- 1Wildlife Conservation Society - India, Bengaluru, India
- 2Endangered Species Management, Wildlife Institute of India, Dehradun, India
- 3Maharashtra Forest Department, Indian Forest Service, Maharashtra, India
Recent studies in the last decade have recorded obligate carnivores adapting to human dominated landscapes. Leopards, amongst other large carnivores, are highly adaptable and survive in a range of environments from the arid regions of Africa and the Middle East to the cold regions of the Russian Far East. They are also highly adaptable in their diet and consequently are present close to and even within high-density human landscapes. These also include the edges of urban areas such as Nairobi and Mumbai. Our study, to better understand the coexistence of leopards and humans, was conducted in 104 km2 of Sanjay Gandhi National Park (SGNP), which is surrounded on three sides by the urban landscape of Mumbai and Thane cities. The study area also included 85 km2 of an adjoining protected area, Tungareshwar Wildlife Sanctuary (TWLS), which is surrounded by a combination of forests, rural areas and agricultural lands. Based on spatial capture—recapture framework we observed that leopard densities in SGNP (26.34 ± 4.96 leopards/100 km2) and TWLS (5.40 ± 2.99 leopards/100 km2) were vastly different. We found that density estimates of wild prey and domestic dogs were higher in SGNP in comparison to TWLS. In both the protected areas (PAs), domestic dogs formed a major proportion of leopard diet and were the single highest species contributors. Our study shows that despite extremely high human density around SGNP (~20,000 people/km2), leopard density is also much higher than the adjoining TWLS which has a comparatively lower surrounding density of people (~1,700 people/km2). Leopard density reported from SGNP is amongst the highest ever reported. This interesting result is probably due to much higher biomass of potential food resources in and around SGNP. Studying this relationship between leopards and their prey (both wild and domestic) in a human dominated landscape will give us valuable insights on human—leopard interactions. The two adjacent and connected PAs are similar ecologically, but differ widely in almost all other aspects, including human densities along the periphery, leopard densities, prey densities as well as management regimes.
Introduction
Large predators in many parts of the world are expanding their distribution ranges (Chapron et al., 2014) and colonizing areas that they were extirpated from in the past (Carter and Linnell, 2016). It was a long-held belief that large carnivores need suitable natural habitats devoid of humans for their survival (Woodroffe, 2000; Carter and Linnell, 2016). However, there is increasing evidence that human-dominated landscapes with ample food resources (such as domestic prey) could allow for the presence of large carnivores (Gehrt et al., 2010; Yirga et al., 2013). In recent decades, carnivores have been widely documented using human-modified spaces. For instance, pumas (Puma concolor) using human modified spaces in Vancouver Island, Canada (Collard, 2012), leopards (Panthera pardus) in Maharashtra, India (Athreya et al., 2013), red foxes (Vulpes vulpes) in London (Cassidy and Mills, 2012), American Black bears (Ursus americans) in Colorado, USA (Lewis et al., 2015) and spotted hyena (Crocuta crocuta) coexisting at high density with people in Wukro district, northern Ethiopia (Yirga et al., 2013). These carnivores are adaptable and can persist in human-dominated areas (Carter and Linnell, 2016).
India is an interesting anomaly in terms of the high diversity of large wildlife present in the second most populous country in the world. The largest global populations of tigers (Panthera tigris) and Asian elephants (Elaphas maximus) are in India (Goodrich et al., 2015; Williams et al., 2020), which is also home to the only population of Asiatic lions (Panthera leo persica; Banerjee et al., 2013; Meena et al., 2021). Among the other large cats, the snow leopards (Panthera uncia) occur in trans-Himalayan region (Sharma et al., 2015). Leopards have a country-wide distribution, ranging from the forests of the Himalayan region (Naha et al., 2018) to the coastal plains (Daniel, 2009) and from the semi-arid landscapes of Rajasthan (Mondal et al., 2012; Kumbhojkar et al., 2019), to forests of Western Ghats (Ramesh et al., 2012) as well as from human-dominated landscapes across the country (Odden et al., 2014; Kshettry et al., 2018; Naha et al., 2018). About 83% of the leopard population exists outside protected areas in India (Jacobson et al., 2016). Leopards in a landscape mosaic of agricultural fields, plantations and human settlements have been observed to feed on domestic prey available in the landscape (Athreya et al., 2016; Kshettry et al., 2018; Naha et al., 2018). They have also been documented at the edges of Indian cities such as Mumbai (Edgaonkar and Chellam, 2002), Guwahati in Assam, (Bharali et al., 2021), Bangalore in Karnataka (Athreya et al., 2015), and Jaipur in Rajasthan (Kumbhojkar et al., 2020). Even though urban cities present very challenging environments, some carnivores utilize the food and shelter available in these environments (Bateman and Fleming, 2012).
Although we are increasingly recording the occurrence of wildlife in urban areas, we currently understand little of the factors contributing to the co-adaptations by humans and wildlife in shared spaces (Gehrt et al., 2010; Carter and Linnell, 2016). Carnivores that thrive in urban and suburban environs are mainly diet generalists (Gehrt et al., 2010; Moss et al., 2016). Some carnivores feed on the organic waste (Lewis et al., 2015) or predate on domestic animals such as dogs, cats and pigs which feed on garbage (Athreya et al., 2016; Yirga et al., 2016). Mountain lions in West-central Alberta (Canada) (Knopff et al., 2014) and spotted hyenas occur in peri-urban spaces in Ethiopia where they are entirely dependent on domestic prey species and the peri-urban waste (Yirga et al., 2016). Abundant, non-seasonal and energy rich food sources in urban areas have positive effects on survival, growth rate and population densities of carnivorous species (Gehrt et al., 2010; Bateman and Fleming, 2012). Medium-sized carnivores have been observed achieving higher population densities in cities compared to their natural habitats due to anthropogenic food sources and shelter (Bateman and Fleming, 2012). However, there has not been a significant ecological assessment of leopard's presence in an urban landscape in India to date. In this study we compare leopard ecology between the urban Sanjay Gandhi National Park situated in the metropolis of Mumbai with the adjoining Tungareshwar Wildlife Sanctuary set in a rural landscape with much lower density of humans. Specifically in this study, we assess leopard density, wild and domestic prey density, and compare the diet of leopards in the two adjacent protected areas (PAs).
Materials and Methods
Study Site
Our study to assess densities of leopards, their prey and leopard diet was carried out in Sanjay Gandhi National Park (SGNP) and Tungareshwar Wildlife Sanctuary (TWLS). Although adjacent to each other, the two protected areas (PAs) differ in many aspects including the management regime (Table 6).
