- 1School of Kinesiology and Health, Capital University of Physical Education and Sports, Beijing, China
- 2Department of Hepatology, Beijing Youan Hospital, Capital Medical University, Beijing, China
Objective: The oral microbiota is the second largest microbiota in the human body and has a significant impact on human health. Recent evidence suggests that dysbiosis of the oral microbiota may be associated with the development of metabolism-associated fatty liver disease (MAFLD). This review aimed to validate the relationship between oral microbial diversity and the development of MAFLD.
Methods: A systematic evaluation was performed based on PRISMA guidelines. Three independent reviewers searched for relevant literature in several databases, including PubMed/Medline, Web of Science, and Scopus, with a search date ranging from the establishment of the databases to June 2024.
Results: A total of 1278 publications were initially screened, including five cross-sectional studies, seven case-control studies, one cohort study, and one retrospective study. These studies included a total of 3335 patients with MAFLD, 254 patients with MASH, and 105 patients with liver cirrhosis. All 14 included studies concluded that there was a correlation or potential correlation between oral microbiota and MAFLD. Seven studies found that the composition of the oral microbiota in MAFLD patients differed from that of healthy controls, and specific oral bacteria may be associated with an increased incidence of MAFLD. At the phylum level, several studies found differences in the abundance of the phyla Firmicutes, Proteobacteria, and Clostridia compared to healthy controls. Additionally, a study on oral fungi found significant differences in the phyla Proteobacteria and in the genus Staphylococcus between patients with MAFLD and healthy controls. At the genus level, Porphyromonas was studied most frequently, with all 8 studies identifying infection with Porphyromonas as a significant risk factor for pathological progression in MAFLD. Furthermore, a dysbiosis in the ratio of Porphyromonas gingivalis./Porphyromonas anomalies may be an important marker of MAFLD progression.
Conclusion: There is an important association between the diversity of oral microbiota composition and MAFLD. This finding suggests the importance of oral health assessment and monitoring for the prevention or intervention of MAFLD.
1 Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a chronic liver disease linked to metabolic stress, resulting from overnutrition and insulin resistance in genetically susceptible individuals (Eslam et al., 2022). According to the diagnostic criteria set out in the 2020 International Consensus Statement of Experts, MAFLD is characterized by the presence of fat accumulation and metabolic dysfunction in the liver. It encompasses the full spectrum of the disease, from simple steatosis without inflammation and fibrosis to stage 4 fibrosis. MAFLD affects up to one-third of the world’s population (Eslam et al., 2022), and its prevalence is increasing in line with the rising rates of type 2 diabetes mellitus and obesity. As the disease progresses, MAFLD can advance to MASH (Metabolic Associated Steatohepatitis), characterized by hepatic inflammation and fibrosis, which significantly raises the risk of cirrhosis and hepatocellular carcinoma. MASH is a more severe form of MAFLD and is a crucial stage in the disease spectrum, often used as a marker for liver disease progression. If left unchecked, MASH can lead to end-stage liver disease, liver cancer, and death, and it may contribute to cardiometabolic disease.
MAFLD and MASH have been shown to be associated with obesity (Gutiérrez-Cuevas et al., 2021), insulin resistance (IR) (Sakurai et al., 2021), glucose abnormalities (Xue et al., 2022), and oxidative stress (Clare et al., 2022). Several recent studies have suggested that the human microbiota plays an important role in the development of MAFLD and its progression to MASH, not only through changes in the composition and relative abundance of gut microbiota but also through dysbiosis of the oral microbiota (Cani, 2018; Flemer et al., 2017). Dysregulation of the oral microbiota may further exacerbate hepatic inflammation, contributing to the transition from simple steatosis to MASH, emphasizing the need to study the role of oral bacteria in disease progression.
The oral microbiota is the second most diverse and numerous microbiota in the human body (Deo and Deshmukh, 2019; Suárez et al., 2021), containing approximately 700 species that form a complex ecological community. This microbiome includes a highly diverse array of viruses, fungi, bacteria, archaea, and protozoa (Caselli et al., 2020). As the oral cavity is considered the main entrance to the body, the microbiota within this ecological niche is likely to affect all parts of the body. When dysbiosis occurs in oral bacteria, it can lead to chronic inflammation, immune dysregulation, and damage to the oral mucosa, using it as a conduit to reach the bloodstream (Suárez et al., 2020). Dysbiosis of the oral microbiota has been associated with the development and progression of MAFLD diseases (Hajishengallis and Chavakis, 2021; Kuraji et al., 2021).
In recent years, there has been an increasing number of studies on the relationship between oral microbiota and MAFLD. One animal study suggested that endotoxemia caused by P.gingivalis is a significant risk factor for the development of MAFLD, and that altered glucose/lipid metabolism may contribute to the progression of the disease (Wang et al., 2022). Additionally, studies have shown that the oral microbiota remains dynamic and is influenced by different lifestyles, such as diet, stress, tobacco consumption, and systemic conditions, which alter the composition and characterization of the oral microbiota, thereby affecting disease progression in MAFLD (Baker and Edlund, 2019; Lee et al., 2021). Given that the oral cavity is a complex microbiological environment, there is a need to further understand the changes in the oral microbial community in the pathological state of MAFLD.
This is the first systematic review to assess the relationship between oral microbiota and MAFLD, The purpose of this article is to explore and compare the composition and diversity of oral microbiota in individuals with MAFLD, drawing insights from published studies to expand knowledge about the role of oral microorganisms in the progression of the disease.
2 Materials and methods
2.1 Protocol and registration
This is a systematic review that was registered with the International Prospective Register of Systematic Reviews and was reported based on the PRISMA statement (Chandler et al., 2019; Stewart et al., 2015). Details of the review protocol were registered on the PROSPERO International Prospective Register of Systematic Reviews in July 2024 (http://www.crd.york.ac.uk/prospero/, registration number: CRD42024531033).
2.2 Eligibility criteria
Literature search was performed according to the PICOS principles. POPULATION: Adult patients diagnosed with MAFLD. Intervention: detection of oral microbiota in the presence of MAFLD. Control: adult population without MAFLD. RESULTS: Association between oral microbiota composition and MAFLD. STUDY DESIGN: Studies evaluating the existence of an association between oral microbiota and the prevalence of MAFLD, the study design could be a case-control study, a cohort study or a cross-sectional study. Exclusion criteria included reviews, conference abstracts, case reports, etc., in which studies did not provide clear data for analyses of the association between oral microbiota and MAFLD.
