- 1Instituto de Pesquisas Cananéia (IPeC), Cananéia, Brazil
- 2Programa de Pós Graduação em Biodiversidade e Conservação da Natureza, Universidade Federal de Juiz de Fora (UFJF), Juiz de Fora, Brazil
- 3Departamento de Zoologia, Laboratório de Ecologia Comportamental e Bioacustica, Universidade Federal de Juiz de Fora (UFJF), Juiz de Fora, Brazil
- 4Department of Mathematics and Statistics, University of Otago, Dunedin, New Zealand
- 5Instituto Aqualie, Juiz de Fora, Brazil
Anthropogenic activities have altered the structure and function of coastal and estuarine ecosystems, affecting the animals that occur in these areas. Predictive models are useful to evaluate the impact of anthropogenic characteristics over species distribution. In this study, we used generalized linear models to assess the influence Valo Grande canal, which allows fresh water to enter the Estuarine Lagunar Complex of Cananéia, has on the occurrence of Guiana dolphins. A population of this species resides in the study area, which comprises many coastal and marine protected areas. Abiotic data and information on species occurrence were gathered between January 2012 and November 2014, during three fieldworks per season, covering four sectors within the estuary. The predictions resulting from generalized linear models indicated that the discharge of fresh water, which decreases salinity in the estuary, has a negative influence on the populations of dolphins in all sectors but, mainly, on sector IV, the closest to Valo Grande Canal. Thus, it is clear that Guiana dolphins presented a heterogeneous distribution within the studied estuary, and the areas of higher concentration of individuals deserve greater attention during the elaboration of conservation strategies.
Introduction
Anthropogenic changes in the environment have brought negative consequences that caused even the protected areas to be susceptible (Filla et al., 2008; Zappes et al., 2009). Triggered by human activities, these changes have been more intense in coastal environments, habitat of several cetaceans that are now vulnerable (Di Beneditto and Rosas, 2008; Crespo et al., 2010; Ross et al., 2011; Gusso-Choueri, 2015). Among the threats to the balance of marine environments, we can highlight: destruction of mangrove swamps, predatory fishing, land and ship pollution, climate change and bioinvasion (Pereira et al., 2014).
In the southern coast of São Paulo, southeast of Brazil, among many conflicts, the closure of Valo Grande canal—built in 1852—is frequently discussed by the locals, in protected areas management boards, or in judicial proceedings, once the estuary has been deeply influenced by the opening of this canal, originally 4.4 m wide, and widening rapidly ever since (Besnard, 1950; Saldanha, 2005; de Souza and de Oliveira, 2016; Prado et al., 2019). Because its margins could not stand the force of the water, they went through a process of erosion, overturning existing houses in the environs of Iguape (Valentin, 2006; de Souza and de Oliveira, 2016). In addition, the rapid siltation that prevented large crafts from navigating in the Valo took the region to economic decadence (Valentin, 2006; Saldanha, 2005).
Today, due to erosion, the canal is more than 300 m wide and 15 m deep at most, meaning that 70% of Ribeira river flow is channeled through it, greatly affecting the ecosystem as a whole, especially regarding the decrease in salinity (Besnard, 1950; Bonetti-Filho and Miranda, 1997; Saldanha, 2005). However, this shift in salinity allowed shoals of manjubas (Anchoviella lepidentostole) to enter the canal and go upstream (Carneiro, 2005; Saldanha, 2005; Souza, 2012). For this reason, this species has taken a greater proportion in comparison to other species, such as crustaceans and saltwater fish that no longer reach the northern part of the estuary (Carneiro, 2005; Saldanha, 2005).
In 1978, the State government decided to build a dam to close Valo Grande canal, causing, once again, changes in the ecosystem (Bonetti-Filho and Miranda, 1997). An improvement in the estuarine water quality was observed, with the rapid reappearance of shellfish, oysters and shrimp (Saldanha, 2005). This recovery, however, contrasted with the floods in the Ribeira river floodplains, which had already been intensively occupied by families and businesses, leading to conflicts among different perspectives on the multiple uses of water resources in the low basin (Souza, 2012).
In 1995, the dam broke, allowing the flow of fresh water, silt and dissolved substances into the Estuarine Lagunar Complex of Cananéia-Iguape, risking its environmental functions of regulation and biodiversity support for the third time (Bonetti-Filho and Miranda, 1997; Bernardes and Miranda, 2001; Souza, 2012).
This estuary is located in the largest continuous remnant of Atlantic Forest and represents one of the most well preserved ecosystems of the Brazilian coast (Souza, 2012). It is legally protected and has more than 40 Environmental Protection Areas at municipal, state and federal levels, which present different levels of restriction of occupation and use (ICMBio, 2015). These protection areas are called Lagamar Mosaic (ICMBio, 2015). In this estuary, there is a residing population of Sotalia guianensis which, according to the Official National List of Endangered Species of Fauna, published in 2014 by Ministry of the Environment, is listed as vulnerable (Monteiro-Filho, 1991; Santos and Rosso, 2008; Havukainen et al., 2011; MMA, 2014; Godoy et al., 2015). Among the threats mammals such as the studied species are subjected to, Begon et al. (2007) and Crespo et al. (2010) evaluate that degradation and habitat loss are the biggest.
In view of the changes described here, the aim of the present study is to assess the influence of the construction of Valo Grande canal on the occurrence and distribution of Guiana dolphins. Among the changes caused by the canal that had already been observed in the Estuarine Lagunar Complex of Cananéia, the decrease in salinity could lead to a probable loss of habitat with a decrease in the occurrence of Guiana dolphins (Sotalia guianensis), since these animals are more observed in areas with higher salinity (Eschrique et al., 2011; Godoy et al., 2015).
Materials and Methods
Study Area and Species
The Estuarine Lagunar Complex of Cananéia, located in the southern coast of the state of São Paulo, is part of the Lagamar Mosaic, the first protected coastal and marine area formally recognized (MMA, 2016). Because of the ecosystem diversity found in the region (beaches, mangrove swamps, estuaries, restingas, rivers, Atlantic Forest) and the fact that it is home to threatened and endemic species, this region integrates the Mata Atlântica Biosphere Reserve and Natural World Heritage Site, recognized by UNESCO since 1991 and 1999, respectively (Cunha-Lignon et al., 2015). It is also an area of major expression of the ecosystem restinga, part of UNESCOS's World Network of Biosphere Reserves (SMA, 2000; UNESCO, 2010). The Estuarine Lagunar Complex of Cananéia is also considered of extreme biological importance, identified as a priority area for conservation (MMA, 2007). The integration between coastal (terrestrial) and marine zones within the unit contributes even further to its protection, considering that a great part of the impact suffered by the marine zone has terrestrial origin (Santos and Schiavetti, 2014).
The Complex is characterized by the presence of three islands: Ilha de Cananéia is separated from the mainland by the Sea of Cubatão (Mar de Cubatão) and separated from Ilha Comprida by a channel called Mar Pequeno and, both islands are separated from Ilha do Cardoso by Trapandé Bay (Baía de Trapandé) (Figure 1).
