
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci. , 10 February 2022
Sec. Human-Wildlife Interactions
Volume 3 - 2022 | https://doi.org/10.3389/fcosc.2022.803381
This article is part of the Research Topic Evaluating Success in Wildlife Conservation and Management View all 14 articles
 Paula A. White1*
Paula A. White1* Blaire Van Valkenburgh2
Blaire Van Valkenburgh2The impact of snaring and human-wildlife conflict (HWC) on large carnivore populations is of growing concern, and yet few empirical data are available. Mortality is the metric most often used, but non-lethal injuries that impact fitness are also important threats. However, because non-lethal injuries to wild carnivores are difficult to detect, they have received little study. Using straightforward forensic examination of the skulls of trophy-hunted lions and leopards from Luangwa Valley (LV) and Greater Kafue Ecosystem (GKE), Zambia, we identified non-lethal injuries consisting of snare damage to teeth and shotgun pellets in skulls. Wire snare entanglement can cause permanent, diagnostic damage to carnivore teeth when individuals bite and pull on the wire. Shotguns are used by poachers, as well as during HWCs to drive off carnivores perceived as threats. Carnivores struck by shotgun pellets can suffer non-lethal, but potentially toxic injuries such as pellets embedded in their skulls. Because poaching and HWC are generally more prevalent near human settlements, we predicted a higher incidence of anthropogenic injuries to carnivores in Luangwa where the human population is larger and more concentrated along protected area edges than Kafue. Contrary to expectation, anthropogenic injuries were more prevalent among lions and leopards in Kafue than Luangwa. Notably, definitive evidence of snare entanglement greatly surpassed previous estimates for these regions. Overall, 37% (41 in 112) of adult male lions (29% in Luangwa, 45% in Kafue) and 22% (10 in 45) of adult male leopards (17% in Luangwa, 26% in Kafue) examined had survived being snared at some point in their lifetime. Among adult male lions, 27% (30 in 112) had old shotgun pellet injuries to their skulls. Our procedure of forensic examination of carnivore skulls and teeth, some of which can be applied to live-captured animals, allows for improved detection of cryptic, non-lethal anthropogenic injuries. Further, our methods represent a consistent and economical way to track changes in the frequency of such injuries over time and between regions, thereby providing a direct measure of the effectiveness of conservation programs that seek to reduce poaching and HWC.
Large carnivore populations are in global decline (Ripple et al., 2014). In Africa, the majority of threats are related to anthropogenic causes including habitat loss, poaching, human-wildlife conflict (HWC), and poorly-regulated trophy hunting (Dickman, 2010; Riggio et al., 2012; Lindsey et al., 2013; Bauer et al., 2016; Jacobson et al., 2016; Wolf and Ripple, 2016; Loveridge et al., 2020). While some topics such as trophy hunting of lions Panthera leo and leopards Panthera pardus, have garnered considerable attention (Loveridge et al., 2007; Packer et al., 2010; Rosenblatt et al., 2014; Creel et al., 2016), fewer studies have attempted to quantify carnivore mortality or injury attributable to other anthropogenic causes, or the effectiveness of programs designed to alleviate anthropogenic threats. Here, we focus on the potential impact on lions and leopards of two human endeavors, wire-snares used both for bush-meat poaching and set as a means of carnivore control (hereafter snaring) and the use of shotguns firing buckshot (a collective term for ammunition consisting of small, spherical metal pellets) to drive off or kill unwelcome predators (a type of HWC). We describe new, low-cost methods of detecting non-lethal injuries from snares and buckshot in free-ranging carnivore populations. Improved detection of these relatively cryptic anthropogenic insults, can, in turn, help determine the efficacy of some anti-poaching and conservation programs aimed at addressing these threats, given that effective programs should result in reduced numbers of injuries over time.
Snaring poses a two-fold threat to large carnivores: indirectly by severely reducing prey populations (Arcese et al., 1995; Fa et al., 2004; Fa, 2007; Lindsey et al., 2013; Wolf and Ripple, 2016; Creel et al., 2018) and directly by inadvertently snaring carnivores as by-catch (Hofer et al., 1993, 1996; Becker et al., 2013a). While various methods have been used to document a decline in prey numbers (Fa, 2007; Henschel et al., 2011; Lindsey et al., 2011; Wolf and Ripple, 2016; Creel et al., 2018), it is more difficult to quantify the number of carnivores killed in snares. Poachers conceal snares making them difficult to find. Victims not removed by poachers decompose quickly and remains may be consumed or scattered by scavengers (Fa, 2007; Lindsey et al., 2011). As a result, attempts to quantify large carnivore mortality due to snaring have produced numbers that are widely felt to be underestimates (MacDonald et al., 2017; Schuette et al., 2018; Loveridge et al., 2020). Moreover, although mortality is the metric most commonly used to measure human impacts, non-lethal injuries also negatively impact individual fitness. Carnivores may escape from snares but can injure themselves in the process. Anecdotal observations of carnivores burdened with snare wire or exhibiting non-lethal injuries, such as snare scars or a missing foot, are not uncommon (Hofer et al., 1993; Yamazaki and Bwalya, 1999; Midlane et al., 2014; Overton et al., 2017; Mweetwa et al., 2018), but have rarely been quantified.
Notably, a few long-term demographic studies involving known individuals have utilized telemetry, camera trap surveys, and observations of injured carnivores, or disappearance of resident individuals, to calculate the incidence of snaring and snare-related mortality (Hofer et al., 1993, 1996; Becker et al., 2013a; Loveridge et al., 2020). Spotted hyena, Crocuta crocuta, in Serengeti National Park, Tanzania, were estimated as having a 10% chance annually of encountering a snare, and a 50% chance of escaping if snared (Hofer et al., 1993, 1996). Lions in the Luangwa Valley (LV), Zambia, had an estimated snaring rate of 11.5% in adult and subadult males and females combined, including 2 of 10 males (20%) over the age of 4 years (Becker et al., 2013a). A radio-collared lion population in Hwange National Park, Zimbabwe, had documented snaring rates of 15.2% among males and females combined, including 16 of 100 (16%) males (Loveridge et al., 2020). Considering that only 5 of the 16 snared males survived, the snaring rate as estimated only from observations of injured individuals (without the assistance of telemetry to document snaring mortalities) would likely have been closer to 5% (5 of 100). Loveridge et al. (2020) also used systematic camera trap surveys to detect occurrence of snaring in the Kavango-Zambezi Transfrontier Conservation Area where their photographs recorded snare injuries in 7 of 452 (1.55%) lions and 85 of 2,037 (4.2%) spotted hyenas. However, not all snare-inflicted damages are readily detected from sightings or images of a live animal. For example, dental damage is unlikely to be observed without handling the animal. Moreover, loose snares may fall off and some injuries may heal (Loveridge et al., 2020). Therefore, estimates based on injuries observed only from a distance or from photographs probably underestimate incidence of snaring.
Like snaring, carnivore mortalities resulting from HWC are difficult to monitor effectively (Groom et al., 2014; Loveridge et al., 2017) and, thus, are likely underestimated given that retaliatory killing by means of snares, spears, firearms, or poison often goes unreported (i.e., “shoot, shovel, and shut-up,” Liberg et al., 2012; Bauer et al., 2016). Wildlife authority scouts and rural pastoralists at times utilize shotguns with buckshot ammunition to chase away (haze) potentially dangerous animals (Marks, 2005; Kavango Zambezi Transfrontier Conservation, 2016, 2020). Hazing with buckshot is intended as a harmless deterrent (Koehler et al., 1990), but serious injury can occur if projectiles strike the animal anywhere in the body, particularly in the eye or face (Clarkson, 1989; Truett, 1993). In addition to direct injury, embedded lead pellets can result in lead absorption and subsequent lead poisoning (McQuirter et al., 2004; Weiss et al., 2017). Incidence of buckshot injury is difficult to document; whether or not an animal was struck may not be known, and people may be reluctant to report that they have injured an animal already considered dangerous. Pellet wounds in the skin quickly heal over and can resemble natural scarring. However, embedded shotgun pellets persist and can be detected during post-mortem examination. Old shotgun pellets have been found in the skulls of stock-raiding jaguars Panthera onca (Rabinowitz, 1986; Hoogesteijn et al., 1991) and man-eating tigers Panthera tigris (Gurung et al., 2008). Shotguns are used also by poachers to hunt game (Siamudaala et al., 2009; Overton et al., 2017) and to deter carnivores that are attracted to racks of drying bushmeat (Brown and Marks, 2007). Unlike snare damage that can be assessed from examining the teeth of living or dead animals, at least some shotgun pellet injuries would likely go undetected without comprehensive post-mortem examination.
