- 1Higher Education Center of Shahid Bakeri, Urmia University, Miyandoab, Iran
- 2Department of Plant Protection, Faculty of Agriculture, Urmia University, Urmia, Iran
- 3Department of Botany, Faculty of Applied Sciences, University of Sri Jayewardenepura, Gangodawila, Sri Lanka
- 4Office of Research Administration, Chiang Mai University, Chiang Mai, Thailand
- 5Center of Excellence in Microbial Diversity and Sustainable Utilization, Chiang Mai University, Chiang Mai, Thailand
- 6Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 7Center for Yunnan Plateau Biology Resources Protection and Utilization, College of Biology and Food Engineering, Qujing Normal University, Qujing, Yunnan, China
- 8Bioinformatics and Biostatistics Core Facility, Institute of Basic Health Sciences, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Bipolaris species exhibit various ecological roles, including plant pathogens, epiphytes, saprophytes, or endophytes, primarily associated with poaceous hosts, including cultivated cereals. Iran is known for its diverse climates and rich flora, which serve as a hotspot for fungal diversity. In this study, to determine the species diversity of Bipolaris associated with members of the Poales and Asparagales plant orders, samples with leaf and stem lesion symptoms were collected from these plants across various locations in Iran between 2010 and 2022. Based on the morphological characteristics and multi-locus phylogeny (ITS−rDNA, GAPDH, and TEF1), nine Bipolaris species were identified: Bipolaris avrinica sp. nov., Bipolaris azarbaijanica sp. nov., Bipolaris banihashemii sp. nov., Bipolaris hedjaroudei sp. nov., Bipolaris hemerocallidis sp. nov., Bipolaris iranica sp. nov., Bipolaris persica sp. nov., Bipolaris crotonis, and Bipolaris salkadehensis. B. crotonis represents a new record for Iran’s funga, while B. salkadehensis has been documented on several new hosts globally. The study provides detailed morphological descriptions and illustrations of all identified species, along with insights into their habitats, distributions, and phylogenetic relationships within the Bipolaris genus. This study also emphasizes the need for further research into fungal biodiversity in Iran and provides significant data on the distribution and host range of Bipolaris species.
1 Introduction
The genus Bipolaris was established by Shoemaker (1959) with Bipolaris maydis as the type species and belongs to the family Pleosporaceae (Pleosporales, Dothideomycetes, Ascomycota) (Manamgoda et al., 2011, 2012, 2014; Raza et al., 2019; Bhunjun et al., 2020). Bipolaris is a dematiaceous hyphomycetous genus, characterized by the production of sympodial conidiophores, straight or curved, and distoseptate conidia with the germination of end cells (Sivanesan, 1987; Manamgoda et al., 2011, 2012, 2014). Although the sexual state name Cochliobolus predates Bipolaris, a proposal to conserve the latter name was made (Rossman et al., 2013).
Bipolaris species exhibit diverse ecological roles as plant pathogens, epiphytes, saprophytes, or endophytes, often associated with grasses and cultivated cereals. These fungi are globally distributed and are significant plant pathogens causing diseases, like leaf spots, foliar blights, and root/foot rots, in various crops (Ellis, 1971; Sivanesan, 1987; Zhang and Li, 2009; Manamgoda et al., 2011, 2012, 2014; Tan et al., 2016; Raza et al., 2019; Bhunjun et al., 2020; Jayawardena et al., 2021; Ferdinandez et al., 2022; Khan et al., 2023; Farr et al., 2024). Certain Bipolaris species cause economically important plant diseases in cereal crops, such as rice brown spot (Bipolaris oryzae), barley and wheat common root rot or spot blotch (Bipolaris sorokiniana), and southern corn leaf blight diseases (B. maydis) (Manamgoda et al., 2014; Bhunjun et al., 2020; Jayawardena et al., 2021). In addition to grasses and cereals, Bipolaris species have been reported on over 60 other genera from various plant families, including Anacardiaceae, Araceae, Euphorbiaceae, Fabaceae, Malvaceae, Rutaceae, and Zingiberaceae, either as saprobes or pathogens (Ellis, 1971; Sivanesan, 1987; Manamgoda et al., 2011, 2012, 2014; Jayawardena et al., 2021; Farr et al., 2024). Furthermore, Bipolaris cynodontis, B. oryzae, and Bipolaris setariae have been identified as causative agents of human infections, such as lung and skin infections, allergic sinusitis, onychomycosis, keratitis, and central nervous system infections, particularly in immunocompromised individuals (da Cunha et al., 2012; Wang et al., 2016; Sharma and Nonzom, 2021). The ecological adaptability of Bipolaris is notable, as it thrives across a broad range of hosts, including grasses, cereals, and different dicotyledonous plants. This adaptability highlights the genus’ capability to colonize diverse environments and exploit varying ecological conditions.
The taxonomy of the Bipolaris genus has historically presented challenges due to its morphological variability and overlapping characteristics with other genera within the family Pleosporaceae. Early classifications were primarily based on morphological traits such as conidial shape, septum ontogeny, germination patterns, hilum morphology, and sexual morph characteristics (Ellis, 1971; Sivanesan, 1987; Alcorn, 1988; Manamgoda et al., 2011, 2012, 2014; Amaradasa et al., 2014; Tan et al., 2014; Hernández-Restrepo et al., 2018). However, the advent of molecular phylogenetics has revolutionized our understanding of Bipolaris taxonomy uncovering cryptic species complexes and providing new insights into the evolutionary relationships within the genus. Historically, the genera Bipolaris, Curvularia, Exserohilum, Johnalcornia, Porocercospora, and Pyrenophora were classified under the helminthosporioid fungi or graminicolous Helminthosporium (Sivanesan, 1987; Alcorn, 1988; Manamgoda et al., 2011, 2012, 2014, 2015; Amaradasa et al., 2014; Tan et al., 2014; Hernández-Restrepo et al., 2018; Marin-Felix et al., 2020). Recent advancements in molecular biology and phylogenetics have led to substantial taxonomic revisions within this group resulting in the recognition of new genera in the family Pleosporaceae (Amaradasa et al., 2014; Manamgoda et al., 2014; Tan et al., 2014). The genus Bipolaris is morphologically similar to Curvularia and shares the same sexual morph, Cochliobolus, which makes their differentiation challenging (Manamgoda et al., 2011, 2014, 2015; Marin-Felix et al., 2017a, b, 2020). However, Bipolaris conidia are generally longer and maintain a uniform curvature along their length, unlike the conidia of Curvularia. Additionally, the Bipolaris species lack stromata structures, as documented in several studies (Manamgoda et al., 2014, 2015; Marin-Felix et al., 2017a, b, 2020). For these reasons, integrating morphological observations with molecular methods is crucial for accurately delineating helminthosporioid fungi, identifying species, and recognizing cryptic species within Bipolaris (Manamgoda et al., 2014, 2015; Tan et al., 2014, 2016; Marin-Felix et al., 2017a, b, 2020; Raza et al., 2019; Ferdinandez et al., 2022). At present, the Index Fungorum (http://www.indexfungorum.org, accessed on 20 October 2024) lists 146 names under the genus Bipolaris, of which approximately 70 species have been reported from the orders Poales and Asparagales, as well as from other monocotyledonous plants (Manamgoda et al., 2014; Farr et al., 2024).
Iran, with its diverse climatic zones and rich flora, represents a hotspot for fungal biodiversity. Despite this ecological importance, the fungal diversity in Iran remains relatively underexplored. In recent years, efforts to study fungal communities in Iran have accelerated driven by advancements in molecular biology and an increasing recognition of Iran's critical role in global biodiversity conservation. To date, 11 species of Bipolaris have been recorded in Iran (Ahmadpour et al., 2011, 2012a, 2012b, 2013, 2014, 2018; Ershad, 2022). However, many of these species were identified based solely on morphological traits raising questions about their accuracy in light of recent molecular taxonomic revisions of Bipolaris species from other regions. This study aims to identify Bipolaris species associated with Poales and Asparagales hosts in Iran by integrating morphological characteristics, ecological observations, and molecular data including ITS−rDNA, GAPDH, and TEF1 sequences.
2 Materials and methods
2.1 Sample collection and fungal isolation
A total of 130 samples exhibiting leaf and stem lesions were collected from various host plants in the orders Poales and Asparagales across different locations in Iran (Isfahan, Mazandaran, and West Azarbaijan Provinces) between 2010 and 2022, and the important collection information was recorded (Rathnayaka et al., 2024). Subsequently, they were brought to the laboratory for further analysis. Small sections, approximately 0.5 × 0.5 cm2, were cut from the interface between healthy and diseased tissue. These sections were disinfected by submerging them in a diluted bleach solution (2% sodium hypochlorite) for 2 min, followed by three thorough rinses in sterile distilled water, and then blotted dry on sterile filter paper. The disinfected sections were then transferred to Potato Dextrose Agar (PDA, 39 g/L, Merck, Germany) plates supplemented with streptomycin sulfate and penicillin G (150 ppm each). The plates were incubated at 23 ± 2°C under cool white fluorescent light with a 12-h photoperiod for 5 days. Fungi growing out from the margins of plant sections were transferred into new PDA plates and purified via single-spore or hyphal tip methods. Furthermore, infected plant samples were incubated in moist chambers at 25°C until formation of conidial mass was observed. The incubated samples were inspected under a stereomicroscope, and single spores were then transferred to PDA at 23°C–25°C using a fine sterile needle. All identified isolates were deposited as pure cultures in the fungal culture collections at the Iranian Research Institute of Plant Protection (IRAN) and Urmia University (FCCUU).
2.2 Morphological characterization
Mycelial disks (5 mm in diameter) were excised from the actively growing margins of 7-day-old cultures and placed on fresh PDA, Corn Meal Agar (CMA, 17 g/L, Quelab, Montreal, Canada), and Malt Extract Agar (MEA, 50 g/L, Quelab, Montreal, Canada) media plates. The plates were incubated in the dark at 25°C for 7 days. Subsequently, the characteristics of the colonies, including color, pattern, and diameter, were observed and recorded. The color of the colonies was recorded using Rayner’s (1970) color charts. The micro-morphological characteristics were observed using 10- to 14-day-old cultures on tap water agar plates with autoclaved wheat straw (TWA–wheat straw) or leaves of the host plant. The cultures were subjected to near-ultraviolet light on a 12-h diurnal cycle at 23°C–25°C, as described by Sivanesan (1987) and Hernández-Restrepo et al. (2018). Fungal structures, such as hyphae, conidiophores, conidiogenous cells, conidia, ascocarps, asci, and ascospores, were measured (20–50 measurements per structure) and photographed using an Olympus AX70 microscope with differential interference contrast (DIC) illumination from slide mounts prepared with either clear lactic acid or lactophenol cotton blue staining solutions. Images were edited with Adobe Photoshop 2020 v. 2.10.8 software (Adobe Inc., San Jose, California). Taxonomic novelties were registered in MycoBank (www.MycoBank.org; Crous et al., 2004).
2.3 DNA extraction, PCR amplification, and sequencing
Total genomic DNA was extracted from the mycelial mass of each isolate harvested from 10-day-old PDA Petri dishes using the method described by Ahmadpour et al. (2021). The internal transcribed spacer (ITS−rDNA) region, parts of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the translation elongation factor-1 alpha (TEF1) genes were amplified using the primer pairs ITS1/ITS4 (White et al., 1990), gpd1/gpd2 (Berbee et al., 1999), and TEF1-983F/TEF1-2218R (Rehner and Buckley, 2005), respectively. Polymerase chain reaction (PCR) was performed in the SimpliAmp™ Thermal Cycler (Applied Biosystems™, Thermo Fisher Scientific Corp., USA) with a final volume of 30 μl. The PCR mixture comprised of 0.4 μM of each primer, 10 μl of a ready master mix (Taq DNA Polymerase 2× Master Mix Red, 2 mM MgCl2, Ampliqon Company, Denmark), and approximately 10 ng of DNA. The PCR amplification conditions were as follows: an initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 45 s, annealing at 62°C–57°C (annealing temperature decreased by 0.5°C per cycle in the first 12 cycles) for 45 s, extension at 72°C for 45 s, and a final extension step at 72°C for 7 min. Amplicons were visualized on a 1% agarose gel stained with FluoroVueTM Nucleic Acid Gel Stain (SMOBIO Technology Inc., China), and the sizes of amplicons were determined using a FluoroBandTM 100 bp+3K Fluorescent DNA Ladder (SMOBIO Technology Inc., China). The amplified products were cleaned and sequenced by Macrogen Corp. (Seoul, South Korea) using the same primer sets that were used for PCR amplification. The sequences derived from this study were submitted to GenBank (Table 1).
2.4 Sequence alignments and phylogenetic analyses
The initial identification of the isolates involved utilizing newly generated sequences of ITS−rDNA, GAPDH, and TEF1 with the NCBI Basic Local Alignment Search Tool (BLAST) (www.ncbi.nlm.nih.gov/blast/). Subsequently, pairwise sequence comparisons were performed between novel species and their closely related taxa using the same BLAST tool. DNA sequences from the type or representative species were obtained from GenBank (Table 1) and used in the analyses. A multi-locus phylogenetic analysis was conducted on a combined dataset comprising the three genes/regions (ITS−rDNA + GAPDH + TEF1). Multiple sequence alignment was done using the online alignment tool MAFFT version 7 (https://mafft.cbrc.jp/alignment/server/) (Katoh et al., 2019). The best-fit substitution models were determined with the Akaike Information Criterion (AIC) in MrModeltest 2.3 (Nylander, 2004). The maximum likelihood (ML) and maximum parsimony (MP) analyses were conducted via the CIPRES Science Gateway portal (accessible at https://www.phylo.org/) (Miller et al., 2012) using RAxML-HPC BlackBox v. 8.2.12 (utilizing the GTR + GAMMA model and 1,000 bootstrapping iterations) (Stamatakis, 2014) and PAUP on ACCESS v. 4.a168 (using the heuristic search option and branch swapping with the tree–bisection–reconnection (TBR) algorithm with 1,000 bootstrapping replicates) (Swofford, 2002) tools, respectively. Descriptive tree statistics [tree length (TL), consistency index (CI), retention index (RI), and homoplasy index (HI)] were calculated for trees generated in the parsimony analysis. Bayesian phylogenetic inference (BI) and Bayesian posterior probabilities (BPP) were conducted in MrBayes v. 3.2.7 (Ronquist et al., 2012) with the Markov chain Monte Carlo (MCMC) method (four chains, 1,000,000 generations, 1,000 sampling frequency, and 25% burn-in phase). In all phylogenetic analyses, Curvularia affinis (CBS 154.34) and Curvularia lunata (CBS 730.96) were used as the outgroup taxa (Manamgoda et al., 2014; Tan et al., 2016; Bhunjun et al., 2020; Ferdinandez et al., 2022). The generated phylogenetic trees were viewed using FigTree v. 1.4.4 (Rambaut, 2019) and further edited using graphic design software, Adobe Illustrator® CC 2020.
2.5 Genealogical Concordance Phylogenetic Species Recognition analysis
Genealogical Concordance Phylogenetic Species Recognition (GCPSR) was used to test for significant recombinant events (Quaedvlieg et al., 2014). Three-locus concatenated datasets (ITS−rDNA + GAPDH + TEF1) with closely related species were used for the analyses. The data were analyzed using SplitsTree 5 software employing the pairwise homoplasy index (PHI or Φw) test (Bruen et al., 2006; Huson and Bryant, 2006). PHI test results indicating a value less than 0.05 (Φw < 0.05) suggest the presence of significant recombination within the dataset. To visualize the relationships between novel taxa and their closely related counterparts, split graphs were constructed using concatenated datasets. The LogDet transformation and split decomposition options were used for this purpose.
