 51 metamorphs were randomly selected from 9 clutches to be weighed, a total of 17 individual offspring from each of the three treatment groups, control, GnRHa and GnRHa + MET. To supplement the assurance colony, 7-11 individuals from 17 clutches (168 offspring in total) were retained at Melbourne Zoo. All 709 remaining offspring were released back into the wild on Mount Baw Baw to augment natural populations for the species (Figure 2C).
2.6 Statistical analyses
The number of male-female pairs ovipositing, and the number of fertile clutches laid were compared between treatment groups using ChiSquare Likelihood Ratio Tests. One-way ANOVAs were used to test for statistical differences among treatment means in; 1) days until oviposition, 2) clutch size, 3) metamorph mass, 4) number of individuals to metamorphosis, and 5) proportion to metamorphosis. Comparisons among treatment means were conducted using each pair student’s t post hoc tests. Prior to ANOVA analysis, to verify homogeneity of variances, Browne-Forsythe equivalence tests were performed. For all response variables variances were equal (P > 0.05) and data were not transformed prior to analysis. All statistical analyses were performed using JMP Pro 16.1.0 software package (SAS Institute Inc.) and statistical significance was accepted at P < 0.05.
3 Results
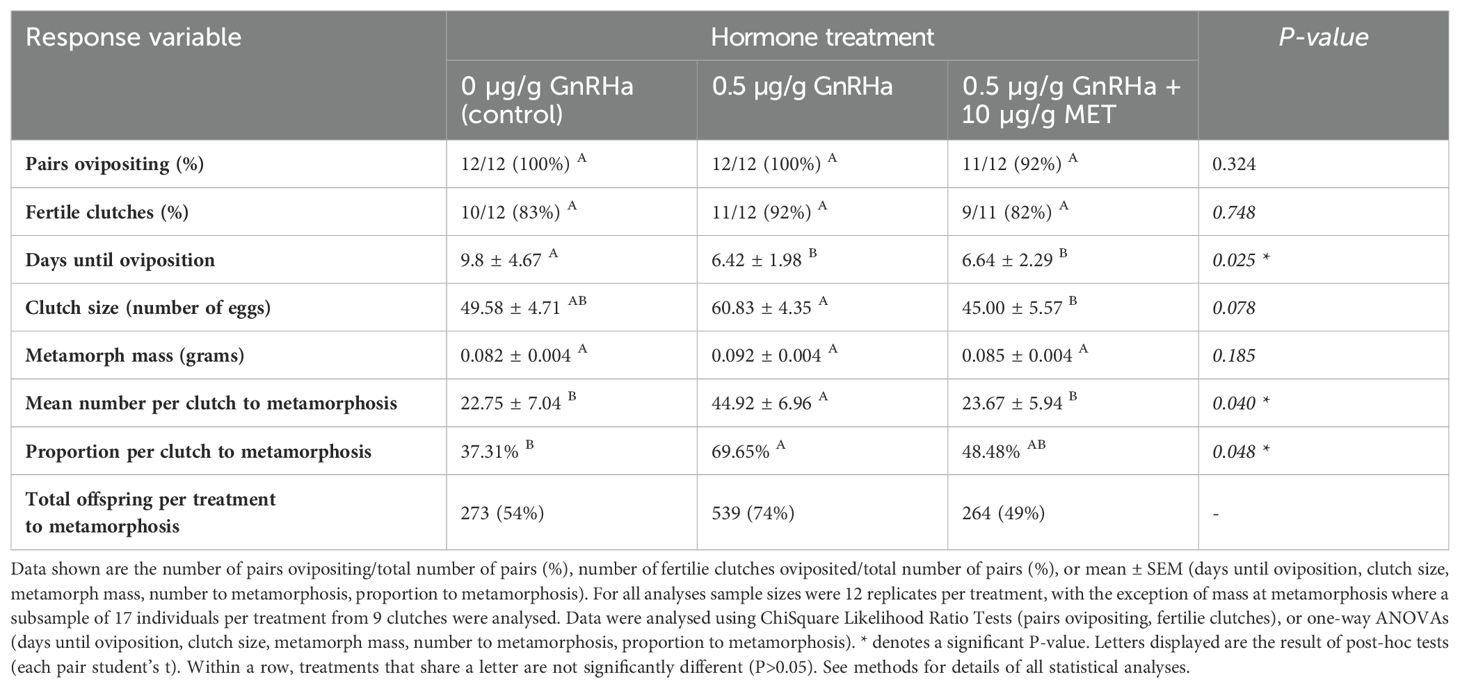
Overall spawning success was high across all experimental treatments, with 92%-100% of male-female pairs ovipositing (Table 1). Of the pairs that successfully oviposited, the percentage of fertile clutches (those producing fertilized embryos) was highest in response to the administration of GnRHa (92%), though differences between treatment groups were not statistically significant (χ2 = 0.580, p = 0.748; Table 1). Hormone treatment had a significant effect on the time taken for pairs to oviposit a clutch of eggs (ANOVA, F-ratio 2,34 = 4.142, p = 0.025). Following hormone administration, pairs in the GnRHa and GnRHa + MET treatment groups took a significantly shorter time to oviposit compared to pairs within the control treatment (each pair student’s t post hoc tests, p < 0.05), which, on average, took an additional 3 days (Table 1). Pairs that oviposited released between 30-80 eggs, with a larger mean clutch size oviposited in response to the administration of GnRHa compared to the GnRHa + MET treatment (each pair student’s t post hoc tests, p < 0.05; Table 1). The mean clutch size released by pairs in the control treatment was not significantly different to either of the hormone treatments (each pair student’s t post hoc tests, p > 0.05; Table 1).
