- 1Key Laboratory of Microecology-immune Regulatory Network and Related Diseases, School of Basic Medicine, Jiamusi University, Jiamusi, Heilongjiang, China
- 2College of Pharmacy, Jiamusi University, Jiamusi, Heilongjiang, China
- 3School of Medical, Huzhou University, Huzhou, Zhejiang, China
Intestinal microbiota and its metabolites are involved in many physiological processes of the human body and play a vital role in maintaining human health. The occurrence of kidney disease can cause intestinal microbiota imbalance, resulting in diarrhea. The change of intestinal microbiota and its metabolites content can aggravate renal function injury, which has a bidirectional regulating effect. The theory of renal-intestinal axis further clarified that the impaired renal function is related to the imbalance of intestinal microorganisms, and the impaired intestinal barrier is related to the accumulation of toxin products. Because of its unique therapeutic advantages, Traditional Chinese Medicine can treat diarrhea by enhancing the growth of beneficial bacteria, inhibiting pathogenic bacteria and immune regulation, and slow down the continuous deterioration of kidney disease. This paper focuses on the relationship between intestinal microbiota and its metabolites and diarrhea, the influence of Traditional Chinese Medicine on intestinal microbiota in the treatment of diarrhea, and the role of intestinal microbiota and its metabolites in the renal-intestinal axis. It provides a theoretical basis for Traditional Chinese Medicine to regulate intestinal microbiota and its metabolites based on the renal-intestinal axis theory to treat nephrology-induced diarrhea, and also provides a new idea and method for Traitional Chinese Medicine to treat nephrology-induced diarrhea.
1 Introduction
As a common gastrointestinal disease, diarrhea not only affects the quality of life of patients, but also poses a certain threat to the health of the body. The intestine is an important nutrient absorption and microbial habitat of the human body, It is also the largest immune system of the human body, known as the “second brain”. The intestinal microbiota is composed of a large number of microbial communities in the intestine and has a symbiotic relationship with the host (Pushpanathan et al., 2019). They jointly maintain the homeostasis and balance of the intestinal environment by affecting the intestinal barrier function, immune response and metabolic activities, thus affecting the health of the host (Kataoka, 2016). The metabolites of intestinal microbiota are produced in the metabolic process of intestinal microbiota, which can directly or indirectly affect the physiological function of the host (Meng et al., 2020). With the deepening of research, the role of intestinal microbiota and its metabolites in maintaining human health has been paid more and more attention (Ramamurthy et al., 2022). The kidney is an important organ of the human body and the “processing center” of water and metabolites in the body. The functional state of the kidney will indirectly affect the microenvironment of the intestine. Damaged kidney function will cause intestinal microbiota imbalance. The disordered intestinal microbiota passes through the damaged intestinal mucosal barrier, causing harmful bacteria to invade and induce chronic inflammation, thus accelerating kidney damage (Kim et al., 2021; Cao et al., 2022). Renal-intestinal axis refers to the physiological and pathological mechanism of the interaction between the kidney and the intestine, and the two effect each other to form a dynamic balance system (Gong et al., 2019; Zhang et al., 2020). Traditional Chinese Medicine (TCM) has a long history of drug use in the treatment of diarrhea and shows unique advantages (Huang et al., 2023; Xue et al., 2023). Therefore, based on renal-intestinal axis theory, this paper summarizes the treatment of diarrhea by regulating the intestinal microbiota and its metabolites of TCM, and explores the role and mechanism of TCM in restoring the micro-ecological balance of the intestine, reducing the inflammatory response, and reducing the level of nephrotoxic metabolites by regulating the intestinal microbiota and its metabolites, and provides a broad application prospect for the treatment of diarrhea with TCM.
