- Department of Biology, University of Miami, Coral Gables, FL, United States
Puya raimondii is the world’s largest bromeliad and a prime example of a “charismatic megaflora”. Indeed, its grand stature and remarkable once-in-a-lifetime flowering event have earned it the name “Queen of the Andes”. Like many Puya species, it is one of the only large-statured plants in high Andean puna ecosystems and an important structural and ecological component throughout its native range, providing food and shelter to a variety of animals. However, its future is threatened by anthropogenic stressors such as climate change and land use. In this article, I provide a review of its life history, ecology, and the most important threats to the plant, including climate change and land use. Despite its importance, status as endangered, and the urgency of its threats, little is being done to forecast its future and protect its scattered populations. I finally call for its conservation through in- and ex-situ initiatives and argue that future intervention will be necessary to ensure its survival.
Introduction
Puya raimondii Harms (Bromeliaceae) is one of the most spectacular alpine plants in the world and a prime example of a “charismatic megaflora” (Figure 1). Indeed, its massive size and grandiose flowering events have earned it the name “Queen of the Andes”. It is an emblematic plant endemic to the high Andes mountains of Peru and Bolivia where it occurs in scattered populations around 4000 meters in elevation (Smith and Downs, 1974; Brako et al., 1993). Like many Andean plants, it is currently under threat by a variety of stressors, including climate change and anthropogenic land use (Lambe, 2009). Here, I provide a brief review of the ecology and conservation of P. raimondii. I review its role in high puna ecosystems of the Andes, highlighting its important interactions with avifauna. I then discuss some of the biggest threats it faces and point towards the need for continued research on this overlooked plant. Finally, I suggest conservation initiatives that would help to ensure the survival of this charismatic megaflora.

Figure 1 (A) Puya raimondii flowering in Huascarán National Park, Peru. (B) A different population during a burn by local livestock farmers. (C) A human in front of a mature plant in full flower. Photos (A, B) reproduced from Sgorbati et al. (2004) with permission. All photos by Sergio Sgorbati.
