- 1Aquatic Ecology, Faculty of Biology, University of Duisburg-Essen, Essen, Germany
- 2Ruhr University Bochum, Faculty of Biology and Biotechnology, Theoretical and Applied Biodiversity Research, Bochum, Germany
- 3Wildlife Conservation Research Unit, Zoology, University of Oxford, Oxford, United Kingdom
- 4Department of Biology, Mekelle University, Mekelle, Ethiopia
Urban spotted hyenas (Crocuta crocuta) in Ethiopia are a prime example of large carnivores coexisting with little to no conflict with people in a human-dominated landscape, providing a valuable waste-removal service. To gain insight in how this urban lifestyle persists across generations, we studied hyena group composition at the city waste dump of Mekelle, a regional capital in northern Ethiopia. We found that hyena cubs and sub-adults foraged with adults in groups of highly variable composition. Young urban hyenas already take part in a fission-fusion dynamic that is also characteristic of hyenas in non-urban environments. They do not seem to learn from only one or few close reference adults. Social network analysis revealed no clusters among these dump-visiting hyenas. The number of counted hyenas is furthermore larger than any hyena clan in non-urban areas. All individuals were more or less equally connected to each other, and each hyena had a few connections, but to different individuals. All cubs and sub-adults were connected to each other, over a maximum of four links. Hyenas shared the abundance of food at the waste dump without overt aggression. A much larger number of urban hyenas shares this waste dump at night than would fit into a single forest fragment, such as those associated with orthodox churches where small groups of hyenas have often been observed to rest at daytime. Hyenas appear to commute from different dens and resting sites located around the city, but we have no information on their behavior and group composition away from the dump. We observed no defense of any part of the dump area by any of the foraging groups. In absence of territorial behavior at this city site, the clan concept does not seem to apply to these urban hyenas. Similar to what has been observed in other urban carnivores, individuals at the waste dump behaved as members of conflict-free foraging groups ostensibly sharing food without aggression. Perhaps this is what most strikingly defines their urbanity.
Introduction
Large carnivore range and numbers are declining worldwide (Wolf and Ripple, 2018), possibly leading to cascading effects and ecosystem degradation (Hoeks et al., 2020). Africa has a relatively intact guild of mammalian carnivores in many places, but the same trends can be observed (Bauer et al., 2015). However, where large carnivore numbers increase, ecosystems can be restored (Atkins et al., 2019), and urban environments potentially offer opportunities. Urban food subsidies can support substantial carnivore populations, provided that conflict with urban humans is limited (Yirga et al., 2017).
Species that successfully adapt to urban life can often occur at higher densities in cities than in the surrounding landscape (Fedriani et al., 2001; Smith and Engeman, 2002). Red foxes (Vulpes vulpes), raccoons (Procyon lotor), and coyotes (Canis latrans) are probably the most iconic urban carnivores and have been documented in cities since the beginning of the twentieth century (Teagle, 1967; Gehrt et al., 2010; Hadidian et al., 2010). Urban carnivores are typically generalistic and opportunistic feeders, hunting synanthropic prey, scavenging from human refuse, and using non-meat food items (Bateman and Fleming, 2012). In urban areas, new dangers, greater resource availability and increased population densities, as well as limited usable space require novel behaviors and adaptations. In red foxes in Saudi Arabia territoriality became absent as they concentrated their activities around ephemeral but rich anthropogenic food sources (Macdonald et al., 1999). Greater resource availability led to shorter hibernation times in North American black bears (Beckmann and Berger, 2003).
In contrast to these examples of rather generalistic urban dwellers less is known about the impact of urban life on the ecology of large obligate carnivores.
In the Horn of Africa, hyenas are widely tolerated by residents, possibly because they are known to clean up organic waste and deceased animals from the streets, thus reducing health risks and bad odor (Gade, 2006; Sonawane et al., 2021). In Tigray state, northern Ethiopia, spotted hyenas (Crocuta crocuta, henceforth hyenas) are known to occur at high densities feeding on urban waste (Yirga et al., 2015b). Wild prey species have been largely depleted, so hyenas are almost completely dependent on anthropogenic food sources and do both scavenge and hunt livestock (Yirga et al., 2015a). The hyena population is estimated at 28,620 across Tigray, with >400 individuals around the regional capital Mekelle where they feed at the city waste dump (Yirga et al., 2015b). Human-hyena coexistence here is almost conflict-free; hyena attacks on humans are extremely rare, the few known cases involve people sleeping or defecating outside at night (Abay et al., 2011). Many livestock holders in Ethiopia keep their sheep, goats and cattle protected in boma enclosures at night, leading to limited depredation. People are rarely aggressive to hyenas, although they may chase them away when they come close (Pers. Obs.).
