- 1School of Earth, Atmospheric and Life Sciences, Faculty of Science, Medicine and Health, University of Wollongong, Wollongong, NSW, Australia
- 2Behavioural Ecology, Department of Biology, Ludwig-Maximilians University of Munich, Munich, Germany
Variation in female mate preferences for male traits remains poorly understood (both among and within females), despite having important evolutionary and conservation implications, particularly for captive breeding. Here, we investigate female mate preferences for male advertisement call frequency, and determine whether preferences vary over repeated trials, in the critically endangered southern corroboree frog, Pseudophryne corroboree. We conducted a series of phonotaxis trials in a six-speaker arena where naïve, captive-bred, virgin females were offered a choice between low, average and high frequency male advertisement calls, with a subset of females tested repeatedly. In the first trial, we found no evidence for a population-level preference for call frequency, but females spent less time in the low call zone than expected by chance. However, our results showed that female mate preferences changed over sequential trials. Females spent significantly more time in the low frequency call zone in the third trial compared to the first trial, and, in the last trial, females exhibited a significant population-level preference for low frequency calls. Subsequently, repeatability estimates of female preferences were low and did not significantly deviate from zero. Our results indicate that female P. corroboree mate preferences can exhibit temporal variation, and suggest that females are more attracted to low call frequencies after repeated exposure. These findings imply that female P. corroboree may become choosier over time, and highlight the potential for mate preferences to exhibit phenotypic plasticity within a single reproductive cycle. Overall, these findings provide the first information on mate preferences in P. corroboree, and emphasize the importance of considering individual variation in mate choice studies. From a conservation perspective, knowledge of individual variation in female mate preferences may be used to conduct behavioral manipulations in captivity that facilitate the breeding of genetically valuable individuals, and improve the success of conservation breeding programs.
Introduction
Female mate preferences for male secondary sexual traits have been intensively studied across a diversity of taxonomic groups (Rosenthal, 2017). Females often prefer males with costly, condition-dependent secondary sexual traits such as conspicuous courtship displays, complex acoustic signals or bright coloration, as these traits function as honest indicators of male quality (Kokko et al., 2003; Rosenthal, 2017). By choosing to mate with high quality males that possess more elaborate traits, females can gain direct “material” benefits (such as resources or paternal care) and/or indirect “genetic” benefits (such as good or compatible genes for offspring) (Neff and Pitcher, 2005; Kuijper et al., 2012). Compared to the large body of evidence investigating female mate preferences at the population level, relatively few studies have considered variation in female preferences for male traits (Jennions and Petrie, 1997; Rosenthal, 2017). In particular, we still know little about whether females within populations vary in their preferences (among-individual variation) or whether individual females are consistent with their preferences over time (within-individual variation) (Bell et al., 2009; Zandberg et al., 2017, 2020). However, empirical research exploring such variation is gaining momentum, with evidence suggesting that individual variation in mate preference may be more common than currently realized (Forstmeier and Birkhead, 2004; Cummings and Mollaghan, 2006; Bell et al., 2009; Fowler-Finn and Rodríguez, 2013; Ah-King and Gowaty, 2016; Zandberg et al., 2017; Aich et al., 2020).
Quantifying the repeatability of mate choice behavior has been identified as an important first step toward understanding how mate preferences vary within populations (Widemo and Sæther, 1999; Brooks and Endler, 2001; Dougherty, 2020). Repeatability is defined as the amount of behavioral variation due to differences between individuals, and is calculated by dividing the among-individual variance by the total phenotypic variance (the sum of among-and within-individual variance) (Bell et al., 2009; Dingemanse and Dochtermann, 2013). Thus, when individuals differ in their average behavior (i.e., leading to high among-individual variance in the sample), and also behave consistently over time (i.e., display low levels of within-individual variance) repeatability is high (Bell et al., 2009). In the context of female mate choice, if females differ in their average choices over time (high among-individual variation), and individual female choices are stable over time (low within-individual variation), repeatability estimates will be high (Bell et al., 2009). Conversely, if individual females are random in their mate choices or exhibit plasticity in mate preferences (high within-individual variation), or, if females are mostly unanimous and consistent with their mate choices over time (low among- and within- individual variation), repeatability estimates will be low (Rosenthal, 2017). Examining the repeatability of mate choice can therefore offer important insights into the evolution of mate preferences, and provide empiricists with information relevant to understanding the particular benefits gained from mate choice decisions (Jennions and Petrie, 1997). Crucially, repeatable variation in mate choice can have major evolutionary consequences as it can directly influence the intensity and direction of sexual selection, which may have indirect flow on effects for population viability and fitness (Jennions and Petrie, 1997; Brooks and Endler, 2001; Cally et al., 2019).
From a conservation perspective, there is growing recognition that knowledge of mate choice, as well as individual variation in mate choice, can facilitate successful conservation breeding programs (CBPs) (Wielebnowski, 1998; Asa et al., 2011; Chargé et al., 2014). CBPs aim to establish viable captive assurance population's ex-situ and provide large numbers of genetically diverse individuals for reintroduction in-situ (Pritchard et al., 2012). Unfortunately, breeding success in captivity can be highly variable, and reproductive failures often occur (Wielebnowski, 1998; Conway, 2011). One reason for this may be that natural mate choice behavior is rarely permitted; either due to a lack of information on the reproductive behavior of a target species, and/or, mate choice is restricted in favor of genetic targets (Asa et al., 2011; Martin-Wintle et al., 2019). Recent work has demonstrated that integrating mate choice into captive breeding protocols can significantly increase behavioral compatibility between mates, resulting in higher mating and reproductive success, and elevated offspring survival post-release (Petrie, 1994; Martin-Wintle et al., 2015; Hartnett et al., 2018; Parrott et al., 2019). Thus, research investigating patterns of female mate choice (including individual variation) in threatened species has real potential to improve captive breeding outcomes. Understanding the mechanisms of female mate choice, and whether females differ in their mate preferences over time, would provide conservation managers with the necessary information to predict and manipulate mate choice in captive environments and anticipate reproductive outcomes (Asa et al., 2011). This type of behavioral research will undoubtedly be an important tool to help improve the captive breeding, genetic management and post-release success of many critically endangered species.
Amphibians are one taxonomic group that stand to benefit substantially from integrative, behavior-based conservation approaches (Kelleher et al., 2018). Amphibians are currently the most threatened vertebrate group (an estimated 41% of species are threatened with extinction), and ex-situ CBPs have been established worldwide to aid in the recovery of critically endangered amphibian species (IUCN, 2020). However, breeding success in amphibian CBPs is often poor (Soorae, 2016), most likely owing to the complexity and diversity of amphibian reproductive ecologies (Haddad and Prado, 2005). Importantly, anuran amphibians have been a model system for the study of sexual selection for over half a century (Wells, 2007), so empiricists have a firm understanding of the mechanisms of female mate choice in this taxonomic group. Surprisingly, however, this vast body of knowledge is seldom integrated into amphibian conservation efforts (Walls and Gabor, 2019).
In nearly all anuran amphibian species, reproduction relies heavily on acoustic communication (Wells, 2007). In most species, males actively advertise to females by calling, and acoustic signals play a crucial role in both mate attraction and mate selection (Gerhardt and Huber, 2002). Females have been shown to discriminate amongst potential mates based on a range of different call characteristics, such as call rate (Laird et al., 2016), call duration (Welch et al., 1998), and call effort (Ward et al., 2013). Dominant call frequency (or call peak frequency) is one component of a male's advertisement call that usually varies among males and is often negatively correlated with body size (larger males have a lower dominant call frequency) (Gerhardt and Huber, 2002; Wells, 2007). Female anurans have been shown to display preferences for lower than average call frequencies, and selection on call frequency is often reported to be weakly directional or stabilizing (Wells, 2007). Females may use male call frequency as a cue to assess male body size or age (Vargas-Salinas et al., 2014), and by discriminating among males based on frequency potentially gain direct or indirect benefits, such as higher fertilization success (Robertson, 1990), or the acquisition of good genes that improve offspring performance and survival (Rausch et al., 2014).
Compared to the extensive research investigating female preferences for male call characteristics in anurans at the population level, among-individual variation in preferences, or the repeatability of such preferences, remain largely unknown. To date, the small number of studies that have examined the repeatability of mate preferences in anurans have yielded mixed results (Gerhardt and Huber, 2002). Some studies have reported that females display repeatable, among-individual variation in preferences for male call characteristics such as call duration (Gerhardt et al., 2000) and call frequency (Jennions et al., 1995; Howard and Young, 1998). Conversely, studies in other species, such as midwife toads (Alytes muletensis), and tungara frogs (Physalaemus pustulosus) have shown that there can be substantial within-individual variation in mate preferences (attributable to random mating and plasticity), resulting in low repeatability estimates (Kime et al., 1998; Lea et al., 2000). Clearly, there is considerable inter- and intra- specific variation in mating preferences in anurans, and further assessment of its repeatability is needed. For threatened species, this knowledge can potentially be utilized by conservation managers to increase the chance of individuals successfully breeding in captivity, or, facilitate social manipulations that maximize reproductive output whilst simultaneously enhancing the genetic management of captive populations.
