- Westwood Professional Services, Westminster, CO, United States
In the past decade, caverniculous bat populations have plummeted due to White-nose syndrome (WNS). Tri-colored bat (Perimyotis subflavus) populations have declined drastically in areas where WNS has been found, leading to the decision to protect tri-colored bats under the federal Endangered Species Act in the United States. At this time, there has not been a thorough review of the literature, nor a concise summary of the tri-colored bat’s life history, diet, threats, or habitat preferences. This absence creates more work for policy makers, federal “Take” permit applicants, and conservationists to find, access, and review critical details of tri-colored bats. A major point of confusion stems from the multiple common names and genera tri-colored bats have been classified under since it was first described a century and a half ago. To address the lack of concise summary, we scoured the scientific literature and compiled nearly a century of data to provide a robust review of the ecology, life history, winter and summer habitats, as well as created maps and figures showing counties where studies have occurred, white-nose syndrome is present, and where bats have been documented. Additionally, this paper highlights data gaps and suggests future research topics that may better inform conservation and management decisions for tri-colored bats.
1 Introduction
Tri-colored bats (Perimyotis subflavus) were first described in 1832 by Frédéric Cuvier, who placed the species in the genus Vespertilio (Hoofer et. al., 2003). Sixty-five years later, in 1897, Gerrit Smith Miller moved the species to the genus Pipistrellus based on their physical similarities to other members of the genus (Miller, 1897). This taxonomic change also led to the use of the common name “eastern pipistrelle”. In the 1980’s, a few studies were published disagreeing where the species classification belonged. Researchers suggested the species was a member of Myotis (Horáček and Hanák, 1985), a member of Parastrellus, and another argued the species belonged in a subgenus of Pipestrellus called Perimyotis (Long 2008). In 2010, genetic data was used to find the closest living relative, the Canyon Bat (Parastrellus hesperus) and was subsequently placed in its own, current genus. “SYNONYMS: Vespertilio subflavus, Pipestrellus subflavus, and Perimyotis subflavus”.
When tri-colored bats were first described, they were abundant in many areas throughout most of eastern North America. More recently, their populations have dramatically declined due to the appearance of White-nose syndrome (WNS) (Ford et al., 2011; Pettit and O’Keefe, 2017; Huebschman, 2019; Cheng et al., 2021) as well as habitat loss (Farrow and Broders, 2011). In September of 2022, the United States Fish and Wildlife Service (USFWS) proposed to list tri-colored bats as endangered under the federal Endangered Species Act, affording an assortment of protections once officially listed. Due to this listing, the numerous name changes, and the various research manuscripts published on this species over the past century, we decided to compile this review. We compiled data to create maps to show where specimens have been reported (Figure 1), compiled reported sex ratios (Figure 2, Supplemental Table 1), and summed studies by ecoregion for summer and winter (Figure 3). This review also covers the overall description of tri-colored bats, their historic and current range, demographics, life history, habitats and roost characteristics in both winter and summer, parasites and predators, as well as known prey, and summarizes topics and regions that would benefit from more research.
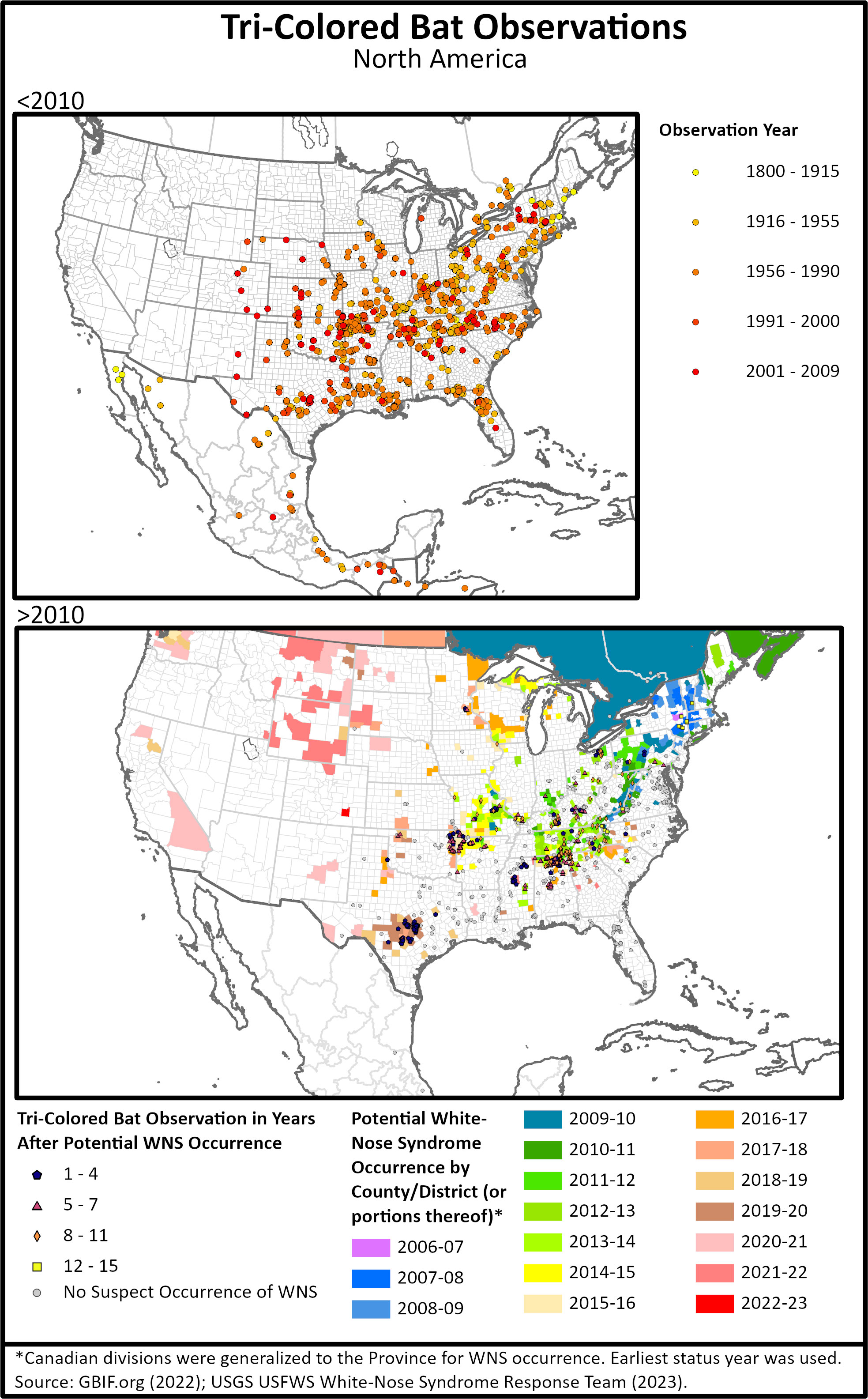
Figure 1 (Top) Tri-colored bat occurrence data from GBIF showing records before 2010 or pre- white-nose-syndrome (WNS) reports, and (Bottom) post WNS reports with years at which WNS was suspected or detected. Areas in Mexico and western edges of the range have not had absence surveys conducted tri-colored bats, so it is unclear if the range is changing.
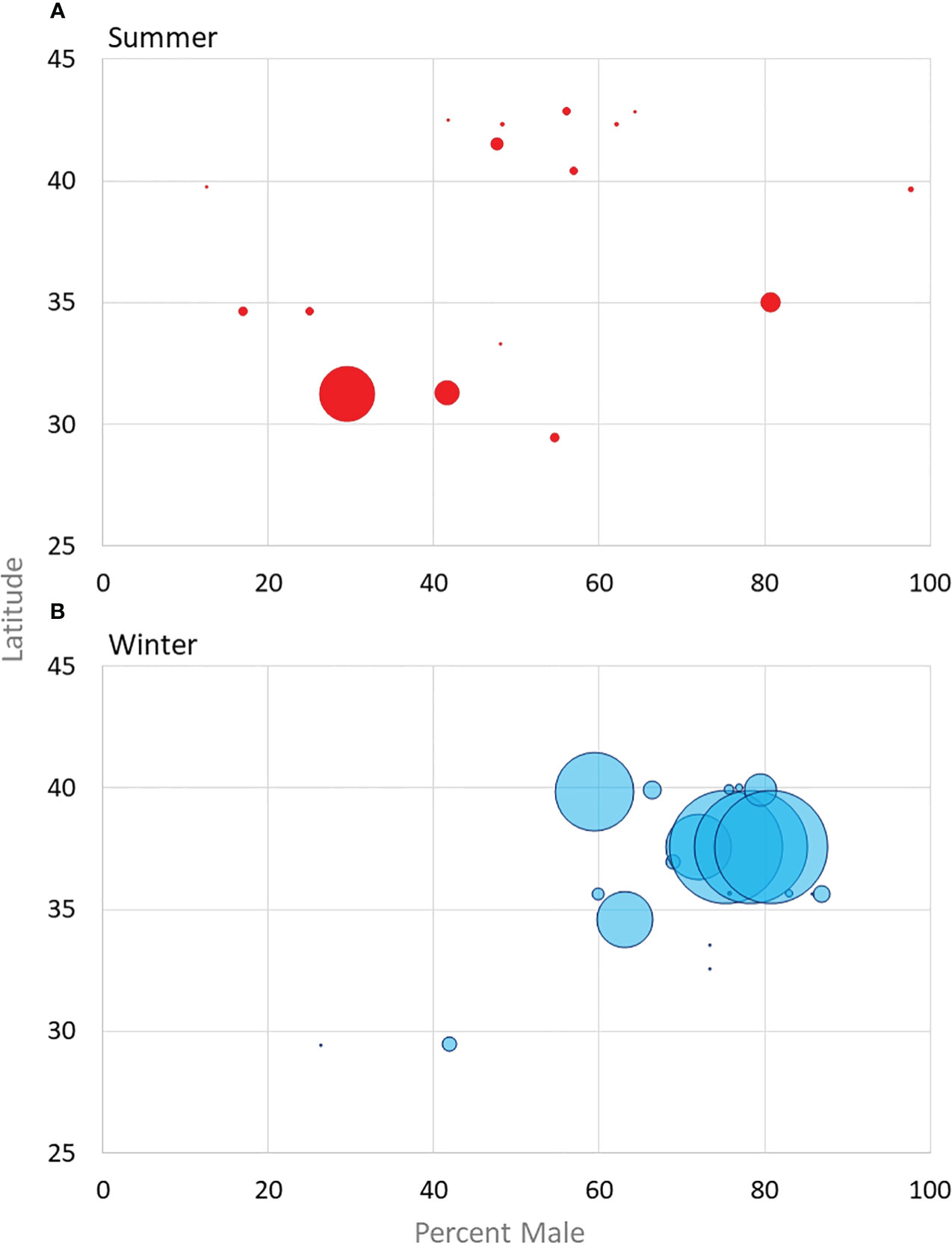
Figure 2 Sex-ratios of males reported from studies with more than 10 bats from both summer habitats (A) (excluding maternity roost studies) and winter hibernacula or fall swarming (B) plotted against overall latitude. Dot size is scaled based on sample size, see Supplemental Table 1 for exact numbers.
