- 1Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, FL, United States
- 2Department of Anthropology, University of Florida, Gainesville, FL, United States
Globally, non-human primates face mounting threats due to unsustainable harvest by humans. There is a need to better understand the diverse drivers of primate harvest and the complex social-ecological interactions influencing harvest in shared human-primate systems. Here, we take an interdisciplinary, systems approach to assess how complex interactions among primate biological and ecological characteristics and human social factors affect primate harvest. We apply our approach through a review and synthesis of the literature on lemur harvest in Madagascar, a country with one of the highest primate species richness in the world coupled with high rates of threatened primate species and populations in decline. We identify social and ecological factors affecting primate harvest, including the characteristics of lemurs that may make them vulnerable to harvest by humans; factors describing human motivations for (or deterrents to) harvest; and political and governance factors related to power and accessibility. We then discuss social-ecological interactions that emerge from: (1) the prevalence of informal institutions (e.g., cultural taboos), (2) adoption of human predatory strategies, (3) synergies with habitat use and habitat loss, and (4) interactions among regional- and local-scale factors (multi-level interactions). Our results illustrate that social-ecological interactions influencing lemur harvest in Madagascar are complex and context-specific, while influenced by a combination of interactions between species-specific characteristics and human social factors. These context-specific interactions may be also influenced by local-level cultural practices, land use change, and effects from regional-level social complexities such as political upheaval and food insecurity. We conclude by discussing the importance of identifying and explicitly accounting for nuances in underlying social-ecological systems and putting forth ideas for future research on primate harvest in shared human-primate systems, including research on social-ecological feedbacks and the application of Routine Activities Theory.
Introduction
The world's non-human primates (hereafter primates) are facing unprecedented pressures from anthropogenic threats, with ~75% of the world's 504 species with populations in decline and ~60% threatened with extinction (Estrada et al., 2017, 2018). The hunting and trapping of primates for direct consumption and trade is a rapidly growing threat across the four main primate distribution regions: Neotropics, Mainland Africa, Madagascar, and Asia (Maldonado et al., 2009; Alves et al., 2010; Nijman et al., 2011; Rovero et al., 2012; Maldonado and Peck, 2013; Fa et al., 2015; Estrada et al., 2017, 2018; Arcus Foundation, 2021). Globally, hunting and trapping threatens 60% of the world's primate species (Estrada et al., 2017). In Mainland Africa, 54% of primate species are threatened by hunting and trapping, while ~40% are impacted in the Neotropics (Estrada et al., 2017, 2018). This threat is more pronounced in Asia and Madagascar, where hunting and trapping threaten 90 and ~70% of the species, respectively (Meijaard et al., 2011; Mittermeier et al., 2013; Schwitzer et al., 2013b; Estrada et al., 2017, 2018).
Although archaeological and ethnographic records suggest that humans are the main predators of primates (Cheney and Wrangham, 1987; Pérez et al., 2005; Urbani, 2005), studies have often ignored the importance of human predation in shaping shared human-primate systems (Urbani, 2005, 2017; Darimont et al., 2015). It has been postulated that the role of humans as predators of primates does not fit within the same theoretical frameworks adopted to understand predation by non-human predators (Vermeij, 2012; Darimont et al., 2015; Urbani, 2017). For instance, human predatory behavior has had a more rapid evolutionary pathway (when compared to that of other predators) relative to the slower evolution of defensive adaptations of prey (Vermeij, 2012). In addition, cultural practices by humans have a level of complexity that may not be adequately addressed in frameworks for non-human predators. Thus, recent studies on the hunting and trapping of primates by humans have aimed to adopt a comprehensive predator-prey framework that incorporates the human dimension within shared human-primate systems (Fuentes and Wolfe, 2002; Fuentes and Hockings, 2010; Fuentes, 2012; Blair et al., 2017b; Riley, 2020: ch. 5). This is critical given the role of humans globally as an unsustainable “apex predator” (Darimont et al., 2015).
Issues of primate harvest should be analyzed holistically and from a systems perspective (Sterling et al., 2010; Blair et al., 2017a,b). Systems thinking calls for an understanding of an entire system, including its individual components, interactions between components, and emergent properties (Ostrom, 2009; Ramage and Shipp, 2009; Sterling et al., 2010; McGinnis and Ostrom, 2014). In the context of biodiversity conservation, systems thinking serves as an approach that integrates human culture and relationships (e.g., traditional ecological knowledge, livelihoods, resource use and valuation) into traditional views of biodiversity (Sterling et al., 2010). A social-ecological system can thus be defined as “a system of bio-geo-physical and social factors that interact regularly,” and that exhibits properties of complex systems, such as feedbacks (see Blair et al., 2017b for definitions of key terms and references therein related to systems thinking).
Moreover, it is critical to understand the underlying drivers of primate harvest and how these drivers interact across scales (Blair et al., 2017a,b). Past research has explored potential drivers of primate harvest and trade including increased access to technology (i.e., guns; Remis and Robinson, 2012), demand for traditional medicine use (Alves et al., 2010), and biomedical trade (Maldonado et al., 2009). There is a growing body of literature that explicitly considers interactions, including feedbacks, between social and ecological components (Blair et al., 2017a). For example, a study in Indonesia found that in communities with strong traditions and taboos against harvesting slow lorises, these primates were found to live side-by-side in human-modified habitats (i.e., in or near villages) (Nijman and Nekaris, 2014). Thus, human social factors (i.e., informal institutions) limiting harvest appeared to contribute positively to slow loris habitat selection. Similarly, in Madagascar, erosion of once prominent taboos against primate harvest has increased the hunting of lemurs for wild meat (i.e., bushmeat; Jenkins et al., 2011), which may feed-back to impact both economic and ecological systems as demand for primates increases and primate populations face rapid declines. Understanding the factors influencing these complex dynamics, including who is consuming primates and why (i.e., diverse motivations), can contribute to the development of interventions that mitigate the drivers and impacts of harvest (Jenkins et al., 2011; Blair et al., 2017a,b).
Madagascar is home to 103 primate species across fifteen genera and is a priority range country for primate conservation (Mittermeier et al., 2013; Schwitzer et al., 2013b; Estrada et al., 2017, 2018). All of Madagascar's primates, the lemurs, are endemic to the country (Myers et al., 2000; Mittermeier et al., 2010), and constitute the highest percentage of threatened primate taxa in the world (Mittermeier et al., 2013; Schwitzer et al., 2013b; Estrada et al., 2017, 2018). Moreover, all lemur species are protected under law; however, illegal hunting and trapping is the second principal threat to lemurs (after habitat loss and degradation from agriculture) and impacts about 70% of extant species (Schwitzer et al., 2013a; Estrada et al., 2017, 2018). Madagascar is also a place with a complex cultural context: the population is comprised of about twenty ethnic groups, two-fifths of the population practices traditional religions, and a system of taboos (including some linked to human-lemur relationships) is dominant throughout the country (Jones et al., 2008; Schwitzer et al., 2013a, 2014; Dresch et al., 2021). Thus, Madagascar is an ideal case for the study of primate harvest and other human-primate interactions (Loudon et al., 2006; Fuentes and Hockings, 2010; Riley, 2020: ch.5).
Here, we put forth a literature review and synthesis on the diverse social and ecological factors, and social-ecological interactions, influencing primate harvest in Madagascar. The concepts of “harvest” and “hunting” of wildlife may vary depending on disciplinary perspectives (i.e., conservation biology, social science, environmental policy) (Bennett et al., 2007). Whether harvest is a threat to specific species or populations (e.g., unsustainable), and whether it is primarily an issue of human livelihood or biodiversity conservation (or both), also varies according to perspective, place, and time (Bennett et al., 2007). For the purposes of this synthesis, and to facilitate integration and dialogue across disciplines, we use the umbrella term “harvest” to refer to any removal of an individual from a population, through hunting or trapping, whether lethal or non-lethal, and for a diverse suite of uses by humans (Estrada et al., 2017; Arcus Foundation, 2021). When referring specifically to the primates of Madagascar in our synthesis, we use the term “lemur.” We summarize the diverse factors influencing lemur harvest, examine key social-ecological interactions, and identify priorities and opportunities for future research. Our study has implications for more holistically understanding and explicitly considering the complex social-ecological interactions influencing primate harvest in Madagascar and beyond.
Literature Search and Synthesis
We developed a list of factors that influence the harvest of primates by humans. To develop our initial list, we built off of key factors presented in a recent social-ecological systems framework proposed to guide studies on hunting and trade in primates (Blair et al., 2017b). Blair et al. (2017b)'s framework and associated variables/factors related to primate hunting and trade are derived from biological and social data and models that build on concepts in ethnoprimatology (i.e., study of the human-primate interface; Fuentes, 2012; Riley and Ellwanger, 2013; Ellwanger, 2017; Riley, 2018, 2020: ch.5). Their flexible framework can be used to explore the roles of diverse human actors, primate species and populations, and governance in primate hunting and trade systems.
