- 1Wilder Institute Calgary Zoo, Calgary, AB, Canada
- 2Rhino Ark, Nairobi, Kenya
- 3International Union for Conservation of Nature (IUCN) Species Survival Commission Conservation Translocation Specialist Group, Calgary, AB, Canada
Conservation translocations have traditionally focused on ecological aspects while overlooking or underestimating the importance of human dimensions. Here, we present a feasibility analysis for a conservation translocation that up front took a holistic approach by investigating both ecological and socio-economic suitability of reinforcing mountain bongo in Eburu National Forest, Kenya. From 2018 to 2019, we set up 50 cameras to detect mountain bongo and searched for secondary signs in a grid overlaying Eburu. We also conducted surveys with 200 households surrounding the forest and interviewed 300 students to understand local perceptions of and interactions with Eburu Forest and their desire for a mountain bongo translocation. We used data from camera trapping and secondary signs in a MaxEnt model to determine the amount and location of available habitat for a bongo conservation translocation. Camera traps recorded only five bongo events in the 2-year study, and MaxEnt models revealed that these antelopes were relegated to less than 2.5 km of available habitat. Socio-economic surveys indicated local support for the conservation of bongo and their habitat, and yet our camera traps uncovered threatening illicit activities that could jeopardize both bongo survival and any attempt at boosting the remnant population with captive-bred individuals. We report how we built on long-term community and stakeholder engagement to mitigate these threats and provide concrete recommendations for how to proceed with a conservation translocation in terms of both the biological aspects and continued efforts to integrate socio-economic needs and community engagement.
Introduction
Direct or indirect human-driven threats lie at the root of demise for the vast majority of imperiled species (IUCN, 2021). It follows that attempts to improve the conservation status of imperiled wildlife should carefully examine and address human interactions with individual species and their ecosystems. Moreover, a human-rights perspective and—more recently—the growing recognition, heightened by the COVID-19 pandemic, that societal well-being and biodiversity are interdependent, have fostered greater integration of human considerations in conservation (Corrigan et al., 2018; Corson et al., 2020; Schneider et al., 2021). Although nature reserves that largely exclude humans remain a valuable, contemporary tool (UNEP-WCMC, 2018; Lewis et al., 2019), emphasis on honoring the needs and rights of local communities in conservation efforts has been growing for decades (Berkes, 2010; Kothari et al., 2013). As a result, a growing number of protected areas and forests are now co-governed by diverse stakeholders or indeed community-led (Macura et al., 2015; Gilmour, 2016; Corrigan et al., 2018).
Despite this overarching trend, planning for specific, management-intensive conservation interventions, such as conservation translocations, has traditionally focused on ecological aspects of the conservation challenge, with implications by or for local communities often ignored or addressed as an after-thought (Brichieri-Colombi and Moehrenschlager, 2016; Rayne et al., 2020; Reed et al., 2021). Conservation translocations are the human-mediated release of organisms for conservation purposes, where source individuals may come from populations under human care or from populations elsewhere in the wild (IUCN, 2013). The feasibility of such programs needs to be carefully evaluated. Pre-eminent priorities include addressing potential threats that may have led to past declines, determining the status of threats currently, and taking mitigative actions that will increase the likelihood of the focal species' growth and sustainability at release sites over time (IUCN, 2013). Integrated planning will involve selection and support of potential release sites and specimen over time, but such planning should be founded within assessments that iteratively address ecological as well as human dimensions (IUCN, 2013; Rayne et al., 2020).
Fortunately, in some locations, conservation translocations are beginning to integrate community considerations, particularly when candidate species or sites are of important cultural or spiritual importance on lands that are co-managed with indigenous societies (McMurdo Hamilton et al., 2020). Particularly in protected areas, however, the perception may arise that consultation with communities is less important given restrictions on human access to such sites, implying limited human interference with ecological conditions required for conservation translocations and limited impact of released wildlife on humans (McMurdo Hamilton et al., 2020). Yet not all parks are created equal, with a wide variety of protected area models balancing ecological integrity with human use (Dudley, 2013). Moreover, implementation or enforcement of restrictions is often difficult, especially in resource-constrained developing economies. Protected area designation may hence not necessarily mean that habitat conditions are sufficiently protected, that ecological conditions would suffice for wildlife reintroductions, that interactions between reintroduced wildlife and humans are unlikely, or that the values and activities of local communities are well-aligned with a conservation translocation and vice versa.
In accordance with the IUCN Guidelines for Reintroductions and Other Conservation Translocations (IUCN, 2013), Kenya's National Recovery and Action Plan for Mountain Bongo (Tragelaphus eurycerus isaaci) identifies involvement of local communities in the conservation of this endemic antelope as a key objective (Kenya Wildlife Service, 2019). The plan recommends managing captive and wild mountain bongo as a global meta-population with the help of conservation translocations. Fewer than 100 mountain bongo survive in the wild, with small populations fragmented between four montane forest areas isolated from each other by 45–75 km wide stretches of farmed and settled lands (Svengren et al., 2017). In contrast, around 500 mountain bongo persist in captivity around the world, including approximately 52 at a captive breeding facility within Kenya (Kenya Wildlife Service, 2019; Mount Kenya Wildlife Conservancy, 2021).
Eburu National Forest, at 87 km2, is the smallest of the remaining bongo-inhabited forests. Gazetted during colonial rule with little regard for local communities, Eburu originally formed part of the much larger Mau Forest Complex. Surrounding non-gazetted forest was lost over time, however, so that by the end of the twentieth century Eburu had become an isolated island of forest surrounded by crowded farms and pastures. By 2001, small-scale agriculture and logging were commonplace inside the forest reserve, resulting in considerable forest loss and degradation (Baldyga et al., 2008; Ministry of Environment Forestry, 2018). Evictions followed, but also considerable work to engage forest-adjacent communities in conservation, enable sustainable use, and foster restoration. Since November 2014, Eburu has been fully encircled by a 43.3 km electric conservation fence intended to protect human life, livestock, and agricultural crops in neighboring communities from wildlife and, conversely, protect the forests from intrusion by livestock, poachers, illegal farming, logging, and charcoal production (Kenya Forest Service, 2017). In combination, these measures have provided hope and evidence for effective protection of the montane forest ecosystem at Eburu.
In this context, Eburu has been identified as target for a captive-to-wild conservation translocation for mountain bongo with a working wild population target of 20 individuals (Kenya Wildlife Service, 2019). We therefore set out to explore biological feasibility via sign surveys and intensive camera trapping, and socio-economic feasibility via focus groups, household surveys, and interviews with school children.
Although our socio-economic surveys indicated a human context favorable to bongo rehabilitation, our camera-trapping revealed threatening illicit activities that astonished local stakeholders. The insights gained inspired a concerted effort to protect forest resources for both mountain bongo and local citizens. We here delineate the interplay between community exclusion and engagement that led Eburu to become suitable for consideration as a translocation site, report the results of our socio-economic and ecological feasibility analysis, recount the events that brought illegal forest use to light, and describe the interventions sparked by the discovery. We conclude with concrete recommendations regarding a conservation translocation of captive-bred mountain bongo to Eburu National Forest.
Materials and Methods
Focal Species
The mountain bongo is a critically endangered, nocturnal, or crepuscular antelope that used to occur in montane forests of Kenya and Uganda but is now endemic to Kenya with small populations surviving on Mt. Kenya, in the Aberdares, the Mau Forest complex, and Eburu Forest (Gibbon et al., 2015; IUCN SSC Antelope Specialist Group, 2017). Large-bodied and spiral-horned, mountain bongo were a popular target for trophy hunters in the past, with hunting licenses issued from 1910 onwards (Prettejohn, 2020). In the 1970s, mountain bongo additionally became the target of live capture for zoos and game parks worldwide (Prettejohn, 2020). Hunting pressure coupled with forest loss and degradation led to population declines and extirpation from various parts of the antelope's range (Mt. Elgon, Mt. Londiani, Cherangani Hills, and Chepalungu Hills; Gibbon et al., 2015; IUCN SSC Antelope Specialist Group, 2017; Kenya Wildlife Service, 2019). Where mountain bongo persist, they are found in rugged terrain with structurally complex vegetation (Estes et al., 2011), and are thought to consume bark, roots, and the leaves of various shrubs, herbs, climbers, and bamboo (Kenya Wildlife Service, 2019). Historic information provided by former hunters suggests that mature bulls are either solitary or lead herds with females, calves and young males, herd size ranging from 4 to 15 or more where browse was plentiful year-round. Out-group males range alone or in pairs (Sheppard et al., in prep.). The maximum number of mountain bongo thought to have existed in Eburu in the 1970s, when populations were still healthy, was 20–30 animals, with a ranging distance of 10 km or less. Like elephants (Loxodonta africana), bongo were observed to repeatedly follow the same trails, making them easy to track, hunt, and snare (Sheppard et al., in prep.).
Prior to this study, the most recent evidence of bongo presence in Eburu stemmed from continuous camera trapping 2006 through 2018 with between three and five cameras set by the Bongo Surveillance Project (BSP) (Prettejohn et al., 2020).
Study Area
Eburu National Forest, located between longitudes 36°07′ and 36°16′ East and latitudes 0°40′ and 0°37′ South in Kenya's Nakuru County, was originally gazetted in 1932 (Boy, 2017). Once an integral part of the much larger Mau Forest Complex, Eburu Forest is now a fenced island, surrounded by agricultural settlements on all sides (Figure 1). The forest is nestled in the rugged terrain of Mt. Eburu, a volcanic massif with two peaks. Indeed, the terrain is so hilly that while the planar area of Eburu Forest is only 87 km2, the surface area increases to 92.6 km2 when factoring in topography. Altitudes range from a low along the fence lines of 2,068 m to a high at Ososogum/Eastern Summit of 2,855 and drive an elevational vegetation gradient, with Acacia trees and leleshwa (Tarchonathus camphoratus) scrubland typical of lower areas, superseded by Dombeya torrida forests higher up that then give way to a mix of Podocarpus spp., Crotalaria spp. and highland bamboo, and finally open moorland. Precipitation averages 700–760 mm annually and generally falls during two seasons: long rains from March to May and short rains between October and November. Temperatures typically range from 24 to 29°C, with the hottest season occurring December to February (Kenya Forest Service, 2017).
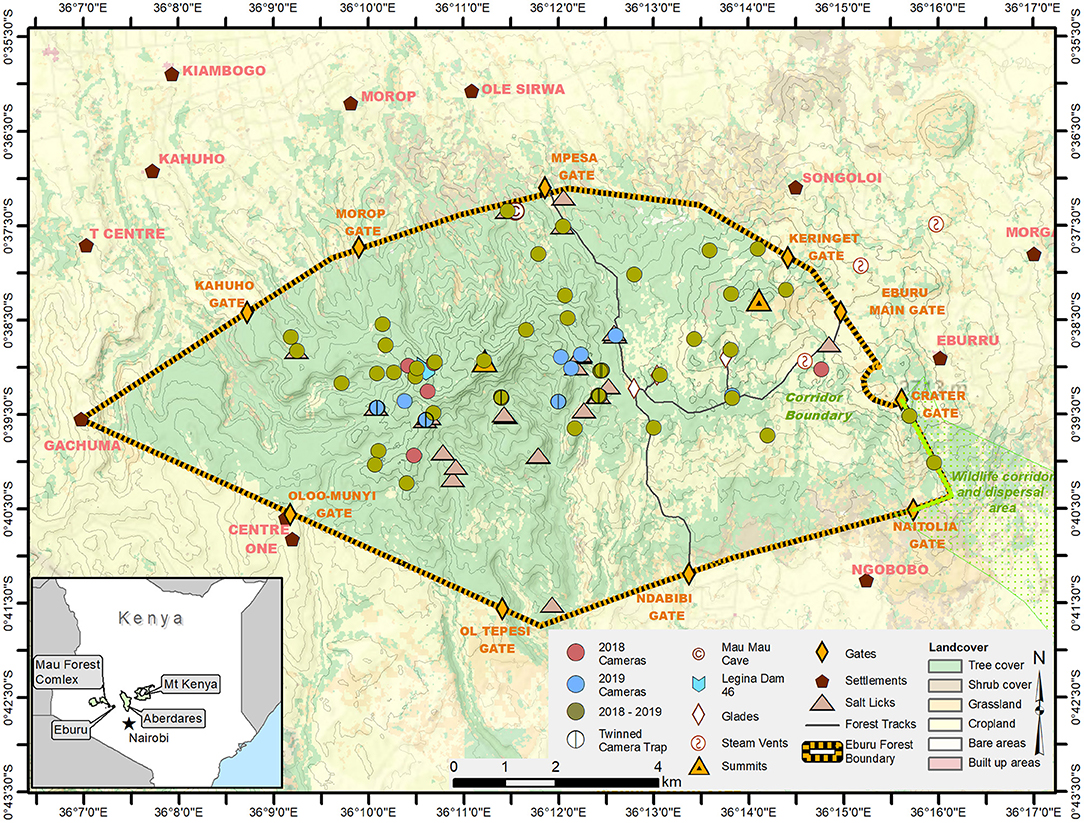
Figure 1. Study area of Eburu National Forest, Kenya, and surrounding human settlements. Green, blue and mauve circles indicate the camera trap locations within the forest. The inset depicts Eburu's location relative to the other forests where mountain bongos still remain in Kenya.