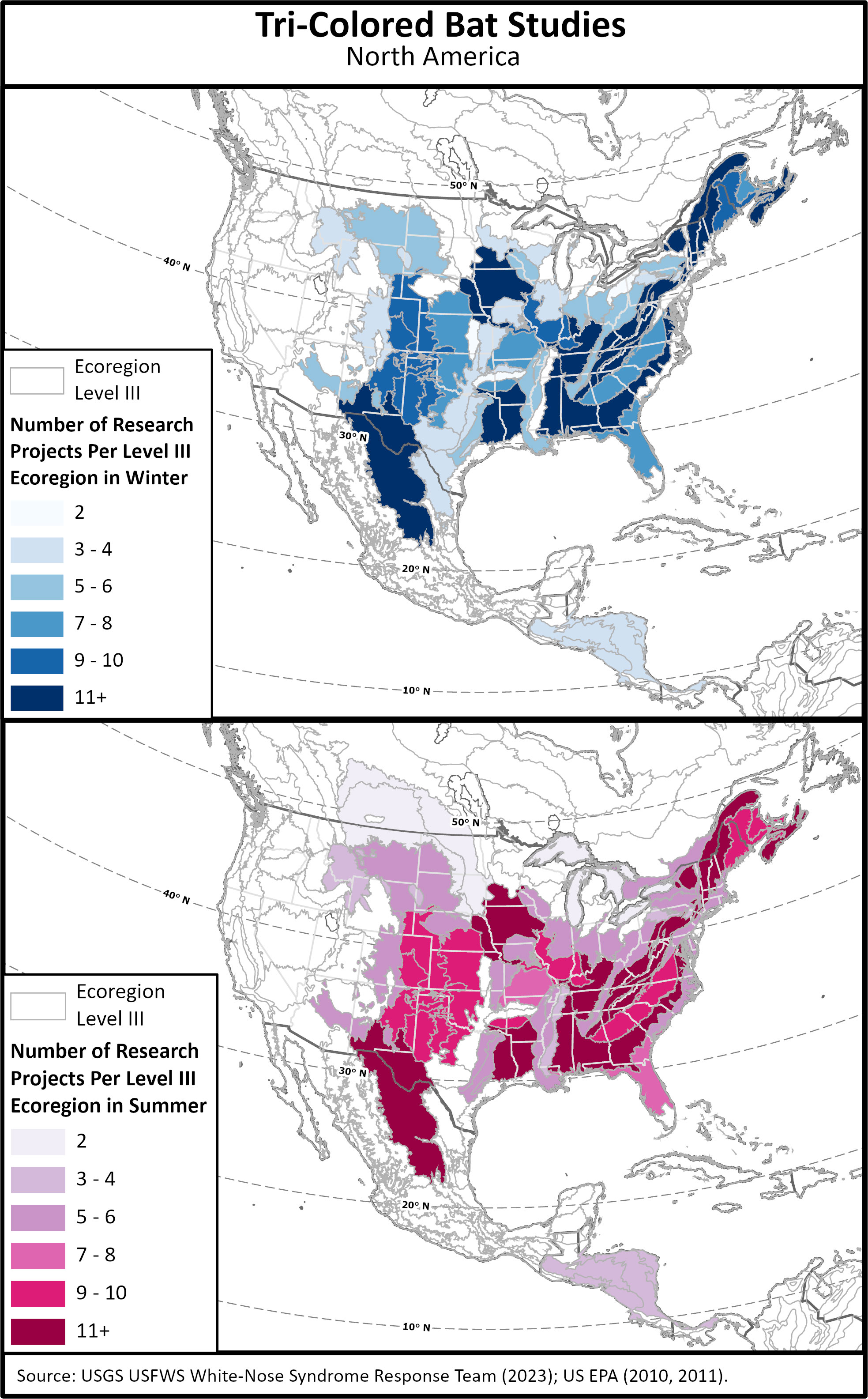
Figure 3 Distribution of published studies conducted on tri-colored bats by Ecoregion Level III for summer and winter. Polygons with no shading or color lack studies.
2 Physical description
The tri-colored bat is a small, caverniculous insectivore weighing 4.0 – 8.0 g (0.2 – 0.3 oz) with a wingspan of 21 – 26 cm (8 – 10 in) and relatively large feet for its size. Tri-colored bats have dark, nearly black wing membranes, a pink tone to their face, ears, and reddish skin covering their radius bone (Fujita and Kunz, 1984; USFWS, 2019). Their fur is yellowish-brown with individual hairs especially on the back having three distinct colors. The base of each hair is dark, with the central portion of the hair being light, yellowish-brown, and dark at the tips (Barbour and Davis, 1969; Fujita and Kunz, 1984).
3 Range and distribution
The range of tri-colored bats extends from eastern Canada, west to Colorado, south to Nicaragua (Medina-Fitoria et al., 2015). Specimens were first collected from Honduras in 1924 (Rinker, 1948) with subsequent observations from Western (Honduras Turcios-Casco et al., 2020) and Central America (McCarthy et al., 1993). Across the species’ range, tri-colored bat population trends have not been historically monitored, so data for regional populations prior to 2006 is from only a few target studies (Supplemental Table 1). In 1962, Ohio was stated to be the region these bats reached their maximum abundance (Davis and Mumford, 1962) and still has populations occurring in all cardinal directions (Figure 1B). Prior to the emergence of WNS in 2006, tri-colored bats were commonly encountered in bat surveys, often in low numbers, in the Northeast and upper Midwest (Figure 1A, Supplemental Table 1). After the arrival of WNS to an area, survey numbers for tri-colored bats declined drastically (Turner et al., 2011; Powers et al., 2015; Cheng et al., 2021; Loeb and Winters, 2022) but are still reported from much of their range (Figure 1B).
Data from 2000 and before show tri-colored bats ranged from eastern Canada, south to Florida, and west to the eastern edges of the Great Plains (Fujita and Kunz, 1984; Briggler and Prather, 2003; Ammerman et al., 2012) and into Central America (McCarthy et al., 1993) (Figure 1). More recently, the range of tri-colored bats has expanded on the western edge, across the United States (Ammerman et al. 2002; Benedict 2004; Ammerman 2005; Geluso et al., 2005; Prendergast et al, 2010) with tri-colored bats showing up in areas with historical survey records including hibernacula where they were previously absent (Figure 1). These areas include central Colorado, eastern New Mexico, and southeastern Wyoming (Geluso et al., 2004; Armstrong et al., 2006; White et al., 2006; Valdez et al., 2009; Adams et al., 2018; Hanttula and Valdez, 2021) (Figure 1A). An analysis of 11 hibernacula over 40 years in Kansas and Oklahoma also shows tri-colored bat abundance having an overall increase over that time (Prendergast et al., 2010). However, published reports in the past decade have not included tri-colored bats from these new western areas (Figure 1B) nor have surveys been published which show significant efforts to find presence or argue absence. Therefore, it is unclear if tri-colored bats have remained in these western expansions. In the southern portion of their range, surveys in Nicaragua captured one tri-colored bat, extending their known range by 160km (100 mi) south (Medina-Fitoria et al., 2015) from previous records in Guatemala (McCarthy et al., 1993). It is unclear whether tri-colored bats were historically present in Nicaragua or if this is part of their range expansion.
4 Demographics
As of August 2023, there are no estimates of the range-wide population of tri-colored bats, and data for birth rates, survival, and maturity are sparse. Hoying and Kunz (1998) report sex ratios of pups born for two consecutive years at one roost: 1981 having 23 males, 14 females and 1982 having 14 males and 15 females. Another study, with small numbers, collected 14 young of year in mist net surveys in Wisconsin, with nine males and five females (Huebschman, 2019). Ten juveniles were captured in mist nets over eight years in the Ouachita Mountains, Arkansas with a 2.3M:1F ratio (Perry et al., 2010). Although these data do not contain even sex ratios, the sample size is rather small, so it is unclear if litters have an overall 1:1 ratio of males and females.
Sex ratios in summer surveys conducted away from maternity colonies are fairly well documented to be skewed male when large numbers of bats are captured (Figure 2). The following male percentages are reported: greater than 50 percent (Powers et al., 2015), 54.5 percent (Jones and Pagels, 1968), 59 percent (Whitaker and Rissler 1992a), 66 percent (Huebschman, 2019; Newman et al., 2021), and 73 percent (Mohr, 1945; Newman et al., 2021) (Figure 2). Conversely, sampling in Tomkins County, New York where bats were shot over feeding grounds, collected 25 females over three years, and one male but it is unclear if maternity colonies were nearby (Wimsatt, 1945). The disparity in males to females in these studies likely show actual population ratios or they could be a sampling anomaly, a product of males and females separating during pup-rearing season or show a difference in foraging preferences between the sexes.
In winter hibernacula, researchers have found similar skewed sex ratio (Supplemental Table 1), with more male tri-colored bats in hibernacula (Mohr, 1945; Davis, 1959; Davis, 1966; Fujita and Kunz, 1984; Sandel et al., 2001) with the heaviest skew as high as 4 males for every 1 female (Fujita and Kunz, 1984) (Figure 2). Studies that report on populations either from mist net surveys or winter roosts have mostly reported male skewed populations. Davis (1959) proposed three hypotheses to explain why more males are found in hibernacula: 1) more males are born 2) more males survive, and 3) females hibernate in other locations. Based on reproduction data from other bat species, Davis (1959) dismissed the possibility that more males are born than females, but the few data available on pups and young-of-year provide some evidence that more males are potentially produced than females (Hoying and Kunz, 1998; Huebschman, 2019). The data Davis (1959, 1966) collected suggests males have a higher survival rate from year to year, which contributes to male skewed populations at winter hibernacula. There are two studies that have reported more females than males during winter (Jones and Pagels, 1968; Jones and Suttkus, 1973); however, these roosts were used throughout the year in Louisiana – where roosts in other studies were only occupied during the winter. Furthermore, of the seven years of data reported in Jones and Suttkus (1973), three years were skewed male, and one year had no bats reported.
5 Life history
Tri-colored bats are generally birthed in litters of two (Lane, 1946; Hoying and Kunz, 1998). By three weeks old bats can fly (Lane, 1946; Hoying, 1983; Fujita and Kunz, 1984) with subadults reported from mist nets by mid-summer, and reproduction possible the following year (Fujita and Kunz, 1984). Winters are spent hibernating. During hibernation, bats lose an average of 1.3g ± 0.1g or roughly 19% of their body weight (Perry and Jordan, 2020). Summers are spent in roosts, with males and females staying mostly separate from one another. The oldest tri-colored bat recorded was a male, 14.2 years of age (Walley and Jarvis, 1971).
Hoying (1983) recaptured one known-age banded female that returned to its place of birth and subsequently gave birth to two normal-sized young. Based on this single record, it was estimated that sexual maturity was reached at three to 11 months of age. However, other data suggest sexual maturity is more commonly reached in the second year (Davis, 1966). Fujita and Kunz (1984) do caution that attainment of sexual maturity in the first year may not be characteristic throughout the range of the tri-colored bat given latitudinal variation in attainment of first molt and bone ossification (epiphysis). The authors emphasized that such a life-history strategy heightens vulnerability to high adult mortality rates, like the rates that occur with WNS.
Mating among tri-colored bats occurs in fall (Hahn, 1908; Cockrum, 1955) when male bats are fertile. Male bats undergo annual changes where their testes are involuted most of the year, but start to enlarge in July, reach maximum size in August, and decrease in weight and size in September until the testes become involuted again by end of November (Krutzsch and Crichton, 1986). Fertilization occurs months later, in spring, using spermatozoa stored in the uterus (Wimsatt, 1945; Fujita and Kunz, 1984). Gestation lasts at least 44 days, after which two pups are usually born (Lane, 1946). Parturition in Missouri was found to extend from early June to early July (LaVal and LaVal, 1980), while in southern Indiana, it was recorded from late June to early July (Cope and Humphrey 1972). Similarly, lactating females have been captured in June and July (Cockrum, 1955; Kunz, 1971). Young have been reported to fly and achieve adult-like flight and foraging ability at about four weeks of age (Lane, 1946), with subadults arriving at hibernacula by early August (Fujita and Kunz, 1984).
