
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 18 February 2025
Sec. Intestinal Microbiome
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1539607
This article is part of the Research TopicGut Microbiota Dynamics and Nutritional Strategies in Porcine Weaning PeriodView all 5 articles
Introduction: Previous studies have suggested that dietary organic iron offers health advantages compared to its inorganic counterpart. However, the effects of iron hydroxy methionine analog chelate (Fe-HMA) supplementation in weaned piglets have not been fully explored. Therefore, this study aimed to investigate the effects of replacing ferrous sulfate with Fe-HMA as the iron source on serum biochemistry, antioxidant capacity, and gut microbiota in weaned piglets.
Methods: One hundred and twenty weaned piglets were randomly allocated to two treatment groups. Each group contained four replicates, with 15 pigs per replicate. Piglets were fed either 100 mg Fe/kg in the form of ferrous sulfate (Fe-sulfate group) or 50 mg Fe/kg in the form of Fe-HMA (Fe-HMA group) as the iron source for 28 days.
Results and discussion: Results showed that supplementing Fe-HMA as an iron source significantly increased the levels of triglycerides and glucose in portal venous serum, albumin in both serum and portal venous serum and decreased serum low-density lipoprotein level in weaned piglets. Additionally, Fe-HMA supplementation significantly reduced serum and liver malondialdehyde levels, while increasing catalase (CAT), glutathione peroxidase (GSH-Px), total superoxide dismutase, and manganese superoxide dismutase levels in serum, as well as GSH-Px and CAT levels in the liver. Moreover, Fe-HMA regulated the intestinal microbiota composition, notably increasing the relative abundance of Proteobacteria and decreasing microbes involved in aromatic_compound_degradation. In conclusion, dietary replacing inorganic iron with Fe-HMA improved metabolic parameters and antioxidant capacity, and regulated gut microbiota composition in weaned piglets.
Iron, an essential trace element, plays a vital role in piglet growth by supporting various physiological processes, including oxygen transport, DNA synthesis, antioxidant enzyme formation, and energy metabolism (Sun et al., 2022). Iron deficiency can lead to vigor reduction, appetite loss, irritability, and pica, which in turn can result in malnutrition and ultimately affect growth (Pasricha et al., 2021). However, the iron reserves in weaned piglets are insufficient to support rapid growth, which can make them susceptible to iron deficiency (Heidbüchel et al., 2019; Ding et al., 2021). In addition, weaning represents a critical phase in piglet production, marked by separation from the mother and the transition to solid feed (Tang et al., 2022). These changes exert stress on piglets, thereby affecting their antioxidant status and nutrient absorption, and potentially hindering growth as their intestine is under development (Cao et al., 2018). Moreover, excessive iron supplementation can exacerbate weaning stress (Ding et al., 2020b; Ding et al., 2021). Therefore, maintaining an appropriate iron level and ensuring adequate iron absorption in weaned piglets is an effective strategy to support their health (Kim et al., 2018).
The National Research Council (NRC, 2012) recommends that the dietary iron requirement is 100 mg/kg in weaned piglets. However, excess iron is often added to diets to ensure sufficient iron intake in practical production (Kim et al., 2018). The iron sources currently can be broadly classified into inorganic and organic forms. The ions in inorganic iron additives, such as ferrous sulfate, are unstable and prone to redox reactions, which can lead to toxicity and oxidative stress (Galaris et al., 2019). Additionally, the low bioavailability of inorganic iron, coupled with the underdeveloped intestines of weaned piglets, can lead to substantial iron excretion with feces, then resulting in iron waste and environmental contamination (Zeng et al., 2023). Organic iron is typically considered to be more efficiently absorbed than inorganic iron (Feng et al., 2019). Studies have shown that organic iron, even at lower doses or half the dose of inorganic iron, has no adverse effects and may even offer benefits in weaned piglets (Feng et al., 2007; Feng et al., 2009). Normally, only ferrous ions can be absorbed by intestine (Shubham et al., 2020). The types of organic iron include simple organic acid iron salts, such as ferrous citrate and ferrous fumarate, as well as chelated iron complexes, in which the iron is bound to amino acids or other organic substances (Ma et al., 2022). Among these, amino acid-chelated iron is especially noteworthy due to its greater stability compared to organic acid iron salts (Predieri et al., 2009). Hydroxy methionine analogs (HMA) can form two chelate rings with metal ions to enhance their stability and absorption (Biagi et al., 2004). It has been reported that Fe-HMA did not cause toxicity in CACO-2 cells, which highlighted its promise as an effective and safe iron supplement (Predieri et al., 2003). The previous study showed that replacing inorganic trace elements with HMA chelate trace elements enhanced serum antioxidant capacity and improved immune function in finishing pigs (Chen et al., 2022). Additionally, a study on juvenile grouper demonstrated that bioavailability of Fe-HMA was approximately 1.3 times greater than that of ferrous sulfate (Huang et al., 2018). Organic iron can also influence intestinal microbiota composition. For instance, dietary supplementation with 84 mg/kg yeast iron increased beneficial bacteria like Firmicutes, Blautia, and Peptococcus levels in the cecum of weaned piglets compared with dietary 104 mg/kg inorganic iron (Zeng et al., 2023). However, the specific effects of Fe-HMA on weaned piglets health have yet to be fully elucidated.
Therefore, this study aims to investigate the effects of replacing ferrous sulfate with Fe-HMA on metabolic parameters, antioxidant capacity, and gut microbiota in weaned piglets, which could provide the basis for the application of Fe-HMA in weaned piglets.
The animal experiment was approved by the Animal Care and Use Committee of Shandong Agricultural University (protocol code SDAUA-2023-327).
A total of 120 healthy 35 d-old weaned piglets (Duroc × Landrace × Large White) with an average body weight of 11.09 ± 0.164 kg were used in a 28-d trial. All piglets were randomly allocated into two groups, each with 4 replicates of 15 pigs per pen in the same room. All pigs were fed a basal diet (Table 1) which was formulated with reference to the National Research Council (2012) to meet or exceed nutritional requirements, except for iron. In the Fe-sulfate group, the diet was supplemented with 100 mg Fe/kg in the form of ferrous sulfate monohydrate, while the Fe-HMA group received 50 mg Fe/kg supplementation in the form of Fe-HMA (Fujian Syno Biotech Co., Ltd., Changsha, China). The supplementation amount was based on the iron content. A three-day acclimation period was provided to help piglets adjust to the living environment before the official trial. The diets were gradually mixed with the experimental diet to ensure a smooth transition to the experimental diet. Throughout the experiment, piglets were housed in a temperature-controlled room (25-28°C) with ad libitum access to feed and water.
At 28 day of the experiment, one piglet from each replicate was selected to collect 10 mL blood and portal venous blood from the jugular vein and dissected thoracic cavity respectively. Serum was obtained from blood samples after centrifugation at 3500 rpm for 15 minutes and was immediately stored at –20°C until analysis.
Approximately 2 g of liver tissue were collected in 2 mL frozen storage tubes, immediately immersed in liquid nitrogen, and then stored at –80°C until antioxidant determination. Cecal chyme were collected into 5 mL sterilized fecal containers for microbiota composition analysis.
