
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci. , 08 May 2024
Sec. Plant Conservation
Volume 5 - 2024 | https://doi.org/10.3389/fcosc.2024.1386204
This article is part of the Research Topic Conserving Plants in a Changing Climate View all articles
 Junaid A. Magray1*
Junaid A. Magray1* Bilal A. Wani1
Bilal A. Wani1 Hanan Javid1*
Hanan Javid1* Tajamul Islam1
Tajamul Islam1 Aijaz H. Ganie2
Aijaz H. Ganie2 Roof Ul Qadir1
Roof Ul Qadir1 Irshad A. Nawchoo1
Irshad A. Nawchoo1Introduction: Phytolacca acinosa Roxb. is a highly valuable multipurpose herb native to the Himalayan region. Unsustainable harvesting of this species due to its diverse uses has resulted in a rapid decline in its population across natural habitats, thereby necessitating its propagation and conservation. To overcome this challenge, the potential of P. acinosa rhizomes for ex situ regeneration was evaluated.
Methods: The current study aims to develop a standard propagation protocol for P. acinosa. Rhizome cuttings derived by splitting whole rhizomes were used to study the effect of various hormones and soil compositions on their sprouting and growth performance.
Results: Soil compositions SC10 and SC5 consisting of soil, sand, pebbles, and vermicompost (1:1:1:1) and soil, sand, and vermicompost (1:1:1), respectively, were the most suitable compositions for the optimum growth of this species. The rhizome segments treated with GA3 (150ppm) induced the highest sprouting percentage (91.67%), with a minimum sprouting time of 23.25 days. The maximum root length (9.25 cm), shoot length (16.5 cm), and leaf number (11.25) were recorded for GA3 (150ppm) treated rhizome cuttings.
Conclutions: Overall, the results of the present study helped in establishing a cost-effective, rapid, efficient, and simple mass propagation method for the target species. The results of this study will serve as a guide for the large-scale cultivation, effective conservation, and sustainable utilization of this economically valuable medicinal herb.
The conservation of plant genetic resources involves two main approaches: in situ, which involves protecting populations in their natural habitat, and ex situ, which involves protecting these resources outside their natural environment. The rising demand from pharmaceutical industries for medicinal plants has led to their increased harvesting from the wild. Anthropogenic threats including overexploitation, habitat degradation, overgrazing, and illegal trade practices have pushed many medicinal plant species to the verge of extinction (Ganie et al., 2019; Chisale et al., 2021; Wani et al., 2022; Wani et al., 2024a). Given the urgency of conserving such species, ex situ conservation methods are recognized as a viable and effective option for the conservation and sustainable utilization of threatened plant species (Dávila-Aranda et al., 2016). This serves as both a backup conservation strategy and, in certain instances, a temporary substitute for in situ conservation.
Vegetative propagation is an important and promising method for mass cultivation of economically important species, especially those posing difficulty when raised through seeds. Propagation through rhizomes is a convenient and effective method for the rapid multiplication of elite plant populations and for conserving their essential genetic characteristics (Lata et al., 2017; Reddy et al., 2022). It is an easy, cost-effective, and successful technique for the large-scale cultivation, conservation, and regeneration of threatened plant species. While seed germination offers the advantage of producing numerous individuals from a single mother plant, its success is constrained by the need for complex stratification treatments and the slow growth of seedlings in most forest understory plant species (Baskin and Baskin, 1998; Luna, 2001). Thus, vegetative propagation in plants, especially alpine species, is considered more important than sexual reproduction (Brožová et al., 2019). Furthermore,vegetative propagation, as compared to sexual reproduction, enables the development of mature individuals within a year of cultivation. It stands out as a highly effective method for conserving economically important overexploited species, contributing to advancements in propagation techniques, and fostering large-scale commercial cultivation.
Phytolacca acinosa Roxb., commonly known as pokeberry,belongs to the family Phytolaccaceae. It is an economically valuable perennial medicinal plant native to the East Asian and Himalayan regions (POWO, 2023). In the traditional medicine system, it is widely employed to treat eye disorders, body aches, swelling, edema, sores, and as a diuretic drug (Basnet and Kalauni, 2020). Rhizomes of P. acinosa contain numerous bioactive compounds, including chochliophilin (A and B), hypaphorine, esculentosides, β-sitosterol, monoglyceride, daucosterol, phytolacacinoside (A, B, and E), phytolaccoside (A, B, and E), esculentoside G, and palmitic acid (Gao et al., 2009; Krishan et al., 2022; Li et al., 2023). Consequently, it serves as an important medicinal herb with diverse pharmacological properties like antibacterial, anti-fungal, anti-inflammatory, antiviral, anti-oxidative, anticancer, immunity-enhancing, anti-parasitic, and insecticidal properties (Gao et al., 2009; Cheng et al., 2017; Bailly, 2021). It has been reported to exhibit cytotoxicity in human cancer cell lines and antimicrobial activity in bacterial culture (Ma et al., 2017, 2019; Krishan et al., 2022). Besides being used as medicine, P. acinosa is also used as a source of food, red dye for coloring wool fabric, and for phytoremediation of heavy metals like cadmium (Cd), lead (Pb), manganese (Mn), and zinc (Zn) (Zhao et al., 2014; Wu et al., 2016; Liu et al., 2022). During the last few decades, climate change, habitat destruction, and unsustainable harvesting of P. acinosa due to its diverse uses have resulted in a rapid decline in its population across natural habitats (Cheng et al., 2017; Magray et al., 2023; Wani et al., 2024b).
