- 1Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biology and Food Engineering, Qujing Normal University, Qujing, China
- 2Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 3Center of Excellence in Microbial Diversity and Sustainable Utilization, Chiang Mai University, Chiang Mai, Thailand
- 4The Key Laboratory for Silviculture and Conservation of the Ministry of Education, Beijing Forestry University, Beijing, China
- 5Engineering Research Center of Southwest Bio-Pharmaceutical Resources, Ministry of Education, Guizhou University, Guiyang, Guizhou, China
- 6The High Efficacy Application of Natural Medicinal Resources Engineering Centre of Guizhou Province (The Key Laboratory of Optimal Utilization of Natural Medicine Resources), School of Pharmaceutical Sciences, Guizhou Medical University, Gui’an, Guizhou, China
- 7Center of Excellence in Biotechnology Research, King Saud University, Riyadh, Saudi Arabia
- 8Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 9Tropical Microbiology Research Foundation, Colombo, Sri Lanka
Apple trees [Malus domestica Borkh. (Rosaceae)] are one of the important temperate fruit crops in China. In comparison to other temperate fruits, such as grapes and pears, fungal studies (in Yunnan) associated with M. domestica are fewer in number. In the present study, we investigated fungi associated with M. domestica in Qujing City, Yunnan Province, China. Samples were collected from apple gardens in different locations. Single spore isolation was carried out to isolate saprobic fungi, while the surface sterilization method was carried out to isolate endophytic fungi. Molecular analyses were carried out to determine the phylogenetic placement of the new collections. Based on the combined methods of morphology and phylogeny, Cytospora qujingensis sp. nov. and Hypoxylon malongense sp. nov. are introduced as novel saprobic and endophytic taxa, respectively. Moreover, Aureobasidium pullulans and Cytospora schulzeri are reported as new geological records from southwestern China. Allocryptovalsa castaneae is reported on M. domestica in China for the first time. The checklist of fungi associated with M. domestica in China is presented.
1 Introduction
Yunnan Province, located in the interior low-latitude plateau in southwestern China, features a complex geography and unique climate. These attributes, in conjunction with its favorable ecological condition, contribute to the high quality of fruit yields in the region (Dong et al., 2023). Yunnan has a diverse range of fruits, which hosts 287 fruit plant species belonging to 118 genera and 49 families from tropical to temperate species, ranking first in China (Zhao et al., 2018). According to the Department of Agriculture and Rural Affairs of Yunnan Province (2021) (accessed 01 April 2024), over 11 million tons of fruits (mostly blueberries, strawberries, raspberries, oranges, and apples) were harvested in 2020.
Apple is one of the four major fruits in the world, rich in minerals and vitamins, with high nutritional value (Sachini et al., 2020). However, large amounts of chemical fertilizers are applied to increase productivity. Extensive use of chemicals causes serious environmental impacts. Farmers are fond of using fungicides since fruits are susceptible to diseases caused by fungal pathogens. Cytospora canker is one of the most widespread canker diseases of Malus domestica (Wang et al., 2024a). For example, 1) Cytospora mali causes severe necrosis of the branches and stems globally (Wang et al., 2007), and 2) Cytospora parasitica is reported to cause canker on apples in China (Ma et al., 2018).
Fungi are diverse in terms of shape, color, lifestyle, and distribution throughout any ecosystem (Bhunjun et al., 2022). Researchers estimated the diversity of fungal species ranging from half a million (May, 2000) to more than 12.5 million (Wu et al., 2019), even reaching 19.35 million (Tedersoo et al., 2021). Hitherto, Species Fungorum (2024) (accession date: 04 September 2024) lists all accepted species of fungi, currently 161,348 species, with only approximately 40,000 named fungal species in GenBank (Hawksworth and Lücking, 2017). An accurate estimate of the number of fungi will provide a better understanding of fungal diversity and biogeography (Hawksworth, 1991; Bhunjun et al., 2022). In traditional taxonomy, species amount was provided based on the host or morphological characters (sexual morph: ascomata, pseudoparaphyses, asci, ascospores; asexual morph: conidiomata, ostioles, conidiophores, conidiogenous cells, conidia). Meanwhile, with the application of DNA sequencing technologies, the reliability of introducing species based on morphological characters was questioned by taxonomists (Wijayawardene et al., 2021b). A vague “dark taxa” poses challenges to researchers in estimating fungal populations; therefore, it is more important to use different molecular approaches to accurately delineate species and to estimate species diversity (Cai et al., 2009; Wijayawardene et al., 2023), such as DNA barcoding (Seifert, 2009), environmental DNA (eDNA) metabarcoding (Tedersoo et al., 2014), metagenomics (Tedersoo et al., 2018), genome sequencing (Baldrian et al., 2022), and phylogenomic analysis (Wijayawardene et al., 2023). Estimating species numbers is critical for species conservation and ecosystem management since changes in fungal diversity can influence ecosystem performance (Genevieve et al., 2019). Our understanding of host specificity is unclear, particularly as new plant species are found and several habitats remain unexplored. Moreover, the knowledge about lifestyle switching between and within species remains unclear and thus needs extensive studies. Furthermore, the possibility of revealing novel fungal species and new host or country records creates more opportunities to discover new compounds, such as antimicrobial agents and enzymes (Suetrong et al., 2023). Researchers demonstrated the importance of revealing novel fungal species from different habitats and substrates, as well as reports of new host or country records. This allows us to enhance our understanding of lifestyle switching of one species in different hosts or the same host in different geographic regions and whether the same species produces new compounds to sustain an individual’s existence when it survives in different habitats, such as from the stratosphere (Wainwright et al., 2003) to the bottom of the Dead Sea (Oren and Gunde-Cimerman, 2012), from the Antarctic glaciers (Freeman et al., 2009) to torrid deserts (Gonçalves et al., 2016), and from the gut of flies (Blackwell, 2017) to deep oceanic sediments (Nagahama et al., 2011), and prevents the occurrence of fungal diseases. For example, 1) Alternaria alternata is a widespread fungus that is regularly isolated from plants as both endophyte and pathogen and in the soil as a saprophyte (Thomma, 2003; DeMers, 2022); and 2) Aureobasidium pullulans is one of the well-adapted saprophytes in the phyllosphere (McCormack et al., 1995; Dik and Elad, 1999) and postharvest diseases (Ippolito and Nigro, 2000) and also reported as an endophyte in symptomless plant organs (Loi et al., 2000).
In previous studies, more than 200 fungal species (including new species and known species) were reported on M. domestica from China (Supplementary Table S1). Alternaria spp. and Penicillium spp. are the most common pathogens associated with the diseases reported from apples (Basson et al., 2019). Cytospora is considered one of the most important pathogenic genera, which can cause canker and dieback disease in apple-producing areas in China, such as Gansu, Shanxi, Liaoning, and Yunnan provinces (Gui et al., 2015). During our field surveys in Qujing City (from January to September 2021), significant disease symptoms on fruits, leaves, and stems have not been observed. Hence, we investigated whether these plants harbor any taxa (endophytes or saprobes) that have been reported as pathogens in previous studies. It is well-established that some species could change their lifestyle due to other factors, such as nutritional requirements and host specificity (Rai and Agarkar, 2016). Furthermore, it was predicted that well-studied host plants in less-studied geographical regions could harbor more novel taxa (Wijayawardene et al., 2022a, b).
In the present study, based on the morphological characteristics and DNA sequence-based phylogenetic analyses, we introduce two novel species, i.e., Cytospora qujingensis sp. nov. (as a saprobe) and Hypoxylon malongense sp. nov. (as an endophyte), respectively. Aureobasidium pullulans (as an endophyte) and Allocryptovalsa castaneae and Cytospora schulzeri (as saprobes) are reported as new geographical or host records in southwestern China.
2 Materials and methods
2.1 Sampling and fungal isolation
Sampling was conducted during the fall season, using a random sampling method. Specifically, healthy and dead leaves and leaf litter of M. domestica were collected to assess fungal presence across different plant tissues from different apple orchards in Qujing City, Yunnan Province, China. The orchards under study were managed by local farmers who applied fungicides regularly as part of their standard pest and disease control practices. All the samples were stored in polythene bags and brought to the microbiology laboratory at Qujing Normal University.
Single spore isolation (for saprobes) was conducted as described by Dai et al. (2017). Stromata or conidiomata were sectioned by hand using a razor blade. The hymenium containing ascospores or conidia mass was removed and placed on a drop of sterile water on a flamed concave microscope slide and separated by sterile needles. The spore suspension was placed drop by drop on potato dextrose agar (PDA) plates, each containing a standard concentration of 39 g/L PDA, and incubated overnight in incubators at 28°C. The germinated spores were photographed and then transferred to a new PDA plate. Mycelium grew within 2 weeks, and the hyphal were transferred to three new PDA plates to get the pure culture for DNA extraction.
The surface sterilization method (for endophytes) was performed following Senanayake et al. (2020) but with a slight change. Plant leaves were cleaned with distilled water and then air-dried for 1 min. The leaf surface was sterilized by soaking in 75% ethanol for 30 s and then 1% sodium hypochlorite for 60 s. Finally, it was rinsed in sterile demineralized distilled water three times. Presterilized forceps were used to transfer the cleaned leaves onto the PDA after they had been chopped into fragments (approximately 5 mm × 5 mm) using a sterile scalpel blade on a sterile glass Petri dish under aseptic conditions within a laminar flow hood to minimize contamination. As for overnight growth in incubators at 28°C, the PDA plates were checked regularly, and individual colonies were transferred to a new PDA plate until pure strains were available for DNA extraction experiments.
2.2 Morphology and preserving
Morphological characteristics were observed and studied using a Leica DM1000 microscope and photographed by differential interference contrast (DIC). A Leica S8AP0 stereomicroscope with an HDMI 200C camera (Leica Corporation, Germany) was used for examining fruiting bodies. An Olympus BX53 compound microscope (Olympus Corporation, Japan) with differential interference contrast (Olympus BX53 DIC compound microscope with an Olympus DP74 camera, Japan) was used to observe and photograph the morphological characteristics. Colonies on PDA were observed and photographed using a Leica S8AP0 stereomicroscope with an HDMI 200C camera under appropriate lighting conditions to capture detailed morphological characteristics. A razor blade was used to obtain thin sections of stromata and conidiomata by hand.
Specimens were preserved at the Mycological Herbarium of Zhongkai University of Agriculture and Engineering (MHZU). Cultures were deposited at the Zhongkai University of Agriculture and Engineering (ZHKUCC). Duplicates of the specimens and type cultures were deposited at the Herbarium of Guizhou Medical University, Guiyang, China (GMB), and Guizhou Medical University Culture Collection (GMBCC) in Guiyang, China, respectively.
2.3 Registration of novel taxa
Index Fungorum Registration Identifiers were obtained from Index Fungorum (2024) (https://www.indexfungorum.org).
2.4 DNA extraction, PCR amplification, and sequencing
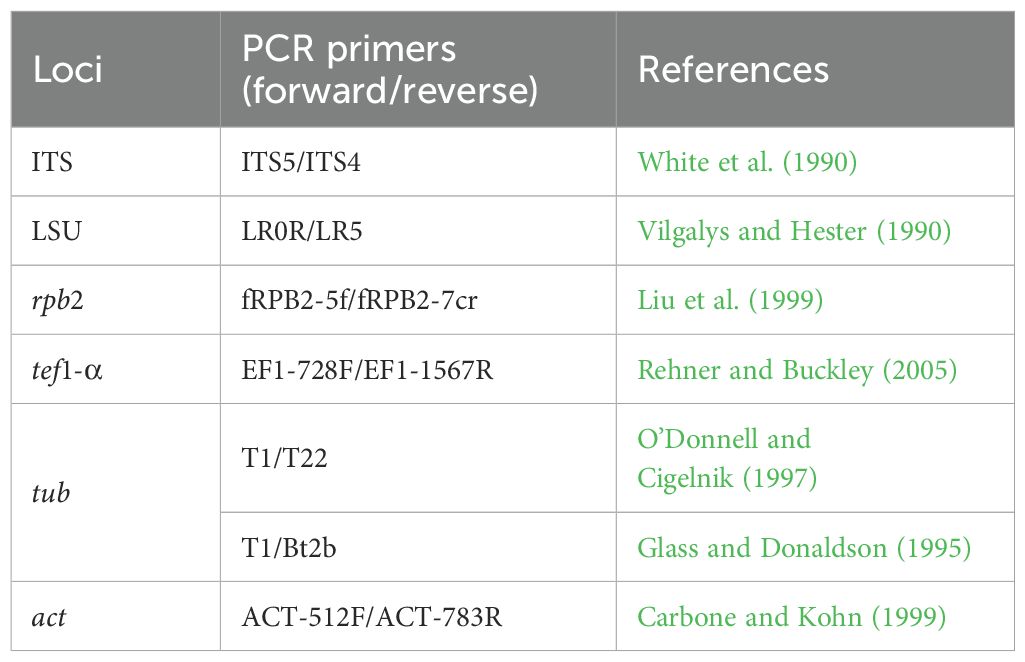
Fresh mycelium was cultured on PDA in incubators at 28°C overnight for 15 days. The genomic DNA was extracted from fresh cultures according to the specifications of the Biospin Fungal Genomic DNA Extraction Kit (bioflux®). Each gene region was separately amplified using both forward and reverse primers, which are provided in Table 1. A final volume of polymerase chain reaction (PCR) was prepared at 25 μL, including 1 μL of DNA template, 1 μL of each forward and reverse primer, 12.5 μL of 2× Taq PCR Master Mix, and 9.5 μL of double-distilled water (ddH2O) as described by Dai et al. (2017). The PCR thermal cycling procedure for amplifying ITS, LSU, and rpb2 was conducted as explained by Ma et al. (2022), while tef1-α, tub, and act genes were conducted as explained by Jia et al. (2024), respectively. The PCR products were obtained in Shanghai Majorbio Bio-Pharm Technology Co., Ltd., and BGI Tech Solutions Co., Ltd. (BGI-Tech, China) for sequencing.
2.5 Phylogenetic analyses
Closely related sequences were downloaded from GenBank based on blast similarity and recent publications (Fan et al., 2020; Maharachchikumbura et al., 2022; Wu et al., 2023; Zhang et al., 2024c) (Supplementary Tables S2–S5). Mafft v.7.215 (http://mafft.cbrc.jp/alignment/server/index.html) (Katoh and Standley, 2013) was used to align the single gene sequence, automatic cutting was done in Trimal.v1.2rev59, and the final improvements were performed in BioEdit v.7.0.5.2 (Hall, 2004) by hand. The combined gene regions of ITS, tef1-α, rpb2, tub, and act (Cytospora); ITS and tub (Allocryptovalsa); ITS and LSU (Aureobasidium); and ITS, LSU, rpb2, and tub (Hypoxylon) regions were performed in BioEdit v. 7.0.5.2 (Hall, 2004) manually. The combined datasets in FASTA format were converted to PHYLIP and NEXUS formats by using ALTER (Alignment Transformation Environment online, http://sing.ei.uvigo.es/ALTER/) (Glez-Peña et al., 2010). The online tool FindModel (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html) was used to determine the best nucleotide substitution model for each partition data (Dai et al., 2022). The phylogenetic trees were constructed by maximum likelihood (ML) and Bayesian analyses.
ML analysis was carried out via the online portal CIPRES Science Gateway v. 3.3 (Miller et al., 2010), using RAxML-HPC v.8 on XSEDE (8.2.12) tool, with the default settings but adapted with the GAMMA nucleotide substitution model and 1,000 rapid bootstrap replicates.
Bayesian analysis was carried out by MrBayes v. 3.0b4 (Ronquist and Huelsenbeck, 2003), and the model of evolution was estimated with MrModeltest v. 2.2 (Nylander, 2004). The posterior probabilities (PP) (Rannala and Yang, 1996; Zhaxybayeva and Gogarten, 2002) were determined by the following Markov chain Monte Carlo sampling (MCMC) in MrBayes v.3.0b4 (Huelsenbeck and Ronquist, 2001). Six simultaneous Markov chains were run for 1,000,000 generations, with trees sampled every 100th generation. The preburn was set to 0.25 and the run was automatically stopped when the mean standard deviation of the split frequency reached below 0.01 (Maharachchikumbura et al., 2015).
Trees were viewed by FigTree v. 1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/) (Rambaut, 2006), and the phylogram was edited by Microsoft Office PowerPoint 2016 (Microsoft Inc., Redmond, WA, USA) and converted to jpg. file by the Adobe PhotoShop CC 2018 software (Jiang et al., 2021).