Of the subset of juveniles that were weighed (n=17 per treatment), frog mass at the time of metamorphosis ranged from 0.05 to 0.12 grams. Mean metamorph mass was statistically similar across the three treatments groups (ANOVA, F-ratio 2,50 = 1.747, p = 0.185; Table 1). Hormone treatment had a significant effect on the mean number of offspring per clutch that developed to metamorphosis (ANOVA, F-ratio 2,34 = 3.542, p = 0.040), which was significantly higher in the GnRHa treatment compared to the two remaining treatments (each pair student’s t post hoc tests, p<0.05; Table 1). Similarly, the proportion of offspring that developed to metamorphosis was significantly different among treatment groups (ANOVA, F-ratio 2,34 = 3.356, p = 0.048). The mean proportion of offspring to metamorphosis from each clutch ranged from 37.3% to 69.7%, with the highest percent exhibited from clutches laid in response to the administration of GnRHa (Table 1). Overall, the cumulative total number of offspring that developed to metamorphosis was 539 (74%) for the GnRHa, compared with 273, (54%) and 264 (49%), in the control and GnRHa + MET treatments, respectively (Table 1).
4 Discussion
The Baw Baw frog wild population is perilously close to extinction and without a dedicated CBP capable of producing high numbers of viable, genetically representative, offspring for translocation the chance of wild recovery will be low. Optimizing reproductive output and effective genetic management are key to the long-term sustainability of the CBP, hence developing tools to ensure these goals are met is critical. Reproductive technologies have emerged as a potential management tool to increase both reproductive output and genetic diversity by providing animals with exogenous hormones to stimulate reproductive events, including spawning (e.g. see Silla et al., 2018). The aim of this study was to build on hormone therapy protocols previously developed for this species (Silla et al., 2023), to test the effect of GnRHa or GnRHa + MET on spawning success in male-female pairs, and on the viability of offspring up until the point of metamorphosis.