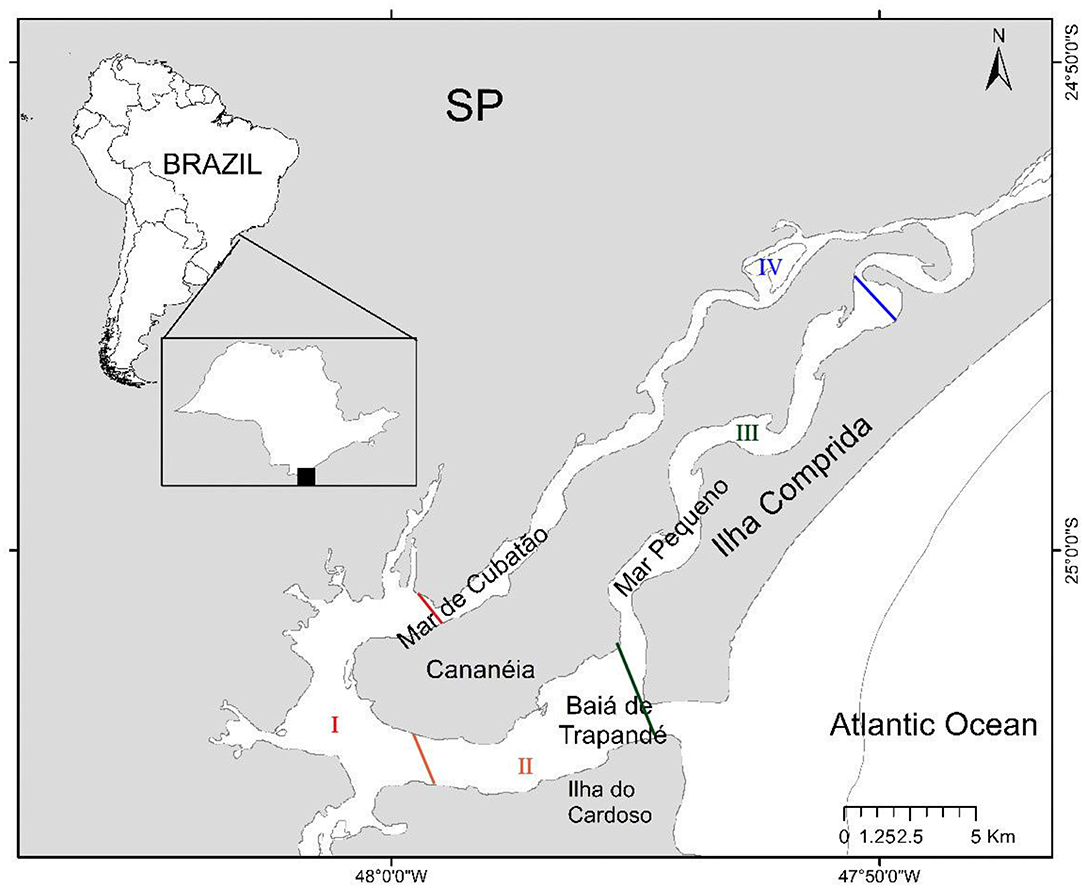
Figure 1. Map of the Estuarine Lagunar Complex of Cananéia, where the fieldwork took place, was divided in four sectors: I, II, III, and IV, according to the area's physiographic characteristics.
In the Cananéia estuary, small groups of Guiana dolphins can be seen daily in several places (Monteiro-Filho, 2000). The species uses this region for feeding and reproduction, and the infants can be seen throughout the year (Monteiro-Filho, 1991; Havukainen et al., 2011; Godoy et al., 2015).
Field Activities
The study area covers all the surroundings of Ilha de Cananéia and was divided in four sectors: I, II, III, and IV, according to the area's physiographic characteristics. Transects were plotted with the aid of GPS TrackMaker 13.5, respecting the distance of 1 km between the transects to avoid oversampling and subsampling. Between January 2012 and November 2014, we conducted three fieldworks per season in each sector, to collect the environmental descriptors and take notes of the dolphins' occurrence.
Whenever weather conditions changed suddenly during our sampling period, fieldworks were interrupted, and the collected data were discarded if less than half of the transects of the sector had been covered.
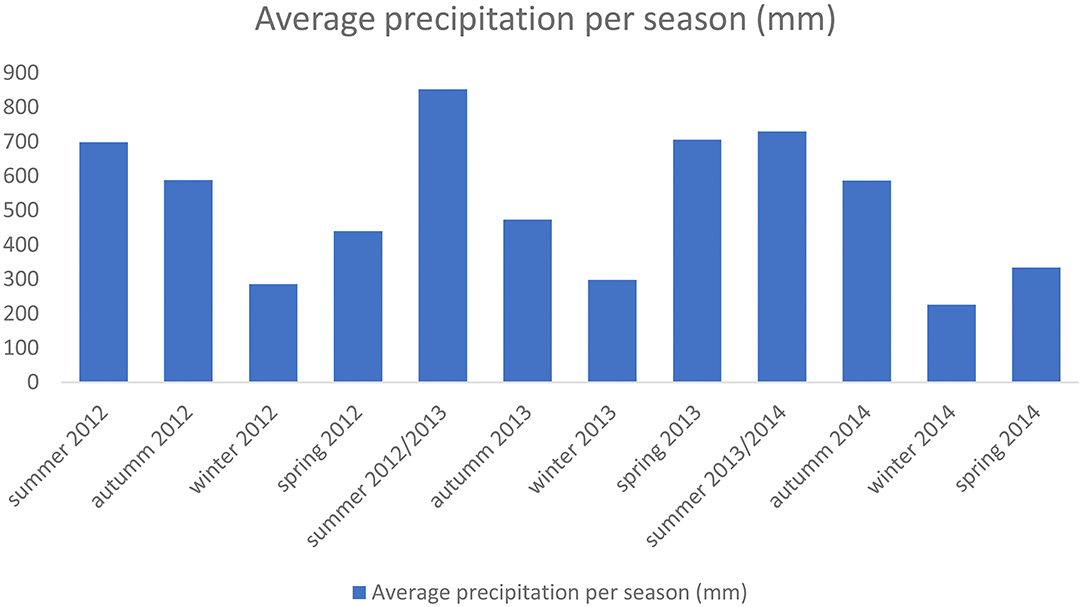
Fieldworks were conducted aboard a craft type Flex-Boat, at low speed (~10 km/h), keeping a minimum distance of 50 m from the dolphins and taking, thus, all precautions to minimize the impact on the animals and not to disturb them (Rezende, 2000; Filla et al., 2008; Filla and Monteiro-Filho, 2009). Every time there was an encounter with a group of estuarine dolphins, its location was recorded within the study area, with the use of GPS, the angle and distance related to the ship. To ensure all areas were sampled at different times of day, fieldworks were conducted in the morning (8 am to 12.30 pm) and afternoon (12.31 pm to 5 pm). Daylight saving time was not adopted. The year was divided into four seasons: spring, summer, autumn and winter, according to the precipitation index, as shown in Figure 2 for the study period (data also made available by the DAEE, 2016).
The flow of the Valo Grande was estimated at 70% of the flow of the Ribeira river, using data from the meteorological station of the Department of Water and Electricity of the state of São Paulo (DAEE, 2016). Valo Grande canal is located 32 km from the northern part of Ilha de Cananéia (Figure 3).