3 Results and discussion
3.1 Phylogenetic analyses
3.1.1 Multigene analyses for Cytospora (Valsaceae, Diaporthales, and Sordariomycetes)
The combined gene regions of ITS, tef1-α, rpb2, tub, and act contained 300 isolates, which comprised 2,249 characters with gaps. Single gene analysis was carried out to compare the topology of the tree and clade stability. Diaporthe vaccinii (CBS 160.32) was used as the outgroup taxon. The best-scoring RAxML tree with a final likelihood value of −53,458.778097 is presented in Figure 1. The matrix had 1,755 distinct alignment patterns, with 23.31% of undetermined characters or gaps. The estimated base frequencies were as follows: A = 0.243243, C = 0.273232, G = 0.242722, and T = 0.229822; the substitution rates were AC = 1.503463, AG = 3.421602, AT = 1.349798, CG = 1.091741, CT = 6.308732, and GT = 1.000000; and the gamma distribution shape parameter alpha = 0.483398. The GTR+I+G model was selected as the best model based on MrModeltest and was used for the Bayesian analysis.
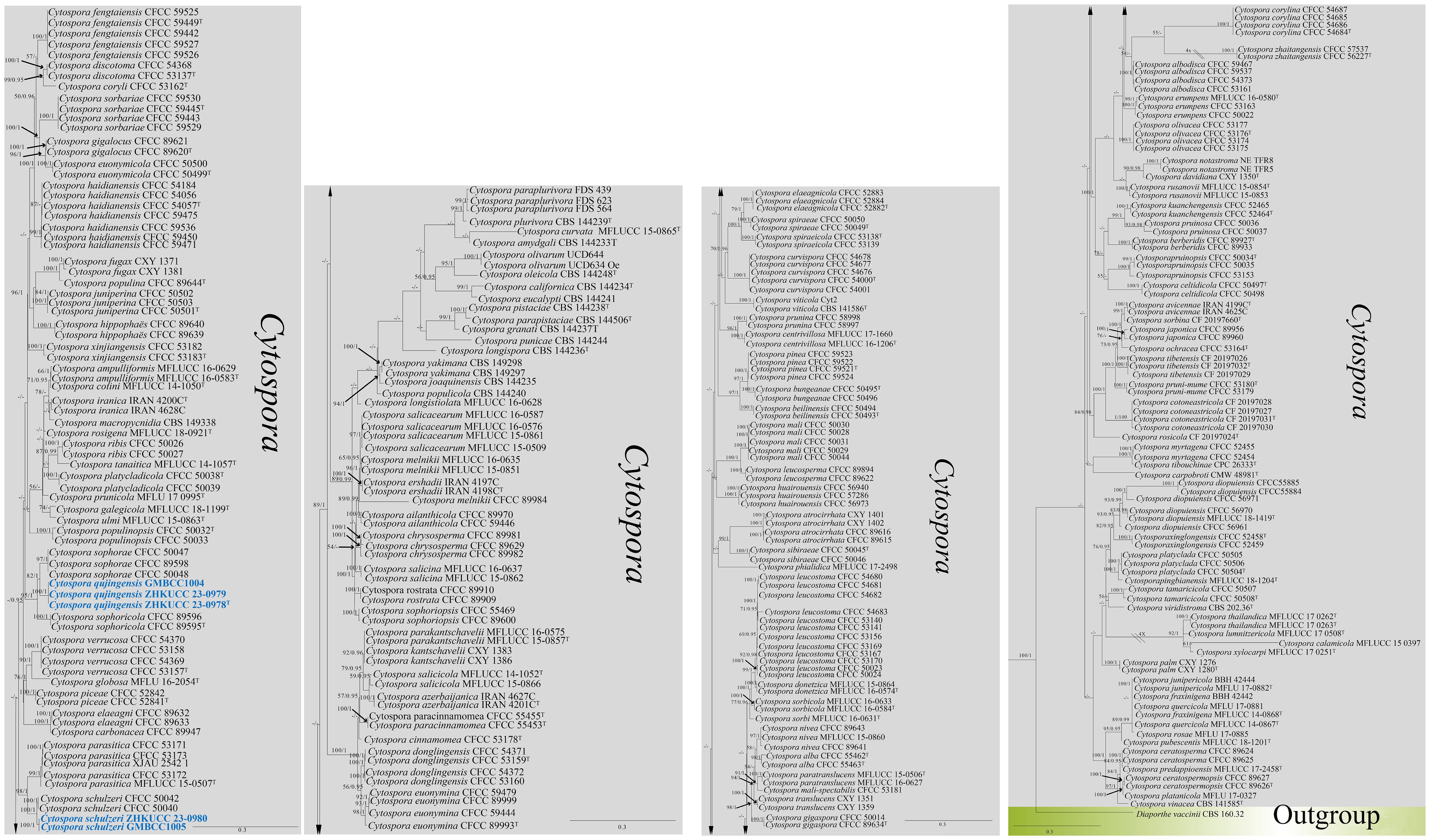
Figure 1. The phylogenetic tree from the best scoring of the RAxML analysis based on combined gene regions (ITS, tef1-α, rpb2, tub, and act) is rooted to Diaporthe vaccinii (CBS 160.32). Bootstrap values for maximum likelihood (MLBP) and Bayesian posterior probabilities (BYPP) equal to or greater than 50% and 0.95 are given at the respective branches. A hyphen (-) means a value lower than 50% (ML) or 0.95 (PP). New isolates are labeled in blue bold and ex-types are indicated in “T” (Supplementary Table S2).
In the phylogenetic analysis, our new isolates [ZHKUCC 23-0978 (ex-type), ZHKUCC 23-0979, and GMBCC1004] formed a monophyletic clade (82% ML, 1.00 PP) sister to Cytospora sophoricola [CFCC 89595 (ex-type) and CFCC 89596] and C. sophorae (CFCC 89598, CFCC 50048, and CFCC 50047). Hence, the taxon which is represented by ZHKUCC 23-0978, ZHKUCC 23-0979, and GMBCC1004 is introduced as a novel species in Cytospora s. str. as C. qujingensis (see taxonomy section for further details). Furthermore, two new strains, ZHKUCC 23-0980 and GMBCC1005, were grouped with C. schulzeri (CFCC 50040 and CFCC 50042) with high statistical values (100% ML, 1.00 PP). Based on phylogenetic analyses and morphological comparisons, we confirmed that these two strains represent C. schulzeri (see taxonomy section for morphological comparison).
3.1.2 Multigene analyses for Allocryptovalsa (Diatrypaceae, Xylariales, and Sordariomycetes)
The combined gene regions of ITS and tub contained 19 isolates, which comprised 1,221 characters with gaps. Single gene analysis was carried out to compare the topology of the tree and clade stability. Eutypella australiensis (STEU-8248) was used as the outgroup taxon. The best-scoring RAxML tree with a final likelihood value of 3,671.144837 is presented in Figure 2. The matrix had 287 distinct alignment patterns, with 25.59% of undetermined characters or gaps. The estimated base frequencies were as follows: A = 0.224076, C = 0.274997, G = 0.237980, and T = 0.262947; the substitution rates were AC = 1.266141, AG = 2.802018, AT = 1.131189, CG = 1.718083, CT = 4.262046, and GT = 1.000000; and the gamma distribution shape parameter alpha = 0.821838. The GTR+I+G model was selected as the best model based on MrModeltest and was used for the Bayesian analysis. In the phylogenetic tree, our two new strains of Allocryptovalsa (ZHKUCC 23-0981 and ZHKUCC 23-0982) were clustered with Allocryptovalsa castaneae [CFCC 52428 (ex-type) and CFCC 52427] with high statistical support (84% ML, 0.99 PP).
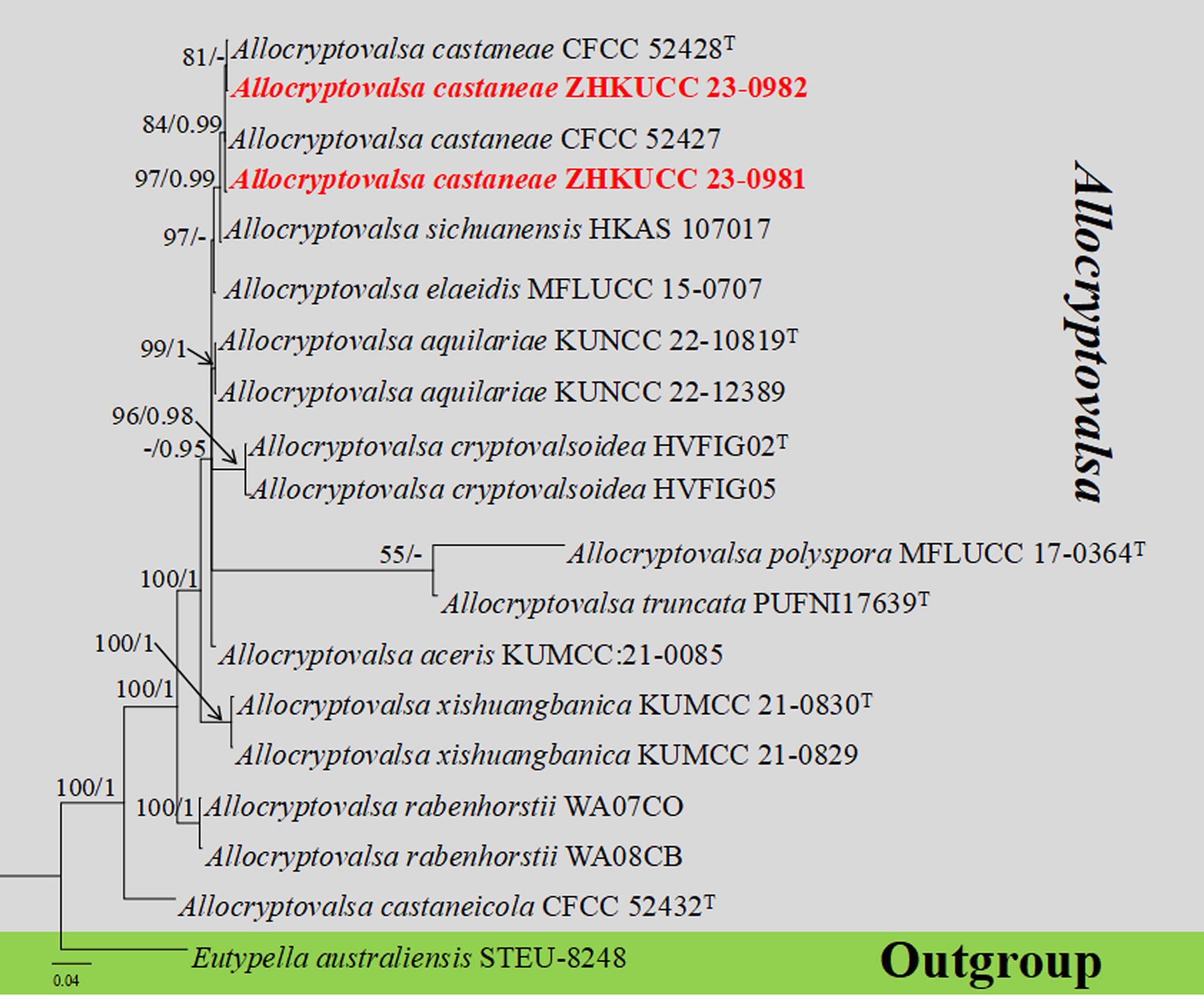
Figure 2. The phylogenetic tree from the best scoring of the RAxML analysis based on combined gene regions (ITS and tub) is rooted to Eutypella australiensis (STEU-8248). Bootstrap values for maximum likelihood (MLBP) and Bayesian posterior probabilities (BYPP) equal to or greater than 50% and 0.95 are given at the respective branches. A hyphen (-) means a value lower than 50% (ML) or 0.95 (PP). New isolates are labeled in red bold and ex-types are indicated in “T” (Supplementary Table S3).
3.1.3 Multigene analyses for Aureobasidium (Saccotheciaceae, Dothideales, and Dothideomycetes)
The combined gene regions of LSU and ITS contained 34 isolates, which comprised 1,410 characters with gaps. Single gene analysis was carried out to compare the topology of the tree and clade stability. Sydowia polyspora (CBS 750.71) was used as the outgroup taxon. The best-scoring RAxML tree with a final likelihood value of −6,098.421647 is presented in Figure 3. The matrix had 298 distinct alignment patterns, with 21.36% of undetermined characters or gaps. The estimated base frequencies were as follows: A = 0.256565, C = 0.222028, G = 0.279511, and T = 0.241896; the substitution rates were AC = 1.507336, AG = 2.554163, AT = 1.625168, CG = 1.056662, CT = 4.675945, and GT = 1.000000; and the gamma distribution shape parameter alpha = 2.840594. The GTR+I+G model was selected as the best model based on MrModeltest and was used for the Bayesian analysis. In the phylogenetic tree, two newly generated strains of Aureobasidium (ZHKUCC 23-0983 and GMBCC1006) were clustered in the clade that comprises Au. pullulans (CBS 584.75 and CBS 146.30), Au. proteae (CBS 114273 and CPC13701), and Au. microstictum (CBS342.66) with high statistical values (91% ML, 1.00 PP). The placement of the abovementioned species agrees with Humphries et al. (2017) and Wu et al. (2023), who performed their analysis based on LSU and ITS gene regions. Thus, we compared the conidial morphologies of the new collection against the other three species and confirmed that our collection belongs to Au. pullulans (see taxonomic key in the taxonomy section).
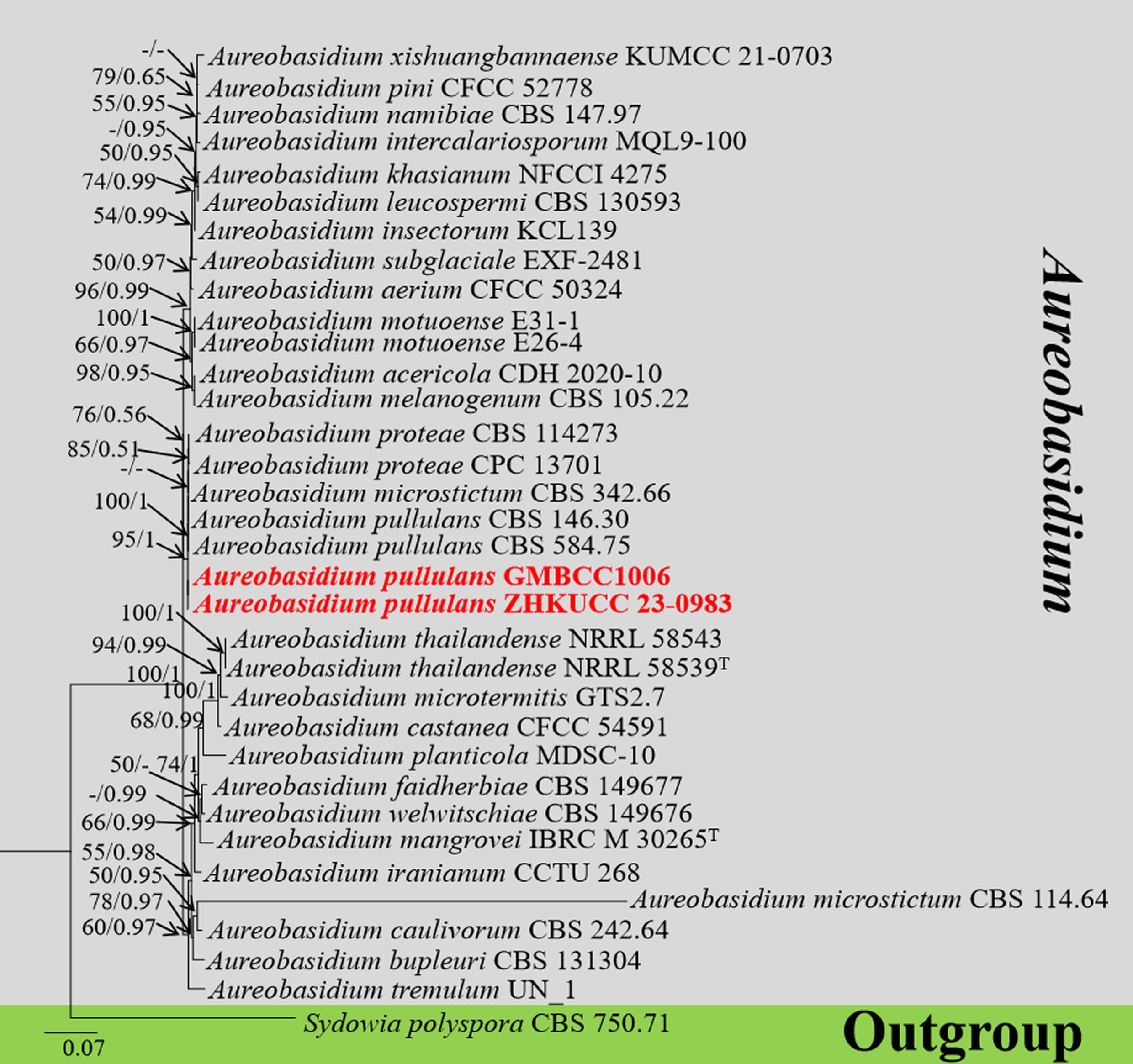
Figure 3. The phylogenetic tree from the best scoring of the RAxML analysis based on combined LSU and ITS is rooted to Sydowia polyspora (CBS 750.71). Bootstrap values for maximum likelihood (MLBP) and Bayesian posterior probabilities (BYPP) equal to or greater than 50% and 0.95 are given at the respective branches. A hyphen (-) means a value lower than 50% (ML) or 0.95 (PP). New isolates are labeled in red bold and ex-types are indicated in “T” (Supplementary Table S4).