2 Intestinal microbiota and its metabolites and diarrhea
2.1 Intestinal microbiota and diarrhea
Intestinal microbiota is mainly composed of beneficial bacteria, harmful bacteria and neutral bacteria, and these microbial communities constitute a complex ecosystem in the intestine to maintain the health of the host body (Li et al., 2021a). Diarrhea, as a manifestation of intestinal dysfunction, is closely related to changes in intestinal microbiota (Shao et al., 2020; Liu et al., 2023). Diarrhea belongs to the category of TCM “diarrhea”, which can be generally divided into six syndrome types: diarrhea of intestinal dampness-heat syndrome, diarrhea of Ganqi Chengpi, diarrhea of spleen and stomach deficiency, diarrhea of stagnation of cold-damp, diarrhea of syndrome of retention of food in stomach, diarrhea of Kidney-Yang Deficiency Syndrome (Zhang et al., 2017; Li et al., 2021b). Studies have shown that diarrhea of intestinal dampness-heat syndrome model can change the structure of intestinal microbiota contents in mice, and the relative abundance of Neisseria were increased. The relative abundance of Lactobacillus, Clostridium and Muribaculum were decreased (Li et al., 2021c). An improper diet combined with high temperature and humidity environments, the abundance of Fusobacteria and Haemophilus in the model group was significantly increased (Qiao et al., 2023a). The intestinal microbiota Alpha diversity index of mice with diarrhea of Ganqi Chengpi was higher than that of normal group. The contents of, Lactobacillus, Bifidobacterium and Ecsherichia coli were significantly higher (Tang et al., 2020). The distribution of intestinal microbiota in patients with diarrhea, spleen and kidney Yang deficiency syndrome and liver stagnation and spleen deficiency syndrome was compared, and Streptococcus was the specific bacteria in the group of liver stagnation and spleen deficiency syndrome (Chao and Zhang, 2020). Intestinal microbiota of patients with diarrhea of Spleen and stomach deficiency, the relative abundance of Firmicutes was decreased, the relative abundance of Proteobacteria, Bacteroidota were increased (You et al., 2020). Bitter-cold purgation method with rhubarb induced to develop diarrhea of Spleen and stomach deficiency model in rats the relative abundance of Ascomycota was increased while the relative abundance of Basidiomycota and Bacteroidota was decreased (Xiao et al., 2023). The diarrhea of stagnation of cold-damp can change the microflora structureare. The relative abundance of Candidatus Arthromitus were decreased, and the relative abundance of Lactobacillus were increased. The Cyanobacteria unidentified specie were the predominant phyla of the stagnation of cold-damp diarrhea mouse model (Wu et al., 2024). In mice with diarrhea of syndrome of retention of food in stomach model, the intestinal microbiota of Bifidobacterium, Lactobacillus, Saccharopolyspora, Sinorhizobium and Ecsherichia coli, decreased significantly, the intestinal microbiota of Proteobacteria, Sphingobium, Actinobacteria and Rhizobium were increased (Guo et al., 2022; Zhou et al., 2022b; Zhou et al., 2023a). Diarrhea of kidney-yang deficiency syndrome can change the structure and function of intestinal microbiota in mice. The relative abundance of Candidatus, Arthromitus, Lactobacillus, Muribaculum, were increased, the relative abundance of Clostridium were decreased (Zhu et al., 2022; Zhou et al., 2024b). (summarized in Figure 1).
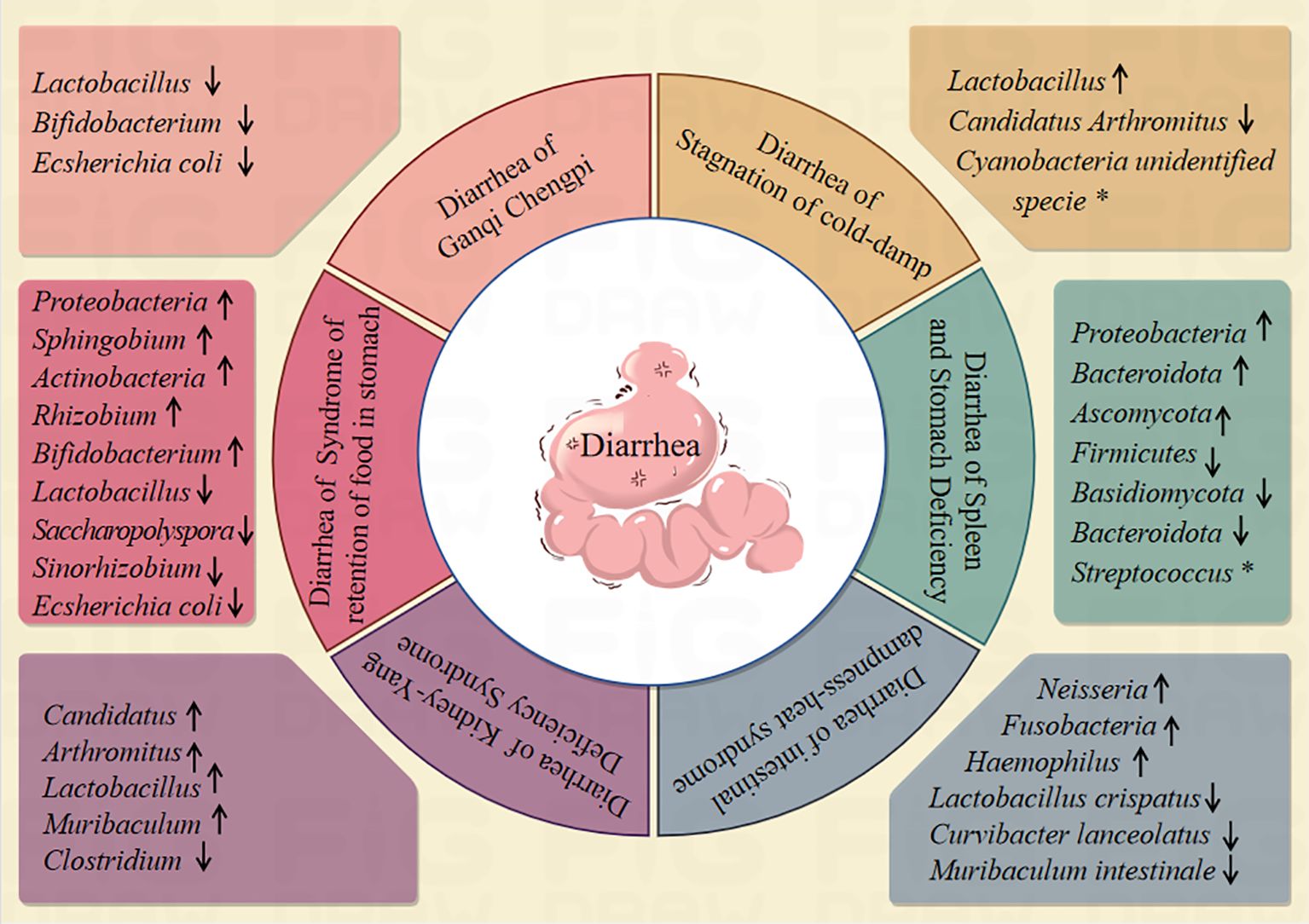
Figure 1. Relationship between diarrhoea and intestinal microbiota. (↑indicates an increase in intestinal microbiota abundance, ↓indicates an decrease in intestinal microbiota abundance, *indicates characteristic microbiota).
