
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci., 08 August 2024
Sec. Animal Conservation
Volume 5 - 2024 | https://doi.org/10.3389/fcosc.2024.1401517
This article is part of the Research TopicAdvances in the Conservation of Neotropical PrimatesView all 6 articles
This study describes the characteristics of forest fragments occupied by a Critically Endangered endemic Peruvian primate, the San Martín titi monkey, Plecturocebus oenanthe (Pitheciidae; Platyrrhini). We selected 45 fragments; 20 had already been surveyed in 2015 by the Proyecto Mono Tocón (six of these had been further split, resulting in 27 fragments); an additional 18 fragments were randomly selected from satellite images. We surveyed these fragments for the presence of P. oenanthe and determined characteristics of the fragments (size, shape, tree density, canopy height) and of the landscape (distance to nearest fragment and road). We also examined changes in the number of fragments and in forest cover between 2015 and 2019. We encountered P. oenanthe in all surveyed fragments except for the smallest one (0.2 ha). Our findings suggest that P. oenanthe can persist in fragments with a wide range of characteristics, particularly with regard to size and tree density. Unless fragmentation continues and overall forest cover in the area diminishes further, the species may be able to persist even in a fragmented landscape, provided that the matrix allows for movements between fragments. However, persistence might not be long-term if groups are not reproductive, populations become too small, and reduced gene flow results in inbreeding.
Anthropogenic habitat loss and fragmentation represent major threats to biodiversity and ecosystems (Fahrig, 2003; Maxwell et al., 2016). Tropical rainforests – the home of the majority of non-human primates – are amongst the most strongly affected ecosystems (Estrada et al., 2017). Habitat loss, i.e., deforestation, results in local, regional or even global extinction of primates and other inhabitants of tropical forests, unless a species is capable of switching to a completely different habitat. In contrast, fragmentation can have variable effects and outcomes, from negative, through neutral to even positive (Ewers and Didham, 2006; Benítez-Malvido and Arroyo-Rodríguez, 2008; Fahrig, 2017). Some primate taxa may be more resilient to habitat fragmentation than others, but this resilience may reach its limits when other disturbances (e.g., the outbreak of diseases) affect their populations (Bicca-Marques et al., 2020; Strier, 2021).
The specific outcome of habitat fragmentation depends on a number of factors including the size and shape of fragments, the distance between fragments, the structure and permeability of the landscape (matrix) surrounding fragments, and the life history and ecological traits of the primate species (Benchimol and Peres, 2014; Galán-Acedo et al., 2019). Larger fragments are likely to provide more and higher quality habitat, which can sustain larger groups or populations. However, while some studies found a relationship between fragment size and presence or abundance of a primate species, others did not (Cristóbal-Azkarate et al., 2005; Arroyo-Rodríguez and Dias, 2010; Marsh et al., 2013). The shape of fragments is likely to influence habitat quality through edge effects: the proportion of a fragment that is affected by edge effects increased with the irregularity of the shape (which results in a larger ratio between the perimeter and the area; Benítez-Malvido and Arroyo-Rodríguez, 2008; see Murcia, 1995, for edge effects in fragments in general). On the other hand, irregularly shaped fragments, particularly when large, may increase the probability that a fragment will be encountered by traveling groups or individuals (Benítez-Malvido and Arroyo-Rodríguez, 2008). The distance between fragments, and the structure and permeability of the landscape will also affect this probability, and the probability that a single group can occupy more than one fragment (Prugh et al., 2008; Pozo-Montuy et al., 2011; Benchimol and Peres, 2014). Roads are another factor that influences the permeability of the landscape; they can limit the movement of primates, and crossing roads may be lethal (Asensio et al., 2017; Hetman et al., 2019). Finally, tree density and canopy cover are obvious characteristics of fragments that directly influence tree-living organisms like primates, as they obtain most food from (plant-based diet) or within (invertebrate and vertebrate prey) trees.
The San Martín titi monkey, Plecturocebus oenanthe (Pitheciidae; Platyrrhini; Primates; previously Callicebus oenanthe; see Byrne et al., 2016 for taxonomic change) is endemic to the San Martín region in Peru (Vermeer and Shanee, 2020). It is categorized as Critically Endangered (IUCN category: CR), due to an estimated population reduction of ≥80% since around 2000 caused by habitat loss (Vermeer and Shanee, 2020). Throughout this region, agricultural activities have led to heavy deforestation and fragmentation. The species’ original geographic range covered 14,686 km2, but by 2013, 55.6% of the original range was lost, and of the remaining 6,500 km2 only about 1,900 km² (13% of the original range) were considered as “good” quality habitat (Shanee et al., 2013). These authors recommended studying the presence of P. oenanthe in marginal habitat, which would include highly fragmented areas. Therefore, in this study we originally aimed at examining fragment occupation by P. oenanthe and characterizing fragments in which the species is present with regard to size, tree density and other variables in comparison to fragments in which the species is not detected. Unexpectedly, titi monkeys occupied all except one of the surveyed forest fragments. Therefore, this paper quantitatively describes the characteristics of occupied fragments and discusses the implications for the conservation of San Martín titi monkeys, in order to identify the range of characteristics under which this species exists in the area, and to identify the minimum values (e.g., fragment size) and maximum values (e.g., distance to nearest fragment) that can be tolerated. As most groups were not habituated to humans, we could not collect data on group size and composition.
