- 1Taronga Institute of Science and Learning, Taronga Conservation Society Australia, Mosman, NSW, Australia
- 2School of Biological Earth and Environmental Sciences, The University of New South Wales, Sydney, NSW, Australia
- 3Stats Central, UNSW Sydney, Sydney, NSW, Australia
- 4BirdLife Australia, Carlton, VIC, Australia
- 5Department of Environment, Land, Water, and Planning, Wodonga, VIC, Australia
- 6Institute of Zoology, Zoological Society of London, London, United Kingdom
- 7School of Biological Sciences, Monash University, Melbourne, VIC, Australia
- 8Department of Genetics, Evolution & Environment, Centre for Biodiversity & Environment Research, University College London, London, United Kingdom
Evaluating the effectiveness of captive breeding programs is central to improving conservation outcomes in released animals. However, few studies have assessed the impact of the strategies and trade-offs involved in husbandry decisions and the selection of traits on the success of breeding programs. This study evaluated a range of husbandry features including an animal's environment, health, and traits of the released individual and its parents involved in the zoo-based Regent Honeyeater breed for release program to optimise individual reproductive success and survivability, leading to improved conservation outcomes in the wild. We analysed 285 birds using a penalised Cox proportional hazard model to assess survival and an ordinal logistic model to evaluate the reproductive success of zoo bred birds released to the wild. Key features identified by the study highlight the importance of having parents that are successful breeders and parents that have an overall higher lifetime reproductive output. However, there were associated quantity-quality trade-offs, as the success of young (i.e., released birds) produced by parents was negatively associated to the number of clutches per year (where one clutch per year was found optimal). The study demonstrated the importance of considering the parental effects on the traits of its offspring beyond its pedigree information and found there was an associated decline in fitness of its offspring with older fathers. Song tutoring using wild Regent Honeyeaters was also important for increased survival post-release. Other important factors are discussed within the review. In general, the study recommended that a multi-faceted approach in the assessment and evaluation of the captive breeding program, to identify markers that will improve conservation outcomes of future releases.
Introduction
Conservation breeding programs continue to play an important role in mitigating the unprecedented rates of species extinction and threats to global biodiversity due to human driven environmental change. Breeding and conservation translocation programs aim to re-establish or supplement animal populations in the wild to enable them to become self-sustaining as threatening processes are addressed. The success of conservation translocation programs not only relies on the number and quality of animals re-introduced but on the long-term commitment to restore and preserve the natural habitat of animals in the wild. Zoos play a crucial role in recovery programs through ex-situ breeding efforts. The Californian condor, Gymnogyps californianus, is often the exemplar of a successful captive breeding and conservation translocation program, whose numbers fell to as low as 22 individuals in 1982 and rose to over 518 birds (in captivity and the wild) after an intensive captive breeding program (Subramanian, 2012; United States Department of the Interior, 2019). Other examples include the black-footed ferret, Mustela nigripes (Biggins et al., 1999), golden lion tamarin, Leontopithecus rosalia (Beck et al., 1991), and red wolf, Canis lupus rufus (Phillips et al., 2003).
The evaluation of captive breeding programs is crucial in our understanding of the effectiveness of the program and the actions that ultimately promote conservation outcomes. Yet, few studies have assessed the impact of the approaches taken at the captive rearing stage which may impact the quality of the released cohort. Decisions in captive breeding programs vary extensively from the genetic, behavioural, physiology and environmental features, which can all shape the condition and success of the release candidate. For that reason, there is the need for empirical evidence to better understand the role in the zoo-based decisions on post-release success.
Conservation breeding programs have been faced with criticism for their low success rates (Fischer and Lindenmayer, 2000). These programs are met with significant challenges including the potential for maladaptive traits to develop as a result of adaptation to captivity (Fraser, 2008). Captive breeding facilities often lack environmental stressors that would be experienced by wild animals such as food shortages, extremes in environmental conditions, interactions with predators or other life-threatening experiences. Whilst extensive genetic or pedigree management is common in breeding programs (Stojanovic et al., 2019), a deeper understanding of fitness and developing key survival skills in released animals can be crucial (Berger-Tal et al., 2020). For example, an animal translocated into the wild is faced by many novel challenges and how it responds to these challenges may largely depend on its behavioural traits, experience and decision-making skills. Therefore, emphasis on an animals' behavioural traits and its resilience should form a major focus in breeding programs (Shier, 2016; Berger-Tal et al., 2020). Additionally, population resilience, where not all individuals respond in the same way to environmental stressors, should also be considered (Watters and Meehan, 2007).
The Regent Honeyeater, Anthochaera phrygia, is a critically endangered passerine with a wild population estimate of 350-400 mature individuals (Garnett et al., 2010; Kvistad et al., 2015). The population has declined from 1,500 in 1992 (Webster and Menkhorst, 1992) and the main threats to the species include habitat loss and fragmentation, degradation of remnant habitat, competition, predation and its small population size (Commonwealth of Australia, 2016).
The Regent Honeyeater inhabits temperate woodlands and open forests of south-east Australia, extending from north-eastern Victoria to south-eastern Queensland (Commonwealth of Australia, 2016). Breeding typically occurs during spring and summer from August to January (Franklin et al., 1989; Commonwealth of Australia, 2016). However, the timing varies across the species range and is thought to be associated with the flowering of food sources including eucalypt e.g., Eucalyptus melliodora & E. sideroxylon and mistletoe, Amyema cambagei (Geering and French, 1998).
Regent Honeyeaters breed in individual pairs or, sometimes, in loose colonies. Males will attract females by singing (Liu et al., 2014) and once fertilisation has occurred, the female will construct the nest with the support of the male (Geering and French, 1998). Once incubation by the female has commenced, the males become less vocal (Geering and French, 1998). Females will typically incubate 2–3 eggs for 14 days and both sexes will feed the young (Oliver, 1998; Commonwealth of Australia, 2016). The birds fledge at ~2 weeks and independence occurs at ~1 month (Liu et al., 2014). At this stage, the male will recommence singing to initiate breeding again. In the wild, males will drive fledglings away, or feed them at a distance from the nest (Higgins et al., 2001); (D. Ingwersen pers obs).
The Taronga Conservation Society has played an important role in the Regent Honeyeater species recovery program, with the reintroduction of 296 captive-bred Regent Honeyeaters in NSW and Victoria, Australia (2000–2017). Post-release monitoring of released birds was coordinated by BirdLife Australia and the Department of Environment, Land, Water and Planning (DEWLP) and Department of Planning, Industry and Environment (DPIE), local groups and other academic institutions and involved extensive support from volunteers.
Rearing animals in a zoo environment allows for management actions such as ensuring genetic diversity and survival at critical life stages. However, it also introduces constraints on the opportunities for learning, which can lead to behavioural changes in captive-bred animals. Zoo-bred Regent Honeyeaters often display undeveloped or atypical variants of wild song (Liu et al., 2014). This has led to speculation that it may influence the attractiveness and breeding success of released birds to their wild conspecifics. To simulate natural learning opportunities, some fledgling Regent Honeyeaters have been exposed to recordings of wild song or housed in the vicinity of wild-origin adults. While song exposure appears to have improved the quality of song (Vecsei, 2015), it has not yet been determined what, if any, influence it has on post-release fitness.
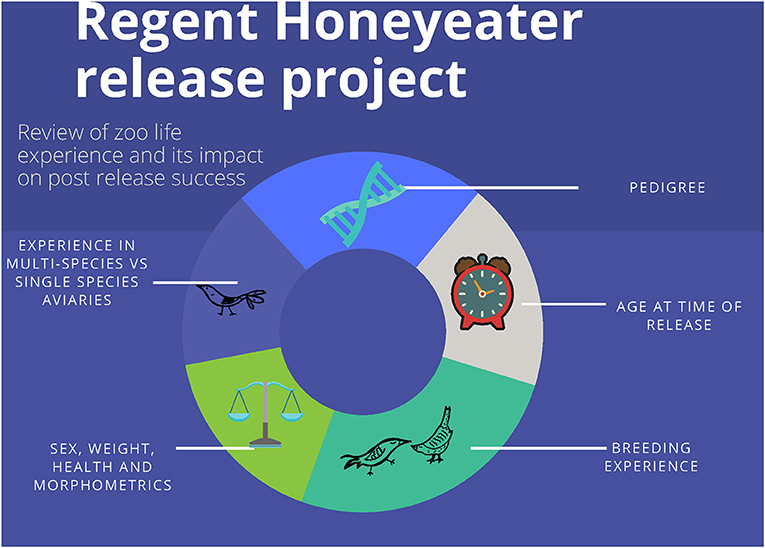
The aim of the study is to evaluate a range of husbandry features including an animal's environment, health, and of the released individual and its parents involved in the captive breeding program for Regent Honeyeaters to better understand how they influence an individual's post-release success (Figure 1). This will allow the development of a captive breeding strategy that will identify individuals that are predicted to have the best opportunity to survive and reproduce in the wild, a key performance criteria of the species Recovery Plan (Commonwealth of Australia, 2016).
The predictions of the study are that:
1. Birds with more time spent in multispecies aviaries i.e., opportunity to interact and compete with other bird species, will outperform birds that are naïve to competition. Food resources in the wild are periodically limiting and under these circumstances naïve birds will have lower survivorship and breeding success.