3.1.4 Multigene analyses for Hypoxylon (Hypoxylaceae, Xylariales, and Sordariomycetes)
The combined gene regions of ITS, LSU, rpb2, and tub contained 150 strains in the sequence analysis, which comprise 3,728 characters with gaps. Single gene analysis was carried out and compared with each species, to compare the topology of the tree and clade stability. Xylaria arbuscula (CBS 126415), X. hypoxylon (CBS 122620), and Biscogniauxia nummularia (MUCL 51395) are set as the outgroup taxa. The best-scoring RAxML tree with a final likelihood value of −84,270.809729 is presented. The matrix had 2,144 distinct alignment patterns, with 33.59% of undetermined characters or gaps. The estimated base frequencies were as follows: A = 0.232029, C = 0.266223, G = 0.261601, and T = 0.240148; the substitution rates were AC = 1.022186, AG = 4.161361, AT = 1.213956, CG = 0.910957, CT = 5.597031, and GT = 1.000000; and the gamma distribution shape parameter alpha = 0.280100 (Figure 4). The GTR+I+G model was selected as the best model based on MrModeltest and was used for the Bayesian analysis. Overall tree topologies based on ML and Bayesian inference (BI) analyses were similar and not significantly different. In the phylogenetic analysis (Figure 4), our new collections [ZHKUCC 23-0984 (ex-type) and GMBCC1007] clustered in clade Hypoxylon and formed an independent lineage sister to Hypoxylon hinnuleum [MUCL 3621 (ex-type), DSM:107932, and DSM:107926] with relatively high statistical support (100% ML, 1.00 PP). Hence, we introduced H. malongense to accommodate our new collection from M. domestica (see taxonomy section for further details).

Figure 4. The phylogenetic tree from the best scoring of the RAxML analysis based on combined gene regions (ITS, LSU, rpb2, and tub) is rooted to Xylaria arbuscula (CBS 126415), X. hypoxylon (CBS 122620), and Biscogniauxia nummularia (MUCL 51395). Bootstrap values for maximum likelihood (MLBP) and Bayesian posterior probabilities (BYPP) equal to or greater than 50% and 0.95 are given at the respective branches. A hyphen (-) means a value lower than 50% (ML) or 0.95 (PP). New isolates are labeled in red bold and ex-types are indicated in “T” (Supplementary Table S5).
3.2 Taxonomy
Cytospora Ehrenb., Sylv. mycol. berol. (Berlin): 28
Index Fungorum Registration Identifier: 7904
Type species: Cytospora chrysosperma (Pers.) Fr., Syst. mycol. (Lundae) 2(2): 542
Cytospora Ehrenb. was typified by C. chrysosperma (Pers.) Fr, and the members were reported as important plant pathogens, saprobes, and endophytes on branches and twigs of a broad range of plants with a worldwide distribution (Fan et al., 2020). Currently, 696 epithets of Cytospora have been listed in Index Fungorum (2024) (accessed 23 June 2024), but many species lack herbarium materials, ex-type, and molecular data. The asexual morph of Cytospora is characterized by pycnidial locules, single or labyrinthine, conidiophores filamentous, conidia hyaline and allantoid; however, the sexual morph shows clavate to elongate obovoid asci, with four or eight hyaline, allantoid ascospores (Spielman, 1983, 1985). In the traditional taxonomy, host-based methods, such as morphological characteristics of the host and shape and size of conidia, were mainly used to define the species of Cytospora (Fan et al., 2020). However, in the past two decades, morphological identification and phylogenetic species recognition concepts have led to the description of several additional new species of Cytospora (Lawrence et al., 2017, 2018; Fan et al., 2020). In this study, we collected two Cytospora species from apples. Morpho-molecular analyses confirmed that one of them is a novel taxon of Cytospora; thus, we introduced our collections [(ZHKUCC 23-0978 (ex-type), ZHKUCC 23-0979, and GMBCC1004] as a novel species, viz., C. qujingensis and collections (ZHKUCC 23-0980 and GMBCC1005) were confirmed as the first record of C. schulzeri on M. domestica from Yunnan Province, southwestern China.
Cytospora qujingensis G.Q. Zhang, Wijayaw., & X.L. Fan, sp. nov.
Index Fungorum Registration Identifier: IF902662

Figure 5. Cytospora qujingensis (MHZU 23-0265, holotype). (A, B) Habit of stromata on a branch. (C) Transverse section through stroma. (D, E) Longitudinal section through stroma. (F–H) Asci in water. (I) Asci in in Melzer’s reagent shows “J-” apical apparatus. (J) Apical apparatus of an ascus. (K) Germinating ascospore. (L–O) Ascospores. (P) Colonies on PDA from above and below. Scale bars: (B) = 1 mm, (C–E) = 0.5 mm, (F–I) = 15 μm, (J–O) = 5 μm, and (P) = 5 cm.
Etymology: Named after the location “Qujing” where the new taxon was first discovered.
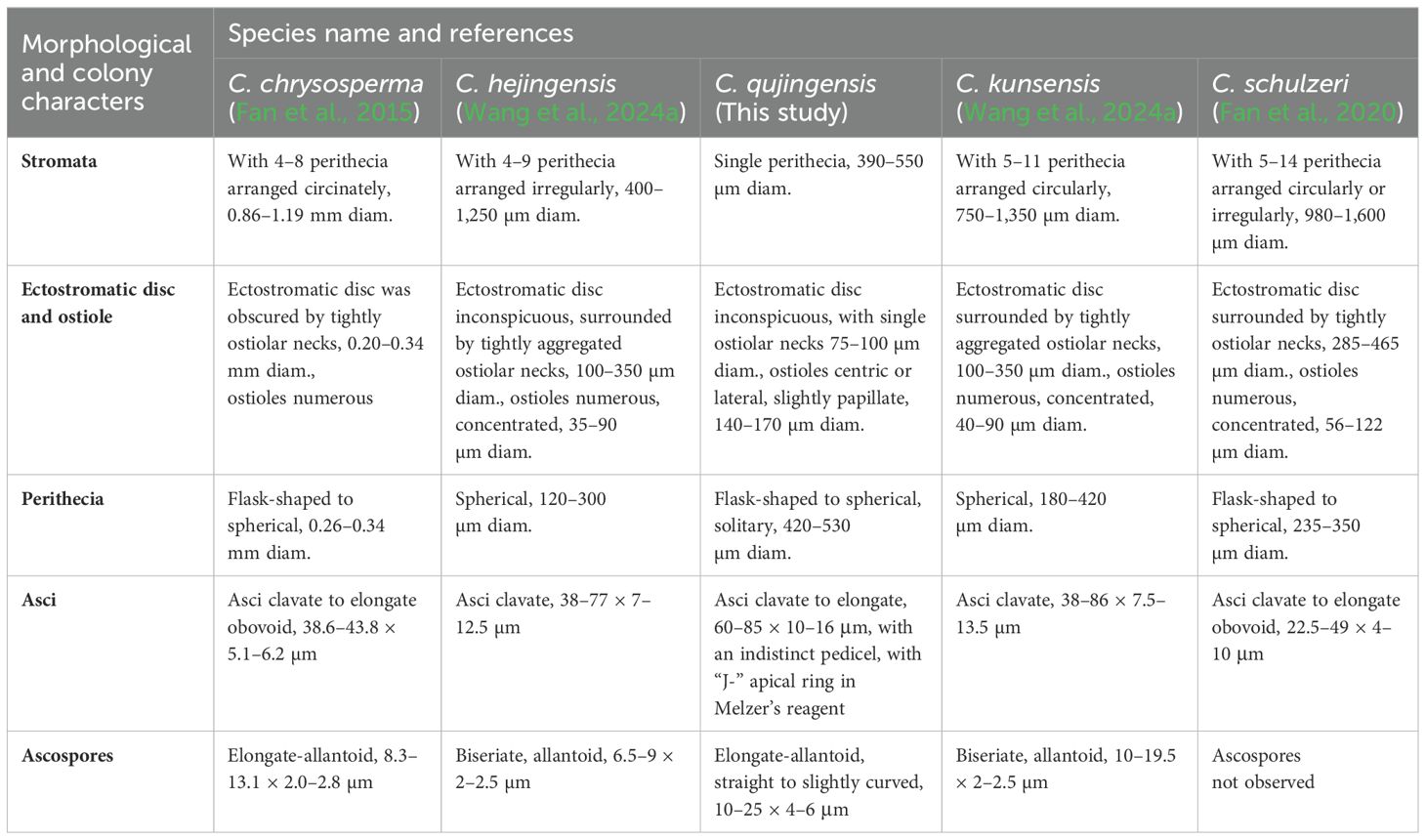
Diagnosis: Cytospora qujingensis differs from other Cytospora species by its asci with “J-” apical ring and larger size of asci (60–85 × 10–16 µm) and ascospores (10–25 × 4–6 µm).
Holotype: MHZU 23-0265
Description: Saprobic on branches of M. domestica in China. Sexual morph: Stromata 390–550 μm in length and 160–330 μm in width (av. = 450 × 230 μm, n = 30), circular to ovoid, brown to black, usually scattered, initially immersed in the bark, slightly to strongly erumpent through the surface of bark when mature. Conceptacle absent. Ectostromatic disc inconspicuous, usually with a single ostiolar neck, 75–100 µm diam. Ostioles 140–170 µm diam., centric or lateral, slightly papillate, dark brown to black. Perithecia 420–530 × 285–300 μm (av. = 470 × 290 μm, n = 5), solitary, flask-shaped to spherical, black, arranged circularly. Paraphyses may be lacking at maturity but usually present, often collapsed and broad. Asci, 60–85 × 10–16 μm (av. = 70 × 12.5 μm, n = 10), eight-spored, unitunicate, clavate to elongate, with an indistinct pedicel, apically rounded and thinned, “J-” apical ring in Melzer’s reagent, refractive, refractive truncated at the top. Ascospores 10–25 × 4–6 μm (av. = 15 × 4.5 μm, n = 25), biseriate to overlapping, or irregularly arranged, elongate-allantoid, straight to slightly curved, slightly constricted at both ends, normally rounded, hyaline, smooth, thin-walled. Asexual morph: not observed.
Culture characteristics: Ascospores germinating on PDA, producing germ tubes from both ends within 24 h. Colonies growing on PDA, reaching 6–9 cm diam. after 7 days at 28°C, white surface, growing up to 6 cm diam. with irregular margins, covering the 9-cm plate after 14 days. In reverse, the cultures are the same as the upper color after 3 days, becoming isabelline to umber after 7–14 days. Colonies are felty with a heterogeneous texture, lacking aerial mycelium.
Material examined: CHINA, Yunnan Province, Qujing City, 25°47′77″N, 62°34′96″E, on the dead branch of M. domestica, 1 October 2021, Guiqing Zhang, Z4 = MHZU 23-0265 (holotype), ex-type: ZHKUCC 23-0978; Ibid. Z4-1 = GMB1004 (isotype), ex-isotype: GMBCC 1004; Ibid. Z14 living culture: ZHKUCC 23-0979.
Notes: Cytospora qujingensis was collected as a saprobe on a dead branch of M. domestica. In the phylogenetic analysis, C. qujingensis grouped with C. sophorae and C. sophoricola (Figure 1), two asexually typified taxa (Fan et al., 2013). Among these two species, our new species shows a closer phylogenetic relationship with C. sophorae (CFCC 50047, CFCC 50048, and CFCC 89598), supported by 82% ML and 1.00 PP statistical support values (Figure 1). However, the representative strains of C. sophorae are not type strains but have been used in the phylogenetic analyses by Jia et al. (2024). The reference strains were isolated from Styphnolobium japonicum (Fabaceae) and Magnolia grandiflora (Magnoliaceae) in the Gansu and Shanxi provinces in China (Fan et al., 2020), whereas our new strains originate from M. domestica (Rosaceae) in Yunnan Province, southwestern China.
Comparative analysis of the base pairs of ITS, tef1-α, rpb2, tub, and act gene regions, our isolate [ZHKUCC 23-0978, (ex-type)] show significant differences with C. sophorae (CFCC 89598, CFCC 50048, and CFCC 50047): 3/489 bp (0.6%), 20/721 bp (2.8%), 23/260 bp (8.8%, including three gaps), 41/508 bp (8.1%, including six gaps), and 4/237 bp (1.7%), respectively. These phylogenetic incongruities, alongside ecological distinctions, form the basis for proposing that ZHKUCC 23-0978 (ex-type), ZHKUCC 23-0979, and GMBCC1004 belong to a new species, Cytospora qujingensis.
Morphologically, C. qujingensis shares similarities with type species C. chrysosperma, and C. schulzeri in terms of asci and ascospore characteristics. However, our isolates can distinguish C. chrysosperma and C. schulzeri (teleomorph Valsa malicola) by their remarkable features, which our isolates with a single perithecial stromata, asci with a “J-” apical ring, refractive, while C. chrysosperma characterized by 4–8 perithecia arranged circinately in black entostromata, and C. schulzeri with 5–14 perithecia, both are not noted as having an apical ring (Fan et al., 2015, 2020) (more details are shown in Table 2). Phylogenetically, both C. chrysosperma and C. schulzeri are not closely related to our new isolates. Recently, Wang et al. (2024a) introduced three novel species of Cytospora, viz. C. hejingensis R. Ma & Ning Jiang, C. kunsensis R. Ma & Ning Jiang, and C. jilongensis R. Ma & Ning Jiang, the first two of which are revealed for their sexual morph while the last one was reported as its asexual morph. However, these three species are not included in our phylogenetic tree. The phylogenetic tree in Wang et al. (2024a) shows that these three species are not closely related to our novel isolates and are accommodated in a separate clade. Furthermore, the morphology among them supported by our new isolates is different from them (Table 2). The inability to obtain the asexual morph of our new isolates under laboratory conditions is a limitation of our study. Future discovery of the sexual morphs of C. sophorae and C. sophoricola would provide insights into whether these species should be considered distinct or a single species. Until then, we recommend treating them as two distinct species.
