
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 15 April 2025
Sec. Clinical Infectious Diseases
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1532289
This article is part of the Research TopicAssessing Nosocomial Fungal Infections: Virulence and Resistance DynamicsView all 3 articles
 Min Kang1
Min Kang1 Yuxuan Wu1
Yuxuan Wu1 Qingquan Shi2
Qingquan Shi2 Zhiqun Wang2
Zhiqun Wang2 Yang Zhang2
Yang Zhang2 Kexin Chen2
Kexin Chen2 Xizhan Xu2
Xizhan Xu2 Man Zhou2
Man Zhou2 Qingfeng Liang2
Qingfeng Liang2 Xinxin Lu1*
Xinxin Lu1*Objective: This study investigated the susceptibility of various Fusarium fungi to five topical antifungal agents: natamycin, voriconazole, chlorhexidine, natamycin combined with chlorhexidine, and voriconazole combined with chlorhexidine. And to explore the clinical feasibility of combination therapy in the treatment of corneal infections caused by F. solani, with the goal of optimizing the treatment regimen for fungal keratitis.
Methods: A total of 194 strains of Fusarium were isolated from the corneas between 2013 and 2024 and identified to the species level using mass spectrometry. The MICs was determined using a commercial microdilution plate to assess the in vitro activity of the drugs used alone and in combination (natamycin/chlorhexidine, voriconazole/chlorhexidine). Additionally, the clinical efficacy was prospectively observed in 5 patients with corneal infections caused by F. solani. The treatment regimens included 5% natamycin combined with 0.04% chlorhexidine, chlorhexidine used alone, or natamycin used alone, with follow-up lasting up to 90 days.
Results: F. solani species complex (FSSC, 46.91%) and the F. fujikuroi species complex (FFSC, 45.88%) were the predominant isolates, with a geographical distribution concentrated in Northern China. The MICs for natamycin in FSSC and FFSC were both 2–8 μg /mL, respectively. The MICs for FSSC and FFSC respectively ranged from 0.25–16 and 1–8 μg/mL for voriconazole and 2 to > 16 μg/mL and 1 to > 16 μg/mL for chlorhexidine. The MICs of natamycin were not significantly different between FSSC and FFSC. However, voriconazole, chlorhexidine, natamycin combined with chlorhexidine, and voriconazole combined with chlorhexidine had significantly higher MICs for FSSC compared with FFSC. Compared with voriconazole, voriconazole combined with chlorhexidine exhibited enhancement of antifungal activity against 100% of tested Fusarium strains. Compared with natamycin, enhancement of antifungal activity of natamycin combined with chlorhexidine was 81.4% for all Fusarium spp., and the activity were significantly lower for F. solani (65.9%) than for non-F. solani species (93.6%). Among the 5 patients, 3 patients received treatment with natamycin combined with chlorhexidine, resulting in clinical cure in 2 patients (in 1-1.5 months), while 1 patient required a corneal transplant due to delayed treatment. One patient treated with natamycin alone and one treated with chlorhexidine alone both achieved clinical cure (in 2-3 months).
Conclusion: Natamycin combined with chlorhexidine and voriconazole combined with chlorhexidine exhibited enhancement of antifungal activity against Fusarium spp. during in vitro sensitivity tests. The findings of this study provide valuable guidance for establishing the epidemiological cutoff and clinical MIC values for Fusarium spp. This study paves the way for future multicenter studies on the treatment of FK with natamycin and chlorhexidine.
Fungal keratitis (FK) is one of the leading causes of monocular blindness in developing countries where manual farming practices are predominant (Halim et al., 2021; Bisen et al., 2024). Infections caused by Fusarium species represent the highest proportion of FK cases (Khurana et al., 2022; Roberts et al., 2023; Skora et al., 2023). The most common route of infection is microtrauma or destructive ocular surface disease (Oliveira Dos Santos et al., 2020).
Fusarium species are ubiquitous filamentous fungi, commonly found in soil, plants, and water (Nucci et al., 2021). Classification of Fusarium spp. is complex; molecular techniques suggest that the medically relevant genus Fusarium consists of several species and subspecies (Sav et al., 2018). Fusarium spp. have shown broad resistance to different classes of antifungal drugs, and susceptibility patterns may vary within species complexes (Slavin et al., 2015). Natamycin or voriconazole has been widely used for the topical treatment of fungal keratitis (Austin et al., 2017; Edwar et al., 2020; Sharma et al., 2022).
Chlorhexidine is a cationic biguanide disinfectant and antiseptic (Hiom et al., 1996; McDonnell and Russell, 1999). Its mechanism of action involves binding to and disrupting bacterial cell membranes, with a wide range of antimicrobial activity (Lima de Sousa et al., 2024). Chlorhexidine affects the fungal plasma membrane and has significant effects elsewhere in the cell (Abbood et al., 2023). It binds to and persists with proteins in the stratum corneum of the epidermis (Sebben, 1983), which has been shown to have a cumulative effect when used repeatedly (Mullany et al., 2006).Recent studies have shown that the combination of natamycin and chlorhexidine or voriconazole and chlorhexidine can also achieve good therapeutic effects (Jiang et al., 2020; Arunga et al., 2021; Raghavan et al., 2021). However, based on published research data, there is currently a lack of systematic in vitro drug susceptibility tests and studies using susceptibility results to guide clinical treatment.
Thus, we designed a commercial broth microdilution plate. The plate includes five commonly used topical antifungal agents (natamycin, voriconazole, chlorhexidine, natamycin combined with chlorhexidine, and voriconazole combined with chlorhexidine). By determining the minimum inhibitory concentrations (MICs) of these agents, we aim to provide clinicians with data that can help guide therapeutic decisions and optimize treatment regimens for patients with FK. Antifungal susceptibility testing to determine the MIC ranges and antifungal activity was conducted on 194 strains of Fusarium isolated from corneal tissues injured during agricultural activities from 2013 to 2024. Through observations of clinical efficacy, we evaluated the feasibility of treating Fusarium keratitis with topical applications of natamycin combined with chlorhexidine and chlorhexidine alone.
A total of 194 strains of Fusarium spp. were isolated from immunocompetent patients with agriculture-related injuries between 2013 and 2024. Most of the isolates were from Northern China. Identification was performed through morphological analysis, mass spectrometry. Fusarium strains were cultured on Potato Dextrose Agar (PDA) at 28°C for 3 days. Following incubation, slide preparations were made and stained with lactophenol cotton blue. Morphological identification (Lu et al., 2023) was performed via microscopic examination. The isolates to be identified were inoculated on PDA medium and cultured at 28°C for 3 days until colonies formed. Hyphae from the edge of the colony were selected with a pointed cotton swab, directly smeared thinly onto the target plate, and dried. 1 μL of 70% formic acid solution was added after drying, followed by 1 μL of matrix solution, which was dried again before mass spectrometry analysis. The solution was primarily a supersaturated solution of α-cyano-4-hydroxycinnamic acid, where the solvent consisted of 50% pure acetonitrile, 47.5% distilled water, and 2.5% trifluoroacetic acid. Identification was performed using the Zhuhai Dir Biotechnology Co., Ltd. fully automated microbial mass spectrometry detection system (Smart MS 5020). An identification score ≥2.0 allows identification to the species/complex level (Huang et al., 2017; Lu, 2021).
Fusarium species were classified as follows (Uemura et al., 2022):
1. F. solani species complex (FSSC) includes strains of F. solani and F. keratoplasticum;
2. F. fujikuroi species complex (FFSC) includes strains of F. verticillioides and F. proliferatum;
3. F. oxysporum species complex (FOSC) consists of only one species, F. oxysporum;
4. F. dimerum species complex (FDSC) consists of only one species, F. dimerum;
5. To more clearly determine the MICs of topical antifungal medications, Fusarium spp. were divided into the F. solani group and the non-F. solani group, including F. verticillioides, F. proliferatum, F. oxysporum, F. keratoplasticum, and F. dimerum.
The antifungal agents included natamycin, natamycin combined with chlorhexidine, voriconazole, voriconazole combined with chlorhexidine, amphotericin B, terbinafine, posaconazole, and itraconazole. Preliminary experiments confirmed the concentration of chlorhexidine in combination with other drugs (8 μg/mL). The detection ranges for the antifungal agents were as follows: natamycin, 0.25–128 μg/mL; natamycin combined with chlorhexidine, 0.25/8–128/8 μg/mL; voriconazole, 0.015–16 μg/mL; voriconazole combined with chlorhexidine, 0.015/8–16/8 μg/mL; amphotericin B, 0.125–16 μg/mL; terbinafine and posaconazole, 0.015–16 μg/mL; and chlorhexidine and itraconazole, 0.03–16 μg/mL. The plates were customized by Zhuhai DL Biotech Co., Ltd. The reagent plates consisted of antifungal agents and a colorimetric agent. The MIC values were determined based on the color changes in the reaction wells. The colorimetric agent was Alamar Blue, which responds to changes in the redox potential caused by fungal growth in the reaction wells. The proliferation of fungi consumes molecular oxygen, causing the colorimetric agent to change color from blue to pink (the oxidized form of Alamar Blue is blue, while the reduced form is pink).
Using a microbroth dilution method (John, 2008), 10 strains of Fusarium were tested, revealing a clear antifungal activity when the concentration of chlorhexidine reached 8 μg/mL in the combined antifungal susceptibility test with F. solani. In contrast, for non-F. solani, a significant antifungal activity was observed when the chlorhexidine concentration reached 1 or 2 μg/mL. Because F. solani accounted for over 50% of the strains studied, a chlorhexidine concentration of 8 μg/mL was selected for combination with natamycin and voriconazole. In the combined drug susceptibility tests, when the concentration of chlorhexidine reached 8 μg/mL, the MICs of natamycin and voriconazole in combination with chlorhexidine were lower than the MICs of the corresponding individual drugs, indicating enhancement of antifungal activity.
