- 1Norwegian Orca Survey, Andenes, Norway
- 2Coastal Ocean Research Institute, Ocean Wise, Vancouver Aquarium, Vancouver, BC, Canada
- 3Fisheries and Oceans Canada, Pacific Biological Station, Nanaimo, BC, Canada
- 4Norwegian Polar Institute, Tromsø, Norway
Identifying mortality sources and mitigation solutions is crucial in species management and conservation. In killer whales (Orcinus orca), mortality events may pose a serious concern for the conservation of small discrete populations, especially if they involve entire groups. This study investigated 19 incidents involving 116 killer whales from a minimum of five populations becoming naturally entrapped in inshore areas of the North Pacific (n = 12) and North Atlantic (n = 7) oceans between 1949 and 2019. Here, we aim to provide an assessment of possible causal factors, lethality and human responses to these events. Site characteristics and group size identified three categories of entrapments. In Category 1, nine cases involved small groups of killer whales (median = 5, range: 1–9) at sites characterized by severe geographic and food constraints. Four cases in Category 2 included larger groups (median= 14, range: 6–19) and entrapment sites with no obvious geographic constraints but at which man-made structures could have acted as deterrents. Five cases assigned to Category 3 involved lone, often young individuals settling in a restricted home range and engaging in interactions with people and boats. Overall, all or some of the killer whales swam out on their own after a mean of 36 d of entrapment (range: 1–172, SD = 51, n = 9 cases), died of nutritional/physiological stress after 58 d (range: 42–90, SD = 21, n = 3 cases) or of injury after ~5 years of daily interactions with boat traffic (n = 1 case). Indication of the killer whales' declining condition or being at risk of injury, and of poor habitat quality, led to the decision to intervene in seven cases where a variety of methods were used to guide or relocate remaining individuals back to open waters after 39 d (SD = 51, range = 8–150). Monitoring protocols, which aided in identifying entrapment situations, and intervention methods which enhanced the health and survival of entrapped killer whales, are discussed.
Introduction
In wildlife management and conservation, identifying causes of mortality is important for status assessment and for implementation of mitigation measures. In cetaceans, entanglement in fishing gear, or bycatch, constitutes a significant concern globally as it leads to substantial mortalities and has potential population-level impacts across a wide range of species (e.g., Read, 2008; Reeves et al., 2013). In some cases, human intervention to rescue single individuals has benefitted the survival, reproduction and even recovery of (sometimes critically) small populations (Kraus et al., 2005; Moore et al., 2010; Robbins et al., 2015; McHugh et al., 2021). Motivated by the desire to enhance animal welfare, and perhaps to conserve species in some cases, responding to cetacean strandings has also become common practice worldwide (Moore et al., 2018).
Natural entrapments, defined as situations in which cetaceans appear unable or unwilling to exit semi-enclosed habitats, represent another source of mortality. For example, entrapment in seasonally forming sea ice has been reported as sporadic but recurrent occurrences in polar regions, involving multiple cetacean species and sometimes large groups of animals (Heide-Jørgensen et al., 2002; Westdal et al., 2017). Other entrapments involve cetaceans found outside of their typical habitat (e.g., a Bryde's whale Balaenoptera edeni entrapped for 100 days in a river of New South Wales, Australia, see Priddel and Wheeler, 1997, 1998) or trapped in confined waters due to falling tide and topographical obstacles (e.g., spinner dolphins Stenella longirostris entrapped in small lagoons on Saipan Island upon falling tide, see Trianni and Kessler, 2002). Regardless of causal factors, entrapped cetaceans often suffer nutritional and physiological stress (Priddel and Wheeler, 1998), to which potential collision with boat traffic, inappropriate human behaviors and stranding may add increased risks (Priddel and Wheeler, 1997; Trianni and Kessler, 2002; Mowat, 2005). Intervention to assist trapped cetaceans in finding their way to open waters may be required, especially if the species is of high conservation concern.
Killer whales (Orcinus orca) live in small discrete populations. For example, although total number of killer whales off the west coast of North America exceeds 1,000 animals, they belong to several discrete populations that differ in their demographics, ecology, behavior and genetics (Barrett-Lennard, 2000; COSEWIC, 2008; Parsons et al., 2013; Ford and Ellis, 2014). The Southern Resident killer whale population numbers fewer than 100 individuals and has been listed as “Endangered” in Canada since 2001 (COSEWIC, 2008) and the so-called “AT1” killer whale population in Alaska had only seven individuals remaining in 2013 and no recruitment since 1984 (Matkin et al., 2012; Allen and Angliss, 2015). Off western Europe, killer whales in Gibraltar have been classified as “Critically Endangered” owing to small population size, low recruitment and isolation from other northeastern Atlantic populations (Esteban et al., 2016). Off the west coast of the British Isles, another small, isolated assemblage of 10 killer whales has shown no growth in 19 years and represents a discrete conservation unit (Beck et al., 2014). Mortalities from entrapments could lead to significant declines in any such small killer whale population but a lack of literature on the subject (except for ice-related cases, see Westdal et al., 2017) has impeded our understanding of these events and of potential management options.
Reviewing available information for 19 cases of natural entrapment of killer whales recorded for the period 1949–2019, this paper examines the characteristics of, and mortality induced by, these events. Using a comparative approach, we identified possible causal factors, behavioral cues indicative of killer whales being entrapped, and intervention methods used. This work aims to achieve a better understanding of these incidents so that they can be more reliably identified in the future and allow for informed management responses.
Materials and Methods
We defined a natural entrapment as a situation where one or more killer whales had remained in an unusual, restricted habitat (Figure 1) for a prolonged period (a few days to years), apparently unable or unwilling to leave. Entrapment events were searched for in the scientific literature, news agencies, databases held by the authors and by contacting people involved in monitoring local killer whale populations. We did not include entrapments in ice, which have recently been reviewed by Westdal et al. (2017).
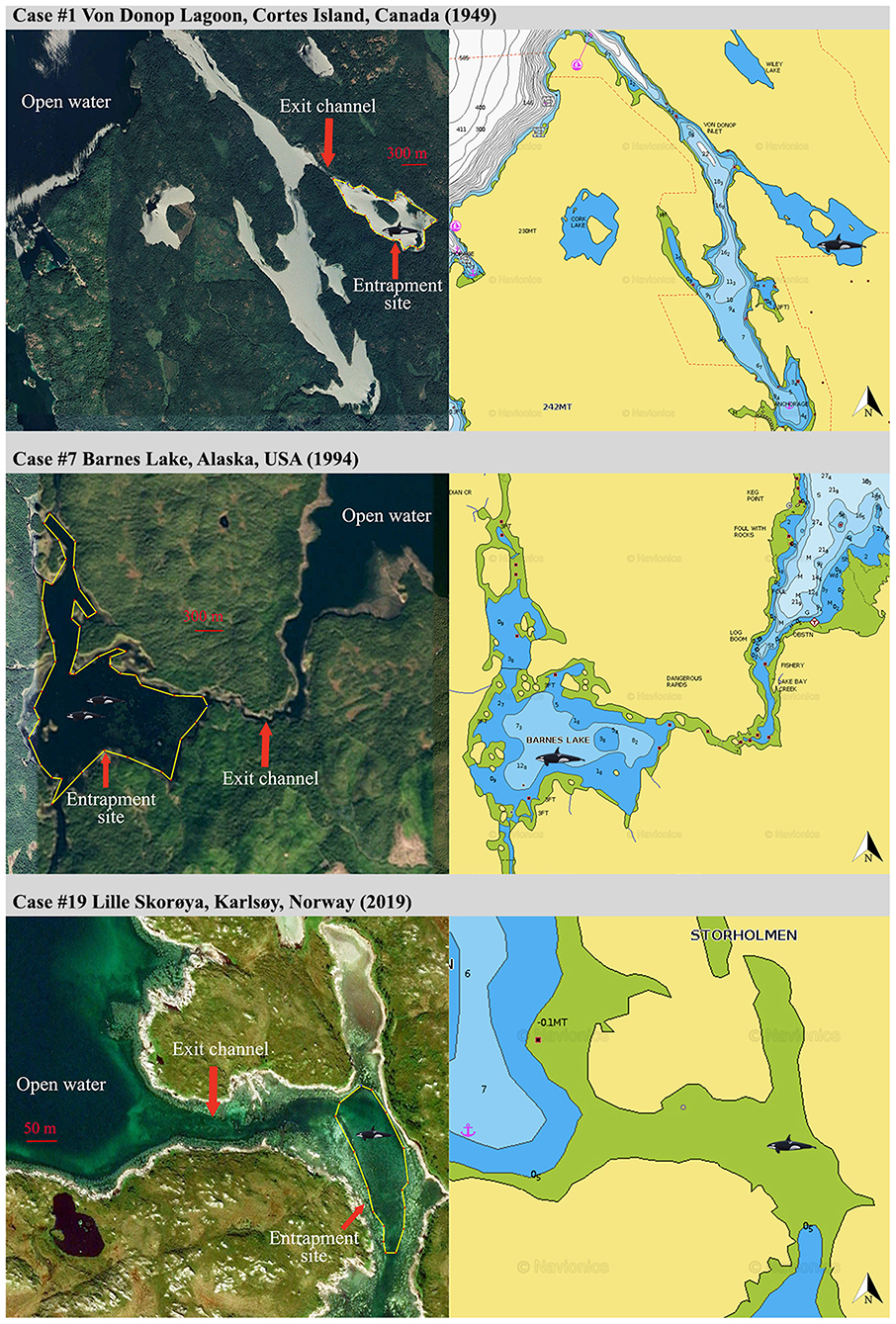
Figure 1. General site configuration (left panels, screengrabs from Google Earth, https://www.google.com/earth/) and corresponding bathymetry (right panels, screengrabs from Navionics, https://www.navionics.com) revealing geographical constraints for a sample of three entrapment cases of Category 1.