2. Successful/compatible breeding pairs that produce more offspring over their lifetime are likely to produce progenies that will have greater capacity to survive post-release as they are produced from parent with higher fitness. There is evidence to suggest that incompatible birds that are forced together are more likely to be stressed and delay reproduction. For example, research on Gouldian finches that were paired with poor-quality mates had 3-4 times higher levels of circulating corticosterone compared with females that were paired with preferred mates (Griffith et al., 2011). The stress hormones were elevated for several weeks in the mismatched pairs which delayed egg laying.
3. Birds that are heavier at release will outperform birds of same sex and age that are lighter, as heavier birds are assumed to have more energy reserves. Having more energy storage means that a bird would be better able to survive, defend nest sites etc., when resources are scarce.
4. Birds that have bred prior to release will have a better breeding success in the wild as they will have had pre-release experience which would improve breeding outcomes (i.e., this assumes that breeding, in part, is a learned behaviour).
5. Birds that are song-tutored and hear the song of wild birds will have greater chances for survival and reproduction. This prediction is based on the current literature which demonstrates the importance of singing in the breeding season (Crates, 2019).
6. Birds with less generational time in captivity will be more successful in breeding and survival as they are closer to wild born individuals and have had fewer generations in which to adapt to captivity.
Materials and Methods
Data Collection
Pre-release Observations
Data from five releases from Victoria were analysed in the study: 2008 (n = 27), 2010 (n = 44), 2013 (n = 38), 2015 (n = 77), and 2017 (n = 101) Total = 287 (Note–data from the birds released in 2000 were not included due to the small sample size n = 9 and because it was from a different site in NSW).
We extracted data using ZIMS, a zoological management software tool (Zoological Information Management System, 2021), on 285 Regent Honeyeaters that met release protocols and were successfully released between 2008 and 2017.
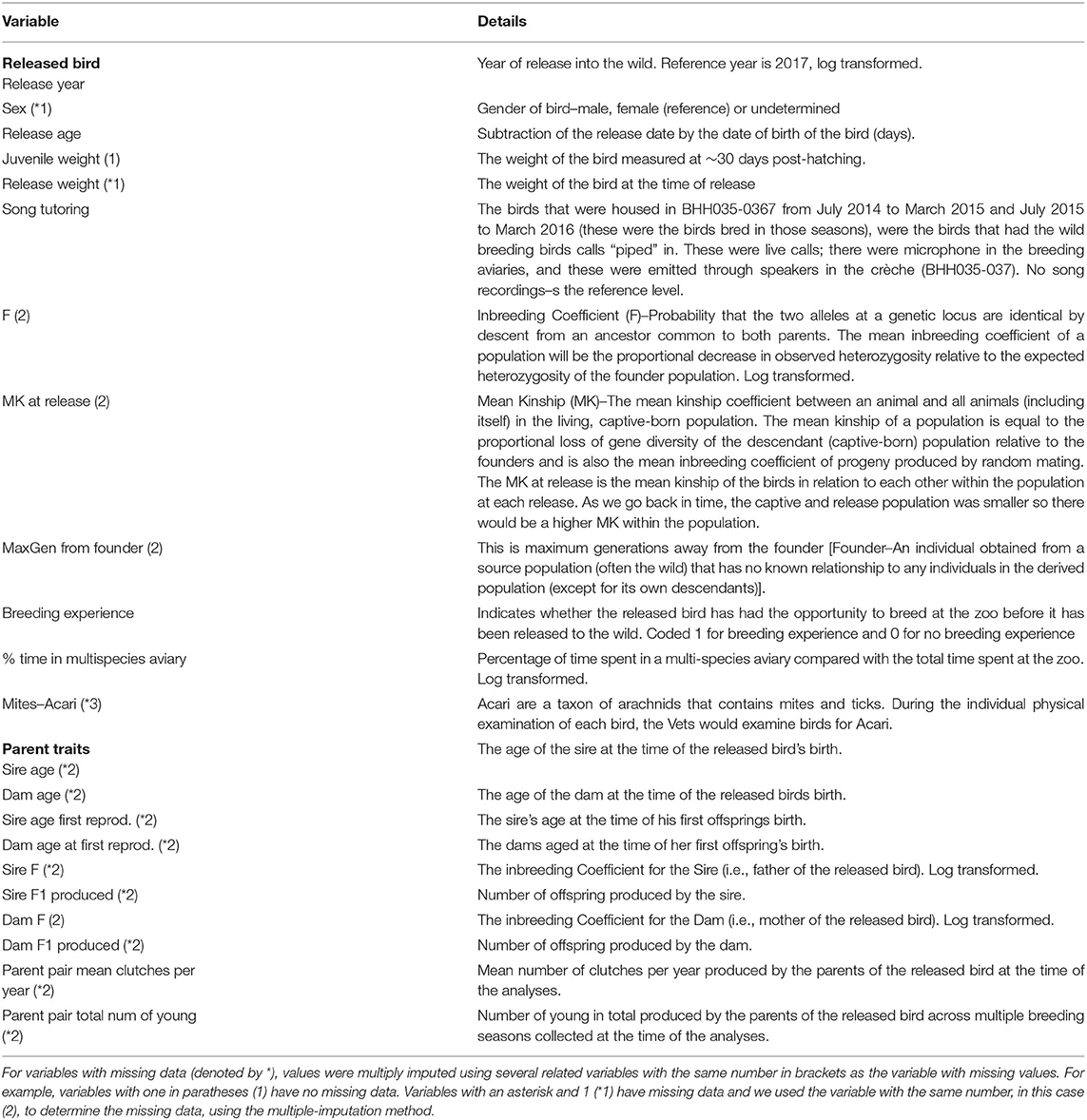
Information relating to the animals' age, environmental conditions, reproduction, health, and pedigree as well as information relating to its parents were used in the study (see Table 1 and Figure 1 for details).
Post-release Observations
All Regent Honeyeaters during 2008–2017 were released to Chiltern-Mt. Pilot National Park, Victoria. All released birds had a colour band over a metal band on one leg (all birds the same each release–to identify the release cohort) and two-colour bands on the other leg. The four-band combination was unique to each bird enabling individually identification in the field. Post-release survival and breeding success was evaluated by visual observations aided by radio tracking. Sixty four percent of birds were fitted with radio transmitters at least once during each release.
Regent Honeyeaters were fitted with BD-2 Holohill transmitters using a backpack style harness. Battery life was ~10 weeks and the weight of the harness and transmitter combined was ~1.7 g. Transmitters were only applied to birds where the transmitter and harness did not exceed 5% of the bird's total body mass i.e., >34 g. However, in 2017 there was an issue with numerous first year birds dying in the first week post-release and some had a body mass near to 34 g. From that point on only birds in their second year of life or older, and weighing more than 40 g, had transmitters attached at the initial release. If a bird was recaptured at 10 weeks or more post-release (and was >34 g) it was considered as eligible for transmitter fitting regardless of its release age.
After the birds had been in the wild for 10 weeks or more regardless of their age (and as long as birds were heavier than 34 g) they had transmitters attached.
Birds were monitored wherever they were detected (through structured surveys and opportunistic sightings), usually within or in close proximity to the National Park but sometimes 50–100 + km away. During the first month post-release birds were monitored daily by observers. In the second month they were monitored 6 days per week, excluding Saturdays. In month 3 monitoring occurred 5 days per week, excluding Saturday and Tuesday, and from month 4 onwards monitoring occurred every second day. During monitoring observers noted if birds were present or absent, or if they were confirmed as dead. Nesting behaviour was recorded, including the identity of individuals in a breeding pair and the reproductive stage. We include up to 19 weeks of post-release information for each individual bird.
Birds wearing working transmitters were located on each monitoring day. For the other birds monitoring was based on locating individuals around known activity areas and the sightings were opportunistic, however, if they were near birds wearing transmitters they would be sighted. Later in the season when birds had settled into breeding pairs, monitoring occurred at the vicinity of the nest or last known location which would allow permit the monitoring of nests. Other birds that were in the vicinity were also monitored during this time.
Data Handling
To study how zoo history and the husbandry variables affected bird survival, we conducted a survival analysis. Monitoring was intensively carried out, and efforts were made to find each bird every week. This was quite successful, such that for most birds (>90%), we have a record of them being resighted each week until they are no longer resighted (died or left the area). As we did not have dates of death for each bird, we used weekly resight records for survival, making the assumption that each bird died in the week following the last week it was resighted (up until the commencement of the breeding season). This is a strong assumption, as some birds may have migrated from the area rather than died. The likelihood that a bird that is not resighted and has migrated, becomes much higher once the breeding season starts, so we do not make this assumption past the start of the breeding season, instead censoring at this point. We therefore assume that birds still alive at the start of breeding season died at some indeterminate time or are still alive.
Reproductive output is low across both wild and captive-bred birds (Taylor et al., 2018) and it is therefore difficult to analyse reproductive success with sufficient power. Therefore, we have used ordinal categories of reproductive stages to examine reproductive success of the captive bred birds. We initially categorised reproductive success into six categories; 0. not resighted after the start of the breeding season, 1. resighted but no nest, 2. nest, 3. incubation, 4. hatching, and 5. fledging success. We fit separate models for males and females. To assess sensitivity of breeding models to survival, we then refit models with only those birds resighted during breeding season (i.e., we remove birds that were not resighted after the start of the breeding season).
