- 1Conservation Science Wildlife Health, San Diego Zoo Wildlife Alliance, Escondido, CA, United States
- 2Department of Fish, Wildlife, and Conservation Biology, Colorado State University, Fort Collins, CO, United States
- 3Northern Rangelands Trust, Isiolo, Kenya
- 4Free the Bears, Phnom Penh, Cambodia
- 5Science for Wildlife, Sydney, NSW, Australia
- 6Save the Elephants, Nairobi, Kenya
Rescue, rehabilitation, and release (‘rescue-rehab-release’) of wildlife is an increasingly widespread practice across ecosystems, largely driven by habitat loss, wildlife exploitation and a changing climate. Despite this, its conservation value has not been realized, in part due to the scarcity of what has been termed “the 4th R”, research. Similar to conservation breeding and headstarting, rescue and rehabilitation entails close association of humans and the wildlife in their care over impressionable and extended periods. However, unlike these interventions, rescue and rehabilitation require an initial, and sometimes sustained, focus on crisis management and veterinary needs which can impede the development of natural behaviors and promote habituation to humans, both of which can compromise post-release survival and recruitment. In this perspective, we discuss the pathways toward, and implications of, behavioral incompetence and highlight opportunities for testable interventions to curtail negative outcomes post-release, without compromising the health or welfare of rescued individuals. We propose that practitioners ‘switch gears’ from triage to fostering behavioral competence as early in the rehabilitation process as is possible, and that research be implemented in order to develop an evidence-base for best practices that can be shared amongst practitioners. We focus on four mammalian species to illustrate specific contexts and considerations for fostering behavioral competence by building on research in the conservation translocation literature. Finally, we discuss a way forward that calls for greater cross-pollination among translocation scenarios involving extended time under human care during developmentally sensitive periods.
Introduction
Wildlife rescue, rehabilitation and release (‘rescue-rehab-release’) is widespread, spanning continents, taxa, and contexts (Guy et al., 2013; Pyke and Szabo, 2018). It can be high-profile and high-stakes when focused on charismatic megafauna or species that may harm humans, and involves considerable labor and financial resources (Molina-López et al., 2017; Englefield et al., 2019; Morgans et al., 2019; Haering et al., 2021). Scenarios prompting rescue-rehab-release vary and are typically reactive, stemming from catastrophic events posing danger to populations or creating unsuitable habitat [e.g., oil spills (Hong et al., 2020), algal blooms (Lefebvre et al., 2016), wildfires (Parrott et al., 2021), drought (Mo et al., 2021)]; and recurring threats that drive defaunation and compromise welfare [e.g., illegal wildlife trade (Moore et al., 2014; Castro Cortés et al., 2022), orphaning, injury, human-wildlife conflict (Marker et al., 2021)], or threats from occupying human-dominated areas [e.g. vehicle collisions, dog attacks (McAlpine et al., 2008; Kwok et al., 2021)]. Crisis translocations like these and others that are reactive (e.g., mitigation translocations) will intensify worldwide with accelerated habitat loss, climate change, and other threats (Pyke and Szabo, 2018; Bradley et al., 2022).
Rescue-rehab-release provides an important touchpoint between wildlife practitioners and the public through ambassador and education programs (Normande et al., 2015; Osterberg et al., 2015; Romero et al., 2019); and through social media, which facilitates sharing of heart-warming stories, engages local and global communities, and engenders support for wildlife (Stokes et al., 2018). Not all rescue-rehab-release has conservation value per se (Cope et al., 2022), but its potential to contribute to species recovery and population health has not been adequately recognized, nor realized (Molony et al., 2006; Pyke and Szabo, 2018; Blair et al., 2020; Paterson et al., 2021).
Releasing wildlife after time under human care (‘HC’) is a shared objective among reactive rescue-rehab-release and proactive conservation strategies like conservation breeding and headstarting, which seek to reinforce or reintroduce free-ranging populations and protect/restore genetic diversity (Thomas et al., 2019) and are distinct from other contexts like mitigation translocations that do not keep animals under HC for lengthy periods (Bradley et al., 2022). The aims of rescue-rehab-release’s later stages overlap with those of proactive breeding/headstarting for translocation, including determining whether wildlife are releasable, preparing them for release, and monitoring post-release (Figure 1). Yet this intersection is infrequently acknowledged or leveraged, and has rarely been documented in the literature. For example, rescue-rehab-release is not considered under the larger umbrella of the IUCN translocation guidelines (IUCN/SSC, 2013), which may contribute to the lack of evidence for appropriate pre-release practices compared to proactive translocations (Alberts, 2007; Guy et al., 2014; Fuller et al., 2021). Pyke and Szabo (2018) articulated the undeniable need to capitalize on research opportunities associated with rescue-rehab-release (the “4 R’s”), outlining how studying rescue-rehab-release could improve understanding of wildlife needs and meaningfully contribute to conservation.