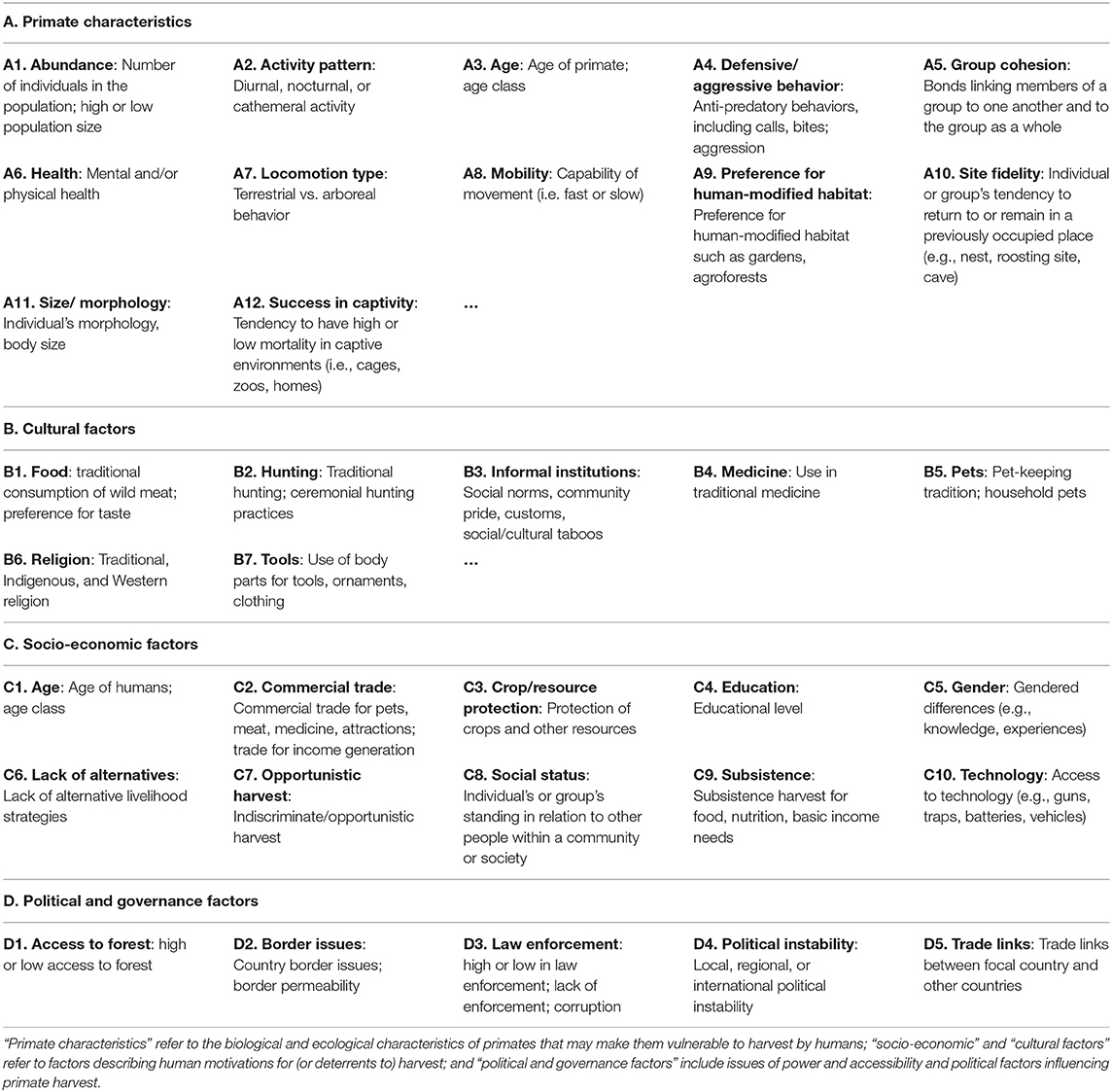
Our list of factors (Table 1 and Supplementary Table 2) used to guide this review and synthesis is organized according to Routine Activities Theory (RAT) (following the organization in Carter et al. (2017)'s social-ecological systems framework). Routine Activities Theory emerged within the field of criminology and provides a framework to assess crime from the potential offender's point of view (Cohen and Felson, 1979; Clarke, 2008). RAT is now increasingly applied in the growing field of conservation criminology to understand issues of illegal hunting and trapping of wildlife by assessing the interactions between the characteristics of suitable targets (i.e., vulnerable or desirable wildlife), motivated offenders (i.e., would-be “poachers”), and level of guardianship (e.g., protected area or community-based management) in time and space (Pires and Clarke, 2012; Pires, 2015; Warchol and Harrington, 2016; Carter et al., 2017). Routine Activities Theory posits that for a crime (e.g., illegal harvest or poaching) to occur, there must be a suitable target (e.g., primates with ecological and biological characteristics that make them vulnerable to harvest), a motivated or likely offender (e.g., actors with diverse cultural and socio-economic motivations), and lack of capable guardianship (e.g., decreased or ineffective governance and political systems). We organized our list of factors affecting primate harvest following these principles (Table 1).
To refine our list and synthesize knowledge on the factors contributing to or hindering the harvest of primates in Madagascar, we conducted a literature search using Web of Science for eight searches of paired terms (19 May 2020): primat* & trade, hunting, poaching, exploitation, conservation, bushmeat, pet, and human-primate conflict. We skimmed the title, abstract, keywords, and objectives of each study and identified 13 (out of 121) studies that were focused on the harvest and/or use of primates in Madagascar. We also included studies focused on wildlife harvest and use generally in Madagascar if primates were included in the study. We examined the References sections of the 13 papers for additional relevant references to include in our review. Moreover, we scanned for articles fitting our key terms in all volumes published in Lemur News (Volumes 1-22; years 1996-2019/20), the newsletter of the Madagascar section of the IUCN/SSC Primate Specialist Group (http://www.primate-sg.org/lemur_news/). Two references, a global overview of the use of primates in traditional medicine (Alves et al., 2010) and a recent study on local attitudes toward aye-ayes (Daubentonia madagascariensis) (Randimbiharinirina et al., 2021), were not part of the original search results but were included in the review. Our search resulted in 51 total papers used for a content analysis (see Supplementary Table 1 for a complete list of references).
We then applied our list by coding each paper through the use of the qualitative analysis software Atlas.ti (https://atlasti.com). We assigned each factor in our initial list a code and proposed, tested, and refined factors through iterative exploration during the coding process (Bernard et al., 2017:ch. 6). For example, if “success in captivity” was identified as a potential factor affecting primate harvest in the nth paper, we returned to the previous papers, tested and coded evidence of that factor, and added it to the list. Table 1 presents the refined list of 34 factors used in our content analysis. “Primate characteristics” refer to the biological and ecological characteristics of primates that may make them vulnerable to harvest by humans; “socio-economic” and “cultural factors” refer to factors describing human motivations for (or deterrents to) harvest; and “political and governance factors” include issues of power and accessibility and political factors influencing primate harvest. We also included a code labeled “Inhibiting” to be co-coded if the specific factor(s) from Table 1 inhibited or hindered the harvest of primates. We used thematic analysis (Bernard et al., 2017; ch. 5) to analyze the coded data and identified emergent themes affecting lemur harvest. We synthesized relevant data under each theme with a focus on interactions between factors.
Results
Summary of Factors
Of the total of 34 factors included in our typology, our analysis of the literature showed evidence for 26 factors affecting lemur harvest in Madagascar (Supplementary Table 2). Eleven different primate characteristics affected lemur harvest. Occurrence of lemur harvest was positively related to abundance, group cohesion, primate health, mobility, success in captivity, and site fidelity. Activity patterns of lemurs, age, defensive behavior, locomotion type, and morphology/body size all affected lemur harvest in mixed ways that depended on the species and specific context. Four cultural factors were identified to affect lemur harvest, including traditional consumption of and preference for wild meat, informal institutions, pet-keeping, and use in traditional medicine. Further research is needed to understand the role of religion in influencing lemur harvest. Moreover, demand from commercial trade, desire to protect crops and meet subsistence needs, and a decline in adequate livelihood earning opportunities in Madagascar promoted lemur harvest. Lemur harvest was also associated with opportunistic harvest (when targeting other resources), high social status, and an increasing availability of modern technologies (e.g., guns). We identified three political and governance factors that promoted lemur harvest, including access to forest, lax law enforcement, and political instability in the country. Considering all types of factors, the most commonly referenced factors across the 51 reviewed articles were subsistence (n = 20 articles), commercial trade (n = 17 articles), and informal institutions (n = 13 articles) (Figure 1). Refer to Supplementary Table 2 for specific examples of how social and ecological factors affect (enhance/promote or hinder/inhibit) harvest of lemurs by humans in Madagascar.