Description, life history, and ecology of Puya raimondii
P. raimondii is endemic to high Andean puna grasslands of Peru and Bolivia between 3000 to 4800 m asl (Figure 2). It occurs mostly in small, isolated, scattered populations usually with a few hundred individuals (Sgorbati et al., 2004; Lambe, 2009). One notable exception, however, is the Titankayocc Regional Conservation Area in Peru (13° 33′ 23″ S, 73° 58′ 56″ W) which could contain upwards of 450,000 plants, nearly half of all P. raimondii individuals (Sgorbati et al., 2004). Populations are typically found on steep, rocky, north facing slopes with maximum solar radiation (Rivera, 1985). P. raimondii typically grows in rocky, slightly acidic, nutrient poor soil or on rocky outcrops.
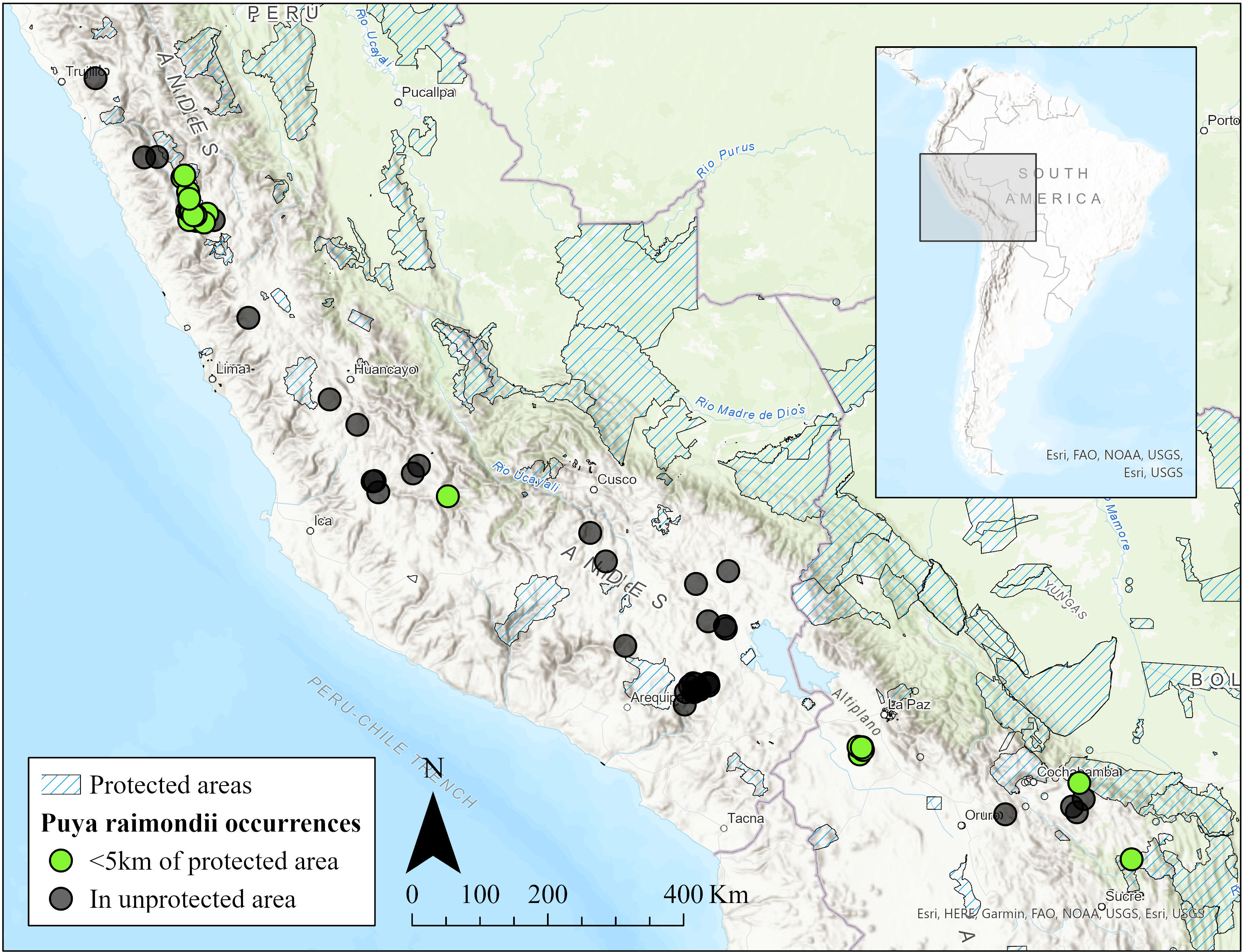
Figure 2 Map of Puya raimondii occurrences and protected areas in Peru and Bolivia. Blue hatching indicates a private or public protected area. Dots are P. raimondii occurrences, with green and black indicating an occurrence within or beyond 5 km of a protected area, respectively. The map was made using ArcGIS Pro 2.8.2 with occurrence records gathered from the Global Biodiversity Information Facility (GBIF).
P. raimondii is the largest bromeliad in the world, exhibiting a globose rosette of hundreds of spine-equipped leaves and an erect stem up to 2 meters tall. It is long-lived, especially for a monocot, and lasts 60-100 years before putting on one of the most spectacular flowering displays in the plant kingdom (Jabaily and Sytsma, 2013). As a monocarp, it produces a once-in-a-lifetime inflorescence that reaches up to 6 meters in length, the most massive of any flowering plant (Givnish et al., 2011). After producing upwards of 100,000 flowers and fruit, its seeds are scattered into the wind, capable of travelling over large distances (Sgorbati et al., 2004; Hornung-Leoni et al., 2013b). It is capable of producing up to 6-12 million seeds with a viability rate up to 80% (Vadillo et al., 2004). However, germination rates in the wild are low as indicated by the rarity of seedlings in P. raimondii stands. After setting seed, the entire plant dies and can remain standing for years, a decades-old remembrance of growth and provisions to the local ecosystem.