The southern corroboree frog, Pseudophryne corroboree, is a terrestrial breeding anuran that is endemic to Kosciuszko National Park in south eastern New South Wales, Australia. Since the 1980s P. corroboree has suffered extreme population declines, predominantly due to lethal effects caused by the introduced amphibian chytrid fungus (Batrachochytrium dendrobatidis) (Hunter et al., 2010). In response to such alarming population declines, a multi-institutional CBP was established in 2003 (OEH NSW, 2012). While P. corroboree can reproduce successfully in captivity, mating success is restricted to a small proportion of advertising males (30–50%) (McFadden et al., 2013). At present, we know very little about patterns of mate choice (McFadden et al., 2013). In nature, we know that P. corroboree males construct shallow terrestrial nests and call to attract potential mates (Osborne, 1991). In captivity, female mate choice has been observed in captive breeding enclosures, where certain males are more successful than others at attracting females and gaining matings, and some females will refrain from mating if they do not encounter a suitable partner (McFadden et al., 2013). It is currently unknown which male characteristics are attractive to female P. corroboree, and what cues females utilize to assess mate quality. Identifying female preferences for male phenotypic cues (as well as whether females vary in their mate preferences), may help maintain viable captive populations of P. corroboree by providing conservation managers with the knowledge needed to conduct captive breeding manipulations that may increase the reproductive success of genetically valuable individuals. The aim of the present study was to gain preliminary insights into patterns of female mate choice in captive southern corroboree frogs. The specific aims were to determine whether females: (1) exhibit preferences for low, average or high frequency male advertisement calls, and (2) vary in their preferences for male call frequency over repeated trials. Given that past work has shown that female body size can influence variation in mate choice in amphibians (Jennions et al., 1995; Neelon et al., 2019), we also considered whether female body size influenced female mate preferences. We examined female mate preferences by conducting repeated phonotaxis choice trials, where females were given the opportunity to choose between low, average or high frequency male calls, with a subset of females tested three times to determine preference repeatability.
Methods
Study Animals
Captive-bred P. corroboree eggs were obtained from Melbourne Zoo, Australia and reared to adulthood at the Ecological Research Centre, University of Wollongong, Australia. Offspring were produced from matings between 6 males and 12 females, resulting in 15–28 unique sire dam pairings, (depending on whether P. corroboree is polyandrous and splits clutches between the nests of multiple males). All frogs were reared individually, under the same, standardized environmental conditions throughout both larval and post-metamorphic life stages. Sex was determined based on body size distributions during the breeding season once sexual maturity was reached (females have a heavier body mass, see McFadden et al., 2013) and all females were visibly gravid. Pseudophryne corroboree females reach sexual maturity ~4–5 years post metamorphosis in the wild, and 3–4 years post metamorphosis in captivity (Hunter, 2000). Females used in the present study (n = 40) were approximately 4 years old (post metamorphosis), had never mated previously, and had not been exposed to calling males. At the time of the study, the body weight of experimental females ranged from 2.70–3.54 grams (mean ± SE = 3.16 ± 0.04). All experimental trials were conducted during the captive breeding season between April 27 and May 9, 2018, when females were visibly gravid. Of note, the timing of the P. corroboree breeding season in captivity is ~6 weeks later than the timing of the natural breeding season in the wild.
Captive Husbandry
During the entire study period, frogs were housed individually in rectangular plastic enclosures (21 cm L x 12 cm W x 12 cm H). Each enclosure contained a base layer of aquarium gravel covered by a layer of sphagnum moss (Brunnings, Australia). Frogs were provided with UV light supplied by a UV-B globe (Reptisun 10.0 T5 High Output 36” bulb; Pet Pacific, Australia) suspended ~20 cm above the enclosures. UV lights were controlled by a timer which was set to a 12-h day/night light cycle. Frogs were also exposed to natural ambient light through a nearby window, which provided them with a natural photoperiod. Frogs were fed 7–10-day old Acheta domestica crickets twice weekly, and once a week crickets were dusted with calcium powder to prevent calcium deficiencies. To prevent the accumulation of nitrogenous waste and detritus, enclosures were flushed twice a week with reverse-osmosis (R.O.) water and sphagnum moss was changed once every four weeks. Frogs were housed in a temperature-controlled room, with temperature cycled annually to reflect natural seasonal changes, including a winter hibernation period. Throughout the year, frogs were kept at temperatures ranging from 5 to 20°C, including an eight-week hibernation period. During hibernation, temperature ranged between 5 and 10°C for a period of 8 weeks and feeding ceased. At the time of experimentation, which occurred during the captive breeding season, frogs were kept at a constant 20°C and on a 12 h day/night light cycle.
Experimental Design
To determine whether female P. corroboree exhibit preferences for male call frequency, and whether any differences in preference were repeatable, we conducted a series of phonotaxis trials. Females were tested inside a hexagonal, six choice arena (120 cm W x 120 cm L; Figure 1). A six-choice hexagon design was used to simulate a natural acoustic environment. This design was employed to more closely simulate natural chorus conditions that involve multiple advertising males, and followed the hexagon design used by Richardson and Lengagne (2010) in their study of female call preference in a treefrog. In nature, P. corroboree males call from nests in small choruses that typically border the edge of ephemeral pools, and advertise to females antiphonally (and females often visit and assess multiple males before mating) (D. Hunter, personal communication). The floor of the hexagon arena was lined with thick plastic corflute (Bunnings, Australia) and the inside walls were lined with 40 mm thick acoustic foam (Dunlop) to limit sound reverberation (see Figure 1). The hexagonal arena was divided into six equal sized “call” zones and a central “no choice” zone (Figure 1). Each call zone contained a speaker (Sony SRS-XB12 Bluetooth speaker) that was positioned on the ground in the angle of the arena, facing the center (see Figure 1). Neighboring speakers were separated by a 60° angle, and were separated by a distance of 50 cm, resembling spacing observed in natural choruses (S. Kelleher, unpublished data). Opposing speakers were separated by a distance of 100 cm. In order to simulate a natural male chorus, two of the six speakers broadcast a high frequency advertisement call, two speakers broadcast an average (medium) frequency call, and two speakers broadcast a low frequency call (see below for details on call frequency and call treatments). Each call frequency was represented on both sides of the hexagonal arena (see Figure 1) to reduce any potential side bias. Of note, similar approaches have been used in other mate choice studies (see Holveck et al., 2011; Vega-Trejo and Backwell, 2017). During each phonotaxis trial, acoustic signals were broadcast from the six speakers antiphonally, but speakers emitting the same call frequency were never played in successive order. Calls were broadcast in a continuous loop, with a constant one-s interval of silence between each successive call for the entire duration of the trial. This continuous call loop ensured that there was no “chorus leader” nor any “chorus followers” after the very first call was played (following Richardson and Lengagne, 2010). Calls alternated successively between the six speakers (with one s silence intervals), meaning that each speaker broadcast a treatment call (comprised of a two-part and one part call, see Figure 2) every 13.5 s, which equates to the approximate average call rate quantified in a captive population of P. corroboree (eight total calls per min, S. Kelleher unpublished data). Before trials began, all speakers were calibrated to 80 dB at the center of the arena (50 cm from each speaker) using a sound decibel meter (Digitech QM-1589 Sound Level Meter). A sound pressure level of 80 dB approximates the sound pressure level of a P. corroboree male calling from a distance of 0.5 m (Pengilley, 1971).
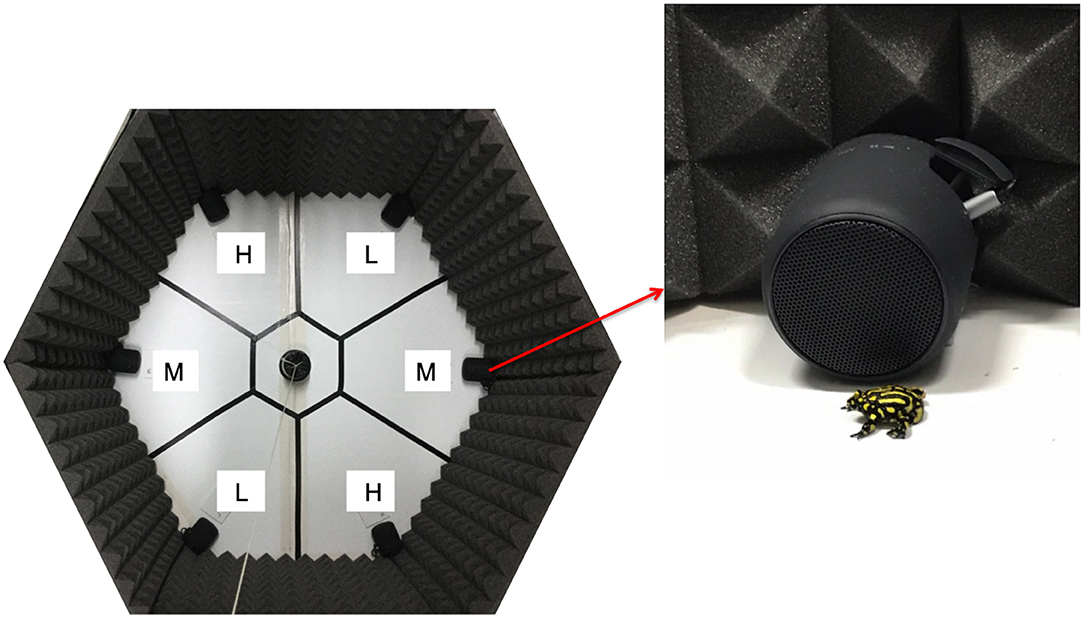
Figure 1. Hexagonal six-choice phonotaxis arena used to test female preferences for male call frequency in P. corroboree. The arena was divided into six call zones—L, low frequency call zone; M, medium (average) frequency call zone; H, high frequency call zone. There was also a central “no choice” zone from which each test subject was released.
