- 1World Wide Fund for Nature India, New Delhi, India
- 2Department of Biodiversity, Wildlife Conservation and Management, University of Mumbai, Mumbai, India
Small population sizes, low densities, and large area requirements make large carnivores particularly sensitive to habitat degradation and land-use change. In fragmented landscapes, many protected areas cannot accommodate viable wildlife populations in themselves, which brings the surrounding human-dominated matrix that may extend wildlife habitats or serve as corridors into focus. Such areas are typically excluded from the conservation portfolio and are subject to rapid land -use change in many areas. This study investigates the occurrence of tigers, sloth bears, leopards and striped hyenas and assesses community use of natural resources and attitudes towards wildlife in a 3,384 km2 portion of semi-arid multiple-use landscape in Western India that also serves as an important wildlife corridor. This area abuts Ranthambore Tiger Reserve, a preeminent protected area in Western India. Sign surveys spanning 1,039.22 km of trails were conducted in 94, 36 km2 grids spanning agricultural land, forests and other land use types to collate information on wildlife occurrence and associated environmental and human factors. Analysis using occupancy models revealed that tiger and sloth bear occurrence probabilities (0.093 ± 0.05), and (0.13 ± 0.02) were considerably lower than those for leopards (0.72 ± 0.22) and striped hyenas (0.91 ± 0.08). Lack of sufficient cover and limited food availability renders these multiple-use habitats poorly suited for tigers and sloth bears, while leopards and hyenas are able to adapt better to multi-use areas. Concurrently, 66 villages were surveyed across the study landscape, where data on broad socio-economic attributes of communities and their attitudes towards wildlife were assessed through questionnaire surveys. More respondents expressed negative attitudes than positive attitudes which vary as a function of education levels, occupation and land holding sizes. Ongoing landscape transformation through mining, agricultural expansion, infrastructure development, and negative attitudes towards wildlife conservation among people living in the agricultural matrix threatens the long-term functionality of these corridors. Therefore, immediate measures are needed to develop and implement corridor conservation strategies and plans, with a focus on land use planning and human-wildlife conflict mitigation. In the absence of decisive and timely action, wildlife populations may increasingly get relegated to fragmented patches, jeopardising their persistence.
Introduction
Large terrestrial carnivores are among the most threatened species globally, having experienced large range contractions and population declines (Wolf and Ripple, 2017). These declines have been especially acute where populations of carnivores or their ungulate prey face high poaching pressures and where their habitats have been diminished, fragmented, and degraded by land use change and other processes (O'Brien et al., 2003; Nyhus and Tilson, 2004; Chapron et al., 2014). With most of the biosphere impacted by human modification (Kennedy et al., 2019), large carnivore populations have increasingly been relegated to protected areas, which have helped sustain breeding populations. Yet given that many protected areas are small, carnivores also extensively use multiple-use forests, agro-ecosystems and sometimes even in peri-urban areas (Athreya et al., 2013; Chapron et al., 2014; Chanchani et al., 2016; Ghosh-Harihar et al., 2019; Bista et al., 2021). A greater emphasis on conservation in multiple-use areas is thus warranted in nations like India where people and wildlife extensively share space within forests, agro-ecosystems, and other land use types (Srivathsa et al., 2019; Puri et al., 2020; Warrier et al., 2020).
Multi-use landscapes encompass diverse land use including agriculture, settlements, mines, grasslands, and forests beyond protected area boundaries. Despite extensive human presence in these areas, they often serve as extensions of available habitats for mammals residing in protected areas (Chapron et al., 2014; Warrier et al., 2020). They may also serve as vital corridors enabling dispersal between source populations maintaining gene flow, and therefore the long-term viability of meta-populations (Saunders et al., 1991; Harris and Silva-Lopez, 1992; Dutta et al., 2015; Thapa et al., 2017; Thatte et al., 2020). As essential as multiple-use areas are for wildlife conservation, they are also areas where wildlife face disproportionate risks of death or injury from retaliation or other anthropogenic causes because of the extensive interface with people and livestock and the high potential for conflict (Gervasi et al., 2014; Acharya et al., 2017). Such interactions may be exacerbated on account of forest fragmentation, which has progressively reduced forest patch size in India often rendering it impermeable for wildlife movement (Jayadevan et al., 2020).
Varying life history and behavioural traits of large carnivores including territoriality and foraging ecology shape whether and where these species have persisted (Kinnaird et al., 2003; Carter and Linnell, 2016; Srivathsa et al., 2020). Four large carnivores, tigers (Panthera tigris), leopards (Panthera pardus), sloth bears (Melursus ursinus), and hyenas (Hyaena hyaena) that co-occur extensively across peninsular India exemplify such variations (Joshi et al., 2013; Chanchani et al., 2016; Majgaonkar et al., 2019; Thatte et al., 2020). Of these, both felines occur across a diverse array of habitats, from dense forests to more arid open woodlands and savannas where they occupy home-ranges from <10 to >2,000 km2 (Simcharoen et al., 2008, 2014; Grant, 2012; Chundawat et al., 2016; Hunter and Barrett, 2019; Naha et al., 2021). Though both felines achieve their highest densities in Protected Areas, they persist in many multi-use landscapes (Joshi et al., 2013; Chanchani et al., 2016; Karanth et al., 2020), including forests and agro-ecosystems, where the smaller leopard, with its wider dietary niche can sometimes occur at relatively high densities as well (Athreya et al., 2013; Stein et al., 2016). However, proclivity to prey on domestic animals, and proximity to people make both felines susceptible to persecution in such landscapes, where there are still critical knowledge gaps about their occurrence (Harihar et al., 2011; Athreya et al., 2013; Stein et al., 2016; Bista et al., 2021). Similarly, there are many knowledge gaps on the ecology of the striped hyena in multi-use areas. This species prefers arid landscapes, where it occupies 14–70 km2 home ranges (Wagner, 2006; AbiSaid and Dloniak, 2015). These are often considered to be of low conservation value in India and continue to be extensively transformed for human-use (Vanak et al., 2017; Majgaonkar et al., 2019). Hyenas often co-occur in human dominated land-use types including agriculture-scrubland mosaics and are known to forage on garbage and carrion near human habitations (Tourani et al., 2012; Alam et al., 2014). Sloth bears have the smallest area requirements among the study species, usually occupying <15 km2 home-ranges (Yoganand et al., 2005; Ratnayeke et al., 2007). Their omnivorous diet is linked to the phenology of flowering and fruiting plant species and these bears are highly myrmecophagous as well (Akhtar et al., 2004; Sukhadiya et al., 2013; Palei et al., 2020; Philip et al., 2021). Sloth bears are heavily dependent on the availability of forest cover and generally shun areas with high levels of anthropogenic disturbance (Ramesh et al., 2012; Das et al., 2014; Puri et al., 2015).
Diverse ecological factors only partially determine the occurrence and persistence of carnivores in multi-use landscapes. Socio-cultural factors, including people's attitudes towards wildlife conservation significantly influence wildlife distribution and survival as well as habitat availability and quality, particularly in multi-use-landscapes where space sharing between people and wildlife is especially pronounced (Redpath et al., 2017; Athreya et al., 2018; Srivathsa et al., 2019; Naha et al., 2020). Attitudes are commonly shaped by costs associated with cohabiting with carnivores, such as human injury and death, livestock predation, and various transaction and psychological costs. When economic and other losses and costs are high and generally unmitigated, the risk of retaliation against wildlife or destruction of wildlife habitats are very real and can be especially deleterious for wildlife occurring in fragmented landscapes with extensive exposure to people and livestock.
Objectives
The main aim of this study is to inform multi-species habitat-use in human-modified landscapes. We assess anthropogenic and ecological factors that govern the habitat-use of four threatened large carnivores in the 3,384 km2 Ramgarh - Ranthambore - Kailadevi and Kuno-Sheopur complexes in North West India. Since ecological factors only partially determine the carnivore occurrence in shared spaces, where local tolerance often plays just as much of a role in carnivore occurrence, we also assess local attitudes towards wildlife across these corridors. Tigers only occur sporadically and there is little information on the status of the other carnivores (Pawar et al., 2019). This region is adjacent to Ranthambore Tiger Reserve, which harbours the most significant source population of tigers in western India (Jhala et al., 2019). This population is isolated from other population clusters in Central India and elsewhere, and thereby genetically distinct (Natesh et al., 2017). Ranthambore and the surrounding landscape also support populations of leopards, sloth bears and hyenas, but little is known about the status of these species beyond the protected area's boundary. By analysing and interpreting ecological and social data that were concurrently collected, this study aims to create a more comprehensive profile of these corridors to inform conservation planning.