3 Results
3.1 Phylogenetic analyses
A total of 85 isolates were obtained from various hosts (Poales and Asparagales plants). All isolates were examined based on their morphology. Representative isolates were then selected from various plant hosts for phylogenetic analyses. PCR amplifications produced DNA fragments of approximately 540 bp for ITS−rDNA, 545 bp for GAPDH, and 850 bp for TEF1. A total of 104 ITS−rDNA, 102 GAPDH, and 95 TEF1 sequences were subjected to multiple sequence alignment (nucleotides + gaps) resulting in 505-, 494-, and 898-character datasets, respectively. A combination of three gene sequences from 104 strains yielded a dataset with 1,897 characters, of which, 1,472 characters were constant, 112 characters were variable and parsimony uninformative, and 313 were parsimony informative. The most parsimonious tree yielded the following metrics: TL = 937, CI = 0.574, RI = 0.842, HI = 0.426. The nucleotide substitution model GTR + I + G was identified by MrModeltest 2.3 for all ITS−rDNA, GAPDH, and TEF1 datasets. The ML, MP, and BI phylogenetic analyses produced trees with similar topology and showed no significant conflicts. The combined dataset analysis of RAxML generated the best-scoring tree (Figure 1) with a final ML optimization likelihood value of −8,235.239283. Estimated base frequencies were as follows: A = 0.229913, C = 0.302740, G = 0.236579, T = 0.230767; substitution rates AC = 1.001686, AG = 2.714677, AT = 1.255930, CG = 0.832074, CT = 6.025850, GT = 1.000000; gamma distribution shape parameter α = 0.731765. Based on morphological characteristics and multi-locus phylogeny (ITS−rDNA, GAPDH, and TEF1), nine Bipolaris species were identified: Bipolaris avrinica sp. nov., B. azarbaijanica sp. nov., B. banihashemii sp. nov., B. hedjaroudei sp. nov., B. hemerocallidis sp. nov., B. iranica sp. nov., B. persica sp. nov., Bipolaris crotonis, and B. salkadehensis. B. crotonis is a new record for Iran’s funga. Also, the phylogenetic relationship of B. salkadehensis with related species was re-defined using sequences from three genomic regions, and several new hosts were identified for this species worldwide. All identified taxa clustered with high statistical support values in the phylogenetic tree (Figure 1). Each species was thoroughly illustrated, described, and discussed in terms of morphology, habitat, distribution, and phylogenetic relationships with other Bipolaris species.
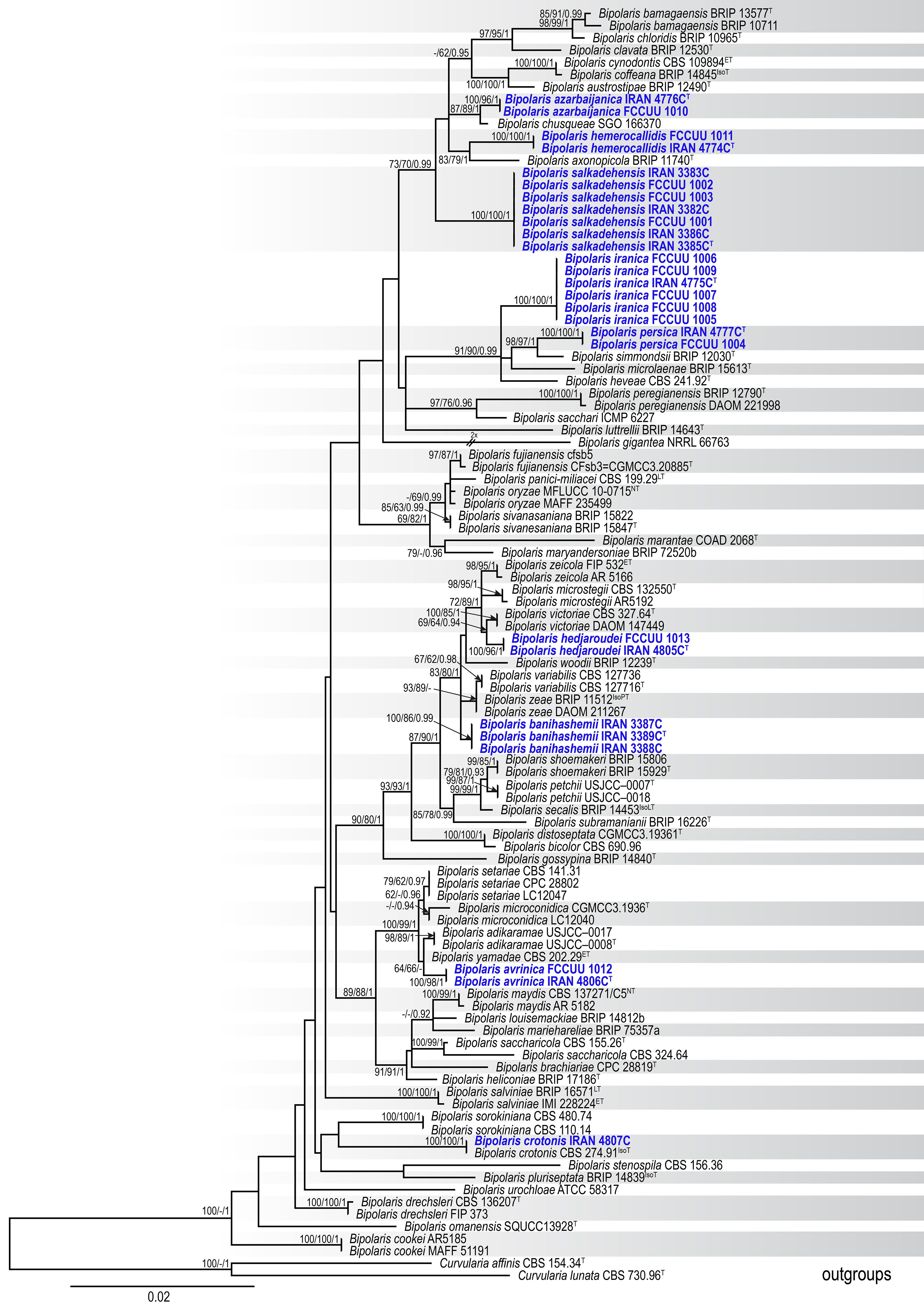
Figure 1. Maximum likelihood (ML) tree of Bipolaris species based on the dataset of ITS−rDNA, GAPDH, and TEF1. Bootstrap support values of the ML and maximum parsimony (MP) (MLBS/MPBS) values ≥60% and Bayesian posterior probabilities (BIPP) ≥0.90 are given at the nodes. The tree is rooted with Curvularia affinis (CBS 154.34) and C. lunata (CBS 730.96), and new species are indicated in blue boldface. The scale bar indicates the number of nucleotide substitutions. T, ET, IsoT, IsoLT, IsoPT, LT and NT indicate ex-type, ex-epitype, ex-isotype, ex-isolectotype, ex-isoparatype, ex-lectotype, and ex-neotype strains, respectively.
3.2 Taxonomy
Bipolaris avrinica A. Ahmadpour, Z. Heidarian, Y. Ghosta, Z. Alavi & F. Alavi, sp. nov. (Figure 2).
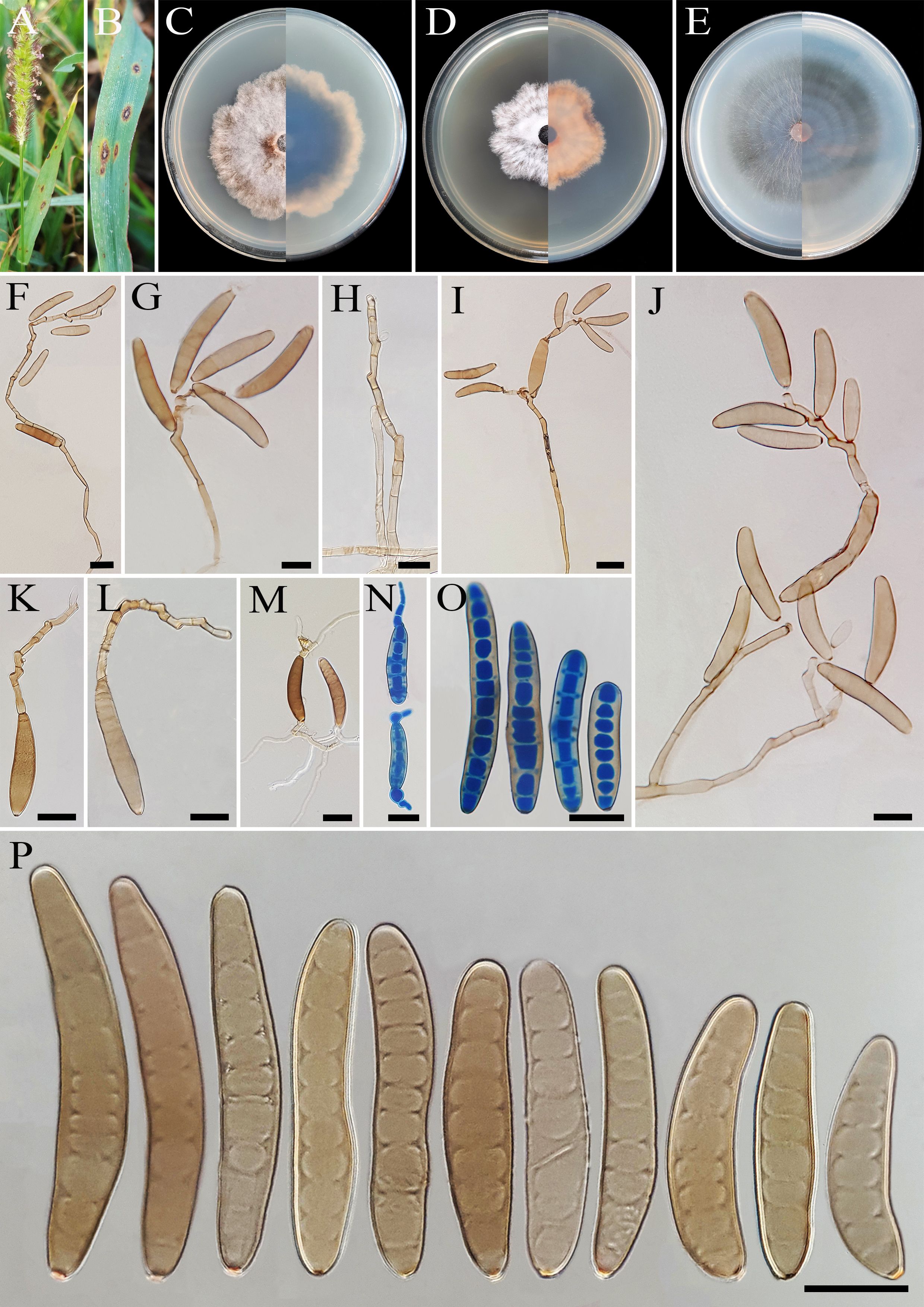
Figure 2. Bipolaris avrinica (IRAN 4806C). (A, B) Lesions on host leaf (Setaria sp.). (C−E) Colonies (front and reverse) on PDA (C), MEA (D), and CMA (E) media after 7 days. (F–H) Conidiophores. (I–L) Conidia with secondary sporulation. (M, N) Germinated conidia. (O, P) Conidia. Scale bars: (F−P) = 20 μm.
MycoBank No: MB 854730
Etymology: The name refers to Avrin Mountain, located in Khoy County, West Azarbaijan Province, where the holotype was collected.
Diagnosis: Differs from Bipolaris adikaramae and B. yamadae by the abundant production of secondary conidiophores and conidia in culture media.
Type: IRAN, West Azarbaijan Province, Khoy County, on infected leaves of Setaria sp. (Poaceae, Poales), 10 September 2020, A. Ahmadpour, (IRAN 18493F, holotype, dried culture; ex-type culture IRAN 4806C).
Description: Lesions on infected leaves of Setaria sp., 1- to 10-mm long, gray color at the center with dark brown margins. Sexual morph: Undetermined. Asexual morph: On TWA Hyphae 2- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (125–)185–500(–600) × 4–6 µm ( ± SD = 342.5 ± 157.5 × 5 ± 1 μm, n = 50), mononematous, semi- to macronematous, arising singly or rarely in groups, unbranched, straight to flexuous, septate, geniculate, pale brown to brown, paler toward the apex, rarely swollen at the base. Secondary conidiophores are frequently formed in culture media and conidia attached to primary conidiophores. Conidiogenous cells (6–)8–21(–16) × 4–7 μm ( ± SD = 14.5 ± 6.5 × 5.5 ± 1.5 μm, n = 50), mono- to polytretic, sympodial proliferation, integrated, terminal or intercalary, subcylindrical to slightly swollen, pale brown to brown, smooth-walled, with thickened and darkened scars. Conidia (45–)50–87(–100) × 10–13 µm ( ± SD = 68.5 ± 18.5 × 12 ± 1 μm, n = 50), pale brown to brown, smooth walled, straight to curved, fusoid to cylindrical, occasionally ellipsoidal, tapering toward rounded ends, (6–)7–10(–11)-distoseptate, germinated mono- or bipolar; hila 1.5- to 2.5-μm wide, inconspicuous, flat, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 50 mm in diameter after 7 days at 25°C in the dark, circular, margin irregular, cottony appearance, gray with white to gray aerial mycelia; reverse gray olivaceous. Colonies on MEA reaching 38-mm diameter, circular, margin irregular, cottony appearance, white with white aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on CMA reaching 52 mm in diameter, circular, margin entire, hairy appearance with concentric rings, gray with sparse white to gray aerial mycelia; reverse olivaceous brown at the center and a hyaline margin.
Additional material examined: IRAN, West Azarbaijan Province, Khoy County, on infected leaves of Setaria sp. (Poaceae, Poales), 10 September 2020, A. Ahmadpour, isolate FCCUU 1012.
Host and distribution: Setaria sp. in Iran (this study).
Notes: Based on the phylogenetic analyses, B. avrinica is closely related to B. adikaramae and B. yamadae (MLBS/MPBS/BIPP = 100/98/1.0) (Figure 1). A comparison of nucleotide differences in ITS−rDNA, GAPDH, and TEF1 indicates that B. avrinica (IRAN 4806C) differs from B. adikaramae (USJCC–0008) by 1/511 bp [0.19%, with one gap (0%)] in ITS−rDNA, 3/550 bp (0.54%) in GAPDH, and 3/763 bp (0.39%) in TEF1 and from B. yamadae (CBS 202.29) by 1/511 bp [0.54%, with one gap (0%)] in ITS−rDNA, 4/480 bp (0.83%) in GAPDH, and 4/763 bp (0.52%) in TEF1. The PHI analysis confirms that B. avrinica shows no significant genetic recombination with closely related species (Φw = > 0.05, Figure 3). Bipolaris avrinica can be differentiated by its abundant production of secondary conidiophores and conidia in cultures, a feature absent in B. adikaramae and B. yamadae. Additionally, B. avrinica has smaller conidia [(45–)50–87(–100) × 10–13 μm] compared to B. yamadae [(60–)65–100(–120) × (12–)14–18 μm] (Manamgoda et al., 2014; Ferdinandez et al., 2022). The production of secondary conidiophores and secondary conidia has been observed in B. cookei and B. microstegii grown on culture media (Manamgoda et al., 2014). However, B. avrinica is phylogenetically distinct from these species (Figure 1). Bipolaris yamadae has been reported from several hosts, including Oryza sp., Euphorbia sp., Panicum spp. (P. capillare, P. implicatum, P. maximum, and P. miliaceum), Saccharum officinarum, and Setaria plicata (Manamgoda et al., 2014; Marin-Felix et al., 2017a; Farr et al., 2024). Bipolaris adikaramae has been isolated from yellow lesions on the leaf of Panicum maximum in Sri Lanka (Ferdinandez et al., 2022). Based on morphological and molecular evidence, we propose B. avrinica as a new species.