Non-urban hyenas live in clans from 6 up to 130 individuals with strong matriarchal hierarchies (Smith and Holekamp, 2019). Clan members recognize each other individually and join in coalitions to cooperatively hunt or defend the territory (Kruuk, 1972). Territoriality is quite central to the clan concept: Defense of an area by members of one clan is the response to intrusion of that area by members of another clan (Kruuk, 1972). Cubs are born in natal dens exclusive to the mother and her 1–2 cubs but are later moved to a communal den shared by all cubs of the clan (Kruuk, 1972). Provisioning of solid food from a mother to her cub at the den is rare. Instead, mothers share food with their cubs directly at kills (Kruuk, 1972; Holekamp and Smale, 1990). Until up to 36 months of age, hyena cubs rely on maternal aid in food acquisition (Watts and Holekamp, 2009). Food acquisition and social behavior are learned by cubs through trial and error and associative learning, where a mother or clan member will additionally reinforce desirable and discourage undesirable behavior, rather than by active teaching of specific skills by a model individual (Holekamp and Smale, 1990, 1998; Holekamp et al., 1997).
Urban hyena cubs need to learn a different skill set compared to their conspecifics in more natural ecosystems: hunting for live prey is less important, interference with competitors such as lions is rare, while proximity to humans is a major determinant. Possibly, daytime avoidance of humans is key to hyena fitness in urban environments (Bohm and Höner, 2015). This could make daytime resting places rather than food the limiting resource for urban hyenas. For hyenas to feed at the Mekelle waste dump they need to commute there from their dens and resting sites, past or through the city. Cubs joining a foraging group to the waste dump need to learn the way, along a safe route, and perhaps learn about spatio-temporal patterns in the availability of different types of edible waste at the dump. They may also need to be protected from other hyenas that could be members of a different clan. We therefore expected that cubs may not randomly join any foraging group but will join a fixed set of known adults (and maybe other cubs) for night-time visits to the waste dump. We examine this by studying foraging group composition and dynamics of hyena cubs.
In this study, we examine how cubs on the waste dump of Mekelle, Ethiopia, are involved in foraging groups and their compositional dynamics, i.e., who they forage with. Our first hypothesis was that cubs are part of foraging groups of a relatively stable composition. Although some fission-fusion dynamics are expected, we hypothesized that cubs would also be stably surrounded by a few adult individuals that they are close to. On basis of the idea that foraging groups arrive from different daytime resting sites, we further hypothesized that analysis of the social network would reveal a strong clustering in the sense of several non-connected foraging groups being present. Creel and Macdonald (1995) hypothesized that reduced resource competition would lead to increased tolerance of conspecifics and thus less aggression in carnivores. As hyenas on the waste dump of Mekelle commute there from different den sites, we nonetheless expected to see traces of this group separation in our social networks.
Materials and Methods
All field work for this study was conducted on the waste dump of the city of Mekelle, with 320,000 inhabitants the largest city and regional capital of Tigray, northern Ethiopia. Hyenas were observed and filmed by two observers out of a vehicle in 9 sessions on 9 consecutive nights in May 2019. For observation and recording, a Nightfox 110R night vision binocular with video function was used. Observation sessions lasted from dusk till dawn (i.e., 10–11 h). The vehicle was parked at either of three observation sites with abundant offal (“plain area,” “chicken excavation,” “fresh pile”) and remained there throughout the whole session. Hyena activity was filmed with night vision equipment throughout the observation session. Immediate individual recognition was not always possible, especially not when crowded with large numbers of hyenas, so it was not always possible to follow every action of every hyena. Video length and filming angle were therefore activity-dependent, not standardized.
The observation sites differed in sort and accessibility of the unloaded meat waste. “Chicken excavation” was a shallow pit where poultry products, including eggs, were easily accessible. “Fresh pile” was a ~2 × 2 × 2 m pit where offal was dumped from the local slaughterhouse. “Plain area” was a patch inside the general household waste area where butchery waste, poultry and eggs were more abundant than in normal household waste, but it was unclear whether this came from an industrial source.
We isolated all videos in which cubs appeared, 120 out of a total of 559. Hereafter, “video” refers to a video with a cub in it, meaning that all adults in those videos are linked to a cub. Videos were from each of the nine observation sessions, there were no nights without cub observations. Individuals were classified as cubs if they were significantly smaller than an adult, had a face with juvenile proportions and showed the fluffy, generally darker fur typical for hyena cubs (for classification see also Supplementary Material 1). Because of the large variation in early development between cubs of different populations, between cubs of different rank within a clan and even between siblings, a cub's size is not a good indication for its age. However, the combination of our criteria selects ages of ca. 7–15 months.