The present study aimed to develop an agro-technique protocol using rhizome cuttings for the vegetative propagation of P. acinosa, a multipurpose medicinal plant native to the Himalaya (Magray et al., 2022). Although the species reproduces through both sexual and asexual means, the time-consuming nature of seed germination and vegetative propagation under natural conditions poses a challenge in generating well-developed seedlings (Magray et al., 2023).Vegetative propagation through rhizomes under in vitro conditions offers an alternative method for rapid and mass multiplication of the target plant and enables the cultivation of plants with the desired clone. Furthermore, different plant growth regulators are widely used in vegetative propagation to stimulate rooting and improve the growth of cuttings (Yoon et al., 2021).
To the best of our knowledge, the vegetative propagation of P. acinosa has not been carried out to date. In this context, the current study was conducted with the following specific objectives: (i) to investigate the effect of different growth hormones on the vegetative propagation potential of P. acinosa; (ii) to determine the effect of different soil compositions on sprouting percentage and related growth parameters; and, based on the scientific insights gained, (iii) to outline a reproducible protocol for vegetative propagation of the target species for its conservation, restoration, and mass production for the pharmaceutical industries. This study may serve as a fundamental guide for the restoration of this valuable plant species in Kashmir Himalaya and also provide scientific insights for the restoration of other valuable medicinal plants growing elsewhere in the world.
The fresh rhizomes of P. acinosa were harvested during February 2022 from the wild populations of Drung and Gogaldara, Jammu and Kashmir, India (Figure 1). The collected rhizomes were washed thoroughly with running tap water and kept in a dark and cool room (4°C) to avoid the emergence of buds until the experiments started. The experiment was conducted at Kashmir University Botanical Garden (KUBG), University of Kashmir, Srinagar (1590m asl; 34.13254722°N, 74.83755278°E), with a humid temperate climate and a high mean annual rainfall of 1005.5 mm (Dad et al., 2021).
Freshly collected rhizomes were cut longitudinally into different sections, each 4 ± 0.5 g and with 2 buds (Figures 2A, B). Prepared rhizome cuttings were disinfected by a surface wash with a 2% solution of the systemic fungicide, Bavistin. The rhizome cuttings were sown in different soil compositions prepared by putting weighed amounts of soil, sand, pebbles, vermicompost (VC), farmyard manure (FYM), and peat in pots (Table 1). In each composition, five replicates of three cuttings were used. The pots were transferred to the pot house under 60% shade, and irrigation was usually performed by daily watering for the first week of the experiment and subsequently when the soil on the surface started drying. The total sprouting percentage was calculated at the culmination of the experiment for all the treatments. Plants were harvested destructively and were analyzed for various morphological parameters like rhizome length, rhizome breadth, plant height, leaf number, leaf length, leaf breadth, and biomass allocation towards above and belowground parts (Figure 2F).

Figure 2 (A–F) Effect of different hormone treatments and soil compositions on different growth parameters of Phytolacca acinosa. (A) Whole rhizome with buds; (B) Rhizome cuttings; (C) Hormone treatment of rhizome cuttings; (D) Harvested material after hormone treatments; (E) Plant growth in different soil compositions; (F) Record of morphological parameters and biomass accumulation.
To study the effect of different growth hormones on the clonal propagation of P. acinosa, the rhizomes were divided longitudinally into 2-6 sections (each 4 ± 0.5 g and 2 buds) with a sterilized sharp knife, depending on the size and number of apical buds on the parental rhizome. The rhizome cuttings were disinfected by a surface wash with a 2% Bavistin solution followed by treatment with different growth hormones including Kinetin, Indole Butyric Acid (IBA), Indole Acetic Acid (IAA), and Gibberellic Acid (GA3) (50ppm, 100ppm, 150ppm, and 200ppm), and distilled water (control) for 48 hours (Figure 2C; Supplementary Material S1). The treated rhizome cuttings were planted in pots containing a mixture of soil and sand in a 1:1 ratio. The pots were placed under partial shade (60%) in pot-house at Kashmir University Botanical Garden and irrigated daily for the first few days (10 days) and after that every 3rd day without fertigation. The experiment consisted of 19 treatments (Supplementary Material S1), each repeated four times in a randomized block design with three cuttings per treatment. The experiment was monitored regularly, and growth parameters including root length, shoot length, leaf number,time taken for sprouting (days), and sprouting percentage were recorded (Figure 2F). The average number of leaves per plant was counted manually, while root length (cm) and shoot length (cm) were measured using a scale.
Data from the experiment were subjected to analysis of variance (ANOVA). The analyses were carried out using IBM SPSS Statistics software (version 23). ANOVA was performed to evaluate the effect of different soil compositions and growth hormones on different growth parameters and morphological traits like sprouting percentage, time taken for sprouting, rhizome length, rhizome breadth, plant height, leaf number, leaf length, leaf breadth, and biomass allocation towards above and belowground parts. Tukey’s test (at p ≤ 0.05) was conducted for statistical mean comparisons.
The sprouting percentage varied significantly among different soil compositions (Table 2). The highest sprouting percentage of rhizome cuttings was recorded in SC10 treatment (100%) consisting of soil, sand, pebbles, vermicompost (1:1:1:1) followed by SC5 (91.67%) having soil, sand, vermicompost in the ratio of 1:1:1, and SC9 (83.33%) treatment having soil, pebbles, vermicompost in 1:1:1 ratio (Table 2; Figure 2E,). The sprouting time of rhizome cuttings also varied significantly with different soil compositions. The shortest sprouting time was recorded in the SC10 treatment (26 days), followed by the SC5 treatment (28.75 days) (Table 2). All morphological parameters also showed significant variation among the various treatments (Table 2; Figures 3A, B). Maximum rhizome length and rhizome breadth of 15.38±0.59cm and 3.13±0.31cm, respectively, were observed for soil composition SC10, followed by composition SC5 (Table 2; Figure 3A). Similarly, maximum plant height (34.2±0.86 cm) and leaf number (49.65±1.03) were recorded in SC10 composition(Table 2; Figure 3A). The leaf length was highest in the SC10 treatment (8.83±0.44 cm), followed by SC5 (7.53±0.51 cm). Likewise, the leaf breadth was highest in the SC5 treatment (2.73±0.4 cm), followed by SC10 (2.63±0.13 cm) (Table 2; Figure 3A). The analysis of variance also exhibited a significant variation in the above and belowground biomass among all the treatments (Table 2). Both the highest aboveground biomass of 11.65±0.66 g and belowground biomass of 31.79±0.88 g were estimated in SC10 composition followed by SC9 with above and belowground biomass of 9.95±0.8 g and 30.05±0.86 g respectively (Table 2; Figure 3B).