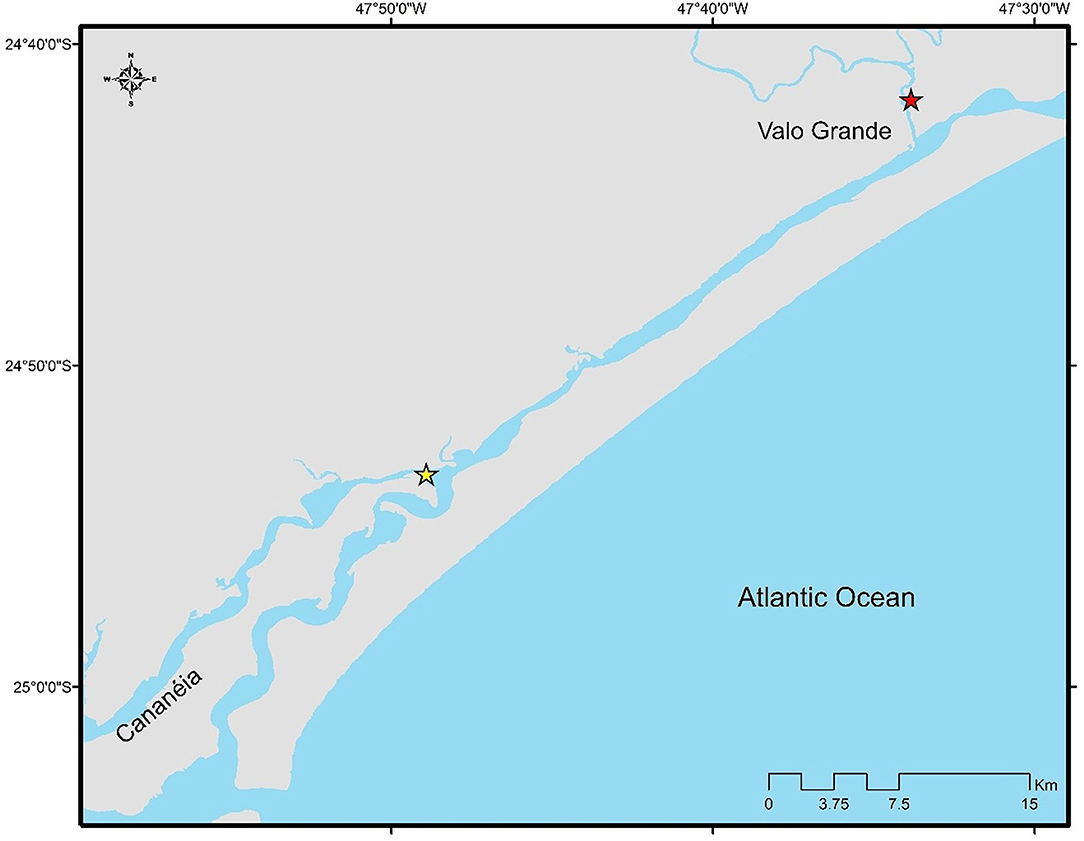
Figure 3. Map indicating the location of the estuary of Valo Grande canal (red star) and the northern part of the sampling area (yellow star).
Data Analysis
Initially, we conducted an exploratory analysis in order to identify possible outliers and remove them from subsequent analyses. Then, in order to assess whether the explanatory variables influenced the occurrence of individuals, a Generalized Linear Model (GLM) was developed, using the stats packages in R software (R Development Core Team., 2009) to analyse which structure would be more suitable for the dataset. The variable “occurrence of dolphins” refers to data counted according to Zuur et al. (2009); variables of this nature are modeled according to Poisson or negative binomial distribution. It is believed that models that use abundance, as opposed to presence and absence models, can present more trustworthy results due to the greater amount of data collected at the same point (Alves, 2015).
All GLMs were better adjusted by the negative binomial distribution. We chose the most adjusted model using Akaike Information Criterion (AIC, Burnham and Anderson, 2002), in which a lower value (smallest unexplained deviation) means a “better” model (Franklin, 2009).
We developed a general model (model 1) with the three predictors, a model by sector (model 2) and by season (model 3) using only the daily flow as an explanatory variable. The Prediction models were developed with the different values of daily flow in Valo Grande through the R software “prediction” function per sector and season. This function shows the average and number of dolphins within each flow value in the different seasons and sectors. We conducted a chi-square test to assess whether there was a significant difference in the numbers of dolphins predicted by the model within each flow value in each of the models. All statistical tests were performed using R software, with a 0.05 significance value.
Results
We conducted 131 surveys, accounting for 330 h of effective effort. During this period, we sighted 5,003 individuals, an average of 38 dolphins per survey (standard deviation = 2.47). The Valo Grande canal flow presented a daily average of 429 m3/s (standard deviation = 146.63) and a maximum of 1,087 m3/s.
The results of the best model 1 are shown in the table below according to the AIC value:
AIC (Poisson): 5259.2
AIC (Binomial Negative Binomial): 2530.2
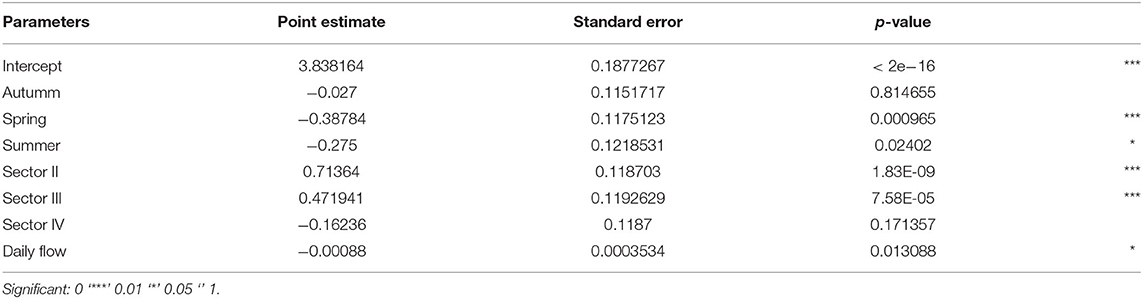
Results show that the occurrence of dolphins throughout the study area was lower in spring and summer, compared to winter (intercept) (Table 1). The opposite was observed in the sectors where there was a significant increase in the occurrence of dolphins (sectors II and III). Also according to the data presented in Table 1, there was a negative significance of Valo Grande daily flow in relation to the number of dolphins in the region.
The statistical summaries of GLMs per sector (model 2) and season (model 3), with the flow of the Valo Grande as the explanatory variable, are presented in Tables 2, 3. Table 2 shows significant results correlating the occurrence of dolphins in sectors III and IV, while results in sectors I and II show that the occurrence of dolphins is not determined by the daily flow of Valo Grande. However, in models that take the four seasons into account (Table 3), summer is the only season in which the occurrence of dolphins does not correlate to Valo Grande flow.
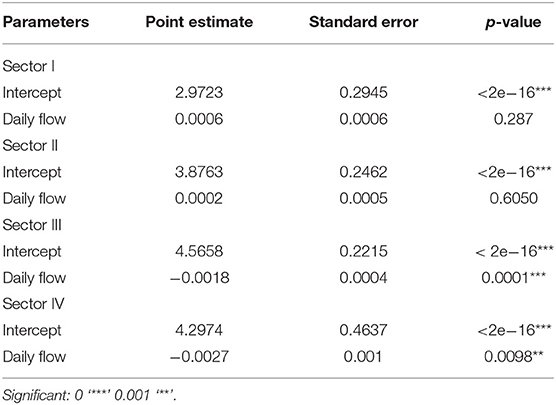
Table 2. Statistical summary of GLMs per sector (I, II, III, and IV) having the flow of the Valo Grande as the explanatory variable: point estimate, standard error and p-value.