2.3 Information sources and search strategy
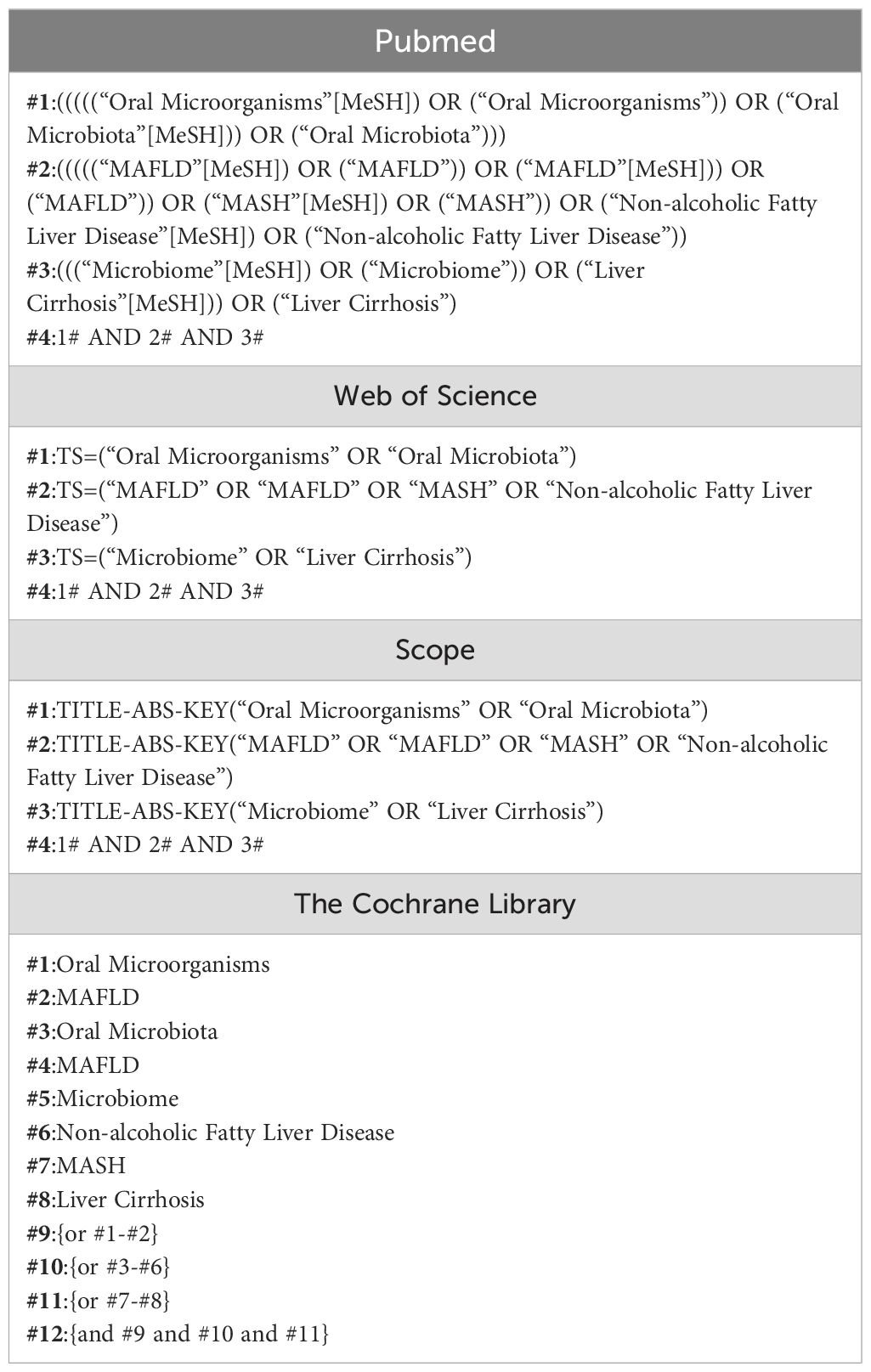
Three independent researchers searched the literature from Pubmed//Medline, Web of Science, Scopus, and the Cochrane Library using the following keyword terms: oral microorganisms, MAFLD, oral microbiota, non-alcoholic fatty liver disease. The search was performed up to 10 June 2024.The search strategy was as follows. The search strategy is shown in Table 1.
2.4 Data collection process
The included literature was analyzed and data extracted by two independent researchers. The included literature was imported into NoteExpress software after literature search to remove duplicates, followed by the first round of screening by reading the title and abstract, and then reading the full text to complete the second round of screening. The extracted data included first author’s name, country, journal, year of publication, number of patients, patient’s age, oral diagnosis, type of microbiota analysis, sample extraction, testing method and main results. In case of disagreement during this period, a third researcher was involved in the decision.
2.5 Quality assessment of included studies
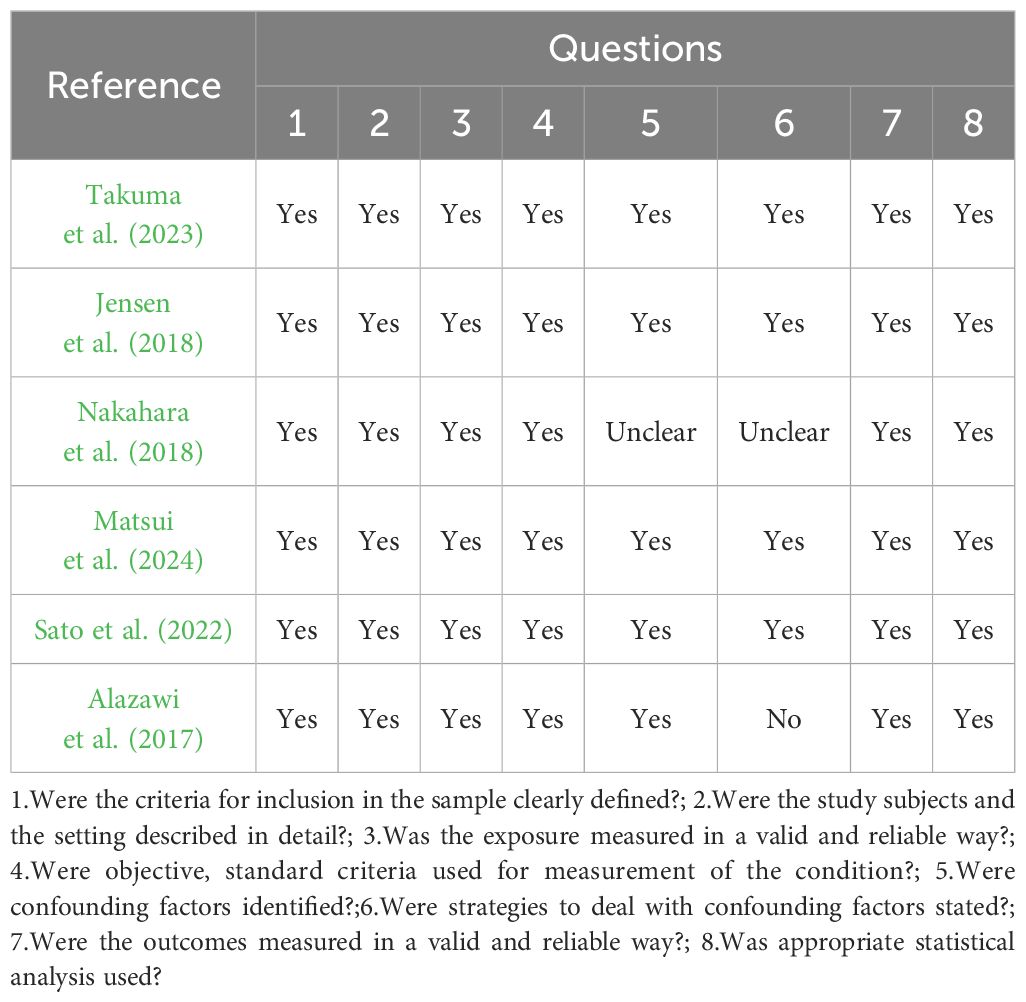
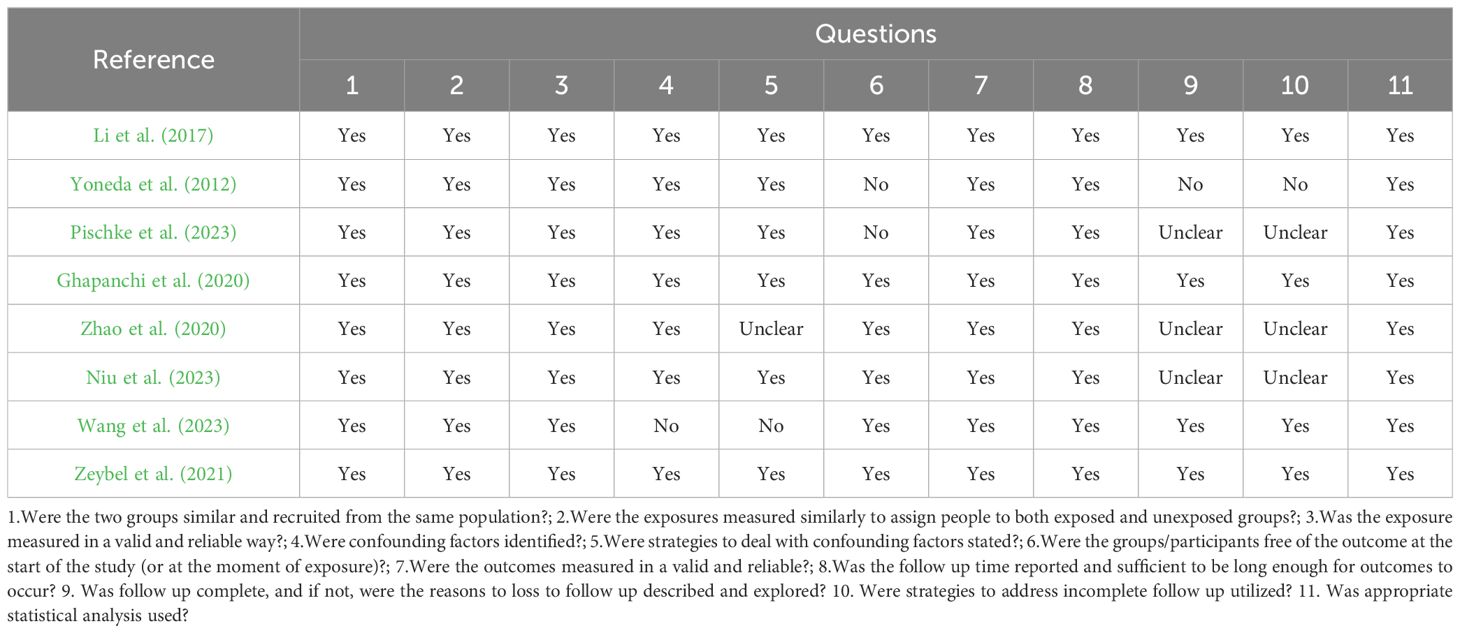
Depending on the type of design of the study, quality was evaluated using the Joanna Briggs Institute (JBI) Analytic Cross-Sectional Study Critical Appraisal Checklist (Moola et al., 2017).The JBI scale is a tool used to assess the quality of a cross-sectional study in terms of the sample frame, participant selection, undertakings and