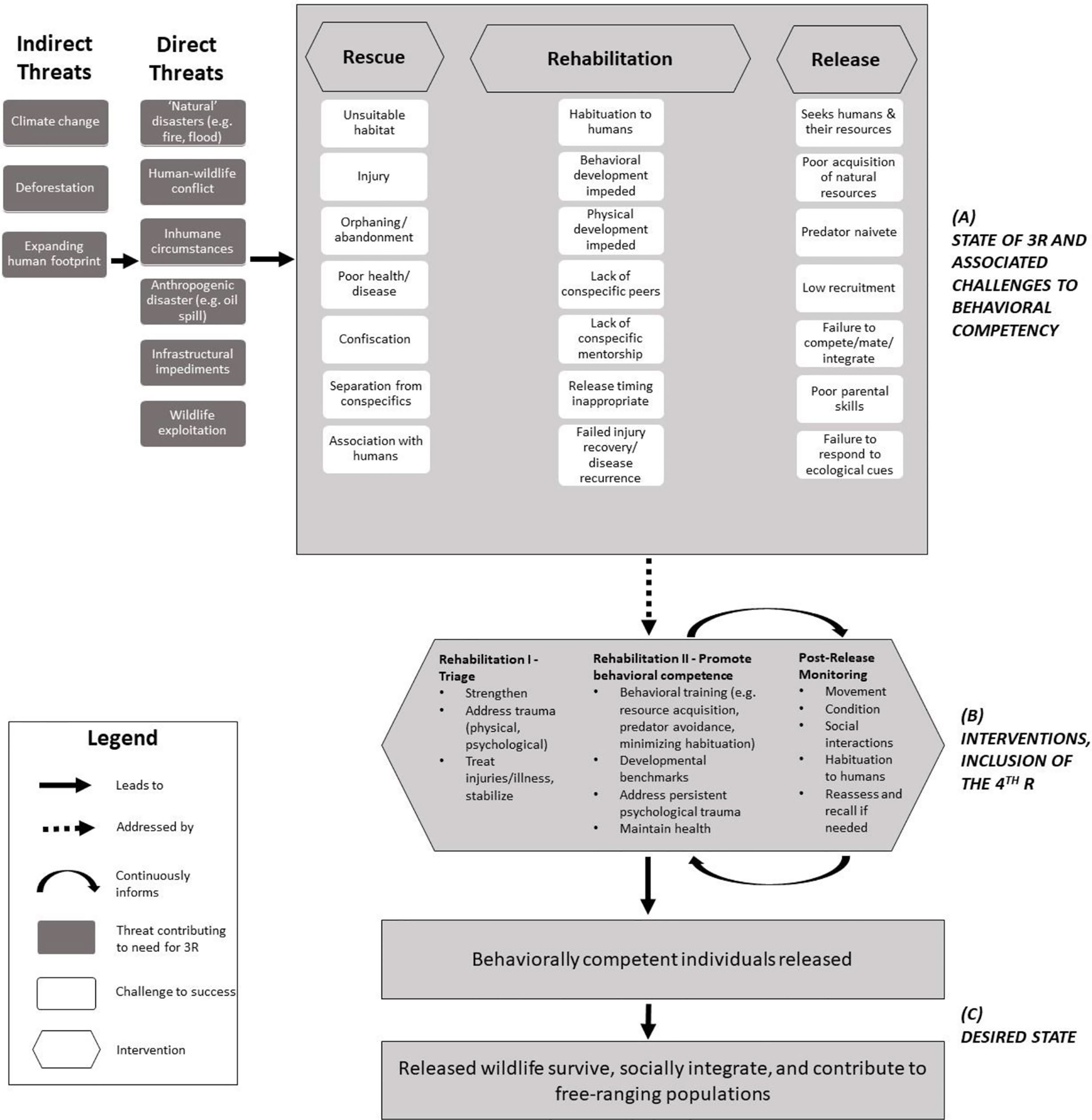
Figure 1 (A) Generalized situation model (CMP, 2020) of rescue-rehab-release. During rehabilitation, several factors may contribute to post-release behavioral incompetence, which may contribute to poor outcomes. These are overlapping considerations for conservation breeding and headstarting. (B) Targeted transition during Rehabilitation from triage activities (I) to interventions that promote behavioral competence (II). Post-release monitoring builds on Rehabilitation II interventions and is iterative and adaptive. Incorporating the 4th R, research, is essential for achieving (C) the desired state for rescued and released wildlife.
HC of wildlife poses unique challenges to post-release success for all conservation translocations (Alberts, 2007; Greggor et al., 2018); however, reactive HC adds numerous challenges. First, from the outset of HC, proactive breeding/headstarting programs benefit from pre-intervention planning and iterative implementation of husbandry and health regimes designed to foster natural behaviors and minimize dependence on humans. This is often not an option with rescued wildlife, and in some cases (e.g., pet trade) rescued animals have been deliberately human-imprinted. Second, rescued wildlife often come into HC after experiencing trauma, which in turn requires caregivers to restore well-being. Especially with juveniles, this may involve caregivers simulating conspecific interaction or providing emotional comfort, which often strengthens bonds with and diminishes fear of humans. Rescued wildlife may learn that humans are not threatening, are a food source, or are conspecific replacements (Jule et al., 2008; Fàbregas et al., 2020), which may be a particular challenge where human-wildlife conflict mitigation strategies employ fear-based deterrents (Mumby and Plotnik, 2018). Third, the consistent resources and safety provided while under HC may inhibit learning of survival-relevant behaviors and dynamic ecological cues. These points are especially concerning for juveniles.
Behavioral incompetence may be dire for released animals, in particular where animals may cause harm to human lives or livelihoods (Gusset, 2009; Bansiddhi et al., 2020). Post-release success can be difficult to achieve, requiring iteration and evaluation, as noted often in the conservation translocation literature (Wolf et al., 1996; IUCN/SSC, 2013; Guy et al., 2014; Berger-Tal et al., 2020). There is a need for adaptive management practices that support releasing behaviorally competent individuals and evaluating post-release outcomes for rehabilitated wildlife (Lander and Gulland, 2003; Guy et al., 2013; Myers and Young, 2018; Campera et al., 2020). This approach may produce leading indicators of success that can be measured earlier and over a shorter term (Morris et al., 2009) than lagging indicators (e.g., survival, recruitment). Vetted leading indicators are a needed complement to lagging indicators, especially given the many resources and sustained monitoring needed to document lagging indicators.
Here, we focus on leveraging rehabilitation to better prepare wildlife for release and, in so doing, maximize contribution to free-ranging populations. This will require species-, and context-specific approaches that incorporate research during rehabilitation. Because behavioral issues are a primary factor in post-release failures for mammals (Berger-Tal et al., 2020), and behavioral conditioning generally improves post-release outcomes in mammals (Tetzlaff et al., 2019), we emphasize fostering the development of behavioral competence during a structured ‘second phase’ of rehabilitation that is implemented as early as possible. While other contexts for wildlife translocations exist (Bradley et al., 2022), we focus on those with the common theme of prolonged time under HC during which wildlife may be studied and their behavior modified. Below, we discuss examples from four mammalian species to illustrate interventions across divergent rescue-rehab-release contexts, acknowledging that any given species may encounter multiple behavioral challenges simultaneously. We use this discussion to promote guideline development that explicitly recognizes the need to switch gears in rehabilitation practices toward behavioral needs, and that draws on commonalities across translocation contexts where wildlife are temporarily under HC (Cope et al., 2022).