For the analysis of group composition and whereabouts of individuals, hyenas needed to be individually identified by their spot patterns and recognized in the videos. A primitive identification key was created to pre-sort the individuals into identification-groups according to the set of patterns in their spots, making comparisons of a new individual to the already identified ones easier (Supplementary Material 1). Since the two sides of a hyena show different spot patterns, each side needs to be treated separately, and each individual has two identification-group memberships: one for its left side, one for its right side. Identified individuals were given a name and an ID number, reported here as C-ID for cubs and A-ID for adults. A total of 26 cubs and 191 adults were identified using this method. For some individuals, we were unable to match left and right-side pictures and it is possible that some individuals have two IDs, one for each side. Both sides are known from 85 individuals (23 cubs, 62 adults). As we focus on cubs, we only used cubs of which both sides were known and therefore three cubs identified by one side only were excluded from all our analyses. For the adults, analyses based on individual recognition were done twice: once with adults with left-sided ID's (which includes double sided IDs), and once with adults with right-sided IDs (which also includes double sided IDs). We found that results did not differ between these analyses, and we choose to report only right-sided adults, so that analyses reported here were performed with a total of 144 individuals (23 cubs + 62 both-sided adults + 59 right sided adults). Adults' IDs are marked with * when both sides are known, with R when only the right side is known.
For each of the videos we recorded date, time, and ID of the individuals. For analysis, we used three parameters:
1. Foraging group size: Numbers of individuals observed together with a focal cub. This was assessed on different temporal grids (per video, per night, and across the study period). Reported foraging group sizes include the focal cub.
2. Foraging group composition: array of individuals (both cubs and adults) seen together with the cub, again assessed on different temporal grids (per video, per night and across the study). The degree of overlap between a cub's foraging groups on different nights, in other words the similarity between a pair of groups, was calculated through the Jaccard-Index in R using the vegan package (Oksanen et al., 2020).
3. Connections between foraging groups: using Cytoscape (ver. 3.7.2), we constructed a social network for all individuals to show the connections between all cubs and adults during the 9-night period. Connections between adults are not included. Randomized null-model networks were created to test for the small-worldness of the networks using the Erdos-Renyi Model (Erdos and Rényi, 1960). The parameters Clustering Coefficient C (where 0 ≤ C ≤ 1) and Betweenness Centrality BWC (where 0 ≤ BWC ≤ 1) were calculated for the social network. The Clustering coefficient is a measure of “how well do my friends know each other,” i.e., how the direct neighbors of a node (here: individual) in a network are themselves interconnected. BWC is a measure of connectivity in the network in that a node with high Betweenness centrality is crucial in connecting different components in the network.
Results
The mean number of observed cubs per night was 5.9 ± 5.4 (mean ± sd) and cubs were observed on 2.3 ± 1.1 nights on average. With all nine nights summed up, the number of videos per cub ranged from 1 (for 3 cubs) to 28 (for C-15). On average each cub was present in 8 ± 7.2 videos throughout the study. We found no regularity in intervals of reoccurrence, such as every night or every other night, for any of the cubs.
Foraging Group Sizes
Per night that a cub was observed on the waste dump its foraging group size was on average 10.7 ± 7.6 individuals (Figure 1). Per-night foraging groups around cups ranged from 2 to 34 individuals.
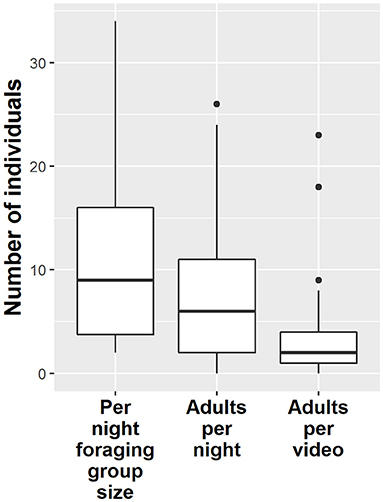
Figure 1. The cubs' per night foraging groups (including adults and other cubs) comprise 10.7 individuals on average (left box). As many foraging groups contained several cubs, the average per night number of adults observed around a cub is lower, at 7.1 individuals (middle box). Per video, the average number of adults is even lower, 2.5, indicating that cubs are not always accompanied by the same group of adults.
Both the range for each cub's foraging group size between different nights and the difference between mean group sizes between cubs are rather large, with some cubs never having a foraging group of more than 10 individuals, while others never had <10 (Supplementary Material 2).
Looking only at the adults accompanying a cub we found that each cub was seen together with an average of 2.5 ± 2.8 adults per video but with 7.1 ± 5.9 adults per whole night (Figure 1).
Composition of Foraging Groups
For each of the 18 cubs that were seen in more than one night, little stability in the composition (i.e., overlap of individuals) above its per-night foraging groups was detected (Table 1). As the cubs were present on 2–5 nights, the mean overlap between all pairs of nights is considered in Table 1. Mean overlap ranged from 0 to 0.13, indicating that in every night a cub was seen it was surrounded by an almost unique set of individuals and was hardly ever observed more than once with any of its peers.