Data handling and analysis was conducted using R-3.6.2 (R Core Team, 2019). To account for missing data (impacting 13 records from 285), we multiply imputed data (Rubin, 1996) 50 times using the mice (Van Buuren and Groothuis-Oudshoorn, 2011) package in R. Table 1 indicates which variables were used for imputation of each missing variable.
Analysis
The study's primary interest is in identifying husbandry variables that drive survival and reproductive success. We used penalised variable selection procedures for all analyses. Penalised methods are an alternative to stepwise methods for model selection (see Friedman et al., 2001). They have been shown to perform well for model selection for both survival analysis (Tibshirani, 1997) and ordinal regression (Wurm et al., 2017). For survival we used a penalised Cox proportional hazard model using the glmnet (Friedman et al., 2010; Simon et al., 2011) package in R. For breeding we used a penalised ordinal regression using the ordinalNet (Wurm et al., 2019) package in R with cumulative link model and probit link. We implemented default model selection procedures in each package, cross validation for survival and AIC for ordinal regression. The models were fit to each of the imputed datasets separately. When models fit to different imputed datasets selected different predictors, we report only predictors which were selected for half or more of the imputed datasets. Partial residual plots of a full model (all predictors) were used to diagnose non-linearity, and for identified non-linear predictors we added quadratic terms. We did not penalise year of release, sex, or whether the bird was outfitted with a transmitter, as we chose to control for these in all models. We do not report coefficients for wearing transmitters, as wearing a transmitter changes the chances of being detected and is therefore confounded. Clustering due to clutches was controlled for in the cross-validation procedure.
Penalised regressions have good properties for selecting variables, however they give biassed estimates of coefficients (Belloni and Chernozhukov, 2013). To estimate unbiased coefficients, we refit the appropriate model with the selected variables but without a penalty using the survival and ordinal packages in R with a random effect for clutch. We report the average of these unbiased estimates across all imputed datasets in the results. No confidence intervals or p-values are given, as inference is incompatible with variable selection, except in special cases (Berk et al., 2013). Highly skewed predictor variables were log transformed prior to analysis. Residual and partial residual plots were checked to identify variables with non-linear relationships, and to check assumptions were met.
It is not straightforward to interpret the output of the models used. Survival models primarily model the hazard, or instantaneous death rate, and how predictors impact upon it. Survival can be calculated from this, but the equation for survival does not lend itself to simple interpretation. Ordinal regressions model a latent continuous variable which is discretised to form ordinal categories, and how predictors impact upon this. Again, the probability of being in any one category can be calculated from this, but it is yet more complicated and cannot be easily interpreted. To aid interpretation we present the models in several ways, all of which are conditional, meaning we look at the impact of changing one predictor while keeping all others constant at some value (mean for continuous variables, and reference category for categorical variables). The figures and tables display how survival and probability of nesting category change when we change the value of one predictor but keep all other predictors in the model constant. It is not meaningful to compare absolute survival or breeding category probabilities across predictors, just within.
Results
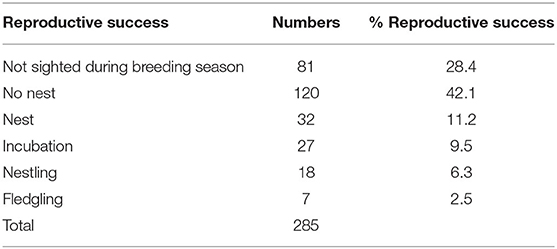
There was a total of 285 individuals analysed in the study: 27 birds in 2008 (9%); 44 birds in 2010 (15%); 37 birds in 2013 (13%); 77 birds in 2015 (27%); and 100 birds in 2017 (35%). Of these 57% were female, 43% were male and the sex of two birds was undetermined. The average age of birds released was 438 days (range 89–3084 days). There were 81 (28.4%) released individuals that were not resighted during the following breeding season and were excluded from breeding analysis (ii) below. One hundred and twenty (42.1%) birds were resighted during the breeding season but did not build a nest and 32 (11.2%) birds were seen to build a nest only. Twenty-seven individuals laid eggs (9.5%), 18 birds had nestlings (6.3%), and seven individuals lead to offspring that had fledged successfully (2.5%).
Survival Analysis
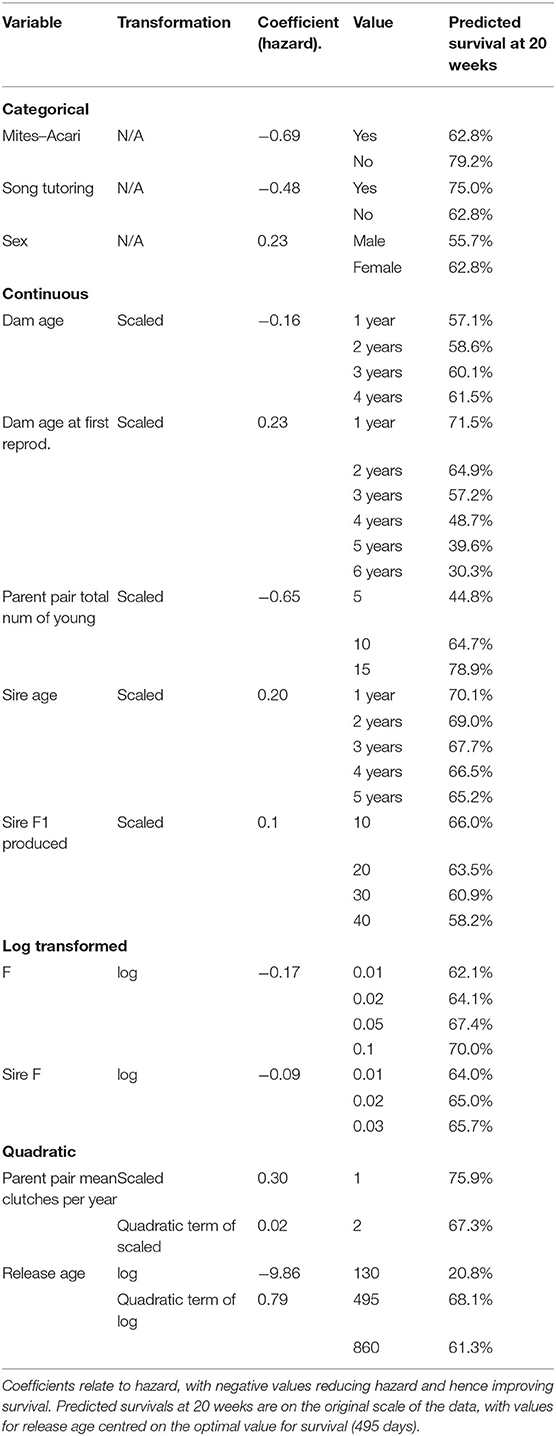
There were 285 individual birds were used in the survival analysis. In addition to year, transmitter and sex, which were not selected for, eleven variables were selected by the model selection procedure, these are: Mites–Acari, Song tutoring, Dam age, Dam age at first reproduction, Parent pair total number of young, Sire age, Sire F1 produced, (log of) F, (log of) Sire F, quadratic of Parent pair mean clutches per year and (log of) Release age. The proportional hazard assumption was not met for the transmitter variable and was hence included as a stratification variable in the unpenalised analysis. This fits separate base hazard for birds with and without transmitters, such that the effect of transmitter is not assumed to be proportional on the hazard. Other variables as still assumed to modify the hazard in the same way for birds with and without transmitters (Figure 2M).