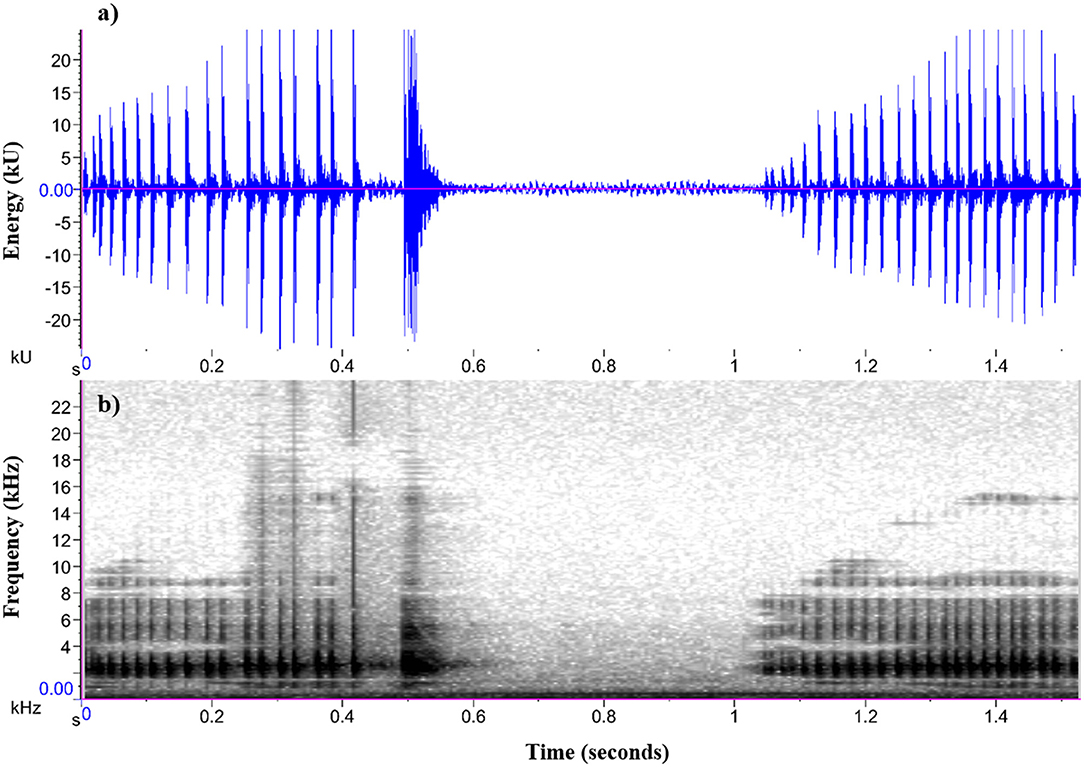
Figure 2. (a) Oscillogram and (b) spectrogram of a male P. corroboree advertisement call (two-part and one-part call) used in call synthesis.
Acoustic signals for playback were synthesized from an advertisement call of a captive P. corroboree male made during the breeding season. The call was recorded in .wav format at a sampling rate of 44.1 kHz and 16-bit resolution, and synthesized using the sound analysis software Audacity (Version 2.2.2). Pseudophryne corroboree males typically produce an advertisement call that consists of an initial two-part call, (which is comprised of a longer first component and then a shorter, pulsatile second component) followed by a second one-part call (see Figure 2). The peak call frequency of the two-part call (both the first and second component) and one-part call is significantly positively correlated (S. Kelleher, unpublished data). The two-part and one-part exemplar call used as a template for call synthesis represented the approximate average call parameters quantified in a captive breeding population of P. corroboree (S. Kelleher, unpublished data). During call synthesis, we kept the temporal structure (call duration, pulse rate) of both the two-part and one-part call constant, but adjusted peak call frequency using the “Change Pitch” audio feature within Audacity. We generated one synthetic exemplar for each of the three call frequency treatments (following Laird et al., 2016). For this study, the peak frequency value for the low call frequency treatment was set at 1,925 Hz, the average (medium) call frequency treatment was set at 2,350 Hz, and the high call frequency treatment was set at 2,775 Hz. These call frequency treatments reflect the approximate minimum, average and maximum male call frequencies observed and quantified in a captive breeding population of P. corroboree (S. Kelleher, unpublished data). Importantly, these call treatments also encompass the previously reported frequency ranges for wild P. corroboree (Pengilley, 1971). Our approach of synthesizing call treatments from a natural exemplar call (that represents captive P. corroboree average call parameters) follows that of previous phonotaxis studies (e.g., Smith and Roberts, 2003; Dreher and Prohl, 2014; Laird et al., 2016), and was taken to ensure that the only parameter that differed between the call stimuli was peak frequency.
Phonotaxis Trials
All females were weighed one week prior to the commencement of the experimental period. Trials were conducted between the h of 09:00 a.m. and 16:00 p.m. inside an artificially illuminated, temperature-controlled experimental room maintained at a constant temperature of 20°C (the same lighting and temperature conditions as the housing room). Trials were conducted during daylight hours under artificial lighting as P. corroboree displays diurnal breeding activity (Pengilley, 1971; Osborne, 1991). To begin a trial, a focal female was first transferred from the housing room to the experimental room. Following a five min acclimation period after moving, the focal female was then placed in the center of the hexagonal experimental arena in the no choice zone, underneath an opaque plastic cup for a further two min. This second acclimation period allowed females to acclimate to the test environment, but the plastic cups muffled sound, preventing females from assessing any calls. After two min, call stimuli began to broadcast through the six speakers, and the plastic cup was raised manually by an observer using a pulley system. Trials commenced when the plastic cup was lifted, which differed in timing for each trial by ~ two to ten seconds. Subsequently, the first call heard by the female once the cup was lifted (and the subsequent call sequence), was randomized for every trial, to one of three continuous call sequences; (1) high, medium, low, (2) medium, low, high, or (3) low, high, medium. This prevented any call sequence confound. Call stimuli continuously played for a trial time of 10 min. Phonotactic behavior was recorded remotely using a high definition digital camera (Panasonic HC-W580M) positioned ~2 m above the arena. During each trial, the observer was shielded from the test subject by a 2 m high opaque curtain. In total, 40 females were tested once, and 18 females were tested three times (with two days in between each repeat trial). Due to time constraints, we were only able to repeatedly test a subset of females. For repeat trials, the arena was rotated clockwise so that the speakers moved one position to the right. This approach ensured that over the three repeat trials each call frequency was broadcast from a different position within the arena, eliminating the potential for directional side biases (possibly due to geomagnetic sensitivity in amphibians, Begall et al., 2013), which could have inflated estimates of repeatability. Additionally, for repeated trials, female test sequence was randomized to control for any order effects. Between every trial the experimental arena was cleaned with ethanol and reverse-osmosis (R.O.) water to remove any potential chemical signals left by previous females. For consistency, cleaning also took place before the first female was tested. To quantify female mate choice behavior, video recordings were analyzed at a later date using the behavioral analysis software JWatcher (Blumstein et al., 2000). Females were considered responsive to the acoustic stimuli if they left the central “no choice” zone. We recorded the proportion of time a female spent in each zone (low, medium or high), and overall preference was defined as the call zone a female spent the maximum amount of time in. Data from the two zones with the same call frequencies were pooled together. It was not possible to record data blind because phonotaxis trials required knowledge of the specific call stimuli presented and repeated trials required knowledge of individual female identity.
Statistical Analysis
Effect of Call Frequency on Female Preference
We analyzed call frequency preference in female frogs (n = 40) using two complementary measures, the proportion of time frogs spent in low, medium and high frequency call zones, and the overall call frequency preference. We arcsine transformed the proportion of time frogs spent in each call zone and used t-tests to evaluate whether the mean proportion of time frogs spent in a given zone deviated from the expected proportion of time (33%, arcsin(sqrt(1/3))). We confirmed that transformed proportion of time data were normally distributed. If females showed neither preference nor avoidance toward a particular call frequency, they should by chance have spent an equal proportion of the total trial time in any one of the three call frequency zones (i.e., 33%). We used binomial tests to evaluate whether females (n = 40) were more likely to choose one particular call frequency (low, medium, high) over any other call frequency based on their overall preference (zone spent the most time in). We tested whether the number of females choosing a given call frequency was higher than expected by chance (33%) given the total number of females which made a choice (n = 40). We Bonferroni adjusted all p-values in the first trial (t-tests and binomial tests) for multiple comparisons.
Repeatability of Female Preferences
For the subset of females tested repeatedly (n = 18), we used generalized linear mixed models (GLMM) to test whether trial number (three level factor: trial 1, 2, and 3) and body weight (grams) affected female preferences, and whether females display repeatable, individual preferences for a specific call frequency (random intercept for female ID). To determine whether female preferences significantly differed among trials we used likelihood ratio tests comparing one model with trial included as a fixed effect, and one model without. We fitted models on the full sample of individuals (18 females tested over three trials and 22 females tested over one trial) to increase power in estimating fixed effects (Martin et al., 2010). For the proportion of time spent in each call frequency zone, we used a Gaussian error distribution and fitted a linear mixed model evaluating the time spent in each zone while controlling for trial number (1, 2, and 3) and an individual's body weight (grams). This approach yielded three models, one for each call frequency: low, medium, or high. We arcsine transformed the response variable proportion of time spent in each zone and inspected model residuals for normality and homogeneity. If the proportion of time spent in a certain frequency call zone changed over trial number, we additionally used t-tests to test whether the mean proportion of time frogs spent in this particular call zone in the first, second, and third trial deviated from the expected proportion of time (33%, arcsin(sqrt(1/3))). For overall preference, we fit a mixed effects model with a binomial error distribution. We evaluated whether individuals that were tested repeatedly (random intercept with 18 levels) were consistent over the three trials in choosing one frequency over the remaining two frequencies. If the response of females was affected by trial number, we further tested whether female choice in trial 1, 2 and 3, respectively, deviated from chance (33%) given the total number of females that made a choice (trial 1: n = 40, trial 2 and 3: n = 18) using a binomial test. We did not Bonferroni adjust p-values in this part of the analysis as trial times are independent.
For all mixed models, we inspected the amount of among-individual variance (Vamong, i.e., the variance explained by individual identity) explained by the model and the residual within-individual variance (Vwithin). If the among-individual variance was >0 we calculated adjusted repeatability (controlling for trial number and body weight) as R = Vamong / (Vamong + Vresidual) [Equation 1] and for binomial data as R = Vamong / (Vamong +(π2 / 3)) [Equation 2] using the R package rptR (Stoffel et al., 2017) which provides means and 95% confidence intervals of repeatability estimates. All statistical analyses were performed in R version 4.0.0 (R Core Team, 2020). Mixed effects models were fit with the R package lme4 (Bates et al., 2015). All data and associated analyses can be found in the Supplementary Material.