Glucose (GLU), total protein (TP), albumin (ALB), urea nitrogen (UREA), triglycerides (TG), total cholesterol (TC), high-density lipoproteins (HDL) and low-density lipoproteins (LDL) in serum and portal venous serum were analyzed on COBUS MIRA Plus fully automated biochemical analyzer (Roche, Indianapolis, USA).
MDA and antioxidants [copper-zinc superoxide dismutase (CuZn-SOD), superoxide dismutase (SOD), Mn superoxide dismutase (Mn-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT)] in serum, portal venous serum and liver were determined using commercial kits from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China) following manufacturer instructions according to previous studies (Sun et al., 2023). Briefly, serum and portal serum were analyzed directly. Liver tissues were homogenized with saline (w/v, 1:9) to obtain supernatant, which was subsequently used for antioxidant indicators determination. The MDA content and antioxidant enzyme activities in liver were normalized to the total protein concentration (per mg protein) of each sample, which was measured using a commercial protein assay kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Total bacterial genomic DNA were extracted from the cecal chyme with the E.Z.N.A.TM stool DNA kit (Omega Bio-Tek, GA, USA). The spectrophotometer and 1% agarose gels electrophoresis were used for monitoring DNA concentration and purity followed by diluting the extracted DNA to 1 ng/mL. The primers 27F (5′-AGRGTTYGATYMTGGCTCAG-3′) and 1492R (5′-RGYTACCTTGTTACGACTT-3′) were adopted to amplify V1-V9 region of the bacteria 16S rRNA gene (Dong et al., 2024; Li et al., 2024). Amplicons were extracted by 2% agarose gels electrophoresis, purified with AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, CA, USA) and quantified with QuantiFluor® dsDNA System (Promega Biotech, WI, USA). Subsequently, SMRTbell libraries were constructed for sequencing on `PacBio Sequel cells. Amplicon sequencing was performed by Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The Uparse pipeline (v.11) was used to cluster high-quality sequences with 97% similarity into identical operational taxonomic units (OTU). Alpha diversity including ACE, Chao 1, Shannon and Simpson indices was revealed by rarefaction analysis using Mothur (v.1.30.2) (Yang et al., 2020). Beta diversity of hierarchical clustering and principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity matrices were performed by Quantitative Insights Into Microbial Ecology (QIIME v.1.9.1) (Li et al., 2024). Wilcoxon rank-sum test and Linear discriminant analysis (LDA) combined effect size measurements (LEfSe) were employed to identify bacterial differences between groups. The identification of bacterial differences between groups was accomplished using both the Wilcoxon rank-sum test and Linear Discriminant Analysis Effect Size (LEfSe). Microbial community functional prediction was conducted using Functional Annotation of Prokaryotic Taxa (FAPROTAX) (Li et al., 2020).
All the indices were determined using individual weaned piglets as the experimental unit. Data normality was assessed using the Shapiro-Wilk test (W > 0.05). All experimental data were analyzed using an independent t-test in SAS 9.6, with significance defined at P < 0.05.
As shown in Table 2, serum ALB was significantly increased while LDL was significantly reduced in Fe-HMA group compared with Fe-sulfate group (P < 0.05). No significant differences were observed in TP, UREA, GLU, TG, TCHO, and HDL between the two groups (P > 0.05).
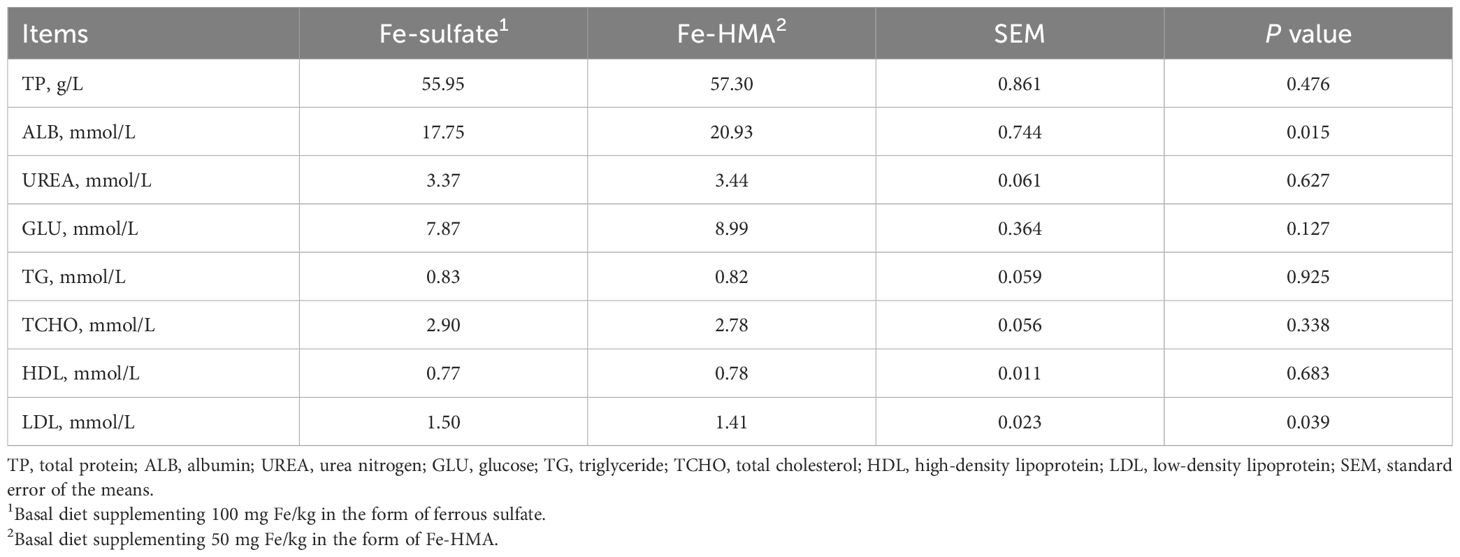
Table 2. Effects of replacing inorganic iron with hydroxy methionine analog chelated iron (Fe-HMA) on serum biochemistry of piglets.
As shown in Table 3, dietary Fe-HMA as the iron source significantly elevated ALB, GLU, TG and HDL levels in portal venous serum compared with Fe-sulfate group (P < 0.05). Meanwhile, compared with Fe-sulfate group, portal venous serum UREA and TCHO showed a tendency increase in Fe-HMA (0.05 < P < 0.10). There were no significant differences in TP and LDL between the two groups (P > 0.05).

Table 3. Effects of replacing inorganic iron with Fe-HMA on portal venous serum biochemistry of piglets.
The MDA content and antioxidant enzyme activity in serum are displayed in Table 4. Fe-HMA significantly reduced MDA content while increasing GSH-Px, CAT, T-SOD and Mn-SOD activities in serum (P < 0.05). No significant difference was observed in CuZn-SOD activity between the two groups (P > 0.05).
As shown in Table 5, a decreased tendency was observed in MDA content in Fe-HMA group (0.05 < P < 0.10). There were no significant differences between the two groups in GSH-Px, CAT, T-SOD, Mn-SOD and CuZn-SOD activities (P > 0.05).