2.2 Metabolites of intestinal microbiota and diarrhea
Metabolites of Intestinal microbiota are produced by intestinal microbiota through metabolism. Metabolites of intestinal microbiota mainly include short-chain fatty acids(SCFAs), choline metabolites, lipids, vitamins, polyamines, etc (Zhang and Wang, 2024), these metabolites play an important physiological function in the intestine and can cause diarrhea. among which SCFAs is one of the important metabolites of intestinal microorganisms. Resistant starch and fiber in the colon are generated after fermentation by anaerobic bacteria, which can reduce the pH of the colon and inhibit the proliferation of pathogens, and mainly contain acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, etc (Shu et al., 2022).
Recent studies have confirmed that SCFAs can indirectly affect the homeostasis of intestinal microbiota by regulating neurotransmitter 5-hydroxytryptamine(5-HT), dopamine and norepinephrine (Dicks, 2022). Bile acids challenge gut microbes by disrupting the stability of macromolecules, which can interfere with RNA secondary structures, cause DNA damage, and promote protein misfolding (Winston and Theriot, 2020). Lipopolysaccharide(LPS) upregulated NLRP3 levels by activating TLR4 and inducing ROS production, resulting in pyroptosis, disruption of the intestinal barrier, ultimately leading to diarrhea (Liu et al., 2024). Trimethylamine oxide (TMAO) is a harmful product of intestinal microbiota metabolism, and its production is closely related to intestinal microbiota. When intestinal microbiota changes, it will directly affect the production of TMAO and cause inflammatory response. Inflammation-related molecules can through intestinal microbiota imbalance cause diarrhea (Guo et al., 2024).
3 Traditional Chinese medicine for the treatment of diarrhea and metabolites of intestinal microbiota
3.1 Mechanism of traditional Chinese medicine in treating diarrhea
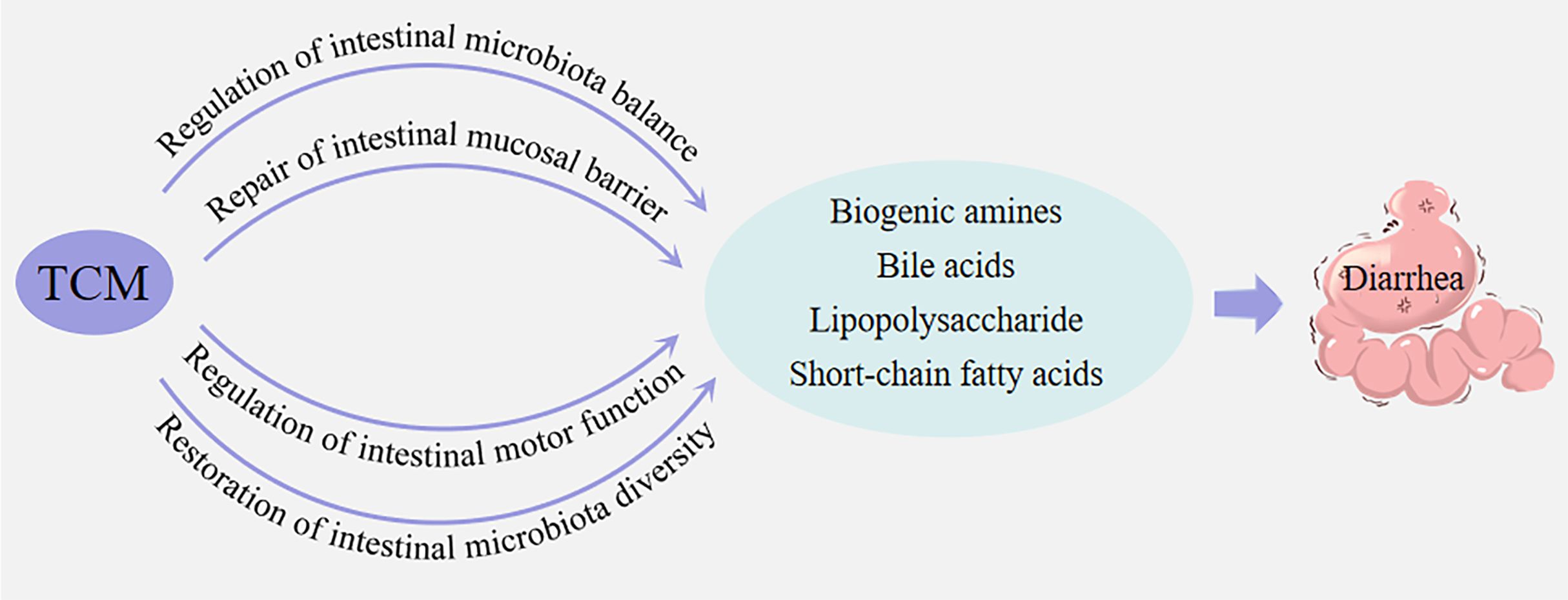
TCM has a long medicinal history in treating diarrhea. TMC believes that different intestinal microbiota are also in an interdependent but mutually restrictive dynamic balance. When this balance changes, the structure and biological characteristics of intestinal microbiota will also change, resulting in the imbalance of intestinal microbiota and thus the occurrence of diseases (Wang et al., 2024). A variety of active ingredients in TCM have bactericidal and bacteriostatic effects. While eliminating pathogenic bacteria, they regulate intestinal microbiota and restore intestinal environmental homeostasis, thereby reducing inflammatory response, repairing intestinal mucosal barrier, and treating diarrhea (He et al., 2018; Li et al., 2022b; Lai et al., 2023). The mechanisms of TMC in treating diarrhea are complex and diverse, it mainly regulates the balance of intestinal microbiota, repairs intestinal mucosal barrier, regulates intestinal motor function, and restores intestinal microbiota diversity.