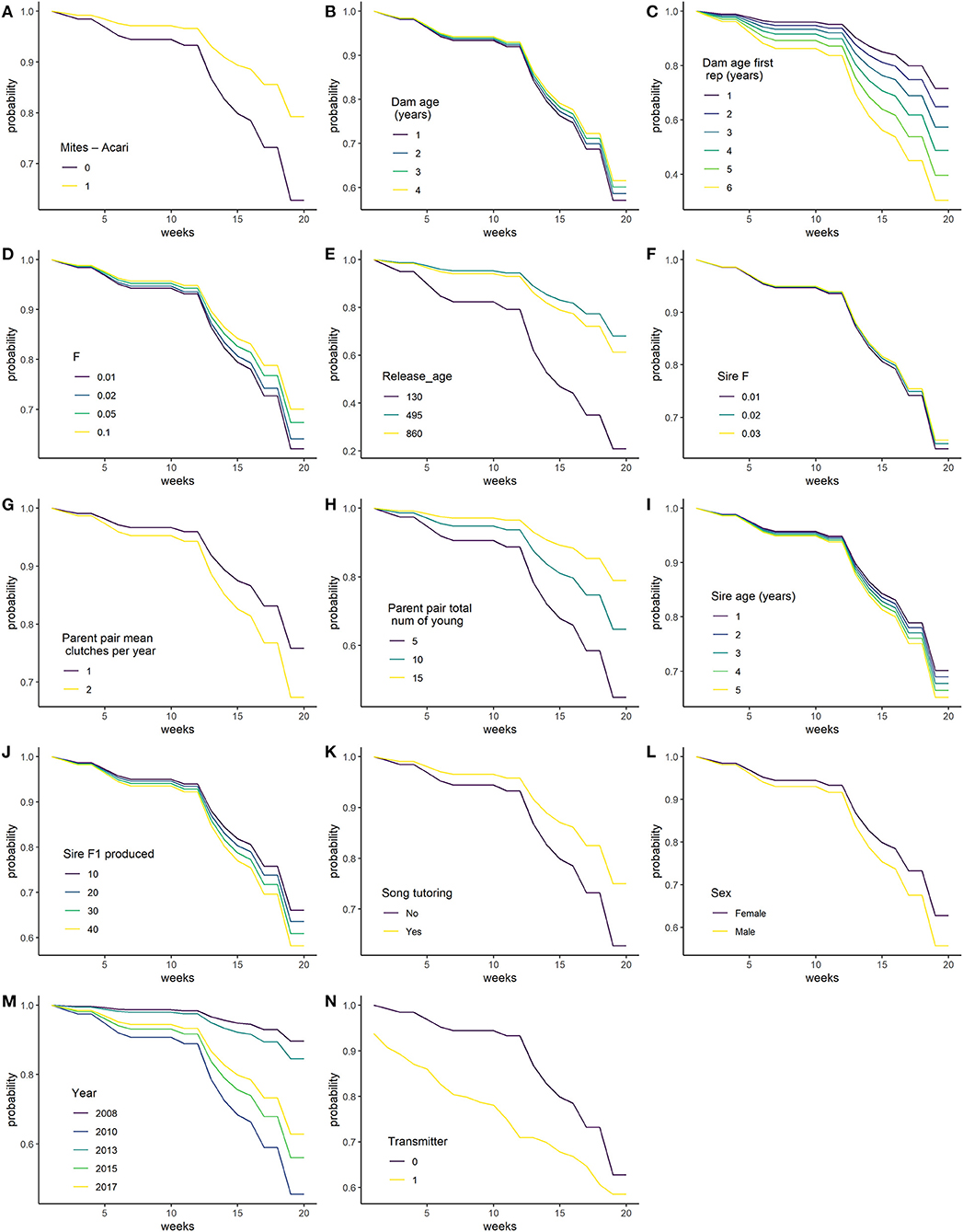
Figure 2. Predicted survival probability of released bird in weeks 1–20, changing each variable in turn, while holding all other continuous variables at their mean and categorical variables at their reference level. (A) Mites - Acari; (B) Dam age (years); (C) Dam age at first reproduction (years); (D) F in breeding coefficient; (E) Release_age (days); (F) Sire F; (G) Parent pair mean clutches per year; (H) Parent pair total number of young; (I) Sire age (years); (J) Sire F1 produced; (K) Song tutoring; (L) Sex; (M) Year; and (N) Transmitter.
Table 2 lists the coefficients of predictors, as well as predicted survival probability at 20 weeks for some values of each variable, holding all other variables constant at their mean or reference value. For the categorical variables, birds with Mites–Acari and birds played song recordings have increased survival. Higher Dam age, Parent pair total number of young, F, and Sire F increase survival, while higher Dam age at first reproduction, Sire age, Sire F1 produced and Parent pair mean clutches per year (within the observed range) is associated with decreased survival. The effect of Release age was quadratic, with optimal survival for birds released at 495 days. Figure 2 shows the effect of changing each variable while holding all constant at one to 20 weeks.
Female Breeding Analysis
Between 162 and 164 female birds are used for analysis (depending on imputed missing data). No variables were found to be predictive of breeding success when analysing all females, nor in the sensitivity analysis when using only those females alive at the start of the breeding season (Supplementary Figure 1).
Male Breeding Analysis
All Released Males
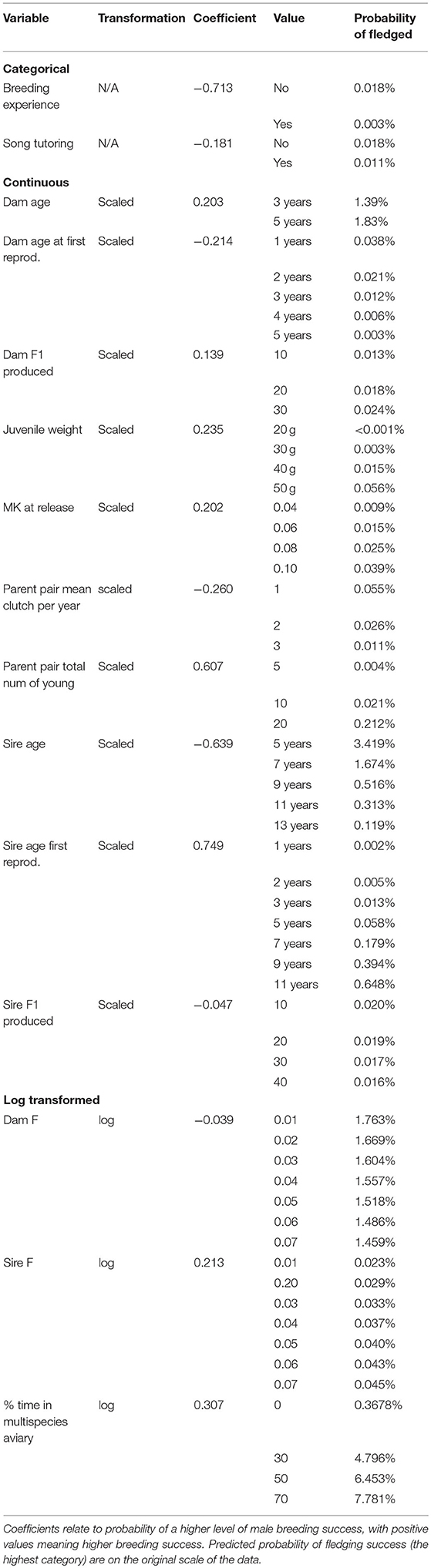
Between 121 and 123 male birds are used for analysis (depending on imputed missing data). Fifteen variables were selected by the model fit to all male birds for half or more of the imputed datasets, these are: Dam age, Dam age at first reproduction, Dam F1 produced, juvenile weight, (log of) Dam F, (log of) % time in multispecies aviary, (log of) Sire F, MK at release, Parent pair mean clutches per year, Parent pair total number of young, Breeding experience, Sire age, Sire age first reproduction, Sire F1 produced and Song tutoring.
For the categorical variables, birds with breeding experience and birds received song tutoring have decreased chance of breeding success. Dam age, Dam F1 produced, juvenile weight, MK at release, Parent pair total number of young, Sire age first reproduction, Sire F and % time in multispecies aviary are positively associated with breeding success, while Dam age at first reproduction, Parent pair mean clutches per year, Sire age, Sire F1 produced, and Dam F are negatively associated with breeding success (Table 3). Figure 3 shows the effect of changing each variable while holding all others constant.
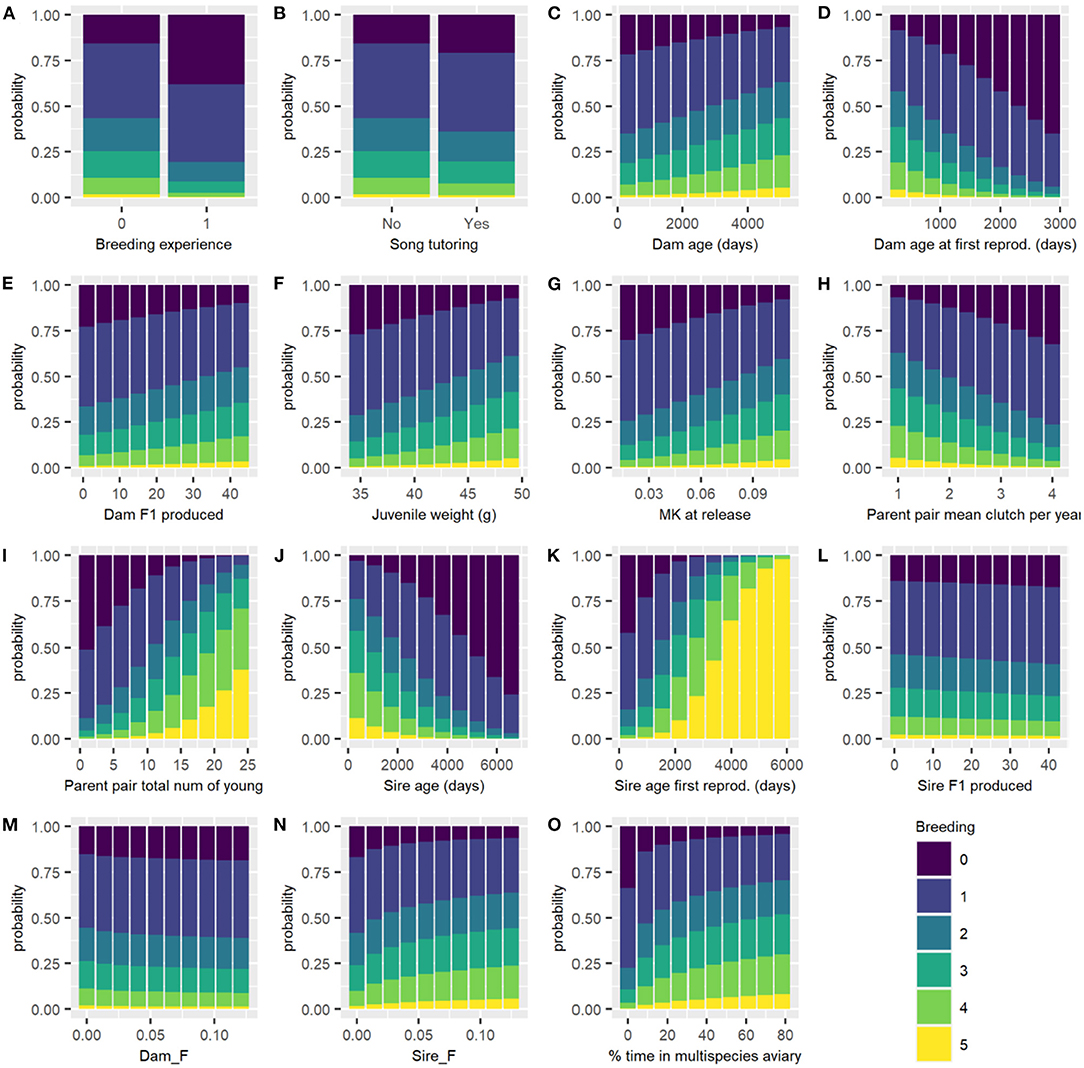
Figure 3. Predicted probability of each breeding category for all males of each breeding category changing each variable in turn, with all other continuous variables held at their mean and categorical variables held at their reference level. Breeding categories: (0) not resighted after the start of the breeding season, (1) resighted but no nest, (2) nest, (3) incubation, (4) hatching, and (5) fledging success. (A) Breeding experience; (B) Song tutoring; (C) Dam age (days); (D) Dam age at first reproduction (days); (E) Dam F1 produced; (F) Juvenile weight (g); (G) MK at release; (H) Parent pair mean clutch per year; (I) Parent pair total number of young; (J) Sire age (days); (K) Sire age at first reproduction (days); (L) Sire F1 produced; (M) Dam F; (N) Sire F; and (O) percentage of time in multispecies aviary.