The SGNP is located within sub-urban Mumbai and Thane districts of Maharashtra state. It is one of the few PAs in the country which falls within the municipal limits of a metropolis, extending over an area of 104 km2 (19° 8′ N, 72° 53′ E and 19° 21′ N, 72° 58′ E). Elevation ranges from 30 to 500 m above mean sea level and the vegetation is categorized as the southern moist deciduous type (Champion and Seth, 1968). Leopard is the apex carnivore in SGNP. Other carnivores found in this landscape are jungle cat (Felis chaus) rusty-spotted cat (Prionailurus rubiginosus), common palm civet (Paradoxurus hermaphroditus), small Indian civet (Viverricula indica), gray mongoose (Herpestes edwardsii) and the ruddy mongoose (Herpestes smithii). Herbivores that occur here include chital (Axis axis), sambar (Rusa unicolor), southern plains langur (Semnopithecus entellus), wild pig (Sus scrofa), bonnet macaque (Macaca radiata), rhesus macaque (Macaca mullata), barking deer (Muntiacus muntjak), Indian chevrotain (Moschiola indica), black-naped hare (Lepus nigricollis nigricollis), and Indian crested porcupine (Hystrix indica) (Edgaonkar and Chellam, 2002; Pradhan, 2002). Cattle, water buffaloes, goats, pigs, and domestic dogs are abundant in the areas to the south of SGNP in the Aarey milk colony (Punjabi et al., 2012). SGNP is one of the most highly visited PAs in the country (Pradhan, 2002). There are about 43 tribal hamlets inside SGNP's boundary represented by the Warli and Mahadev Koli tribes (Landy, 2017; Nair et al., 2021). People from the city use parts of SGNP mainly for recreational activities.
In our study we also included a few forest patches and other wooded areas adjacent to SGNP (Figure 1) where leopard presence was observed. These were the Aarey Milk Colony (12.8 km2) which is a largely human-modified forest. The Aarey Milk Colony consists of more than 30 cattle production units with a total capacity of more than 15,000 head of cattle (Punjabi et al., 2012). Other similar areas adjoining SGNP which we included were Indian Institute of Technology-Powai campus (2.20 km2) and Dadasaheb Phalke Film City (1.77 km2) which are located along the southern boundary of SGNP. The total area surveyed in the SGNP landscape was approximately 120 km2.
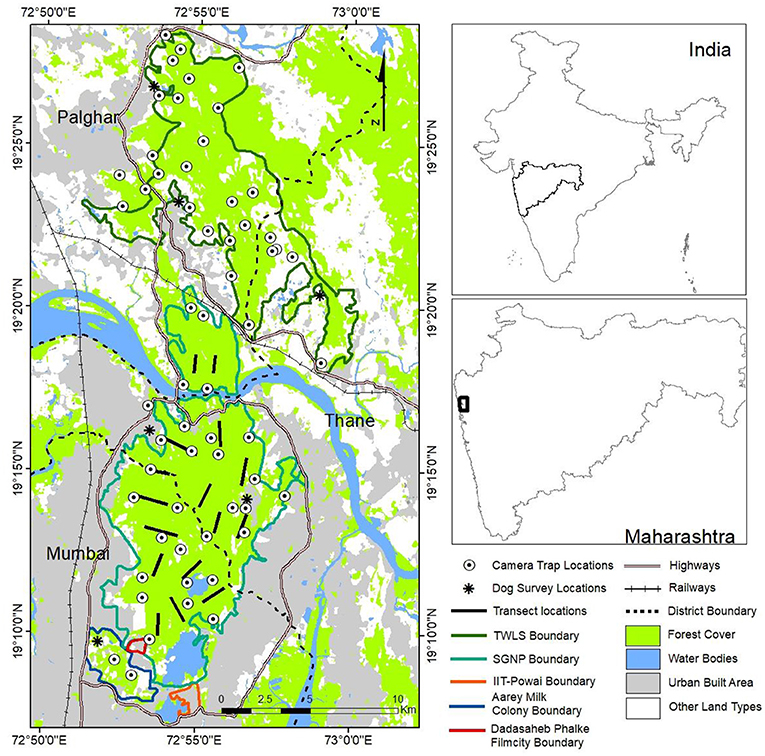
Figure 1. A map of Sanjay Gandhi National Park and Tungareshwar Wildlife Sanctuary showing camera trap locations, line transects and dog survey locations.
The TWLS, (19°23′38″N and 72°58′9″E) is located in the Palghar and Thane districts of Maharashstra. It is contiguous with SGNP along its southern boundary (Figure 1). The total area of TWLS is 85.70 km2, which was sampled in its entirety in this study. TWLS supports southern moist teak bearing forests, southern moist mixed deciduous forests and western sub-tropical hill forests (Champion and Seth, 1968). The highest point of TWLS is at an altitude of 663 meters above mean sea level. The terrain is mostly hilly and undulating. Some of the mammals that occur in TWLS include leopard, jungle cat, rusty-spotted cat, wild pig, common palm civet, small Indian civet, southern plains langur, bonnet macaques, gray mongoose, black-naped hare, and barking deer.
The local inhabitants of this area belong to the Warli and Mahadev Koli tribes. Minimum human density along the periphery of TWLS is 1,700 persons/km2. The major threats faced by TWLS are encroachment and illicit firewood collection.
Estimating Leopard Density
We used camera trap surveys within a spatial capture-recapture framework (Royle et al., 2017) to estimate leopard densities in the two PAs. In SGNP, camera trap surveys were carried out from 22nd February 2015 to 14th April 2015, and in TWLS they were conducted from 26th April 2016 to 6th June 2016. Although these two PAs were sampled in two consecutive years due to logistic constraints and limitations, there was no major change in habitat or management regime in the period that would have affected our findings. Camera trap locations were selected so that each individual leopard within the study area would be exposed to the camera trap array. Camera trap locations were selected to maximize the probability of photographic capture, based on leopard signs and at junctions of forest trails. In both sites, camera trap locations were approximately 2-3 km from each other, to ensure that we obtained spatial recaptures of individuals (multiple individuals each captured in multiple locations), which is critical for spatial capture-recapture modeling. In SGNP, camera traps were placed in three blocks: Block 1 had 9 locations which were active for 15 nights; block 2 had 10 locations which were active for 15 nights and block 3 had 12 locations which were active for 14 nights. TWLS had two blocks: block 1 had 16 locations active for 20 nights and block 2 had 12 locations active for 15 nights. This spatio-temporal schedule of camera trap effort was fully accounted for in the analysis using the trap deployment matrix. To obtain images of both flanks, a pair of self- triggered camera traps (Cuddeback Attack and Cuddeback C1) was placed at each camera trap location, set two-three feet from the ground. Because the study was carried out in a human use area where the risk of camera theft was high, we set the camera traps at 17:00 and removed them at 07:30 each day of the survey.