3.1.1 Regulation of intestinal microbiota balance
By clearing heat and detoxifying, warming the middle and dispelling cold, strengthening the spleen and stomach, TCM regulates the intestinal microecological environment, promotes the growth of beneficial bacteria and inhibits the reproduction of harmful bacteria, so as to restore the balance of intestinal microbiota (Li et al., 2022). Gegen Qinlian decoction can reverse the decrease in the richness of intestinal microbiota, significantly increase the relative abundance of SCFA-producing bacteria, and regulate intestinal microbiota (Liu et al., 2019; Li et al., 2021d). “Butuyajie” formula has the effects of regulating soil tonifying tower, clearing heat and detoxification, astringent and diarrhea, regulating the imbalance of intestinal microbiota in rats with diarrhea, increasing the abundance of Firmicutes and decreasing the abundance of Bacteroidetes and Proteobacteria, thus achieving the effect of treating diarrhea (Xiao et al., 2023).
3.1.2 Repair of intestinal mucosal barrier
Intestinal mucosa is an important place for direct contact and communication between the internal environment and the outside world, and is the first line of defense against invasion of pathogens and harmful substances. The integrity of intestinal mucosal barrier is a prerequisite for maintaining normal intestinal function and health. Xianglian pills can restore the intestinal microbiota of mice with diarrhea, increase the expression of tight-link protein and the content of SCFAs, reduce the level of pro-inflammatory factors, alleviate intestinal mucosal damage, and improve diarrhea symptoms (Yang et al., 2021). Pingwei san can reduce inflammatory cell infiltration in the colon, promote the expression of aquaporins and tight junction markers, and play a therapeutic role in rhub-induced spleen-deficiency diarrhea in rats by protecting the intestinal barrier and regulating the imbalance of intestinal microbiota (Fan et al., 2023). TCM such as Zingiber officinale Rosc., Coptis chinensis Franch., and Panax quinquefolius L. and so on, also have the effect of reducing intestinal inflammation and repairing intestinal mucosal barrier (Zhou et al., 2021; Zhou et al., 2023; Kim et al., 2024).
3.1.3 Regulation of intestinal motor function
The main function of gastrointestinal motility is to regulate the passage of food and its residues for optimal digestion and absorption. imbalance between the mechanisms of absorption and secretion in the intestinal tract with loss of excess fluid in the stools, and stimulates peristaltic activity in the small intestines, leading to changes in intestinal mucosa permeability to electrolytes, it will cause diarrhea (Jabri et al., 2016). TCM is often used to regulate the intestinal movement function of Qi-regulating and digestion drugs, such as Baohe pill decoction and Weichang’an pills, to alleviate the occurrence of diarrhea by regulating qi and guiding stagnation and promoting intestinal peristalsis (Wei et al., 2018; Zhou et al., 2022a).
3.1.4 Restoration of intestinal microbiota diversity
The diversity of intestinal microbiota is a key factor in maintaining the homeostasis of intestinal microbial environment. It not only helps to repair intestinal microbiota structural disorders, but also promotes body health by increasing the abundance of beneficial bacteria and reducing the proportion of harmful bacteria (Xie et al., 2019; Miao et al., 2023). Chinese yam can improve diarrhea by increasing the diversity of intestinal microbiota, increasing the abundance of beneficial bacteria, improving the disorder of intestinal microbiota, and increasing the level of SCFAs (Zhang et al., 2019). Poria cocos Polysaccharide can improve αlpha diversity and beta diversity of intestinal microbiota mice with antibiotic-induced diarrhea, thus achieving the therapeutic effect on diarrhea (Xu et al., 2023). Qiweibaizhu powder can increase the abundance of Actinobacteria, Bacteroidetes and Proteobacteria in the intestinal mucosa of antibiotic-associated diarrhea mice, and restore the richness and diversity of intestinal microbiota (Hui et al., 2020).
3.2 The relationship between traditional Chinese medicine in the treatment of diarrhea and metabolites of intestinal microbiota
There is a close relationship between the treatment of diarrhea with TCM and the metabolites of intestinal microbiota. Mainly embodied in TCM can adjust the structure and function of intestinal microbiota, affect intestinal metabolites generated, and the amount and type of change. Through the antibacterial, anti-inflammatory and immune regulation functions of the active ingredients of TCM, they directly or indirectly act on intestinal microbiota, inhibit the growth of harmful bacteria, promote the reproduction of beneficial bacteria, and achieve the homeostatic balance of metabolites, thus achieving the purpose of treating diarrhea.