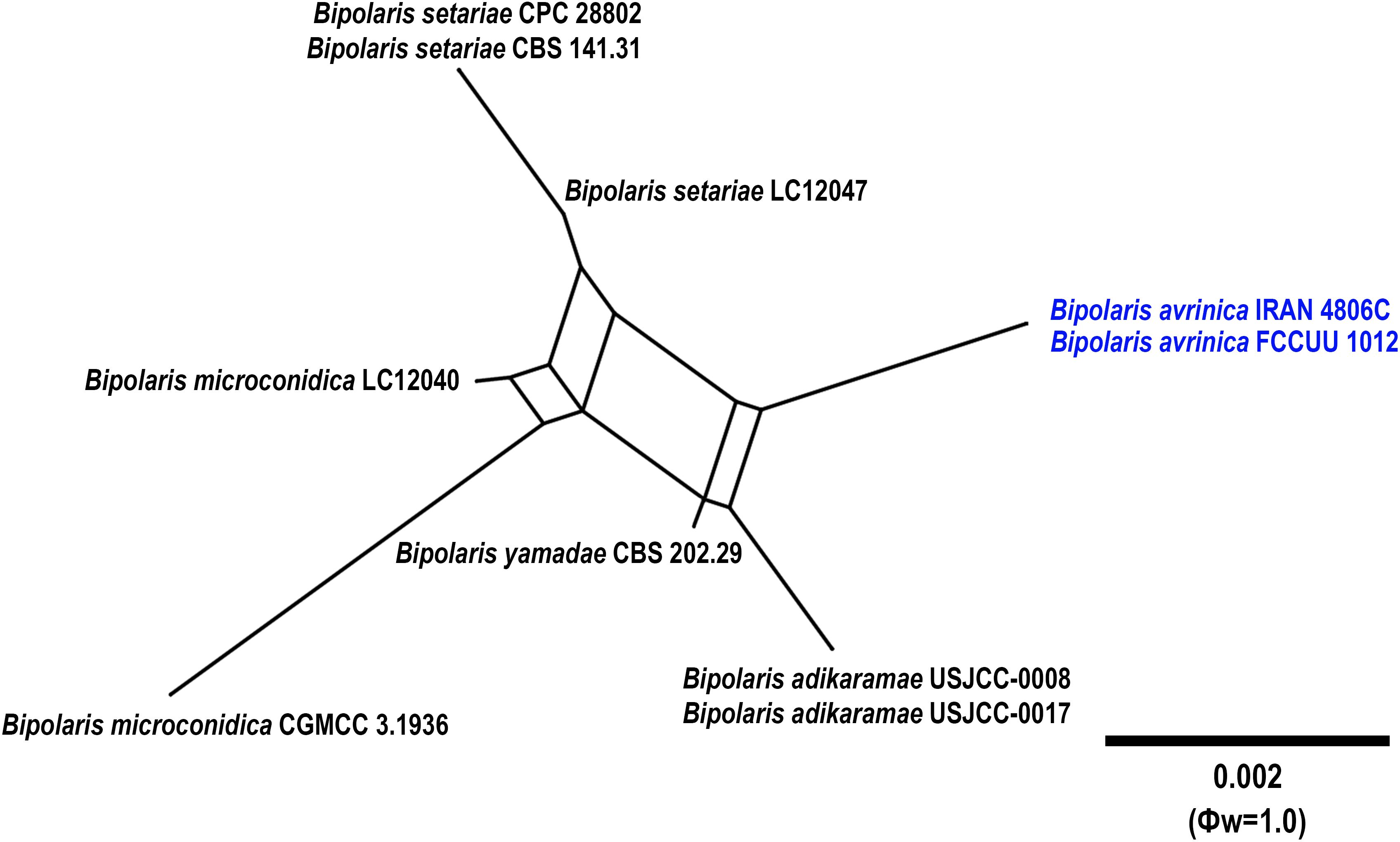
Figure 3. Split graphs showing the results of PHI test of Bipolaris avrinica with their most closely related species (Φw = 1.0). The new taxa are shown in bold blue.
Bipolaris azarbaijanica A. Ahmadpour, Z. Heidarian, Y. Ghosta, Z. Alavi & F. Alavi, sp. nov. (Figure 4).
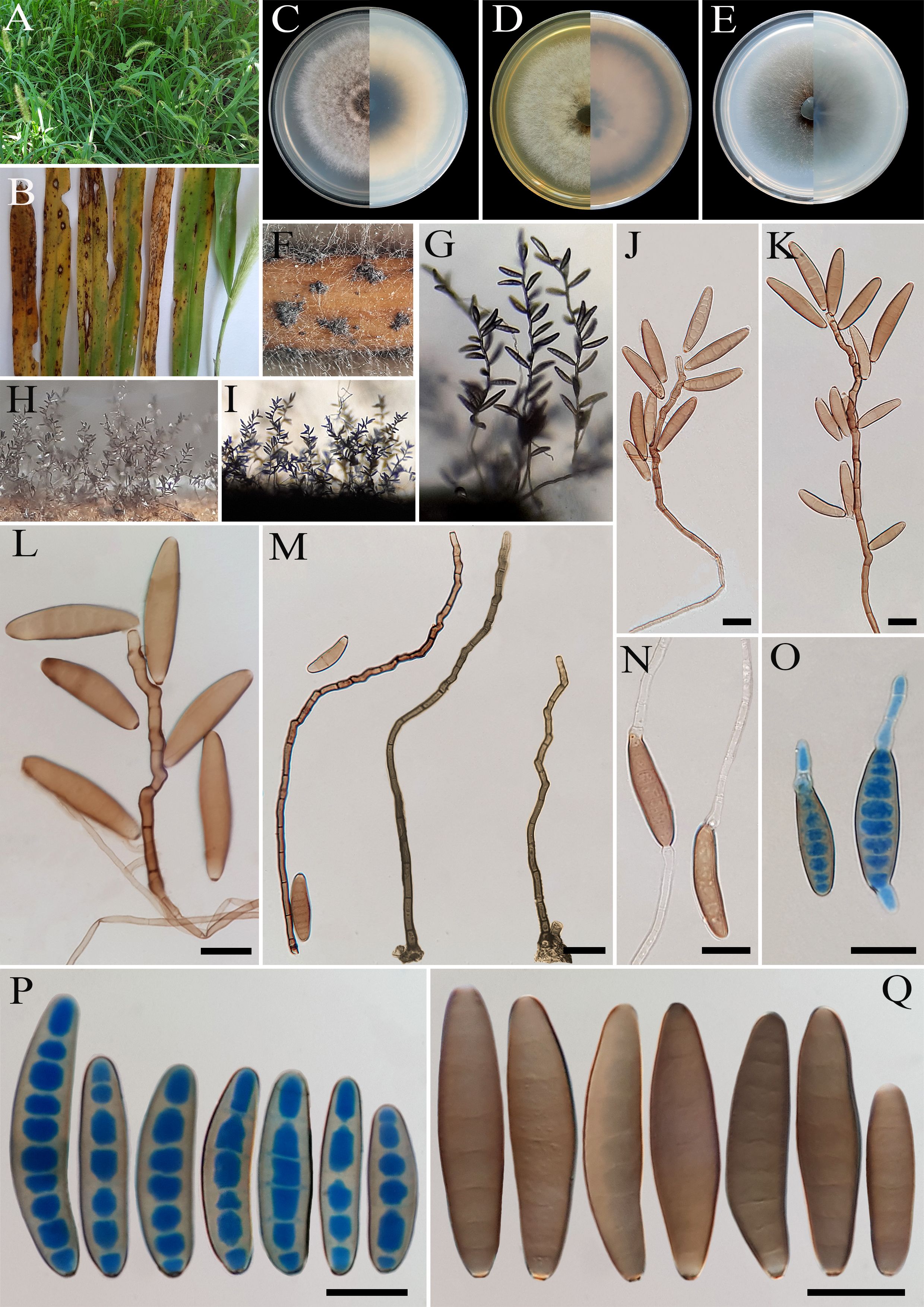
Figure 4. Bipolaris azarbaijanica (IRAN 4776C). (A, B) Lesions on host leaf (Setaria sp.). (C−E) Colonies (front and reverse) on PDA (C), MEA (D), and CMA (E) media after 7 days. (F−I) Sporulation pattern on TWA medium. (J–M) Conidiophores. (N, O) Germinated conidia. (P, Q) Conidia. (J−Q) Scale bars = 20 μm.
MycoBank No: MB 854731
Etymology: The name refers to the West Azarbaijan Province, where the holotype was collected.
Diagnosis: Differs from Bipolaris chusqueae by the shape (fusoid to cylindrical) and size (longer and wider) of conidia.
Type: IRAN, West Azarbaijan Province, Salmas County, on leaves of Setaria sp. (Poaceae, Poales), 10 September 2015, A. Ahmadpour/Z. Heidarian, (IRAN 18208F, holotype, dried culture; ex-type culture IRAN 4776C).
Description: Leaf spots on Setaria sp., 1- to 10-mm long, gray at the center with a red-brown margin. Sexual morph: Undetermined. Asexual morph: On TWA: Hyphae 3- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (112–)140–300(–450) × 5–7 µm ( ± SD = 220 ± 80 × 6 ± 1 μm, n = 50), mononematous, semi- to macronematous, arising singly or in groups, unbranched, straight to flexuous, septate, geniculate, pale brown to brown, paler toward the apex, swollen at the base. Conidiogenous cells (8–)10–22(–25) × 5–8 μm ( ± SD = 16 ± 6 × 6.5 ± 1.5 μm, n = 50), mono- to polytretic, sympodial proliferation, integrated, terminal or intercalary, subcylindrical to slightly swollen, pale brown to brown, smooth walled to slightly verruculose, with thickened and darkened scars. Conidia (44–)50–80(–84) × 11–15 µm ( ± SD = 65 ± 15 × 13 ± 2 μm, n = 50), pale brown to brown, smooth walled, straight to slightly curved, broadly fusoid to cylindrical, occasionally ellipsoidal to clavate, tapering toward the rounded ends, apical and basal cells paler than the median cells, (4–)5–9(–10)-distoseptate, germination mono- or bipolar; hila 2- to 3-μm wide, flat to slightly protuberant, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 73 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, gray at the center with white to gray aerial mycelia, white at the margin; reverse olivaceous gray at the center, margin pale brown. Colonies on MEA reaching 68 mm in diameter, circular, margin entire, cottony appearance, gray at the center, white at the margin with white aerial mycelia; reverse brown to pale brown. Colonies on CMA reaching 65 mm in diameter, circular, margin entire, hairy appearance, olivaceous gray with sparse white to gray aerial mycelia; reverse olivaceous gray at the center and a hyaline margin.
Additional material examined: Iran, West Azarbaijan Province, Salmas County, on leaves of Setaria sp. (Poaceae, Poales), 10 September 2015, A. Ahmadpour/Z. Heidarian, isolate FCCUU 1010.
Host and distribution: Setaria sp. in Iran (this study).
Notes: Bipolaris azarbaijanica is phylogenetically closely related to B. chusqueae (MLBS/MPBS/BIPP = 100/96/1.0) (Figure 1). The pairwise DNA sequence comparison revealed that B. azarbaijanica is distinct from B. chusqueae. A comparison of nucleotide differences in ITS−rDNA and GAPDH indicates that B. azarbaijanica (IRAN 4776C) differs from B. chusqueae (SGO 166370) by 3/525 bp (0.57%) in ITS−rDNA and 6/531 bp (1.12%) in GAPDH. The PHI analysis confirms that B. azarbaijanica has no significant genetic recombination with closely related species (Φw = > 0.05, Figure 5). Morphologically, B. azarbaijanica can be differentiated by the shape of the conidia (broadly fusoid to cylindrical vs. subcylindrical to narrowly clavate in B. chusqueae), and longer and wider conidia [(44–)50–80(–84) × 11–15 µm vs. (17–)26–50(–68) × 10–12(–15) μm in B. chusqueae] (Lebeuf et al., 2023). Bipolaris chusqueae has been reported from Chusquea cumingii (Bambusoideae, Poales) in Chile (Lebeuf et al., 2023).
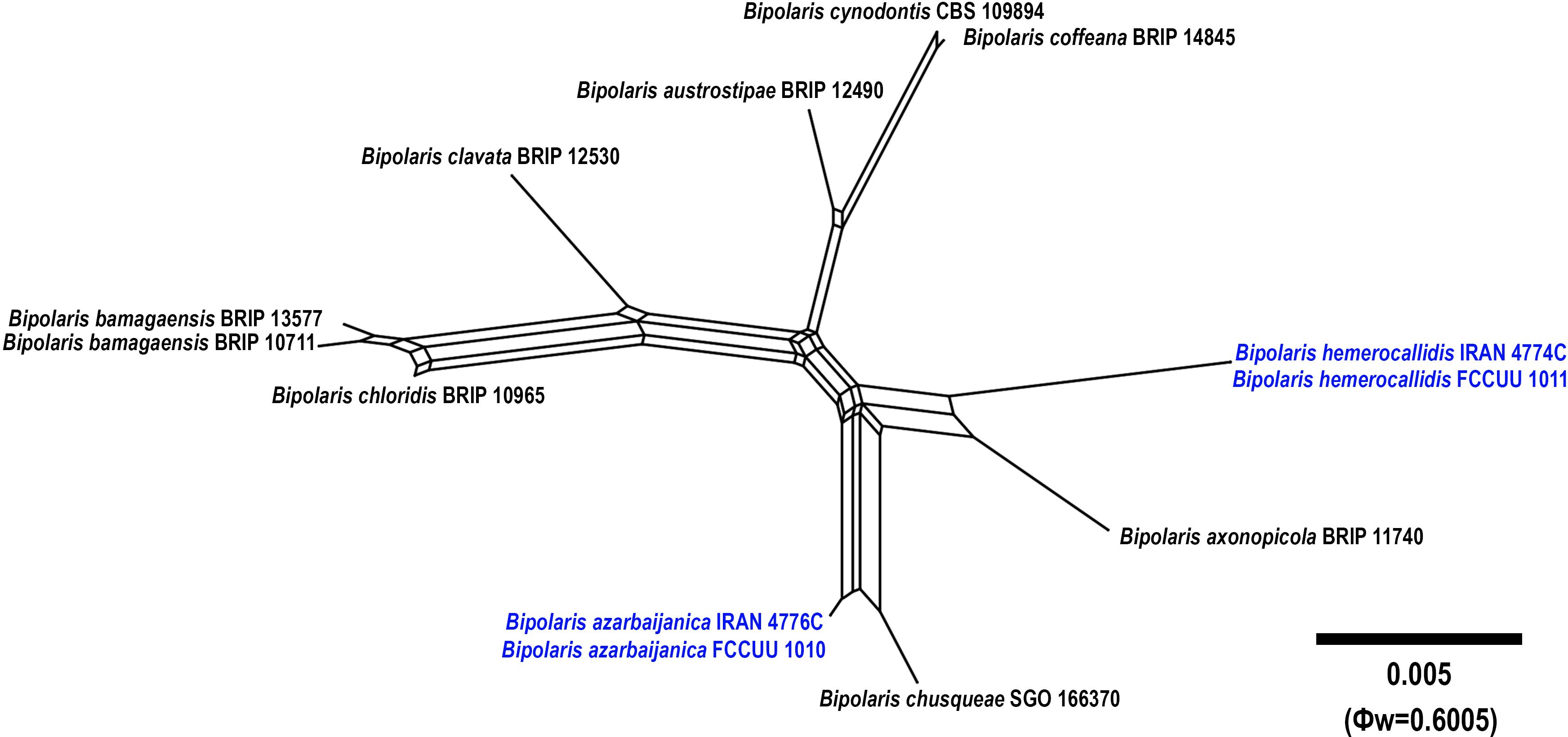
Figure 5. Split graphs showing the results of PHI test of Bipolaris azarbaijanica and B. hemerocallidis with their most closely related species (Φw = 0.6005). The new taxa are shown in bold blue.
Bipolaris banihashemii A. Ahmadpour, Z. Heidarian, Y. Ghosta, Z. Alavi & F. Alavi, sp. nov. (Figure 6).
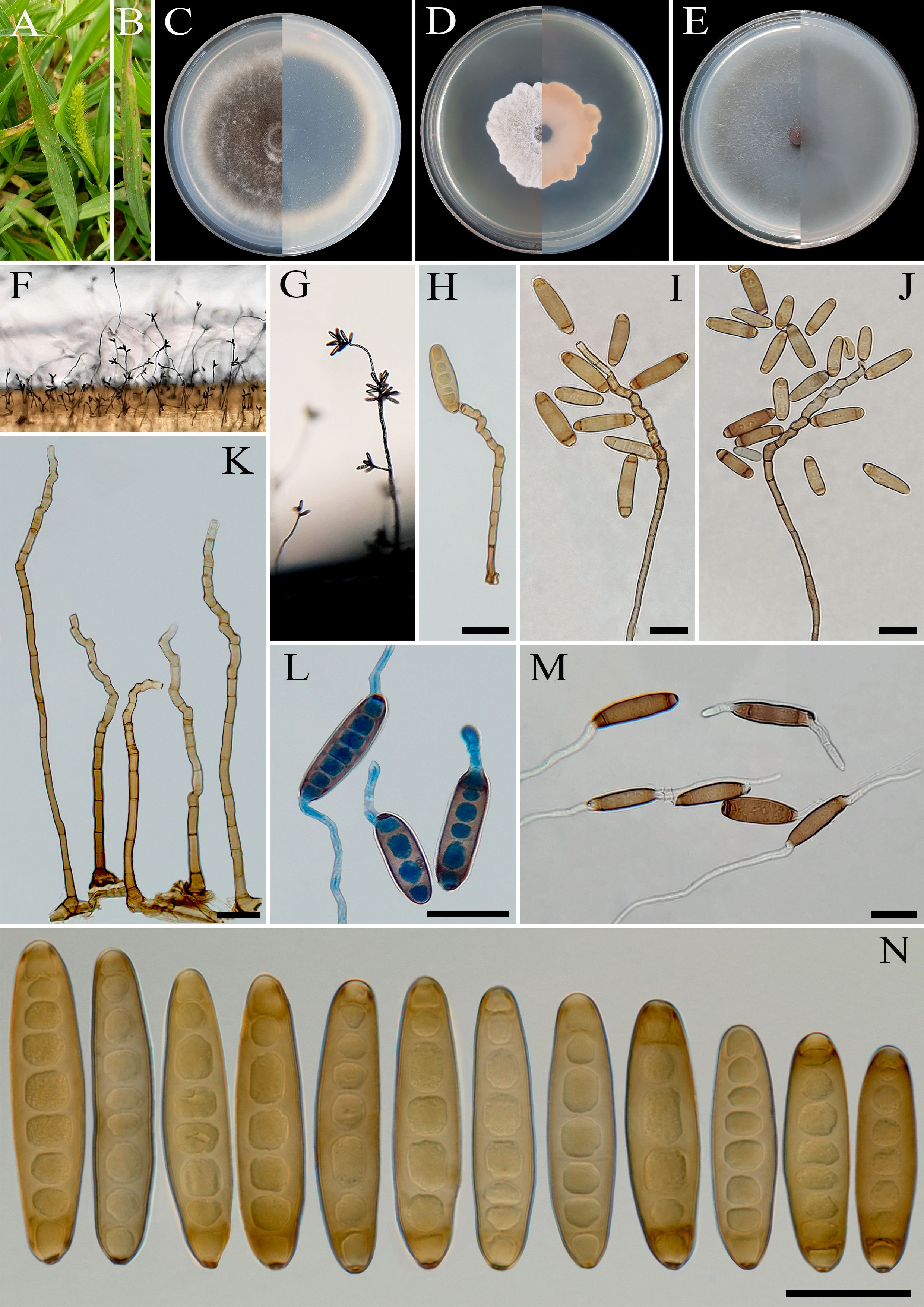
Figure 6. Bipolaris banihashemii (IRAN 3389C). (A, B) Lesions on host leaf (Setaria sp.). (C−E) Colonies (front and reverse) on PDA (C), MEA (D), and CMA (E) media after 7 days. (F, G) Sporulation pattern on TWA medium. (H−K) Conidiophores. (L, M) Germinated conidia. (N) Conidia. (H−N) Scale bars = 20 μm.