6 Winter habitat
Tri-colored bats hibernate in caves, crevices, mines, and man-made structures including bridges, buildings, culverts, dams, and sewers (Goehring, 1954; Davis, 1964b; Jones and Pagels, 1968; Lacki and Bookhout, 1983; Fujita and Kunz, 1984; Whitaker and Rissler, 1992b; Lance et al., 2001; Ferrara and Leberg, 2005; Dixon, 2011; Damm and Geluso, 2008; Meierhofer et al., 2019; Newman et al., 2021) throughout their range. However, in Indiana (Whitaker and Rissler, 1992a; Whitaker and Rissler, 1992b) and southern portions of their range in the United States, tri-colored bats have additionally been recorded in flight on warmer nights (Andersen et al., 2022) and roosting in hardwoods (Newman et al., 2021). Tri-colored bats are reported to enter hibernacula to begin hibernation earlier than most other bat species, between late July–August, and depart later than other species in late April–May (Rysgaard, 1942; Davis, 1959; LaVal and LaVal, 1980) with some males being reported to stay months longer (Davis, 1959).
While in hibernacula, tri-colored bats spend their time hibernating with bouts of activity. Individuals can be more sedentary during hibernation compared to other bats (Rysgaard, 1942; Davis, 1964b); however, they do appear to awaken at similar intervals to big brown bats (Eptesicus fuscus), little brown bats (Myotis lucifugus) (Brack and Twente, 1985; Twente et al., 1985), and gray bats (Myotis grisescens) (Jackson et al., 2022a). Inside one cave, two individuals in a study of 20 remained in the same spot for 13 weeks but half of the study sample had relocated by eight weeks (Davis, 1964b). Inside a cave in Missouri, 20 separate hibernation periods lasted more than 50 days, and three periods potentially lasting more than 90 (Brack and Twente, 1985). Remaining still for long periods is an energetically conservative hibernation strategy that appears to be adopted by tri-colored bats, especially by individuals with less fat mass (McGuire et al., 2021). In Tennessee, tri-colored bat activity was monitored outside of their hibernacula. Tri-colored bats had higher frequencies of activity in mid and late hibernation periods, compared to the co-occurring Indiana bats (Myotis sodalist) and gray bats (M. grisescens) (Jackson et al., 2022a).
Data collected in Tennessee for bat skin temperatures during hibernation drop to an average of 14.4°C but rise to 28.99°C during arousal periods (Jackson et al., 2022b) with similar ranges found in Texas, overall, which had a slightly higher average 15.07°C and noted differences by ecoregion (Leivers et al., 2019; Meierhofer et al., 2019). Furthermore, in Louisiana and Texas, where winters are milder, tri-colored bats were recorded in flight throughout warmer winter nights with no clear preference to vegetation coverage (Andersen et al., 2022). Consistent with these data, average length of hibernation periods are reported to increase as temperature decreases. On average tri-colored bats hibernated for 15.2 days at 12°C and 25.3 days at 10°C (Brack and Twente, 1985).
Mark and recapture studies during the August swarming period, and studies that tracked bats during winter months (Newman et al., 2021) suggest strong fidelity to hibernacula. Similarly, Rysgaard captured one bat in 1940, banded it and released it 128.7 km (80 mi) away and it returned to nearly the same spot in the cave it was first taken (Rysgaard, 1942). Data shows a range in hibernation strategies, with some specimens found hibernating alone (Lacki and Bookhout, 1983; Fujita and Kunz, 1984) and some hibernating in small groups (e.g., Sandel et al., 2001). Prior to the arrival of WNS to an area, overwintering bats tend to select deeper parts of caves where ambient temperatures tend to stay relatively constant (Lacki and Bookhout, 1983; Fujita and Kunz, 1984). Use of small caves, less than 50 m (164 ft) deep, have been used as hibernacula (Briggler and Prather, 2003; Dixon, 2011) as well as urban sewers (Goehring, 1954). Griffin (1940) found that males and females did not segregate during hibernation. Most studies that report male and female counts have found skewed sex ratios in hibernating populations (Figure 2) which could be caused by the three hypotheses Davis proposed, discussed earlier, as well as differences of male and female selection of hibernacula (Davis, 1959; Fujita and Kunz, 1984), latitudinal migration of females to more southern areas which is supported by the correlation of harshness of winter with the percentage of males (Davis, 1959), and or the skewed survival rate which leads to male abundance in overall populations (Davis, 1966; Jones and Pagels, 1968). Additionally, studies that capture flying bats in or near hibernacula (Jackson et al., 2022a) may be observing behavioral differences if males are more active than females.
McNab (1974) proposed hibernation roost selection would inversely correlate temperature and body size, and data support this proposal. A study focused on bats in abandoned coal mines in Ohio found tri-colored bats roosting further into mine tunnels than other species, suggesting they prefer more stable temperatures and conditions (Lacki and Bookhout, 1983); a similar conclusion was made from hibernating tri-colored bats in Minnesota (Swanson and Evans, 1936). However, across Texas, temperatures from winter roosts ranged between 5°C and 19°C, with no significant differences detected between ecoregions (level III) (Meierhofer et al., 2019). In northeast Iowa, Dixon (2011) reported a preference for caves with vertical entrances (likely a function of the karst topography in which the caves occurred) and smaller caves (i.e., less than or equal to 50 m (164 ft) in length), but larger caves were also used. In Arkansas, Briggler and Prather (2003) found a preference for larger caves with east-facing aspects and an avoidance of caves on steep slopes. They found that larger caves on shallow slopes had a greater buffer capacity from weather conditions, a wider variety of temperature profiles within a season, and little variation between seasons, conditions that appeared to favor consistent use by tri-colored bats. Temperatures at roost sites in hibernacula where WNS is present range between 2–10° C (Johnson et al., 2016; Loeb and Winters, 2022) which is lower than roosts in hibernacula without WNS documented in Texas (Leivers et al., 2019). Lab-based studies further elucidate microclimate impacts to hibernating bats, with humidity, temperature, and fat content synergistically effecting individual energy expenditure during hibernation (McGuire et al., 2021).
7 Spring emergence and migration
In the spring, tri-colored bats depart hibernacula later than most other species in late April–May (LaVal and LaVal, 1980; Whitaker and Rissler, 1992a). In most areas, tri-colored bats migrate from hibernacula to summer roosting sites. In the north-central and northeastern portions of the range, bats typically migrate latitudinally and or northward to summer grounds (Fraser et al., 2012; Samoray et al., 2019), however, in Florida, migration south to summer habitat is also reported (Smith et al., 2022). There are also a few documented cases where bats roost near or in winter hibernacula during the summer in Louisiana (Jones and Pagels, 1968; Ferrara and Leberg, 2005) which has less severe winters compared to the northern range. Documented migration distances range from 52.8 km (32.8 mi) (Griffin, 1940) to extensive latitudinal migrations, with males prone to migrate longer distances than females (Fraser et al., 2012). Tracking studies have shown bats can travel in direct paths between hibernacula and summer roosts, with the longest distance documented being 243 km (151 mi) (Samoray et al., 2019). Griffin (1940) documented 136.8km (85 mi). A single tri-colored bat was observed 103.5km (64.3 mi) from shore, but it is not clear if it flew out, or was transported on a ship (Thornton et al., 2023). There are also reports of males and non-reproductive females roosting during summer at winter hibernacula (Jones and Pagels, 1968; Whitaker and Rissler, 1992a) as well as remaining in torpor until midsummer (Davis, 1959).
8 Summer habitat
Summer habitat consists of woodlands, where tri-colored bats have their maternity colonies and roost in trees. In the Midwest, oak (Quercus spp.) abundance correlated with greater abundances of tri-colored bats (Veilleux et al., 2003; Perry and Thill, 2007). There is evidence from Indiana and Ohio suggesting upland habitats are preferred over riparian and bottomland habitats (Veilleux et al., 2003) and that forests that are periodically thinned/managed for understory growth have increased bat activity (Silvis et al., 2016). Overall forest structure also appears to influence habitat preference by tri-colored bates (Yates and Muzika, 2006). Tri-colored bats have been reported from tree cavities of: American sweetgum (Liquidambar styraciflua), swamp chestnut oaks (Quercus michauxii), tupelo (Nyssa spp.) tulip tree (Liriodendron tulipifera), sweetbay (Magnolia virginiana), loblolly pine (Pinus taeda), red maple (Acer rubrum), and American holly (Ilex opaca) (Newman et al., 2021).
Woodland coverage is greatest in eastern Iowa along the Mississippi River and decreases westward (Leatherberry et al., 2006). The woodland habitat present, however, is generally highly fragmented by agriculture (Jackson et al., 1996). It is not clear how fragmentation affects tri-colored bats. However, tri-colored bat activity was not statistically affected by landscape fragmentation or urbanization alone, in and around Washington DC, West Virginia, with higher activity observed in fragmented, urban parks compared to fragmented, rural parks (Johnson et al., 2008). Similarly, acoustic surveys sponsored by Iowa Department of Natural Resources (Blanchong, 2017) showed tri-colored bat presence was likely across most of central and western Iowa, where woodland cover is relatively minimal in the state due to agriculture.
Mist net surveys found peak tri-colored bat capture occurred 31–60 minutes after sunset (Huebschman, 2019). Observations in a maternity roost found similar findings with bats flying in the roost roughly 15 minutes before sunset, and all foraging bats in flight within 45 minutes of sunset (Lane, 1946). Summer flying occurs between May and September (Lane, 1946; Davis, 1966; Huebschman, 2019), with more narrow periods likely occurring in northern extents of the range compared to the south.
Throughout their range, mature females gather in maternity colonies, separate from males, which appear to roost singly (Lane, 1946; Fujita and Kunz, 1984; Perry and Thill, 2007). Maternity colonies are generally small, with reported numbers ranging from single females, the average of four individuals (Veilleux and Veilleux, 2004a), 15 individuals (Whitaker, 1998, Hoying and Kunz, 1998) 19 individuals (Hoying and Kunz, 1998) and the largest of 75 recorded in a windowless barn (Lane, 1946) (Supplemental Table 1). Roost fidelity among females appears to be high both within summers and between summers, and there is some evidence of fidelity to natal roost sites (Veilleux and Veilleux, 2004b). Individual and geographic variations exist in the date of parturition, with earlier dates being recorded in the southern portion of their range compared to northern populations (Cockrum, 1955). Lactating females have been reported captured in June (Cockrum, 1955; Kunz, 1971; Hoying and Kunz, 1998; Miller, 2003; Huebschman, 2019) and July (Kunz, 1971; Hoying and Kunz, 1998). Newly born pups cling to their mothers during forage flights and are held in a sack formed by the wing and tail membranes, until they are independent enough to be left in the roost (Lane, 1946). However, it has been observed that if a disturbance occurs in the roost, mothers will carry larger young and fly (Lane, 1946). Young-of-year have been captured in mist nets as early as July 31 in Wisconsin (Huebschman, 2019) which aligns with data reporting pups being able to fly and leaving roosts between July and August in Massachusetts (Hoying and Kunz, 1998).