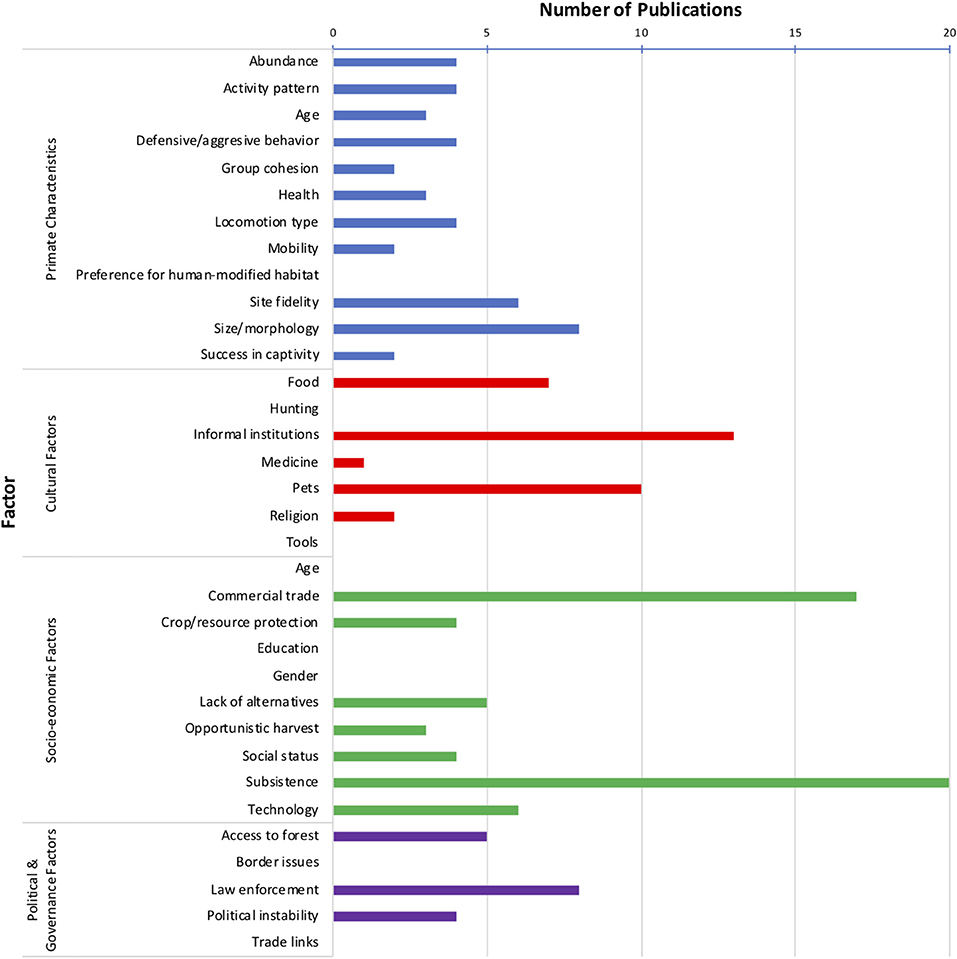
Figure 1. Number of reviewed publications (total reviewed = 51) in which each factor affecting lemur harvest in Madagascar was identified. Refer to Table 1 for descriptions of each factor.
Social-Ecological Interactions
Here, we detail social-ecological interactions that we identified from our synthesis and discuss how they enhance or hinder the harvest of lemurs by humans. We discuss social-ecological interactions that emerge from: (1) the prevalence of informal institutions, (2) human predatory strategies, (3) synergies with habitat use and habitat loss, and (4) interactions among regional- and local-scale factors (multi-level interactions). We indicate factors (and thus, any interactions between factors) using the labeling in Table 1.
The Role of Informal Institutions
Informal institutions, including social norms, community pride, customs, and social and cultural taboos, shape primate harvest in complex ways. A system of informal institutions known as fady are cultural taboos prevalent throughout Madagascar (van Gennep, 1904 in Jones et al., 2008; Rudd, 1960 in Jones et al., 2008; Anania et al., 2019/20). Fady are powerful forces that profoundly shape Malagasy culture and include a range of prohibitions that, if not observed, can result in diverse consequences spanning social disapproval and supernatural punishments (Jones et al., 2008). There are various examples documented in the literature in which adherence to fady have resulted in the protection of species and habitats in Madagascar (see Jones et al., 2008 and references therein). There are several fady that involve taboos against harvesting primates. Within a single region, the role of fady in deterring harvest of lemurs is variable. In the late 1980s in northern Madagascar, for example, lemurs appeared to be protected by a fady against their hunting in Ankarana Massif Special Reserve, but not in the adjacent Analamera Special Reserve; the reasons for this variation are not reported (B3) (Hawkins et al., 1990). Moreover, fady appear to limit the harvest of Milne-Edwards's sifaka (Propithecus edwardsi) in the southern region of Ranomafana National Park (southeastern Madagascar) (Wright et al., 2005 in Dunham et al., 2008), while hunting of this species occurs in the northern region (Lehman et al., 2006 in Dunham et al., 2008). In the Ranobe forest of southwest Madagascar, fady do not seem to contribute to restrictions on lemur hunting, and residents (consisting primarily of farmers) hunt lemurs opportunistically and for subsistence purposes (C7; C9) (Gardner and Davies, 2014). Urban residents of western Madagascar cite fady as the main reason for not consuming lemurs, as well as bush pigs, fruit bats, goats, and domestic pigs (B3) (Randrianandrianina et al., 2010). However, illegal harvest of lemurs still occurs to supply demand for lemur meat in urban areas, though demand is thought to be low based on current understanding of lemur harvests gathered from interviews (B1; C2) (Randrianandrianina et al., 2010). Fady might influence the ownership of pet lemurs throughout urban and rural areas of Madagascar, particularly prohibition of keeping lemurs as pets, but further research is needed to understand this phenomenon (B3; B5) (see Reuter et al., 2016a, 2018).
We identified complex interactions between taboos, harvest, and the “human-like” characteristics of some lemur species (e.g., large body size, human-like posture, and morphology). This is a key interaction between a social factor and various biological/ecological factors, as it links informal institutions with lemur morphology and behavior. For example, a study on the consumption of wild meat in the Alaotra-Mangoro region of eastern Madagascar found a positive relationship between lemur species that locals considered taboo to eat and those that had more “human-like” characteristics, notably large diurnal lemurs in the family Indriidae (A11; B1; B3) (Jenkins et al., 2011). This aversion to consuming lemurs with human-like features may stem from the notion that larger lemur species embody human ancestors (e.g., eastern wooly lemur- Avahi laniger), and from stories of large lemurs helping warn humans against enemies who entered the forest (A11; B1; B3) (Jones et al., 2008). At the Beza Mahafaly Special Reserve in southern Madagascar, local people (composed of Mahafaly, Antandroy, and Tanala peoples) do not kill ring-tailed lemurs (Lemur catta) and Verreaux's sifaka (Propithecus verreauxi) because, according to origin myths, they were once human, and causing these species harm results in bad luck (B3) (Loudon et al., 2006). Local Mahafaly people acknowledge the morphological and behavioral similarities between the aforementioned diurnal lemur species and humans, notably a reduced rostrum, grasping hands, reliance on vision, and group living (A5; A11; B3) (Loudon et al., 2006). Moreover, residents note that these species live in families and “fight among each other”; the perceived uniqueness of these aggressive, human-like behaviors partly contributed to Mahafaly people's self-imposed restrictions on hunting ring-tailed lemurs and Verreaux's sifakas (A4; A5; B3) (Loudon et al., 2006). In eastern Madagascar, smaller species like the brown lemur (Eulemur fulvus) have a less “human-like face and stance” compared to other diurnal lemur species and are a preferred wild meat (A11; B1) (Jones et al., 2008; Jenkins et al., 2011).
Recent data on increases in hunting of large diurnal lemurs suggest that taboos against hunting various species may be eroding. Social change- driven by wealth disparities, human migration, and growing food insecurity throughout Madagascar- may drive site- and context-specific taboo erosion (A11; B3; C6; C9) (Figure 2) (Sautter, 1980 in García and Goodman, 2003; Jenkins et al., 2011; Sauther et al., 2013). For example, although there is a fady against eating golden-crowned sifakas (Propithecus tattersalli) by sympatric humans throughout their range, immigrants to the areas who do not observe the fady tend to hunt them (Meyers, 1996; Vargas et al., 2002). Taboo erosion in some regions also leads to increased harvesting of lemurs for trade as pets and meat (B3; C2) (Mutschler et al., 2001; Gould and Sauther, 2016).

Figure 2. Interactions among regional- and local-scale factors contributing to harvest of lemurs in Madagascar (multi-level interactions). Positive feedbacks (amplifying effects) on local harvest of lemurs by humans may result from political/governance (e.g., political instability, reduced agricultural investments) and socioeconomic (e.g., increased food insecurity, human migration) factors originating at the regional scale. The figure highlights opportunities for future studies on primate harvest in Madagascar (and beyond) to explicitly consider the impacts of regional factors on interactions with site-specific cultural factors and species-specific vulnerabilities to harvest. Figure adapted from Brashares et al. (2014).
Other types of fady promote lemur harvest. For example, one fady promotes the persecution of aye-ayes (Daubentonia madagascariensis) in some parts of Madagascar due to their reputation as evil omens. This fady, along with local characterizations of aye-ayes as crop pests, results in their killing when sighted near villages (B3; C3; C7) (Albignac, 1987 in Simons and Meyers, 2001; Quinn and Wilson, 2004; Koenig, 2005 in Loudon et al., 2006; Jones et al., 2008). A recent study, however, revealed the heterogeneity in local attitudes toward aye-ayes across 11 villages in northern Madagascar (Randimbiharinirina et al., 2021). The researchers found that negative attitudes toward aye-ayes in some villages stemmed from vague accounts unfounded in the ecology of this lemur species, while positive and neutral attitudes in other villages were based on the observed behavior of aye-ayes as providers of pest-control services and curiosity about the species (B3; C3) (Randimbiharinirina et al., 2021). The identification of this variable social-ecological relationship across the study area provides opportunities for targeted conservation initiatives that highlight the beneficial value of aye-ayes as providers of pest-control services, and offers a nuanced approach that considers local and site-specific values.