3.2.1 Regulates the generation of SCFAs
Changes in the structure of intestinal microbiota can affect the production of metabolites. SCFAs are metabolites of intestinal microbiota and are important agents of interaction between host and intestinal microbiota (Parada Venegas et al., 2019; Qiao et al., 2023b; Gallardo et al., 2024). Pomegranate peel polyphenols can generate abundant SCFAs by promoting the generation of SCFAs, improving the intestinal environment and alleviating the occurrence of diarrhea (Shi et al., 2022). Shenling Baizhu powder can reduce lipid metabolism disorders, regulate intestinal microbiota imbalance, significantly increase the content of acetic acid, butyric acid and valeric acid in SCFAs, and treat diarrhea (Qiao et al., 2024). In addition, TCM such as Atractylodes macrocephala and Aucklandia lappa can improve the structure of intestinal microbiota, reduce the expression of 5-HT and butyric acid, increase the expression of the 5-hydroxytryptamine-4 receptor and 5-hydroxytryptamine transporter protein in colon tissue, and alleviate diarrhea (Li et al., 2024).
3.2.2 Influence on the production of biogenic amines
Biogenic amines and other metabolites can promote intestinal peristalsis and increase intestinal permeability (Kuo et al., 2024). The heat-clearing antidote in TCM can relieve diarrhea symptoms by inhibiting the growth of harmful bacteria and reducing the production of harmful metabolites such as biological amines. TCM such as Aqueous cinnamon extract direct inhibition Both gene and protein levels of the colonic 5-HT synthetase, Tryptophan Hydroxylase 1 were also decreased in cinnamon extract treated irritable bowel syndrome(IBS) rats (Yu et al., 2023). Si-Ni-San can improve the abnormal intestinal microbiota induced by chronic restraint stress and inhibit the expression of dopamine β hydroxylase and c-fos in rat ventricles induced by chronic restraint stress. Inhibition of abnormal energy metabolism and decreased expression of occlusive hormone, The content of enterochromaffin cells, mast cells and 5-HT was inhibited (Chen et al., 2022).
3.2.3 Regulate the metabolic pathway of bile acids
Bile acids, the general name of cholanates in bile, play an important role in intestinal motility, lipid digestion and bacterial growth. Bile acids can induce accelerated colon movement, improve visceral sensitivity, and ultimately lead to an increase in the content of bile acids in stool (Ticho et al., 2019; Guo et al., 2022). Some components of TCM can improve the intestinal environment by regulating the metabolic pathway of intestinal microbiota, affecting the generation and metabolism of metabolites such as bile acids. Qiwei Baizhu powder to anti-diarrheal by increased the levels of deoxycholic acid and beta-muricholicacid and decreased those of taurocholate acid, tauro-alpha-muricholic acid, and tauro-beta-muricholic acid (Xie et al., 2022). The anti-diarrhea effect of Aconite aqueous was associated with significantly increased fecal taurocholic acid, deoxycholic acid, lithocholic acid, glycochenodeoxycholic acid, dehydro-lithocholic acid, and 12-ketolithocholic acid restoring bile acids homeostasis (Zhang et al., 2023).
3.2.4 Regulation of lipopolysaccharide synthesis
LPS is an important component of the outer membrane of gram-negative bacteria. When it enters the blood circulation, it will cause mild inflammatory response of the body. LPS not only acts on epithelial cells, but also regulates the immune response of the body, thus maintaining the immune homeostasis of the body, and has a regulatory effect on multiple tissues of the body. The combination of Persicaria hydropiper (L.) can regulate the production of LPS and maintain the mucosal barrier of intestinal microbiota, thus achieving the purpose of treating diarrhea (Cheng et al., 2024). Huosha oral liquid can improve the symptoms of irritable bowel syndrome diarrhea patients and reduce the levels of serum diamine oxidase, D-lactate and endotoxin, so as to play a role in the intervention of diarrhea (Lin et al., 2021). (summarized in Figure 2).