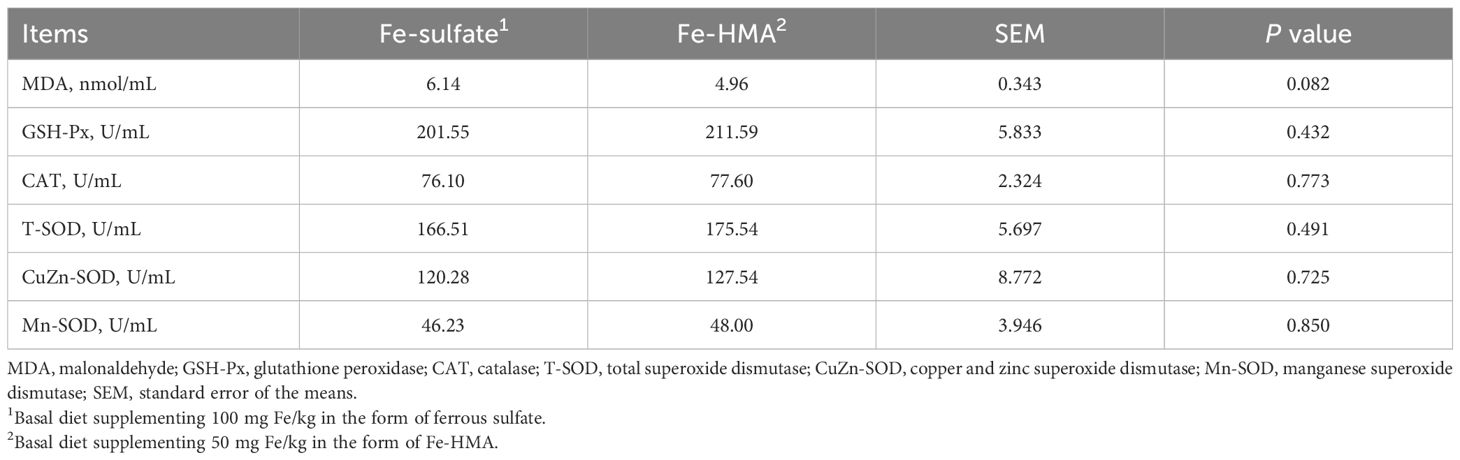
Table 5. Effects of replacing inorganic iron with Fe-HMA on portal venous serum antioxidants of piglets.
As shown in Table 6, MDA content in liver was significantly decreased in Fe-HMA group (P < 0.05). Meanwhile, Fe-HMA significantly increased GSH-Px and CAT activities in the liver (P < 0.05). No significant differences were found in T-SOD, CuZn-SOD and Mn-SOD activities in liver between the two groups (P > 0.05).
The Venn diagram showed that a total of 628 OTUs were identified in the cecal contents of weaned piglets (Figure 1A). There were 509 equivalent OTUs between the two treatments, whereas 90 unique OTUs were in Fe-sulfate and 29 in Fe-MHA, respectively. Rarefaction curves of pooled samples were provided at an OTU definition of 97% identity and it tended to reach plateau with the number of reads sampled increased indicating an adequate sequence number (Figure 1B). No significance was observed between the two groups in alpha diversity indices of ACE index (Figure 1C), Chao 1 index (Figure 1D), Shannon index (Figure 1E) and Simpson index (Figure 1F) (P > 0.05). In the beta diversity analysis, hierarchical clustering and PCoA analysis based on bray_curtis distance (Figure 1G) were used to measure the similarity and differences of bacterial community composition in cecal contents between the two treatments. Hierarchical clustering tree at OUT level exhibited that the differences of cecal microbial flora in Fe-HMA group are smaller, which was also revealed by PCoA plot (Figure 1H) with the obviously separated samples between the two groups.
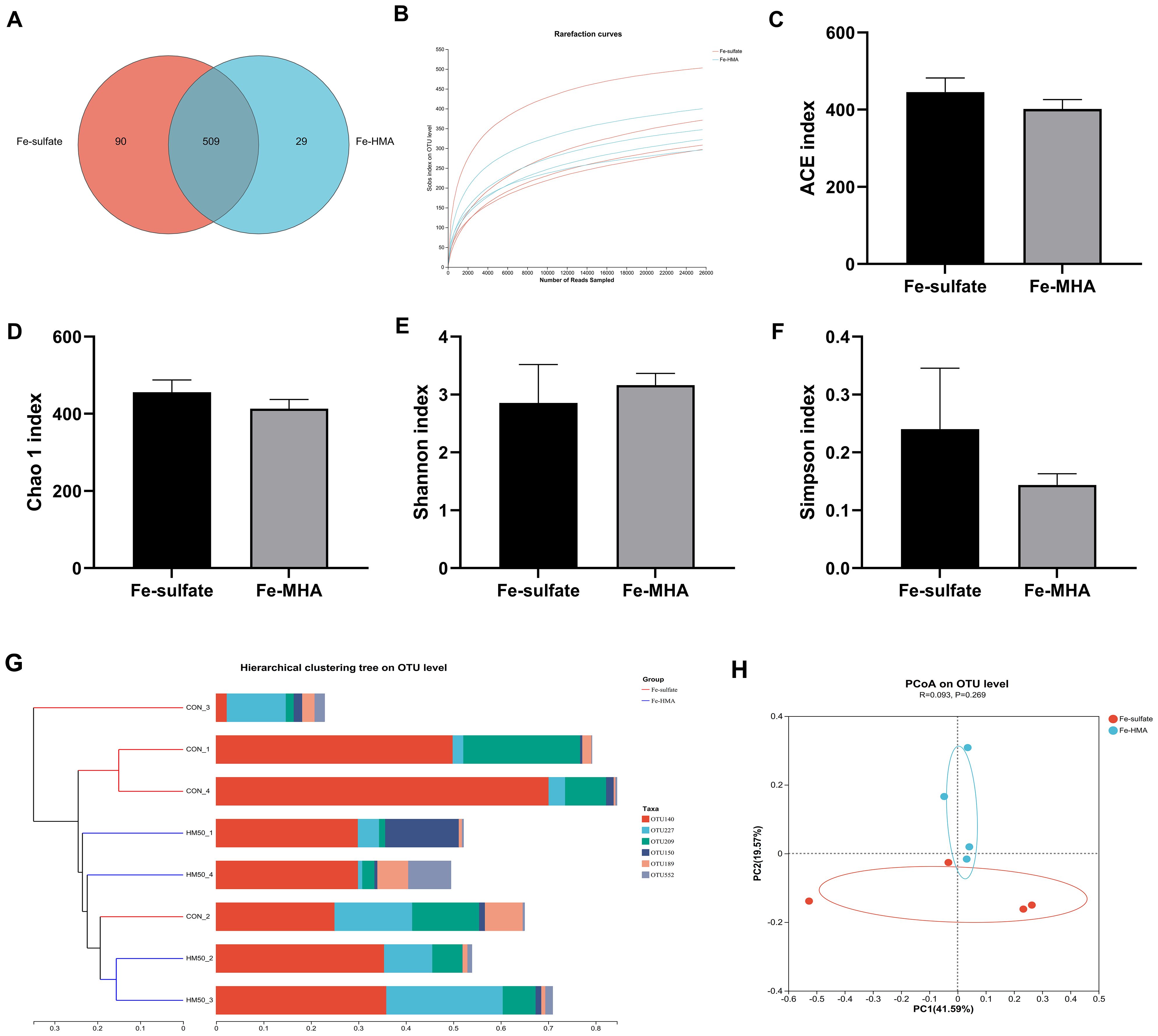
Figure 1. Effects of replacing inorganic iron with hydroxy methionine analog chelated iron (Fe-HMA) on cecal microbial diversity and composition of piglets. (A) Venn diagram; (B) Rarefaction curves; (C) ACE index; (D) Chao 1 index; (E) Shannon index; (F) Simpson index; (G) Hierarchical clustering tree on OTU level; (H) Principal coordinate analysis (PCoA) based on Bray–Curtis distances. Fe-sulfate, piglets fed the basal diet with 100 mg Fe/kg in the form of ferrous sulfate monohydrate; Fe-HMA, piglets fed the basal diet with 50 mg Fe/kg in the form of Fe-HMA. Values are depicted as means ± SEM (standard error).