Summer roosts of tri-colored bats are mostly located in canopy foliage and epiphytes, however bats have been observed roosting on bridges (Keeley and Tuttle, 1999), culverts (Meierhofer et al., 2022), other human structures (Lane, 1946), and in overwintering sites (Jones and Pagels, 1968, Whitaker and Rissler, 1992a) with roost characteristics differing by region. In northern regions, like Nova Scotia, roost site selection in one study appeared to be based on greater covering of bony beard lichen (Usnea trichodea) (Poissant et al., 2010). In more southern regions, roosts occur in dead hardwood leaves, pine needles, or Spanish moss (Tillandsia usneoides) (Menzel et al., 1999; Veilleux et al., 2003; Perry and Thill, 2007; O’Keefe et al., 2009; Shute et al., 2021). Another study reports height as a factor in roost selection, with use of dead leaf clusters in oaks ranging from 6.6 meters to 21.9 meters off the ground the Missouri Ozarks (Hammesfahr et al., 2022). In Indiana, tri-colored bats have been documented roosting in foliage, not in tree hollows, with oaks preferred (Veilleux et al., 2003). Non-reproductive tri-colored bats in North Carolina were found roosting only in forested stands older than 72 years, mainly at lower elevations, and closer to non-linear openings and water (O’Keefe et al., 2009). Similarly, in Arkansas, all 47 roosts recorded were in tree canopies, not trunks, with half of female roosting in pine trees, the other half were located in dead leaves of deciduous trees, mainly mature oaks (more than 50 years old) which is also where more than 90 percent of male roosts were located as well (Perry and Thill, 2007). Studies have also encountered maternity colonies in barns (Lane, 1946; Cope et al., 1960; Fujita and Kunz, 1984; Cope et al., 1991; Winchell and Kunz, 1996) and other man-made structures. However, radio-telemetry data led Harvey et al. (2011) to conclude that most tri-colored bats roost in trees in summer and rarely occur in buildings.
Interannual fidelity to roosting areas has been documented for females (Allen, 1921; Veilleux and Veilleux, 2004a). Males appear to select tree sizes randomly, but females seem to prefer larger trees (Perry and Thill, 2007). Upland habitats were preferred over riparian and bottomland habitats, possibly because oaks offer more preferred roosts. Roost-site fidelity for tri-colored bats averages about 3.9 days, with switching occurring between nearby roosts, and average foraging distances or distance-to foraging grounds averaged 1.8 ± 0.1 km (1.1 ± 0.1 mi; maximum of 4.4 km [2.7 mi]) (Whitaker, 1998).
9 Fall migration and swarming
Tri-colored bats are reported to enter hibernacula caves earlier than most bat species, between late July–August (Davis, 1964a; Whitaker and Rissler, 1992a). However, there are also data showing tri-colored bats flying at hibernacula entrances in September and October in Indiana (Whitaker and Rissler, 1992a) and throughout winter months in Texas (Sandel et al., 2001). The large number of marked bats recaptured during the August swarming period suggests strong fidelity to specific hibernacula (Newman et al., 2021). Furthermore, the hibernation ecology of tri-colored bats makes them particularly susceptible to long winters. Therefore, migration from the northern extent of the species’ range to more southern hibernacula is posited as preferable or advantageous for some individuals (Fraser et al., 2012). In Florida, migration north to overwintering sites was documented using isotopes, with almost half of the studied animals migrating more than 100km (62 mi) (Smith et al., 2022). Migration north may indicate that southern extents of the tri-colored bat range are too warm for overwintering (Smith et al., 2022).
10 Effects of temperature on bat migration and flight activity
There is a positive correlation between bat activity and nightly temperature, shown repeatedly in studies both seasonally (O’Farrell and Bradley, 1970; Avery, 1985; Rydell, 1991; Johnson et al., 2011) and nightly (Lacki, 1984; Hayes, 1997; Vaughan et al., 1997; Gaisler et al., 1998; Shiel and Fairley, 1998). Temperatures below 10 – 13°C [50 – 55°F]) are likely to result in reduced bat activity among all bat species (USFWS, 2011), which is now used a guidance for wind turbine operators (USFWS, 2011). Flight activity during migration is also reduced at cooler temperatures and higher windspeeds (Johnson et al., 2011).
11 Annual survival
Davis (1959); Davis (1966) has a multiyear study at two caves in West Virginia (1952–1955) which focused on recovering banded bats year after year. Recovery rate (Davis, 1966) was higher for males (38%) than for females (14%) which could be due to survivability or bats changing hibernation locations. Overall, Davis’ data suggests a female bat will add approximately 2.06 reproductive females to the population over their lifespan. Similarly, Perry and Jordan (2020) report five years of recapture data in Arkansas with more advanced analyses. Their findings suggest annual survival rates between 0.101 to 0.706 for tri-colored bats. Hoying and Kunz (1998) report mortality rates at roughly 50% for pups before leaving the roost. Similarly, a technical study (Forbes, 2012) in Canada, reported that mortality in yearlings is high when exposed to WNS.
12 White-nose syndrome
The fungal pathogen Pseudogymnoascus destructans (Pd) which manifests itself as WNS (Lorch et al., 2011; Warnecke et al., 2012) is the most lethal threat facing nine species of bats in North America (reviewed in Cheng et al., 2021). Hibernating bats with WNS have an increase in the frequency and duration of arousal bouts during hibernation which causes the wasting of energy stores needed to survive hibernation (Reeder et al., 2012; Verant et al., 2014). Bats in hibernacula infected with WNS have more activity than non-infected hibernacula, including flights over the landscape in cooler temperatures (Bernard and McCracken, 2017). In addition to observed fatalities at hibernacula, WNS has also been linked to decreased regional populations (Turner et al., 2011; Ingersoll et al., 2013; Ingersoll et al., 2016; Perry and Jordan, 2022), cave-bat abundance in summer habitats (Dzal et al., 2010; Brooks, 2011; O’Keefe et al., 2019, Deeley et al., 2021; Johnson et al., 2021; Loeb and Winters, 2022; Perea et al., 2022) as well as winter hibernacula (Lacki et al., 2015; Powers et al., 2015; Perry and Jordan, 2020).
There is promising data suggesting that some populations of tri-colored bats that have survived the initial impact of the fungus are adopting alternative behaviors and hibernation strategies, selecting cooler roosting microclimates (Johnson et al., 2016; Loeb and Winters, 2022) and that cooling hibernacula can attract cave-hibernating bats affected by WNS (Turner et al., 2022). There is also promising data suggesting that fungicide aerosol application may improve survivability (Gabriel et al., 2022) as well as vaccines and some management strategies (Bernard et al., 2019). However, hibernacula location and ecology must be considered because many arthropods and organisms endemic to cave systems may respond poorly to fungicides and other treatments (Bernard et al., 2019).
13 Predators and parasites
Predation by an assortment of animals including house cats (Felix domesticus), raccoons (Procyon lotor) (Hoying and Kunz, 1998), hoary bats (Lasiurus cinereus) (Bishop, 1947; Wine et al., 2019), leopard frogs (Rana pipiens) (Creel, 1963), and voles (Microtus ochragaster) (Martin, 1961) have been reported, but there are likely many more vertebrate predators of this species. Potential predation by a dark fishing spider (Dolomedes tenebrosus) was observed at a winter hibernaculum in Texas (Leivers et al., 2021).
Endoparasites collected from tri-colored bats include Apicomplexans, Helminths, Nematodes and Trematodes. An Apicomplexan Eimeria heidti was described from tri-colored bats (McAllister et al., 2011; McAllister et al., 2014), Macy collected and described the Helminth species Lecilhodendrium breckenridgei (Macy, 1936) and Acanthatrium pipistrelli (Macy, 1940) from tri-colored bats and Acanthatrium lunatum (Williams, 1957; Pistole, 1988), Ascarops slrongylina (Williams, 1957), Paralecithodendrium transversum (Pistole, 1988), Physocephalus sexalatus (Macy, 1931; Williams, 1957), Plagiorchris micracanthos (Williams, 1957; Nickel and Hansen, 1967), and Ochoterenatrema breckenridgei (McAllister et al., 2016). The nematode Longibucca lasiura is reported from tri-colored bat stomachs (Elsea, 1953; Measures, 1994). Trematodes Acanthatrium eptesi were recovered from small intestines (Ashley and Rabalais, 1980)
Ectoparasites include Acarini Hypoaspis sp. (Palmer & Gunier, 1975), Macrocheles sp. (Palmer and Gunier, 1975), Diptera Liancalus genualis (Palmer and Gunier, 1975), Chiggers Euschoengastia pipistrelle (Jones et al., 1952; Whitaker and Mumford, 1971; Zajkowska et al., 2018; McAllister et al., 2021), E. staffordi (Zajkowska et al., 2018), Leptotrombidium myotis (Zajkowska et al., 2018), and Perissopalla flagellisetula (McAllister et al., 2016; Zajkowska et al., 2018), Coccidium Eimeria macyi (Wheat, 1975; McAllister et al., 2016),
14 Diet
Reports of tri-colored bat diet suggest they are generalists, feeding on an assortment of arthropods including small beetles (Coleoptera), flies (Diptera), moths (Lepidoptera), stoneflies (Tricoptera), true bugs (Hemiptera and Homoptera), spiders (Aranaea), and wasps (Hymenoptera) (Fujita and Kunz, 1984; Menzel et al., 2002; Carter et al., 2003; Feldhamer et al., 2009; Kaarakka et al., 2013; Bernard et al., 2021). Evaluation of stomach contents and fecal matter showed Coleoptera and Hemiptera (specifically subclade Homoptera) from more than 50% of the 24 specimens evaluated, with Hymenoptera, and Lepidoptera occurring in 30%–50% (Griffith and Gates, 1985). Weinkauf et al. (2018) used fecal samples and identified unknown Leaf beetles, Hairy fungus beetles (Typhaea stercorea), Rove beetles (Aleochara sp.), Midges (Dicrotendipes sp.), Mosquitoes (Aedes vexans and Anopheles punctipennis), Case-bearing moths (Coleophora sp.), and Twirler moths (Telphusa latifasciella). In West Virginia, summer diets were composed of mostly even proportions of Coleoptera, Hemitpera, Lepidotpera, Diptera, Hymenoptera and Tricoptera with some samples including Neuroptera and Araneae (Carter et al., 2003). In winter, when bats have been observed flying outside of hibernacula in Indiana, no feeding occurred even though flying insects were present (Whitaker and Rissler, 1992b), however in Tennessee active, tri-colored bats were documented to be feeding on a wide-selection of arthropods (Bernard et al., 2021).
15 Data gaps and future studies
In our review, we were able to identify a few areas where data are needed. Due to the range of intensity and severity of winters across the tri-colored bat’s range, many completed research projects on bat behaviors and survival should be repeated across the range to ensure conclusions can be adopted for appropriate, local, management decisions. Research efforts elucidating the overall effects on different behaviors and survival rates will also help local conservation efforts.
Life history studies are also incomplete and should be prioritized so that REAs, population growth, and population responses can be modelled correctly. Three studies to date were able to collect long term survivability data but they are isolated to Arkansas (Perry and Jordan, 2020), South Carolina (Loeb and Winters, 2022), and West Virginia (Davis, 1966). These finding may or may not be representative of patterns in areas with longer winters in the north, or warmer, shorter winters in the south. Changes in life expectancy when exposed to pollution are also needed, as data show mercury can be present in bats (Yates et al., 2014). Similarly, survivability in man-made structures like city sewers, culverts, and bridges is needed, especially since these structures have great potential for localized WNS treatments.
Although there are some long-term studies at hibernacula which have recorded abundance of tri-colored bats, long term mark, release, recapture (MRR) studies that last at least a full generation are needed. Our recommendation is that MRR studies be conducted throughout the latitudinal range, as well as in different winter intensities and lengths, which will aid in creating rates for life expectancy and survivability. Current resource equivalency models (Stephenson et al., 2022) utilize the findings from West Virginia (Davis, 1966), which has less severe winters than Nova Scotia, Wisconsin, or Oklahoma. Those data also do not reflect the life expectancy of bats in WNS infected regions.