Materials and Methods
Study Area
The study area encompasses Sawai Madhopur, Boondi, Tonk, and Karauli districts of the north-west Indian state of Rajasthan and the Sheopur district of the central Indian state of Madhya Pradesh. It lies under varying administrative jurisdictions including the Ranthambore Tiger Reserve, National Chambal Wildlife Sanctuary, Sheopur Forest Division and various land managed by revenue departments and gram sabhas (village councils) of both aforementioned states. In terms of its terrain, the study area is heterogenous. Undulating terrain comprising low rugged hills and gorges characterise Kailadevi and Ranthambore (elevation 200–500 m) (Singh et al., 2021). The surrounding areas have extensive plains where mustard, rape seed, soyabean, wheat and chickpeas are cultivated. The north-flowing Chambal river bisects the study area, and its banks are characterised by intricately carved scrubby ravines, which are a mosaic of stunted-thorny forests and farmlands. The dry deciduous forests of Sheopur Division and Kuno - Palpur national park are distributed over an undulating terrain as well. Temperatures range from winter lows of about 2°C to a summer maximum of over 47°C (Singh et al., 2021). The area receives ~700 mm of precipitation each year, largely in the monsoons between June and August. Dhonk (Anogiesus pendula) is the dominant tree in the dry deciduous forests, with stands of kadaya (Sterculia urens), salai (Boswellia serrata), raunj (Acacia leucophloea), amaltas (Cassia fistula), palash (Butea monosperma), tendu (Diospyros melanoxylon), gurjan (Lannea coromandelica), and jamun (Syzygium cumini) trees also populating these forests (Singh et al., 2021). The open scrublands contain shrubs such as Euphorbia spp., with extensive areas dominated by the invasive Mesquite (Prosopis juliflora) as well. The flora and fauna of Ranthambore, Kailadevi and Sheopur blocks are similar. The National Chambal Wildlife Sanctuary comprises predominantly scrubby ravine habitat, which are interspersed with farmlands in the areas surrounding the sanctuary. These form the connecting habitats between the Ranthambore, Kailadevi and Sheopur blocks (Ranganathan, 2017; Shah et al., 2015). The area harbours a carnivore guild that includes six felids, three canids, four viverrids, and one mustelid, ursid and hyena species each. The local communities primarily belong to agro-pastoral communities, such as the Gujjars and Meenas that both practise agriculture and raise livestock (primarily buffaloes) for dairy production.
Study Design and Data Collection
Occupancy Sampling
Carnivores frequently use human trails and paths to move through forests, scrubland and human-dominated areas, and therefore, sampling for animal signs on trails is an efficient method of surveying large areas for carnivores (Karanth and Suinquist, 2000; Wilson and Delahay, 2001; Thorn et al., 2010). Given the wide expanse of the study area, we delineated 94, 36 km2 grids for surveys (Figure 1). Within each grid, we carried out foot-surveys along trails that were on average ~10 km in length (Supplementary Table 4) during February 2019 and collected data on detection (1) and non-detection (0) of target species along 1 km segment of each trail (Hines et al., 2010). Our total survey effort was 921 km.
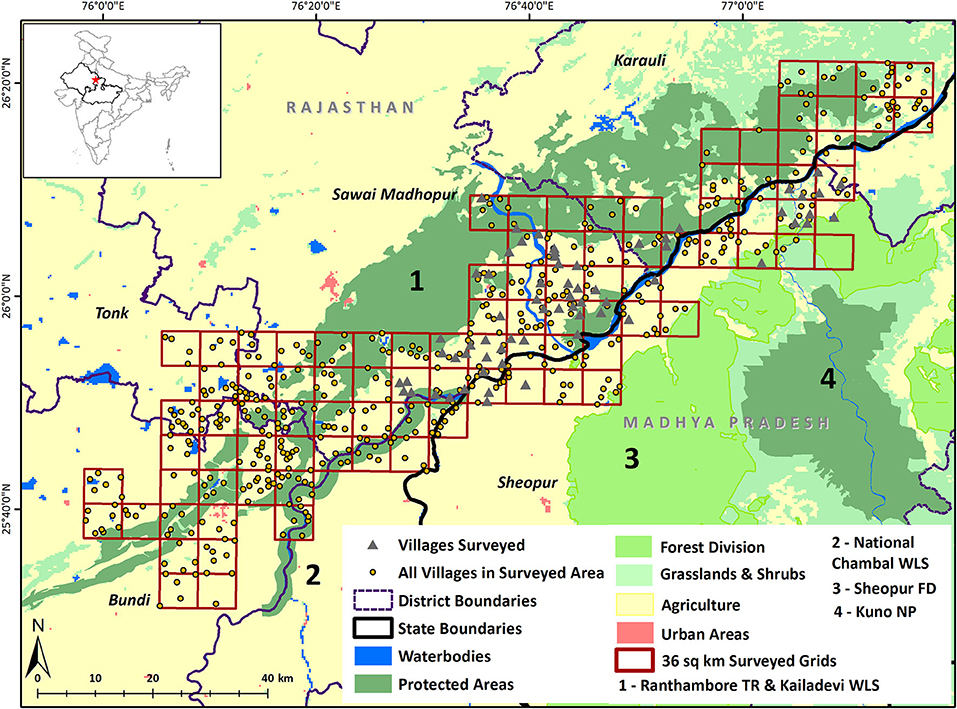
Figure 1. The study landscape abuts the Ranthambore Tiger Reserve, and encompasses a multi-use landscape corridor that connects Ranthambore and Kuno National Parks.
Prior to walking the trails, all the surveyors were trained on pugmark, scat, and scrape-mark identification for the four target species. This was done by walking trails within Ranthambore National Park, along trails known to be regularly used by all four target species, along with forest department staff, and senior WWF staff who were well trained in animal sign identification. Each trail was sampled by two observers, along with at least one forest department personnel, and care was taken to ensure that each trail sampling team had at least one person who was experienced in species sign identification. If there was ambiguity on pugmark or scrape-mark identification then the sign was discarded. All carnivore scats were genetically identified to confirm species. Scats were collected using swabs (HiMedia Inc.) and stored in vials containing Longmire's buffer (Longmire et al., 1997). DNA was extracted using QIAamp DNA Stool Mini Kit (QIAGEN Inc.) and species was identified using a combination of a generic carnivore primer and a felid specific primer (Farrell et al., 2000; Mukherjee et al., 2016).
We also collected information on relevant covariates: wild ungulate (prey) presence for tigers, leopards and hyenas, food resources (termite and ant signs, and fruiting trees) for sloth bears, human disturbances (people seen, lopping signs, grass/bamboo cut, vehicles, trash on the trail), construction/mining, and livestock. We recorded observer footprint visibility and signs of livestock along the trails as both could obscure wildlife signs. Additionally, we derived remotely sensed covariates (such as NDVI, forest cover, agricultural land, built-up area, waterbodies, terrain-ruggedness, built-up area and human population) but only retained those with correlation r < 0.7 (Supplementary Table 1; Supplementary Figure 1).
Social Survey
Semi-structured questionnaire surveys were conducted across 1,962 households in 66 villages. At least 25% of all the households in each village were sampled, where a single respondent 18 years or older participated in these verbal surveys after providing prior consent. Houses were selected at random across the village starting near the centre and moving outwards in at-least three different directions. Our survey was divided into 3 main sections, the first was focussed on social and demographic characteristics, such as education, occupation, land, and livestock holdings. Education was divided into 5 levels p1- illiterate, 2-Primary school, 3-Secondary school (grade - 6–10), 4-Higher Secondary school (grade 11–12), and 5-Graduate/Post-graduate degree]. Occupation of the respondents was divided into 5 categories (1-Agriculture, 2-Livestock husbandry, 3-Business, 4-Other and 5-labourer). Land-holding size of the respondents was taken in Bhigas (1 Acre = ~1.6 Bhigas in the study landscape) and along with livestock holding sizes and composition. The second section focused on natural resource dependency and local perceptions of wildlife populations. Natural resource use of various forest produce, including timber, leaves, fruits, vegetables, firewood, glue, and medicinal plants, which was measured in kilograms collected per day. And the last section pertained to the attitudes of the respondents towards wildlife. These involved open-ended questions about their encounters and relationships with wildlife, the damages, costs, or lack thereof incurred by them, and their perceptions towards living alongside said wildlife. The attitude related questions focused solely on large carnivores and herbivores and whether these species were important for ecosystems and warranted protection. Surveys were conducted by WWF's community-conservation project staff and community resource persons associated with WWF, local to the study area. Further details on the social surveys can be found in Appendix 1.
Data Analysis
Occupancy Analysis
The occupancy model used accounts for spatially clustered animal-sign data along trails (Hines et al., 2010). The key parameter of interest from these surveys was occupancy/habitat use (Ψ) probability for all four focal species. Given that a trail segment is occupied, there are two additional parameters of interest that relate to finer scale spatial use by the target species – the probability that a segment is used given that the previous segment was not used (θ), and the probability that a segment is used given that the previous segment is used (θ'). We adopted a two-stage modelling strategy wherein we first assessed variation in detection probability as a function of combinations of relevant covariates. For this step, we maintained a “global” model structure for Ψ (e.g., Karanth et al., 2011) (Table 1). In the next stage, we carried forward the best supported detection model from the first step and used additive and interactive combinations of the covariates to build between 21 and 44 models per species to assess factors influencing species' habitat use in Program MARK (White and Burnham, 1999) (Table 2). We evaluated model support using Akaike Information Criterion adjusted for small sample sizes (Burnham and Anderson, 2002).
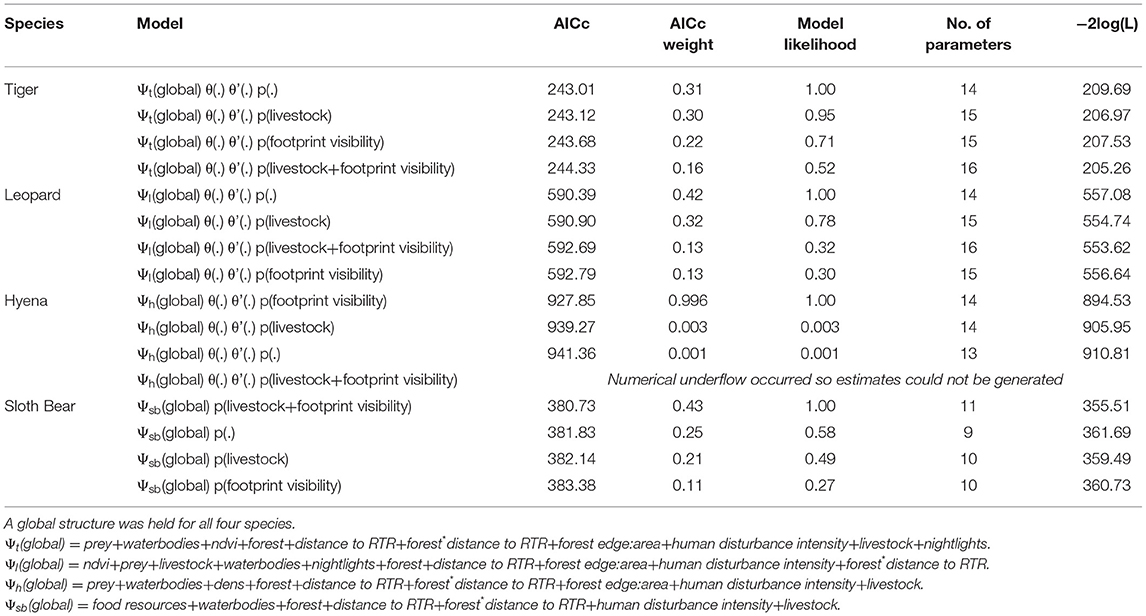
Table 1. Model comparisons to estimate detection probability as a function of observer footprint visibility and livestock.