Human Predatory Strategies
A number of lemur species have adopted ecological and behavioral adaptations which also contribute to predator avoidance (e.g., nocturnal activity patterns, arboreality, group cohesion) (Vermeij, 2012; Urbani, 2017). However, humans as predators have in turn adapted several novel harvesting strategies in response to these behavioral adaptations in an evolving predator-prey arms race (Vermeij, 2012; Urbani, 2017). For example, researchers posit that diurnality is a main ecological trait characterizing the extinct lemurs of Madagascar, and that diurnal behavior, along with large body size, enhances lemur vulnerability to harvest by humans (A2; A11) (Godfrey and Irwin, 2007). However, small, extant nocturnal lemur species (e.g., Avahi occidentalis and Lepilemur edwardsi) are often susceptible to harvest by manual removal from nesting sites during the day (A2; A10; A11) (García and Goodman, 2003). In other cases, entire trees are cut down to extract nocturnal lemurs resting in tree cavities (A2; A7; A10) (Reuter et al., 2016c). Nocturnal mouse lemurs (Microcebus spp.) nest in tree cavities and hollow branches of dead octopus trees (Didierea madagascariensis), or in leaf nests in the canopy when tree cavities are unavailable (Gardner and Davies, 2014). In southwest Madagascar, groups of sleeping mouse lemurs are harvested during the day by hand, but entire trees may be removed if manual access is difficult (A2; A5; A7; A10) (Gardner and Davies, 2014). If the mouse lemurs are active when found during the daytime, they are captured using a pole covered in untreated latex of the plants Euphorbia stenoclada or Folotsia grandiflora (Gardner and Davies, 2014).
Human predatory behavior interacts with lemur locomotion types (i.e., terrestrial or arboreal behavior). Primates that range primarily in the forest canopy are considered more evasive to hunting than ground-dwelling species (Rovero et al., 2012). In the Makira forest of northeastern Madagascar, however, people build bridges with snares (called laly totoko) which connect fruiting trees to forest fragments to trap arboreal frugivores (A7; A10) (Golden, 2009; Schwitzer et al., 2013a). Noose rope traps placed at ground level and baited with fruit ensnare other more ground-dwelling, diurnal lemurs, including common brown lemurs (Eulemur fulvus), ring-tailed lemurs (Lemur catta), and white-headed lemurs (Eulemur albifrons) (Goodman and Raselimanana, 2003; Borgerson, 2015). Although sifakas (Propithecus spp.) and ring-tailed lemurs (Lemur catta) can climb trees, pursuit hunting by use of dogs exhausts them and increases their chance of capture close to the ground by people (A7; A8) (Goodman and Raselimanana, 2003; Gardner and Davies, 2014).
As alluded to above, interactions between human predatory strategies and lemur site fidelity (i.e., an individual or group's tendency to return to or remain in a previously occupied place, such as in a feeding or roosting site) generally promote lemur harvest. Frugivorous lemurs that are restricted to acquiring food from specific trees during the fruiting season (austral winter) are targets for capture (A10) (Golden, 2009; Schwitzer et al., 2013a; Borgerson, 2015). Hunters build bridges with snares across forest fragments, which forces lemurs to cross in order to access the fruiting trees. The predictability of the foraging habits of frugivorous primates at specific sites makes them susceptible to harvest (Hill and Padwe, 2000 in Borgerson, 2015). In the Masoala Peninsula, the predictable travel paths of white-headed lemurs (Eulemur albifrons) and red ruffed lemurs (Varecia rubra) to seasonally fruiting trees eases their capture (A10). In Vohimana Reserve (estern Madagascar) people construct snares with a noose made from bicycle brake cables and lure lemurs into the snare with fruit (e.g., guava, Psidium spp.) (Anania et al., 2019/20). Nocturnal species roosting in tree cavities during the day are also susceptible to manual harvest by people with knowledge of nesting sites (A2; A10) (García and Goodman, 2003). Hunters prey on fat-tailed dwarf lemurs (Cheirogaleus medius) nesting in the forest canopy during the day by poking their nests with a pole to wake them (Gardner and Davies, 2014).
Access to technology, such as guns, has changed the nature of lemur harvest in Madagascar in recent years (C10). Guns facilitate the harvest of large numbers of lemurs by only a few people, and anecdotal reports suggest that use of firearms requires individuals to have the financial means to access them (A1; C10) (Jenkins et al., 2011; Reuter et al., 2016c). Wealthy, urban individuals travel into rural areas to hunt lemurs with guns, although rural people rely on traditional trapping methods (Golden, 2009; Jenkins et al., 2011). There is evidence of lemur hunting by commercial shotguns or locally made models in Ankarafantsika National Park, northwestern Madagascar (García and Goodman, 2003). People may also use a combination of technologies, including blowpipes, slingshots, and snares, to hunt lemurs and other wildlife (Anania et al., 2019/20).
Synergies With Habitat Use and Habitat Loss
People carry out opportunistic and indiscriminate harvest of lemurs when conducting other extractive activities in lemur habitat (C7; D1). Selective logging has been identified as one of the most prevalent forms of habitat degradation in Madagascar, resulting in the opportunistic harvest of lemurs by loggers (Schwitzer et al., 2013a). In the Vohimana forest (eastern Madagascar) timber extraction (e.g., hardwood logging), slash-and-burn agriculture (tavy), and charcoal production act in synergy with hunting through the use of snares to threaten lemurs and their habitat (Anania et al., 2019/20). An increasing number of lemur traps have also been documented in Tsimanampetsotse National Park in southwestern Madagascar (Sauther et al., 2013). This is one of the few protected areas of Ceonozoic limestone habitat, and the resident ring-tailed lemur (Lemur catta) populations are threatened by opportunistic hunting for food (adults) and to supply the pet trade (infants) while people extract trees for cattle forage, construction materials, pirogues, and firewood to fuel the production of bricks in the city of Ankoronga (C2; C7; C9) (Sauther et al., 2013). Increases in the illegal hunting of lemurs in the Jardin Botanique B study site in Ankarafantsika National Park (northwestern Madagascar) are partly attributed to the increasing numbers of people who enter the park and dig holes to collect maciba (Dioscorea maciba), fish illegally in Lake Ravelobe, and harvest trees (Henkel et al., 2019/20). Within Ankarafantsika National Park (northwestern Madagascar) there is an official zone designated for the legal collection of raffia palms (Raffia) (García and Goodman, 2003). About 20 or more raffia fiber harvest camps are occupied each year and the remains of consumed wildlife in some camps have been found to consist primarily of lemurs (C7; C9; D1) (García and Goodman, 2003). Similarly, activities such as livestock grazing and the collection of forest products (medicinal plants, fibers, wood for fuel and construction) in multiple-use protected areas (e.g., Ranobe PK32, southwest Madagascar) result in the opportunistic hunting of lemurs for subsistence (C7; C9; D1) (Gardner and Davies, 2014).
Increased rates of deforestation in Madagascar, primarily driven by the need to meet economic and subsistence needs at the household level, have resulted in high degrees of habitat fragmentation (Schwitzer et al., 2013a). Fragmented, patchy areas lead to increased encounters with lemurs, increases in hunting opportunities (e.g., to meet subsistence needs, supply wildlife trade), and in turn potential disease transmission (C2; C7; C9; D1) (Barrett and Ratsimbazafy, 2009; Gilles and Reuter, 2014; LaFleur et al., 2016, 2018, 2019). The decline in the populations of red-collared brown lemurs (Eulemur collaris) in Saint Luce (southeast Madagascar) is attributed to hunting, although a population persists in the littoral forest fragments of the area (Roberts et al., 2019/20). However, a proposed mining plan threatens to clear the forest fragments, and thus would restrict dispersal and reduce the viability of the red-collared brown lemur population (Temple et al., 2012 in Roberts et al., 2019/20). Populations of ring-tailed lemurs (Lemur catta) persisting in unprotected, isolated forest fragments throughout Madagascar are also vulnerable to increased encounter rates with humans, and thus opportunistic harvest; this is complicated by the synergistic effects of taboo erosion in some ring-tailed lemur habitat (B3; C7; D1) (Gould and Sauther, 2016). Conversely, decreased lemur harvest rates in some regions (e.g., Ranobe OK32 protected area) may be a result of declines in lemur abundance due to forest degradation (A1; D1) (Gardner and Davies, 2014), and in some areas, such as in the Beza Mahafaly Special Reserve (southwest Madagascar), lemur species are still protected by the synergistic effects of hunting taboos and ancestral forests that are protected against deforestation (B3; D1) (Loudon et al., 2006).