The circos plot at phylum level showed that the primary dominant phylum of all samples was Firmicutes while the secondary dominant phylum was Bacteroidetes (Figure 2A). However, the Proteobacteria proportions were significantly elevated by Fe-HMA supplementation compared to Fe-sulfate (Figure 2B). The bacterial community bar plots of top 10 genera and species are shown in Figure 2C (at genus level) and Figure 2E (at species level). As shown in Figure 2C, Lactobacillus was the most prevalent genus of all samples. The proportion of Gemmiger, Dorea, Subdoligranulum, Lachnospira, unclassified_o_Rickettsiales, Acinetobacter, Rickettsia, unclassified_f_Erysipelotrichaceae and Exiguobacterium were significantly increased in Fe-HMA group while unclassified_f_Prevotellaceae was significantly decreased (P < 0.05) (Figure 2D). The primary dominant species was Lactobacillus_amylovorus in all samples (Figure 2E). Fe-HMA supplementation significantly increased the richness of Gemmiger_formicilis, Mediterraneibacter_faecis, Dorea_longicatena, Subdoligranulum_variabile, Lachnospira_eligens, unclassified_o:Rickettsiales, Rickettsia_conorii, unclassified_f:Erysipelotrichaceae, unclassified_g:Acinetobacter and Exiguobacterium_acetylicum and decreased unclassified_f:Prevotellaceae and Parabacteroides_merdae (P < 0.05) (Figure 2F).
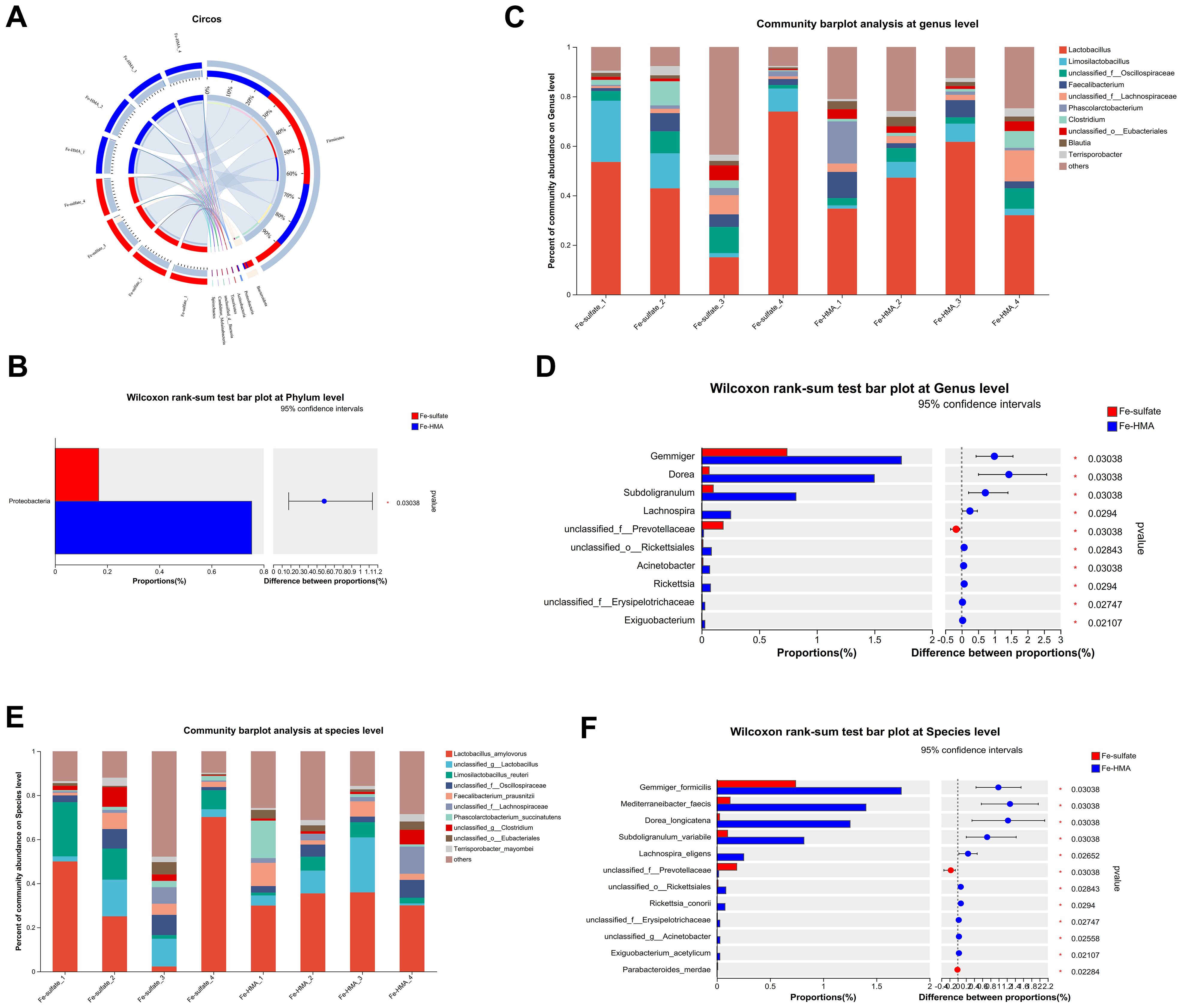
Figure 2. Effects of replacing inorganic iron with Fe-HMA on cecal microbial community at phylum, genus and species level. (A) Circos diagram at phylum level; (B) Wilcoxon rank-sum test bar plot at phylum level. (C) Community barplot analysis at genus level; (D) Wilcoxon rank-sum test bar plot at genus level; (E) Community barplot analysis at species level; (F) Wilcoxon rank-sum test bar plot at species level. Fe-sulfate, piglets fed the basal diet with 100 mg Fe/kg in the form of ferrous sulfate monohydrate; Fe-HMA, piglets fed the basal diet with 50 mg Fe/kg in the form of Fe-HMA.