Loeb and Winters (2022) found hibernating tri-colored bats change roosting behavior in response to WNS, selecting cooler temperatures in caves. They also hypothesized warmer regions, such as caves in Florida, may not reach cool enough temperatures. In general, southern caves may be too warm for healthy bats to overwinter (Smith et al., 2022). There are also data showing tri-colored bats arriving in roosts in the middle of winter, but it is not clear if these bats were hibernating nearby or actively feeding and not hibernating (Whitaker & Rissler 1992, Lacki et al., 2015, Cervone et al., 2016). Research into the length of hibernation, temperature of hibernacula, feeding patterns in winter or non-breeding seasons, and mortality rates by WNS is needed to further understand population threats across the range of tri-colored bats.
Hoying and Kunz (1998) are the only group to report on sex of pups prior to flight, but with a small sample size. More data are needed to establish if this species is born with any sex-skewed bias to better model population growth potentials. Due to the location of most maternity roosts, and the risk to the bat pups being handled early on, data collection on captive colonies would likely be best, with supplemental data coming from any wild, deceased, pregnant specimens.
It is clear that summer roosts are segregated by sex, but more information is needed if tri-colored bats remain segregated during feeding, migration, and other activities. These data are important for population monitoring, estimating reproductive potential, risk assessment – especially with wind turbines, and calculating mitigation acres in USFWS approved “Resource Equivalency Analyses” (Stephenson et al., 2022).
Wind facilities are being erected at varying rates across the range of tri-colored bats, which means there is an urgent need to clarify: how tri-colored bats are affected by wind turbines; when tri-colored bats are most likely to interact with turbines – including the hours after sunset and the months, as well as if there are safe ways to deter deleterious interactions. Promising data has been collected showing deterrence using ultrasonic acoustics to keep bats from bridges (Wetzel and Roby, 2023) but implementation at wind facilities has not been published.
Studies focusing on assorted aspects of tri-colored bat biology and ecology have been conducted throughout their range (Figure 3). We grouped studies by Ecoregion Level III (US EPA, 2011) which shows noticeable absences of Summer studies in many of the ecoregions surrounding the Great Lakes; the Arkansas Valley, Cross Timbers, Edwards Plateau, Flint Hills, Western Gulf Coastal Plain, Southern Texas Plains, and Southern Florida Coastal Plain; the majority of ecoregions in Canada as well as Mexico, except for the Chihuahuan Desert, and most of Central America (Figure 3). Winter studies are also absent in much of the ecoregions in Canada, Mexico and Central America, as well as the Arkansas Valley, Flint Hills, Mississippi Valley Loess Plains, Gulf Coast Plains, and much of the central East Coast (Figure 3). Furthermore, overall data are lacking for: quantifiable summer habitat characteristics including tree stand age, density, and landscape heterogeneity; location of maternal colony locations; migration and foraging flight elevation; regional and overall population estimates, and; habitat management strategies to boost populations. There is also a dearth of data on which species predate tri-colored bats and overall causes of mortality outside of WNS. By understanding the natural history of tri-colored bats, both range wide and within sub-regions, conservationists, policy makers, and land managers will be able to make informed decisions and hopefully increase tri-colored bat populations to healthy, secure numbers.
16 Methods
We searched scholar.google.com using keywords “Tri-colored Bats”, “Eastern Pipestrelle”, “Vespertilio subflavus”, “Pipestrellus subflavus”, and “Perimyotis subflavus”. These keywords were also paired with “Ecology”, “Habitat”, “Hibernacula”, “Migration”, “Parasites”, “Predators”, “Roost”, and “Sex Ratios”. Cursory searches were also done using “murciélago” + “subflavus” paired with “hibernación”, “bosque”, “dieta”, “ migración”, and “cueva” to identify potential papers published in Spanish. Searches were done sporadically between September 2021 – August 2023. While the authors reviewed each manuscript, pertinent citations were also pursued to add to this review as well as papers suggested by reviewers. Admittedly dissertations, gray literature, and research theses are important and valuable to understanding topics, but they can also be reviewed by non-experts and lead to misinterpretations of data which is why we chose to exclude these publications from our review. Data published by Federal and State agencies in the United States were also left out of this review.
While reviewing each study, we extracted data where studies were conducted, years the studies were done, time of year, and the number of tri-colored bats reported and their sexes if present. Authors of papers that did not list counties were contacted for more information. In studies where authors did not respond, but maps were present, we used approximation for locations to the best of our ability. These studies are marked with a ~ in Supplemental Table 1. These data were then utilized to create reference maps for counties and territories with published studies, the unique ID we assigned the study, and the season in which the study was performed, “Study Location Layer”. Our work did not control for separate publications which used the same data or that sampled areas at the same time as other authors. In ArcGIS Pro, we ran “Select by Location” to isolate the Ecoregions Level III layer (US EPA, 2010). We then ran “Union” to relate the “Studies Location Layer” with the ecoregions layer using the selection results. Data from the new layer were exported, then filtered in Excel. Using a “SUMIF” query, the sum of the count of research projects within each ecoregion using the unique values list as the criteria was created, which was used for symbology and categorizations for Figure 3. Studies that reported sex ratios were extracted, along with the latitude of the county or territory they were conducted to create a graph (Figure 2). Data from the following studies were also used to create Figures 2, 3 (Muir and Polder, 1960; Kunz and Schlitter, 1968; Blankespoor and Ulmer, 1970; Czaplewski, 1979; Hilton and Best, 2000; Broders et al., 2001; Trousdale and Beckett, 2002; Broders et al., 2003; Kalcounis-Rueppell et al., 2007; Quinn and Broders, 2007; Gilley and Kennedy, 2010; Helms, 2010; White et al., 2016).
Occurrence data were downloaded from GBIF.org (2022) to show historical and current ranges (Figure 1). Data were filtered by removing observations without specific latitude and longitude. White-nose syndrome distribution data were downloaded from White-nose syndrome Response Team (2023). Observations were plotted in ArcPro to create range maps for pre and post white nose syndrome that grouped observations in areas without white-nose syndrome using similar dates as Ford et al. (2011).
Author contributions
Each author of this paper contributed to the design, review, and or figures. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Westwood for allowing us time to work on this project and the three reviewers who gave exceptionally helpful critiques. We would also like to offer our apologies to any teams that have published papers on tri-colored bats that did not make it into this review. Portions of this work were included in a Wind Facility habitat conservation plan prepared by SC and EP, which was formally submitted to the USFWS in 2023.
Conflict of interest
All authors were employed by Westwood Professional Services.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2023.1204901/full#supplementary-material
References
Adams R. A., Stoner B., Nespoli D., Bexell S. M. (2018). New records of tricolored bats (Perimyotis subflavus) in colorado, with first evidence of reproduction. Western North Am. Nat. 78, 212–215. doi: 10.3398/064.078.0213
Ammerman L. K. (2005). Noteworthy records of the eastern pipistrelle, Perimyotis subflavus, and silver-haired bat, Lasionycteris noctivagans, (Chiroptera: Vespertilionidae) from the Chisos Mountains, Texas. Texas J. Sci. 57, 202–208.
Ammerman L. K., Rodriguez R. M., Higginbotham J. L., Matthews A. K. (2002). Recent records of bats from the lower canyons of the Rio Grande River of West Texas. Texas J. Sci. 54, 369–375.
Andersen B. R., McGuire L. P., Wigley T. B., Miller D. A., Stevens R. D. (2022). Habitat associations of overwintering bats in managed pine forest landscapes. Forests 13 (5), 803. doi: 10.3390/f13050803
Armstrong D. M., Adams R. A., Taylor K. E. (2006). New records of the eastern pipistrelle (Pipistrellus subflavus) in Colorado. Western North Am. Nat. 66, 268. doi: 10.3398/1527-0904(2006)66[268:NROTEP]2.0.CO;2
Ashley D. C., Rabalais F. C. (1980). Brief note: Helminth parasites of pipistrellus subflavus from Ohio. Ohio J. Sci. 80, 64–64.
Barbour R. W., Davis W. H. (1969). Bats of America (Lexington: The University Press of Kentucky), 286.
Benedict R. A. (2004). Reproductive activity and distribution of bats in NEBRASKA. Western North American Naturalist. 64, 231–248.
Bernard R. F., Evans J., Fuller N. W., Reichard J. D., Coleman J. T., Kocer C. J., et al. (2019). Different management strategies are optimal for combating disease in East Texas cave versus culvert hibernating bat populations. Conserv. Sci. Pract. 1 (10), e106. doi: 10.1111/csp2.106
Bernard R. F., McCracken G. F. (2017). Winter behavior of bats and the progression of white-nose syndrome in the southeastern United States. Ecol. Evol. 7 (5), 1487–1496. doi: 10.1002/ece3.2772
Bernard R. F., Willcox E. V., Jackson R. T., Brown V. A., McCracken G. F. (2021). Feasting, not fasting: winter diets of cave hibernating bats in the United States. Front. Zoology 18, 1–13. doi: 10.1186/s12983-021-00434-9
Bishop S. C. (1947). Curious behavior of a hoary bat. J. Mammalogy 28, 293–294. doi: 10.1093/jmammal/28.3.293a
Blanchong J. (2017). Iowa acoustic bat surveys (Iowa State University Research and Demonstration Farms Progress Reports). 2016
Blankespoor H. D., Ulmer M. J. (1970). Helminths from six species of iowa bats. Proceedings of the Iowa Academy of Science. 77, 201–206.
Brack V., Twente J. W. (1985). The duration of the period of hibernation of three species of vespertilionid bats. Institute of Field studies. Can. J. Zoology 63 (12), 2952–2954. doi: 10.1139/z85-442
Briggler J. T., Prather J. W. (2003). Seasonal use and selection of caves by the Eastern Pipistrelle bat (Pipistrellus subflavus). Am. Midland Nat. 149, 406–412. doi: 10.1674/0003-0031(2003)149[0406:SUASOC]2.0.CO;2
Broders H. G., McAlpine D. F., Forbes G. J. (2001). Status of the eastern pipistrelle (Pipistrellus subflavus) (Chiroptera: Vespertilionidae) in New Brunswick. Northeastern Nat. 8, 331–336. doi: 10.1656/1092-6194(2001)008[0331:SOTEPP]2.0.CO;2
Broders H. G., Quinn G. M., Forbes G. J. (2003). Species status, and the spatial and temporal patterns of activity of bats in Southwest Nova Scotia, Canada. Northeastern Nat. 10 (4), 383–398. doi: 10.1656/1092-6194(2003)010[0383:SSATSA]2.0.CO;2
Brooks R. T. (2011). Declines in summer bat activity in central New England 4 years following the initial detection of white-nose syndrome. Biodiversity Conserv. 20, 2537–2541. doi: 10.1007/s10531-011-9996-0
Carter T. C., Menzel M., Saugey D. (2003). Population trends of solitary foliage-roosting bats. Monitoring trends in bat populations of the United States and territories: problems and prospects US Geological Survey, Biological Resources Discipline, Information and Technology Report Springfield (VA: National Information Services USGS/BRD/ITR-2003-003).
Cheng T. L., Reichard J. D., Coleman J. T., Weller T. J., Thogmartin W. E., Reichert B. E., et al. (2021). The scope and severity of white-nose syndrome on hibernating bats in North America. Conserv. Biol. 35, 1586–1597. doi: 10.1111/cobi.13739
Cockrum E. L. (1955). Reproduction in North American bats (Transactions of the Kansas Academy of Science), 458–487.