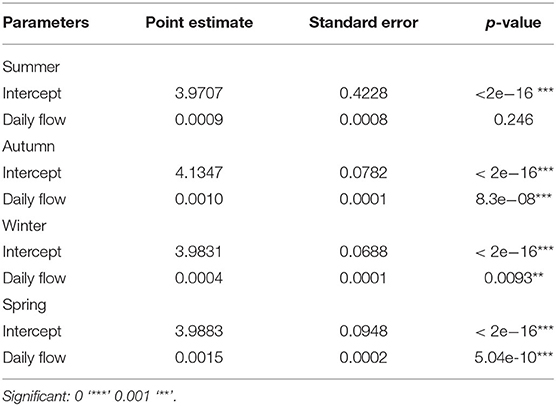
Table 3. Statistical summary of GLMs by season (summer, autumn, winter and spring) having the flow of the Valo Grande as the explanatory variable: point estimate, standard error and p-value.
Predictive Models
The average number of dolphins predicted by models 2 and 3 for each daily flow of Valo Grande canal (minimum, medium, maximum) per sector and season are represented in Figures 4, 5.
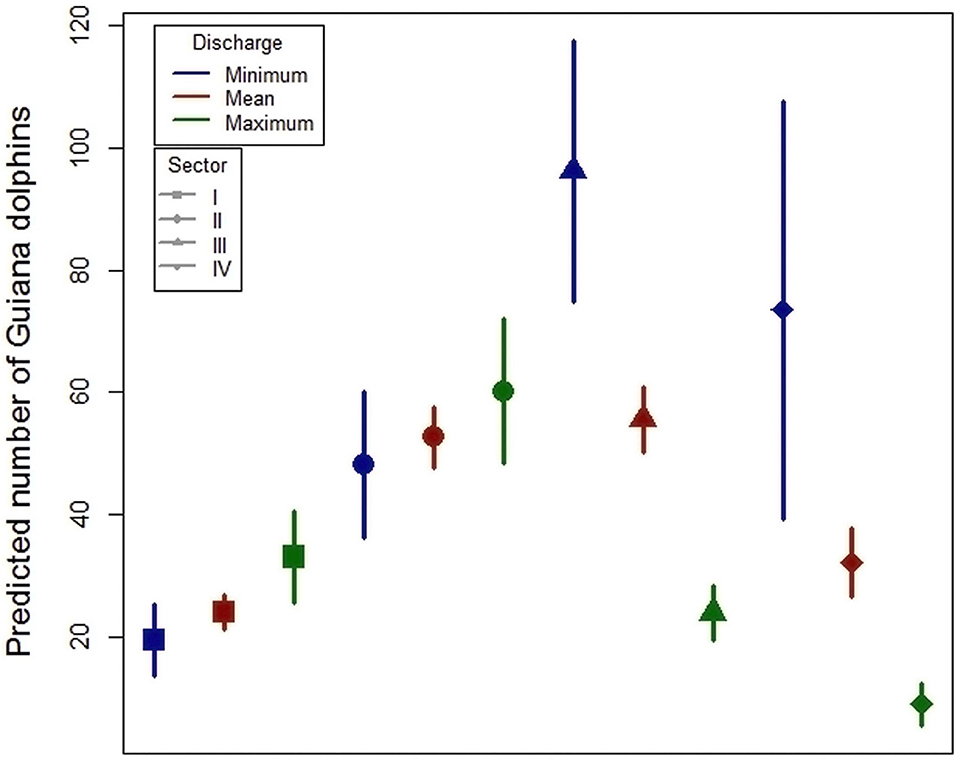
Figure 4. Average number of Guiana dolphins predicted for different flows of Valo Grande canal (minimum, medium and maximum), in different sectors.
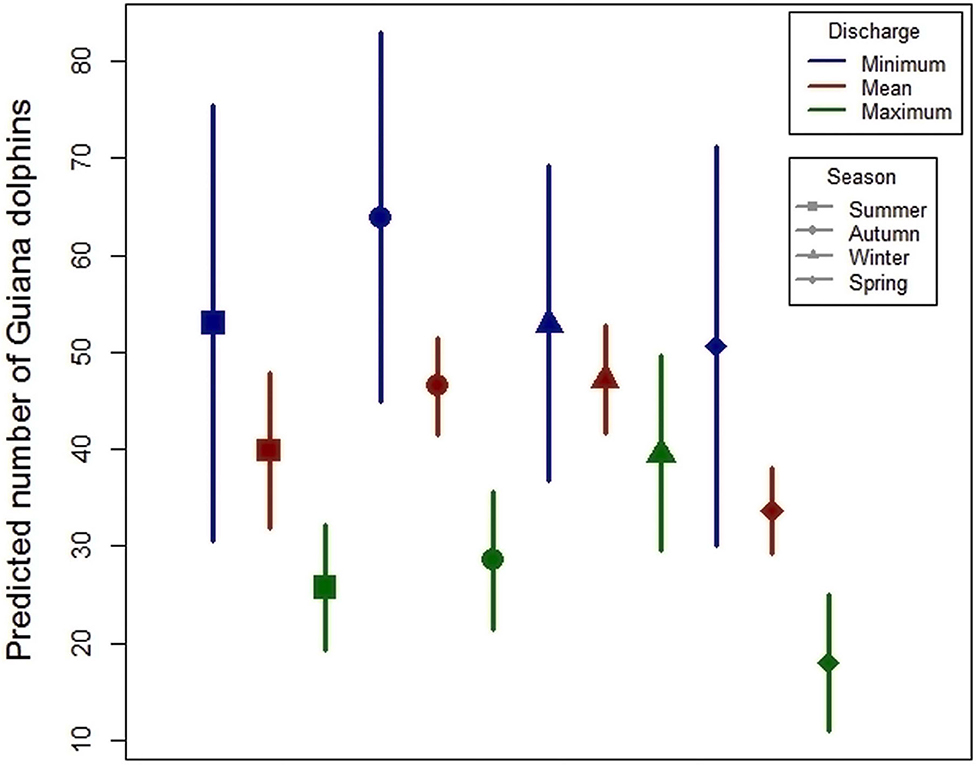
Figure 5. Average number of Guiana dolphins predicted for different flows of Valo Grande canal (minimum, medium and maximum) in different seasons.
The results pointed to an increase in the average number of dolphins following a decrease of Valo Grande canal flow. In sector III, the maximum flow was approximately twice the minimum flow, while in sector IV it increased approximately four times. In sector I, during winter and spring, the increase in the number of dolphins in relation to the increase of flow was not significant.
The predicted value of the number of dolphins resulting from the predictive models within each season, separated by sector, are presented in Appendix A. The predicted value of the number of dolphins resulting from the predictive models within each sector, separated by season, are presented in Appendix B.
Discussion
Results of Generalized Linear Models pointed to a negative influence of Valo Grande canal daily flow on the number of dolphins in the study area. The predictive models showed that in sectors II, III, and IV there was an increase in the number of dolphins with the decrease of Valo Grande canal average flow. This increase was crescent from sectors II to IV, corresponding to the proximity of these sectors to Valo Grande canal. Sector I was the only one that had a non-significant decrease in number of dolphins with the increase of the canal flow, according to data presented in Appendix A,B. The results presented in the predictive model confirm that the decrease in flow would increase the dolphins' spatial distribution in the region, especially in sector IV, the closest to Valo Grande canal.