Here, we present a new method for determining an individual's history of snare entanglement that relies on diagnostic tooth wear that differs markedly from natural tooth wear (Van Valkenburgh and White, 2021). Carnivores often use their teeth to bite or pull on the wire when attempting to free themselves from a snare, and can damage their teeth in the process. Tooth damage is permanent, and consequently, diagnostic “snare wear” of teeth can be detected even if the snare or wound is no longer present. In addition, we document old embedded shotgun pellets in carnivore skulls that provide evidence of non-lethal shotgun injuries presumed to result from firearm poaching and/or HWC. Applying these methods, we report on and compare the frequency of non-lethal injuries due to snares and shotgun pellets in lions and leopards in LV and Greater Kafue Ecosystem (GKE), Zambia. Although our study relied on skulls from trophy-hunted individuals, the methods of detecting snare entanglement could be applied to tranquilized animals.
Poaching and HWC are generally positively correlated with proximity of human settlements to protected areas (Arcese et al., 1995; Siamudaala et al., 2009; Lindsey et al., 2011; Watson et al., 2013, 2015; Winterbach et al., 2014). The human population is larger in LV than GKE (CIESIN., 2018). Moreover, the LV's protected area network consists of four disjunct national parks (NPs) interspersed with numerous game management areas (GMAs) (Figure 1). The Luangwa River traverses the length of the protected area complex and delineates the official boundary between many of the NPs and GMAs. This design has created hard edges to protected areas with associated roads, infrastructure and human settlements located directly on the NP boundaries (Wittemyer et al., 2008; Watson et al., 2013). In contrast, the GKE contains a single large NP with a more limited road network, and the most highly populated towns are located outside of the GKE proper (Overton et al., 2017). Human encroachment into GMAs is occurring in both the LV and GKE (Watson et al., 2015). However, because of the size and distribution of the human population in LV, we hypothesized that incidences of anthropogenic injuries in carnivores relating to poaching and HWC would be more prevalent in LV than in GKE.
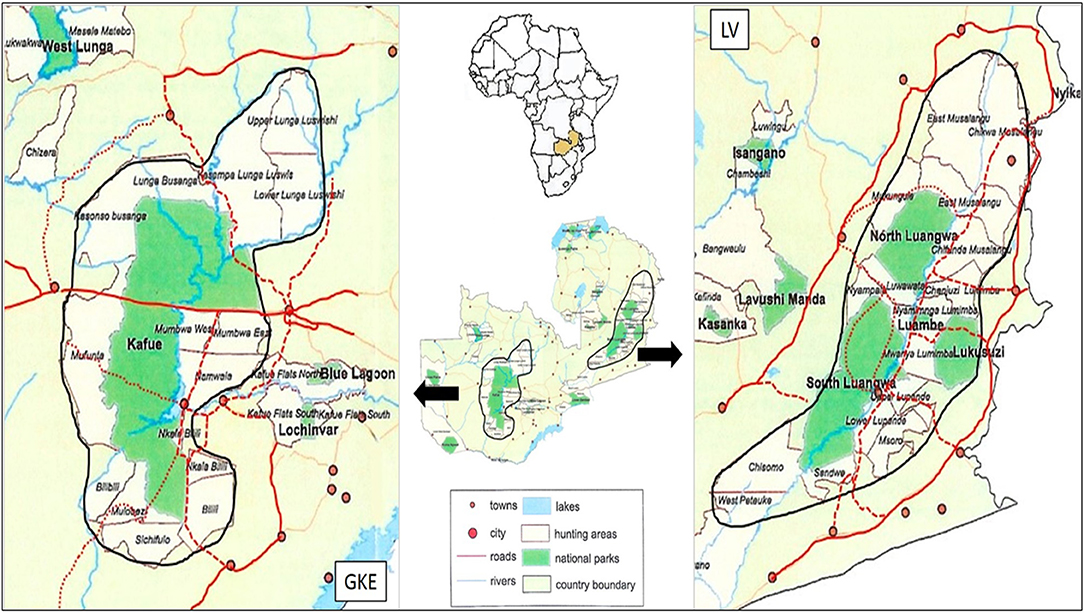
Figure 1. Location map of Zambia showing the Luangwa Valley (LV) and the Greater Kafue Ecosystem (GKE). Enlarged maps illustrate the different designs of the two regions with respect to locations of NPs and GMAs. Shown are the main paved (solid red), main unpaved (dashed red) and minor unpaved (dotted red) roads in proximity to protected area boundaries. Not shown are networks of largely seasonal, unpaved roads located in NPs and GMAs.
The study took place in two regions in Zambia; LV and the GKE (Figure 1). The LV's four NPs total 15,630 km2 and adjacent GMAs total 46,408 km2 for a combined protected area of 62,038 km2 (Astle, 1999). The GKE's single large (22,400 km2) NP and adjacent GMAs that total 44,147 km2 represent a combined protected area of 66,547 km2 (Siamudaala et al., 2009). Within each region, animals routinely cross between NPs and GMAs. Legal trophy hunting for lions and leopards occurs in most GMAs.
Between 2007 and 2012, skulls and teeth of trophy-hunted male lions and leopards were examined and photographed as part of a larger study on carnivores conducted in partnership with Department of National Parks and Wildlife (formerly Zambia Wildlife Authority, Research/Employment Permit No. #008872). For each skull, digital images were recorded of the left and right lateral sides, anterior, occipital, and dorsal views using a Nikon 35 mm D3300 digital camera with Nikkor AF70–300 mm f/4.5–6.3 lens (Nikon USA Inc., Melville, New York, USA) and stored on SD cards. Stored images were later re-examined on a computer and display monitor at which time the full extent of anthropogenic damages was assessed. Oral histories of hunts and photographs obtained from hunters, and direct examination of carcasses and hides, were used to confirm the presence of snares or snare scars. Snare scars can be differentiated from marks left by overly tight radio-collars as follows: a neck snare typically leaves a much narrower (<1 cm wide) scar encircling the entire neck where the wire has been. If the snare wire has cut the skin and created a deeper wound, then the scar may be wider, but the irregular scar tissue on the healed edges of the wound are in contrast to the broader swath (approx. >4 cm wide) of rubbing wear that can occur from the smooth band of an overly-tight radio-collar which may rub in only a few places around the neck. Details on applying these methods to live-captured, tranquilized animals are provided as Supplementary Material (Supplementary Document 1).
Our sample consisted of 112 lions and 45 leopards distributed between LV and GKE (Table 1).
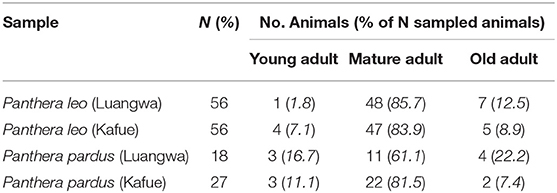
Table 1. Age distribution of sampled carnivores from Luangwa Valley and Greater Kafue Ecosystem, Zambia.
Because the probability of a carnivore having encountered a snare and/or having been involved in HWC is likely to increase with age, comparisons between distinct populations need to control for differences in age structure.
We estimated ages of sampled carnivores using established methods for each species and assigned individuals to one of three age classes. Lions were estimated as young adult <5 years, mature adult ≥5– <7 years, or old adult ≥7 years based on tooth wear along the entire tooth row and closure of the interfrontal suture (Schaller, 1972; Smuts et al., 1978; White and Belant, 2016). Leopards were estimated as young adult <5 years, mature adult ≥5– <7 years, or old adult ≥7 years based on tooth wear of the upper and lower canines (C1, c1) and premolars (P3, p3,4) after Stander (1997). We tested for difference in age structure of sampled lions and leopards from LV and GKE using chi-square performed in SPSS v.26 with significance levels set at P < 0.050.
Wire snares are known to cause damage to teeth when ensnared carnivores bite or pull at the wire as they try to free themselves (puma Puma concolor, Logan et al., 1999; red fox Vulpes vulpes, Muńoz-Igualada et al., 2010; coyote Canis latrans, Garvey and Patterson, 2014). In lions, tooth damage diagnostic of biting on wire (fencing) was first noticed among captive-bred animals during a study of correlations between tooth pulp ratios and lion age (White et al., 2016). The same pattern of tooth damage was subsequently noted among wild lions that had snare scars (Zambia Lion Project, unpublished data).
For each skull examined, we recorded incidents of the diagnostic tooth damage that results from repeated biting and pulling on a wire snare, most notably abnormal V-shaped horizontal notches on the posterior edge of the upper and lower canines (Figure 2). This pattern of tooth damage differs markedly from natural tooth wear that occurs with age in these species (lion—Smuts et al., 1978; Whitman and Packer, 2007; leopard—Stander, 1997) and that was described previously for this same sample of specimens (Van Valkenburgh and White, 2021). The severity of tooth damage likely depends on physical aspects of the snare itself, such as wire gauge, where on the body the animal was snared, how long the snare was in place, and whether the animal was able to reach the wire to bite and pull on it.