Table 2. Model comparisons to determine influence of ecological and anthropogenic covariates on species's occupancy/habitat use.
For three of the four focal species, θ' values were roughly four times higher than θ values, indicating pronounced spatial structuring in the animal sign data from these trail surveys. Since sloth bear detections were extremely sparse, the correlated detection model did not converge and we generated estimated parameters using the single species occupancy model instead (Srivathsa et al., 2019). Given that the average home ranges of tigers, leopards and striped hyenas exceeds 36 km2, the occupancy parameter (Ψ) is interpreted as “habitat-use probability” (Wagner, 2006; Simcharoen et al., 2008, 2014; Wagner et al., 2008; Grant, 2012; Chundawat et al., 2016). Sloth bear home ranges are on average smaller than 36 km2 (Joshi et al., 1995; Yoganand et al., 2005; Ratnayeke et al., 2007), and hence, Ψ is interpreted as the occupancy probability.
Social Data Analysis
Attitudes towards wildlife was the primary response variable for our social surveys (Dickman and Hazzah, 2016), which was scored on a five point likert scale. Responses that entailed wildlife as needing to be eradicated or controlled lethally were scored as emphatically negative (1). When responses tended to characterise wildlife around them predominantly as a nuisance, these were scored as negative (2). Responses that were ambivalent towards wildlife, revealing neither preferences (or benefits) nor antagonism (or costs) were assigned to the neutral category (3). When responses spoke favourably of wildlife or testified the importance of sustaining wildlife to protect their habitats from which important ecosystem services emanate, a positive score was assigned (4). And last, responses that expressed that the wildlife around enriches the experiences of their daily lives, and their lives and the landscape would be irreversibly damaged without them were scored as emphatically positive (5). These were rescaled to a 3-point scale for the ANOVA analysis (1-negative, 2-positive, 3-neutral). For further details on how responses were scored, and other social data were collected please refer to Supplementary Table 2.
Predictor variables were education level, occupation, livestock holdings, land holdings, and natural resource dependency. We conducted a one-way ANOVA for livestock holdings, land holdings and natural dependency, and a Kruskal-Wallis sum rank test for education level and occupation categories followed by the Dunn's post-hoc test, in R (version 4.0.3), to ascertain if there were variations among these groups of predictor variables.
Results
Animal-Habitat Relationships
We encountered 57 tiger signs, which included 46 pugmarks, 6 scats, 3 scrape marks and 1 direct sighting, 149 leopard signs were encountered, including 95 pugmarks, 48 scats, 3 scrape marks and 2 claw mark signs on trees. Four hundred and forty six signs of striped hyenas were encountered, including 389 pugmarks, 55 scats, and 2 direct sightings. Of the 91 sloth bear signs encountered, there were 30 pugmarks, 30 scats, 27 digging signs, and 2 claw mark signs on trees. Tiger, leopard, hyena, and sloth bear signs were encountered in 14, 45, 69, and 32 of the ninety four 36 km2 grids, respectively. The naïve occupancy of the four target species was therefore 0.15 for tigers, 0.48 for leopards, 0.73 for hyenas, and 0.34 for sloth bears.
Observer footprint visibility and livestock sign encounter rates did not influence detection probability for tigers and leopards (Table 1). However, the footprint visibility was found to significantly explain variation in hyena sign detection, with higher detection probability in segments where footprints were more visible (β = 2.03, CI = 0.72–3.34). Estimated model averaged detection probabilities were 0.57 (SE = 0.19) for tigers, 0.74 (SE = 0.55) for leopards. For sloth bears and hyenas, segment specific detection probabilities were estimated as a function of covariates. Segment specific p estimates for sloth bears ranged between 0.14 (SE = 0.02) and 0.16 (SE = 0.02), while those for hyenas ranged between 0.63 (SE = 0.14) and 0.75 (SE = 0.14). Estimated habitat-use/occupancy (Ψ) probabilities were 0.09 (SE = 0.07; CI = 0.02–0.32) for tigers, 0.75 (SE = 0.21; CI = 0.25–0.97) for leopards, 0.93 (SE = 0.09; CI = 0.47–0.99) for hyenas, and 0.19 (SE = 0.21; CI = 0.02–0.78) for sloth bears (Table 2).
Forest area and distance from Ranthambore had the strongest influence on tiger habitat-use probability at a site (Ψ) as indicated by the best-supported model (Table 3; Figure 2). Tigers were 2.5 times more likely to occur in forests from 0 to 2 km from Ranthambore, than greater distances from the park boundary; the probability of tiger occurrence beyond 10 km from the park boundary was negligible but >0. While forest cover also positively influenced leopard and striped hyena habitat-use probability, both species were far more widespread across the study area than tigers (Table 3; Figure 2). Leopard habitat-use too declined with increasing distance from Ranthambore, with leopards 1.5 times more likely to occur between 0 and 5 km from Ranthambore, than 5 and 10 km from the park boundary, and less than a third as likely to occur 10–15 km from Ranthambore. The striped hyena was the most widespread of the four focal species, with the highest habitat-use probability, nearly three orders of magnitude higher than leopards across the study area (Table 3; Figure 2). The open, sparsely vegetated landscape is used extensively by hyenas, even though its natural scrubby habitats are now interspersed with farmlands. Sloth bears showed a high fidelity to forests, and the species occurrence probabilities drop sharply beyond the protected area boundaries. Sloth bear occupancy was positively associated with the availability of ants, termites, and fruiting trees, and declined with increasing human disturbance (Table 3; Figure 3).
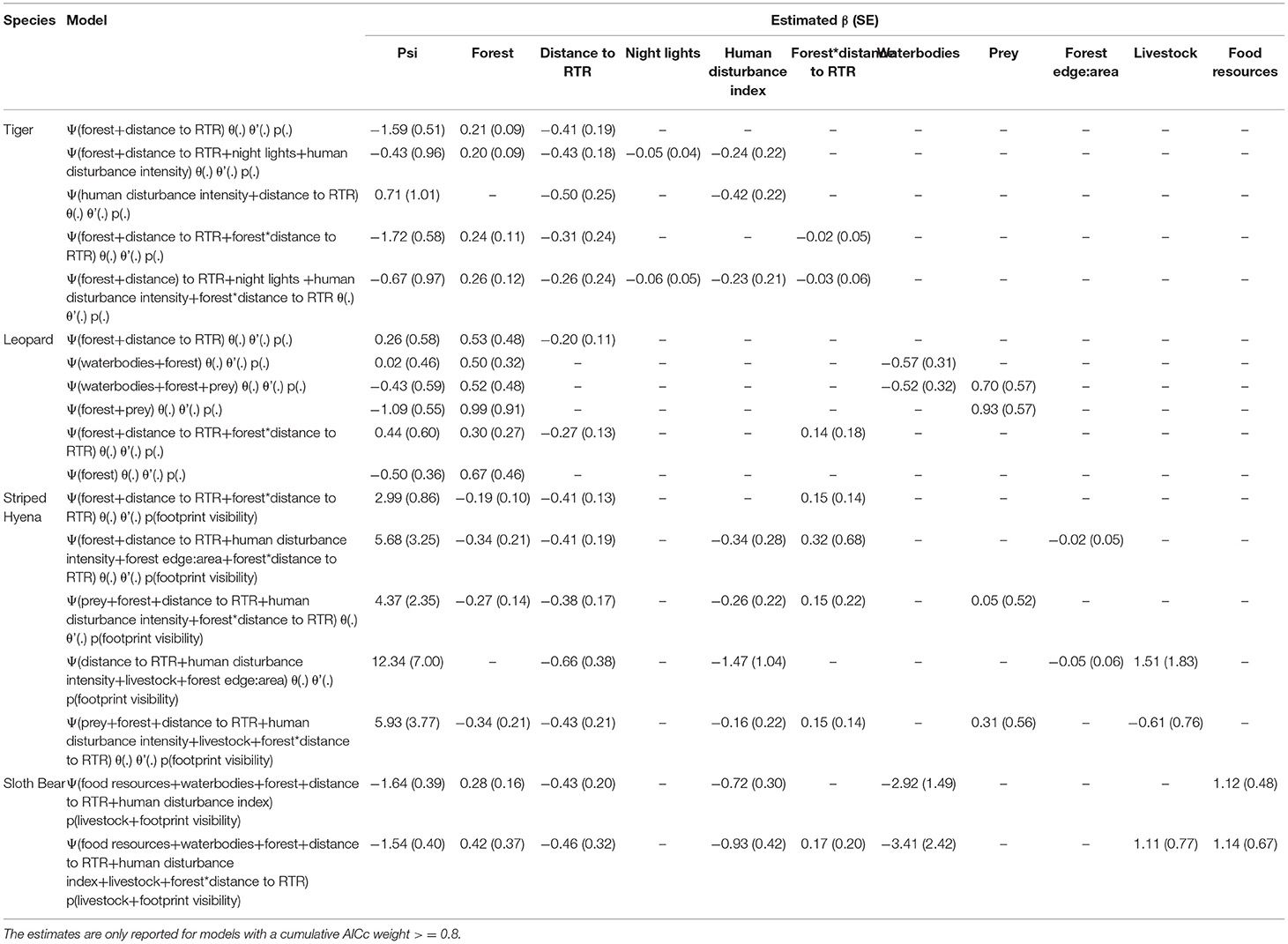
Table 3. Estimates of coefficients along with standard error for covariates influencing the species' occupancy/habitat use.