To further explore the most discriminant biomarker in the cecal microbiota between the two groups, LEfSe analysis was conducted (Figure 3). The results showed that Prevotellamassilia enriched in Fe-sulfate group, and Blautia, Mediterraneibacter, Dorea, Coprococcus, Gemmiger and Subdoligranulum were more abundant in Fe-HMA group at genus level. Meanwhile, Prevotellamassilia_timonensis was enriched in Fe-sulfate group, and Mediterraneibacter_faecis, Coprococcus_comes, Dorea_longicatena, Gemmiger_formicilis and Subdoligranulum_variabile were more abundant in Fe-HMA group at species level.
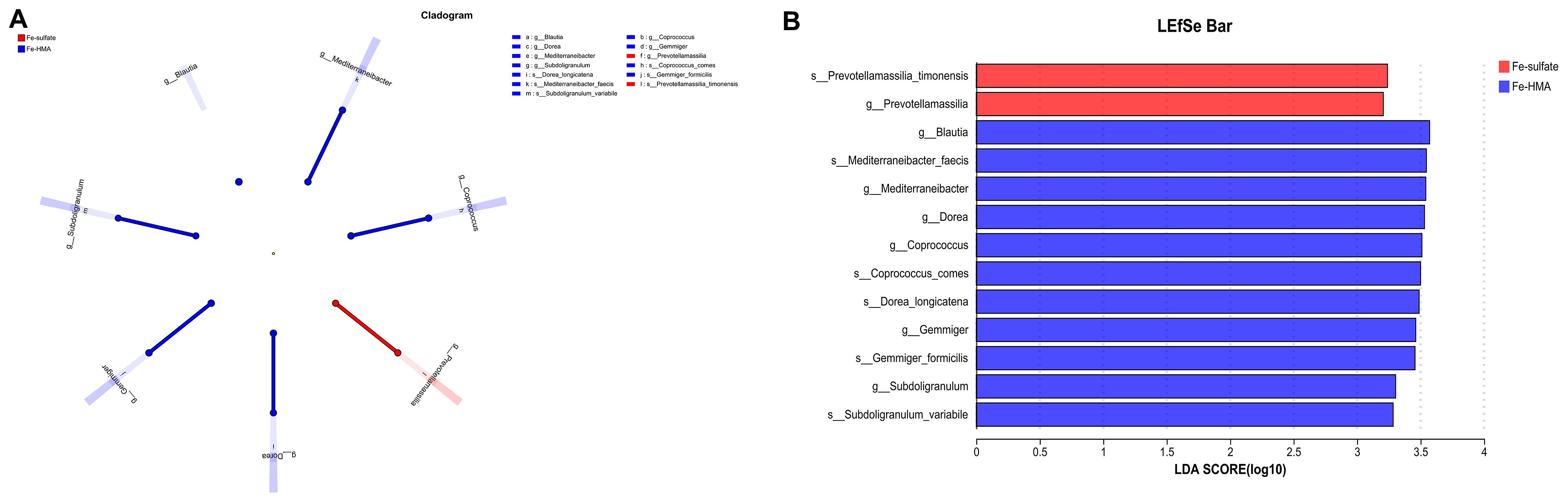
Figure 3. Effects of replacing inorganic iron with Fe-HMA on LEfSe analysis of piglets. (A) LEfSe multilevel taxonomic hierarchical tree diagram; (B) Histogram of LDA scores of microbiota with a threshold value of 4, showing features with differential abundance between groups. Fe-sulfate, piglets fed the basal diet with 100 mg Fe/kg in the form of ferrous sulfate monohydrate; Fe-HMA, piglets fed the basal diet with 50 mg Fe/kg in the form of Fe-HMA.
The heatmap of predicted microbial function by FAPROTAX is shown in Figure 4A. Around 32 enriched functions were identified between the two groups in weaned piglets. Among these, three functions (aerobic_chemoheterotrophy, intracellular_parasites, and aromatic_compound_degradation) were significantly enriched in the Fe-HMA group (P < 0.05) (Figure 4B)
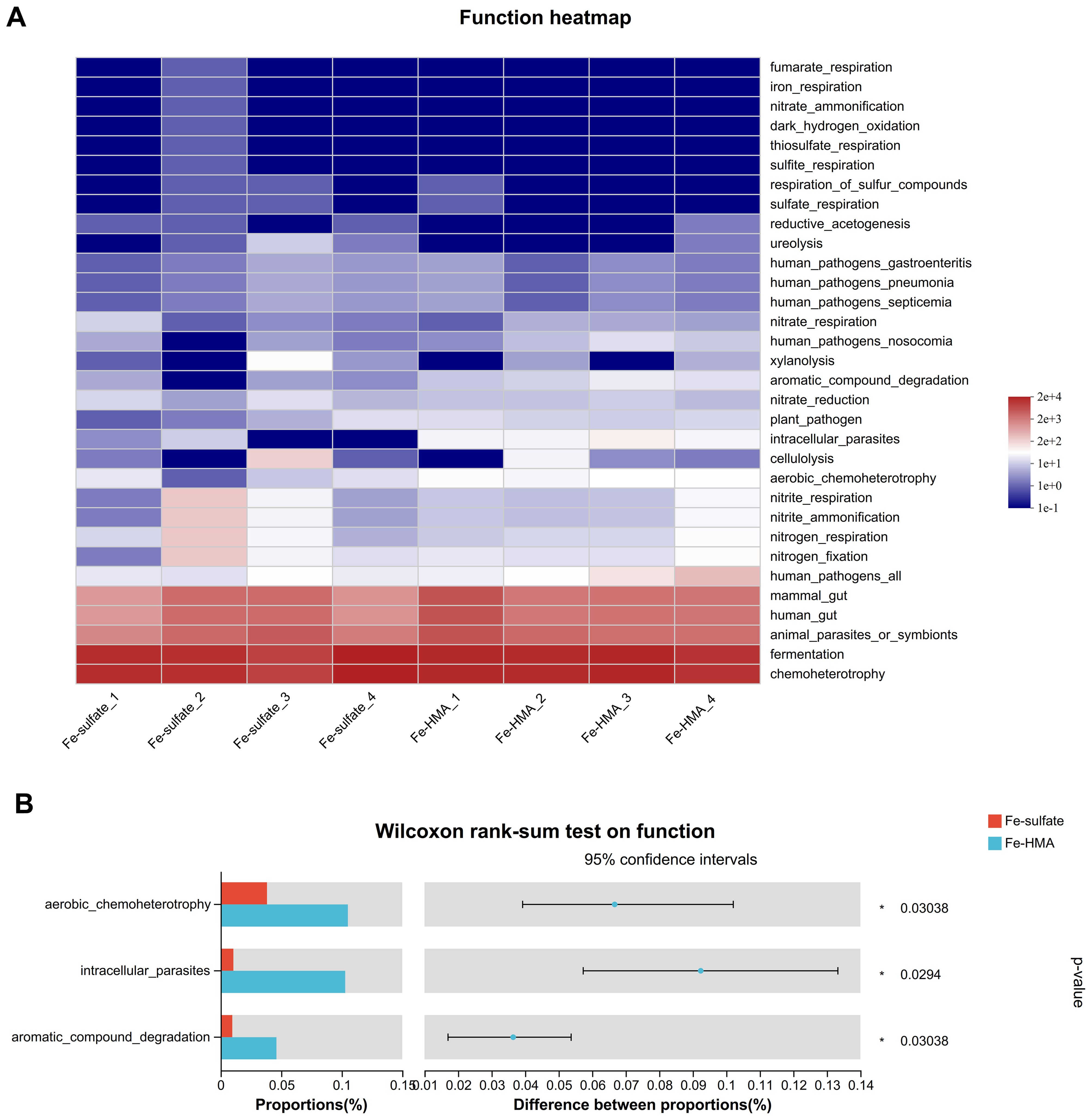
Figure 4. Effects of replacing inorganic iron with Fe-HMA on bacterial relative abundance of predicted function. (A) Heatmap of top 32 predicted microbial functions; (B) Wilcoxon rank-sum test bar plot on predicted function of gut bacteria. Fe-sulfate, piglets fed the basal diet with 100 mg Fe/kg in the form of ferrous sulfate monohydrate; Fe-HMA, piglets fed the basal diet with 50 mg Fe/kg in the form of Fe-HMA.
