- 1Agroscope, Environmental Analytics, Zürich, Switzerland
- 2Ithaka Institute, Arbaz, Switzerland
- 3Ithaka Institute, Goldbach, Germany
- 4Agroscope, Climate and Agriculture Group, Zürich, Switzerland
Pyrogenic carbon capture and storage (PyCCS), which comprises the production of biomass, its pyrolysis, and the non-oxidative use of the biochar to create carbon sinks, has been identified as a promising negative emission technology with co-benefits by improving soil properties. Using biochar as a soil additive becomes increasingly common as farmers seek methods for soil improvement and climate change adaptation. Concurrently, there is growing interest in quantifying soil organic carbon (SOC) at the level of individual plots to remunerate farmers for their good agricultural practices and the resulting (temporary) carbon dioxide removal (CDR). However, methods currently applied in routine analysis quantify SOC, irrespective of its speciation or origin, and do not allow to distinguish biochar-C from SOC. As certification of PyCCS-derived CDR is already established using another quantification method (i.e., analysis of biochar-C content, tracking and registration of its application, and offsetting of carbon expenditures caused by the PyCCS process), the analysis of biochar-C as part of SOC may result in double counting of CDR. Hence, the objectives of this review are (1) to compare the physicochemical properties and the quantities of biochar and SOC fractions on a global and field/site-specific scale, (2) to evaluate the established methods of SOC and pyrogenic carbon (PyC) quantification with regard to their suitability in routine analysis, and (3) to assess whether double counting of SOC and biochar C-sinks can be avoided via analytical techniques. The methods that were found to have the potential to distinguish between non-pyrogenic and PyC in soil are either not fit for routine analysis or require calibration for different soil types, which is extremely laborious and yet to be established at a commercial scale. Moreover, the omnipresence of non-biochar PyC in soils (i.e., from forest fires or soot) that is indistinguishable from biochar-C is an additional challenge that can hardly be solved analytically. This review highlights the risks and limits of only result-based schemes for SOC certification relying on soil sampling and analysis. Carbon sink registers that unite the (spatial) data of biochar application and other forms of land-based CDR are suggested to track biochar applications and to effectively avoid double counting.
1 Introduction
Rising temperatures, water scarcity, prolonged droughts, and unexpected weather events are intensifying more than ever in recent history. These impacts are not surprising as the current atmospheric greenhouse gas (GHG) concentration is higher than at any point in the last 800,000 years. Altogether, global net anthropogenic GHG emissions were 59 ± 6.6 Gt CO2eq in 2019, which was 12% higher than in 2010 (Canadell et al., 2021). To accelerate climate mitigation activities, a global framework was set to limit global warming to well below 2°C and pursue efforts to limit it to 1.5°C (United Nations, 2015). This goal can no longer be achieved by reducing emissions alone, and carbon dioxide removal (CDR) is necessary to transfer carbon (C) from the atmosphere into non-atmospheric C-sinks (Smith et al., 2020).
The European Commission recognizes the importance of industrial negative emission technologies, such as direct air capture with carbon storage (DACCS) and nature-based solutions for CDR. The buildup of soil organic matter (SOM) is a crucial element of nature-based solutions, which can be achieved through reduced tillage, reduced drainage, cover crops, and several other methods, including biochar (cf. definition in Table 1) application to soil (Whitman et al., 2010; Blanco-Canqui et al., 2020; COWI, EI, and IEEP, 2020; Don et al., 2024). In agriculture, biochar is used, among others, as a plant nutrient carrier, compost additive, animal bedding material, and soil conditioner to alleviate nutrient losses, stimulate buildup of soil organic carbon (SOC), counteract soil erosion, and improve soil water retention and long-term soil fertility under a changing climate (Blanco-Canqui et al., 2020; Schmidt et al., 2021). The production and non-oxidative application of biochar itself is considered a negative emission technology, often referred to as PyCCS (Schmidt et al., 2021; Lefebvre et al., 2023). In 2022, a still rather modest 100,000 t of CO2 was removed from the atmosphere via PyCCS in Europe, but this number is expected to increase to up to 225 Mt. of CO2 annually by 2036 (EBI, 2023). Globally, PyCCS may contribute to 6–35% of the negative emissions needed by 2,100 without generating undesirable side effects through land use change (Werner et al., 2021).
A certification of negative emissions is necessary for their financing, which requires the respective carbon removal technique to be quantifiable, deliver additional climate benefits, strive to store carbon for a long time, and contribute to effectively removing carbon from the atmosphere (COWI, EI, and IEEP, 2020). Accordingly, the EU suggested four QUantification, Additionality, Long-term storage, sustainabilITY (QU. A. L. ITY) criteria for industrial carbon removal certification methodologies (European Commission, 2022). Today, these carbon removals are certified by private companies according to their own guidelines or companies relying on independent third-party certification, e.g., the European Biochar Certificate (EBC)‘s C-Sink Certificate (EBC, 2021). Either way, negative emission certificates are generated and sold on the voluntary market. A core element of a well-functioning market is that each negative emission can be certified only once. Avoiding double counting is essential to maintaining the integrity of the carbon removal systems and to collecting correct data for greenhouse gas inventories. Double counting of negative emissions may occur when more than one entity claims the same negative emission (Schneider et al., 2015; COWI, EI, and IEEP, 2020), e.g., when biochar is certified as a biochar C-sink and as part of SOC increase, as detailed below.
For biochar, the certification process involves the quantification of its organic carbon (Corg) content and molar H-to-Corg ratio. Once soil application is confirmed, a fixed portion of biochar-C cannot be burned or oxidized by other means anymore (e.g., 74–93% when molar H to Corg ratio < 0.4) and the certification process is completed (Woolf et al., 2010; Budai et al., 2013; IPCC, 2019; EBC 2021–2023; Rodrigues et al., 2023). In contrast, result-based SOC certification schemes usually include repeated soil sampling and quantification of SOC to assess this (temporary) C-sink (Paul et al., 2023). However, as discussed in detail below, the quantification of SOC typically also includes biochar-C and other pyrogenic carbon (PyC) contained in pyrogenic carbonaceous materials (PCM, cf. Table 1) such as soot or wildfire-derived char. Therefore, e.g., the EBC C-Sink guidelines for certifying biochar C-sinks require that when farmers purchase certified biochar, they sign a contract that they are not participating in a SOC certification program to avoid double counting (EBC, 2021). This is a weak and hardly verifiable requirement that may be difficult to adhere to with the increasing interest in SOC certification and expanding the use of biochar. In addition, the prevailing inconsistencies in SOC monitoring protocols and the lack of standardized protocols in some regions could intensify the double-counting risk (Smith et al., 2020; Oldfield et al., 2022). Therefore, the extent to which non-pyrogenic SOC and non-biochar PyC can be quantified in routine soil analysis in the presence of biochar should be evaluated. Routine analysis here means that the method can be implemented with a high degree of automation and cost-effectively on a large number of samples to enable a low-cost certification process. In Europe, costs for SOC determination can be as low as European €10 or less per sample according to the information provided by commercial laboratories and farmers. To date, many chemical, thermal, physical, spectroscopic, and molecular techniques have been developed for SOC and PyC differentiation and quantification (Hammes et al., 2007; Zimmermann and Mitra, 2017). Hence, this review aims to
i. Compare the physicochemical properties and the quantities of biochar and SOC fractions on a global and field/site-specific scale,
ii. Evaluate the established methods of SOC quantification, and
iii. Discuss existing analytical methods for PyC quantification and their suitability in routine analysis of biochar-C content in agricultural soils.
This review also aims to evaluate whether double counting of SOC and biochar C-sinks can be avoided via analytical techniques and to derive conclusions for reliable approaches for CDR certification when biochar is applied to soil.
2 Methods
Literature research was performed on Web of Science and Google Scholar covering the past 20 years. However, several articles published between 1990 and 1999 were also included due to their importance for the discussion. The following keywords were used during the article search: biochar; pyrogenic carbon continuum; soil organic carbon; soil organic carbon analysis; soil; biochar separation; biochar quantification in soil; pyrogenic carbon analysis; pyrogenic carbonaceous material; biochar carbon sink certification; carbon dioxide removal, CDR; double counting; carbon credits; pyrogenic carbon stocks; and fluxes. Care was taken to prioritize peer-reviewed publications and those articles that were written in English. However, several methods-related documents published in German were also included due to their importance in discussing analytical developments. Studies related to the PyC analysis in atmospheric samples were excluded. Priority was given to the recent PyC analysis involved with biochar-C analysis. After the initial screening, the articles were manually classified into different review sections.
3 Carbon speciation, stocks, and fluxes in soil and biochar
Biochar contains a wide range of organic and mineral components, whose mass fractions and speciation are determined by both the feedstock and the pyrolysis process parameters, such as temperature (Keiluweit et al., 2010; Singh et al., 2012; Bird et al., 2015; Rathnayake et al., 2020). Carbon speciation includes aliphatic and (polycyclic) aromatic hydrocarbons that form graphene-like sheets but may also comprise some residual non-pyrogenic compounds, e.g., from lignin; ash content can be in the range of 3–90% (Keiluweit et al., 2010; Hardie et al., 2014; Xiao and Chen, 2017; Ippolito et al., 2020).