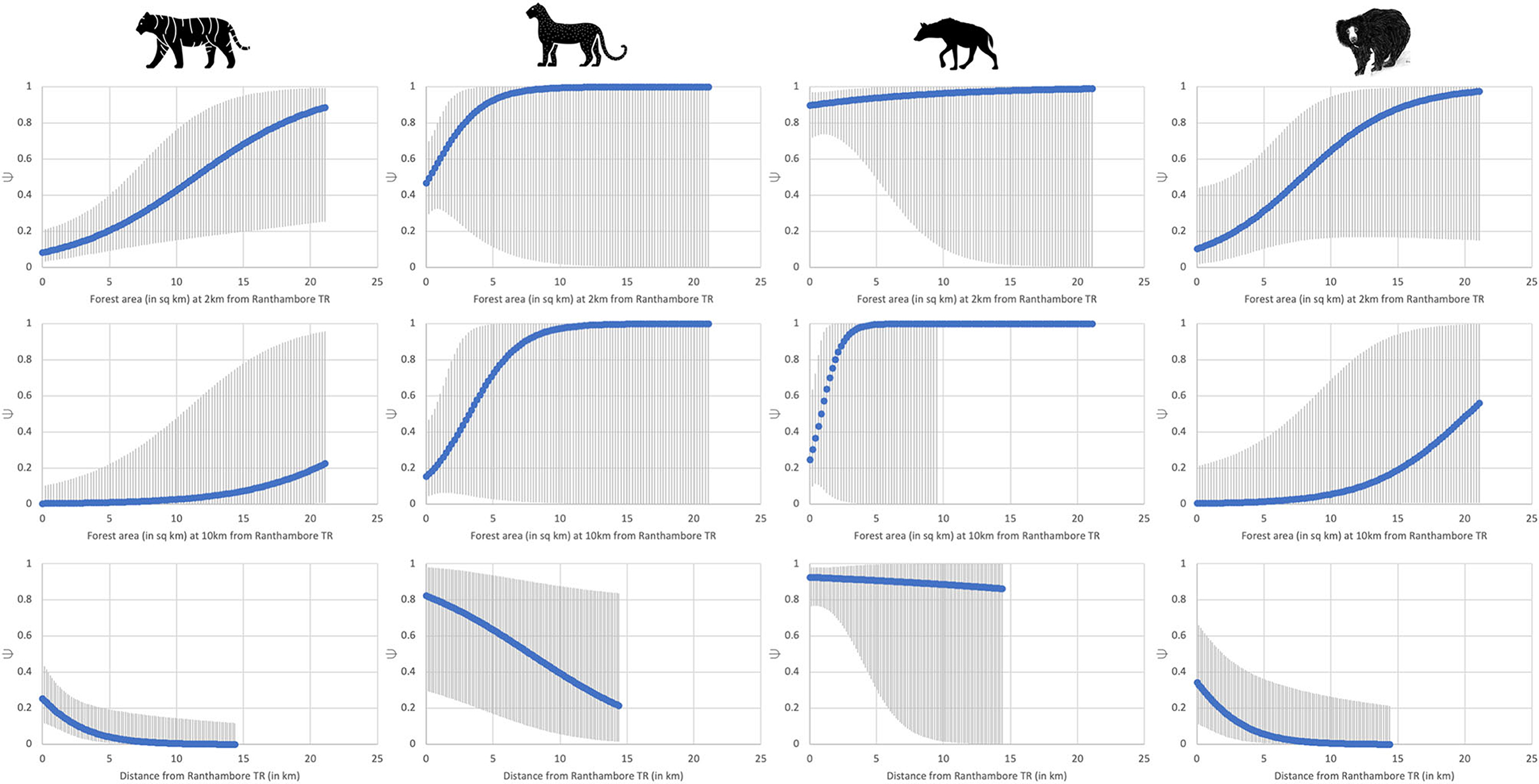
Figure 2. All four target species' Ψ were positively associated with forest area, with tiger habitat-use significantly associated with forest area. Ψ decreased with increasing distance from Ranthambore Tiger Reserve for all four species.
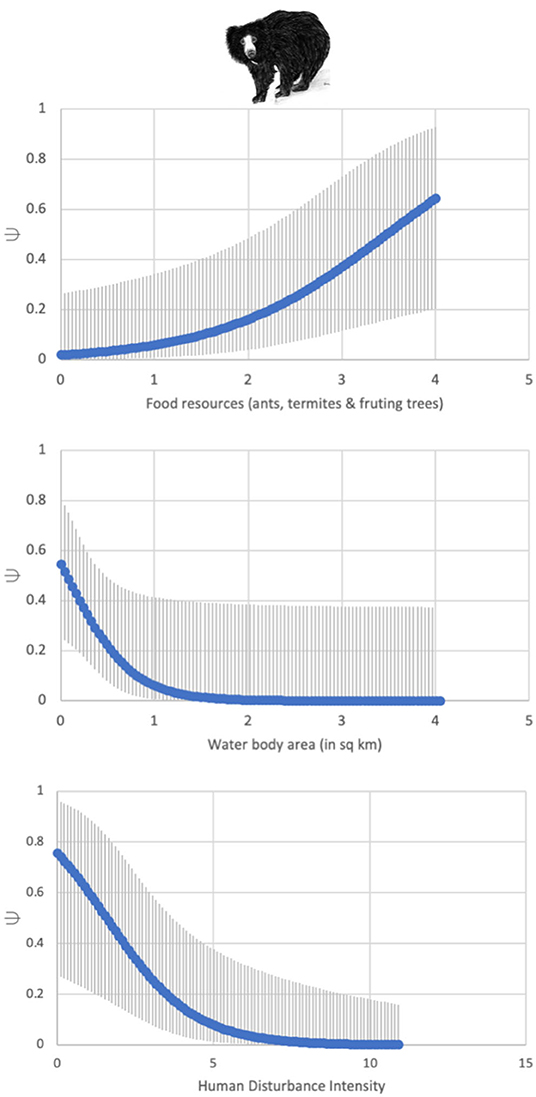
Figure 3. Sloth bear occupancy increases with food resources (ants, termites, and fruiting trees), and water availability, but decreases with increasing human disturbance. Both food availability and human disturbance have a significant influence of sloth bear occupancy across these multi-use areas.
Social Profile and Attitudes Towards Wildlife Among Local Communities
Overall, 1,955 residents of 66 villages, with a cumulative population of about 6,000 people were interviewed. Agriculture is the primary occupation for most of the local population (77% of respondents), with a much lower proportion of people engaged in pastoralism, labour, business, other livelihoods and being unemployed (7, 2, 2, 5, and 7%, respectively). Only 5% of respondents were college level educated or higher, 7% higher secondary educated, with 24% with either a primary or secondary level of education, while 40% were illiterate. Thirty four percent of the respondents owned livestock, buffaloes were the predominant livestock species reared (62.7%), followed by cattle (29.7%) and goats (9.2%). Less than one percent of people reported keeping other domestic animals – i.e., poultry, camels, or dogs. The average number of livestock per household was 4.12 (SD = 15.12). The average land holding of respondents was 5.35 (SD = 7.21) bighas (1 acre = ~3.25 Bighas in the study area), and a sizable proportion (20%) of the respondents surveyed were landless. Respondents reported extracting six primary types of resources from the region's forests: fuelwood, grass, and leaves (fodder), tubers and vegetables, fruit, glue, medicinal plants, and timber for house construction. Some of these items (the first two) were extracted daily in many households, while other items were extracted less frequently. The mean quanta of resources cumulatively extracted (all values converted to daily estimates) was 7 kg (SD = 11), but there was considerable variation among households (Figure 4). Fuelwood was prominent among resources accessed daily, and only 45.8% of households reported having LPG connexions.