Case studies and discussion
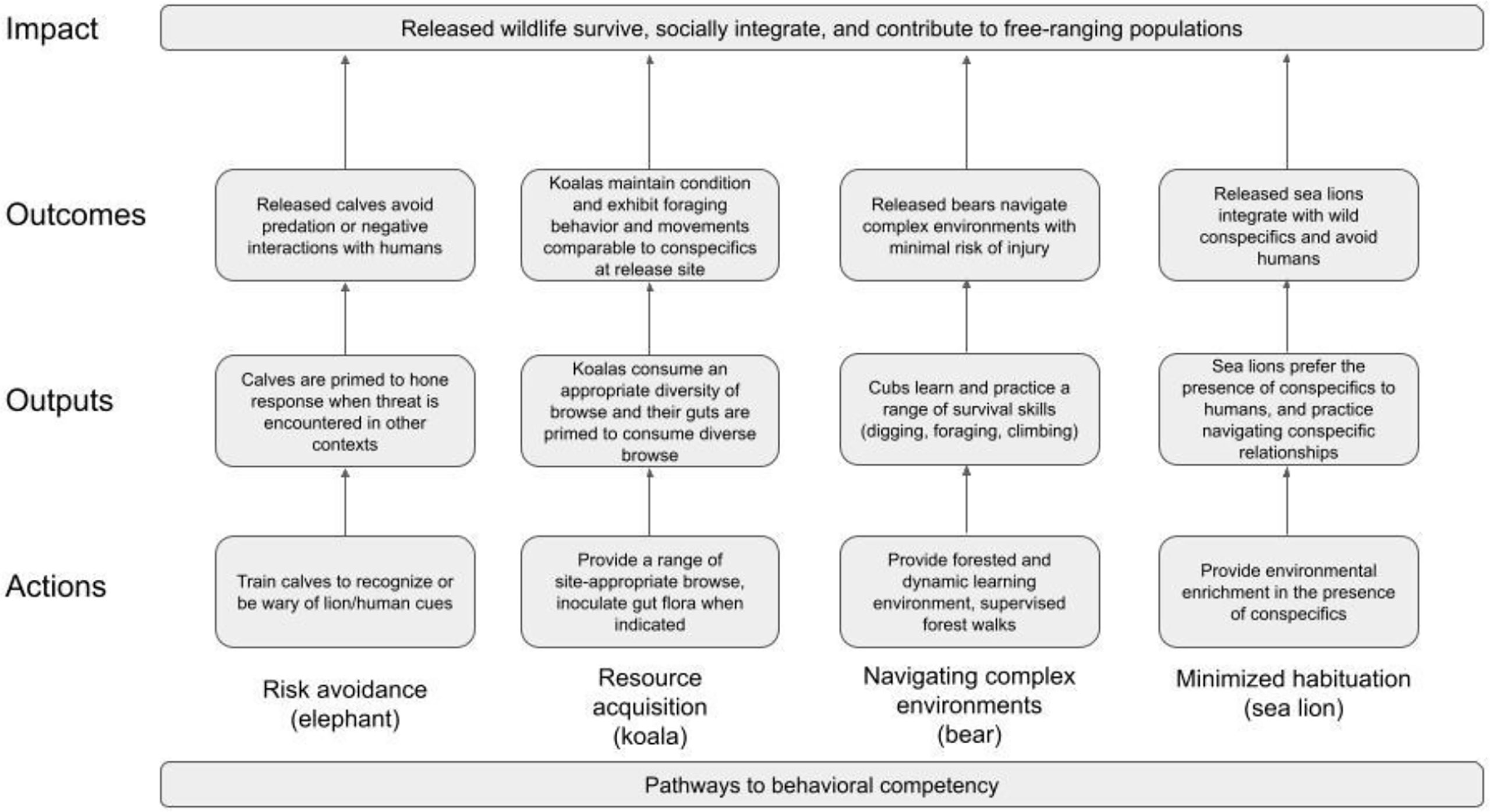
Theory of change logic (CMP, 2020) for example interventions for African savannah elephant (Loxodonta africana), koala (Phascolarctos cinereus), Asiatic black bear (Ursus thibetanus), and California sea lion (Zalophus californianus) is depicted in Figure 2. All of these species are rescued for reasons related to threats to individual animals (e.g., koala: vehicle strikes; sea lion: disease; elephant: stranding; bear: illegal wildlife trade). Koalas and sea lions are also rescued for habitat-scale threats to populations (koala: bushfires, floods; sea lion: algal blooms) (Adams-Hosking et al., 2011; Gallahar et al., 2021; Parrott et al., 2021). The ages of rescue vary, with koalas, bears and sea lions rescued at all life stages and elephants as juveniles. Thus, developmentally sensitive periods are well represented, and learning/unlearning are clear intervention opportunities (Tetzlaff et al., 2019).
Risk avoidance (African savannah elephant)
Releasing naïve individuals vulnerable to predation is a common problem in the breeding/headstarting literature (Griffin et al., 2000). It has been addressed through predator recognition training and aversive conditioning (Rowell et al., 2020; Edwards, 2021; Morris et al., 2021), with many instances of success. For example, Shier and Owings (2006) paired predator alarm calls with predator exposures in captive black-tailed prairie dogs, increasing antipredator behaviors and post-release survival, while (Blumstein et al., 2019) exposed bettongs and bilbies to low densities of feral cats prior to reintroduction to elicit antipredator behaviors.
Elephants, threatened by habitat loss and illegal trade, spend juvenile and subadult years learning from conspecifics to identify and respond to threats (Moss, 1988; McComb et al., 2011). Throughout their range, these threats also underpin the orphaning/stranding of juveniles, prompting rescue with the hope of eventual release. However, spending those years under HC can impede learning to avoid lions (Shannon et al., 2022) or humans, leading to undesirable outcomes (e.g., crop-raiding or occurring in proximity to lions/humans). As with other social species (Shier and Owings, 2006), learning antipredator behavior from knowledgeable conspecifics is not always possible under HC. In such cases, a next-best approach may be priming calves to respond to predator cues, followed by opportunities to learn from knowledgeable conspecifics upon release.