4 The role of intestinal microbiota and its metabolites in renal-intestinal axis
4.1 Renal-intestinal theory
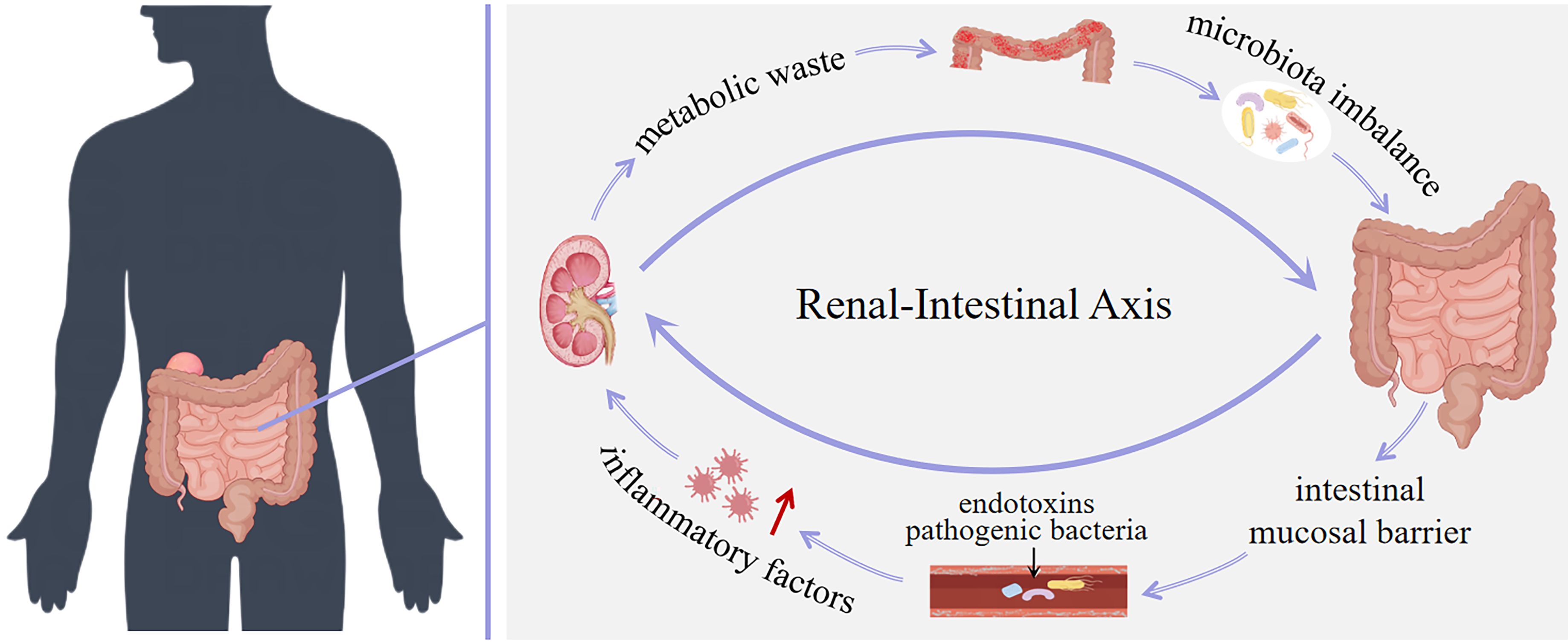
The concept of “enterorenal syndrome” was first proposed in 2011, and this theory reveals the close connection between the kidney and the intestinal in several key biological processes such as inflammation, immunity, and metabolism. It not only promoted the formation of the renal-intestinal axis theory, but also created a new therapeutic perspective to treat a variety of kidney diseases by adjusting the intestinal microbiota (Evenepoel et al., 2017). According to the renal-intestinal axis theory, the intestine is not only a key place for the digestion and absorption of nutrients, but also the largest immune organ in the human body. The intestinal immune system consists of intestinal microorganisms, epithelial cells and immune cells, and their interactions jointly maintain the defense mechanism against pathogens (Qian et al., 2016). The renal-intestinal axis theory has been thoroughly investigated recently from a variety of angles, including immunology, chemoinformatics, and molecular biology, confirming the link between the intestine and the kidney. As our understanding of the relationship between intestinal microbiota and its metabolites and kidney disease has deepened, it has become clear that both the intestinal microbiota and its metabolites are crucial for keeping the human body in a healthy state and that there is a significant correlation between the development of kidney disease and changes in the intestinal microenvironment. At present, based on the renal-intestinal axis theory, it is clear that the interaction between kidney and intestine is bidirectional, which provides a new scientific basis and treatment strategy for the diagnosis and treatment of kidney diseases (Li et al., 2023a; Xie et al., 2024a; Zhou et al., 2024a).
4.2 The role of intestinal microbiota and its metabolites in renal-intestinal axis
The metabolites of intestinal microbiota and play a crucial role in the renal-intestinal axis. As a bridge of information communication between the kidney and the intestine, they affect the health and functional stability between the kidney and the intestine through various mechanisms such as affecting metabolic pathways, maintaining intestinal barrier function, and regulating immune responses (Li et al., 2023; Li et al., 2024). On the one hand, the metabolic waste produced by the body in patients with kidney disease cannot be excreted in time, which leads to accumulation in blood and tissues. These harmful substances penetrate into the intestinal cavity through the mesenteric vessel wall, leading to imbalance of intestinal microbiota and its metabolites. On the other hand, the imbalance of intestinal microbiota and its metabolites destroys the intestinal mucosal barrier, damages intestinal epithelial cells, increases intestinal permeability, and intensifies the intestinal absorption of harmful substances. At the same time, pathogenic bacteria and endotoxins enter the blood circulation, making the level of inflammatory factors in the blood significantly increase, inducing systemic inflammation, aggravating the kidney burden, and aggravating the development of kidney disease. Again and again, the two formed a vicious circle. In conclusion, intestinal microbiota and its metabolites play an important role in renal-intestinal axis homeostasis (Li et al., 2022; Li et al., 2023b). (summarized in Figure 3).