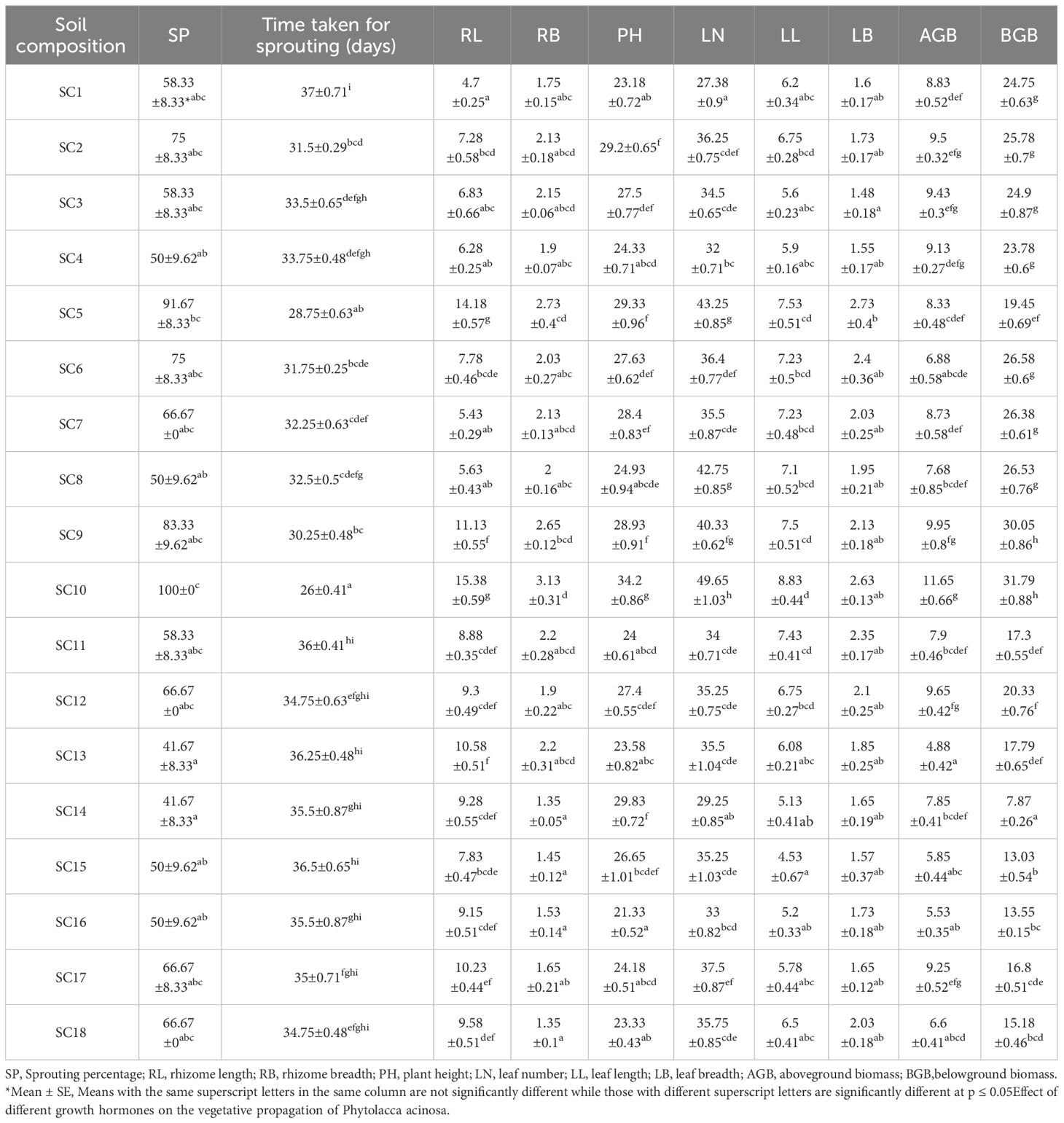
Table 2 Effect of different soil combinations on growth and morphological parameters of rhizome cuttings.