P. raimondii plays an important role in the ecology and structure of high elevation puna ecosystems, providing food and shelter for many bird species. At least six hummingbird species have been reported visiting the flowers of P. raimondii, which all act as potential pollinators: the Andean Hillstar (Oreotrochilus estella), the Black Metaltail (Metallura phoebe), the Giant Hummingbird (Patagona gigas), the Green-headed Hillstar (Oreotrochilus stolzmanni), the Shining Sunbeam (Aglaeactis cupripennis), and the Sparkling Violetear (Colibri coruscans) (Salinas et al., 2007; Hornung-Leoni et al., 2013a). In contrast, some birds such as the Peruvian Sierra Finch (Phrygilus punensis) steal nectar and eat the anthers and sepals of the flowers, and thus does not serve a role in pollination (Hornung-Leoni et al., 2013a). Aside from alimentation, P. raimondii creates important habitat. Dense P. raimondii stands provide cover for seed-eating birds while they forage amongst grasses, and the tightly packed leaves of the plant are a common roosting place for birds, sheltered from frigid temperatures and wind (Dorst, 1957). Various bird species, including the Black-winged Ground Dove (Metriopelia melanoptera), the Rufous-collared Sparrow (Zonotrichia capensis), and the Creamy-breasted Canastero (Asthenes dorbignyi) build their nests in the foliage of P. raimondii, using the recurved spines as convenient anchor points and as a line of defense against potential predators (Dorst, 1957). In summary, P. raimondii is a valuable resource for puna avifauna thanks to its prolonged flowering event, its abundant, sugar-rich nectar (Hornung-Leoni et al., 2013a), and its large, protective foliage suitable for nestbuilding.
P. raimondii foliage, however, can also be a danger to birds. Indeed, several species of birds have been found dead within the plant, ensnared by the rigid spines on its leaves (Dorst, 1957; Rees and Roe, 1980). Because the spines are recurved and point inwards, a struggling animal that attempts to unhook itself from one spine can be hooked by another one further towards the leaf base. Eventually, the hopeless animal would die and decompose within the foliage of the plant, providing rich fertilizer. For this reason, P. raimondii could be acting as a “protocarnivorous” plant, adapted to take advantage of its animal inhabitants (Rees and Roe, 1980). This unique nutrient acquisition strategy is seen in at least three other bromeliad species (Givnish, 2015), but further research should be conducted to substantiate this hypothesis.
Threats
P. raimondii faces many threats that jeopardize its existence, and like many Andean plants, climate change is perhaps its most important threat. In the puna, precipitation is predicted to drop from 10% to 30% by 2100, leading to longer and more intense dry seasons. At the same time, temperature is predicted to rise by 4 to 5 ˚C, on average, by 2100 (Rolando et al., 2017). An assessment of the current and projected distribution of P. raimondii found that suitable habitat for the plant will be reduced by 22% or 44% in the climate scenarios of Representative Concentration Pathways 4.5 or 8.5, respectively (Quispe Rojas and Elias Nuñez, 2020). In other words, even a moderate, hopeful climate scenario may reduce the range of P. raimondii by one fifth, and an extreme scenario would reduce it almost by half. This stark reduction in range mirrors that of other Andean plants, as one study found that the average range reduction of thousands of Andean plant species in a no dispersal scenario was 45% (Ramirez-Villegas et al., 2014). To complicate things further, suitable habitat would shift upwards in elevation as temperatures increase. In order for P. raimondii to stay within its preferred climate, it would have to migrate upwards in elevation, sometimes colonizing completely new habitat. A vertical migration rate of 9 meters per year will likely be necessary for trees to keep up with rising temperatures in the tropical Andean forests (Feeley et al., 2011). Similar rates of migration, if not higher ones, may be required for puna vegetation because higher elevations are likely to experience faster warming than lower ones (Urrutia and Vuille, 2009). The long generational times and disjunct populations of P. raimondii will make it especially challenging for populations to migrate, and it is unlikely that it will be able to keep up with rising temperatures. In addition, the reproductive biology of P. raimondii is poorly understood, and there is potential for normal precipitation patterns in the puna to be disrupted which could lead to fewer reproductive events or decreased germination success. Furthermore, the challenges posed by climate change may be compounded by human land-use (Feeley and Silman, 2010). Indeed, higher elevation areas that would otherwise become suitable for colonization by P. raimondii may already be occupied by agriculture or other land-use, and therefore be inaccessible to the plant.