The information related to the identified cases of entrapment was compiled in tables. Duration of the entrapments was calculated considering the first sighting day as Day 0 (Table 1). The ecotypes, populations, individual identities (when known), group size and group composition of the killer whales involved were documented (Table 2; Supplementary Table 1). To identify any common factors potentially contributing to entrapment, we compared the environmental characteristics across cases (summarized in Table 3, detailed for each site in Supplementary Table 2; Figure 1). Specifically, we assessed surface area, maximum depths and distance to open water at the entrapment site. We also investigated the presence of geographical constraints by measuring minimum width (i.e., narrowest point), minimum depth (i.e., shallowest point at low tide) and speed of currents in identified constrictions, if any (Table 3; Supplementary Table 2). As a means to standardize measurements, widths at the narrowest points were measured from shore to shore at high waters. However, we expect that only a portion of a channel may be effectively navigable by large body-sized cetaceans like killer whales. Therefore, our measurements should be regarded as underestimating the narrowness of channels. Measurements were done using the measuring tools and the bathymetry and currents data in Navionics (https://www.navionics.com), except for surface areas calculated with the polygon measuring tool in Google Earth Pro (https://www.google.com/earth/). Historical data on water level at low and high tides were retrieved from national agencies and used to calculate the tidal fluctuation throughout the entrapment period. Other features at entrapment sites such as salinity, prey availability and presence of man-made obstacles were also assessed whenever possible.
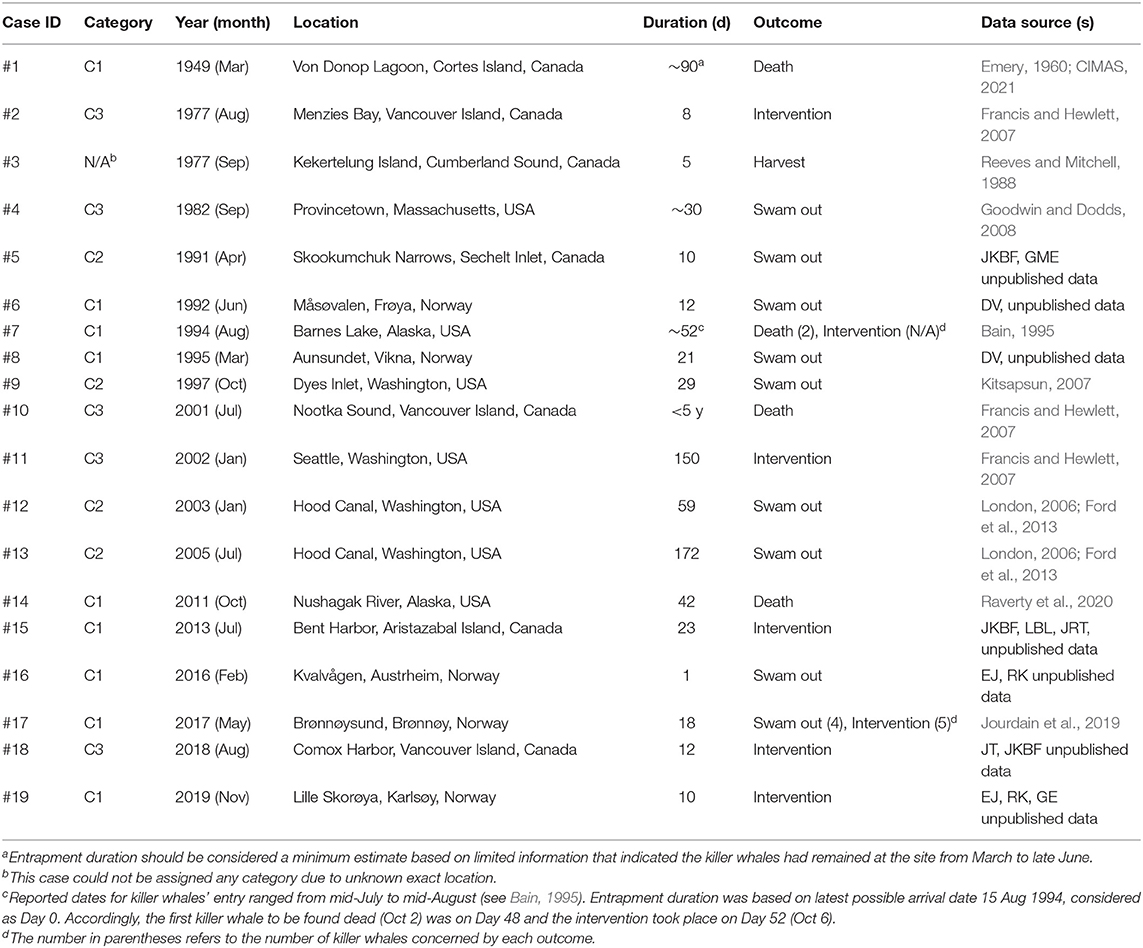
Table 1. Summary of the 19 cases of natural killer whale entrapment 1949–2019, assigned categories (see Methods) and associated sources of information from which the data presented hereafter in the various sections of this study were derived.
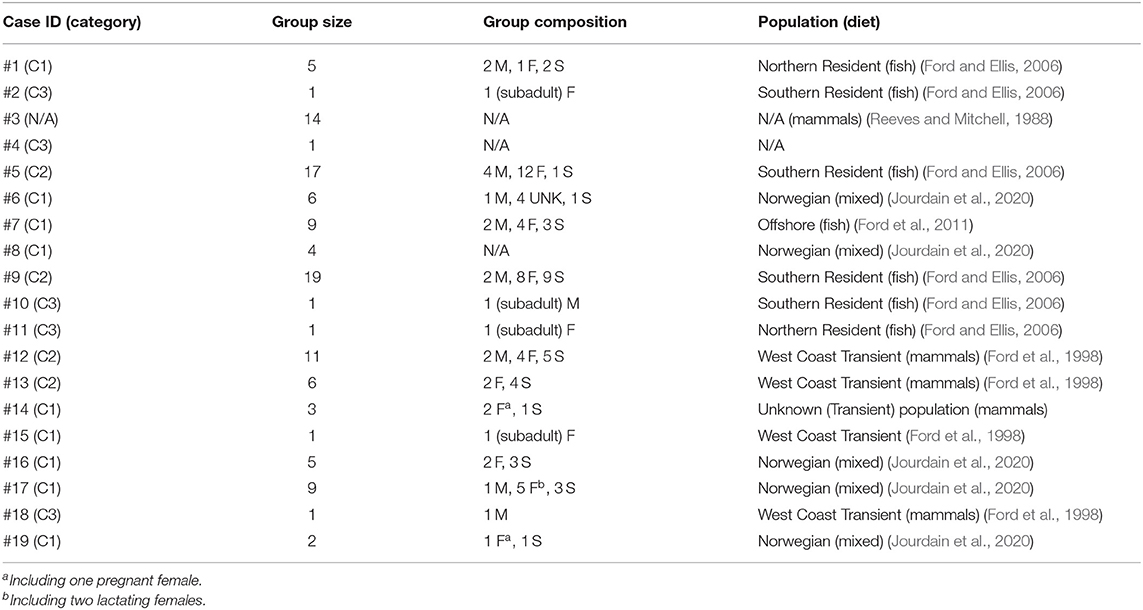
Table 2. Characteristics of the killer whale groups and individuals (M: adult male, F: adult female, S: subadult of unknown sex, UNK: individuals of undetermined sex and age-class) that have become entrapped in the 19 cases recorded in 1949–2019 and included in this study.