5 Traditional Chinese medicine treats diarrhea caused by kidney disease by regulating intestinal microbiota and its metabolites
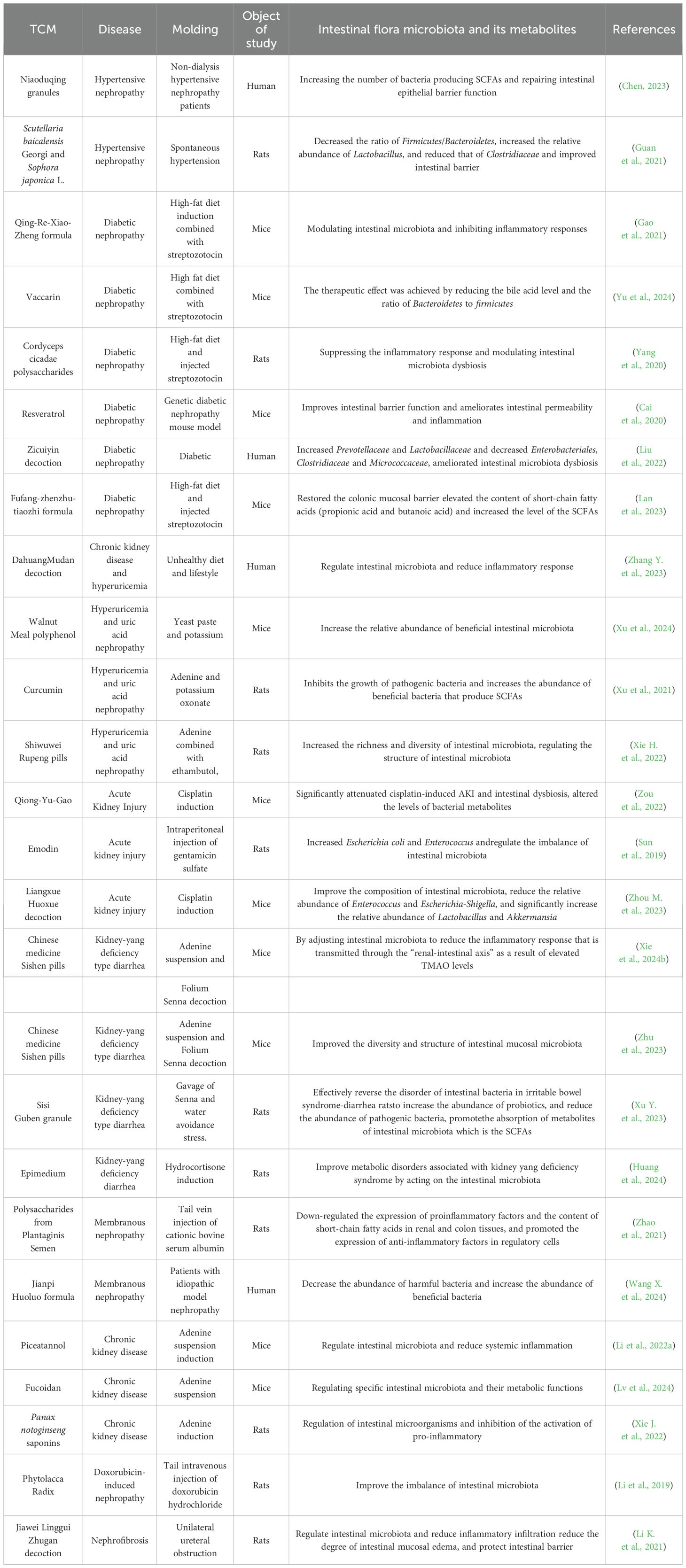
Because of the characteristics of multi-component, multi-target and so on, TCM has shown unique advantages in the treatment of diseases. Kidney disease can induce diarrhoea. TCM compound preparations can regulate intestinal microbiota and its metabolites through the renal-intestinal axis pathway, repair intestinal barrier damage, accelerate the excretion of toxins in the body, and slow down the further development of kidney disease (Zhou et al., 2023b; Li et al., 2024). (as shown in Table 1).
6 Summary and prospect
With the increasing attention paid to the theory of renal-intestinal axis in the treatment of nephropathy, scientific research has begun to explore the essence of renal-intestinal axis from the fields of immunology, molecular biology, chemical informatics, etc., and confirmed the multifaceted and multi-level connection between the kidney and the intestine. With the study of the interaction between intestinal microbiota and its metabolites and nephropathy induced diarrhea, it is found that the intervention of intestinal microbiota and its metabolites is a potential target for the treatment of kidney disease. Due to its unique therapeutic advantages, based on this characteristic target, TCM can achieve the purpose of treating diarrhea by regulating the balance of intestinal microbiota, changing the content of intestinal microbiota and its metabolites, and promoting the repair of intestinal mucosa, thereby slowing down the development of kidney disease. At present, the molecular mechanism of TCM treating the imbalance of intestinal microbiota and its metabolites in the renal-intestinal axis is still unclear. Therefore, it is necessary to further explore the theoretical research of renal-intestinal axis and the specific mechanisms of diarrhoea induced through kidney disease treated with TCM, so as to further guide the clinical application.
Author contributions
TZ: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Investigation, Visualization, Writing – original draft. ZL: Investigation, Visualization, Writing – original draft. CL: Supervision, Writing – review & editing. HZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Technological Innovation Team Construction Project of Heilongjiang Provincial Department of Education (2021-KYYEF-0638), Joint Guidance Project of Natural Science Foundation of Heilongjiang Province (LH2022H093), Heilongjiang Province in Special funding for postdoctoral (LBH-Q20185), The “Dongji” Academic Team of Jiamusi University (DJXSTD202414).