Cytospora schulzeri Sacc. & P. Syd., Syll. fung. (Abellini) 14(2): 918
Index Fungorum Registration Identifier: 140461
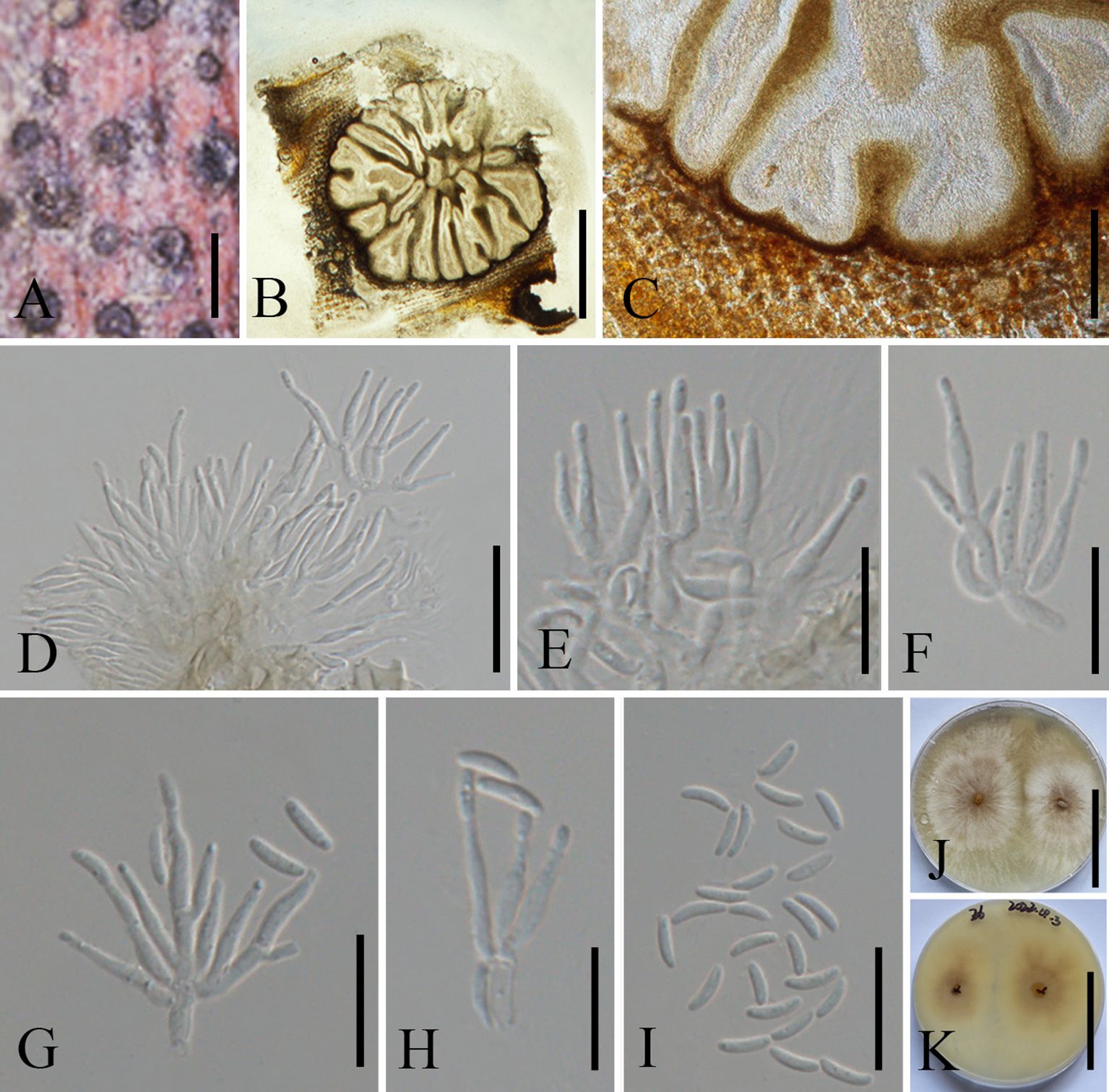
Figure 6. Cytospora schulzeri on a dead stem of Malus domestica (MHZU 23-0266). (A) Habit of conidiomata on a branch. (B) Transverse section through conidiomata. (C) Pycnidial wall. (D–H) Immature and mature conidia attached to conidiogenous cells. (I) Conidia. (J, K) Colonies on PDA from above and below (J: above, K: below). Scale bars: (A) = 300 μm, (B) = 2 mm, (C) = 100 μm, (D–H) = 15 μm, (I) = 10 μm, and (J, K) = 5 cm.
Description: Saprobic on a dead branch of M. domestica in China. Sexual morph: See Fan et al. (2020). Asexual morph: Pycnidial stromata ostiolate, scattered, immersed in bark, erumpent through the surface of bark, flat, discoid, with multiple locules. Conceptacle absent. Ectostromatic disc 250–450 µm diam., circular to ovoid, brown, with one to five ostioles per disc. Ostioles numerous, 55–105 μm diam., arranged circularly, black, at the same level as the disc. Locules numerous, 900–1550 µm diam., irregular, arranged circularly with common walls. Conidiomata wall comprising a few layers of cells of textura angularis, with innermost layer brown, outer layer brown to dark brown. Conidiophores 10–20 × 1.5–2.0 μm, hyaline, unbranched, filamentous, thin-walled. Conidiogenous cells 9–16 × 1–2 μm (av. = 13.5 × 1.5 μm, n = 20), enteroblastic polyphialidic. Conidia 4.5–7 × 1–2 μm (av. = 5.5 × 1.5 μm, n = 20), allantoid, aseptate, hyaline, smooth, thin-walled.
Culture characteristics: Conidia germinating on PDA, producing germ tubes from both ends within 24 h. Colonies fast growing, reaching up to 6 cm in diam. after 7 days and entirely covering the 9-cm plate after 14 days, centrally white and olivaceous gray at the margin, becoming olivaceous black at the center. Colonies are slightly fluffy, thin with a uniform texture; sterile.
Material examined: CHINA, Yunnan Province, Qujing City, 25°47′77″N, 62°34′96″E, on a dead branch of M. domestica, 1 October 2021, Guiqing Zhang, Z6 = MHZU 23-0266, living culture: ZHKUCC 23-0980; Ibid. Z6-1 = GMB1005, living culture: GMBCC1005.
Known host and distribution: Known on M. pumila from Hebei, Ningxia, and Gansu provinces, China (Fan et al., 2020); on Chestnut from Hebei, China (Jiang et al., 2020); on M. spectabilis from Tibet, China (Li et al., 2024a); and on M. domestica from Yunnan Province, China (this study).
Notes: Our second cytospora-like taxon morphologically resembles C. schulzeri (CFCC 50040 and CFCC 50042), and this was confirmed in the phylogenetic analysis (Figure 1, ZHKUCC 23-0980 and GMBCC1005). Cytospora schulzeri is a common pathogen that causes apple canker disease in China (Wei, 1979; Zhuang, 2005). Cytospora schulzeri was reported as a saprobe on M. pumila from Hebei, Ningxia, and Gansu provinces (Fan et al., 2020); as pathogens that caused canker disease in Castanea mollissima and branches of M. spectabilis from Hebei Province and Tibet, respectively (Jiang et al., 2020; Li et al., 2024a). In this study, samples were collected from branches of M. domestica and identified as a saprobic fungus. Hence, this is the first record of C. schulzeri on M. domestica from Yunnan Province, southwestern China.
Allocryptovalsa Senwanna, Phookamsak & K.D. Hyde, in Senwanna, Phookamsak, Doilom, Hyde & Cheewangkoon, Mycosphere 8(10): 1839
Index Fungorum Registration Identifier: 553857
Type species: Allocryptovalsa polyspora Senwanna, Phookamsak & K.D. Hyde, in Senwanna, Phookamsak, Doilom, Hyde & Cheewangkoon, Mycosphere 8(10): 1840
Allocryptovalsa Senwanna, Phookamsak & K.D. Hyde, typified with A. polyspora Senwanna, Phookamsak & K.D. Hyde. Zhu et al. (2021) regarded that the members of Allocryptovalsa show a cosmopolitan distribution (i.e., in Australia, China, Germany, India, Thailand, and the United States). Allocryptovalsa was originally introduced to accommodate two species, i.e., A. cryptovalsoidea and A. rabenhorstii (basionym: Valsa rabenhorstii Nitschke), which was characterized by immersed perithecia, polysporous asci, and allantoid ascospores (Senwanna et al., 2017). Subsequently, Konta et al. (2020) and Hyde et al. (2020) introduced A. elaeidis from Elaeis guineensis and A. truncata isolated from decaying twigs of unidentified plants, respectively. Zhu et al. (2021) introduced two new species within this genus, A. castaneae and A. castaneicola, which were isolated from C. mollissima in China. Hitherto, three additional new species have been reported in Allocryptovalsa. Allocryptovalsa xishuangbanica was discovered on dead branches collected from China (Maharachchikumbura et al., 2022); A. aceris was isolated on dead twigs of Acer palmatum (Aceraceae) from China (Senanayake et al., 2023); and A. aquilariae was isolated from dead twigs of Aquilaria sinensis from China (Chethana et al., 2023). In this study, we collected A. castaneae and it is the first host record in China.
Allocryptovalsa castaneae N. Jiang & X.L. Fan, Frontiers in Microbiology 12(no. 646262): 5
Index Fungorum Registration Identifier: 837777

Figure 7. Allocryptovalsa castaneaea (MHZU 23-0264). (A) Malus domestica branch. (B) Habit of stromata on a branch. (C) Transverse section through stroma. (D) Longitudinal section through the stroma. (E–H) Asci. (I) Ascospores. (J) Germinating ascospore. (K, L) Colonies on PDA from above and below [(K) above, (L) below]. Scale bars: (B) = 2 mm, (C) = 250 μm, (D) = 1.2 mm, (E–H) = 30 μm, (I, J) = 5 μm, and (K, L) = 4 cm.
Description: Saprobic on branches of M. domestica in China. Sexual morph: Stromata 2.5–3.5 mm diam., scattered to gregarious, immersed in the bark, erumpent through the surface of bark, with 8–13 perithecia arranged irregularly. Ectostromatic disc 0.2–0.6 mm diam., circular to oblong, brown, with more than eight ostioles arranged circularly per disc. Ostioles numerous, 100–250 µm diam., gregarious, umbilicate, 4-sulcate dark brown to black, at the same level as the disc. Perithecia 210–280 µm diam., outer surface coated with yellow, powdery entostroma, black, flask-shaped, perithecial necks erumpent in groups. Asci 120–220 × 12–24 µm (av. = 155 × 18 µm, n = 10), unitunicate, polysporous, clavate to elongate obovoid, long pedicellate, apically rounded, thin-walled. Ascospores 7.5–10.5 × 2.5–3.5 µm (av. = 9 × 3 µm, n = 20), aseptate, elongate-allantoid, slightly curved, pale yellowish to pale brown at maturity, smooth, thin-walled. Asexual morph: undetermined.
Culture characteristics: Colonies are initially white, uniform, becoming dark after 2 weeks.
Material examined: CHINA, Yunnan Province, Qujing City, 25°47′77″N, 62°34′96″E, 1 October 2021, on a dead branch of M. domestica, Guiqing Zhang, Z7 = MHZU 23-0264, living culture: ZHKUCC 23-0981; Ibid. Z1, living culture: ZHKUCC 23-0982.
Known host and distribution: Known on C. mollissima from Hebei Province and on Juglans regia in Yunnan Province, Chuxiong Yi Autonomous Prefecture (Zhu et al., 2021); M. domestica in Yunnan province, southwestern China (this study).
Notes: Allocryptovalsa castaneae is a taxon with a distinctive orange ectostromatic disc, and short pedicellate asci, which was described by Zhu et al. (2021). However, our new collection has black ectostromatic disc and slightly broader asci with a longer pedicel. Nevertheless, based on phylogenetic analysis (Figure 2), we confirmed that the new collections (i.e., ZHKUCC 23-0981 and ZHKUCC 23-0982) are A. castaneae. Allocryptovalsa castaneae has been known on C. mollissima in Hebei and on J. regia in Chuxiong Yi Autonomous Prefecture, Yunnan Province, China. Therefore, we report a new host record of A. castaneae on M. domestica from Qujing City, Yunnan, China.
Aureobasidium Viala & G. Boyer, Rev. gén. Bot. 3: 371
Index Fungorum Registration Identifier: 7297
Type species: Aureobasidium vitis Viala & G. Boyer, Rev. gén. Bot. 3: 371
Aureobasidium Viala & G. Boyer, typified with Au. vitis [current name: Au. pullulans (de Bary & Löwenthal) G. Arnaud]. Aureobasidium species are often called black yeast because they produce melanin during growth, and species of Aureobasidium with a yeast-like morph have a wide range of distribution and diverse life modes, including saprobes, endophytes, and pathogens (Lee et al., 2021; Wang et al., 2022). Recently, based on morphology characters, phylogenetic analysis, and biochemistry, more species of this genus have been introduced. So far, 66 epithets have been recorded in Index Fungorum (2024) (accessed 23 June 2024). Aureobasidium pullulans can produce pullulan polysaccharides, which are with the properties of water retention, barrier formation, regeneration, whitening, hydrating, and repairing, and it also serves as an ingredient in cosmetic formulas (Wu et al., 2023). In this study, we collected an Aureobasidium species from M. domestica. Morpho-molecular analyses confirmed that it is Au. pullulans.
Aureobasidium pullulans (de Bary & Löwenthal) G. Arnaud, Annals d’École National d’Agric. de Montpellier, Série 2 16(1-4): 39
Index Fungorum Registration Identifier: 101771
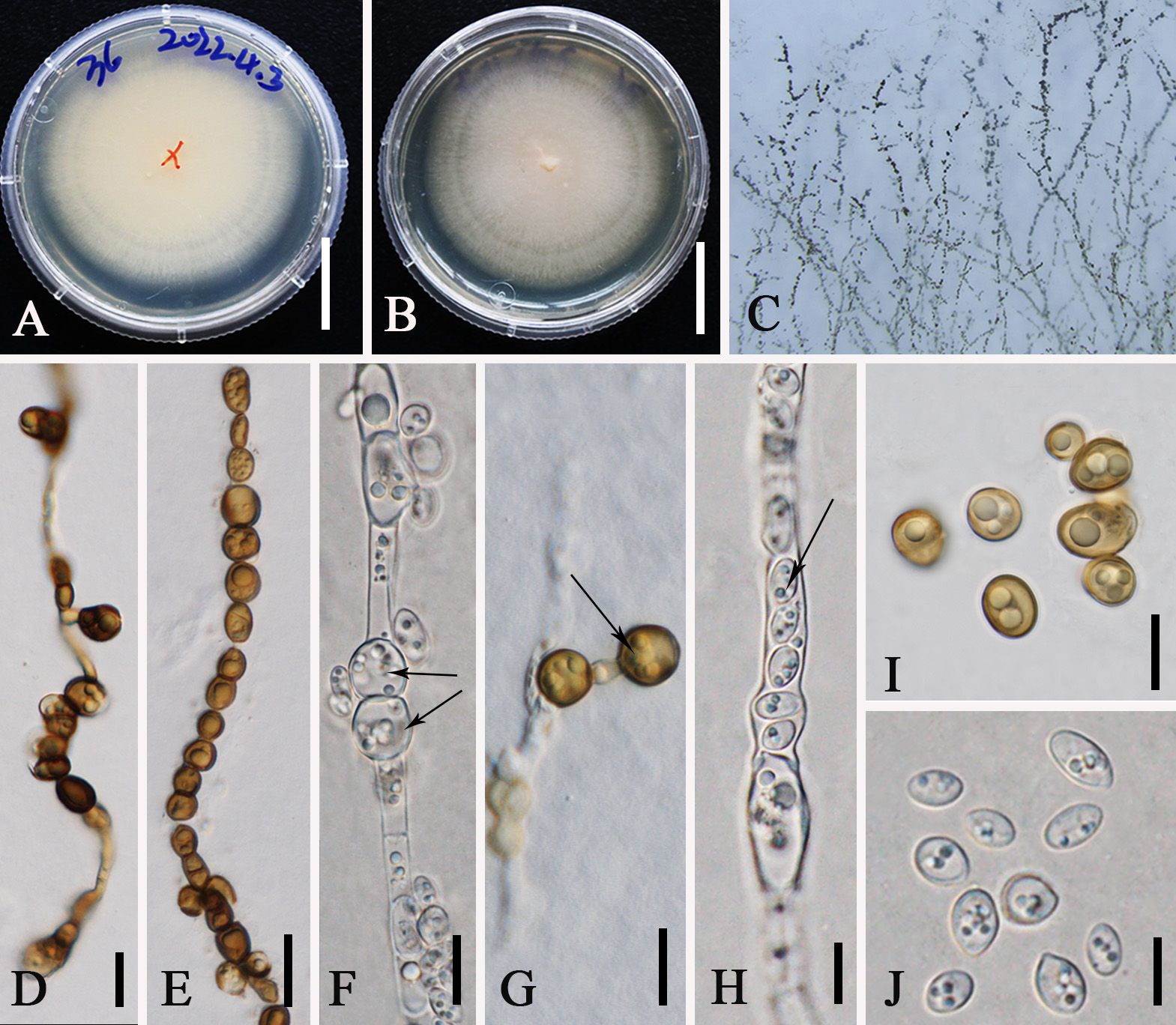
Figure 8. Aureobasidium pullulans (ZHKUCC 23-0983). (A, B) Colonies on PDA from above and below [(A) below, (B) above] (C) Melanized hyphae. (D) Melanized hyphae developing into arthrospores and chlamydospores. (E) Melanized hyphae/clamydospores. (F) Intercalary clamydospores. (G) Terminal clamydospores. (H) Endoconidia. (I) Chlamydospores. (J) Conida. Scale bars: (A, B) = 3 cm, (D, G) = 15 µm, (E) = 20 μm, and (F–J) = 10 μm.
Description: Endophytic in a leaf of M. domestica in China. Sexual morph: not observed. Asexual morph on PDA: Vegetative hyphae hyaline to brown, smooth to slightly roughened, thin-walled, 4–12 µm wide, constricted at septa, disarticulating to dark brown chlamydospores, 7–13 × 8–10 µm, intercalary or terminal. Conidiogenous cells undifferentiated, intercalary or terminal, or arising as short lateral branches on hyaline hyphae. Conidia 5−10 × 3−5 µm (av. = 8 × 4 μm, n = 20), one-celled, ellipsoidal and variable in shape and size, hyaline homogeneous to sectored, yeast-like to filamentous growth, smooth. Secondary conidia smaller. Endoconidia 5 × 3 µm, occasionally produced in an intercalary cell and released into a neighboring empty cell.
Culture characteristics: Colonies on PDA at 28°C attaining approximately 50 mm diam. after 7 days, appearing smooth and slimy due to abundant sporulation, pinkish, reverse pinkish. Initially white, uniform, becoming pink after 2 weeks. Hyphae are hyaline, smooth, thin-walled, with transverse septa.