Anthropogenic fire is another serious threat to P. raimondii. Humans have used fire for millennia throughout much of the Andes to clear native vegetation and make way for pasture, however P. raimondii has no natural protection against fire. Thus, anthropogenic fires easily destroy the plants, and entire populations can be lost in a single blaze. Although fires are an occasional natural disturbance in the puna, the frequency and intensity at which they occur now is much higher than it was historically (Crausbay and Martin, 2016). Furthermore, decreased precipitation due to climate change may exacerbate the intensity of fires in the dry season. Livestock farmers also destroy plants directly in fear that the spiny leaves might ensnare livestock (Lambe, 2009; Aquino et al., 2018). However, evidence for livestock becoming trapped in P. raimondii leaves is scarce and more research should be conducted to properly assess the risk that the plant poses to livestock. Finally, people occasionally harvest P. raimondii for practical uses. Dry parts of the plant are used for fuel, inflorescences and young leaves for animal fodder, dried inflorescences for ceremonial use, and dry leaves and stems for fences and thatch roofs (Hornung-Leoni and Sosa, 2004). Although harvesting can be done in a sustainable manner, overexploitation of the plant for practical uses could reduce populations over time.
Aside from direct anthropogenic threats, uneven demographics of P. raimondii populations are another stressor for the plant. Multiple studies have found that within populations there was a low percentage of seedlings compared to juveniles and adults, indicating populations in decline (Salazar Castillo and Villasante Benavides, 2012; Montesinos Tubée, 2014; Aquino et al., 2018). In contrast, another study found a large percentage of seedlings compared to juveniles and adults, providing no evidence of diminishing populations. The differences here could likely be attributed to the specific anthropogenic stressors each population faces. Protecting these existing populations, especially those with a high proportion of seedlings, will be important to avoid population decline.
Studies on the genetics of P. raimondii present contrasting results but indicate that genetic diversity threatens the plant in certain unprotected areas. Sgorbati et al. (2004) found genetic similarity among populations to be as high as 98.3% and within populations to be at least 99.38%, indicating extremely low genetic diversity. However, their study sampled plants in unprotected populations with high human impact. More recent studies have found moderate to high genetic diversity, especially in protected areas such as Huascarán National Park (Hornung-Leoni et al., 2013b; Tumi et al., 2022). Thus, low genetic diversity threatens populations of P. raimondii but only when combined with existing anthropogenic disturbances. This indicates the need for more comprehensive conservation strategies, specifically those aimed at restoring its genetic diversity in unprotected areas.
Protection status and ways forward
P. raimondii is currently considered endangered in Peru and by the International Union for the Conservation of Nature (IUCN) (Lambe, 2009). In contrast, it is not listed in Bolivia’s most recent Red List for Andean plants (Ministerio de Medio Ambiente y Agua, 2012). In Peru, it is legally protected (Decreto Supremo No 043-2006-AG), which prohibits the extraction, collection, possession, transport, or export of any specimen. This law, however, is irregularly enforced outside of protected areas. Even within some protected areas, Peru permits indirect land use such as livestock farming and resource extraction that may destroy or otherwise jeopardize P. raimondii populations. Protecting P. raimondii from anthropogenic land use is necessary for the species’ survival, especially within protected areas.