Results showed that spawning success was high across all treatment groups. Overall, the percentage of fertile clutches oviposited was highest in pairs administered GnRHa (92%), and lowest in pairs administered GnRHa + MET (82%), though differences between treatment groups were not statistically significant. Both hormone treatment groups (GnRHa and GnRHa + MET) took significantly less time to oviposit compared to the control pairs, which on average took an additional three and a half days to oviposit. The administration of GnRHa has been used to successfully induce spawning in male-female pairs/groups, as well as gamete release in isolated animals, in a diversity of amphibian species (Uteshev et al., 2013; Sherman et al., 2008; Jacobs et al., 2016; Otero et al., 2023; Silla and Byrne, 2021; Silla et al., 2018; Silla, 2011; Guy et al., 2020). The administration of GnRHa mimics natural GnRH-1 molecules, binding to receptors on the anterior pituitary to stimulate the endogenous synthesis and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) (Vu and Trudeau, 2016). Over the past decade, there has been growing interest in the administration of GnRHa in combination with a dopamine antagonist (domperidone, pimozide, or metoclopramide), which is hypothesized to attenuate the stimulatory effect of GnRHa by limiting dopaminergic inhibition (Vu and Trudeau, 2016). The combined administration of GnRHa plus metoclopramide, a method also referred to as AMPHIPLEX, has been successfully used to induce spawning in a number of species, including northern leopard frogs, tiger frogs, Argentine horned frogs, Cranwell’s horned frogs, and common lesser escuercito (Trudeau et al., 2010; Godome et al., 2021). However, studies on the northern leopard frog, American bullfrog, and Panamanian golden frog report statistically similar spawning rates in frogs administered GnRHa + MET compared with GnRHa alone at an optimal dose (Bronson et al., 2021; Nascimento et al., 2015; Vu et al., 2017). Our previous research inducing spawning in the Baw Baw frog, reported no statistical difference in the spawning success of pairs administered GnRHa compared to pairs receiving GnRHa + MET, though a higher proportion of pairs in the GnRHa + MET treatment oviposited (Silla et al., 2023). In the present study, sample sizes were almost doubled and, consistent with research in the northern leopard frog, American bullfrog, and Panamanian golden frog (Bronson et al., 2021; Nascimento et al., 2015; Vu et al., 2017), our data show no difference in spawning rates between hormone treatments.
While a growing number of studies are investigating the use of hormone therapies to induce spawning and improve captive-breeding outcomes for amphibians, there remains a lack of data quantifying outcomes beyond oviposition and fertilization. As such, there is limited understanding of the degree to which specific protocols might influence (either improve, or potentially compromise) offspring fitness (such as body size and survival rates between developmental stages) and lead to shifts in population viability (Silla and Byrne, 2024). For the first time, we monitored offspring throughout development to the point of metamorphosis, across each treatment group. Our results demonstrate that significantly more offspring developed to metamorphosis in the GnRHa treatment group compared to the control or GnRHa + MET treatments. Both the mean number of offspring metamorphosing, and the proportion of offspring developing to metamorphosis was significantly higher in the GnRHa group. The GnRHa treatment resulted in 74% of eggs metamorphosing (total eggs=539), compared to approximately 50% in the control and GnRHa + MET treatments (total eggs = 273 and 264, respectively). Additional research will now be required to determine the mechanism by which the addition of MET resulted in fewer offspring reaching metamorphosis compared to GnRHa alone. Overall, the administration of GnRHa resulted in a 25% increase in offspring generation, equating to several hundred more offspring available for release to augment natural populations for the species on Mount Baw Baw. Our results highlight the importance of measuring a suite of response variables across different developmental life-stages to properly assess the outcome of hormone therapies. Future research aims to expand the number of fitness-determining traits quantified to include growth trajectories, morphology, and behavioral performance (Silla and Byrne, 2024).
While the proportion of eggs developing to metamorphosis was significantly different between treatment groups, metamorph weight was statistically similar, and individual variation in weight was extremely low. These results are consistent with the notion that Baw Baw frog larvae must reach a body-size threshold in order to trigger metamorphic onset (Gilbert et al., 2020). Previous research in this species found that rearing temperature and food availability influenced time to metamorphosis, but that there was no effect on body mass or length (Gilbert et al., 2020). The Wilbur–Collins model for amphibian metamorphosis explains that the range in body size and time to metamorphosis for a given species are determined by a minimum body size that must be obtained and a maximum body size that will not be exceeded at metamorphosis (Wilbur and Collins, 1973). The model predicts that amphibian species inhabiting stochastic environments will have a wide range of possible sizes at metamorphosis, while species exploiting relatively stable environments during larval development will have a narrower range. The narrow range in size at metamorphosis for Baw Baw frogs reported in the present study, and by Gilbert et al. (2020), suggests that conditions during larval development on Mount Baw Baw have historically been relatively stable. As natural conditions become increasingly stochastic, Baw Baw frog tadpoles may face new selective pressures and a narrow metamorphic body size range may have negative fitness consequences for remnant wild populations. Consequently, developing effective protocols for larval rearing within the conservation breeding program is likely to become increasingly valuable into the future.