Ethical Note
All procedures outlined in this study were approved by the University of Wollongong Animal Ethics Committee (Protocol Number AE17/14).
Results
In all trials (first trial and repeated trials), all females left the no choice zone and exhibited positive phonotactic behavior to the acoustic stimuli broadcast in the experimental arena. Females crawled freely toward the speakers, with the majority of females remaining within 1 cm of one of the speakers, or directly touching it, indicating that they were responsive to the calls. During the first trial, a subset of females visited up to five speakers, though most females (62%) visited a single speaker. In the first trial, females spent significantly less time on average in the low frequency call zone than expected by chance (t-test: t = −3.44, df = 39, p = < 0.01; Figure 3). The mean proportion of time spent in the medium frequency call zone did not deviate from chance (t-test: t = −2.07, df = 39, p = 0.14; Figure 3), nor did the mean time spent in the high frequency call zone (t-test: t = −0.1, df = 39, p = 1; Figure 3). Based on overall preference (the zone females spent the maximum amount of time in), there was no evidence that females preferred low, medium or high male call frequencies (binomial test: n = 40, plow = 0.95, pmedium = 1, phigh = 0.39).
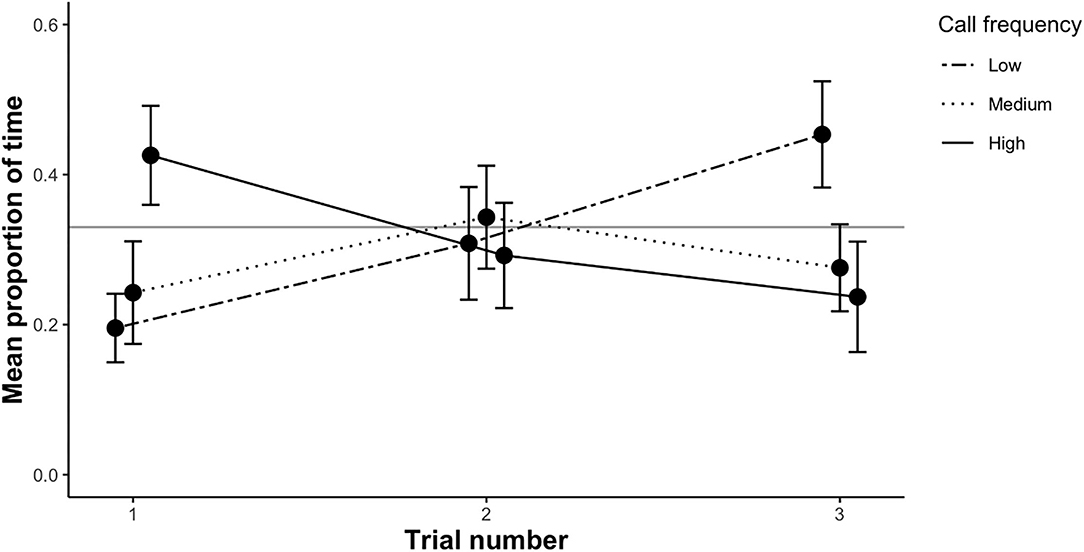
Figure 3. The mean proportion of time (±SE) female P. corroboree spent in each of the three call zones (low, medium and high) during each of the three trial rounds. Sample size for each trial as follows—trial 1: n = 40, trial 2: n= 18, trial 3: n = 18. The proportion of time spent in each call zone was compared to an expected value of 0.33, represented by the gray horizontal line.
Effect of Trial on Female Preference for Call Frequency
We re-tested a subset of females (n = 18) for a second and third time. The mean proportion of time females spent in the low frequency call zone differed significantly among the three trials (X2 = 8.31, df = 2, p = 0.016; Figure 3). Females spent significantly more time in the low frequency call zone in the third trial compared to the first trial (β = 0.32, SE = 0.11, p < 0.01). The mean proportion of time spent in the low frequency call zone was 24% in the first trial, which was significantly less than expected by chance (t = −3.44, df = 39, p = < 0.01). This increased to 31% in the second trial (t = −1.14, df = 17, p = 0.27) and to 45% in the third trial (t = 0.84, df = 17, p = 0.41). The proportion of time spent in the medium frequency zone was not affected by trial number (X2 = 0.74, df = 2, p = 0.69; Figure 3), nor was the proportion of time spent in the high frequency zone (X2 = 3.34, df = 2, p = 0.19; Figure 3).
Based on overall preference (the call zone females spent the maximum amount of time in), preference for low frequency calls significantly differed among the three trials (X2 = 9.67, df = 2, p < 0.01; Figure 4) with females preferring low frequency calls more often in the third trial than in the first trial (β = 2.42, SE = 0.94, p < 0.01). The number of females preferring low frequency calls did not deviate from chance (0.33) in the first trial (binomial test: n = 10 out of ntotal = 40, p = 0.32) and second trial (binomial test: n = 6 out of ntotal =18, p = 1), but was significantly higher than chance in the third trial (binomial test: n = 11 out of ntotal = 18, p = 0.01). Female preference for medium frequency calls was unaffected by trial number (X2 = 2.4, df = 2, p = 0.3; Figure 4) as was the case for high frequency calls (X2 = 5.23, df = 2, p = 0.07; Figure 4). However, there was a trend that preference for high frequency calls was lower in the third compared to the first trial (β = −1.43, SE = 0.74, p = 0.05; Figure 4).
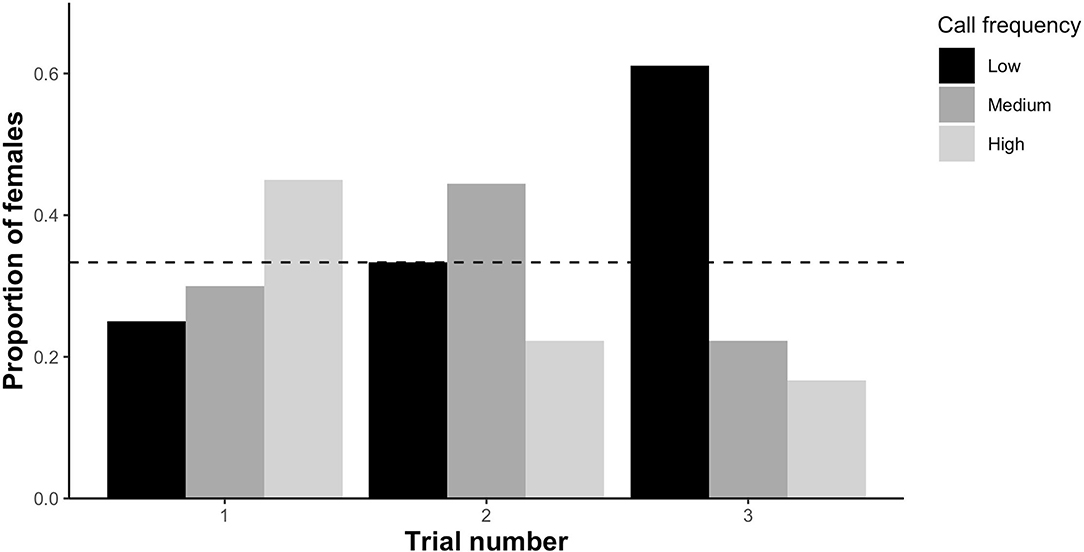
Figure 4. The proportion of female P. corroboree choosing low, medium or high male call frequencies during each of the three trial rounds. Female overall preference was determined as the zone each female spent the most time in. Sample size for each trial as follows—trial 1: n = 40, trial 2: n = 18, trial 3: n = 18. Female overall preference was compared to an expected value of 0.33, represented by the dotted horizontal line.
Effect of Body Weight on Female Preference for Call Frequency
There was no significant effect of female body weight on the proportion of time spent in the low, medium or high frequency call zones (all p values > 0.05). There was also no significant effect of body weight on overall preference (call zone spent the maximum amount of time in) (all p values > 0.05).
Repeatability of Female Preferences
For females that were tested repeatedly (n = 18), the among-individual variance components for the proportion of time spent in low (Vamong = 0.02), medium (Vamong = 0.01) and high frequency call zones (Vamong = 0) were very low. Accordingly, there was no significant repeatability (adjusted repeatability controlling for body size and trial number) in the proportion of time females spent in the low (R = 0.14 [0, 0.48]), or medium frequency call zones (R = 0.07 [0, 0.41]). Based on overall preference (the call zone females spent the maximum amount of time in) there was no evidence for repeatable preferences for low (Vamong = 1.41, R = 0.25 [0, 0.99]), medium (Vamong = 1.02, R = 0.18 [0, 0.92]) or high (Vamong = 0) frequency calls.
Discussion
Compared to the overwhelming number of studies investigating population-level mate preferences, research investigating individual variation in female mate preferences has remained limited. This is surprising as variation in mate preferences at both the among- and within-individual level can have major implications for the development of sexual selection theory, and threatened species conservation. Here, we investigated whether female P. corroboree exhibited preferences for male calls that differed in frequency, and whether individual females varied in their preferences over three repeated trials. For the first trial, our results showed that females did not exhibit a population-level preference for call frequency, yet, the mean proportion of time females spent in the low frequency call zone was lower than expected by chance. We found evidence that female mate preferences were significantly affected by trial number, whereby time spent in the low frequency call zone increased over repeated trials, and low frequency calls were significantly preferred in the last trial. Subsequently, there was no significant repeatability in preference based on any of the measures used. Overall, these findings suggest that female P. corroboree mate preferences for call frequency are dynamic, and highlight that individual females' may alter their mate preferences within a single breeding season, providing evidence for plasticity in female mate preferences.