Strains were inoculated onto PDA and incubated at 28°C for 5 d. A suitable amount of spores was scraped and resuspended in 5 mL of diluent (containing 0.1% Tween and 0.85% sterile saline). The cell concentration was adjusted to 1–5×108 cfu/mL. A 100-μL aliquot of this suspension was added to 10 mL of RPMI 1640 solution supplemented with glucose, achieving a final Fusarium spore concentration of 1–5×104 cfu/mL. To each well of a 96-well plate, 100 μL of the suspension was added. After incubating the plates at 35°C for 48 h, the MIC was determined by identifying the lowest concentration of antifungal agent required to achieve 100% inhibition of fungal growth compared to the control (indicated by a purple or red color). The criteria for synergy were based on a custom susceptibility plate; synergy was confirmed when the MIC of the combination of natamycin or voriconazole with 8 μg/mL chlorhexidine was lower than that of natamycin or voriconazole alone. We also included the Aspergillus flavus strain ATCC 204304 for quality control in the analysis. The methods for the antifungal susceptibility tests were based on CLSI M38-A2 (John, 2008).
Patients were enrolled in September 2023 during their first visit to our center. The inclusion criterium was a positive culture for Fusarium spp. The diagnostic criteria included the presence of hyphal structures in corneal scrapings, fungal hyphae images visible during in vivo confocal microscopy (IVCM), Fusarium spp. found in the culture of the corneal specimen, and susceptibility testing performed. Treatment involved a combination of 5% natamycin and 0.04% chlorhexidine eye drops along with 0.04% chlorhexidine eye drops alone. Follow-up assessments were conducted at 7, 14, 21, 30, 60, and 90 d. The observation endpoints were clinical cure (Rahman et al., 1998), defined by corneal epithelial healing, complete control of infection, no recurrence within 2 months of follow-up, or the need for corneal transplantation. Four patients with Fusarium keratitis were enrolled.
Statistical analyses were conducted using R version 4.4.1 (R Foundation, Vienna, Austria). Categorical variables were expressed as frequency and percentage. The MIC50/MIC90 and frequency of studied isolates were determined (Soleimani et al., 2023). Mann–Whitney U test was used to compare resistance by region and the MIC values between the F. solani and non-F. solani groups. Kruskal–Wallis’s test and Dunn’s test were used to analyze MIC values among different complexes. P < 0.05 was considered statistically significant.
A total of 194 Fusarium strains were isolated from 194 agricultural immunocompetent workers between 2013 and 2024, all of whom had injuries from crops. The most common isolate was F. solani (n = 85, 43.81%) followed by F. verticillioides (n = 47, 24.23%), F. proliferatum (n = 42, 21.65%), F. keratoplasticum (n = 6, 3.09%), F. dimerum (n = 9, 4.64%), and F. oxysporum (n = 5, 2.58%). The isolates were categorized into their respective species complexes (Figure 1A), with FSSC being the most common (n = 91, 46.91%) followed by FFSC (n = 89, 45.88%), FDSC (n = 9, 4.64%), and FOSC (n = 5, 2.58%). Geographically, Fusarium spp. was concentrated in Beijing, Hebei, Inner Mongolia, and Liaoning, almost located in Northern China (Supplementary Appendix Figure 1). No significant temporal or seasonal differences in frequency were observed among the Fusarium species complexes (Figures 1B, C). The incidence of Fusarium spp. peaked from March to June and from September to December, with significant increases observed in autumn and spring (Figure 1C).
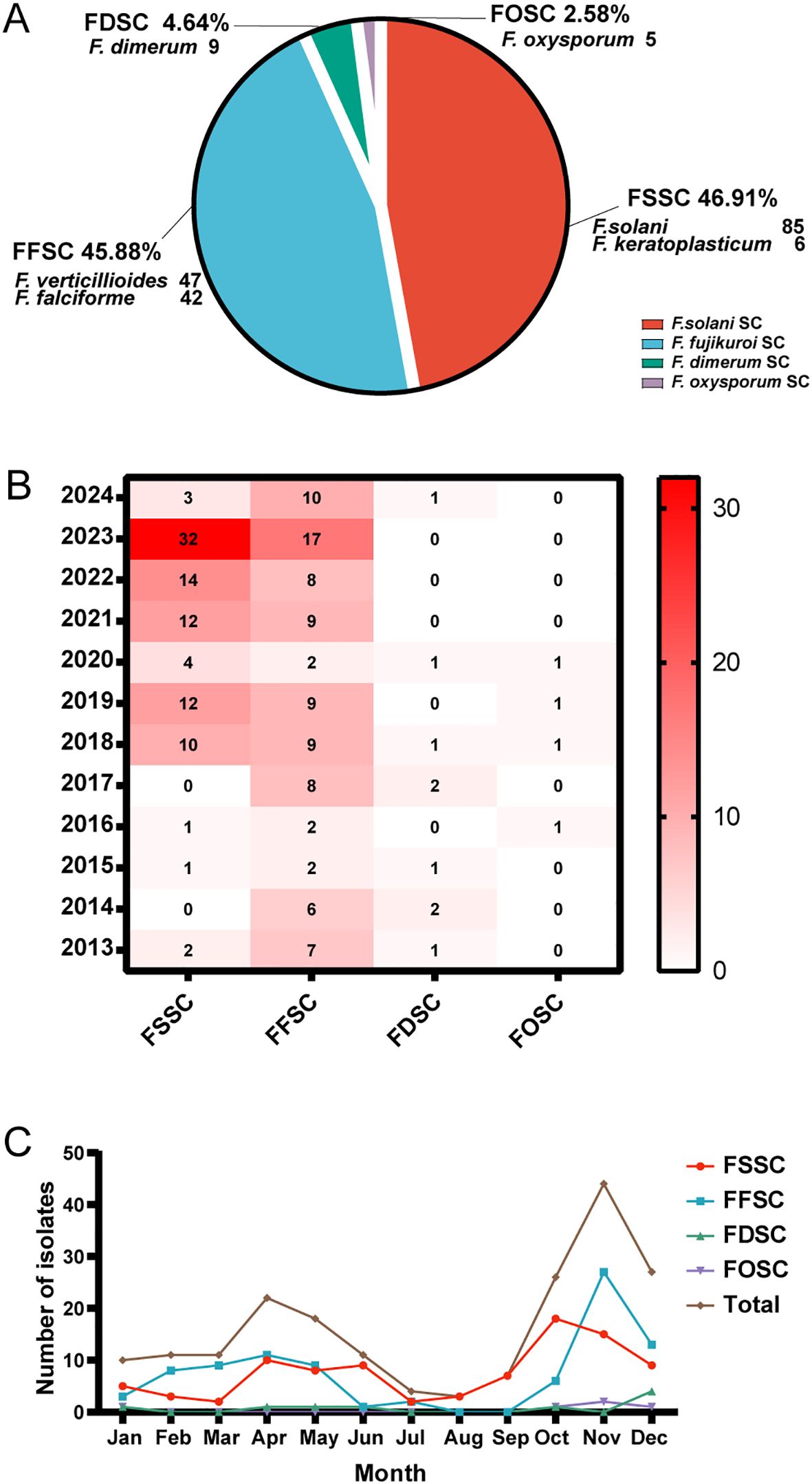
Figure 1. Classification of Fusarium isolates and their annual and seasonal distributions. (A) Distribution of 194 Fusarium isolates. SC = species complex. (B) Annual distribution heat map of Fusarium spp. complexes from 2013 to 2024. The number of Fusarium species complexes are shown per year within the squares of the heat map. The color scale indicates number of isolates and the darker the red color, the higher the number of isolates while white represents 0 isolates. (C) Seasonal distributions of Fusarium spp. complexes. Total represents the number of Fusarium isolates.
In vitro susceptibility tests for Fusarium isolates showed that the MIC values of natamycin combined with chlorhexidine (<0.12/8-8/8μg/mL) was significantly lower than that of natamycin (8-8μg/mL) and the MICs of voriconazole combined with chlorhexidine (<0.015-4μg/mL)was lower than that of voriconazole (0.25-8μg/mL) (P<0.001, P<0.001) (Figures 2A, B; Supplementary Appendix Table 1).

Figure 2. Comparison of the MIC values of four antifungal drugs for Fusarium species. Violin plots illustrating the distributions of MICs for (A) natamycin (NAT) vs. natamycin combined with chlorhexidine (NAT+CHX), (B) voriconazole (VOR) vs. voriconazole combined with chlorhexidine (VOR+CHX) against Fusarium species. Asterisks denote statistical significance levels: ***P < 0.001.
The MICs of natamycin were lower for FDSC compared with FSSC, FFSC, and FOSC (P < 0.001, P < 0.001, and P < 0.01, respectively). Natamycin combined with chlorhexidine showed significantly higher MIC values in FSSC than in FFSC (P < 0.01) (Table 1; Figure 3A).