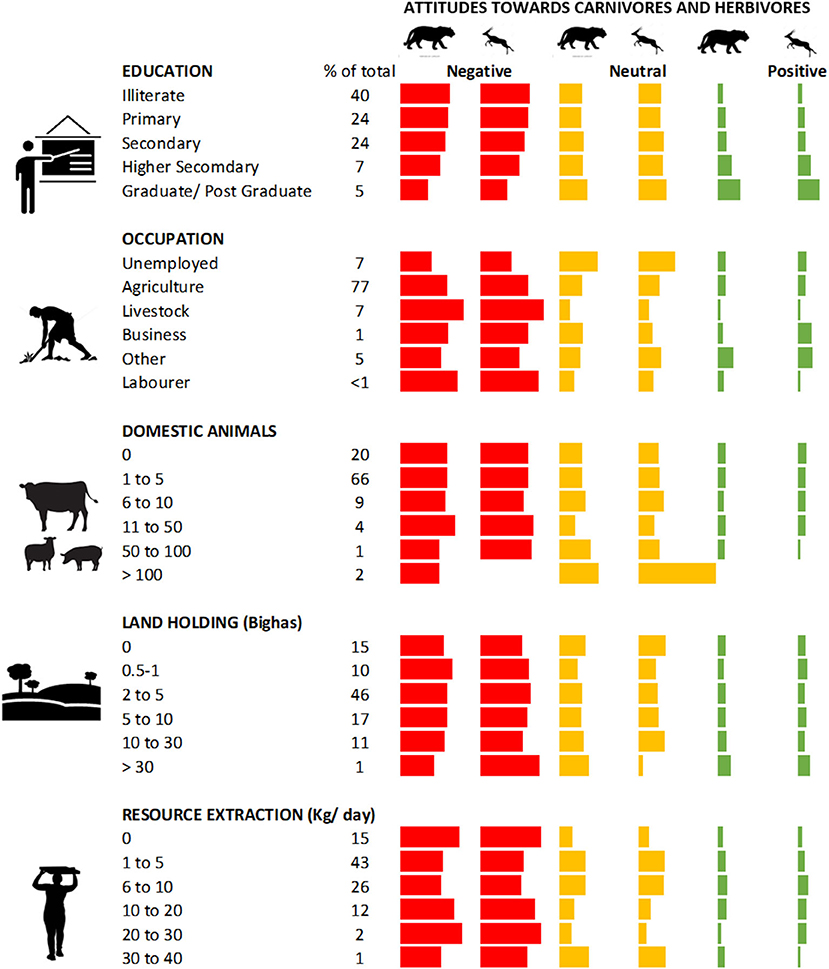
Figure 4. Attribution of attitudes towards carnivores and herbivores on a three-point likert scale to respondents, summarised under various socio-economic and livelihood categories. Values are all proportions of the total respondents in each category. The percent of total respondents (n = 1,962) in each category are reported as percent values.
Twenty-eight percent of respondents believed that livestock numbers had increased in the region, 62% believed it had decreased and 10% believed it was stable. Sixty eight percent of people surveyed believed that carnivore populations had increased across the landscape, 25% believed carnivore populations had decreased and 7% believed it was stable. Sixty five percent of respondents believed that wild herbivore populations had increased across the landscape, 23% believed it had decreased, and 12% believed it had remained stable across the landscape. Responses towards wildlife, both carnivores and herbivores, generally seemed to reflect that negative or ambivalent attitudes were expressed considerably more than positive attitudes. However, there appear to be some differences across categories of respondents based on education, occupation, and some other parameters.
Among the occupation categories, unemployed respondents expressed significantly more ambivalent attitudes towards wildlife than employed respondents in all other categories. Pastoralists were significantly more negative towards both carnivores and herbivores than those engaged in other forms of occupation. While those engaged in agriculture and labour were significantly more negative towards herbivores than respondents in other forms of employment. The views of respondents in business and other forms of employment did not significantly differ from other respondents (Supplementary Figure 2). Twenty to thirty percent of respondents who had completed senior secondary school, or college (graduation and beyond) expressed negative attitudes significantly more than those whose terminal education was up till secondary school or lower (Supplementary Figure 2). Views towards wildlife also differed among respondents with varying land-holdings; respondents with larger land holdings expressed positive views towards wildlife more than those with smaller land holdings (Figure 4). These observations or differences were also statistically validated (Supplementary Tables 3, 5–8).
Discussion
The importance of multi-use landscapes, as wildlife habitat and for connectivity, is increasingly being recognised. Such landscapes are used by carnivores to different extents and in different ways, and carnivores have behaviourally adapted to occurring in human-land use types to varying levels (Chapron et al., 2014; Majgaonkar et al., 2019). Our findings about heterogenous occurrences of four large carnivores and associated actors in this multi-use landscape both provide several new insights to guide connectivity planning, and raise some questions for future research to identify strategies to sustain wildlife in this landscape, while also building community support for conservation.
First, low probabilities of occurrence of tigers and sloth bears in much of the study area, especially at greater distances from Ranthambore TR, suggests that these species don't use the study area extensively as habitat, but may still use it for dispersal. In contrast, extensive striped hyena occurrence indicates that the species uses the area as habitat, and is likely a corridor dweller, given that areas with scrub and agriculture provide suitable habitats. While leopard occurrence was more widespread than sloth bears and tigers, it was largely associated with forest tracts, with only low occurrence probability in agricultural areas, which is the dominant land-use. Figure 5 shows how the variability of space use between these four large carnivores maps out across these multi-use corridors. These results suggest the need for at least two overlapping conservation strategies. For corridor dwellers, like the striped hyena, conservation value of non-protected areas and small scrub patches that provide refuge and prevent persecution is high, despite being embedded in a human-modified surrounding. Such species often have more-or-less continuous distribution and breeding populations within the areas identified as movement corridors for larger species, like the tiger. Given that tiger use of the corridor may be more infrequent, it is crucial that conservation efforts focus on keeping the corridor functional for their dispersal to nearby habitat patches in north-west India. This is imperative for the recovery of this isolated tiger sub-population. So long as land use types that are permeable to movement (including) agriculture are maintained, the priority may be to influence the design and location of infrastructure projects, mines, and urban development, to ensure that impediments like major highways do not severely erode connectivity (Thatte et al., 2020).

Figure 5. Estimated probabilities of habitat-use/occupancy for all four focal species. Striped hyenas are widespread across the landscape, followed by leopards, sloth bears, and tigers. Leopards are largely concentrated around the forest edges and sparse across most of the landscape whereas sloth bears and tigers are restricted to the edges of the forest areas and are negligibly present across the landscape.
Second, recent evidence from North India (Warrier et al., 2020) and Central India (Sarkar et al., 2021) suggest that tigers may spend extended periods of time in multi-use corridors. Yet, this appears not to be the case in our study area in Western India. How has this come to be? Our finding that carnivore use is largely being restricted to the areas around Ranthambore, especially for tigers and sloth bears, suggests that the matrix may be hostile for these species (Ranganathan et al., 2008). This could be an outcome of several factors in combination. Numerous studies have demonstrated that the spatial distribution of predators is primarily affected by the spatial distribution of their wild prey (Carter et al., 2019). For instance, prey densities are the key determinant for tiger presence across a 38,000 km2 landscape in Southern India (Karanth et al., 2011). However, in this study landscape, the presence of wild prey did not determine the habitat-use of leopards, tigers, and striped hyenas. Since wild prey have a limited distribution across the agricultural matrix, relative to protected areas and reserve forests (for example Ranthambore and Sheopur), they may support dispersing animals, but are likely too sparsely distributed for tigers to include large agricultural tracts in their territories. Domestic animals that are preyed on by tigers and other carnivores may also be an attractant, especially closer to forest edges. Prey distribution may be sparse both because cover may be sparse for many months of the year, and because wild ungulates may be hunted outside protected areas. Further, the availability of forest cover is crucial for carnivores, particularly ambush predators like tiger and leopard, and for concealment against anthropogenic risks especially in multi-use landscapes. While there are extensive tracts of scrub habitats in the ravines along the Chambal and smaller patches embedded elsewhere, it appears that these patches may be too disturbed or too small to provide refuges for large carnivores, other than hyenas. Alternately, wildlife in these areas may be especially vulnerable to poaching. This contrasts with other regions, where leopards can be found at densities as high as 4.8/100 km2 in agro-ecosystems (Athreya et al., 2013), and their presence is strongly associated with irrigated crop fields and high livestock densities (Majgaonkar et al., 2019; Warrier et al., 2020). The crops of arid Rajasthan and western Madhya Pradesh do not provide the same cover as sugarcane plantations elsewhere in India.
Resource availability and cover played out differently for sloth bears and hyenas, with the former showing a positive relationship with fruiting trees, ants and termites within its forest habitats, and the latter being widely distributed. The hyena's relative success in this multi-use landscape may be attributed, at least in part, to ubiquitously high livestock densities, which provide ample opportunity for scavenging (Majgaonkar et al., 2019). Our analysis did not yield hyena occurrence to be strongly associated with livestock presence. However, several cattle carcass disposal are distributed across the landscape, which we did not document and hyenas are known to routinely visit these, an aspect that needs further investigation. Striped hyenas are not a forest dwelling species but still showed higher occurrence probabilities near Ranthanmbore's boundaries. This may be because more secure den sites are to be found within the rugged slopes of the reserve, than in agricultural fields or the ravines, which are more disturbed. Hyenas are also known to den in agricultural fields in this landscape, but it is unknown if they are able to successfully rear pups in disturbed areas. By increasing habitat diversity and food resources, low-density urbanisation may benefit some species, while being permeable for travelling and foraging (Riley et al., 2010). However, anthropogenic disturbance within these urban and peri-urban areas can reduce habitat quality and suitability (Hansen et al., 2005; McKinney, 2008).
Third, observed patterns of carnivore occurrence in the landscape are also a reflection of their populations within the region's protected areas. Ranthambore remains the sole source population of tigers in this landscape. Reddy et al. (2012) indicated tiger movement between Ranthambore, Kuno and Madhav National Parks, as well as in the opposite direction, indicating that these areas contained source populations that have been extirpated in the recent past. Evidence of this is seen in the low occurrence probabilities of tigers around the Kuno-Sheopur forest block, even as probabilities were higher around Ranthambore Tiger Reserve. The same may apply for sloth bears and leopards, though specific data is lacking. A more detailed study on the population ecology of sloth bears, leopards and hyenas in this region would be extremely useful in this regard.