description of the results, confounding factor considerations, measurement instruments, data collection methods, statistical analysis methods, and interpretation of the results, respectively, for study quality were developed. According to the JBI tool, each study was categorized as: ‘High Quality’ (HQ) - high scores in all assessed domains; ‘Moderate Quality’ (MQ) - good performance in most domains with minor deficiencies; “low quality” (LQ)-poor performance in multiple domains and questionable credibility; and “very low quality” (VQ)-serious quality deficiencies; “Distal Quality” (UQ)-Some domains are scored unclearly or with questionable credibility. When using the JBI scale, the quality of the study was assessed on an item-by-item basis and scores were recorded (e.g., “yes,” “no,” or “unclear”) to synthesize the quality of the study. Two authors assessed each study independently and any disagreements were discussed with a third researcher.
3 Results
3.1 Literature search
The search strategy is shown in Figure 1, and after the first round of search, a total of 1,271 studies were retrieved from Medline (n = 254), Pubmed (n = 227), Web of Science (n = 323), Embase (n = 192), and Scopus (n = 275), and 7 studies were retrieved by other methods. After deletion of duplicates, 965 studies remained. By reading the titles, abstracts, and according to the inclusion and exclusion criteria, 152 studies remained, and further reading of the full text resulted in the inclusion of 14 studies.
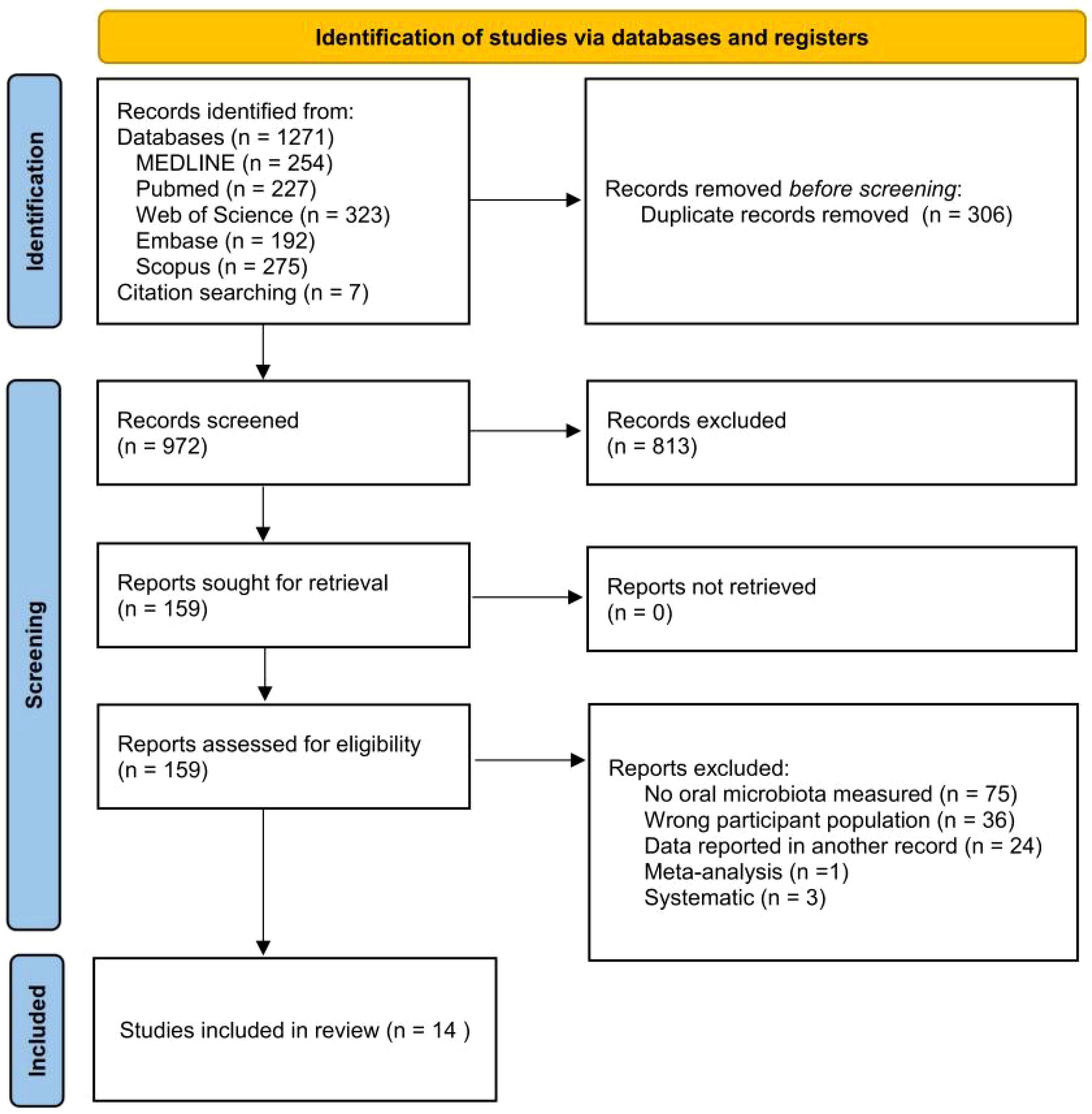
Figure 1. PRISMA flowchart diagram. From Page et al. (2021).