Sensitivity Analysis–Subset of Birds That Were Resighted During Breeding Season
When re-estimating coefficients for the variables selected above for male breeding, with just those males that were resighted during breeding season, we obtained the results in Table 4.
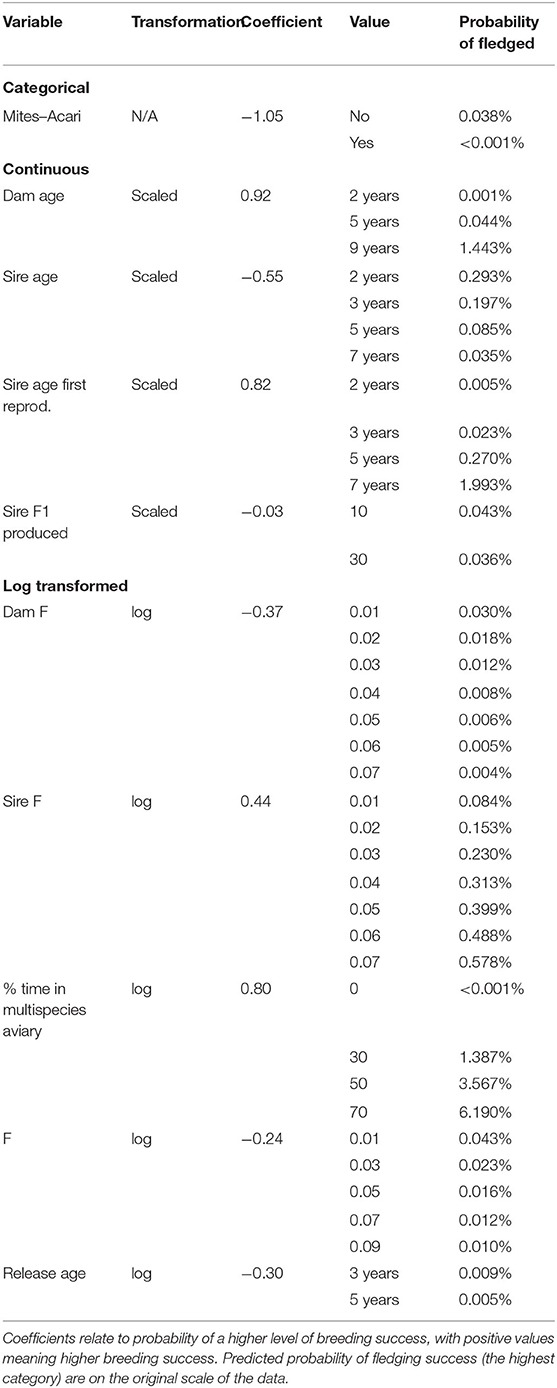
Table 4. Output from ordinal regression model for (transformed) variables for the subset of males resighted during the male breeding season.
Between 78 and 79 male birds are used for analysis (depending on imputed missing data). Ten variables were selected by the model fit to birds resighted during the breeding season for half or more of the imputed datasets, these are: Mites–Acari, Dam age, Sire age, Sire age first reproduction, Sire F1 produced, (log of) Dam F, (log of) Sire F, (log of) % time in multispecies aviary, (log of) F, (log of) Release age. Seven variables selected in this subset of males were also selected in analysis of all males, these are Dam age, Sire age, Sire age first reproduction, Sire F1 produced, Dam F, Sire F and % time in multispecies aviary. For all these variables, the models fit to both datasets agreed in terms of the direction of the effect (whether changing the variable increases or decreases breeding success), though the magnitude of the effect differed. In addition, in the analysis of only those males sighted during breeding season, higher Release age, higher F and having Mites–Acari are associated with decreased breeding success.
Discussion
Developing an evidence-based framework for the assessment of captive breeding programs is important as it identifies markers, management actions or outputs that lead to the increased performance of the candidate and/or cohort post-release and ultimately promotes conservation outcomes. Yet, few studies have assessed the impact of the approaches taken at the captive rearing stage which may impact the quality of the released cohort. Decisions in captive breeding programs vary extensively from the genetic, behavioural, physiology and environmental features, which can all shape the condition and success of the release candidate. For that reason, there is the need for empirical evidence to better understand the role in the zoo-based decisions on post-release success.
Challenges associated with behaviour, including learning, socialisation and mating (Berger-Tal et al., 2020) need to be continually evaluated in breeding programs and identify individuals with a high likelihood of success post-release. In the current study we examined survival and reproductive success of Regent Honeyeaters released as part of the captive breeding program for the species. We identified several important features that should be considered when optimising the breeding program and suggest a multifaceted approach to improving survival and reproductive outcomes post-release. These key findings include the importance of considering the traits, experience and success of the parent birds, as well as the husbandry practises such as the positive effects of being housed in a large, multi-species aviary and hearing the vocalisations of wild Regent Honeyeaters.
Below we address the predictions of the study.
1. Birds with more time spent in multispecies aviaries i.e., opportunity to interact with other bird species, will outperform birds that are naïve to competition. Food resources in the wild are periodically limiting and under these circumstances naïve birds will have lower survivorship and breeding success.
The housing conditions for male Regent Honeyeaters was important to its reproductive success post-release. Specifically, providing Regent Honeyeaters with the opportunity to spend time in a large, complex multispecies aviary, where lots of other bird species co-habitat in a close to “real world” environment, was found to be important to male reproductive success. The model shows that if males spent no time in a multispecies aviary, then there was <0.001% probability of successfully fledging offspring compared to those that spent 70% of their time in multispecies aviaries which showed a 7.8% probability of fledgling their young, thus there is a higher probability level of breeding success with increased time in a multispecies aviary (Table 4; Figure 4). We hypothesise that it enables birds to develop their skills in a close to natural setting and provides opportunity for a range of interactions including competition with other birds for resources, as well as space for extended flight. In the wild, birds have to compete with larger nectarivores and noisy miners for resources (Commonwealth of Australia, 2016), so learning these skills before release is critical to male breeding success.
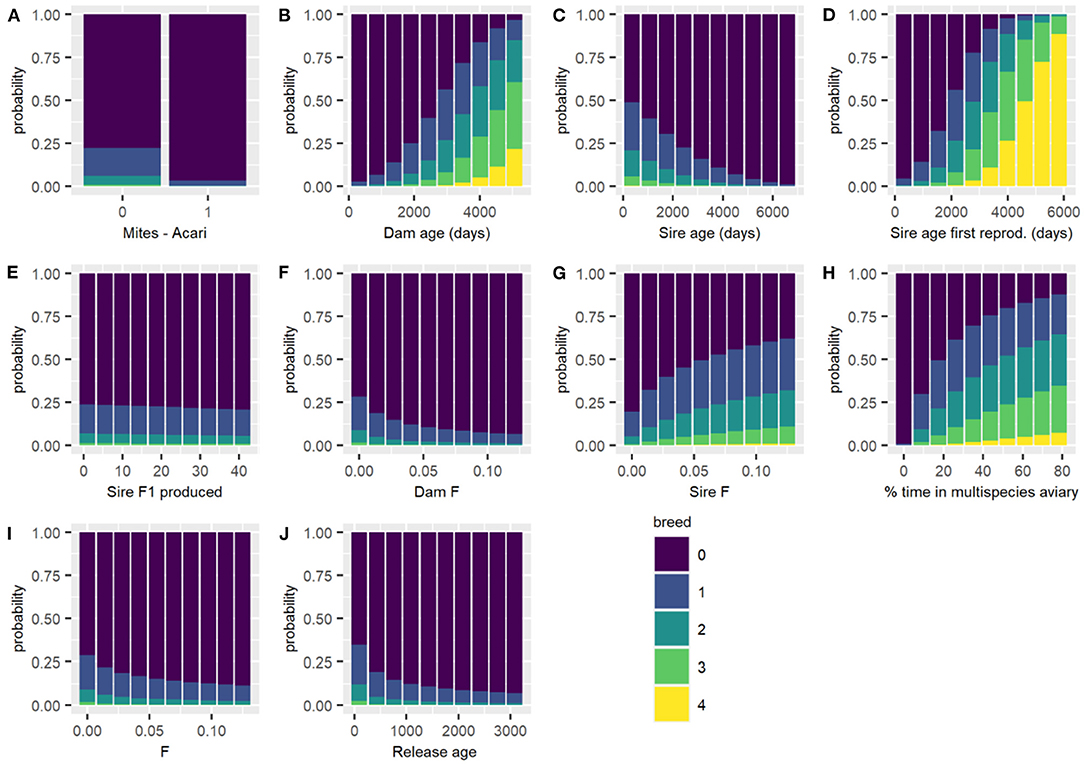
Figure 4. Predicted probability of each breeding category for the subset of male birds that were resighted during breeding season for each breeding category changing each variable in turn, with all other continuous variables held at their mean and categorical variables held at their reference level. Breeding categories: (1) resighted but no nest, (2) nest, (3) incubation, (4) hatching, and (5) fledging success. (A) Mites - Acari; (B) Dam age (days); (C) Sire age (days); (D) Sire age at first reproduction (days); (E) Sire F1 produced; (F) Dam F; (G) Sire F; (H) percentage of time in multispecies aviary; (I) F inbreeding coefficient; and (J) Release age (days).