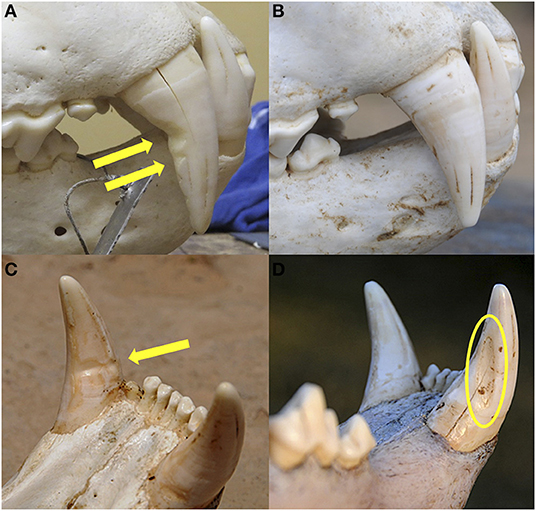
Figure 2. Lion upper canine teeth of (A) a snared lion showing characteristic horizontal notches (arrows) resulting from biting and pulling on wire, and (B) a lion of similar age with no evidence of having been snared showing normal age-related tooth wear. Lion lower canine teeth showing (C) horizontal notch characteristic of snare damage, and (D) near-vertical groove (shown in oval) that is a result of normal age-related tooth wear from occlusion with the upper canine.
Each lion and leopard skull was scored as having (1) no evidence of snare damage to their dentition, (2) some damage to dentition that was consistent with snaring but could not be positively attributed to a snare, or (3) definite tooth damage consistent with snaring coupled with physical evidence that the animal had been snared at some point in its life. Confirming physical evidence on lions included presence of a snare or an old snare scar (Figure 3). Leopards (carcasses and hides) were inspected directly for evidence of snare scars which are far less conspicuous on this species due to their cryptic coat patterns and softer fur (Zambia Lion Project, unpublished data). We compared incidence of snaring in lions and leopards among regions using chi-square tests performed in SPSS v.26 with significance levels set at P < 0.050.

Figure 3. Old scars (arrows) caused by wire snares (A) encircling a lion's neck and (B) a lion's left front foot. (C) Lion with healed stump of left hind foot lost to a snare and snare scar encircling right hind leg above the hock.
Old shotgun pellet injuries were identified by the presence of pellets visibly embedded in the skull, shallow circular indentations containing evidence of pellet fragments, metal marks, and resultant bony inflammation (Figure 4). The presence of fragmented pellets, metal marks, and inflammation are directly associated with lead pellets (as opposed to steel or copper), and make shotgun pellet injuries easily discernible from naturally-occurring injuries attributable to prey-handling and intraspecific conflict (Van Valkenburgh and White, 2021). Damage to skulls that was associated with time of death was readily determined (e.g., gunshot wound with unhealed splintered bone) and excluded from analyses.
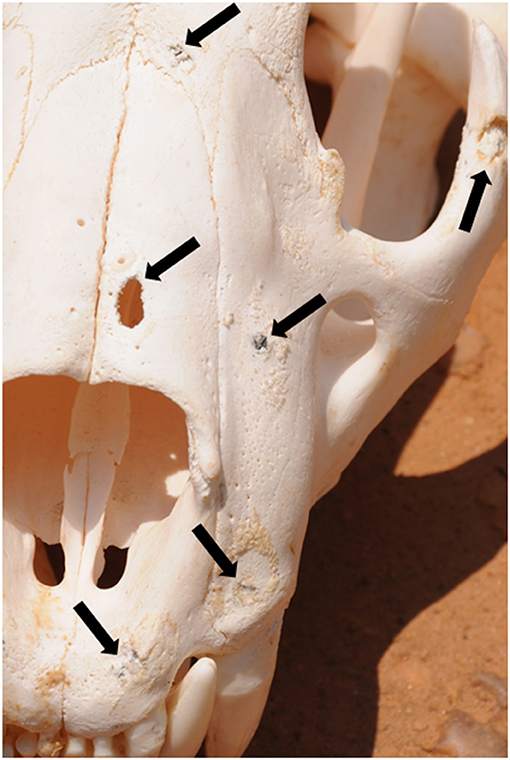
Figure 4. Lion skull with old embedded shotgun pellets and lead fragments (arrows) in premaxilla, maxilla, frontal, and jugal bones and penetrating hole through nasal bone. Note chronic bony inflammation (raised circular granular areas) associated with embedded pellets. Gray metal marks left by the passing pellet are visible at the upper edge of the penetrating hole through the nasal bone.
Poachers in Zambia are known to utilize rudimentary, locally-made muzzleloaders that fire crudely-fashioned lead projectiles, such as 1 cm lengths of rebar, or battery lead melted down into crude slugs (Brown and Marks, 2007) that can injure or kill large carnivores. However, we did not detect any instance of non-lethal cranial injury from locally-made large projectiles. Here, we deal only with shotguns that fire uniformly spherical lead pellets (buckshot).
Shotgun pellet injuries were found only in lions. We compared incidence of shotgun pellet injuries in LV vs. GKE lions using chi-square performed in SPSS v.26 with significance levels set at P < 0.050.
The number of anthropogenic injuries per skull was recorded as follows: an individual with snare damage alone was counted as having survived one incident. Similarly, an individual with shotgun pellet damage alone was counted as having survived one incident. An individual with both snare damage and shotgun pellet damage was counted as having survived two incidents because we assumed that each type of injury was sustained during an independent event. However, it is possible that snare and shotgun pellet injuries occurred concurrently during a single event (see section Discussion). Regardless of the sequence of occurrence, double injuries represent additive insults in terms of their negative impact on individual fitness.
Human population size and trend data in LV and GKE from 2000 to 2015 were obtained from the Population Estimation Service v.4 (CIESIN., 2018) based on the number of people residing in each region recorded at 5-year intervals. Human population in LV was larger than in GKE for each interval and showed a faster rate of increase over the time period (Supplementary Table 1), although encroachment into GMAs was high in both LV and GKE (Watson et al., 2015). Human population growth is especially prevalent along protected area edges where greater employment opportunities from tourism exist along with other infrastructure benefits such as clinics and schools (Wittemyer et al., 2008). Due to reserve design, the LV contains more roads and more settlements along protected area edges than does the GKE (Figure 1). Previously, Watson et al. (2013) reported a strong correlation between occurrence of snaring and areas of human development in LV especially along the shared borders of NPs and GMAs. In contrast, a study in the GKE found that the nearest human population centers (and many of the recognized poaching hotspots) were located in nearby towns or on the outer perimeters of the GMAs rather than on the NP boundary (Overton et al., 2017). Therefore, we anticipated greater incidences of anthropogenic injuries in carnivores in LV than GKE.
The majority of hunted lions (85%) and leopards (73%) were mature adults, i.e., ≥5 years old, with 11% of lions and 13% of leopards having estimated ages of ≥7 years, i.e., old adults (Table 1). There was no significant difference in age distribution between regions either for lions (X2 = 2.144, df = 2, P = 0.342) or leopards (X2 = 2.639, df = 2, P = 0.267).
Snare damage in lions was high in both regions with 37% (41 of 112) of individuals showing definitive evidence of having been snared at some point during their lifetime (Table 2). Leopards had a lower incidence of snare damage than lions, with definitive evidence of snaring found in 10 of 45 (22%) leopards (Table 2).
In GKE, 25 of 56 lions (45%) showed definitive snare damage to their dentition accompanied by existing snares (n = 3) or scars (n = 22). An additional 4 (7%) had tooth damage that may have been snare-related although this could not be confirmed, while 27 (48%) GKE lions did not show any tooth damage or scars indicative of snaring (Table 2). In LV, 16 of 56 lions (29%) had definitive snare damage to their teeth accompanied by existing snares (n = 2) or scars (n = 14). Four additional lions (7%) had tooth damage that may have been snare-related, although this could not be confirmed. In LV, 36 (64%) lions had no tooth damage or scars indicative of snaring (Table 2). Considering only the cases in which snare (or no damage) was definitive, there was a trend for a higher incidence of snared lions in GKE compared with LV, and the difference approached significance (X2 = 3.261, df = 2, P = 0.071).
Among leopards, there was a higher incidence of snared leopards in GKE (26%) than in LV (17%), although the difference was not significant (X2 = 0.556, df = 2, P = 0.456) (Table 2). Cases of possible, but unconfirmed, snare damage in leopards were the same in GKE (11%) and LV (11%). Overall, 67% of leopards showed no evidence of having been snared.
Evidence of non-lethal shotgun pellet injuries was found only among lions. Of the 112 lions sampled, 30 individuals (27%) had old shotgun pellets embedded in their skulls. Similar to the regional patterns of snare damage, GKE lions had a higher incidence of past shotgun pellet injuries than LV lions, but the difference was not significant. Seventeen of 56 (30%) GKE lions incurred shotgun pellet damage whereas the same was true for only 13 of 56 (23%) LV lions (X2 = 0.728, df = 2, P = 0.300) (Table 2).