SOC comprises a wide range of carbonaceous moieties, including carbon derived from readily decomposable plant debris and microbial biomass (i.e., cellulose, sugars, proteins, and lipids), lignin, waxes, resins, tannins, and secondary metabolites from plants, humic substances, and PCM (Baldock and Skjemstad, 2000; Six et al., 2002; Lehmann and Kleber, 2015; Reisser et al., 2016). Pyrogenic carbonaceous material in soil exhibits overlapping physicochemical properties to that of biochar and is of both natural (e.g., wildfire char) and anthropogenic (soot) origins (Knicker, 2011; Santín et al., 2017). The continuum of PCM in soil, their formation pathways, initial reservoirs, and some basic physicochemical characteristics are indicated in Figure 1.
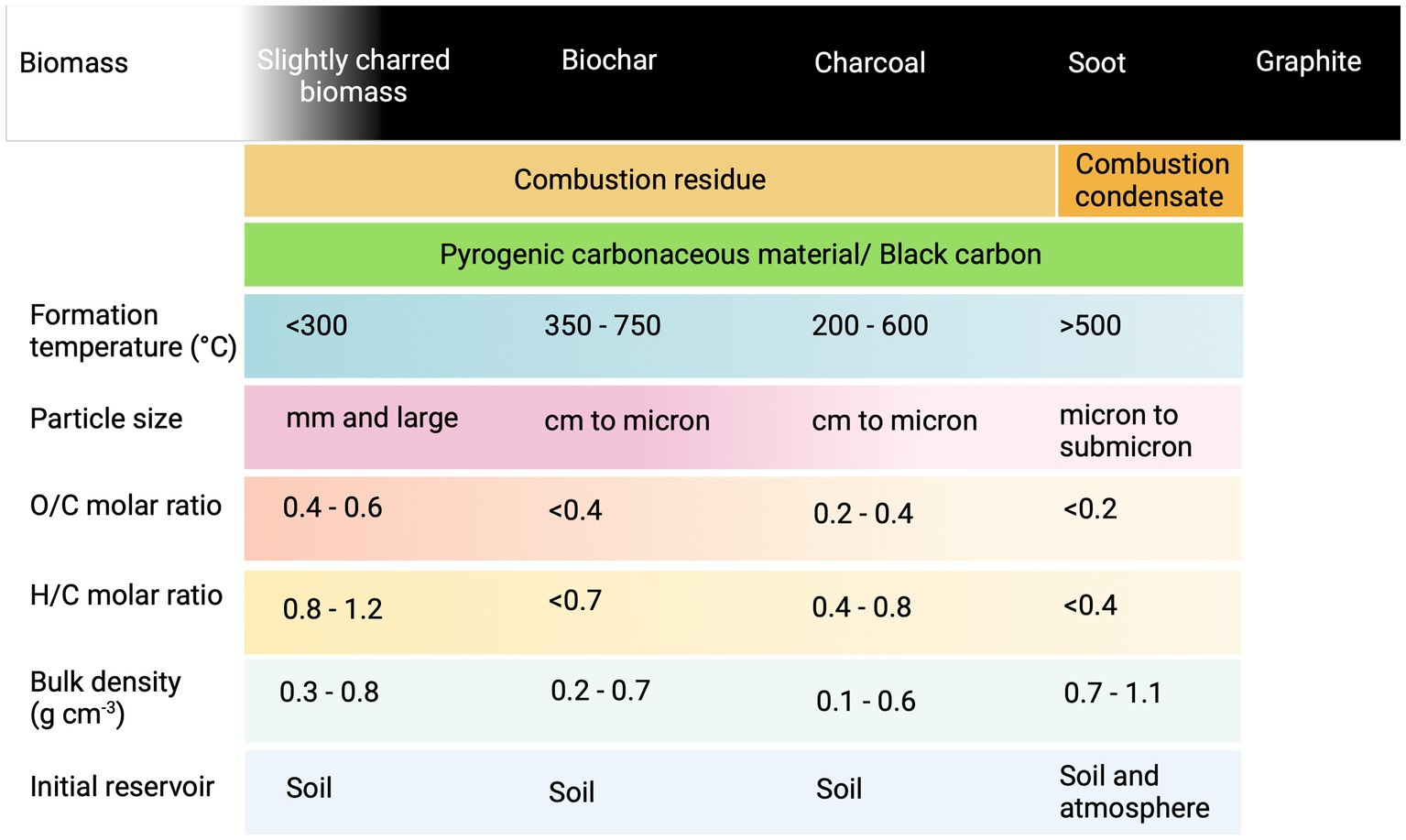
Figure 1. Continuum of pyrogenic carbonaceous materials (PCM) in soil (Hedges et al., 2000; Masiello, 2004; Wiedemeier, 2014; Bird et al., 2015; Santín et al., 2017; Wagner et al., 2018). This figure was created with BioRender.com.
Both on a global level and the level of an individual plot of land, both non-pyrogenic and pyrogenic SOC are present and that might interfere during biochar-C analysis. Global PyC stocks due to PCM in soil range from 54 to 212 Gt (Supplementary Table S1). Depending on the fire intensity in the past, the PyC fraction in SOC varies globally, but also varies for land use type and soil texture, with agricultural land and clay soils rather showing higher PyC (Reisser et al., 2016). Apart from biochar, PCM enters soils via direct and indirect routes such as wild or manmade fires (land clearing, burning of crop residues, and unintended fires as a result of peatland drainage) and atmospheric depositions from incomplete combustion (soot), which is more prominent in upper soil horizons (Sanderman et al., 2021). Tillage, erosion, and surface runoff activities induce the horizontal and vertical movements of PCM in soils (Qi et al., 2017; Jiménez-González et al., 2021). The annual global input flux of PyC to the terrestrial environment via vegetation fires and fossil fuel burning ranges from 40 to 383 Mt. Yr−1 and 2 to 12 Mt. Yr−1, respectively. The annual global biochar input flux is approximately 0.1 Mt. Yr−1 (Supplementary Table S1), and PyC loss due to remineralization ranges from 103 to 207 Mt. Yr−1 (Bird et al., 2015).
Depending on the soil types and PyC analytical methods used, the PyC content in soils could vary from 1 to 37% of SOC (Table 2). The application of 1–10 t ha−1 biochar to mineral soils with low-to-medium SOC content (it is uncommon to apply biochar to organic soils or minerals soil with high SOC) results in 2–18% of SOC being biochar-C nominally (Figure 2). According to that, the initial non-biochar PCM content in soil (4.3–5.6 t ha−1) could be higher than a low biochar input (0.7 t ha−1 of biochar-C for 1 t biochar) to the soil.
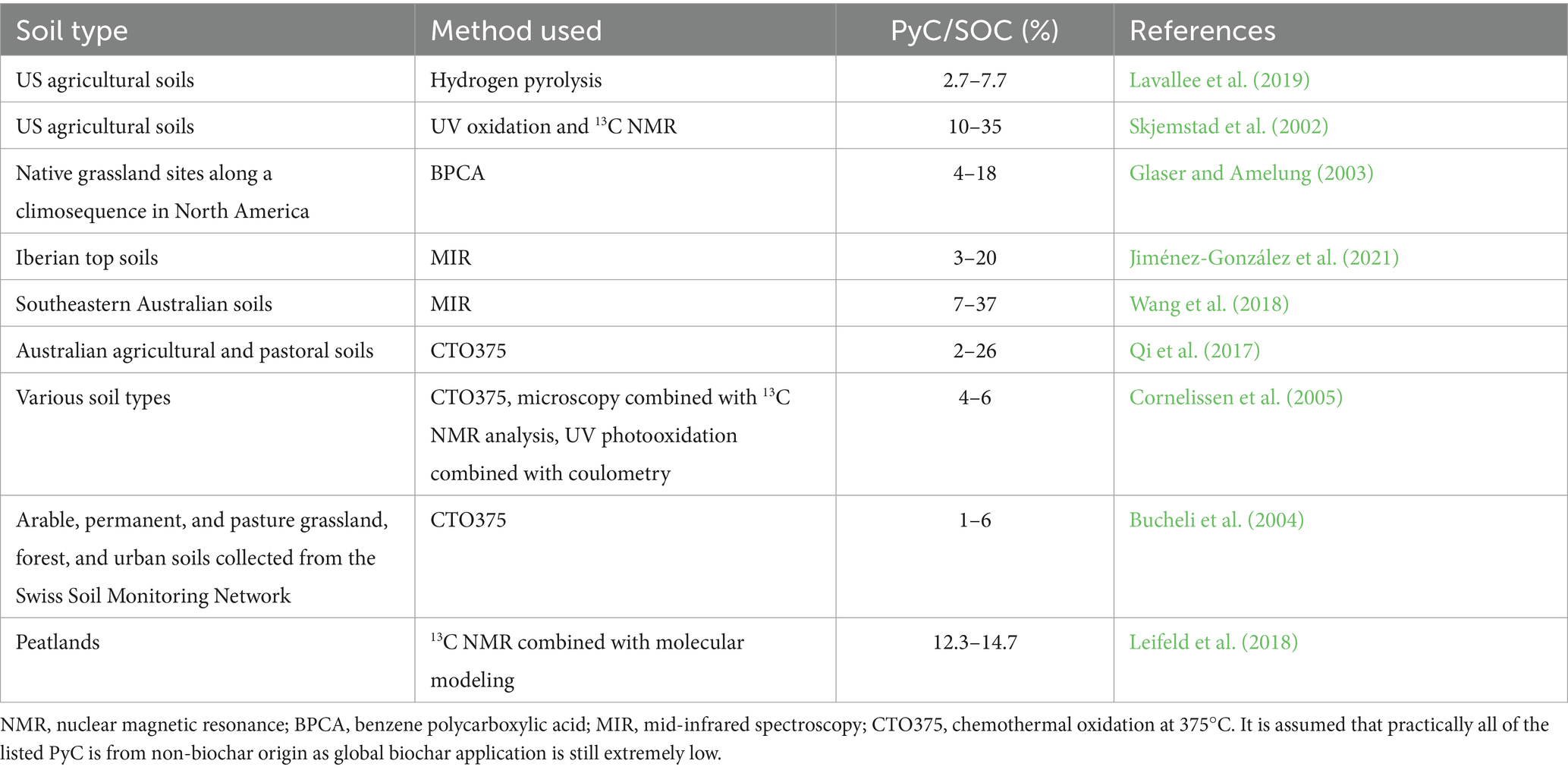
Table 2. The percentage of PyC in total SOC in different soils as reported in previous studies using different analytical methods.