Each leopard individual was identified based on its unique rosette pattern and assigned a unique individual identification number (Karanth et al., 2017). After careful processing and validation of the camera trap image and associated data, we prepared the following input files for spatial capture-recapture analysis: the trap deployment file (with details of the spatial location of each camera trap location and the temporal schedule of trap deployment at each location); the captures file (with details of which animal was captured at which camera trap location on which occasion); and the state space (mask) file, specifying the area within which activity centers of individual leopards could possibly be located buffered to a distance of eight km from the outermost trap locations, so that animals at the edge of the state space had virtually no probability of being photo-captured in our trap array.
All statistical analyses were carried out using package secr (Efford, 2021) within the R statistical environment (R Core Team, 2021). We fit four plausible models to each data set, where baseline detection probability g0 and the movement parameter σ were each modeled either as constant or as differing between sexes (using the hybrid finite mixtures approach, as sex was unknown for some individuals). As no model received clear support from the data (Table 1), we derived estimates of real parameters (g0, σ, density, pmix) and unconditional standard errors using model-averaging (Burnham and Anderson, 2002; Cade, 2015).
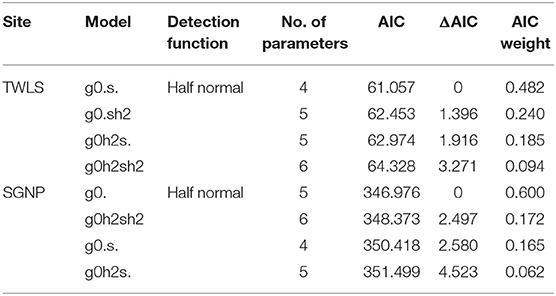
Table 1. Model selection results for the spatial capture-recapture modeling of leopard photo-captures in SGNP and TWLS conducted in 2015 and 2016 respectively.
Wild Prey Density
We used line transect sampling (Buckland et al., 2001, 2015) to estimate densities of wild prey species. Sixteen transects samplers were randomly marked in SGNP and each transect was surveyed five (three morning replicates and two evening replicates) times during January and February 2015. A total walk effort of 120 km was expended during the surveys. The line transect data were analyzed using program DISTANCE (Thomas et al., 2010). The analysis involved data exploration, selection of right truncation distances and fitting of different detection functions (half normal, hazard rate, uniform; see (Buckland et al., 2001, 2015) to the data in order to estimate average detection probability. The best model was selected based on the lowest Akaike Information Criteria (AIC) values (Burnham and Anderson, 2002).
Although we initiated line transect surveys in TWLS in 2016, these had to be abandoned due to extremely low encounter rates of wild prey. We instead assessed the relative abundance (encounters/km) of wild prey based on direct sightings and sign encounters during foot surveys conducted within 4 km2 grid cells superimposed across TWLS. A total walk effort of 87.3 kms was expended during these foot surveys.
Estimating Densities of Domestic Dogs
Domestic dogs are important prey for leopards in rural and semi-urban regions in India (Athreya et al., 2016; Kumbhojkar et al., 2020). To obtain estimates of dog densities we carried out dog density estimation using photographic surveys within a capture-recapture framework, at three different locations at the periphery of both SGNP as well as TWLS. These locations were selected taking into consideration logistical constraints and to represent the area on the periphery of both PAs. Survey locations were selected based on an initial reconnaissance survey, and were near garbage dumping sites, water bodies, feeding sites and human settlements. To avoid violation of the assumption of geographic and demographic closure (Amstrup et al., 2010), the sampling interval for the surveys was kept short. We covered a relatively large area to ensure that the perimeter to area ratio was small (Punjabi et al., 2012). Surveys were carried out by teams of two persons on a motorbike with a hand-held camera with a telephoto lens, who would traverse a predetermined route on a motorcycle, visiting each pre-identified survey location, scan for dogs within a 30–50 m radius, and carefully photograph both flanks of individual dogs found. The surveys were conducted over four sampling occasions except for Aarey Milk Colony where only three surveys were conducted. We used natural markings, scars, tail shapes, among other attributes, to individually identify photo-captured dogs. Data were analyzed using the Huggins (1989) conditional likelihood models using program MARK (White and Burnham, 1999), to estimate dog abundance. To estimate density, the surveyed areas were buffered by a width based on Vanak and Gompper (2010) study, yielding estimated densities for six locations across the two PAs.
Leopard Diet
Leopard scats were collected along roads and trails in both the PAs. A total of about 180 km each were walked in each of the two PAs. The scats were sun-dried and then washed under running water through a sieve. Hair, nails, and claws were collected from each scat sample and were sun-dried. Twenty-five hair samples were selected randomly from each scat and used for identification of prey. Individual prey species were identified under a microscope based on the medullary patterns of the hair (Athreya et al., 2016; Kshettry et al., 2018) using available reference slides. To determine the adequacy of sample size, we plotted a species accumulation curve based on the scat samples. The data obtained were analyzed to calculate relative frequencies of occurrence of individual prey species in leopard diet and prey selectivity of leopards was assessed based on the equation given by Chakrabarti et al. (2016).
Management Regimes of the Two Protected Areas
To understand the two protected areas at their management level. Information was collected on various aspects such as staff strength, revenue and tourist visitation rates etc. from the Forest Department staff at SGNP and TWLS.
Results
Leopard Density
In SGNP, a camera trap effort of 422 trap nights yielded a total of 92 photographs of leopards from which 31 individuals (10 males, 17 females, and 4 individuals whose sex could not be determined) were identified. The leopard density in SGNP during 2015 was estimated to be 26.34 ± 4.96 (SE) leopards/100 km2. Humans had the highest camera trap encounter rate of 29.15/100 trap nights, despite our traps being active only at night and the figure excluding captures of the research team and forest department staff.
In TWLS nine images of leopards were obtained from the trap effort of 429 trap nights. Five leopard individuals (two males, two females, and one individual whose sex could not be determined) were identified. Leopard density was estimated to be 5.40 ± 2.99 (SE) leopards/100 km2. The camera trap encounter rate of humans was 7.92 humans/100 trap nights, which was highest amongst all the species photographed in TWLS (Table 2).
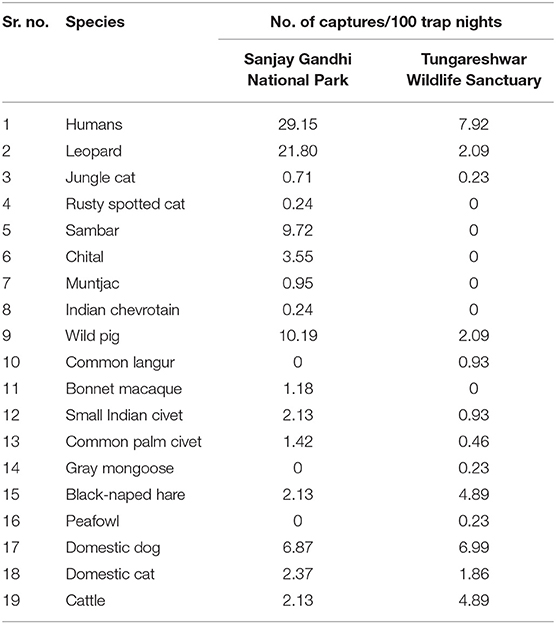
Table 2. Photo-capture rates of species photo-captured in Sanjay Gandhi National Park and Tungareshwar Wildlife Sanctuary in 2015 and 2016, respectively.