Table 3. Characteristics of the sites where killer whales became entrapped in the North Pacific and North Atlantic Oceans in 1949–2019 for cases assigned to Categories 1 and 2.
Group size (and behavior) of the killer whales involved, and environmental features at entrapment sites were then used as criteria to assign each case to one of the three following categories:
Category 1: Lone or small groups of killer whales and entrapment sites characterized by severe geographic and food constraints (Figure 1).
Category 2: Larger groups of killer whales and entrapment sites with no obvious geographic or immediate food constraints.
Category 3: Single individuals that established a limited home range (“box”) despite the lack of geographical constraints and engaged with boats and humans or, at the very least, accepted close contact with people.
Outcomes were assessed for all entrapment events. Management responses, in terms of the process that led to the decision of taking action and the method of intervention (if any) were reviewed (Table 4).
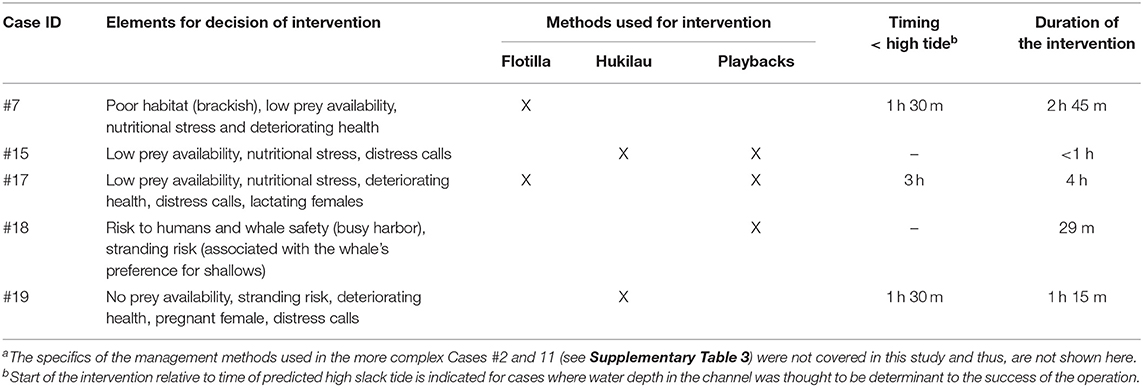
Table 4. Summary of the key elements considered in the process of decision making and methods used to direct the killer whales back toward open waters for fivea of the seven entrapments that led to human intervention.
Results
We compiled 19 events of natural entrapments involving a total of 116 killer whales from a minimum of five populations for the period 1949–2019: 12 in the eastern North Pacific and seven in the North Atlantic (Tables 1, 2; Supplementary Table 1; Figures 1, 2). Entrapments occurred throughout the year, with no obvious global or local seasonal pattern (Table 1).
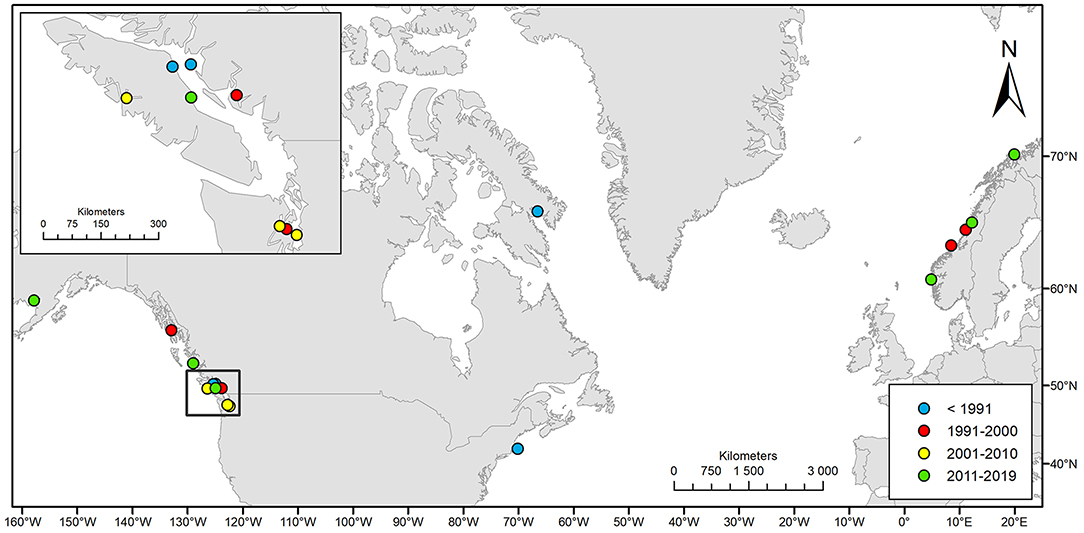
Figure 2. Geographic distribution of the 19 cases of natural killer whale entrapments 1949–2019 included in this study.
Characteristics of Entrapped Killer Whales
Entrapped groups numbered 1–19 killer whales (median = 6) and involved all sex and age classes (Table 2). In the eastern North Pacific, five cases involved the mammal-eating ecotype (Bigg's or Transient) of killer whales (Cases #12–15, 18) while seven involved fish-eating (Resident and Offshore) ecotypes (Cases # 1, 2, 5, 7, 9–11) (Table 2). Ringed seal (Phoca hispida) claws found in the stomach of two of the killer whales entrapped in eastern Canada in Case #3 suggested they were a mammal-eating ecotype (see Reeves and Mitchell, 1988) (Table 2). Killer whales that became entrapped in Norway (Cases #6, 8, 16, 17, 19) were likely fish-eating or having a mixed diet that also included mammals (see Jourdain et al., 2020) (Table 2). The killer whales involved in Cases #16 and #17 were known to feed on marine mammals in addition to fish (EJ unpublished data). The latter group of killer whales became entrapped twice in Norway, 1 year apart (Cases #16, 17; Table 1, see Supplementary Table 1 for individual identifications). In the North Pacific, the male killer whale T73B was also involved in two entrapment cases (Cases #12, 18; Table 1, see Supplementary Table 1 for identifications).
Cases of Natural Entrapments 1949–2019
Category 1
Nine cases (47%) involving lone or small groups of killer whales (median = 5, range: 1–9) fell in this category (Tables 1, 2). Most of these entrapment sites consisted of a small bay (often < 1.5 km2) separated from more open water by a narrow channel or, in Case #14, a river (100 m to 100 km in length), with sections that had maximum depths of ~2 m or less at low tide (Table 3; Supplementary Table 2; Figure 1). Tidal oscillations varied from 1.32 to 6.72 m throughout the entrapment period (Table 3; Supplementary Table 2). In some cases, for which the killer whales' first day at the entrapment site was reliably known, maximum water levels decreased in the days that followed entrapment (Cases #6, 17, 19) (Figure 3). Tidal flow combined with substantial variations in water depths and the presence of rock outcrops and boulders resulted in strong currents and even rapids in channels for some sites (e.g., Cases #1, 7; Table 3; Supplementary Table 2). Limited prey availability and low salinity were identified at eight and three entrapment sites, respectively (Table 3; Supplementary Table 2).