Material examined: CHINA, Yunnan Province, Qujing City, Malus plantation, 25°47′77″N, 62°34′96″E, on the leaves of M. domestica, 1 October 2021, Guiqing Zhang, Z24, living culture: ZHKUCC 23-0983. Ibid. Z36, living culture: GMBCC1006
Known host and distribution: Known on M. domestica in Poland, Europe (Mirzwa-Mróz and Wińska-Krysiak, 2011); M. pumila in Canada (Ginns, 1986); Hylocereus polyrhizus and Hylocereus undatus (Taylor and Hyde, 2003; Ding et al., 2021); Pinus thunbergii (Jayawardena et al., 2018); Trachycarpus fortune (Wu et al., 2017); Vitis sp (Grabowski, 2007); Pinus in China (Ding et al., 2021); and M. domestica in Yunnan Province, China (this study).
Notes: In this study, Au. pullulans was isolated as an endophyte in the leaves of M. domestica. Previously, it was reported from the leaves of M. domestica which was grown at an experimental orchard in Germany (Rühmann et al., 2013). Moreover, it shows a broad range of geographical distribution from cold to warm climates and wet/humid regions to arid ones (Bozoudi and Tsaltas, 2018). Phylogenetically, our new collections (ZHKUCC 23-0983, GMBCC1006) clustered in the clade that comprises Au. pullulans, Au. proteae, and Au. microstictum. Morphologically, our collections closely resemble Au. pullulans, in having yeast-like colonies covered with a slimy mass of spores and same-size conidia [5–10 × 3–5 µm (av. = 8 × 4 µm, n = 20)] and possess chlamydospores and endoconidia. However, our collections can be distinguished from Au. proteae and Au. microstictum by the presence of chlamydospores and endoconidia (Yoshikawa and Yokoyama, 1987; Crous et al., 2011). Thus, we confirmed our new collections (ZHKUCC 23-0983 and GMBCC1006) to belong to Au. pullulans, and this is the first report of Au. pullulans from Yunnan, southwestern China.
Hypoxylon Bull., Hist. Champ. Fr. (Paris) 1(1): 168
Index Fungorum Registration Identifier: 2456
Type species: Hypoxylon coccineum Bull., Hist. Champ. Fr. (Paris) 1(1): 174
Species of Hypoxylon are often isolated as saprobes and endophytes of angiospermous plants (Halecker et al., 2020; Ma et al., 2022). The sexual morph of Hypoxylon is characterized by hemispherical, cushion-shaped stromata, immersed locules, periphysate ostiolate opening, eight-spored, uniseriate asci with an amyloid apical ring, one-celled ascospores (Rogers, 2018), while asexual morphs are characterized by different branching patterns of conidia, conidiogenous structure with virgariella-like branching patterns on natural substrate and artificial medium (Ju and Rogers, 1996). In this study, we collected a Hypoxylon species from apples. Morpho-molecular analyses confirmed that it is a novel taxon of Hypoxylon; thus, we introduce H. malongense.
Hypoxylon malongense G.Q. Zhang, Wijayaw., & Q.R. Li, sp. nov.
Index Fungorum Registration Identifier: IF902663
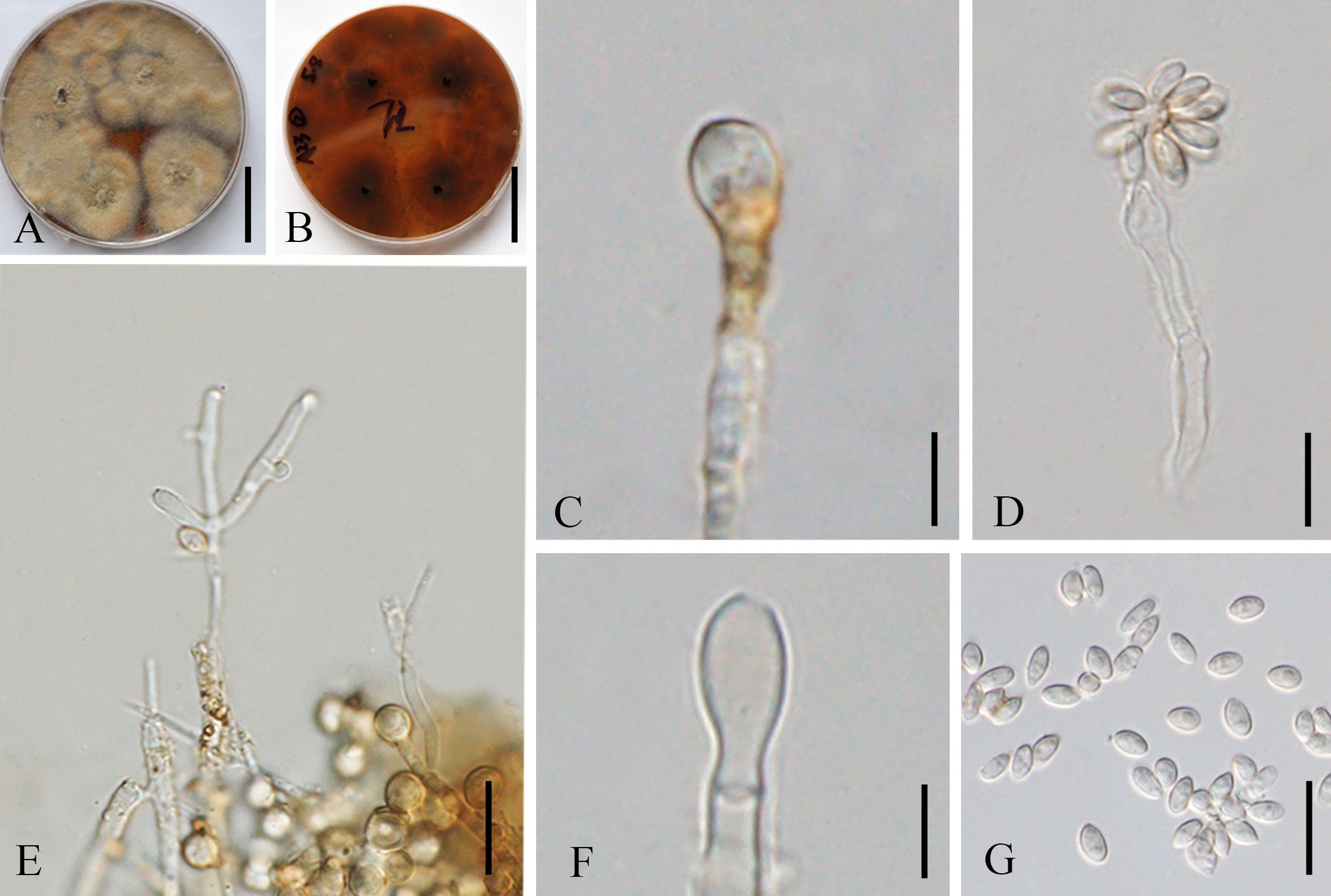
Figure 9. Hypoxylon malongense (ZHKUCC 23-0984, ex-type). (A, B) Colonies on PDA from above and below [(A) below, (B) above]. (C, E, F) Conidiophores and conidiogenous cells. (D) Anamorph from structure with nodulisporium-like branching patterns. (G) Conidia. Scale bars: (A, B) = 3 cm, (C) = 5 µm, (D, F, G) = 10 µm, and (E) = 15 µm.
Etymology: Named after the location “Malong” where the new taxon was first discovered.
Ex-type: ZHKUCC 23-0984
Description: Endophytic in the leaf of M. domestica in China. Sexual morph: not observed. Asexual morph on PDA: Conidiogenous structure on PDA with nodulisporium‐like branching patterns to unbranched. Conidiophores simple, septate, thin, sparingly branched or unbranched, straight to slightly curved, hyaline to yellow-brown, smooth walled to sometimes verruculose. Conidiogenous cells 9–19 × 2–4 μm (av. = 15 × 3 μm, n = 10), enteroblastic, phialidic, terminal or lateral, subcylindrical, straight or slightly curved, hyaline to pale yellow‐brown, smooth to finely verruculose, denticulate and protuberant conidiogenous loci, thickened. Conidia 4.1–7 × 2.0–3.0 μm (av. = 5.0 × 2.5 μm, n = 20), aseptate, rarely oblong, ellipsoidal to subglobose, apex obtuse, base truncate or bluntly rounded, straight or slightly curved, hyaline, smooth, mostly with minute guttules.
Culture characteristics: Colonies on PDA covering a 9-cm Petri dish after 3 weeks at 28°C, under 24 h dark, at first whitish becoming pale brown powdery, with scarce white mycelium that form rays toward the margins; yellowish-brown from below. Sporulating regions scattered over the entire surface of the colony.
Material examined: CHINA, Yunnan Province, Qujing City, M. domestica plantation, 25°47′77″N, 62°34′96″E, endophytic in the living leaves of M. domestica, 1 October 2021, Guiqing Zhang, Z60, ex-type: ZHKUCC 23-0984. Ibid. Z72, isotype: GMBCC1007.
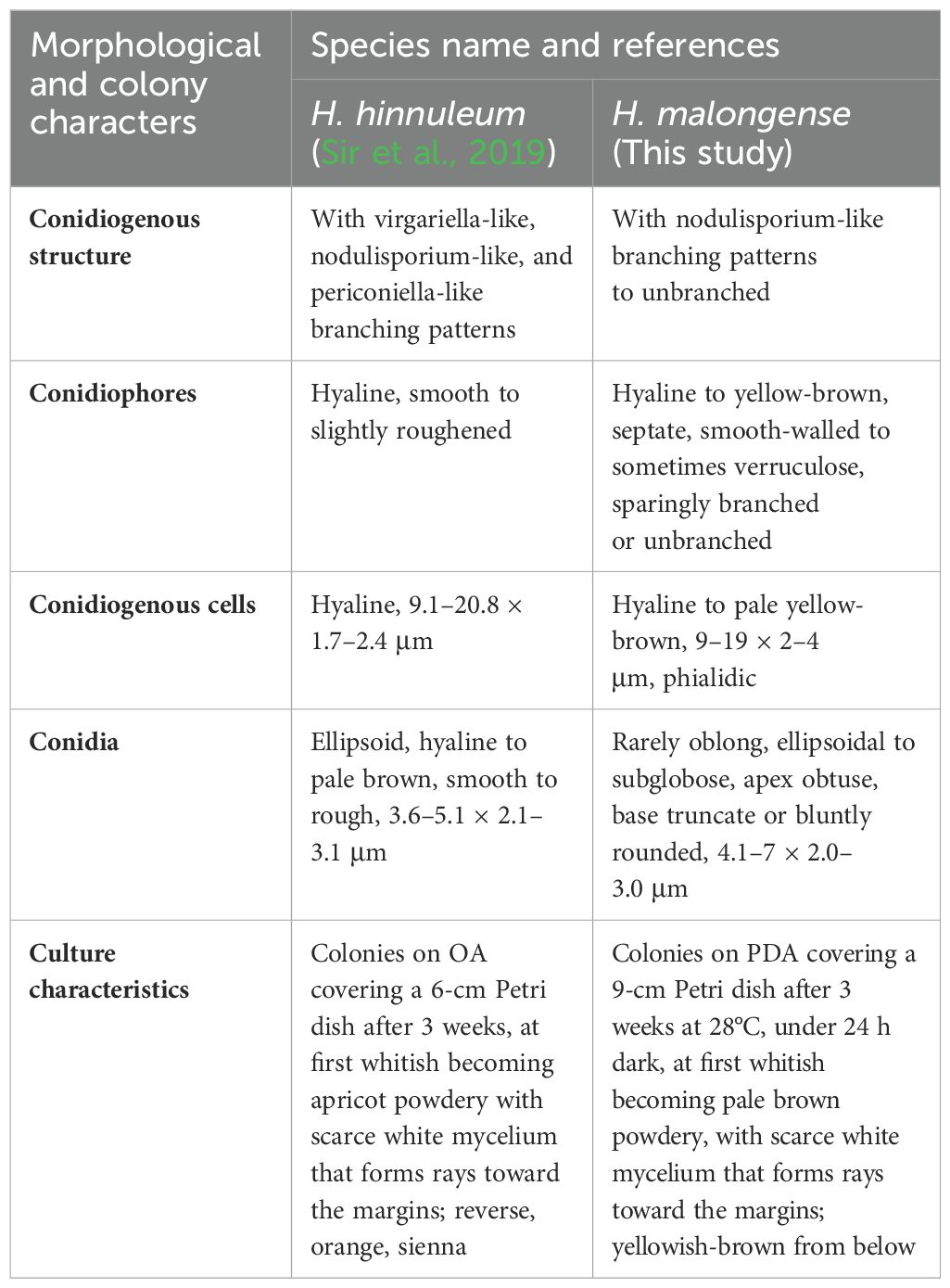
Notes: The multilocus phylogenetic analyses indicate that our new isolates H. malongense [ZHKUCC 23-0984 (ex-type) and GMBCC1007] form an independent lineage sister to H. hinnuleum [MUCL 3621 (ex-type), DSM:107932, and DSM:107926] with high statistical support (100% ML, 1.00 PP) (Figure 4). Morphologically, H. malongense can be distinguished from H. hinnuleum by its distinct differences in conidiogenous structure and conidia (Table 3). Our isolation is characterized by having nodulisporium-like branching patterns or unbranched conidiogenous structure, with ellipsoidal to subglobose conidia. However, H. hinnuleum is characterized by virgariella-like, nodulisporium-like, and periconiella-like branching patterns, with ellipsoid conidia. Therefore, more morphological differences between the new taxon and H. hinnuleum are listed in Table 3. Based on both morphology and phylogeny, we established this species as a novel taxon within Hypoxylon.
3.3 Discussion
3.3.1 Rich and underexplored fungal diversity in Yunnan
Yunnan Province in China is rich in fungal diversity, and annually, a significant number of species are introduced (e.g., Feng and Yang, 2018; Wijayawardene et al., 2021a, b; He and Zhao, 2022; Hongsanan et al., 2023; Du et al., 2023; Tian et al., 2024). These novel species have been reported from well-studied hosts (e.g., 1. from coffee fide Lu et al., 2022; 2. from bamboo fide Dai et al., 2022; Han et al., 2024a, b; 3. from Pará Rubber fide Xu et al., 2024; 4. from Macadamia fide Zhang et al., 2024a), well-studied microhabitats (e.g., freshwater fungi fide Shen et al., 2022; Li et al., 2024b), and unusual or understudied microhabitats (e.g., fungi from bats fide Liu et al., 2023; fungi from dead American bullfrog larvae fide Yang et al., 2023a). Furthermore, some species have been introduced from well-studied and complex genera, such as Colletotrichum, and confirmed the unexplored and rich fungal diversity in the region (e.g., Colletotrichum gardeniae Q. Zhang et al. fide Zhang et al., 2023).
Extensive exploration of fungal diversity in Qujing City, Yunnan Province has been carried out since 2019 and more than 16 novel fungal species have been introduced from different hosts in different studies (Yasanthika et al., 2020; Doilom et al., 2021; Monkai et al., 2021; Dissanayake et al., 2022; Wijayawardene et al., 2022b; Yang et al., 2023b, 2024; Wang et al., 2024b; Zhang et al., 2024b; Zhou et al., 2024). The present study is a continuation of a long-term study of discovering fungal diversity in an understudied geographical region, Qujing City. Malus species are an important fruit crop that is widely cultivated in Qujing City and its adjacent villages (e.g., Tongquan Town, Maguohe Town, and Wangjiashizhuang Town). There are several varieties of Malus species commonly cultivated in this region. Even though Malus species are extensively cultivated, there are no proper studies carried out to understand the mycobiota inhabiting its phyllosphere and mycosphere. This study has been carried out to fulfil this requirement. We have not observed any significant diseases; thus, we focused on identifying common saprobic and endophytic taxa associated with Malus species. In total, we have isolated 60 species from different localities of Qujing, and most taxa belong to Alternaria sp. and Fusarium sp.
In the present study, two novel species (C. qujingensis and H. malongense) and three new records (C. schulzeri, A. castaneae, and Au. pullulans) have been compiled. Based on detailed morphological studies, complemented by phylogenetic analyses based on ITS, tef1-α, rpb2, tub, and act sequence data (Figure 1), C. qujingensis is introduced as a novel saprobe taxon, while collections (ZHKUCC 23-0980 and GMBCC1005) revealed to be hitherto known species, namely, C. schulzeri, which was first reported from southwestern China. However, H. malongense was isolated as novel endophytic fungi on M. domestica from Qujing, Yunnan, based on morphological descriptions coupled with phylogenetic analyses (ITS, LSU, rpb2, and tub loci regions). Furthermore, Zhu et al. (2021) reported A. castaneae on C. mollissima and J. regia from Hebei and Yunnan provinces, respectively. Herein, we report our collections (ZHKUCC 23-0981 and ZHKUCC 23-0982) as a new host record of A. castaneae isolated from M. domestica. Moreover, the ITS and LSU regions are used to construct the phylogenetic tree of Aureobasidium, and the placements in Figure 3 in our study agree with Humphries et al. (2017) and Wu et al. (2023), who performed their analysis based on LSU and ITS gene regions. The morphological characteristics of each related species in the phylogenetic tree are compared in our study. Thus, we regard our collections (ZHKUCC 23-0983 and GMBCC1006) as the first report of Au. pullulans on M. domestica from Yunnan, China. Both the geographical and host distribution of fungus provide new insights into its ecological preferences and contribute to our knowledge of its regional distribution (Li et al., 2024b).