The San Martín titi monkey is one of the currently recognized 25 species of the genus Plecturocebus. Titi monkeys of this genus occupy a wide range of habitats, from evergreen rainforests (both terra firme and flooded), to semi-deciduous dry forests (Bicca-Marques and Heymann, 2013). In contrast to this obvious ecological variability, titi monkeys are uniform in their social system: all species are pair living, with groups consisting of the adult breeding pair and usually one to three offspring; occasionally, a third adult may be present (Bicca-Marques and Heymann, 2013). Offspring generally disperse when reaching maturity (Bossuyt, 2002). Titi monkeys show very high levels of paternal care (Wright, 1984). Most groups of P. oenanthe contain between 2 and 4 individuals, but groups of up to 8 individuals have been seen; mean group size varies between 2.9 and 3.6 (Shanee et al., 2024). P. oenanthe feeds primarily on fruits, but also includes leaves and other non-reproductive plant parts, flowers, invertebrates, and vertebrates in its diet (DeLuycker, 2007, 2012, 2024). Shanee et al. (2024) provide additional information on the biology of P. oenanthe.
The two study areas are located close to the city of Moyobamba. Area 1 covers 1790.7 ha and Area 2 covers 2545.2 ha (Figure 1). The climate in the Moyobamba area corresponds to the category Af in the Köppen-Geiger classification. Mean annual temperature area is 20.7°C and annual precipitation is 2021 mm (Climate-data.org, 2024). The natural vegetation in the Moyobamba area corresponds to humid premontane tropical forest in the Holdridge classification system (Romero Herrada, 2018). Agricultural activities in the area include coffee agroforestry; non-shaded coffee; pineapple, yuca and banana plantations; rice fields and grazing pastures. The main difference between the two areas is the presence of a conservation area in the southeast of Area 1 and an asphalted main two-lane road that borders Area 2 to its north.
A forest fragment was defined as an isolated area of forest that contained trees with a diameter at breast height (dbh) ≥10 cm and taller than 8 m, surrounded by a matrix. The matrix can include agricultural areas, pastures, shrubland and vegetation with trees that did not meet the forest fragment criteria, water bodies, and swamps. Fragments were considered separate fragments if they were divided by a road or wide track with none or minimal connectivity between the branches above the road.
We surveyed a total of 45 forest fragments. This included 20 fragments surveyed in 2015 by Proyecto Mono Tocón, 10 per area. Land use changes resulted in further fragmentation of six fragments (five split into two, and one into three fragments), resulting in 27 fragments. We selected an additional 18 fragments through stratified random sampling using area as the selection criterion:<3, 3-6, 6-9, >9 ha (Buja and Menza, 2013) from satellite images (Google Earth; Spot 6 imagery generously provided by the Peruvian Space Agency, CONIDA). Google Earth images used were from as close to the time of the study as possible. The CONIDA images used were from 2019, or if this image had cloud cover, from 2018. Area 1 contained fragments 1 to 21 (1-10 first evaluated in 2015 and 11-21 first evaluated in 2019), and Area 2 fragments 22 to 38 (22-31 first evaluated in 2015 and 32-38 first evaluated in 2019; fragment numbers represent our enumeration and do not have a specific meaning) (Figure 2). The surveyed fragments represent 13% of all fragments (349) in Areas 1 and 2 (Supplementary Figure 1).
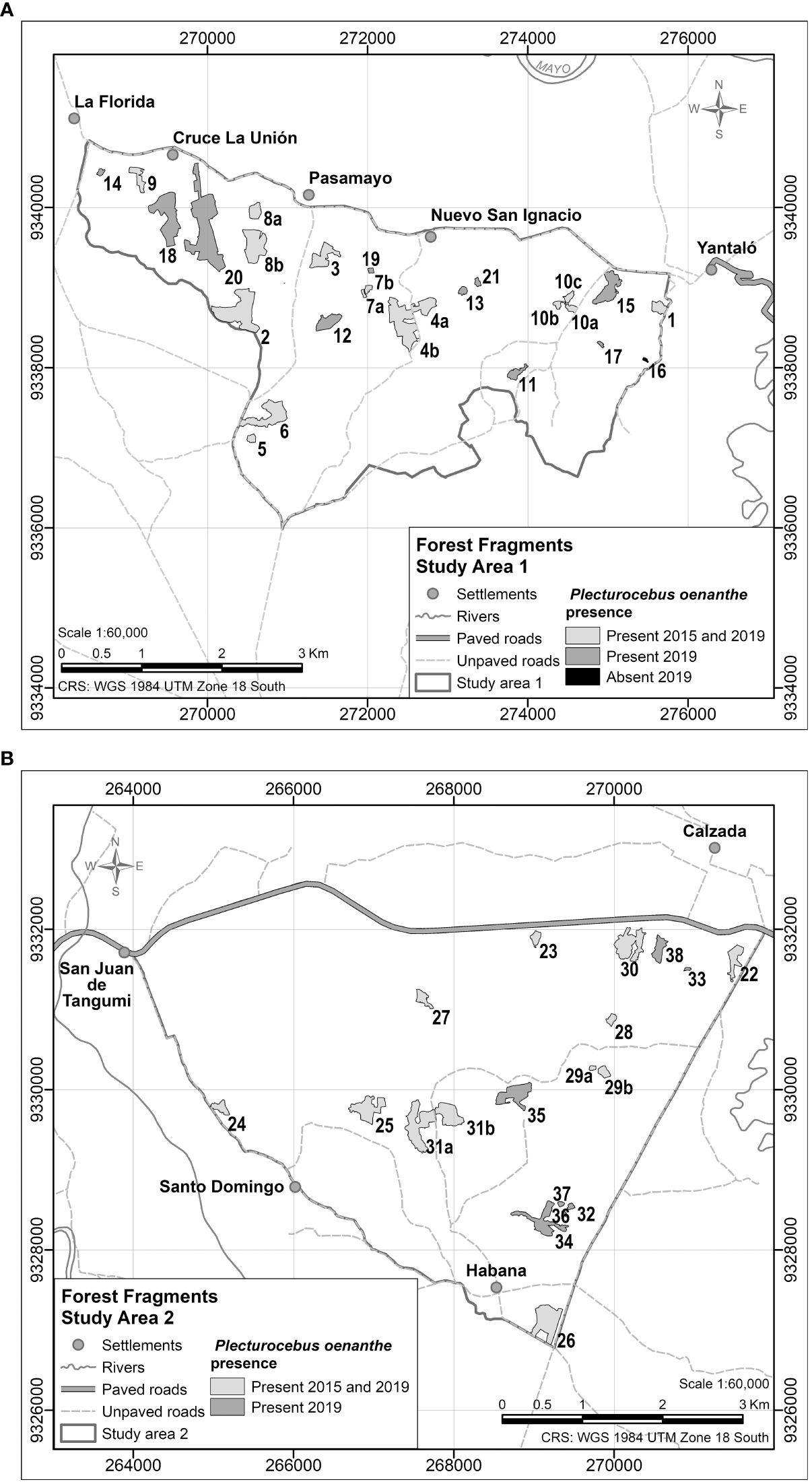
Figure 2. Geographic location of surveyed fragments in Area 1 (A) and Area 2 (B). Fragments with the same numeral, but labelled a, b or c represent fragments that belonged to single fragments in 2015 and were subsequently split into two or three fragments.