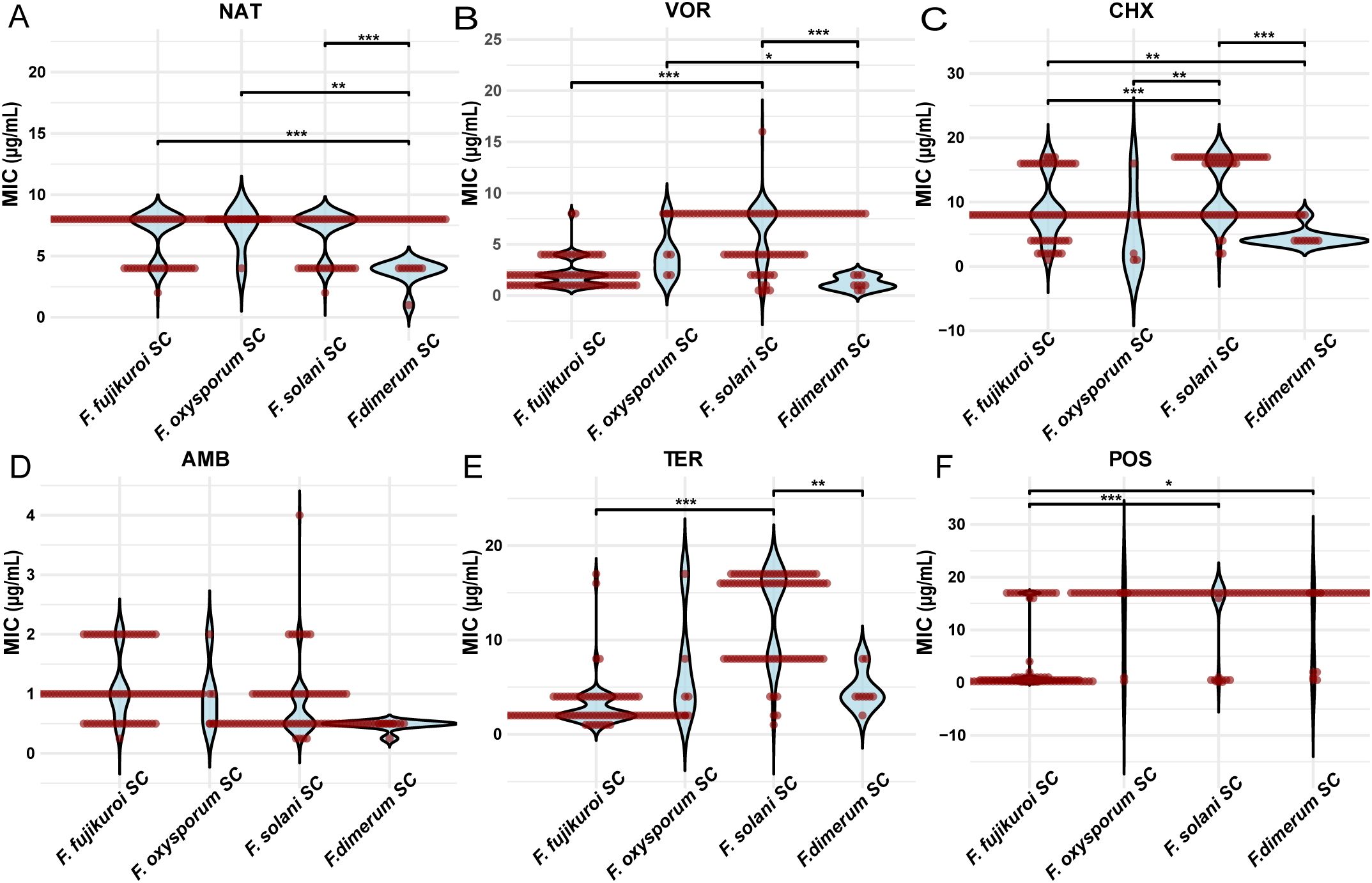
Figure 3. Comparison of the MIC values of six antifungal drugs for different Fusarium species complexes. MIC distributions of (A) natamycin (NAT), (B) voriconazole (VOR), (C) chlorhexidine (CHX), (D) amphotericin B (AMB), (E) terbinafine (TER), and (F) posaconazole (POS) across FSSC, FFSC, FDSC and FOSC. Asterisks denote statistical significance levels: *P < 0.05, **P < 0.01, and ***P < 0.001.
Voriconazole had significantly higher MIC values in FSSC compared to FFSC and FDSC (P < 0.001 and P < 0.001, respectively). Voriconazole combined with chlorhexidine also showed significantly higher MIC values in FSSC compared to FFSC (P < 0.001; Table 1; Figure 3B).
The MIC values of chlorhexidine were significantly higher in FSSC than in FFSC. Specifically, MIC exceeded 16 μg/mL for 28 strains in FSSC but only four strains in FFSC (P < 0.001; Table 1; Figure 3C).
Amphotericin B had significantly higher MIC values in FFSC compared with FSSC (P < 0.001) and FDSC (P < 0.001). Terbinafine showed significantly higher MIC values in FSSC compared with FFSC (P < 0.001) and FDSC (P < 0.01). Posaconazole had significantly higher MIC values in FSSC compared with FFSC (P < 0.001). For itraconazole, 97.9% of Fusarium isolates had MIC values exceeding 16 μg/mL (Table 1; Figures 3D-F).
The MIC values for F.solani and non-F. solani respectively ranged from 2–8 and 1–8 μg/mL for natamycin; 0.5–8 and 0.25–16 μg/mL for voriconazole; 2 to > 16 and 1 to > 16 μg/mL for chlorhexidine; < 0.12/8 to 8/8 and < 0.12/8 to 8/8 μg/mL for natamycin combined with chlorhexidine; and < 0.015/8 to 4/8 and < 0.015/8 to 2/8 μg/mL for voriconazole combined with chlorhexidine (Table 2). The MIC values of natamycin, voriconazole, chlorhexidine, natamycin combined with chlorhexidine (8μg/mL), and voriconazole combined with chlorhexidine (8μg/mL) were significantly higher in the F. solani group than in the non-F. solani group (P < 0.05, P < 0.001, P < 0.001, P < 0.001, and P < 0.001, respectively; Figures 4A–E). Compared with natamycin, natamycin combined with chlorhexidine exhibited enhancement of antifungal activity against 81.4% of 194 Fusarium strains. For F. solani, the enhancement of antifungal activity was 65.9% (56/85), significantly lower than that for non-F. solani (93.6%, 102/109; P < 0.01). For F. solani, 45.9% (39/85) of clinical isolates showed the lowest concentration in natamycin combined with chlorhexidine (8μg/mL), significantly lower than that for non-F. solani (68.8%, 75/109; P < 0.01; Supplementary Appendix Table 3). In contrast, compared with voriconazole, voriconazole combined with chlorhexidine (8μg/mL) exhibited a 100% enhancement of antifungal activity. For F. solani, 48.2% (41/85) of clinical isolates showed the lowest concentration, significantly lower than that for non-F. solani (76.1%, 83/109; P < 0.001; Supplementary Appendix Table 3).
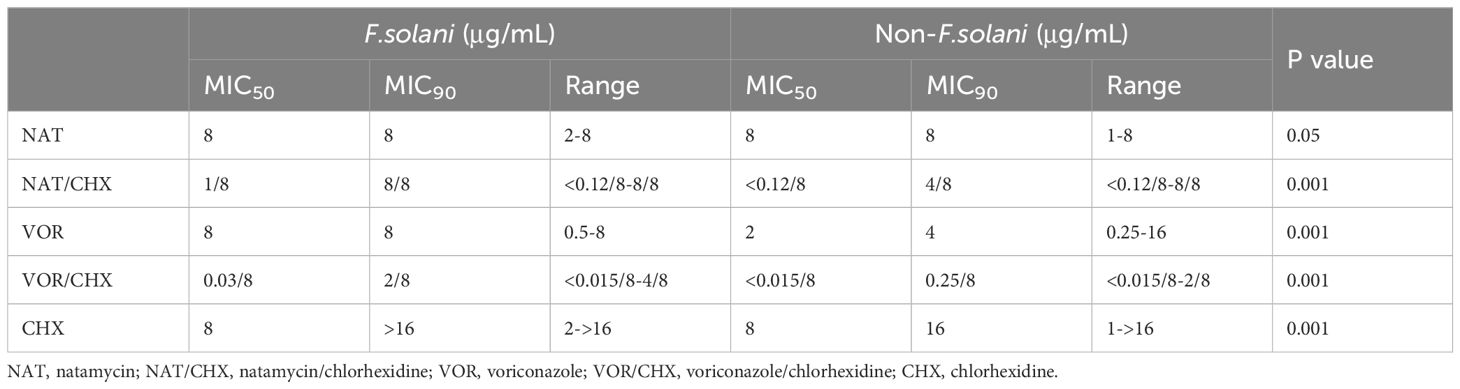
Table 2. MIC50, MIC90, and MIC ranges of ophthalmic antifungal drugs against F. solani and non-F.solani species.