3.3.2 Species that can switch life modes; insights from this study
Fungi play key roles in ecosystems as saprobes, endophytes, and pathogens, but the role of an individual species in nature is still unknown (Schmit and Mueller, 2007). Recently, more research focused on the properties of endophytic fungi. For example, the secondary metabolites produced by endophytes can be employed in biotechnology such as in the pharmaceutical industry (Strobel and Daisy, 2003; Mapook et al., 2022). Mattoo and Nonzom (2021) mentioned that the endophyte could switch to be a pathogen lifestyle when it became more widespread upon the host getting old (e.g., Colletotrichum tropicale fide Rojas et al., 2010). For example, Pestalotiopsis palmarum, Ceratocystis paradoxa, and Ganoderma lucidum have been reported as endophytes, pathogens, or saprobes on coconut, which suggested that those fungi may switch their lifestyles from endophytes to pathogens or saprobes (Bhunjun et al., 2022; Tian et al., 2024). Furthermore, Knapp et al. (2018) claimed that a saprobic ancestral lifestyle of endophytes would be the reason for its ability to produce enzymes. Bhunjun et al. (2024) argued that the endophytic lifestyle is the ancestor of fungi, implying that some endophytes are host-specific, while others are connected to a wide range of hosts. A better understanding of fungi lifestyle switching could help us fill the gaps on fungi diversity and secondary metabolite diversity produced by fungi. Glauser et al. (2009) predicted that the production of secondary metabolite can be triggered by the presence of other fungi and pathogens.
In the present study, we isolated two Cytospora species, and both of them are isolated as saprobic taxa on M. domestica. More than 100 species of Cytospora are causal agents (or associated with) of the stem canker and dieback of woody and coniferous plants (e.g., Populus and Salix) (Fan et al., 2020). Initially, C. schulzeri was reported as a pathogen that can cause black spots on the infected apple trees in China (Wei, 1979; Zhuang, 2005). Recently, it was reported as a saprobe on M. pumila and a pathogen on C. mollissima and M. spectabilis in China (Fan et al., 2020; Jiang et al., 2020; Li et al., 2024a). However, we have not observed any disease symptoms that are similar to symptoms caused by C. schulzeri on Malus spp. in our study areas. Thus, we conclude that C. schulzeri is only a saprobic taxon in this region. More collections are needed to confirm whether C. qujingensis and C. schulzeri can exist as endophytes. Furthermore, pathogenicity tests need to be carried out to check whether both species are latent pathogens of M. domestica.
Species of Diatrypaceae were less reported as pathogens (Trouillas et al., 2011) or endophytes in petioles and woody tissue (Carroll, 1986; de Errasti et al., 2010), but most of them were predominantly saprobes inhibiting the wood and bark of various angiosperms. Hitherto, Allocryptovalsa species have not been reported as pathogens. Nevertheless, we conclude that further collection of different hosts in this region would provide insightful data to conclude the host switching and life mode changes of the taxon.
Aureobasidium pullulans was initially found as a saprobic yeast-like taxon but subsequently reported as an endophyte on the flesh of sweet cherries (Schena et al., 2003) and a pathogen isolated from the environment of a patient’s bone marrow (Liu et al., 2020). Ginns (1986) isolated this species on M. pumila from Canada, and later, Mirzwa-Mróz and Wińska-Krysiak (2011) recorded Au. pullulans on M. domestica from Europe. Previously, Ding et al. (2021) reported Au. pullulans from P. thunbergii (which causes brown spot needle blight) in China. In our study, we report Au. pullulans as an endophyte on M. domestica from China for the first time. Furthermore, many yeast species, including the yeast-like species, Au. pullulans, have been reported as effective antagonists against postharvest diseases in fruit. Aureobasidium pullulans is an effective biocontrol agent against postharvest diseases in various fruits, including apples (Castoria et al., 2001).
Hypoxylon malongense was isolated as an endophyte, from M. domestica in Qujing in the present study. Hypoxylon species are generally regarded as saprobe, but some of them are known to have an endophytic phase in their life cycle (viz., H. rubiginosum, H. guilanense) (Halecker et al., 2020). For example, H. monticulosum and H. submonticulosum have been isolated as endophytes from the stems of Litsea akoensis var. chitouchiaoensis (Lauraceae) and raspberry (Rubus idaeus), respectively (Burgess et al., 2017; Cheng et al., 2020). Furthermore, H. fuscum, H. truncatum, and H. investiens have been reported to exist with an endophytic lifestyle and produce abundant secondary metabolites (Gu et al., 2007; Basnet et al., 2019; Yuan et al., 2019). Some species in this genus are found as pathogens on woody plants. For example, H. macrocarpum was reported as the causal agent of wood rot (Hu and Wright, 2022). More studies indicated that Hypoxylon can depend on a wide range of hosts (Ma et al., 2022; Zhu et al., 2023). However, whether there is lifestyle switching, more samples are needed to demonstrate the mechanisms of switching between and within species. Anyway, illustrations and morphological descriptions of the asexual morph of this genus in the future are also needed. More research is still needed to figure out the species of our collections whether they contain secondary metabolites to further determine their classification placements. However, we believe it is essential to document and make an inventory of the distribution of species to understand the biogeographical and evolutionary patterns.
3.3.3 Necessity of inventorying and continuous updating of Malus-associated fungi against the geographical distribution
Nevertheless, the fungal diversity and impact of fungal pathogens on the agricultural and forest industries are poorly studied in China. As Shen et al. (2018) summarized, fungal diversity will be affected by plant species, environmental conditions, sampling time, and isolation and extraction methods.
Shen et al. (2018) indicated the remarkable fungal diversity on the apple surfaces. Dai et al. (2021) listed all pathogenic fungi on apples (containing apple root, shoot, leaf, flower, and fruit). There exist 65 types of apple disease in China, and 46 of them are caused by 149 different kinds of pathogenic fungal species. A search involving the keywords “Malus domestica” and “China” retrieved 47 fungi species present in the USDA Fungal Databases (accessed on 20 September 2024). Furthermore, extra retrieval involving the keywords “Malus domestica” and “China” retrieved 19 titles of research articles that had been published since 2018 in the Scopus Database (accessed on 20 December 2024). We scanned more than 100 available articles about fungi species (including saprobes, pathogens, and endophytes) associated with M. domestica reported in China in Chinese and English from 2000 to present. Hitherto known fungi species isolated on M. domestica from China, including saprobic, pathogenic, and endophytic fungi, was presented in a checklist (Supplementary Table S1), which will expand our knowledge of fungi associated with M. domestica and will prove timely and informative to a researcher’s future work.
Some culturable taxa like Alternaria, Aspergillus, Cladosporium, and Penicillium have been identified by previous studies (Magnani et al., 2007; Tadych et al., 2012). Granado et al. (2008) pointed out that the molecular approach, involving ribosomal DNA sequencing, to identify yeasts and sterile fungi, allows to spot unculturable fungi on apple fruits in the future. Bulgari et al. (2012) reported some uncultured species on Malus, viz., Bradyrhizobium, Bradyrhizobium, and Legionella. Our current study showed that Malus species are rich in culturable fungal diversity. However, we have not regarded the unculturable fungal diversity in this study. Hence, we conclude that more studies need to be carried out to collect more data to recognize the distribution of Malus taxa, and for precise identification based on the morpho-molecular analyses, barcoding current pathogens and potential pathogens would also be essential. Furthermore, more samples are required to determine whether fungi exist as a saprobe or endophyte and have the potential to be pathogens as well. In the future, studies based on omics approaches would be helpful in discovering novel taxa including unculturable taxa (Wijayawardene et al., 2023).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
G-QZ: Conceptualization, Data curation, Methodology, Software, Writing – original draft. Z-ML: Formal analysis, Methodology, Writing – review & editing. X-LF: Investigation, Writing – review & editing. Q-RL: Investigation, Writing – review & editing. JK: Visualization, Writing – review & editing. NS: Visualization, Writing – review & editing. AE: Visualization, Writing – review & editing. IM: Visualization, Writing – review & editing. D-QD: Conceptualization, Supervision, Writing – review & editing. NW: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We are grateful to “Yunnan Revitalization Talents Support Plan” (“High-End Foreign Experts” Program and “Young Talents” Program), the National Natural Science Foundation of China (No. NSFC 32460002), Yunnan Provincial Department of Science and Technology “Zhihui Yunnan” plan (202403AM140023), Mee-mann Chang Academician Workstation in Yunnan Province (Grant No. 202205AF150002), Yunnan Province Young and Middle-Aged Academic and Technical Leaders Reserve Talents Program (Grant No. 202305AC350252), and General Programs of the Provincial Department of Science and Technology (Grant No. 202101BA070001-076) and the Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River for support. The authors express their high appreciation to the Researchers supporting Project Number (RSPD2025R741), King Saud University, Riyadh, Saudi Arabia. JK and NS are partially supported by Chiang Mai University, Thailand.
Acknowledgments
We are grateful to Program of Doctoral Innovation Research Team from Qujing Normal University for the support. G-QZ thanks Dr. Dhanushka Wanasinghe for his valuable comments on the notes of Cytospora and is grateful to the Faculty of Science and Graduate School, Chiang Mai University, for supporting the TA/RA M.Sc. Scholarship. The authors would like to thank Dr. Shaun Pennycook for his advice and patient explanation of nomenclature.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1517908/full#supplementary-material
Supplementary Table 1 | Checklist of fungi associated with Malus domestica/Malus pumila in China.
Supplementary Table 3 | The names, isolate numbers, and corresponding GenBank accession numbers of the taxa used in Figure 1.
Supplementary Table 4 | The names, isolate numbers, and corresponding GenBank accession numbers of the taxa used in Figure 2.
Supplementary Table 5 | The names, isolate numbers, and corresponding GenBank accession numbers of the taxa used in Figure 3.
Supplementary Table 6 | The names, isolate numbers, and corresponding GenBank accession numbers of the taxa used in Figure 4.
References
Baldrian, P., Větrovský, T., Lepinay, C., Kohout, P. (2022). High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Divers. 114, 539–547. doi: 10.1007/s13225-021-00472-y
Basnet, B. B., Chen, B., Suleimen, Y. M., Ma, K., Guo, S., Bao, L., et al. (2019). Cytotoxic secondary metabolites from the endolichenic fungus Hypoxylon fuscum. Planta Medica 85, 1088–1097. doi: 10.1055/a-0957-3567
Basson, E., Meitz-Hopkins, J. C., Lennox, C. L. (2019). Morphological and molecular identification of fungi associated with south African apple core rot. Eur. J. Plant Pathol. 153, 849–868. doi: 10.1007/s10658-018-1601-x
Bhunjun, C. S., Niskanen, T., Suwannarach, N., Wannathes, N., Chen, Y. J., McKenzie, E. H., et al. (2022). The numbers of fungi: are the most speciose genera truly diverse? Fungal Divers. 114, 387–462. doi: 10.1007/s13225-022-00501-4
Bhunjun, C. S., Phukhamsakda, C., Hyde, K. D., McKenzie, E. H., Saxena, R. K., Li, Q. (2024). Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 125, 73–98. doi: 10.1007/s13225-023-00516-5
Blackwell, M. (2017). Made for each other: ascomycete yeasts and insects. The fungal kingdom, American Society for Microbiology, Washington, D.C., 945–962. doi: 10.1128/9781555819583.ch46
Bozoudi, D., Tsaltas, D. (2018). The multiple and versatile roles of Aureobasidium pullulans in the vitivinicultural sector. Fermentation 4, 85. doi: 10.3390/Fermentation4040085
Bulgari, D., Bozkurt, A. I., Casati, P., Çağlayan, K., Quaglino, F., Bianco, P. A. (2012). Endophytic bacterial community living in roots of healthy and ‘Candidatus Phytoplasma Mali’-infected apple (Malus domestica, Borkh.) trees. Antonie Van Leeuwenhoek 102, 677–687. doi: 10.1007/s10482-012-9766-3
Burgess, K., Ibrahim, A., Sørensen, D., Sumarah, M. W. (2017). Trienylfuranol A and trienylfuranone A–B: metabolites isolated from an endophytic fungus, Hypoxylon submoniticulosum, in the raspberry Rubus idaeus. J. Antibiot. 70, 721–725. doi: 10.1038/ja.2017.18
Cai, L., Hyde, K. D., Taylor, P. W. J., Weir, B. S., Waller, J. M., Abang, M. M., et al. (2009). A polyphasic approach for studying Colletotrichum. Fungal Divers. 39, 183–204.
Carbone, I., Kohn, L. M. A. (1999). method for designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia 91, 553–556. doi: 10.1080/00275514.1999.12061051
Carroll, G. C. (1986). The biology of endophytism in plants with particular reference to woody perennials. Microbiol. Phyll., 203–222.
Castoria, R., De Curtis, F., Lima, G., Caputo, L., Pacifico, S., De Cicco, V. (2001). Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: study on its modes of action. Postharvest Biol. Tec. 22, 7–17. doi: 10.1016/S0925-5214(00)00186-1
Cheng, M. J., Wu, M. D., Aung, T., Liao, H. R., Khamthong, N., Hsieh, S. Y. (2020). Metabolites from the endophytic fungus Hypoxylon monticulosum. Chem. Nat. Compd. 56, 1170–1172. doi: 10.1007/s10600-020-03258-x
Chethana, K. W., Rathnayaka, A. R., Samarakoon, B. C., Wu, N., Wijesinghe, S. N., Yasanthika, W. A., et al. (2023). AJOM new records and collections of fungi: 151–200. Asian J. Mycol. 6, 89–243. doi: 10.5943/ajom/6/2/7
Crous, P. W., Summerell, B. A., Swart, L., Denman, S., Taylor, J. E., Bezuidenhout, C. M., et al. (2011). Fungal pathogens of Proteaceae. Persoonia-Mol. Phyl. Evol. Fungi 27, 20–45. doi: 10.3767/003158511X606239
Dai, D. Q., Phookamsak, R., Wijayawardene, N. N., Li, W. J., Bhat, D. J., Xu, J. C., et al. (2017). Bambusicolous fungi. Fungal Divers. 82, 1–105. doi: 10.1007/s13225-016-0367-8
Dai, D. Q., Wijayawardene, N. N., Dayarathne, M. C., Kumla, J., Han, L. S., Zhang, G. Q., et al. (2022). Taxonomic and phylogenetic characterizations reveal four new species, two new asexual morph reports, and six new country records of bambusicolous Roussoella from China. J. Fungi 8, 532. doi: 10.3390/jof8050532
Dai, P. B., Zhang, R., Sun, G. Y. (2021). A checklist of pathogenic fungi on apple in China. Mycosystema 40, 936–964. doi: 10.13346/j.mycosystema.210017
de Errasti, A., Carmarán, C. C., Novas, M. V. (2010). Diversity and significance of fungal endophytes from living stems of naturalized trees from Argentina. Fungal Divers. 41, 29–40. doi: 10.1007/s13225-009-0012-x
DeMers, M. (2022). Alternaria alternata as endophyte and pathogen. Microbiology 168, 1153. doi: 10.1099/mic.0.001153
Department of Agriculture and Rural Affairs of Yunnan Province (2021). Annual fruit industry development report of Yunnan Province. Available online at: https://nync.yn.gov.cn/html/2021/nongyechanyebaogao_0730/389317.html?cid=4978 (Accessed April 1 2024).