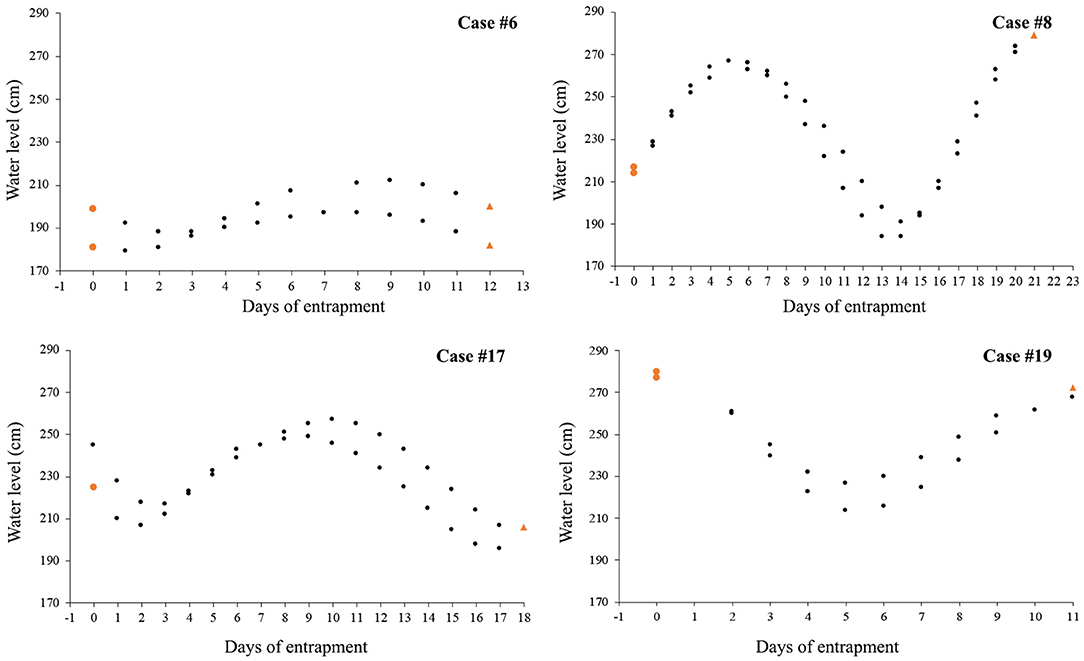
Figure 3. Variation over time of water level (in cm) at high tide after killer whales first appeared at the entrapment site; tidal data are only shown for the four cases in Category 1 for which entrance day could reliably be identified (colored circles and triangles indicate water level on the day of killer whales' entry and departure, respectively).
Category 2
Four cases (21%) involving larger groups of killer whales (median = 14, range: 6–19) were assigned to this category (Tables 1, 2). All of these incidents took place in relatively deep inlets with areas of 15–350 km2 that likely contained adequate food supply, as confirmed for Cases #12 and 13 (Table 3; Supplementary Table 2). Although one inlet had high current flow and tidal rapids of up to 18 kn at its entrance except at slack tide (Case #5), there was >10 m depth at low water throughout the channel (Supplementary Table 2). At the entrances of the other two entrapment inlets, there was a floating bridge across the larger (Cases #12, 13) and two suspended bridges across the smaller (Case #9) (Table 3; Supplementary Table 2).
Category 3
Five cases (26%) were assigned to this category (Table 1; Supplementary Table 3). In four of these, the individual killer whales were juveniles (Table 2; Supplementary Table 3). Known identity and social affiliation for individuals in Cases #10 and #11 combined with extensive sighting history available for their natal groups identified that close relatives (including the mother in Case #11) had died some time before their first sighting as loners (Francis and Hewlett, 2007; Supplementary Table 3). This could have caused the two young whales to be left behind and, due to lack of experience, to settle in locations not normally part of their typical range. Interactions with humans may have been promoted by the need for contact in this highly social species. The young age of individuals involved in Cases #2 and #4 (Table 2; Supplementary Table 3) suggested that similar factors may have played a role in their entrapment. Reasons why the adult male T73B had remained in Comox Harbor for 12 d could not be reliably identified.
Outcomes of Killer Whale Entrapments
In Category 1, killer whales remained entrapped for an average of 30 d (SD = 27, range: 1–90, n = 9), leading to various outcomes (Table 1): in four cases, all or some of the entrapped killer whales swam out on their own after 1–21 d (mean = 11, SD = 8); in three cases one or several killer whales died after 42–90 d (mean = 58, SD = 21); in four cases, human intervention resulted in all remaining live killer whales returning to open waters after 10–52 d (mean = 26, SD = 18).
In Category 2, the animals remained entrapped for an average of 68 d (SD = 72, range: 11–172, n = 4). All survived and swam away with no intervention (Table 1).
The five cases in Category 3 indicated that, without intervention, entrapment situations with lone individuals may last for weeks or even years (Table 1; Supplementary Table 3). In Case #2, Miracle was live captured after 8 d for medical treatment and display in an aquarium. In Case #4, Elsa swam out after a month, though her survival could not be confirmed. In Case #10, Luna died after nearly 5 years remaining in the area of Nootka Sound in close contact with humans and daily boat traffic. In Case #11, and after 5 months of consistent observations of declining condition, Springer was captured for medical treatment before being successfully relocated to her natal group. In Case #18, human intervention successfully enticed male killer whale T73B out of Comox Harbor after 12 d of entrapment (Table 4).
Re-sightings confirmed long-term survival and resumed normal socialization patterns of rescued killer whales, except for NKW-788 and her calf (Case #19) which had not yet been resighted at the time of writing. Post-release calf production was confirmed for females from Cases #7 (Dahlheim et al., 2000), #11 (Towers et al., 2020) and #17 (EJ, unpublished data).
Human Interventions
Basis for Decision of Intervention
For cases in Category 1, the decision to intervene was based largely on low prey availability (Table 3; Supplementary Table 2) and signs of behavioral stress and declining body condition (Table 4; Figure 4). In Case #7, the death of two individuals confirmed that the condition of survivors was likely to continue deteriorating (Bain, 1995). The production of loud, repeated distress calls for several hours at a time was interpreted as further indicator of stress (see Kuczaj et al., 2015) in Cases #15, 17, and 19. The increased metabolic demands of the lactating and pregnant females (Oftedal, 1997) in Cases #17 and 19 (Table 2), respectively, heightened concern for the whales (Table 4). In Category 3, signs of sickness and also deteriorating health led to the decision to capture the entrapped individuals (Cases #2 and 11), for rehabilitation and relocation in Case #11 (Supplementary Table 3). Risks to the safety of humans and the entrapped killer whale contributed to the decision to intervene in Case #18 where the whale was observed physically engaging with fishing gear and the anchor lines of vessels (Table 4; JRT, unpublished data).
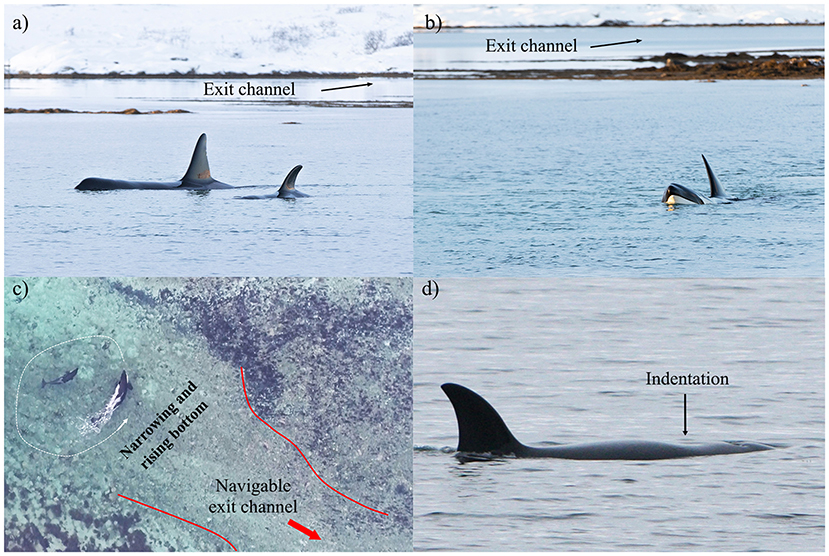
Figure 4. Observations that may be used as cues of killer whales being entrapped: (a) long periods spent motionless at the surface intermittent with (b) stereotyped pacing back and forth in front of the mouth of the exit channel leading to (c) consistent turnarounds due to sudden narrowing and rising bottom (Case #19); (d) indentation behind the blowhole indicative of a decreased blubber thickness and nutritional stress (Case #15).