Our finding that P. corroboree females did not display a population-level mate preference for call frequency during the first trial was surprising because there is a large body of empirical evidence that female anurans prefer average to low call frequencies, a trait known to correlate with male body size and reliably signal male quality (Gerhardt and Huber, 2002; Wells, 2007). Previous work in anurans has demonstrated that phenotypic variation in female preferences for male call frequency can often be attributed to size-assortative mating. For example, in the African painted reed frog (Hyperolius marmoratus), females differ in their preferences for male call frequency due to size-dependent discriminatory abilities, most likely related to differences in the frequency tuning of the basilar papilla in female ears (Jennions et al., 1995). Similarly, in the smooth toadlet, (Uperoleia laevigata), females prefer the call frequency of males that are ~70% of their own body weight, as this allows for proper alignment of the cloaca during gamete release, which facilitates successful fertilization (Robertson, 1990). However, these reasons are unlikely to explain the lack of population-level preference observed in the first trial because we found no evidence that female body size affected the likelihood of choosing any particular call frequency. An alternative reason why we failed to detect a population level preference for call frequency during the first trial may relate to the way we produced our call stimuli. As we manipulated a natural exemplar call taken from an individual male, we can't be certain that we would have found similar results if we had manipulated the calls of a different male. Arguably, call frequency might only be important for phonotaxis when presented in combination with certain call trait values that are difficult to characterize. To address this issue, future preference tests with P. corroboree should make replicated sets of manipulated trait values using different male calls for each trial (e.g., Oliva et al., 2018). Until this work has been conducted, explanations for a lack of a population-level preference in the first trial will remain speculative. Unexpectedly, we found that females in the first trial spent less time in the low frequency zone than predicted by chance. This result implies that females may have been initially avoiding males with low frequency calls, or, spending less time assessing them. While the reason for this result remains unclear, one possibility is that higher frequency calls were easier to detect in a noisy environment, as has been previously shown in both anurans (Bee, 2008; Parris et al., 2009) and birds (Lohr et al., 2003; Cardoso and Atwell, 2011).
Critically, however, we found that the time females spent in the low frequency call zone significantly differed among subsequent trials, and, by the third trial, females significantly preferred low frequency calls. These findings suggest that females may have initially been selecting males at random (based on overall preference), but as the breeding season progressed, females altered their preferences, resulting in a directional population-level preference for low frequency advertisement calls. This finding is particularly noteworthy as it is in line with a growing body of evidence that individual female mate preferences can exhibit phenotypic plasticity over sequential trials (Gabor and Halliday, 1997; Qvarnström et al., 2000; Wacker et al., 2016). This draws added attention to the potential for female mate preferences to display significant temporal variation within a single reproductive season.
One explanation for the change in female mate preference over sequential trials may be that females become choosier over time, as previously reported in other taxa (Forsgren, 1997; Gabor and Halliday, 1997; Uetz and Norton, 2007). Female choosiness (commitment to finding and assessing males) (Jennions and Petrie, 1997; Brooks and Endler, 2001) can vary due to a multitude of factors, including female age (Atwell and Wagner, 2014), physiological condition (Judge et al., 2014) and the risk of predation (Atwell and Wagner, 2015). A female's current reproductive state can also heavily influence an individual's degree of choosiness within a short time period (Lynch et al., 2005). Empirical evidence in numerous taxonomic groups, including anurans, has demonstrated that as individual females transition through different stages within their reproductive cycle, fluctuations in circulating hormone levels can mediate changes to preference thresholds for male signals (permissiveness), and alter a female's degree of choosiness, resulting in plasticity in mate choice (Lynch et al., 2005, 2006). For instance, in female midwife toads (A. muletensis), reproductive state has been shown to influence mating preferences, whereby ovulating females are highly receptive and more discriminatory, compared to gravid or mated females, who were less receptive and less choosy (Lea et al., 2000). Plasticity in mate choice attributable to changes in reproductive state has also been reported in tungara frogs (P. pustulosus) where females are initially selective, but as they approach the end of their reproductive cycle become more permissive and less choosy as they are constrained by a dwindling window of time in which they must oviposit their eggs (Lynch et al., 2005; Baugh and Ryan, 2009).
In our study, it is possible that during the first trial P. corroboree females were not in the optimal physiological state to mate (despite visually appearing gravid) and were thus receptive to male calls yet behaved relatively indiscriminately (based on overall population-level preference). As the females used in this study had never been previously exposed to male acoustic signals, it is possible that auditory stimulation from male calls during the first trial triggered a cascade of hormonal changes that altered receptivity (Wilczynski and Lynch, 2011). Indeed, there is strong evidence that sexual arousal in female amphibians is heavily reliant on hearing male acoustic signals, and that male calls alter female reproductive state by modulating changes in estrogen levels, which eventually triggers ovulation (Wilczynski and Lynch, 2011). Thus, after the first trial, hormonally primed P. corroboree females may have then become more discriminatory (as their reproductive state changed), resulting in a significant population-level preference for low call frequency by the last trial. Overall, there is considerable potential for female P. corroboree to vary in their choosiness within a single reproductive cycle. Pseudophryne corroboree have a breeding season that extends over multiple weeks (Osborne, 1991), so females have the time and opportunity to invest in mate choice (Wells, 2007). In nature, females are likely to visit the nests of multiple males prior to mating, as has been observed in other Pseudophryne species (Byrne and Keogh, 2007). This is also corroborated by observations of P. corroboree in captivity, where females can spend days to weeks assessing several males before selecting a mate (S. Kelleher, unpublished data). Although the exact time frame from ovulation to oviposition is currently unknown, it is likely that female P. corroboree do not ovulate until after they have entered a breeding site and are engaged in amplexus with a chosen male, as observed in three closely related sister species with similar reproductive ecologies, Pseudophryne bibronii, Pseudophryne dendyi and Pseudophryne semimarmorata (Woodruff, 1976). Additionally, there is some evidence for sequential polyandry in P. corroboree (Pengilley, 1973; McFadden et al., 2013), so females may be able to release a partial clutch of eggs, whilst retaining the remaining eggs for subsequent matings, as has previously been reported in other Pseudophryne species (Woodruff, 1976; Byrne and Keogh, 2009). Therefore, female P. corroboree may not be subjected to the same time constraints as reported in other anuran species that decrease their choosiness during ovulation (such as explosive or seasonal breeders that typically ovulate before entering a breeding site and are then committed to oviposition within hours or days to avoid a loss of egg fertilization capacity) (Lea et al., 2000; Lynch et al., 2006; Baugh and Ryan, 2009). Future studies should endeavor to test these ideas experimentally. This could be achieved by conducting manipulative experiments where female reproductive state is controlled by administering hormones and female phonotactic responses are measured (for example see Lynch et al., 2006).
An alternative explanation is that female P. corroboree mate preferences may change over sequential trials due to increased experience in evaluating male signals (Wagner et al., 2001; Caro et al., 2010). It is well-established that female mate preferences and degree of choosiness can be highly dependent on a female's prior social experience and past exposure to male signals (also referred to as experience-mediated plasticity) (Fowler-Finn and Rodríguez, 2012a,b). Numerous empirical studies have reported that experience-mediated plasticity can result in acquired, weakened or even reversed mate preferences due to effects on preference thresholds and choosiness (Walling et al., 2008; Fowler-Finn and Rodríguez, 2012a). For example, in field crickets (Teleogryllus oceanicus) naïve females that had never been previously exposed to male calls were receptive, but less discriminate in mate choice compared to females that had prior experience with acoustic signals, which were highly discriminate (Bailey and Zuk, 2008). Similarly, in wolf spiders, inexperienced females showed no directional mate preferences for ornamented males, but females who had previously been exposed to a variety of male phenotypes developed a preference for ornamented males (Hebets and Vink, 2007). Taken together, these studies emphasize that mate preferences can differ dramatically between experienced and inexperienced females, and that prior experience may actually be required before females can develop a preference for particular males (Bailey and Zuk, 2008). As the females used in the present study were virgins, with no prior experience with male acoustic signals, it is highly plausible that preferences changed over repeated trials due to effects associated with experience-mediated plasticity. As experience-mediated plasticity can also result in reversed preferences (Walling et al., 2008), this line of reasoning may also explain why females initially appeared to avoid low call frequencies, but then preferred these calls in the last trial. To determine the effect of previous experience in P. corroboree, future work should consider repeating this experiment with females previously exposed to male signals. Experience-mediated effects may be attributed to increased mate sampling that occurs over sequential trials. Theoretical models predict that female choosiness increases as females' sample and assess a greater number of males, resulting in stronger directional selection on male traits (Muniz and Machado, 2018). For example, if females adopt a “best of N males” sampling tactic, where they assess a number of potential mates (N) and choose between them, sexual selection is predicted to be most intense when females sample above a critical threshold of males, and, when females can only sample a small number of males, preferences are harder to detect (Benton and Evans, 1998; Muniz and Machado, 2018). In P. corroboree, females may have become choosier in subsequent trials because there were more opportunities for mate sampling, resulting in a population-level preference for low frequency male calls in the final trial. It is also important to note that when females sample mates simultaneously, and over various time points (as is generally the case in frog choruses), they may make comparative mate choices based on the relative attractiveness of the available mates to each other, irrespective of their absolute preference (Lea and Ryan, 2015; Zandberg et al., 2020). This comparative evaluation of potential mates may facilitate temporal plasticity in mate preferences, depending on the availability and relative attractiveness of the potential partners that are sampled (Lea and Ryan, 2015; Neelon and Höbel, 2017; Zandberg et al., 2020). Consideration of relative mate preferences may be of particular importance to CBPs as the specific males available to be sampled in a captive setting could influence female mate choice decisions (Neelon and Höbel, 2017). Additionally, as for many mate preference studies based on auditory cues alone, females were unable to find a male and subsequently mate (i.e., they were not rewarded by assessing and finding a male). Thus, it is possible that patterns of mate preference may differ when females can physically interact with potential mates, and decide to mate or not.