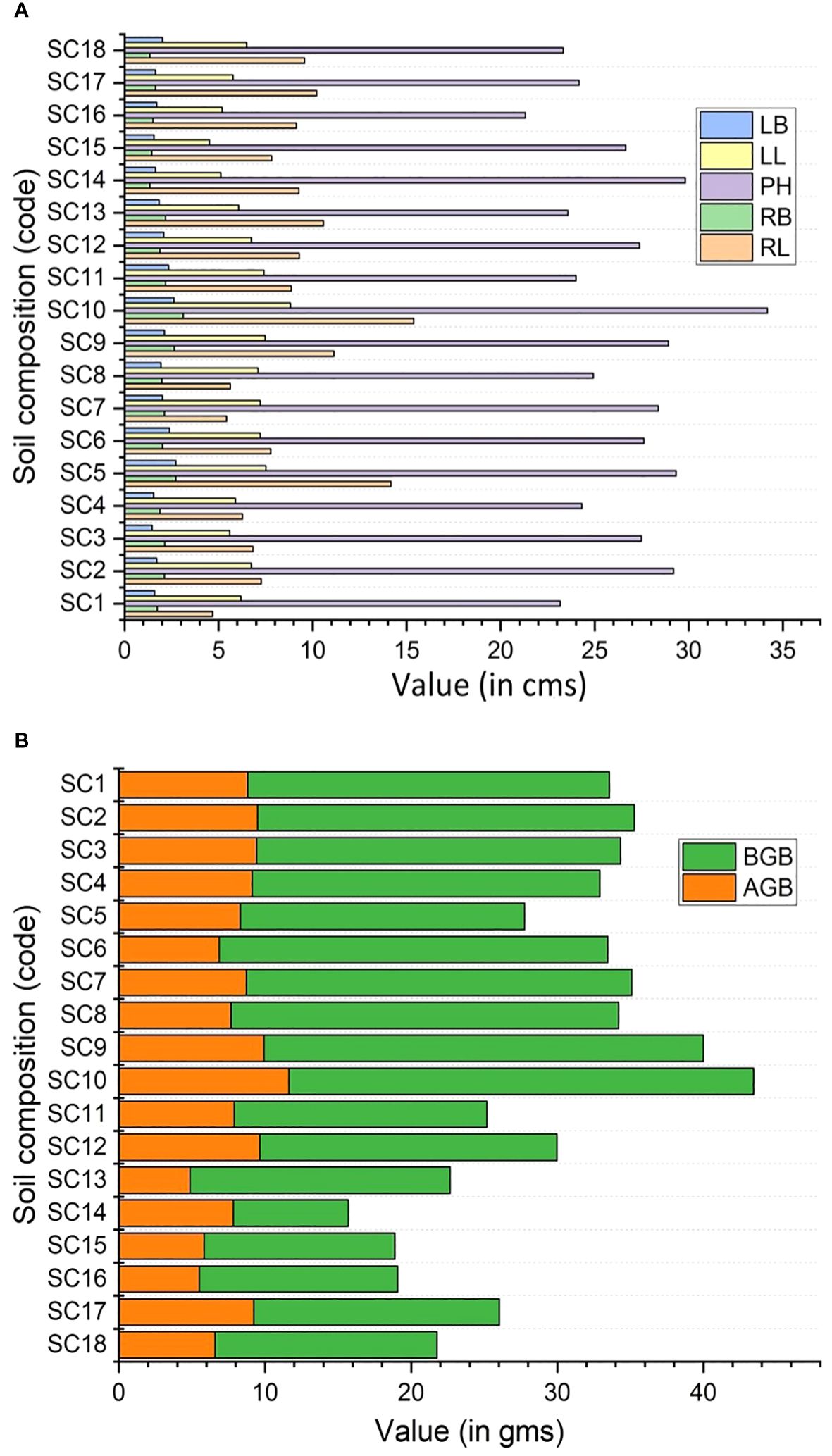
Figure 3 (A, B) Effect of soil composition on morphological parameters and biomass allocation of Phytolacca acinosa. (A) RL, rhizome length; RB, rhizome breadth; PH, plant height; LN, leaf number; LL, leaf length; LB, leaf breadth; (B) AGB, aboveground biomass; BGB, belowground biomass.
The rhizome cuttings treated with different phytohormones showed significant variation in all the growth parameters (Table 3; Figures 2D, F). The highest sprouting percentage (91.67%) was observed in the rhizome divisions treated with GA3 (150ppm), followed by (83.33%) treated with GA3 (100ppm) as compared to the control with 33.3% of sprouting (Table 3, Figure 2D). The auxins used (IAA and IBA) failed to stimulate the sprouting of rhizome cuttings. The sprouting time varied among different treatments, ranging from 5.25 to 9.25 (Table 3). GA3 at a concentration of 150ppm was most effective in reducing the sprouting time of rhizome cuttings. GA3 (150ppm) was most effective in reducing the sprouting time of rhizome cuttings. The highest sprouting percentage was recorded in 150ppm GA3 (91.67%) and 100ppm GA3 (83.33%); sprouting time was also attained rapidly with 23.25 and 25 days, respectively; however, the sprouting percentage of 33.33% in control was completed with a sprouting time of 35.75 days (Table 3). Further, treatment of rhizome cuttings with gibberellic acid also significantly increased the shoot length, root length, and leaf number on plants. The maximum number of leaves (11.25±0.63) was observed in GA3 (150ppm) followed by 50ppm GA3 (9.75±0.63). The highest root length (9.25±0.48 cm) and shoot length (16.5±0.65 cm) were also recorded in rhizome cuttings treated with GA3 (150ppm) (Table 3).