Testable interventions for juvenile elephants to enhance antipredator behavior toward lions and unfamiliar humans may follow precedents of aversive conditioning. Pairing olfactory or auditory cues that signal the presence of lions or unknown humans with stimuli known to elicit attention may prime calves to be more alert when they encounter those cues post-release. Hazing (e.g., with horn blasts, drone buzzes) (Petracca et al., 2019) may further encourage negative association or refine antipredator responses. Leading indicators that such conditioning is effective include increased avoidance and defensive behavior. Lagging indicators include survival into adulthood and appropriate avoidance responses when encountering lions or humans post-release (Edwards, 2021).
Resource acquisition (koala)
Prior exposure to foods needed upon release is critical to promoting behavioral competence. An accepted approach includes exposing animals to diverse foods and providing opportunities to practice handling them (Harvey 2018, Stoinski and Beck, 2004), ideally matched to resources at release sites (Stamps and Swaisgood, 2007). Recently, equipping wildlife to forage effectively includes considering gut microbiota due to greater understanding of its role in behavior (Ezenwa et al., 2012; Davidson et al., 2020), including food preference (Yang et al., 2020). Blyton et al. (2019) showed that altering gut microbiota via inoculations changes koala browse preference. Yang et al. (2020) linked diet training in Yangtze sturgeon to shifts in gut microbial communities, which in turn were linked to diet preference.
Once on a trajectory of recovery, koalas are threatened by catastrophic climate change-driven bush-fires and habitat loss, with megafires resulting in the rescue, rehabilitation and planned release of scores of individuals. Koalas are dietary specialist obligate folivores that consume only a small selection of available tree species, and may vary from one another in the species they select (McAlpine et al., 2008; Gallahar et al., 2021). The microbiome is an important consideration for translocation success since it is linked to general health and condition and varies based on site and habitat (Blyton et al., 2019; Brice et al., 2019; Littleford-Colquhoun et al., 2022). It is not yet understood how a koala’s established preferences for certain species of browse in situ influence subsequent behavior and condition in HC, where diet is likely to change due to several factors including caregivers lacking prior knowledge of in situ diet and limited access to browse collection sites. Furthermore, diet changes and associated gut microbiota changes under HC could impact digestibility and browse selection post-release.
Given this growing understanding of the importance of microbiota to wildlife health (Williams et al., 2018), rehabilitators have begun to address poor body condition during rehabilitation using “poop shakes”, fecal material from healthy koalas, to reinoculate the gut and assist in digestion. Such an intervention, paired with exposure to different browse types at release sites, may affect survival behaviors like selecting appropriate browse. Candidate leading indicators of success include a change in food preferences or consumption, improved condition and fecal outputs, and similarity in gut composition with koalas at rescue sites. Lagging indicators include survival, species-typical movements and home range re-establishment. Since rescue and release sites cannot always be matched, a potential additional lagging indicator of intervention efficacy is similarity in gut microbial composition between released and wild koalas at the same site.
Navigating complex environments (Asiatic black bear)
For solitary mammal species, how to navigate complex and dynamic habitats is often learned during a period of prolonged parental rearing. Thus, orphaning and human-rearing of such species in facilities without physical complexity, dynamic ecological cues, or appropriate conspecific mentors can result in behavioral incompetence (Stoinski and Beck, 2004). However, evidence suggests these disadvantages can be minimized by increasing the complexity and size of physical learning environments (Stamps and Swaisgood, 2007) and providing species-appropriate environmental enrichment (Reading et al., 2013).
Asiatic black bears, threatened by habitat degradation and illegal trade (including the pet trade), are largely solitary with a ~2.5-year maternal care period. Rescued cubs often arrive at sanctuaries in ill health after experiencing trauma, necessitating triage-care. While many rescues result in life-long HC, release into the wild can improve welfare, reduce pressure on limited resources and contribute to population health. Behavioral incompetence resulting from HC may result in injury, illness or death post-release if bears do not know how to obtain adequate resources in complex forested and seasonally dynamic environments, compete for territory and resources, and avoid conflict with humans.
Testable interventions to foster a diverse repertoire of behavioral skills include designing forested enclosures with enrichment like deadfall logs, climbing structures, and puzzle feeders; and exposure to complex forest environments during critical developmental stages. Forest walking–the practice of caregivers accompanying rescued cubs into unfenced, forested-habitat to expose them to their natural range–is an approach to behavioral training that has been successful for multiple bear species (Fredriksson, 2005; Ashraf et al., 2008; Steinmetz et al., 2021). Leading indicators of efficacy include increased exploratory behavior, refuge seeking in trees, and increased behavioral diversity. Beyond post-release survival, lagging indicators could include few to no injuries in the years following release, species-appropriate ranging patterns (Abidin et al., 2018), absence of conflict with humans and recruitment into a wild population. While rescue of bear cubs has been ongoing for decades, and hundreds are under HC, release is a nascent endeavor and evidence-based behavioral interventions are desperately needed.
Minimizing habituation (California sea lion)
Habituation toward humans and perceiving them as a food source is a challenge faced by many translocation projects (Beringer et al., 2004). In addition to minimizing unnecessary interactions with humans under HC, caregivers can facilitate strong conspecific relationships as an alternative to humans (Fàbregas et al., 2020). Social relationships play an important role in post-release success for translocations (Shier and Swaisgood, 2012; Snijders et al., 2017; Goldenberg et al., 2019). Opportunities to develop relationships and hone social skills may provide an alternative to seeking human attention during rehabilitation.
Every year, thousands of California sea lions are stranded with various ailments and are transferred to facilities for rehabilitation. Sea lions thrive in HC and are quick to associate food and other rewards with humans (Cox et al., 1996). Thus, habituation to humans is a universal challenge facing sea lion rehabilitators, and each year some individuals are deemed unsuitable for release due to concern they will become nuisance animals. In addition to practices like avoiding talking and limiting human presence, offering conspecific alternatives to humans is a promising and testable intervention. This may include group housing, exposure to enrichment items in group settings, and site design to promote navigating dominance hierarchies and group hunting of live fish. Leading indicators of success for these interventions include reduced incidence of gazing toward, tracking, and approaching humans. Lagging indicators include absence of nuisance behaviors post-release and contribution to recruitment.