2. Successful/compatible breeding pairs that produce more offspring over their lifetime are likely to produce progenies that will have greater capacity to survive post-release as they are produced from parent with higher fitness.
An important indicator of an individual's survival and male reproductive success in released Regent Honeyeaters is the lifetime reproductive output of its parents. The predicted survival at 20 weeks for a bird with average features in all other variables is 44.8% if the parent pair had five offspring, while with parents that had 15 offspring, the same bird would have predicted survival of 78.9% (Table 2). Although reproductive output is constrained by animal management decisions, parents with higher fitness produce offspring with greater survival and reproductive success. However, there is a quantity-quality trade-off; our study indicates that the optimal number of clutches per year is one, which means offspring (i.e., released candidates) benefit from parents who only reproduce, in captivity, once per year (i.e., 75.9% predicted survival). Offspring born to parents that had two clutches in a season had lower predicted survival (i.e., 67.3%), and survival decreases with increasing number of clutches. In a natural setting, Regent Honeyeaters will typically produce a single clutch and may produce a second clutch if conditions are favourable (SWIFFT, 2020) or if the initial nest failed (Taylor et al., 2018). As offspring fitness is related to resource investment, the study suggests that there is a decline in the resource allocation in progeny from the second, third and fourth clutch, even in a captive setting.
We found that both maternal and paternal effects influence the traits of offspring. Delayed first time breeding in fathers improves the reproductive success of male offspring, but the opposite is true for mothers, where delayed first-time breeding in mothers is not beneficial to breeding or the survival of its offspring. Mothers who bred earlier in life had offspring with greater chances for survival post-release. The present study reports that first time mothers that breed at age one in captivity, produce offspring that have a predicted survival of 71.5% compared to mothers that first reproduce at age six, who produce young that are predicted to survive to 20 weeks post-release at 30.3% (Table 2). It is unclear why delayed first time breeding in mothers impacts success, as the timing of breeding opportunities is typically a management decision based on suitable pairings and available husbandry space. However, it might be that late first-time breeders in mothers is a signal for poorer outcomes in offspring. In general, the average age of first breeding differs slightly between the sexes with females first breeding at ~2 years of age and males at 3 years (Gillespie, 2013). Controlling for the age at first breeding, breeding success and survival is enhanced when mothers are older and when fathers are younger.
Most studies have focused on the maternal effects as these tend to influence the early embryonic development and major developmental pathways (Bettegowda et al., 2008), however, paternal influence is also important (Ducatez et al., 2012). In fact, several paternal effects such as paternal age at mating and stress can affect the phenotypic traits of the offspring (Ducatez et al., 2012). We found that in Regent Honeyeaters, older fathers produce offspring with slight reduced survivability and breeding success (Predicted survival at 20 weeks with sire age is 70.1% at 1 year and 65.2% at 5 years; see Table 2). In wild birds of other species, the suspected shortening of telomeres in offspring (i.e., repeating DNA sequences found at the ends of chromosomes) is related to the traits of its ageing father (Bauch et al., 2019), and there is a strong indication that telomere length impacts health and longevity (Eisenberg, 2019). Interestingly, there is no similar effect of the maternal age at conception, on the telomere length of its offspring (Eisenberg, 2019). Further investigations on the telomere length in Regent Honeyeaters would prove interesting. Additionally, this indicates that in male Regent Honeyeaters, there is a potential loss in reproductive quality as males age.
3. Birds that are heavier at release will outperform birds of same sex and age that are lighter, as heavier birds are assumed to have more energy reserves. Having more energy storage means that a bird would be better able to survive, defend nest sites etc., when resources are scarce.
One of our predictions was that heavier birds would have better chances to succeed in the wild, particularly if resources were poor at the time of release. We found that release weight of birds does not seem to impact survival or reproductive success. Released birds fall within the normal weight range of wild birds [released birds: 32.8–51.6 g; wild birds 32.6–53.0 g (Regent Honeyeater recovery team, unpublished data)]. Interestingly, we identified juvenile weight, which is correlated with release weight (ρ = 0.76), as a potential marker of future breeding success in males, where a heavier juvenile male would be more likely have to an increase in breeding success. We suspect that because the variables are correlated and are difficult to distinguish using variable selection methods, it may explain the discrepancy.
4. Birds that have bred prior to release will have a better breeding success in the wild as they will have had pre-release experience which would improve breeding outcomes (i.e., this assumes that breeding, in part, is a learned behaviour).
Contrary to our initial study predictions, prior breeding experience of the released birds did not impact the survival and reproductive success of released Regent Honeyeaters. However, only 11% (32 out 285) of released Regents had prior breeding experience, so this could have affected our ability to detect the impact of this trait. Given that age at release was found to be important for post-release survival, it might be more of a priority to release birds at an optimal age, rather than holding them for longer to gain breeding experience.
We identified that the optimal release age for survival is 495 days which is when birds are close to one and half years old (Table 2). The average age of Regent Honeyeaters released by this re-introduction program lies close to this at 438 days and ranged from 89 to 3,084. Indeed, there would be obvious logistical implications associated with either delaying the release age of birds or trying to release all birds at the same age. Whilst it might be tempting to release young birds, increasing release numbers and frequency, the evidence suggests that birds below 1 year of age have lower survivability. Similarly, older birds that have spent longer periods in captivity may have greater difficulty learning or adapting to novel experiences, which could reduce survival following release (Canessa et al., 2014). In the wild, the life expectancy of Regent Honeyeaters is ~10 years (BirdLife, 2020).
5. Birds that are song-tutored and hear the song of wild birds will have greater chances for survival and reproduction. This prediction is based on the current literature which demonstrates the importance of singing in the breeding season (Crates, 2019).
Song exposure, through virtual or wild-origin tutors was associated with increased survival of released birds. The Regent honeyeater displays geographic variation in song characteristics and an individual's vocalisations change over time (Powys, 2010). They are also known to improvise and imitate the sounds of other species and incorporate components of these into their vocal repertoire (Liu et al., 2014; Vecsei, 2015). Singing in Regent Honeyeaters functions in courtship and nest protection (Crates, 2019). We found that released candidates that were tutored i.e., heard the song of wild Regents as fledglings showed higher propensity to survive; where birds that were tutored were had a 75.0% predicted survival at 20 weeks compared to 62.8% survival for birds that didn't receive song tutoring (Table 2). We found a negative association between song tutoring and male reproduction; however, this association did not hold when individuals that were not resighted during the breeding season were removed from the analysis. Regent Honeyeaters that can sing the full repertoire of wild individuals may be better able to attract mates and establish pair-bonds, negotiate conspecific interactions, and maintain social cohesion of flocks (Crates et al., 2021). We suggest tracking birds in the field over longer periods time to understand if there is behavioural modification of song structure over time and associated breeding/survival success.
6. Birds with less generational time in captivity will be more successful in breeding and survival as they are closer to wild born individuals and have had fewer generations in which to adapt to captivity.
Individuals with less generational time in captivity would be expected to retain more wild behaviours and show less adaptation to captivity (for example see Grueber et al., 2017). However, we found no effect of generational time on improved conservation outcomes in Regent Honeyeaters. This may indicate that the traits reported in this current study haven't been affected by time in captivity, or the effects of being further removed from the founder may occur, the more time that passes (see Woodworth et al., 2002).
In a captive breeding program, pedigree information is used to carefully pair individuals to reduce inbreeding. Our findings highlight the importance of reducing the inbreeding coefficient of the dam (Dam F) to maximise breeding success (see Tables 3, 4). The study further reported that increases in genetic relatedness (MK at release) led to greater chances of reproductive success (Table 3). These subtle changes in genetics suggest that even small changes in genetic relatedness and mean kinship are important and warrants further investigations (note: that the F inbreeding coefficient was within the guidelines of the studbook, F = < 0.125). More detailed genetic and behavioural analysis is required to understand the social dynamics of Regent Honeyeaters, and the potential implications of captive social structure on post-release success.