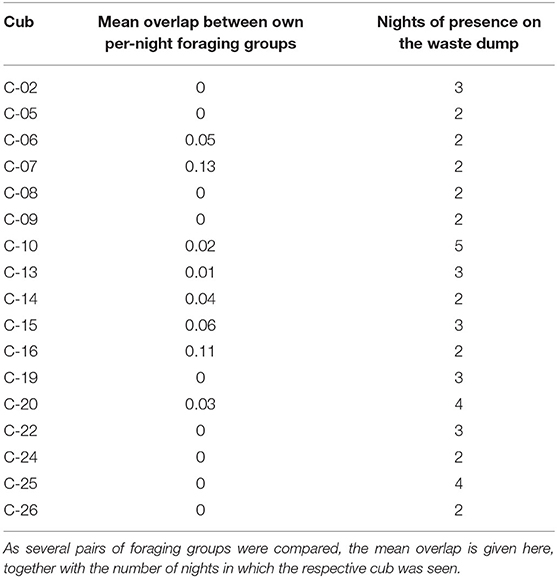
Table 1. Degree of overlap (Jaccard similarity) between per-night foraging groups for each cub that was present more than one night.
The composition of the group of adults around a cub (note here: only adults, not all individuals in the per-video group) differs substantially between the videos of one night for each cub. The overlap between per-video adults for cubs that were seen in multiple videos in at least 2 nights ranged from J = 0 (for several cubs) to J = 0.27 in C-25's group of 20 June.
Overlap between the per-period foraging groups as indicated by the Jaccard-Index is rather low for most pairwise (i.e., pairs of cubs) comparisons, with a mean similarity of J = 0.1. There are 10 pairs of per-period foraging groups that had an overlap of J > 0.5. In 3 of these pairs, the per-period foraging groups are based only on one night in which the 2 respective cubs were also observed together, so the high overlap is based on the cubs and their respective foraging group of that night spending much time together at the observation site (Supplementary Material 3).
Connection Between Foraging Groups
Combining all connections of a cub to another cub or an adult on a per-period level shows how the per-period foraging groups around each cub are connected to each other (Figure 2). Those 37 adults outside of the circle are only connected to one cub, all 84 adults on the circles outline have links to at least two cubs. Only one cub, C-21, was isolated from the other cubs on a per-period level, the other 22 cubs are all linked with an average of 6.2 ± 3.3 connections to other cubs. Over a maximum of 4 links, every cub is connected to all other cubs.

Figure 2. Network of how the cubs are connected to other individuals (excluding adult-adult connections) over nine nights, i.e., a period-wide network of all 144 observed individuals. Cubs are represented by green dots, adults by blue dots. Orange lines indicate that the pair was seen together more than once, the thicker the orange line the more shared occurrences the pair has.
There are 7 adult—cub pairs that were together twice and 2 adult—cub pairs that were together 3 times (orange connecting lines in Figure 2). The pairs that were together 3 times are cub C-15 and adult A-100*, and cub C-10 and adult A-48*. Adult A-100* is also connected to C-20 by two sightings and to 8 other cubs by one sighting. Apart from its 3 shared sightings with C-10, A-48* was only seen once with the 9 other cubs it is connected to. The adult A-188* is the only adult that has three cubs with which it was seen twice each: C-15, C-16, and C-20.
The cubs have on average 21.2 ± 11 connections to adults. Adults have only 2.9 ± 2.1 connections to cubs. The Clustering coefficient C for the network is 0.395. Average Shortest Path Length is 2.7, resulting in a small-world topography for this network. BWC is rather low, ranging only from 0 to 0.29 (median: 0.001). The 15 most central individuals in terms of BWC are all cubs, with C-15 as the most central one (BWC = 0.29). The adult with the highest Betweenness Centrality is A-213* (BWC = 0.025). However, it does not have the most connections to cubs, instead, this BWC indicates that it has connections to cubs that themselves have many connections. Similarly, A-141* is only linked to 5 cubs but has the third highest BWC of adults (BWC = 0.019), as it is connected to C-19, C-25, C-10, C-09, and C-13, all among the most central cubs.
Discussion
With spotted hyenas in Mekelle coexisting with people without conflict, we wanted to know how their urban lifestyle affects social foraging behavior and how cubs take part in this. Our findings of reduced territorial behavior and hardly observable borders of social groups at the waste dump are in line with previous findings on how urbanity and food provisioning by humans can affect carnivores (see Newsome et al., 2015 for a review).
The first hypothesis of this study was that foraging groups to which cubs belong would have a rather stable composition of individuals in the sense that every time a cub is seen, it would be surrounded by the same individuals. We reject this hypothesis because the association between cubs and adults was not persistent over time. In fact, the foraging groups of cubs observed on multiple nights were so different, that many cubs were not seen more than once with any putative relative. Moreover, no clear temporal patterns were found in the reoccurrence of cubs. This could however be affected by this study only covering 9 consecutive nights and further observations could uncover some regularities.
A caveat with presence video data is that individuals could be missed or recorded but with insufficient detail to allow individual identification. However, our finding of compositional variability is so strong that the known level of uncertainty in our data would not affect our conclusions.