Dik, A. J., Elad, Y. (1999). Comparison of antagonists of Botrytis cinerea in greenhouse-grown cucumber and tomato under different climatic conditions. Eur. J. Plant Pathol. 105, 123–137. doi: 10.1023/A:1008778213278
Ding, X., Lin, S., Zhao, R., Ye, J. (2021). First report of brown spot needle blight on Pinus thunbergii caused by Aureobasidium pullulans in China. Plant Dis. 105, 4166. doi: 10.1094/PDIS-11-20-2435-PDN
Dissanayake, L. S., Samarakoon, M. C., Mortimer, P. E., Lu, Y. Z., Li, Q. R., Hyde, K. D., et al. (2022). Morpho-molecular characterization of two novel amphisphaeriaceous species from Yunnan, China. Phytotaxa 446, 144–158. doi: 10.11646/phytotaxa.446.3.1
Doilom, M., Hyde, K. D., Dong, W., Liao, C. F., Suwannarach, N., Lumyong, S. (2021). The plant family Asteraceae is a cache for novel fungal diversity: Novel species and genera with remarkable ascospores in Leptosphaeriaceae. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.660261
Dong, L., Wang, L. D., Zhao, X. C., Zhao, J., Zhao, J. J., Li, Y. P. (2023). The status quo and countermeasures of fruit industry brand development of Yunnan Province. J. Agric. 13, 102–106. doi: 10.11923/j.issn.2095-4050.cjas2021-0199
Du, T. Y., Dai, D. Q., Mapook, A., Lu, L., Stephenson, S. L., Suwannarach, N., et al. (2023). Additions to Rhytidhysteron (Hysteriales, Dothideomycetes) in China. J. Fungi. 9, 148. doi: 10.3390/jof9020148
Fan, X. L., Bezerra, J. D. P., Tian, C. M., Crous, P. W. (2020). Cytospora (Diaporthales) in China. Persoonia 45, 1–45. doi: 10.3767/Persoonia.2020.45.01
Fan, X. L., Liang, L. Y., Ma, R., Tian, C. M. (2013). Morphological and phylogenetic studies of Cytospora (Valsaceae, Diaporthales) isolates from Chinese scholar tree, with description of a new species. Mycoscience 55, 252–259. doi: 10.1016/j.myc.2013.10.001
Fan, X. L., Tian, C. M., Yang, Q., Liang, Y. M., You, C. J., Zhang, Y. B. (2015). Cytospora from Salix in northern China. Mycotaxon 129, 303–315. doi: 10.5248/129.303
Feng, B., Yang, Z. (2018). Studies on diversity of higher fungi in Yunnan, southwestern China: A review. Plant Divers. 40, 165–171. doi: 10.1016/j.pld.2018.07.001
Freeman, K. R., Martin, A. P., Karki, D., Lynch, R. C., Mitter, M. S., Meyer, A. F., et al. (2009). Evidence that chytrids dominate fungal communities in high-elevation soils. Proc. Natl. Acad. Sci. 106, 18315–18320. doi: 10.1073/pnas.0907303106
Genevieve, L., Pierre-Luc, C., Roxanne, G. T., Amélie, M., Danny, B., Vincent, M., et al. (2019). Estimation of fungal diversity and identification of major abiotic drivers influencing fungal richness and communities in northern temperate and boreal Quebec forests. Forests 10, 1096. doi: 10.3390/f10121096
Ginns, J. H. (1986). Compendium of plant disease and decay fungi in Canada 1960–1980. Canada. Agricul., 1813. doi: 10.5962/bhl.title.58888
Glass, N. L., Donaldson, G. C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61, 1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995
Glauser, G., Gindro, K., Fringeli, J., De Joffrey, J. P., Rudaz, S., Wolfender, J. L. (2009). Differential analysis of mycoalexins in confrontation zones of grapevine fungal pathogens by ultrahigh pressure liquid chromatography/time-of-flight mass spectrometry and capillary nuclear magnetic resonance. J. Agr. Food Chem. 57, 1127–1134. doi: 10.1021/jf803353
Glez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fdez-Riverola, F., Posada, D. (2010). ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 38, 14–18. doi: 10.1093/nar/gkq321
Gonçalves, V. N., Cantrell, C. L., Wedge, D. E., Ferreira, M. C., Soares, M. A., Jacob, M. R., et al. (2016). Fungi associated with rocks of the Atacama Desert: taxonomy, distribution, diversity, ecology and bioprospection for bioactive compounds. Environ. Microbiol. 18, 232–245. doi: 10.1111/1462-2920.13005
Grabowski, M. (2007). The study of new fungus species causing apple sooty blotch. Folia Hortic. 19, 89–97. doi: 10.5555/20083070265
Granado, J., Thürig, B., Kieffer, E., Petrini, L., Flieβbach, A., Tamm, L., et al. (2008). Culturable fungi of stored ‘golden delicious’ apple fruits: a one-season comparison study of organic and integrated production systems in Switzerland. Microb. Ecol. 56, 720–732. doi: 10.1007/s00248-008-9391-x
Gu, W., Ge, H. M., Song, Y. C., Ding, H., Zhu, H. L., Zhao, X. A., et al. (2007). Cytotoxic benzo fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J. Nat. Prod. 70, 114–117. doi: 10.1021/np0604127
Gui, T. R., Kong, B. H., Ma, X. L., Ji, P., Yang, Y. J., Shi, A. X., et al. (2015). Studies on biological characters and pathogenicity of isolates of Cytospora from apple tree in Yunnan. J. Agric. Sci. 05, 2096–2102. doi: 10.16213/j.cnki.scjas.2015.05.046
Halecker, S., Wennrich, J. P., Rodrigo, S., Andrée, N., Rabsch, L., Baschien, C., et al. (2020). Fungal endophytes for biocontrol of ash dieback: the antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol. 45, 100918. doi: 10.1016/j.funeco.2020.100918
Hall, T. (2004). BioEdit. Ibis Therapeutics, Carlsbad, CA 92008, USA. Available online at: http://www.mbio.ncsu.edu/BioEdit/bioedit.html (Accessed 18 March 2005).
Han, L. S., Liu, C., Dai, D. Q., Promputtha, I., Elgorban, A., Al-Rejaie, S., et al. (2024b). Five new species, two new sexual morph reports, and one new geographical record of Apiospora (Amphisphaeriales, Sordariomycetes) isolated from bamboo in Yunnan, China. Front. Cell. Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1476066
Han, L. S., Wijayawardene, N. N., Liu, C., Han, L. H., Promputtha, I., Li, Q., et al. (2024a). Paramphibambusabambusicola gen. et. sp. nov., Arecophila xishuangbannaensis and A. zhaotongensis spp. nov. in Cainiaceae from Yunnan, China. MycoKeys 104, 113–132. doi: 10.3897/mycokeys.104.117872
Hawksworth, D. L. (1991). The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol. Res. 95, 641–655. doi: 10.1016/S0953-7562(09)80810-1
Hawksworth, D. L., Lücking, R. (2017). Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectrum. 5, 1110–1128. doi: 10.1128/microbiolspec.funk-0052-2016
He, X., Zhao, C. L. (2022). Diversity of wood-decaying fungi in Wuliangshan area, Yunnan Province, PR China. Diversity 14, 131. doi: 10.3390/d14020131
Hongsanan, S., Phookamsak, R., Bhat, D. J., Wanasinghe, D. N., Promputtha, I., Suwannarach, N., et al. (2023). Exploring ascomycete diversity in Yunnan, China I: resolving ambiguous taxa in Phaeothecoidiellaceae and investigating conservation implications of fungi. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1252387
Hu, J., Wright, G. C. (2022). Canker and wood rot pathogens present in young lemon orchards in south-west Arizona. Plant Pathol. 71, 411–425. doi: 10.1111/ppa.13476
Huelsenbeck, J. P., Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755. doi: 10.1093/bioinformatics/17.8.754
Humphries, Z., Seifert, K. A., Hirooka, Y., Visagie, C. M. (2017). A new family and genus in Dothideales for Aureobasidium-like species isolated from house dust. IMA Fungus 8, 299–315. doi: 10.5598/imafungus.2017.08.02.05
Hyde, K. D., Norphanphoun, C., Maharachchikumbura, S. S. N., Bhat, D. J., Jones, E. B. G., Bundhun, D., et al. (2020). Refined families of Sordariomycetes. Mycosphere 11, 305–1059. doi: 10.5555/20210251481
Index Fungorum (2024). Available online at: http://www.indexfungorum.org/names/Names.asp (Accessed 23 June 2024).
Ippolito, A., Nigro, F. (2000). Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot. 19, 715–723. doi: 10.1016/S0261-2194(00)00095-8
Jayawardena, R. S., Purahong, W., Zhang, W., Wubet, T., Li, X. H., Liu, M., et al. (2018). Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 90, 1–84. doi: 10.1007/s13225-018-0398-4
Jia, A., Chen, B., Lu, H., Xing, Y., Li, B., Fan, X. (2024). Multigene phylogeny and morphology reveal three new species of Cytospora isolated from diseased plant branches in Fengtai District, Beijing, China. MycoKeys 101, 163–189. doi: 10.3897/mycokeys.101.116272
Jiang, H. B., Phookamsak, R., Hyde, K. D., Mortimer, P. E., Xu, J. C., Kakumyan, P., et al. (2021). A taxonomic appraisal of bambusicolous fungi in Occultibambusaceae (Pleosporales, Dothideomycetes) with new collections from Yunnan Province, China. Life 11, 932. doi: 10.3390/life11090932
Jiang, N., Yang, Q., Fan, X. L., Tian, C. M. (2020). Identification of six Cytospora species on Chinese chestnut in China. MycoKeys 62, 1–25. doi: 10.3897/mycokeys.62.47425
Ju, Y. M., Rogers, J. D. (1996). A revision of the genus Hypoxylon. In American Phytopathological Society (St. Paul, MN, USA: APS Press).
Katoh, K., Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Knapp, D. G., Németh, J. B., Barry, K., Hainaut, M., Henrissat, B., Johnson, J., et al. (2018). Comparative genomics provides insights into the lifestyle and reveals functional heterogeneity of dark septate endophytic fungi. Scie. Rep. 8, 6321. doi: 10.1038/s41598-018-24686-4
Konta, S., Maharachchikumbura, S. S. N., Senanayake, I. C., Mckenzie, E. H. C., Stadler, M., Boonmee, S., et al. (2020). A new genus Allodiatrype, five new species and a new host record of diatrypaceous fungi from palms (Arecaceae). Mycosphere 11, 239–268. doi: 10.5943/mycosphere/11/1/4
Lawrence, D. P., Holland, L. A., Nouri, M. T., Travadon, R., Abramians, A., Michailides, T. J., et al. (2018). Molecular phylogeny of Cytospora species associated with canker diseases of fruit and nut crops in California, with the descriptions of ten new species and one new combination. IMA Fungus 9, 333–370. doi: 10.5598/imafungus.2018.09.02.07
Lawrence, D. P., Travadon, R., Pouzoulet, J., Rolshausen, P. E., Wilcox, W. F., Baumgartner, K. (2017). Characterization of Cytospora isolates from wood cankers of declining grapevine in North America, with the descriptions of two new Cytospora species. Plant Pathol. 66, 713–725. doi: 10.1111/ppa.12621
Lee, D. H., Cho, S. E., Oh, J. Y., Cho, E. J., Kwon, S. (2021). A novel species of Aureobasidium (Dothioraceae) recovered from Acer pseudosieboldianum in Korea. J. Asia Pac. Biodivers. 14, 657–661. doi: 10.1016/j.japb.2021.08.005
Li, J. T., Li, J. R., Jiang, N. (2024a). Identification of Cytospora species isolated from branch canker diseases of woody plants in Tibet, China. Forests 15, 121. doi: 10.3390/f15010121
Li, L., Zhao, Z. X., Gui, H., Wang, X. A., Xing, P., Karunarathna, S. C., et al. (2024b). Environmental factors shaping the culturable freshwater fungi diversity of four lakes in Yunnan Province, China. Diversity 16, 612. doi: 10.3390/d16100612
Liu, X., Li, X., Bozorov, T. A., Ma, R., Ma, J., Zhang, Y., et al. (2020). Characterization and pathogenicity of six Cytospora strains causing stem canker of wild apple in the Tianshan Forest, China. For. Pathol. 50, 12587. doi: 10.1111/efp.12587
Liu, X. F., Tibpromma, S., Hughes, A., Chethana, K., Wijayawardene, N. N., Dai, D. Q., et al. (2023). Culturable mycota on bats in central and southern Yunnan Province, China. Mycosphere 14, 497–662. doi: 10.5943/mycosphere/14/1/7
Liu, Y., Whelen, S., Hall, B. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molec. Biol. Evolu. 16, 1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092
Loi, M. C., Ballero, M., Loddo, M., Sponga, F., Ariu, A. (2000). Variazioni della presenza endofitica in specie vegetali della sardegna. II contributo. Micologia Italiana 29, 11–16.
Lu, L., Karunarathna, S. C., Dai, D. Q., Xiong, Y. R., Suwannarach, N., Stephenson, S. L., et al. (2022). Description of four novel species in Pleosporales associated with coffee in Yunnan, China. J. Fungi. 8, 1113. doi: 10.3390/jof8101113
Ma, R., Liu, Y. M., Yin, Y. X., Tian, C. M. (2018). A canker disease of apple caused by Cytospora parasitica recorded in China. For. Pathol. 48, 12416. doi: 10.1111/efp.12416
Ma, H., Song, Z., Pan, X., Li, Y., Yang, Z., Qu, Z. (2022). Multi-gene phylogeny and taxonomy of Hypoxylon (Hypoxylaceae, Ascomycota) from China. Diversity 14, 37. doi: 10.3390/d14010037
Magnani, R. F., De Souza, G. D., Rodrigues-Filho, E. (2007). Analysis of alternariol and alternariol monomethyl ether on flavedo and albedo tissues of tangerines (Citrus reticulata) with symptoms of Alternaria brown spot. J. Agr. Food Chem. 55, 4980–4986. doi: 10.1021/jf0704256
Maharachchikumbura, S. S. N., Hyde, K. D., Jones, E. B. G., McKenzie, E. H. C., Huang, S. K., Abdel-Wahab, M. A., et al. (2015). Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 72, 199–301. doi: 10.1007/s13225-015-0331-z
Maharachchikumbura, S. S., Wanasinghe, D. N., Elgorban, A. M., Al-Rejaie, S. S., Kazerooni, E. A., Cheewangkoon, R. (2022). Brunneosporopsis yunnanensis gen. et sp. nov. and Allocryptovalsa xishuangbanica sp. nov., New Terrestrial Sordariomycetes from Southwest China. Life 12, 635. doi: 10.3390/life12050635
Mapook, A., Hyde, K. D., Hassan, K., Kemkuignou, B. M., Čmoková, A., Surup, F., et al. (2022). Ten decadal advances in fungal biology leading towards human well-being. Fungal Divers. 116, 547–614. doi: 10.1007/s13225-022-00510-3
Mattoo, A. J., Nonzom, S. (2021). Endophytic fungi: understanding complex cross-talks. Symbiosis 83, 237–264. doi: 10.1007/s13199-020-00744-2
McCormack, P., Wildman, H. G., Jeffries, P. (1995). The influence of moisture on the suppression of Pseudomonas syringae by Aureobasidium pullulans on an artificial leaf surface. FEMS Microbiol. Ecol. 16, 159–165. doi: 10.1111/j.1574-6941.1995.tb00279.x
Miller, M. A., Pfeiffer, W., Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE), IEEE, Piscataway, NJ, USA. 1–8. doi: 10.1109/GCE.2010.5676129
Mirzwa-Mróz, E., Wińska-Krysiak, M. (2011). Diversity of sooty blotch fungi in Poland. Acta Sci. Pol. Hortorum Cultus. 10, 191–200.
Monkai, J., Wanasinghe, D. N., Jeewon, R., Promputtha, I., Phookamsak, R. (2021). Morphological and phylogenetic characterization of fungi within Bambusicolaceae: Introducing two new species from the Greater Mekong Subregion. Mycol. Prog. 20, 721–732. doi: 10.1007/s11557-021-01694-9
Nagahama, T., Takahashi, E., Nagano, Y., Abdel-Wahab, M. A., Miyazaki, M. (2011). Molecular evidence that deep-branching fungi are major fungal components in deep-sea methane cold-seep sediments. Environ. Microbiol. 13, 2359–2370. doi: 10.1111/j.1462-2920.2011.02507.x
O’Donnell, K., Cigelnik, E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7, 103–116. doi: 10.1006/mpev.1996.0376
Oren, A., Gunde-Cimerman, N. (2012). “Fungal life in the dead sea,” in Biology of marine Fungi (Springer, Berlin, Heidelberg), 115–132. doi: 10.1007/978-3-642-23342-5_6
Rai, M., Agarkar, G. (2016). Plant–fungal interactions: what triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 42, 428–438. doi: 10.3109/1040841X.2014.958052
Rambaut, A. (2006). FigTree. Tree drawing tool version 1.4. 0 (University of Edinburgh: Institute of Evolutionary Biology, Edinburgh, UK).