Of note, the percentage of pairs ovipositing reported in the present study (92-100%) was substantially higher than the percentage of pairs ovipositing in the previous breeding season under the same hormone treatments (33-71%) (Silla et al., 2023). The age and body condition of animals was comparable between years, so differences are not expected to be a result of parental phenotype. While the provision of environmental conditions, including breeding enclosure habitat/substrate, food availability, temperature, and photoperiod, remained constant between years, there were two abiotic changes that may have contributed to the differences observed. First male-female pairs in the present study entered the breeding tanks on October 10, 2023, two weeks later than pairs in our previous study, which entered the breeding tanks on September 26, 2022. Frogs in both years were exposed to the same temperature regime and were warmed after an over-wintering period at the same time. The increase in spawning success in the present study may reflect a beneficial effect of a longer warming period to prime the gonads and prepare broodstock for reproduction. The second difference between the two years, was that breeding enclosures were initially irrigated with Reverse Osmosis (RO) water, which was changed to a carbon-filtered water system prior to the present study. Filtration via reverse osmosis is a purification process that removes dissolved solids, including salts, from source water. While species such as the Lake Oku clawed frog (Xenopus longipes) are sensitive to dissolved solids and larvae only thrive in RO water (Michaels et al., 2015)), other species develop osmotic imbalance in pure RO and carbon-filtered, or reconstituted RO water is recommended (Odum and Zippel, 2008). Previous research has shown that fertilization success of the terrestrial-breeding anuran Pseudophryne guentheri is improved by 20-30% in higher osmolality solutions (25-100mOsm kg-1; generated by serial addition of amphibian saline) compared to pure water (3 mOsm kg-1), reflective of the natural terrestrial fertilization environment of this species (Silla, 2013). The mode of reproduction for the Baw Baw frog similarly involves a terrestrial oviposition site. If female Baw Baw frogs have the ability to assess the osmolality of the environment, as observed in other species (Haramura, 2008), then the provision of RO water may have been suboptimal and resulted in fewer females ovipositing compared to the present study. Overall, the differences in spawning success observed between years highlights the importance of a holistic approach to amphibian captive breeding. While reproductive technologies, specifically hormone therapies, can be used to overcome impediments to breeding that are often observed in captive amphibians, the outcomes of hormone therapies are enhanced when they are used in concert with the provision of naturalistic environmental conditions that have been optimized on a species-specific basis (Silla et al., 2021). The provision of environmental conditions should be viewed as an evolving process as a deeper understanding of species’ requirements are elucidated.
5 Conclusions
Our study adds to a growing body of research reporting the effectiveness of utilizing reproductive technologies to improve reproductive outcomes for amphibian conservation management. We have highlighted the need for research that quantifies the impact of hormone therapies on a suite of fitness-determining traits that encompass a greater temporal span in order to gain a more comprehensive understanding of outcomes. Further research in this area will allow us to gain a better understanding of the impact of protocol refinement on individual fitness and the long-term viability of populations, which will ultimately allow us to progress the field of amphibian reproductive technologies to maximize the chance of beneficial conservation outcomes (see Silla and Byrne, 2024). Critically, the present study has taken a step to bridge knowledge gaps concerning how specific hormone therapies may impact offspring fitness by monitoring individuals through to metamorphosis and quantifying size at metamorphosis. Overall, male-female pairs that were administered GnRHa produced a significantly higher total number of eggs and proportion of eggs that developed to metamorphosis, resulting in several hundred more metamorphs available to augment wild populations. We maintain that amphibian CBPs must optimize reproductive output and retention of genetic diversity to ensure the best chance of recovering threatened wild amphibian populations and recommend the continued use of GnRHa to enhance conservation-breeding outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Zoos Victoria Animal Ethics Commitee ZV22009. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
DeG: Investigation, Project administration, Writing – original draft, Writing – review & editing. DaG: Project administration, Writing – review & editing. PB: Conceptualization, Supervision, Writing – review & editing. AS: Conceptualization, Supervision, Formal analysis, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The support of the following funding grants are acknowledged; the Australian Research Council Discovery Early Career Researcher Award (DE210100812) awarded to AS with ancillary funding from Zoos Victoria and the Garry White Foundation awarded to DeG.