Serum biochemistry can reflect metabolic status and liver health in animals. ALB, a liver-secreted protein that maintains osmolality and transports nutrients, serves as the indicator of the liver’s protein synthesis capacity (Rothschild et al., 1988; Regmi et al., 2018). Iron plays a critical role in ATP synthesis, which provides the necessary energy for ALB secretion (Rothschild et al., 1988). A previous study demonstrated that dietary supplementation with iron chelates with lysine and glutamic acid at 400 mg/kg and 800 mg/kg significantly increased serum ALB levels in broilers (Han et al., 2022). In the present study, elevated ALB levels in both serum and portal serum of weaned piglets suggest that Fe-HMA may enhance liver function by providing highly bioavailable iron, compared to the inorganic iron group (Regmi et al., 2018). However, the content of LDL is associated with atherosclerotic diseases (Penson et al., 2020). In the present study, Fe-HMA reduced serum LDL level in weaned piglets, contributing to maintaining organism health (Penson et al., 2020). The hepatic portal vein, a major vein that enters the liver at the hilum hepatis after merging with the mesenteric veins from the digestive tract, plays a critical role in nutrient absorption and hormone metabolism (Han et al., 2021; He et al., 2023). In this study, the levels of TG and GLU in portal vein serum were elevated in the Fe-HMA group, suggesting Fe-HMA supplementation increased intestinal absorption of nutrients. Overall, Fe-HMA improved the serum biochemical profile of weaned piglets, contributing to improved health.
The weaning of pigs is usually accompanied by oxidative stress, which can be influenced by iron (Ma et al., 2014; Ding et al., 2021). A study showed that hydroxy methionine analogs could improve the antioxidant capacity of organisms (Kuang et al., 2012). MDA, a key indicator of oxidative stress, was significantly reduced in both serum and liver in the Fe-HMA group, indicating a decrease in lipid peroxidation levels in piglets (Zheng et al., 2013; Sun et al., 2023). However, CAT, SOD and GSH-Px are essential enzymes in animal antioxidant system (Feng et al., 2009; Zeng et al., 2023). Studies have reported organic iron can significantly enhance the antioxidant capacity of animals. For instance, supplementing yeast iron as the iron source in weaned piglets’ diets increased activities of CAT, GSH-Px, SOD and T-AOC in serum compared to inorganic iron (Zeng et al., 2023). Meanwhile, organic iron source, even at a lower dosage than inorganic iron, can improve antioxidant capacity. A study showed 90 mg/kg of glycine-chelated iron significantly elevated hepatic SOD and SDH relative mRNA expressions in weaned piglets compared to the 120 mg/kg ferrous sulfate addition (Feng et al., 2009). In this study, Fe-HMA increased serum activities of CAT, GSH-Px, T-SOD and Mn-SOD, as well as hepatic GSH-Px and CAT, indicating that Fe-HMA can enhance antioxidant capacity.
The bacteria in the intestine act as a barrier against pathogens, and their changes are closely linked to the health of piglets. Similar to dietary addition of iron made by saccharomyces cerevisiae (Zeng et al., 2023), dietary Fe-HMA supplementation had no significant effects on alpha indices including Simpson, Shannon and Chao1 compared with ferrous sulfate in the present study. The predominant bacteria at phylum level in the piglet cecum is Firmicutes (Chen et al., 2020). In this study, Firmicutes constituted the largest proportion of the gut microbiota in weaned piglets, indicating that the type of iron source had little effect on the prevalence of this dominant phylum. However, the iron source influenced the composition of other microbial communities within the gut. The Wilcoxon rank-sum test showed that the differential microorganism in the gut of piglets supplemented with the two iron sources at the phylum level was Proteobacteria, a potential pathogenic bacteria containing the most genera of bacteria that catalyze the oxidation of iron (Hedrich et al., 2011). The previous study showed that the abundance of Proteobacteria in cecal increased in weaned piglets fed with diet supplemented with a high level of 3000 mg/kg FeSO4 (Ding et al., 2020a). The organic iron supplementation has been shown to increase Proteobacteria relative abundance in intestine. A previous study showed that oral iron administration 25 mg/d Fe (ferrous glycine) improved the abundance of Proteobacteria in ileum, cecum and colon in the fourth day of newborn piglets (Dong et al., 2023). Zeng et al. (2023) reported that dietary supplemented 104 mg/kg and 124 mg/kg iron made by Saccharomyces cerevisiae increased the abundance of Proteobacteria in cecum. Similarly, the addition of Fe-HMA to the diet elevated the relative abundance of Proteobacteria in cecum of weaned piglets in the present study. Gemmiger, Dorea, and Subdoligranulum are the butyrate-producing genera (Lu et al., 2023; Ma et al., 2024; Visuthranukul et al., 2024). Butyrate is known for its anti-inflammatory properties and its ability to ameliorate intestinal inflammatory diseases (Zhang et al., 2021). A previous study showed that oral administration of Escherichia coli significantly reduced the abundance of Gemmiger in the colon of weaned piglets (Wang et al., 2024). Similarly, the abundance of Gemmiger_formicilis and Subdoligranulum was reported to decrease in the intestines of patients with inflammatory bowel disease (Ning et al., 2023; Visuthranukul et al., 2024). However, Fe-HMA supplementation increased the abundance of Gemmiger, Dorea, and Subdoligranulum in this study, suggesting its potential role in alleviating intestinal inflammation. Uremic toxins, including indolyl sulfate, p-Cresol sulfate and phenylacetylglutamine, can be produced by the metabolism of aromatic amino acids (Vacca et al., 2020). However, the proportion of microorganisms with aromatic_compound_degradation function increased in Fe-HMA group in this study, which may lead to lower toxins produced by aromatic compounds. Overall, Fe-HMA supplementation altered the intestinal bacterial composition in weaned piglets, which may impact intestinal health. However, their specific implications in intestine required further investigation.