We found no significant repeatability (consistent, individual differences) in female mate preferences, based on any of the measures used. Females were generally unanimous in their mate preferences by the last trial (low among-individual variation) and females were inconsistent in their preferences across repeated trials (high within-individual variation), resulting in low repeatability estimates. Despite providing no evidence for repeatability, this finding is particularly intriguing as it draws attention to the potential for individual P. corroboree females to be receptive to a broader range of male phenotypes, and vary substantially in their mate preferences within a relatively short time period. In line with this finding, a growing number of studies in frogs (Lea et al., 2000; Lynch et al., 2005, 2006), birds (Qvarnström et al., 2000), fish (Tinghitella et al., 2013; Wacker et al., 2016) and invertebrates (Filice and Long, 2017; Kelly, 2018) have demonstrated that there can be substantial within-individual variation in female mate choice, attributed to phenotypic plasticity (Ah-King and Gowaty, 2016; Rosenthal, 2017). This growing body of evidence suggests that mate choice plasticity might be highly adaptive, as inflexible mate choice behavior has the potential to be costly in dynamic environments where the quantity and quality of potential mates can vary markedly (Qvarnström et al., 2000). In amphibians in particular, breeding is inextricably linked to climatic conditions which are often highly variable (Wells, 2007), so females may need to exhibit reversible plasticity in their mating decisions to ensure mating success under various conditions, such as when preferred mates are scarce (Fowler-Finn and Rodríguez, 2012b; Tinghitella et al., 2013). Subsequently, we expect that further empirical research investigating individual variation in mate preferences in amphibians will reveal that plasticity in mate preferences and subsequent choice is more widespread than currently realized, which contradicts the widely held view that female mate choice behavior in amphibians is often stereotyped and uniform (Baugh and Ryan, 2009).
Overall, the information gained from this study provides preliminary insights into the reproductive behavior of one of Australia's most critically endangered vertebrate species. Perhaps most importantly, our study shows that female P. corroboree exhibit strong behavioral responses to synthesized acoustic stimuli, and are highly receptive to the calls presented to them (even without the presence of a live male). This strong phonotactic behavior indicates that there is good potential to manipulate female mate choice in captivity to achieve reproductive outcomes that benefit conservation breeding (Fisher et al., 2003). To date, this type of work has focused on mammals, such as captive harvest mice, pygmy loris' and striped face dunnarts. In these species, male olfactory cues (scent markings) have been used to manipulate female preferences and determine optimal pairings in captivity (Fisher et al., 2003; Roberts and Gosling, 2004; Parrott et al., 2019). Whether similar approaches can be taken with anurans is an exciting avenue for future research. Interestingly, our results suggest that female P. corroboree exhibit a population-level preference for low frequency male advertisement calls after repeated exposure. This finding indicates that male call frequency may play a role in female mate choice decisions in P. corroboree and provides conservation managers with the first information on female mate preferences (and predictors of male attractiveness) in P. corroboree. However, our results simultaneously highlight the possibility for there to be within-individual variation in female preferences throughout a single breeding season. These findings emphasize that a single snap shot measurement of mate preference might not provide conservation managers with accurate information to be able to predict and manipulate mate preferences in captive populations. Conservation managers may need to consider the potential for mate preferences to exhibit plasticity in order to successfully incorporate mate choice into CBP management. For instance, if individual preferences vary throughout the breeding season, it might be useful to rotate the males presented to females at different critical time points in a breeding cycle. For example, if females are less choosy at the start of their reproductive cycle, they may be more permissive in their mate choices. As choosiness increases, females may need to be presented with a different set of potential mates. Similarly, if mate preferences are altered by experience-mediated plasticity, younger, inexperienced females may initially be less selective and more willing to mate with less attractive but genetically valuable males, compared to older, experienced females. If this is the case, there may be value in keeping a proportion of captive females unexposed to male signals, as this could allow conservation managers to conduct breeding manipulations that may increase the reproductive success of genetically valuable individuals, and subsequently, increase the genetic variation (and possible adaptive potential) of captive populations (Asa et al., 2011). Overall, further work is needed in P. corroboree to build on these preliminary insights and more comprehensively understand individual variation in mate choice before incorporating this knowledge into captive management. Nevertheless, our findings provide a platform for continued research into patterns and mechanisms of mate choice, which will advance our knowledge of P. corroboree reproductive ecology and inform management practices.
More broadly, our findings add to the growing body of evidence in amphibians and other vertebrates that individual female mate preferences can vary within relatively short time scales, such as a single reproductive cycle. Our study advances our understanding of female reproductive behavior by emphasizing the importance of considering individual variation when investigating mate preferences. Future mate choice studies should endeavor to test individuals repeatedly, as population-level patterns may mask important sources of variation (Dougherty, 2020). Such individual variation is not only important for advancing our understanding of the proximate and ultimate control of female mate choice behavior, but is also likely to have major implications for the application of mate choice to conservation breeding programs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
This study was reviewed and approved by the University of Wollongong Animal Ethics Committee (Protocol Number AE17/14).
Author Contributions
SK, PB, and AS conceived the study. SK and PB ran the experiments. SK analyzed all behavioral data and wrote the manuscript with input from all authors. ND advised on the statistical analyses. AH conducted the statistical analyses. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the Australian Research Council (Linkage Grant LP170100351) awarded to PB and AS and the University of Wollongong SMAH Small Project Grant (262 27 0976) awarded to PB and AS. This study was also supported by the Holsworth Research Endowment – Equity Trustees Charitable Foundation and the Ecological Society of Australia, and a Frog and Tadpole Study Group of New South Wales student grant awarded to SK. This work was conducted while SK was in receipt of an Australian Government Research Training Program (RTP) Scholarship. AH was supported by the German Science Foundation (DFG, HE 8857/1-1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Emma McInerney for assistance with data collection during the present study. Thanks also goes to Brittany Mitchell for assistance with animal husbandry. We thank Melbourne Zoo for providing the southern corroboree eggs used in this present study. We acknowledge Michael McFadden from Taronga Zoo's Herpetofauna Division, as well as Deon Gilbert from Zoos Victoria, for offering advice on the husbandry requirements for captive southern corroboree frogs. We also acknowledge the support of NSW Department of Planning, Industry and Environment (DPIE) Threatened Species Officer Dr David Hunter, who facilitates the integration of ex situ and in situ conservation efforts for this species.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.748104/full#supplementary-material
References
Ah-King, M., and Gowaty, P. A. (2016). A conceptual review of mate choice: stochastic demography, within-sex phenotypic plasticity, and individual flexibility. Ecol. Evol. 6, 4607–4642. doi: 10.1002/ece3.2197
Aich, U., Bonnet, T., Fox, R. J., and Jennions, M. D. (2020). An experimental test to separate the effects of male age and mating history on female mate choice. Behav. Ecol. 31, 1353–1360. doi: 10.1093/beheco/araa092
Asa, C. S., Traylor-Holzer, K., and Lacy, R. C. (2011). Can conservation-breeding programmes be improved by incorporating mate choice? Int Zoo Yearbook 45, 203–212. doi: 10.1111/j.1748-1090.2010.00123.x
Atwell, A., and Wagner, W. E. (2014). Female mate choice plasticity is affected by the interaction between male density and female age in a field cricket. Anim. Behav. 98, 177–183. doi: 10.1016/j.anbehav.2014.10.007
Atwell, A., and Wagner, W. E. (2015). Along came a spider who sat down beside her: perceived predation risk, but not female age, affects female mate choosiness. Behav. Process. 115, 143–148. doi: 10.1016/j.beproc.2015.04.002
Bailey, N. W., and Zuk, M. (2008). Acoustic experience shapes female mate choice in field crickets. Proc. R. Soc. B. Biol. Sci. 275, 2645–2650. doi: 10.1098/rspb.2008.0859
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J Statist Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Baugh, A. T., and Ryan, M. J. (2009). Female túngara frogs vary in commitment to mate choice. Behav. Ecol. 20, 1153–1159. doi: 10.1093/beheco/arp120
Bee, M. A. (2008). Finding a mate at a cocktail party: spatial release from masking improves acoustic mate recognition in grey treefrogs. Anim. Behav. 75, 1781–1791. doi: 10.1016/j.anbehav.2007.10.032