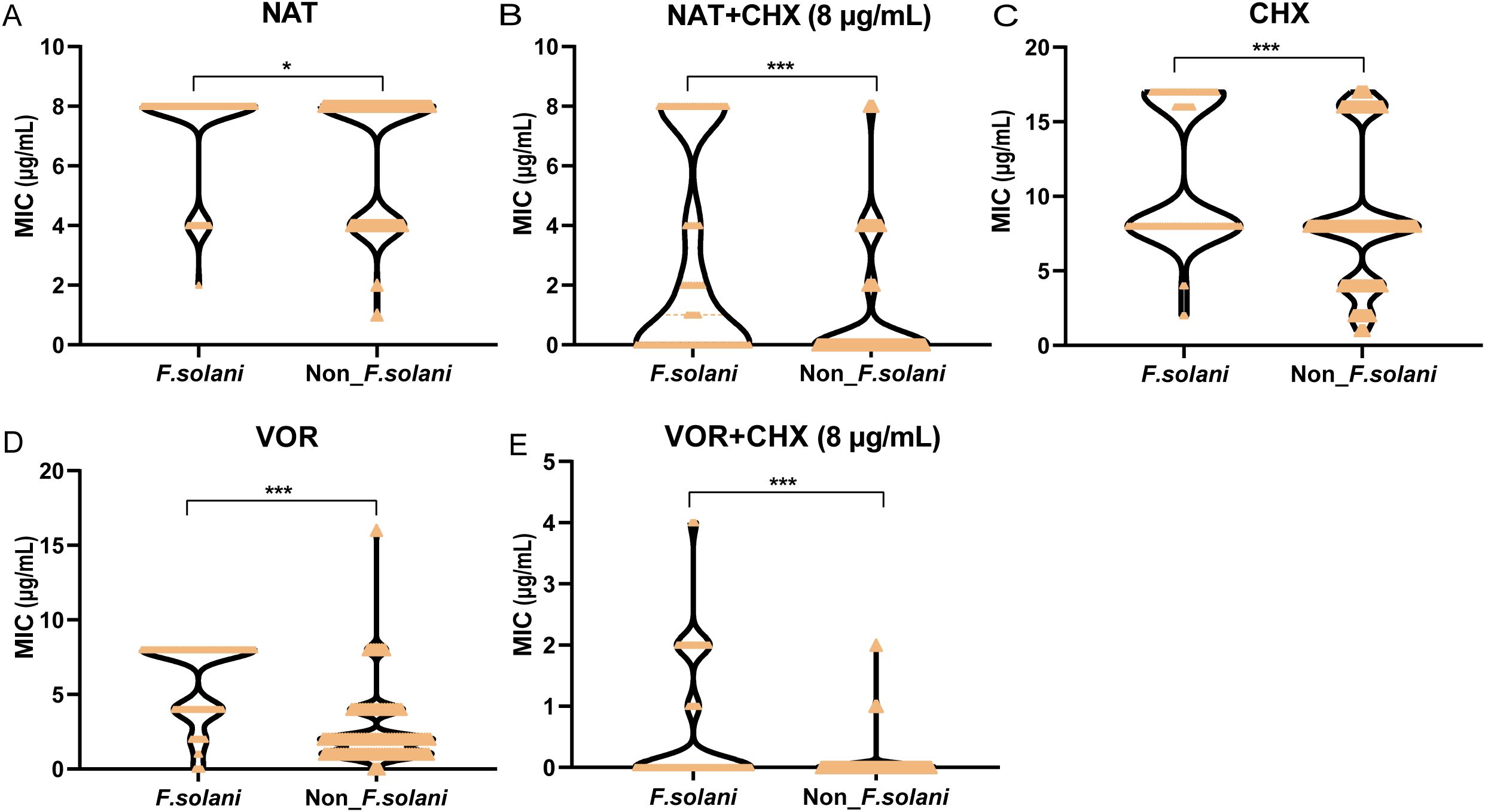
Figure 4. Comparison of topical medication efficacy between F. solani and non-F. solani species. Violin plots illustrating the distributions of MICs for (A) natamycin (NAT), (B) natamycin combined with chlorhexidine (NAT+ CHX), (C) chlorhexidine (CHX), (D) voriconazole (VOR), and (E) voriconazole combined with chlorhexidine (VOR+ CHX) against F.solani and non-F. solani species. CHX concentration in combination drug was fixed at 8 µg/mL. Asterisks denote statistical significance levels: *P < 0.05, and ***P < 0.001.
In Northern China, topical voriconazole formulations are limitedly available, and natamycin ophthalmic solution is often the first-line of treatment chosen by clinicians for Fusarium keratitis. This study was a single-center study, and only 5 patients diagnosed with F. solani keratitis were collected during the study period without other antifungal treatments. Clinical data from 5 patients is a limitation of this study since statistically significant conclusions can’t be established. Among five patients, three patients (Case 1-Case 3) received topical treatment with 5% natamycin combined with 0.04% chlorhexidine, and one patient (Case 4) received only natamycin, while one patient (Case 5) received only chlorhexidine. The results are detailed below.
A 64-year-old female farmer presented to our hospital in September with eye pain and redness after being scratched by a bean sprout in her left eye. Slit lamp examination revealed conjunctival and ciliary congestion without scleral congestion, corneal ulcer, infiltration, stromal edema, and wrinkling of the posterior elastic layer with poorly defined borders. IVCM examination showed epithelial loss with numerous fungal hyphal structures in the ulcer area. The edges of the ulcer demonstrated significant inflammatory cell infiltration. The patient was clinically diagnosed with Fusarium keratitis in the left eye (Figures 5A–C). Treatment with 5% natamycin combined with 0.04% chlorhexidine eye drops was administered every hour for 1 month. The conjunctival congestion and edema subsided, the corneal lesions disappeared, and complete epithelial healing was achieved, leaving only superficial opacification. IVCM examination showed complete healing of the corneal epithelial ulcer, fibroblast proliferation in the stroma, and no remaining fungal hyphal structures (Figures 5D–F). Thus, the patient achieved clinical cure.
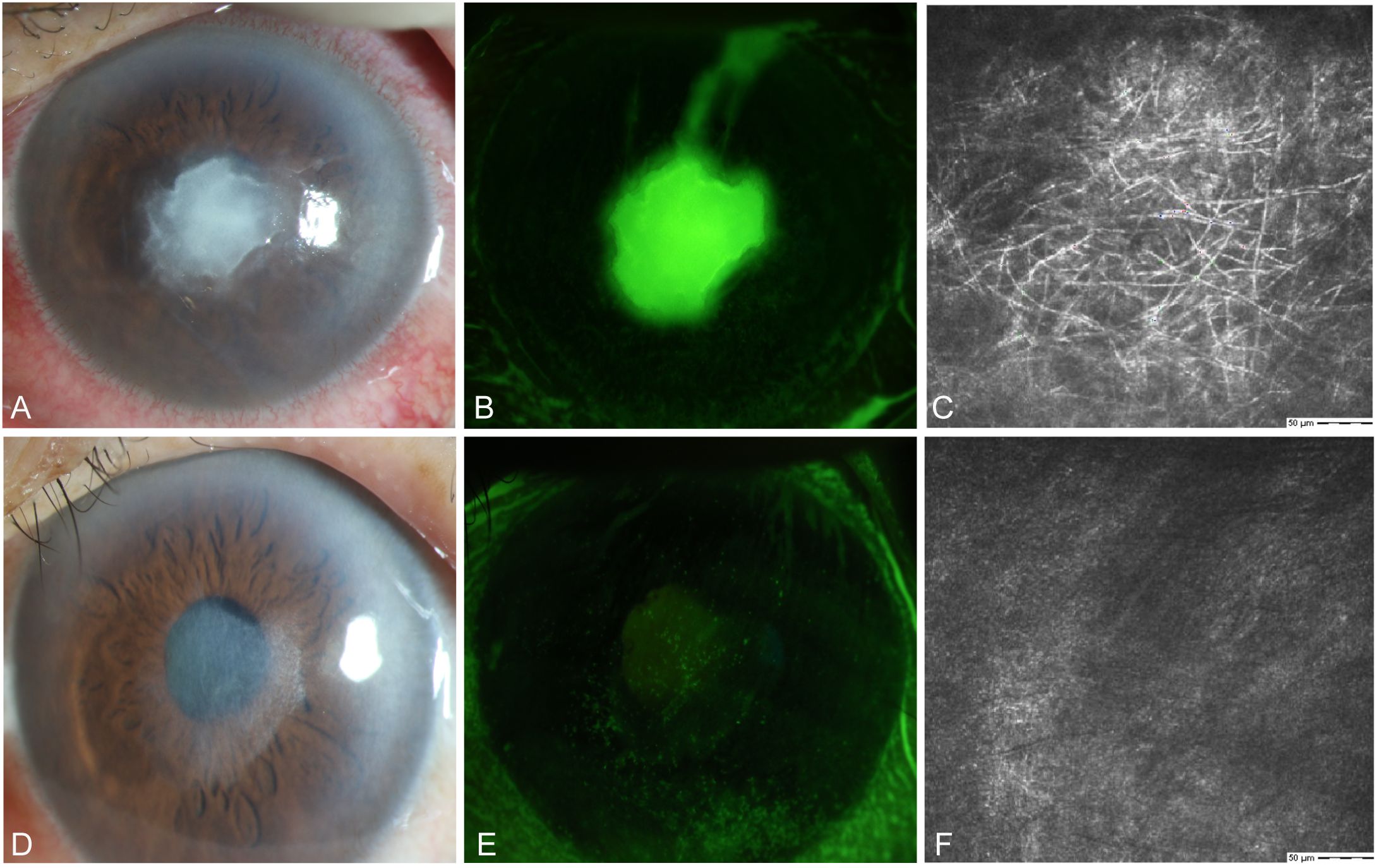
Figure 5. Slit-lamp and IVCM images acquired during patient (Case 1) treatment. (A) Slit-lamp examination images obtained at initial diagnosis showing grayish-white dense infiltrates and feathery margins. (B) Corneal fluorescein staining images showing epithelial ulcers. (C) IVCM images obtained at initial diagnosis showing fungus-like structures. (D, E) Slit-lamp examination and corneal fluorescein staining images obtained after treatment showing corneal ulcer healing. (F) IVCM examination images showing corneal epithelial healing and absence of mycelial structures.
A 50-year-old female presented with the sensation of a foreign body in her right eye after riding an electric bicycle. The patient was clinically diagnosed with right eye Fusarium keratitis (Figures 6A–C). Treatment with 5% natamycin combined with 0.04% chlorhexidine eye drops was administered every hour for 1.5 months. The conjunctival congestion and edema subsided, and complete epithelial healing was achieved, leaving only superficial opacification. IVCM examination showed complete healing of the corneal epithelial ulcer, fibroblast proliferation in the stroma, and no remaining fungal hyphal structures (Figures 6D–F). Thus, the patient achieved clinical cure.