Multi-Level Interactions
Multi-level interactions, or interactions between social and ecological factors at multiple organizational levels (e.g., individual actors, institutions) (Hull et al., 2015), affect lemur harvest and result in key feedbacks and impacts across scales (Figure 2). Madagascar has undergone high political instability at a regional level in recent years, which interacts with protected area management to affect local rates of illegal harvest (D3; D4). In 2009, Madagascar underwent a political crisis (military coup) which had far-reaching impacts on both local livelihoods and the environment. In the immediate aftermath, there was a significant decrease of international support for environmental programs, among others (Schwitzer et al., 2014). Although the World Bank continued to provide support for protected areas, inappropriate allocation and management of funds occurred, in part due to the decrease in government control throughout the country (Schwitzer et al., 2014). The political instability and decreased support for environmental management efforts resulted in a rapid increase in illegal harvest and trafficking of wildlife and wildlife products, including lemurs (a positive feedback or amplifying effect, D3; D4) (Barrett and Ratsimbazafy, 2009; Schwitzer et al., 2014). For example, in the Jardin Botanique B study site in Ankarafantsika National Park (northwestern Madagascar), there was an increase in human encroachment into the forest following the 2009 political crisis that resulted in increases in illegal hunting (as well as illegal fishing and extraction of other forest resources), partly driving the decline of the golden-brown mouse lemur (Microcebus ravelobensis) population (D1; D3; D4) (Henkel et al., 2019/20).
Madagascar's rapidly growing human population (which increased sevenfold since the early 1910s) resulted in increased environmental pressures to meet the growing demand for food (Schwitzer et al., 2013a). Ongoing and increasing issues of food insecurity at a regional level, notably the lack of domestic meat options, also caused a positive feedback (amplifying effect) that drove up local harvest of wildlife in Madagascar (C6; C9) (Figure 2) (Schwitzer et al., 2013a; Reuter et al., 2016b). Lemurs comprise an important part of the diets of rural inhabitants throughout Madagascar, where subsistence hunting is increasing to feed this rapidly growing population (C6; C9) (Goodman, 1993; Dunham et al., 2008; Golden, 2009; Jenkins et al., 2011; Razafimanahaka et al., 2012; Sauther et al., 2013; Gardner and Davies, 2014; Borgerson, 2016; Borgerson et al., 2016; Reuter et al., 2016b,c). Because the opportunity or capacity to engage in sustainable agricultural activities (including livestock production) in and near protected areas is limited, people increasingly rely on wild meat resources to supply their dietary needs (C6; C9) (Goodman and Raselimanana, 2003; Golden, 2009; Schwitzer et al., 2013a; Gardner and Davies, 2014). This is exemplified within protected areas of southwest Madagascar, where hunting for wild meat occurs as an indirect result of limited land allocated for farming, reducing the prospects of income-generation from agriculture (C6; C9) (Schwitzer et al., 2013a; Gardner and Davies, 2014). Moreover, on the Masoala peninsula in northeast Madagascar, poverty, poor household health, and child malnutrition are strong predictors of trapping and consuming lemurs for subsistence (C9) (Borgerson et al., 2016). Furthermore, lemur harvest and trade via opportunistic means is increasing throughout rural Madagascar to meet basic household income needs (C7; C9) (Gardner and Davies, 2014; Reuter et al., 2016c). In some cases, meat consumption occurs in the home and the surplus is sold in local markets (C2; C9) (Gardner and Davies, 2014).
Discussion
In this study, we identify and synthesize the diverse social and ecological factors and social-ecological interactions that affect lemur harvest in Madagascar. We highlight and discuss social-ecological interactions that emerge from informal institutions, human innovation and predatory strategies, synergies with habitat use and habitat loss, and interactions between social and ecological factors across scales. Our study highlights the value of identifying social-ecological interactions and provides implications for the sustainable management of human-primate systems. Many of the key themes that emerged in our synthesis on Madagascar, such as the role of informal institutions, interactions between harvest and habitat loss, and complex role of political and governance factors are also relevant to other human-primate systems of the world that face similar challenges with co-managing primate conservation and human livelihood needs (Riley, 2007; Parathian and Maldonado, 2010; Starr et al., 2010; McLennan et al., 2017; Hockings et al., 2020).
Studies from other regions also provide insights into further lines of inquiry that may be relevant to explore in Madagascar in the future. For example, a study on the harvest and trade of slow lorises (genus Nycticebus) in Vietnam used ethnographic approaches to identify key differences in knowledge of slow loris habitat between men and women (Thạch et al., 2018). Women had knowledge of loris roosting sites due to their encounters with sleeping lorises in bamboo stands when collecting non-timber forest products during the day, while men noted encountering active lorises in cashew and other plantations while engaging in nighttime hunting trips (Thạch et al., 2018). Gendered differences in knowledge regarding slow loris ecology and behavior such as those identified in Vietnam provide context for understanding and potentially mitigating differences in local-scale opportunistic harvest of primates.
In another study in the Cantanhez National Park, Guinea Bissau, a social-ecological approach revealed complex interactions between humans and chimpanzees in their shared habitat (Hockings et al., 2020). Here Nalú and Balanta peoples share habitat and wild food resources (i.e. fruit) with chimpanzees (Hockings et al., 2020). Nalú and Balanta communities observe informal institutions that protect chimpanzees from hunting and consumption. These informal institutions stem from the notion that chimpanzees are thought to have previously been human and currently share many similarities with people (Casanova et al., 2014 in Hockings et al., 2020). This protection may not hold, however, when people seek to retaliate against crop raiding (e.g., of orange fruits), resulting in the occasional killing of chimpanzees (Hockings et al., 2020). Hockings et al. (2020) suggest that there should be active management of the plant species that are consumed by both people and primates, particularly in degraded and deforested areas. Thus, building on established informal institutions, local values, knowledge of shared resource use, and emergent social-ecological relationships can simultaneously benefit people, chimpanzees, and the shared forest habitat.
Our synthesis and approach provide the foundation for further exploration of key research questions dealing with often overlooked and underappreciated feedbacks in social-ecological systems (coupled human and natural systems; Miller et al., 2012; Hull et al., 2015; Larrosa et al., 2016). These include (1) what are potential unintended social-ecological feedbacks (surprises) of primate harvest and overharvest on human-primate systems? (2) What is the role of delayed effects (time lags) in the emergence and impacts of social-ecological feedbacks resulting from primate harvest over time? Addressing these questions requires prioritizing the collection and analysis of long-term data in established research sites, and collaborative research resulting from interdisciplinary teams (Black and Copsey, 2014; Pooley et al., 2014; Hull et al., 2015; Blair et al., 2017a,b). Policy makers and conservation practitioners interested in identifying and understanding the emergence of social-ecological feedbacks in systems where humans and lemurs co-occur, including how regional policies may affect local human-lemur interactions, may benefit from adapting the schematic presented in Figure 2 (see Brashares et al., 2014 for inspiration for the figure and further examples). The figure can be used as a guide to organize regional-scale factors, while local- and site-specific factors, including species-specific primate vulnerabilities, can be “plugged in” to the diagram to illustrate potential feedbacks affecting primate harvest. Furthermore, it is critical to consider multilevel analyses that elucidate local- to national-level dynamics and the challenges of site-specific and limited conditions for biodiversity conservation toward efficient allocation of scarce conservation resources (see Horning, 2008).
Our study also highlights the value of using Routine Activities Theory (RAT) to frame research on wildlife harvest. By seeking to understand illegal harvest events from the actor's point of view, we can better consider the nuances of site-specific cultural and socio-economic systems in relation to species vulnerability and existing governance structures. This is particularly important in Madagascar, where all lemur species are protected by law and hunting and trapping is their second principal threat (after habitat loss and degradation from agriculture) (Schwitzer et al., 2013a; Estrada et al., 2017, 2018). Thus, RAT provides a promising avenue for designing studies that focus on why individuals may be motivated to harvest primates, and linking those motivations to the biological and ecological characteristics of primates that may make them vulnerable and desirable for harvest. Moreover, the approach also integrates issues of governance by explicitly considering the factors that may result in effective or ineffective guardianship of wildlife (Carter et al., 2017). We facilitate the application of RAT by providing researchers with a flexible list of considerations for study design (Table 1).
This study adds to the growing body of research on interdisciplinary approaches toward understanding wildlife harvest that consider site- and context-specific complexities (Duffy et al., 2016; Blair et al., 2017a,b; Carter et al., 2017; Thạch et al., 2018). We build on the list of factors proposed by Blair et al. (2017b) to analyze primate hunting and trade systems. Blair et al. (2017b)'s social-ecological systems framework and associated factors are derived from concepts in ethnoprimatology. Ethnoprimatology can be defined as the study of the human-primate interface, and combines human economic, social, and political elements with the objective biological approaches of “traditional primatology” (Fuentes and Wolfe, 2002; Fuentes and Hockings, 2010; Fuentes, 2012; Malone et al., 2014). The ethnoprimatological approach is thus complementary to and inherently a systems approach, as humans and other primates are seen as co-creating and shaping shared social-ecological systems. As such, a goal is to understand system complexity (e.g., role of feedbacks) through disentangling different components of a system and their interactions. Such an approach allows for the convergence of “anthropogenic realities” into the lives of non-human primates (Malone et al., 2014).