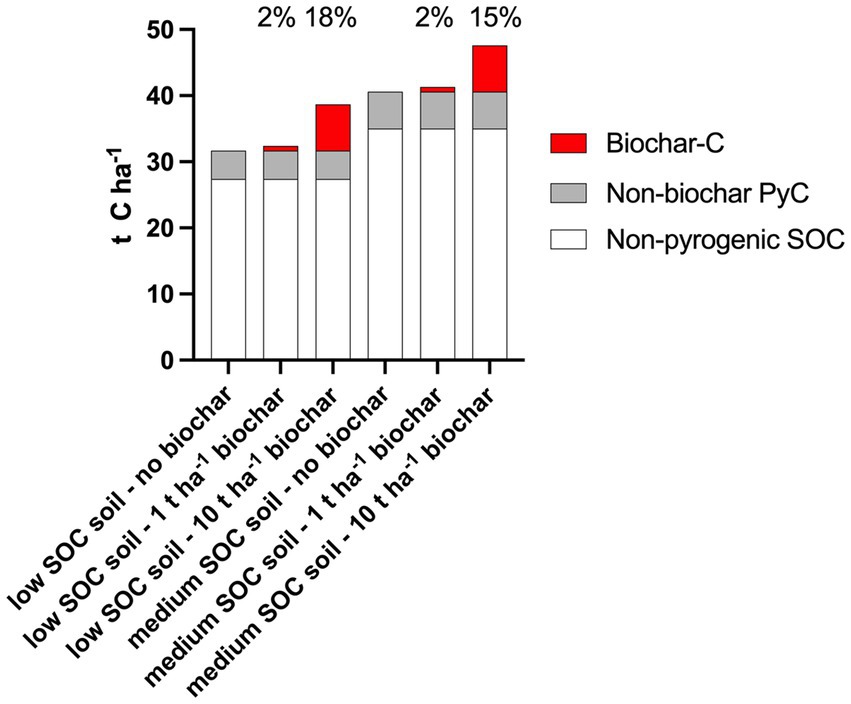
Figure 2. An exemplary illustration of the soil organic carbon (SOC) stocks and its composition from non-pyrogenic SOC, non-biochar pyrogenic carbon (PyC), and biochar carbon after application of 0, 1, and 10 t ha−1 biochar with 0.7 g g−1 carbon (biochar-C) content. The low (31.7 t ha−1) and medium (40.6 t ha−1) SOC stock values were based on the SOC concentrations in the top 20 cm of Swiss arable soils (Leifeld et al., 2005). Non-biochar PyC was assumed to be 13.7% of the SOC (Reisser et al., 2016). Percentages indicate the contribution of biochar to total SOC (i.e., analytically determined SOC including pyrogenic SOC).
4 Current methods to quantify SOC in routine analysis
4.1 Dry oxidation methods
The most widely used methods for determining SOC are based on dry oxidation. For this purpose, the sample is heated to a defined temperature, oxidized by pure oxygen, and the resulting CO2 is quantified. Inorganic carbon, i.e., carbonates, must be removed by acid treatment prior to measurement and deducted to accurately calculate the Corg (VDLUFA, 1991; DIN EN 15936). Dry oxidation-based methods are simple, easy to automate, and have high throughput for smaller sample amounts (i.e., milligram levels). However, in principle, this method is similar to the method to measure biochar-C; e.g., the analytical guidelines of the EBC define the application of the DIN 51732, a dry oxidation procedure for solid fuels (Bachmann et al., 2016; Bird et al., 2017; EBC, 2012–2023). Given that the standard method for quantifying SOC and biochar-C analysis are practically the same, the SOC method will account for biochar as SOC.
4.2 Wet oxidation methods
Soil organic carbon can also be quantified by wet oxidation. Here, a mixture of potassium dichromate and sulfuric acid (sulfochromic oxidation) is added to the sample, followed by measuring the residual oxidizing agent (Agroscope, 2020) or the newly formed Cr (III) (ISO14235). While ISO14235 was withdrawn, sulfochromic oxidation is still used; e.g., it is the mandatory reference method in Switzerland (Agroscope, 2020) and the Soil Survey Standard Method in New South Wales, Australia (Department of Sustainable Natural Resources, New South Wales, 1990). The advantage of wet chemical approaches is that organic carbon (including amorphous organic carbon and PyC) is oxidized in a very targeted manner, whereas carbonates remain unaffected and are thus not detected. However, biochar or any other non-biochar PyC present in soil can be oxidized under the conditions applied in the Swiss reference method. Thus, biochar-C will be at least partially detected as SOC (Agroscope, 2020). In addition, Hardy and Dufey (2017) clearly showed that wet oxidation according to the Walkley–Black method will at least partially oxidize charcoal, with a potential impact of charcoal aging on its resistance to wet oxidation.
4.3 Analytical precision and minimal detectable difference (MDD)
For the Walkley and Black method of wet oxidation, the Global Soil Laboratory Network quantified a coefficient of variation (CV) of 2.7% when the sample contains 1% organic carbon (FAO, 2019). Assuming soils with low (31.7 t ha−1) and medium (40.6 t ha−1) SOC contents in the upper 20 cm [cf. Figure 2, soil data for Switzerland (Leifeld et al., 2005)], the addition of 0.7 t ha−1 biochar-C (e.g., 1 t of biochar with 70% C content) increases SOC by 2.2 and 1.7%, respectively. This change is lower than the CV and thus could not be accurately measured. For the dry combustion method used in the elemental analyzer, Fliessbach et al. (2021) reported a 1–2% CV for a sample containing 0.73–2.6% of C; i.e., again, the addition of 1 t ha−1 biochar would not be recognized analytically.
However, the accuracy of SOC determination is not solely determined by analytical precision of organic carbon quantification but also, e.g., by spatial heterogeneity as well as the variability of soil bulk density that is needed to derive SOC stocks (t C ha−1) from Corg (%C; Poeplau et al., 2017, 2020; Wiesmeier et al., 2020). Even under reasonably optimized conditions (100 samples per plot of 1–2 ha) to be applied for a sampling of scientific long-term experiments, Schrumpf et al. (2011) quantified an MDD of 1–2.5 t ha−1of SOC for cropland and grassland sites, respectively. This further confirms that a single application of 1 t biochar (70% Corg content) would not be detectable, while larger applications (e.g., 3 t ha−1 biochar) and/or repeated application will quickly pass this level of MDD within the typical timeframes of 3–5 years (Wiesmeier et al., 2020) between repeated SOC quantification to detect stock changes. However, the MDD is likely to be higher for routine analysis when less than 100 samples are taken per plot for economic reasons.
5 Methods to quantify PyC contents in soils, their prospects, and limitations in the quantification of soil biochar-C content in routine analyses
Pyrogenic carbon in soil consists of a continuum of materials between the partly charred material and highly graphitized soot-like structures without clear-cut boundaries (Figure 1) (Schmidt et al., 2001; Knicker, 2011). The existing methods for quantifying, isolating, and characterizing PCM in soil attempt to differentiate the inorganic material, thermally unaltered organic carbon, and PyC using chemical, thermal, physical, spectroscopic, or molecular marker techniques (Bird, 2015). Due to technical limitations and variations in treatment severities, different methods isolate and characterize different fractions of the PyC continuum that are unique for the applied methodologies (Zimmermann and Mitra, 2017). Previous literature has thoroughly discussed these analytical methods in quantifying pyrogenic and non-pyrogenic fractions of SOC (Schmidt et al., 2001; Hammes et al., 2007; Wiedemeier et al., 2013; Bird, 2015; Hardy et al., 2022). Hence, this section and Table 3 only briefly summarize the prospects and limitations of currently available PyC analytical methods and evaluate their suitability for quantifying soil biochar-C in the presence of non-biochar PyC in soil with the goal of allowing quantification of non-biochar SOC in routine analysis.