Ross et al. (2011) listed 10 principles that characterize the habitat needs for small cetaceans, contributing to delineate boundaries and priority habitats. One of the principles is that the priority habitat must take into account the external connections needed to ensure its integrity, since many anthropogenic actions in coastal waters are strongly impacting small cetaceans. Among these actions, the regulation of fluvial ecosystems charge and habitat loss have been threats to them (Begon et al., 2007). Examples of these threats are presented by Nabi et al. (2021) in their bibliographic review of habitat changes and habitat loss caused by the regulation of river flow on Indus River Dolphin populations (Platanista gangetica minor) and conservation strategies of Yangtze Finless Porpoise (Neophocaena asiaeorientalis).
In the southeast of Brazil, the opening of Valo Grande canal took place in 1852, and completely changed the fluvial charge of Ribeira river, leading to the construction of a dam, which was kept closed from 1978 to 1995, when it broke down (Souza, 2012; de Souza and de Oliveira, 2016; Prado et al., 2019). Nowadays, Valo Grande canal is responsible for 70% of the flow coming from the higher portion of Ribeira river (Alto do rio Ribeira). Many studies have been trying to assess the changes suffered by this environment, which is part of a region considered of extreme biological importance, identified as a priority area for conservation (MMA, 2007), with more than 40 Protection Areas.
According to Nascimento et al. (2008), the re-opening of Valo Grande canal caused an increase in the flow, which led to the appearance of sandy islands and the formation of sand spots at the bottom of the lagunar canal. In a study conducted by Mahiques et al. (2009), in the central part of the complex, there was a change in the depositional pattern: before the opening of Valo Grande canal there was a predominance of sands and nowadays, the deposited sediments present more than 65% of silt and 8% of clay. According to the same author, today, the presence of metal in the sediments within the Cananéia estuary comes from the Ribeira river hydrological system that used to be low when the canal was closed, but has increased substantially after the opening as a consequence of the discharge of heavy metals from ancient existing mining companies of Ribeira river toward the inner part of the estuary (Mahiques et al., 2009; Coelho, 2011; Saito and Oliveira, 2012; Salgado et al., 2021).
The flow of Valo Grande canal directly influences salinity and water transparency in the sectors assessed in this study (Bernardes and Miranda, 2001; Coelho, 2011). Salinity, on the other hand, is an important factor for the species distribution, especially in estuaries (Begon et al., 2007). This difference in salinity has brought consequences for the northern region of the estuary and in places closer to Valo Grande canal, observed from macrophytic banks found today within and around mangrove forests, suffering thus a reduction of areas covered by mangrove swamps (Cunha-Lignon et al., 2015; Sampaio et al., 2021). The APA CIP Management Plan (Environmental Protection Area of Cananéia-Iguape-Peruíbe) also points to a loss of mangrove swamps habitats in the northern part, resulting from changes caused by the construction of the canal, which has come to be considered one of the factors of greatest impact on mangrove and estuarine species of the region (ICMBio, 2016). Once acknowledged its importance for the food chains in estuaries and marine areas, the destruction of mangrove swamps could lead to the decline of coastal and estuarine fishing in the region (Pereira et al., 2014).
The southern part of the study area–Trapandé Bay, the area of highest concentration of Guiana dolphins, located >50 km from Valo Grande canal–is still influenced by the freshwater intake, which alters salinity (Geise et al., 1999; Santos and Rosso, 2007; Coelho, 2011). Studies with cetaceans also confirmed that salinity influences the spatial distribution of some species that live along the coast and estuaries (Dias et al., 2009; Stutz Reis, 2013; Godoy et al., 2015; Kanaji et al., 2016).
The region's ichthyofauna has also changed because of the freshwater intake in the Complex (Maciel, 2001). In his study of the region, using various methods for catching fish between 1996 and 1997, the freshwater species were more abundant in the region influenced by Valo Grande canal, especially in the rainy season, and the typically marine species were more abundant in areas nearer the adjacent ocean, where salinity was higher. However, Contente (2013) did not find any species, using data from before the opening of Valo Grande canal. According to Maciel (2001), the flow of Ribeira river is a tensor that modulates the structure of fish communities in the region
Studies on habitat use by cetaceans show that environmental characteristics effectively influence distribution and abundance of food resources, which, in turn, influence species distribution in a given area (Ballance, 1992; Davis et al., 1998; Hastie et al., 2004; Torres et al., 2008; Rupil et al., 2018). Therefore, the environmental variables used in most studies (depth, temperature, salinity, declivity, among others) once related to the abundance of preys, are indirectly linked to the presence of cetaceans, known to be sensible to these resources abundance (Baumgartner et al., 2001; Learmonth et al., 2006; MacLeod, 2009; Lambert et al., 2014; de Boer et al., 2014; Godoy et al., 2020). Most preys of Guiana dolphins are found in the estuary because they're from salt and brackish water, showing this region's importance for this species (Zanelatto, 2001; Oliveira et al., 2008; Lopes et al., 2012; Rupil et al., 2018). However, the decrease in salinity directly affects the occurrence of marine fish that enter the estuary to reproduce and feed, due to physiological limitations, since most species can only stand small variations of salinity (Maciel, 2001; Pascke and Lanzendorf, 2017).
Although the lower limit of salinity and the upper limit of exposure time for dolphins in low salinity environments are unknown, variations in salinity can alter the availability of prey and also cause physiological changes. In spite of the fact that cetaceans have a complex osmoregulation mechanism, some studies with Tursiops truncatus found physiological evidence of blood changes and skin lesions due to exposure to low salinity (Xu et al., 2013; Ewing et al., 2017; McClain et al., 2020; Takeshita et al., 2021).
To prevent the flow of freshwater in the estuary, the Public Prosecutor's Office of the state of São Paulo has determined that Valo Grande has to be closed again. The decision has been appealed and is currently under trial. If the final sentence is for its closure, a long term monitoring of the fauna and flora will be needed, in order to: (1) evaluate the recovery of areas of Manguezal; (2) carry out the survey of the estuary's ichthyofauna to confirm the decrease of freshwater species and consequent increase of marine species; (3) monitor dolphins in order to verify the increase in their occurrence in the areas closest to Valo Grande; (4) identify the exact cause of illness or death of Guiana dolphins using necropsy, virtopsy, pathology, histopathology, microbiology, and biochemistry techniques to assess the animal's health and understand how the various environmental factors can cause morbidity or mortality (as proposed by Nabi et al., 2021) and (5) monitor accumulation of heavy metals in the ecological system.
Although the closure of Valo Grande is expected to bring ecological benefits, it will possibly also have negative social impacts, since floods, which also came to occur with the open Valo, were more frequent when it was closed. These floods affected the local population living on the floodplains, leading to huge financial losses (Souza, 2012; Prado et al., 2019). Therefore, in case of closure, support to the communities in the floodplain areas of Ribeira river will be necessary. A community group may have to be formed with researchers, managers of Protection Areas, representatives of local communities, politicians and public prosecutors to discuss this social issue. To prevent the social impacts, the relocation of the population, dredging of the original course of the river or installation of a floodgate in the Valo could be proposed. In the case of installation of a floodgate, the biologists working in the area under recovery will need to be consulted to advise when it could be opened, since to re-enter of fresh water in the system can affect directly or indirectly the areas in recovery.
It is interesting to highlight that the collaboration between managers of Protection Areas and researchers makes the information resulting from research relevant to the management and directly applicable during the decision-making processes. Managers and researchers tend to agree on the importance of most themes and the need to respond to fundamental questions about the main threats to protected areas management (Cvitanovic et al., 2013).