Each cub was on average surrounded by 2.5 adults per video it was seen in. However, the number of per-night adults, i.e., the total number of adults the cub was seen with throughout a whole night is 7.1. A cub seems to have a larger “pool” of adults it is with on the waste dump during a night, but it doesn't spend all its time during a night with all of them. The overlap between adults accompanying a cub on a per-video level is low; rather than staying with 1 or 2 adults, with other adults fissioning and fusing over time, the composition of the set of adults around a cub is almost unique in every video.
Per-night data showed another interesting pattern: although many adults were only seen once with a cub, those that were seen multiple times with a cub seemed to play important roles. Only those adults were observed providing food, cleaning, disciplining or grooming cubs. These adults, known by both their body sides, are also among the most connected in the per-period network and among those that were seen in the most nights. For example, adult A-48* put meat in front of C-10 and they were together in 3 out of C-10's 5 nights. This sort of provisioning behavior is usually only observed by mothers to their own cub (Holekamp and Smale, 1990). In the absence of maternity data, we posit that cubs may have “reference adults” that care for the cub, even though the cub also joins foraging groups without their “reference adult.”
The lack of exclusive association of a cub with one adult may reflect that cubs learn their urban lifestyle by local enhancement (see Thorpe, 1956; Heyes and Galef Jr, 1996). With local enhancement, an individual learns not by observing a model individual performing an action but develops knowledge or skills when its attention is drawn to a certain aspect of the environment by other individuals in a specific location. However, it is possible that cubs are more strongly associated with particular adults at younger ages not represented in our data set.
We further hypothesized that the waste dump is exploited by several non-connected hyena foraging groups and that these would emerge as clusters in our social network analysis. These clusters would be highly intra-connected but hardly inter-connected. However, our social network on a per-period level showed that all individuals are more or less equally connected with each other and no clusters emerged. Betweenness Centrality is low for all individuals, meaning that there are no individuals connecting everyone to everyone; each individual has a few connections and each to very different individuals. Based on these findings, and on the fact that cubs are all connected to each other, one could argue that all individuals behave as if they are part of one single super-clan while on the waste dump, or even that the clan concept does not apply to these urban hyenas. This is further developed below.
Wild hyena clans have never been recorded to exceed 130 individuals, with an average of 29 (Holekamp and Dloniak, 2010), but clan size is mainly limited by prey abundance (Holekamp and Dloniak, 2010). Even though the supply of food in our study may vary over time and can be sparse during fasting periods (Yirga et al., 2012), the waste dump offers a continuous and abundant food source. There are more, smaller waste dumps in the surroundings of Mekelle, plus waste and domestic animals in the streets. Sonawane et al. (2021) calculated that each hyena around Mekelle consumes more than 900 kg of carcass waste per year. Yirga et al. (2017) counted more than 400 hyenas around Mekelle in calling station surveys. We identified 144 individuals but observed many more that we could not identify. Our camera trap data give an average of 254 hyenas entering the waste dump per night. The actual number could be lower as some individuals may enter and leave the waste dump several times during a single night. The number could however also be higher as not all points of entry to the dump could be covered with our camera traps. Schramme (2015) genetically analyzed hyena scat samples and suggested that all hyenas around Mekelle are remarkably closely related. However, relatedness within clans is usually not remarkably higher than between clans because of high male mediated gene-flow (van Horn et al., 2004), so the genetic analysis by Schramme (2015) is not sufficiently informative.
The mean number of connections and the ratio of realized vs. possible connections in the per-period social network is low, which means that the network is not densely intra-connected. Mean overlap between the cubs' per-period foraging groups is very low. This would not be expected in a resource-rich environment; many carnivores and hyenas as such turn most gregarious when prey is abundant, and factors that lead to fission are often related to competition (Smith et al., 2008). Moreover, Yirga et al. (2015b) suggested that hyenas on the waste dump were “congregations from various directions, apparently fusing without aggression.” Yirga et al. (2017) also found that bones at multiple den sites in the surroundings had been collected from the waste dump, indicating that hyenas foraging at the waste dump occupy different dens.
Spatial structure of the human-used landscape around Mekelle is organized such that there is a huge city waste dump with highly abundant food, that can actually nourish hundreds of hyenas. However, the same urban landscape provides no equally large single area where hundreds of hyenas could rest during the daytime. Suitable resting sites and locations for dens are spread over a number of smaller areas around Mekelle. We speculate that this particular spatial structure is key to the observed social structure of Mekelle's urban hyenas: Individuals are sharing members of flexible, loosely connected, conflict-free foraging groups. We know that such groups come from different directions to exploit the waste dump during the night (but without any recognizable temporal pattern) and that they take off in different directions when they leave the dump. Where exactly they go and rest during the daytime requires further study.