The realised population sizes and long-term persistence of large carnivores in protected areas within human dominated landscapes may intrinsically be linked to the hostility of the surrounding matrix (Ranganathan et al., 2008; Chapron et al., 2014). While communities in the matrix could threaten the survival of carnivores through direct hunting pressure within protected areas, risks to animals outside protected area boundaries and within may also play a critical role in the survival and recovery of metapopulations. Our attitudes assessment suggests predominantly negative attitudes towards both large carnivores and wild ungulates in this landscape. Such attitudes can engender hostility towards wildlife (Dickman and Hazzah, 2016), but we do not have specific evidence to link it to poaching or other forms of retaliation, which are usually silent crimes. Given the high level of reliance on rain-fed and subsistence agriculture and small livestock holdings, such attitudes may stem from the loss of crops and livestock, with limited or no mitigation. Although this is only a preliminary analysis, it suggests that in addition to habitat suitability and ecological resistance for wildlife presence and movement, social resistance may also have played a role in the species fortunes within the matrix and may determine future conservation outcomes. Our results indicate that increased literacy, which potentially shifts people away from agriculture and animal rearing towards other occupations – may potentially engender more positive attitudes (Holmern et al., 2007; Karanth and Nepal, 2012; Karanth et al., 2019). Thus, interventions that aim to raise literacy levels among local communities both through the formal education process and sustained awareness campaigns could help engender more positive attitudes towards wildlife. Such activities, though important, are unlikely to yield conservation dividends in themselves unless root causes of unfavourable attitudes towards wildlife are thoroughly assessed and systematically addressed, for example, through well-functioning crop and livestock compensation and insurance programs.
Directions for Future Research
Even as our study advances knowledge of wildlife use of a significant and understudied corridor and some attributes of communities in the region, it has several limitations which can guide the design of future research. First, a single season study only provides a snapshot of wildlife use of the area, which may be dynamic, following cropping cycles (e.g., Warrier et al., 2020). A longer-term dataset is also a priority given significant land use change—primarily through agricultural expansion, sand mining and ravine flattening in critical portions of the corridor (Ranganathan, 2017). Future studies can provide specific spatially explicit guidance. Second the use of other sampling methods in addition to sign surveys—for example camera traps and genetic analysis of scat—would potentially have added data and strengthened inference from the occupancy models, and potentially also cast more light on the origin, movement, and numbers of individually marked animals within the study area (tigers, leopards, and hyenas). These methods would also provide reliable information on other species of conservation importance in the corridor including caracals (Caracal caracal), wolves (Canis lupus) and various small cats like the rusty-spotted cat (Prionailurus rubiginosus) and Asiatic Wild Cat (Felis silvestris ornata). Future socio-ecological studies could further investigate the interface and relationships between human-wildlife conflict and wildlife occurrence and tolerance (e.g., Warrier et al., 2021). Additionally, more open-ended interviews, focal group discussions and qualitative data analysis could further illuminate the factors underlying tolerance and cohabitation (Ogra, 2009; Austin et al., 2010). Furthermore, information on compensation, including the status of current claims and an assessment of the efficiency of compensation payment mechanisms is also important.
The state of Rajasthan does not currently have a policy for crop compensation and property damage (Karanth and Kudalkar, 2017). Continued economic losses to wildlife have been seen to drive people to engage in retaliatory killing in some areas (Holmern et al., 2007). There also appears to be a lack of understanding or assessment of crop damage by the forest administration in Rajasthan. Economic losses to wildlife may be a major driving factor of the locals' negative attitudes towards wildlife in this multi-use landscape. Effective assessments of crop damage and compensation mechanisms for damage by wildlife may go a long way towards quelling negative attitudes towards wildlife, and promote greater tolerance, and in turn allow wildlife conservation to receive a higher priority in these multi-use areas.
A diverse array of unique and threatened wildlife cohabits with people with a litany of social problems including poverty, low education levels and gender inequality. This makes setting aside resources for wildlife preservation in this region, an extremely challenging proposition, similar to much of the developing world. However, prioritising development at the expense of natural habitats and the wildlife they harbour not only imperils wildlife but also compromises the ecosystem services that local people also depend on. Therefore, prioritising natural habitat conservation across multi-use habitats is imperative, to facilitate wildlife conservation alongside socio-economic development, and co-existence in healthy ecosystems.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
PT and PC conceived and designed the study. TM, NB, and AB designed the social surveys. PK, SK, TM, NB, PT, and PC collected the data. SS, PC, and PK analysed the data and wrote and edited the manuscript. SS, PT, and PC provided feedback on the manuscript. YS and AB planned and supervised the project and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The funding for this project was provided by WWF-India's Tiger Conservation Project in the Western India Tiger Landscape.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the World Wide Fund for Nature-India, Rajasthan Forest Department and Madhya Pradesh Forest Department for permission to conduct the study and to field staff of Ranthambore Tiger Reserve, National Chambal Wildlife Sanctuary and Sheopur Forest Division for conducting the sign surveys with our team for facilitating the surveys in several locations. Community resource persons: Ummed Bhairwa, Sonu Verma, Gajanand Sharma, Kailash Sharma, and Dharmendra Gujjar of WWF-India's Western India Tiger Landscape contributed for coordination with forest staff and field data collection. Our volunteers: Archita Sharma, Kaushal Chauhan, Rutuja Bhatade, Aakash Bhushan, Malvika Colvin, Soham Pattekar, Vinayaka Hegde, Pawan Gadenna, Ravneesh Singh Klair, Deval Kadam, Dinkar Samore, Sudheer Dewarpalli, and Mugdha Govande for their assistance in the field. We also thank the donors of the WWF's Tiger Conservation in the Western India Tiger Landscape for their generous support. We would also like to thank Uma Ramkrishnan and her lab at the National Center for Biological Sciences for the genetic identification of our scat samples collected in the field.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.787431/full#supplementary-material
References
AbiSaid, M., and Dloniak, S. M. D. (2015). Hyaena hyaena. The IUCN Red List of Threatened Species 2015, e.T10274A45195080.
Acharya, K. P., Paudel, P. K., Jnawali, S. R., Neupane, P. R., and Koehl, M. (2017). Can forest fragmentation and configuration work as indicators of human–wildlife conflict? Evidences from human death and injury by wildlife attacks in Nepal. Ecol. Indic. 80, 74–83. doi: 10.1016/j.ecolind.2017.04.037
Akhtar, N., Bargali, H. S., and Chauhan, N. P. S. (2004). Sloth bear habitat use in disturbed and unprotected areas of Madhya Pradesh, India. Ursus 15, 203–211. doi: 10.2192/1537-6176(2004)015<0203:SBHUID>2.0.CO;2
Alam, M., Khan, J., Kushawa, S., Agrawal, R., Pathak, B., and Kumar, S. (2014). Assessment of suitable habitat of near threatened striped hyena (Hyaena hyaena Linnaeus, 1758) using remote sensing and geographic information system. Asian J. Geoinform. 14, 1–10. (accessed July 16, 2014).
Athreya, V., Odden, M., Linnel, J. D. C., Krishnaswamy, J., and Karanth, U. (2013). Big cats in our backyards: persistence of large carnivores in a human-dominated landscape in India. PLoS ONE 8:e57872. doi: 10.1371/journal.pone.0057872
Athreya, V., Pimpale, S., Borkar, A. S., Surve, N., Chakravarty, S., Ghosalkar, M., et al. (2018). Monsters or Gods? Narratives of large cat worship in western India. Cat News 67, 23–26. (accessed June 8, 2018).
Austin, Z., Smart, J. C. R., Justine Irvine, R., and White, P. C. L. (2010). Identifying conflicts and opportunities for collaboration in the management of a wildlife resource: a mixed-methods approach. Wildlife Res. 37, 647–657. doi: 10.1071/WR10057
Bista, A., Chanchani, P., Subedi, N., and Bajracharya, S. B. (2021). The peri-urban leopards of Kathmandu: assessing determinants of presence and predation on domestic animals. Oryx. 56, 91–100. doi: 10.1017/S0030605320000423
Burnham, K. P., and Anderson, D. R. (2002). A practical information-theoretic approach. Model Select. Multimodel Infer. 2, 70–71.
Carter, N. H., Levin, S. A., and Grimm, V. (2019). Effects of human-induced prey depletion on large carnivores in protected areas: lessons from modeling tiger populations in stylized spatial scenarios. Ecol. Evol. 9, 11298–11313. doi: 10.1002/ece3.5632
Carter, N. H., and Linnell, J. D. C. (2016). Co-adaptation is key to coexisting with large carnivores. Trends Ecol. Evol. 31, 575–578. doi: 10.1016/j.tree.2016.05.006
Chanchani, P., Noon, B. R., Bailey, L. L., and Warrier, R. A. (2016). Conserving tigers in working landscapes. Conserv. Biol. 30, 649–660. doi: 10.1111/cobi.12633
Chapron, G., Kaczensky, P., Linnell, J. D. C., Von Arx, M., Huber, D., Andrén, H., et al. (2014). Recovery of large carnivores in Europe's modern human-dominated landscapes. Science 346, 1517–1519. doi: 10.1126/science.1257553
Chundawat, R. S., Sharma, K., Gogate, N., Malik, P. K., and Vanak, A. T. (2016). Size matters: scale mismatch between space use patterns of tigers and protected area size in a tropical dry forest. Biol. Conserv. 197, 146–153. doi: 10.1016/j.biocon.2016.03.004
Das, S., Dutta, S., Sen, S., Babu, S., Kumara, H. N., and Singh, M. (2014). Identifying regions for conservation of sloth bears through occupancy modelling in north-eastern Karnataka, India. Ursus 25, 111–120. doi: 10.2192/URSUS-D-14-00008.1
Dickman, A. J., and Hazzah, L. (2016). “Money, myths and man-eaters: complexities of human–wildlife conflict,” in editor F. Angelici. Problematic Wildlife (Cham: Springer).