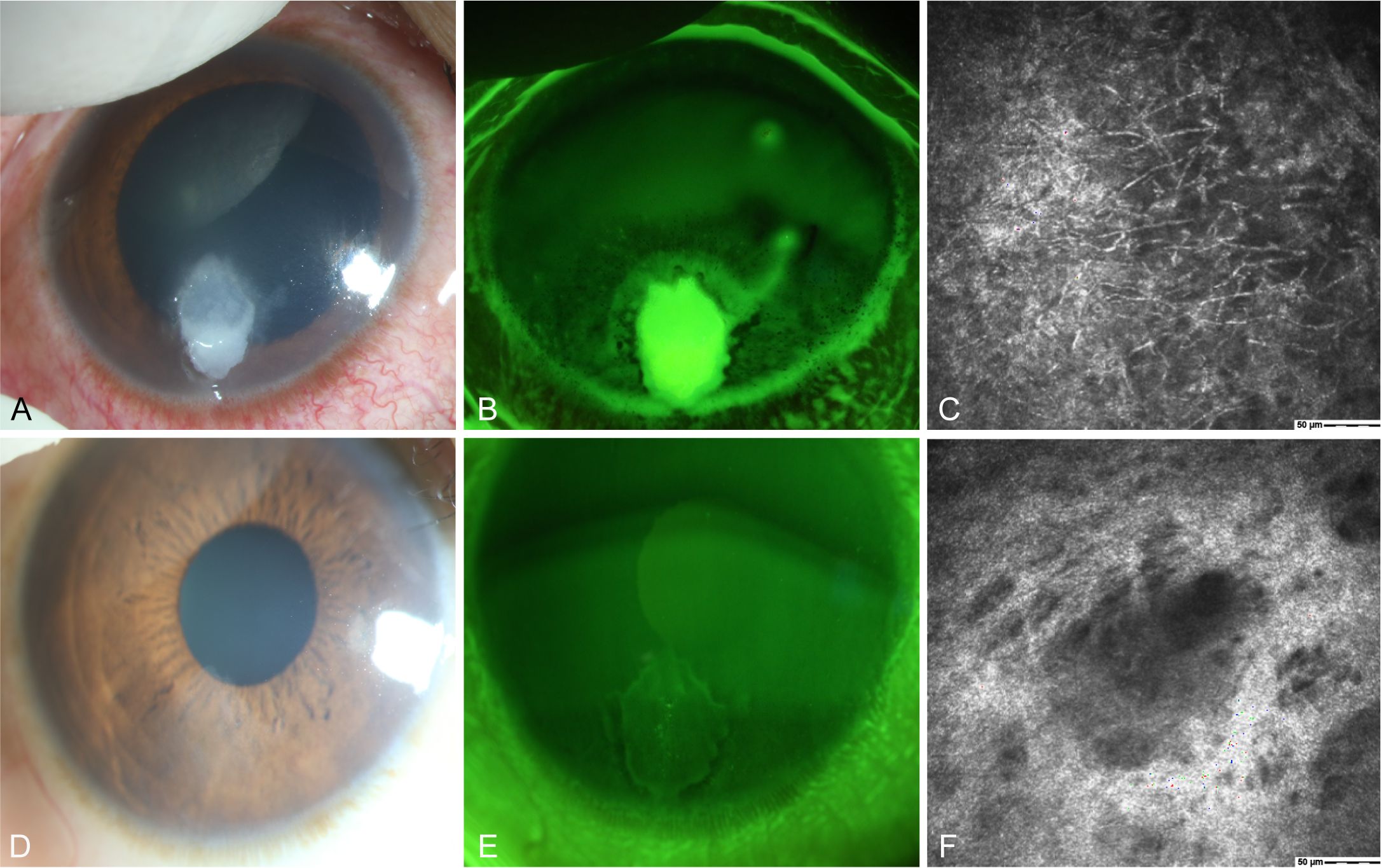
Figure 6. Slit-lamp and IVCM images acquired during patient (Case 2) treatment. (A) Slit-lamp examination images obtained at initial diagnosis showing grayish-white dense infiltrates and feathery margins. (B) Corneal fluorescein staining images showing epithelial ulcers. (C) IVCM images obtained at initial diagnosis showing fungus-like structures. (D, E) Slit-lamp examination and corneal fluorescein staining images obtained after treatment showing corneal ulcer healing. (F) IVCM examination images showing corneal epithelial healing and absence of mycelial structures.
A 57-year-old male with an unknown occupation presented with a total corneal ulcer. IVCM examination revealed fungal hyphae within approximately 400 microns of the stromal layer (Figures 7A, B, D). Following 2 weeks of treatment with 5% natamycin in combination with 0.04% chlorhexidine eye drops every hour (Figure 7C), the disease progressed in an uncontrolled manner, and a subsequent corneal transplantation was carried out.
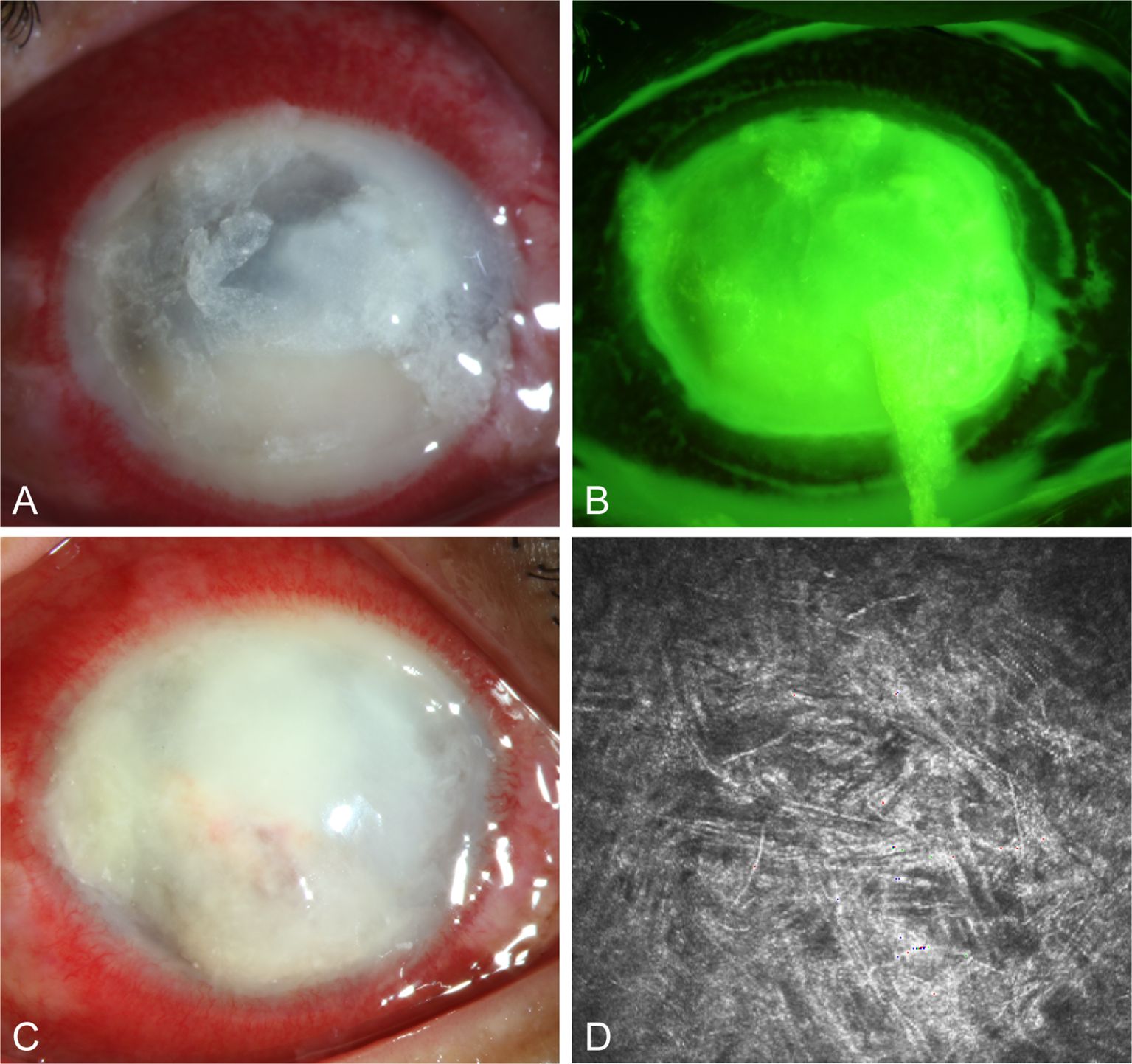
Figure 7. Slit-lamp and IVCM images acquired during patient (Case 3) treatment. (A, B) Slit-lamp examination and corneal fluorescein staining images obtained at initial diagnosis showing total corneal ulcer. (C) Slit-lamp examination image obtained after therapy showing total corneal ulcer. (D) IVCM image obtained initial diagnosis showing fungus-like structures.
A female farmer presented in October with redness and pain in her right eye after being scratched by corn leaves. Slit lamp examination revealed conjunctival and ciliary congestion without scleral congestion, corneal ulceration, turbidity, stromal edema, and wrinkling of the posterior elastic layer with poorly defined borders. IVCM examination showed epithelial loss with numerous fungal hyphal structures in the ulcer area along with significant inflammatory cell infiltration at the ulcer margins. The patient was clinically diagnosed with Fusarium keratitis in the right eye (Figures 8A–C). Treatment with 0.04% chlorhexidine eye drops was administered every hour for 3 months. Follow-up IVCM examination showed gradual healing of the epithelial defect, fibroblast proliferation in the stroma, and no remaining fungal hyphal structures. Thus, the patient achieved clinical cure.