We found individual lions with both snare and shotgun pellet injuries in LV and in GKE. Again, the numbers were greater in GKE where nine of 25 lions (36%) with definite snare injuries also carried evidence of old shotgun pellet wounds. Thus, nine of the total 56 (16%) GKE lions examined had incurred non-lethal injuries from both snares and shotgun pellets during their lifetime. By comparison, four of 16 (25%) snared lions in the LV also had evidence of old shotgun pellet wounds. Thus, four of the total 56 (7%) LV lions had incurred non-lethal injuries from both snares and shotgun pellets during their lifetime (Table 2). When considering all incidents of anthropogenic damage (snares and shotgun pellets) combined, GKE lions sustained significantly more anthropogenic injuries (n = 42 incidents) than did LV lions (n = 29 incidents) (X2 = 6.502, df = 2, P = 0.011) (Table 2).
Among lions and leopards, age was a significant factor in the likelihood of an animal having snare damage. More than one-third (35%) of all lions in the mature age class and two-thirds (67%) of all lions in the old age class showed definitive evidence of having been snared during their lifetime. In contrast, none of the five young lions showed evidence of snaring (X2 = 8.499, df = 2, P = 0.014) (Figure 5A) (Supplementary Table 2). In both LV and GKE, there was no significant increase in the percentage of lions with shotgun injury with age (Figure 5B) (Supplementary Table 3). Unlike snare damage that was found only in mature and older age classes, embedded shotgun pellets were found in the skull of one of the five young lions sampled (Figure 5B) (Supplementary Table 3).
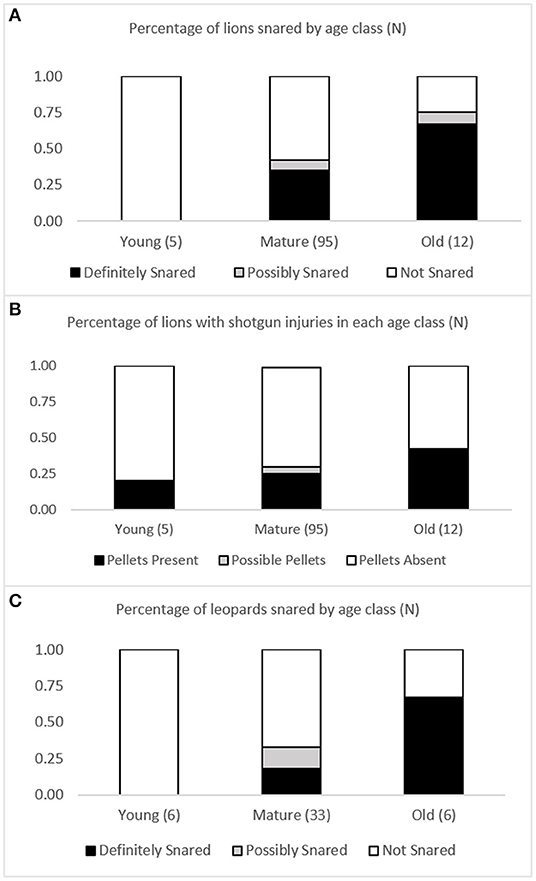
Figure 5. Incidence of non-lethal anthropogenic injuries in each age class. (A) Percentage of lions snared in each age class, (B) percentage of lions with shotgun pellets in each age class, (C) percentage of leopards snared in each age class.
Among leopards, nearly one-fifth (18%) of all leopards in the mature age class, and two-thirds (67%) of old leopards, had definitive evidence of having been snared during their lifetime. In contrast, none of the six young leopards showed evidence of snaring (X2 = 7.746, df = 2, P = 0.021) (Figure 5C) (Supplementary Table 4).
Large African carnivores must contend with escalating challenges to their survival in the face of increasing human populations and associated pressures that include poaching and direct conflicts with humans. A comprehensive assessment of the scale and spatio-temporal patterns of anthropogenic-related injuries and mortality is key to combating these threats. However, the incidence of events is almost certainly under-reported because not all snared carnivores will be discovered, and because some injuries are harder to detect than others.
Our study utilized a previously overlooked source of data—the skulls of trophy-hunted individuals—to better quantify incidence of poaching and HWC. Our samples represent animals hunted throughout the GMAs in both study areas, and there was no difference in age structure between lions and leopards sampled in LV vs. GKE. Therefore, we assume that our sampled animals are representative of mature or older adult males in the general population. Using simple forensic examination, we identified tooth damage diagnostic of snaring and the presence of embedded shotgun pellets in the skulls of a large sample of carnivores that lived in Zambia between ca. 2000–2012. Applying this novel yet straightforward methodology, we detected a far greater incidence of anthropogenic injury to carnivores than has been reported previously.
For example, at least 37% of adult male lions sampled had been snared at some point in their lifetimes (29% in LV; 45% in GKE), a frequency that exceeds that reported previously by Becker et al. (2013a) for lions in a portion of the LV. Becker et al. (2013a) found that 20% (two of 10) of male lions >4 years old in the LV had been caught in wire snares in an 18-month period (June 2009 to December 2010) that overlaps with our sampled time interval. Becker et al.'s 2013a study was focused in a core area of the LV and detection of snare entanglement was augmented by residents' and tourists' reports to conservation programs involved in animal rescue, i.e., live-capture and snare removal (South Luangwa Conservation Society; Zambia Carnivore Programme). Because the incidence of snaring is positively correlated with rural settlements especially along the borders of NPs and GMAs (Watson et al., 2013, 2015), it might be expected that Becker et al. (2013a) would have found higher rates of snared lions in the core area of the LV compared with our sample of animals from throughout the GMAs.
Contrary to that expectation, we detected rates of snare entanglement in LV lions that were higher than reported previously, a difference that we attribute primarily to our method of post-mortem dental assessment. Adult male lions roam over large areas especially in hunting blocks (GMAs) (Loveridge et al., 2010a), which may increase their likelihood of encountering snares. Becker et al. (2013a) found that a higher proportion of snared lions in South Luangwa National Park (located within the LV) were males compared to females, and believed the males were encountering snares in the neighboring GMAs where snaring was concentrated (South Luangwa Conservation Society., 2010, 2011; Becker et al., 2013a). Similarly, Loveridge et al. (2020) found incidence of snared carnivores to be higher in hunting areas than in NPs. This is consistent with our finding of high rates of snared male lions sampled from GMAs. However, at least some of our sampled lions undoubtedly utilized a combination of NP and GMA habitats (Becker et al., 2013a,b; Midlane et al., 2015; Creel et al., 2016).
Of course, there are other contributing factors that may have influenced the difference in reported rates. Our study involved a larger number of lions (112 adult male lions of which 56 were from the LV). The sampling periods also varied. Our sampling began in 2007 and ended in 2012, and the snared lions in our study were mostly mature (≥5–7 year) or older males. Thus, our sampled animals were born and lived between ca. 2000–2012. It is possible that snaring rates in the LV declined between 2000 and 2012, and that the lower numbers of snared lions reported by Becker et al. (2013a) toward the end of that period (June 2009–Dec 2010) reflect that decline. However, Becker et al. (2013a) evaluated snaring trends in a 13,775 km2 area of the southern LV from December 2005 to November 2010 and found no substantive evidence that snaring was on the decline. Thus, we maintain that the higher snaring rates reported in our study are a better estimate of anthropogenic impacts because our method was more effective at detecting an individual's history of snaring especially when injuries, such as unusual tooth wear, are not readily apparent except by close examination.
Our findings on snaring rates in GKE lions were even more alarming; 45% of the GKE lions sampled had been snared at some point in their lifetime. To our knowledge, ours is the first study to quantify snare injuries of lions originating from throughout the GKE although others (Midlane et al., 2014; Overton et al., 2017; Schuette et al., 2018; Vinks et al., 2021a) have reported sightings of Kafue lions with non-lethal snare injuries. Schuette et al. (2018) recorded 79 incidences of snare-related injuries to large carnivores in the northern Kafue NP (NKNP) “over the past 5 years” (presumably 2013–2018), but did not specify number of incidents per species, nor whether recorded incidents included repeated sightings of the same individuals. Subsequently, Vinks et al. (2021a) reported that 80% (n = 5) of the snare injuries that they encountered in NKNP lions from 2013 to 2018 were young adult (4–5.99 year old) males. Again, while it is possible that snaring in GKE declined after 2012, more recent investigations suggest that poaching increased in the region (Overton et al., 2017; Mkanda et al., 2018).