Montane forests like Eburu act as critical water catchment areas and jointly supply millions of households with water. Rivers emanating from Eburu and the nearby Mau Forest Complex are also the lifeline of conservation and tourism areas downstream, including key lakes such as Lake Naivasha and Lake Nakuru (Albertazzi et al., 2018).
Human presence in Eburu dates to at least the late Middle and Upper Pleistocene 120,000 to 45,000 years ago (Van Baelen et al., 2019). Indigenous hunter-gather groups were largely assimilated or displaced by waves of pastoral immigrants approximately 3,000 and 2,000 years ago and finally the arrival of the Massai in the eighteenth century. Forest-dwelling hunter-gatherer groups that had persisted to this time became collectively known as Ogiek. Livestock diseases and smallpox introduced with the arrival of Europeans in the nineteenth century devastated Masaai communities and cleared Eburu for settlement by Europeans, who established large, sparsely settled farms in the area in the early twentieth century (Boy, 2017). Under British colonial rule, the Indigenous Ogiek were evicted from Eburu and the larger Mau Forest ecosystem on multiple occasions between 1911 and the 1930s (Sang, 2001) but continue to this day to engage in beekeeping and other forest activities on the land that their ancestors once walked. Post-independence in 1963, the de-colonization of the “White Highlands” of Kenya resulted in waves of in-migration of different tribal groups including Kikuyu, Kipsigis, Kamba, and Luhya, and further evictions of Ogiek from gazetted forests. Today the area surrounding the reserve is surrounded by densely packed farms and pastures (Boy, 2017).
In the late 1990s and early 2000s, Eburu Forest Reserve was on the verge of collapse, with a rampant red cedar harvest making way for cultivation (Figure 2). Smoke could be seen across the landscape as charcoal burning was rampant (E. Kihiu, pers. commun., 2021). Recurrent forest fires caused by human activities, including charcoal burning and beekeeping, destroyed extensive forest cover in the lower and middle forest belts. In the upper forest areas, illegal settlements led to the conversion of prime indigenous forest into cultivated areas with annual crops. Kiosks were established inside the reserve where customers could buy chapati, mandazi, and bush meat including mountain bongo (J. Kiruy, pers. commun., 2021). The crisis situation in Eburu Forest led the Government to carry out an eviction of the illegal settlers residing in the forest in 2006 (Centre on Housing Rights Eviction, 2007; Church, 2015).
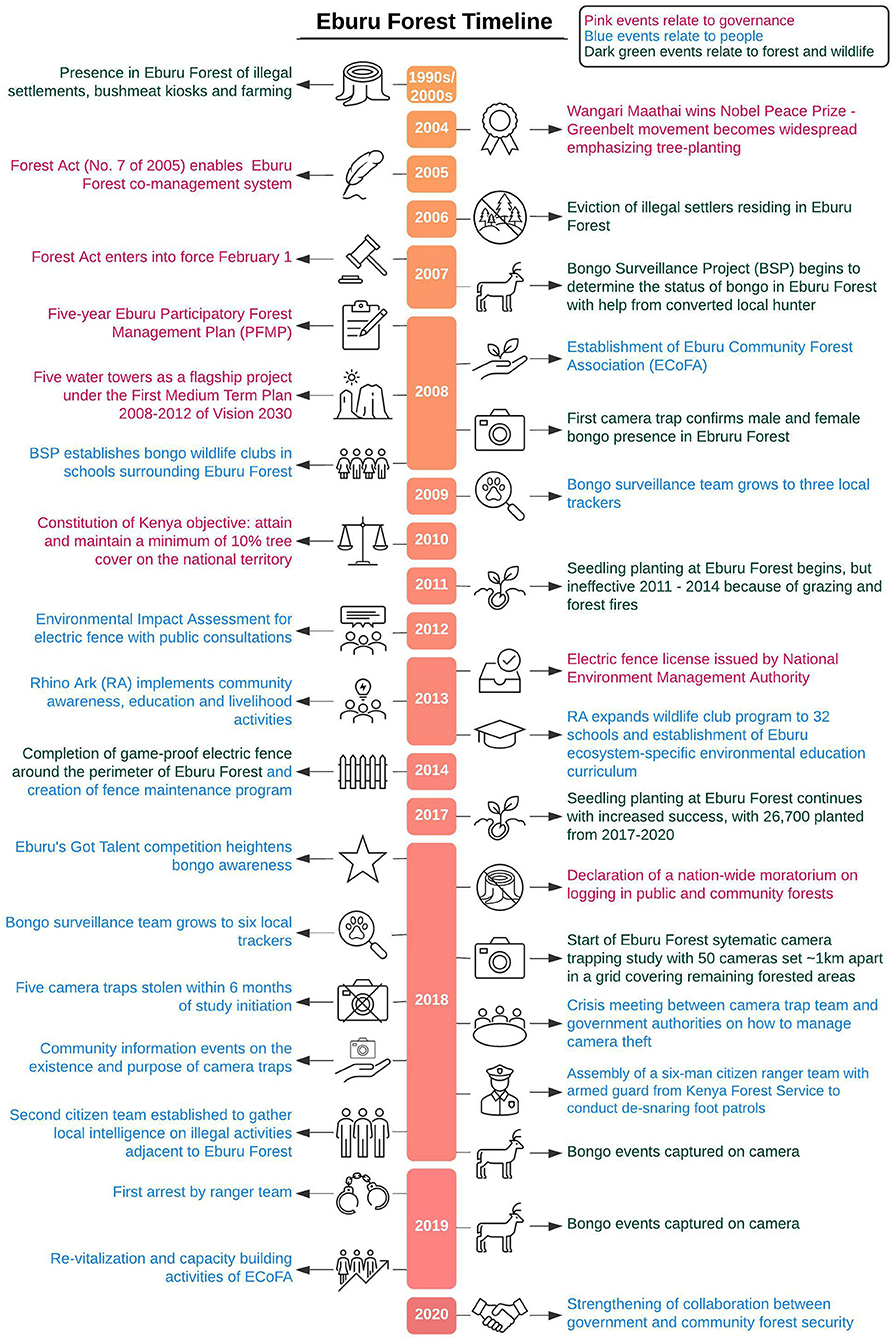
Figure 2. Eburu forest timeline highlighting key conservation-relevant events from the 1990's to 2020. Events in pink relate to governance, events in blue to impacts on local people and events in dark green to impacts on the forest and wildlife.
In the 15 intervening years since then, community engagement has turned around the fate of the forest. We provide an overview of relevant events in Figure 2. Milestones across this time period include a policy change, with the Kenyan government ushering in a forest co-management system in 2005 with the new Forest Act (No. 7 of 2005), which was entered into force on February 1, 2007. In line with the new Act, a 5-year Eburu Participatory Forest Management Plan (PFMP) was prepared in 2008 (Mutune et al., 2015). It was one of the first PFMPs prepared in the country. The PFMP coincided with the establishment of one of Kenya's earliest community forest associations at Eburu Forest (ECoFA) that same year (Mutune et al., 2015). Though the ECoFA started well, with strong financial backing, mismanagement and a lack of funds saw the association reduced to a skeleton structure until re-vitalization and capacity building activities commenced in 2019.
Beginning in 2007, the BSP, a Kenyan NGO, began to determine the status of bongo in Eburu by engaging a notorious local hunter to search for secondary signs of the species. In 2008, a camera trap unequivocally confirmed both male and female bongo presence in the forest (Prettejohn et al., 2020). The team grew to three local trackers by 2009, and two to five cameras were installed on a continuous basis until 2018 when the current study commenced. Starting in 2008, BSP also worked with local schools to establish bongo wildlife clubs that allowed school children to learn about this endemic species in their neighborhood (P. Munene, pers. commun., 2021).
Simultaneously, forest restoration had grown in the public conscience in Kenya through the work of The Greenbelt Movement, which commenced in 1977 and grew into a widespread movement after its founder, Wangari Maathai won a Nobel Peace Prize in 2004 (The Nobel Peace Prize, 2004). Growing nationwide awareness on the need to protect and rehabilitate indigenous forest led to a flagship project focused on Kenya's five most prominent water towers (montane water catchment areas), including the Mau Forest complex of which Eburu formally forms part, under the government's “First Medium Term Plan 2008–2012 of Vision 2030” (Government of Kenya, 2008). Two years later, the 2010 Constitution of Kenya set as an objective the attainment and maintenance of at least 10% tree cover in the nation. In Eburu Forest, rehabilitation efforts began in earnest in 2011 with the planting of seedlings thanks to the Greenbelt Movement and other organizations with a reforestation mandate. Unfortunately, these efforts were initially largely unsuccessful due to poor seedling survival rates as a result of forest fires and cattle grazing (J. Kiruy, pers. commun., 2021).
Around this time, Rhino Ark Kenya Charitable Trust was invited by government institutions and forest-adjacent communities to commence a comprehensive stakeholder process for the establishment of a perimeter fence around Eburu Forest. An Environmental Impact Assessment (EIA) study that included extensive public consultations started in mid-2012. On August 14, 2012, an Eburu Leaders' Sensitization Workshop was held in Naivasha, followed by four community sensitization meetings held in locations adjacent to the forest, which saw the participation of 486 local community members. In addition, questionnaires were administered to 119 individuals from the local communities, private ranches, non-governmental organizations (NGOs), and government departments (Kenya Wildlife Service, 2012).
Following the submission of the EIA report, the National Environment Management Authority issued a license to build the proposed electric fence on February 18, 2013 (letter Ref. No. NEMA/EIA/5/2/927). The first fencing post was placed in March 2013 and the 43.3 km long electric fence was completed in November 2014. Fence construction was undertaken with labor contracted from the forest-adjacent communities to provide local job opportunities and build ownership of the fence. Once completed, a fence maintenance program was established. The most skillful and dedicated 12 community members involved in the fence construction were offered permanent jobs as fence attendants, whereby each of them is responsible for maintaining an approximately 4 km section of the fence (Kenya Wildlife Service, 2014).
Once the fence was in place, rehabilitation efforts became more effective: 26,700 seedlings planted 2017–2020 have enjoyed a survival rate of 20% (D. Chege, pers. commun., 2021).
Concurrently with the fence construction, Rhino Ark started implementing a wide-range of community awareness, education, and livelihood development activities including the rehabilitation of water sources and water projects; the promotion of conservation-based enterprises, such as beekeeping and the growing of fruit trees; and the promotion of more sustainable energy sources, such as biogas and the use of portable kilns to convert crop residue into charcoal (Rhino Ark, 2016).
Building on the bongo wildlife clubs established by BSP, Rhino Ark expanded the program to a total of 32 primary and secondary schools, and designed an environmental education curriculum specific to the Eburu ecosystem. The curriculum was soon adopted by the Kenya Institute of Curriculum Development (Rhino Ark, 2013). In 2018, bongo awareness was heightened when 28 wildlife clubs competed in a talent competition—“Eburu's Got Talent”—that saw them perform poems, songs, and dance routines about the mountain bongo, which subsequently featured on local radio shows.
2018 also saw the declaration of a nation-wide moratorium on logging in public and community forests to allow for a comprehensive review of forest resource management and illegal logging in Kenya (Tobiko, 2018). The moratorium, which to the general public signaled that they should stay out of gazetted forests, has remained in place ever since with limited concessions made in 2020 for mature and over-mature timber plantations (Tobiko, 2020). No timber plantations exist in Eburu (Boy, 2017), which since 2017 has been exclusively managed as a conservation area (Kenya Forest Service, 2017).