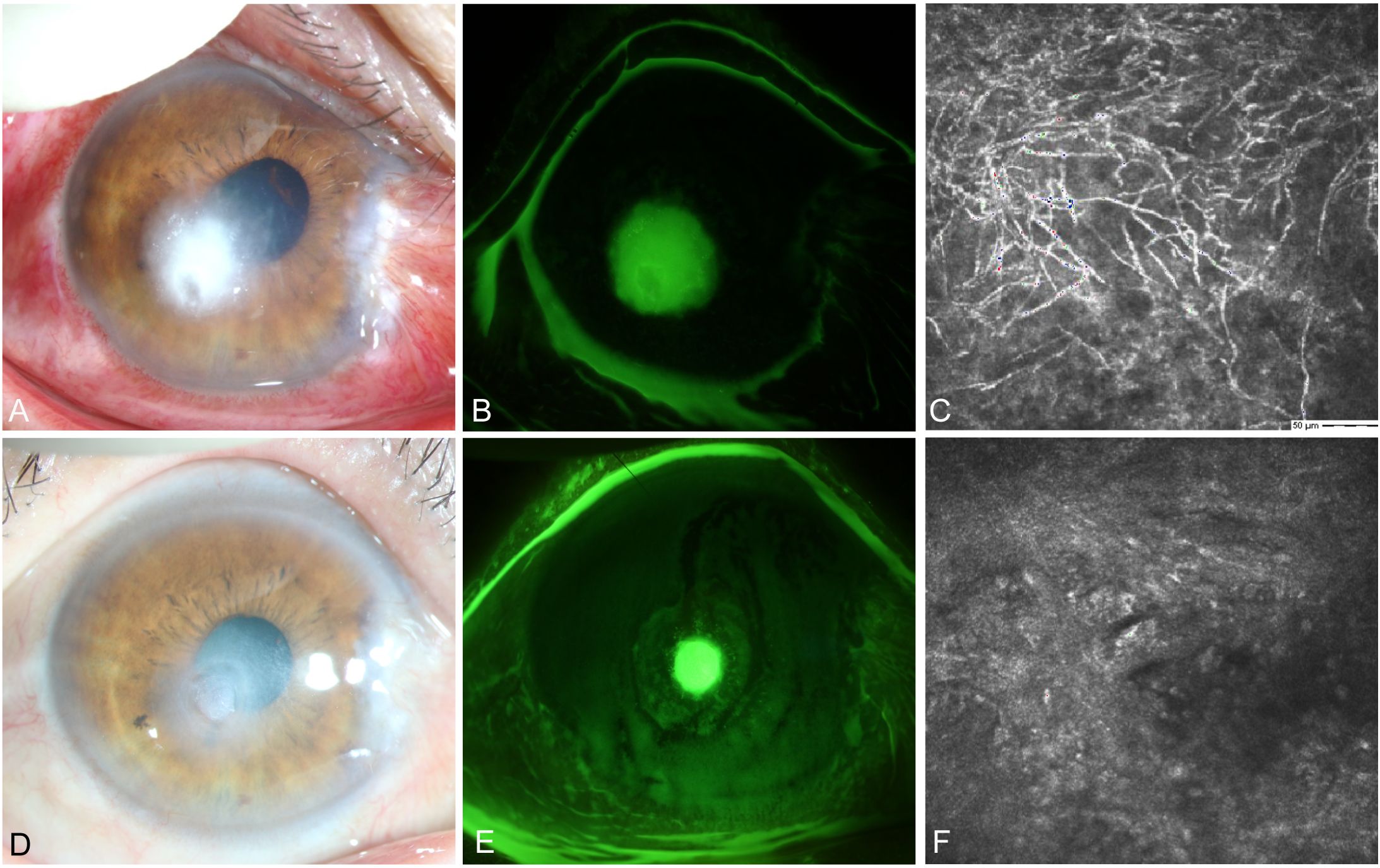
Figure 8. Slit-lamp and IVCM images acquired during patient (Case 4) treatment. (A) Slit-lamp examination images obtained at initial diagnosis showing grayish-white dense infiltrates and feathery margins. (B) Corneal fluorescein staining images showing epithelial ulcers. (C) IVCM images obtained at initial diagnosis showing fungus-like structures. (D, E) Slit-lamp examination and corneal fluorescein staining images obtained after treatment showing corneal ulcer healing. (F) IVCM examination images showing corneal epithelial healing and absence of mycelial structures.
A female farmer presented with redness and pain in her right eye, with no history of corneal trauma or foreign body involvement. Slit lamp examination revealed conjunctival congestion and edema, corneal ulceration and infiltration (Figure 9A) leading to the clinical diagnosis of right eye Fusarium keratitis. Treatment was implemented using 5% natamycin eye drops every hour for a duration of 2 months. Follow-up slit-lamp examination revealed that conjunctival congestion and edema subsided and corneal lesions disappeared (Figure 9B). The patient achieved clinical cure.
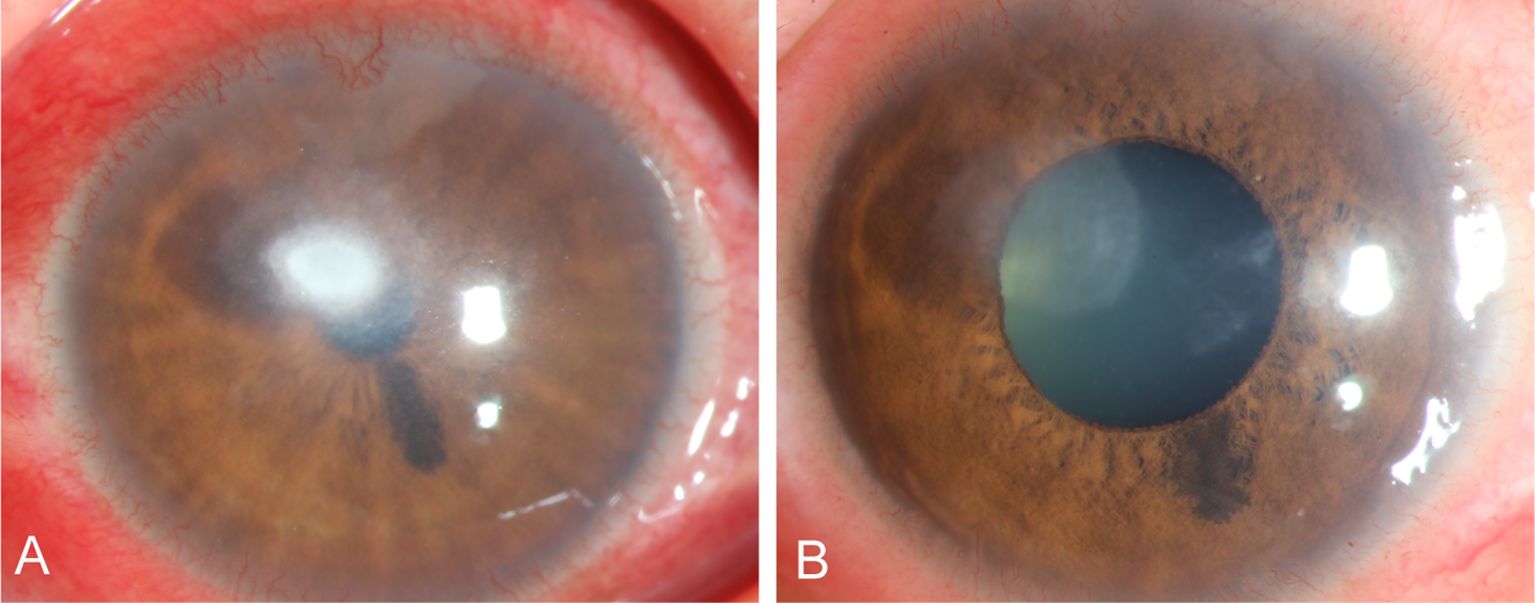
Figure 9. Slit-lamp images acquired during patient (Case 5 ) treatment. (A) Slit-lamp examination images obtained at initial diagnosis showing total corneal ulcer. (B) Slit-lamp examination obtained after treatment showing corneal ulcer healing.
In this study, among 3 patients (Cases 1 - 3) receiving treatment of natamycin combined with chlorhexidine, two achieved clinical cure within 1 - 1.5 months. One patient’s drug treatment failed due to a delayed diagnosis. Additionally, there was one case where a patient treated with only natamycin was cured within two months, and yet another patient, who was treated solely with chlorhexidine, achieved clinical cure within three months.
The specific Fusarium strains isolated from FK specimens depend on factors such as the geographic region, environment, and climate. Most cases in this study were patients with normal immune function from the northern regions of China, where the peak incidence of Fusarium spp. occurs in autumn. Among the isolates, F. solani was the most common (43.8%) followed by F. verticillioides (24.2%) and F. proliferatum (21.6%). In contrast, a 2008 study conducted in Shandong Province, China identified F. solani as the most common species followed by F. verticillioides and F. oxysporum (Xie et al., 2008), while F. falciforme and F. keratoplasticum were identified as the primary species in a 2022 study conducted in Taiwan (Huang et al., 2022). A 2010 study conducted in the United States indicated that contact lens-related keratitis was primarily caused by F. solani and F. oxysporum (Sun et al., 2010). In a study conducted in the Netherlands from 2005–2016, F. oxysporum was predominant followed by F. solani (Oliveira Dos Santos et al., 2020). A 2024 multicenter study conducted in Romania found that F. solani was the most common filamentous fungus in infectious keratitis, particularly during busy farming seasons (Balasoiu et al., 2024). Despite these differences in the predominant Fusarium species in keratitis across different regions, F. solani remains the primary species associated with agricultural injuries in most areas. Thus, for clinical decision-making, it is crucial to inquire about injury history and promptly identify the Fusarium spp. involved.