Cope J. B., Humphrey S. R. (1972). Reproduction of the bats myotis keenii and pipistrellus subflavus in indiana. Bat Res. News 13 (9).
Cope J. B., Baker W., Confer J. (1960). Breeding colonies of four species of bats of Indiana. Proc. Indiana Acad. Sci., 262–266.
Cope J. B., Whitaker J. O. Jr., Gummer S. L. (1991). Duration of bat colonies in Indiana. Proc. Indiana Acad. Sci., 199–202.
Damm J. P., Geluso K. (2008). Use of a mine by eastern pipistrelles (Perimyotis subflavus) in east central Nebraska. Western North Am. Nat. 68, 382–389. doi: 10.3398/1527-0904(2008)68[382:UOAMBE]2.0.CO;2
Davis W. H. (1959). Disproportionate sex ratios in hibernating bats. J. Mammalogy 40, 16. doi: 10.2307/1376111
Davis W. H. (1964a). Fall swarming of bats at Dixon Cave, Kentucky. Natl. Speleological Soc. Bull. 26, 82–83.
Davis W. H. (1964b). Winter Awakening Patterns in the Bats Myotis lucifugus and Pipistrellus subflavus. J. Mammalogy 45, 645. doi: 10.2307/1377349
Davis W. H. (1966). Population dynamics of the bat Pipistrellus subflavus. J. Mammalogy 47, 383–396. doi: 10.2307/1377679
Davis W. H., Mumford R. E. (1962). Ecological notes on the bat Pipistrellus subflavus. Am. Midland Nat., 394–398. doi: 10.2307/2422744
Deeley S., Johnson J. B., Ford W. M., Gates J. E. (2021). White-nose syndrome-related changes to mid-atlantic bat communities across an urban-to-rural gradient. BMC zoology 6 (1), 12.
Dixon J. W. (2011). The role of small caves as bat hibernacula in Iowa. J. Cave Karst Stud. 73, 21–27. doi: 10.4311/jcks2010lsc0145
Dzal Y., McGuire L. P., Veselka N., Fenton M. B. (2010). Going, going, gone: The impact of white- nose syndrome on the summer activity of the little brown bat (Myotis lucifugus). Biol. Lett. 7 (3): 392–394. doi: 10.1098/rsbl.2010.0859
Elsea J. R. (1953). Observations on the morphology and biology of Longibucca eptesica n. sp. (Nematoda: Cylindrocorporidae) parasitic in the bat. Proc. Helminthological Soc. Washington 20, 65–76.
Farrow L. J., Broders H. G. (2011). Loss of forest cover impacts the distribution of the forest-dwelling tri-colored bat (Perimyotis subflavus). Mamm. Biol. 76, 172–179. doi: 10.1016/j.mambio.2010.04.004
Feldhamer G. A., Carter T. C., Whitaker J. O. (2009). Prey consumed by eight species of insectivorous bats from southern Illinois. Am. Midland Nat. 162, 43–51. doi: 10.1674/0003-0031-162.1.43
Ferrara F. J., Leberg P. L. (2005). Influence of investigator disturbance and temporal variation on surveys of bats roosting under bridges. Wildlife Soc. Bull. 33 (3), 1113–1122. doi: 10.2193/0091-7648(2005)33[1113:IOIDAT]2.0.CO;2
Forbes G. (2012). Final report on wildlife monitoring – Highway 7 fence project (Roads and Wildlife).
Ford W. M., Britzke E. R., Dobony C. A., Rodrigue J. L., Johnson J. B. (2011). Patterns of acoustical activity of bats prior to and following white-nose syndrome occurrence. J. Fish Wildlife Manage. 2, 125–134. doi: 10.3996/042011-JFWM-027
Fraser E. E., McGuire L. P., Eger J. L., Longstaffe F. J., Fenton M. B. (2012). Evidence of latitudinal migration in tri-colored bats, perimyotis subflavus. PLoS One 7, e31419. doi: 10.1371/journal.pone.0031419
Gabriel K. T., McDonald A. G., Lutsch K. E., Pattavina P. E., Morris K. M., Ferrall E. A., et al. (2022). Development of a multi-year white-nose syndrome mitigation strategy using antifungal volatile organic compounds. PLoS One 17 (12), e0278603. doi: 10.1371/journal.pone.0278603
Gaisler J., Zukal J., Rehak Z., Homolka M. (1998). Habitat preference and flight activity of bats in a city. J. Zoology 244, 439–445. doi: 10.1111/j.1469-7998.1998.tb00048.x
Geluso K. N., Benedict R. A., Kock F. L. (2004). Seasonal activity and reproduction in bats of east-central Nebraska. Biology Faculty Publications. 45.
Geluso K., Mollhagen T. R., Tigner J. M., Bogan M. A. (2005). Westward expansion of the eastern pipistrelle (Perimyotis subflavus) in the United States, including new records from New Mexico, South Dakota, and Texas. Western North Am. Nat. 65, 405–409.
Gilley L. M., Kennedy M. L. (2010). A test of mist-net configurations in capturing bats over stream corridors. Acta Chiropterologica 12, 363–369. doi: 10.3161/150811010X537954
Goehring H. H. (1954). Perimyotis subflavus obscurus, Myotis keenii, and Eptesicus fuscus fuscus hibernating in a storm sewer in central Minnesota. J. Mammalogy 35, 434–436. doi: 10.2307/1375980
Griffith L. A., Gates J. E. (1985). Food habits of cave-dwelling bats in the central appalachians. J. Mammalogy 66, 451–460. doi: 10.2307/1380919
Hahn W. L. (1908). Some habits and sensory adaptations of cave-inhabiting bats. Biol. Bull. 15, 135–164. doi: 10.2307/1536066
Hammesfahr A., Rega-Brodsky C. C., Womack-Bulliner K., Whitney J. (2022). Roost characteristics of a tricolored bat Perimyotis subflavus in the Missouri Ozarks. Trans. Kansas Acad. Sci. 125, 159–164. doi: 10.1660/062.125.0307
Hanttula M. K., Valdez E. W. (2021). First record and diet of the tri-colored bat (Perimyotis subflavus) from Guadalupe Mountains National Park and Culberson County, Texas. Western North Am. Nat. 81, 131–134. doi: 10.3398/064.081.0111
Hayes J. P. (1997). Temporal variation in activity of bats and the design of echolocation-monitoring studies. J. Mammalogy 78, 514–524. doi: 10.2307/1382902
Helms J. S. (2010). A little bat and a big city: Nocturnal behavior of the tricolored bat (Perimyotis subflavus) near Indianapolis airport (Indiana State University).
Hilton C. D., Best T. L. (2000). Gastrointestinal helminth parasites of bats in Alabama. Occasional Papers North Carolina Museum Natural Sci. North Carolina Biol. Survey 12, 57–66.
Hoofer S. R., Van Den Bussche R. A., Horáček I. (2003). Generic status of the American pipistrelles (Vespertilionidae) with description of a new genus. J. mammalogy 87, 981–992. doi: 10.1644/05-MAMM-A-425R1.1
Horáček I., Hanák V. (1985). Generic status of Pipistrellus savii (Bonaparte 1837) and remarks on systematics of the genus Pipistrellus. Bat Res. News 26, 62.
Hoying K. M. (1983). Growth and development of the eastern pipistrelle bat, Perimyotis subflavus (Boston University).
Hoying K. M., Kunz T. H. (1998). Variation in size at birth and post-natal growth in the insectivorous bat Perimyotis subflavus (Chiroptera: Vespertilionidae). J. Zoology 245, 15–27. doi: 10.1111/j.1469-7998.1998.tb00067.x
Huebschman J. J. (2019). Bats in southwest Wisconsin during the era of white-nose syndrome. Northeastern Nat. 26, 168–182. doi: 10.1656/045.026.0115
Ingersoll T. E., Sewall B. J., Amelon S. K. (2013). ) Improved analysis of long-term monitoring data demonstrates marked regional declines of bat populations in the eastern United States. PLoS One 8 (6), e65907. doi: 10.1371/journal.pone.0065907
Ingersoll T. E., Sewall B. J., Amelon S. K. (2016). Effects of white-nose syndrome on regional population patterns of 3 hibernating bat species. Conserv. Biol. 30 (5), 1048–1059. doi: 10.1111/cobi.12690
Jackson L. S., Thompson C. A., Dinsmore J. J. (1996). The Iowa breeding bird atlas (University of Iowa Press).
Jackson R. T., Willcox E. V., Bernard R. F. (2022b). Winter torpor expression varies in four bat species with differential susceptibility to white-nose syndrome. Sci. Rep. 12 (1), 5688. doi: 10.1038/s41598-022-09692-x
Jackson R. T., Willcox E. V., Zobel J. M., Bernard R. F. (2022a). Emergence activity at hibernacula differs among four bat species affected by white-nose syndrome. Ecol. Evol. 12 (7), e9113. doi: 10.1002/ece3.9113
Johnson C., Brown D. J., Sanders C., Stihler C. W. (2021). Long-term changes in occurrence, relative abundance, and reproductive fitness of bat species in relation to arrival of White-nose Syndrome in West Virginia, USA. Ecol. Evol. 11, 12453–12467. doi: 10.1002/ece3.7991
Johnson J. B., Gates J. E., Ford W. M. (2008). Distribution and activity of bats at local and landscape scales within a rural–urban gradient. Urban Ecosyst. 11, 227–242. doi: 10.1007/s11252-008-0055-x
Johnson J. B., Gates J. E., Zegre N. P. (2011). Monitoring seasonal bat activity on a coastal barrier island in Maryland, USA. Environ. Monit. Assess. 173, 685–699. doi: 10.1007/s10661-010-1415-6
Johnson J. S., Scafini M. R., Sewall B. J., Turner G. G. (2016). Hibernating bat species in Pennsylvania use colder winter habitats following the arrival of white-nose syndrome. Conserv. Ecol. Pennsylvania’s bats, 181–199.
Jones J. K., Loomis R. B., Krutzsch P. H., Webb O. L. (1952). New records of bats from Northeastern Kansas, with notes on the bat chigger, euschongastia pipistrelli (Acarina, Trombiculidae). Trans. Kansas Acad. Sci. (1903) 55, 312–314. doi: 10.2307/3626238
Jones C., Pagels J. (1968). Notes on a population of Perimyotis subflavus in Southern Louisiana. J. Mammalogy 49, 134–139. doi: 10.2307/1377741
Jones C., Suttkus R. D. (1973). Colony structure and organization of Perimyotis subflavus in southern Louisiana. J. Mammalogy 54, 962–968. doi: 10.2307/1379091
Kaarakka H. M., Pelton E. M., Redell D. (2013). Eastern Pipistrelle (Perimyotis subflavus) species guidance (Wisconsin Department of Natural Resources).
Kalcounis-Rueppell M. C., Payne V. H., Huff S. R., Boyko A. L. (2007). Effects of wastewater treatment plant effluent on bat foraging ecology in an urban stream system. Biol. Conserv. 138, 120–130. doi: 10.1016/j.biocon.2007.04.009
Keeley B., Tuttle M. (1999). Bats in American bridges: Resource publication no. 4 (Bat Conservation International, Inc).
Krutzsch P. H., Crichton E. G. (1986). Reproduction of the male eastern pipistrelle, Perimyotis subflavus, in the north-eastern United States. Reproduction 76, 91–104. doi: 10.1530/jrf.0.0760091
Kunz T. H. (1971). Reproduction of some vespertilionid bats in central Iowa. Am. Midland Nat. 86, 477. doi: 10.2307/2423638
Kunz T. H., Schlitter D. A. (1968). An annotated checklist of bats from Iowa. Trans. Kansas Acad. Sci. (1903) 71, 166. doi: 10.2307/3627368
Lacki M. J. (1984). Temperature and humidity-induced shifts in the flight activity of little brown bats. Ohio Journal of Science 84 (5), 264–266.