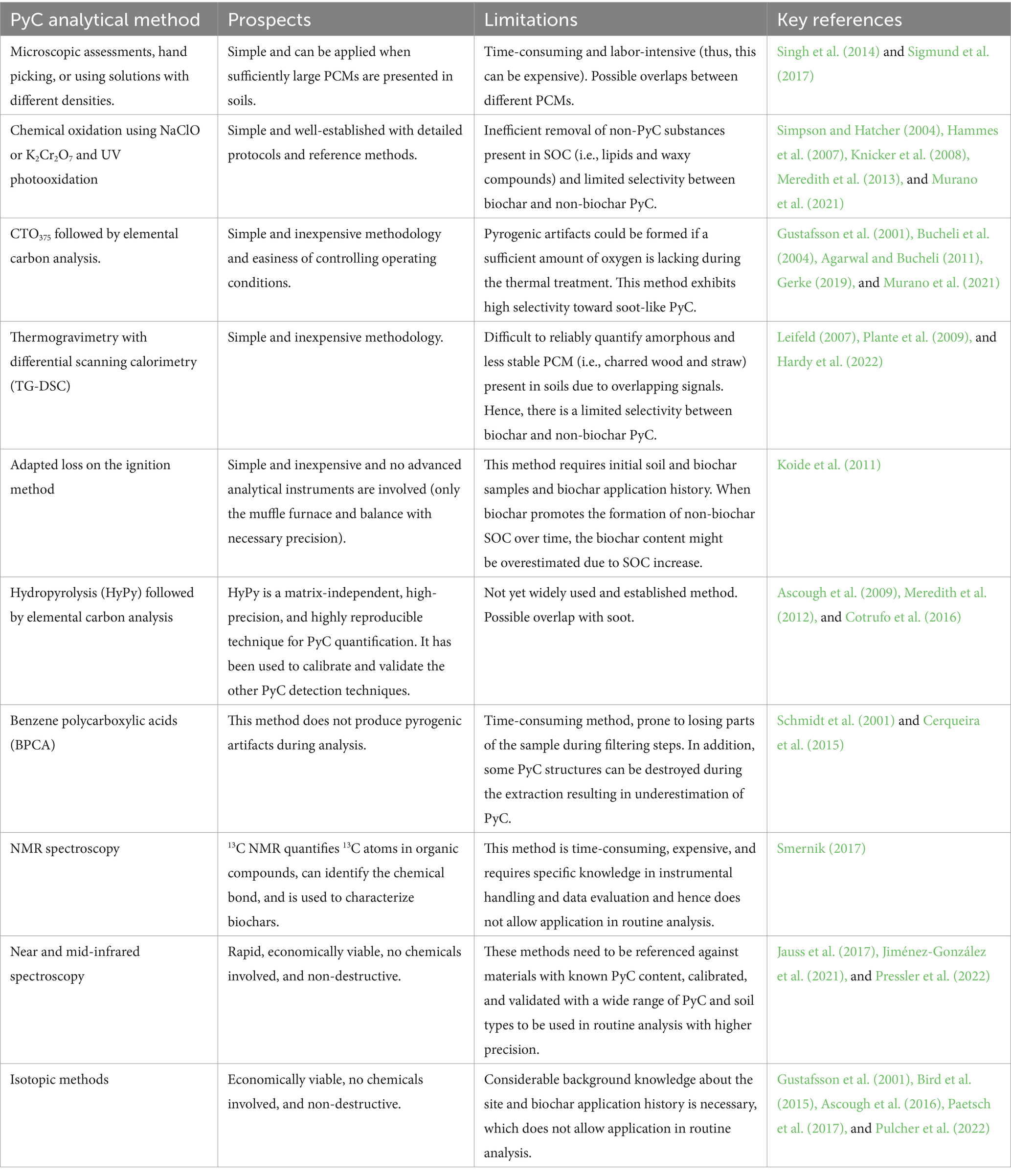
Table 3. Prospects and limitations of existing PyC analytical methods in distinguishing soil applied biochar-carbon from non-biochar PyC and from amorphous SOC.
Physical techniques used for the biochar separation from the soil are based on its visual appearance or differences in biochar material size or density compared to other non-biochar soil organic and mineral matter. When bigger biochar particles (> 2 mm) are applied to the soil, it is easier to physically separate by hand picking or sieving and by combining with microscopic techniques for further identification and verification (Spokas, 2013; Paetsch et al., 2017). However, due to the similar color (i.e., black) and depending on the abundance of non-biochar PCM in the soil, there can be biases to the overall quantified biochar content by hand picking or their visual appearance. Biochar in soil is subject to physical disintegration; i.e., particle size is reduced over time (Spokas et al., 2014; Sigmund et al., 2023), which will result in an underestimation of the soil biochar content. Alternately, biochar can be separated by flotation due to its bulk density, which is lower than soil mineral matter. Liquids with different densities, such as water (Sigmund et al., 2017) or sodium polytungstate solution (Singh et al., 2014), can be used. However, biochar in soil may form biochar mineral complexes whose density might be similar to that of bulk soil (Archanjo et al., 2017; Yang et al., 2021). Due to the overlapping bulk density, skeletal density, envelope density, and porosity values of biochar and other non-biochar PCM (Santín et al., 2017), the sensitivity and precision of density separation methods can be lower. Physical methods can be extremely time-consuming and labor-intensive, and sample losses could occur during sample handling. Hence, physical techniques are irrelevant to biochar quantification in routine analysis. However, they may be useful in research to gain aged biochar for analysis or experiments.
The use of chemical techniques for quantifying biochar in soil depends on the oxidative resistance of the biochar carbon material compared to the other non-biochar SOC material. The methods include the use of NaClO, K2Cr2O7, or UV-based oxidation methods. However, they could not effectively oxidize hydrophobic non-biochar SOC and may at least partially oxidize PyC (Hammes et al., 2007; Knicker et al., 2008; Meredith et al., 2013; Murano et al., 2021). Furthermore, the K2Cr2O7 oxidation method exhibited good reproducibility and recovery for the chemical oxidation-resistant elemental carbon (COREC) content in plant char (i.e., plant material charred at 350°C under oxic conditions) mixed with HF-treated soil. Nevertheless, that method has not yet been validated for the biochar produced from various sources and soils with various amounts of non-biochar PCM.
Chemothermal oxidation method at 375°C (CTO375) followed by elemental carbon analysis is a relatively simple, inexpensive technique to isolate and quantify PyC in soils (Gustafsson et al., 2001; Agarwal and Bucheli, 2011). However, pyrogenic artifacts could be formed if a sufficient amount of oxygen is lacking during the thermal treatment, lignin may partly survive the treatment, and some of the PyC can be oxidized entirely during the oxidation step, whereby the extent may vary for different soils as well as for PCM types (Agarwal and Bucheli, 2011; Gerke, 2019; Murano et al., 2021). Thermogravimetric analysis and differential scanning calorimetry (TGA-DSC) offer the possibility to identify thermal signatures specific for SOC and biochar/other non-biochar PCMs. However, biochars show different signatures depending on the pyrolysis temperature, and also, soils may vary in their background signal (Leifeld, 2007; Hardy et al., 2022). Hence, unless the baseline soil (without biochar) exhibited distinct thermal signatures compared to that of biochar, it is harder to quantify biochar content in soil reliably. This method still needs to be validated for different biochars, different application rates, and soils with various amounts of native PCM and mineral compositions.
Hydropyrolysis (HyPy) is a thermal technique with high reproducibility that can be used to quantify the most stable fraction of PCM present in soil (Meredith et al., 2012). It measures the fraction of PyC that contains >7 aromatic ring structures with an H/C molar ratio of less than 0.5 (stable polycyclic aromatic carbon—SPAC) (Meredith et al., 2012). Wildfire chars exhibit considerably lower SPAC content (i.e., <30–40% on a dry ash-free basis) than biochar samples (i.e., SPAC content up to 75% on a dry ash-free weight basis [Santín et al., 2017]). Using HyPy may result in an underestimation of biochar’s contribution to SOC as biochar also contains non-SPAC carbon, especially when produced at rather low temperatures, which might be certified as a temporary C-sink (Schmidt et al., 2022). Thus, HyPy still might not fully exclude double counting of CDR. In contrast, HyPy might overestimate the contribution of biochar-C to SOC for soil with high natural PCM content.
Koide et al. (2011) recently used an adapted loss on the ignition (heating sample at 550°C for 4 h) method to determine the soil biochar content. This method is simple and inexpensive, and no advanced analytical instruments are involved, only the muffle furnace and balance with the necessary precision. However, this method has to date only tested for extremely high biochar application rates (20–25 t ha−1) and requires reference samples of both soil and biochar, which both hinders its application in routine analysis. Nakhli et al. (2019) significantly improved the loss-on-ignition method by looking at two different temperatures, but the need for the reference sample remains.
Benzene polycarboxylic acids (BPCA) is a molecular marker method used in soil PyC analysis (Schmidt et al., 2001). This method determines the condensed aromatic structure in PCM and does not produce pyrogenic artifacts during analysis. The conventional BPCA method is time-consuming, prone to losing parts of the sample during filtering steps, and highly variable due to the different GC instrumental conditions and calibrations used (Hammes et al., 2007; Wiedemeier et al., 2016). Overestimation may arise from quantifying humic acid compounds such as PyC (Chang et al., 2018; Gerke, 2019).