The fitness of both wild and captive reared individuals must be considered when evaluating the success of a breed for release program, as well as the availability of suitable habitat for release. Translocations with wild sourced individuals have higher likelihood of success than translocations from captive bred individuals (Fischer and Lindenmayer, 2000) but this method is not always possible. In the wild, Regent Honeyeaters have a low contemporary reproductive output, with the range-wide nest survival between 2015 and 2017 being 31.7% (Crates et al., 2018). In this study, the Regent Honeyeater captive breed for release program reported 8.8% success of birds reaching the nestling or fledgling stage (i.e., 25/285) (Table 5), which is lower than the reported value for wild birds. However, it is important to bear in mind that north-east Victoria has always been observed to show a lower success rate than regions in NSW (e.g., Geering and French, 1998). Another marker to evaluate the success of the breeding program is the resighting of released birds beyond 12 months post-release. For the captive-bred birds, by the end of 2019 a total of 14.7% (42/285) of individuals had been resighted 12 months or more after release. This compares favourably to the resighting of colour banded wild birds, with post-12-month resighting rates around 9% (Regent Honeyeater recovery team unpublished data). Further, for a release program to be successful, observations of mixing and breeding between captive and wild birds is required. Post-release information has observed captive-bred females successfully breeding with wild males and in August 2019 there was a confirmed sighting of a captive male, released in 2017, that had paired with a wild female and successfully fledging two birds, providing support to the success of the breeding program (Regent Honeyeater recovery team unpublished data). A reality of conservation breeding programs is that ideal habitat for release often is no longer available, particularly when habitat loss is a threatening process involved in the species' decline, and compromises must be made. While Chiltern-Mt. Pilot National Park has historically seen lower nest success than other parts of the Regent Honeyeater range, it was one of four known key breeding areas for the species at the time of the development of the breeding program (Commonwealth of Australia, 2016), and was selected as the most suitable release site. A 2020 release took place in other key breeding habitat in NSW and others are intended in the future. Similar evaluation to the current study will be critical in determining the effect of release site on the success of individuals, and if the parameters we have identified as influencing fitness are applicable when birds are released into different habitat.
Other Considerations
In the wild, there is a male bias in the sex ratio for Regent Honeyeaters with an estimated 1.18 males per female (Crates et al., 2018). In the survival analysis, it was evident that female outperformed males at 20 weeks post-release and this may be a consequence of the sex bias in the wild (Figure 2). What was interesting was that female breeding analysis did not identify any of the features as being predictive of breeding success (see Supplementary Figure 1). Perhaps this highlights the need to undertake mate choice studies to understand more deeply female breeding behaviour and the factors that may be likely to affect it.
Birds with and without transmitters are not directly comparable, as wearing a transmitter makes it more likely that observers will find the bird each week. This should mean that if transmitters had no effect, we would see better survival for birds with transmitters, as birds are more likely to be found alive. However, we observe the opposite (Figure 2M), birds with transmitters have poorer predicted survival. This suggests wearing of transmitters may lead to worsesurvival outcomes.
Birds with previous exposure to mites (i.e., Acari) showed mixed results, with Regent Honeyeaters showing higher levels of survival post-release but showed decreased breeding success. Other research suggests the lack of exposure of captive populations to pathogens occurring in the wild may potentially impair survivability of released animals due to reduced immunity (Kołodziej-Sobocińska, 2019). Whilst the results on the importance to prior exposure to parasites on released Regent Honeyeaters is unclear, it does suggest that it is important to include parasite exposure in future captive breeding reviews.
Captive breeding programs serve as an increasingly valuable tool for species recovery. In this study, the use of evidence-based research to drive husbandry decision-making in captive breeding programs will lead to the refinement and improvement of zoo practises for captive rearing of Regent Honeyeaters and subsequently aims to increase the post-release success of the breeding program (see Figure 5). Through this analysis, it is evident that there is no one single contributing factor that promotes survivability or reproductive success in individual birds but rather there are multiple features that are important. We recommend that information relating to the husbandry practises on Regent Honeyeaters is continually reviewed and analysed so that we can understand and learn about the impact of zoo practises on individuals and continually optimise breeding programs to ultimately release fitter birds that have better conservation outcomes for the species.

Figure 5. Breeding and release guidelines developed as part of the review of the zoo life experiences for Regent Honeyeaters.
We also acknowledge that whilst every effort is being taken to optimise the breeding program for Regent Honeyeaters, the main threatening processes i.e., deforestation, habitat fragmentation and predation need to be addressed if the species has the potential to become self-sustaining and have improved conservation outcomes.
Data Availability Statement
The datasets used and analyzed during the current study will be made available by the corresponding author upon reasonable request.
Ethics Statement
All animals used in this study were managed in accordance with strict animal ethics protocols outlined by the Australian Bird and Bat Banding Scheme (permit # 2633/1), and the Victoria Department of Environment, Land, Water and Planning (scientific licences 10004378, 10005368, 10006686, 10007088, 10007525, 10008288, and animal ethics permits AEC 09.17, AEC 10.33, AEC 12.23, AEC 14.07, AEC 14.22 and AEC 17.003) and AEC 4b0511, AEC5b0819, and EAPA48705.
Author Contributions
JT, BP, and AE conceived the project and obtained funding. DI, GJ, and GT collected the field data. ES and DW were involved in the zoo aspect of the project. GP analysed the data. All authors were involved in the writing of the manuscript, development of ideas and conclusions and gave final approval for publication.
Funding
This work was supported by the Taronga Conservation Society and the Winifred Violet Scott Charitable Trust. Partial funding for field data collection was provided by NERC, Natural Environment Research Council. The Regent Honeyeater Recovery Program is funded from the NSW Department of Planning, Industry and Environment and the NSW governments Environmental Trust, the Victorian Department of Environment, Land, Water and Planning, and BirdLife Australia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank W. van der Ven, D. McAllister, and M. Rios Meza for their assistance with data extraction. We would like to acknowledge M. Van Sluys and S. Brice from the Welfare, Conservation and Science Division, M. Shiels, R. Matkovics, and the Bird department, L. Vogelnest, P. Thompson, and the Veterinary Wildlife Hospital team at the Taronga Conservation Society Australia for the helpful exchange of ideas, consultation and implementation of results derived from the review. We would like to acknowledge input from members of the recovery team and staff. We would also like to acknowledge all the community volunteers who assisted with thousands of hours of monitoring bird's post-release.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.669563/full#supplementary-material
Supplementary Figure 1. Coefficient paths and model selection criteria for one imputation of the data. Candidate models are indexed by the parameter lambda, which shrinks coefficients toward exactly zero as it increases (bottom plot). The top plots show the model selection criteria (cross validation or AIC). The model with the lowest value of the model selection criteria is chosen (vertical dashed line), with predictors with non-zero coefficients making up the final model. In both breeding models for females, the model chosen is the one without any predictors.
References
Bauch, C., Boonekamp, J. J., Korsten, P., Mulder, E., and Verhulst, S. (2019). Epigenetic inheritance of telomere length in wild birds. PLoS Genet. 15:e1007827. doi: 10.1371/journal.pgen.1007827
Beck, B. B., Kleiman, D. G., Dietz, J. M., Castro, I., Carvalho, C., Martins, A., et al. (1991). Losses and reproduction in reintroduced golden lion tamarins, Leontopithecus rosalia. Dodo J. Jersey Wildlife Preservation Trust 27, 50–61.
Belloni, A., and Chernozhukov, V. (2013). Least squares after model selection in high dimensional sparse models. Bernoulli 19, 521–547. doi: 10.3150/11-BEJ410
Berger-Tal, O., Blumstein, D. T., and Swaisgood, R. R. (2020). Conservation translocations: a review of common difficulties and promising directions. Anim. Conserv. 23, 121–131. doi: 10.1111/acv.12534
Berk, R., Brown, L., Buja, A., Zhang, K., and Zhao, L. (2013). Valid post-selection inference. Ann. Stat. 41, 802–837. doi: 10.1214/12-AOS1077
Bettegowda, A., Lee, K. B., and Smith, G. W. (2008). Cytoplasmic and nuclear determinants of the maternal-to-embryonic transition. Reprod. Fertil. Dev. 20, 45–53. doi: 10.1071/RD07156
Biggins, D. E., Vargas, A., Godbey, J. L., and Anderson, S. H. (1999). Influence of prerelease experience on reintroduced black-footed ferrets (Mustela nigripes). Biol. Conserv. 89, 121–129. doi: 10.1016/S0006-3207(98)00158-X
BirdLife, A. (2020). Regent Honeyeater Identification Guide. Available online at: https://www.birdlife.org.au/documents/WL-Regent_Honeyeater-identification-brochure.pdf (accessed November 10, 2020).
Canessa, S., Hunter, D., Mcfadden, M., Marantelli, G., Mccarthy, M., and Mccallum, H. (2014). Optimal release strategies for cost-effective reintroductions. J. Appl. Ecol. 51, 1107–1115. doi: 10.1111/1365-2664.12253
Commonwealth of Australia (2016). National Recovery Plan for the Regent Honeyeater. Commonwealth of Australia.
Crates, R. (2019). Mimicry in regent honeyeaters: is it really mimicry after all? The Whistler 13, 50–55.