3.2 Description of the studies
The 14 included studies were published between 2012 and 2024 and consisted of 5 cross-sectional studies, 7 case-control studies, 1 cohort study, and 1 retrospective study. The studies were conducted in Japan (n=4), United Kingdom (n=2), China (n=4), Turkey (n=1), Iran (n=1), Denmark (n=1), and Germany (n=1). Regarding the type of liver disease, eight studies (57%) investigated the oral microbiological composition of patients with MAFLD (Alazawi et al., 2017; Zhao et al., 2020; Li et al., 2018; Nakahara et al., 2018; Niu et al., 2023; Sato et al., 2022; Wang et al., 2023; Zeybel et al., 2021), four studies investigated the status of oral microbiology in patients with MASH (Matsui et al., 2024; Nakahara et al., 2018; Pischke et al., 2023; Takuma et al., 2023) and two studies investigated the composition of oral microbiology in patients with liver cirrhosis (Ghapanchi et al., 2020; Jensen et al., 2018). All studies assessed the oral microbiological status of the chronic liver disease population and included a total of 3335 patients with MAFLD, 254 patients with MASH and 105 patients with liver cirrhosis (see Table 2).
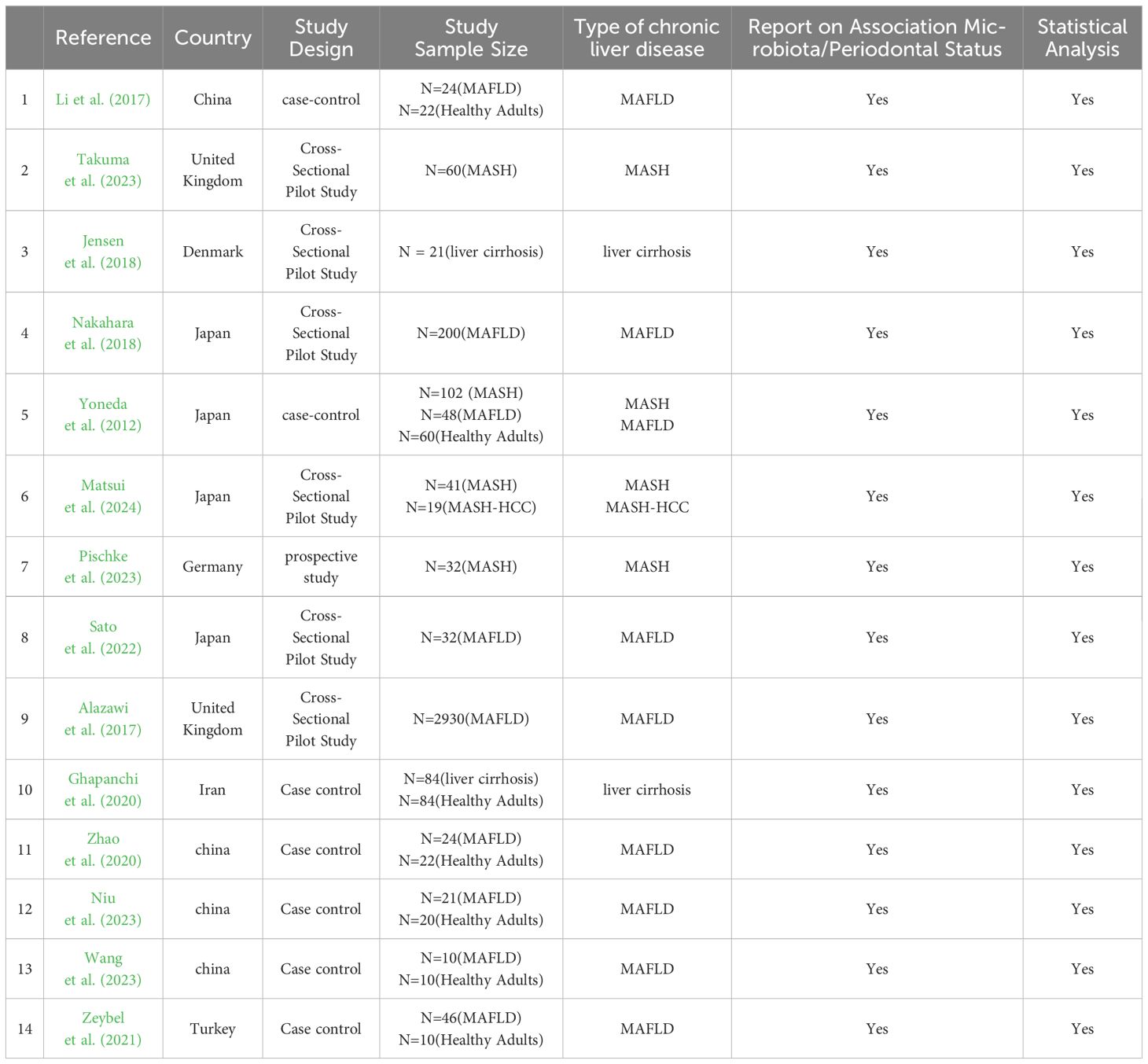
Table 2. General characteristics of the selected studies investigating the association between oral microbiota and MAFLD.
3.3 Quality assessment and risk of bias of included studies
According to JBI Critical Appraisal Checklist for Analytical Cross-Sectional, four studies fulfilled all required questions (Jensen et al., 2018; Matsui et al., 2024; Sato et al., 2022; Takuma et al., 2023). Two studies satisfied seven questions (Alazawi et al., 2017; Nakahara et al., 2018). The most unanswered questions were related to strategies to deal with confounding factors as shown in Table 3.
Three studies attended to most requirements according to the JBI checklist for cohort studies, suiting eleven questions (Ghapanchi et al., 2020; Li et al., 2018; Zeybel et al., 2021). Three studies satisfied eight questions (Zhao et al., 2020; Pischke et al., 2023; Yoneda et al., 2012) (see Table 4). Two studies satisfied nine questions (Niu et al., 2023; Wang et al., 2023) (see Table 4).
3.4 Oral microbiota and MAFLD
Eight studies investigated the association between oral microbiota and MAFLD, consistently finding correlations between specific phyla or genera of oral microbiota and the disease. Adamo et al. (2023) discovered that, compared to healthy controls, MAFLD patients had a higher relative abundance of Firmicutes and a lower abundance of Bacteroidetes in their saliva. Additionally, the proportions of Clostridium, Porphyromonas, and Veillonella were higher, while the proportions of Prevotella were significantly lower in the oral microbiota of MAFLD patients.
Nakahara et al. (2018) found that periodontal pathogens and intestinal bacteria do not directly influence hepatocellular carcinoma (HCC). However, HCC has a direct impact on the salivary periodontal bacterial species, including Porphyromonas gingivalis, Tannerella forsythia, Fusobacterium nucleatum, and Prevotella intermedia.
P.g infection is an important risk factor for pathological progression in MAFLD. Increase in the monounsaturated/saturated fatty acid ratio may be an important change that facilitates progression of MAFLD.
Sato et al. (2022) aimed to demonstrate a close association between MAFLD, particularly liver stiffness, and the presence of Porphyromonas gingivalis. Yumiko Nagao et al. Alazawi et al. (2017) found that the frequency of type 4 Porphyromonas gingivalis infection increased with the progression of fibrosis stages. Alazawi et al. (2017) identified that antibodies to two specific bacteria, Selenomonas noxia (OR=1.13) and Streptococcus oralis (OR=1.14), are closely associated with steatosis (OR=1.14).
Zhao et al. (2020) revealed increased abundances of bacteria associated with oral infections and decreased abundances of potentially beneficial aerobic bacteria. Niu et al. (2023) findings illustrate the potential correlation between core mycobiome and the development of MAFLD and could propose potential therapeutic strategies.
Zeybel et al. (2021) compared MAFLD patients with varying disease severity to healthy individuals and found significant differences in oral microbiota composition. Patients with mild MAFLD had significantly lower abundances of specific species in Firmicutes (Porphyromonas spp. and Fusobacterium rectum), anaplastic bacilli (Porphyromonas przewalskii), and actinomycetes (Matharomycetes). Moderate MAFLD patients showed increased abundances in Firmicutes (Fusobacterium) and decreased in anaplastic bacilli (Campylobacter and Haemophilus pusillus). Severe MAFLD patients exhibited reduced abundances in Anaplasma (Porphyromonas endodontiae and Prevotella), Aspergillus (Haemophilus pusillus), and increased Actinobacteria (Actinobacillus johnsonii). Additionally, both severe and moderate steatosis patients had significantly reduced sputum Haemophilus abundances compared to the healthy population (see Table 5).