Natamycin is the most commonly used topical antifungal agent for FK in clinical practice; natamycin is the only drug currently approved by the United States Food and Drug Administration for the treatment of FK (Dennyson Savariraj et al., 2021; Arboleda and Ta, 2024). In the present study, the MIC values of natamycin ranged from 1–8 μg/mL, consistent with the range of 0.5–16 μg/mL reported by Tuft et al. (2024). In this study, the MIC was higher in the F. solani group than in the non-F. solani group, indicating worse prognosis for F. solani FK compared with non-F. solani FK. For the species complexes, the MIC of FDSC was significantly lower than those of the other complexes.
The MIC values of voriconazole differed from those of natamycin and showed greater variability among the species complexes. The MIC50 value of FSSC for voriconazole was higher than that of non-FSSC (8 vs. 2 μg/mL). Similarly, the MIC of voriconazole in the F. solani group was higher than that in the non-F. solani group (8 vs. 2 μg/mL). Similarly, other studies have found a relatively high MIC of voriconazole for FSSC (Walther et al., 2017; Oliveira Dos Santos et al., 2020), which was associated with delayed corneal epithelial healing and a higher incidence of complications compared with other species complexes (Oechsler et al., 2013).
Chlorhexidine is a common, inexpensive, safe, and effective antimicrobial agent with good antibacterial properties (Ward et al., 2023). Chlorhexidine binds to cell membranes to prevent pathogen adhesion and promote the release of their contents (Letzelter et al., 2019). In this study, the MIC values of chlorhexidine were significantly higher for FSSC compared to FFSC, with the MIC of chlorhexidine for F. solani being higher in the F. solani group than in the non-F. solani group, consistent with the findings of Oliveira Dos Santos et al. (2019). Considering the differences in the sensitivity of different Fusarium spp. to chlorhexidine, in the clinical treatment of Fusarium keratitis, detailed species identification should be prioritized before using chlorhexidine to optimize the treatment plan and improve the success rate. Such species identification is crucial for reducing treatment delays and improving prognosis.
In vitro testing showed significant enhancement of antifungal activity for combinations of natamycin with chlorhexidine and voriconazole with chlorhexidine. The enhancement of antifungal activity of natamycin combined with chlorhexidine was 81.4% in Fusarium species but only 28.9% in F. solani, significantly lower than that in the non-F. solani group (52.6%). This further demonstrates that accurate species identification is essential when using natamycin for treatment. The combination of voriconazole with chlorhexidine showed a 100% enhancement of antifungal activity. Based on 20 strains of Fusarium spp. collected from the skin, ear canals, and corneas of patients in Zhejiang, China, Jiang et al. (2020) found that the synergistic activity of natamycin combined with chlorhexidine was 10%, different from our findings. This suggests that factors such as the infection site, geographic location, and prior treatment can lead to variability in drug sensitivity. All strains in this study were isolated from patients with untreated agricultural injuries. Chlorhexidine is an inexpensive and easily accessible drug for the treatment of patients with FK. When combined with topical antifungal drugs, chlorhexidine can shorten the treatment time, facilitate patient management, and reduce the economic burden on patients.
Treatment with 5% natamycin combined with 0.04% chlorhexidine demonstrated good efficacy in cases 1 and 2 since the corneal lesions healed rapidly.Therefore, the combination of natamycin and chlorhexidine showed good therapeutic potential against Fusarium infections. The desired effect is observed in the limited data, which can provide a basis for subsequent research. The patient in case 3 presented with full corneal ulceration, after the opportunity for early drug treatment had passed. This led to uncontrolled progression of the disease, and the patient ultimately required a corneal transplant. This case highlights the importance of early diagnosis and treatment. Currently, there is no voriconazole eye drop formulation available in Northern China; if natamycin proves ineffective, voriconazole combined with chlorhexidine could be considered. In case 4, clinical cure was achieved solely with chlorhexidine; thus, in the absence of antifungal agents, chlorhexidine eye drops can be used to treat FK. Hoffman et al. (2022) showed that participants in the natamycin group had significantly better visual acuity at 90 days than the chlorhexidine group, and that natamycin was associated with faster epithelial reformation and slightly smaller scar or infiltrate size from day 7 onwards. Arunga et al. (2021) have mentioned that chlorhexidine 0.2% was found to be a useful adjunctive topical antifungal in cases of fungal keratitis not responding to natamycin 5%. This suggests that the combination of chlorhexidine and natamycin may help enhance natamycin antifungal susceptibility to Fusarium, potentially shortening the duration of treatment. This study will continue to accumulate data and further conduct multicenter research on the combination of natamycin and chlorhexidine.
Our study still had several limitations. First, the sample size for clinical efficacy observation was limited. This is because our laboratory is a regional tertiary hospital, and it is relatively difficult to access patients who have not previously received drug treatment. Although we obtained the MIC values of natamycin and the combination of natamycin and chlorhexidine, the clinical efficacy observation only included 5 cases, which may not be representative of the overall patient population. Despite these limitations, larger cohorts can help to confirm our findings and provide stronger evidence for the treatment of Fusarium keratitis.
Natamycin combined with chlorhexidine and voriconazole combined with chlorhexidine exhibited enhancement of antifungal activity against Fusarium spp. during in vitro sensitivity tests. The findings of this study provide valuable guidance for establishing the epidemiological cutoff and clinical MIC values for Fusarium spp. This study paves the way for future multicenter studies on the treatment of FK with natamycin and chlorhexidine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee (TRECKY2021–024). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
MK: Conceptualization, Formal Analysis, Writing – original draft. YW: Methodology, Writing – original draft. QS: Software, Writing – original draft. ZW: Data curation, Writing – original draft. YZ: Data curation, Writing – original draft. KC: Data curation, Writing – original draft. XX: Data curation, Writing – original draft. MZ: Data curation, Writing – original draft. QL: Writing – review & editing. XL: Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Key Research and Development Program, grant number 2019YFC1200702.
Thanks to theZhuhai DL Biotech Co., Ltd. for great help throughout the entire project. The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1532289/full#supplementary-material
Supplementary Appendix Figure 1 | The geographic distribution of the Fusarium species complex. The F. solani species complex has the widest distribution, covering 17 provinces (17/20, 85.0%), while the F. fujikuroi species complex covers 14 provinces (14/20, 70%), both concentrated in Beijing, Hebei, Inner Mongolia, and Liaoning. They are mainly distributed in Northern China. The pie chart size is directly proportional to the number of strains in the area.
Supplementary Apppendix Table 1 | Antifungal susceptibility pattern of Fusarium isolates. (Excel-formatted table).
Supplementary Apppendix Table 2 | Antifungal susceptibility pattern of Different Fusarium spp. Complexes. (Excel-formatted table).
Supplementary Apppendix Table 3 | Antifungal susceptibility pattern of F. solani and Non-F. solani. (Excel-formatted table).
Abbood, H. M., Hijazi, K., Gould, I. M. (2023). Chlorhexidine resistance or cross-resistance, that is the question. Antibiotics (Basel) 12, 798. doi: 10.3390/antibiotics12050798
Arboleda, A., Ta, C. N. (2024). Overview of mycotic keratitis. Cornea 43, 1065–1071. doi: 10.1097/ICO.0000000000003559
Arunga, S., Mbarak, T., Ebong, A., Mwesigye, J., Kuguminkiriza, D., Mohamed-Ahmed, A. H. A., et al. (2021). Chlorhexidine gluconate 0.2% as a treatment for recalcitrant fungal keratitis in Uganda: a pilot study. BMJ Open Ophthalmol. 6, e000698. doi: 10.1136/bmjophth-2020-000698
Austin, A., Lietman, T., Rose-Nussbaumer, J. (2017). Update on the management of infectious keratitis. Ophthalmology 124, 1678–1689. doi: 10.1016/j.ophtha.2017.05.012
Balasoiu, A. T., Balasoiu, M., Zlatian, O. M., Ghenea, A. E. (2024). Bacterial and fungal keratitis in a tertiary care hospital from Romania. Microorganisms 12, 787. doi: 10.3390/microorganisms12040787
Bisen, A. C., Sanap, S. N., Agrawal, S., Biswas, A., Mishra, A., Verma, S. K., et al. (2024). Etiopathology, epidemiology, diagnosis, and treatment of fungal keratitis. ACS Infect. Dis. 10, 2356–2380. doi: 10.1021/acsinfecdis.4c00203
Dennyson Savariraj, A., Salih, A., Alam, F., Elsherif, M., AlQattan, B., Khan, A. A., et al. (2021). Ophthalmic sensors and drug delivery. ACS Sens 6, 2046–2076. doi: 10.1021/acssensors.1c00370
Edwar, L., Janna, Y. M., Rozaliyani, A., Louisa, M. (2020). Therapeutic response time of topical voriconazole 1% and intrastromal voriconazole 0.05% versus topical natamycin 5% monotherapy in Fusarium keratitis in rabbit. Mycoses 63, 1128–1132. doi: 10.1111/myc.13160
Halim, I., Singh, P., Sarfraz, A., Kokkayil, P., Pati, B. K., Thakuria, B., et al. (2021). Fungal Keratitis Due to Fusarium lichenicola: A Case Report and Global Review of Fusarium lichenicola Keratitis. J. Fungi (Basel) 7, 889. doi: 10.3390/jof7110889
Hiom, S. J., Furr, J. R., Russell, A. D., Hann, A. C. (1996). The possible role of yeast cell walls in modifying cellular response to chlorhexidine diacetate. Cytobios 86, 123–135.