Rannala, B., Yang, Z. (1996). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. Evol. 43, 304–311. doi: 10.1007/BF02338839
Rehner, S. A., Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97, 84–98. doi: 10.1080/15572536.2006.11832842
Rogers, J. D. (2018). The Xylariaceae systematic, biological and evolutionary aspects. Mycologia 71, 1–42. doi: 10.1080/00275514.1979.12020984
Rojas, E. I., Rehner, S. A., Samuels, G. J., Van Bael, S. A., Herre, E. A., Cannon, P., et al. (2010). Colletotrichum gloeosporioides sl associated with Theobroma cacao and other plants in Panama: multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 102, 1318–1338. doi: 10.3852/09-244
Ronquist, F., Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Rühmann, S., Pfeiffer, J., Brunner, P., Szankowski, I., Fischer, T. C., Forkmann, G., et al. (2013). Induction of stilbene phytoalexins in grapevine (Vitis vinifera) and transgenic stilbene synthase-apple plants (Malus domestica) by a culture filtrate of Aureobasidium pullulans. Plant Physiol. Bioch. 72, 62–71. doi: 10.1016/j.plaphy.2013.03.011
Sachini, R., Steffens, C. A., Martin, M. S., Schveitzer, B., Fenili, C. L., Petri, J. L. (2020). Mineral contents in the skin and flesh of fruits of apple cultivars. Rev. Bras. Frutic. 42, e-572. doi: 10.1590/0100-29452020572
Schena, L., Nigro, F., Pentimone, I., Ligorio, A., Ippolito, A. (2003). Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biol. Tec. 30, 209–220. doi: 10.1016/S0925-5214(03)00111-X
Schmit, J. P., Mueller, G. M. (2007). An estimate of the lower limit of global fungal diversity. Biodivers. Conserv. 16, 99–111. doi: 10.1007/s10531-006-9129-3
Seifert, K. A. (2009). Progress towards DNA barcoding of fungi. Mol. Ecol. Resour. 9, 83–89. doi: 10.1111/j.1755-0998.2009.02635.x
Senanayake, I. C., Rathnayaka, A. R., Marasinghe, D. S., Calabon, M. S., Gentekaki, E., Lee, H. B., et al. (2020). Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 11, 2678–2754. doi: 10.5943/mycosphere/11/1/20
Senanayake, I. C., Rossi, W., Leonardi, M., Weir, A., McHugh, M., Rajeshkumar, K. C., et al. (2023). Fungal diversity notes 1611–1716: taxonomic and phylogenetic contributions on fungal genera and species emphasis in south China. Fungal Divers. 122, 161–403. doi: 10.1007/s13225-023-00523-6
Senwanna, C., Phookamsak, R., Doilom, M., Hyde, K. D., Cheewangkoon, R. (2017). Novel taxa of Diatrypaceae from Para rubber (Hevea brasiliensis) in northern Thailand; introducing a novel genus Allocryptovalsa. Mycosphere 8, 1835–1855. doi: 10.5943/mycosphere/8/10/9
Shen, H. W., Bao, D. F., Bhat, D. J., Su, H. Y., Luo, Z. L. (2022). Lignicolous freshwater fungi in Yunnan Province, China: an overview. Mycology 13, 119–132. doi: 10.1080/21501203.2022.2058638
Shen, Y., Nie, J., Li, Z., Li, H., Wu, Y., Dong, Y., et al. (2018). Differentiated surface fungal communities at point of harvest on apple fruits from rural and peri-urban orchards. Sci. Rep. 8, 2165. doi: 10.1038/s41598-017-17436-5
Sir, E. B., Becker, K., Lambert, C., Bills, G. F., Kuhnert, E. (2019). Observations on Texas Hypoxylons, including two new Hypoxylon species and widespread environmental isolates of the H. croceum complex identified by a polyphasic approach. Mycologia 111, 832–856. doi: 10.1080/00275514.2019.1637705
Species Fungorum (2024). Available online at: https://www.speciesfungorum.org/ (Accessed 4 September 2024).
Spielman, L. J. (1983). Taxonomy and biology of Valsa species on hardwoods in North America, with special reference to species on maples. Cornell University, New York, USA.
Spielman, L. J. (1985). A monograph of Valsa on hardwoods in North America. Biodivers. Conserv. 63, 1355–1378. doi: 10.1139/b85-190
Strobel, G., Daisy, B. (2003). Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. R. 67, 491–502. doi: 10.1128/mmbr.67.4.491-502.2003
Suetrong, S., Preedanon, S., Kobmoo, N., Srihom, C., Somrithipol, S., Saengkaewsuk, S., et al. (2023). Unravelling the hidden diversity of cave mycobiota in Thailand’s Satun Geopark. Sci. Rep. 13, 19162. doi: 10.1038/s41598-023-43316-2
Tadych, M., Bergen, M. S., Johnson-Cicalese, J., Polashock, J. J., Vorsa, N., White, J. F. (2012). Endophytic and pathogenic fungi of developing cranberry ovaries from flower to mature fruit: diversity and succession. Fungal Divers. 54, 101–116. doi: 10.1007/s13225-012-0160-2
Taylor, J. E., Hyde, K. D. (2003). Microfungi of Tropical and Temperate Palms (Hong Kong: Fungal Divers Press), 459.
Tedersoo, L., Bahram, M., Põlme, S., Kõljalg, U., Yorou, N. S., Wijesundera, R., et al. (2014). Global diversity and geography of soil fungi. Science 346, 1256688. doi: 10.1126/science.1256688
Tedersoo, L., Mikryukov, V., Anslan, S., Bahram, M., Khalid, A. N., Corrales, A., et al. (2021). The Global Soil Mycobiome consortium dataset for boosting fungal diversity research. Fungal Divers. 111, 573–588. doi: 10.1007/s13225-021-00493-7
Tedersoo, L., Sánchez-Ramírez, S., Koljalg, U., Bahram, M., Döring, M., Schigel, D., et al. (2018). High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 90, 135–159. doi: 10.1007/s13225-018-0401-0
Thomma, B. P. (2003). Alternaria spp.: from general saprophyte to specific parasite. Molecul. Plant Pathol. 4, 225–236. doi: 10.1046/j.1364-3703.2003.00173.x
Tian, X. G., Bao, D. F., Karunarathna, S. C., Jayawardena, R. S., Hyde, K. D., Bhat, D. J., et al. (2024). Taxonomy and phylogeny of ascomycetes associated with selected economically important monocotyledons in China and Thailand. Mycosphere 15, 1–274. doi: 10.5943/mycosphere/15/1/1
Trouillas, F. P., Pitt, W. M., Sosnowski, M. R., Huang, R., Peduto, F., Loschiavo, A., et al. (2011). Taxonomy and DNA phylogeny of Diatrypaceae associated with Vitis vinifera and other woody plants in Australia. Fungal Divers. 49, 203–223. doi: 10.1007/s13225-011-0094-0
Vilgalys, R., Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wainwright, M., Wickramasinghe, N. C., Narlikar, J. V., Rajaratnam, P. (2003). Microorganisms cultured from stratospheric air samples obtained at 41 km. FEMS Microbiol. Lett. 218, 161–165. doi: 10.1111/j.1574-6968.2003.tb11513.x
Wang, S., Jiang, N., Ma, R. (2024a). Morphology and phylogeny reveal three new species of Cytospora associated with tree cankers in China. J. Fungi 10, 139. doi: 10.3390/jof10020139
Wang, C. B., Jiang, N., Zhu, Y. Q., Xue, H., Li, Y. (2022). Aureobasidium aerium (Saccotheciaceae, Dothideales), a new yeast-like fungus from the air in Beijing, China. Phytotaxa 544, 185–192. doi: 10.11646/PHYTOTAXA.544.2.4
Wang, X. L., Kang, Z. S., Huang, L. L., Yang, P. (2007). Pathogen identification of Valsa canker on pear tree: evidences from rDNA-ITS sequences and cultural characteristics. Mycosystema 26, 517–527. doi: 10.5555/20083016649
Wang, L., Su, J., Muhammad, A., Zhao, C. (2024b). Two new wood-inhabiting fungal species (Polyporales, Basidiomycota) from Yunnan Province, China. Phytotaxa 647, 1–8. doi: 10.11646/phytotaxa.647.1.1
Wei, J. C. (1979). Identification of fungus handbook, Shanghai, China (Shanghai: Shanghai Sci Technol Pr).
White, T. J., Bruns, T., Lee, S. J., Taylor, J. L. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: Guide to Methods Appl. 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wijayawardene, N. N., Boonyuen, N., Ranaweera, C. B., de Zoysa, H. K., Padmathilake, R. E., Nifla, F., et al. (2023). OMICS and other advanced technologies in mycological applications. J. Fungi 9, 688. doi: 10.3390/jof9060688
Wijayawardene, N. N., Dai, D. Q., Zhu, M. L., Wanasinghe, D. N., Kumla, J., Zhang, G. Q., et al. (2022b). Fungi associated with dead branches of Magnolia grandiflora: A case study from Qujing, China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.954680
Wijayawardene, N. N., Dissanayake, L. S., Li, Q. R., Dai, D. Q., Xiao, Y., Wen, T. C., et al. (2021a). Yunnan–Guizhou Plateau: a mycological hotspot. Phytotaxa 523, 1–31. doi: 10.11646/PHYTOTAXA.523.1.1
Wijayawardene, N. N., Phillips, A. J., Pereira, D. S., Dai, D. Q., Aptroot, A., Monteiro, J. S., et al. (2022a). Forecasting the number of species of asexually reproducing fungi (Ascomycota and Basidiomycota). Fungal Divers. 14, 463–490. doi: 10.1007/s13225-022-00500-5
Wijayawardene, N. N., Phillips, A. J., Tibpromma, S., Dai, D. Q., Selbmann, L., Monteiro, J. S., et al. (2021b). Looking for the undiscovered asexual taxa; case studies from lesser studied life modes and habitats. Mycosphere 12, 1186–1229. doi: 10.5555/20220020706
Wu, F., Feng, Z., Wang, M., Wang, Q. (2023). Proposal of four new Aureobasidium species for exopolysaccharide production. J. Fungi 9, 447. doi: 10.3390/jof9040447
Wu, B., Hussain, M., Zhang, W., Stadler, M., Liu, X., Xiang, M. (2019). Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 10, 127–140. doi: 10.1080/21501203.2019.1614106
Wu, J. B., Zhan, R. L., Liu, F., Cang, J. M. (2017). First report of a stem and fruit spot of Pitaya caused by Aureobasidium pullulans in China. Plant Dis. 101, 249–250. doi: 10.1094/PDIS-05-16-0702-PDN
Xu, R. F., Karunarathna, S. C., Phukhamsakda, C., Dai, D. Q., Elgorban, A. M., Suwannarach, N., et al. (2024). Four new species of Dothideomycetes (Ascomycota) from Pará Rubber (Heveabrasiliensis) in Yunnan Province, China. MycoKeys 103, 71. doi: 10.3897/mycokeys.103.117580
Yang, Y., Jiang, Q., Li, Q., Yang, J., Cha, L., Cheng, L., et al. (2023b). Molecular systematics and taxonomic analyses of three new wood-inhabiting fungi of Hyphoderma (Hyphodermataceae, Basidiomycota). J. Fungi 9, 1044. doi: 10.3390/jof9111044
Yang, Y., Li, R., Jiang, Q., Zhou, H., Muhammad, A., Wang, H., et al. (2024). Phylogenetic and taxonomic analyses reveal three new wood-inhabiting fungi (Polyporales, Basidiomycota) in China. J. Fungi 10, 55. doi: 10.3390/jof10010055
Yang, E. F., Lu, W., Tibpromma, S., Dai, D. Q., Gao, Y., Promputtha, I., et al. (2023a). Three interesting fungi from American bullfrog larvae (Rana catesbeiana) in Yunnan, China. Phytotaxa 587, 251–268. doi: 10.11646/phytotaxa.587.3.4
Yasanthika, E., Dissanayake, L. S., Wanasinghe, D. N., Karunarathna, S. C., Mortimer, P. E., Samarakoon, B. C., et al. (2020). Lonicericola fuyuanensis (Parabambusicolaceae) a new terrestrial pleosporalean ascomycete from Yunnan Province, China. Phytotaxa 446, 103–113. doi: 10.11646/phytotaxa.446.2.3
Yoshikawa, M., Yokoyama, T. (1987). Leaf blight of day lily caused by Aureobasidium microstictum (Bubak) WB Cooke. J. Phytopathol. 53, 606–615. doi: 10.3186/jjphytopath.53.606
Yuan, C., Yang, H. X., Guo, Y. H., Fan, L., Zhang, Y. B., Li, G. (2019). New α-pyrones from an endophytic fungus, Hypoxylon investiens J2. RSC Adv. 9, 27419–27423. doi: 10.1039/C9RA05308E
Zhang, X., Karunarathna, S. C., Tibpromma, S., Du, T. Y., Elgorban, A. M., Lumyong, S., et al. (2024a). Morphology and phylogenetic analyses reveal Neopalmiascoma gen. nov. (Bambusicolaceae, Pleosporales) on Macadamia integrifolia in Yunnan Province, China. Phytotaxa 633, 230–240. doi: 10.11646/phytotaxa.633.3.3
Zhang, H., Mao, Y. T., Ma, M. X., Tao, G. C., Wei, T. P., Jiang, Y. L. (2024c). Culturable endophytic Sordariomycetes from Rosa roxburghii: New species and lifestyles. J. Sys. Evol. 62, 637–676. doi: 10.1111/jse.13035
Zhang, Q., Nizamani, M. M., Feng, Y., Yang, Y. Q., Jayawardena, R. S., Hyde, K. D., et al. (2023). Genome-scale and multi-gene phylogenetic analyses of Colletotrichum spp. host preference and associated with medicinal plants. Mycosphere 14, 1–106. doi: 10.5943/mycosphere/14/si2/1
Zhang, G. Q., Wijayawardene, N. N., Han, L. H., Kumla, J., Suwannarach, N., Li, Q., et al. (2024b). Three novel woody litter inhabiting fungi in Didymosphaeriaceae, Phaeoseptaceae and Synnemasporellaceae from Zhujiangyuan Nature Reserve, Yunnan Province, PR China. MycoKeys 106, 173–200. doi: 10.3897/mycokeys.106.123105
Zhao, J., Dai, J. J., Feng, Z. J., Zhang, R. K., Fang, Y. Q. (2018). Current situation and trend analysis of fruit greening in Yunnan. J. Yunnan Agricul. 10, 92–93.
Zhaxybayeva, O., Gogarten, J. P. (2002). Bootstrap, Bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genomics 3, 4. doi: 10.1186/1471-2164-3-4
Zhou, Q., Jiang, Q., Yang, X., Yang, J., Zhao, C., Zhao, J. (2024). Phylogenetic and taxonomic analyses of five new wood-inhabiting fungi of Botryobasidium, Coltricia and Coltriciella (Basidiomycota) from China. J. Fungi 10, 205. doi: 10.3390/jof10030205
Zhu, H. Y., Pan, M., Wijayawardene, N. N., Jiang, N., Ma, R., Dai, D. Q., et al. (2021). The hidden diversity of diatrypaceous fungi in China. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.6462
Zhu, A. H., Song, Z. K., Wang, J. F., Guan, H. W., Ma, H. X. (2023). Multi-locus phylogeny and morphology reveal two new species of Hypoxylon (Hypoxylaceae, Xylariales) from Motuo, China. Microorganisms 12, 72. doi: 10.3390/microorganisms12010072
Keywords: apple tree, fungal diversity, life mode, phylogeny, taxonomy, two novel species
Citation: Zhang G-Q, Li Z-M, Fan X-L, Li Q-R, Kumla J, Suwannarach N, Elgorban AM, Moussa IM, Dai D-Q and Wijayawardene NN (2025) Fungi from Malus in Qujing, China: two new species, three new records, and insights into potential host jumping and lifestyle switching. Front. Cell. Infect. Microbiol. 15:1517908. doi: 10.3389/fcimb.2025.1517908
Received: 27 October 2024; Accepted: 06 February 2025;
Published: 11 March 2025.
Edited by:
Sinang Hongsanan, Shenzhen University, ChinaReviewed by:
Marasinghe Wadige Diana Sandamali, Smith College, United StatesChihiro Kadooka, Sojo University, Japan
Copyright © 2025 Zhang, Li, Fan, Li, Kumla, Suwannarach, Elgorban, Moussa, Dai and Wijayawardene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Qin Dai, Y2ljaWRhaWRvbmdxaW5AZ21haWwuY29t; Nalin N. Wijayawardene, bmFsaW53aWpheWF3YXJkZW5lQHlhaG9vLmNvbQ==
†These authors have contributed equally to this work
 Gui-Qing Zhang
Gui-Qing Zhang Zhu-Mei Li1†
Zhu-Mei Li1† Xin-Lei Fan
Xin-Lei Fan Qi-Rui Li
Qi-Rui Li Jaturong Kumla
Jaturong Kumla Nakarin Suwannarach
Nakarin Suwannarach Abdallah M. Elgorban
Abdallah M. Elgorban Ihab M. Moussa
Ihab M. Moussa Dong-Qin Dai
Dong-Qin Dai Nalin N. Wijayawardene
Nalin N. Wijayawardene