MycoBank No: MB 854732
Etymology: Named in honor of Dr. Zia Banihashemi, emeritus Professor of Shiraz University, Iran, who significantly contributed to the knowledge of mycology and plant pathology in Iran.
Diagnosis: Differs from Bipolaris variabilis and B. zeae by the size of conidiophores and the shape and size of the conidia.
Type: IRAN, West Azarbaijan Province, Khoy County, on infected leaves of Setaria sp. (Poaceae, Poales), 20 September 2010, A. Ahmadpour, (IRAN 18244F, holotype, dried culture; ex-type IRAN 3389C).
Description: Leaf spots on Setaria sp., 1- to 5-mm long, gray at the center with red-brown margins. Sexual morph: Undetermined. Asexual morph: On TWA Hyphae 3- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (150–)260–400(–450) × 5–7 µm ( ± SD = 330 ± 70 × 5 ± 1 μm, n = 50), mononematous, semi- to macronematous, arising singly or in groups, unbranched, straight to flexuous, septate, geniculate, pale brown to brown, paler toward the apex, swollen at the base. Conidiogenous cells (7–)9–23(–28) × 5–8 μm ( ± SD = 16 ± 7 × 6.5 ± 1.5 μm, n = 50), mono- to polytretic, sympodial proliferation, integrated, terminal or intercalary, subcylindrical to slightly swollen, pale brown to brown, smooth walled to slightly verruculose, with thickened and darkened scars. Conidia (28–)38–62(–68) × 9–13 µm ( ± SD = 50 ± 18.5 × 11 ± 2 μm, n = 50), golden brown, smooth walled, straight, cylindrical to fusoid, occasionally ellipsoidal, tapering toward rounded ends, end cells often cut off by a thick dark septum, (4–)5–8(–9)-distoseptate, germination mono- or bipolar; hila 2- to 3-μm wide, truncate, slightly protruding, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 67 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, olivaceous green at the center, white at the margin with white to gray aerial mycelia; reverse gray olivaceous to olivaceous black with a hyaline margin. Colonies on MEA reaching 35 mm in diameter, circular, margin irregular, cottony appearance, white with white aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on CMA reaching 62 mm in diameter, circular, margin entire, hairy appearance, olivaceous gray with sparse white to gray aerial mycelia; reverse olivaceous gray at the center and a hyaline margin.
Additional materials examined: IRAN, West Azarbaijan Province, Khoy County, on infected leaves of Setaria sp. (Poaceae, Poales), 20 September 2010, A. Ahmadpour, isolate IRAN 3388C; ibid. on infected leaves of Setaria sp. (Poaceae, Poales), 20 September 2010, A. Ahmadpour, isolate IRAN 3387C.
Host and distribution: Setaria sp. in Iran (this study).
Notes: Based on multi-locus phylogenetic analyses, B. banihashemii clustered closely with B. variabilis and B. zeae (MLBS/MPBS/BIPP = 100/86/0.99) (Figure 1). A comparison of nucleotide differences in ITS−rDNA, GAPDH, and TEF1 indicates that B. banihashemii (IRAN 3389C) differs from B. variabilis (CBS 127716) by 1/548 bp [0.18%, with one gap (0%)] in ITS−rDNA, 4/577 bp (0.69%) in GAPDH, and 1/642 bp (0.15%) in TEF1 and from B. zeae (BRIP 11512) by 3/577 bp (0.52%) in GAPDH and 2/712 bp (0.28%) in TEF1. The PHI analysis confirms that B. banihashemii has no significant genetic recombination with closely related species (Φw = > 0.05, Figure 7). Bipolaris variabilis can be differentiated by having longer conidiophores (up to 1,600 μm vs. up to 450 μm in B. banihashemii), shape of conidia (verruculose walled, straight or slightly curved, globose to obclavate conidia vs. smooth walled, straight, cylindrical to fusoid conidia in B. banihashemii), and wider conidia (10–19.5 vs. 9–13 μm in B. banihashemii) (Marin-Felix et al., 2017a). Bipolaris zeae differs from B. banihashemii in producing shorter conidiophores (up to 370 vs. 450 μm in B. banihashemii) and longer and wider conidia [(30–)40–80(–120) × 12–18(–21) μm vs. (28–)38–62(–68) × 9–13 µm in B. banihashemii] (Sivanesan, 1987; Manamgoda et al., 2014).
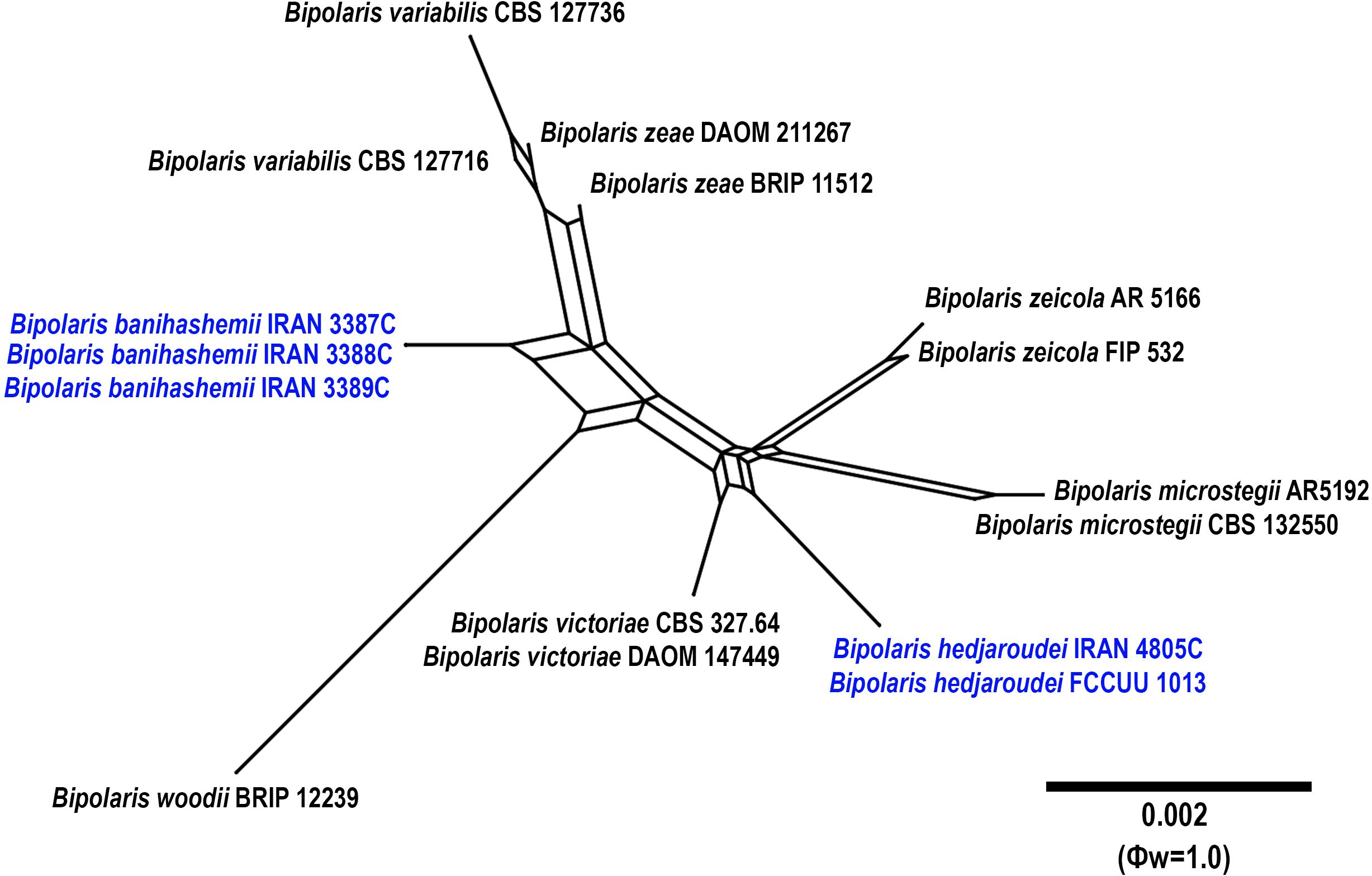
Figure 7. Split graphs showing the results of PHI test of Bipolaris banihashemii and B. hedjaroudei with their most closely related species (Φw = 1.0). The new taxa are shown in bold blue.
Bipolaris hedjaroudei A. Ahmadpour, Z. Heidarian, Y. Ghosta, Z. Alavi & F. Alavi, sp. nov. (Figure 8).
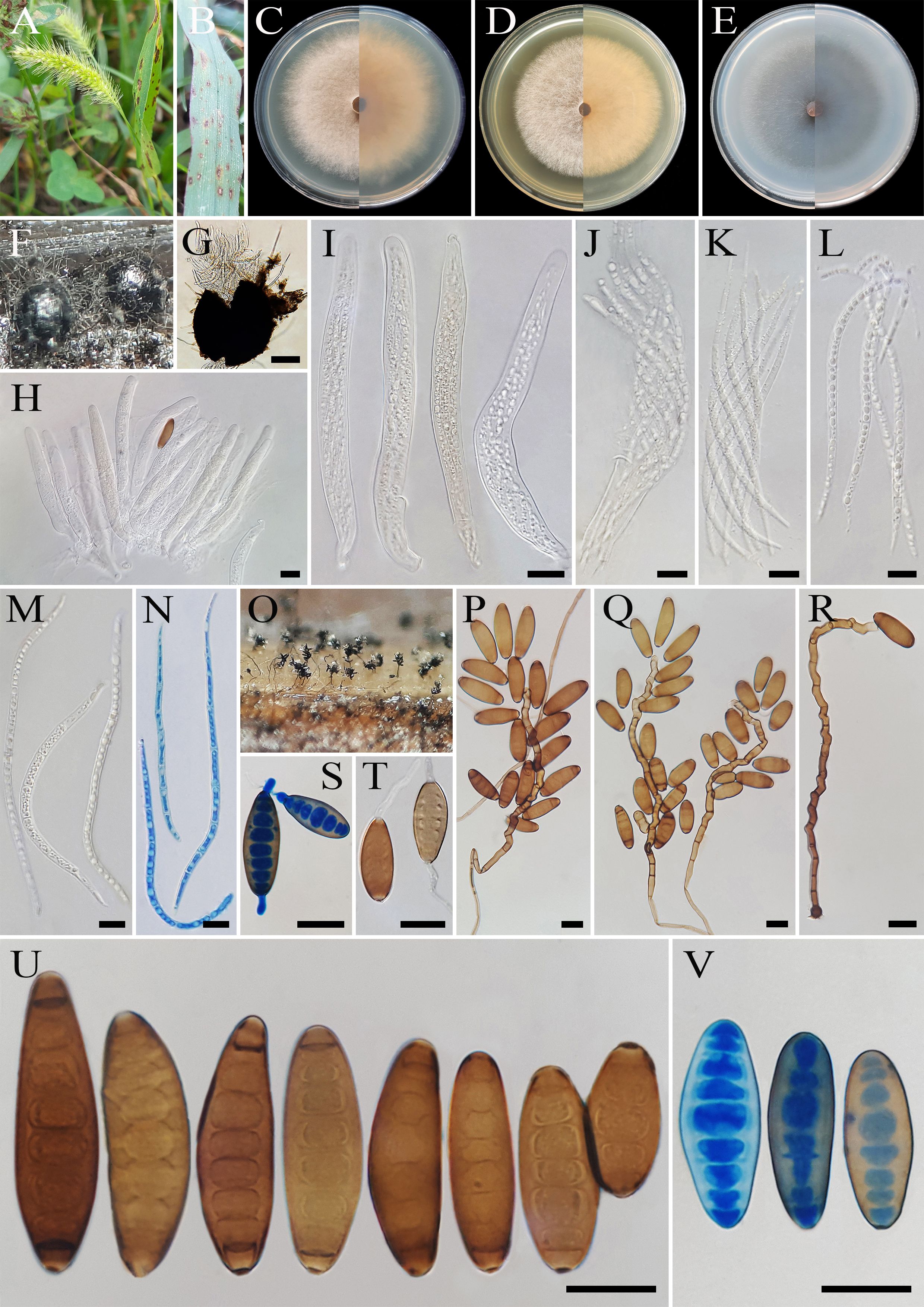
Figure 8. Bipolaris hedjaroudei (IRAN 4805C). (A, B) Lesions on host leaf (Setaria sp.). (C−E) Colonies (front and reverse) on PDA (C), MEA (D), and CMA (E) media after 7 days. (F, G) Ascomata on TWA medium containing leaves of the host plant. (H−N) Asci and ascospores. (O) Sporulation pattern on TWA medium. (P–R) Conidiophores. (S, T) Germinated conidia. (U, V) Conidia. (G) Scale bars = 100 μm. (H–V) Scale bars = 20 μm.
MycoBank No: MB 854733
Etymology: Named in honor of Dr. Ghorbanali Hedjaroud, emeritus Professor of Tehran University, who significantly contributed to the knowledge of mycology in Iran.
Diagnosis: Differs from Bipolaris microstegii, B. victoriae, B. zeicola, and B. woodii by having longer conidiophores, smaller conidia, and production of sexual morph (homothallic species) in culture media.
Type: IRAN, West Azarbaijan Province, Khoy County, on infected leaves of Setaria sp. (Poaceae, Poales), 10 September 2020, A. Ahmadpour, (IRAN 18492F, holotype, dried culture; ex-type IRAN 4805C).