Lacki M. J., Bookhout T. A. (1983). A survey of bats in Wayne National Forest, Ohio. Ohio Journal of Science. 1, 45–50.
Lacki M. J., Dodd L. E., Toomey R. S., Thomas S. C., Couch Z. L., Nichols B. S. (2015). Temporal changes in body mass and body condition of cave-hibernating bats during staging and swarming. J. fish wildlife Manage. 6 (2), 360–370. doi: 10.3996/042015-JFWM-033
Lance R. F., Hardcastle B. T., Talley A., Leberg P. L. (2001). Day-roost selection by Rafinesque's big-eared bats (Corynorhinus rafinesquii) in Louisiana forests. J. Mammalogy 82 (1), 166–172. doi: 10.1644/1545-1542(2001)082<0166:DRSBRS>2.0.CO;2
Lane H. K. (1946). Notes on Perimyotis subflavus subflavus (F. Cuvier) during the season of parturition. Proc. Pennsylvania Acad. Science. JSTOR, 57–61.
LaVal R. K., LaVal M. L. (1980). Ecological studies and management of Missouri bats, with emphasis on cave-dwelling species. Missouri Department of Conservation: Terrestrial Series 8, 1–53.
Leatherberry E. C., Moser W. K., Perry C., Woodall C., Jespen E., Pennington S., et al. (2006). Iowa’s forests 1999-2003 (Part A). St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Research Station, 84.
Leivers S. J., Lee E. H., Fuller N. W. (2021). Tri-Colored Bat (Perimyotis subflavus) Predation by a Dark Fishing Spider (Dolomedes tenebrosus) in East Texas. Southeastern Nat. 20 (3), 98. doi: 10.1656/058.020.0312
Leivers S. J., Meierhofer M. B., Pierce B. L., Evans J. W., Morrison M. L. (2019). External temperature and distance from nearest entrance influence microclimates of cave and culvert-roosting tri-colored bats (Perimyotis subflavus). Ecol. Evol. 9, 14042–14052. doi: 10.1002/ece3.5841
Loeb S. C., Winters E. A. (2022). Changes in hibernating tricolored bat (Perimyotis subflavus) roosting behavior in response to white-nose syndrome. Ecol. Evol. 12, e9045. doi: 10.1002/ece3.9045
Lorch J. M., Meteyer C. U., Behr M. J., Boyles J. G., Cryan P. M., Hicks A. C., et al. (2011). Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480, 376–378. doi: 10.1038/nature10590
Macy R. W. (1931). New bat trematodes of the genera Plagiorchis, Limatulum, and Dicrocoelium. J. Parasitol. 18, 28–33. doi: 10.2307/3271740
Macy R. W. (1936). ) A new bat trematode Lecithodendrium brackenridgei, with a key to the species of the genus. Zentralblatt fur Bakteriologie Parasitenkunde Infektionskrankheiten und Hygiene Abt I (Originale) 136, 236–237.
Macy R. W. (1940). Description of three new trematodes with notes on other species of Acanthatrium (Lecithodendriidae), and a key to the genus. J. Parasitol. 26, 279–286. doi: 10.2307/3272100
Martin R. L. (1961). Vole predation on bats in an Indiana cave. J. Mammalogy 42, 540–541. doi: 10.2307/1377384
McAllister C. T., Burt S., Seville R. S., Robison H. W. (2011). A new species of eimeria (Apicomplexa: Eimeriidae) from the eastern Pipistrelle, perimyotis subflavus (Chiroptera: Vespertilionidae), in Arkansas. J. Parasitol. 97, 896–898. doi: 10.1645/GE-2761.1
McAllister C. T., Connior M. B., Bursey C. R., Durden L. A., Seville R. S., Robison H. W., et al. (2016). Parasites (Coccidia, Trematoda, Acari) of tri-colored bats, Perimyotis subflavus (Chiroptera: Vespertilionidae): New geographical records for Oklahoma. Proceedings of the Oklahoma Academy of Science. 96, 63–69.
McAllister C. T., Durden L. A., Greiman S. E. (2021). Euschoengastia pipistrelli (Acari: Trombiculidae) from American Perimyotis, Perimyotis subflavus (Chiroptera: Vespertilionidae): Novel Stereoscopic and Scanning Electron Microscopy. J. Parasitol. 107, 125–128. doi: 10.1645/20-139
McAllister C., Seville R., Arlen R., Connior M. (2014). A new species of Eimeria (Apicomplexa: Eimeriidae) from tri-colored bats, Perimyotis subflavus (Chiroptera: Vespertilionidae), from the Ouachitas of Arkansas. Acta Parasitologica 59, 690–693. doi: 10.2478/s11686-014-0297-0
McCarthy T. J., Davis W. B., Hill J. E., Jones J. K. Jr., Cruz G. A. (1993). Bat (MamMalia: Chiroptera) records, early collectors, and faunal lists for northern Central America. Ann. Carnegie Museum 62, 191–228. doi: 10.5962/p.226650
McGuire L. P., Johnson E. M., Frick W. F., Boyles J. G. (2021). Temperature alone is insufficient to understand hibernation energetics. J. Exp. Biol. 224 (14), e1–e6. doi: 10.1242/jeb.239772
McNab B. K. (1974). The behavior of temperate cave bats in a subtropical environment. Ecology 55, 943–958. doi: 10.2307/1940347
Measures L. N. (1994). Synonymy of Longibucca eptesica with Longibucca lasiura (Nematoda: Rhabditoidea) and New Host and Geographic Records. J. Parasitol. 80, 486. doi: 10.2307/3283424
Medina-Fitoria A., Saldaña O., Martínez J. G., Aguirre Y., Silva W., Chávez M., et al. (2015). Nuevos reportes sobre los murciélagos (MamMalia: Chiroptera) de Nicaragua, América Central, con la adición de siete nuevos registros de especies. Mastozoología neotropical 22 (1), 43–54.
Meierhofer M. B., Leivers S. J., Pierce B. L., Powers G. W., Evans J. W., Morrison M. L. (2022). Structural and environmental predictors of tricolored bat presence and abundance in texas caves. J. Mammalogy 103 (2), 407–414.
Meierhofer M. B., Johnson J. S., Leivers S. J., Pierce B. L., Evans J. W., Morrison M. L. (2019). Winter habitats of bats in Texas. PLoS One 14 (8), e0220839. doi: 10.1371/journal.pone.0220839
Menzel M. A., Krishon D. M., Carter T. C., Laerm J. (1999). Notes on tree roost characteristics of the northern yellow bat (Lasiurus intermedius), the Seminole bat (L. seminolus), the evening bat (Nycticeius humeralis), and the eastern pipistrelle (Perimyotis subflavus). Florida Scientist, 185–193.
Menzel M. A. Jr., Menzel J. M., Carter T. C., Whitaker J. O. Jr., Ford W. M. (2002). Notes on the late summer diet of male and female eastern pipistrelles (Perimyotis subflavus) at Fort Mountain State Park, Georgia. Georgia J. Sci. 60, 170–180.
Miller G. S. (1897). Revisions of the North American bats of the family vespertilionid [a] e (US Government Printing Office).
Miller D. A. (2003). Species diversity, reproduction, and sex ratios of bats in managed pine forest landscapes of Mississippi. Southeastern Nat. 2, 59–72. doi: 10.1656/1528-7092(2003)002[0059:SDRASR]2.0.CO;2
Mohr C. E. (1945). Sex ratios of bats in Pennsylvania. Proceedings of the Pennsylvania Academy of Science. 65–69.
Muir T. J., Polder E. (1960). Notes on hibernating bats in Dubuque County caves. Proceedings of the Iowa Academy of Science. 602–606.
Newman B. A., Loeb S. C., Jachowski D. S. (2021). Winter roosting ecology of tricolored bats (Perimyotis subflavus) in trees and bridges. J. Mammalogy 102, 1331–1341. doi: 10.1093/jmammal/gyab080
Nickel P. A., Hansen M. F. (1967). Helminths of bats collected in Kansas, Nebraska and Oklahoma. Am. Midland Nat., 481–486. doi: 10.2307/2485245
O’Farrell M. J., Bradley W. G. (1970). Activity patterns of bats over a desert spring. J. Mammalogy 51, 18–26. doi: 10.2307/1378527
O’Keefe J. M., Loeb S. C., Lanham J. D., Hill H. S. Jr. (2009). Macrohabitat factors affect day roost selection by eastern red bats and eastern pipistrelles in the southern Appalachian Mountains, USA. For. Ecol. Manage. 257, 1757–1763. doi: 10.1016/j.foreco.2009.01.037
O’Keefe J. M., Pettit J. L., Loeb S. C., Stiver W. H. (2019). White-nose syndrome dramatically altered the summer bat assemblage in a temperate Southern Appalachian Forest. Mamm. Biol. 98, 146–153. doi: 10.1016/j.mambio.2019.09.005
Palmer D. B. Jr., Gunier W. J. (1975). ) A preliminary survey of arthropods associated with bats and bat caves in Missouri and two counties of Oklahoma. J. Kansas Entomological Soc., 524–531.
Perea S., Yearout J. A., Ferrall E. A., Morris K. M., Pynne J. T., Castleberry S. B. (2022). Seven-year impact of white-nose syndrome on tri-colored bat (Perimyotis subflavus) populations in Georgia, USA. Endangered Species Resour. 48, 99–106. doi: 10.3354/esr01189\
Perry R. W., Carter S. A., Thill R. E. (2010). Temporal patterns in capture rate and sex ratio of forest bats in Arkansas. Am. Midland Nat. 164 (2), 270–282. doi: 10.1674/0003-0031-164.2.270
Perry R. W., Jordan P. N. (2020). Survival and persistence of tricolored bats hibernating in Arkansas mines. J. Mammalogy 101, 535–543. doi: 10.1093/jmammal/gyaa016
Perry R. W., Jordan P. N. (2022). Changes in the forest bat community after arrival of white-nose syndrome in the Ouachita Mountains of Arkansas. Southeastern Nat. 21 (2), 107–115. doi: 10.1656/058.021.0204
Perry R. W., Thill R. E. (2007). Tree roosting by male and female Eastern Pipistrelles in a forested landscape. J. Mammal 88, 974–981. doi: 10.1644/06-MAMM-A-215R.1
Pettit J. L., O’Keefe J. M. (2017). Impacts of white-nose syndrome observed during long-term monitoring of a midwestern bat community. J. Fish Wildlife Manage. 8, 69–78. doi: 10.3996/102016-JFWM-077
Pistole D. H. (1988). A survey of helminth parasites of chiropterans from Indiana. Proc. Helminthological Soc. Washington 55, 270–274.
Poissant J. A., Broders H. G., Quinn G. M. (2010). Use of lichen as a roosting substrate by Perimyotis subflavus, the tricolored bat, in Nova Scotia. Ecoscience 17, 372–378. doi: 10.2980/17-4-3352
Powers K. E., Reynolds R. J., Orndorff W., Ford W. M., Hobson C. S. (2015). Post-White-nose syndrome trends in Virginia’s cave bats. 2008–2013.
Prendergast J. A., Jensen W. E., Roth S. D. (2010). Trends in abundance of hibernating bats in a karst region of the southern Great Plains. Southwestern Nat. 55.3, 331–339. doi: 10.1894/MRD-10.1
Quinn G. M., Broders H. G. (2007). Roosting and foraging ecology of eastern pipistrelle (Perimyotis subflavus) bats in SW Nova Scotia (Unpublished report to Nova Scotia Habitat Conservation Fund c/o NS Department of Natural Resources).