Feasibility Analysis
The design of our feasibility analysis, including biological survey methods and protocols for interviewing adults and children, was reviewed and approved in advance of implementation by Kenya's National Commission for Science, Technology and Innovation (NACOSTI). Implementation occurred under NACOSTI permit #17458 and in close, continued collaboration with the Kenya Forest Service, Kenya Wildlife Service, and Eburu's Community Forest Association. The study involved camera trapping and sign surveys targeted at capturing information about mountain bongo, and focus groups, household and student surveys to gain understanding of local ecological knowledge, forest use, and conservation attitudes. While NACOSTI did not require a formal ethics review, all interviews followed the University of Guelph (Canada) ethics guidelines. The Kenya Forest Service in partnership with the Eburu Community Forest Association (ECoFA), with mandated jurisdiction over the co-management of Eburu Forest, guided all interactions with the forest-adjacent communities. In line with the Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity (Secretiariat of the Convention on Biological Diversity, 2011), the Kenya Forest Service requires that a prior informed consent process is duly implemented with regard to community stakeholders surrounding forest resources nation-wide.
Camera Trapping and Sign Surveys
To document the remaining bongo population, its habitat preferences and threats to survival, an existing team of three trackers from the BSP was increased to six in 2018 and trained to undertake systematic camera trapping from 06 March 2018 to 27 November 2019. The trackers, all of them reformed poachers, were selected based on their abundant knowledge of the forest. Fifty camera traps were set to cover all remaining forested areas within the 43 km electric game-proof fence, at an average elevation of 2,548 m (± 113 m) (Figure 1). Although there was an attempt to set the cameras at approximately 1 km straight-line distance in a grid, the terrain made this challenging and, in many instances regular spacing could not be achieved. Non-baited cameras were attached to trees or posts around knee height of the trackers. However, due to the undulating terrain, some cameras set on steep slopes were placed very high or low on a standing tree, or the angle of the camera was tipped so that the field of view was angled toward the trail. At grid points where trackers detected secondary signs of bongo—including tracks, droppings, and scratching posts—cameras were twinned for increased capture success (Burton et al., 2011). Cameras were active 24 h/day with a 9 s delay for a 21-month period and regularly checked to switch SD cards and batteries. On the way to and from cameras, trackers also looked for and mapped secondary signs of bongo and any evidence of human disturbance, including illegal logging, charcoal kilns, leg-traps, and neck snares. Kilns, traps, and snares were dismantled when found. The trackers were highly skilled and recorded only unambiguous secondary signs of mountain bongo. Six months into the study, concerns were raised about forest security after five cameras had been stolen. In response, six community information events (approximately 100–250 audience members per event) were completed in October 2018 on the existence and purpose of the camera traps.
Focus Groups
Four focus groups, each with approximately 10 men and 10 women, were conducted in March 2018 as an entry-level assessment tool with the aim of gaining a sense of locals' interactions with the forest, and of their beliefs, knowledge and attitudes regarding the forest, mountain bongo, and nature conservation. One settlement was selected from each of the four forest-adjacent Locations (sub-district administrative units) where larger settlements existed. Participants comprised community members with dwellings closest to the forest edge, and with an interest, history, or knowledge of forest issues, and were selected by local community conservation leaders. Although a set of guiding questions was at hand (Supplementary Data Sheet 1), the focus groups did not follow a rigorous structure. Instead, open discussion and information sharing among participants was encouraged. These discussions helped inform questions for a more detailed and structured household survey (see below). Focus groups also served to determine general features of the area, including gender relationships and other societal norms, by observing who was able to speak within each group, and the depth of information shared.
Household Surveys
Building on information gained in focus groups, we undertook a household survey via structured interviews with individuals from 200 households (male and female) near Eburu Forest from November to December 2018 (Supplementary Data Sheet 2). Questions addressed local residents' interactions with Eburu Forest before and since perimeter fencing, traditional and local ecological knowledge about the forest ecosystem and mountain bongo, and attitudes toward conservation and a potential conservation translocation. Clearance to conduct the household survey was first sought from the four administrative Location Chiefs that oversee communities surrounding Eburu Forest. Survey participants were selected using stratified random sampling based on distance from forest edge (≤ 1, 1–2, >2 km), gender of respondent (male vs. female), and the relative population size of each of the four administrative units (known as “Locations”) that neighbor Eburu Forest. Each participant was informed of the survey's purpose, that participation was voluntary, that they could decline answering any individual question, and how long the survey would take. Interviews were conducted in the respondent's preferred language (Kiswahili, English, Ma, or Kikuyu) and participants were given a bar of laundry soap as a token of appreciation upon completion.
Student Surveys
Because translocation efforts for large mammals generally require a decade or more of intensive management, we also involved future community leaders in the study by conducting a survey among school students (Supplementary Data Sheet 3). We first secured permission from the relevant administrative chiefs and reviewed the survey's purpose and questionnaire with the headmasters and teachers of all participating schools. We then conducted standardized interviews between March and April 2019 with 300 youths, half of whom were members in their school's wildlife club. At the time, 32 schools had a bongo wildlife club. Student respondents were representatively sampled from 37 schools (24 primary schools; 13 secondary schools) based on the size and gender composition of the school population, and were asked to answer a total of 16 questions that addressed general environmental knowledge and knowledge specific to mountain bongo.
Data Analysis
We reviewed all bongo images captured by camera traps to determine the number, sex, and age (adult vs. immature) of individuals. A new bongo camera event occurred when there were more than 60 min between pictures, or if a different bongo crossed in front of the camera, based on individual identifying features (stripe pattern, horns, age, sex, etc.). We also took note of camera captures of other antelope species to gain an idea of relative frequency of occurrence, of potential predators, and of wildlife overall to assess faunal biodiversity.
All bongo camera captures (N = 5) and data on secondary signs (N = 40) were combined (N = 45) to examine environmental suitability via a maximum entropy model (MaxEnt). MaxEnt uses presence vs. background data and biophysical covariates to predict the suitability of the area for species presence (Phillips et al., 2004). Given the low total number of presence observations and the possibility that they reflect the movements of only a few individuals, the modeling approach we used is equivalent to a resource selection function that contrasts use vs. availability (Boyce et al., 2002; Griffin et al., 2021).
We derived environmental variables from a cloud-free Sentinel 2 image, taken on December 20, 2019 (USGS, 2020), and a Digital Elevation Model (DEM) obtained from ASTERGTM (USGS, 2011). We used PCI Geomatica Software (PCI-Geomatica, 2017) and the raster package in R (Hijmans, 2020; R Core Team, 2020) to derive the following landscape variables from the Sentinel 2 image: Normalized Difference Vegetation Index (NDVI), Atmospheric Resistant Vegetation Index (ARVI), Brightness, Greenness, and Wetness values generated by a tasseled cap analysis, albedo, and NDVI homogeneity (a texture measure that relates to habitat structure) (Jensen, 2007); we used the DEM to derive elevation, slope, aspect and Terrain Roughness Index (TRI—mean of the absolute differences between the value of a cell and the value of its eight surrounding cells) (Wilson et al., 2007). We also used the RCMRD 2016 Land Use Land Cover map derived from Sentinel 2 Global Land Cover data (RCMRD-SERVIR, 2017) to calculate edge density (all landcover transitions in the landscape in relation to the landscape area), as edge habitat has been identified as potentially important for bongo (Estes et al., 2011). Justifications for inclusion of the explanatory variables are provided in the Supplementary Materials (Supplementary Table 1).
Because the input raster data were collected at different resolutions (Sentinel2 = 10 m, vegetation = 20 m, and DEM = 30 m), we resampled the landscape variables to 30 m resolution using bi-linear resampling. Based on extensive surveys and local knowledge of the area, we also derived distance to seasonal and permanent water, distance to forest roads, and distance to salt licks. Before including environmental variables in our model, we ran variance inflation factor (VIF) tests using the “vif” function in the R package usdm to determine multicollinearity and included only variables with a VIF <2 (Naimi et al., 2014).
We ran the MaxEnt model using the “maxent” function (version 3.4.1) in the R package dismo (version 1.3.3) using the recommended settings (Phillips et al., 2004, 2005, 2006; Elith et al., 2011). By default, MaxEnt retains only one species record per grid cell, and thus reduced our sample size from 45 to 37 bongo evidence locations. MaxEnt selects background data at random and can provide internal model evaluation via a jackknife of calibration data, each time predicting probability of occurrence for the omitted calibration grid cells (Phillips et al., 2005). To additionally evaluate model accuracy more rigorously, we divided presence points and the search effort area from which absence points were drawn into two periods. The first two thirds of the mountain bongo presence points served as training data for our baseline model, which covered the period between 06 March 2018 and 18 April 2019 at 9:06 am. The last third of the bongo presence points served model testing, and covered the period from 18 April 2018 9:07 am to 27 November 2019. The precise time point for period separation was dictated by two secondary signs discovered (but not necessarily created) within 30 min of each other on the same day. Search effort polygons for each period where generated by buffering the trackers' search-associated movements and collected GPS points by 30 m. We then calibrated a baseline model with data from Period 1 and used it to predict the probability of bongo presence in the Period 2 search effort polygon for period 2 presence data (13 grid cells) and 130 randomly selected background grid cells. We then used the “evaluate” function in the R package dismo (Phillips et al., 2005; Hijmans et al., 2017) to compute the area under the curve (AUC) of the receiver operating characteristic (ROC), averaged over 30 model runs (each with a different random set of background calibration points). AUC provides a measure of prediction accuracy for presence-absence data that is independent of the threshold used to transform probabilistic into binary predictions of occurrence (Fielding and Bell, 1997). Although use of AUC in the context of presence-background models has been criticized (Li and Guo, 2021), it's use in our case seemed justified, as background data were limited to the search area and hence more likely to represent true absence than unconstrained background data.
We also compared the baseline model with a model calibrated using all combined presence points from Periods 1 and 2 and absences drawn from within the total search effort area across both periods. We then projected the combined model into the full study extent (the entire Eburu Forest rather than just search polygons) over 30 iterations. Next, we averaged the resulting probability of occurrence surfaces over the 30 individual model outcomes to obtain a final predictive surface of suitable habitat. We considered grid cells as suitable bongo habitat where the averaged probability of occurrence exceeded 0.6.
Because the primary aim of our model was prediction (to get a sense of the total area within Eburu Forest suitable for mountain bongo) rather than inference, we did not attempt to test for spatial autocorrelation in model residuals. We expected spatial structuring in the explanatory variables to account for the vast majority of spatial structure in bongo presence points. Any residual spatial autocorrelation, e.g., due to presence records reflecting movements of just a few individual mountain bongo, is not a major concern for prediction (Boyce, 2006; Hawkins et al., 2007). Indeed, attempts to account for such autocorrelation via, for example, inclusion of an autocovariate among explanatory variables often result in higher AUC values (Dormann et al., 2007; McPherson and Jetz, 2007), so our approach likely yields more conservative estimates of model accuracy.
To evaluate threats to bongo, we mapped all human disturbances in Eburu as recorded by the trackers. Threats were classified into two categories. Category 1 included direct threats to bongo, i.e., evidence of poaching such as leg traps and neck snares. Category 2 included threats to bongo habitat, such as evidence of charcoal production, logging, illegal camps, firewood collection, donkey tracks, and marijuana fields. Threats were mapped by year for 2018, 2019, and 2020. We also calculated the average distance of each threat category to the Eburu fence line as a proxy for the degree of forest penetration of the detected human threats, i.e., the greater distance from fence line means greater time spent (illegally) in the forest, and hence greater risk of detection.
Responses to the household survey were analyzed by determining the proportion of respondents who mentioned specific topics. Proportions were straightforward to derive for multiple choice questions. For open-ended questions, responses were manually classified into emerging themes (e.g., finance, culture/spirituality, ecosystem, security, education, etc.) or sentiments (e.g., pride, recognition, future benefits, management concerns, etc.) to then derive the frequency with which each was mentioned across respondents. We used two-sample Z-tests to determine if proportions differed between men and women (Quinn and Keough, 2002).
For student surveys, questions that had one or more correct answers were scored for each student based on the number of accurate responses given. For other questions, we again simply determined the proportion of students who mentioned specific topics. We then calculated a total score for each student as well as separate summary scores for questions pertaining to basic ecology (five questions), deeper ecological knowledge (three questions), nature's benefit to humans (two questions), conservation concepts (two questions), and knowledge of Eburu (four questions). To test for differences between students inside and outside of wildlife Clubs, we used two-sample t-tests for accuracy scores (Quinn and Keough, 2002).
Results
Local Ecological Knowledge, Attitudes, and Forest Interactions
A total of 36 women and 45 men took part in focus group interviews, with each group reasonably gender-balanced. The focus group interviews offered perspective on the shared basis of knowledge about forest perceptions and usage, traditional and cultural forest relationships, conservation attitudes, and mountain bongo. Through the focus groups, we learned of widespread and sophisticated conservation attitudes, support for the perimeter fence surrounding the forest, and that many people claimed to be from other areas and, as such, did not have strong cultural ties to the forest. Those with the longest tenure in the area—the Ogiek and Kikuyu group members—spoke of prayer caves inside Eburu Forest, and of the harvest taboo of an endemic species, the Meru oak (Vitex kenensis). When asked how people felt about a bongo translocation, they immediately responded by clapping enthusiastically, or expressing profound support. We learned that the majority of citizens surrounding Eburu Forest were farmers and the issue universally mentioned for all four focus groups was the need for improved water supply to their farms.