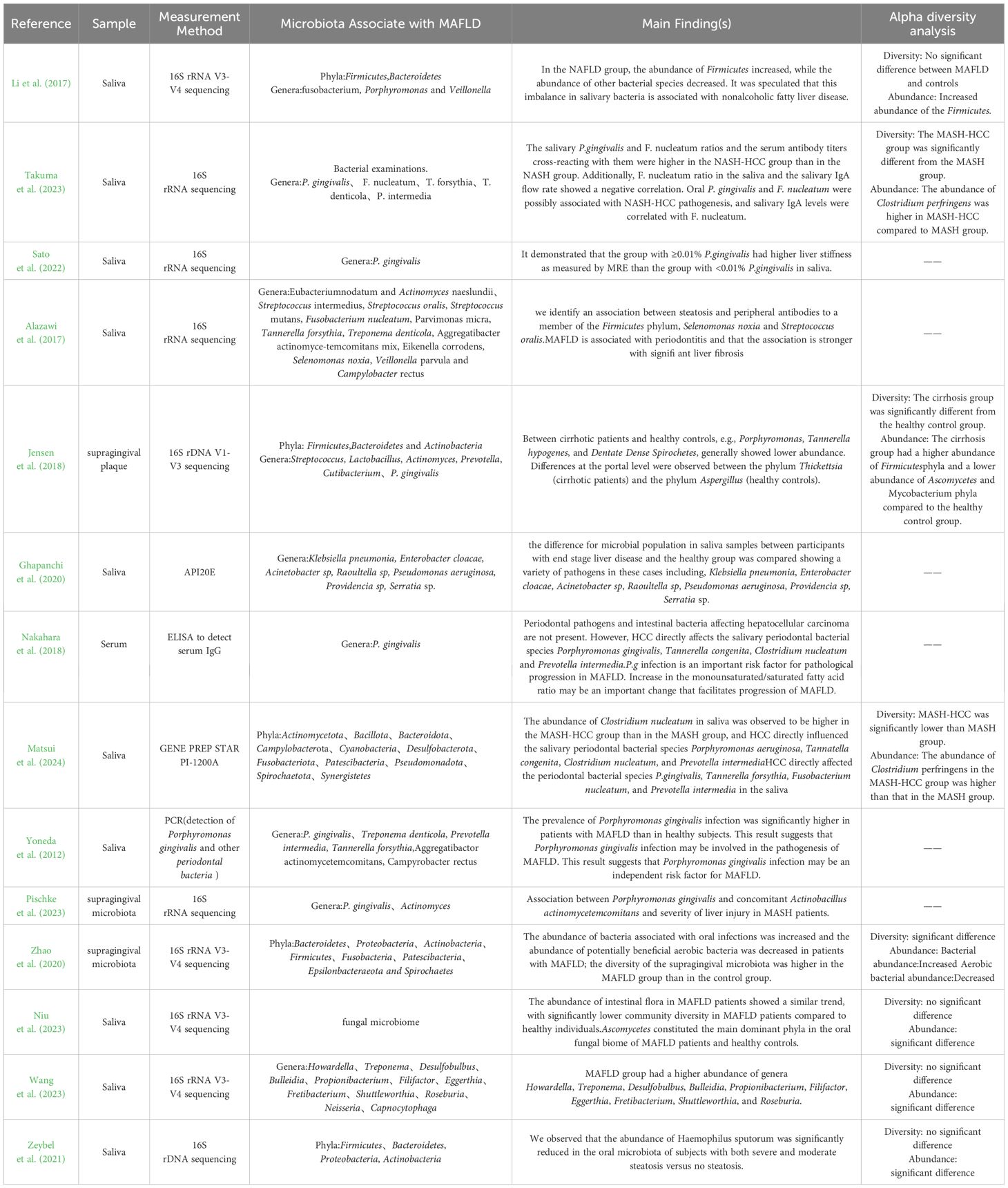
Table 5. Outcomes of the selected studies investigating the association between oral microbiota and MAFLD.
3.5 Oral microbiota and MASH and liver cirrhosis
Four studies examined the oral microbiota in patients with MASH (Matsui et al., 2024; Nakahara et al., 2018; Pischke et al., 2023; Takuma et al., 2023), and two studies investigated the oral microbiota composition in patients with liver cirrhosis (Ghapanchi et al., 2020; Jensen et al., 2018). Takuma et al. (2023) (2023) compared the oral microbiota between MASH and liver cancer groups, finding that the proportions of Porphyromonas gingivalis and Fusobacterium nucleatum were significantly higher in the liver cancer group. Matsui et al. (2024) recruited 41 MASH patients and 19 MASH-HCC patients to analyze the periodontal bacteria and oral microbiota composition, discovering that liver cancer directly affected the levels of P. gingivalis, Tannerella forsythia, F. nucleatum, and Prevotella intermedia in saliva, although periodontal pathogens and oral microbiota did not directly impact liver cancer progression (Matsui et al., 2024). Jensen et al. (2018) found that patients with liver cirrhosis had generally lower abundances of P. gingivalis, T. forsythia, and Treponema denticola compared to healthy controls. Ghapanchi et al. (2020) reported significantly higher levels of Escherichia coli in patients with liver cirrhosis compared to healthy individuals (see Table 5).
4 Discussion
Oral microbiota, recognized as the second largest microbiota in the human body, significantly influences human health. Maintaining the homeostasis of oral microorganisms helps the body resist adverse external stimuli. In contrast, dysregulation can lead to both oral and systemic diseases (Qi et al., 2021). Studies have established a close association between oral microbes and the development of various conditions, including digestive diseases, cardiovascular diseases, bone-related diseases, and Alzheimer’s disease (Bastos et al., 2011; Hu et al., 2017). In recent years, the link between salivary oral microbiota and metabolic-associated fatty liver disease (MAFLD) has garnered increasing research attention. Most studies focus on elucidating the mechanisms of MAFLD, enhancing predictive diagnostics, and identifying potential interventional biomarkers through oral microbes (Åberg and Helenius-Hietala, 2022; Nagao et al., 2014). Given the critical role of oral microbiota in relation to MAFLD, this study provides a systematic review of the current literature, aiming to deepen the understanding of the possible relationship between oral health and MAFLD.
This review analyzed a total of 14 studies, which collectively involved 3,335 patients diagnosed with MAFLD, along with 254 patients with MASH and 105 patients with liver cirrhosis. Each study indicated a correlation or potential correlation between specific phyla or genera of oral microbiota and MAFLD. Notably, seven studies demonstrated that the composition of the oral microbiota in patients with MAFLD was distinct from that of healthy controls, suggesting a link between particular oral bacteria and an elevated risk of developing MAFLD. Regarding phyla, several studies reported significant differences in the abundance of Firmicutes, Ascomycota, and Clostridia in comparison to healthy controls (R. Li et al., 2017; Matsui et al., 2024; Nakahara et al., 2018; Takuma et al., 2023). Additionally, research focused on oral fungi found notable differences in the phyla Ascomycota and the genus Staphylococcus among individuals with MAFLD compared to healthy individuals. Among the genera studied, Porphyromonas emerged as a significant focus, with all ten studies indicating a relationship between Porphyromonas and the progression of MAFLD, highlighting its relevance in this area of investigation.