Protected areas are invaluable bastions for P. raimondii. Tumi et al. (2022) list four protected areas centered on P. raimondii populations: Huascaran National Park, the Calipuy National Sanctuary, the Nor Yauyos Cochas Landscape Reserve, and the Titankayocc Regional Conservation Area. Of these, Titankayocc is the only one that was specifically established to protect P. raimondii and is home to the largest population, representing around half of all individuals (Sgorbati et al., 2004; MINAM, 2019). The other three protected areas all acknowledge P. raimondii in their current master plans (SERNANP, 2015; SERNANP, 2017; SERNANP, 2022), but aside from mentioning the intent to protect the plant and engage in periodic surveys, there are no specific research or conservation agendas. In Bolivia, the “Flavio Machicado Viscarra” Wildlife Sanctuary was established to protect P. raimondii and other local flora and fauna (SERNAP, 1987) and is to my knowledge the only protected area in the country that specifically targets P. raimondii.
In addition to protecting existing populations of P. raimondii, many should be expanded or new populations established. P. raimondii’s long generational time and single flowering event would complicate this task due to the limited opportunities for seed collection in the wild. However, plants grown in captivity have taken a fraction of the time to reach maturity than they do in the wild. For example, one individual grown at the University of California Botanical Garden at Berkeley flowered after 28 years in 1986 (Lineham, 1987), and another flowered after just 24 years in 2014 (Maclay, 2014). P. raimondii’s ability to thrive in a novel landscape and climate compared to its natural habitat highlights the potential that botanical gardens have in raising the plant ex situ. However, to my knowledge, there is not a single program dedicated to cultivating this species in nurseries, highlighting the dearth of attention given to P. raimondii in plant conservation.
In the wild, P. raimondii populations must quickly migrate upwards in elevation in order to stay within their climatic niche. However, many tropical plants are not able to migrate fast enough to keep up with climate warming (Feeley et al., 2023). Augmenting P. raimondii populations with additional plants or establishing new populations would require plantings to be strategically located upslope from existing populations. To ensure that P. raimondii keeps up with a required migration rate of 9 meters per year (Urrutia and Vuille, 2009), new plantings should occur, for example, 90 meters above existing individuals every 10 years. Planting new individuals of P. raimondii at this rate would be necessary in order to ameliorate its slow response to climate change. Few studies have investigated the feasibility of assisted migration on tropical alpine plants, therefore little is known about how successful such a program would be. Still, one study found high survivorship of seedlings (between 77% and 98% survival) for 12 cloud forest tree species in areas above the species’ current elevational distributions (García-Hernández et al., 2019). Assisted migration of P. raimondii would ultimately rely on the survivorship of seedlings, the suitability of the habitat in terms of soil type and microclimate, and its interactions with other organisms. Leveraging existing ecological knowledge of the plant would be necessary to inform its assisted migration. However, like all plant introductions to new habitats, research into potential negative interactions with novel coexisting species would be beneficial.
Despite P. raimondii’s large latitudinal range (8°4’17” S - 18°24’55” S, Figure 2), the plant is only found in isolated patches, often several dozens of kilometers from the nearest populations. The reason for this patchiness is not well understood. Indeed, we still lack a complete understanding of which specific environmental niche P. raimondii occupies. More research investigating the precise soil, microclimate, and hydrological conditions that P. raimondii requires will be necessary to fully understand which areas are suitable for potential outplantings. Only when we have a more comprehensive knowledge of P. raimondii’s environmental niche can we augment existing populations or establish new ones. Similarly, it is unknown which environmental cues P. raimondii uses to induce flowering, which usually happens as mass events, with multiple individuals flowering at once. It is clear that more research needs to be done on the basic biology and life history of P. raimondii.
Conclusion
The high Andes hold some of the most biodiverse ecosystems on the planet with high conservation priorities (Myers et al., 2000). The “Queen of the Andes” deserves more attention in the world of plant conservation due to its aesthetic appeal, importance in puna ecosystems, and its bleak future in the face of global change. Unfortunately, to my knowledge, there are few existing initiatives to protect the species in the wild and no concerted efforts to cultivate the species ex-situ. Restoring existing populations and establishing new ones will be necessary to slow the decline of the species and give it some chance of survival. Its success in living collections outside of its native range, like at the UC Botanical Garden, highlights the potential for ex-situ conservation of this plant. The fact that P. raimondii can thrive in gardens offers a glimmer of hope and further underscores the importance of botanical gardens in plant conservation. Protecting P. raimondii would not only ensure the survival of the plant, but also the myriad species associated with it.