In addition to snare damage, we found lions both from LV and GKE with old shotgun pellets embedded in their skulls. As was true of snaring, the incidence of non-lethal shotgun pellet injury was more prevalent in GKE lions (30%) than in LV lions (23%), which is consistent with the greater use of shotguns by poachers in GKE (Overton et al., 2017). Programs in LV (e.g., Lewis, 2007; South Luangwa Conservation Society., 2010) that use incentives and seizures of illegal weapons to reduce the number of firearms and snares may have influenced the lower rates of snaring and shotgun pellet injuries found in LV lions. Anti-poaching and incentive programs operate in GKE as well, although efforts there were less intensive or widespread than in LV during our study period (Siamudaala et al., 2009; Overton et al., 2017). Poachers in LV were apparently aware of the greater risk of detection when discharging a firearm in proximity to anti-poaching bases and tourist camps (South Luangwa Conservation Society., 2012; Watson et al., 2013). In contrast, hunting with firearms is prevalent in the vast, less-patrolled landscape of the GKE (Overton et al., 2017). The increased availability and use of affordable LED flashlights allows poachers to shoot at night (Bowler et al., 2020) and may have increased the incidence of shotgun poaching and buckshot injury in GKE.
The motivating factors behind shotgun pellet injuries to lions are unclear. Poachers may have been deliberately hunting lions to obtain body parts (Overton et al., 2017; Williams et al., 2017; Everatt et al., 2019a), chasing away lions attracted to bushmeat (Brown and Marks, 2007), or shooting at lions opportunistically while hunting other prey (Overton et al., 2017). Although we found no evidence of shotgun pellet injuries among the leopard skulls that we examined, Overton et al. (2017) reported firearm poaching of leopards and cheetahs in GKE. Other possible sources of non-lethal shotgun pellet injuries to lions are rural pastoralists and the wildlife authority, both of whom sometimes fire buckshot to chase away animals perceived as threats. Lacking additional information, such as a known history of hazing of an individual lion, it was not possible to determine which source(s) were responsible for the shotgun pellet injuries documented in our sample.
Some lions were found to have suffered both snare and shotgun pellet injuries. Occurrence of these double injuries was more prevalent among GKE lions (16%) than LV lions (7%). The sequence of double injuries is unknown, and multiple scenarios are plausible; snare damage, especially debilitating injury such as a missing foot, could negatively impact an individual's hunting ability, thereby leading to increased likelihood of HWC and subsequent shotgun pellet injury. Three of the lions in our sample were each missing a foot, and two of these also had shotgun pellets embedded in their skulls. Conversely shotgun pellet injuries, especially to the eyes or facial muscles, could lead individuals to subsequently be attracted to easy prey such as livestock or animals caught in snares. Prior to our study period, three lions in LV shot for depredating livestock were found to have old snare wounds (Loveridge et al., 2010b). Five of 10 jaguars in Belize killed for preying on livestock had old shotgun wounds or pellets in the skull compared with 17 jaguars not associated with livestock predation whose post-mortem examination showed no evidence of previous injuries. At least three of the non-livestock predating jaguars were radio-collared animals known to travel without incident near pastures and villages containing livestock and dogs (Rabinowitz, 1986); thus, it appears that the livestock predating and non-livestock predating jaguars were similarly susceptible to armed ranchers. Ten of 19 livestock-killing jaguars in Venezuela had old gunshot wounds, including a cat blinded in one eye by buckshot in the face (Hoogesteijn et al., 1991). Similarly, 10 of 18 human-killing tigers in Nepal had physical impairments, including some with old gunshot wounds (Gurung et al., 2008). In Zambia, poachers shoot at lions that are attracted to bushmeat and set snares for predators around temporary camps in the bush (Brown and Marks, 2007); thus, some lions may have incurred double injuries simultaneously.
Compared with our findings for lions, we found fewer but still high incidences of snaring among leopards in both regions, with a trend toward higher incidence in GKE compared with LV. Overall, 22% of Zambian leopards showed definitive evidence of having been snared (17% in LV; 26% in GKE). There are several potential explanations for the relatively lower frequency of snare injury found in leopards. Although leopards occur in the same regions and habitats as do other large carnivores, e.g., lion, spotted hyena, and wild dog Lycaon pictus for which snaring by-catch is well-known (Becker et al., 2013a; Loveridge et al., 2020; Vinks et al., 2021b), leopards may be less likely to encounter a snare due to behavioral differences. However, leopards feed on ungulate species targeted by wire-snare poachers (Henschel et al., 2011; Creel et al., 2018; Strampelli et al., 2018), and may scavenge on animals killed in snares (Strampelli et al., 2018). Snares are set in lines or clusters (Noss, 1998; Becker et al., 2013a), and predators that investigate struggling prey or carrion are susceptible to themselves becoming snared (Knopff et al., 2010; Everatt et al., 2019b). Therefore, despite the rarity of reports, leopards are presumably vulnerable to snaring like other large African carnivores.
Leopards may be less likely to survive an encounter with a snare (Swanepoel et al., 2015; Loveridge et al., 2020). In India, only three of 113 (2.7%) snared leopards escaped from the snare wire on their own; 59 were dead in the snare, while the remaining 51 were detected and rescued (Gubbi et al., 2021). In LV, two adult leopards found dead had snare wires tightened around their torsos; the resultant wounds had penetrated their abdominal cavities and exposed their intestines (Zambia Lion Project, unpublished data). Snaring mortalities of leopards have been documented elsewhere in Africa [Equatorial Guinea (Fa and Garcí Yuste, 2001), Zimbabwe (Lindsey et al., 2011)].
Another possibility is that our ability to detect snare injuries varies among species. In particular, a leopard's soft fur and cryptic coloration may conceal smaller wounds and scars. While camera traps in Kavango-Zambezi Transfrontier Conservation Area detected multiple cases of snared lions and spotted hyenas, only a single leopard out of 386 identified individuals was noted as having a possible snare scar (Loveridge et al., 2020). Leopards with non-lethal snare injuries were encountered in NKNP by Schuette et al. (2018), and yet a subsequent (2013–2019) camera trap study to investigate leopard mortality in NKNP (Vinks et al., 2021b) detected no snare injuries among 63 identified individuals. Similarly, a camera trap study of leopards in LV did not detect any snare injuries among 43 identified individuals and concluded that snared leopards in LV were uncommon relative to snared lions and wild dogs (Rosenblatt et al., 2016). By comparison, our method found that 26% of male leopards in GKE and 17% of male leopards in LV had survived a snare encounter during their lifetime.
Given the prevalence of wire-snare poaching throughout Africa (Lindsey et al., 2013) and leopards' apparent vulnerability, the scarcity of reports of snared leopards is unlikely to reflect actual by-catch. It is more likely that individuals either die or that snare injuries on leopards are difficult to detect, especially when relying on observations of live animals at a distance or in camera trap photographs. Our findings of diagnostic snare damage to teeth in 10 of 45 (22%) Zambia leopards examined indicates that incidence of snaring is much more common in this species than is currently known, and adds to a growing concern regarding the impact of snaring by-catch on large carnivore populations (Lewis and Phiri, 1998; Becker et al., 2013a; Strampelli et al., 2018; Loveridge et al., 2020).
In both lions and leopards, age was significantly correlated with a history of snare entanglement, with the highest rates of injuries seen among old (≥7 years) cats. Each age class faces variable risks (e.g., dispersal, establishing territory, losing dominance status, and becoming nomadic) over the course of a lifetime (Patterson et al., 2004; Elliot et al., 2014; Loveridge et al., 2016), and thus, the chance of being snared or engaging in HWC is cumulative. Older males are likely to have traversed larger areas, and may ultimately wind up occupying less favorable habitats (Loveridge et al., 2010), closer to protected area boundaries and human settlements where they are more likely to encounter snares (Watson et al., 2013).
Overall, our data suggest that between 2000 and 2012 anthropogenic threats to lions and leopards were greater in GKE than in LV despite a larger human population, including along protected area edges, in LV. Human population size may not be the most accurate predictor of poaching pressure. For example, data on livestock numbers or proportion of an area used for livestock farming may be a more accurate measure of potential HWC risk. However, the majority (82%) of rural people in Zambia keep at least some types of small livestock, such as chickens (Lubungu and Mofya-Mukuka, 2012). The finer-scale pattern of pastoral land use, which is complex and subject to the influences of weather, politics, economics, and more (Watson et al., 2015), is outside the scope of this study. In addition to the differences in anti-poaching programs between GKE and LV, specific poaching practices likely vary between regions. Poacher type (subsistence vs. commercial) dictates amount of time spent hunting and type of weapon used (Solly, 2007). Fewer people spending more time devoted to poaching could have a greater impact on carnivores (Fa and Garcí Yuste, 2001; Fa et al., 2004), especially if they are using guns as well as snares. The number of snares set per trap-line, array of snare sets, and gauge of snare wire also may influence rates of injuries to carnivores (Noss, 1998; Becker et al., 2013a; Creel et al., 2018).