Wild Prey Density
In SGNP a total of eight potential leopard prey species (chital, sambar, barking deer, wild pig, common langur, bonnet macaque, gray jungle fowl, and red spur fowl) were encountered on line transects. Densities were estimated only for chital, sambar, bonnet macaque, and common langur as the other species lacked adequate sample sizes to fit the detection function. The half normal function with cosine adjustment terms was found to be the best fit model for chital, sambar, and bonnet macaque and uniform function with cosine adjustments was the best model for common langur (Table 3).
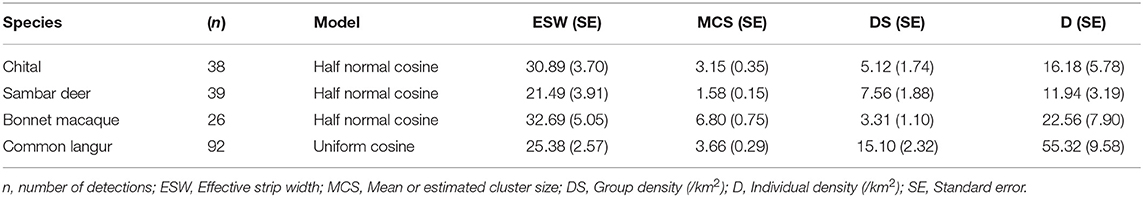
Table 3. Individual and group densities of major wild prey species of leopards estimated in Sanjay Gandhi National Park, Mumbai, Maharashtra in 2015.
Common langur occurred at the highest density followed by bonnet macaque, chital, and sambar (Table 3). Rhesus macaque and Indian chevrotain were not encountered on the line transects although they occur in the study area (based on personal sightings and camera trap photo captures).
In TWLS a total effort of 87.3 km was expended during the foot surveys during which we obtained only one direct sighting each of wild pigs and black-naped hare, eight sightings of bonnet macaques and three of northern plains langur. Barking deer pellet groups were seen on two occasions. The sign encounter rate of wild pigs, primates (including bonnet macaques and northern plains langur) and black-naped hares were 0.3, 0.5, and 0.09/km, respectively.
Domestic Dog Density
Domestic dogs occurred at an average density of 17.26 ± 0.69 (SE) /km2 in the areas sampled around SGNP and 7.7 ± 3.4 dogs/km2 around TWLS (Table 4).
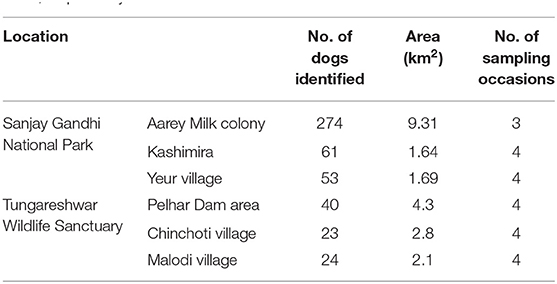
Table 4. Summary of photo-captures of domestic dogs in three locations in Sanjay Gandhi National Park and Tungareshwar Wildlife Sanctuary in 2015 and 2016, respectively.
Leopard Diet
Thirteen prey species were found in 97 leopard scats obtained in SGNP and seven prey species were identified from the 23 leopard scats collected from TWLS. The species accumulation curve flattened out at 13 species at 55 scat samples for SGNP (Figure 2), but the sample size for TWLS was too small to plot the species accumulation curve. Biomass consumed per scat was calculated using generalized model given by Chakrabarti et al. (2016) as shown in Table 5.
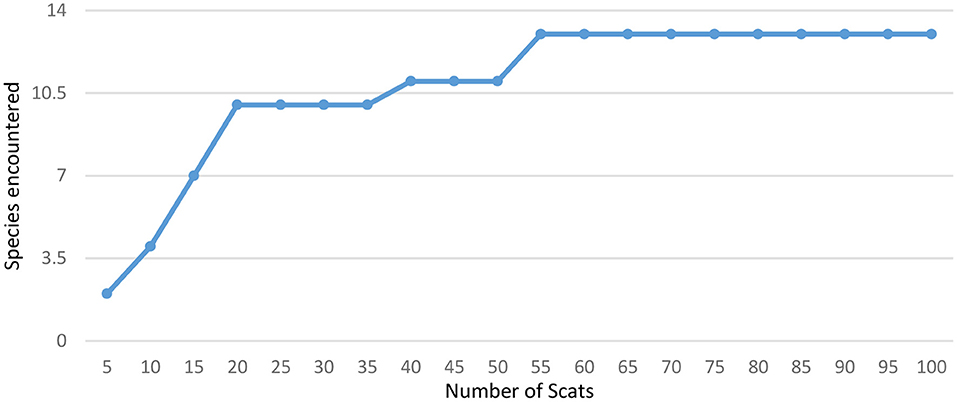
Figure 2. Accumulation curve for prey species found in leopard scats collected from Sanjay Gandhi National Park in 2015.
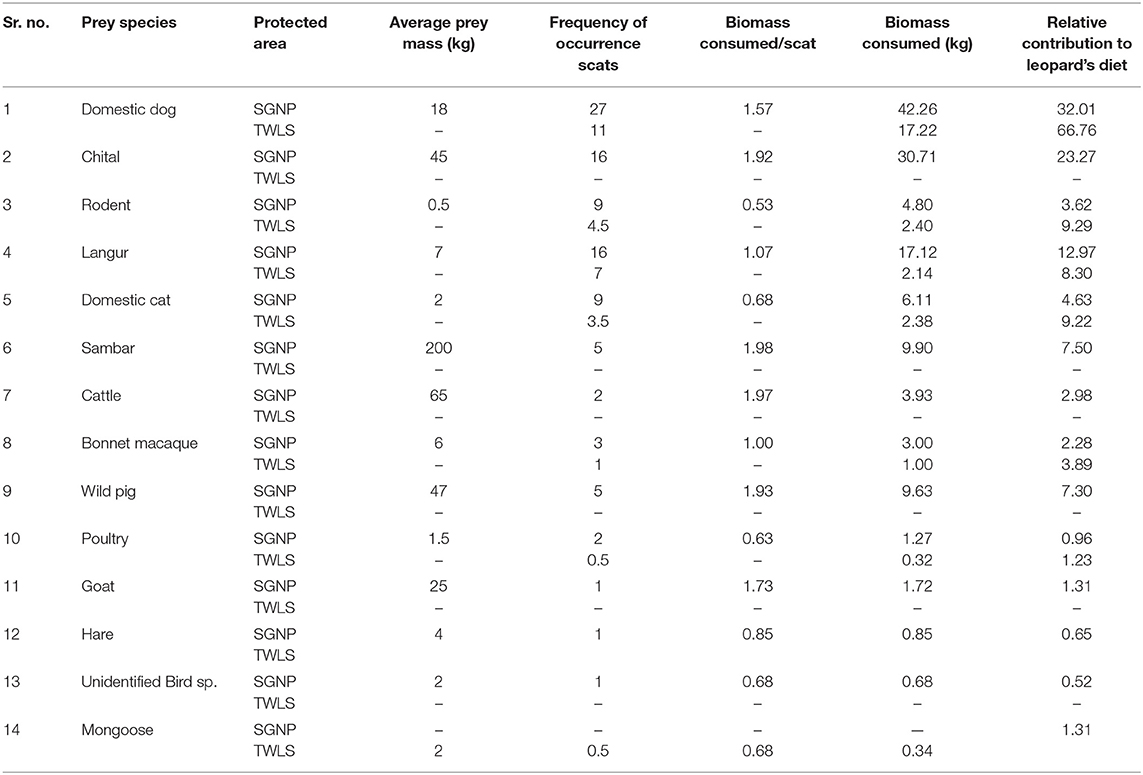
Table 5. Frequency of prey species found in leopard's scats, biomass consumed and relative contribution of each prey consumed in the study area of Sanjay Gandhi National Park (n = 97) in 2015 and Tungareshwar Wildlfie Sanctuary (n = 23) in 2016.