Of the 200 people who participated in household surveys, there were 93 women and 107 men aged 14–82, with the mean, median, and mode of age equal to 40 (see Supplementary Table 2A for more demographic information on the respondents). When the participants were presented with a set of wildlife images (Supplementary Data Sheet 4), 68.5% were able to correctly identify a mountain bongo among other antelopes, although 74.5% had never seen one in person. Local people were largely in favor of increasing the mountain bongo forest population using captive-bred bongo (94.5%). Respondents identified several potential personal advantages of a bongo re-stocking event, including the opportunity to see and/or learn more about bongo (63%), additional employment (60%), and growth in tourism (54%). Responses diverged distinctly along gender lines, with women expressing more interest in the educational benefits (73% women vs. 59% men, z = 2.113, p < 0.05), and men more vocal about the economic benefits (31% men vs. 15% women, z = 2.626, p < 0.05).
Local residents' interactions with Eburu Forest were complex, which is noteworthy as few respondents had long-standing family roots in the area (only 4% of respondents came from families who had lived in the area for at least three generations). The forest had cultural importance for 76% of respondents, including for religious practices (41%), medicinal plants (25% but with significant difference by gender: men 31%, women 18%, z = −2.046, p < 0.05), traditional honey production (24%), and youth initiation practices (22%). Residents also commented on ecosystem services provided by Eburu Forest in the form of economic activities including logging for fuelwood (74%), timbers (59%—men 69%, women 47% z = 3.133, p < 0.05) and charcoal for cooking (50%—men 61%, women 38%, z = −3.261, p < 0.05), grazing areas for cattle (60%), as well as water provision (47%), improved farming (31%), and climate moderation (27%). The importance of a healthy ecosystem was an answer that eclipsed all others (75% of respondents). Under half (43%) observed changes to their forest-based activities since the completion of the electrified, game proof fence in 2014. By restricting residents' access to the forest, the fence had hindered access to fuelwood or charcoal for cooking (22%) and reduced cattle grazing (22%). Most residents, however, supported the fence (84%) and reported that the fence protects farms and livestock from wildlife damage (70%), protects the forest from being destroyed by logging or charcoal production (46%) and increases security or reduces theft (24%). Moreover, 38% of respondents stated that they were participating in forest recovery schemes through indigenous tree planting efforts.
The students interviewed (148 female, 152 male) ranged in age from 11 to 21 (mean age 15), had experienced 8–17 (mean 11) years of schooling and attained either Standard 8 (last year of primary school) or Form 4 (last year of secondary school) (Supplementary Table 2B). Among students, wildlife club members vs. non-members showed no noticeable difference in scores for knowledge on basic ecology (t = 1.608, p = 0.109), deeper ecological knowledge (t = 1.047, p = 0.296), nature's benefits for humans (t = 0.958, p = 0.339), or knowledge of Eburu (t = 1.358, p = 0.175), but differed significantly in scores for conservation concepts (t = 3.460, p < 0.05) and total scores (t = 3.391, p < 0.05); wildlife club members had higher average scores for conservation concepts (mean = 62.9%) and total scores (mean = 57.6%) than non-members (51 and 54%, respectively). More than half the students correctly defined mountain bongo as herbivores (54%) and described bongo as brown or red with white stripes (52.7%); many (45%) fittingly suggested that bongo live in forests. Overall, students had a good understanding of basic ecological knowledge (90% average score on these questions), an average understanding of conservation concepts (57%) and deeper ecological knowledge (51%), and a low understanding of the benefits of nature to humans (31%) or knowledge about Eburu (30%).
Hidden Human Dimensions
Given the fence, Kenya Forest Service guards and a national moratorium on logging, Eburu Forest was expected to be relatively devoid of human disturbance. As soon as the tracker team commenced installing camera traps within the forest, however, they began detecting signs of illegal hunting and logging. When reported to Kenya Forest Service and other forest security stakeholders, these observations were initially largely dismissed. The theft and destruction of five cameras within the first 6 months of the study, however, elevated attention by illustrating the scale of illegal activities, and led to stakeholder cohesion in forest security efforts. Over the course of 2018 and 2019, a total of 19 camera traps were stolen or destroyed by poachers (2018: 2 in May, 3 in July, 1 in December; 2019: 2 in January, 7 in February, 4 in March; Figure 3).
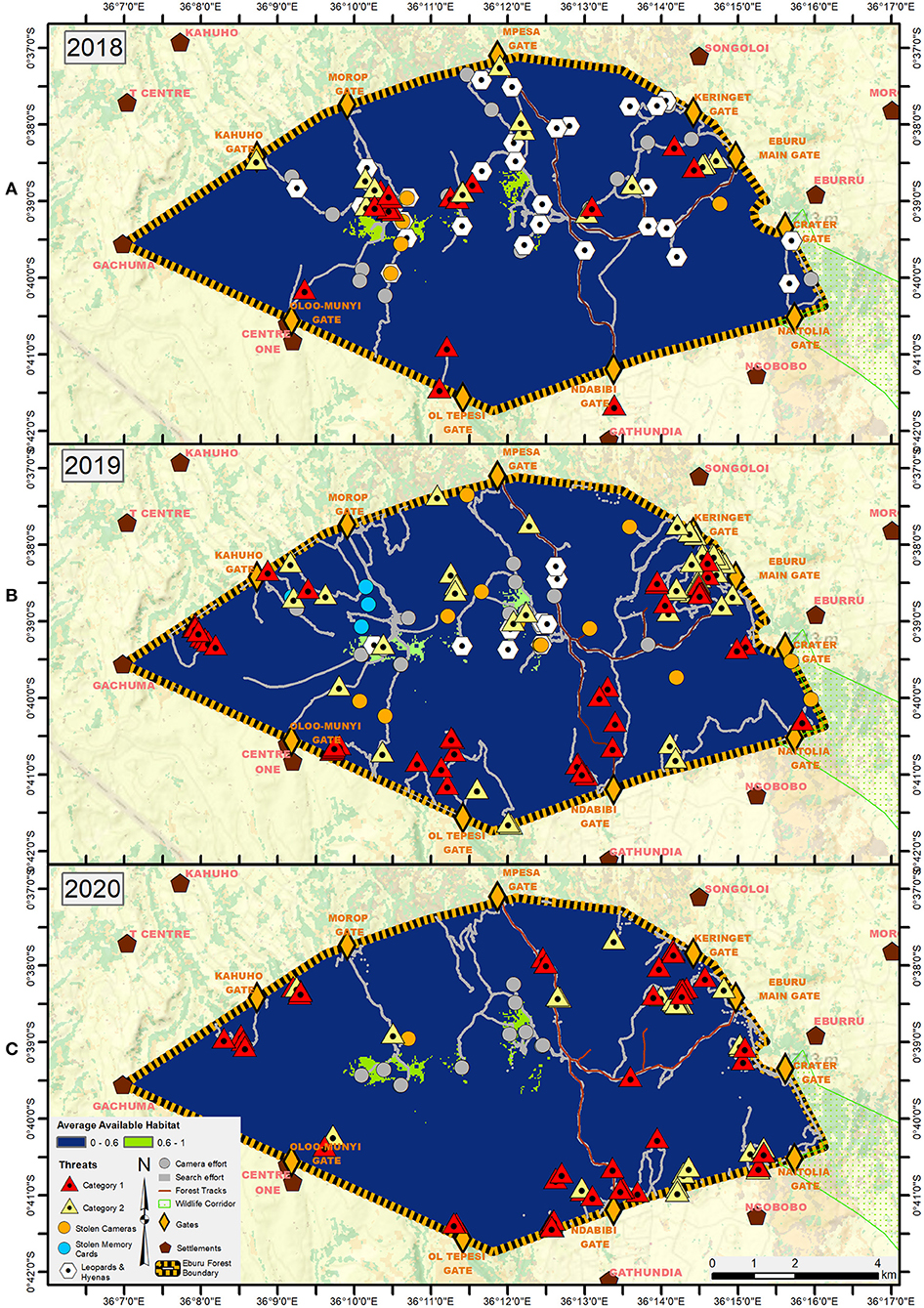
Figure 3. Threats detected in Eburu forest in (A) 2018, (B) 2019, and (C) 2020, including direct threats to bongo (Threat Category 1: evidence of poaching such as leg traps and neck snares), threats to bongo habitat (Threat Category 2: evidence of charcoal production, logging, illegal camps, firewood collection, donkey tracks, and marijuana fields), interference with camera traps, and signs of potential predators (leopards, hyenas). In 2020, the impact of COVID-19 on field operations demanded focus on data for mountain bongo and human threats, so we do not have reliable predator data for 2020.
Strategic Intervention
Recognition of the presence of serious environmental crime inspired forest security stakeholders to disguise camera traps to evade poachers by wedging the cameras within chiseled recesses of upright, rotting logs, and the assembly of a six-man citizen ranger team together with an armed guard from Kenya Forest Service to conduct de-snaring foot patrols (Figure 2). Beginning in mid-2018, the team conducted regular multi-day patrols over 10–15 days per month in the forest. Each day, the team walked for approximately 6–7 h covering different forest sections on a rotating basis to dismantle and record the location of any illegal items observed. In addition, another citizen team was established to gather local intelligence on illegal activities from their home settlements adjacent to the forest. The identities of the six men involved are kept confidential, with reports gathered at a command center by a neutral non-resident.
The community trackers and joint forest security patrols discovered more than 60 leg traps/neck snares, 15 charcoal kilns, and 25 firewood and timber cache sites over 2018/2019 (Figures 3A,B). As a result of these efforts, a retaliatory physical assault was carried out on one of the community trackers by four of his neighbors, angry at the constraints being placed on their illegal forest activities. The four men were arrested and charged (cases are before the courts at this time). Five other culprits caught on hidden cameras were cautioned or arrested by the wildlife authorities, and a notorious poaching gang disbanded toward the end of 2019. The collaboration between government and community forest security forces strengthened further in 2020 with 92 leg traps/neck snares, 35 charcoal kilns, 10 fuelwood collection sites, and 11 rafter harvesting sites detected and dismantled (Figures 2, 3C). Ten joint forest security stakeholder meetings were held—roughly one per month.
Remaining Mountain Bongo at Eburu
Over the course of the 8972.79 days of camera trapping effort, camera traps recorded approximately 600,000 images, 182,781 of which were wildlife images, the remainder false triggers. Camera traps recorded only five bongo events, captured on three cameras, with all captures being of single adult males (Figure 4). Two bongo events were captured on Camera 29 on November 15, 2018 at 19:40 and November 16, 2018 at 04:34; one bongo event was captured on Camera 3a on April 1, 2019 at 19:33 h; and two bongo events were captured on Camera 33 on March 4, 2019 at 20:22, and April 21, 2019 at 23:16 (Figure 2). Cameras 3a, 33, and 29 were 1.5–2 km apart as the crow flies, separated by deep ravines (1.5 to 3.5 km apart considering the topography of the area). An additional 40 bongo secondary signs were observed during the study (11 in 2018, 29 in 2019).