Dutta, T., Sharma, S., Maldonado, J. E., Panwar, H. S., and Seidensticker, J. (2015). Genetic variation, structure, and gene flow in a sloth bear (Melursus ursinus) meta-population in the Satpura-Maikal landscape of central India. PLoS ONE 10:e0123384. doi: 10.1371/journal.pone.0123384
Farrell, L. E., Roman, J., and Sunquist, M. E. (2000). Dietary separation of sympatric carnivores identified by molecular analysis of scats. Mol. Ecol. 9, 1583–1590. doi: 10.1046/j.1365-294x.2000.01037.x
Gervasi, V., Nilsen, E. B., Odden, J., Bouyer, Y., and Linnell, J. D. C. (2014). The spatio-temporal distribution of wild and domestic ungulates modulates lynx kill rates in a multi-use landscape. J. Zool. 292, 175–183. doi: 10.1111/jzo.12088
Ghosh-Harihar, M., An, R., Athreya, R., Borthakur, U., Chanchani, P., Chetry, D., et al. (2019). Protected areas and biodiversity conservation in India. Biol. Conserv. 237, 114–124. doi: 10.1016/j.biocon.2019.06.024
Grant, T. (2012). Leopard Population Density, Home Range Size and Movement Patterns in a Mixed Land Use Area of the Mangwe District of Zimbabwe (Master thesis). Grahamstown, South Africa: Rhodes University. Available online at: https://core.ac.uk/download/pdf/145047042.pdf (accessed February 2012).
Hansen, A. J., Knight, R. L., Marzluff, J., O'Brien, M., Powell, S., Brown, K., et al. (2005). Effects of exurban development on biodiversity: patterns, mechanisms, and research needs. Ecol. Appl. 15, 1893–1905. doi: 10.1890/05-5221
Harihar, A., Pandav, B., and Goyal, S. P. (2011). Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. J. Appl. Ecol. 48, 806–814. doi: 10.1111/j.1365-2664.2011.01981.x
Harris, L. D., and Silva-Lopez, G. (1992). “Forest fragmentation and the conservation of biological diversity,” in Conservation Biology, eds P. L. Fiedler and S. K. Jain (Boston, MA: Springer).
Hines, J. E., Nichols, J. D., Royle, J. A., MacKenzie, D. I., Gopalaswamy, A. M., Kumar, N. S., et al. (2010). Tigers on trails: occupancy modeling for cluster sampling. Ecol. Appl. 20, 1456–1466. doi: 10.1890/09-0321.1
Holmern, T., Nyahongo, J., and Røskaft, E. (2007). Livestock loss caused by predators outside the Serengeti National Park, Tanzania. Biol. Conserv. 135, 518–526. doi: 10.1016/j.biocon.2006.10.049
Jayadevan, A., Nayak, R., Karanth, K. K., Krishnaswamy, J., DeFries, R., Karanth, K. U., et al. (2020). Navigating paved paradise: evaluating landscape permeability to movement for large mammals in two conservation priority landscapes in India. Biol. Conserv. 247:108613. doi: 10.1016/j.biocon.2020.108613
Jhala, Y. V., Qureshi, Q., and Nayak, A. K. (2019). Status of tigers, co-predators and prey in India 2018. Summary Report. National Tiger Conservation Authority, Government of India, New Delhi and Wildlife Institute of India, Dehradun. TR No./2019/05. Available online at: https://ntca.gov.in/assets/uploads/Reports/AITM/Status_Tigers_India_summary_2018.pdf (accessed July 22 2019).
Joshi, A., Vaidyanathan, S., Mondo, S., Edgaonkar, A., and Ramakrishnan, U. (2013). Connectivity of tiger (Panthera tigris) populations in the human-influenced forest mosaic of central India. PLoS ONE 8:e77980. doi: 10.1371/journal.pone.0077980
Joshi, A. R., Garshelis, D. L., and Smith, J. L. (1995). Home ranges of sloth bears in Nepal: implications for conservation. J. Wildlife Manage. 59, 204–214. doi: 10.2307/3808932
Karanth, K. K., Jain, S., and Weinthal, E. (2019). Human–wildlife interactions and attitudes towards wildlife and wildlife reserves in Rajasthan, India. Oryx 53, 523–531. doi: 10.1017/S0030605317001028
Karanth, K. K., and Kudalkar, S. (2017). History, location, and species matter: insights for human–wildlife conflict mitigation from India. Hum. Dimensions Wildlife 22, 331–346. doi: 10.1080/10871209.2017.1334106
Karanth, K. K., and Nepal, S. K. (2012). Local residents perception of benefits and losses from protected areas in India and Nepal. Environ. Manage. 49, 372–386. doi: 10.1007/s00267-011-9778-1
Karanth, K. U., Gopalaswamy, A. M., Kumar, N. S., Vaidyanathan, S., Nichols, J. D., and MacKenzie, D. I. (2011). Monitoring carnivore populations at the landscape scale: Occupancy modelling of tigers from sign surveys. J. Appl. Ecol. 48, 1048–1056. doi: 10.1111/j.1365-2664.2011.02002.x
Karanth, K. U., Kumar, N. S., and Karanth, K. K. (2020). Tigers against the odds: applying macro-ecology to species recovery in India. Biol. Conserv. 252:108846. doi: 10.1016/j.biocon.2020.108846
Karanth, K. U., and Suinquist, M. (2000). Behavioural correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagarahole, India. J. Zool. 250, 255–265. doi: 10.1111/j.1469-7998.2000.tb01076.x
Kennedy, C. M., Oakleaf, J. R., Theobald, D. M., Baruch-Mordo, S., and Kiesecker, J. (2019). Managing the middle: a shift in conservation priorities based on the global human modification gradient. Glob. Chang. Biol. 25, 811–826. doi: 10.1111/gcb.14549
Kinnaird, M. F., Sanderson, E. W., O'Brien, T. G., Wibisono, H. T., and Woolmer, G. (2003) Deforestation trends in a tropical and landscape implications for endangered large mammals. Conserv. Biol. 17, 245–257. doi: 10.1046/j.1523-1739.2003.02040.x
Longmire, J. L., Maltbie, M., and Baker, R. J. (1997). Use of ‘lysis buffer' in DNA isolation and its implications for museum collections. Museum Texas Tech Univ. 163, 1–3. doi: 10.5962/bhl.title.143318
Majgaonkar, I., Vaidyanathan, S., Srivathsa, A., Shivakumar, S., Limaye, S., and Athreya, V. (2019). Land-sharing potential of large carnivores in human-modified landscapes of western India. Conserv. Sci. Pract. 1:e34. doi: 10.1111/csp2.34
McKinney, M. L. (2008). Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst. 11, 161–176. doi: 10.1007/s11252-007-0045-4
Mukherjee, S., Athreya, R., Karunakaran, P. V., and Choudhary, P. (2016). Ecological Species Sorting in Relation to Habitat Structure in the Small Cat Guild of Eaglenest Wildlife Sanctuary, Arunachal Pradesh. Sálim Ali Centre for Ornithology and Natural History, Coimbatore, Tamil Nadu. Technical Report No.PR-182. 52 pp.
Naha, D., Dash, S. K., Chettri, A., Chaudhary, P., Sonker, G., and Heurich, M. (2020): Landscape predictors of human-leopard conflicts within multi-use areas of the Himalayan region. Sci. Rep. 10:11129. doi: 10.1038/s41598-020-67980-w.
Naha, D., Dash, S. K., Kupferman, C., Beasly, J. C., and Sathyakumar, S. (2021). Movement behavior of a solitary large carnivore within a hotspot of human-wildlife conflicts in India. Sci. Rep. 11:3862. doi: 10.1038/s41598-021-83262-5
Natesh, M., Atla, G., Nigam, P., Jhala, Y. V., Zachariah, A., Borthakur, U., et al. (2017). Conservation priorities for endangered Indian tigers through a genomic lens. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-09748-3
Nyhus, P., and Tilson, R. (2004). Agroforestry, elephants, and tigers: balancing conservation theory and practice in human-dominated landscapes of Southeast Asia. Agric. Ecosyst. Environ. 104, 87–97. doi: 10.1016/j.agee.2004.01.009
O'Brien, T. G., Kinnaird, M. F., and Wibisono, H. T. (2003). Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 6, 131–139. doi: 10.1017/S1367943003003172
Ogra, M. (2009). Attitudes toward resolution of human–wildlife conflict among forest-dependent agriculturalists near Rajaji National Park, India. Hum. Ecol. 37, 161–177. doi: 10.1007/s10745-009-9222-9
Palei, H. S., Debata, S., and Sahu, H. K. (2020). Diet of sloth bear in an agroforest landscape in eastern India. Agroforest. Syst. 94, 269–279. doi: 10.1007/s10457-019-00389-1
Pawar, D., Nelson, H. P., Pawar, D. R., and Khanwilkar, S. (2019). Estimating leopard panthera Pardus fusca (Mammalia: Carnivora: Felidae) abundance in Kuno Wildlife Sanctuary, Madhya Pradesh, India. J. Threat. Taxa 11, 13531–13544. doi: 10.11609/jott.4774.11.5.13531-13544
Philip, R., Bhatnagar, C., and Koli, V. K. (2021). Feeding ecology of the vulnerable sloth bear (Melursus ursinus) in and around Mount Abu Wildlife Sanctuary, Rajasthan, India. Int. J. Environ. Stud. 1–11. doi: 10.1080/00207233.2021.1941668
Puri, M., Srivathsa, A., Karanth, K. K., Kumar, N. S., and Karanth, U. K. (2015). Multiscale distribution models for conserving widespread species: the case of sloth bear Melursus ursinus in India. Divers. Distrib. 21, 1087–1100. doi: 10.1111/ddi.12335
Puri, M., Srivathsa, A., Karanth, K. U., Patel, I., and Kumar, N. S. (2020). The balancing act: maintaining leopard-wild prey equilibrium could offer economic benefits to people in a shared forest landscape of central India. Ecol. Indic. 110:105931. doi: 10.1016/j.ecolind.2019.105931
Ramesh, T., Kalle, R., Sankar, K., and Qureshi, Q. (2012). Factors affecting habitat patch use by sloth bears in Mudumalai Tiger Reserve, Western Ghats, India. Ursus 23, 78–85. doi: 10.2192/URSUS-D-11-00006.1
Ranganathan, J., Chan, K. M., Karanth, K. U., and Smith, J. L. D. (2008). Where can tigers persist in the future? A landscape-scale, density-based population model for the Indian subcontinent. Biol. Conserv. 141, 67–77. doi: 10.1016/j.biocon.2007.09.003
Ranganathan, P. (2017). The Effects of Land Use Change on Carnivore Use of Wildlife Dispersal Routes in Ranthambhore Tiger Reserve, India (PhD thesis). Duke University. Available online at: https://dukespace.lib.duke.edu/dspace/bitstream/handle/10161/14134/Ranganathan_MPreport_Final.pdf?sequence=1 (accessed April 13, 2017).