Domestic dogs were found to be the highest contributors to leopard's diet. The biomass contributed by domestic dogs was at 32.01 and 66.76% in SGNP and TWLS, respectively. Wild prey formed 53.97% of the leopard's diet in SGNP and 13.5% in TWLS.
Management Regimes of the Two Protected Areas
SGNP is surrounded by an extremely high density of humans (20,000 persons/km2) while TWLS, even though connected to SGNP, is set in a lower human density, rural landscape (1,700 persons/km2). The three senior-most managers are the same for the two PAs but SGNP has 109 staff spread over three forest ranges (administrative units) whereas TWLS, with a similar area to SGNP, has 30 staff and one forest range. The tourist footfall in 2016 was approximately 1.6 million in SGNP and approximately 90,000 in TWLS. The revenue generated from this and other allied activities therefore was also very different (Table 6).
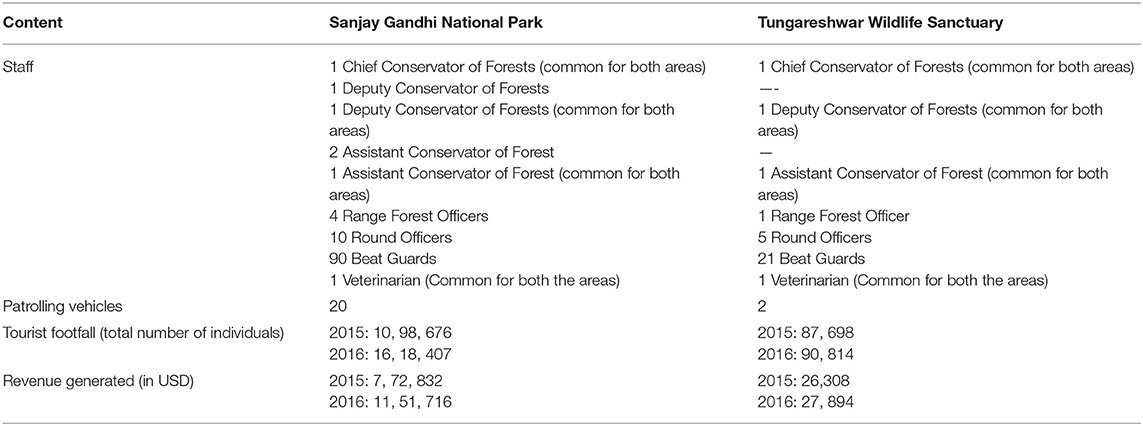
Table 6. Comparison of management structure and revenue between Sanjay Gandhi National Park and Tungareshwar Wildlife Sanctuary.
Discussion
Our study revealed unprecedented leopard density (26.34 ± 4.96 leopards/100 km2) despite extremely high human density (over 20,000 people/km2) along the periphery of an urban PA–SGNP. In contrast, the rural landscape surrounding TWLS having much lower human density (1,700 people /km2) along its periphery had a lower leopard density (5.40 ± 2.99 leopards/100 km2). Such high densities were not reported even in PAs of India where the numbers ranged from 12.04 ± 2.98/100 km2 (Achanakmar Tiger Reserve, Mandal et al., 2017) to 14.99 ± 6.9/100 km2 (Rajaji Tiger Reserve, Harihar et al., 2009). Even in human dominated landscapes of western Maharashtra and Rajasthan, reported leopard densities were 6.4 ± 0.78/100 km2 (Athreya et al., 2013) and 6.38 ± 2.4/100 km2 (Sharma, 2017), respectively. The estimate of leopard density from our study area is amongst the highest recorded leopard densities from India.
It is often thought that wild carnivores do not occur at high densities near dense human habitations, however recent studies have shown that there are highly adaptable large carnivores that can share space with high density of humans (Yirga et al., 2013; Odden et al., 2014). Interestingly, in both the PAs, humans had the highest photo encounter rate. Our camera traps deployed between dusk and dawn, found human encounter rates to be the highest among all the species in the PAs. The tourist footfall in SGNP was 1–1.6 million per year (2015–2016) whereas TWLS had ~90,000 tourists (2015–2016). There have been no attacks on people due to leopards reported (based on Maharashtra Forest Department records) from October 2013 to June 2016. This is unique in the world where a large carnivore, is occurring at high density in a PA situated in a metropolis.
The high density of leopards in SGNP, as compared to other PAs, can be attributed to lack of larger predators, few threats, intensive management (Table 6) and, most importantly, high food availability (Fuller et al., 2010; Singh et al., 2016) consisting both of wild and domestic prey. Leopards are the apex predators of this landscape with the last tiger having been killed at the southern boundary of SGNP in 1929 (Prater, 1929). A study conducted in Sariska Tiger Reserve in Rajasthan showed that leopard density reduced from 7.6 ± 0.6 leopards/100 km2 to 3.1 ± 0.4 leopards/100 km2 following reintroduction of tigers (Mondal et al., 2012). The other possible reasons for high densities such as reduction of threats due to effectiveness of management regimes could not be assessed during our study. Further studies should be carried out to assess the stark difference in management regimes between both these PAs. Results from our leopard prey estimation study indicate that food availability could be an important factor contributing to the high leopard densities in this landscape. Densities of obligate carnivores like leopards are strongly linked to the availability of food resources and habitat (Karanth et al., 2004; Knopff et al., 2014; Filla et al., 2017). The results from leopard scat analyses highlight the importance of dogs in leopard's diet. In TWLS, despite lower domestic dog density than SGNP, domestic dogs constituted 66.76% to the leopard's diet. This was higher than SGNP which had 32.01% of domestic dogs in the leopard's diet. The differences in leopard densities, dog densities, wild prey densities, as well as the contribution of domestic dogs to leopard diet in the two PAs present a discrepancy that we have not been able to fully resolve. While densities of dogs are lower in TWLS, so are the densities of leopards as well as wild prey (so low, in fact, that we were unable to derive estimates). It is certainly plausible that in SGNP, the availability of domestic dogs over and above wild prey leads to high leopard densities, while in TWLS, extremely low densities of wild prey lead to a very high representation of dogs in leopard diet, without accompanying numerical responses (Holling, 1959) by leopards. To corroborate our speculation on the effects of wild and domestic prey density on leopard diet, and therefore on leopard density, further studies are required.