Vegetative reproduction offers an efficient means for mass multiplication of germplasm and cultivation for species exhibiting poor regeneration through sexual means (Deepak et al., 2016; Sreekissoon et al., 2021). The frequent weather changes at high elevations result in reproductive failures at various developmental stages of plants (Carbognani et al., 2018). Phytolacca acinosa, a typical sub-alpine plant, exhibits poor seed germination within its wild habitats (Magray et al., 2023). The present study demonstrated multi-approaches for the propagation of P. acinosa through rhizome cuttings to compensate for this reproductive bottleneck. In vegetative propagation, the application of plant growth regulators and various chemicals has been extensively employed to enhance the rooting and subsequent growth of rhizome cuttings (Nadeem et al., 2000; Butola and Badola, 2007; Bisht and Bhatt, 2014). During the present study, the vegetative growth and sprouting percentage of the plant was found to be highest at intermediate hormone concentrations (Table 3). The best results were recorded for GA3 150ppm and kinetin 150ppm.Gibberellic acid is an important phytohormone, as it is at the core of many plant growth and developmental processes. Gibberellic acid (GA3)-treated rhizome cuttings showed a significant increase in root and shoot development (Ma and Huang, 2016). It is reported to enhance the sprouting percentage and improve the growth and survival of vegetatively propagated plants under in vitro conditions (Shabir et al., 2010; Hassan et al., 2020). In blueberries, gibberellic acid induces plant growth and enhances leaf development as compared to the control (Zang et al., 2016). Consistent with earlier studies (Tuna et al., 2008; Wen et al., 2010; Bose et al., 2013), GA3 application enhanced various plant growth characteristics like leaf number, root length, and shoot length in P. acinosa. Our results conform to those of Shabir et al. (2010), who also reported a significant increase in growth-related parameters in Inula racemosa when treating rhizomes with GA3.Gibberellins can influence plant growth by accelerating the movement of cytokinins to developing buds and also by altering carbohydrate metabolism (Salimi et al., 2010). Further, similar to our findings, cytokinins have been reported to increase the shoot length and leaf number in Phytolacca dodecandra (Chishimba et al., 1999). Kinetin also improved the shooting and leaf numbers in Phytolacca americana (Trunjaruen et al., 2022). The efficacy of applying cytokinins (kinetin) and GA3 in promoting sprouting responses, root and shoot development among rhizome explants has been reported in several plant species, such as Pongamia pinnata (Sugla et al., 2007); Jurinea dolomiaea (Banday et al., 2014); Talinum triangulare (Swarna and Ravindhran, 2013); Picrorhiza kurroa (Patial et al., 2012) and Euphorbia wallichi (Hassan et al., 2020). In Nardostachys jatamansi, vegetative propagation by rhizome splitting was found to be successful and proved to be a better and more rapid means of multiplication, as well as higher production than cultivation through seedlings (Nautiyal and Nautiyal, 2004).
The current study revealed that different soil combinations significantly affected the sprouting and growth performance of P. acinosa. Rhizome cuttings exhibited maximum sprouting percentage with minimum sprouting time in soil:sand:pebbles:vermicompost (1:1:1:1) composition (Table 2). In natural habitats, the species is mainly found growing along roadsides and forest margins with a soil texture consisting mainly of soil, sand, and pebbles. Porous sandy soil is best for successful mass cultivation as it allows the plant to develop a thick, deep root system (Chauhan, 1999). According to Banday et al. (2014), porous sandy soil allows enough aeration and easy penetration of roots and, therefore, is suitable for propagating and developing robust rootstock in Jurinea dolomiaea. Many researchers (Devkota and Jha, 2009; Deshmukh, 2010; Hassan et al., 2020) also reported that sandy loam soils result in maximum growth and higher yields in various economically important medicinal plant species. Similarly, in Paris polyphylla, maximum sprouting and rooting were found in sandy soils (Kavita et al., 2015). Chauhan and Bhatt (2000) found that sand particles, being larger than soil particles, hold more oxygen and less water due to rapid drainage, resulting in improved aeration compared to compactly packed soil particles.
Our study also reflected a positive growth response of rhizomes to adding vermicompost. Rhizome cuttings planted in the soil, sand, and pebbles mixed with vermicompost lead to a significant increase in morphological parameters such as rhizome length, rhizome breadth, plant height, leaf number, and leaf dimensions. The aboveground and belowground biomass also increased significantly in the soil:sand:pebbles:vermicompost (1:1:1:1) combination (SC10), indicating the importance of vermicompost in generating a higher biomass yield. Our results are in line with those obtained for Angelica glauca and Heracleum candicans (Butola and Badola, 2007), which reported a significant influence of sandy loam soil mixed with vermicompost on the cultivation and growth parameters of these species. The addition of vermicompost to soil enhanced germination, root biomass, and pod number in pea crops (Bhadauria et al., 2014). In Polygonatum cirrhifolium, vermicompost addition to the soil leads to increased biomass production (Lohani et al., 2011). Plant growth is significantly impacted by the availability of soil resources, particularly nutrients (Bazzaz and Bazzaz, 1996). Several researchers (Lohani et al., 2011; Rather et al., 2022) also reported an increase in plant biomass with the application of vermicompost. The composted organic matter supplies key nutrients like nitrogen and phosphorus essential for the proper growth and development of plants (Sánchez et al., 2017). Incorporating organic materials such as compost, manure, and animal dung into the soil not only boosts crop yield but also contributes to maintaining soil fertility for longer periods of time. Additionally, organic fertilizers enhance both soil nutrient retention and water-holding capacity (Ali et al., 2018). These fertilizers also promote microbial growth and activity, which is crucial for breaking down soil nutrients and making them readily available to plants (Chen, 2006).
The development of efficient integrated procedures for the vegetative propagation of plants is of great importance for their cultivation, regeneration, and conservation. Considering the urgent need to restore the diminished natural populations of P. acinosa, our research could serve as a foundational reference. This could enable conservationists to implement a multifaceted approach in devising prompt restoration and regeneration strategies for this multipurpose medicinal herb.