Beyond the regional differences, the overall rates of snare and shotgun pellet injuries detected were sobering. Our data documented tooth damage that was definitively attributable to snaring in 37% of Zambia's adult male lions and 22% of adult male leopards from the LV and GKE regions combined. This equates to nearly two out of five male lions and one out of four male leopards becoming ensnared during their lifetime, and we know this is an underestimate as we report only on individuals with detectable tooth damage and who survived being snared; we have no record of animals that died undetected in snares. Further, not all individuals captured in snares bite on the wire, and thus our estimated rates of non-lethal snare injuries derived from dental damage are minima. The LV and GKE are two of the most stringently protected wildlife areas in Zambia, recognized as priorities for conservation and as important strongholds for large carnivores (IUCN., 2006; Riggio et al., 2012; Midlane et al., 2014; Kavango Zambezi Transfrontier Conservation, 2018; Loveridge et al., 2020). Nevertheless, snaring and HWC continue to pose major conservation challenges in both regions (Overton et al., 2017; Mkanda et al., 2018; Conservation South Luangwa., 2020).
By generating more accurate estimates of snaring rates, our method can help reveal “cryptic” poaching (Liberg et al., 2012). Likewise, while HWC is recognized as a primary threat to carnivores, empirical data on the number of problem animals killed and injured are scant (Loveridge et al., 2010b; Bauer et al., 2016; Jacobson et al., 2016; IUCN., 2018). Data gleaned from skulls (trophy-sourced, animals killed in HWC, natural mortalities) can be used to identify regions where snaring and shotgun poaching pose a significant threat, and where HWC protocols that involve hazing with buckshot might benefit from greater scrutiny and review. Our technique of detecting snare damage to teeth and body (snare scars) is equally applicable to examination of tranquilized large carnivores during live-capture for research purposes (Supplementary Document 1), and thus, can inform on rates of snare injuries in populations without trophy hunting.
In Van Valkenburgh and White (2021), we reported on naturally-occurring tooth wear, tooth breakage, and cranial injuries in trophy-hunted carnivores. We proposed that standardized photographs of the skulls and teeth of hunted carnivores be required to be collected and archived for purposes of scientific investigations and population-level comparisons of carnivore health. Our ability to identify snare and shotgun pellet damage from skulls adds considerable value to this proposal, and we reiterate our recommendation for collection and archiving of standardized photographs. Obtaining as much information as possible from trophy-hunted (as well as live-captured) individuals represents an important contribution to long-term population conservation of carnivores by identifying natural changes, such as natural tooth wear and breakage that relate to diet, and human-caused injuries that may otherwise go undetected. Simple forensic examination of carnivore teeth and skulls is a low-cost means of monitoring changes in frequency of these threats and can further serve to quantify the effectiveness of select conservation efforts.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the animal study because this study did not involve the handling, sampling, or use of any live specimens. All material was examined and photographic data collected by one of us (PW) in Zambia as part of a larger study on age estimation of carnivores in partnership with Department of National Parks and Wildlife (formerly Zambia Wildlife Authority, Research/Employment Permit No. #008872).
PW and BV: conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored and reviewed drafts of the paper, and approved the final draft. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Department of Parks and Wildlife (formerly Zambia Wildlife Authority) for permission to conduct field research. We are grateful to the professional hunters, taxidermists, and clients who provided logistical support, hunt information, and access to trophies. We also thank two reviewers whose comments greatly improved the paper.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.803381/full#supplementary-material
Arcese, P., Hando, J., and Campbell, K. (1995). “Historical and present-day anti-poaching efforts in Serengeti,” in Serengeti II: Dynamics, Management, and Conservation of an Ecosystem, eds A. R. E. Sinclair and P. Arcese (Chicago, IL: University of Chicago Press), 506–533.
Astle, W. L (1999). A History of Wildlife Conservation and Management in the mid-Luangwa Valley, Zambia. Bristol: British Empire and Commonwealth Museum.
Bauer, H., Packer, C., Funston, P. F., Henschel, P., and Nowell, K. (2016). Panthera leo. The IUCN Red List of Threatened Species 2016: e.T15951A115130419.
Becker, M., McRobb, R., Watson, F., Droge, E., Kanyembo, B., Murdoch, J., et al. (2013a). Evaluating wire-snare poaching trends and the impacts of by-catch on elephants and large carnivores. Biol. Conserv. 158, 26–26. doi: 10.1016/j.biocon.2012.08.017
Becker, M. S., Watson, F. G. R., Droge, E., Leigh, K., Carlson, R. S., and Carlson, A. A. (2013b). Estimating past and future male loss in three Zambian lion populations. J. Wildl. Manage. 77, 128–142. doi: 10.1002/jwmg.446
Bowler, M., Beirne, C., Tobler, M. W., Anderson, M., DiPaola, A., Fa, J. E., et al. (2020). LED flashlight technology facilitates wild meat extraction across the tropics. Front. Ecol. Environ. 18, 2242 doi: 10.1002/fee.2242
Brown, T., and Marks, S. A. (2007). “Livelihoods, hunting and the game meat trade in Northern Zambia,” in Bushmeat and Livelihoods: Wildlife Management and Poverty Reduction, Conservation Science and Practice Series No.2. eds G. Davies, and D. Brown (Oxford: Blackwell Publishing Ltd.), 92–105. doi: 10.1002/9780470692592.ch6
CIESIN. (2018). Center for International Earth Science Information Network. Available online at: http://www.ciesin.org (accessed December 31, 2020).
Clarkson, P. L (1989). “The twelve gauge shotgun: a bear deterrent and protection weapon,” in Bear-People-Conflicts—Proceedings of a Symposium on Management Strategies (Yellowknife: Department of Renewable Resources, Government of the Northwest Territories), 55–60.
Creel, S., Matandiko, W., Schuette, P., Rosenblatt, E., Sanguinetti, C., Banda, K., et al. (2018). Changes in African large carnivore diets over the past half-century reveal the loss of large prey. J. Appl. Ecol. 55, 2908–2916. doi: 10.1111/1365-2664.13227
Creel, S., M'Soka, J., Droege, E., Rosenblatt, E., Becker, M., Matandiko, W., et al. (2016). Assessing the sustainability of African lion trophy hunting, with recommendations for policy. Ecol. Appl. 26, 1–43. doi: 10.1002/eap.1377
Dickman, A. J (2010). Complexities of conflict: the importance of considering social factors for effectively resolving human-wildlife conflict. Anim. Conserv. 13, 458–466. doi: 10.1111/j.1469-1795.2010.00368.x
Elliot, N. B., Cushman, S. A., MacDonald, D. W., and Loveridge, A. J. (2014). The devil is in the dispersers: predictions of landscape connectivity change with demography. J. Appl. Ecol. doi: 10.1111/1365-2664.12282
Everatt, K. T., Kokes, R., and Lopez Pereira, C. (2019a). Evidence of a further emerging threat to lion conservation; targeted poaching for body parts. Biodiv. Conserv. 28, 4099–4114. doi: 10.1007/s10531-019-01866-w
Everatt, K. T., Moore, J. F., and Kerley, G. I. H. (2019b). Africa's apex predator, the lion, is limited by interference and exploitative competition with humans. Glob. Ecol. Conserv. 20, e00758. doi: 10.1016/j.gecco.2019.e00758
Fa, J. E (2007). “Bushmeat markets – white elephants or red herrings?,” in Bushmeat and Livelihoods: Wildlife Management and Poverty Reduction, Conservation Science and Practice Series No.2, eds G. Davies and D. Brown (Oxford: Blackwell Publishing Ltd.), 47–60. doi: 10.1002/9780470692592.ch3
Fa, J. E., and Garcí Yuste, J. E. (2001). Commercial bushmeat hunting in the Monte Mitra forest, Equatorial Guinea: extent and impact. Anim. Biodiv. Conserv. 24, 31–52.
Fa, J. E., Ryan, S. F., and Bell, D. J. (2004). Hunting vulnerability, ecological characteristics and harvest rates of bushmeat species in afrotropical forests. Biol. Conserv. 121, 167–176. doi: 10.1016/j.biocon.2004.04.016
Garvey, M. E., and Patterson, B. R. (2014). Evaluation of cable restraints to live-capture coyotes (Canis latrans) in Southern Ontario, Canada. Can. Wildl. Biol. Manage. 3, 22–29.
Groom, R. J., Funston, P. J., and Mandisodza, R. (2014). Surveys of lions Panthera leo in protected areas in Zimbabwe yield disturbing results: what is driving the population collapse? Oryx 48, 385–393. doi: 10.1017/S0030605312001457
Gubbi, S., Kolekar, A., and Kumara, V. (2021). Quantifying wire snares as a threat to leopards in Karnataka, India. Trop. Conserv. Sci. 14, 1–8. doi: 10.1177/19400829211023264
Gurung, B., David Smith, J. L., McDougal, C., Karki, J. B., and Barlow, A. (2008). Factors associated with human-killing tigers in Chitwan National Park, Nepal. Biol. Conserv. 141, 3069–3078. doi: 10.1016/j.biocon.2008.09.013
Henschel, P., Hunter, L. T. B., Coad, L., Abernethy, K. A., and Mühlenberg, M. (2011). Leopard prey choice in the Congo Basin rainforest suggests exploitative competition with human bushmeat hunters. J. Zool. 285, 11–20. doi: 10.1111/j.1469-7998.2011.00826.x
Hofer, H., Campbell, K. L. I., East, M. L., and Huish, S. A. (1996). “The impact of game meat hunting on target and non-target species in the Serengeti,” in The Exploitation of Mammal Populations, eds V. J. Taylor and N. Dunstone, (London: Chapman and Hall), 117–146. doi: 10.1007/978-94-009-1525-1_9
Hofer, H., East, M. L., and Campbell, K. L. I. (1993). Snares, commuting hyaenas and migratory herbivores: humans as predators in the Serengeti. Symp. Zool. Soc. Lond. 65, 347–366.