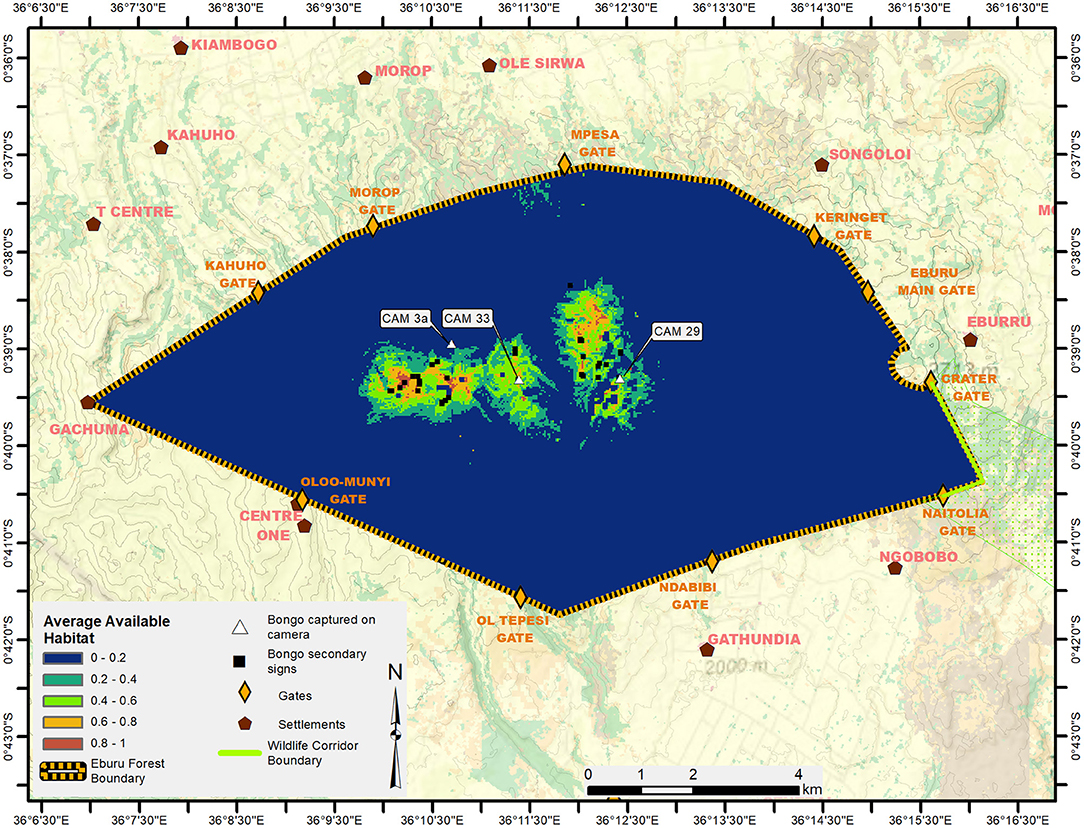
Figure 4. Environmental suitability for mountain bongo in Eburu Forest based on MaxEnt modeling, with warmer colours representing higher suitability. The camera labels highlight the locations in which mountain bongo were detected on camera traps and the black squares indicate where secondary signs of bongo were observed.
Other antelope captured on camera traps in Eburu Forest in 2018 and 2019 were bushbuck (Tragelaphus scriptus; 143,994 images), red duiker (Cephalophus harveyi; 7,809 images), waterbuck (Kobus ellipsiprymnus; 96 images), and Kirk's dikdik (Madoqua kirki; 65 images). Bushbuck and red duiker were the most widespread antelope species, recorded on all and at 33 camera traps, respectively, including the three cameras at which bongo were recorded. Kirk's dikdik were recorded at six cameras and waterbuck at one. Additionally, 72 secondary antelope signs were observed: 54 bushbuck (29 in 2018, 25 in 2019), 13 duikers (4 in 2018, 9 in 2019), and 2 dikdik (both in 2019).
Potential bongo predators recorded on camera traps were leopards (Panthera pardus; 98 images) and spotted hyenas (Crocuta crocuta; 769 images). Between 2018 and 2019, leopards were found at 14 camera traps and hyenas at 21 cameras. Leopards were found on the same or twinned camera at all three locations were cameras recorded bongo; hyenas overlapped at two of the three locations. An additional 17 predator secondary signs were observed: 4 leopard (2 in 2018, 2 in 2019) and 11 hyenas (6 in 2018, 5 in 2019).
Along with the antelope and potential bongo predator species, a total of 33 mammal species were recorded on camera traps in Eburu Forest in 2018 and 2019, including 12 carnivore species, 3 non-human primates, 7 rodent, and 9 ungulate species, as well as domestic dogs and humans. The number of species recorded at each camera ranged from 3 to 22, with an average 10.7 mammal species per camera trap (SE ± 0.6). Waterbuck were the only mammal species recorded at a single camera trap site, and bushbuck were the only mammal species found at every camera trap site (Supplementary Table 3). Humans were recorded on 18 of the camera traps.
Environmental Suitability
The minimum elevation at which bongo evidence was recorded was 2321 m. All camera captures of mountain bongo occurred in dense forest (mixed montane forest including Podocarpus spp. and Crotalaria spp.) combined with highland bamboo (Yushania alpina) at an average elevation of 2,544 m (SE ± 45 m). Secondary bongo signs were also found in the same dense vegetation layer at an average elevation of 2,574 m (SE ± 105 m).
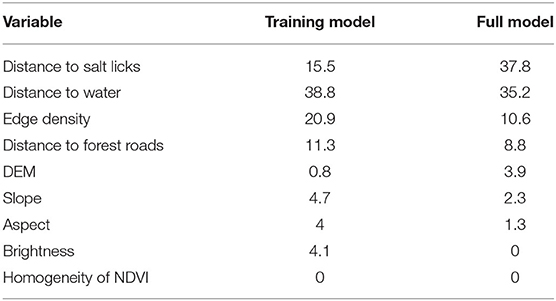
For the MaxEnt environmental suitability models, VIF analysis reduced the environmental variables retained to distance to roads, distance to salt licks, distance to forest roads, edge density, aspect, brightness, DEM, homogeneity of NDVI, and slope. Model accuracy was high with average AUC = 0.964 for the jackknifed calibration data and 0.942 (±0.014 standard deviation) for the Period 2 testing area. Individual explanatory variables ranked similarly in their contribution to model fit in both the base model and the model calibrated with combined data, as judged by gains and losses in jackknifed AUC. The MaxEnt output provides the permutation importance of each variable in the model, where for each variable, the values of that variable on training presence and background data are randomly permuted and the model re-evaluated on this permuted data to examine the resulting percentage drop in training AUC. In both models, permutation importance was highest for distance to salt licks (15.5 and 37.8% for the base and full model, respectively) and distance to seasonal and permanent water (38.8 and 35.2%, respectively). Other variables of importance were edge density and distance to roads (Table 1).
Figure 4 shows the final averaged habitat model for mountain bongo in Eburu. The amount of available habitat (p > 0.6) was small at 2.16 km2 (2.3 km2 when considering the hilly topography), with only 2.5% of Eburu Forest classified as suitable.
Human threats to bongo occurred in or close to suitable bongo habitat at the start of the study in 2018 (Figure 3A) with distance to the fence line averaging 2.2 km ± 0.8 for threat Category 1 and 1.8 km ± 1 for threat Category 2. Threats then gradually moved out toward the fence line in 2019 (Figure 3B) with average distances equaling 0.8 km ± 0.6 and 0.9 km ± 1, respectively. This trend continued into 2020 (Figure 3C); with respective average distances at 0.6 km ± 0.5 and 0.8 km ± 0.7.
Discussion
The frequency of conservation translocations has increased exponentially over the last 30 years, and projections suggest that such increases will continue in the future to prevent regional or global extinction of species, to restore faltering populations, or to improve the ecological function of ecosystems (Moehrenschlager et al., 2013; Armstrong et al., 2018; Swan et al., 2018). While conservation translocations may enjoy wide-spread public support where recovery entails proximate positive outcomes for local communities (Williams and Haines, 2021), situations where the species of interest, or their protection, threaten local livelihood can result in vehement opposition that precludes the initiation of conservation translocations or their long-term success (Vaske et al., 2013; Gray et al., 2017). While biological considerations are often the focus of conservation translocation studies, the viability of managed populations is frequently linked to socio-economic, political, or legal factors (Riley and Sandström, 2016).
Holistic feasibility assessments for conservation translocations that from the onset consider community context and perspectives alongside ecological parameters are explicitly recommended by the IUCN Guidelines for Reintroductions and Other Conservation Translocations (IUCN, 2013). Nonetheless they remain rare but are gaining momentum (Leiper et al., 2018; McMurdo Hamilton et al., 2020; Rayne et al., 2020). The analysis presented here provides a valuable case study that illustrates both the complexities and necessity of a holistic approach.
Focus group discussions and structured interviews with community members or key informants are common tools for gaining insights on the local populations' interaction with the environment and associated needs, wants, attitudes and aspirations (Bajracharya et al., 2005; Nyumba et al., 2018). While it is known that such tools may fail to reveal practices that are illegal or perceived to be so, this can be mitigated to a degree by assuring participants of their anonymity and that no incriminating information would be shared with government and law enforcement agencies (Gavin et al., 2010; Solomon et al., 2015). Even where confidences can be gained, however, illicit practices may not come to light if constrained to a small proportion of the population and conducted in sufficient secrecy that the general public is unaware.
In Eburu, the vast majority of survey participants supported conservation of the forest, the electrified fence with its dual purpose of protecting humans from wildlife and vice versa, and the idea of bolstering local mountain bongo numbers with the help of conservation translocations. Yet camera trapping and sign surveys, intended to collect ecological information for the feasibility study, quickly revealed that a small number of community members were actively undermining forest conservation efforts by illegally hunting and logging within the forest reserve.
Some illicit activity was to be expected: a previous study on resource-use conflict had noted that a small proportion of the forest-adjacent community extracted charcoal and timber from Eburu without permission, or had expressed disgruntlement vis-a-vis the fence and permit restrictions on firewood and life stock grazing (Makhanu, 2015). Interference of the fence with access to charcoal, fuel wood and grazing was also mentioned by 22% of participants in our study. Neither study, however, predicted poaching, nor the tenacity with which perpetrators would defend their illegal activities.
That the discovery of illegal activities was at first met by incredulity among the stakeholders responsible for forest security is undoubtedly not unique to Eburu Forest (see e.g., Dureuil et al., 2018; Sabuhoro et al., 2020). Although the concept of “paper parks” exists (officially gazetted protected areas that are not or are poorly enforced—Bruner et al., 2001), the presence of visible protective measures, such as Eburu's electric perimeter fence and eight Kenya Forestry Service stations manned by guards, can lull people into the belief that protective measures are effective. Any observations reported to the contrary are then dismissed as isolated incidents that pose no overall threat. In our study of Eburu, it took the consecutive theft of multiple pieces of expensive and, in Kenya, hard-to-come-by field equipment, to shake forest security stakeholders out of this complacency.
With multiple stakeholders involved, the resulting awareness might have descended into a mutual blame game. Thanks to a quick intervention that gathered all relevant agencies in an emergency meeting, the result instead has been the building of mutual trust, commitment and coordination, now reinforced monthly at joint forest security meetings. These meetings serve to remind the different stakeholders of their shared goals, exchange information and ideas, and closely coordinate efforts. They have given rise to two innovative approaches that involve citizens in mitigating conservation threats, have led to some of the illicit actors being successfully reprimanded, and have been effective at pushing potentially harmful activities away from primary mountain bongo habitat toward the vicinity of the fence.
Confidence in a joint ability to tackle the crisis and ideas to involve ordinary community members in the response would have been less forthcoming without the knowledge—gained during focus groups and household interviews—that the population at large is supportive of forest conservation. Hence while it was our ecological monitoring efforts that inadvertently revealed the problem, planned community consultation contributed to the solution. This illustrates the value of soliciting community perspectives up front alongside ecological investigations rather than as an afterthought.
Broad community support for forest conservation and mountain bongo likely reflects, at least in part, the diverse and durable efforts over the past two decades by various organizations to raise environmental awareness and engage the communities bordering Eburu in habitat restoration. Although intimate knowledge of and appreciation for an ecosystem and its gifts and services is common in communities that have a long-standing association with a particular landscape or environment (Pretty et al., 2009), the relatively recent arrival of much of the population surrounding Eburu might have made environmental respect less inherent. It is noteworthy, therefore, that when asked about the benefits of Eburu Forest, 75% of household survey respondents mentioned ecosystem health, and that the majority of adults and children knew what mountain bongo look like even though most had never seen one.
Similarly, the fact that a forest island, which seemed doomed by degradation just two decades ago (Church, 2015), was found to teem with a diversity of wildlife (33 mammal species documented through camera traps), including top predators, speaks to the success of protective measures taken. These measures combined legal barriers (official protected status), physical barriers (presence of guards, fence), and social barriers (community awareness and engagement). Any instrument on its own is unlikely to have been as effective. Protected status alone rarely is. Community engagement alone can be, particularly where community-wide cultural or spiritual ties align with a conservation ethic (Davies et al., 2013), a scenario not applicable to Eburu. Physical barriers on their own are also unlikely to suffice, as most can be overcome or circumvented. Guards cannot be everywhere, and Eburu's electric fence, for example, is game-proof rather than human-proof, not infrequently breached by short-circuiting, and under constant need for maintenance (Otungah et al., 2008). Seeing the fence built and maintained, however, helped signal to surrounding communities that talk around the importance of conservation and around ensuring the community's' safety from wildlife was sincere, and that the organizations involved can be trusted to follow through on promises (McLeish, 2020). The health of Eburu Forest, and with it the prospects for a conservation translocation of mountain bongo being feasible, have hence benefitted from a long-term investment in readying both the ecosystem and surrounding human communities for the potential release of a critically endangered species.