Ratnayeke, S., van Manen, F. T., and Padmalal, U. K. G. K. (2007). Home ranges and habitat use of sloth bears Melursus Ursinus Inornatus in Wasgomuwa National Park, Sri Lanka. Wildlife Biol. 13, 272–284. doi: 10.2981/0909-6396(2007)13[272:HRAHUO]2.0.CO;2
Reddy, P. A., Gour, D. S., Bhavanishankar, M., Jaggi, K., Hussain, S. M., Harika, K., et al. (2012). Genetic evidence of tiger population structure and migration within an isolated and fragmented landscape in northwest India. PLoS ONE 7:e29827. doi: 10.1371/journal.pone.0029827
Redpath, S. M., Linnell, J. D. C., Festa-Bianchet, M., Boitani, L., Bunnefeld, N., Dickman, A., et al. (2017). Don't forget to look down – Collaborative approaches to predator conservation. Biol. Rev. 92, 2157–2163. doi: 10.1111/brv.12326
Riley, S. P. D., Gehrt, S. D., and Cypher, B. L. (2010). “Urban carnivores: final perspectives and future directions,” in Urban Carnivores: Ecology, Conflict, and Conservation, eds S. D. Gehrt, S. P. D. Riley, and B. L. Cypher (Baltimore, MD: The Johns Hopkins University Press), 223–232.
Sarkar, M. S., Niyogi, R., Masih, R. L., Hazra, P., Maiorano, L., and John, R. (2021). Long-distance dispersal and home range establishment by a female sub-adult tiger (Panthera tigris) in the Panna landscape, central India. Eur. J. Wildl. Res. 67:54. doi: 10.1007/s10344-021-01494-2
Saunders, D. A., Hobbs, R. J., and Margules, C. R. (1991). Biological consequences of ecosystem fragmentation: a review. Conserv. Biol. 5, 18–32. doi: 10.1111/j.1523-1739.1991.tb00384.x
Shah, S., Nayak, S., Gurjar, R., and Borah, J. (2015). Beyond the Realms of Ranthambhore: The Last Abode for Arid Zone Tigers. Delhi: WWF-India.
Simcharoen, A., Savini, T., Gale, G. A., Simcharoen, S., Duangchantrasiri, S., Pakpien, S., et al. (2014). Female tiger Panthera tigris home range size and prey abundance: Important metrics for management. Flora Fauna Int. 48, 370–377. doi: 10.1017/S0030605312001408
Simcharoen, S., Barlow, A. C. D., Simcharoen, A., and Smith, J. L. D. (2008). Home range size and daytime habitat selection of leopards in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Biol. Conserv. 144, 2242–2250. doi: 10.1016/j.biocon.2008.06.015
Singh, R., Pandey, P., Qureshi, Q., Sankar, K., Krausman, P. R., and Goyal, S. P. (2021). Philopatric and natal dispersal of tigers in a semi-arid habitat, western India. J. Arid Environ. 184:104320. doi: 10.1016/j.jaridenv.2020.104320
Srivathsa, A., Majgaonkar, I., Sharma, S., Singh, P., Punjabi, G. A., Chawla, M. M., et al. (2020). Opportunities for prioritizing and expanding conservation enterprise in India using a guild of carnivores as flagships. Environ. Res. Lett. 15:064009. doi: 10.1088/1748-9326/ab7e50
Srivathsa, A., Puri, M., Karanth, K., Patel, I., and Kumar, N. S. (2019). Examining human–carnivore interactions using a socio-ecological framework: sympatric wild canids in India as a case study. R. Soc. Open Sci. 6:182008. doi: 10.1098/rsos.182008
Stein, A. B., Athreya, V., Gerngross, P., Balme, G., Henschel, P., Karanth, U., et al. (2016). Panthera pardus. The IUCN Red List of Threatened Species 2016, e. T15954A50659089. Available online at: https://library.wcs.org/doi/ctl/view/mid/33065/pubid/PUB19066.aspx (accessed July 11 2015).
Sukhadiya, D., Joshi, J. U., and Dharaiya, N. (2013). Feeding ecology and habitat use of sloth bear (Melursus ursinus) in Jassore Wildlife Sanctuary, Gujarat, India. Indian J. Ecol. 40, 14–18. (accessed March 20, 2013).
Thapa, K., Wikramanayake, E., Malla, S., Acharya, K. P., Lamichhane, B. R., Subedi, N., et al. (2017). Tigers in the Terai: strong evidence for meta-population dynamics contributing to tiger recovery and conservation in the Terai Arc Landscape. PLoS ONE 12:e0177548. doi: 10.1371/journal.pone.0177548
Thatte, P., Chandramouli, A., Tyagi, A., Patel, K., Baro, P., Chhattani, H., et al. (2020). Human footprint differentially impacts genetic connectivity of four wide-ranging mammals in a fragmented landscape. Divers. Distribut. 26, 299–314. doi: 10.1111/ddi.13022
Thorn, M., Green, M., Bateman, P. W., Cameron, E. Z., Yarnell, R. W., and Scott, D. M. (2010). Comparative efficacy of sign surveys, spotlighting and audio playbacks in a landscape-scale carnivore survey. South Afr. J. Wildlife Res. 40, 77–86. doi: 10.3957/056.040.0113
Tourani, M., Moqanaki, E. M., and Kiabi, B. H. (2012). Vulnerability of striped hyaenas, Hyaena hyaena, in a human-dominated landscape of Central Iran. Zool. Middle East 56, 133–136. doi: 10.1080/09397140.2012.10648948
Vanak, A. T., Ankila Hiremath, J., Krishnan, S., Ganesh, T., and Rai, N. D. (2017). “Filling in the (forest) blanks: The past, present and future of India's savanna grasslands,” in Transcending Boundaries: Reflecting on Twenty Years of Action and Research at ATREE. Ashoka Trust for Research in Ecology and the Environment, Banglore, India.
Wagner, A. P. (2006). Behavioral Ecology of the Striped Hyena (Hyaena hyaena). Bozeman, MT: Montana State University.
Wagner, A. P., Frank, L. G., and Creel, S. (2008). Spatial grouping in behaviourally solitary striped hyenas, Hyaena hyaena. Anim. Behav. 75, 1131–1142. doi: 10.1016/j.anbehav.2007.08.025
Warrier, R., Noon, B. R., and Bailey, L. L. (2020). Agricultural lands offer seasonal habitats to tigers in a human-dominated and fragmented landscape in India. Ecosphere 11:e03080. doi: 10.1002/ecs2.3080
Warrier, R., Noon, B. R., and Bailey, L. L. (2021). A framework for estimating human-wildlife conflict probabilities conditional on species occupancy. Front. Conserv. Sci. 2:679028. doi: 10.3389/fcosc.2021.679028
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139. doi: 10.1080/00063659909477239
Wilson, G. J., and Delahay, R. J. (2001). A review of methods to estimate the abundance of terrestrial carnivores using field signs and observation. Wildlife Res. 28:151. doi: 10.1071/WR00033
Wolf, C., and Ripple, W. J. (2017). Range contractions of the world's large carnivores. R. Soc. Open Sci. 4:170052. doi: 10.1098/rsos.170052
Keywords: large carnivores, habitat-use, multi-use corridor, tigers, hyenas, sloth bears, leopards, community attitudes
Citation: Kannan P, Salaria S, Khan S, Mark T, Baberwal N, Bhatnagar A, Shethia Y, Thatte P and Chanchani P (2022) Assessing Carnivore Occurrence and Community Attitudes Towards Wildlife in a Multi-Use Arid Landscape Corridor. Front. Conserv. Sci. 2:787431. doi: 10.3389/fcosc.2021.787431
Received: 30 September 2021; Accepted: 27 December 2021;
Published: 18 January 2022.
Edited by:
Camilla Sandström, Umeå University, SwedenReviewed by:
Sathyakumar Sambandam, Wildlife Institute of India, IndiaYash Veer Bhatnagar, Nature Conservation Foundation, India
Copyright © 2022 Kannan, Salaria, Khan, Mark, Baberwal, Bhatnagar, Shethia, Thatte and Chanchani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pranav Chanchani, cHJhbmF2QHd3ZmluZGlhLm5ldA==
†These authors have contributed equally to this work
 Prameek Kannan
Prameek Kannan Saloni Salaria
Saloni Salaria Siddique Khan
Siddique Khan Tanuj Mark
Tanuj Mark Navin Baberwal1
Navin Baberwal1 Pranav Chanchani
Pranav Chanchani