To calculate perimeter and area, we walked around the fragments and tracked the perimeter with a Garmin GPS (GPSmap 62s). From the GPS data, we calculated the area and the perimeter of the polygon with QGIS 3.10 (QGIS.org, 2020). If we could not walk around an entire fragment, we completed the polygon from satellite images (see 2.3).
We calculated the shape index (SI) of forest fragments with the SAGA Vector Polygon tool (Conrad et al., 2015) in QGIS 3.10 (QGIS.org, 2020) according to the following formula
where P is the perimeter and A is the area (Patton, 1975). An SI of 1 indicates a perfect circle; the higher SI, the more complex the shape.
We determined sampling points with ArcMap 10.6 (ESRI 2018). We drew a buffer of 20 m from the fragment border to avoid sampling at the often very low and dense vegetation along the border (Figure 3). We then drew a single transect or several transects, according to fragment size and shape, that maximized the number of sampling points separated by 20 m intervals. Sometimes difficult terrain or very dense vegetation made it impossible to follow drawn transects; in such cases, alternative transects were improvised in the field. In very small fragments, we could not completely exclude the 20 m buffer from the fragment border from sampling.

Figure 3. View of the border of a forest fragment. Note the low and dense vegetation along most parts of the border. Photo © Eckhard W. Heymann.
At each sample point, we employed the point-centered quadrat method (Krebs, 1999). We measured the distance to the nearest tree (including woody palms) with a dbh ≥10 cm; in the case of multiple stems, we measured the distance from the sampling point to the center between the stems. We added the radius of trees, calculated from their circumference, to the measured distance.
We visually estimated canopy height to the nearest meter. CLRV and ZLCC independently estimated height; if there was a disagreement, we repeated the height estimation together to reach a consensus.
We could not employ the point-centered quadrat method in four fragments because the fragment was too small (two fragments), the owner had set out shooting traps to hunt terrestrial rodents, or the fragment was very swampy. In these cases, we used existing trails for data collection.
We determined fragment occupation by visiting fragments at 06:30 h a.m., searching for titi monkeys and listening for their vocalizations. If we did not see or hear monkeys by 09:30 h a.m., we played a recording of their loud call; depending on the fragment size, we made the playback inside the fragment or from the edge. Playbacks readily attract titi monkeys to the speaker and provoke them to call back (Aldrich et al., 2008). If we did not detect monkeys after a maximum of three visits, we considered titi monkeys as being absent from the respective forest fragment.
From satellite images (see 2.3), we calculated the distance to the nearest forest fragment (edge to edge), distance to nearest paved and unpaved road, the total number of forest fragments per area, and the forest cover per study area. We compared changes in the number of fragments and in forest cover between 2015 and 2019.
To find out whether Areas 1 and 2 differed in any way that could have influenced the overall findings, we compared the characteristics statistically. For normally distributed data, we employed the T-test, for non-normally distributed data the Mann-Whitney U test (MWU test). We performed these tests in Statistica v.14 (TIBCO Software Inc, 2020).
Titi monkeys were present in 44 out of 45 surveyed forest fragments (Figures 2, 4). In 2019, they were present in all fragments already occupied in 2015, and in all fragments except one first surveyed in 2019. We detected titi monkeys during the first visit in 40 fragments, in five during the second visit (Supplementary Table 1).