Intervention Methods
Seven cases (#2, 7, 11, 15, 17, 18, 19) led to human intervention (Tables 1, 4). Interventions in the rarer and more extreme cases involving young killer whales in poor health (Category 3) were very complex. Consequently, the methods of capture (Cases #2, 11), rehabilitation and relocation (Case #11) for these cases were not described in this study, although a chronology of main events can be found in Supplementary Table 3. The other five interventions used a variety of methods and combinations thereof to drive the entrapped killer whales back to open waters (Table 4). Vessels were used as observation platforms and to deploy equipment in all cases, but flotillas of vessels (described in Jourdain et al., 2019) were used to herd and guide the whales out of the entrapment site only in two of these five interventions (Cases #7, 17; Table 4; Figure 5). “Hukilaus,” consisting of a long surface line interspersed at intervals with weighted, vertical lines (modified from Figure 15 in Norris and Dohl, 1980) were successfully used in two other cases (Cases #15, 19; Table 4; Figure 6; Supplementary Video 4). Playbacks of killer whale calls recorded from the same killer whale population were used in three cases, as a single means (Case #18) or in combination with a hukilau (Case #15) and a flotilla of vessels (Case #17) (Table 4). To maximize water depth in the constricted channel and increase the animals' likelihood of successfully leaving the site of entrapment, interventions (Cases #7, 15, 17, 19) were cautiously started before high slack tide when relevant (Table 4). During interventions, recorded whale responses included tighter grouping (Cases #7, 17), boat/hukilau avoidance (Cases #7, 15, 17, 19; Supplementary Video 4), fluke slaps (Case #7), spy-hops (Case #17) and loud vocalizations (Case #19). Killer whales temporarily stopped or attempted to turn around at sections of the channel where there was significant narrowing, faster currents, rising shallows, and kelp beds (Bain, 1995, EJ unpublished data; Supplementary Video 4). For two interventions (Cases #17, 19), the whales were constantly monitored for their behavior state and positions from the flight monitor of an aerial drone, to verify a calm and grouped state, and to adjust the progression of the operation (described in Jourdain et al., 2019).
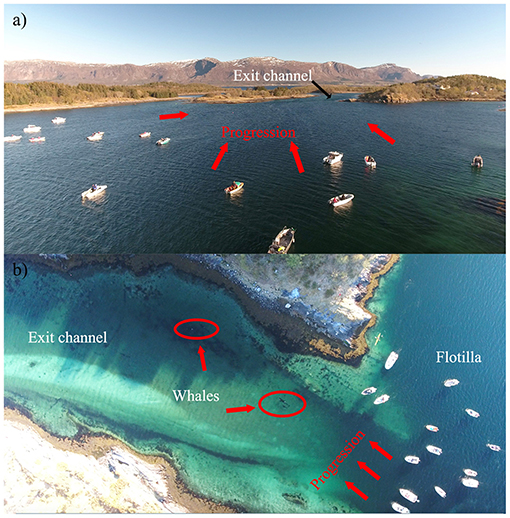
Figure 5. (a) Photograph showing the coordinated line of boats used in Case #17 as a means to guide the entrapped killer whales toward the exit channel; (b) Live aerial imagery (drone), used throughout the intervention in this case (and Case #19), allowed for constant monitoring of behavior (stress, group cohesion) and to carefully adjust the progression of the operation.
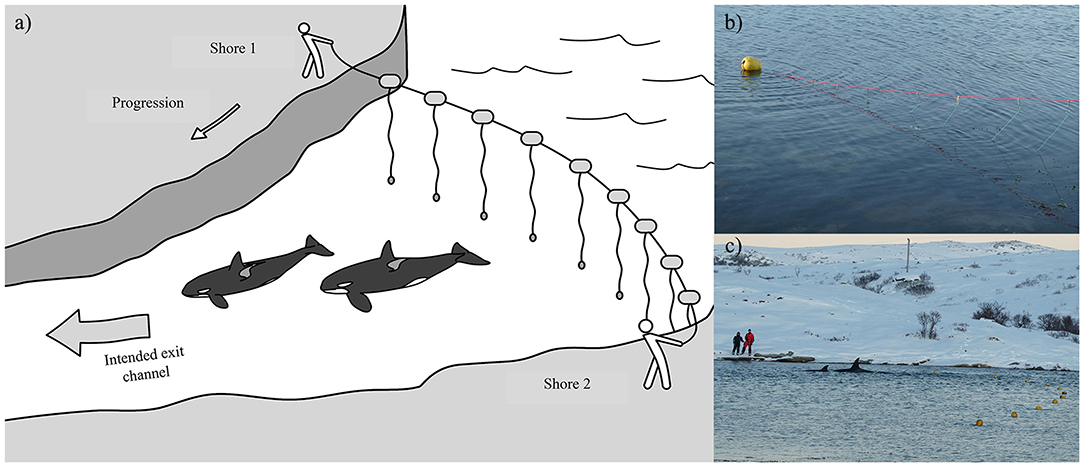
Figure 6. (a) Illustration of how a “hukilau” was used in Cases #15 and 19 as a means to guide the entrapped killer whales toward the exit channel that led to open water (Illustration: Frédérique Lucas); (b) Structure of the hukilau, made of a main line with floats stretched across the bay and attached 2 m-sinking hanging lines every meter (Case #19); (c) Photograph showing the team stationed on the opposite shore moving killer whales from the entrapment site by pulling the hukilau toward the intended exit channel (Case #19) (Photographs: Tor Johansen, Fiskeridirektoratet).
Discussion
By compiling information for 19 natural killer whale entrapments, we provide the first characterization of these events (Table 1; Figures 1, 2). Comparing the configuration of entrapment sites and characteristics of entrapped individuals across cases identifies features that may facilitate entrapment (Table 3; Supplementary Table 2). Our results show that entrapments are rare but recurrent and may happen in any coastal region frequented by killer whales, regardless of their dietary habits or ecotype.
Why Do Killer Whales Become Entrapped?
Prey distribution and foraging behavior may contribute to explaining why killer whales have become entrapped in cases of Categories 1 and 2. Both fish- and mammal-eating killer whales are known to forage in nearshore waters (Ford and Ellis, 2014). Deep nearshore areas associated with high relief topography may enhance foraging efficiency in fish-eating killer whales by providing the opportunity to trap fish-prey against the bottom (Heimlich-Boran, 1988; Nøttestad, 2002; Wright et al., 2017). Nearshore waters, typical pinniped habitat, are also used by mammal-eating killer whales both in Norway (Jourdain et al., 2017) and in western North America (Heimlich-Boran, 1988; Ford and Ellis, 2014; Towers et al., 2019). Our results confirm that both fish- and mammal-eating ecotypes occasionally become entrapped (Table 2).
In killer whales, foraging behavior is culturally transmitted, often leading to different groups adopting different diets, foraging strategies and patterns of occurrence, even in sympatry (Riesch et al., 2012; Ford and Ellis, 2014). Thus, some individuals and groups may be using nearshore waters more frequently than others and may be more likely to become entrapped. This idea is supported by the observation that the same killer whales were found entrapped on two occasions in Norway (Cases #16 and 17) and in the North Pacific (Cases #12 and 18) (Supplementary Table 1). Alternatively, it may be argued that these specific individuals may be more accustomed to extreme shallows and complex shorelines and are thus less likely to become entrapped. In contrast, individuals and groups unaccustomed to navigating complex or confined inner coastal waterways, such as the Offshore killer whales involved in the Barnes Lake incident (Case #7, Table 2), may be more susceptible to entrapment. Regardless, topography and bathymetry appear to be factors that increase the chance of killer whales becoming entrapped, a conclusion supported by the striking similarities documented among many of the cases identified in this study.