Therefore, we can conclude through the predictive models that the flow coming from Valo Grande influences negatively the distribution of Guiana dolphins in the surroundings of Ilha de Cananéia, especially in the areas that are closer to the Valo Grande canal. In view of this, a thorough assessment of the pros and cons regarding the closing of Valo Grande is imminent, taking into account, however, that the loss of speccies of fauna and flora in certain regions of the estuary will have an inestimable value, often with no possibility of recovery.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because the data were obtained through observations of animals in their natural environment.
Author Contributions
DF and AA contributed to the conception and design of the study. DF organized the database. HP performed the statistical analysis. DF and HP wrote sections of the manuscript. All authors contributed to the manuscript review, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Instituto de Pesquisas Cananéia (IPeC) and Instituto Aqualie for their logistics support, the Graduate Programme in Ecology (PGEcol) at the Federal University of Juiz de Fora, Minas Gerais (UFJF). DF received financial support from CAPES (2013–2016) and the Programa Petrobras Ambiental.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.852104/full#supplementary-material
References
Alves, T. P (2015). Padrão de ocupação de hábitat das espécies dominantes de characiformes do Lago Guaíba (Rio Grande do Sul, Brasil) em função de parâmetros ambientais naturais (master's thesis). Porto Alegre: Pontifícia Universidade Católica do Rio Grande Do Sul.
Ballance, L. T (1992). Habitat use patterns and ranges of the bottlenose dolphin in the Gulf of California, Mexico. Mar. Mamm. Sci. 8, 262–274. doi: 10.1111/j.1748-7692.1992.tb00408.x
Baumgartner, M. F., Mullin, K. D., May, L. N., and Leming, T. D. (2001). Cetacean habitats in the northern Gulf of Mexico. Fish. Bull. 99, 219–239.
Begon, M., Townsend, C. R., and Harper, J. L. (2007). Ecologia de Indivíduos a Ecossistemas. Porto Alegre: Artmed.
Bernardes, M. E. C., and Miranda, L. B. D. (2001). Circulação estacionária e estratificação de sal em canais estuarinos: simulação com modelos analíticos. Revista Brasileira de Oceanografia. 49, 115–132. doi: 10.1590/S1413-77392001000100010
Besnard, W (1950). Considerações gerais em torno da questão lagunar de Cananéia-Iguape II. Boletim. Inst. Paul. Oceanogr. 1, 3–28. doi: 10.1590/S0100-42391950000200001
Bonetti-Filho, J., and Miranda, L. B. (1997). Estimativa da descarga de água doce no Sistema Estuarino Lagunar de Cananéia-Iguape. Rev. Bras. Oceanogr. 45, 89–94. doi: 10.1590/S1413-77391997000100009
Burnham, K. P., and Anderson, D. R. (2002). Information and likelihood theory: a basis for model selection and inference. Model selection and multimodel inference: a practical information theoretic approach, 2nd ed. New York, NY: Springer, 49–97.
Carneiro, R. R. S (2005). A pesca da manjuba (Anchoviella lepidentostole) e o canal do Valo Grande: uma relação de (des) continuidades em Iguape-SP. (master's thesis). São Paulo (SP): Universidade de São Paulo.
Coelho, L. V (2011). Estudo biogeoquímico do fósforo no complexo estuarino-lagunar de Cananéia-Iguape (SP): influência do Valo Grande e fluxo bêntico. (dissertation). São Paulo (SP): Instituto Oceanográfico, Universidade de São Paulo.
Contente, R. F (2013). Padrões ecológicos locais e multidecadais da ictiofauna do estuário Cananéia-Iguape. (master's thesis). São Paulo (SP): Universidade de São Paulo.
Crespo, E. A., Alarcón, D., Alonso, M., Bazzalo, M., Borobia, M., Cremer, M., et al. (2010). Report of the working group on major threats and conservation. Latin Am. J. Aquat. Mamm. 8, 47–56. doi: 10.5597/lajam00153
Cunha-Lignon, M., Almeida, R., Lima, N. G. B., Galvani, E., Menghini, R. P., Coelho-Jr, C., et al. (2015). Monitoramento de Manguezais: abordagem integrada frente às alterações ambientais. In: Congresso Brasileiro de Unidades de Conservação - CBUC, Curitiba. Anais do VIII CBUC - Trabalhos Técnicos 2015. Curitiba: Fundação Grupo Boticário. p 1–17.
Cvitanovic, C., Wilson, S. K., Fulton, C. J., Almany, G. R., Anderson, P., Babcock, R. C., et al. (2013). Critical research needs for managing coral reef marine protected areas: Perspectives of academics and managers. J. Environ. Manag. 114, 84–91. doi: 10.1016/j.jenvman.2012.10.051
DAEE. (2016). Departamento de Águas e Energia Elétrica. http://www.hidrologia.daee.sp.gov.br/ (accessed January 29, 2016).
Davis, R. W., Fargion, G. S., May, M., Leming, T. D., Baumgartner, M., Evans, W. E., et al. (1998). Physical habitat of cetaceans along the continental slope in the North-Central and Western Gulf of Mexico. Mar. Mamm. Sci. 14, 490–507. doi: 10.1111/j.1748-7692.1998.tb00738.x
de Boer, M. N., Simmonds, M. P., Reijnders, P. J., and Aarts, G. (2014). The influence of topographic and dynamic cyclic variables on the distribution of small cetaceans in a shallow coastal system. PloS ONE. 9, e86331. doi: 10.1371/journal.pone.0086331
de Souza, T. D. A., and de Oliveira, R. C. (2016). Alterações ambientais no complexo estuarino-lagunar de Cananeia-Iguape: a influência do canal artificial do “Valo Grande”. Boletim de Geografia. 34, 30–44. doi: 10.4025/bolgeogr.v34i3.23474
Di Beneditto, A. P. M., and Rosas, F. C. W. (2008). “Mortalidade”, in Biologia, ecologia e conservação do boto-cinza, Monteiro-Filho, E.L.A., and Monteiro, K.D.K.A. São Paulo, SP: Páginas & Letras Editora e Gráfica. p. 211–222.
Dias, L. A., Herzing, D., and Flach, L. (2009). Aggregations of Guiana dolphins (Sotalia guianensis) in Sepetiba Bay, Rio de Janeiro, south-eastern Brazil: distribution patterns and ecological characteristics. J. Mar. Biolog. Assoc. 89, 967–973. doi: 10.1017/S0025315409000782
Eschrique, S. A., Coelho, L. H., Oliveira, E. N., and Braga, E. S. (2011). “Qualidade da água como ferramenta na gestão ambiental de estuários –exemplo do litoral sul de São Paulo”, in Simpósio Brasileiro de Oceanografia. Anais, Santos. p. 01–06
Ewing, R. Y., Mase-Guthrie, B., McFee, W., Townsend, F., Manire, C. A., Walsh, M., et al. (2017). Evaluation of serum for pathophysiological effects of prolonged low salinity water exposure in displaced bottlenose dolphins (Tursiops truncatus). Front Vet Sci. 4, 80. doi: 10.3389/fvets.2017.00080
Filla, G. F., Atem, A. C. G., Bisi, T. L., Oliveira, L. V., Domit, C, Gonçalves, M., et al. (2008). Proposal for creation of a ‘zoning with regulation of use in the Cananéia Estuarine-Lagoon Complex' aiming the conservation of the estuarine dolphin, Sotalia guianensis (van Bénéden) (Cetacea, Delphinidae). Pan-Am. J. Aquat. Sci. 3, 75–83.