Description: On infected leaves of Setaria sp., leaf lesions 1- to 10-mm long, gray at the center and red-brown at the margins. Sexual morph: On sterile leaves of Setaria sp. in TWA medium Ascomata pseudothecial, (300–)400–550(–600) × (290–)300–500(–550) μm ( ± SD = 475 ± 75 × 400 ± 100 μm, n = 20), solitary, scattered, superficial or slightly embedded, globose to subglobose or oval, dark brown to black, unilocular with a short ostiolate neck, with long brown setae and conidiophores bearing conidia developing on the upper part of the ascoma. Ostiolar neck 10–20× 8–12 μm ( ± SD = 15 ± 5 × 10 ± 2 μm, n = 20), conical, central, filled with masses of hyaline cells frequently covering the apex of the neck. Peridium (ascomata wall) 30- to 35-μm wide, composed of layers of pigmented thick-walled cells. Pseudoparaphyses 2- to 3-μm wide, hyaline, septate, filamentous, simple to branched. Asci (136–)150–200(–212) × (15–)17–20 (–22) μm ( ± SD = 150 ± 50 × 18.5 ± 1.5 μm, n = 20), with eight ascospores coiled in a tight helix, bitunicate, cylindrical to clavate, occasionally obclavate–fusoid, straight or curved, with short pedicel. Ascospores 170–250 × 5–7 μm ( ± SD = 210± 40 × 6 ± 1 μm, n = 50), hyaline, filiform to flagelliform, tapering toward the rounded ends, tightly coiled inside the ascus, 7–13 septate, with a thin mucilaginous sheath visible in water mounts. Asexual morph: On TWA Hyphae 2- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (125–)175–250(–325) × 5–7 µm ( ± SD = 212.5 ± 37.5 × 6 ± 1 μm, n = 50), mononematous, semi- to macronematous, arising mostly singly or rarely in groups, unbranched, straight to flexuous, septate, geniculate, pale brown to brown, paler toward the apex, swollen at the base. Conidiogenous cells (6–)8–22(–25) × 5–8 μm ( ± SD = 15 ± 7 × 6.5 ± 1.5 μm, n = 50), mono- to polytretic, sympodial proliferation, integrated, terminal or intercalary, subcylindrical to slightly swollen, pale brown to brown, smooth walled to slightly verruculose, with thickened and darkened scars. Conidia (25–)32–60(–62) × 15–17 µm ( ± SD = 46 ± 14 × 16 ± 1 μm, n = 50), brown to dark brown, smooth walled, straight to slightly curved, broadly fusiform, occasionally ellipsoidal to obclavate, tapering toward the rounded ends, apical and basal cells paler than the median cells, end cells often cut off by a thick dark septum, (4–)5–8(–9)-distoseptate, germinated mono- or bipolar; hila 2- to 3-μm wide, conspicuous, brown, slightly protuberant, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 65 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, velvety, gray at the center and white at the margin, with gray to white aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on MEA reaching 61 mm in diameter, circular, margin entire, cottony appearance, white with white aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on CMA reaching 61 mm in diameter, circular, margin entire, hairy appearance, gray with sparse white to gray aerial mycelia; reverse olivaceous brown at the center and a hyaline margin.
Additional material examined: Iran, West Azarbaijan Province, Khoy County, on infected leaves of Setaria sp. (Poaceae, Poales), 10 September 2020, A. Ahmadpour, isolate FCCUU 1013.
Host and distribution: Setaria sp. in Iran (this study).
Notes: Bipolaris hedjaroudei is phylogenetically closely related to B. victoriae, B. microstegii, B. zeicola, and B. woodii (Figure 1). Pairwise sequence similarity analyses of three genomic regions in B. hedjaroudei distinguished it from closely related taxa. A comparison of nucleotide differences in ITS−rDNA, GAPDH, and TEF1 indicates that B. hedjaroudei (IRAN 4805C) differs from B. microstegii (CBS 132550) by 2/507 bp (0.39%) in ITS−rDNA, 5/539 bp (0.92%) in GAPDH, and 8/763 bp [1.04%, with two gaps (0%)] in TEF1; from B. victoriae (CBS 327.64) by 1/480 bp (0.20%) in GAPDH and 6/763 bp [0.78%, with two gaps (0%)] in TEF1; from B. woodii (BRIP 12239) by 2/512 bp (0.39%) in ITS−rDNA, 9/550 bp [1.63%, with one gap (0%)] in GAPDH, and 8/761 bp [1.05%, with two gaps (0%)] in TEF1; and from B. zeicola (FIP 532) by 2/437 bp [0.45%, with one gap (0%)] in ITS−rDNA, 1/480 bp (0.20%) in GAPDH, and 6/763 bp [0.78%, with two gaps (0%)] in TEF1. The PHI analysis confirms that B. hedjaroudei has no significant genetic recombination with closely related species (Φw = > 0.05, Figure 7). Bipolaris hedjaroudei can be differentiated by having longer conidiophores (up to 325 μm vs. up to 250 μm in B. victoriae, up to 270 μm in B. zeicola, up to 250 μm in B. woodii) and smaller conidia [(25–)32–60(–62) × 15–17 µm vs. (25–)55–90(–110) × (10–)12–16(–19) μm in B. victoriae, (45–)65–90(–105) × (10–)15–19(–22) μm in B. zeicola, (60–)69–76(–86) × (10–)12.5–13.5(–15) μm in B. woodii] (Manamgoda et al., 2014; Tan et al., 2016). Bipolaris microstegii differs from B. hedjaroudei in producing secondary conidiophores and conidia, longer conidiophores (up to 750 μm vs. up to 325 μm in B. hedjaroudei), and accentuated septa (Manamgoda et al., 2014). Bipolaris victoriae and B. zeicola have been reported on various poaceous hosts and caused destructive diseases in oat and maize, respectively (Manamgoda et al., 2014; Farr et al., 2024). Bipolaris hedjaroudei is a homothallic species that forms sexual morph abundantly on TWA medium containing host leaves after 21–30 days. In contrast, B. victoriae and B. zeicola are heterothallic species, and the sexual morph of B. microstegii and B. woodii has not been recorded yet (Manamgoda et al., 2014; Tan et al., 2016).
Bipolaris hemerocallidis A. Ahmadpour, Z. Heidarian, Y. Ghosta, Z. Alavi & F. Alavi, sp. nov. (Figure 9).
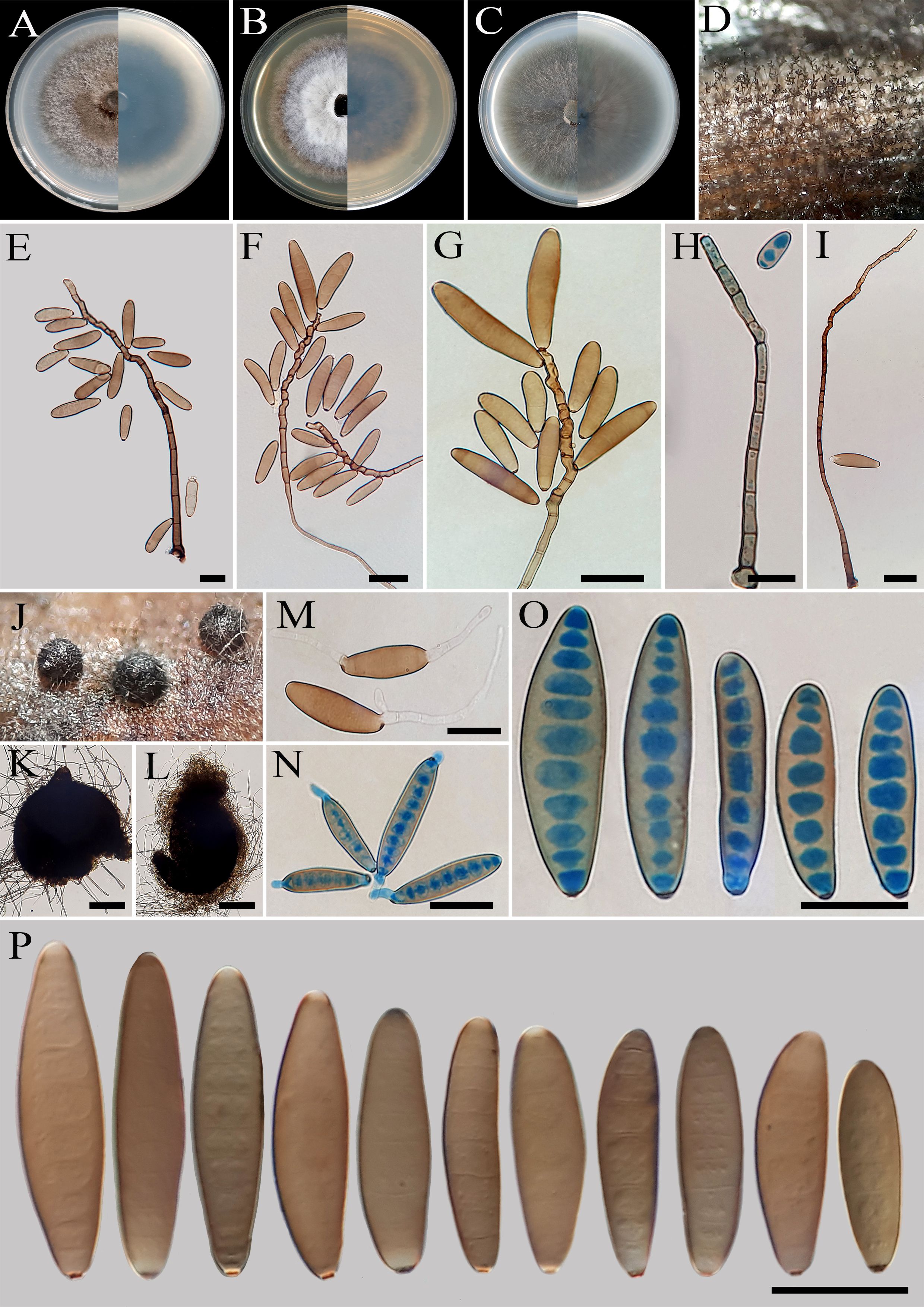
Figure 9. Bipolaris hemerocallidis (IRAN 4774C). (A−C) Colonies (front and reverse) on PDA (A), MEA (B), and CMA (C) media after 7 days. (D) Sporulation pattern on TWA medium. (E–I) Conidiophores. (J−L) Sterile ascomata on TWA medium containing leaves of the host plant. (M, N) Germinated conidia. (O, P) Conidia. (K, L) Scale bars = 50 μm, (E–I), (M–P) Scale bars = 20 μm.
MycoBank No: MB 854734
Etymology: Named after the host genus, Hemerocallis, from which the holotype was collected.
Diagnosis: Differs from Bipolaris axonopicola by having longer conidiophores.
Type: IRAN, Isfahan Province, Isfahan County, Flower Garden, on leaves of Hemerocallis fulva (Asphodelaceae, Asparagales), 7 October 2013, A. Ahmadpour/Z. Heidarian, (IRAN 18206F, holotype, dried culture; ex–type IRAN 4774C).
Description: Associated with leaves of Hemerocallis fulva. Sexual morph: Undetermined. Asexual morph: On TWA Hyphae 2- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (180–)230–550(–600) × 5–7 µm ( ± SD = 390 ± 160 × 6 ± 1 μm, n = 50), mononematous, semi- to macronematous, arising singly or in groups, unbranched, straight to flexuous, septate, geniculate, pale brown to brown, paler toward the apex, swollen at the base. Conidiogenous cells (5–)7–21(–25) × 5–8 μm ( ± SD = 14± 7 × 6.5 ± 1.5 μm, n = 50), mono- to polytretic, sympodial proliferation, integrated, terminal or intercalary, subcylindrical to slightly swollen, pale brown to brown, smooth walled to slightly verruculose, with thickened and darkened scars. Conidia (38–)40–52(–60) × 9–11 µm ( ± SD = 46 ± 6 × 10 ± 1 μm, n = 50), pale brown to brown, smooth walled, straight to slightly curved, broadly fusoid to cylindrical, occasionally ellipsoidal to clavate, tapering toward the rounded ends, apical and basal cells paler than the median cells, (4–)5–9(–10)-distoseptate, germinated mono- or bipolar; hila 2- to 3-μm wide, truncate, slightly protruding, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 65 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, olivaceous green at the center with white to gray aerial mycelia, white at the margin; reverse olivaceous gray to olivaceous black. Colonies on MEA reaching 55 mm in diameter, circular, margin entire, cottony appearance, olivaceous gray to gray, with white to gray aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on CMA reaching 60 mm in diameter, circular, margin entire, hairy appearance, olivaceous gray with sparse white to gray aerial mycelia; reverse olivaceous brown at the center with a hyaline margin. Sterile ascomata were produced on TWA medium containing leaves of the host plant. However, these structures remained sterile (without asci and ascospores) after 3–6 months of incubation.
Additional material examined: IRAN, Isfahan Province, Isfahan County, Flower Garden, on leaves of Hemerocallis fulva (Asphodelaceae, Asparagales), 7 October 2013, A. Ahmadpour/Z. Heidarian, isolate FCCUU 1011.
Host and distribution: Associated with leaves of Hemerocallis fulva in Iran (this study).
Notes: Bipolaris hemerocallidis is phylogenetically close to B. axonopicola (MLBS/MPBS/BIPP = 100/100/1.0) (Figure 1). The pairwise DNA sequence comparison revealed that B. hemerocallidis is distinct from B. axonopicola. A comparison of nucleotide differences in ITS−rDNA, GAPDH, and TEF1 indicates that B. hemerocallidis (IRAN 4774C) differs from B. axonopicola (BRIP 11740) by 6/532 bp [1.12%, with four gaps (0%)] in ITS−rDNA, 17/546 bp (3.11%) in GAPDH and 4/788 bp (0.50%) in TEF1. The PHI analysis confirms that B. hemerocallidis has no significant genetic recombination with closely related species (Φw = > 0.05, Figure 5). Bipolaris hemerocallidis can be differentiated by having longer conidiophores (up to 600 μm vs. up to 250 μm in B. axonopicola) (Tan et al., 2016). Bipolaris axonopicola is only known on Axonopus fissifolius (Poaceae) in Australia (Tan et al., 2016). In this study, B. hemerocallidis was isolated from the leaves of Hemerocallis fulva (Asphodelaceae, Asparagales) in the greenhouse.
Bipolaris iranica A. Ahmadpour, Z. Heidarian, Y. Ghosta, Z. Alavi & F. Alavi, sp. nov. (Figure 10).

Figure 10. Bipolaris iranica (IRAN 4775C). (A, B) Lesions on host leaf (Cynodon dactylon). (C−E) Colonies (front and reverse) on PDA (C), MEA (D), and CMA (E) media after 7 days. (F) Sporulation pattern on TWA medium. (G–L) Conidiophores. (M, N) Germinated conidia. (O) Conidia. (J−O) Scale bars = 20 μm.
MycoBank No: MB 854735
Etymology: Named after the country “Iran” where the holotype was collected.
Diagnosis: Differs from Bipolaris heveae, B. microlaenae, and B. simmondsii by having much longer conidiophores and accentuated transverse septa.
Type: IRAN, West Azarbaijan Province, Khoy County, on infected leaves of Cynodon dactylon (Poaceae, Poales), 20 September 2010, A. Ahmadpour, (IRAN 18207F, holotype, dried culture; ex–type IRAN 4775C).
Description: Leaf lesions on Arundo donax, Cynodon dactylon, Echinochloa colona, Hordeum vulgare, Sorghum halepense, and Triticum aestivum, 1- to 10-mm long. Sexual morph: Undetermined. Asexual morph: On TWA Hyphae 2- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (125–)187–480(–550) × 7–8 µm ( ± SD = 337.5 ± 146.5 × 7.5 ± 0.5 μm, n = 50), mononematous, macronematous, arising singly or in groups, simple, straight to flexuous, septate, geniculate, with cell walls thicker than vegetative hyphae, pale brown to brown, paler toward the apex, basal cell swollen and darker than the other cells, up to 10 μm in diameter Conidiogenous cells (8–)10–24(–28) × 6–8 μm ( ± SD = 17 ± 7 × 7 ± 1 μm, n = 50), mono- to polytretic, proliferating sympodially, integrated, terminal or intercalary, subcylindrical to slightly swollen, pale brown to dark brown, smooth walled to slightly verruculose, with thickened and darkened scars. Conidia (70–)85–100(–110)× 15–20 µm ( ± SD = 92.5 ± 7.5 × 17.5 ± 2.5 μm, n = 50), brown to dark brown, smooth walled, straight to curved, mostly navicular to fusoid, rarely cylindrical to clavate, taper toward rounded ends, apical and basal cells paler than the median cells, septa accentuated at maturity, (6–)8–11(–13)-distoseptate, germinated mono- or bipolar; hila 2- to 3-μm wide, flat to slightly protuberant, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 58 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, olivaceous gray at the center with white to gray aerial mycelia, white at the margin; reverse olivaceous gray to olivaceous black with a hyaline margin. Colonies on MEA reaching 42 mm in diameter, circular, margin entire, cottony appearance, white with white aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on CMA reaching 66 mm in diameter, circular, margin entire, olivaceous gray with sparse gray aerial mycelia; reverse olivaceous gray at the center and a hyaline margin.
Additional materials examined: IRAN, West Azarbaijan Province, Miyandoab County, on infected leaves of Sorghum halepense (Poaceae, Poales), 11 July 2013, A. Ahmadpour/Z. Heidarian, isolate FCCUU 1005; ibid. on infected leaves of Echinochloa colona (Poaceae, Poales), 23 September 2014, A. Ahmadpour/Z. Heidarian, isolate FCCUU 1007; West Azarbaijan Province, Urmia County, on infected leaves of Arundo donax (Poaceae, Poales), 20 September 2014, A. Ahmadpour/Z. Heidarian, isolate FCCUU 1006; West Azarbaijan Province, Bukan County, on infected leaves of Hordeum vulgare (Poaceae, Poales), 20 October 2014, A. Ahmadpour/Z. Heidarian, isolate FCCUU 1008; West Azarbaijan Province, Khoy County, on infected leaves of Triticum aestivum (Poaceae, Poales), 22 May 2021, A. Ahmadpour, isolate FCCUU 1009.