In our study sites, extremely high biomass of potential domestic prey species for the leopard is mainly associated with humans. Globally, carnivore species in peri-urban and urban landscapes show similar patterns of feeding on domestic prey (Yirga et al., 2016; Kumbhojkar et al., 2020). Domestic dogs and other domestic species subsist on anthropogenic waste (Bhalla et al., 2021). Abundance of such domestic prey in human dominated landscape causes higher densities of predators (Yirga et al., 2013; Athreya et al., 2016). The present study highlighted the importance of domestic dogs (both feral and domestic in this landscape) from leopard's diet. Other studies in India (Edgaonkar and Chellam, 2002; Athreya et al., 2016; Kumbhojkar et al., 2020) also document this relationship between leopards and domestic dogs. Edgaonkar and Chellam's (2002) study in SGNP showed domestic dogs to be the principal prey for leopards. Although leopards thrive at higher densities in this modified landscape, it remains to be seen if the prey-predator dynamics are affected long term by human-associated domestic prey.
Studies documenting carnivores utilizing human modified landscapes present novel conservation challenges (Bateman and Fleming, 2012; Loock et al., 2018; Riley et al., 2021). Our study highlights leopard persistence at extremely high densities in an urban PA. Human dominated areas provide carnivores with cost-effective and energy-rich food resources which increases their survival and densities. But along with rewards these human dominated habitats also present the carnivores with risks and threats (Bateman and Fleming, 2012). The rapid development and urbanization of Mumbai and Thane could prove a potential threat to future leopard populations. Linear intrusions like national and state highways, already present along the periphery of the two PAs can serve as a barrier for dispersal of carnivores (Poessel et al., 2014; Riley et al., 2014). There is a need for further research to understand threats to leopards associated with this habitat.
Conclusion
We observed that leopards occur at greater densities in SGNP landscape as compared to other studies from India. This high density is likely to be a result of high abundance of wild as well as domestic prey and absence of competition from similar sized predators. In TWLS, where we observed a low density of leopards and wild prey as compared to SGNP, domestic dogs contributed maximum to the leopard's diet. Further studies should be carried out in this landscape to understand the prey-predator dynamics and human influence on the same. This will help us understand the complex relationship between humans and leopards in this landscape.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation on request.
Author Contributions
NS, VA, SS, and KS conceived the study. VG provided support to carry out the work. NS collected the data along with staff provided by VG. NS and DJ analyzed the data. SS, VA, and KS gave necessary inputs during data analysis. NS and VA led the writing of the manuscript. DJ, SS, and KS provided guidance and critical reviews during the writing process. All authors have contributed significantly to the draft of the manuscript and given their approval for publication.
Funding
This research study was funded by Wildlife Institute of India, Maharashtra Forest Department, and Wildlife Conservation Society-India.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared committee with one of the authors, VA, at time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the staff at SGNP and TWLS for their immense support and enthusiasm during field work. We would like to thank the three reviewers for their valuable inputs. We thank our funders for supporting this work. We would like to thank our field assistants Shubhash, Parshu Mama, and Kamal. We also acknowledge the help offered by a long list of volunteers from Mumbai and Thane who have contributed to this project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.787031/full#supplementary-material
References
Amstrup, S. C., McDonald, T. L., and Manly, B. F., (eds.). (2010). Handbook of Capture-Recapture Analysis. Princeton, NJ: Princeton University Press. doi: 10.1515/9781400837717
Athreya, V., Odden, M., Linnell, J. D., Krishnaswamy, J., and Karanth, K. U. (2016). A cat among the dogs: leopard Panthera pardus diet in a human-dominated landscape in western Maharashtra, India. Oryx 50, 156–162. doi: 10.1017/S0030605314000106
Athreya, V., Odden, M., Linnell, J. D., Krishnaswamy, J., and Karanth, U. (2013). Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PLoS ONE 8, e57872. doi: 10.1371/journal.pone.0057872
Athreya, V., Srivathsa, A., Puri, M., Karanth, K. K., Kumar, N. S., and Karanth, K. U. (2015). Spotted in the news: using media reports to examine leopard distribution, depredation, and management practices outside protected areas in Southern India. PLoS ONE 10, e0142647. doi: 10.1371/journal.pone.0142647
Banerjee, K., Jhala, Y. V., Chauhan, K. S., and Dave, C. V. (2013). Living with lions: the economics of coexistence in the Gir forests, India. PLoS ONE 8, e49457. doi: 10.1371/journal.pone.0049457
Bateman, P. W., and Fleming, P. A. (2012). Big city life: carnivores in urban environments. J. Zool. 287, 1–23. doi: 10.1111/j.1469-7998.2011.00887.x
Bhalla, S. J., Kemmers, R., Vasques, A., and Vanak, A. T. (2021). ‘Stray appetites’: a socio-ecological analysis of free-ranging dogs living alongside human communities in Bangalore, India. Urban Ecosyst. 24, 1245–1258. doi: 10.1007/s11252-021-01097-4
Bharali, K. K., Sharma, D. K., Sahariah, D., Singh, Y. L., and Bhobora, C. (2021). Nowhere to live: squeezing habitat and human-leopard conflicts in Maligaon, Guwahati, Assam. Appl. Ecol. Environ. Sci. 9, 138–143 doi: 10.12691/aees-9-2-3
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., Borchers, D. L., and Thomas, L. (2001). Introduction to Distance Sampling: Estimating Abundance of Biological Populations. Oxford: Oxford University Press.
Buckland, S. T., Rexstad, E. A., Marques, T. A., and Oedekoven, C. S. (2015). Distance Sampling: Methods and Applications, Vol. 431. New York, NY: Springer. doi: 10.1007/978-3-319-19219-2
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer.