In addition to the design of interventions, their cadence and strength should be planned in consideration of release environments (Homberger et al., 2014; Nogueira et al., 2014). Released wildlife will encounter fluctuations in resource availability, predation threat, and conspecific interaction. Priming animals to respond appropriately to changing ecological cues may better position them to adjust to periods of scarcity or assess degrees of threat. This may at times conflict with welfare aims to ensure wildlife under HC always feel secure, but is in line with the Five Domains of animal welfare that encompass broader ranges of experiences, including negative experiences (Mellor, 2017).
Way forward
A common feature uniting the examples above, and simultaneously making a strong case for their integration across rescue-rehab-release, conservation breeding, and headstarting, is small sample size and species specificity (Shaw et al., 2021; Cope et al., 2022). The fragmented and separated nature of translocation programs (i.e., welfare vs. conservation organizations) exacerbate this challenge. The efficacy of behavioral interventions is difficult to determine from one project alone; establishing meta-programs comprising a community of practitioners that build evidence for guidelines will improve outcomes for released wildlife. In turn, guidelines established through such collaborations would provide starting points for ecologically similar but less frequently rescued/lesser understood species (Alberts, 2007).
To facilitate knowledge exchange, rescue-rehab-release projects should implement the same standards of research fundamental to proactive translocations (Pyke and Szabo, 2018; Cope et al., 2022). There have been important research contributions from rescue-rehab-release, but this space is as yet largely untapped (Guy et al., 2013). In addition to scrutiny of rehabilitation protocols and an intentional focus on behavioral competence, post-release monitoring, where lagging indicators of success can be documented, is essential to understand if existing protocols are effective and what interventions are needed. This has been a recurring theme in the proactive conservation translocation literature (Berger-Tal et al., 2020), and should be equally valued in rescue-rehab-release. As post-release monitoring in conservation translocations has made clear, failures are to be expected and provide direction for improvement. Dedicated scenario planning to identify potential post-release problems drawing on lessons learned and experiences of other programs would more clearly define research needs. Incorporating lessons learned from both failures and successes, as well as setting realistic expectations for the supporting public, will improve outcomes for wildlife and garner long-term support for the complex, iterative, and often messy process of wildlife release.
We recognize that resource scarcity–including resources available for research–remains a persistent challenge to establishing effective strategies for wildlife under HC. However, we argue that the development and testing of leading/lagging indicators can facilitate more effective use of limited resources. Determining measurable release criteria indicative of behavioral competence may help identify unreleasable individuals earlier in the rehabilitation pipeline, thereby driving targeted resource distribution. Additionally, extensive post-release monitoring may become unnecessary or may be more strategically deployed after validating the reliability of leading and lagging indicators.
The examples above highlight interventions that are relevant across mammalian species and are supported in the wider conservation translocation literature, though we acknowledge that many other interventions merit testing. A greater embrace of intervention validation in the rescue-rehab-release realm that can be shared across translocation contexts will greatly improve post-release outcomes. Such a direction would ensure returning rescued animals to the wild benefits conservation and makes best use of resources available for wildlife.
Author contributions
SG, MO, JMP, KL, MH, and HN drafted the first version of this manuscript. All authors contributed to discussions on the topic and provided comments and edits on drafts.
Funding
JMP was supported by the National Science Foundation Postdoctoral Fellowship in Biology Program [grant number 2109816].
Acknowledgments
We thank Hariyo Wibisono, Mathias Tobler, and Eric Gaglione for helpful discussions during early stages of drafting this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abidin M. K. Z., Mohammed A. A., Nor S. M. (2018). Home-range and activity pattern of rehabilitated malayan sun bears (Helarctos malayanus) in the tembat forest reserve, terengganu (Selangor, Malaysia: AIP Publishing), 020036. doi: 10.1063/1.5027951
Adams-Hosking C., Grantham H. S., Rhodes J. R., McAlpine C., Moss P. T. (2011). Modelling climate-change-induced shifts in the distribution of the koala. Wildl. Res. 38, 122. doi: 10.1071/WR10156
Alberts A. C. (2007). Behavioral considerations of headstarting as a conservation strategy for endangered Caribbean rock iguanas. Appl. Anim. Behav. Sci. 102, 380–391. doi: 10.1016/j.applanim.2006.05.037
Ashraf N. V. K., Dadda T., Boro P., Akhtar N. (2008). Walking with bears: rehabilitation of Asiatic black bears in arunachal pradesh. Wildlife Trust of India. Available at: https://www.wti.org.in/wp-content/uploads/2017/03/pub_walking_bears.pdf
Bansiddhi P., Brown J. L., Thitaram C. (2020). Welfare assessment and activities of captive elephants in Thailand. Animals 10, 919. doi: 10.3390/ani10060919
Berger-Tal O., Blumstein D. T., Swaisgood R. R. (2020). Conservation translocations: a review of common difficulties and promising directions. Anim. Conserv. 23, 121–131. doi: 10.1111/acv.12534
Beringer J., Mabry P., Meyer T., Wallendorf M., Eddleman W. R. (2004). Post-release survival of rehabilitated white-tailed deer fawns in Missouri. Wildlife. Soc. Bull. 32, 732–738. doi: 10.2193/0091-7648(2004)032[0732:PSORWD]2.0.CO;2
Blair C. D., Muller L. I., Clark J. D., Stiver W. H. (2020). Survival and conflict behavior of American black bears after rehabilitation. Jour. Wild. Mgmt. 84, 75–84. doi: 10.1002/jwmg.21783
Blumstein D. T., Letnic M., Moseby K. E. (2019). In situ predator conditioning of naive prey prior to reintroduction. Phil. Trans. R. Soc B. 374, 20180058. doi: 10.1098/rstb.2018.0058
Blyton M. D. J., Soo R. M., Whisson D., Marsh K. J., Pascoe J., Le Pla M., et al. (2019). Faecal inoculations alter the gastrointestinal microbiome and allow dietary expansion in a wild specialist herbivore, the koala. Anim. Microbiome. 1, 6. doi: 10.1186/s42523-019-0008-0
Bradley H. S., Tomlinson S., Craig M. D., Cross A. T., Bateman P. W. (2022). Mitigation translocation as a management tool. Conserv. Biol. 36, e13667. doi: 10.1111/cobi.13667
Brice K. L., Trivedi P., Jeffries T. C., Blyton M. D. J., Mitchell C., Singh B. K., et al. (2019). The koala (Phascolarctos cinereus) faecal microbiome differs with diet in a wild population. PeerJ 7, e6534. doi: 10.7717/peerj.6534
Campera M., Brown E., Imron M. A., Nekaris K. A. I. (2020). Unmonitored releases of small animals? the importance of considering natural dispersal, health, and human habituation when releasing a territorial mammal threatened by wildlife trade. Biol. Conserv. 242, 108404. doi: 10.1016/j.biocon.2019.108404
Castro Cortés A. A., Brieva C., Witte C. (2022). Implications of wildlife trafficking on the health and conservation efforts of an endangered turtle species in Colombia. Conservat. Sci. Prac. 4, e595. doi: 10.1111/csp2.595
Cope H. R., McArthur C., Dickman C. R., Newsome T. M., Gray R., Herbert C. A. (2022). A systematic review of factors affecting wildlife survival during rehabilitation and release. PloS One 17, e0265514. doi: 10.1371/journal.pone.0265514
Cox M., Gaglione E., Prowten P., Noonan M., Aquatic mammals (1996). Food preferences communicated via symbol discrimination by a California Sea lion (Zalophus californianus) 22, 9.