Hosts and distribution: Arundo donax, Cynodon dactylon, Echinochloa colona, Hordeum vulgare, Sorghum halepense, and Triticum aestivum in Iran (this study).
Notes: Based on the results of phylogenetic analyses (Figure 1), B. iranica isolates clustered well in a separate lineage with 100% ML, 100% MP bootstrap, and 1.0 BI posterior probability values, representing a new taxon. The pairwise DNA sequence comparison revealed that B. iranica is distinct from related taxa, B. heveae, B. microlaenae, and B. simmondsii. A comparison of nucleotide differences in ITS−rDNA, GAPDH, and TEF1 indicates that B. iranica (IRAN 4775C) differs from B. heveae (CBS 241.92) by 5/539 bp [0.92%, with four gaps (0%)] in ITS−rDNA, 11/490 bp (2.24%) in GAPDH and 6/770 bp (0.77%) in TEF1; from B. microlaenae (BRIP 15613) by 2/534 bp (0.37%) in ITS−rDNA, 11/543 bp (2.02%) in GAPDH, and 12/788 bp (1.52%) in TEF1; and from B. simmondsii (BRIP 12030) by 3/536 bp [0.55%, with one gap (0%)] in ITS−rDNA, 13/536 bp (2.42%) in GAPDH, and 7/788 bp (0.88%) in TEF1. The PHI analysis confirms that B. iranica has no significant genetic recombination with closely related species (Φw = > 0.05, Figure 11). Bipolaris iranica is morphologically similar to B. heveae, B. microlaenae, and B. simmondsii; however, it can be distinguished by its much longer conidiophores (up to 550 μm vs. up to 335 μm in B. heveae, and up to 240 μm in B. simmondsii), and its accentuated septa, which are absent in B. heveae, B. microlaenae, and B. simmondsii (Manamgoda et al., 2014; Tan et al., 2016). Bipolaris heveae is known to cause diseases on rubber trees (Hevea brasiliensis) across various tropical countries, including Cambodia, the Dominican Republic, Ghana, Guatemala, Haiti, Honduras, Indonesia, Mexico, Nigeria, the Philippines, Sri Lanka, and the United States. Unlike B. iranica, this pathogen does not infect grass species and is restricted to its specific host plant (Manamgoda et al., 2014; Farr et al., 2024). Furthermore, B. microlaenae and B. simmondsii have only been documented in Australia, where they cause leaf spots on Zoysia macrantha (Tan et al., 2016).
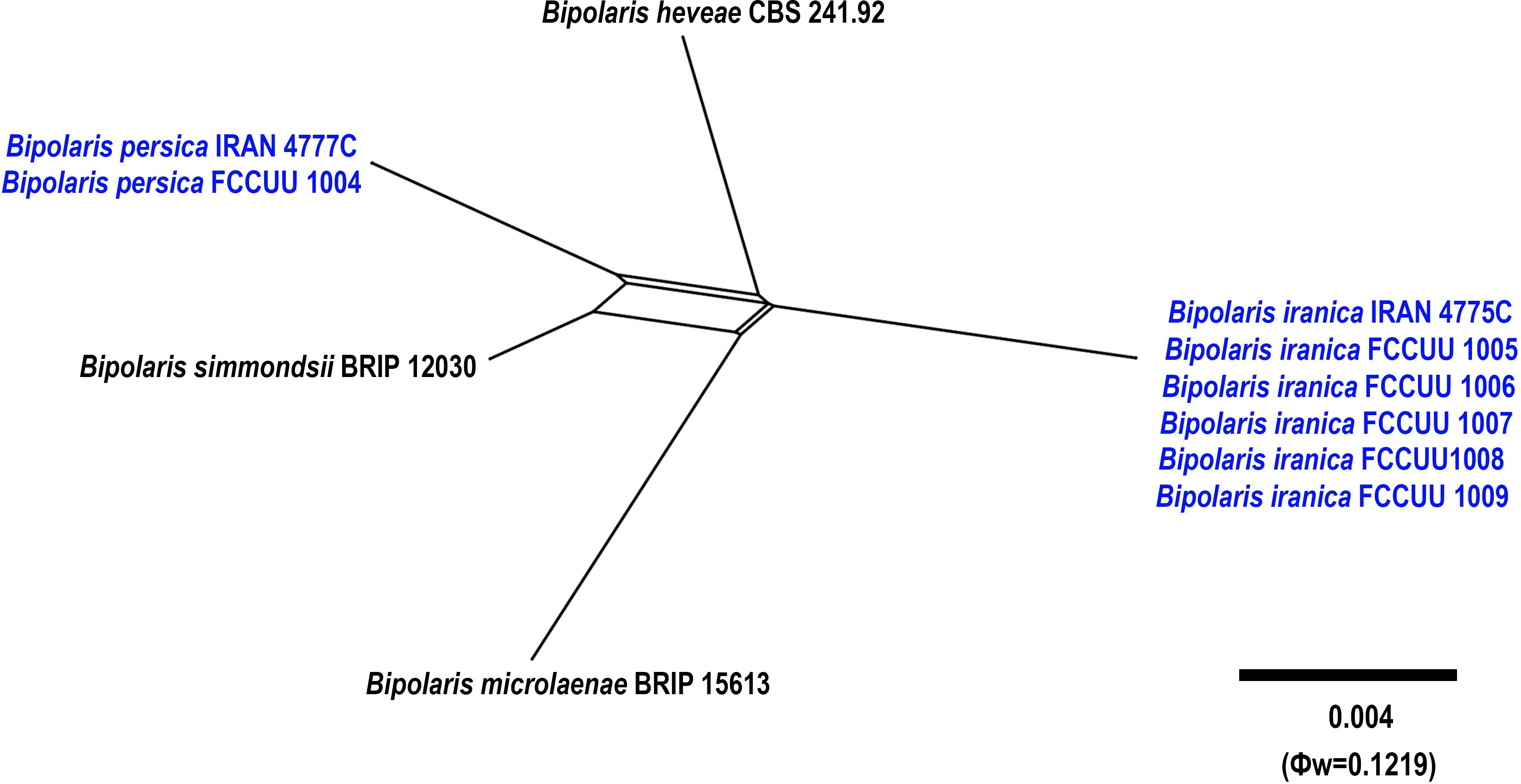
Figure 11. Split graphs showing the results of PHI test of Bipolaris iranica and B. persica with their most closely related species (Φw = 0.1219). The new taxa are shown in bold blue.
Bipolaris persica A. Ahmadpour, Z. Heidarian, Y. Ghosta, Z. Alavi & F. Alavi, sp. nov. (Figure 12)

Figure 12. Bipolaris persica (IRAN 4777C). (A, B) Lesions on host leaf (Cynodon dactylon). (C−E) Colonies (front and reverse) on PDA (C), MEA (D), and CMA (E) media after seven days. (F) Sporulation pattern on TWA medium. (G–I) Conidiophores. (K, L) Germinated conidia. (J, M, N) Conidia. (G−N) Scale bars = 20 μm.
MycoBank No: MB 854736
Etymology: The name refers to the old name of Iran, Persia, from where the holotype was collected.
Diagnosis: Differs from Bipolaris heveae, B. microlaenae, and B. simmondsii by having longer conidiophores and accentuated transverse septa.
Type: IRAN, West Azarbaijan Province, Mahabad County, on infected leaves of Cynodon dactylon (Poaceae, Poales), 20 June 2015, A. Ahmadpour, (IRAN 18209F, holotype, dried culture; ex-type IRAN 4777C).
Description: Leaf spots on Cynodon dactylon, 1- to 10-mm long, with dark brown spots. Sexual morph: Undetermined. Asexual morph: On TWA Hyphae 2- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (210–)250–330(–350) × 6–7 µm ( ± SD = 290 ± 40 × 6.5 ± 0.5 μm, n = 50), mononematous, macronematous, arising singly or in groups, simple, straight to flexuous, septate, geniculate, with cells wall thicker than those of vegetative hyphae, pale brown to brown, paler toward the apex, basal cell swollen and darker than the other cells, up to 10 μm in diameter. Conidiogenous cells (7–)10–25(–30) × 6–8 μm ( ± SD = 17.5 ± 7.5 × 7 ± 1 μm, n = 50), mono- to polytretic, proliferating sympodially, integrated, terminal or intercalary, subcylindrical to slightly swollen, pale brown to brown, smooth walled to slightly verruculose, with thickened and darkened scars. Conidia (62–)80–95(–100)× 13–20 µm ( ± SD = 87.5 ± 7.5 × 16.5 ± 3.5 μm, n = 50), brown to dark brown, smooth walled, curved, mostly navicular to fusoid, tapering toward the rounded ends, apical and basal cells paler than the median cells, septa accentuated at maturity, (6–)7–10(–11)-distoseptate, germinated mono- or bipolar; hila 2- to 3-μm wide, flat, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 68 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, olivaceous gray with white to gray aerial mycelia, sporulation abundant; reverse olivaceous gray at the center with hyaline margin. Colonies on MEA reaching 58 mm in diameter, circular, margin entire, cottony appearance, gray at the center, white at the margin with white to gray aerial mycelia; reverse olivaceous gray with hyaline margin. Colonies on CMA reaching 53 mm in diameter, circular, margin entire, olivaceous green with white to gray aerial mycelia; reverse olivaceous gray to olivaceous black and a hyaline margin.
Additional material examined: IRAN, West Azarbaijan Province, Mahabad County, on infected leaves of Cynodon dactylon (Poaceae, Poales), 20 June 2015, A. Ahmadpour, isolate FCCUU 1004.
Host and distribution: Cynodon dactylon in Iran (this study).
Notes: Bipolaris persica is phylogenetically closely related to B. heveae, B. iranica, B. microlaenae, and B. simmondsii (Figures 1, 11). Pairwise DNA sequence comparison revealed that B. persica is distinct from its closely related taxa. A comparison of nucleotide differences in ITS−rDNA, GAPDH, and TEF1 indicates that B. persica (IRAN 4777C) differs from B. heveae (CBS 241.92) by 1/500 bp [0.20%, with one gap (0%)] in ITS−rDNA, 13/481 bp (2.70%) in GAPDH, and 7/731 bp (0.95%) in TEF1; from B. iranica (IRAN 4775C) by 4/500 bp [0.80%, with three gaps (0%)] in ITS−rDNA, 15/504 bp (2.97%) in GAPDH, and 8/744 bp (1.07%) in TEF1; from B. microlaenae (BRIP 15613) by 4/500 bp [0.80%, with three gaps (0%)] in ITS−rDNA, 17/516 bp (3.29%) in GAPDH, and 8/744 bp (1.07%) in TEF1; and from B. simmondsii (BRIP 12030) by 4/500 bp [0.80%, with three gaps (0%)] in ITS−rDNA, 9/545 bp (1.65%) in GAPDH, and 3/744 bp (0.40%) in TEF1. The PHI analysis further confirms that B. iranica has no significant genetic recombination with closely related species (Φw = > 0.05, Figure 11). Morphologically, Bipolaris persica can be differentiated from closely related taxa by its longer conidiophores (up to 350 μm vs. up to 240 μm in B. simmondsii), and by its accentuated septa, which are absent in B. heveae, B. microlaenae, and B. simmondsii (Manamgoda et al., 2014; Tan et al., 2016). However, B. persica shares overlapping morphological characteristics and the same host with B. iranica, complicating their differentiation. Unlike B. persica, B. iranica has a broader host range, including Arundo donax, Cynodon dactylon, Echinochloa colona, Hordeum vulgare, Sorghum halepense, and Triticum aestivum (this study). Consequently, using molecular tools is essential for accurately distinguishing Bipolaris species and identifying any cryptic species.
Bipolaris crotonis Sivan., Trans. Br. mycol. Soc. 84(3): 404 (1985) (Figure 13).

Figure 13. Bipolaris crotonis (IRAN 4807C). (A, B) Lesions on host leaf (Eleusine indica). (C−E) Colonies (front and reverse) on PDA (C), MEA (D), and CMA (E) media after 7 days. (F−I) Sporulation pattern on TWA medium. (J–N) Conidiophores. (O, P) Germinated conidia. (Q, R) Conidia. (J−R) Scale bars = 20 μm.
Description: Leaf spots on Eleusine indica, gray at the center and dark brown margins. Sexual morph: Undetermined. Asexual morph: On TWA Hyphae 3- to 6-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (60–)100–300(–325) × 5–7 µm ( ± SD = 200± 100 × 6 μm, n = 50), mononematous, semi- to macronematous, arising singly or mostly in groups, unbranched, straight to flexuous, septate, geniculate, pale brown to brown, paler toward the apex, swollen at the base. Conidiogenous cells (9–)11–26(–30) × 5–8 μm ( ± SD = 18.5 ± 7.5 × 6.5 ± 1.5 μm, n = 50), mono- to polytretic, proliferating sympodially, integrated, terminal or intercalary, subcylindrical to slightly swollen, hyaline to pale brown, smooth walled to slightly verruculose, with thickened and darkened scars. Conidia (62–)75–100(–120) × 17–25 µm ( ± SD = 87.5 ± 12.5 × 21 ± 4 μm, n = 50), straight, brown to dark golden brown, smooth walled, broadly ellipsoidal, fusoid to obclavate, tapering toward the rounded ends, apical and basal cells paler than the median cells, (5–)7–9(–11)-distoseptate, germinated mono- or bipolar; hila 2- to 3-μm wide, truncate, slightly protruding, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 45 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, velvety, gray at the center and white at the margin, with sparse gray aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on MEA reaching 40 mm in diameter, circular, margin entire, cottony appearance, gray at the center, white at the margin, with floccose aerial mycelia; reverse brown to pale brown from the center to the margin. Colonies on CMA reaching 50 mm in diameter, hairy appearance, olivaceous gray, with sparse gray aerial mycelia; reverse olivaceous gray at the center and a hyaline margin.
Material examined: IRAN, Mazandaran Province, Nour County, from leaf spots of Eleusine indica (Poaceae, Poales), 10 September 2022, Hashemlou E., living culture IRAN 4807C.
Hosts: Croton sp., Eleusine indica (Sivanesan, 1987; Manamgoda et al., 2014; Farr et al., 2024).
Distribution: Australia, Iran (this study), Papua New Guinea, Samoa, and Vanuatu (Sivanesan, 1987; Manamgoda et al., 2014; Farr et al., 2024).
Notes: Bipolaris crotonis is morphologically similar and phylogenetically related to B. sorokiniana (Figure 1) and can be differentiated by having longer conidia [(51–)60–110(–138) × (14–)20–25(–32) µm vs. (31–)40–72(–100) × 15–25(–27) μm in B. sorokiniana] (Sivanesan, 1987; Manamgoda et al., 2014). This species is heterothallic, and sexual morph can be developed by crossing compatible isolates in Sach’s agar medium (Manamgoda et al., 2014). Tan et al. (2014) reported that B. eleusines is phylogenetically similar to B. crotonis and synonymized it under B. crotonis based on nomenclatural priority. Bipolaris crotonis was first reported on decaying leaves of Croton sp. (Euphorbiaceae) (Sivanesan, 1985; Manamgoda et al., 2014) and was later identified on Eleusine indica (Poaceae) (Tan et al., 2014; Bhunjun et al., 2020). This species has also been reported as the causal agent of black point disease in wheat on the North China Plain (Xu et al., 2018) and as an endophytic fungus associated with Dillenia indica, an ethnomedicinal plant (Prasher and Kumar, 2021). To the best of our knowledge, this is the first report of B. crotonis in Iran.
Bipolaris salkadehensis Ahmadpour & Heidarian, Mycotaxon 120: 302 (2012) (Figure 14).

Figure 14. Bipolaris salkadehensis (IRAN 3382C). (A) Lesions on host culms (Scirpus acutus). (B−D) Colonies (front and reverse) on PDA (B), MEA (C), and CMA (D) media after 7 days. (E, F) Sporulation pattern on TWA medium. (G–I) Conidiophores. (J, K) Germinated conidia. (L) Conidia. (G−L) Scale bars = 20 μm.