Here we speculate that the limited size of daytime resting areas around the city necessitates fusion of relatively small commuting foraging groups at the waste dump. Once at the spacious dump, no single foraging group would be able to exclude all other foraging groups from the entire waste dump or even from particular resource-rich areas. In presence of highly abundant food, it would also not pay to risk injury in territorial disputes. The waste dump allows all to feed to satiation in absence of territorial behavior. This mechanism was first hypothesized by Creel and Macdonald (1995) and was observed in several urban dwellers, such as red foxes (Macdonald et al., 1999), badgers (Davison et al., 2009), and raccoons (Smith and Engeman, 2002). Breck et al. (2019) observed urban coyotes to be more bold and exploratory compared to their conspecifics in rural areas. Similarly, the hyenas on the waste dump were observed to engage in object play and were surprisingly bold toward the observation vehicle and the observers inside. In such a system where satiation and absence of conflict seems guaranteed, it may become rather irrelevant which foraging group any individual decides to join. We propose that this social closeness or lack of aggression among foraging groups fosters a more loose and flexible foraging group membership than would be possible for hyenas in non-urban areas that are members of one specific clan. Cubs, subadults and immigrating males could learn this social closeness, as frequent encounters of individuals that include ritualized meeting ceremonies are crucial in maintaining individual relationships. Continuity would be important in this scenario; groups of wild hyenas that maintained friendly relationships with each other but that got socially separated following a time of spatial separation, resorted to clan wars after reunion (Boydston et al., 2003). As clans are at least partially defined as groups that exhibit territorial defense (Kruuk, 1972), one can argue that the clan concept does not apply to hyena groups at sites where territoriality is absent. We feel that this is definitely the case for Mekelle's urban hyenas at the city waste dump. We however cannot exclude the possibility that these urban hyenas show territorial behavior at daytime resting sites and dens, that seem to come in more limited supply, at least in terms of area per site, around Mekelle. We therefore recommend further research, identifying all hyenas individually and monitoring them across the human-used landscape, to fully establish social structure. Additionally, a series of nine consecutive nights of observations is merely a snapshot in time. With our data we were able to confirm several mechanisms proposed to act on carnivores in urban contexts, but we cannot make any statements about how factors like reduced territoriality vary over time, for example seasons. An especially interesting next step from this study would be to examine how the patterns of foraging groups we found here change during the Christian fasting month in Ethiopia, as meat waste on the dump is drastically reduced during this period (Yirga et al., 2012).
We further speculate that conflict-free human-hyena coexistence is not related to specific learned behavior, but rather to food availability. Hyenas follow natural behavior by congregating at the most easily accessible food patch at night. As long as this is waste, and as long as there are no humans on the waste dump during the night, conflict with humans will be limited. The most parsimonious explanation doesn't need to involve cultural transmission, as hyenas do not need to learn skills that are not already available in their opportunistic and generalistic repertoire. This does not mean, however, that hyenas are not influenced by human activity. Hyenas in a Kenyan nature reserve changed their activity patterns toward more nocturnal roaming as a consequence of increased human activity (Boydston et al., 2003). With reduced food competition, even hyenas in the wild turn more gregarious (Smith et al., 2008). This is consistent with our findings.
Yirga et al. (2012) pointed out that hyenas around Mekelle turn to more hunting and livestock depredation when offal on the waste dump becomes sparse. If access to waste was reduced, hyenas might try harder to enter livestock bomas. The present offal in food waste enables a larger population of hyenas to live around Mekelle than what would be expected in a more natural ecosystem. A sudden reduction in accessible food waste would leave a large population of predators without sufficient food sources, potentially leading to devastating consequences for both livestock and humans in the region (Newsome et al., 2015). In the region, people feel responsible for protecting their livestock and blame themselves for incidents of depredation (Yirga et al., 2012; Baynes-Rock, 2013). However, this may be a specificity of the integrated type of farming in Ethiopia, where every farmer needs a pair of oxen to plow and therefore keeps a small herd of livestock that is relatively easy to protect. Livestock also contribute protein to people's diets, but in the highlands there are no extensive herding practices by specialized pastoralists, a context with a higher risk of depredation. Land use is therefore a determining factor in the future development of the urban hyena population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because data was obtained solemnly through uninvasive observation of the study subjects.
Author Contributions
FS and MV designed the study and analyzed the data. FS wrote the first version of the manuscript. GY supervised and co-organized the field work. FS, HB, and MV edited and finalized the manuscript. All authors contributed to interpretation of the results.