Hoffman, J. J., Yadav, R., Sanyam, S. D., Chaudhary, P., Roshan, A., Singh, S. K., et al. (2022). Topical chlorhexidine 0.2% versus topical natamycin 5% for the treatment of fungal keratitis in Nepal: A randomized controlled noninferiority trial. Ophthalmology 129, 530–541. doi: 10.1016/j.ophtha.2021.12.004
Huang, Y., Chang, Z., Bai, J., Zhu, M., Zhang, M. X., Wang, M., et al. (2017). The research and application of pretreatment method for matrix-assisted laser desorption ionization-time of flight mass spectrometry identification of filamentous fungi. Chin. Med. J. 97, 2345–2349. doi: 10.3760/cma.j.issn.0376-2491.2017.30.013
Huang, T. E., Ou, J. H., Hung, N., Yeh, L. K., Ma, D. H., Tan, H. Y., et al. (2022). Fusarium keratitis in Taiwan: molecular identification, antifungal susceptibilities, and clinical features. J. Fungi (Basel) 8, 476. doi: 10.3390/jof8050476
Jiang, T., et al. (2020). The combined utilization of Chlorhexidine and Voriconazole or Natamycin to combat Fusarium infections. BMC Microbiol. 20, 275. doi: 10.1186/s12866-020-01960-y
John, H. (2008). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard-Second Edition. (Wayne, PA, USA: Clinical and Laboratory Standards Institute(CLSI)), M38A2E.
Khurana, A., Kumar, A., Chauhan, L. (2022). Clinical profile and treatment outcomes of Fusarium keratitis. Indian J. Ophthalmol. 70, 852–859. doi: 10.4103/ijo.IJO_999_21
Letzelter, J., Hill, J. B., Hacquebord, J. (2019). An overview of skin antiseptics used in orthopaedic surgery procedures. J. Am. Acad. Orthop Surg. 27, 599–606. doi: 10.5435/JAAOS-D-18-00105
Lima de Sousa, T., Dourado, D., Rodrigues, J. S., de Souza Reboucas, J., Montes, M., Formiga, F. R. (2024). Treatment of periodontal disease: does drug delivery matter? Front. Bioeng Biotechnol. 12, 1427758. doi: 10.3389/fbioe.2024.1427758
Lu, X. (2021). Application Manual of Microbial Mass Spectrometry Technology (Beijing: People’s Medical Publishing House).
Lu, H., Xu, H., Feng, C. (2023). Medical Mycological Examination and Atlas (Shanghai: Shanghai Scientific & Technical Publishers).
McDonnell, G., Russell, A. D. (1999). Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12, 147–179. doi: 10.1128/CMR.12.1.147
Mullany, L. C., Darmstadt, G. L., Khatry, S. K., Katz, J., LeClerq, S. C., Shrestha, S., et al. (2006). Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet 367, 910–918. doi: 10.1016/S0140-6736(06)68381-5
Nucci, M., Barreiros, G., Akiti, T., Anaissie, E., Nouer, S. A. (2021). Invasive fusariosis in patients with hematologic diseases. J. Fungi (Basel) 7, 815. doi: 10.3390/jof7100815
Oechsler, R. A., Feilmeier, M. R., Miller, D., Shi, W., Hofling-Lima, A. L., Alfonso, E. C. (2013). Fusarium keratitis: genotyping, in vitro susceptibility and clinical outcomes. Cornea 32, 667–673. doi: 10.1097/ICO.0b013e318277ac74
Oliveira Dos Santos, C., Kolwijck, E., van der Lee, H. A., Tehupeiory-Kooreman, M. C., Al-Hatmi, A. M. S., Matayan, E., et al. (2019). In vitro activity of chlorhexidine compared with seven antifungal agents against 98 fusarium isolates recovered from fungal keratitis patients. Antimicrob. Agents Chemother. 63, e02669-02618. doi: 10.1128/AAC.02669-18
Oliveira Dos Santos, C., Kolwijck, E., van Rooij, J., Stoutenbeek, R., Visser, N., Cheng, Y. Y., et al. (2020). Epidemiology and clinical management of fusarium keratitis in the Netherlands, 2005-2016. Front. Cell Infect. Microbiol. 10, 133. doi: 10.3389/fcimb.2020.00133
Raghavan, A., Nair, A. V., N., K., Venkatapathy, N., Rammohan, R. (2021). Voriconazole in the successful management of a case of Acanthamoeba-Cladosporium keratitis. Am. J. Ophthalmol. Case Rep. 22, 101107. doi: 10.1016/j.ajoc.2021.101107
Rahman, M. R., Johnson, G. J., Husain, R., Howlader, S. A., Minassian, D. C. (1998). Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br. J. Ophthalmol. 82, 919–925. doi: 10.1136/bjo.82.8.919
Roberts, D., Salmon, J., Cubeta, M. A., Gilger, B. C. (2023). Phase-Dependent Differential In Vitro and Ex Vivo Susceptibility of Aspergillus flavus and Fusarium keratoplasticum to Azole Antifungals. J. Fungi (Basel) 9, 966. doi: 10.3390/jof9100966
Sav, H., Rafati, H., Oz, Y., Dalyan-Cilo, B., Ener, B., Mohammadi, F., et al. (2018). Biofilm formation and resistance to fungicides in clinically relevant members of the fungal genus fusarium. J. Fungi (Basel) 4, 16. doi: 10.3390/jof4010016
Sebben, J. E. (1983). Surgical antiseptics. J. Am. Acad. Dermatol. 9, 759–765. doi: 10.1016/S0190-9622(83)70192-1
Sharma, N., Bagga, B., Singhal, D., Nagpal, R., Kate, A., Saluja, G., et al. (2022). Fungal keratitis: A review of clinical presentations, treatment strategies and outcomes. Ocul Surf 24, 22–30. doi: 10.1016/j.jtos.2021.12.001
Skora, M., Obloza, M., Tymecka, M., Kalaska, B., Gurgul, M., Kaminski, K. (2023). Studies on antifungal properties of methacrylamido propyl trimethyl ammonium chloride polycations and their toxicity in vitro. Microbiol. Spectr. 11, e0084423. doi: 10.1128/spectrum.00844-23
Slavin, M., van Hal, S., Sorrell, T. C., Lee, A., Marriott, D. J., Daveson, K., et al. (2015). Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin. Microbiol. Infect. 21, 490 e1–490 10. doi: 10.1016/j.cmi.2014.12.021
Soleimani, M., Izadi, A., Khodavaisy, S., Dos Santos, C. O., Tehupeiory-Kooreman, M. C., Ghazvini, R. D., et al. (2023). Fungal keratitis in Iran: Risk factors, clinical features, and mycological profile. Front. Cell Infect. Microbiol. 13, 1094182. doi: 10.3389/fcimb.2023.1094182
Sun, Y., Chandra, J., Mukherjee, P., Szczotka-Flynn, L., Ghannoum, M. A., Pearlman, E. (2010). A murine model of contact lens-associated fusarium keratitis. Invest. Ophthalmol. Vis. Sci. 51, 1511–1516. doi: 10.1167/iovs.09-4237
Tuft, S., Stone, N. R.H., Burton, M. J., Johnson, E. M., Borman, A. M. (2024). Antifungal susceptibility profiles for fungal isolates from corneas and contact lenses in the United Kingdom. Eye (Lond) 38, 529–536. doi: 10.1038/s41433-023-02719-1
Uemura, E. V. G., Barbosa, M. D. S., Simionatto, S., Al-Harrasi, A., Al-Hatmi, A. M. S., Rossato, L. (2022). Onychomycosis caused by fusarium species. J. Fungi (Basel) 8, 360. doi: 10.3390/jof8040360
Walther, G., Stasch, S., Kaerger, K., Hamprecht, A., Roth, M., Cornely, O. A., et al. (2017). Fusarium keratitis in Germany. J. Clin. Microbiol. 55, 2983–2995. doi: 10.1128/JCM.00649-17
Ward, A. B., Harris, P. A., Argo, C. M., Watson, C., Neacsu, M., Russell, W. R., et al. (2023). Homemade Nucleic Acid Preservation Buffer Proves Effective in Preserving the Equine Faecal Microbiota over Time at Ambient Temperatures. Anim. (Basel) 13, 3107. doi: 10.3390/ani13193107
Keywords: fungal keratitis, Fusarium spp., antifungal susceptibility, chlorhexidine, clinical efficacy observation
Citation: Kang M, Wu Y, Shi Q, Wang Z, Zhang Y, Chen K, Xu X, Zhou M, Liang Q and Lu X (2025) Antifungal susceptibility and clinical efficacy of chlorhexidine combined with topical ophthalmic medications against Fusarium species isolated from corneal samples. Front. Cell. Infect. Microbiol. 15:1532289. doi: 10.3389/fcimb.2025.1532289
Received: 21 November 2024; Accepted: 26 March 2025;
Published: 15 April 2025.
Edited by:
Letizia Angiolella, Sapienza University of Rome, ItalyReviewed by:
Kyung-Tae Lee, Jeonbuk National University, Republic of KoreaCopyright © 2025 Kang, Wu, Shi, Wang, Zhang, Chen, Xu, Zhou, Liang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxin Lu, bHV4aW54aW4yMDA5QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.