Description: Culm spots on Scirpus acutus, 1- to 20-mm long, with dark brown spots. Sexual morph: Undetermined. Asexual morph: on TWA Hyphae 3- to 5-μm wide, pale brown to brown, smooth, septate, branched. Conidiophores (225–)260–400(–590) × 6–7 µm ( ± SD = 330 ± 70 × 6.5 ± 0.5 μm, n = 50), mononematous, semi- to macronematous, arising singly or in groups, unbranched, straight to flexuous, septate, geniculate, pale brown to brown, paler toward the apex, swollen at the base. Conidiogenous cells (8–)10–24(–30) × 5–8 μm ( ± SD = 16 ± 6 × 6.5 ± 1.5 μm, n = 50), smooth walled, mono- to polytretic, proliferating sympodially, integrated, terminal or intercalary, subcylindrical to slightly swollen, subhyaline or pale brown to brown. Conidia (32–)52–70(–93) × 11–15 µm ( ± SD = 61 ± 9 × 13 ± 2 μm, n = 50), brown to dark brown, smooth walled to slightly verruculose, straight to slightly curved, subcylindrical to fusoid, occasionally obclavate to clavate, tapering toward the rounded apex, median cells brown to dark brown, apical and basal cells paler than the median cells being subhyaline to pale brown, end cells often cut off by a thick dark septum, (3–)5–8(–10)-distoseptate, germinated mono- or bipolar; hila 2- to 4-μm wide, flat to slightly protuberant, thickened, and darkened. Stroma, chlamydospores, and microconidiation were not observed.
Culture characteristics: Colonies on PDA reaching 60 mm in diameter after 7 days at 25°C in the dark, circular, margin entire, olivaceous gray to olivaceous brown with white to gray aerial mycelia; reverse gray olivaceous to olivaceous black at the center, white at the margin. Colonies grow more slowly on MEA, reaching 40 mm in diameter, circular, with irregular margin, gray at the center, white at the margin, with floccose aerial mycelia; reverse brown. Colonies on CMA reaching 51 mm in diameter, hairy appearance, olivaceous gray, with sparse gray aerial mycelia; reverse olivaceous gray.
Materials examined: IRAN, West Azarbaijan Province, Khoy County, on infected culms of Scirpus acutus (Cyperaceae, Poales), 10 September 2020, A. Ahmadpour, living culture IRAN 3382C; ibid. on infected leaves of Sparganium erectum (Typhaceae, Poales), 20 September 2010, A. Ahmadpour, living culture BI 1 = IRAN 3385C; ibid. on infected leaves of Cladium mariscus (Cyperaceae, Poales), 20 September 2010, A. Ahmadpour, living culture BI 4 = IRAN 3386C; ibid. on infected leaves of Setaria sp. (Poaceae, Poales), 25 September 2013, A. Ahmadpour, living culture FCCUU 1002; West Azarbaijan Province, Miyandoab County, on infected leaves of Sorghum halepense (Poaceae, Poales), 10 July 2013, A. Ahmadpour/Z. Heidarian, living culture IRAN 3383C; ibid. on infected leaves of Arundo donax (Poaceae, Poales), 10 July 2013, A. Ahmadpour/Z. Heidarian, living culture FCCUU 1001; ibid. on infected leaves of Hordeum vulgare (Poaceae, Poales), 10 May 2014, A. Ahmadpour/Z. Heidarian, living culture FCCUU 1003.
Hosts: Arundo donax, Cladium mariscus, Hordeum vulgare, Scirpus acutus, Setaria sp., Sorghum halepense, and Sparganium erectum (Ahmadpour et al., 2012a; Farr et al., 2024; this study).
Distribution: Iran (Ahmadpour et al., 2012a; this study).
Notes: Bipolaris salkadehensis was originally reported from infected leaves of Sparganium erectum (Sparganiaceae) and Cladium mariscus (Cyperaceae) with brown oval to elliptical lesions based on morphological characteristics and molecular data obtained from ITS−rDNA sequences (Ahmadpour et al., 2012a). In this study, two additional genes, GAPDH and TEF1, were sequenced for the ex-type isolate (Bi 1 = IRAN 3385C) as well as for other isolates from various plant hosts (Table 1) and used in phylogenetic analyses. Bipolaris salkadehensis isolates clustered well in a separate lineage with 100% ML, 100% MP bootstrap, and 1.0 BI posterior probability values (Figure 1). This species is morphologically similar to B. cynodontis and B. setariae (Sivanesan, 1987; Ahmadpour et al., 2012a). However, the conidia of B. cynodontis are smaller in size (30–75 × 10–16 μm), have three to nine (commonly seven to eight) distoseptate, and without cut-off in end cells (Sivanesan, 1987; Ahmadpour et al., 2012a). The conidia of B. setariae are fusoid to navicular, pale to mid golden brown, 5–10 distoseptate, 45–100 (mostly 50–70) × 10–15 μm, without cut-off in end cells, and lighter than those of B. salkadehensis (Sivanesan, 1987; Ahmadpour et al., 2012a). Zibani et al. (2025) have reported that B. salkadehensis exhibits low virulence as a pathogen of corn in Algeria. To the best of our knowledge, Arundo donax, Hordeum vulgare, Scirpus acutus, Setaria sp., and Sorghum halepense are newly identified hosts for this species.
4 Discussion
Advancements in molecular biology and phylogenetics have revolutionized our understanding of Bipolaris taxonomy and phylogeny. Techniques, such as DNA sequencing and phylogenetic analysis have enabled the identification of cryptic species and elucidated evolutionary relationships within the genus (Manamgoda et al., 2012, 2014; Tan et al., 2016; Raza et al., 2019; Bhunjun et al., 2020; Ferdinandez et al., 2022). Nevertheless, challenges persist in defining species boundaries and integrating morphological and molecular data. The results of this study revealed the presence of seven previously undocumented Bipolaris species from Iran, representing significant additions to the fungal biodiversity of the region. Comprehensive morphological characteristics and molecular phylogenetic analyses distinguish these new species from known taxa within the Bipolaris genus. Moreover, the study reports new records of Bipolaris species (B. crotonis) and identifies additional plant hosts for B. salkadehensis, highlighting the ecological diversity and host specificity of these fungi in Iranian ecosystems. Understanding their distribution, host range, and pathogenicity is crucial for developing effective disease management strategies and safeguarding agricultural crops in Iran.
Numerous Bipolaris species exhibit similar morphological characteristics making identification based solely on morphology unreliable and often ambiguous. Factors, such as environmental conditions, host plants, substrate, and culture media, further influence the morphological characteristics of Bipolaris species (Sivanesan, 1987; Alcorn, 1988; Manamgoda et al., 2012, 2014, 2015; Tan et al., 2014, 2016; Marin-Felix et al., 2017a, b, 2020). Accurate identification and understanding of genetic and pathogenic variability are essential for developing effective control measures and breeding programs. Molecular analyses, particularly using ITS−rDNA, GAPDH, and TEF1 loci, have proven invaluable in addressing these challenges, offering more precise species delimitation within the Bipolaris genus (Berbee et al., 1999; Manamgoda et al., 2012, 2014, 2015; Tan et al., 2014, 2016; Marin-Felix et al., 2017a, b, 2020). While ITS−rDNA has limitations in distinguishing closely related species, GAPDH has been shown to be a more informative genetic marker and is recommended as a critical locus and supplementary barcode for accurately identifying closely related Bipolaris species (Manamgoda et al., 2012, 2014, 2015; Madrid et al., 2014; Tan et al., 2014, 2016; Marin-Felix et al., 2017a, b, 2020; Bhunjun et al., 2020). Comparative analyses of taxa have consistently demonstrated that the GAPDH gene region provides greater resolution, as indicated by multiple prior investigations (Berbee et al., 1999; Manamgoda et al., 2012, 2014, 2015; Madrid et al., 2014; Tan et al., 2014, 2016; Ferdinandez et al., 2022). In our study, B. persica and B. iranica exhibit overlapping morphological features complicating their differentiation. Therefore, molecular tools are essential for accurate differentiation among Bipolaris species.
The sexual morph of the fungus Bipolaris is rare in natural environments but has been observed under controlled laboratory conditions (Sivanesan, 1987; Manamgoda et al., 2011, 2014). The majority of Bipolaris species are heterothallic, with their sexual reproduction determined by mating-type idiomorphs, MAT1-1 and MAT1-2 (Turgeon, 1998; Yun et al., 1999; Lu et al., 2011). In heterothallic species, the presence of both mating types is necessary for the development of sexual structures. The only previously known homothallic species within the Bipolaris genus is B. luttrellii (synonym: Cochliobolus luttrellii), which contains both MAT1-1 and MAT1-2 idiomorphs (Turgeon, 1998; Yun et al., 1999; Lu et al., 2011). This study identifies B. hedjaroudei as another homothallic species capable of forming a sexual morph on TWA medium supplemented with host leaves (Figure 8) making it the second known homothallic species in the genus. MAT genes are particularly useful for studying the evolution of reproductive strategies and sexual mechanisms, as they appear to evolve faster than ITS−rDNA and GAPDH sequence regions (Turgeon, 1998). Additionally, due to their high interspecies variability and low intraspecies variability, MAT genes have been proposed as potential markers for defining species boundaries (Yun et al., 1999). Phylogenetic analyses of MAT genes, along with ITS−rDNA and GAPDH sequences, provide valuable insights into the evolutionary history of reproductive strategies (Turgeon, 1998). Yun et al. (1999) also developed a specific PCR assay for amplifying MAT idiomorphs using the TAIL–PCR technique. Further research should focus on identifying and characterizing the mating type idiomorphs in B. hedjaroudei and other newly identified species in this study.
Most of the newly identified Bipolaris species in this study, including Bipolaris avrinica, B. azarbaijanica, B. banihashemii, and B. hedjaroudei, were isolated from Setaria species. This plant genus is among the most significant weeds affecting field crops in Iran. Some species of Bipolaris (B. bicolor, B. setariae, and B. sorokiniana on Eleusine indica; B. euphorbiae on Euphorbia heterophylla; B. eleusines on Echinochloa crus-galli; B. sorghicola on Sorghum halepense; B. yamadae on various poaceous plants; and B. microstegii, B. panici-miliacei, and B. zeicola on Microstegium vimineum) are known to cause diseases in weeds. These fungi have shown potential for use as herbicides and have been validated as effective mycoherbicides in several studies (Figliola et al., 1988; Winder and Van Dyke, 1990; Kleczewski and Flory, 2010; Manamgoda et al., 2011; Zhang et al., 2014, 2022; Omar and Naqiuddin, 2020; Tan et al., 2022, 2024; Xiao et al., 2022; Khan et al., 2023). Additional research is needed to investigate the host range, pathogenicity, and potential applications of these newly discovered species as mycoherbicides on Setaria spp. and other plants within the Poaceae family. Recent studies suggest that certain phytopathogenic fungi can switch hosts and infect nearby plants (Rai and Agarkar, 2016). Weeds can serve as reservoirs for pathogens that threaten economically important crops. Additionally, environmental changes may drive certain fungi, previously considered mildly pathogenic, to evolve into more aggressive pathogens in new hosts (Manamgoda et al., 2011; Rai and Agarkar, 2016; Hernández-Restrepo et al., 2018). This study also revealed that B. iranica and B. salkadehensis have wide host ranges, being isolated from barley, wheat, and various weed species. Hence, the accurate identification of Bipolaris species associated with cereal crops and their weedy hosts is crucial for effective disease management and for ensuring stable crop production.
Understanding fungal biodiversity, such as Bipolaris species, is crucial for understanding diverse ecosystems globally. Exploring their diversity, distribution, and ecological functions enhances our understanding of plant health and ecosystem dynamics on a worldwide scale. This comprehensive study highlights the rich diversity of Bipolaris species and the complex relationships between fungi and their environments providing insights that are valuable for developing strategies to manage plant diseases and conserve fungal biodiversity in different regions.
5 Conclusions
This study analyzed 85 Bipolaris isolates collected from various hosts, in the Poales and Asparagales plant orders, across different locations in Iran between 2010 and 2022. Seven new species (B. avrinica, B. azarbaijanica, B. banihashemii, B. hedjaroudei, B. hemerocallidis, B. iranica, and B. persica) were discovered, along with two new records to Iran’s funga (B. crotonis and B. salkadehensis), identified through a combination of morphological characteristics and multi-locus phylogenetic analyses (ITS−rDNA, GAPDH, and TEF1). Investigating the diversity, distribution, and ecology of Bipolaris species in Iran is crucial for understanding their role in plant diseases, ecosystem interactions, and the development of effective disease management strategies in agriculture. This detailed study provides valuable insights into the diverse Bipolaris species in Iran paving the way for future research into plant diseases and fungal biodiversity in the country.
Data availability statement
The datasets presented in this study can be found in the online repository https://www.ncbi.nlm.nih.gov/genbank/ and the accession numbers are mentioned in Table 1.
Author contributions
AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition, Project administration, Resources, Supervision. ZH: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Methodology, Resources, Visualization. YG: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology. ZA: Investigation, Methodology, Software, Formal analysis, Writing – review & editing. FA: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. DM: Data curation, Validation, Writing – review & editing, Formal analysis, Visualization. JK: Data curation, Writing – review & editing, Formal analysis. SK: Data curation, Writing – review & editing, Formal analysis, Funding acquisition, Visualization. PR: Data curation, Writing – review & editing, Formal analysis. NS: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by Iran’s National Elite Foundation (Grant No: 15/80035), the Research Deputy of Urmia University, and Chiang Mai University.
Acknowledgments
The authors thank Esmaeil Hashemlou (Department of Plant Protection, Faculty of Agriculture, Tehran University, Iran) for sampling assistance. SK thanks the National Natural Science Foundation of China (No. NSFC 32260004), the Yunnan Revitalization Talents Support Plan (Young Talents Program and High-End Foreign Experts Program), and the Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmadpour, A., Castell-Miller, C., Javan-Nikkhah, M., Naghavi, M. R., Dehkaei, F. P., Leng, Y., et al. (2018). Population structure, genetic diversity, and sexual state of the rice brown spot pathogen Bipolaris oryzae from three Asian countries. Plant Pathol. 67, 181–192. doi: 10.1111/ppa.12714
Ahmadpour, A., Donyadoost-Chelan, M., Heidarian, Z., Javan-Nikkhah, M. (2011). New species of Bipolaris and Curvularia on grass species in Iran. Rostaniha 12, 39–49. doi: 10.22092/botany.2011.101430
Ahmadpour, A., Ghosta, Y., Poursafar, A. (2021). Novel species of Alternaria section Nimbya from Iran as revealed by morphological and molecular data. Mycologia 113, 1073–1088. doi: 10.1080/00275514.2021.1923299
Ahmadpour, A., Heidarian, Z., Donyadoost-Chelan, M., Javan-Nikkhah, M., Tsukiboshi, T. (2012a). A new species of Bipolaris from Iran. Mycotaxon 120, 301–307. doi: 10.5248/120.301
Ahmadpour, A., Heidarian, Z., Karami, S., Pordel, A., Jabbarifar, S. M., Tsukiboshi, T., et al. (2013). New species of Bipolaris and Curvularia on poaceous plants in Iran (3). Rostaniha 14, 216–228. doi: 10.22092/botany.2014.101288
Ahmadpour, A., Heidarian, Z., Karami, S., Tsukiboshi, T., Zhang, M., Javan-Nikkhah, M. (2012b). New species of Bipolaris and Curvularia on grass species in Iran. Rostaniha 13, 69–82. doi: 10.22092/botany.2012.101374
Ahmadpour, A., Javan-Nikkhah, M., Naghavi, M. R., Dehkaei, F. P. (2014). Morphological and phylogenetic investigation of Bipolaris oryzae and some species of Bipolaris obtained from rice and grass weeds. Iran. J. Plant Pathol. 50, 123–135.
Alcorn, J. L. (1988). The taxonomy of Helminthosporium species. Annu. Rev. Phytopathol. 26, 37–56. doi: 10.1146/annurev.py.26.090188.000345
Al Dughaishi, S., Maharachchikumbura, S. S., Al-Sadi, A. (2018). Bipolaris Omanensis, a novel saprobic species of Bipolaris from Oman based on morphology and sequence data. Phytotaxa 385, 23–30. doi: 10.11646/phytotaxa.385.1.3
Amaradasa, B. S., Madrid, H., Groenewald, J. Z., Crous, P. W., Amundsen, K. (2014). Porocercospora seminalis gen. et comb. nov. the causal organism of buffalograss false smut. Mycologia 106, 77–85. doi: 10.3852/13-147
Andrie, R. M., Schoch, C. L., Hedges, R., Spatafora, J. W., Ciuffetti, L. M. (2008). Homologs of ToxB, a host-selective toxin gene from Pyrenophora tritici-repentis, are present in the genome of sister-species Pyrenophora bromi and other members of the Ascomycota. Fungal Genet. Biol. 45, 363–377. doi: 10.1016/j.fgb.2007.10.014
Berbee, M. L., Pirseyedi, M., Hubbard, S. (1999). Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91, 964–977. doi: 10.1007/s13225-020-00446-6
Bhunjun, C. S., Dong, Y., Jayawardena, R. S., Jeewon, R., Phukhamsakda, C., Bundhun, D., et al. (2020). A polyphasic approach to delineate species in Bipolaris. Fungal Divers. 102, 225–256. doi: 10.1007/s13225-020-00446-6
Bruen, T. C., Philippe, H., Bryant, D. (2006). A simple and robust statistical test for detecting the presence of recombination. Genetics 172, 2665–2681. doi: 10.1534/genetics.105.048975
Crous, P. W., Gams, W., Stalpers, J. A., Robert, V., Stegehuis, G. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Stud. Mycol. 50, 19–22.