Nonetheless, our ecological analysis determined that Eburu Forest holds only roughly 2.2 km2 of suitable environment for mountain bongo. This is a fraction of the area typically used by mountain bongo herds given historic observations that 8-10 bongo might range over 10 km (Sheppard et al., in prep.). Assuming 10 km can be interpreted as a diameter, this translates to ~78.5 km2. At face value, this might suggest that reinforcement of the remaining bongo population at Eburu with captive-bred individuals is not feasible without first implementing extensive habitat restoration to expand the area suitable for release.
Our environmental suitability analysis, however, may be misleading. The rarity of camera captures (3 bongo events among 182,781 wildlife images), and the fact that each capture involved lone males suggests strongly that our observations of bongo habitat use are limited to the movements of at best 2–3 lonesome survivors. Remnant individuals or populations may not utilize the best or all available habitat, and may in fact be pushed into sub-optimal areas given human-induced threats (Namgail et al., 2007; Shanee, 2009; Fowler et al., 2012). Moreover, habitat selection by individuals does not necessarily reflect limiting factors relevant at population-scale (Germain and Arcese, 2014; Dunn and Angermeier, 2016), and this may be particularly pertinent for individuals that would ordinarily reside in herds. It therefore seems unwise to conclude that the environmental conditions that correspond to the locations currently frequented by the few individuals remaining in Eburu represent the best or only suitable habitat for bongos in this forest. In the Aberdares, for example, where the size of surviving forest is much larger than Eburu (225,224 vs. 8,715 ha), and provides a less human-penetrated core, the country's most intact mountain bongo population (with an estimated 40–50 individuals; Kenya Wildlife Service, 2019) resides at lower elevations, and in areas with reduced extremes in terrain (DSh, pers. observ.).
This is not to say that habitat restoration would not be helpful. Clearly, ongoing reforestation of denuded areas with native tree species is most welcome and should continue for multiple reasons, including forest health and regeneration, the benefits it may ultimately bring to bongo, increased resilience of ecosystem services, and continued engagement and pride of community members in conservation actions. The practice is well-established in Eburu, with 3,600 indigenous seedlings planted across 12.2 ha in 2019, and 4,250 seedlings across 16 ha in 2020 (J. Kiruy, pers. commun., August 18, 2021). For direct benefit to bongo in terms of both habitat and community support, it may be useful to specifically include plants favored by mountain bongo as forage among the those grown by school or community nurseries and planted during community events.
A conservation translocation of mountain bongo to Eburu, however, need not and likely should not wait until additional suitable habitat has been created. With so few mountain bongo left, and a likely lack of females, reinforcement is urgent. Reinforcing existing populations, even if very small, is generally considered easier than reintroducing species to locations from where they have vanished completely (Champagnon et al., 2012; Martin et al., 2012; Hardy et al., 2018). This is even more critical for translocations of captive-born individuals who are less likely to have the essential survival skills than animals that are sourced and translocated from other wild populations. The remaining wild individuals in Eburu can still serve to anchor released animals near release sites, to prevent dangerous post-release dispersal, and to illustrate key survival behaviors in terms of foraging, activity periods, and anti-predator behaviors (Moehrenschlager and Lloyd, 2016). Moreover, timing of reinforcements has been identified as an important factor in determining success, with earlier onset yielding better long-term results (Hardy et al., 2018).
We fully acknowledge that Eburu Forest in its current state, and even if fully restored within its fenced boundary, is too small to host a mountain bongo population of sufficient size to sustain itself in the long-term. The Mountain Bongo Task Force has set a tentative target size for the wild population at Eburu of 20 individuals (Kenya Wildlife Service, 2019), which is in line with historic population estimates for the area (Sheppard et al., in prep.). Populations of this size were likely viable in Eburu in the past thanks to connectivity with populations in the larger Mau Forest Complex. The insular nature of Eburu Forest as it exists today, however, means that a population that small would need to be carefully managed (as is planned) to avoid the detriments associated with small size, including demographic and environmental stochasticity, potential allele effects, genetic drift, and inbreeding depression (Caughley, 1994).
As the first reinforced wild population within an artificially managed mountain bongo meta-population, however, a small population at Eburu could be immensely valuable, particularly in terms of developing effective release strategies. Moreover, established populations could be seen as a potential “stepping stone” site which not only serves as a destination for naïve captive-born animals, but indeed as an eventual source for wild-born behaviorally superior animals that could be translocated to other protected areas (Lloyd et al., 2019).
Several aspects of the site render it favorable for a conservation translocation.
First, illegal activities within the forest, although still ongoing, are now understood and actively being managed with considerable success. Their increasing confinement toward the edge of the forest indicates that perpetrators fear the increased chance of detection associated with the longer time required to penetrate deeper into the forest and more pristine habitat. The electric fence and recently established Kenya Wildlife Service outpost, combined with citizen engagement in forest security, help deter all but the most tenacious minority of offenders.
Second, political will and stakeholder support exist, as illustrated by the considerable effort put into joint forest security measures by both the Kenya Forest Service and Kenya Wildlife Service and earmarking of Eburu as a potential translocation site (Kenya Wildlife Service, 2019). The two government agencies are further supported in Eburu Forest by an engaged and organized Community Forest Association, plus technical and financial support from conservation NGOs including Rhino Ark, BSP, and Eburru Rafiki. Forest-adjacent communities are enthusiastic about a conservation translocation, with 94.5% of household heads interviewed in favor of bongo reinforcement. Although for some the enthusiasm is tied to expectations of economic benefits through tourism, many also recognize a more intrinsic, educational value to boosting bongo presence. Moreover, hopes for tourism might in fact materialize, as Eburu is not far from Lake Naivasha, which consistently attracts large numbers of visitors (Abiya, 1996; Njiru et al., 2017). Initial interest in Eburu might be enticed by tales of elusive bongo, and subsequent visits by the rugged, beautiful, volcanic habitat the bongo calls home.
Thirdly, despite the rugged terrain, Eburu also offers a more accessible site for a bongo conservation translocation than alternative locations. Excellent existing rural and forest roads provide access to areas in Eburu in close proximity to suitable habitat and so would facilitate transport of captive-bred individuals to soft-release pens.
Finally, much of Eburu's advantage lies precisely in being small. Conservation translocations aim for species or ecosystem benefits, but risks need to be considered not only for the released individuals but also remaining conspecifics, other species, or human communities. The limited size of Eburu Forest permits a conservation translocation here to effectively test and impact an entire but well-contained ecosystem, facilitating genuine insights on how a reinforced population interacts with other species, including predators and competitors, and thus providing room for adaptive management (Moehrenschlager and Lloyd, 2016). Moreover, reinforcements do carry risks for remaining wild individuals, such as pathogen transfers or maladaptive genetic swamping (Champagnon et al., 2012), although these very rarely manifest in conservation translocations (Novak et al., 2021). With clearly very few wild individuals remaining at Eburu, the overall risk to wild mountain bongo would be minimal should such unintended consequences occur, and mistakes could be rectified before undertaking reinforcements in the Aberdares, at Mt. Kenya or in the Mau Complex proper, where larger wild populations persist.
To avoid mistakes in the first place, however, careful consideration should be given to the selection of individuals for release with regards to behavioral suitability, genetic and physical health, including the absence of pathogens and parasites (Champagnon et al., 2012; IUCN, 2013). Given the apparent absence of female mountain bongo in Eburu and what is known about historic herd composition, we recommend that an initial release involve 3–6 females. We also recommend release of one mature and potentially one immature male, as lack of recent camera trap evidence could suggest that the previously observed male individual(s) have since died. Experience in zoos suggests that males can be paired (C. Magner, pers. commun., 2021), and age-differentiated pairs have been observed in the wild (Bosley, 2003).
The bongo individuals selected for release should be acclimatized to each other and to Eburu Forest in on-site soft release pens for a month or more. Once released, the individuals should be carefully monitored by an established network of approximately 20 camera traps serviced by the now experienced tracker team in habitat identified as most suitable. In addition, a tree hideout could be constructed near the release sight to facilitate unobtrusive, live observation, and feeding stations set up to gradually wean released animals off the diet they were accustomed to in captivity.
Subsequent releases of up to a total of 30 individuals over 3–5 years, depending on post-release mortality and availability of individuals for release, might aim to maintain the population at a sex-ratio of 3–4 females to 1 male of varying age and maturity, again carefully selected for health and behavioral aptitude. Once a herd has established and is actively reproducing, conservation translocations can be limited to the occasional transfer of individuals from and to either captive or stabilized wild populations for maintenance of genetic diversity. All releases should be followed by careful and long-term monitoring. Monitoring will be critical in determining if herd bonds form and last, if reproduction occurs, if young fall prey to opportunistic predators, and if poachers try to take advantage of tame or at least somewhat human-accustomed mountain bongo.
Monitoring should not, however, be limited to biological aspects. Although the IUCN Guidelines for Reintroductions and Other Conservation Translocations focus on monitoring biological goals (IUCN, 2013), it is equally important to monitor socio-economic aspects of a conservation translocation. Releases involve decisions regarding the selection and support of individuals and sites and these considerations should incorporate human dimensions iteratively in an adaptive management process. Monitoring not only includes ecological parameters, but also needs to assess human dimensions on an ongoing basis (Moehrenschlager and Lloyd, 2016). For success in re-establishing a small mountain bongo population in Eburu, community support will be critical for decades to come. Regular attitude checks via repeated household surveys every 2–3 years will be important, and note should be taken of any bongo-related comments that arise in discussion at the Community Forest Association or other community discussion fora.
Additionally, community spirit might need to be actively fostered. Community festivities, such as a repeat of the “Eburu's Got Talent” competition that allowed schools to showcase their knowledge about mountain bongo, are one option. Another is to ensure that community members experience tangible benefits from their conservation efforts. Assisting the Community Forest Association through capacity building, working toward facilitating a sustainable honey cooperative, and public recognition and support for local conservation champions are three initiatives we are currently pursuing. Helping to promote eco-tourism and providing teachers with additional material to encourage student knowledge about the Eburu ecosystem are two additional alternatives.
Implementing sustainable use of Eburu Forest compatible with a conservation translocation may initially require forest-internal zoning that combines a strictly protected bongo sanctuary off-limits to humans with a surrounding buffer zone where honey harvesting, back-country camping, and leisure hikes are permitted. Such zoning might be seasonally dynamic and become unnecessary once a bongo population has become well-established and any threat of poaching minimized. Both formal and citizen-based patrols and data gathering will be key to continuously monitoring threats. Participatory monitoring might be encouraged via an ecosystem-specific citizen science monitoring App or a bongo “hotline” for people to call in and share relevant observations. Such participatory monitoring will not only help enforce zoning and keep an eye on threats, but also serves to reinforce local pride and ownership over the conservation endeavor (de Araujo Lima Constantino et al., 2012; Evans et al., 2018).
Although areas with low human presence and impact persist, wildlife around the globe for the most part exists within close proximity to humans. A recent study found that the median distance to edge in areas of low human impact is merely 6 km worldwide (Jacobson et al., 2019). Therefore, to give conservation translocations the best chance at success, their planning, implementation, and follow-up must take a holistic approach that carefully considers, shapes, and mitigates both biological and socio-economic factors throughout. We believe that our study convincingly demonstrates the importance of such a holistic approach in the feasibility assessment and planning phase, and are pleased to report that it is already serving as a model for the feasibility analysis at a different potential release site for mountain bongo at the Ragati and Chehe Forest Stations on the slopes of Mt. Kenya. We hope that we have also provided useful pointers on how to integrate biological with socio-economic considerations during implementation and subsequent monitoring and evaluation. Given the ubiquity of humans on this planet, it is paramount that any form of conservation management take impacts by and on humankind into account and actively influence these for mutual benefits to local communities, wider society, and wildlife.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
DSh is responsible for study design, contributed to the underlying research for this article, and together with her field assistants, collected the camera and sign data, and conducted the interviews. TB-C and DSt looked after data analysis, tables and figures. DSt also identified and filled citation gaps. CL is a member of the Mountain Bongo Task Force and provided much of the local history and context in the introduction, methods, and discussion sections of the paper. AM chairs the Conservation Translocation Specialist Group (CTSG) of the International Union for the Conservation of Nature (IUCN) Species Survival Commission (SSC) and helped with framing this paper and recommendations regarding a translocation of Mountain Bongo. JM provided guidance and supervision for much of the work and did the majority of writing. Each author wrote or contributed to sections of the manuscript and manuscript revisions. All authors have read and approved the submitted version.