The 13C nuclear magnetic resonance (NMR) quantifies 13C atoms in organic compounds and can identify the chemical bonds (Smernik, 2017). Both soil and biochar contain aliphatic-C and aryl-C. Hence, this method cannot differentiate biochar and non-biochar SOC, e.g., in humic acids. Methods based on IR are promising due to their simplicity, low cost, and potential to simultaneously determine multiple parameters beyond SOC, e.g., inorganic carbon and total nitrogen with one measurement (Baldock et al., 2013). However, their widespread application requires comprehensive reference databases that comprise spectra and calibrations for all types of soil that shall be investigated. In Australia, a national model for an MIR-based SOC determination was built based on 20,495 soil samples from 4,526 locations across the country (Baldock et al., 2013). Such comprehensive reference databases might even allow us to distinguish biochar and non-biochar PyC, as the site-specific reference spectra might also cover the different background concentrations of PyC. However, this needs to be proven separately. It also needs to be verified in detail to what extent the semi-persistent carbon fraction of biochar can also be detected by NIRS/MIRS as part of the biochar-C pool. In essence, IR-based methods are promising and have the potential to even reduce costs per sample, but require a tremendous investment to build the reference that would have to be accomplished by public actors, like the Commonwealth Scientific and Industrial Research Organization in Australia.
Isotopic carbon chemistry can also be used to delineate the biochar-C in the soil if their 13C isotopic signatures are substantially different from the soils they applied (Bird et al., 2015). However, in reality, framers may apply biochar produced from various feedstock materials into the soil, originating potentially from both C3 and C4 plants with contrasting isotopic signatures, which hinders the application of isotope-based methods in routine analyses (Chalk and Smith, 2022).
Generally, all of these methods require considerable background knowledge about the site and biochar application history, partly high-tech equipment, or, in the case of near or mid-infrared (NIR/MIR) spectroscopy a site/soil-specific reference library, which is not yet routinely available. Thus, in the foreseeable future, it will hardly be possible to assess the MDD between biochar and other non-biochar PCM in biochar-applied soils with sufficient sensitivity, selectivity, and precision using currently available PyC analytical methods in routine analysis.
6 C-sink registry
An analytical solution to quantify non-biochar SOC is in itself only necessary as long as there is no confirmed or independently verifiable information as to whether, and if so at what dosage, biochar-C has been applied to a specific piece of land. Once such information is available, total SOC can be determined with state-of-the-art routine analysis (cf. Section 3), and the already-certified biochar-C could be simply subtracted arithmetically. Advanced biochar-based C-sink certification methods require the registration of biochar C-sinks in a public carbon sink registry containing all necessary information, such as biochar-C content and molar H to Corg ratio, the amount and date of applied biochar, and localization of the biochar C-sink (EBC, 2021; Etter et al., 2021; Puro.earth, 2024). However, farmers can apply biochar without C-sink certification and without entry into a carbon registry. In that case, no double accounting would occur when the total SOC of the soil is analyzed. However, if biochar C-sinks are valorized for CO2-emission offsets on the voluntary market without registering the localized biochar application, the risk of double accounting is high. Therefore, biochar-based C-sinks may not be certified without listing on a public C-sink registry to make the above information available. When SOC-based C-sinks are certified, it is necessary to query the state or regional C-sink registry to verify how much C-sink-certified biochar was applied during the SOC certification period in order to subtract the already-certified C-sink from the SOC C-sink certification.
Tracking systems are already established as a part of C-sink registers or in energy attribute certificates, which are issued to confirm the use of renewable energy (NREL, 2015). In addition, registers are used for certificates from emission avoidance and forestry; e.g., carbon credits created within the clean development mechanism had to be listed in the international transaction log provided by the United Nations Framework Convention on Climate Change (Lovell, 2010). Here, avoiding double use of certificates is the major goal, and spatial information is not included, which would be added information to C-sink registers. Currently, dedicated C-sink registers are offered, e.g., by C Capsule (Sheffield, United Kingdom), Puro.earth (Helsinki, Finland), and the Global Carbon Register Foundation (Arbaz, Switzerland). All providers mentioned above offer global applicability but differ in the details of the registered information. Each register may contain entries from all over the world. Thus, a single register query is currently insufficient to obtain the necessary information. This could be overcome either through geographical exclusivity of the registers or through a global (e.g., International Organization for Standardization (ISO)) standard for data structure and query procedures of such registers so that even information that may be distributed across different databases can be retrieved in a uniform manner. Geographical exclusivity could be achieved in the form of national registers run by federal offices or independent non-profit, non-governmental institutions (foundations) with a governmental mandate that would exclude the operation of any other non-connected register organization in the country. A description of the certification process for biochar and SOC C-sink using a carbon sink register to avoid double counting is explained in more detail in the Supplementary material with the aid of a hypothetical example (Supplementary Text 1).
7 Conclusion
Biochar production and biochar application to agricultural soils to build up SOC are two synergistic CDR methods. However, their combination is a challenge to the monitoring, reporting, and verification (MRV) of SOC buildup as there is a significant risk of double counting when biochar is applied, and SOC buildup is remunerated based on soil sampling and quantification of organic carbon. Based on this review, standard analytical methods for soil carbon are fit for purpose but cannot distinguish between SOC and PyC including biochar. More advanced methods can distinguish PyC from non-pyrogenic SOC but are complex, expensive, and not suitable for routine analysis. Moreover, none of these PyC analytical methods can sufficiently distinguish biochar-C from non-biochar PyC present in soil. Thus, there is a considerable risk of double counting of CDR when the production of biochar-C is certified and, at the same time, SOC certification based on result-based payments with sampling and organic carbon quantification performed on land to which biochar was applied. Hence, this risk can efficiently be addressed at the governance level using the following two steps: (1) all biochar-based carbon sinks shall be registered in a public C-sink register, and (2) when certifying SOC increase as CDR, the public C-sink register shall be consulted to control that no certified biochar was applied to the respective field during the certification period. If biochar was applied, the SOC increase needs to be corrected arithmetically for the already-certified biochar-C. Similarly, SOC measurements taken for SOC-based CDR certification should be registered and geo-referenced to improve their MRV and to enable proper global carbon accounting considering the different types, permanence, and potential control periods of C-sinks. We, therefore, suggest the use of C-sink registers as a cost-effective tool to avoid double counting of CDR and improve overall carbon accounting for climate change mitigation. These registers should be implemented on regional, national, or supranational (e.g., EU) levels and should claim exclusivity for this respective area.
Author contributions
DR: Investigation, Writing – original draft. H-PS: Writing – review & editing. JL: Writing – review & editing. DB: Writing – review & editing. TB: Writing – review & editing. NH: Conceptualization, Funding acquisition, Writing – review & editing.
Glossary
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Road4Schemes project under the H2020 European Joint Programme SOIL (EJP-SOIL, 862695) project. Open access funding by Agroscope.
Conflict of interest
H-PS and NH are members of the scientific advisory board of Carbon Standards International AG. H-PS is co-founder of the Global Carbon Register Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fclim.2024.1343516/full#supplementary-material
References
Agarwal, T., and Bucheli, T. D. (2011). Adaptation, validation and application of the chemo-thermal oxidation method to quantify black carbon in soils. Environ. Pollut. 159, 532–538. doi: 10.1016/j.envpol.2010.10.012
Agroscope. (2020). Schweizerische Referenzmethoden der Forschungsanstalten Agroscope, Bestimmung des organisch gebundenen Kohlenstoffs (Corg), Version 1.2. Available at: https://ira.agroscope.ch/en-US/publication/46276 (Accessed August 03, 2023).
Archanjo, B. S., Mendoza, M. E., Albu, M., Mitchell, D. R. G., Hagemann, N., Mayrhofer, C., et al. (2017). Nanoscale analyses of the surface structure and composition of biochars extracted from field trials or after co-composting using advanced analytical electron microscopy. Geoderma 294, 70–79. doi: 10.1016/j.geoderma.2017.01.037
Ascough, P. L., Bird, M. I., Brock, F., Higham, T. F. G., Meredith, W., Snape, C. E., et al. (2009). Hydropyrolysis as a new tool for radiocarbon pre-treatment and the quantification of black carbon. Quat. Geochronol. 4, 140–147. doi: 10.1016/j.quageo.2008.11.001
Ascough, P. L., Bird, M. I., Meredith, W., and Snape, C. E. (2016). Dates and fates of pyrogenic carbon: using spectroscopy to understand a “missing” global carbon sink. Spectrosc. Eur. 28, 6–9.
Bachmann, H. J., Bucheli, T. D., Dieguez-Alonso, A., Fabbri, D., Knicker, H., Schmidt, H. P., et al. (2016). Toward the standardization of biochar analysis: the COST action TD1107 Interlaboratory comparison. J. Agric. Food Chem. 64, 513–527. doi: 10.1021/acs.jafc.5b05055
Baldock, J. A., Hawke, B., Sanderman, J., and Mac Donald, L. M. (2013). Predicting contents of carbon and its component fractions in Australian soils from diffuse reflectance mid-infrared spectra. Soil Res. 51, 577–595. doi: 10.1071/SR13077
Baldock, J. A., and Skjemstad, J. O. (2000). Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org. Geochem. 31, 697–710. doi: 10.1016/S0146-6380(00)00049-8
Bird, M. (2015). “Test procedures for biochar analysis in soils,” in Biochar for Environmental Management. New York: Routledge, 711–748.
Bird, M., Keitel, C., and Meredith, W. (2017). “Analysis of biochars for C, H, N, O and S by elemental analyzer” in Biochar: A guide to analytical methods. eds. B. Singh, M. Camps-Arbestain, and J. Lehmann (Clayton: CSIRO Publishing), 39–50.