Our results also have meaningful implications for conservation and management of primates in a critical era in which there are more complex threats to their survival than ever before (Estrada et al., 2017). Identifying diverse social and ecological factors influencing primate harvest, and key social-ecological interactions, is an initial step toward understanding and mitigating the mounting threats that primates- and the people that rely on them- face in shared human-primate systems worldwide. Our results highlighting the complexity of interacting factors that influence primate harvest suggests that a “one size fits all” approach to primate harvest mitigation efforts is unlikely to be successful (Ostrom, 2007; Horning, 2008). Furthermore, efforts that focus solely on blanket regulatory mechanisms while ignoring deeper cultural and political underpinnings that are driving primate harvest and trade may be misplaced. Madagascar in particular is at a crucial point at present to mitigate issues of human-primate interactions, especially given record rates of primate species declines driven by anthropogenic threats (Schwitzer et al., 2013b; Estrada et al., 2017, 2018). Primate conservation efforts in Madagascar have historically overlooked the role of local cultural knowledge and informal institutions (Jones et al., 2008). Strategies such as co-management, community-based management, and participatory approaches may help bridge this gap. Our results showing the multi-level interactions suggest that using solely locally-driven management approaches may also be ineffective. Nested governance structures (such as those described in Ostrom, 1990, 2007; Marshall, 2008) may be a more promising model, which would allow for critical coordination across local, regional, and global institutional levels.
Author Contributions
CR and VH contributed to the conception and design of the study. CR and DM contributed to data collection. CR wrote the first draft of the manuscript. DM and VH wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Jessica Kahler, Elizabeth Pienaar, John Richard Stepp, and Eleanor Sterling for valuable comments during the conceptualization of this study. We also thank two reviewers for helpful comments that improved the quality of the manuscript. We are grateful to five anonymous peer reviewers who reviewed earlier versions of this manuscript. Funding for this study was provided by the Department of Wildlife Ecology and Conservation, the Tropical Conservation and Development Program, and a Richard Jones Outstanding New Faculty Research award from the University of Florida.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.776897/full#supplementary-material
References
Alves, R. R. N., Souto, W. M. S., and Barboza, R. R. D. (2010). Primates in traditional folk medicine: a world overview. Mammal Rev. 40, 155–180. doi: 10.1111/j.1365-2907.2010.00158.x
Anania, A., Giubilato, T., MacDonald, M., Sauvadet, L., Corsetti, S., Rasolondraibe, E., et al. (2019/20). Inventory of the lemur community in the Vohimana reserve, eastern Madagascar. Lemur. News 22, 38–44.
Arcus Foundation (2021). State of the Apes: Killing, Capture, Trade and Ape Conservation. Cambridge: Cambridge University Press.
Barrett, M. A., and Ratsimbazafy, J. (2009). Luxury bushmeat trade threatens lemur conservation. Nature 461, 470–470. doi: 10.1038/461470a
Bennett, E. L., Blencowe, E., Brandon, K., Brown, D., Burn, R. W., Cowlishaw, G., et al. (2007). Hunting for consensus: reconciling bushmeat harvest, conservation, and development policy in west and Central Africa. Conserv. Biol. 21, 884–887. doi: 10.1111/j.1523-1739.2006.00595.x
Bernard, H. R., Wutich, A., and Ryan, G. W. (2017). Analyzing Qualitative Data: Systematic Approaches. Los Angeles, CA: SAGE Publications.
Black, S. A., and Copsey, J. A. (2014). Purpose, process, knowledge and dignity in interdisciplinary projects. Conserv. Biol. 28, 1139–1141. doi: 10.1111/cobi.12344
Blair, M. E., Le, M. D., and Sterling, E. J. (2017a). Multidisciplinary studies of wildlife trade in primates: challenges and priorities. Am. J. Primatol. 79:e22710. doi: 10.1002/ajp.22710
Blair, M. E., Le, M. D., Thạch, H. M., Panariello, A., Vu, N. B., Birchette, M. G., et al. (2017b). Applying systems thinking to inform studies of wildlife trade in primates. Am. J. Primatol. 79:22715. doi: 10.1002/ajp.22715
Borgerson, C. (2015). The effects of illegal hunting and habitat on two sympatric endangered primates. Int. J. Primatol. 36, 74–93. doi: 10.1007/s10764-015-9812-x
Borgerson, C. (2016). Optimizing conservation policy: The importance of seasonal variation in hunting and meat consumption on the Masoala Peninsula of Madagascar. Oryx 50, 405–418. doi: 10.1017/S0030605315000307
Borgerson, C., Mckean, M. A., Sutherland, M. R., and Godfrey, L. R. (2016). Who hunts lemurs and why they hunt them. Biol. Conserv. 197, 124–130. doi: 10.1016/j.biocon.2016.02.012
Brashares, J. S., Abrahms, B., Fiorella, K. J., Golden, C. D., Hojnowski, C. E., Marsh, R. A., et al. (2014). Wildlife decline and social conflict. Science 345, 376–378. doi: 10.1126/science.1256734
Carter, N. H., López-Bao, J. V., Bruskotter, J. T., Gore, M., Chapron, G., Johnson, A., et al. (2017). A conceptual framework for understanding illegal killing of large carnivores. Ambio 46, 251–264. doi: 10.1007/s13280-016-0852-z
Casanova, C., Sousa, C., and Costa, S. (2014). Are animals and forests forever? Perceptions of wildlife at Cantanhez Forest National Park, Guinea Bissau. Memórias 16, 69-104.
Cheney, D., and Wrangham, R. (1987). “Predation,” in Primate Societies, ed. B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, and T. T. Struhsaker (London: University of Chicago Press), 227–329.
Clarke, R. V. (2008). “Situational crime prevention,” in Environmental Criminology and Crime Analysis, eds R. Wortley and L. Mazerolle (London: Rutledge) 178–194.
Cohen, L., and Felson, M. (1979). Social change and crime rate trends: a routine activity approach. Am. Sociol. Rev. 44, 588–608. doi: 10.2307/2094589
Darimont, C. T., Fox, C. H., Bryan, H. M., and Reimchen, T. E. (2015). The unique ecology of human predators. Science 349, 858–860. doi: 10.1126/science.aac4249
Dresch, J., Southall, A. W., Deschamps, H. J., Covell, M. A., and Kent, R. K. (2021). Madagascar. Encyclopedia Britannica. Available online at: https://www.britannica.com/place/Madagascar.
Duffy, R., St. John, F. A. V., Buscher, B., and Brockington, D. (2016). Toward a new understanding of the links between poverty and illegal wildlife hunting. Conserv. Biol. 30, 14–22. doi: 10.1111/cobi.12622
Dunham, A. E., Erhart, E. M., Overdorff, D. J., and Wright, P. C. (2008). Evaluating effects of deforestation, hunting, and El Niño events on a threatened lemur. Biol. Conserv. 141, 287–297. doi: 10.1016/j.biocon.2007.10.006
Ellwanger, A. L. (2017). “Ethnoprimatology,” in International Encyclopedia of Primatology, ed. A. Fuentes (Hoboker, NJ: John Wiley and Sons), 361–369. doi: 10.1002/9781119179313.wbprim0178
Estrada, A., Garber, P. A., Mittermeier, R. A., Wich, S., Gouveia, S., Dobrovolski, R., et al. (2018). Primates in peril: the significance of Brazil, Madagascar, Indonesia and the Democratic Republic of the Congo for global primate conservation. PeerJ. 6:e4869. doi: 10.7717/peerj.4869
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Di Fiore, A., et al. (2017). Impending extinction crisis of the world's primates: why primates matter. Sci. Adv. 3:e1600946. doi: 10.1126/sciadv.1600946
Fa, J. E., Olivero, J., Farfán, M. Á., Márquez, A. L., Duarte, J., Nackoney, J., et al. (2015). Correlates of bushmeat in markets and depletion of wildlife. Conserv. Biol. 29, 805–815. doi: 10.1111/cobi.12441
Fuentes, A. (2012). Ethnoprimatology and the anthropology of the human-primate interface. Annu. Rev. Anthropol. 41, 101–117. doi: 10.1146/annurev-anthro-092611-145808
Fuentes, A., and Hockings, K. J. (2010). The ethnoprimatological approach in primatology. Am. J. Primatol. 72, 841–847. doi: 10.1002/ajp.20844
Fuentes, A., and Wolfe, L. D. (2002). Primates Face to Face: the Conservation Implications of Human-Nonhuman Primate Interconnections. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511542404
García, G., and Goodman, S. M. (2003). Hunting of protected animals in the Parc National d'Ankarafantsika, north-western Madagascar. Oryx 37, 115–118. doi: 10.1017/S0030605303000206
Gardner, C. J., and Davies, Z. G. (2014). Rural bushmeat consumption within multiple-use protected areas: qualitative evidence from southwest Madagascar. Hum. Ecol. 42, 21–34. doi: 10.1007/s10745-013-9629-1
Gilles, H. R., and Reuter, K. E. (2014). The presence of diurnal lemurs and human-lemur interactions in the buffer zone of the Ankarana National Park. Lemur News 18, 27–30.
Godfrey, L. R., and Irwin, M. T. (2007). The evolution of extinction risk: past and present anthropogenic impacts on the primate communities of madagascar. Folia Primatol. 78, 405–419. doi: 10.1159/000105152
Golden, C. D. (2009). Bushmeat hunting and use in the Makira Forest, north-eastern Madagascar: a conservation and livelihoods issue. Oryx 43:386. doi: 10.1017/S0030605309000131
Goodman, S. M. (1993). A reconnaissance of Ile Sainte Marie, Madagascar: The status of the forest, avifauna, lemurs and fruit bats. Biol. Conserv. 65, 205–212. doi: 10.1016/0006-3207(93)90054-5
Goodman, S. M., and Raselimanana, A. (2003). Hunting of wild animals by Sakalava of the Menabe region: a field report from Kirindy-Mite. Lemur News 8, 4–6.