Davidson G. L., Raulo A., Knowles S. C. L. (2020). Identifying microbiome-mediated behaviour in wild vertebrates. Trends Ecol. Evol. 35, 972–980. doi: 10.1016/j.tree.2020.06.014
Edwards M. C. (2021). How to train your wildlife: A review of predator avoidance training. Appl. Anim. Behav. Sci. 7, 105170. doi: 10.1016/j.applanim.2020.105170
Englefield B., Candy S., Starling M., McGreevy P. (2019). The demography and practice of australians caring for native wildlife and the psychological, physical and financial effects of rescue, rehabilitation and release of wildlife on the welfare of carers. Animals 9, 1127. doi: 10.3390/ani9121127
Ezenwa V. O., Gerardo N. M., Inouye D. W., Medina M., Xavier J. B. (2012). Animal behavior and the microbiome. Science 338, 198–199. doi: 10.1126/science.1227412
Fàbregas M. C., Fosgate G. T., Ganswindt A., Bertschinger H., Hofmeyr M., Meyer L. C. R. (2020). Rehabilitation method affects behavior, welfare, and adaptation potential for subsequent release of orphaned white rhinoceros. Acta Ethol. 23, 105–114. doi: 10.1007/s10211-020-00343-w
Fredriksson G. (2005). “Conservation threats facing sun bears, helarctos malayanus, in Indonesia and experiences with sun bear rehabilitations in East kalimantan, Indonesia,” in Rehabilitation and release of bears (Koln, Germany: Zoologischer Garten Koln), 35–42.
Fuller R., Venter B., Venter L., Venter J., Blue S. K. (2021). Guidelines for the rehabilitation, release, and post-release assessment of chacma baboons (Papio ursinus) for reinforcement. Primate. Conserv. 35, 1–18.
Gallahar N., Leigh K., Phalen D. (2021). Koala tree selection in a mixed-tenure landscape and post-fire implications. Wildlife. Res. 48, 737–755. doi: 10.1071/WR20206
Goldenberg S. Z., Owen M. A., Brown J. L., Wittemyer G., Oo Z. M., Leimgruber P. (2019). Increasing conservation translocation success by building social functionality in released populations. Global Ecol. Conserv. 18, e00604. doi: 10.1016/j.gecco.2019.e00604
Greggor A. L., Vicino G. A., Swaisgood R. R., Fidgett A., Brenner D., Kinney M. E., et al. (2018). Animal welfare in conservation breeding: Applications and challenges. Front. Vet. Sci. 5. doi: 10.3389/fvets.2018.00323
Griffin A. S., Blumstein D. T., Evans C. S. (2000). Training captive-bred or translocated animals to avoid predators. Conserv. Biol. 14, 1317–1326. doi: 10.1046/j.1523-1739.2000.99326.x
Gusset M. (2009). “A framework for evaluating reintroduction success in carnivores: Lessons from African wild dogs,” in Reintroduction of top-order predators. Eds. Hayward M. W., Somers M. J. (Oxford, UK: Wiley-Blackwell), 307–320. doi: 10.1002/9781444312034.ch14
Guy A. J., Curnoe D., Banks P. B. (2013). A survey of current mammal rehabilitation and release practices. Biodivers. Conserv. 22, 825–837. doi: 10.1007/s10531-013-0452-1
Guy A. J., Curnoe D., Banks P. B. (2014). Welfare based primate rehabilitation as a potential conservation strategy: does it measure up? Primates 55, 139–147. doi: 10.1007/s10329-013-0386-y
Haering R., Wilson V., Zhuo A., Stathis P. (2021). A survey of veterinary professionals about their interactions with free-living native animals and the volunteer wildlife rehabilitation sector in new south Wales, Australia. Aust. Zool. 41, 254–282. doi: 10.7882/AZ.2020.045
Harvey B. D (2018). Influences on Foraging preferences of the endangered pacific pocket mouse(Perognathus longimembris pacificus): Implications for a novel conservation strategy. Masters Thesis. University of California Los Angeles.
Homberger B., Jenni L., Duplain J., Lanz M., Schaub M. (2014). Food unpredictability in early life increases survival of captive grey partridges (Perdix perdix) after release into the wild. Biol. Conserv. 177, 134–141. doi: 10.1016/j.biocon.2014.06.023
Hong S., Yoon S. J., Kim T., Ryu J., Kang S.-G., Khim J. S. (2020). Response to oiled wildlife in the management and evaluation of marine oil spills in south Korea: A review. Regional. Stud. Mar. Sci. 40, 101542. doi: 10.1016/j.rsma.2020.101542
IUCN/SSC (2013). Guidelines for reintroductions and other conservation translocations (Gland, Switzerland: IUCN Species Survival Commission).