Acknowledgments
The authors would like to pay respect to the Wurundjeri people of the Kulin Nation as the traditional custodians and cultural knowledge holders of the land on which this research was conducted at Melbourne Zoo. We also acknowledge the Gunaikurnai people as the traditional custodians of the Mount Baw Baw area where wild populations of our study species are located, and offspring generated from this research were released. We acknowledge the support of amphibian conservation staff at Melbourne Zoo, particularly Tom Fair and Ryan Moes. We also thank Dr Michael Magrath for his valuable feedback on earlier versions of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bronson E., Guy E. L., Murphy K. J., Barrett K., Kouba A. J., Poole V., et al. (2021). Influence of oviposition-inducing hormone on spawning and mortality in the endangered Panamanian golden frog (Atelopus zeteki). BMC Zoology 6, 17. doi: 10.1186/s40850-021-00076-8
Browne R. K., Silla A. J., Upton R., Della-Togna G., Marcec-Greaves R., Shishova N. V., et al. (2019). Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology 133, 187–200. doi: 10.1016/j.theriogenology.2019.03.035
Burns T. (2021). Impacts of amphibian chytrid fungus on the Baw Baw frog. Doctoral dissertation, (VIC, Australia: Deakin University).
Clemann N., Swan M. (2023). Frogs of Victoria: A Guide to Identification, Ecology and Conservation (CSIRO Publishing: Melbourne).
Clulow J., Upton R., Trudeau V., Clulow S. (2019). Amphibian assisted reproductive technologies: moving from technology to application. In: Comizzoli P., Brown J., Holt W. (eds) Reprod. Sci. Anim. Conserv. Advances in Experimental Medicine and Biology, vol 1200. Cham, Switzerland: Springer, 413–463. doi: 10.1007/978-3-030-23633-5_14
Della Togna G., Howell L. G., Clulow J., Langhorne C. J., Marcec-Greaves R., Calatayud N. E. (2020). Evaluating amphibian biobanking and reproduction for captive breeding programs according to the Amphibian Conservation Action Plan objectives. Theriogenology 150, 412–431. doi: 10.1016/j.theriogenology.2020.02.024
Gilbert D. J., Magrath M. J., Byrne P. G. (2020). Warmer temperature and provision of natural substrate enable earlier metamorphosis in the critically endangered Baw Baw frog. Conserv. Physiol. 8, coaa030. doi: 10.1093/conphys/coaa030
Godome T., Sintondji S. W., Azon M. T. C., Tossavi C. E., Ouattara N. I., Fiogbe E. D. (2021). Artificial reproduction and embryogeny of the tiger frog hoplobatrachus occipitalis (Günther 1858). Proc. Zoological Soc. India: Springer 74, 43–51. doi: 10.1007/s12595-020-00341-7
Guy E. L., Gillis A. B., Kouba A. J., Barber D., Poole V., Marcec-Greaves R. M., et al. (2020). Sperm collection and cryopreservation for threatened newt species. Cryobiology 94, 80–88. doi: 10.1016/j.cryobiol.2020.04.005
Haramura T. (2008). Experimental test of spawning site selection by Buergeria japonica (Anura: Rhacophoridae) in response to salinity level. Copeia 2008 (1), 64–67. doi: 10.1643/CH-06-091
Harding G., Griffiths R. A., Pavajeau L. (2016). Developments in amphibian captive breeding and reintroduction programs. Conserv. Biol. 30, 340–349. doi: 10.1111/cobi.2016.30.issue-2
Hollis G. J. (2004). Ecology and conservation biology of the Baw Baw frog Philoria frosti (Anura: Myobatrachidae): distribution, abundance, autoecology and demography. (Doctoral dissertation). University of Melbourne, Department of Zoology, VIC, Australia.
Hollis G. J. (2011). National Recovery Plan for the Baw Baw Frog Philora Frosti (VIC, Australia: Department of Sustainability and Environment Melbourne).