In Category 1, entrapment sites consisted of a confined area (commonly < 1.5 km2) separated from open water by an often sinuous, narrow and shallow channel (Table 3; Supplementary Table 2; Figure 1). Although the killer whales may have entered while pursuing prey, it was not possible to identify why they were reluctant to leave using the reverse course. Possibly, a number of geographical features (e.g., topography, bathymetry, sea floor composition) and associated factors (e.g., strong currents, kelp beds) combined to impair the whales' ability to navigate (e.g., distortion of echolocation, see Sundaram et al., 2006). Killer whales showed most reluctance to move through sections characterized by sudden narrowing and/or a rapidly rising bottom during attempts to drive the whales to open waters, which supports this hypothesis (Bain, 1995, see Results and Supplementary Video 4). Such features were also linked to entrapments and strandings in other cetaceans (Priddel and Wheeler, 1997; Trianni and Kessler, 2002; Sundaram et al., 2006). In some cases, kelp beds presented an apparent physical or acoustic block to open waters (e.g., Case #7). Furthermore, the only two observations of killer whales attempting to exit the entrapment site on their own only at slack tide (Case #17, see Jourdain et al., 2019) suggested that currents in the constricted channel may also play a role in impeding the movements of entrapped killer whales. Decreasing water level in the channel overtime owing to tidal fluctuations may have, in some cases, further constrained the whales' willingness or even ability (e.g., Case #19) to leave site (Figure 3; Supplementary Video 4).
In cases of Categories 2 and 3, the factors that promoted entrapment are less clear. Possibly, the presence of structures such as bridges (Supplementary Table 2) which, although not physical barriers, may have acted as psychological barriers in Category 2. For example, although Hood Canal (Cases #12, 13) did not feature extreme narrows or shallows, it was suggested that the killer whales may have entered when the floating bridge across the inlet was opened so vessels could transit but were reluctant to leave when the bridge was closed (Ford et al., 2013). A combination of unfortunate events (e.g., death of a close companion or relative, sickness) may have led to young, inexperienced whales becoming separated from their natal social group and promoted entrapment for cases of Category 3.
Lethality of Killer Whale Entrapments
Site characteristics appeared to render some entrapments more lethal than others, as reflected by the distinction between cases of Category 1 and Category 2. A large body of water (Category 2), which is more likely to contain a prey supply (e.g., Cases #12, 13; Table 3; Supplementary Table 2) could mitigate the otherwise negative effects of temporary entrapment. However, at sites characterized by a small surface area (Category 1), the lack of prey emerged as a main concern in all cases (Table 3; Supplementary Table 2). Indeed, although the whales' entry into confined waters was at least in some cases likely due to foraging, overall prey availability in such areas was inadequate to sustain long-term health. Evidence suggests that killer whales can survive without food for up to ~75 days (Ford and Ellis, 1999). In this study, the entrapments that resulted in confirmed mortality indicated killer whales may die following a 42 to 90-day period of poor nutrition. Nutritional stress was confirmed as a contributing factor in the deaths of the killer whales in Nushagak River (Raverty et al., 2020). Mortality after 42 d of entrapment in Nushagak River suggests that survival may be further compromised when the entrapment occurs in fresh or brackish waters. As in other cetaceans exposed to fresh water for an extended period (e.g., Priddel and Wheeler, 1997), killer whales in Nushagak River (and Barnes Lake) had a skin condition characterized by severe degenerative changes caused by fungal and bacterial overgrowth (Bain, 1995; Raverty et al., 2020). Such compromised condition, known to have lethal consequences in cetaceans (Duignan et al., 2020), was confirmed to have contributed to mortality in this specific case (Raverty et al., 2020).
Management of Killer Whale Entrapments
If an entrapment is suspected, an investigation may be required to determine if an intervention is warranted. Because the configuration of the entrapment site seems to most affect the outcome of an entrapment, site characteristics (surface area, food supply, salinity) and geographic constraints should be assessed. Second, behavior patterns should be monitored for signs of entrapment, including for lone individuals in unrestricted areas (Category 3). Behavioral signs of entrapment include strong preference for certain parts of the entrapment site (Bain, 1995; Jourdain et al., 2019), atypical behavior (e.g., stereotyped pacing back and forth in a small area, long periods spent motionless at the surface, see Figure 4), lack of feeding activity (most cases) and possible intermittent production of loud repeated calls as an apparent attempt to connect with conspecifics (Kuczaj et al., 2015; Jourdain et al., 2019) (Table 4). If there is evidence for entrapment, monitoring should further aim at detecting any signs of nutritional stress or impaired health. These may include (but are not limited to) indentation behind the blowhole (the so-called “peanut-head” condition, Figure 4d), lethargy, abnormal skin conditions (see Figure 7 in Raverty et al., 2020), or an acetone-like smell as the whale(s) exhales (indicative of ketosis i.e., starving condition). Using an aerial drone to monitor the whales may provide detailed information and reduce disturbance. For example, aerial imagery in Cases #17 and 19 was particularly useful in detecting the presence of lactating and pregnant females (Table 2). Signs of declining condition and/or the presence of individuals at higher risk of nutritional stress (e.g., pregnant and lactating females) may increase the need to intervene. As human attention and boat traffic may pose an increased risk of stress and injury to the animals, implementing regulative measures is advised. Maintaining space and quiet at the entrapment site is also likely to enhance the whales' willingness to explore the area and find their way out on their own.
As means to guide the whales back to open waters, a coordinated line of boats (Figure 5) with intermittent banging of metal hollow pipes held in the water (Bain, 1995), a “hukilau” (modified from Figure 15 in Norris and Dohl, 1980; Figure 6; Supplementary Video 4) and/or underwater playbacks of killer whale calls (Jourdain et al., 2019) may be used (Table 4). Although all three methods have proved effective, they may be more or less appropriate in different situations. Each method varies in its complexity and the levels of resources required. While a line of boats moving slowly from the head of the bay toward the exit channel may be used to successfully herd killer whales in large bays (Figure 5), using a hukilau (Figure 6; Supplementary Video 4) may be as efficient and easier to operate in smaller bodies of water. Alternatively, playing killer whale sounds may be just as effective. However, as killer whales respond differently to various playback stimuli (e.g., Filatova et al., 2011), prior knowledge of the entrapped killer whales' identity (ecotype and population) is essential to determine what type of calls should be used to obtain the desired effect of attracting (rather than deterring) the whales. Perhaps most importantly, the timing of the operation relative to that of predicted high tide may be critical to the success of the operation. Specifically, to maximize water depth in the exit channel and presumably the killer whales' willingness (and ability, in the case of extreme shallows) to enter it, the start of the operation should be planned so that the whales can reach the mouth of the channel just before or at high slack tide. Tidal fluctuations may also be important to consider: if the trend is a declining water level in the coming days or weeks, a sooner rather than later intervention is best (Figure 3). Last, to minimize the stress induced to the animals (and risk of stranding) during the intervention and to prevent anything that could distract the whales away from taking the intended exit course, human traffic not necessary to the intervention, both on the water and on the surrounding shoreline, should be avoided as much as possible.
In the particular case of a lone killer whale in poor health, capture for medical care and rehabilitation may be necessary. In addition, when the situation involves a young inexperienced individual, relocation may be needed to reunite the whale with other members of its natal or social group. For example, the young female Springer (Case #11) was relocated to Johnstone Strait in July 2002 (Supplementary Table 3), an area frequented by her natal group during summer. After release, she eventually reunited with her population and resumed normal social patterns (Francis and Hewlett, 2007). The case of Luna (#10) further supports this. Out of the typical range of his population, Luna had only a slim chance of making it back to his natal group on his own and, without any intervention, he did not. Even though he was apparently healthy and able to feed himself in an unrestricted area, his need for social contact led him to develop interactions with people and boats and to build a “psychological box” in which he stayed for nearly 5 years (Supplementary Table 3, Francis and Hewlett, 2007). Comparable to situations with solitary sociable cetaceans (see Nunny and Simmonds, 2018), this case was surrounded by controversy, politics and management issues.