Bird, M. I., Wynn, J. G., Saiz, G., Wurster, C. M., and McBeath, A. (2015). The pyrogenic carbon cycle. Annu. Rev. Earth Planet. Sci. 43, 273–298. doi: 10.1146/annurev-earth-060614-105038
Blanco-Canqui, H., Laird, D. A., Heaton, E. A., Rathke, S., and Acharya, B. S. (2020). Soil carbon increased by twice the amount of biochar carbon applied after 6 years: field evidence of negative priming. GCB Bioenergy 12, 240–251. doi: 10.1111/gcbb.12665
Brown, R., del Campo, B., Boateng, A. A., Garcia-Perez, M., Masek, O., Lehmann, J., et al. (2015). “Fundamentals of biochar production” in Biochar for environmental management: Science, technology and implementation (New York: Routledge), 39–61.
Bucheli, T. D., Blum, F., Desaules, A., and Gustafsson, Ö. (2004). Polycyclic aromatic hydrocarbons, black carbon, and molecular markers in soils of Switzerland. Chemosphere 56, 1061–1076. doi: 10.1016/j.chemosphere.2004.06.002
Budai, A., Zimmerman, A. R., Cowie, A. L., Webber, J. B. W., Singh, B. P., Glaser, B., et al. (2013). Biochar carbon stability test method: an assessment of methods to determine biochar carbon stability. Available at: https://biochar-international.org/wp-content/uploads/2018/06/IBI_Report_Biochar_Stability_Test_Method_Final.pdf (Accessed August 01, 2023).
Canadell, J. G., Monteiro, P. M. S., Costa, M. H., Da, L. C., Cox, P. M., Eliseev, A. V., et al. (2021). Global carbon and other biogeochemical cycles and feedbacks. Available at: https://hal.science/hal-03336145 (Accessed August 02, 2023).
Cerqueira, W. V., Rittl, T. F., Novotny, E. H., and Netto, A. D. (2015). High throughput pyrogenic carbon (biochar) characterisation and quantification by liquid chromatography. Anal. Methods 7, 8190–8196. doi: 10.1039/C5AY01242B
Chalk, P., and Smith, C. J. (2022). 13C methodologies for quantifying biochar stability in soil: a critique. Eur. J. Soil Sci. 73, 1–12. doi: 10.1111/ejss.13245
Chang, Z., Tian, L., Li, F., Zhou, Y., Wu, M., Steinberg, C. E. W., et al. (2018). Benzene polycarboxylic acid — a useful marker for condensed organic matter, but not for only pyrogenic black carbon. Sci. Total Environ. 626, 660–667. doi: 10.1016/j.scitotenv.2018.01.145
Cornelissen, G., Gustafsson, O. R., Bucheli, T. D., Jonker, M. T. O., Koelmans, A. A., and Van Noort, P. C. M. (2005). Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 39, 6881–6895. doi: 10.1021/es050191b
Cotrufo, M. F., Boot, C., Abiven, S., Foster, E. J., Haddix, M., Reisser, M., et al. (2016). Quantification of pyrogenic carbon in the environment: an integration of analytical approaches. Org. Geochem. 100, 42–50. doi: 10.1016/j.orggeochem.2016.07.007
COWI, EI, and IEEP. (2020). Analytical support for the operationalisation of an EU carbon farming initiative: Lessons learned from existing result-based carbon farming schemes and barriers and solutions for implementation within the EU. Report to the European Commission, DG Climate. Available at: https://climate.ec.europa.eu/document/download/b0fc5b79-92b3-4ec1-89ba-3846158e904a_en?filename=policy_forest_carbon_report_en.pdf (Accessed July 15, 2023).
Department of Sustainable Natural Resources, New South Wales. (1990). Soil survey standard test method organic carbon. Available at: https://www.environment.nsw.gov.au/resources/soils/testmethods/oc.pdf (Accessed August 05, 2023).
DIN 51732. Testing of solid fuels – determination of total carbon, hydrogen and nitrogen content – instrumental methods. Available at: https://www.din.de/de/mitwirken/normenausschuesse/nmp/veroeffentlichungen/wdc-beuth:din21:205570833 (Accessed September 25, 2023).
DIN EN 15936. Soil, waste, treated biowaste and sludge – determination of total organic carbon (TOC) by dry combustion. Available at: https://www.din.de/de/mitwirken/normenausschuesse/naw/wdc-beuth:din21:344580989 (Accessed September 25, 2023).
Don, A., Seidel, F. J. L., Leifeld, J., Kätterer, T., Martin, M., Pellerin, S., et al. (2024). Carbon sequestration in soils and climate change mitigation – definitions and pitfalls. Glob. Chang. Biol. 30:e16983. doi: 10.1111/gcb.16983
EBC. (2021). Certification of the carbon sink potential of biochar, Ithaka Institute, Arbaz, Switzerland. Available at: https://www.european-biochar.org/media/doc/139/c_en_sink-value_2-1.pdf (Accessed August 02, 2023).
EBC. (2012-2023). European biochar certificate - guidelines for a sustainable production of biochar.’ carbon standards international (csi), frick, switzerland. Available at: https://www.european-biochar.org/media/doc/2/version_en_10_3.pdf (Accessed August 25, 2023).
EBI. (2023). European biochar industry consortium e.V., Freiburg European biochar market report 2022/23. Available at: https://www.biochar-industry.com/market-overview/ (Accessed October 26, 2023).
Etter, H., Vera, A., Aggarwal, C., Delaney, M., and Manley, S. (2021). Methodology for biochar utilization in soil and non-soil applications: Version 1.0. Available at: https://verra.org/wp-content/uploads/2021/08/210803_VCS-Biochar-Methodology-v1.0-.pdf (Accessed October 26, 2023).
European Commission. (2022). Commission staff working document: Impact assessment report accompanying the document proposal for a regulation of the european parliament and of the council establishing a union certification framework for carbon removals. SWD. Available at: https://data.consilium.europa.eu/doc/document/ST-15557-2022-ADD-3/en/pdf (Accessed November 21, 2023).
FAO (2019). Standard operating procedure for soil organic carbon Walkley-black method titration and colorimetric method. Available at: https://www.fao.org/3/ca7471en/ca7471en.pdf (Accessed October 25, 2023).
Fliessbach, A., Tresch, S., and Steffens, M. (2021). Review on the techniques and requirements for monitoring stock changes of soil organic carbon. Available at: https://www.bafu.admin.ch/dam/bafu/en/dokumente/boden/externe-studien-berichte/review-on-the-techniques-and-requirements-for-monitoring-stock-changes-of-soil-organic-carbon.pdf.download.pdf/PoBourgeois_PROJEKT3.pdf (Accessed October 26, 2023).
Gerke, J. (2019). Black (pyrogenic) carbon in soils and waters: a fragile data basis extensively interpreted. Chem. Biol. Technol. Agric. 6, 1–8. doi: 10.1186/s40538-019-0151-6
Glaser, B., and Amelung, W. (2003). Pyrogenic carbon in native grassland soils along a climosequence in North America. Global Biogeochem. Cycles 17, 1–8. doi: 10.1029/2002gb002019
Gustafsson, Ö., Bucheli, T. D., Kukulska, Z., Andersson, M., Largeau, C., Rouzaud, J. N., et al. (2001). Evaluation of a protocol for the quantification of black carbon in sediments. Global Biogeochem. Cycles 15, 881–890. doi: 10.1029/2000GB001380
Hagemann, N., Spokas, K., Schmidt, H. P., Kägi, R., Böhler, M. A., and Bucheli, T. D. (2018). Activated carbon, biochar and charcoal: linkages and synergies across pyrogenic carbon’s ABCs. Water (Switzerland) 10, 1–19. doi: 10.3390/w10020182
Hammes, K., Schmidt, M. W. I., Smernik, R. J., Currie, L. A., Ball, W. P., Nguyen, T. H., et al. (2007). Comparison of quantification methods to measure fire-derived (black-elemental) carbon in soils and sediments using reference materials from soil, water, sediment and the atmosphere. Global Biogeochem. Cycles 21, 1–18. doi: 10.1029/2006GB002914
Hardie, M., Clothier, B., Bound, S., Oliver, G., and Close, D. (2014). Does biochar influence soil physical properties and soil water availability? Plant Soil 376, 347–361. doi: 10.1007/s11104-013-1980-x
Hardy, B., Borchard, N., and Leifeld, J. (2022). Identification of thermal signature and quantification of charcoal in soil using differential scanning calorimetry and benzene polycarboxylic acid (BPCA) markers. Soil 8, 451–466. doi: 10.5194/soil-8-451-2022
Hardy, B., and Dufey, J. E. (2017). Geoderma the resistance of centennial soil charcoal to the “Walkley-black” oxidation. Geoderma 303, 37–43. doi: 10.1016/j.geoderma.2017.05.001
Hedges, J. I., Eglinton, G., Hatcher, P. G., Kirchman, D. L., Arnosti, C., Derenne, S., et al. (2000). The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem. 31, 945–958. doi: 10.1016/S0146-6380(00)00096-6
IPCC. (2019). Appendix 4 method for estimating the change in mineral soil organic carbon stocks from biochar amendments: basis for future methodological development. In IPCC, 2019 refinement to the 2006 IPCC guidelines for National Greenhouse gas Inventories. Available at: https://www.ipcc-nggip.iges.or.jp/public/2019rf/pdf/4_Volume4/19R_V4_Ch02_Ap4_Biochar.pdf (Accessed September 25, 2023).