Vegetative propagation is one of the most effective methods to regenerate and sustain plant diversity. The knowledge of vegetative reproduction requirements, growth hormone effects, and soil compositions is important for mass cultivation, restoration, and ex situ conservation of medicinally valuable species. Our study is the first report on the successful propagation of P. acinosa through rhizomes. The current study revealed that the application of hormones, especially Gibberellic acid and Kinetin, proved effective in reducing sprouting time and promoting sprouting percentage, rhizome, and shoot length in rhizome cuttings. The plants also showed maximum growth performance in highly porous, loosely packed soils consisting of soil, sand, pebbles, and vermicompost (1:1:1:1). The addition of vermicompost to the soil enhanced the vegetative growth of the plant. Thus, propagation through rhizome cuttings proves a convenient and cost-effective method for large-scale cultivation and conservation of the target species under ex situ conditions. The present study provides a basic guide for the regeneration and conservation of P. acinosa, along with scientific insights for the restoration of threatened endemic biodiversity elsewhere in the world. Its implications are significant for achieving successful germination, and large-scale cultivation and serve as a sustainable approach for utilizing and conserving the target species, thereby reducing its exploitation in wild habitats.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JM: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. BW: Writing – review & editing. HJ: Data curation, Formal analysis, Writing – review & editing. TI: Data curation, Formal analysis, Writing – review & editing. AG: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft. RQ: Writing – original draft. IN: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We are grateful to the research scholars of PRBGPR Laboratory, Department of Botany, University of Kashmir, for their kind assistance and support during the present study. Author (JM) highly acknowledges the Maulana Azad National Fellowship (MANF), Govt. of India, for funding under MANF-201920-JKO416200268, during the study period.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SP declared a past co-authorship with the author TI.
The reviewer ZW declared a past co-authorship with the author TI to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2024.1386204/full#supplementary-material
Ali M., Khan F., Ahmad W., Ishaq M., Saeed M. (2018). Enhancing wheat productivity and soil physical properties of water eroded agricultural land through integrated nutrient management. Soil Environ. 37, 21–27. doi: 10.25252/SE
Bailly C. (2021). Medicinal properties and anti-inflammatory components of Phytolacca (Shanglu). Digital Chin. Med. 4, 159–169. doi: 10.1016/j.dcmed.2021.09.001
Banday A., Nawchoo I. A., Kaloo Z. A., Shabir P. A., Rather A. A. (2014). Efficient propagation of an endangered medicinal plant Jurinea dolomiaea Boiss in the North Western Himalaya using rhizome cuttings under ex situ conditions. J. Plant Breed. Crop Sci. 6, 114–118. doi: 10.5897/JPBCS
Baskin C. C., Baskin J. M. (1998). Seeds: ecology, biogeography, and, evolution of dormancy and germination (San Diego, CA, USA: Academic Press).
Basnet A., Kalauni S. K. (2020). Phytochemical screening and biological activity analysis of some selected medicinal plants of Ilam district of Nepal. Curr. Perspect. Medicinal Aromatic Plants 3, 61–73. doi: 10.38093/cupmap.765409
Bazzaz F. A., Bazzaz F. (1996). Plants in changing environments: linking physiological, population, and community ecology (UK: Cambridge University Press).
Bhadauria T., Kumar P., Maikhuri R., Saxena K. G. (2014). Effect of application of vermicompost and conventional compost derived from different residues on pea crop production and soil faunal diversity in agricultural system in Garhwal Himalayas India. Natural Sci. 6, 433–446. doi: 10.4236/ns.2014.66042
Bisht A. S., Bhatt A. B. (2014). Effect of hormonal and soil treatment on the growth performance of valuable medicinal plant Acorus calamus Linn. World J. Pharm. Pharm. Sci. 3, 1156–1168. Available at: http://www.wjpps.com/admin/assets/article_issue/1399554513.pdf.
Bose S. K., Yadav R. K., Mishra S., Sangwan R. S., Singh A. K., Mishra B., et al. (2013). Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol. Biochem. 66, 150–158. doi: 10.1016/j.plaphy.2013.02.011
Brožová V., Koutecký P., Doležal J. (2019). Plant apomixis is rare in Himalayan high-alpine flora. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-019-50907-5
Butola J. S., Badola H. K. (2007). Vegetative propagation of Angelica glauca and Heracleum candicans. J. Trop. Medicinal Plants 8, 85–91.
Carbognani M., Tomaselli M., Petraglia A. (2018). Different temperature perception in high-elevation plants: new insight into phenological development and implications for climate change in the alpine tundra. Oikos 127, 1014–1023. doi: 10.1111/oik.04908
Chauhan N. S. (1999). Medicinal and aromatic plants of Himachal Pradesh (New Delhi, India: Indus publishing).
Chauhan V. S., Bhatt J. C. (2000). “Agriculture in Uttarakhand: From subsistence towards self-sufficiency,” in Uttarakhand statehood: dimensions of development. Eds. Sati M. C., Sati S. P. (Indus Pub. Co, New Delhi), 168–180.
Chen J. H. (2006). “The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility,” in International workshop on sustained management of the soil-rhizosphere system for efficient crop production and fertilizer use, vol. 16. (Land Development Department, Bangkok Thailand), 1–11.
Cheng Y., Li P., Yang Y., Zhang J., Yan F., Wang H. (2017). Microsatellites for Phytolacca acinosa (Phytolaccaceae), a traditional medicinal herb 1. Appl. Plant Sci. 5, 1700028. doi: 10.3732/apps.1700028
Chisale H. L., Chirwa P. W., Babalola F. D., Manda S. O. (2021). Perceived effects of climate change and extreme weather events on forests and forest-based livelihoods in Malawi. Sustainability 13, 11748. doi: 10.3390/su132111748
Chishimba W. K., Lingumbwanga E. S., Chongo G. M. (1999). In vitro propagation of a tropical berry, Phytolacca dodecandra. J. Trop. For. Sci. 11, 316–319. Available at: https://www.jstor.org/stable/43582531.
Dad J. M., Muslim M., Rashid I., Reshi Z. A. (2021). Time series analysis of climate variability and trends in Kashmir Himalaya. Ecol. Indic. 126, 107690. doi: 10.1016/j.ecolind.2021.107690
Dávila-Aranda P., Rodríguez-Arévalo I., García-Rojas L., Lecona-Rodríguez A. (2016). “Ethnobotany and ex situ conservation of plant genetic resources in México,” in Ethnobotany of Mexico (Springer, New York, NY), 475–489.