Conclusion
We have shown that natural entrapments of killer whales are rare but recurrent occurrences that can lead to mortality. Fortunately, fairly simple, inexpensive and humane steps can be taken to assist the whales in most cases, provided that situations can be identified, assessed and then addressed before the onset of irreversible physiological stress, disease, injury or even stranding. Our investigation revealed an important lack of reporting in the peer-reviewed literature, including for other species of cetaceans. However, protocols on how to manage these special cases could enhance the survival of individuals/groups of significant importance in the cases of populations of high conservation concern (e.g., Beck et al., 2014; Allen and Angliss, 2015; Esteban et al., 2016). Driven by climate change and shifting prey distributions, killer whales and other cetaceans may increasingly venture into new and unfamiliar habitats. This, combined with increasing public awareness (e.g., social media and global communication), means that natural entrapments may become both more frequent and more frequently reported. This paper outlines approaches for identifying and managing killer whale entrapments, which may also be applicable to managing entrapments in other species of cetaceans.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the animal study because this study does not involve any physical capture or handling of animals.
Author Contributions
EJ, LB-L, GE, JF, and JT conceived the study and developed the methodology. EJ, GE, RK, and JT did the measurements of entrapment sites. EJ led the writing of the manuscript. All authors collected and processed data from a number of attended cases, searched for and compiled information relative to unattended cases, discussed the results and implications, contributed critically to the manuscript at all stages, and gave final approval for publication.
Funding
Open access funding provided by the Sea World and Busch Gardens Conservation Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Jørgen Wiig, Katrine Jill, Marius Vågenes, Roy Hilmar Svendsen, and Rolf Henning Myrmel for providing additional information and/or material related to Case #16. We thank Jane Watson for commenting on earlier drafts, which greatly improved the manuscript. Frédérique Lucas made Figure 6a for specific inclusion in this study. We thank Tor Johansen (Norwegian Directorate of Fisheries) for sharing his photographs and footage of the intervention in Case #19, some of which was featured in Figure 6 and in Supplementary Video 4.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.707616/full#supplementary-material
References
Allen, B. M., and Angliss, R. P. (2015). Alaska Marine Mammal Stock Assessments, 2014. Seattle, WA: U.S. Department of Commerce, NOAA Technical Memorandum. NMFS-AFSC-301.304 p.
Bain, D. (1995). Killer Whales (Orcinus orca) in Barnes Lake, Alaska: A Preliminary Report [Unpublished report]. Vallejo, CA: Marine World Foundation.
Barrett-Lennard, L. G. (2000). Population Structure and Mating Patterns of Killer Whales (Orcinus orca) as Revealed by DNA Analysis (Ph.D. dissertation), University of British Columbia.
Beck, S., Foote, A. D., Kotter, S., Harries, O., Mandleberg, L., Stevick, P. T., Whooley, P., and Durban, J. W. (2014). Using opportunistic photo-identification to detect a population decline of killer whales (Orcinus orca) in British and Irish waters. J. Marine Biol. Assoc. U. K. 94, 1327–1333. doi: 10.1017/S0025315413001124
CIMAS (2021). Orcas in Von Donop Lagoon, ca. 1949. Cortes Island Museum and Archives Society. Available online at: https://cortesmuseum.com/orcas-in-von-donop-lagoon-c1949-by-lynne-jordan/ (accessed April 12, 2021).
COSEWIC (2008). COSEWIC Assessment and Update Status Report on the Killer Whale Orcinus orca, Southern Resident Population, Northern Resident Population, West Coast Transient Population, Offshore Population and Northwest Atlantic / Eastern Arctic Population, in Canada. Ottawa: Committee on the Status of Endangered Wildlife in Canada, viii + 65p.
Dahlheim, M., Bain, D., Sims, C., and Demaster, D. (2000). “Alaska fisheries science center processed report 2000-06,” in Southern Resident Killer Whale Workshop, Seattle, WA. 17p.
Duignan, P. J., Stephens, N. S., and Robb, K. (2020). Fresh water skin disease in dolphins: a case definition based on pathology and environmental factors in Australia. Sci. Rep. 10:21979. doi: 10.1038/s41598-020-78858-2
Emery, M. (1960). Did Killer Whales Commit Suicide? Mystery of Von Donop Lagoon. Daily Colonist. Victoria, BC: Victoria Press Ltd., p.3.
Esteban, R., Verborgh, P., Gauffier, P., Giménez, J., Martín, V., Pérez-Gil, M., et al. (2016). Using a multi-disciplinary approach to identify a critically endangered killer whale management unit. Ecol. Indicat. 66, 291–300. doi: 10.1016/j.ecolind.2016.01.043
Filatova, O. A., Fedutin, I. D., Burdin, A. M., and Hoyt, E. (2011). Responses of Kamchatkan fish-eating killer whales to playbacks of conspecific calls. Marine Mammal Sci. 27, E26–E42. doi: 10.1111/j.1748-7692.2010.00433.x
Ford, J. K. B., and Ellis, G. M. (1999). Transients: Mammal-Hunting Killer Whales of British Columbia, Washington, and Southeastern Alaska. Vancouver, BC and Seattle, WA: UBC Press and University of Washington Press.
Ford, J. K. B., and Ellis, G. M. (2006). Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Marine Ecol. Progress Series 316, 185–199. doi: 10.3354/meps316185
Ford, J. K. B., and Ellis, G. M. (2014). “You are what you eat: foraging specializations and their influence on the social organization and behavior of killer whales,” in Primates and Cetaceans: Field Research and Conservation of Complex Mammalian Societies, eds. J. Yamagiwa and L. Karczmarski. (Tokyo, Japan: Springer), 75–98.
Ford, J. K. B., Ellis, G. M., Barrett-Lennard, L. G., Morton, A. B., Palm, R. S., and Balcomb, K. C. (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471. doi: 10.1139/z98-089
Ford, J. K. B., Ellis, G. M., Matkin, C. O., Wetklo, M. H., Barrett-Lennard, L. G., and Withler, R. E. (2011). Shark predation and tooth wear in a population of Northeastern Pacific killer whales. Aquatic Biol. 11, 213–224. doi: 10.3354/ab00307
Ford, J. K. B., Stredulinsky, E. H., Towers, J. R., and Ellis, G. M. (2013). Information in Support of the Identification of Critical Habitat for Transient Killer Whales (Orcinus orca) Off the West Coast of Canada. Research Document 2012/155, iv + 46 p.
Francis, D., and Hewlett, G. (2007). Operation orca: Springer, Luna and the Struggle to Save West Coast Killer Whales. Madeira Park, BC: Harbour Publishing Co. Ltd.
Goodwin, L., and Dodds, M. (2008). Lone Rangers. A Report on Solitary Dolphins and Whales Including Recommendations for Their Protection. Marine Connection. Uckfield: Marine Connection, 48p.
Heide-Jørgensen, M. P., Richard, P., Ramsay, M., and Akeeagok, S. (2002). Three recent ice entrapments of Arctic cetaceans in West Greenland and the eastern Canadian High Arctic. NAMMCO Sci. Publ. 4, 143–148. doi: 10.7557/3.2841
Heimlich-Boran, J. R. (1988). Behavioral ecology of killer whales (Orcinus orca) in the Pacific Northwest (USA). Can. J. Zool. 66, 565–578. doi: 10.1139/z88-084
Jourdain, E., Andvik, C., Karoliussen, R., Ruus, A., Vongraven, D., and Borgå, K. (2020). Isotopic niche differs between seal and fish-eating killer whales (Orcinus orca) in northern Norway. Ecol. Evolut. 10, 4115–4127. doi: 10.1002/ece3.6182
Jourdain, E., Karoliussen, R., Curé, C., Massenet, M., Barrett-Lennard, L. G., and Ellis, G. M. (2019). A case of natural killer whale (Orcinus orca) entrapment in northern Norway: from assessment to rescue. Aquatic Mammals 45, 14–20. doi: 10.1578/AM.45.1.2019.14
Jourdain, E., Vongraven, D., Bisther, A., and Karoliussen, R. (2017). First longitudinal study of seal-feeding killer whales (Orcinus orca) in Norwegian coastal waters. PLoS ONE 12:e0180099. doi: 10.1371/journal.pone.0180099
Kitsapsun (2007). Dyes Inlet orcas – Ten Years Later. Available online at: http://data.kitsapsun.com/projects/story/dyes-inlet-orcas-ten-years-later/ (accessed May 1, 2021).