Funding
MV and GY gratefully acknowledge funding of our cooperation through project 57345350, of the DAAD program P.R.I.M.E. (Postdoctoral Researchers International Mobility Experience). The field work was partly financed by a DAAD PROMOS scholarship to FS by Ruhr-Universität Bochum. We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank the University of Mekelle, Ethiopia for providing field cars, and Gebresadik Gebreyesus from the Mekelle City Administration for allowing us to work on the waste dump. Many thanks also to the students helping in the field: Sven Beckerwerth, Brhane Aregehey, Anika Hirz and Kai Möx. We would like to thank the two reviewers for their valuable inputs.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.866836/full#supplementary-material
References
Abay, G. Y., Bauer, H., Gebrihiwot, K., and Deckers, J. (2011). Peri-urban spotted hyena (Crocuta crocuta) in Northern Ethiopia. Diet, economic impact, and abundance. Eur. J. Wildl. Res. 57, 759–765. doi: 10.1007/s10344-010-0484-8
Atkins, J. L., Long, R. A., Pansu, J., Daskin, J. H., Potter, A. B., Stalmans, M. E., et al. (2019). Cascading impacts of large-carnivore extirpation in an African ecosystem. Science 364, 173–177. doi: 10.1126/science.aau3561
Bateman, P. W., and Fleming, P. A. (2012). Big city life: Carnivores in urban environments. J. Zool. 287, 1–23. doi: 10.1111/j.1469-7998.2011.00887.x
Bauer, H., Chapron, G., Nowell, K., Henschel, P., Funston, P., Hunter, L. T. B., et al. (2015). Lion (Panthera leo) populations are declining rapidly across Africa, except in intensively managed areas. PNAS 112, 14894–14899. doi: 10.1073/pnas.1500664112
Baynes-Rock, M. (2013). Local tolerance of hyena attacks in East Hararge Region, Ethiopia. Anthrozoos 26, 421–433. doi: 10.2752/175303713X13697429464438
Beckmann, J. P., and Berger, J. (2003). Rapid ecological and behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. J. Zool. 261, 207–212. doi: 10.1017/S0952836903004126
Boydston, E. E., Kapheim, K. M., Watts, H. E., Szykman, M., and Holekamp, K. E. (2003). Altered behaviour in spotted hyenas associated with increased human activity. Anim. Conserv. 6, 207–219. doi: 10.1017/S1367943003003263
Breck, S. W., Poessel, S. A., Mahoney, P., and Young, J. K. (2019). The intrepid urban coyote: a comparison of bold and exploratory behavior in coyotes from urban and rural environments. Sci. Rep. 9:2104. doi: 10.1038/s41598-019-38543-5
Creel, S., and Macdonald, D. W. (1995). Sociality, group size, and reproductive suppression among carnivores. Adv. Stud. Behav. 24, 203–257. doi: 10.1016/S0065-3454(08)60395-2
Davison, J., Huck, M., Delahay, R. J., and Roper, T. J. (2009). Restricted ranging behaviour in a high-density population of urban badgers. J. Zool. 277, 45–53. doi: 10.1111/j.1469-7998.2008.00509.x
Erdos, P., and Rényi, A. (1960). On the evolution of random graphs. Publ. Math. Inst. Hung. Acad. Sci 5, 17–60.
Fedriani, J. M., Fuller, T. K., and Sauvajot, R. M. (2001). Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography 24, 325–331. doi: 10.1034/j.1600-0587.2001.240310.x
Gade, D. W. (2006). Hyenas and humans in the Horn of Africa. Geogr. Rew. 96, 609–632. doi: 10.1111/j.1931-0846.2006.tb00519.x
Gehrt, S. D., Riley, S. P. D., and Cypher, B. L, . (eds) (2010). Urban Carnivores. Ecology, Conflict, and Conservation. Baltimore: JHU Press.
Hadidian, J., Prange, S., Rosatte, R., Riley, S. P., and Gehrt, S. D. (2010). “Raccoons (Procyon lotor)”, in Urban Carnivores. Ecology, Conflict, and Conservation, eds S. D. Gehrt, S. P. D. Riley, B. L. Cypher (Baltimore: JHU Press), 35–47.
Heyes, C. M., and Galef, B. G. Jr (1996). Social Learning in Animals: The Roots of Culture. San Diego: Academic Press.
Hoeks, S., Huijbregts, M. A. J., Busana, M., Harfoot, M. B. J., Svenning, J.-C., and Santini, L. (2020). Mechanistic insights into the role of large carnivores for ecosystem structure and functioning. Ecography 43, 1752–1763. doi: 10.1111/ecog.05191
Holekamp, K. E., and Dloniak, S. M. (2010). Intraspecific variation in the behavioral ecology of a tropical carnivore, the spotted hyena. Adv. Stud. Behav. 42, 189–229. doi: 10.1016/S0065-3454(10)42006-9
Holekamp, K. E., and Smale, L. (1990). Provisioning and food sharing by lactating spotted hyenas, Crocuta crocuta (Mammalia: Hyaenidae). Ethology 86, 191–202. doi: 10.1111/j.1439-0310.1990.tb00429.x
Holekamp, K. E., and Smale, L. (1998). Behavioral development in the spotted hyena. Bioscience 48, 997–1005. doi: 10.2307/1313456
Holekamp, K. E., Smale, L., Berg, R., and Cooper, S. M. (1997). Hunting rates and hunting success in the spotted hyena (Crocuta crocuta). J. Zool. 242, 1–15. doi: 10.1111/j.1469-7998.1997.tb02925.x
Kruuk, H. (1972). The Spotted Hyena. A Study Of Predation And Social Behavior. Chicago: Univ. of Chicago Press.