Filla, G. F., and Monteiro-Filho, E. L. A. (2009). Group structure of sotalia guianensis in the bays within the coast of Paraná, south of Brazil. J. Mar. Biolog. Assoc. 89, 985–993. doi: 10.1017/S0025315409002926
Franklin, J (2009). Mapping Species Distributions: Spatial Inference and Prediction. Cambridge: Cambridge University Press.
Geise, L., Gomes, N., and Cerqueira, R. (1999). Behavior, habitat use and population size of Sotalia fluviatilis (Gervais, 1853) (Cetacea, Delphinidae) in the Cananéia estuary region, São Paulo, Brazil. Revista Brasileira de Biologia. 59, 183–194. doi: 10.1590/S0034-71081999000200002
Godoy, D. F., Andriolo, A., and Filla, G. F. (2015). The influence of environmental variables on estuarine dolphins (Sotalia guianensis) spatial distribution and habitat used in the Estuarine Lagunar Complex of Cananéia, southeastern Brazil. Ocean Coastal Manag. 106, 68–76. doi: 10.1016/j.ocecoaman.2015.01.013
Godoy, D. F., Mendonça, J. T., and Andriolo, A. (2020). Occurrence of Guiana dolphin (Sotalia guianensis) in southeast of Brazil: Driven by prey distribution or human fishing activity?. Aquat Conserv. 30, 1910–1921. doi: 10.1002/aqc.3367
Gusso-Choueri, P. K (2015). Uso de bagre amarelo (Cathorops spixii) como modelo biológico de exposição e efeito de contaminantes no Complexo Estuarino-Lagunar Cananéia-Iguape-Peruíbe. (master's thesis). Curitiba (PR): Universidade Federal do Paraná.
Hastie, G. D., Wilson, B., Wilson, L. J., Parsons, K. M., and Thompson, P. M. (2004). Functional mechanisms underlying cetacean distribution patterns: hotspots for bottlenose dolphins are linked to foraging. Marine Biol. 144, 397–403. doi: 10.1007/s00227-003-1195-4
Havukainen, L., Monteiro-Filho, E. L. A., and Filla, G. F. (2011). Population density of Sotalia guianensis (Cetacea: Delphinidae) in the Cananéia region, Southeastern Brazil. Rev. Biol. Trop. 59, 1275–1284.
ICMBio. (2015). Instituo Chico Mendes de Conservação da Biodiversidade. Available online at: http://www.icmbio.gov.br/portal/mosaicosecorredoresecologicos/moscaicos-reconhecidos-oficialmente/1870-mosaico-de-unidades-de-conservacao-donlitoral-sul-de-sao-paulo-e-do-litoral-do-parana-lagamar> (accessed August 08, 2015).
ICMBio. (2016). Plano de Manejo da Área de Proteção Ambiental Cananéia Iguape Peruibe. Available online at: http://www.icmbio.gov.br/portal/images/stories/DCOM_plano_de_manejo_Apa_Cananeia_Iguape_peribe_SP_20160418.pdf. (accessed April 15, 2016).
Kanaji, Y., Okazaki, M., Watanabe, H., and Miyashita, T. (2016). Biogeography of small odontocetes in relation to wide-scale oceanographic structure in the North Pacific Ocean. Fisher. Oceanogra. 25, 119–132. doi: 10.1111/fog.12140
Lambert, E., Pierce, G. J., Hall, K., Brereton, T., Dunn, T. E., Wall, D., et al. (2014). Cetacean range and climate in the eastern North Atlantic: future predictions and implications for conservation. Global Change Biol. 20, 1782–1793. doi: 10.1111/gcb.12560
Learmonth, J. A., Macleod, C. D., Santos, M. B., Pierce, G. J., Crick, H. Q. P., and Robinson, R. A. (2006). Potential effects of climate change on marine mammals. Oceanogra. Marine Biol. 44, 431. doi: 10.1201/9781420006391.ch8
Lopes, X. M., Da Silva, E., Bassoi, M., Dos Santos, R. A., and De Oliveira Santos, M. C. (2012). Feeding habits of Guiana dolphins, Sotalia guianensis, from south-eastern Brazil: new items and a knowledge review. J. Mar. Biolog. Assoc. 92, 1723–1733. doi: 10.1017/S0025315412000495
Maciel, N. A. L (2001). Composição, abundância e distribuição espaço-temporal da ictiofauna do complexo estuarino-lagunar de Iguape-Cananéia-São Paulo-Brasil. (master's thesis). São Paulo (SP): Universidade de São Paulo.
MacLeod, C. D (2009). Global climate change, range changes and potential implications for the conservation of marine cetaceans: a review and synthesis. Endanger. Species Res. 7(2), 125–136. doi: 10.3354/esr00197
Mahiques, M. M. D., Burone, L., Figueira, R. C. L., Lavenére-Wanderley, A. A. D. O., Capellari, B., Rogacheski, C. E., et al. (2009). Anthropogenic Influences in a Lagoonal Environment: a Multiproxy Approach at the Valo Grande Mouth, Cananéia-Iguape System (SE Brazil). Braz. J. Oceanogr. 57 325–337. doi: 10.1590/S1679-87592009000400007
McClain, A. M., Daniels, R., Gomez, F. M., Ridgway, S. H., Takeshita, R., Jensen, E. D., et al. (2020). Physiological effects of low salinity exposure on bottlenose dolphins (Tursiops truncatus). J. Zoo Wildl. Med. 1, 61–75. doi: 10.3390/jzbg1010005
MMA. (2007). Ministério do Meio Ambiente. Portaria n° 9, de 23 de janeiro de 2007. Disponível em: http://www.icmbio.gov.br/ (Acessado em 24 de julho de 2013).
MMA. (2014). Ministério do Meio Ambiente. Lista Nacional Oficial das Espécies da Fauna Brasileira Ameaçadas de Extinção. Portaria n. 444, de 17 de dezembro de 2014. Diário oficial da república federativa do brasil, brasilia, df. seção 245, 121–126.
MMA. (2016). Ministério do Meio Ambiente. Disponível em: http://www.mma.gov.br/biodiversidade/biodiversidade-aquatica/zona-costeira-e-marinha/unidades-de-conservacao-e-mosaicos Acessado em: 05 de março de 2016.
Monteiro-Filho, E. L. A (2000). Group organization in the dolphin Sotalia guianensis in an estuary of southeastern Brazil. Ciência e Cultura. Ciênc. Cult. 52, 97–101.
Monteiro-Filho, E. L. D. A (1991). Comportamento de caça e repertório sonoro do golfinho Sotalia brasiliensis (Cetacea: Delphinidae) na região de Cananéia, Estado de São Paulo. (master's thesis). Campinas (SP): Unicamp.
Nabi, G., Ahmad, S., McLaughlin, R. W., Hao, Y., Khan, S., Ahmad, N., et al. (2021). Deteriorating Habitats and Conservation Strategies to Repopulate the Endangered Indus River Dolphin (Platanista gangetica minor); a Lesson Learned From the Conservation Practices of the Yangtze Finless Porpoise (Neophocaena asiaeorientalis). Front. Mar. Sci. doi: 10.3389/fmars.2021.561905
Nascimento, D. R. D Jr, Giannini, P. C. F., Tanaka, A. P. B., and Guedes, C. C. F. (2008). Mudanças morfológicas da extremidade NE da Ilha Comprida (SP) nos últimos dois séculos. Geologia USP. Série Científica. 8, 25–39. doi: 10.5327/Z1519-874X2008000100003
Oliveira, M. R., Rosas, F. C. W., Pinheiro, P. C., and Dos Santos, R. A. (2008). “Alimentação,” in: Biologia, ecologia e conservação do boto-cinza, Monteiro-Filho, E. L. A., Monteiro, K. D. K. A., (eds). São Paulo, SP: Páginas and Letras Editora e Gráfica. p. 91–102.