Funding
Funding was provided by the Calgary Zoo Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Our gratitude goes to the communities surrounding Eburu Forest, the Kenya Forest Services, Kenya Wildlife Service, and Location Chiefs for their cooperation, to the Bongo Surveillance Program's team of trackers, specifically Solomon Muriithi, Eric Shibi, Muriithi Kosen, Geoffrey Muthemba, James Chege, Peter Njoroge, and Elijah Ribiro Mwai, to Mike Prettejohn, Peter Munene and colleagues at Rhino Ark, and to Patrick Mwangi for his invaluable assistance in coordinating the field work. We also thank the two reviewers for their valuable feedback on an earlier version of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.788267/full#supplementary-material
References
Abiya, I. O.. (1996). Towards sustainable utilization of Lake Naivasha, Kenya. Lakes Reserv. 2, 231–242. doi: 10.1111/j.1440-1770.1996.tb00067.x
Albertazzi, S., Bini, V., Lindon, A., and Trivellini, G. (2018). Relations of power driving tropical deforestation: a case study from the Mau Forest (Kenya). Belgeo 2, 1–19. doi: 10.4000/belgeo.24223
Armstrong, D. P., Seddon, P. J., and Moehrenschlager, A. (2018). “Reintroduction,” in Encyclopedia of Ecology, 2nd Edn., ed B. D. Fath (Oxford, UK: Elsevier), 458–466. doi: 10.1016/B978-0-12-409548-9.10589-5
Bajracharya, S. B., Furley, P. A., and Newton, A. C. (2005). Effectiveness of community involvement in delivering conservation benefits to the Annapurna Conservation Area, Nepal. Environ. Conserv. 32, 239–247. doi: 10.1017/S0376892905002298
Baldyga, T. J., Miller, S. N., Driese, K. L., and Gichaba, C. M. (2008). Assessing land cover change in Kenya's Mau Forest region using remotely sensed data. Afr. J. Ecol. 46, 46–54. doi: 10.1111/j.1365-2028.2007.00806.x
Berkes, F.. (2010). Devolution of environment and resources governance: trends and future. Environ. Conserv. 37, 489–500. doi: 10.1017/S037689291000072X
Bosley, L. F.. (2003). International Studjournal for Bongo Antelope (Tragelaphus eurycerus isaaci). Fort Worth, TX: Fort Worth Zoo.
Boy, G.. (2017). The Mau Eburu Forest: A Visitors' Guide. Nairobi: The Rhino Ark Kenya Charitable Trust.
Boyce, M. S.. (2006). Scale for resource selection functions. Divers. Distrib. 12, 269–276. doi: 10.1111/j.1366-9516.2006.00243.x
Boyce, M. S., Vernier, P. R., Nielsen, S. E., and Schmiegelow, F. K. A. (2002). Evaluating resource selection functions. Ecol. Modell. 157, 281–300. doi: 10.1016/S0304-3800(02)00200-4
Brichieri-Colombi, T. A., and Moehrenschlager, A. (2016). Alignment of threat, effort, and perceived success in North American conservation translocations. Conserv. Biol. 30, 1159–1172. doi: 10.1111/cobi.12743
Bruner, A. G., Gullison, R. E., Rice, R. E., and da Fonseca, G. A. B. (2001). Effectiveness of parks in protecting tropical biodiversity. Science 291, 125. doi: 10.1126/science.291.5501.125
Burton, A. C., Sam, M. K., Kpelle, D. G., Balangtaa, C., Buedi, E. B., and Brashares, J. S. (2011). Evaluating persistence and its predictors in a West African carnivore community. Biol. Conserv. 144, 2344–2353. doi: 10.1016/j.biocon.2011.06.014
Caughley, G.. (1994). Directions in conservation biology. J. Anim. Ecol. 63, 215–244. doi: 10.2307/5542
Centre on Housing Rights and Eviction (2007). Submission to the United Nations Committee on Economic, Social and Cultural Rights on Occasion of Pre-Sessional Working Group Discussion. Centre on Housing Rights and Eviction.
Champagnon, J., Elmberg, J., Guillemain, M., Gauthier-Clerc, M., and Lebreton, J.-D. (2012). Conspecifics can be aliens too: a review of effects of restocking practices in vertebrates. J. Nat. Conserv. 20, 231–241. doi: 10.1016/j.jnc.2012.02.002
Church, C.. (2015). Mau Eburru the 'smoking' Mountain Regenerates. SWARA April–June 2015 Edn. Nairobi: East African Wildlife Society (EAWLS).
Corrigan, C., Bingham, H., Shi, Y., Lewis, E., Chauvenet, A., and Kingston, N. (2018). Quantifying the contribution to biodiversity conservation of protected areas governed by indigenous peoples and local communities. Biol. Conserv. 227, 403–412. doi: 10.1016/j.biocon.2018.09.007
Corson, C., Flores-Ganley, I., Worcester, J., and Rogers, S. (2020). From paper to practice? Assembling a rights-based conservation approach. J. Polit. Ecol. 27, 1128–1147. doi: 10.2458/v27i1.23621
Davies, J., Hill, R., Walsh, F. J., Sandford, M., Smyth, D., and Holmes, M. C. (2013). Innovation in management plans for community conserved areas: experiences from Australian indigenous protected areas. Ecol. Soc. 18 (2). doi: 10.5751/ES-05404-180214
de Araujo Lima Constantino, P., Carlos, H. S. A., Ramalho, E. E., Rostant, L., Marinelli, C. E., Teles, D., et al. (2012). Empowering local people through community-based resource monitoring: a comparison of Brazil and Namibia. Ecol. Soc. 17, 22. doi: 10.5751/ES-05164-170422
Dormann, C. F., McPherson, J. M., Araújo, M. B., Bivand, R., Bolliger, J., Carl, G., et al. (2007). Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628. doi: 10.1111/j.2007.0906-7590.05171.x
Dudley, N.. (2013). Guidelines for Applying Protected Area Management Categories Including IUCN WCPA Best Practice Guidance on Recognising Protected Areas and Assigning Management Categories and Governance Types. Gland: IUCN.
Dunn, C. G., and Angermeier, P. L. (2016). Development of habitat suitability indices for the candy darter, with cross-scale validation across representative populations. Trans. Am. Fish. Soc. 145, 1266–1281. doi: 10.1080/00028487.2016.1217929
Dureuil, M., Boerder, K., Burnett Kirsti, A., Froese, R., and Worm, B. (2018). Elevated trawling inside protected areas undermines conservation outcomes in a global fishing hot spot. Science 362, 1403–1407. doi: 10.1126/science.aau0561
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., and Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57. doi: 10.1111/j.1472-4642.2010.00725.x
Estes, L. D., Mwangi, A. G., Reillo, P. R., and Shugart, H. H. (2011). Predictive distribution modeling with enhanced remote sensing and multiple validation techniques to support mountain bongo antelope recovery. Anim. Conserv. 14, 521–532. doi: 10.1111/j.1469-1795.2011.00457.x
Evans, K., Guariguata, M. R., and Brancalion, P. H. S. (2018). Participatory monitoring to connect local and global priorities for forest restoration. Conserv. Biol. 32, 525–534. doi: 10.1111/cobi.13110
Fielding, A. H., and Bell, J. F. (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49. doi: 10.1017/S0376892997000088
Fowler, N. L., Center, A., and Ramsey, E. A. (2012). Streptanthus bracteatus (Brassicaceae), a rare annual woodland forb, thrives in less cover: evidence of a vanished habitat? Plant Ecol. 213, 1511–1523. doi: 10.1007/s11258-012-0109-2
Gavin, M. C., Solomon, J. N., and Blank, S. G. (2010). Measuring and monitoring illegal use of natural resources. Conserv. Biol. 24, 89–100. doi: 10.1111/j.1523-1739.2009.01387.x
Germain, R. R., and Arcese, P. (2014). Distinguishing individual quality from habitat preference and quality in a territorial passerine. Ecology 95, 436–445. doi: 10.1890/13-0467.1
Gibbon, G. E. M., Bindemann, M., and Roberts, D. L. (2015). Factors affecting the identification of individual mountain bongo antelope. PeerJ 3, e1303. doi: 10.7717/peerj.1303
Gilmour, D.. (2016). Forty Years of Community-Based Forestry: A Review of its Extent and Effectiveness. Rome: Food and Agriculture Organization of the United Nations.
Government of Kenya (2008). Kenya Vision 2030: First Medium Term Plan 2008-2012. Nairobi: Ministry of State for Planning, National Development and Vision 2030.
Gray, T. N. E., Crouthers, R., Ramesh, K., Vattakaven, J., Borah, J., Pasha, M. K. S., et al. (2017). A framework for assessing readiness for tiger Panthera tigris reintroduction: a case study from eastern Cambodia. Biodivers. Conserv. 26, 2383–2399. doi: 10.1007/s10531-017-1365-1
Griffin, L. P., Casselberry, G. A., Hart, K. M., Jordaan, A., Becker, S. L., Novak, A. J., et al. (2021). A novel framework to predict relative habitat selection in aquatic systems: applying machine learning and resource selection functions to acoustic telemetry data from multiple shark species. Front. Mar. Sci. 8, 358. doi: 10.3389/fmars.2021.631262
Hardy, M. A., Hull, S. D., and Zuckerberg, B. (2018). Swift action increases the success of population reinforcement for a declining prairie grouse. Ecol. Evol. 8, 1906–1917. doi: 10.1002/ece3.3776
Hawkins, B. A., Diniz-Filho, J. A. F., Mauricio Bini, L., De Marco, P., and Blackburn, T. M. (2007). Red herrings revisited: spatial autocorrelation and parameter estimation in geographical ecology. Ecography 30, 375–384. doi: 10.1111/j.0906-7590.2007.05117.x
Hijmans, R. J.. (2020). raster: Geographic Data Analysis and Modeling. R Package Version 3.4-5 ed. Available online at: https://CRAN.R-project.org/packages/raster (accessed October 6, 2020).
Hijmans, R. J., Phillips, S., Leathwick, J., and Elith, J. (2017). dismo: Species Distribution Modeling. R Package Version 1(4), 1-1 ed. Available online at: https://cran.r-project.org/web/packages/dismo/ (accessed October 6, 2020).
IUCN (2021). The IUCN Red List of Threatened Species. Version 2021-1: Summary Statistics. Available online at: https://www.iucnredlist.org/resources/summary-statistics (accessed August 8, 2021).
IUCN SSC Antelope Specialist Group. (2017). Tragelaphus eurycerus ssp isaaci. The IUCN Red List of Threatened Species. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T22057A50197212.en (accessed May 28, 2021).
Jacobson, A. P., Riggio, J. M, Tait, A., and E. M. Baillie, J. (2019). Global areas of low human impact (‘Low Impact Areas') and fragmentation of the natural world. Sci. Rep. 9, 14179. doi: 10.1038/s41598-019-50558-6
Jensen, J. R.. (2007). Remote Sensing of the Environment: An Earth Resource Perspective. Upper Saddle River, NJ: Pearson Prentice Hall.
Kenya Forest Service Rhino Ark, and Eburru Community Forest Association.. (2017). Eburu Participatory Forest Management Plan 2017–2021. Unpublished Report.
Kenya Wildlife Service (2019). National Recovery and Action Plan for the Mountain Bongo (Tragelaphus eurycerus isaaci) in Kenya (2019–2023). Nairobi: Kenya Wildlife Service.
Kenya Wildlife Service Rhino Ark, and Kenya Forest Service. (2012). Environmental and Social Impact Assessment Project Report for the Proposed Mau Eburu Electric Fence. Nairobi: Kenya Wildlife Service.
Kenya Wildlife Service Rhino Ark, and Kenya Forest Service. (2014). Handjournal on the Management of Electric Fence. Nairobi: Kenya Wildlife Service.
Kothari, A., Camill, P., and Brown, J. (2013). Conservation as if people also mattered: policy and practice of community-based conservation. Conserv. Soc. 11, 1–15. doi: 10.4103/0972-4923.110937
Leiper, I., Zander, K. K., Robinson, C. J., Carwadine, J., Moggridge, B. J., and Garnett, S. T. (2018). Quantifying current and potential contributions of Australian indigenous peoples to threatened species management. Conserv. Biol. 32, 1038–1047. doi: 10.1111/cobi.13178
Lewis, E., MacSharry, B., Juffe-Bignoli, D., Harris, N., Burrows, G., Kingston, N., et al. (2019). Dynamics in the global protected-area estate since 2004. Conserv. Biol. 33, 570–579. doi: 10.1111/cobi.13056
Li, W., and Guo, Q. (2021). Plotting receiver operating characteristic and precision-recall curves from presence and background data. Ecol. Evol. 11, 10192–10206. doi: 10.1002/ece3.7826
Lloyd, N. A., Hostetter, N. J., Jackson, C. L., Converse, S. J., and Moehrenschlager, A. (2019). Optimizing release strategies: a stepping-stone approach to reintroduction. Anim. Conserv. 22, 105–115. doi: 10.1111/acv.12448
Macura, B., Secco, L., and Pullin, A. S. (2015). What evidence exists on the impact of governance type on the conservation effectiveness of forest protected areas? Knowledge base and evidence gaps. Environ. Evid. 4, 24. doi: 10.1186/s13750-015-0051-6
Makhanu, R.. (2015). Assessment of Factors That Contribute to Forest Resource Use Conflicts: A Case of Eburu Forest, Kenya. Master of Arts, University of Nairobi.