Author contributions
RF: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I thank Sergio Sgorbati for graciously providing photos for the article. I also acknowledge the researchers who have dedicated time and effort to studying P. raimondii. I also thank P. Velásquez-Noriega and one reviewer for providing helpful feedback on the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aquino W., Condo F., Condo J., Yllaconz R. (2018). Distribucion geografica y poblacional de Puya raimondii Harms en el distrito de Huarochiri, Provincia de Huarochiri, Lima, Peru. Biologist (Lima) 16, 25–33. doi: 10.24039/rtb2018161219
Brako L., Zarucchi J. L., Raven P. H. (1993). Catalogue of the flowering plants and gymnosperms of Peru. (St. Louis: Missouri Botanical Garden).
Crausbay S. D., Martin P. H. (2016). Natural disturbance, vegetation patterns and ecological dynamics in tropical montane forests. J. Trop. Ecol. 32, 384–403. doi: 10.1017/S0266467416000328
Dorst J. (1957). The Puya stands of the Peruvian high plateaux as a bird habitat. Ibis 99, 594–599. doi: 10.1111/j.1474-919X.1957.tb03051.x
Feeley K. J., Bernal-Escobar M., Fortier R., Kullberg A. T. (2023). Tropical trees will need to acclimate to rising temperatures—But can they? Plants 12. doi: 10.3390/plants12173142
Feeley K. J., Silman M. R. (2010). Land-use and climate change effects on population size and extinction risk of Andean plants. Glob. Chang. Biol. 16, 3215–3222. doi: 10.1111/j.1365-2486.2010.02197.x
Feeley K. J., Silman M. R., Bush M. B., Farfan W., Cabrera K. G., Malhi Y., et al. (2011). Upslope migration of Andean trees. J. Biogeogr. 38, 783–791. doi: 10.1111/j.1365-2699.2010.02444.x
García-Hernández M., de los Á., Toledo-Aceves T., López-Barrera F., Sosa V. J., Paz H. (2019). Effects of environmental filters on early establishment of cloud forest trees along elevation gradients: Implications for assisted migration. For Ecol. Manage 432, 427–435. doi: 10.1016/j.foreco.2018.09.042
Givnish T. J. (2015). New evidence on the origin of carnivorous plants. Proc. Natl. Acad. Sci. U.S.A. 112, 10–11. doi: 10.1073/pnas.1422278112
Givnish T. J., Barfuss M. H. J., van Ee B., Riina R., Schulte K., Horres R., et al. (2011). Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: Insights from an eight-locus plastid phylogeny. Am. J. Bot. 98, 872–895. doi: 10.3732/ajb.1000059
Hornung-Leoni C., Sosa V. (2004). Uses of the giant bromeliad, Puya raimondii. J. Bromeliad Soc. 54, 3–8.
Hornung-Leoni C. T., González-Gómez P. L., Troncoso A. J. (2013a). Morphology, nectar characteristics and avian pollinators in five Andean Puya species (Bromeliaceae). Acta Oecologica 51, 54–61. doi: 10.1016/j.actao.2013.05.010
Hornung-Leoni C. T., Sosa V., Simpson J., Gil K. (2013b). Genetic variation in the emblematic Puya raimondii ( Bromeliaceae ). Crop Breed. Appl. Biotechnol. 13, 67–74. doi: 10.1590/S1984-70332013000100008
Jabaily R. S., Sytsma K. J. (2013). Historical biogeography and life-history evolution of Andean Puya (Bromeliaceae). Botanical J. Linn. Soc. 171, 201–224. doi: 10.1111/j.1095-8339.2012.01307.x
Lambe A. (2009). Puya raimondii, queen of the Andes. IUCN Red List Threatened Species. doi: 10.2305/IUCN.UK.2009-2.RLTS.T168358A6482345.en
Maclay K. (2014). UC Botanical Garden readies for rare, spectacular Puya raimondii flowering. Berkeley News.