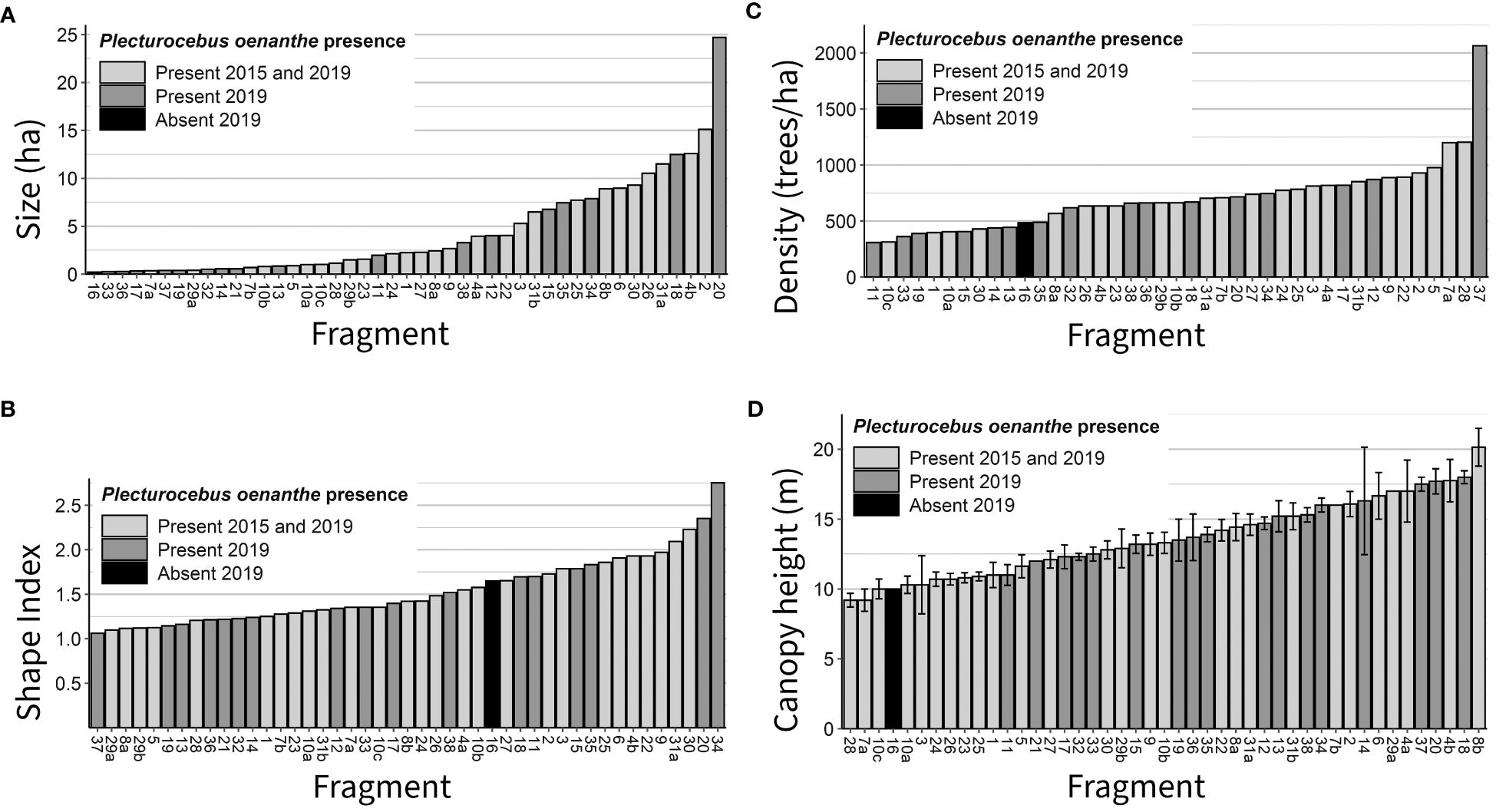
Figure 4. Presence/absence of Plecturocebus oenanthe in surveyed fragments in relation to fragment size (A), shape index (B), tree density (C) and mean canopy height (D).
Surveyed fragments ranged in size from 0.2 to 24.7 ha (Figure 4A). We encountered P. oenanthe in all fragments except the smallest one. The median size of all surveyed forest fragments was 2.3 ha (IQR: 0.7-7.5 ha); median fragment size did not differ between Area 1 (2.1 ha; IQR: 0.7-6.8 ha) and Area 2 (2.3 ha; IQR: 0.5-7.7 ha) (MWU test: U=238.50, Zadj=-0.184, pexact=0.847; Supplementary Figure 2A).
The shape index (SI) ranged from 1.06 to 2.75 (Figure 4B). The median SI was 1.42 (IQR: 1.24-1.79); the median SI did not differ between Area 1 (1.41; IQR: 1.25-1.73) and Area 2 (1.42; IQR: 1.21-1.86) (Supplementary Figure 2B). 91% of all the surveyed fragments had an SI<2; hence albeit small, most fragments are rounded.
Tree density ranged from 308 to 2065 trees/ha (Figure 4C). The median tree density was 663 trees/ha (IQR: 485-819); the median tree density did not differ between Area 1 (663 trees/ha; IQR: 405-819) and Area 2 (683.5 trees/ha; IQE: 634-783) (MWU test: U=176.00, Zadj=-0.801, pexact=0.427; Supplementary Figure 2C).
Canopy height ranged between 9 and 20 m (Figure 4D). Mean canopy height was 13.6 (SD: ± 2.7) m. Mean canopy height did not differ between Area 1 (14.0 m ± 3.1 m) and Area 2 (13.2 m ± 2.3 m; T-test: t=0.904, p>0.1 (Supplementary Figure 2D).
Most surveyed fragments were relatively close to a neighboring fragment (median: 29 m, IQR: 7-44 m) (Supplementary Figure 1). The median distance to the nearest fragment did not differ between Area 1 (15 m; IQR: 5-78 m) and Area 2 (34 m; IQR 9-43 m) (MWU test: U=231.00, Zadj=-0.356, pexact=0.724).
Distance to the nearest road (paved or unpaved) ranged between 0 and 724 m (median: 157 m; IQR: 44-355 m) (Supplementary Figure 1). The median distance to the nearest road did not differ between Area 1 (153 m; IQR: 44-340 m) and Area 2 (202 m; IQR 37-397 m) (MWU test: U=229.50, Zadj=-0.391, pexact=0.691).