Macdonald, D. W., Courtenay, O., Forbes, S., and Mathews, F. (1999). The red fox (Vulpes vulpes) in Saudi Arabia: loose-knit groupings in the absence of territoriality. J. Zool. 249, 383–391. doi: 10.1111/j.1469-7998.1999.tb01207.x
Newsome, T. M., Dellinger, J. A., Pavey, C. R., Ripple, W. J., Shores, C. R., Wirsing, A. J., et al. (2015). The ecological effects of providing resource subsidies to predators. Glob. Ecol. Biogeogr. 24, 1–11. doi: 10.1111/geb.12236
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., and Legendre, P. (2020). vegan: Community Ecology Package. R package version 2.5-6. 20192020.
Schramme, E. (2015). Social structure of spotted hyena (Crocuta crocuta) populations around Mekelle city in Tigrey, Ethiopia. [master's thesis]. [Antwerpen], Universiteit Antwerpen.
Smith, H. T., and Engeman, R. M. (2002). An extraordinary raccoon, Procyon lotor; density at an urban park. Can. Field Nat. 116, 636–639. Available online at: https://digitalcommons.unl.edu/icwdm_usdanwrc/487
Smith, J., and Holekamp, K. E. (2019). “Spotted hyenas”, in Encyclopedia of Animal Behavior, ed. J. C. Choe (Amsterdam; Boston; Heidelberg; London; New York; Qxford: Elsevier Academic Press), 190–208. doi: 10.1016/B978-0-12-809633-8.20749-8
Smith, J. E., Kolowski, J. M., Graham, K. E., Dawes, S. E., and Holekamp, K. E. (2008). Social and ecological determinants of fission–fusion dynamics in the spotted hyaena. Anim. Behav. 76, 619–636. doi: 10.1016/j.anbehav.2008.05.001
Sonawane, C., Yirga, G., and Carter, N. H. (2021). Public health and economic benefits of spotted hyenas Crocuta crocuta in a peri-urban system. J. Appl. Ecol. 58, 2892–2902. doi: 10.1111/1365-2664.14024
van Horn, R. C., Engh, A. L., Scribner, K. T., Funk, S. M., and Holekamp, K. E. (2004). Behavioural structuring of relatedness in the spotted hyena (Crocuta crocuta) suggests direct fitness benefits of clan-level cooperation. Mol. Ecol. 13, 449–458. doi: 10.1046/j.1365-294X.2003.02071.x
Watts, H. E., and Holekamp, K. E. (2009). Ecological determinants of survival and reproduction in the spotted hyena. J. Mammal. 90, 461–471. doi: 10.1644/08-MAMM-A-136.1
Wolf, C., and Ripple, W. J. (2018). Rewilding the world's large carnivores. Roy. Soc. Open Sci. 5:172235. doi: 10.1098/rsos.172235
Yirga, G., Iongh, H. H., de Leirs, H., Gebrehiwot, K., Deckers, J., and Bauer, H. (2015a). Food base of the spotted hyena (Crocuta crocuta) in Ethiopia. Wildl. Res. 42, 19–24. doi: 10.1071/WR14126
Yirga, G., Iongh, H. H., de Leirs, H., Gebrihiwot, K., Deckers, J., and Bauer, H. (2012). Adaptability of large carnivores to changing anthropogenic food sources. Diet change of spotted hyena (Crocuta crocuta) during Christian fasting period in northern Ethiopia. J. Anim. Ecol. 81, 1052–1055. doi: 10.1111/j.1365-2656.2012.01977.x
Yirga, G., Leirs, H., Iongh, H. H., de Asmelash, T., Gebrehiwot, K., Deckers, J., et al. (2015b). Spotted hyena (Crocuta crocuta) concentrate around urban waste dumps across Tigray, northern Ethiopia. Wildl. Res. 42, 563–569. doi: 10.1071/WR14228
Keywords: carnivores, Crocuta crocuta, predators, social structure, territoriality, Urban ecology
Citation: Struller F, Bauer H, Yirga G and Vos M (2022) Growing Up Urban: Hyena Foraging Groups and Social Structure at a City Waste Dump. Front. Conserv. Sci. 3:866836. doi: 10.3389/fcosc.2022.866836
Received: 31 January 2022; Accepted: 31 March 2022;
Published: 27 April 2022.
Edited by:
Dhananjaya Katju, American University, United StatesReviewed by:
Bill Bateman, Curtin University, AustraliaJulie K. Young, Utah State University, United States
Copyright © 2022 Struller, Bauer, Yirga and Vos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthijs Vos, TWF0dGhpanMudm9zQHJ1Yi5kZQ==
 Franziska Struller
Franziska Struller Hans Bauer
Hans Bauer Gidey Yirga2,4
Gidey Yirga2,4 Matthijs Vos
Matthijs Vos