Crous, P. W., Wingfield, M. J., Guarro, J., Cheewangkoon, R., van der Bank, M., Swart, W. J., et al. (2013). Fungal Planet description sheets: 154–213. Persoonia 31, 188–296. doi: 10.3767/003158513X675925
da Cunha, K. C., Sutton, D. A., Fothergill, A. W., Cano, J., Gené, J., Madrid, H., et al. (2012). Diversity of Bipolaris species in clinical samples in the United States and their antifungal susceptibility profiles. J. Clin. Microbiol. 50, 4061–4066. doi: 10.1128/JCM.01965-12
Ershad, D. (2022). Fungi and fungal analogues of Iran (Iran: Ministry of Agriculture, Agricultural Research, Education and Extension Organization, Iranian Research Institute of Plant Protection).
Farr, D. F., Rossman, A. Y., Castlebury, L. A. (2024). United States National Fungus Collections Fungus-Host Dataset. Available online at: https://fungi.ars.usda.gov/ (Accessed October 20 2024).
Ferdinandez, H. S., Manamgoda, D. S., Udayanga, D., Deshappriya, N., Munasinghe, M. S., Castlebury, L. A. (2022). Molecular phylogeny and morphology reveal two new graminicolous species, Bipolaris adikaramae sp. nov and B. petchii sp. nov., with new records of fungi from cultivated rice and weedy grass hosts. Mycol. Prog. 21, 59. doi: 10.1007/s11557-022-01809-w
Figliola, S. S., Camper, N. D., Ridings, W. H. (1988). Potential biological control agents for goosegrass (Eleusine indica). Weed Sci. 36, 830–835. doi: 10.1002/ps.6742
Hernández-Restrepo, M., Madrid, H., Tan, Y. P., da Cunha, K. C., Gene, J., Guarro, J., et al. (2018). Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia 41, 71–108. doi: 10.3767/persoonia.2018.41.05
Huson, D. H., Bryant, D. (2006). Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267. doi: 10.1093/molbev/msj030
Jayawardena, R. S., Hyde, K. D., de Farias, A. R. G., Bhunjun, C. S., Ferdinandez, H. S., Manamgoda, D. S., et al. (2021). What is a species in fungal plant pathogens? Fungal Divers. 109, 239–266. doi: 10.1007/s13225-021-00484-8
Katoh, K., Rozewicki, J., Yamada, K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 108, 1–7. doi: 10.1093/bib/bbx108
Khan, N. A., Asaf, S., Ahmad, W., Jan, R., Bilal, S., Khan, I., et al. (2023). Diversity, lifestyle, genomics, and their functional role of Cochliobolus, Bipolaris, and Curvularia species in environmental remediation and plant growth promotion under biotic and abiotic stressors. J. Fungi. 9, 254. doi: 10.3390/jof9020254
Kleczewski, N. M., Flory, S. L. (2010). Leaf blight disease on the invasive grass Microstegium vimineum caused by a Bipolaris sp. Plant Dis. 94, 807–811. doi: 10.1094/PDIS-94-7-0807
Lane, B., Stricker, K. B., Adhikari, A., Ascunce, M. S., Clay, K., Flory, S. L., et al. (2020). Large-spored Drechslera gigantea is a Bipolaris species causing disease on the invasive grass Microstegium vimineum. Mycologia 112, 921–931. doi: 10.1080/00275514.2020.1781495
Lebeuf, R., Landry, J., Ammirati, J. F., Aronsen, A., Cantillo, T., Castillo, R., et al. (2023). Fungal systematics and evolution: FUSE 9. Sydowia 75, 313–377. doi: 10.12905/0380.sydowia75-2023-313
Lourenço, C. C., Alves, J. L., Guatimosim, E., Colman, A., Barreto, R. W. (2017). Bipolaris marantae sp. nov., a novel Helminthosporoid species causing foliage blight of the garden plant Maranta leuconeura in Brazil. Mycobiology 45, 123–128. doi: 10.5941/MYCO.2017.45.3.123
Lu, S. W., Yun, S. H., Lee, T., Turgeon, B. G. (2011). Altering sexual reproductive mode by interspecific exchange of MAT loci. Fungal Genet. Biol. 48, 714–724. doi: 10.1016/j.fgb.2011.04.006
Madrid, H., da Cunha, K. C., Gené, J., Dijksterhuis, J., Cano, J., Sutton, D. A., et al. (2014). Novel Curvularia species from clinical specimens. Persoonia 33, 48–60. doi: 10.3767/003158514X683538
Manamgoda, D. S., Cai, L., Bahkali, A. H., Chukeatirote, E., Hyde, K. D. (2011). Cochliobolus: an overview and current status of species. Fungal Divers. 51, 3–42. doi: 10.1007/s13225-011-0139-4
Manamgoda, D. S., Cai, L., Bahkali, A. H., Chukeatirote, E., Hyde, K. D. (2012). A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Divers. 12, 131–144. doi: 10.1007/s13225-012-0189-2
Manamgoda, D. S., Rossman, A. Y., Castlebury, L. A., Chukeatirote, E., Hyde, K. D. (2015). A taxonomic and phylogenetic re-appraisal of the genus Curvularia (Pleosporaceae): human and plant pathogens. Phytotaxa 212, 175–198. doi: 10.11646/phytotaxa.212.3.1
Manamgoda, D. S., Rossman, A. Y., Castlebury, L. A., Crous, P. W., Madrid, H., Chukeatirote, E., et al. (2014). The genus bipolaris. Stud. Mycol. 79, 221–288. doi: 10.1016/j.simyco.2014.10.002
Marin-Felix, Y., Groenewald, J. Z., Cai, L., Chen, Q., Marincowitz, S., Barnes, I., et al. (2017a). Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 86, 99–216. doi: 10.1016/j.simyco.2017.04.002
Marin-Felix, Y., Hernández-Restrepo, M., Crous, P. W. (2020). Multi-locus phylogeny of the genus Curvularia and description of ten new species. Mycol. Prog. 19, 559–588. doi: 10.1007/s11557-020-01576-6
Marin-Felix, Y., Senwanna, C., Cheewangkoon, R., Crous, P. W. (2017b). New species and records of Bipolaris and Curvularia from Thailand. Mycosphere 8, 1556–1574. doi: 10.5943/mycosphere/8/9/11
Miller, M. A., Pfeiffer, W., Schwartz, T. (2012). The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources (Paper presented at: Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the extreme to the campus and beyond (ACM). 41: 1-8. doi: 10.1145/2335755.2335836
Nylander, J. A. A. (2004). MrModeltest v2.0. Program distributed by the author. Uppsala Sweden: Evol. Biol. Centre. 1-2. doi: 10.4236/bio.2004.48074
Omar, Z. R., Naqiuddin, M. (2020). Bipolaris sorokiniana: A potential indigenous plant pathogen to control goosegrass (Eleusine indica) in oil palm plantations. J. Oil Palm Res. 32, 219–227. doi: 10.21894/jopr.2020.0018
Prasher, I., Kumar, V. (2021). Diversity of endophytic fungi associated with Dillenia indica L, an ethnomedicinal plant. Curr. Res. Environ. Appl. Mycol. 11, 532–559. doi: 10.5943/cream/11/1/35
Quaedvlieg, W., Binder, M., Groenewald, J. Z., Summerell, B. A., Carnegie, A. J., Burgess, T. I., et al. (2014). Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 33, 1–40. doi: 10.3767/003158514X681981
Rai, M., Agarkar, G. (2016). Plant–fungal interactions: what triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 42, 428–438. doi: 10.3109/1040841X.2014.958052
Rambaut, A. (2019). FigTree, a graphical viewer of phylogenetic trees. Available online at: http://tree.bio.ed.ac.uk/software/figtree (Accessed 20 October 2024).
Rathnayaka, A. R., Tennakoon, D. S., Jones, G. E., Wanasinghe, D. N., Bhat, D. J., Priyashantha, A. H., et al. (2024). Significance of precise documentation of hosts and geospatial data of fungal collections, with an emphasis on plant-associated fungi. N. Z. J. Bot. 31, 1–28. doi: 10.1080/0028825X.2024.2381734
Raza, M., Zhang, Z. F., Hyde, K. D., Diao, Y. Z., Cai, L. (2019). Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Divers. 99, 1–104. doi: 10.1007/s13225-019-00434-5
Rehner, S. A., Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF-1a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97, 84–98. doi: 10.3852/mycologia.97.1.84
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Rossman, A. Y., Manamgoda, D. S., Hyde, K. D. (2013). Proposal to conserve the name Bipolaris against Cochliobolus (Ascomycota: Pleosporales: Pleosporaceae). Taxon 62, 1331–1332. doi: 10.12705/626.21
Sharma, B., Nonzom, S. (2021). New record of Bipolaris cynodontis: an emerging human pathogen causing superficial mycosis in North India. Skin Appendage Disord. 7, 292–297. doi: 10.1159/000513339
Shoemaker, R. A. (1959). Nomenclature of Drechslera and Bipolaris, grass parasites segregated from Helminthosporium. Canad. J. Bot. 37, 879–887. doi: 10.1080/07060660609507377
Sivanesan, A. (1987). Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol. Pap. 158, 1–261. doi: 10.2307/3759472
Sivanesan, A. (1985). New species of Bipolaris. Trans. Br. Mycol. Soc 84, 403–421. doi: 10.1016/S0007-1536(85)80003-6
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Swofford, D. L. (2002). PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4 (Sunderland, Massachusetts, USA: Sinauer Associates).
Tan, Y. P., Crous, P. W., Shivas, R. G. (2016). Eight novel Bipolaris species identified from John L. Alcorn’s collections at the Queensland Plant Pathology Herbarium (BRIP). Mycol. Prog. 15, 1203–1214. doi: 10.1007/s11557-016-1240-6
Tan, M., Ding, Y., Bourdôt, G. W., Qiang, S. (2024). Evaluation of Bipolaris yamadae as a bioherbicidal agent against grass weeds in arable crops. Pest Manage. Sci. 80, 166–175. doi: 10.1002/ps.7630
Tan, M., Ding, R., Huang, Q., Qiang, S. (2022). Evaluation of Bipolaris panici-miliacei as a bioherbicide against Microstegium vimineum. Biocontrol Sci. Technol. 32, 178–195. doi: 10.1080/09583157.2021.1977240
Tan, Y. P., Madrid, H., Crous, P. W., Shivas, R. G. (2014). Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australas. Plant Dis. 43, 589–603. doi: 10.1007/s13313-014-0315-6
Turgeon, B. G. (1998). Application of mating type gene technology to problems in fungal biology. Annu. Rev. Phytopathol. 36, 115–137. doi: 10.1146/annurev.phyto.36.1.115
Vu, D., Groenewald, M., De Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., et al. (2019). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 92, 135–154. doi: 10.1016/j.simyco.2018.05.001
Wang, L., Al-Hatmi, A. M., Lai, X., Peng, L., Yang, C., Lai, H., et al. (2016). Bipolaris oryzae, a novel fungal opportunist causing keratitis. Diagn. Microbiol. Infect. Dis. 85, 61–65. doi: 10.1016/j.diagmicrobio.2015.11.020
White, T., Bruns, T., Lee, S., Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols: a guide to methods and applications. Eds. Innis, M., Gelfand, D., Shinsky, J., White, T. (Academic Press, New York), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Winder, R. S., Van Dyke, C. G. (1990). The pathogenicity, virulence, and biocontrol potential of two Bipolaris species on johnsongrass (Sorghum halepense). Weed Sci. 38, 89–94. doi: 10.1017/S0043174500056162
Xiao, W., Li, J., Zhang, Y., Guo, Y., Fang, W., Valverde, B. E., et al. (2022). A fungal Bipolaris bicolor strain as a potential bioherbicide for goosegrass (Eleusine indica) control. Pest Manage. Sci. 78, 1251–1264. doi: 10.1002/ps.6742
Xu, K. G., Jiang, Y. M., Li, Y. K., Xu, Q. Q., Niu, J. S., Zhu, X. X., et al. (2018). Identification and pathogenicity of fungal pathogens causing black point in wheat on the North China Plain. Indian J. Microbiol. 58, 159–164. doi: 10.1007/s12088-018-0709-1
Yun, S. H., Berbee, M. L., Yoder, O. C., Turgeon, B. G. (1999). Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proc. Nat. Acad. Sci. 96, 5592–5597. doi: 10.1073/pnas.96.10.559
Zhang, J., Duan, G., Yang, S., Yu, L., Lu, Y., Tang, W., et al. (2022). Improved bioherbicidal efficacy of Bipolaris eleusines through herbicide addition on weed control in paddy rice. Plants 11, 2659. doi: 10.3390/plants11192659
Zhang, J., Li, M. (2009). A new species of Bipolaris from the halophyte Sesuvium portulacastrum in Guangdong Province, China. Mycotaxon 109, 289–300. doi: 10.5248/109.289
Zhang, Q., Meng, Y., Zhao, W., Wang, Q., Wang, X., Xue, L., et al. (2024). Bipolaris fujianensis sp. nov., an emerging pathogen of sapling shoot blight on Chinese Fir, and its sensitivity to fungicides. Plant Dis. 108, 1025–1032. doi: 10.1094/PDIS-07-23-1254-RE
Zhang, J., Peng, G., Duan, G., Zhou, Y., Yang, S., Yu, L. (2014). Bipolaris eleusines, a potential mycoherbicide candidate for control of barnyardgrass (Echinochloa crus-galli). Biocontrol Sci. Technol. 24, 839–846. doi: 10.1080/09583157.2014.891724
Keywords: helminthosporioid fungi, morphology, seven novel species, phylogeny, Pleosporaceae, taxonomy
Citation: Ahmadpour A, Heidarian Z, Ghosta Y, Alavi Z, Alavi F, Manamgoda DS, Kumla J, Karunarathna SC, Rampelotto PH and Suwannarach N (2025) Morphological and phylogenetic analyses of Bipolaris species associated with Poales and Asparagales host plants in Iran. Front. Cell. Infect. Microbiol. 15:1520125. doi: 10.3389/fcimb.2025.1520125
Received: 30 October 2024; Accepted: 05 February 2025;
Published: 18 March 2025.
Edited by:
Sinang Hongsanan, Shenzhen University, ChinaReviewed by:
Sudhir Navathe, Agharkar Research Institute, IndiaKhalid Hameed, Duke University, United States
Copyright © 2025 Ahmadpour, Heidarian, Ghosta, Alavi, Alavi, Manamgoda, Kumla, Karunarathna, Rampelotto and Suwannarach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdollah Ahmadpour, YS5haG1hZHBvdXJAdXJtaWEuYWMuaXI=; Nakarin Suwannarach, c3V3YW5fNDYyQGhvdG1haWwuY29t
†ORCID: Abdollah Ahmadpour, orcid.org/0000-0002-4697-2230
Zeinab Heidarian, orcid.org/0000-0002-1775-9027
Youbert Ghosta, orcid.org/0000-0003-4038-2448
Zahra Alavi, orcid.org/0000-0002-6990-9795
Fatemeh Alavi, orcid.org/0000-0003-3655-2264
Dimuthu S. Manamgoda, orcid.org/0000-0002-1936-8556
Jaturong Kumla, orcid.org/0000-0002-3673-6541
Samantha C. Karunarathna, orcid.org/0000-0001-7080-0781
Pabulo Henrique Rampelotto, orcid.org/0000-0002-8992-9697
Nakarin Suwannarach, orcid.org/0000-0002-2653-1913
 Abdollah Ahmadpour
Abdollah Ahmadpour Zeinab Heidarian
Zeinab Heidarian Youbert Ghosta
Youbert Ghosta Zahra Alavi
Zahra Alavi Fatemeh Alavi
Fatemeh Alavi Dimuthu S. Manamgoda3†
Dimuthu S. Manamgoda3† Jaturong Kumla
Jaturong Kumla Samantha C. Karunarathna
Samantha C. Karunarathna Pabulo Henrique Rampelotto
Pabulo Henrique Rampelotto Nakarin Suwannarach
Nakarin Suwannarach