In conclusion, Fe-HMA supplementation improved metabolic parameters, enhanced antioxidant capacity, and modulated gut microbiota composition in weaned piglets compared with Fe-sulphate. These improvements also supported liver function and intestinal nutrient absorption, potentially contributing to overall health. This study also revealed the promise of Fe-HMA as a superior iron source in piglet nutrition.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1192598.
The animal study was approved by Animal Care and Use Committee of Shandong Agricultural University (protocol code SDAUA-2023-327). The study was conducted in accordance with the local legislation and institutional requirements.
YF: Formal analysis, Writing – original draft. GZ: Software, Writing – review & editing. YL: Data curation, Formal analysis, Visualization, Writing – review & editing. XY: Writing – review & editing. NJ: Writing – review & editing. WL: Resources, Writing – review & editing. WY: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Shandong Province Modern Agricultural Industry Technology System Project (SDAIT-08-04) and Shandong Province Key R&D Program (Action Plan for Scientific and Technological Innovation for Rural Revitalization and Enhancement, 2023TZXD041).
Author WL was employed by the company Fujian Syno Biotech Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Biagi, G., Cinti, E., Ferruzza, S., Mordenti, A., Predieri, G., Tegoni, M. (2004). Metal chelates of 2-hydroxy-4-methylthiobutanoic acid in animal feeding: characterization, in vitro and in vivo investigations (DEU). Available online at: https://cris.unibo.it/handle/11585/8418 (Accessed November 10, 2024).
Cao, S. T., Wang, C. C., Wu, H., Zhang, Q. H., Jiao, L. F., Hu, C. H. (2018). Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets1. J. Anim. Sci. 96, 1073–1083. doi: 10.1093/jas/skx062
Chen, J., Wang, H., Ma, Y., Zhang, Y., Wang, S., Zang, J. (2022). Effects of the methionine hydroxyl analog chelated microminerals on growth performance, antioxidant status, and immune response of growing–finishing pigs. Anim. Sci. J. 93, e13730. doi: 10.1111/asj.13730
Chen, S., Wu, X., Wang, X., Shao, Y., Tu, Q., Yang, H., et al. (2020). Responses of intestinal microbiota and immunity to increasing dietary levels of iron using a piglet model. Front. Cell Dev. Biol. 8. doi: 10.3389/fcell.2020.603392
Ding, H., Han, J., Feng, J. (2020a). PSII-38 Tolerable upper intake levels of iron damage the intestine and alter the intestinal flora in weaned piglets. J. Anim. Sci. 98, 381. doi: 10.1093/jas/skaa278.670
Ding, H., Yu, X., Chen, L., Han, J., Zhao, Y., Feng, J. (2020b). Tolerable upper intake level of iron damages the intestine and alters the intestinal flora in weaned piglets. Metallomics 12, 1356–1369. doi: 10.1039/d0mt00096e
Ding, H., Zhao, Y., Yu, X., Chen, L., Han, J., Feng, J. (2021). Tolerable upper intake level of iron damages the liver of weaned piglets. J. Anim. Physiol. Anim. Nutr. 105, 668–677. doi: 10.1111/jpn.13521
Dong, Z., Liu, S., Deng, Q., Li, G., Tang, Y., Wu, X., et al. (2023). Role of iron in host-microbiota interaction and its effects on intestinal mucosal growth and immune plasticity in a piglet model. Sci. China Life Sci. 66, 2086–2098. doi: 10.1007/s11427-022-2409-0
Dong, J., Zhao, Z., Wang, Z., Li, S., Zhang, Y., Sun, Z., et al. (2024). Impact of deoxynivalenol on rumen function, production, and health of dairy cows: Insights from metabolomics and microbiota analysis. J. Hazard Mater 465, 133376. doi: 10.1016/j.jhazmat.2023.133376
Feng, X., Jiang, S., Zhang, F., Wang, R., Zhao, Y., Zeng, M. (2019). Siderophore (from synechococcus sp. PCC 7002)-chelated iron promotes iron uptake in caco-2 cells and ameliorates iron deficiency in rats. Mar. Drugs 17, 709. doi: 10.3390/md17120709
Feng, J., Ma, W. Q., Xu, Z. R., He, J. X., Wang, Y. Z., Liu, J. X. (2009). The effect of iron glycine chelate on tissue mineral levels, fecal mineral concentration, and liver antioxidant enzyme activity in weanling pigs. Anim. Feed Sci. Technol. 150, 106–113. doi: 10.1016/j.anifeedsci.2008.07.004
Feng, J., Ma, W. Q., Xu, Z. R., Wang, Y. Z., Liu, J. X. (2007). Effects of iron glycine chelate on growth, haematological and immunological characteristics in weanling pigs. Anim. Feed Sci. Technol. 134, 261–272. doi: 10.1016/j.anifeedsci.2007.02.005
Galaris, D., Barbouti, A., Pantopoulos, K. (2019). Iron homeostasis and oxidative stress: an intimate relationship. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1866, 118535. doi: 10.1016/j.bbamcr.2019.118535
Han, M., Fu, X., Xin, X., Dong, Y., Miao, Z., Li, J. (2022). High dietary organic iron supplementation decreases growth performance and induces oxidative stress in broilers. Animals-basel 12, 1604. doi: 10.3390/ani12131604
Han, Y.-H., Onufer, E. J., Huang, L.-H., Sprung, R. W., Davidson, W. S., Czepielewski, R. S., et al. (2021). Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science. doi: 10.1126/science.abe6729
He, F., Jin, X., Wang, C., Hu, J., Su, S., Zhao, L., et al. (2023). Lactobacillus rhamnosus GG ATCC53103 and Lactobacillus plantarum JL01 improved nitrogen metabolism in weaned piglets by regulating the intestinal flora structure and portal vein metabolites. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1200594
Hedrich, S., Schlömann, M., Johnson, D. B. (2011). The iron-oxidizing proteobacteria. Microbiology 157, 1551–1564. doi: 10.1099/mic.0.045344-0
Heidbüchel, K., Raabe, J., Baldinger, L., Hagmüller, W., Bussemas, R. (2019). One iron injection is not enough—iron status and growth of suckling piglets on an organic farm. Animals 9, 651. doi: 10.3390/ani9090651
Huang, Q.-C., Wang, E.-L., Kwaku, A., Dong, X.-H., Tan, B.-P., Chi, S.-Y., et al. (2018). Iron bioavailability of different sources in juvenile grouper epinephelus coioides. Aquac Res. 49, 2799–2807. doi: 10.1111/are.13742
Kim, J. C., Wilcock, P., Bedford, M. R. (2018). Iron status of piglets and impact of phytase superdosing on iron physiology: a review. Anim. Feed Sci. Technol. 235, 8–14. doi: 10.1016/j.anifeedsci.2017.11.001
Kuang, S.-Y., Xiao, W.-W., Feng, L., Liu, Y., Jiang, J., Jiang, W.-D., et al. (2012). Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol. 32, 629–636. doi: 10.1016/j.fsi.2011.12.012
Li, Y., Liu, Y., Mu, C., Zhang, C., Yu, M., Tian, Z., et al. (2024). Magnolol-driven microbiota modulation elicits changes in tryptophan metabolism resulting in reduced skatole formation in pigs. J. Hazard Mater 467, 133423. doi: 10.1016/j.jhazmat.2024.133423
Li, Y., Zhao, X., Zhang, L., Zhan, X., Liu, Z., Zhuo, Y., et al. (2020). Effects of a diet supplemented with exogenous catalase from penicillium notatum on intestinal development and microbiota in weaned piglets. Microorganisms 8, 391. doi: 10.3390/microorganisms8030391
Lu, X., Ma, J., Li, R. (2023). Alterations of gut microbiota in biopsy-proven diabetic nephropathy and a long history of diabetes without kidney damage. Sci. Rep. 13, 12150. doi: 10.1038/s41598-023-39444-4
Ma, O., Dutta, A., Bliss, D. W., Nakatsu, C. H., Weaver, C. M., Whisner, C. M. (2024). Identifying gut microbiome features that predict responsiveness toward a prebiotic capable of increasing calcium absorption: A pilot study. Calcif Tissue Int. 114, 513–523. doi: 10.1007/s00223-024-01201-8
Ma, J., Liu, S., Piao, X., Wang, C., Wang, J., Lin, Y., et al. (2022). Dietary supplementation of ferrous glycine chelate improves growth performance of piglets by enhancing serum immune antioxidant properties, modulating microbial structure and its metabolic function in the early stage. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.876965
Ma, J., Wen, X., Mo, F., Wang, X., Shen, Z., Li, M. (2014). Effects of different doses and duration of iron supplementation on curing iron deficiency anemia: an experimental study. Biol. Trace Elem Res. 162, 242–251. doi: 10.1007/s12011-014-0115-4
National Research Council (2012). Nutrient Requirements of Swine (11th rev. ed.). Washington, D.C.: The National Academies Press.