Crates, R., Langmore, N., Ranjard, L., Stojanovic, D., Rayner, L., Ingwersen, D., et al. (2021). Loss of vocal culture and fitness costs in a critically endangered songbird. Proc. R. Soc. B Biol. Sci. 288:20210225. doi: 10.1098/rspb.2021.0225
Crates, R., Rayner, L., Stojanovic, D., Webb, M., Terauds, A., and Heinsohn, R. (2018). Contemporary breeding biology of critically endangered regent honeyeaters: implications for conservation. Ibis 161, 521–532. doi: 10.1111/ibi.12659
Ducatez, S., Baguette, M., Stevens, V. M., Legrand, D., and Freville, H. (2012). Complex interactions between paternal and maternal effects: parental experience and age at reproduction affect fecundity and offspring performance in a butterfly. Evolution 66, 3558–3569. doi: 10.1111/j.1558-5646.2012.01704.x
Eisenberg, D. T. A. (2019). Paternal age at conception effects on offspring telomere length across species-What explains the variability? PLoS Genet. 15:e1007946. doi: 10.1371/journal.pgen.1007946
Fischer, J., and Lindenmayer, D. B. (2000). An assessment of the published results of animal relocations. Biol. Conserv. 96, 1–11. doi: 10.1016/S0006-3207(00)00048-3
Franklin, D., Menkhorst, P., and Robinson, J. (1989). Ecology of the regent honeyeater Xanthomyza phrygia. Emu 89, 140–154. doi: 10.1071/MU9890140
Fraser, D. (2008). The role of the veterinarian in animal welfare. Animal welfare: too much or too little? Abstracts of the 21st symposium of the Nordic committee for veterinary scientific cooperation (NKVet). Vaerlose, Denmark. September 24–25, 2007. Acta Vet. Scand. 50(Suppl. 1), S1–12. doi: 10.1186/1751-0147-50-S1-S5
Friedman, J., Hastie, T., and Tibshirani, R. (2001). The Elements of Statistical Learning. New York, NY: Springer.
Friedman, J., Hastie, T., and Tibshirani, R. (2010). Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22. doi: 10.18637/jss.v033.i,01
Garnett, S., Szabo, J., and Dutson, G. (2010). The Action Plan for Australian Birds. Melbourne, VIC: CSIRO Publishing.
Geering, D., and French, K. (1998). Breeding biology of the regent honeyeater Xanthomyza phrygia in the Capertee Valley, New South Wales. Emu 98, 104–116. doi: 10.1071/MU98011
Gillespie, J. (2013). Regent Honeyeater Husbandry Guidelines, Anthochaera phrygia, Revised 2013. Taronga Conservation Society. Available online at: http://www.geckodan.com/wp-content/uploads/2017/12/Regent-Honeyeater-pdf (accessed November 10, 2020).
Griffith, S. C., Pryke, S. R., and Buttemer, W. A. (2011). Constrained mate choice in social monogamy and the stress of having an unattractive partner. Proc. Biol. Sci. 278, 2798–2805. doi: 10.1098/rspb.2010.2672
Grueber, C. E., Reid-Wainscoat, E. E., Fox, S., Belov, K., Shier, D. M., Hogg, C. J., et al. (2017). Increasing generations in captivity is associated with increased vulnerability of Tasmanian devils to vehicle strike following release to the wild. Sci. Rep. 7:2161. doi: 10.1038/s41598-017-02273-3
Higgins, P., Peter, J., and Steele, W. (2001). Handbook of Australian, New Zealand and Antarctic Birds. Melbourne, VIC: Oxford University Press.
Kołodziej-Sobocińska, M. (2019). Factors affecting the spread of parasites in populations of wild European terrestrial mammals. Mammal Res. 64, 301–318. doi: 10.1007/s13364-019-00423-8
Kvistad, L., Ingwersen, D., Pavlova, A., Bull, J. K., and Sunnucks, P. (2015). Very low population structure in a highly mobile and wide-ranging endangered bird species. PLoS ONE 10:e0143746. doi: 10.1371/journal.pone0143746
Liu, S. C., Gillespie, J., Atchison, N., and andrew, P. (2014). The recovery programme for the Regent honeyeaterAnthochaera phrygia:an example of conservation collaboration in Australia. Int. Zoo Yearbook 48, 83–91. doi: 10.1111/izy12040
Oliver, D. (1998). Ecology and Conservation of the Endangered Regent Honeyeater, Xanthomyza phrygia, in northern NSW (Ph.D. thesis). Armidale, NSW: University of New England.
Phillips, M. K., Henry, V. G., and Kelly, B. T. (2003). Restoration of the Red Wolf [Online]. Available online at: https://digitalcommons.unl.edu/icwdm_usdanwrc/234 (accessed November 10, 2020).
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Rubin, D. (1996). Multiple imputation after 18+ years. J. Am. Stat. Assoc. 91, 473–489. doi: 10.1080/01621459.1996.10476908
Shier, D. (2016). “Manipulating animal behavior to ensure reintroduction success,” in Conservation Behavior: Applying Behavioral Ecology to Wildlife Conservation and Management, eds D. Saltz, and O. Berger-Tal (Cambridge: Cambridge University Press), 275–304.
Simon, N., Friedman, J., Hastie, T., and Tibshirani, R. (2011). Regularization paths for cox's proportional hazards model via coordinate descent. J. Stat. Softw. 39, 1–13. doi: 10.18637/jss.v039.i05
Stojanovic, D., Cook, H. C. L., Sato, C., Alves, F., Harris, G., Mckernan, A., et al. (2019). Pre-emptive action as a measure for conserving nomadic species. The Journal of Wildlife Management, 83, 64–71. doi: 10.1002/jwmg.21575
SWIFFT (2020). Regent Honeyeater, State Wide Integrated Flora and Fauna Teams. Available online at: https://www.swifft.net.au/cb_pages/sp_regent_honeyeater.php (accessed October 17, 2020).
Taylor, G., Ewen, J. G., Clarke, R. H., Blackburn, T. M., Johnson, G., and Ingwersen, D. (2018). Video monitoring reveals novel threat to Critically Endangered captive-bred and released Regent Honeyeaters. Emu Austral Ornithol. 118, 304–310. doi: 10.1080/01584197.2018.1442227
Tibshirani, R. (1997). The lasso method for variable selection in the Cox model. Stat. Med. 16, 385–395. doi: 10.1002/(SICI)1097-0258(19970228)16:4<385::AID-SIM380>3.0.CO;2-3
United States Department of the Interior (2019). California Condor Recovery Program 2019. Annual Population Status.
Van Buuren, S., and Groothuis-Oudshoorn, K. (2011). MICE: multivariate imputation by chained equations R. J. Stat. Softw. 45, 1–67. doi: 10.18637/jss.v045.i03
Vecsei, M. (2015). Juvenile Song Learning in Regent Honeyeaters, Anthochaera phrygia, at Taronga Zoo, Australia. Sydney, NSW: Faculty of Science, Department of Biological Sciences Macquarie University, Sydney, NSW Australia in partial fulfilment of the requirements for the degree of Master of Research.
Watters, J. V., and Meehan, C. L. (2007). Different strokes: can managing behavioral types increase post-release success? Appl. Animal Behav. Sci. 102, 364–379. doi: 10.1016/j.applanim.2006.05.036
Webster, R., and Menkhorst, P. (1992). The regent honeyeater (Xanthomyza phrygia): population status and ecology in Victoria and New South Wales (Technical Report Series No. 26). Heidelberg, VIC: Arthur Rylah Institute for Environmental Research, Department of Conservation and Environment.
Woodworth, L. M., Montgomery, M. E., Briscoe, D. A., and Frankham, R. (2002). Rapid genetic deterioration in captive populations: causes and conservation implications. Conserv. Genet. 3, 277-288. doi: 10.1023/A:1019954801089
Wurm, M., Rathouz, P., and Hanlon, B. (2019). ordinalNet: Penalized Ordinal Regression. R package version 2.6.
Wurm, M. J., Rathouz, P. J., and Hanlon, B. M. (2017). Regularized ordinal regression and the ordinalNet R package. arXiv arXivpreprint:1706.05003.
Zoological Information Management System. (2021). Species360 Zoological Information Management System (ZIMS). Available online at: https://zims.isis.org
Keywords: reintroduction species, conservation biology, captive breeding, breeding program, Honeyeaters
Citation: Tripovich JS, Popovic G, Elphinstone A, Ingwersen D, Johnson G, Schmelitschek E, Wilkin D, Taylor G and Pitcher BJ (2021) Born to Be Wild: Evaluating the Zoo-Based Regent Honeyeater Breed for Release Program to Optimise Individual Success and Conservation Outcomes in the Wild. Front. Conserv. Sci. 2:669563. doi: 10.3389/fcosc.2021.669563
Received: 19 February 2021; Accepted: 28 April 2021;
Published: 15 June 2021.
Edited by:
Oded Berger-Tal, Ben-Gurion University of the Negev, IsraelReviewed by:
Nir Sapir, University of Haifa, IsraelRobert Heinsohn, Australian National University, Australia
Alison L. Greggor, San Diego Zoo Institute for Conservation Research, United States
Copyright © 2021 Tripovich, Popovic, Elphinstone, Ingwersen, Johnson, Schmelitschek, Wilkin, Taylor and Pitcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joy S. Tripovich, anRyaXBvdmljaEB6b28ubnN3Lmdvdi5hdQ==
 Joy S. Tripovich
Joy S. Tripovich Gordana Popovic
Gordana Popovic Andrew Elphinstone
Andrew Elphinstone Dean Ingwersen4
Dean Ingwersen4 Benjamin J. Pitcher
Benjamin J. Pitcher