Forest fragments in the matrix around the surveyed fragments were mostly small (Table 1; Supplementary Figure 3). The number of forest fragments increased in both Area 1 and 2. The median size of forest fragments in Area 1 decreased from 1.3 ha in 2015 to 1.0 ha in 2019, and remained unchanged in Area 2 (0.8 ha in both 2015 and 2019). Forest cover diminished between 2015 and 2019 in both Area 1 and 2 (Table 1).
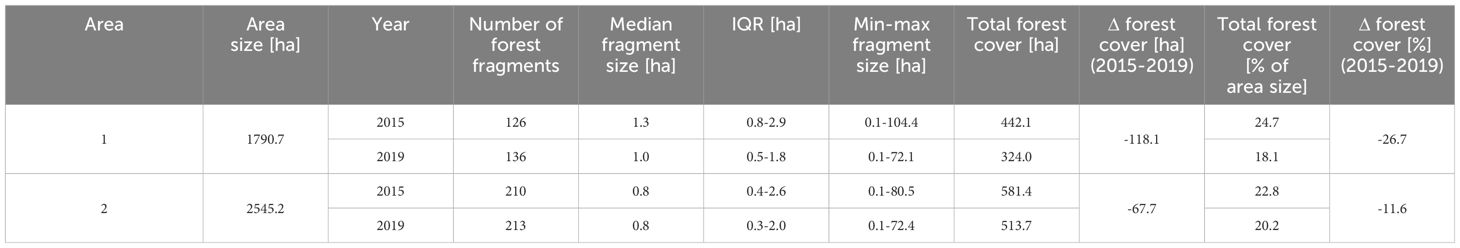
Table 1. Changes in the number of fragments, fragment size and forest cover in Areas 1 and 2 between 2015 and 2019.
Quite unexpectedly, our study found P. oenanthe to be present in all but one of the surveyed fragments. Also, titi monkeys were present in 2019 in all fragments surveyed in 2015, despite further fragmentation and reduction in forest cover between 2015 to 2019 (Table 1). Forest fragments varied strongly in all characteristics that we considered, except for shape. This indicates that the critically endangered P. oenanthe seems to be able to adapt to and to persist in a fragmented habitat embedded in an agricultural matrix, at least in the short term.
In our study, P. oenanthe occupied fragments as small as 0.3 ha. This is similar to dusky titi monkeys, Plecturocebus moloch, which occupy fragments as small as 0.5 ha (Michalski and Peres, 2005). Presence in such small fragments does not necessarily mean that a group occupies this fragment only. Rather, it is conceivable that titi monkeys occupy two or more neighboring fragments. However, we detected titi monkeys in the majority of cases upon the first visit to a fragment, which makes it plausible that they were indeed residing in the respective fragment only. In any case, residing in and travelling between different fragments remains an option. Such travelling would be facilitated if distances between fragments are short (Fahrig, 2017), which is the case in our study. This would allow titi monkeys to employ landscape complementation (i.e., fragments provide different resources, e.g., food in one, sleeping sites in another) and landscape supplementation (i.e., fragments provide supplementary resources, e.g., different food species) (Dunning et al., 1992).
The type and quality of vegetation around fragments will also be critical, as it determines the degree of isolation of forest fragments, matrix permeability and connectivity (Prugh et al., 2008; Pozo-Montuy et al., 2011; Benchimol and Peres, 2014). In our study areas, shrubland, young secondary forest, agroforestry coffee plantations, and banana plantations or cocoa plantations would allow for above ground or arboreal travelling, while unshaded coffee plantations, pineapple plantations and pastures could only be crossed via terrestrial travelling. So far, terrestrial foraging but not terrestrial travelling has been observed in P. oenanthe (DeLuycker, 2007). However, other Plecturocebus species regularly forage and travel on the ground, including travelling between forest fragments (Martínez and Wallace, 2011; Souza-Alves et al., 2019). Travelling between forest fragments, both above and on the ground, has been reported in different species of howler monkeys, genus Alouatta (e.g (Asensio et al., 2009; Arroyo-Rodríguez and Dias, 2010; Serio-Silva et al., 2019). On the way to surveying fragments, we observed titi monkeys feeding on bananas, mandarins and “taperiba”, Spondias mombin (Anacardiaceae) in farmland; moving through agroforestry areas, shrubland, timber plantations; and resting in a large tree with vines in an agroforestry area. Farmers reported having seen the titi monkeys feeding on farm fruit and they even left fruit out for them. They also reported titi monkeys moving through agroforestry areas and feeding on “guava”, Psidium guajava (Fabaceae), trees that provide shade for the coffee.
Apart from finding sufficient resources in fragments or complementing/supplementing resources by occupying two or more fragments, travelling between fragments is also extremely important for the social dynamics of these pair-living primates. Upon maturity, titi monkeys of both sexes usually disperse from their family group (Bossuyt, 2002; Van Belle et al., 2016). This may become increasingly more difficult if travelling between fragments is impeded, or if no unoccupied fragments are available. This could increase group size and – in the long term – change the social and mating system. Guyana sakis, Pithecia pithecia, and golden-faced sakis, Pithecia chrysocephala, usually live in small family groups with a single breeding pair (Norconk, 2007), but on an island and in an isolated forest fragment, respectively, groups contain multiple adult males and females (Setz and Gaspar, 1997; Norconk, 2006).
All species of the genus Plecturocebus, including P. oenanthe, occupy small home ranges, mostly <10 ha, and even in continuous forest, home ranges can be as small as 1.7 ha (Supplementary Table 1). This may dispose members of this genus to persist in small fragments, provided these harbor sufficient resources, both food and safe places for sleeping and resting. Whether the shape of fragments is an important factor, too, must remain open. In our study, most fragments were round (SI 1.2-1.4), which minimizes the amount of edge (Benítez-Malvido and Arroyo-Rodríguez, 2008).