Ning, L., Zhou, Y.-L., Sun, H., Zhang, Y., Shen, C., Wang, Z., et al. (2023). Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts. Nat. Commun. 14, 7135. doi: 10.1038/s41467-023-42788-0
Pasricha, S.-R., Tye-Din, J., Muckenthaler, M. U., Swinkels, D. W. (2021). Iron deficiency. Lancet 397, 233–248. doi: 10.1016/S0140-6736(20)32594-0
Penson, P. E., Pirro, M., Banach, M. (2020). LDL-C: lower is better for longer—even at low risk. BMC Med. 18, 320. doi: 10.1186/s12916-020-01792-7
Predieri, G., Beltrami, D., Pattacini, R., Parisi, M. L., Sinicropi, A., Valensin, D., et al. (2009). Structural studies in solution and in the solid state on the zinc chelate of 2-hydroxy-(4-methylthio)butanoic acid, an effective mineral supplement in animal feeding. Inorg Chim. Acta 362, 1115–1121. doi: 10.1016/j.ica.2008.05.027
Predieri, G., Tegoni, M., Cinti, E., Leonardi, G., Ferruzza, S. (2003). Metal chelates of 2-hydroxy-4-methylthiobutanoic acid in animal feeding: preliminary investigations on stability and bioavailability. J. Inorg Biochem. 95, 221–224. doi: 10.1016/S0162-0134(03)00067-9
Regmi, N., Wang, T., Crenshaw, M. A., Rude, B. J., Liao, S. F. (2018). Effects of dietary lysine levels on the concentrations of selected nutrient metabolites in blood plasma of late-stage finishing pigs. J. Anim. Physiol. Anim. Nutr. 102, 403–409. doi: 10.1111/jpn.12714
Rothschild, M. A., Oratz, M., Schreiber, S. S. (1988). Serum albumin. Hepatology 8, 385–401. doi: 10.1002/hep.1840080234
Shubham, K., Anukiruthika, T., Dutta, S., Kashyap, A. V., Moses, J. A., Anandharamakrishnan, C. (2020). Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. Technol. 99, 58–75. doi: 10.1016/j.tifs.2020.02.021
Sun, L. M., Yu, B., Luo, Y. H., Zheng, P., Huang, Z., Yu, J., et al. (2023). Effect of small peptide chelated iron on growth performance, immunity and intestinal health in weaned pigs. Porc Health Manag 9, 32. doi: 10.1186/s40813-023-00327-9
Sun, L., Yu, B., Luo, Y., Zheng, P., Huang, Z., Yu, J., et al. (2022). Effects of different sources of iron on growth performance, immunity, and intestinal barrier functions in weaned pigs. Agriculture 12, 1627. doi: 10.3390/agriculture12101627
Tang, X., Xiong, K., Fang, R., Li, M. (2022). Weaning stress and intestinal health of piglets: a review. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1042778
Vacca, M., Celano, G., Calabrese, F. M., Portincasa, P., Gobbetti, M., De Angelis, M. (2020). The controversial role of human gut lachnospiraceae. Microorganisms 8, 573. doi: 10.3390/microorganisms8040573
Visuthranukul, C., Sriswasdi, S., Tepaamorndech, S., Chamni, S., Leelahavanichkul, A., Joyjinda, Y., et al. (2024). Enhancing gut microbiota and microbial function with inulin supplementation in children with obesity. Int. J. Obes. 48, 1696–1704. doi: 10.1038/s41366-024-01590-8
Wang, Y., Zhang, Z., Du, M., Ji, X., Liu, X., Zhao, C., et al. (2024). Berberine alleviates ETEC-induced intestinal inflammation and oxidative stress damage by optimizing intestinal microbial composition in a weaned piglet model. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1460127
Yang, G., Yan, Y., Zhang, L., Ruan, Z., Hu, X., Zhang, S., et al. (2020). Porcine circovirus type 2 (PCV2) and Campylobacter infection induce diarrhea in piglets: Microbial dysbiosis and intestinal disorder. Anim. Nutr. 6, 362–371. doi: 10.1016/j.aninu.2020.05.003
Zeng, Y., Jiang, L., Zhou, B., Liu, Y., Wang, L., Hu, Z., et al. (2023). Effect of high efficiency digestion and utilization of organic iron made by saccharomyces cerevisiae on antioxidation and caecum microflora in weaned piglets. Animals 13, 498. doi: 10.3390/ani13030498
Zhang, M., Wang, Y., Zhao, X., Liu, C., Wang, B., Zhou, J. (2021). Mechanistic basis and preliminary practice of butyric acid and butyrate sodium to mitigate gut inflammatory diseases: a comprehensive review. Nutr. Res. 95, 1–18. doi: 10.1016/j.nutres.2021.08.007
Keywords: antioxidant, gut microbiota, metabolic parameters, organic iron, weaned piglets
Citation: Fu Y, Zhou G, Liu Y, Yuan X, Jiao N, Lu W and Yang W (2025) Changes of metabolic parameters, antioxidant capacity, and gut microbiota in response to substitution of ferrous sulfate with iron hydroxy methionine analog chelate in weaned piglets. Front. Cell. Infect. Microbiol. 15:1539607. doi: 10.3389/fcimb.2025.1539607
Received: 04 December 2024; Accepted: 24 January 2025;
Published: 18 February 2025.
Edited by:
Yong Zhuo, Sichuan Agricultural University, ChinaCopyright © 2025 Fu, Zhou, Liu, Yuan, Jiao, Lu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiren Yang, d3J5YW5nQHNkYXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.