Oral microbial diversity is crucial for maintaining health and varies with the physiological state of the host (Giordano-Kelhoffer et al., 2022). Increased microbiome richness and diversity are recognized as markers of healthy ecosystems, particularly within oral microbial ecosystems (Falony et al., 2019). Alpha diversity analyses of oral microbiota, which include both richness and diversity dimensions, utilize commonly used richness indices such as Chao1 and ACE, and diversity indices such as Shannon and Simpson (L. Wang et al., 2019).Among the studies included in this review, eight conducted Alpha diversity analysis (Zhao et al., 2020; Jensen et al., 2018; Li et al., 2017; Matsui et al., 2024; Niu et al., 2023; Takuma et al., 2023; Wang et al., 2023; Zeybel et al., 2021), but results varied across studies. Five of these studies compared the oral microbiota of patients with MAFLD to that of healthy controls, with only one study showing a significant difference in diversity between the two groups. However, in terms of abundance, all five studies demonstrated that the diversity of the gingival microbiota was higher in the MAFLD group compared to the healthy control group. This discrepancy may be due to the sampling methodologies; four studies derived their samples from saliva, whereas the study showing a significant difference used supragingival microbiota. The oral cavity contains different sites that provide specific niches for microbial colonization characterized by varying oxygen levels, nutrient availability, and mechanical stress conditions. Consequently, the type of sample used could significantly impact the results when assessing the link between oral microbiota and MAFLD. Additionally, Alpha diversity analysis was performed on the MASH-HCC group and the MASH group, revealing significant differences between the two groups in terms of both diversity and abundance. The abundance of C. nucleatum was notably higher in the MASH-HCC group than in the MASH group, suggesting a potential role of C. nucleatum in the progression of NASH to hepatocellular carcinoma. In conclusion, while healthy subjects did not show differences in the diversity of salivary flora, they did show differences in the abundance of certain flora, such as Phylum Firmicutes and Clostridium nucleatum, compared to MAFLD patients. MASH shows differences in both diversity and abundance when compared to MASH-HCC. Given that sampling in the included studies was predominantly from saliva, with only two studies sampling from supragingival microbiota, further research is needed to validate the impact of different sampling methods on the differences observed in the flora.
Another finding of this review is that a dysregulated ratio of Firmicutes to Bacteroidetes may be an important marker of MAFLD progression (Stojanov et al., 2020). A lower ratio of these phyla is considered characteristic of oral and intestinal health. Previously, numerous studies have identified dysbiosis in the intestinal flora of patients with MAFLD and have suggested that this imbalance is associated with steatosis and fibrosis in the liver (Abenavoli et al., 2023; Jasirwan et al., 2021). Similarly, the dysregulation of the ratio of Bacteroides Firmicutesto Bacteroides anthropophilus was also confirmed in oral microbiology. Li et al. (2017) found this dysregulation in the oral microbiological analyses of patients with MAFLD and suggested that it was a contributing factor in the pathogenesis of MAFLD induced by the intestinal flora. Additionally, Zhao et al. (2020) observed a lower percentage of the Firmicutesphylum to anabolic phylum in the supragingival plaque of the control group compared to the MAFLD group (61.41% and 72.38%, respectively). Moreover, specific strains within the Firmicutesphylum, such as Lactobacillus and Streptococcus, were found to have increased abundance in patients with MAFLD, potentially exacerbating the metabolic imbalance and inflammatory response. Overall, these studies suggest that the dysregulation of the Firmicutes phylum to Anthrobacteria phylum ratio is not only a crucial hallmark of MAFLD but may also play a key role in its pathogenesis and progression by influencing the gut-hepatic axis mechanisms. Future studies should continue to explore the specific mechanisms of this disproportion and evaluate potential interventions to treat MAFLD by modulating both the gut and oral microbiota.
Porphyromonas, a Gram-negative anaerobic bacterium, is widely recognized as a primary causative agent of chronic periodontitis (Darveau, 2010). Beyond oral health, Porphyromonas is considered a confounding risk factor for systemic diseases such as cardiovascular disease, diabetes mellitus (DM), preterm birth, and rheumatoid arthritis (Figuero et al., 2011; Pizzo et al., 2010; Seymour et al., 2007; Wada and Kamisaki, 2010). Yoneda et al. (2012) discovered that the prevalence of Porphyromonas infections was significantly higher in patients with MAFLD than in healthy subjects. Similarly, Takuma et al. (2023) found that the proportion of Porphyromonas and Clostridium nucleatum in saliva was higher in the NASH-HCC group compared to the NASH group. Satsuki Sato observed that the group with ≥0.01% Porphyromonas in their saliva had a higher proportion of Porphyromonas gingivalis than the group with less than 0.01%. Additionally, the Aeromonas gingivalis group with less than 0.01% exhibited greater liver stiffness as measured by Magnetic Resonance Elastography (MRE).Several studies have demonstrated that oral administration of Porphyromonas gingivalis can lead to dysbiosis of the intestinal flora, decreased expression of ileocecal connexin genes, and impaired intestinal barrier function. This disruption may subsequently result in systemic endotoxemia, insulinemia, and hepatic steatosis (Ohtsu et al., 2019; Sohn et al., 2022). Furthermore, an increase in Porphyromonas DNA has been detected in the liver following oral administration of the bacteria. Collectively, this body of evidence suggests that Porphyromonas gingivalis infection may be an independent risk factor for MAFLD/MASH.
The potential translocation ability of the oral microbiota through the circulation system or following the flow of food and fluids into the digestive system could explain its presence in the gut and its role in MAFLD (Sun et al., 2020). Tonetti et al. (2013) mentioned that oral microbiota may contribute to the development of MAFLD through two mechanisms. First, oral microbiota might reach the intestines, disrupt the intestinal barrier, and affect the liver through the ‘gut-liver axis,’ leading to a chronic inflammatory response and promoting lipid deposition in the liver. Second, oral microbiota and their metabolites could enter the bloodstream via periodontal pockets, or the inflammatory reactions caused by oral bacteria might release inflammatory mediators into the bloodstream, contributing to systemic inflammation and liver damage.
The limitations of this systematic review include the relatively low number of participants and the small number of published studies meeting the inclusion criteria. While the studies employed various methods and sample types to analyze the oral microbiota, this variability should not necessarily be viewed as a limitation. Different regions of the oral cavity, such as saliva or supragingival plaque, can provide valuable insights, and the use of diverse detection methods—such as 16S rRNA sequencing or PCR—offers complementary advantages. For example, 16S rRNA sequencing, which relies on PCR amplification of specific ribosomal RNA fragments, is useful for broad bacterial community profiling, whereas other PCR methods may target specific microbial species. Each method has its own strengths, and a combination of approaches may be necessary in certain cases. Future research should focus on increasing sample sizes and exploring the complementary use of multiple methods to gain a more comprehensive understanding of the relationship between oral microbiota and MAFLD.
Many studies did not adequately address potential confounding factors that could influence the relationship between oral microbiota and MAFLD. These confounding factors include, but are not limited to, participants’ age, gender, dietary habits, smoking status, and overall health conditions. Failure to effectively control these confounding factors can lead to biased or spurious associations, affecting the reliability of the study results. For instance, some studies may not consider participants’ dietary habits, which can significantly impact the composition of the oral microbiota. To enhance the accuracy and reliability of future research, it is crucial to carefully identify and control these confounding factors and use appropriate statistical methods to adjust for these variables.
The type of sample used (e.g., saliva, supragingival plaque) can significantly influence the study results, as different oral sites may harbor distinct microbial communities. The oral cavity is a complex environment where various sites have different oxygen levels, nutrient availability, and mechanical stress conditions, leading to variations in microbial distribution and species. Therefore, differences in sampling techniques add complexity to interpreting the results. For example, saliva samples may better reflect the overall oral microbiota, whereas supragingival plaque samples may better represent local microbial communities. To obtain more reliable results when assessing the link between oral microbiota and MAFLD, future research should standardize sampling techniques and consider the characteristics of microbial communities at different oral sites.
To our knowledge, this is the first literature systematic review that evaluated the possible relationships between oral microbiota and MAFLD. They confirmed our hypothesis that there is relationship between the composition and diversity of oral microbiota with MAFLD. Further experimental investigations are necessary to confirm the conclusions presented in this systematic review. These results point to the importance to develop studies that could considerably aid the clinical management of MAFLD. Therefore, Assessment of the oral microbiota’s helps predict and prevent MAFLD.