Hunter D., Clemann N., Coote D., Gillespie G., Hollis G., Scheele B., et al. (2018). “Frog declines and associated management response in south-eastern mainland Australia and Tasmania,” in Status of conservation and decline of amphibians (New Zealand and Pacific Islands, Australia), 38–58.
IUCN (2023). Table 1a: Number of species evaluated in relation to the overall number of described species, and numbers of threatened species by major groups of organisms. IUCN Red List.
Jacobs L. E., Robertson J. M., Kaiser K. (2016). Variation in male spermiation response to exogenous hormones among divergent populations of Red-eyed Treefrogs. Reprod. Biol. Endocrinol. 14, 1–7. doi: 10.1186/s12958-016-0216-3
Malone B. S. (1985). Status, distribution, and ecology of the Baw Baw frog (philoria frosti): a report to department of conservation, forests, and lands, Victoria and world wildlife fund Australia (Department of Conservation, Forests, and Lands). Arthur Rylah Institute for Environmental Research Heidelberg, VIC, Australia.
Mcfadden M. S., Gilbert D., Bradfield K., Evans M., Marantelli G., Byrne P. (2018). 11 The role of ex-situ amphibian conservation in Australia. Status of Conservation and Decline of Amphibians (Australia: New Zealand, and Pacific Islands), 125.
Michaels C. J., Tapley B., Harding L., Bryant Z., Grant S., Sunter G., et al. (2015). Breeding and rearing the critically endangered lake Oku clawed frog (Xenopus longipes Loumont and Kobel 1991). Amphibian Reptile Conserv. 9, 100–110.
Nascimento N. F., Silva R. C., Valentin F. N., Paes Mdcf D. S. M., Nakaghi L. (2015). Efficacy of buserelin acetate combined with a dopamine antagonist for spawning induction in the bullfrog (Lithobates catesbeianus). Aquaculture Res. 46, 3093–3096.
Odum R., Zippel K. (2008). Amphibian water quality: approaches to an essential environmental parameter. Int. Zoo Yearbook 42, 40–52. doi: 10.1111/j.1748-1090.2008.00053.x
Otero Y., Calatayud N. E., Arcia I. D., Mariscal D., Samaniego D., Rodríguez D., et al. (2023). Recovery and Characterization of Spermatozoa in a Neotropical, Terrestrial, Direct-Developing Riparian Frog (Craugastor evanesco) through Hormonal Stimulation. Animals 13, 2689. doi: 10.3390/ani13172689
Sherman C. D., Uller T., Wapstra E., Olsson M. (2008). Within-population variation in ejaculate characteristics in a prolonged breeder, Peron’s tree frog, Litoria peronii. Naturwissenschaften 95, 1055–1061. doi: 10.1007/s00114-008-0423-7
Silla A. J. (2011). Effect of priming injections of luteinizing hormone-releasing hormone on spermiation and ovulation in Gϋnther's toadlet, Pseudophryne guentheri. Reprod. Biol. Endocrinol. 9, 1–9. doi: 10.1186/1477-7827-9-68
Silla A. J. (2013). Artificial fertilization in a terrestrial toadlet (Pseudophryne guentheri): effect of medium osmolality, sperm concentration and gamete storage. Reproduction Fertility Dev. 25, 1134–1141. doi: 10.1071/RD12223
Silla A. J., Byrne P. G. (2019). The role of reproductive technologies in amphibian conservation breeding programs. Annu. Rev. Anim. Biosci. 7, 499–519. doi: 10.1146/annurev-animal-020518-115056
Silla A. J., Byrne P. G. (2021). Hormone-induced ovulation and artificial fertilization in four terrestrial-breeding anurans. Reproduction Fertility Dev. 33, 615–618. doi: 10.1071/RD20243
Silla A. J., Byrne P. G. (2024). The importance of quantifying fitness-determining traits throughout life to assess the application of reproductive technologies for amphibian species recovery. Front. Conserv. Sci. 5, 1378624. doi: 10.3389/fcosc.2024.1378624
Silla A. J., Calatayud N. E., Trudeau V. L. (2021). Amphibian reproductive technologies: approaches and welfare considerations. Conserv. Physiol. 9, coab011. doi: 10.1093/conphys/coab011
Silla A. J., Hobbs R. J., Gilbert D. J., Goodall D., Parrott M. L., Lee A., et al. (2023). Application of reproductive technologies to the critically endangered baw baw frog, philoria frosti. Animals 13, 2232. doi: 10.3390/ani13132232
Silla A. J., Kouba A. J. (2022). “Integrating Reproductive technologies into the conservation toolbox for the recovery of amphibian species,” in Reproductive Technologies and Biobanking for the Conservation of Amphibians. Eds. Silla A. J., Kouba A. J., Heatwole H., CSIRO, Melbourne, Australia
Silla A. J., Langhorne C. J. (2022). Protocols for hormonally induced spermiation, and the cold storage, activation, and assessment of amphibian sperm. In Silla A. J., Kouba A. J., Heatwole H. (Eds) Reproductive Technologies and Biobanking for the Conservation of Amphibians. CSIRO, Melbourne, Australia.