Hoogesteijn, R., Hoogesteijn, A., and Mondolfi, E. (1991). “Jaguar predation vs. conservation: cattle mortality by felines on three ranches in the Venezuelan Llanos,” in Symposium: Mammals as Predators, Zool. Soc. Lond. Regent's Park (London).
IUCN. (2006). Conservation strategy for the lion in Eastern and Southern Africa. IUCN SSC Cat Specialist Group. Available online at: https://www.environment.gov.za/sites/default/files/docs/pantheraleo_conservationstrategy_regionalafrica.pdf (accessed January 2, 2008).
IUCN. (2018). Guidelines for the Conservation of Lions in Africa. IUCN SSC Cat Specialist Group. Version 1.0. Muri; Bern: IUCN.
Jacobson, A. P., Gerngross, P., Lemeris, J.r., Schoonover, R. F., Anco, C., et al. (2016). Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ 4:e1974. doi: 10.7717/peerj.1974
Kavango Zambezi Transfrontier Conservation Area (2016). Human Wildlife Conflict Mitigation Measures. Available online at: https://www.kavangozambezi.org/en/publications-2019 (accessed October 8, 2021).
Kavango Zambezi Transfrontier Conservation, Area (2018). The Kavango-Zambezi Transfrontier Conservation Area Carnivore Strategy 2018–2022, ed K.C.C. Coalition. Available online at: http://www.kavangozambezi.org (accessed October 8, 2021).
Kavango Zambezi Transfrontier Conservation, Area (2020). A Manual for Reducing and Mitigating Human-Large Predator Conflict. Available online at: https://www.kavangozambezi.org/en/publications-2020 (accessed October 8, 2021).
Knopff, K. H., Knopff, A. A., and Boyce, M. S. (2010). Scavenging makes cougars susceptible to snaring at wolf bait stations. J. Wildl. Manage. 74, 644–653. doi: 10.2193/2009-252
Koehler, A. E., Marsh, R. E., and Salmon, T. P. (1990). “Frightening methods and devices/stimuli to prevent mammal damage – a review,” in Proceedings of the Fourteenth Vertebrate Pest Conference 1990. 50. Available online at: http://digitalcommons.unl.edu/vpc14/50 (accessed October 13, 2020).
Lewis, D. M (2007). “Can wildlife and agriculture coexist outside protected areas in Africa? A hopeful model and a case study from Zambia,” in Bushmeat and Livelihoods: Wildlife Management and Poverty Reduction, Conservation Science and Practice Series No.2, eds G. Davies and D. Brown (Oxford: Blackwell Publishing Ltd.), 177–196. doi: 10.1002/9780470692592.ch11
Lewis, D. M., and Phiri, A. (1998). Wildlife snaring – an indicator of community response to a community-based conservation project. Oryx 32, 111–121. doi: 10.1046/j.1365-3008.1998.d01-21.x
Liberg, O., Chapron, G., Wabakken, P., Christian Pedersen, H., Thompson Hobbs, N., and Sand, H. (2012). Shoot, shovel and shut up: cryptic poaching slows restoration of a large carnivore in Europe. Proc. Royal Soc. B 279, 910–915. doi: 10.1098/rspb.2011.1275
Lindsey, P. A., Balme, G., Becker, M., Begg, C., Bento, C., Bocchino, C., et al. (2013). The bushmeat trade in African savannas: impacts, drivers, and possible solutions. Biol. Conserv. 160, 80–96. doi: 10.1016/j.biocon.2012.12.020
Lindsey, P. A., Romañach, S. S., Tambling, C. J., Chartier, K., and Groom, R. (2011). Ecological and financial impacts of illegal bushmeat trade in Zimbabwe. Oryx 45, 96–111. doi: 10.1017/S0030605310000153
Logan, K. A., Sweanor, L. L., Smith, J. F., and Hornocker, M. G. (1999). Capturing pumas with foot-hold snares. Wildl. Soc. Bull. 27, 201–208.
Loveridge, A. J., Hemson, G., Davidson, Z., and MacDonald, D. W. (2010a). “African lions on the edge: reserve boundaries as ‘attractive sinks',” in Biology and Conservation of Wild Felids, eds D. W. MacDonald and A. J. Loveridge (Oxford: Oxford University Press), 283–304.
Loveridge, A. J., Kuiper, T., Parry, R. H., Sibanda, L., Hunt, J. H., Stapelkamp, B., et al. (2017). Bells, bomas and beefsteak: complex patterns of human-predator conflict at the wildlife-agropastoral interface in Zimbabwe. PeerJ 5, e2898. doi: 10.7717/peerj.2898
Loveridge, A. J., Searle, A. W., Murindagomo, F., and MacDonald, D. W. (2007). The impact of sport-hunting on the population dynamics of an African lion population in a protected area. Biol. Conserv. 134, 548–558. doi: 10.1016/j.biocon.2006.09.010
Loveridge, A. J., Sousa, L. L., Seymour-Smith, J., Hunt, J., Coals, P., O'Donnell, H., et al. (2020). Evaluating the spatial intensity and demographic impacts of wire-snare bush-meat poaching on large carnivores. Biol. Conserv. 244, 108504. doi: 10.1016/j.biocon.2020.108504
Loveridge, A. J., Valeix, M., Elliot, N. B., and MacDonald, D. W. (2016). The landscape of anthropogenic mortality: how African lions respond to spatial variation in risk. J. Appl. Ecol. 54, 815–825. doi: 10.1111/1365-2664.12794
Loveridge, A. J., Wang, S. W., Frank, L. G., and Seidensticker, J. (2010b). “People and wild felids: conservation of cats and management of conflicts,” in eds Biology and Conservation of Wild Felids, eds D. W. MacDonald and A. J. Loveridge (Oxford: Oxford University Press), 161–195.
Lubungu, M., and Mofya-Mukuka, R. (2012). The Status of the Smallholder Livestock Sector in Zambia. IAPRI Technical Report No. 1, Parliamentary Committee on Agriculture, Lusaka.
MacDonald, D. W., Loveridge, A. J., Dickman, A., Johnson, P. J., Jacobsen, K. S., and du Preez, B. (2017). Lions, trophy hunting and beyond: knowledge gaps and why they matter. Mam. Rev. 47, 247–253. doi: 10.1111/mam.12096
Marks, S. A (2005). Large Mammals and a Brave People: Subsistence Hunters in Zambia. New Brunswick; London: Transaction Publishers.
McQuirter, J. L., Rothenberg, S. J., Dinkins, G. A., Kondrashov, V., Manalo, M., and Todd, A. C. (2004). Change in blood lead concentration up to 1 year after a gunshot wound with a retained bullet. Amer. J. Epidemiol. 159, 683–692. doi: 10.1093/aje/kwh074
Midlane, N., O'Riain, M. J., Balme, G. A., and Hunter, L. T. B. (2015). To track or to call: comparing methods for estimating population abundance of African lions Panthera leo in Kafue National Park. Biodiv. Conserv. 24, 1311–1327. doi: 10.1007/s10531-015-0858-z
Midlane, N., O'Riain, M. J., Balme, G. A., Robinson, H. S., and Hunter, L. T. B. (2014). On tracks: a spoor-based occupancy survey of lion Panthera leo distribution in Kafue National Park, Zambia. Biol. Conserv. 172, 101–108. doi: 10.1016/j.biocon.2014.02.006
Mkanda, F. X., Munthali, S., Milanzi, J., Chifunte, C., Kaumba, C., Muswema, N., et al. (2018). ‘The Giant Sleeps Again?' – Resource, Protection and Tourism of Kafue National Park, Zambia. Parks 24.1. May 2018. doi: 10.2305/IUCN.CH.2018.PARKS-24-1FXM.en
Muńoz-Igualada, J., Shivik, J. A., Dominguez, F. G., Gonzalez, L. M., Moreno, A. A., Olalla, M. F., et al. (2010). Traditional and new cable restraint systems to capture fox in central Spain. J. Wildl. Manage. 74, 181–187. doi: 10.2193/2008-603
Mweetwa, T., Christianson, D., Becker, M., Creel, S., Rosenblatt, E., Merkle, J., et al. (2018). Quantifying lion (Panthera leo) demographic response following a three-year moratorium on trophy hunting. PLoS ONE 13, e0197030. doi: 10.1371/journal.pone.0197030
Noss, A. J (1998). The impacts of cable snare hunting on wildlife populations in the forests of the Central African Republic. Conserv. Biol. 12, 390–398. doi: 10.1046/j.1523-1739.1998.96027.x
Overton, J., Davies, S., Nguluka, L., Chibeya, D., Nsende, E., Sompa, B., et al. (2017). The Illegal Bushmeat Trade in the Greater Kafue Ecosystem, Zambia - Drivers, Impacts and Solutions. FOA/Department of National Parks and Wildlife/Panthera/Game Rangers International, Zambia.