5 Conclusions
The link between the diversity of oral microbiota and MAFLD underscores the critical role of assessing and monitoring oral health in the prevention and management of MAFLD. This connection highlights the potential benefits of prioritizing oral healthcare in strategies aimed at addressing MAFLD.
Author contributions
MH: Methodology, Writing – original draft. XZ: Writing – review & editing. RZ: Resources, Supervision, Writing – original draft. YS: Investigation, Writing – original draft. JZ: Writing – review & editing. JW: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abenavoli, L., Scarlata, G. G. M., Scarpellini, E., Boccuto, L., Spagnuolo, R., Tilocca, B., et al. (2023). Metabolic-dysfunction-associated fatty liver disease and gut microbiota: from fatty liver to dysmetabolic syndrome. Medicina 59, 594. doi: 10.3390/medicina59030594
Åberg, F. O., Helenius-Hietala, J. (2022). Oral health and liver disease: bidirectional associations—A narrative review. Dentistry J. 10, 16. doi: 10.3390/dj10020016
Adamo, T., Ghiani, G., Guerriero, E. (2023). Recovering feasibility in real-time conflict-free vehicle routing. Comput. Ind. Eng. 183, 10. doi: 10.1016/j.cie.2023.109437
Alazawi, W., Bernabé, E., Tai, D., Janicki, T. J., Kemos, P., Samsuddin, S., et al. (2017). Periodontitis is associated with significant hepatic fibrosis in patients with non-alcoholic fatty liver disease. PloS One 12, e0185902. doi: 10.1371/journal.pone.0185902
Baker, J. L., Edlund, A. (2019). Exploiting the oral microbiome to prevent tooth decay: has evolution already provided the best tools? Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03323
Bastos, J., d., A., Diniz, C. G., Bastos, M. G., Vilela, E. M., Silva, V. L., et al. (2011). Identification of periodontal pathogens and severity of periodontitis in patients with and without chronic kidney disease. Arch. Oral. Biol. 56, 804–811. doi: 10.1016/j.archoralbio.2010.12.006
Cani, P. D. (2018). Human gut microbiome: hopes, threats and promises. Gut 67, 1716–1725. doi: 10.1136/gutjnl-2018-316723
Caselli, E., Fabbri, C., D’Accolti, M., Soffritti, I., Bassi, C., Mazzacane, S., et al. (2020). Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. 20, 120. doi: 10.1186/s12866-020-01801-y
Chandler, J., Cumpston, M., Li, T., Page, M. J., Welch, V. (2019). Cochrane handbook for systematic reviews of interventions (Hoboken: Wiley).
Clare, K., Dillon, J. F., Brennan, P. N. (2022). Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J. Clin. Transl. Hepatol. 10, 939–946. doi: 10.14218/jcth.2022.00067
Darveau, R. P. (2010). Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8, 481–490. doi: 10.1038/nrmicro2337
Deo, P. N., Deshmukh, R. S. (2019). Oral microbiome: Unveiling the fundamentals. J. Oral. Maxillofac. Pathology: JOMFP 23, 122–128. doi: 10.4103/jomfp.JOMFP_304_18
Eslam, M., El-Serag, H. B., Francque, S., Sarin, S. K., Wei, L., Bugianesi, E., et al. (2022). Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat. Rev. Gastroenterol. Hepatol. 19, 638–651. doi: 10.1038/s41575-022-00635-5
Falony, G., Vandeputte, D., Caenepeel, C., Vieira-Silva, S., Daryoush, T., Vermeire, S., et al. (2019). The human microbiome in health and disease: hype or hope. Acta Clinica Belgica 74, 53–64. doi: 10.1080/17843286.2019.1583782
Figuero, E., Sánchez-Beltrán, M. C., Cuesta-Frechoso, S., Tejerina, J. M. R., del Castro, J. A., Gutiérrez, J. M., et al. (2011). Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J. periodontology 82, 1469–1477. doi: 10.1902/jop.2011.100719
Flemer, B., Warren, R. D., Barrett, M. P., Cisek, K., Das, A., Jeffery, I. B., et al. (2017). The oral microbiota in colorectal cancer is distinctive and predictive. Gut 67, 1454–1463. doi: 10.1136/gutjnl-2017-314814
Ghapanchi, J., Bazargani, A., Khorshidi, H., Erfani, M., Rezazadeh, F., Azad, A., et al. (2020). Isolation and identification of non- commensal pathogenic bacteria in the saliva of patients candidate for liver transplant: A cross sectional study in Shiraz, South of Iran. J. Dentistry 21, 81–86. doi: 10.30476/DENTJODS.2019.77854
Giordano-Kelhoffer, B., Lorca, C., March Llanes, J., Rábano, A., del Ser, T., Serra, A., et al. (2022). Oral microbiota, its equilibrium and implications in the pathophysiology of human diseases: A systematic review. Biomedicines 10, 1803. doi: 10.3390/biomedicines10081803
Gutiérrez-Cuevas, J., Santos, A., Armendariz-Borunda, J. (2021). Pathophysiological molecular mechanisms of obesity: A link between MAFLD and NASH with cardiovascular diseases. Int. J. Mol. Sci. 22, 11629. doi: 10.3390/ijms222111629
Hajishengallis, G., Chavakis, T. (2021). Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21, 426–440. doi: 10.1038/s41577-020-00488-6
Hu, J., Iragavarapu, S., Nadkarni, G. N., Huang, R., Erazo, M., Bao, X., et al. (2017). Location-specific oral microbiome possesses features associated with CKD. Kidney Int. Rep. 3, 193–204. doi: 10.1016/j.ekir.2017.08.018
Jasirwan, C. O. M., Muradi, A., Hasan, I., Simadibrata, M., Rinaldi, I. (2021). Correlation of gut Firmicutes/Bacteroidetes ratio with fibrosis and steatosis stratified by body mass index in patients with non-alcoholic fatty liver disease. Bioscience microbiota Food Health 40, 50–58. doi: 10.12938/bmfh.2020-046
Jensen, A., Ladegaard Grønkjær, L., Holmstrup, P., Vilstrup, H., Kilian, M. (2018). Unique subgingival microbiota associated with periodontitis in cirrhosis patients. Sci. Rep. 8, 10718. doi: 10.1038/s41598-018-28905-w
Kuraji, R., Sekino, S., Kapila, Y. L., Numabe, Y. (2021). Periodontal disease–related nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An emerging concept of oral-liver axis. Periodontology 87, 204–240. doi: 10.1111/prd.12387
Lee, Y.-H., Chung, S. W., Auh, Q.-S., Hong, S.-J., Lee, Y.-A., Jung, J., et al. (2021). Progress in oral microbiome related to oral and systemic diseases: an update. Diagnostics 11, 1283. doi: 10.3390/diagnostics11071283
Li, R., Liu, W., Zhang, P., Huang, J., Chen, Y., Luo, G., et al. (2017). Analysis in saliva microbiome composition in patients with NAFLD and normal subjects. J. Pract. Med. 33 (21), 3593–3597. doi: 10.3969/j.issn.1006-5725.2017.21.024
Li, F., Sun, G., Wang, Z., Wu, W., Guo, H., Peng, L., et al. (2018). Characteristics of fecal microbiota in non-alcoholic fatty liver disease patients. Sci. China Life Sci. 61, 770–778. doi: 10.1007/s11427-017-9303-9
Matsui, T., Morozumi, T., Yamamoto, Y., Kobayashi, T., Takuma, R., Yoneda, M., et al. (2024). Relationship of metabolic dysfunction-associated steatohepatitis-related hepatocellular carcinoma with oral and intestinal microbiota: A cross-sectional pilot study. Medicina 60, 1150. doi: 10.3390/medicina60071150
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., et al. (2017). “Systematic reviews of etiology and risk,” in Joanna Briggs Institute reviewer’s manual, vol. 5. (The Joanna Briggs Institute Adelaide, Australia), 217–269.