Silla A. J., Mcfadden M., Byrne P. G. (2018). Hormone-induced spawning of the critically endangered northern corroboree frog Pseudophryne pengilleyi. Reproduction Fertility Dev. 30, 1352–1358. doi: 10.1071/RD18011
Silla A. J., Mcfadden M. S., Byrne P. G. (2019). Hormone-induced sperm-release in the critically endangered Booroolong frog (Litoria booroolongensis): effects of gonadotropin-releasing hormone and human chorionic gonadotropin. Conserv. Physiol. 7, coy080. doi: 10.1093/conphys/coy080
Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S., Fischman D. L., et al. (2004). Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786. doi: 10.1126/science.1103538
Trudeau V. L., Schueler F. W., Navarro-Martin L., Hamilton C. K., Bulaeva E., Bennett A., et al. (2013). Efficient induction of spawning of Northern leopard frogs (Lithobates pipiens) during and outside the natural breeding season. Reprod. Biol. Endocrinol. 11, 1–9. doi: 10.1186/1477-7827-11-14
Trudeau V. L., Somoza G. M., Natale G. S., Pauli B., Wignall J., Jackman P., et al. (2010). Hormonal induction of spawning in 4 species of frogs by coinjection with a gonadotropin-releasing hormone agonist and a dopamine antagonist. Reprod. Biol. Endocrinol. 8, 1–9. doi: 10.1186/1477-7827-8-36
Uteshev V., Shishova N., Kaurova S., Manokhin A., Gakhova E. (2013). Collection and cryopreservation of hormonally induced sperm of pool frog (Pelophylax lessonae). Russ J. Herpetol 20, 105–109.
Vu M., Trudeau V. L. (2016). Neuroendocrine control of spawning in amphibians and its practical applications. Gen. Comp. Endocrinol. 234, 28–39. doi: 10.1016/j.ygcen.2016.03.024
Vu M., Weiler B., Trudeau V. L. (2017). Time-and dose-related effects of a gonadotropin-releasing hormone agonist and dopamine antagonist on reproduction in the northern leopard frog (Lithobates pipiens). Gen. Comp. Endocrinol. 254, 86–96. doi: 10.1016/j.ygcen.2017.09.023
Keywords: amphibian, captive breeding, conservation, gamete-release, oviposition, offspring, reproductive technologies, hormone therapy
Citation: Gilbert DJ, Goodall D, Byrne PG and Silla AJ (2024) Hormone therapy improves conservation breeding outcomes in the critically endangered Baw Baw frog, Philoria frosti. Front. Conserv. Sci. 5:1464730. doi: 10.3389/fcosc.2024.1464730
Received: 15 July 2024; Accepted: 11 September 2024;
Published: 04 October 2024.
Edited by:
José Luis Ros-Santaella, Czech University of Life Sciences Prague, CzechiaReviewed by:
Melina Alicia Velasco, Universidad Nacional de La Plata, ArgentinaAllison Julien, Fort Worth Zoo, United States
Rosaria Meccariello, University of Naples Parthenope, Italy
Copyright © 2024 Gilbert, Goodall, Byrne and Silla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deon J. Gilbert, ZGdpbGJlcnRAem9vLm9yZy5hdQ==
 Deon J. Gilbert
Deon J. Gilbert Damian Goodall
Damian Goodall Phillip G. Byrne
Phillip G. Byrne Aimee J. Silla
Aimee J. Silla