Tree density varied strongly between fragments. However, we must point out that the number of sampling points for the point-centered quadrat method is lower than theoretically requested (Ganzhorn et al., 2011). Nevertheless, since the potential error applies to practically all fragments, the results still suggest that P. oenanthe can persist in forest fragments with quite divergent tree densities. The same holds true for canopy height.
Distance to roads also varied strongly; some fragments were immediately next to a road while others were a several hundred meters away. Thus, nearby roads do not appear to deter titi monkeys from living in forest fragments. While roads can constrain travel between fragments and crossing roads may be lethal (Asensio et al., 2017; Hetman et al., 2019), Galán-Acedo et al. (2019) did not find any significant effect of road abundance in fragmented landscape on primate populations.
In summary, our study demonstrates that P. oenanthe can persist in a highly fragmented habitat, seemingly tolerating a broad range of fragment characteristics, giving hope for the future of this Critically Endangered species. Thus, the major threat may not consist in forest fragmentation per se, but in forest loss, as suggested by Galán-Acedo et al. (2019) for primates in general. Nevertheless, there is currently no point for a re-evaluation of this categorization. First, detailed data are needed to see whether population densities remain stable or continue to decrease. Second, data are needed on social dynamics (group size and composition). Unfortunately, we have data neither on population density nor on group size and composition, to see whether these variables have changed since 2015. Also, we do not know whether all groups or only some are reproductive, which would be crucial for the long-term persistence in fragments and for the stability of the population. Despite continued occupation of all fragments surveyed in 2015 and 2019 and occupation of all fragments added in 2019, we cannot rule out that reproduction is impaired and population size is decreasing. However, observations on the presence of infants and juveniles suggest that reproduction can take place in groups living in fragments (DeLuycker, 2007: two infants during study period in one group in a 3 ha fragment; Hodges, 2020: two groups with one infant each in fragments of 2.15 ha and 4.15 ha, respectively; Huashuayo-Llamocca, 2017: one juvenile in group living in a 4 ha fragment). Only the collection of demographic data during more detailed and long-term monitoring of fragment occupation can resolve this issue. Third, data are needed on the productivity of forest fragments, to evaluate whether they can provide enough food resources for P. oenanthe in the long run. In any case, the reduction in forest cover in the two study areas from 2015 to 2019 is an alarming signal. Prevention of further habitat loss, along with maintaining (or increasing) connectivity between fragments, remains a conservation priority. Modelling connectivity under scenarios with increasing fragmentation or scenarios with reforestation will help to guide conservation efforts (Schaffer-Smith et al., 2016), but ground-based evaluation of populations, fragment occupation and permeability of the matrix in different parts of the distributional area of P. oenanthe remain crucial.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the study was conducted as part of the research and conservation activities of the Proyecto Mono Tocón.
CLRV: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft. ZLCC: Investigation, Writing – original draft. EWH: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the German Academic Exchange Service (DAAD) through the PROMOS program and by Global Wildlife Conservation (grant no.5149.029-0211).
We are grateful to the Proyecto Mono Tocón (PMT) in Moyobamba, Peru, for support and collaboration, particularly to Maribel Monica Taco Huallpa and Julio César Tello Alvarado. We thank the Comisión Nacional de Investigación y Desarrollo Aeroespacial (CONIDA), in Lima, Peru, for providing satellite images. Finally, we are thankful to three reviewers and to Editor Dr. Carlos R. Ruiz-Miranda for their constructive comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2024.1401517/full#supplementary-material
Aldrich B. C., Molleson L., Nekaris K. A. I. (2008). Vocalizations as a conservation tool: an auditory survey of the Andean titi monkey Callicebus oenanthe Thomas 1924 (Mammalia: Primates: Pitheciidae) at Tarangue, Northern Peru. Contributions Zool. 77, 1–6. doi: 10.1163/18759866-07701001
Arroyo-Rodríguez V., Dias P. A. D. (2010). Effects of habitat fragmentation and disturbance on howler monkeys: a review. Am. J. Primatol. 72, 1–16. doi: 10.1002/ajp.20753
Asensio N., Arroyo-Rodríguez V., Dunn J. C., Cristóbal-Azkarate J. (2009). Conservation value of landscape supplementation for howler monkeys living in forest patches. Biotropica 41, 768–773. doi: 10.1111/j.1744-7429.2009.00533.x
Asensio N., Murillo-Chacon E., Schaffner C. M., Aureli F. (2017). The effect of roads on spider monkeys’ home range and mobility in a heterogeneous regenerating forest. Biotropica 49, 546–554. doi: 10.1111/btp.12441
Benchimol M., Peres C. A. (2014). Predicting primate local extinctions within “real-world” forest fragments: A pan-neotropical analysis. Am. J. Primatol. 76, 289–302. doi: 10.1002/ajp.22233
Benítez-Malvido J., Arroyo-Rodríguez V. (2008). “Habitat fragmentation, edge effects and biological corridors in tropical ecosystems,” in Encyclopedia of Life Support Systems (EOLSS). Eds. Claro K.D., Oliveira P. S., Rico-Gray V., Ramirez A., Almeida A. A., Bonet A., Scarano F. R., Consoli F. L., Morales F. J., Naoki J., Costello J. A., Sampaio M. V., Quesada M., Morris M. R., Palacios M., Ramirez N., Marcal O., Ferraz R. H., Marquis R. J., Parentoni R. R., Lüttge U. (Eolss Publishers Oxford, Oxford).