Deepak K. G. K., Suneetha G., Surekha C. (2016). A simple and effective method for vegetative propagation of an endangered medicinal plant Salaciaoblonga Wall. J. Natural Medicines 70, 115–119. doi: 10.1007/s11418-015-0932-6
Deshmukh B. S. (2010). Ex situ conservation studies on Ethno-Medicinal rare, endemic plant species from western Ghats of Maharashtra. Int. J. Pharma Biosci. 1, 1–2.
Devkota A., Jha P. K. (2009). Variation in growth of Centella asiatica along different soil composition. Bot. Res. Int. 2, 55–60.
Ganie A. H., Tali B. A., Khuroo A. A., Reshi Z. A., Nawchoo I. A. (2019). Impact assessment of anthropogenic threats to high-valued medicinal plants of Kashmir Himalaya, India. J. Nat. Conserv. 50, 125715. doi: 10.1016/j.jnc.2019.125715
Gao H. M., Liu J. X., Wang Z. M., Wang W. H. (2009). Phytolacacinoside A, a new triterpenoid saponin from Phytolacca acinosa Roxb. J. Asian Natural products Res. 11, 433–438. doi: 10.1080/10286020902849266
Hassan A., Nawchoo I. A., Yaqoob U., Mohi-ud-din G. G. (2020). Conservation strategies of Euphorbia wallichii Hook. f—A species with cryptocotylar seeds. Proc. Natl. Acad. Sciences India Section B: Biol. Sci. 90, 1067–1074. doi: 10.1007/s40011-020-01177-z
Kavita D., Adhikari R. S., Pande V., Kumar M., Pratibha R. (2015). Vegetative propagation of an endangered medicinal plant of Himalayan region, Paris polyphylla Smith. Int. J. Curr. Microbiol. Appl. Sci. 4, 660–665.
Krishan R., Sharma R. K., Sharma S. S. (2022). Assessment of seed biology of the Himalayan medicinal herb Phytolacca acinosa Roxb., the Indian pokeweed, from the perspective of longevity, conservation and propagation. Nucleus 65, 331–339. doi: 10.1007/s13237-022-00404-4
Lata H., Chandra S., Khan I. A., ElSohly M. A. (2017). “Micropropagation of cannabissativa L.—an update,” in Cannabis sativa L.-botany and biotechnology (Springer, Cham), 285–297.
Li B., Wang Y., Wang C., Peng D., Su H., Shi C., et al. (2023). Two new triterpene glycosides with antiproliferative activities on HepG2 from Phytolacca acinosa fruit fermentation broth. Natural Product Res. 37, 2327–2334. doi: 10.1080/14786419.2022.2042284
Liu C., Lin H., Dong Y., Li B. (2022). Increase of P and Cd bioavailability in the rhizosphere by endophytes promoted phytoremediation efficiency of Phytolacca acinosa. J. Hazardous Materials 431, 128546. doi: 10.1016/j.jhazmat.2022.128546
Lohani N., Kumar R., Tewari L. M., Joshi G. C. (2011). Effect of different organic treatments on ex situ conservation of Polygonatum cirrhifolium Royle. Int. J. Biodiversity Science Ecosystem Serv. Manage. 7, 134–140. doi: 10.1080/21513732.2011.625370
Luna T. (2001). Propagation protocol for Devil’s club (Oplopanax horridus). Native Plants J. 2, 106–108. doi: 10.3368/npj.2.2.106a
Ma X., Huang B. (2016). Gibberellin-stimulation of rhizome elongation and differential GA-responsive proteomic changes in two grass species. Front. Plant Sci. 7, 905. doi: 10.3389/fpls.2016.00905
Ma X. P., Lou H. Y., Zhang W. F., Song J. R., Li Y., Pan W. D. (2019). Triterpenoids from phytolacca acinosa. Chem. Natural Compounds 55, 292–295. doi: 10.1007/s10600-019-02670-2
Ma X. P., Zhang W. F., Yi P., Lan J. J., Xia B., Jiang S., et al. (2017). Novel flavones from the root of phytolacca acinosa roxb. Chem. Biodiversity 14, e1700361. doi: 10.1002/cbdv.201700361
Magray J. A., Wani B. A., Ganie A. H., Nawchoo I. A., Javid H. (2023). Effects of pre-sowing treatments and seed sources on seed germination of Phytolacca acinosa Roxb. J. Appl. Res. Medicinal Aromatic Plants 34, 100478. doi: 10.1016/j.jarmap.2023.100478
Magray J. A., Wani B. A., Islam T., Ganie A. H., Nawchoo I. A. (2022). Phyto-ecological analysis of Phytolacca acinosa Roxb. assemblages in Kashmir Himalaya, India. Front. Forests Global Change 5, 976902. doi: 10.3389/ffgc.2022.976902
Nadeem M., Palni L. M. S., Purohit A. N., Pandey H., Nandi S. K. (2000). Propagation and conservation of Podophyllum hexandrum Royle: an important medicinal herb. Biol. Conserv. 92, 121–129. doi: 10.1016/S0006-3207(99)00059-2
Nautiyal M. C., Nautiyal B. P. (2004). Agrotechniques for high altitude medicinal & aromatic plants (High Altitude Plants Physiology Research Centre).
Patial V., Devi K., Sharma M., Bhattacharya A., Ahuja P. S. (2012). Propagation of Picrorhiza kurroa Royle ex Benth: An important medicinal plant of Western Himalaya. J. Medicinal Plants Res. 6, 4848–4860. doi: 10.5897/JMPR
POWO (2023) Plants of the world online. Facilitated by the royal botanic gardens, kew. Available at: http://www.plantsoftheworldonline.org/.