Cade, B. S. (2015). Model averaging and muddled multimodel inferences. Ecology 96, 2370–2382. doi: 10.1890/14-1639.1
Carter, N. H., and Linnell, J. D. (2016). Co-adaptation is key to coexisting with large carnivores. Trends Ecol. Evol. 31, 575–578. doi: 10.1016/j.tree.2016.05.006
Cassidy, A., and Mills, B. (2012). “Fox Tots Attack Shock”: urban foxes, mass media and boundary-breaching. Environ. Commun. J. Nat. Cult. 6, 494–511. doi: 10.1080/17524032.2012.716370
Chakrabarti, S., Jhala, Y. V., Dutta, S., Qureshi, Q., Kadivar, R. F., and Rana, V. J. (2016). Adding constraints to predation through allometric relation of scats to consumption. J. Anim. Ecol. 85, 660–670. doi: 10.1111/1365-2656.12508
Champion, S. H., and Seth, S. K. (1968). A Revised Survey of the Forests of India. New Delhi: Manager of Publications.
Chapron, G., Kaczensky, P., Linnell, J. D., Von Arx, M., Huber, D., Andrén Lopez-Bao, J. V., et al. (2014). Recovery of large carnivores in Europe's modern human-dominated landscapes. Science 346, 1517–1519. doi: 10.1126/science.1257553
Collard, R. C. (2012). Cougar-human entanglements and the biopolitical un/making of safe space. Environ. Plan. D Soc. Space 30, 23–42. doi: 10.1068/d19110
Edgaonkar, A., and Chellam, R. (2002). Food habit of the leopard, Panthera pardus, in the Sanjay Gandhi National Park, Maharashtra, India. Mammalia 2002, 353–360. doi: 10.1515/mamm.2002.66.3.353
Efford, M. (2021). secr: Spatially Explicit Capture-Recapture Models. R package version 4.4.5. Available online at: https://CRAN.R-project.org/package=secr. (accessed July 20, 2021).
Filla, M., Premier, J., Magg, N., Dupke, C., Khorozyan, I., Waltert, M., et al. (2017). Habitat selection by Eurasian lynx (Lynx lynx) is primarily driven by avoidance of human activity during day and prey availability during night. Ecol. Evol. 7, 6367–6381. doi: 10.1002/ece3.3204
Fuller, T., Destefano, S., and Warren, P. S. (2010). “Carnivore behaviour and ecology, and relationship to urbanization,” in Urban Carnivores: Ecology, Conflict, and Conservation, eds S. D. Gehrt, S. P. D. Riley, and B. L. Cypher (Baltimore, MD: Johns Hopkins University Press), 13–20.
Gehrt, S. D., Riley, S. P., and Cypher, B. L. (2010). Urban Carnivores: Ecology, Conflict, and Conservation. Baltimore, MD: Johns Hopkins University Press.
Goodrich, J., Lynam, A., Miquelle, D., Wibisono, H., Kawanishi, K., Pattanavibool, A., et al. (2015). Panthera tigris. The IUCN Red List of Threatened Species 2015.
Harihar, A., Pandav, B., and Goyal, S. P. (2009). Density of leopards (Panthera pardus) in the Chilla Range of Rajaji National Park, Uttarakhand. India. Mammalia 73, 68–71. doi: 10.1515/MAMM.2009.007
Holling, C. S. (1959). Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398. doi: 10.4039/Ent91385-7
Huggins, R. M. (1989). On the statistical analysis of capture experiments. Biometrika 76, 133–140. doi: 10.1093/biomet/76.1.133
Jacobson, A. P., Gerngross, P., Lemeris, J. R. Jr, Schoonover, R. F., Anco, C., Breitenmoser-Würsten, C., et al. (2016). Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ 4, e1974. doi: 10.7717/peerj.1974
Karanth, K. U., Nichols, J. D., Harihar, A., Miquelle, D. G., Kumar, N. S., and Dorazio, R. M. (2017). “Field practices: assessing tiger population dynamics using photographic captures,” in Methods For Monitoring Tiger and Prey Populations, eds K. U. Karanth and J. D. Nichols (Singapore: Springer), 191–224. doi: 10.1007/978-981-10-5436-5_10
Karanth, K. U., Nichols, J. D., Kumar, N. S., Link, W. A., and Hines, J. E. (2004). Tigers and their prey: predicting carnivore densities from prey abundance. Proc. Natl. Acad. Sci. U.S.A. 101, 4854–4858. doi: 10.1073/pnas.0306210101
Knopff, A. A., Knopff, K. H., Boyce, M. S., and Clair, C. C. S. (2014). Flexible habitat selection by cougars in response to anthropogenic development. Biol. Conserv. 178, 136–145. doi: 10.1016/j.biocon.2014.07.017
Kshettry, A., Vaidyanathan, S., and Athreya, V. (2018). Diet selection of leopards (Panthera pardus) in a human-use landscape in North-Eastern India. Trop. Conserv. Sci. 11, 1940082918764635. doi: 10.1177/1940082918764635
Kumbhojkar, S., Yosef, R., Benedetti, Y., and Morelli, F. (2019). Human-leopard (Panthera pardus fusca) co-existence in Jhalana forest reserve, India. Sustainability 11, 3912. doi: 10.3390/su11143912
Kumbhojkar, S., Yosef, R., Kosicki, J. Z., Kwiatkowska, P. K., and Tryjanowski, P. (2020). Dependence of the leopard Panthera pardus fusca in Jaipur, India, on domestic animals. Oryx 55, 1–7. doi: 10.1017/S0030605319001145
Landy, F. (2017). “Urban leopards are good cartographers: human-non human and spatial conflicts at Sanjay Gandhi National Park, Mumbai,” in Places of Nature in Ecologies of Urbanism, eds A. Rademacher and K. Sivaramakrishnan (Hong Kong: Hong Kong University Press), 67–85. doi: 10.5790/hongkong/9789888390595.003.0004
Lewis, D. L., Baruch-Mordo, S., Wilson, K. R., Breck, S. W., Mao, J. S., and Broderick, J. (2015). Foraging ecology of black bears in urban environments: guidance for human-bear conflict mitigation. Ecosphere 6, 1–18. doi: 10.1890/ES15-00137.1
Loock, D. J., Williams, S. T., Emslie, K. W., Matthews, W. S., and Swanepoel, L. H. (2018). High carnivore population density highlights the conservation value of industrialised sites. Sci. Rep. 8, 1–9. doi: 10.1038/s41598-018-34936-0
Mandal, D., Basak, K., Mishra, R. P., Kaul, R., and Mondal, K. (2017). Status of leopard panthera pardus and striped hyena Hyaena hyaena and their prey in achanakmar tiger reserve, Central India. J. Zool. Stud. 4, 34–41.