Martin, T. G., Nally, S., Burbidge, A. A., Arnall, S., Garnett, S. T., Hayward, M. W., et al. (2012). Acting fast helps avoid extinction. Conserv. Lett. 5, 274–280. doi: 10.1111/j.1755-263X.2012.00239.x
McLeish, D.. (2020). Eburu forest in Kenya back from the brink. ConservationMag. Cape Town: ConservationMag.
McMurdo Hamilton, T., Canessa, S., Clark, K., Gleeson, P., Mackenzie, F., Makan, T., et al. (2020). Applying a values-based decision process to facilitate comanagement of threatened species in Aotearoa New Zealand. Conserv. Biol. 35, 1162–1173. doi: 10.1111/cobi.13651
McPherson, J. M., and Jetz, W. (2007). Effects of species' ecology on the accuracy of distribution models. Ecography 30, 135–151. doi: 10.1111/j.2006.0906-7590.04823.x
Ministry of Environment and Forestry (2018). Taskforce Report on Forest Resources Management and Logging Activities in Kenya. Kenya: Ministry of Environment and Forestry.
Moehrenschlager, A., and Lloyd, N. A. (2016). “Release considerations and techniques to improve conservation translocation success,” in Reintroduction of Fish and Wildlife Populations, eds. D. S. Jachowski, J. J. Millspaugh, P. L. Angermeier and R. Slotow. (Berkeley, CA: University of California Press), 245–280. doi: 10.1525/9780520960381-013
Moehrenschlager, A., Shier, D. M., Moorhouse, T. P., and Stanley Price, M. R. (2013). “Righting past wrongs and ensuring the future,” in Key Topics in Conservation Biology Vol. 2. (Oxford, UK: Wiley-Blackwell), 405–429. doi: 10.1002/9781118520178.ch22
Mount Kenya Wildlife Conservancy. (2021). Keeping up with the mountain bongo. Mount Kenya Wildlife Conservancy News. Q2 2021 ed. Nanyuki: Mount Kenya Wildlife Conservancy.
Mutune, J., Wahome, R., and Mungai, D. (2015). Local participation in community forest associations: a case study of Sururu and Eburu Forests, Kenya. Int. J. Afr. Asian Stud. 13, 84–94. Available online at: https://iiste.org/Journals/index.php/JAAS/article/view/25611
Naimi, B., Hamm, N. A. S., Groen, T. A., Skidmore, A. K., and Toxopeus, A. G. (2014). Where is positional uncertainty a problem for species distribution modelling? Ecography 37, 191–203. doi: 10.1111/j.1600-0587.2013.00205.x
Namgail, T., Fox, J. L., and Bhatnagar, Y. V. (2007). Habitat shift and time budget of the Tibetan argali: the influence of livestock grazing. Ecol. Res. 22, 25. doi: 10.1007/s11284-006-0015-y
Njiru, J., Waithaka, E., and Aloo, P. A. (2017). An overview of the current status of Lake Naivasha fishery: challenges and management strategies. Open Fish Sci. J. 10, 1–11. doi: 10.2174/1874401X01710010001
Novak, B. J., Phelan, R., and Weber, M. (2021). U.S. conservation translocations: over a century of intended consequences. Conserv. Sci. Pract. 3, e394. doi: 10.1111/csp2394
Nyumba, T. O., Wilson, K., Derrick, C. J., and Mukherjee, N. (2018). The use of focus group discussion methodology: insights from two decades of application in conservation. Methods Ecol. Evol. 9, 20–32. doi: 10.1111/2041-210X.12860
Otungah, B. M., Warutere, J. K., Ogombe, F., Githui, J., Wambani, C., Kabugi, H., et al. (2008). Aberdare Fence Management Strategic Plan (AFMSP) 2008–2018. Unpublished Report.
Phillips, S. J., Anderson, R. P., and Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Phillips, S. J., Dudik, M., and Schapire, R. E. (2004). “A maximum entropy approach to species distribution modeling,” in Proceedings of the Twenty-First International Conference on Machine Learning (New York, NY: ACM Press), 655–662. doi: 10.1145/1015330.1015412
Phillips, S. J., Dudík, M., and Schapire, R. E. (2005). Maxent software for modeling species niches and distributions. Version 3.4.1 ed. Available online at: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed October 6, 2020).
Prettejohn, M., Shears, J., and Comport, P. (2020). Bongo Surveillance Project 2020 Review. UK: Lulu Press Inc.
Pretty, J., Adams, B., Berkes, F., de Athayde, S. F., Dudley, N., Hunn, E., et al. (2009). The intersections of biological diversity and cultural diversity towards integration. Conserv. Soc. 7, 100–112. doi: 10.4103/0972-4923.58642
Quinn, G. P., and Keough, M. J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press. doi: 10.1017/CBO.9780511806384
R Core Team. (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rayne, A., Byrnes, G., Collier-Robinson, L., Hollows, J., McIntosh, A., Ramsden, M., et al. (2020). Centring indigenous knowledge systems to re-imagine conservation translocations. People Nat. 2, 512–526. doi: 10.1002/pan.3.10126
RCMRD-SERVIR. (2017). Kenya Sentinel2 Land Use Land Cover. Available online at: http://geoportal.rcmrd.org/layers/servir%3Akenya_sentinel2_lulc2016 (accessed October 4, 2019).
Reed, G., Brunet, N. D., Longboat, S., and Natcher, D. C. (2021). Indigenous guardians as an emerging approach to indigenous environmental governance. Conserv. Biol. 35, 179–189. doi: 10.1111/cobi.13532
Rhino Ark Ministry of Environment Water, and Natural Resources, and Ministry of Education, Science, and Technology.. (2013). Conservation Education Curriculum for Eburu Primary Schools. Nairobi: Rhino Ark Kenya Charitable Trust.
Rhino Ark. (2016). Eburu Ecosystem Conservation Project: Rapid Impact Assessment Report. Nairobi: Rhino Ark Kenya Charitable Trust.
Riley, S. J., and Sandström, C. (2016). “Human dimensions insights for reintroductions of fish and wildlife populations,” in Reintroduction of Fish and Wildlife Populations, 1st Edn., eds D. S. Jachowski, J. J. Millspaugh, P. L. Angermeier, and R. Slotow (Oakland, CA: University of California Press), 55–77. doi: 10.1525/9780520960381-006
Sabuhoro, E., Wright, B. A., Powell, R. B., Hallo, J. C., Layton, P. A., and Munanura, I. E. (2020). Perceptions and behaviors of Indigenous populations regarding illegal use of protected area resources in East Africa's mountain gorilla landscape. Environ. Manage. 65, 410–419. doi: 10.1007/s00267-020-01254-z
Sang, J. K.. (2001). “Kenya: The Ogiek in Mau Forest,” in Indigenous Peoples and Protected Areas in Africa: From Principles to Practice, eds. J. Nelson and L. Hossack (Utrecht, Netherlands: Forest Peoples Programme), 111–194.
Schneider, F. D., Matias, D. M., Burkhart, S., Drees, L., Fickel, T., Hummel, D., et al. (2021). Biodiversity conservation as infectious disease prevention: why a social-ecological perspective is essential. Glob. Sustain. 4, 1–14. doi: 10.1017/sus.2021.11
Secretiariat of the Convention on Biological Diversity. (2011). Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity. Montreal, QC: Canada.
Shanee, S.. (2009). Modelling spider monkeys Ateles spp. Gray, 1825: ecological responses and conservation implications to increased elevation. J. Threat. Taxa 1, 450–456. doi: 10.11609/JoTT.o2189.450-6
Solomon, J. N., Gavin, M. C., and Gore, M. L. (2015). Detecting and understanding non-compliance with conservation rules. Biol. Conserv. 189, 1–4. doi: 10.1016/j.biocon.2015.04.028
Svengren, H., Prettejohn, M., Bunge, D., Fundi, P., and Björklund, M. (2017). Relatedness and genetic variation in wild and captive populations of mountain bongo in Kenya obtained from genome-wide single-nucleotide polymorphism (SNP) data. Glob. Ecol. Conserv. 11, 196–206. doi: 10.1016/j.gecco.2017.07.001
Swan, K. D., Lloyd, N. A., and Moehrenschlager, A. (2018). Projecting further increases in conservation translocations: a Canadian case study. Biol. Conserv. 228, 175–182. doi: 10.1016/j.biocon.2018.10.026
The Nobel Peace Prize. (2004). NobelPrize.org. Nobel Prize Outreach AB 2021. Available online at: https://www.nobelprize.org/prizes/lists/all-nobel-peace-prizes/ (accessed September 21, 2021).
Tobiko, K.. (2018). Extension of the Moratorium on Logging Activies in Public and Community Forests. Nairobi: Ministry of Environment and Forestry.
Tobiko, K.. (2020). Re: Moratorium on Logging in Public and Community Forests. Nairobi: Ministry of Environment and Forestry.
UNEP-WCMC IUCN, and NGS.. (2018). Protected Planet Report 2018. Cambridge UK; Gland; Washington, DC: UNEP-WCMC, IUCN, and NGS.
USGS (2011). ASTGTM: ASTER Global Digital Elevation Model V002. U.S./Japan ASTER Science Team. Available online at: https://lpdaac.usgs.gov/node/1079 (accessed November 15, 2019).
USGS (2020). Earth Explorer. Available online at: http://earthexplorer.usgs.gov/ (accessed June 12, 2020).
Van Baelen, A., Wilshaw, A., Griffith, P., Noens, G., Maíllo-Fernández, J.-M., Foley, R. A., et al. (2019). Prospect farm and the middle and later Stone Age occupation of Mt. Eburru (Central Rift, Kenya) in an East African context. Afr. Archaeol. Rev. 36, 397–417. doi: 10.1007/s10437-019-09342-0
Vaske, J. J., Roemer, J. M., and Taylor, J. G. (2013). Situational and emotional influences on the acceptability of wolf management actions in the Greater Yellowstone Ecosystem. Wildl. Soc. Bull. 37, 122–128. doi: 10.1002/wsb.240
Williams, S., and Haines, J. (2021). “Scarlet macaw reintroduction on the Nicoya Peninsula of Costa Rica,” in Global Conservation Translocation Perspectives: 2021. Case Studies from Around the Globe, ed P. Soorae (Gland: IUCN SSC Conservation Translocation Specialist Group, Environment Agency—Abu Dhabi and Calgary Zoo, Canada), 133–136.
Keywords: poaching, reinforcement, endemic species, Tragelaphus eurycerus isaaci, local communities, household surveys, camera trapping, illegal activities
Citation: Sheppard DJ, Brichieri-Colombi TA, Stark DJ, Lambrechts C, Moehrenschlager A and McPherson JM (2022) When Ecological Analysis Reveals Hidden Human Dimensions: Building on Long-Term Community Participation to Enable a Conservation Translocation of Mountain Bongo in Kenya. Front. Conserv. Sci. 2:788267. doi: 10.3389/fcosc.2021.788267
Received: 01 October 2021; Accepted: 23 December 2021;
Published: 31 January 2022.
Edited by:
Carlos R. Ruiz-Miranda, State University of the North Fluminense Darcy Ribeiro, BrazilReviewed by:
Katia Maria P. M. B. Ferraz, University of São Paulo, BrazilEwan MacDonald, University of Oxford, United Kingdom
Copyright © 2022 Sheppard, Brichieri-Colombi, Stark, Lambrechts, Moehrenschlager and McPherson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donna J. Sheppard, ZG9ubmFzQGNhbGdhcnl6b28uY29t
 Donna J. Sheppard
Donna J. Sheppard Typhenn A. Brichieri-Colombi
Typhenn A. Brichieri-Colombi Danica J. Stark
Danica J. Stark Christian Lambrechts
Christian Lambrechts Axel Moehrenschlager
Axel Moehrenschlager Jana M. McPherson
Jana M. McPherson