Reeder D. M., Frank C. L., Turner G. G., Kurta A., Britzke E. R., Darling S. R., et al. (2012). Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS One 7 (6), e38920. doi: 10.31371/journal.pone.0038920
Rinker G. C. (1948). A bat (Pipistrellus) record from Honduras. J. Mammalogy 29, 179–180. doi: 10.1093/jmammal/29.2.179
Rydell J. (1991). Seasonal use of illuminated areas by foraging northern bats Eptesicus nilssoni. Ecography 14, 203–207. doi: 10.1111/j.1600-0587.1991.tb00653.x
Rysgaard G. N. (1942). A study of the cave bats of Minnesota with especial reference to the large brown bat, Eptesicus fuscus fuscus (Beauvois). Am. Midland Nat. 28, 245–267. doi: 10.2307/2420702
Samoray S. T., Cotham S. N., Gumbert M. W. (2019). Spring migration behavior of a Perimyotis subflavus (tri-colored bat) from Tennessee. Southeastern Nat. 18. doi: 10.1656/058.018.0302
Sandel J. K., Benatar G. R., Burke K. M., Walker C. W., Lacher T. E., Honeycutt R. L. (2001). Use and selection of winter hibernacula by the Eastern Pipistrelle (Pipistrellus subflavus) in Texas. J. Mammalogy 82, 173–178. doi: 10.1644/1545-1542(2001)082<0173:UASOWH>2.0.CO;2
Smith L. M., Gore J. A., Doonan T. J., Campbell C. J. (2022). Tricolored bats at a southern range edge exhibit partial migration northward in autumn. Movement Ecol. 10 (1), 56.
Shiel C. B., Fairley J. S. (1998). Activity of Leisler’s bat Nyctalus leisleri (Kuhl) in the field in south-east county Wexford, as revealed by a bat detector Biology and Environment: Proceedings of the Royal Irish Academy. 105–112, JSTOR.
Shute K. E., Loeb S. C., Jachowski D. S. (2021). Summer roosting ecology of the northern yellow bat and tri-colored bat in coastal South Carolina. Southeastern Nat. 20, 459–476. doi: 10.1656/058.020.0306
Silvis A., Gehrt S. D., Williams R. A. (2016). Effects of shelterwood harvest and prescribed fire in upland Appalachian hardwood forests on bat activity. For. Ecol. Manage. 360, 205–212. doi: 10.1016/j.foreco.2015.10.010
Swanson G., Evans C. (1936). The hibernation of certain bats in Southern Minnesota. J. Mammalogy 17, 39–43. doi: 10.2307/1374548
Thornton J. E. B., Richlen M. E., McDonald T. B., Bell J. T. (2023). Opportunistic offshore sighting of a tricolored bat (Perimyotis subflavus). Southeastern Nat. 22 (1), N9–N12. doi: 10.1656/058.022.0106
Trousdale A. W., Beckett D. C. (2002). Bats (MamMalia: Chiroptera) recorded from mist-net and bridge surveys in southern Mississippi. Officers Mississippi Acad. Sci. 47, 185.
Turcios-Casco M. A., Mejía-Suazo C. J., Bautista D. J. O., Ávila-Palma H. D. (2020). Noteworthy records of the Geoffroy’s tailless bat and the Eastern Pipistrelle in Copán, western Honduras (Mammalia Chiroptera). Biodiversity J. 2), 527–534. doi: 10.31396/Biodiv.Jour.2020.11.2.527.534
Turner G. G., Reeder D., Coleman J. T. (2011). A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats, with a look at the future. Update white-nose syndrome bats. Bat Res. News 52, 13.
Turner G. G., Sewall B. J., Scafini M. R., Lilley T. M., Bitz D., Johnson J. S. (2022). Cooling of bat hibernacula to mitigate white-nose syndrome. Conservation Biology 36, 2. doi: 10.1111/cobi.13803
Turner G. G., Sewall B. J., Scafini M. R., Lilley T. M., Bitz D., Johnson J. S. (2022). Cooling of bat hibernacula to mitigate white-nose syndrome. Conserv. Biol. 36 (2), e13803.
Twente J. W., Twente J., Brack V. (1985). The duration of the period of hibernation of three species of vespertilionid bats. II. Laboratory studies. Can. J. Zoology 63 (12), 2955–2961.
US EPA (2010) NA_CEC_Eco_Level3. Available at: http://www.epa.gov/wed/pages/ecoregions.htm.
US EPA (2011) Level III ecoregions of Central and South America. Available at: ftp://ftp.epa.gov/wed/ecoregions/sa/sa_eco.zip.
USFWS (2011) Indiana bat section 7 and section 10 guidance for wind energy projects. Available at: http://www.fws.gov/midwest/endangered/mammals/inba/pdf/inbaS7and10WindGuidanceFinal26Oct2011.pdf.
USFWS (2019) Conserving South Carolina’s at-risk species: Tri-colored bats. Available at: https://www.fws.gov/southeast/pdf/fact-sheet/tri-colored-bat.pdf.
Valdez E. W., Geluso K., Foote J., Allison-Kosior G., Roemer D. M. (2009). Spring and winter records of the Eastern Pipistrelle (Perimyotis subflavus) in Southeastern New Mexico. Western North Am. Nat. 69, 396–398. doi: 10.3398/064.069.0315
Vaughan N., Jones G., Harris S. (1997). Habitat use by bats (Chiroptera) assessed by means of a broad-band acoustic method. J. Appl. Ecol., 716–730. doi: 10.2307/2404918
Veilleux J. P., Veilleux S. L. (2004a). Colonies and reproductive patterns of tree-roosting female eastern pipistrelle bats in Indiana. Proceedings of the Indiana Academy of Science. 60–65.
Veilleux J. P., Veilleux S. L. (2004b). Intra-annual and interannual fidelity to summer roost areas by female Eastern Pipistrelles, Perimyotis subflavus. Am. Midland Nat. 152, 196–200. doi: 10.1674/0003-0031(2004)152[0196:IAIFTS]2.0.CO;2
Veilleux J. P., Whitaker J. O., Veilleux S. L. (2003). Tree-roosting ecology of reproductive female eastern pipistrelles, Perimyotis subflavus, in Indiana. J. Mammalogy 84, 1068–1075. doi: 10.1644/BEM-021
Verant M. L., Meteyer C. U., Speakman J. R., Cryan P. M., Lorch J. M., Blehert D. S. (2014). White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Phys. 14 (10). doi: 10.1186/s12899-014-0010-4
Walley H. D., Jarvis W. L. (1971). Longevity record for Pipistrellus subflavus. Illinois Acad. Sci. 64, 305.
Warnecke L., Turner J. M., Bollinger T. K., Lorch J. M., Misra V., Cryan P. M., et al. (2012). Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl. Acad. Sci. 109, 6999–7003. doi: 10.1073/pnas.1200374109
Weinkauf C. J., Comer C. E., Conway W. C., Farrell C. (2018). Dietary composition of four common chiropteran species in a bottomland hardwood forest. Acta Chiropterologica 20, 195–205. doi: 10.3161/15081109ACC2018.20.1.015
Wetzel T., Roby P. (2023). Bats use of bridges and culverts (No. cmr 23-008). Missouri (Department of Transportation. Construction and Materials Division).
Wheat B. E. (1975). Eimeria macyi sp. n. (Protozoa: Eimeriidae) from the eastern pipistrelle, Pipistrellus subflavus, from Alabama. J. Parasitol., 920–922.
White-nose syndrome Response Team (2023). White nose syndrome occurrence by County/District (USGS). Available at: https://sciencebase.usgs.gov/geoserver/wns_status/wfs.
Whitaker J. O. Jr. (1998). Life history and roost switching in six summer colonies of eastern pipistrelles in buildings. J. Mammalogy 79 (2), 651–659. doi: 10.2307/1382995
Whitaker J. O. Jr., Mumford R. E. (1971). Notes on a collection of bats taken by mist-netting at an Indiana cave (American Midland Naturalist), 277–279.
Whitaker J. O. Jr., Rissler L. J. (1992a). Seasonal activity of bats at Copperhead Cave. In Proc. Indiana Acad. Sci. 101, 127–134). doi: 10.2307/2426321
Whitaker J. O. Jr., Rissler L. J. (1992b). Winter activity of bats at a mine entrance in Vermillion County, Indiana (American Midland Naturalist:52–59).
White J. A., Lemen C. A., Freeman P. W. (2016). Acoustic Detection Reveals Fine-Scale Distributions of Myotis lucifugus, Myotis septentrionalis, and Perimyotis subflavus in Eastern Nebraska. Western North Am. Nat. 76, 27–35. doi: 10.3398/064.076.0105
White J. A., Moosman P. R. Jr., Kilgore C. H., Best T. L. (2006). First record of the eastern pipistrelle (Pipistrellus subflavus) from southern New Mexico. Southwestern Nat. 51, 420–422. doi: 10.1894/0038-4909(2006)51[420:FROTEP]2.0.CO;2
Williams R. R. (1957). Some helminth parasites from bats with descriptions of two new species of Acanthatrium Faust 1919 (trematoda: Lecithodendriidae) (The Ohio State University).
Wimsatt W. A. (1945). Notes on breeding behavior, pregnancy, and parturition in some vespertilionid bats of the eastern United States. J. Mammalogy 26, 23–33. doi: 10.2307/1375029
Winchell J. M., Kunz T. H. (1996). Day-roosting activity budgets of the eastern pipistrelle bat, Pipistrellus subflavus (Chiroptera: Vespertilionidae). Can. J. Zoology 74, 431–441. doi: 10.1139/z96-050
Wine M., Bowen C. A., Green D. M. (2019). Interspecific aggression between a hoary bat (Lasiurus cinereus) and a tricolored bat (Perimyotis subflavus) in Northern Arkansas. Southeastern Nat. 18, N37–N40. doi: 10.1656/058.018.0407
Yates D. E., Adams E. M., Angelo S. E., Evers D. C., Schmerfeld J., Moore M. S., et al. (2014). Mercury in bats from the northeastern United States. Ecotoxicology 23, 45–55. doi: 10.1007/s10646-013-1150-1
Yates M. D., Muzika R. M. (2006). Effect of forest structure and fragmentation on site occupancy of bat species in Missouri Ozark forests. J. Wildlife Manage. 70, 1238–1248. doi: 10.2193/0022-541X(2006)70[1238:EOFSAF]2.0.CO;2
Keywords: bats, demographics, Perimyotis subflavus, eastern pipistrelle, ecology, sex-ratio
Citation: McCoshum SM, Pratt EL, Lent KC and Boisen EM (2023) Literature review of tri-colored bat natural history with implications to management. Front. Conserv. Sci. 4:1204901. doi: 10.3389/fcosc.2023.1204901
Received: 12 April 2023; Accepted: 17 August 2023;
Published: 21 September 2023.
Edited by:
David R. Breininger, University of Central Florida, United StatesReviewed by:
Krizler Tanalgo, University of Southern Mindanao, PhilippinesMelissa Meierhofer, University of Helsinki, Finland
Copyright © 2023 McCoshum, Pratt, Lent and Boisen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaun M. McCoshum, bWNjb3Noc21AZ21haWwuY29t; c2hhdW4ubWNjb3NodW1Ad2VzdHdvb2Rwcy5jb20=
 Shaun M. McCoshum
Shaun M. McCoshum Erica L. Pratt
Erica L. Pratt