Bicca-Marques J. C., Chaves Ó. M., Hass G. P. (2020). Howler monkey tolerance to habitat shrinking: Lifetime warranty or death sentence? Am. J. Primatol. 82, e23089. doi: 10.1002/ajp.23089
Bicca-Marques J. C., Heymann E. W. (2013). “Ecology and behavior of titi monkeys, genus Callicebus,” in Evolutionary biology and conservation of titis, sakis and uacaris. Eds. Veiga L. M., Barnett A. A., Ferrari S. F., Norconk M. A. (Cambridge University Press, Cambridge), 196–207.
Bossuyt F. (2002). Natal dispersal of titi monkeys (Callicebus moloch) at Cocha Cashu, Manu National Park, Peru. Am. J. Phys. Anthropol. 117, 47.
Buja K., Menza C. (2013). Sampling design tool for ArcGIS – Instruction Manual (Silver Spring, MD: NOAA).
Byrne H., Rylands A. B., Carneiro J. C., Lynch Alfaro J. W., Bertuol F., da Silva M. N. F., et al. (2016). Phylogenetic relationships of the New World titi monkeys (Callicebus): first appraisal of taxonomy based on molecular evidence. Front. Zool. 13, 1–26. doi: 10.1186/s12983-016-0142-4
Climate-data.org. (2024). Climate: Moyobamba. Available online at: https://en.climate-data.org/south-america/peru/san-martin/moyobamba-4364.
Conrad O., Bechtel B., Bock M., Dietrich H., Fischer E., Gerlitz L., et al. (2015). System for automated geoscientific analyses (SAGA) v. 2.1.4. Geoscientific Model. Dev. 8, 1991–2007. doi: 10.5194/gmd-8-1991-2015
Cristóbal-Azkarate J., Veà J. J., Asensio N., Rodríguez-Luna E. (2005). Biogeographical and floristic predictors of the presence and abundance of mantled howlers (Alouatta palliata mexicana) in rainforest fragments at Los Tuxtlas, Mexico. Am. J. Primatol. 67, 209–222. doi: 10.1002/(ISSN)1098-2345
DeLuycker A. M. (2007). The ecology and behavior of the Rio Mayo titi monkey (Callicebus oenanthe) in the Alto Mayo, northern Peru (Washington University: PhD thesis).
DeLuycker A. M. (2012). Insect prey foraging strategies in Callicebus oenanthe in Northern Peru. Am. J. Primatol. 74, 450–471. doi: 10.1002/ajp.22002
DeLuycker A. M. (2024). Diet and feeding ecology of the Critically Endangered San Martín titi monkey (Plecturocebus oenanthe) in Peru. Int. J. Primatol. 45, 104–126. doi: 10.1007/s10764-021-00256-w
Dunning J. B., Danielson B. J., Pulliam H. R. (1992). Ecological processes that affect populations in complex landscapes. Oikos 65, 169–175. doi: 10.2307/3544901
Estrada A., Garber P. A., Rylands A. B., Roos C., Fernandez-Duque E., Di Fiore A., et al. (2017). Impending extinction crisis of the world’s primates: why primates matter. Sci. Adv. 3, e1600946. doi: 10.1126/sciadv.1600946
Ewers R. M., Didham R. K. (2006). Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 81, 117–142. doi: 10.1017/S1464793105006949
Fahrig L. (2003). Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Syst. 34, 487–515. doi: 10.1146/annurev.ecolsys.34.011802.132419
Fahrig L. (2017). Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 48, 1–23. doi: 10.1146/annurev-ecolsys-110316-022612
Galán-Acedo C., Arroyo-Rodríguez V., Cudney-Valenzuela S. J., Fahrig L. (2019). A global assessment of primate responses to landscape structure. Biol. Rev. 94, 1605–1618. doi: 10.1111/brv.12517
Ganzhorn J. U., Rakotondranary S. J., Ratovomanana Y. R. (2011). “Habitat description and phenology,” in Field and laboratory methods in primatology. A practical guide. Eds. Setchell J. M., Curtis D. J. (Cambridge University Press, Cambridge), 51–68.
Hetman M., Kubicka A. M., Sparks T. H., Tryjanowski P. (2019). Road kills of non-human primates: A global view using a different type of data. Mammal Rev. 49, 276–283. doi: 10.1111/mam.12158
Hodges S. (2020). Paternal activity budgets of the Peruvian San Martín titi monkey (Plecturocebus oenanthe) in response to habitat destruction, seasonality, thermal stress, and infant care. Primate Conserv. 34, 1–12. Available at: http://www.primate-sg.org/storage/pdf/PC34_Hodges_P_oenanthe.pdf.
Huashuayo-Llamocca R. (2017). Algunos aspectos ecológicos del mono tocón Callicebus oenanthe (Primates: Pitheciidae) durante la temporada seca, en un bosque secundario fragmentado del área protegida “Morro de Calzada”, Moyobamba - San Martín. Agosto 2014 - Enero 2015 (Universidad Nacional San Luis Gonzaga de Ica).
Marsh L. K., Chapman C. A., Arroyo-Rodríguez V., Cobden A., Dunn J. C., Gabriel D., et al. (2013). “Primates in fragments 10 years later: once and future goals,” in Primates in fragments: Complexity and resilience. Eds. Marsh L. K., Chapman C. A. (Springer, New York), 505–525.
Martínez J., Wallace R. B. (2011). First observations of terrestrial travel for Olalla’s titi monkey (Callicebus olallae). Neotropical Primates 18, 49–52. Available at: http://www.primate-sg.org/storage/pdf/NP18.2_Martinez__Wallace_pp.49-52.pdf.