Nagao, Y., Kawahigashi, Y., Sata, M. (2014). Association of Periodontal Diseases and Liver Fibrosis in Patients With HCV and/or HBV infection. Hepatitis Monthly 14, e23264. doi: 10.5812/hepatmon.23264
Nakahara, T., Hyogo, H., Ono, A., Nagaoki, Y., Kawaoka, T., Miki, D., et al. (2018). Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 53, 269–280. doi: 10.1007/s00535-017-1368-4
Niu, C.-g., Tu, Y., Jin, Q., Chen, Z., Yuan, K.-y., Wang, M., et al. (2023). Mapping the human oral and gut fungal microbiota in patients with metabolic dysfunction-associated fatty liver disease. Front. Cell. Infection Microbiol. 13. doi: 10.3389/fcimb.2023.1157368
Ohtsu, A., Takeuchi, Y., Katagiri, S., Suda, W., Maekawa, S., Shiba, T., et al. (2019). Influence of Porphyromonas gingivalis in gut microbiota of streptozotocin-induced diabetic mice. Oral. Dis. 25, 868–880. doi: 10.1111/odi.2019.25.issue-3
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi: 10.1136/bmj.n71
Pischke, S., Shiprov, A., Peters, U., Schulze zur Wiesch, J., Kluwe, J., Westphal, T., et al. (2023). High prevalence of periodontal disease in patients with NASH- possible association of poor dental health with NASH severity. Ann. Hepatol. 28 (2), 100887. doi: 10.1016/j.aohep.2022.100887
Pizzo, G., Guiglia, R., Russo, L. L., Campisi, G. (2010). Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur. J. Intern. Med. 21, 496–502. doi: 10.1016/j.ejim.2010.07.011
Qi, Y., Wu, H.-m., Yang, Z., Zhou, Y., Jin, L., Yang, M.-f., et al. (2021). New insights into the role of oral microbiota dysbiosis in the pathogenesis of inflammatory bowel disease. Digestive Dis. Sci. 67, 42–55. doi: 10.1007/s10620-021-06837-2
Sakurai, Y., Kubota, N., Yamauchi, T., Kadowaki, T. (2021). Role of insulin resistance in MAFLD. Int. J. Mol. Sci. 22, 4156. doi: 10.3390/ijms22084156
Sato, S., Kamata, Y., Kessoku, T., Shimizu, T., Kobayashi, T., Kurihashi, T., et al. (2022). A cross-sectional study assessing the relationship between non-alcoholic fatty liver disease and periodontal disease. Sci. Rep. 12, 13621. doi: 10.1038/s41598-022-17917-2
Seymour, G., Ford, P., Cullinan, M., Leishman, S., Yamazaki, K. (2007). Relationship between periodontal infections and systemic disease. Clin. Microbiol. infection 13, 3–10. doi: 10.1111/j.1469-0691.2007.01798.x
Sohn, J., Li, L., Zhang, L., Settem, R. P., Honma, K., Sharma, A., et al. (2022). Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4+ T cells. Mol. Oral. Microbiol. 37, 42–52. doi: 10.1111/omi.12359
Stewart, L. A., Clarke, M., Rovers, M., Riley, R. D., Simmonds, M., Stewart, G., et al. (2015). Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA 313, 1657–1665. doi: 10.1001/jama.2015.3656
Stojanov, S., Berlec, A., Strukelj, B. (2020). The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 8, 1715. doi: 10.3390/microorganisms8111715
Suárez, L. J., Arboleda, S., Angelov, N., Arce, R. M. (2021). Oral versus gastrointestinal mucosal immune niches in homeostasis and allostasis. Front. Immunol. 12. doi: 10.3389/fimmu.2021.705206
Suárez, L. J., Garzón, H., Arboleda, S., Rodríguez, A. (2020). Oral dysbiosis and autoimmunity: from local periodontal responses to an imbalanced systemic immunity. A review. Front. Immunol. 11. doi: 10.3389/fimmu.2020.591255
Sun, J., Tang, Q., Yu, S., Xie, M., Xie, Y., Chen, G., et al. (2020). Role of the oral microbiota in cancer evolution and progression. Cancer Med. 9, 6306–6321. doi: 10.1002/cam4.v9.17
Takuma, R., Morozumi, T., Yamamoto, Y., Kobayashi, T., Matsui, T., Yoneda, M., et al. (2023). Association between non-alcoholic steatohepatitis-related hepatocellular carcinoma and periodontopathic bacteria: a cross-sectional pilot study. Appl. Sciences-Basel 13, 3893. doi: 10.3390/app13063893
Tonetti, M. S., Van Dyke, T. E., workshop*, w. g. o. t. j. E. A (2013). Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAPWorkshop on Periodontitis and Systemic Diseases. J. periodontology 84, S24–S29. doi: 10.1902/jop.2013.1340019
Wada, K., Kamisaki, Y. (2010). Roles of oral bacteria in cardiovascular diseases—from molecular mechanisms to clinical cases: involvement of Porphyromonas gingivalis in the development of human aortic aneurysm. J. Pharmacol. Sci. 113, 115–119. doi: 10.1254/jphs.09R22FM
Wang, T., Ishikawa, T., Sasaki, M., Chiba, T. (2022). Oral and gut microbial dysbiosis and non-alcoholic fatty liver disease: the central role of Porphyromonas gingivalis. Front. Med. (Lausanne) 9. doi: 10.3389/fmed.2022.822190
Wang, M., Yan, L., Qiao, C.-Y., Zheng, C., Niu, C.-g., Huang, Z., et al. (2023). Ecological shifts of salivary microbiota associated with metabolic-associated fatty liver disease. Front. Cell. Infection Microbiol. 13. doi: 10.3389/fcimb.2023.1131255
Wang, L., Yin, G., Guo, Y.-j., Zhao, Y., Zhao, M., Lai, Y., et al. (2019). Variations in oral microbiota composition are associated with a risk of throat cancer. Front. Cell. Infection Microbiol. 9. doi: 10.3389/fcimb.2019.00205
Xue, Y., Xu, J., Li, M., Gao, Y. (2022). Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index-related parameters. Front. Endocrinol. (Lausanne) 13. doi: 10.3389/fendo.2022.951689
Yoneda, M., Naka, S., Nakano, K., Wada, K., Endo, H., Mawatari, H., et al. (2012). Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 12, 16. doi: 10.1186/1471-230x-12-16
Zeybel, M., Arif, M., Li, X., Altay, O., Yang, H., Shi, M., et al. (2021). Multiomics analysis reveals the impact of microbiota on host metabolism in hepatic steatosis. Advanced Sci. 9, e2104373. doi: 10.1002/advs.202104373
Keywords: oral microbiota, oral microbiology, metabolism-associated fatty liver disease, systematic review, health
Citation: Huang M, Zhang X, Zhou R, Song Y, Zhang J and Wu J (2024) Advances in the study of oral microbiota and metabolism associated fatty liver disease: a systematic review. Front. Cell. Infect. Microbiol. 14:1491696. doi: 10.3389/fcimb.2024.1491696
Received: 05 September 2024; Accepted: 21 October 2024;
Published: 12 November 2024.
Edited by:
Soumyadev Sarkar, Arizona State University, United StatesReviewed by:
Shelley Sardul Singh, Intellia Therapeutics, Inc., United StatesTripti Nair, University of Southern California, United States
Copyright © 2024 Huang, Zhang, Zhou, Song, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wu, d3VqaWFuY3VwZXNAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Mingming Huang
Mingming Huang Xinbi Zhang
Xinbi Zhang Rui Zhou1
Rui Zhou1 Yingzhe Song
Yingzhe Song Jing Zhang
Jing Zhang Jian Wu
Jian Wu