Ippolito, J. A., Cui, L., Kammann, C., Wrage-Mönnig, N., Estavillo, J. M., Fuertes-Mendizabal, T., et al. (2020). Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar 2, 421–438. doi: 10.1007/s42773-020-00067-x
ISO14235. (1998). soil quality — determination of organic carbon by sulfochromic oxidation. Available at: https://www.iso.org/standard/23140.html (Accessed October 06, 2023).
Jauss, V., Sullivan, P. J., Sanderman, J., Smith, D. B., and Lehmann, J. (2017). Pyrogenic carbon distribution in mineral topsoils of the northeastern United States. Geoderma 296, 69–78. doi: 10.1016/j.geoderma.2017.02.022
Jiménez-González, M. A., De la Rosa, J. M., Aksoy, E., Jeffery, S., Oliveira, B. R. F., and Verheijen, F. G. A. (2021). Spatial distribution of pyrogenic carbon in Iberian topsoils estimated by chemometric analysis of infrared spectra. Sci. Total Environ. 790:148170. doi: 10.1016/j.scitotenv.2021.148170
Keiluweit, M., Nico, P. S., and Johnson, M. G. (2010). Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 44, 1247–1253. doi: 10.1021/es9031419
Knicker, H. (2011). Pyrogenic organic matter in soil: Its origin and occurrence, its chemistry and survival in soil environments. Quaternary Int. 243, 251–263. doi: 10.1016/j.quaint.2011.02.037
Knicker, H., Wiesmeier, M., and Dick, D. P. (2008). A simplified method for the quantification of pyrogenic organic matter in grassland soils via chemical oxidation. Geoderma 147, 69–74. doi: 10.1016/j.geoderma.2008.07.008
Koide, R. T., Petprakob, K., and Peoples, M. (2011). Quantitative analysis of biochar in field soil. Soil Biol. Biochem. 43, 1563–1568. doi: 10.1016/j.soilbio.2011.04.006
Lavallee, J. M., Conant, R. T., Haddix, M. L., Follett, R. F., Bird, M. I., and Paul, E. A. (2019). Selective preservation of pyrogenic carbon across soil organic matter fractions and its influence on calculations of carbon mean residence times. Geoderma 354:113866. doi: 10.1016/j.geoderma.2019.07.024
Lefebvre, D., Fawzy, S., Aquije, C. A., Osman, A. I., Draper, K. T., and Trabold, T. A. (2023). Biomass residue to carbon dioxide removal: quantifying the global impact of biochar. Biochar 5:65. doi: 10.1007/s42773-023-00258-2
Lehmann, J., and Joseph, S. (2015). Biochar for environmental management: Science, technology and implementation. New York: Routledge.
Lehmann, J., and Kleber, M. (2015). The contentious nature of soil organic matter. Nature 528, 60–68. doi: 10.1038/nature16069
Leifeld, J. (2007). Thermal stability of black carbon characterised by oxidative differential scanning calorimetry. Organ. Geochem. 38, 112–127. doi: 10.1016/j.orggeochem.2006.08.004
Leifeld, J., Alewell, C., Bader, C., Krüger, J. P., Mueller, C. W., Sommer, M., et al. (2018). Pyrogenic carbon contributes substantially to carbon storage in intact and degraded northern peatlands. L. Degrad. Dev. 29, 2082–2091. doi: 10.1002/ldr.2812
Leifeld, J., Bassin, S., and Fuhrer, J. (2005). Carbon stocks in Swiss agricultural soils predicted by land-use, soil characteristics, and altitude. Agricult. Ecosyst. Environ. 105, 255–266. doi: 10.1016/j.agee.2004.03.006
Lovell, H. C. (2010). Governing the carbon offset market. Wiley Interdiscip. Rev. Clim. Chang. 1, 353–362. doi: 10.1002/wcc.43
Masiello, C. A. (2004). New directions in black carbon organic geochemistry. Mar. Chem. 92, 201–213. doi: 10.1016/j.marchem.2004.06.043
Meredith, W., Ascough, P. L., Bird, M. I., Large, D. J., Snape, C. E., Song, J., et al. (2013). Direct evidence from hydropyrolysis for the retention of long alkyl moieties in black carbon fractions isolated by acidified dichromate oxidation. J. Anal. Appl. Pyrolysis 103, 232–239. doi: 10.1016/j.jaap.2012.11.001
Meredith, W., Ascough, P. L., Bird, M. I., Large, D. J., Snape, C. E., Sun, Y., et al. (2012). Assessment of hydropyrolysis as a method for the quantification of black carbon using standard reference materials. Geochim. Cosmochim. Acta 97, 131–147. doi: 10.1016/j.gca.2012.08.037
Michelsen, H. A., Colket, M. B., Bengtsson, P., D’Anna, A., Desgroux, P., Haynes, B. S., et al. (2020). A review of terminology used to describe soot formation and evolution under combustion and pyrolytic conditions. Am. Chem. Soc. Nano 14, 12470–12490. doi: 10.1021/acsnano.0c06226
Murano, H., Liu, G., Wang, Z., Tanihira, Y., Asahi, T., and Isoi, T. (2021). Quantification methods of pyrogenic carbon in soil with soil as a complex matrix: comparing the CTO-375 and Cr2O7 methods. Soil Sci. Plant Nutr. 67, 380–388. doi: 10.1080/00380768.2021.1925960
Nakhli, S. A. A., Panta, S., Brown, J. D., Tian, J., and Imhoff, P. T. (2019). Quantifying biochar content in a field soil with varying organic matter content using a two-temperature loss on ignition method. Sci. Total Environ. 658, 1106–1116. doi: 10.1016/j.scitotenv.2018.12.174
NREL. (2015). Renewable electricity: how do you know you are using it? Available at: https://www.nrel.gov/docs/fy15osti/64558.pdf (Accessed January 20, 2024).
Oldfield, B. E. E., Eagle, A. J., Rubin, R. L., Rudek, J., and Gordon, D. R. (2022). Crediting agricultural soil carbon sequestration; regional consistency is necessary for carbon credit integrity. Science 375, 1222–1225. doi: 10.1126/science.abl7991
Paetsch, L., Mueller, C. W., Rumpel, C., Angst, Š., Wiesheu, A. C., Girardin, C., et al. (2017). A multi-technique approach to assess the fate of biochar in soil and to quantify its effect on soil organic matter composition. Org. Geochem. 112, 177–186. doi: 10.1016/j.orggeochem.2017.06.012
Paul, C., Bartkowski, B., Dönmez, C., Don, A., Mayer, S., Steffens, M., et al. (2023). Carbon farming: are soil carbon certificates a suitable tool for climate change mitigation? J. Environ. Manag. 330:117142. doi: 10.1016/j.jenvman.2022.117142
Plante, A. F., Fernández, J. M., and Leifeld, J. (2009). Geoderma application of thermal analysis techniques in soil science. Geoderma 153, 1–10. doi: 10.1016/j.geoderma.2009.08.016
Poeplau, C., Jacobs, A., Don, A., Vos, C., Schneider, F., Wittnebel, M., et al. (2020). Stocks of organic carbon in German agricultural soils—key results of the first comprehensive inventory. J. Plant Nutr. Soil Sci. 183, 665–681. doi: 10.1002/jpln.202000113
Poeplau, C., Vos, C., and Don, A. (2017). Soil organic carbon stocks are systematically overestimated by misuse of the parameters bulk density and rock fragment content. Soil 3, 61–66. doi: 10.5194/soil-3-61-2017
Pressler, Y., Boot, C. M., Abiven, S., Lugato, E., and Francesca Cotrufo, M. (2022). Continental-scale measurements of soil pyrogenic carbon in Europe. Soil Res. 60, 103–113. doi: 10.1071/SR19396
Pulcher, R., Balugani, E., Ventura, M., Greggio, N., and Marazza, D. (2022). Inclusion of biochar in a C dynamics model based on observations from an 8-year field experiment. Soil 8, 199–211. doi: 10.5194/soil-8-199-2022
Puro.earth (2024). Biochar Methodology, version 3. Available at: https://7518557.fs1.hubspotusercontent-na1.net/hubfs/7518557/Supplier%20Documents/Puro.earth%20Biochar%20Methodology.pdf (Accessed March 24, 2024).