Jule K. R., Leaver L. A., Lea S. E. G. (2008). The effects of captive experience on reintroduction survival in carnivores: A review and analysis. Biol. Conserv. 141, 355–363. doi: 10.1016/j.biocon.2007.11.007
Kwok A., Haering R., Travers S. K., Stathis P. (2021). Trends in wildlife rehabilitation rescues and animals fate across a six-year period in new south Wales, Australia. PloS One 16, 1–27. doi: 10.1371/journal.pone.0257209
Lander M. E., Gulland F. M. D. (2003). Rehabilitation and post-release monitoring of steller Sea lion pups raised in captivity. Wildlife. Soc. Bull. 31, 1047–1053.
Lefebvre K. A., Quakenbush L., Frame E., Huntington K. B., Sheffield G., Stimmelmayr R., et al. (2016). Prevalence of algal toxins in alaskan marine mammals foraging in a changing arctic and subarctic environment. Harmful. Algae. 55, 13–24. doi: 10.1016/j.hal.2016.01.007
Littleford-Colquhoun B. L., Weyrich L. S., Hohwieler K., Cristescu R., Frère C. H. (2022). How microbiomes can help inform conservation: landscape characterisation of gut microbiota helps shed light on additional population structure in a specialist folivore. Anim. Microbiome. 4, 12. doi: 10.1186/s42523-021-00122-3
Marker L., Honig M., Pfeiffer L., Kuypers M., Gervais K. (2021). Captive rearing of orphaned African wild dogs (Lycaon pictus) in Namibia: A case study. Zoo Biol. Zoo 41 (2), 21662. doi: 10.1002/zoo.21662
McAlpine C. A., Rhodes J. R., Bowen M. E., Lunney D., Callaghan J. G., Mitchell D. L., et al. (2008). Can multiscale models of species’ distribution be generalized from region to region? a case study of the koala. J. Appl. Ecol. 45, 558–567. doi: 10.1111/j.1365-2664.2007.01431.x
McComb K., Shannon G., Durant S. M., Sayialel K., Slotow R., Poole J., et al. (2011). Leadership in elephants: the adaptive value of age. Proc. R. Soc. B. 278, 3270–3276. doi: 10.1098/rspb.2011.0168
Mellor D. (2017). Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 7, 60. doi: 10.3390/ani7080060
Molina-López R. A., Mañosa S., Torres-Riera A., Pomarol M., Darwich L. (2017). Morbidity, outcomes and cost-benefit analysis of wildlife rehabilitation in Catalonia (Spain). PloS One 12, e0181331. doi: 10.1371/journal.pone.0181331
Molony S. E., Dowding C. V., Baker P. J., Cuthill I. C., Harris S. (2006). The effect of translocation and temporary captivity on wildlife rehabilitation success: An experimental study using European hedgehogs (Erinaceus europaeus). Biol. Conserv. 130, 530–537. doi: 10.1016/j.biocon.2006.01.015
Moore R., Wihermanto, Nekaris K. (2014). Compassionate conservation, rehabilitation and translocation of Indonesian slow lorises. Endang. Species. Res. 26, 93–102. doi: 10.3354/esr00620
Morgans C. L., Santika T., Meijaard E., Ancrenaz M., Wilson K. A. (2019). Cost-benefit based prioritisation of orangutan conservation actions in Indonesian Borneo. Biol. Conserv. 238, 108236. doi: 10.1016/j.biocon.2019.108236
Mo M., Roache M., Davies J., Hopper J., Pitty H., Foster N., et al. (2021). Estimating flying-fox mortality associated with abandonments of pups and extreme heat events during the austral summer of 2019–20. Pac. Conserv. Biol 28 (2), 124–39. doi: 10.1071/PC21003
Morris D. W., Kotler B. P., Brown J. S., Sundararaj V., Ale S. B. (2009). Behavioral indicators for conserving mammal diversity. Ann. New York. Acad. Sci. 1162, 334–356. doi: 10.1111/j.1749-6632.2009.04494.x
Morris V., Pitcher B. J., Chariton A. (2021). A cause for alarm: Increasing translocation success of captive individuals through alarm communication. Front. Conserv. Sci. 2. doi: 10.3389/fcosc.2021.626311
Moss C. J. (1988). Elephant memories: Thirteen years in the life of an elephant family (Chicago: University of Chicago Press).
Mumby H. S., Plotnik J. M. (2018). Taking the elephants’ perspective: Remembering elephant behavior, cognition and ecology in human-elephant conflict mitigation. Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00122
Myers P. J., Young J. K. (2018). Post-release activity and habitat selection of rehabilitated black bears. Human-Wildlife. Interact. 12, 322–337.