Begall, S., Malkemper, E. P., Cerveny, J., Nemec, P., and Burda, H. (2013). Magnetic alignment in mammals and other animals. Mamm. Biol. 78, 10–20. doi: 10.1016/j.mambio.2012.05.005
Bell, A. M., Hankison, S. J., and Laskowski, K. L. (2009). The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. doi: 10.1016/j.anbehav.2008.12.022
Benton, T. G., and Evans, M. R. (1998). Measuring mate choice using correlation: the effect of female sampling behaviour. Behav. Ecol. Sociobiol. 44, 91–98. doi: 10.1007/s002650050520
Blumstein, D. T., Evans, C. S., and Daniel, J. C. (2000). JWatcher (Version 1.0). Available online at: http://www.jwatcher.ucla.edu/
Brooks, R., and Endler, J. A. (2001). Female guppies agree to differ: phenotypic and genetic variation in mate-choice behavior and the consequences for sexual selection. Evolution 55, 1644–1655. doi: 10.1111/j.0014-3820.2001.tb00684.x
Byrne, P. G., and Keogh, J. S. (2007). Terrestrial toadlets use chemosignals to recognize conspecifics, locate mates and strategically adjust calling behaviour. Anim. Behav. 74, 1155–1162. doi: 10.1016/j.anbehav.2006.10.033
Byrne, P. G., and Keogh, J. S. (2009). Extreme sequential polyandry insures against nest failure in a frog. Proc. R. Soc. B. Biol. Sci. 276, 115–120. doi: 10.1098/rspb.2008.0794
Cally, J. G., Stuart-Fox, D., and Holman, L. (2019). Meta-analytic evidence that sexual selection improves population fitness. Nat. Commun. 10:2017. doi: 10.1038/s41467-019-10074-7
Cardoso, G. C., and Atwell, J. W. (2011). On the relation between loudness and the increased song frequency of urban birds. Anim. Behav. 82, 831–836. doi: 10.1016/j.anbehav.2011.07.018
Caro, S. P., Sewall, K. B., Salvante, K. G., and Sockman, K. W. (2010). Female Lincoln's sparrows modulate their behavior in response to variation in male song quality. Behav. Ecol. 21, 562–569. doi: 10.1093/beheco/arq022
Chargé, R., Teplitsky, C., Sorci, G., and Low, M. (2014). Can sexual selection theory inform genetic management of captive populations? A review. Evol. Appl. 7, 1120–1133. doi: 10.1111/eva.12229
Conway, W. G. (2011). Buying time for wild animals with zoos. Zoo Biol. 30, 1–8. doi: 10.1002/zoo.20352
Cummings, M., and Mollaghan, D. (2006). Repeatability and consistency of female preference behaviours in a northern swordtail, Xiphophorus nigrensis. Anim. Behav. 72, 217–224. doi: 10.1016/j.anbehav.2006.01.009
Dingemanse, N. J., and Dochtermann, N. A. (2013). Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. doi: 10.1111/1365-2656.12013
Dougherty, L. R. (2020). Designing mate choice experiments. Biol. Rev. 95, 759–781. doi: 10.1111/brv.12586
Dreher, C. E., and Prohl, H. (2014). Multiple sexual signals: calls over colors for mate attraction in an aposematic, color-diverse poison frog. Front. Ecol. Evol. 2:22. doi: 10.3389/fevo.2014.00022
Filice, D. C. S., and Long, T. A. F. (2017). Phenotypic plasticity in female mate choice behavior is mediated by an interaction of direct and indirect genetic effects in Drosophila melanogaster. Ecol. Evol. 7, 3542–3551. doi: 10.1002/ece3.2954
Fisher, H. S., Swaisgood, R. R., and Fitch-Snyder, H. (2003). Odor familiarity and female preferences for males in a threatened primate, the pygmy loris Nycticebus pygmaeus: applications for genetic management of small populations. Naturwissenschaften 90, 509–512. doi: 10.1007/s00114-003-0465-9
Forsgren, E. (1997). Mate sampling in a population of sand gobies. Anim. Behav. 53, 267–276. doi: 10.1006/anbe.1996.0374
Forstmeier, W., and Birkhead, T. R. (2004). Repeatability of mate choice in the zebra finch: consistency within and between females. Anim. Behav. 68, 1017–1028. doi: 10.1016/j.anbehav.2004.02.007
Fowler-Finn, K. D., and Rodríguez, R. L. (2012a). The evolution of experience-mediated plasticity in mate preferences. J. Evol. Biol. 25, 1855–1863. doi: 10.1111/j.1420-9101.2012.02573.x
Fowler-Finn, K. D., and Rodríguez, R. L. (2012b). Experience-mediated plasticity in mate preferences: mating assurance in a variable environment. Evolution 66, 459–468. doi: 10.1111/j.1558-5646.2011.01446.x
Fowler-Finn, K. D., and Rodríguez, R. L. (2013). Repeatability of mate preference functions in Enchenopa treehoppers (Hemiptera: Membracidae). Anim. Behav. 85, 493–499. doi: 10.1016/j.anbehav.2012.12.015
Gabor, C. R., and Halliday, T. R. (1997). Sequential mate choice by multiply mating smooth newts: females become more choosy. Behav. Ecol. 8, 162–166. doi: 10.1093/beheco/8.2.162
Gerhardt, H. C., and Huber, F. (2002). Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago, IL: The University of Chicago Press.
Gerhardt, H. C., Tanner, S. D., Corrigan, C. M., and Walton, H. C. (2000). Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav. Ecol. 11, 663–669. doi: 10.1093/beheco/11.6.663
Haddad, C. F. B., and Prado, C. P. A. (2005). Reproductive modes in frogs and their unexpected diversity in the Atlantic Forest of Brazil. BioScience 55, 207–217. doi: 10.1641/0006-3568(2005)055[0207:RMIFAT]2.0.CO;2
Hartnett, C. M., Parrott, M. L., Mulder, R. A., Coulson, G., and Magrath, M. J. L. (2018). Opportunity for female mate choice improves reproductive outcomes in the conservation breeding program of the eastern barred bandicoot (Perameles gunnii). Appl. Anim. Behav. Sci. 199, 67–74. doi: 10.1016/j.applanim.2017.10.008
Hebets, E. A., and Vink, C. J. (2007). Experience leads to preference: experienced females prefer brush-legged males in a population of syntopic wolf spiders. Behav. Ecol. 18, 1010–1020. doi: 10.1093/beheco/arm070
Holveck, M. J., Geberzahn, N., and Riebel, K. (2011). An experimental test of condition-dependent male and female mate choice in zebra finches. PLoS ONE. 6:e23974. doi: 10.1371/journal.pone.0023974
Howard, R. D., and Young, J. R. (1998). Individual variation in male vocal traits and female mating preferences in Bufo americanus. Anim. Behav. 55, 1165–1179. doi: 10.1006/anbe.1997.0683
Hunter, D. (2000). The Conservation and Demography of the Southern Corroboree Frog (Pseudophryne corroboree). Canberra: University of Canberra.
Hunter, D. A., Speare, R., Marantelli, G., Mendez, D., Pietsch, R., and Osborne, W. (2010). Presence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in threatened corroboree frog populations in the Australian alps. Dis. Aquat. Org. 92, 209–216. doi: 10.3354/dao02118
IUCN (2020). Table 1a: Number of Species Evaluated in Relation to Overall Number of Described Species, and Numbers of Threatened Species by Major Groups of Organisms. IUCNRed List Version 2020–2022. Available online at: http://www.iucnredlist.org
Jennions, M. D., Backwell, P. R. Y., and Passmore, N. I. (1995). Repeatability of mate choice: the effect of size in the African painted reed frog, Hyperolius marmoratus. Anim. Behav. 49, 181–186. doi: 10.1016/0003-3472(95)80165-0
Jennions, M. D., and Petrie, M. (1997). Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327. doi: 10.1017/S0006323196005014
Judge, K. A., Ting, J. J., and Gwynne, D. T. (2014). Condition dependence of female choosiness in a field cricket. J. Evol. Biol. 27, 2529–2540. doi: 10.1111/jeb.12509
Kelleher, S. R., Silla, A. J., and Byrne, P. G. (2018). Animal personality and behavioral syndromes in amphibians: a review of the evidence, experimental approaches, and implications for conservation. Behav. Ecol. Sociobiol. 72:79. doi: 10.1007/s00265-018-2493-7
Kelly, C. D. (2018). The causes and evolutionary consequences of variation in female mate choice in insects: the effects of individual state, genotypes and environments. Curr. Insect Sci. 27, 1–8. doi: 10.1016/j.cois.2018.01.010
Kime, N. M., Rand, A. S., Kapfer, M., and Ryan, M. J. (1998). Consistency of female choice in the Tungara frog: a permissive preference for complex characters. Anim. Behav. 55, 641–649. doi: 10.1006/anbe.1997.0752
Kokko, H., Brooks, R., Jennions, M. D., and Morley, J. (2003). The evolution of mate choice and mating biases. Proc. R. Soc. B. Biol. Sci. 270, 653–664. doi: 10.1098/rspb.2002.2235
Kuijper, B., Pen, I., and Weissing, F. J. (2012). A guide to sexual selection theory. Annu. Rev. Ecol. Evol. 43, 287–311. doi: 10.1146/annurev-ecolsys-110411-160245
Laird, K. L., Clements, P., Hunter, K. L., and Taylor, R. C. (2016). Multimodal signaling improves mating success in the green tree frog (Hyla cinerea), but may not help small males. Behav. Ecol. Sociobiol. 70, 1517–1525. doi: 10.1007/s00265-016-2160-9
Lea, A. M., and Ryan, M. J. (2015). Irrationality in mate choice revealed by tungara frogs. Science 349, 964–966. doi: 10.1126/science.aab2012
Lea, J., Halliday, T., and Dyson, M. (2000). Reproductive stage and history affect the phonotactic preferences of female midwife toads, Alytes muletensis. Anim. Behav. 60, 423–427. doi: 10.1006/anbe.2000.1482
Lohr, B., Wright, T. F., and Dooling, R. J. (2003). Detection and discrimination of natural calls in masking noise by birds: estimating the active space of a signal. Anim. Behav. 65, 763–777. doi: 10.1006/anbe.2003.2093
Lynch, K. S., Crews, D., Ryan, M. J., and Wilczynski, W. (2006). Hormonal state influences aspects of female mate choice in the Túngara Frog (Physalaemus pustulosus). Horm. Behav. 49, 450–457. doi: 10.1016/j.yhbeh.2005.10.001
Lynch, K. S., Rand, A. S., Ryan, M. J., and Wilczynski, W. (2005). Plasticity in female mate choice associated with changing reproductive states. Anim. Behav. 69, 689–699. doi: 10.1016/j.anbehav.2004.05.016
Martin, J. G. A., Nussey, D. H., Wilson, A. J., and Reale, D. (2010). Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol. Evol. 2, 362–374. doi: 10.1111/j.2041-210X.2010.00084.x
Martin-Wintle, M. S., Shepherdson, D., Zhang, G., Zhang, H., Li, D., Zhou, X., et al. (2015). Free mate choice enhances conservation breeding in the endangered giant panda. Nat. Commun 6:10125. doi: 10.1038/ncomms10125
Martin-Wintle, M. S., Wintle, N. J. P., Díez-León, M., Swaisgood, R. R., and Asa, C. S. (2019). Improving the sustainability of ex situ populations with mate choice. Zoo Biology. 38, 119–132. doi: 10.1002/zoo.21450
McFadden, M., Hobbs, R., Marantelli, G., Harlow, P., Banks, C., and Hunter, D. (2013). Captive management and breeding of the critically endangered southern corroboree frog (Pseudophryne corroboree) (Moore 1953) at Taronga and Melbourne Zoos. Amphibian Reptile Conserv. 5, 70–87.