Pascke, M. S., and Lanzendorf, F. N. (2017). Diferença entre peixes de água salgada e peixes de água doce. Maiêutica-Ciências Biológicas. 5.
Pereira, D. S., Agassi, E. S. M., Poffo, I. R. F., and Ferreira, R. B. (2014). Cadernos de Educação Ambiental (18): Pesca Sustentável. Editora: SMA/SP - Secretaria de Meio Ambiente do Estado de São Paulo. São Paulo
Prado, H. M., Schlindwein, M. N., Murrieta, R. S. S., Nascimento, D. R. D., Souza, E. P. D., Cunha-Lignon, M., et al. (2019). The Valo Grande channel in the Cananéia-Iguape estuary-lagoon complex (SP, Brazil): Environmental history, ecology, and future perspectives. Ambiente and Sociedade. 22. doi: 10.1590/1809-4422asoc0182r2vu19l4td
R Development Core Team. (2009). A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Rezende, F (2000). Bioacústica e alterações acústico comportamentais de Sotalia fluviatilis guianensis (CETACEA, DELPHINIDAE) frente à atividade de embarcações na Baía de Trapandé, Cananéia, SP. (dissertation). São Carlos (SP): Universidade Federal de São Carlos.
Ross, P. S., Barlow, J., Jefferson, T. A., Hickie, B. E., Lee, T., Macfarquhar, C., et al. (2011). Ten guiding principles for the delineation of priority habitat for endangered small cetaceans. Marine Policy. 35, 483–488. doi: 10.1016/j.marpol.2010.11.004
Rupil, G. M., Bogoni, J. A., Barbosa, L., Marcondes, M. C. C., and Farro, A. P. C. (2018). Climate influences on Guiana dolphin diet along the Brazilian coast. Scientia Marina. 82, 159–168. doi: 10.3989/scimar.04775.27A
Saito, R. T., and Oliveira, T. S. (2012). Implicações ambientais e sociais do canal do valo grande no Sistema Estuarino Lagunar Cananéia/Iguape-SP. Anuário da Produção Acadêmica Docente. 5, 153–170.
Saldanha, I. R. R (2005). Espaços, recursos e conhecimento tradicional dos pescadores de manjuba (Anchoviella lepidentostole) em Iguape/SP. São Paulo. (dissertation). São Paulo (SP): Universidade de São Paulo.
Salgado, L. D., Filla, G. F., and Carvalho-Neto, F. S. (2021). Concentrations of Pb, Cd and Zn in sediments of an estuarine complex affected by ancient mining activities in Southeast Brazil. J. Integr. Coast. Zone Manag. 20, 233–247. doi: 10.5894/rgci-n192
Sampaio, J. A. G., Reis, C. R. G., Lignon, M. C., Nardoto, G. B., and Salemi, L. F. (2021). Interactive effects of abiotic and biotic factors drive aquatic plant colonization in subtropical mangroves. Res. Squ. doi: 10.21203/rs.3.rs-309998/v1
Santos, C. Z., and Schiavetti, A. (2014). Spatial analysis of protected areas of the coastal/marine environment of Brazil. J. Nat. Conserv. 22, 453–461. doi: 10.1016/j.jnc.2014.05.001
Santos, M. C. O., and Rosso, S. (2007). Ecological aspects of marine tucuxi dolphins (Sotalia guianensis) basec on group size and composition in the Cananéia Estuary, Southern Brazil. Aquat. Mamm. 6, 71–82. doi: 10.5597/lajam00110
Santos, M. C. O., and Rosso, S. (2008). Social organization of marine tucuxi dolphins, Sotalia guianensis, in the Cananéia estuary of southeastern Brazil. J. Mammal. 89, 347–355. doi: 10.1644/07-MAMM-A-090R2.1
SMA. (2000). Secretaria do Estado do Meio Ambiente. São Paulo: Atlas das Unidades de Conservação Ambiental do Estado de São Paulo.
Souza, E. P (2012). Canal do Valo Grande: Governança das águas estuarinas na perspectiva da aprendizagem social. (master's thesis). São Paulo (SP): Universidade de São Paulo.
Stutz Reis, S (2013). Uso do hábitat pelo boto-cinza Sotalia guianensis (Van Benédén, 1864) (Cetacea: Delphinidae) na Baía de Benevente, ES, Brasil (dissertation). Juiz de Fora (MG): Universidade Federal de Juiz de Fora.
Takeshita, R., Balmer, B. C., Messina, F., Zolman, E. S., Thomas, L., Wells, R. S., et al. (2021). High site-fidelity in common bottlenose dolphins despite low salinity exposure and associated indicators of compromised health. PLoS ONE 16, e0258031. doi: 10.1371/journal.pone.0258031
Torres, L. G., Read, A. J., and Halpin, P. (2008). Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity. Ecol. Applicat. 18, 1702–1717. doi: 10.1890/07-1455.1
UNESCO. (2010). United Nations Educational, Scientific and Cultural Organization. Available online at: http://www.unesco.org
Valentin, A (2006). Uma civilização do arroz: agricultura, comércio e subsistência no Vale do Ribeira (1800-1880) (Doctoral dissertation) São Paulo: Universidade de São Paulo.
Xu, S., Yang, Y., Zhou, X., Xu, J., Zhou, K., and Yang, G. (2013). Adaptive evolution of the osmoregulation-related genes in cetaceans during secondary aquatic adaptation. BMC Evolut. Biol. 13, 1–9. doi: 10.1186/1471-2148-13-189
Zanelatto, R. C (2001). Dieta do boto-cinza Sotalia fluviatilis (Cetacea, Delphinidae) no Complexo Estuarino da Baía e Paranaguá e sua relação com a ictiofauna estuarina. (master's thesis). Curitiba (PR): Universidade Federal do Paraná.
Zappes, C. A., Andriolo, A., Silva, F. O., and Monteiro-Filho, E. L. A. (2009). Potential conflicts between fishermen and Sotalia guianensis (Van Bénéden, 1864) (Cetacea, Delphinidae) in Brazil. Sitientibus Série Ciências Biologicas. 9, 208–214. doi: 10.13102/scb8013
Keywords: generalized linear model, Guiana dolphin, habitat modifications, anthropic activities, predictive model, marine mammal
Citation: Godoy DF, Pavanato H and Andriolo A (2022) Planning Conservation Strategies of Guiana Dolphin Related to Canal Flow and Habitat Changes in the Estuarine Lagunar Complex of Cananéia. Front. Conserv. Sci. 3:852104. doi: 10.3389/fcosc.2022.852104
Received: 19 January 2022; Accepted: 19 April 2022;
Published: 25 May 2022.
Edited by:
Govindhaswamy Umapathy, Centre for Cellular and Molecular Biology (CCMB), IndiaReviewed by:
Maria Angelica Miglino, University of São Paulo, BrazilGhulam Nabi, Hebei Normal University, China
Copyright © 2022 Godoy, Pavanato and Andriolo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Ferro de Godoy, ZGFueWZnb2RveUB5YWhvby5jb20uYnI=
 Daniela Ferro de Godoy
Daniela Ferro de Godoy Heloise Pavanato4
Heloise Pavanato4 Artur Andriolo
Artur Andriolo