Kraus, S. D., Brown, M. W., Caswell, H., Clark, C. W., Fujiwara, M., Hamilton, P. K., et al. (2005). North Atlantic right whales in crisis. Science 309, 561–562. doi: 10.1126/science.1111200
Kuczaj, S. A., Frick, E. E., Jones, B. L., Lea, J. S., Beecham, D., and Schnöller, F. (2015). Underwater observations of dolphin reactions to a distressed conspecific. Learn. Behav. 43, 289–300. doi: 10.3758/s13420-015-0179-9
London, J. M. (2006). Harbor Seals in Hood Canal: Predators and Prey (PhD dissertation), University of Washington.
Matkin, C. O., Durban, J. W., Saulitis, E. L., Andrews, R. D., Straley, J. M., Matkin, D. R., et al. (2012). Contrasting abundance and residency patterns of two sympatric populations of Transient killer whales (Orcinus orca) in the Northern Gulf of Alaska. Fishery Bull. 110, 143–155.
McHugh, K. A., Barleycorn, A. A., Allen, J. B., Bassos-Hull, K., Lovewell, G., Boyd, D., et al. (2021). Staying alive: long-term success of bottlenose dolphin interventions in southwest Florida. Front. Marine Sci. 7:624729. doi: 10.3389/fmars.2020.624729
Moore, K. M., Simeone, C. A., and Brownell Jr, R. L. (2018). “Strandings,” in Encyclopedia of Marine Mammals. 3rd Edn, eds B. Wursig, J. G. M. Thewissen, and K. Kovacs (Cambridge, MA: Academic Press), 945–951.
Moore, M., Walsh, M., Bailey, J., Brunson, D., Gulland, F., Landry, S., et al. (2010). Sedation at sea of entangled North Atlantic right whales (Eubalaena glacialis) to enhance disentanglement. PLoS ONE 5:e9597. doi: 10.1371/journal.pone.0009597
Norris, K. S., and Dohl, T. P. (1980). Behavior of the Hawaiian spinner dolphin, Stenella longirostris. Fishery Bull. 77, 821–849.
Nøttestad, L. (2002). Killer Whales (Orcinus orca L.) and Saithe (Pollachius virens L.) Trap Herring (Clupea harengus L.) in Shallow Water by Taking Advantage of Steep Bottom Topography. Hillerød, Denmark: International Council for the Exploration of the Sea.
Nunny, L., and Simmonds, M. P. (2018). A global reassessment of solitary-sociable dolphins. Front. Vet. Sci. 5:331. doi: 10.3389/fvets.2018.00331
Oftedal, O. T. (1997). Lactation in whales and dolphins: evidence of divergence between baleen-and toothed-species. J. Mammary Gland Biol. Neoplasia 2, 205–230. doi: 10.1023/A:1026328203526
Parsons, K. M., Durban, J. W., Burdin, A. M., Burkanov, V. N., Pitman, R. L., Barlow, J., et al. (2013). Geographic patterns of genetic differentiation among killer whales in the Northern North Pacific. J. Heredity 104, 737–754. doi: 10.1093/jhered/est037
Priddel, D., and Wheeler, R. (1997). Rescue of a Bryde's whale Balaenoptera edeni entrapped in the Manning River, New South Wales: unmitigated success or unwarranted intervention? Austr. Zool. 30, 261–271. doi: 10.7882/AZ.1997.002
Priddel, D., and Wheeler, R. (1998). Hematology and blood chemistry of a Bryde's whale, Balaenoptera edeni, entrapped in the Manning River, New South Wales, Australia. Marine Mammal Sci. 14, 72–81. doi: 10.1111/j.1748-7692.1998.tb00691.x
Raverty, S., St. Leger, J., Noren, D. P., Burek Huntington, K., Rotstein, D. S., Gulland, F. M., et al. (2020). Pathology findings and correlation with body condition index in stranded killer whales (Orcinus orca) in the northeastern Pacific and Hawaii from 2004 to 2013. PLoS ONE 15:e0242505. doi: 10.1371/journal.pone.0242505
Read, A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mammal. 89, 541–548. doi: 10.1644/07-MAMM-S-315R1.1
Reeves, R. R., Mcclellan, K., and Werner, T. B. (2013). Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endangered Species Res. 20, 71–97. doi: 10.3354/esr00481
Reeves, R. R., and Mitchell, E. (1988). Distribution and seasonality of killer whales in the Eastern Canadian Arctic. Rit Fiskideildar 11, 136–160.
Riesch, R., Barrett-Lennard, L. G., Ellis, G. M., Ford, J. K. B., and Deecke, V. B. (2012). Cultural traditions and the evolution of reproductive isolation: ecological speciation in killer whales? Biol. J. Linnean Soc. 106, 1–17. doi: 10.1111/j.1095-8312.2012.01872.x
Robbins, J., Knowlton, A. R., and Landry, S. (2015). Apparent survival of North Atlantic right whales after entanglement in fishing gear. Biol. Conserv. 191, 421–427. doi: 10.1016/j.biocon.2015.07.023
Sundaram, B., Poje, A. C., Veit, R. R., and Nganguia, H. (2006). Acoustical dead zones and the spatial aggregation of whale strandings. J. Theor. Biol. 238, 764–770. doi: 10.1016/j.jtbi.2005.06.022
Towers, J. R., Pilkington, J. F., Gisborne, B., Wright, B. M., Ellis, G. M., Ford, J. K. B., et al. (2020). Photo-Identification Catalogue and Status of the Northern resident Killer Whale Population in 2019. Canadian Technical Report of Fisheries and Aquatic Sciences. 3371. iv + 69 p.
Towers, J. R., Sutton, G. J., Shaw, T. J. H., Malleson, M., Matkin, D., Gisborne, B., Forde, J., Ellifrit, D., Ellis, G. M., Ford, J. K. B., and Doniol-Valcroze, T. (2019). Photo-Identification Catalogue, Population Status, and Distribution of Bigg's Killer Whales Known From Coastal Waters of British Columbia, Canada. Canadian Technical Report of Fisheries and Aquatic Sciences. 3311. vi + 299 p.
Trianni, M. S., and Kessler, C. C. (2002). Incidence and strandings of the spinner dolphin, Stenella longirostris, in Saipan Lagoon. Micronesica Agana 34, 249–260.
Westdal, K. H., Higdon, J. W., and Ferguson, S. H. (2017). Review of killer whale (Orcinus orca) ice entrapments and ice-related mortality events in the Northern hemisphere. Polar Biol. 40, 1467–1473. doi: 10.1007/s00300-016-2019-6
Wright, B. M., Ford, J. K. B., Ellis, G. M., Deecke, V. B., Shapiro, A. D., Battaile, B. C., and Trites, A. W. (2017). Fine-scale foraging movements by fish-eating killer whales (Orcinus orca) relate to the vertical distributions and escape responses of salmonid prey (Oncorhynchus spp.). Movement Ecol. 5:3. doi: 10.1186/s40462-017-0094-0
Keywords: natural entrapment, killer whale, cetacean, management, conservation
Citation: Jourdain E, Barrett-Lennard LG, Ellis GM, Ford JKB, Karoliussen R, Towers JR and Vongraven D (2021) Natural Entrapments of Killer Whales (Orcinus orca): A Review of Cases and Assessment of Intervention Techniques. Front. Conserv. Sci. 2:707616. doi: 10.3389/fcosc.2021.707616
Received: 10 May 2021; Accepted: 31 July 2021;
Published: 24 August 2021.
Edited by:
Shermin De Silva, Trunks & Leaves Inc., United StatesReviewed by:
Sheryl Hamilton, University of Tasmania, AustraliaAndrew Butterworth, University of Bristol, United Kingdom
Copyright © 2021 Jourdain, Barrett-Lennard, Ellis, Ford, Karoliussen, Towers and Vongraven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eve Jourdain, ZXZlam91cmRhaW5AeWFob28uZnI=
 Eve Jourdain
Eve Jourdain Lance G. Barrett-Lennard2
Lance G. Barrett-Lennard2 John K. B. Ford
John K. B. Ford Jared R. Towers
Jared R. Towers Dag Vongraven
Dag Vongraven