Packer, C., Brink, H., Kissui, B. M., Maliti, H., Kushnir, H., and Caro, T. (2010). Effects of trophy hunting on lion and leopard populations in Tanzania. Conserv. Biol. 25, 142–153. doi: 10.1111/j.1523-1739.2010.01576.x
Patterson, B. D., Kasiki, S. M., Selempo, E., and Kays, R. W. (2004). Livestock predation by lions (Panthera leo) and other carnivores on ranches neighbouring Tsavo National Park, Kenya. Biol. Conserv. 119, 507–516. doi: 10.1016/j.biocon.2004.01.013
Rabinowitz, A. R (1986). Jaguar predation on domestic livestock in Belize. Wildl. Soc. Bull. 14, 170–174.
Riggio, J., Jacobson, A., Dollar, L., Bauer, H., Becker, M., Dickman, A., et al. (2012). The size of savannah Africa: a lion's (Panthera leo) view. Biodiv. Conserv. 22, 17–35. doi: 10.1007/s10531-012-0381-4
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., et al. (2014). Status and ecological effects of the world's largest carnivores. Science 343, 1241484. doi: 10.1126/science.1241484
Rosenblatt, E., Becker, M. S., Creel, S., Droge, E., Mweetwa, T., Schuette, P. A., et al. (2014). Detecting declines of apex carnivores and evaluating their causes: an example with Zambian lions. Biol. Conserv. 180, 176–186. doi: 10.1016/j.biocon.2014.10.006
Rosenblatt, E., Creel, S., Becker, M. S., Merkle, J., Mwape, H., Schuette, P., et al. (2016). Effects of a protection gradient on carnivore density and survival: an example with leopards in the Luangwa valley, Zambia. Ecol. Evol. 6, 3772–3785. doi: 10.1002/ece3.2155
Schaller, G. B (1972). The Serengeti Lion: A Study of Predator-Prey Relations (Wildlife Behaviour & Ecology Series WBE. Chicago, IL; London: University of Chicago Press.
Schuette, P., Namukonde, N., Becker, M. S., Watson, F. G. R., Creel, S., Chifunte, C., et al. (2018). Boots on the ground: in defense of low-tech, inexpensive, and robust survey methods for Africa's under-funded protected areas. Biodiv. Conserv. 27, 2173–2191. doi: 10.1007/s10531-018-1529-7
Siamudaala, V. M., Nyirenda, V. R., and Saiwana, L. M. (2009). Effectiveness of Law Enforcement on Wildlife Crimes in the Kafue ecosystem in Zambia. Chilanga: Zambia Wildlife Authority.
Smuts, G. L., Anderson, J. L., and Austin, J. C. (1978). Age determination of the African lion (Panthera leo). J. Zool. Lond. 185, 115–146. doi: 10.1111/j.1469-7998.1978.tb03317.x
Solly, H (2007). “Cameroon: from free gift to valued commodity – the bushmeat commodity chain around the Dja Reserve,” in Bushmeat and Livelihoods: Wildlife Management and Poverty Reduction. Conservation Science and Practice Series No.2. eds G. D. Davies and D. Brown (Oxford: Blackwell Publishing Ltd.), 61–72. doi: 10.1002/9780470692592.ch4
Stander, P. E (1997). Field age determination of leopards by tooth wear. Afri. J. Ecol. 35, 156–161. doi: 10.1111/j.1365-2028.1997.068-89068.x
Strampelli, P., Andresen, L., Everatt, K. T., Somers, M. J., and Rowcliffe, J. M. (2018). Habitat use responses of the African leopard in a human-disturbed region of rural Mozambique. Mamm. Biol. 89, 14e20. doi: 10.1016/j.mambio.2017.12.003
Swanepoel, L. H., Somers, M. J., van Hoven, W., Schiess-Meier, M., Owen, C., Snyman, A., et al. (2015). Survival rates and causes of mortality of leopards Panthera pardus in southern Africa. Oryx 49, 595–603. doi: 10.1017/S0030605313001282
Truett, J. C., (1993). Guidelines for Oil and Gas Operations in Polar Bear Habitats. US Dept. Int. Minerals Management Service, Alaska OCS Region, OCS Study MMS 93-0008, p. 111.
Van Valkenburgh, B., and White, P. A. (2021). Naturally-occurring tooth wear, tooth fracture, and cranial injuries in large carnivores from Zambia. PeerJ 9, e11313. doi: 10.7717/peerj.11313
Vinks, M. A., Creel, S., Rosenblatt, E., Becker, M. S., Schuette, P., Goodheart, B., et al. (2021b). Leopard Panthera pardus density and survival in an ecosystem with depressed abundance of prey and dominant competitors. Oryx. 2021, 1–10. doi: 10.1017/S0030605321000223
Vinks, M. A., Creel, S., Schuette, P., Becker, M. S., Rosenblatt, E., Sanguinetti, C., et al. (2021a). Response of lion demography and dynamics to the loss of preferred larger prey. Ecol. Appl. 31, e02298. doi: 10.1002/eap.2298
Watson, F., Becker, M. S., McRobb, R., and Kanyembo, B. (2013). Spatial patterns of wire-snare poaching: implications for community conservation in buffer zones around National Parks. Biol. Conserv. 168, 1–9. doi: 10.1016/j.biocon.2013.09.003
Watson, F. G. R., Becker, M. S., Milanzi, J., and Nyirenda, M. (2015). Human encroachment into protected area networks in Zambia: implications for large carnivore conservation. Reg. Environ. Change 15, 415–429. doi: 10.1007/s10113-014-0629-5
Weiss, D., Tomasallo, C. D., Meiman, J. G., Alarcon, W., Graber, N. M., Bisgard, K. M., et al. (2017). Elevated blood lead levels associated with retained bullet fragments – United States, 2003–2012. MMWR Morb. Mortal Wkly. Rep. 66, 130–133. doi: 10.15585/mmwr.mm6605a2
White, P. A., and Belant, J. L. (2016). Individual variation in dental characteristics for estimating age of African lions. Wildl. Biol. 22, 71–77. doi: 10.2981/wlb.00180
White, P. A., Ikanda, D., Ferrante, L., Chardonnet, P., Mesochina, P., and Cameriere, R. (2016). Age estimation of African lions Panthera leo by ratio of tooth areas. PLoS ONE 11, e0153648. doi: 10.1371/journal.pone.0153648
Whitman, K. L., and Packer, C. (2007). A Hunter's Guide to Aging Lions in Eastern and Southern Africa. Longbeach, CA: Safari Press.
Williams, V. L., Loveridge, A. J., Newton, D. J., and MacDonald, D. W. (2017). A roaring trade? The legal trade in Panthera leo bones from African to East-Southeast Asia. PLoS ONE 12, e0185996. doi: 10.1371/journal.pone.0185996
Winterbach, H. E. K., Winterbach, C. W., and Somers, M. J. (2014). Landscape suitability in Botswana for the conservation of its six large African carnivores. PLoS ONE 9, e100202. doi: 10.1371/journal.pone.0100202
Wittemyer, G., Elsen, P., Bean, W. T., Burton, A. C. O., and Brashares, J. S. (2008). Accelerated human population growth at protected area edges. Science 321, 123–126. doi: 10.1126/science.1158900
Wolf, C., and Ripple, W. J. (2016). Prey depletion as a threat to the world's large carnivores. R. Soc. Open Sci. 3, 160252. doi: 10.1098/rsos.160252
Keywords: carnivore, human-wildlife conflict, pathology, snare, teeth, wildlife forensics, Zambia
Citation: White PA and Van Valkenburgh B (2022) Low-Cost Forensics Reveal High Rates of Non-lethal Snaring and Shotgun Injuries in Zambia's Large Carnivores. Front. Conserv. Sci. 3:803381. doi: 10.3389/fcosc.2022.803381
Received: 27 October 2021; Accepted: 07 January 2022;
Published: 10 February 2022.
Edited by:
Camilla Sandström, Umeå University, SwedenReviewed by:
Bogdan Cristescu, Cheetah Conservation Fund, NamibiaCopyright © 2022 White and Van Valkenburgh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula A. White, cGF3QGNhcm5pdm9yZWNvbnNlcnZhdGlvbi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.