Qi, F., Naidu, R., Bolan, N. S., Dong, Z., Yan, Y., Lamb, D., et al. (2017). Pyrogenic carbon in Australian soils. Sci. Total Environ. 586, 849–857. doi: 10.1016/j.scitotenv.2017.02.064
Rathnayake, D., Maziarka, P., Ghysels, S., Mašek, O., Sohi, S., and Ronsse, F. (2020). How to trace back an unknown production temperature of biochar from chemical characterization methods in a feedstock independent way. J. Anal. Appl. Pyrolysis 151:104926. doi: 10.1016/j.jaap.2020.104926
Reisser, M., Purves, R. S., Schmidt, M. W. I., and Abiven, S. (2016). Pyrogenic carbon in soils: a literature-based inventory and a global estimation of its content in soil organic carbon and stocks. Front. Earth Sci. 4, 1–14. doi: 10.3389/feart.2016.00080
Rodrigues, L., Budai, A., Elsgaard, L., Hardy, B., Keel, S. G., Mondini, C., et al. (2023). The importance of biochar quality and pyrolysis yield for soil carbon sequestration in practice. Eur. J. Soil Sci. 74, 1–11. doi: 10.1111/ejss.13396
Sanderman, J., Baldock, J. A., Dangal, S. R. S., Ludwig, S., Potter, S., Rivard, C., et al. (2021). Soil organic carbon fractions in the Great Plains of the United States: an application of mid-infrared spectroscopy. Biogeochemistry 156, 97–114. doi: 10.1007/s10533-021-00755-1
Santín, C., Doerr, S. H., Merino, A., Bucheli, T. D., Bryant, R., Ascough, P., et al. (2017). Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Sci. Rep. 7, 11233–11211. doi: 10.1038/s41598-017-10455-2
Schmidt, H. P., Abiven, S., Hageman, N., and Meyer Zu Drewer, J. (2022). Permanence of soil applied biochar. An executive summary for global biochar carbon sink certification. Arbaz, Switzerland. Available at: www.biochar-journal.org/en/ct/109
Schmidt, H. P., Kammann, C., Hagemann, N., Leifeld, J., Bucheli, T. D., Sánchez Monedero, M. A., et al. (2021). Biochar in agriculture – a systematic review of 26 global meta-analyses. GCB Bioenergy 13, 1708–1730. doi: 10.1111/gcbb.12889
Schmidt, M. W. I., Skjemstad, J. O., Czimczik, C. I., and Prentice, K. M. (2001). Comparative analysis of black carbon in soils. Global Biogeochem. Cycles 15, 163–167. doi: 10.1029/2000GB001284
Schneider, L., Kollmuss, A., and Lazarus, M. (2015). Addressing the risk of double counting emission reductions under the UNFCCC. Clim. Chang. 131, 473–486. doi: 10.1007/s10584-015-1398-y
Schrumpf, M., Schulze, E. D., Kaiser, K., and Schumacher, J. (2011). How accurately can soil organic carbon stocks and stock changes be quantified by soil inventories? Biogeosci. Discus. 1, 1193–1212. doi: 10.5194/bg-8-1193-2011
Sigmund, G., Bucheli, T. D., Hilber, I., Micić, V., Kah, M., and Hofmann, T. (2017). Effect of ageing on the properties and polycyclic aromatic hydrocarbon composition of biochar. Environ Sci Process Impacts 19, 768–774. doi: 10.1039/c7em00116a
Sigmund, G., Schmid, A., Schmidt, H. P., Hagemann, N., Bucheli, T. D., and Hofmann, T. (2023). Small biochar particles hardly disintegrate under cryo-stress. Geoderma 430:116326. doi: 10.1016/j.geoderma.2023.116326
Simpson, M. J., and Hatcher, P. G. (2004). Overestimates of black carbon in soils and sediments. Naturwissenschaften 91, 436–440. doi: 10.1007/s00114-004-0550-8
Singh, B. P., Cowie, A. L., and Smernik, R. J. (2012). Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 46, 11770–11778. doi: 10.1021/es302545b
Singh, B., Fang, Y., Cowie, B. C. C., and Thomsen, L. (2014). NEXAFS and XPS characterisation of carbon functional groups of fresh and aged biochars. Org. Geochem. 77, 1–10. doi: 10.1016/j.orggeochem.2014.09.006
Six, J., Conant, R. T., Paul, E. A., and Paustian, K. (2002). Stabilization mechanisms of SOM implications for C saturation of soils.Pdf. Plant Soil 241, 155–176. doi: 10.1023/A:1016125726789
Skjemstad, J. O., Reicosky, D. C., Wilts, A. R., and McGowan, J. A. (2002). Charcoal carbon in US agricultural soils. Soil Sci. Soc. Am. J. 66, 1249–1255. doi: 10.2136/sssaj2002.1249
Smernik, R. J. (2017). “Analysis of biochars by 13C nuclear magnetic resonance spectroscopy” in Biochar: A guide to analytical methods (Clayton: CSIRO Publishing), 151–161.
Smith, P., Soussana, J. F., Angers, D., Schipper, L., Chenu, C., Rasse, D. P., et al. (2020). How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Glob. Chang. Biol. 26, 219–241. doi: 10.1111/gcb.14815
Spokas, K. A. (2013). Impact of biochar field aging on laboratory greenhouse gas production potentials. GCB Bioenergy 5, 165–176. doi: 10.1111/gcbb.12005
Spokas, K. A., Novak, J. M., Masiello, C. A., Johnson, M. G., Colosky, E. C., Ippolito, J. A., et al. (2014). Physical disintegration of biochar: an overlooked process. Environ. Sci. Technol. Lett. 1, 326–332. doi: 10.1021/ez500199t
United Nations (2015). Paris Agreement. Available at: https://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (Accessed August 20, 2023).
VDLUFA (1991). Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungs-anstalten eV). Methodenbuch, Band I: Die Untersuchung von Böden. Available at: http://www.methodenbuch.de/index.php?option=com_content&view=article&id=7&Itemid=108&lang=de (Accessed August 11, 2023).
Wagner, S., Rudolf, J., and Stubbins, A. (2018). Dissolved black carbon in aquatic ecosystems. Limnol. Oceanogr. Lett. 3, 168–185. doi: 10.1002/lol2.10076
Wang, X., Sanderman, J., and Yoo, K. (2018). Climate-dependent topographic effects on pyrogenic soil carbon in southeastern Australia. Geoderma 322, 121–130. doi: 10.1016/j.geoderma.2018.02.025
Werner, C., Lucht, W., Gerten, D., and Kammann, C. (2021). Potential of land-neutral negative emissions through biochar sequestration. Earth’s Futur. 10:2583. doi: 10.1029/2021EF002583
Whitman, T., Scholz, S. M., and Lehmann, J. (2010). Biochar projects for mitigating climate change: an investigation of critical methodology issues for carbon accounting. Carbon Manag. 1, 89–107. doi: 10.4155/cmt.10.4
Wiedemeier, D. B. (2014). New insights into pyrogenic carbon by an improved benzene Polycarboxylic acid molecular marker method. Available at: https://www.zora.uzh.ch/id/eprint/105332/1/2014_Thesis_Daniel_Wiedemeier_III%20.pdf (Accessed August 29, 2022).
Wiedemeier, D. B., Hilf, M. D., Smittenberg, R. H., Haberle, S. G., and Schmidt, M. W. I. (2013). Improved assessment of pyrogenic carbon quantity and quality in environmental samples by high-performance liquid chromatography. J. Chromatogr. A 1304, 246–250. doi: 10.1016/j.chroma.2013.06.012
Wiedemeier, D. B., Lang, S. Q., Gierga, M., Abiven, S., Bernasconi, S. M., Früh-Green, G. L., et al. (2016). Characterization, quantification and compound-specific isotopic analysis of pyrogenic carbon using benzene polycarboxylic acids (Bpca). J. Vis. Exp. 2016, 1–9. doi: 10.3791/53922
Wiesmeier, M., Mayer, S., Carsten, P., Katharina, H., Axel, D., Uwe, F., et al. (2020). CO2 certificates for carbon sequestration in soils: methods, management practices and limitations. Leibniz Cent. Landsc. Res. doi: 10.20387/BONARES-NE0G-CE98
Woolf, D., Amonette, J. E., Street-Perrott, F. A., Lehmann, J., and Joseph, S. (2010). Sustainable biochar to mitigate global climate change. Nat. Commun. 1, 1–9. doi: 10.1038/ncomms1053
Xiao, X., and Chen, B. (2017). A direct observation of the fine aromatic clusters and molecular structures of biochars. Environ. Sci. Technol. 51, 5473–5482. doi: 10.1021/acs.est.6b06300
Yang, F., Xu, Z., Huang, Y., Tsang, D. C. W., Ok, Y. S., Zhao, L., et al. (2021). Stabilization of dissolvable biochar by soil minerals: release reduction and organo-mineral complexes formation. J. Hazard. Mater. 412:125213. doi: 10.1016/j.jhazmat.2021.125213
Keywords: pyrogenic carbon capture and storage, carbon sink certification, carbon dioxide removal, pyrogenic carbonaceous material, black carbon, monitoring, reporting, verification
Citation: Rathnayake D, Schmidt H-P, Leifeld J, Bürge D, Bucheli TD and Hagemann N (2024) Quantifying soil organic carbon after biochar application: how to avoid (the risk of) counting CDR twice? Front. Clim. 6:1343516. doi: 10.3389/fclim.2024.1343516
Edited by:
Carlos Paulo, SRK Consulting, CanadaReviewed by:
José María De La Rosa, Spanish National Research Council (CSIC), SpainAbhishek Kumar, University of California, Davis, United States
Puja Khare, Council of Scientific and Industrial Research (CSIR), India
Jorge Paz-Ferreiro, RMIT University, Australia
Copyright © 2024 Rathnayake, Schmidt, Leifeld, Bürge, Bucheli and Hagemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolas Hagemann, bmlrb2xhcy5oYWdlbWFubkBhZ3Jvc2NvcGUuYWRtaW4uY2g=; aGFnZW1hbm5AaXRoYWthLWluc3RpdHV0Lm9yZw==
 Dilani Rathnayake
Dilani Rathnayake Hans-Peter Schmidt
Hans-Peter Schmidt Jens Leifeld4
Jens Leifeld4 Nikolas Hagemann
Nikolas Hagemann