MINAM (2019). Plan Maestro Area de Conservacion Regional Bosque de Puya raimondii Titankayocc 2021-2026 (Peru: Lima).
Ministerio de Medio Ambiente y Agua (2012). LIBRO ROJO de la Flora amenazada de Bolivia. Vol. I (Zona Andina. La Paz). p. 600.
Montesinos Tubée D. B. (2014). Inventory and conservation status of Puya raimondii (Bromeliaceae) in Moquegua, Peru. Chloris Chilensis 17, 1–9.
Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1080/21564574.1998.9650003
Quispe Rojas W. R., Elias Nuñez E. (2020). Distribución potencial de Puya raimondii Harms en futuros escenarios del cambio climático. J. High Andean Res. 22, 170–181. doi: 10.18271/ria.2020.605
Ramirez-Villegas J., Cuesta F., Devenish C., Peralvo M., Jarvis A., Arnillas C. A. (2014). Using species distributions models for designing conservation strategies of Tropical Andean biodiversity under climate change. J. Nat. Conserv. 22, 391–404. doi: 10.1016/j.jnc.2014.03.007
Rees W. E., Roe N. A. (1980). Puya raimondii (Pitcairnioideae, Bromeliaceae) and birds: an hypothesis on nutrient relationships. Can. J. Bot. 58, 1262–1268. doi: 10.1139/b80-157
Rolando J. L., Turin C., Ramírez D. A., Mares V., Monerris J., Quiroz R. (2017). Key ecosystem services and ecological intensification of agriculture in the tropical high-Andean Puna as affected by land-use and climate changes. Agric. Ecosyst. Environ. 236, 221–233. doi: 10.1016/j.agee.2016.12.010
Salazar Castillo J., Villasante Benavides F. (2012). Distribución geográfica y situación actual de Puya raimondii Harms en la Región de Arequipa - Perú. Octubre 2009 – Marzo 2011. Quaderni di Botanica Ambientale Applicata 23, 31–39.
Salinas L., Arana C., Suni M. (2007). El néctar de especies de Puya como recurso para picaflores Altoandinos de Ancash, Perú. Rev. Peru Biol. 14, 129–134. doi: 10.15381/rpb.v14i1.2166
Sgorbati S., Labra M., Grugni E., Barcaccia G., Galasso G., Boni U., et al. (2004). A survey of genetic diversity and reproductive biology of Puya raimondii (Bromeliaceae), the endangered queen of the Andes. Plant Biol. 6, 222–230. doi: 10.1055/s-2004-817802
Tumi L., Ge X. J., Prado G. E., Cosacov A., García V. H., Arakaki M., et al. (2022). Genetic diversity and genetic structure of Puya raimondii (Bromeliaceae) for its conservation in the Peruvian Andes. Rev. Peru Biol. 29. doi: 10.15381/rpb.v29i2.22557
Urrutia R., Vuille M. (2009). Climate change projections for the tropical Andes using a regional climate model: Temperature and precipitation simulations for the end of the 21st century. J. Geophys. Res. Atmos. 114, 1–15. doi: 10.1029/2008JD011021
Keywords: Bromeliaceae, Bolivia, climate change, plant conservation, Peru, Puna
Citation: Fortier RP (2024) Queen of the Andes: the ecology and conservation of Puya raimondii. Front. Conserv. Sci. 5:1349553. doi: 10.3389/fcosc.2024.1349553
Received: 04 December 2023; Accepted: 15 January 2024;
Published: 29 January 2024.
Edited by:
Brenda Molano-Flores, Illinois Natural History Survey (INHS), United StatesReviewed by:
Harold Rusbelth Quispe Melgar, Asociación ANDINUS, PeruPaola Velásquez Noriega, Museo Nacional de Historia Natural (MNHN), Bolivia
Copyright © 2024 Fortier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riley P. Fortier, Rm9ydGllci5yaWxleUBnbWFpbC5jb20=
 Riley P. Fortier
Riley P. Fortier