Gould, L., and Sauther, L. M. (2016). Going, going, gone… Is the iconic ring-tailed lemur (Lemur catta) headed for imminent extirpation. Primate Conserv. 30, 89–101. doi: 10.1159/isbn.978-3-318-03025-9
Hawkins, A., Chapman, P., Ganzhorn, J., Bloxam, Q., Barlow, S., and Tonge, S. (1990). Vertebrate conservation in Ankarana special reserve, Northern Madagascar. Biol. Conserv. 54, 83–110. doi: 10.1016/0006-3207(90)90136-D
Henkel, H., Zimmermann, E., Klein, A., Randrianambinina, B., Rasoloharijana, S., Rakotondravony, R., et al. (2019/20). Indications of a potential alarming population decline in the golden-brown mouse lemur (Microcebus ravelobensis) in a long-term study site in the Ankarafantsika National Park. Lemur News 22, 51–53.
Hill, K., and Padwe, J. (2000). “Sustainability of Ache Hunting in the Mbaracayú Reserve, Paraguay,” in Hunting for Sustainability in Tropical Forests, eds J. G. Robinson, and E. L. Bennett (New York, NY: Columbia University Press), 79–105.
Hockings, K. J., Parathian, H., Bessa, J., and Frazão-Moreira, A. (2020). Extensive overlap in the selection of wild fruits by chimpanzees and humans: implications for the management of complex social-ecological systems. Front. Ecol. Evol. 8:123. doi: 10.3389/fevo.2020.00123
Horning, N. R. (2008). Madagascar's biodiversity conservation challenge: from local- to national-level dynamics. Env. Sci. 5:2. doi: 10.1080/15693430801912246
Hull, V., Tuanmu, M., and Liu, J. (2015). Synthesis of human-nature feedbacks. Ecol. Soc. 20:17. doi: 10.5751/ES-07404-200317
Jenkins, R. K., Keane, A., Rakotoarivelo, A. R., Rakotomboavonjy, V., Randrianandrianina, F. H., Razafimanahaka, H. S., et al. (2011). Analysis of patterns of bushmeat consumption reveals extensive exploitation of protected species in eastern Madagascar. PLoS ONE 6:27570. doi: 10.1371/journal.pone.0027570
Jones, J. P., Andriamarovololona, M. M., and Hockley, N. (2008). The Importance of taboos and social norms to conservation in Madagascar. Conserv. Biol. 22, 976–986. doi: 10.1111/j.1523-1739.2008.00970.x
Koenig, P. (2005). Découverte d'une dépouille de Aye-aye (Daubentonia madagascariensis) dans le nord-ouest de Madagascar. Lemur News 10, 6–7.
LaFleur, M., Clarke, T., Reuter, K., Schaefer, M., and Terhorst, C. (2019). Illegal trade of wild-captured lemur catta within Madagascar. Folia Primatol. 90, 199–214. doi: 10.1159/000496970
LaFleur, M., Clarke, T. A., Reuter, K., and Schaeffer, T. (2016). Rapid decrease in populations of wild ring-tailed lemurs (Lemur catta) in Madagascar. Folia Primatol. 87, 320–330. doi: 10.1159/000455121
LaFleur, M., Gould, L., Sauther, M., Clarke, T., and Reuter, K. (2018). Restating the case for a sharp population decline in Lemur catta. Folia Primatol. 89, 295–304. doi: 10.1159/000489676
Larrosa, C., Carrasco, L. R., and Milner-Gulland, E. J. (2016). Unintended feedbacks: challenges and opportunities for improving conservation effectiveness. Conserv. Lett. 9, 316–326. doi: 10.1111/conl.12240
Lehman, S. M., Ratsimbazafy, J., Rajaonson, A., and Day, S. (2006). Decline of Propithecus diadema edwardsi and Varecia variegata variegata (Primates: Lemuridae) in South-East Madagascar. Oryx 40, 108–111. doi: 10.1017/s0030605306000019
Loudon, J. E., Sauther, M. L., Fish, K. D., Hunter-Ishikawa, M., and Ibrahim, Y. J. (2006). One reserve, three primates: applying a holistic approach to understand the interconnections among ring-tailed lemurs (Lemur catta), Verreaux's sifaka (Propithecus verreauxi), and humans (Homo sapiens) at Beza Mahafaly Special Reserve, Madagascar. Ecol. Env. Anthropol. 2, 54–74.
Maldonado, A., Nijman, V., and Bearder, S. (2009). Trade in night monkeys Aotus spp. in the Brazil-Colombia-Peru tri-border area: International wildlife trade regulations are ineffectively enforced. Endanger. Species Res. 9, 143–149. doi: 10.3354/esr00209
Maldonado, A., and Peck, M. (2013). The role of primate conservation to fight the illegal trade in primates: the case of the owl monkeys in the Colombian-Peruvian Amazon. Folia Primatol. 84, 299–300.
Malone, N., Wade, A. H., Fuentes, A., Riley, E. P., Remis, M., and Robinson, C. J. (2014). Ethnoprimatology: critical interdisciplinarity and multispecies approaches in anthropology. Cri. Anthropol. 34, 8–29. doi: 10.1177/0308275X13510188
Marshall, G. R. (2008). Nesting, subsidiarity, and community-based environmental governance beyond the local level. Int. J. Commons. 2, 75–97. doi: 10.18352/ijc.50
McGinnis, M. D., and Ostrom, E. (2014). Social-ecological system framework: Initial changes and continuing challenges. Ecol. Soc. 19:30. doi: 10.5751/ES-06387-190230
McLennan, M. R., Spagnoletti, N., and Hockings, K. J. (2017). The implications of primate behavioural flexibility for sustainable human-primate coexistence in anthropogenic habitats. Int. J. Primatol. 38, 105–121. doi: 10.1007/s10764-017-9962-0
Meijaard, E., Buchori, D., Hadiprakarsa, Y., Utami-Atmoko, S. S., Nurcahyo, A., Tjiu, A., et al. (2011). Quantifying killing of orangutans and human-orangutan conflict in Kalimantan, Indonesia. PLoS ONE 6:e27491. doi: 10.1371/journal.pone.0027491
Miller, B. W., Caplow, S. C., and Leslie, P. W. (2012). Feedbacks between conservation and social-ecological systems. Conserv. Biol. 26, 218–227. doi: 10.1111/j.1523-1739.2012.01823.x
Mittermeier, R. A., Louis Jr, E. E., Richardson, M., Schwitzer, C., Langrand, O., Rylands, A. B., et al. (2010). Lemurs of Madagascar, 3rd edition, Tropical Field Guide Series. Arlington: Conservation International.
Mittermeier, R. A., Schwitzer, C., Johnson, S., and Ratsimbazafy, J. (2013). “Introduction,” in Lemurs of Madagascar: A Strategy for their Conservation 2013–2016, ed. C. Schwitzer, et al. (Bristol: IUCN SSC Primate Specialist Group, Bristol Conservation and Science Foundation, and Conservation International), 5–11.