Maxwell S. L., Fuller R. A., Brooks T. M., Watson J. E. (2016). Biodiversity: The ravages of guns, nets and bulldozers. Nature 536, 143–145. doi: 10.1038/536143a
Michalski F., Peres C. A. (2005). Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol. Conserv. 124, 383–396. doi: 10.1016/j.biocon.2005.01.045
Murcia C. (1995). Edge effects in fragmented forests: implications for conservation. Trends Ecol. Evol. 10, 58–62. doi: 10.1016/S0169-5347(00)88977-6
Norconk M. (2006). Long-term study of group dynamics and female reproduction in Venezuelan Pithecia pithecia. Int. J. Primatol. 27, 653–674. doi: 10.1007/s10764-006-9030-7
Norconk M. A. (2007). “Saki, uakaris, and titi monkeys: Behavioral diversity in a radiation of primate seed predators,” in Primates in perspective. Eds. Campbell C.J. Fuentes A., MacKinnon K. C., Panger M., Bearder S. K. (Oxford University Press, New York), 123–138.
Patton D. R. (1975). A diversity index for quantifying habitat “edge”. Wildlife Soc. Bull. 3, 171–173. Available at: https://www.jstor.org/stable/3781151.
Pozo-Montuy G., Serio-Silva J. C., Bonilla-Sánchez Y. M. (2011). Influence on the landscape matrix on the abundance of arboreal primates in fragmented landscapes. Primates 52, 139–147. doi: 10.1007/s10329-010-0231-5
Prugh L. R., Hodges K. E., Sinclair A. R., Brashares J. S. (2008). Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. 105, 20770–20775. doi: 10.1073/pnas.0806080105
QGIS.org. (2020). QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available at: https://www.qgis.org.
Romero Herrada R. J. (2018). Caracterización y estructura del hábitat del mono tocón de San Martín (Plecturocebus oenanthe Thomas 1924) en el Morro de Calzada (San Martín, Perú) (Universidad Nacional Mayor San Marcos: Tesis titular).
Schaffer-Smith D., Swenson J. J., Boveda-Penalba A. J. (2016). Rapid conservation assessment for endangered species using habitat connectivity models. Environ. Conserv. 43, 221–230. doi: 10.1017/S0376892915000405
Serio-Silva J. C., Ramírez-Julián R., Eppley T. M., Chapman C. A. (2019). “Terrestrial locomotion and other adaptive behaviors in howler monkeys (Alouatta pigra) living in forest fragments,” in Movement ecology of Neotropical forest mammals: Focus on social animals. Eds. Reyna-Hurtado R., Chapman C. A. (Springer, Cham), 125–140.
Setz E. Z. F., Gaspar D. A. (1997). Scent-marking behavior in free-ranging golden-faced saki monkeys, Pithecia pithecia chrysocephala: sex differences and context. J. Zool. London 241, 603–611. doi: 10.1111/j.1469-7998.1997.tb04852.x
Shanee S., Aldrich B., Pacheco V., Serrano-Villavicencio J. (2024). Callicebus oenanthe (Primates: Pitheciidae). Mamm. Species 56, 1–13. doi: 10.1093/mspecies/seae002
Shanee S., Tello-Alvarado J. C., Vermeer J., Bóveda-Penalba A. J. (2013). GIS risk assessment and GAP analysis for the Andean titi monkey (Callicebus oenanthe). Primate Conserv. 26, 17–23. doi: 10.1896/052.026.0111
Souza-Alves J. P., Mourthe I., Hilário R. R., Bicca-Marques J. C., Rehg J., Gestich C. C., et al. (2019). Terrestrial behavior in titi monkeys (Callicebus, Cheracebus, and Plecturocebus): potential correlates, patterns, and differences between genera. Int. J. Primatol. 40, 553–572. doi: 10.1007/s10764-019-00105-x
Strier K. B. (2021). The limits of resilience. Primates 62, 861–868. doi: 10.1007/s10329-021-00953-3
TIBCO Software Inc. (2020). Data Science Workbench, version 14. Available online at: http://tibco.com.
Van Belle S., Fernandez-Duque E., Di Fiore A. (2016). Demography and life history of wild red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador: A 12-year study. Am. J. Primatol. 78, 204–215. doi: 10.1002/ajp.22493
Vermeer J., Shanee S. (2020). Plecturocebus oenanthe. The IUCN Red List of Threatened Species 2020, e.T3553A17975319. doi: 10.2305/IUCN.UK.2020-3.RLTS.T3553A17975319.en
Keywords: habitat fragmentation, fragment occupation, neotropics, Pitheciidae, terrestriality, conservation, Amazonia, Peru
Citation: Rubio Vargas CL, Cerón Cancharis ZL and Heymann EW (2024) Characterization of forest fragments occupied by the critically endangered and endemic San Martín titi monkey (Plecturocebus oenanthe). Front. Conserv. Sci. 5:1401517. doi: 10.3389/fcosc.2024.1401517
Received: 15 March 2024; Accepted: 20 June 2024;
Published: 08 August 2024.
Edited by:
Carlos R. Ruiz-Miranda, Universidade Estadual do Norte Fluminense, BrazilReviewed by:
Karen B. Strier, University of Wisconsin-Madison, United StatesCopyright © 2024 Rubio Vargas, Cerón Cancharis and Heymann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eckhard W. Heymann, ZWhleW1hbkBnd2RnLmRl
†Present addresses: Carina Linda Rubio Vargas, School of Natural Sciences, Faculty of Science and Engineering, Macquarie University, Sydney, Australia
Zoila Lasmit Cerón Cancharis, CATIE - Tropical Agricultural Research and Higher Education Center, Cartago, Turrialba, Costa Rica
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.