Meena, V., Johnson, P. J., Zimmermann, A., Montgomery, R. A., and Macdonald, D. W. (2021). Evaluation of human attitudes and factors conducive to promoting human-lion coexistence in the Greater Gir landscape, India. Oryx 55, 589–598. doi: 10.1017/S0030605319000760
Mondal, K., Gupta, S., Bhattacharjee, S., Qureshi, Q., and Sankar, K. (2012). Response of leopards to re-introduced tigers in Sariska Tiger Reserve, Western India. Int. J. Biodivers. Conserv. 4, 228–236. doi: 10.5897/IJBC12.014
Moss, W. E., Alldredge, M. W., Logan, K. A., and Pauli, J. N. (2016). Human expansion precipitates niche expansion for an opportunistic apex predator (Puma concolor). Sci. Rep. 6, 39639. doi: 10.1038/srep39639
Naha, D., Sathyakumar, S., and Rawat, G. S. (2018). Understanding drivers of human-leopard conflicts in the Indian Himalayan region: Spatio-temporal patterns of conflicts and perception of local communities towards conserving large carnivores. PLoS ONE 13, e0204528. doi: 10.1371/journal.pone.0204528
Nair, R., Dhee P.atil, O., Surve, N., Andheria, A., Linnell, J. D., and Athreya, V. (2021). Sharing spaces and entanglements with big cats: the Warli and their Waghoba in Maharashtra, India. Front. Conserv. Sci. 2, 683356. doi: 10.3389/fcosc.2021.683356
Odden, M., Athreya, V., Rattan, S., and Linnell, J. D. (2014). Adaptable neighbours: movement patterns of GPS-collared leopards in human dominated landscapes in India. PLoS ONE 9, e112044. doi: 10.1371/journal.pone.0112044
Poessel, S. A., Burdett, C. L., Boydston, E. E., Lyren, L. M., Alonso, R. S., Fisher, R. N., et al. (2014). Roads influence movement and home ranges of a fragmentation-sensitive carnivore, the bobcat, in an urban landscape. Biol. Conserv. 180, 224–232. doi: 10.1016/j.biocon.2014.10.010
Pradhan, M. S. (2002). Common Vertebrate Species of Sanjay Gandhi National Park, Borivali, Mumbai. Zoological Survey of India.
Prater, S. H. (1929). On the Occurrence of Tigers on the Island of Bombay and Salsette. J. Bombay Nat. Hist. Soc. 33, 973–974.
Punjabi, G. A., Athreya, V., and Linnell, J. D. (2012). Using natural marks to estimate free-ranging dog Canis familiaris abundance in a MARK-RESIGHT framework in suburban Mumbai, India. Trop. Conserv. Sci. 5, 510–520. doi: 10.1177/194008291200500408
R Core Team (2021). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Availalble online at: https://www.R-project.org/.
Ramesh, T., Kalle, R., Sankar, K., and Qureshi, Q. (2012). Dietary partitioning in sympatric large carnivores in a tropical forest of Western Ghats, India. Mammal Study 37, 313–321. doi: 10.3106/041.037.0405
Riley, S. P., Serieys, L. E., Pollinger, J. P., Sikich, J. A., Dalbeck, L., Wayne, R. K., et al. (2014). Individual behaviors dominate the dynamics of an urban mountain lion population isolated by roads. Curr. Biol. 24, 1989–1994. doi: 10.1016/j.cub.2014.07.029
Riley, S. P., Sikich, J. A., and Benson, J. F. (2021). Big cats in the big city: Spatial ecology of mountain lions in greater Los Angeles. J. Wildl. Manag. 85, 1527–1542. doi: 10.1002/jwmg.22127
Royle, J. A., Gopalaswamy, A. M., Dorazio, R. M., Nichols, J. D., Jathanna, D., Parameshwaran, R., et al. (2017). “Concepts: assessing tiger population dynamics using capture-recapture sampling,” in Methods for Monitoring Tiger and Prey Populations, eds K. U. Karanth and J. D. Nichols (Singapore: Springer), 163–189. doi: 10.1007/978-981-10-5436-5_9
Sharma, R. K. (2017). A Country side carnivore- Aspect of leopard ecology at Jawai, Rajasthan (Master's thesis). Wildlife Institute of India, Dehradun, India.
Sharma, R. K., Bhatnagar, Y. V., and Mishra, C. (2015). Does livestock benefit or harm snow leopards? Biol. Conserv. 190, 8–13. doi: 10.1016/j.biocon.2015.04.026
Singh, A., Mukherjee, A., Dookia, S., and Kumara, H. N. (2016). High resource availability and lack of competition have increased population of a meso-carnivore-a case study of golden jackal in Keoladeo National Park, India. Mammal Res. 61, 209–219. doi: 10.1007/s13364-016-0267-z
Thomas, L., Buckland, S. T., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., et al. (2010). Distance software: design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47, 5–14. doi: 10.1111/j.1365-2664.2009.01737.x
Vanak, A. T., and Gompper, M. E. (2010). Interference competition at the landscape level: the effect of free-ranging dogs on a native mesocarnivore. J. Appl. Ecol. 47, 1225–1232. doi: 10.1111/j.1365-2664.2010.01870.x
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139. doi: 10.1080/00063659909477239
Williams, C., Tiwari, S. K., Goswami, V. R., de Silva, S., Kumar, A., Baskaran, N., et al. (2020). Elephas Maximus. The IUCN Red List of Threatened Species 2020.
Woodroffe, R. (2000). Predators and people: using human densities to interpret declines of large carnivores. Anim. Conserv. 3, 165–173.
Yirga, G., Ersino, W., De Iongh, H. H., Leirs, H., Gebrehiwot, K., Deckers, J., et al. (2013). Spotted hyena (Crocuta crocuta) coexisting at high density with people in Wukro district, northern Ethiopia. Mammal. Biol. 78, 193–197. doi: 10.1016/j.mambio.2012.09.001
Keywords: leopard, human-carnivore interactions, Mumbai, domestic dogs, carnivore, density, city
Citation: Surve NS, Sathyakumar S, Sankar K, Jathanna D, Gupta V and Athreya V (2022) Leopards in the City: The Tale of Sanjay Gandhi National Park and Tungareshwar Wildlife Sanctuary, Two Protected Areas in and Adjacent to Mumbai, India. Front. Conserv. Sci. 3:787031. doi: 10.3389/fcosc.2022.787031
Received: 30 September 2021; Accepted: 20 January 2022;
Published: 10 March 2022.
Edited by:
Alexandra Zimmermann, University of Oxford, United KingdomReviewed by:
Pavel Kindlmann, Charles University, CzechiaFrédéric Landy, Université Paris Nanterre, France
Copyright © 2022 Surve, Sathyakumar, Sankar, Jathanna, Gupta and Athreya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikit Sanjay Surve, bmlraXQuc3VydmVAZ21haWwuY29t
 Nikit Sanjay Surve
Nikit Sanjay Surve Sambandam Sathyakumar
Sambandam Sathyakumar Kalyanasundaram Sankar2
Kalyanasundaram Sankar2 Devcharan Jathanna
Devcharan Jathanna