Rather A. M., Rashid S., Sultan R., Nawchoo I. A., Wani I. A., Kamili A. N. (2022). Hurdles and clarifications for the cultivation of saffron in Jammu and Kashmir. J. Agric. Food Res. 10, 100344. doi: 10.1016/j.jafr.2022.100344
Reddy M. C., Indu K., Bhargavi C., Rajendra M. P., Babu B. H. (2022). A review on vegetative propagation and applications in forestry. J. Plant Dev. Sci. 14, 265–272.
Salimi K., Afshari R. T., Hosseini M. B., Struik P. C. (2010). Effects of gibberellic acid and carbon disulphide on sprouting of potato minitubers. Scientia Hortic. 124, 14–18. doi: 10.1016/j.scienta.2009.12.026
Sánchez Ó.J., Ospina D. A., Montoya S. (2017). Compost supplementation with nutrients and microorganisms in composting process. Waste Manage. 69, 136–153. doi: 10.1016/j.wasman.2017.08.012
Shabir P. A., Nawchoo I. A., Wani A. A. (2010). Development of vegetative and sexual multiplication protocol for commercialization of Inularacemosa Hook.f.—a critically endangered medicinal plant of N. W. Himalaya. Natural Sci. 8, 246–252.
Sreekissoon A., Plačková L., Doležal K., Finnie J. F., Van Staden J. (2021). In vitro and ex vivo vegetative propagation and cytokinin profiles of Sceletiumtortuosum (L.) NE Br.: a South African medicinal plant. Plant Cell Tissue Organ Culture (PCTOC) 145, 191–202. doi: 10.1007/s11240-020-02001-2
Sugla T., Purkayastha J., Singh S. K., Solleti S. K., Sahoo L. (2007). Micropropagation of Pongamia pinnata through enhanced axillary branching. In Vitro Cell. Dev. Biology-Plant 43, 409–414. doi: 10.1007/s11627-007-9086-x
Swarna J., Ravindhran R. (2013). In vitro organogenesis from leaf and transverse thin cell layer derived callus cultures of Talinum triangulare (Jacq.) Willd. Plant Growth Regul. 70, 79–87. doi: 10.1007/s10725-012-9780-5
Trunjaruen A., Luecha P., Taratima W. (2022). Micropropagation of pokeweed (Phytolacca americana L.) and comparison of phenolic, flavonoid content, and antioxidant activity between pokeweed callus and other parts. PeerJ 10, e12892. doi: 10.7717/peerj.12892
Tuna A. L., Kaya C., Dikilitas M., Higgs D. (2008). The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 62, 1–9. doi: 10.1016/j.envexpbot.2007.06.007
Wani Z. A., Bhat J. A., Negi V. S., Satish K. V., Siddiqui S., Pant S. (2022). Conservation Priority Index of species, communities, and habitats for biodiversity conservation and their management planning: a case study in Gulmarg Wildlife Sanctuary, Kashmir Himalaya. Front. Forests Global Change 5, 995427. doi: 10.3389/ffgc.2022.995427
Wani Z. A., Pant S., Bhat J. A., Shukla G. (2024b). Distribution and survival of medicinal and aromatic plants is threatened by the anticipated climate change. Trees Forests People 16, 100549. doi: 10.1016/j.tfp.2024.100549
Wani Z. A., Pant S., Bhat J. A., Tariq M., Siddiqui S., Alshaharni M. O. (2024a). Bibliometric analysis of studies on threat assessment and prioritization of species for conservation. Front. Forests Global Change 7, 1374120. doi: 10.3389/ffgc.2024.1374120
Wen F. P., Zhang Z. H., Bai T., Xu Q., Pan Y. H. (2010). Proteomics reveals the effects of gibberellic acid (GA3) on salt-stressed rice (Oryza sativa L.) shoots. Plant Sci. 178, 170–175. doi: 10.1016/j.plantsci.2009.11.006
Wu M., Zhao Z. L., Zhan K. H., Chang X. L. (2016). Study on absorption and desorption of Phytolacca acinosa. China Food Additives 6, 102–107.
Yoon A., Oh H. E., Kim S. Y., Park Y. G. (2021). Plant growth regulators and rooting substrates affect growth and development of Salix koriyanagi cuttings. Rhizosphere 20, 100437. doi: 10.1016/j.rhisph.2021.100437
Zang Y. X., Chun I. J., Zhang L. L., Hong S. B., Zheng W. W., Xu K. (2016). Effect of gibberellic acid application on plant growth attributes, return bloom, and fruit quality of rabbiteye blueberry. Scientia Hortic. 200, 13–18. doi: 10.1016/j.scienta.2015.12.057
Keywords: rhizome, conservation, Phytolacca acinosa, Himalaya, propagation, soil composition
Citation: Magray JA, Wani BA, Javid H, Islam T, Ganie AH, Qadir RU and Nawchoo IA (2024) Vegetative propagation of Phytolacca acinosa Roxb. by rhizome cuttings: a step towards conservation and cultivation approach. Front. Conserv. Sci. 5:1386204. doi: 10.3389/fcosc.2024.1386204
Received: 14 February 2024; Accepted: 22 April 2024;
Published: 08 May 2024.
Edited by:
Shreekar Pant, Baba Ghulam Shah Badshah University, IndiaReviewed by:
Zishan Ahmad Wani, Baba Ghulam Shah Badshah University, IndiaCopyright © 2024 Magray, Wani, Javid, Islam, Ganie, Qadir and Nawchoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junaid A. Magray, anVuYWlkbWFncmF5Nzg2QGdtYWlsLmNvbQ==; Hanan Javid, YmhhdGhhbmFuMTIzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.