Mutschler, T., Randrianarisoa, A. J., and Feistner, A. T. (2001). Population status of the Alaotran gentle lemur Hapalemur griseus alaotrensis. Oryx 35, 152–157. doi: 10.1046/j.1365-3008.2001.00167.x
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Nijman, V., and Nekaris, K. (2014). Traditions, taboos and trade in slow lorises in Sundanese communities in southern Java, Indonesia. Endanger. Species Res. 25, 79–88. doi: 10.3354/esr00610
Nijman, V., Nekaris, K. A. I., Donati, G., Bruford, M., and Fa, J. (2011). Primate conservation: measuring and mitigating trade in primates. Endanger. Species Res. 13, 159–161. doi: 10.3354/esr00336
Ostrom, E. (1990). Governing the Commons: The Evolution of Institutions for Collective Action. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511807763
Ostrom, E. (2007). A diagnostic approach for going beyond panaceas. Proc. Natl. Acad. Sci. U.S.A. 1041, 5181–15187. doi: 10.1073/pnas.0702288104
Ostrom, E. (2009). A general framework for analyzing sustainability of social-ecological systems. Science 325, 419–422. doi: 10.1126/science.1172133
Parathian, H. E., and Maldonado, A. M. (2010). Human-nonhuman primate interactions amongst Tikuna people: perceptions and local initiatives for resource management in Amacayacu in the Colombian Amazon. Am. J. Primatol. 72, 855–865. doi: 10.1002/ajp.20816
Pérez, V. R., Godfrey, L. R., Nowak-Kemp, M., Burney, D. A., Ratsimbazafy, J., and Vasey, N. (2005). Evidence of early butchery of giant lemurs in Madagascar. J. Hum. Evol. 49, 722–742. doi: 10.1016/j.jhevol.2005.08.004
Pires, S., and Clarke, R. V. (2012). Are parrots CRAVED? An analysis of parrot poaching in Mexico. J. Res. Crime Delinq. 49, 122–146. doi: 10.1177/0022427810397950
Pires, S. F. (2015). A CRAVED Analysis of Multiple Illicit Parrot Markets in Peru and Bolivia. Eur. J. Crim. Pol. Res. 21, 321–336. doi: 10.1007/s10610-014-9264-4
Pooley, S. P., Mendelsohn, J. A., and Milner-Gulland, E. J. (2014). Hunting down the chimera of multiple disciplinarity in conservation science. Conserv. Biol. 28, 22–32. doi: 10.1111/cobi.12183
Quinn, A., and Wilson, D. E. (2004). Daubentonia madagascariensis. Mamm. Species 740, 1–6. doi: 10.1644/740
Ramage, M., and Shipp, K. (2009). Systems Thinkers. London: Springer, London, England. doi: 10.1007/978-1-84882-525-3
Randimbiharinirina, R. D., Richter, T., Raharivololona, B. M., Ratsimbazafy, J. H., and Schüßler, D. (2021). To tell a different story: unexpected diversity in local attitudes towards Endangered Aye-ayes Daubentonia madagascariensis offers new opportunities for conservation. People Nat. 3, 484–498. doi: 10.1002/pan3.10192
Randrianandrianina, F. H., Racey, P. A., and Jenkins, R. K. (2010). Hunting and consumption of mammals and birds by people in urban areas of western Madagascar. Oryx 44, 411–415. doi: 10.1017/S003060531000044X
Razafimanahaka, J. H., Jenkins, R. K., Andriafidison, D., Randrianandrianina, F., Rakotomboavonjy, V., Keane, A., et al. (2012). Novel approach for quantifying illegal bushmeat consumption reveals high consumption of protected species in Madagascar. Oryx 46, 584–592. doi: 10.1017/S0030605312000579
Remis, M. J., and Robinson, C. A. (2012). Reductions in primate abundance and diversity in a multiuse protected area: synergistic impacts of hunting and logging in a Congo Basin forest. Am. J. Primatol. 74, 602–612. doi: 10.1002/ajp.22012
Reuter, K. E., Clarke, T. A., Lafleur, M., Ratsimbazafy, J., Kjeldgaard, F. H., Rodriguez, L., et al. (2018). Exploring the role of wealth and religion on the ownership of captive lemurs in Madagascar using qualitative and quantitative data. Folia Primatol. 89, 81–96. doi: 10.1159/000477400
Reuter, K. E., Gilles, H., Wills, A. R., and Sewall, B. J. (2016a). Live capture and ownership of lemurs in Madagascar: extent and conservation implications. Oryx 50, 344–354. doi: 10.1017/S003060531400074X
Reuter, K. E., Randell, H., Wills, A. R., Janvier, T. E., Belalahy, T. R., and Sewall, B. J. (2016c). Capture, movement, trade, and consumption of mammals in Madagascar. Plos ONE 11:150305. doi: 10.1371/journal.pone.0150305
Reuter, K. E., Randell, H., Wills, A. R., and Sewall, B. J. (2016b). The consumption of wild meat in Madagascar: Drivers, popularity and food security. Environ. Conserv. 43, 273–283. doi: 10.1017/S0376892916000059
Riley, E. P. (2007). The human-macaque interface: conservation implications of current and future overlap and conflict in Lore Lindu National park. Sulawesi, Indonesia. Am. Anthropol. 109, 473–484. doi: 10.1525/aa.2007.109.3.473
Riley, E. P. (2018). The maturation of ethnoprimatology: theoretical and methodological Pluralism. Int. J. Primatol. 39, 705–729. doi: 10.1007/s10764-018-0064-4
Riley, E. P. (2020). The Promise of Contemporary Primatology. New York, NY: Routledge. doi: 10.4324/9781138314269
Riley, E. P., and Ellwanger, A. L. (2013). “Methods in Ethnoprimatology: Exploring the Human-Non-Human Primate Interface,” in Primate Ecology and Conservation: A Handbook of Techniques, eds. E. J. Sterling, N. Bynum, and M. E. Blair (Oxford: Oxford University Press), 128–150. doi: 10.1093/acprof:oso/9780199659449.003.0008
Roberts, S. H., Racevska, E., and Donati, G. (2019/20). Observation of the natural re-colonisation of a littoral forest fragment by the Endangered red-collared brown lemur (Eulemur collaris) in southeast Madagascar. Lemur News 22, 24–26.
Rovero, F., Mtui, A. S., Kitegile, A. S., and Nielsen, M. R. (2012). Hunting or habitat degradation? Decline of primate populations in Udzungwa Mountains, Tanzania: An analysis of threats. Biol. Conserv. 146, 89–96. doi: 10.1016/j.biocon.2011.09.017
Sauther, M., Cuozzo, F., Jacky, I. Y., Fish, K., Lafleur, M., Ravelohasindrazana, L., et al. (2013). Limestone cliff-face and cave use by wild ring-tailed lemurs (Lemur catta) in southwestern Madagascar. Madag. Conserv. Dev. 8:5. doi: 10.4314/mcd.v8i2.5
Sautter, G. (1980). “Société, nature, espace dans l'ouest malgache,” in Changements sociaux dans l'Ouest Malgache, ed G. Sautter, et al. (Paris: Orstom), 5–33.
Schwitzer, C., Baker-Médard, M., Dolch, R., Golden, C., Irwin, M., et al. (2013a). “Factors Contributing to Lemur Population Decline on a National Scale, and Proposed Immediate and Longer-Term Mitigation Actions,” in Lemurs of Madagascar: A Strategy for their Conservation 2013-2016, ed. C. Schwitzer, C. et al. (Bristol: IUCN SSC Primate Specialist Group, Bristol Conservation and Science Foundation, and Conservation International), 34–51.
Schwitzer, C., Mittermeier, R. A., Davies, N., Johnson, R. J, and Razafindramanana, J. (2013b). Lemurs of Madagascar: A Strategy for Their Conservation 2013–2016. Bristol: IUCN SSC Primate Specialist Group, Bristol Conservation and Science Foundation, and Conservation International.
Schwitzer, C., Mittermeier, R. A., Johnson, S. E., Donati, G., Irwin, M., Peacock, H., et al. (2014). Averting Lemur Extinctions amid Madagascar's Political Crisis. Science 343:842. doi: 10.1126/science.1245783
Simons, E. L., and Meyers, D. M. (2001). Folklore and beliefs about the aye aye (Daubentonia madagascariensis). Lemur News 6, 11–16.
Starr, C., Nekaris, K. A.-I., Streicher, U., and Leung, L. (2010). Traditional use of slow lorises Nycticebus bengalensis and N. pygmaeus in Cambodia: an impediment to their conservation. Endanger. Species Res. 12, 17–23. doi: 10.3354/esr00285
Sterling, E. J., Gómez, A., and Porzecanski, A. L. (2010). A systemic view of biodiversity and its conservation: processes, interrelationships, and human culture. BioEssays 32, 1090–1098. doi: 10.1002/bies.201000049
Temple, H. J., Anstee, S., Ekstrom, J., Pilgrim, J. D., Rabenantoandro, J., Ramanamanjato, J. B., et al. (2012). Forecasting the Path Towards a Net Positive Impact on Biodiversity for Rio Tinto QMM. Gland: IUCN.
Thạch, H. M., Le, M. D., Vu, N. B., Panariello, A., Sethi, G., Sterling, E. J., et al. (2018). Slow loris trade in Vietnam: Exploring diverse knowledge and values. Folia Primatol. 89, 45–62. doi: 10.1159/000481196
Urbani, B. (2005). The targeted monkey: a reevaluation of predation on New World primates. J. Anthropol. Sci. 83, 89–109.
Urbani, B. (2017). “Humans as primate predators,” in The International Encyclopedia of Primatology, ed. A. Fuentes (Chichester, West Sussex: Wiley Blackwell) 1–3. doi: 10.1002/9781119179313.wbprim0258
van Gennep, A. (1904). Tabou Et Totémisme à Madagascar: Étude Descriptive Et Théorique (Classic Reprint). Paris: Ernest Leroux.
Vargas, A., Jiménez, I., Palomares, F., and Palacios, M. J. (2002). Distribution, status, and conservation needs of the golden-crowned sifaka (Propithecus tattersalli). Biol. Conserv. 108, 325–334. doi: 10.1016/S0006-3207(02)00117-9
Vermeij, G. J. (2012). The limits of adaptation: Humans and the predator-prey arms race. Evolution 66, 2007–2014. doi: 10.1111/j.1558-5646.2012.01592.x
Keywords: coupled human and natural systems (CHANS), ethnoprimatology, feedbacks, human-wildlife interactions, hunting, primate conservation, social-ecological systems, wildlife trade
Citation: Rivera CJ, Mayo D and Hull V (2021) Social-Ecological Interactions Influencing Primate Harvest: Insights From Madagascar. Front. Conserv. Sci. 2:776897. doi: 10.3389/fcosc.2021.776897
Received: 14 September 2021; Accepted: 31 October 2021;
Published: 23 November 2021.
Edited by:
Joelisoa Ratsirarson, University of Antananarivo, MadagascarReviewed by:
Qiang Dai, Chinese Academy of Sciences (CAS), ChinaHong Mingsheng, China West Normal University, China
Copyright © 2021 Rivera, Mayo and Hull. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian J. Rivera, christian.rivera@ufl.edu
 Christian J. Rivera
Christian J. Rivera