Muniz, D. G., and Machado, G. (2018). Mate sampling influences the intensity of sexual selection and the evolution of costly sexual ornaments. J. Theor. Biol. 447, 74–83. doi: 10.1016/j.jtbi.2018.03.026
Neelon, D. P., and Höbel, G. (2017). Social plasticity in choosiness in green tree frogs, Hyla cinerea. Behav. Ecol. 28, 1540–1546. doi: 10.1093/beheco/arx103
Neelon, D. P., Rodríguez, R. L., and Höbel, G. (2019). On the architecture of mate choice decisions: preference functions and choosiness are distinct traits. Proc. R. Soc. B. Biol. Sci. 286:20182830. doi: 10.1098/rspb.2018.2830
Neff, B. D., and Pitcher, T. E. (2005). Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38. doi: 10.1111/j.1365-294X.2004.02395.x
OEH NSW (2012). National Recovery Plan for the Southern Corroboree Frog, Pseudophryne corroboree, and the Northern Corroboree Frog, Pseudophryne pengilleyi. Hurstville: Office of Environment and Heritage (NSW).
Oliva, M. V., Kaiser, K., Robertson, J. M., and Gray, D. A. (2018). Call recognition and female choice in a treefrog with a multicomponent call. Ethology. 124, 331–337. doi: 10.1111/eth.12736
Osborne, W.S. (1991). The biology and Management of the Corroboree Frog (Pseudophyrne corroboree) in NSW. Species Management Report 8. Hurstville, NSW: NSW National Parks and Wildlife Service.
Parris, K. M., Velik-Lord, M., and North, J. M. A. (2009). Frogs call at a higher pitch in traffic noise. Ecol. Soc. 14:25. doi: 10.5751/ES-02687-140125
Parrott, M. L., Nation, A., and Selwood, L. (2019). Female mate choice significantly increases captive breeding success, and scents can be frozen to determine choice, in the stripe-faced dunnart. Appl. Anim. Behav. Sci. 214, 95–101. doi: 10.1016/j.applanim.2019.03.006
Pengilley, R. K. (1971). Calling and associated behaviour of some species of Pseudophryne (Anura: Leptodactylidae). J. Zool. 163, 73–92. doi: 10.1111/j.1469-7998.1971.tb04525.x
Pengilley, R. K. (1973). Breeding biology of some Species of Pseudophryne (Anura: Leptodactylidae) of the Southern Highlands, New South Wales. Aust. Zool. 18, 15–30.
Petrie, M. (1994). Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371, 598–599. doi: 10.1038/371598a0
Pritchard, D. J., Fa, J. E., Oldfield, S., and Harrop, S. R. (2012). Bring the captive closer to the wild: redefining the role of ex situ conservation. Oryx 46, 18–23. doi: 10.1017/S0030605310001766
Qvarnström, A., Pärt, T., and Sheldon, B. C. (2000). Adaptive plasticity in mate preference linked to differences in reproductive effort. Nature 405, 344–347. doi: 10.1038/35012605
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rausch, A. M., Sztatecsny, M., Jehle, R., Ringler, E., and Hödl, W. (2014). Male body size and parental relatedness but not nuptial colouration influence paternity success during scramble competition in Rana arvalis. Behaviour 151, 1869–1884. doi: 10.1163/1568539X-00003220
Richardson, C., and Lengagne, T. (2010). Multiple signals and male spacing affect female preference at cocktail parties in treefrogs. Proc. R. Soc. B. Biol. Sci. 277, 1247–1252. doi: 10.1098/rspb.2009.1836
Roberts, S. C., and Gosling, L. M. (2004). Manipulation of olfactory signaling and mate choice for conservation breeding: a case study of harvest mice. Conserv. Biol. 18, 548–556. doi: 10.1111/j.1523-1739.2004.00514.x
Robertson, J. G. M. (1990). Female choice increases fertilization success in the Australian frog, Uperoleia laevigata. Anim. Behav. 39, 639–645. doi: 10.1016/S0003-3472(05)80374-4
Rosenthal, G. G. (2017). Mate Choice: The Evolution of Sexual Decision Making from Microbes to Humans. Princeton: Princeton University Press. doi: 10.2307/j.ctt1vwmhb0
Smith, M. J., and Roberts, J. D. (2003). An experimental examination of female preference patterns for components of the male advertisement call in the quacking frog, Crinia georgiana. Behav. Ecol. Sociobiol. 55, 144–150. doi: 10.1007/s00265-003-0691-3
Soorae, P. S. (Ed.). (2016). Global Re-Introduction Perspectives: 2016. Case-Studies From Around the Globe. Gland: IUCN/SSC Re-introduction Specialist Group and Abu Dhabi, UAE: Environment Agency-Abu Dhabi.
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Tinghitella, R. M., Weigel, E. G., Head, M., and Boughman, J. W. (2013). Flexible mate choice when mates are rare and time is short. Ecol. Evol. 3, 2820–2831. doi: 10.1002/ece3.666
Uetz, G. W., and Norton, S. (2007). Preference for male traits in female wolf spiders varies with the choice of available males, female age and reproductive state. Behav. Ecol. Sociobiol. 61, 631–641. doi: 10.1007/s00265-006-0293-y
Vargas-Salinas, F., Quintero-Ángel, A., Osorio-Domínguez, D., Rojas-Morales, J. A., Escobar-Lasso, S., Gutiérrez-Cárdenas, P. D. A., et al. (2014). Breeding and parental behaviour in the glass frog Centrolene savagei (Anura: Centrolenidae). J. Nat. Hist. 48, 1689–1705. doi: 10.1080/00222933.2013.840942
Vega-Trejo, R., and Backwell, P. R. Y. (2017). Testing female preferences under more natural conditions: a case study on a fiddler crab. Behav. Ecol. Sociobiol. 71:81. doi: 10.1007/s00265-017-2314-4
Wacker, S., Östlund-Nilsson, S., Forsgren, E., Newport, C., and Amundsen, T. (2016). Mate choice plasticity in a coral reef fish. Behav. Ecol. 27, 1331–1342. doi: 10.1093/beheco/arw050
Wagner, W. E., Smeds, M. R., and Wiegmann, D. D. (2001). Experience affects female responses to male song in the variable field cricket Gryllus lineaticeps (Orthoptera, Gryllidae). Ethology 107, 769–776. doi: 10.1046/j.1439-0310.2001.00700.x
Walling, C. A., Royle, N. J., Lindström, J., and Metcalfe, N. B. (2008). Experience-induced preference for short-sworded males in the green swordtail, Xiphophorus helleri. Anim. Behav. 76, 271–276. doi: 10.1016/j.anbehav.2008.03.008
Walls, S. C., and Gabor, C. R. (2019). Integrating behavior and physiology into strategies for amphibian conservation. Front. Ecol. Evol. 7:234. doi: 10.3389/fevo.2019.00234
Ward, J. L., Love, E. K., Vélez, A., Buerkle, N. P., O'Bryan, L. R., and Bee, M. A. (2013). Multitasking males and multiplicative females: dynamic signalling and receiver preferences in Cope's grey treefrog. Anim. Behav. 86, 231–243. doi: 10.1016/j.anbehav.2013.05.016
Welch, A. M., Semlitsch, R. D., and Gerhardt, H. C. (1998). Call duration as an indicator of genetic quality in male gray tree frogs. Science 280, 1928–1930. doi: 10.1126/science.280.5371.1928
Widemo, F., and Sæther, S. A. (1999). Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends in Ecol. Evol. 14, 26–31. doi: 10.1016/S0169-5347(98)01531-6
Wielebnowski, N. C. (1998). “Contributions of behavioral studies to captive management and breeding of rare and endangered mammals,” in Behavioral Ecology and Conservation Biology, ed T. Caro (New York, NY: Oxford University Press Inc.).
Wilczynski, W., and Lynch, K. S. (2011). Female sexual arousal in amphibians. Horm. Behav. 59, 630–636. doi: 10.1016/j.yhbeh.2010.08.015
Woodruff, D. S. (1976). Courtship, reproductive rates, and mating system in three Australian Pseudophryne (Amphibia, Anura, Leptodactylidae). J. Herpetol. 10, 313–318. doi: 10.2307/1563068
Zandberg, L., Gort, G., van Oers, K., and Hinde, C. A. (2017). Direct fitness benefits explain mate preference, but not choice, for similarity in heterozygosity levels. Ecol. Lett. 20, 1306–1314. doi: 10.1111/ele.12827
Keywords: captive breeding program, conservation, plasticity, repeatability, reproductive behavior
Citation: Kelleher SR, Silla AJ, Hertel AG, Dingemanse NJ and Byrne PG (2021) Mate Preference Plasticity in a Critically Endangered Frog: Implications for Conservation Breeding. Front. Conserv. Sci. 2:748104. doi: 10.3389/fcosc.2021.748104
Received: 27 July 2021; Accepted: 18 October 2021;
Published: 16 November 2021.
Edited by:
Yonggang Nie, Institute of Zoology, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Lysanne Snijders, Wageningen University and Research, NetherlandsBaowei Zhang, Anhui University, China
Copyright © 2021 Kelleher, Silla, Hertel, Dingemanse and Byrne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shannon R. Kelleher, c2hhbm5vbnJhZWtlbGxlaGVyQGdtYWlsLmNvbQ==
 Shannon R. Kelleher
Shannon R. Kelleher Aimee J. Silla1
Aimee J. Silla1 Anne G. Hertel
Anne G. Hertel Phillip G. Byrne
Phillip G. Byrne