Nogueira S. S. C., Abreu S. A., Peregrino H., Nogueira-Filho S. L. G. (2014). The effects of feeding unpredictability and classical conditioning on pre-release training of white-lipped peccary (Mammalia, tayassuidae). PloS One 9, e86080. doi: 10.1371/journal.pone.0086080
Normande I. C., Luna F. D. O., Malhado A. C. M., Borges J. C. G., Viana Junior P. C., Attademo F. L. N., et al. (2015). Eighteen years of antillean manatee trichechus manatus manatus releases in Brazil: lessons learnt. Oryx 49, 338–344. doi: 10.1017/S0030605313000896
Osterberg P., Samphanthamit P., Maprang O., Punnadee S., Brockelman W. Y. (2015). Gibbon (Hylobates lar) reintroduction success in phuket, Thailand, and its conservation benefits: Gibbon reintroductions in phuket. Am. J. Primatol. 77, 492–501. doi: 10.1002/ajp.22367
Parrott M. L., Wicker L. V., Lamont A., Banks C., Lang M., Lynch M., et al. (2021). Emergency response to australia’s black summer 2019–2020: The role of a zoo-based conservation organisation in wildlife triage, rescue, and resilience for the future. Animals 11, 1515. doi: 10.3390/ani11061515
Paterson J. E., Carstairs S., Davy C. M. (2021). Population-level effects of wildlife rehabilitation and release vary with life-history strategy. J. Nat. Conserv. 61, 125983. doi: 10.1016/j.jnc.2021.125983
Petracca L. S., Frair J. L., Bastille-Rousseau G., Hunt J. E., Macdonald D. W., Sibanda L., et al. (2019). The effectiveness of hazing African lions as a conflict mitigation tool: implications for carnivore management. Ecosphere 10, e02967. doi: 10.1002/ecs2.2967
Pyke G. H., Szabo J. K. (2018). Conservation and the 4 rs, which are rescue, rehabilitation, release, and research: Conservation and the 4 rs. Conserv. Biol. 32, 50–59. doi: 10.1111/cobi.12937
Reading R. P., Miller B., Shepherdson D. (2013). The value of enrichment to reintroduction success. Zoo Biol. 32, 332–341. doi: 10.1002/zoo.21054
Romero F., Espinoza A., Sallaberry-Pincheira N., Napolitano C. (2019). A five-year retrospective study on patterns of casuistry and insights on the current status of wildlife rescue and rehabilitation centers in Chile. Rev. Chil. Hist. Nat. 92, 6. doi: 10.1186/s40693-019-0086-0
Rowell T. A. A. D., Magrath M. J. L., Magrath R. D. (2020). Predator-awareness training in terrestrial vertebrates: Progress, problems and possibilities. Biol. Conserv. 252, 108740. doi: 10.1016/j.biocon.2020.108740
Shannon G., Cordes L. S., Slotow R., Moss C., McComb K. (2022). Social disruption impairs predatory threat assessment in African elephants. Animals 12, 495. doi: 10.3390/ani12040495
Shaw R. C., Greggor A. L., Plotnik J. M. (2021). The challenges of replicating research on endangered species. AB&C 8, 240–246. doi: 10.26451/abc.08.02.10.2021
Shier D. M., Owings D. H. (2006). Effects of predator training on behavior and post-release survival of captive prairie dogs (Cynomys ludovicianus). Biol. Conserv. 132, 126–135. doi: 10.1016/j.biocon.2006.03.020
Shier D. M., Swaisgood R. R. (2012). Fitness costs of neighborhood disruption in translocations of a solitary mammal. Conserv. Biol. 26, 116–123. doi: 10.1111/j.1523-1739.2011.01748.x
Snijders L., Blumstein D. T., Stanley C. R., Franks D. W. (2017). Animal social network theory can help wildlife conservation. Trends Ecol. Evol. 32, 567–577. doi: 10.1016/j.tree.2017.05.005
Stamps J. A., Swaisgood R. R. (2007). Someplace like home: Experience, habitat selection and conservation biology. Appl. Anim. Behav. Sci. 102, 392–409. doi: 10.1016/j.applanim.2006.05.038
Steinmetz R., Phumanee W., Phoonjampa R., Weingdow S. (2021). First attempt at rehabilitation of Asiatic black bear cubs to the wild in Thailand. J. Threat. Taxa. 13, 18411–18418. doi: 10.11609/jott.6343.13.6.18411-18418
Stoinski T. S., Beck B. B. (2004). Changes in locomotor and foraging skills in captive-born, reintroduced golden lion tamarins (Leontopithecus rosalia rosalia). Am. J. Primatol. 62, 1–13. doi: 10.1002/ajp.20002
Stokes R., Tully G., Rosati A. G. (2018). Pan African sanctuary alliance: securing a future for the African great apes. Int. Zoo Yb. 52, 173–181. doi: 10.1111/izy.12174
Tetzlaff S. J., Sperry J. H., DeGregorio B. A. (2019). Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis. Biol. Conserv. 236, 324–331. doi: 10.1016/j.biocon.2019.05.054
Thomas P., Boyer D., Oehler D., Silver S., Perrotti L. (2019). “Headstarting as a conservation strategy for threatened and endangered species,” in Scientific foundations of zoos and aquariums. Eds. Kaufman A.., Bashaw M., Maple T. Scientific Foundations of Zoos and Aquariums: Their Role in Conservation and Research (Cambridge: Cambridge University Press), 91–111. doi: 10.1017/9781108183147.004
Williams C. L., Caraballo-Rodríguez A. M., Allaband C., Zarrinpar A., Knight R., Gauglitz J. M. (2018). Wildlife-microbiome interactions and disease: exploring opportunities for disease mitigation across ecological scales. Drug Discovery Today.: Dis. Models 28, 105–115. doi: 10.1016/j.ddmod.2019.08.012
Wolf C. M., Griffith B., Reed C., Temple S. A. (1996). Avian and mammalian translocations: Update and reanalysis of 1987 survey data. Conserv. Biol. 10, 1142–1154. doi: 10.1046/j.1523-1739.1996.10041142.x
Keywords: wildlife rescue, rehabilitation, behavioral competence, post-release monitoring, behavioral training, reintroduction biology
Citation: Goldenberg SZ, Parker JM, Chege SM, Greggor AL, Hunt M, Lamberski N, Leigh KA, Nollens HH, Ruppert KA, Thouless C, Wittemyer G and Owen MA (2022) Revisiting the 4 R’s: Improving post-release outcomes for rescued mammalian wildlife by fostering behavioral competence during rehabilitation. Front. Conserv. Sci. 3:910358. doi: 10.3389/fcosc.2022.910358
Received: 01 April 2022; Accepted: 11 July 2022;
Published: 10 August 2022.
Edited by:
Julie Old, Western Sydney University, AustraliaReviewed by:
Bill Bateman, Curtin University, AustraliaCopyright © 2022 Goldenberg, Parker, Chege, Greggor, Hunt, Lamberski, Leigh, Nollens, Ruppert, Thouless, Wittemyer and Owen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shifra Z. Goldenberg, c2dvbGRlbmJlcmdAc2R6d2Eub3Jn
 Shifra Z. Goldenberg
Shifra Z. Goldenberg Jenna M. Parker1,2
Jenna M. Parker1,2 Alison L. Greggor
Alison L. Greggor Nadine Lamberski
Nadine Lamberski Hendrik H. Nollens
Hendrik H. Nollens Kirstie A. Ruppert
Kirstie A. Ruppert Chris Thouless
Chris Thouless George Wittemyer
George Wittemyer Megan A. Owen
Megan A. Owen