Acknowledgments
We extend our deepest admiration and respect to researchers in this field. If any relevant studies or papers by scientists in this area have not been cited, we offer our sincere apologies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cai, T. T., Ye, X. L., Li, R. R., Chen, H., Wang, Y. Y., Yong, H. J., et al. (2020). Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.01249
Cao, C., Zhu, H., Yao, Y., Zeng, R. (2022). Gut dysbiosis and kidney diseases. Front. Med. 9. doi: 10.3389/fmed.2022.829349
Chao, G., Zhang, S. (2020). The characteristics of intestinal flora of IBS-D with different syndromes. Immunity Inflammation disease 8, 615–628. doi: 10.1002/iid3.348
Chen, H. Y., Liu, J., Weng, D. Z., Yan, L., Pan, C. S., Sun, K., et al. (2022). Ameliorative effect and mechanism of Si-Ni-San on chronic stress-induced diarrhea-irritable bowel syndrome in rats. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.940463
Chen, Z. (2023). Clinical effect of Niaoduqing granules combined with omesartan ester tablets in the treatment of hypertensive nephropathy. Chin. J. Clin. Rational Drug Use 16, 28–32. doi: 10.15887/j.cnki.13-1389/r.2023.31.008
Cheng, X., Zhu, Y., Huang, J., Li, Y., Jiang, X., Yang, Q. (2024). A neutral polysaccharide from Persicaria hydropiper (L.) spach ameliorates lipopolysaccharide-induced intestinal barrier injury via regulating the gut microbiota and modulating AKT/PI3K/mTOR and MAPK signaling pathways. J. Ethnopharmacol. 320, 117403. doi: 10.1016/j.jep.2023.117403
Dicks, L. M. T. (2022). Gut bacteria and neurotransmitters. Microorganisms 10, 1838. doi: 10.3390/microorganisms10091838
Evenepoel, P., Poesen, R., Meijers, B. (2017). The gut-kidney axis. Pediatr. Nephrol. 32, 2005–2014. doi: 10.1007/s00467-016-3527-x
Fan, Y., Zhao, Q., Wei, Y., Wang, H., Ga, Y., Zhang, Y., et al. (2023). Pingwei san ameliorates spleen deficiency-induced diarrhea through intestinal barrier protection and gut microbiota modulation. Antioxidants 12, 1122. doi: 10.3390/antiox12051122
Gallardo, P., Izquierdo, M., Viver, T., Bustos-Caparros, E., Piras, D., Vidal, R. M., et al. (2024). A metagenomic approach to unveil the association between fecal gut microbiota and short-chain fatty acids in diarrhea caused by diarrheagenic Escherichia coli in children. Microbial. Cell. 11, 116–127. doi: 10.15698/mic2024.04.820
Gao, Y., Yang, R., Guo, L., Wang, Y., Liu, W. J., Ai, S., et al. (2021). Qing-Re-Xiao-Zheng formula modulates gut microbiota and inhibits inflammation in mice with diabetic kidney disease. Front. Med. 8. doi: 10.3389/fmed.2021.719950
Gong, J., Noel, S., Pluznick, J. L., Hamad, A. R. A., Rabb, H. (2019). Gut microbiota-kidney cross-talk in acute kidney injury. Semin. Nephrol. 39, 107–116. doi: 10.1016/j.semnephrol.2018.10.009
Guan, Y., Chen, K., Quan, D., Kang, L., Yang, D., Wu, H., et al. (2021). The combination of Scutellaria baicalensis Georgi and Sophora japonica L. ameliorate renal function by regulating gut microbiota in spontaneously hypertensive rats. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.575294
Guo, M., Fang, L., Chen, M., Shen, J., Tan, Z., He, W. (2024). Dysfunction of cecal microbiota and CutC activity in mice mediating diarrhea with kidney-yang deficiency syndrome. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1354823
Guo, X., Okpara, E. S., Hu, W., Yan, C., Wang, Y., Liang, Q., et al. (2022). Interactive relationships between intestinal flora and bile acids. Int. J. Mol. Sci. 23, 8343. doi: 10.3390/ijms23158343
Guo, K., Yan, Y., Zeng, C., Shen, L., He, Y., Tan, Z. (2022). Study on Baohe pills regulating intestinal microecology and treating diarrhea of high-fat and high-protein diet mice. BioMed. Res. Int. 2022, 6891179. doi: 10.1155/2022/6891179
He, L., Liu, Y., Guo, Y., Shen, K., Hui, H., Tan, Z. (2018). Diversity of intestinal bacterial lactase gene in antibiotics-induced diarrhea mice treated with Chinese herbs compound Qi Wei Bai Zhu San. 3 Biotech. 8, 4. doi: 10.1007/s13205-017-1024-y
Huang, R., Chen, Z., Ding, K., Sun, E., Huang, Y., Wei, Y., et al. (2024). Study on the intervention effect of Epimedium before and after suet-oil-processed on kidney yang deficiency rats based on intestinal flora and fecal metabolomics. J. Pharm. Biomed. Analysis 240, 115957. doi: 10.1016/j.jpba.2023.115957
Huang, L., Wu, Y., Xiao, N., Tan, Z. (2023). Progress in research on treatment of intestinal dysbacteriosis diarrhea with traditional Chinese medicine. Chin. J. Microecol. 35, 615–620. doi: 10.13381/j.cnki.cjm.202305022
Hui, H., Wu, Y., Zheng, T., Zhou, S., Tan, Z. (2020). Bacterial characteristics in intestinal contents of antibiotic-associated diarrhea mice treated with Qiweibaizhu powder. Med. Sci. monitor. 26, e921771. doi: 10.12659/MSM.921771
Jabri, M. A., Rtibi, K., Sakly, M., Marzouki, L., Sebai, H. (2016). Role of gastrointestinal motility inhibition and antioxidant properties of myrtle berries (Myrtus communis L.) juice in diarrhea treatment. Biomed. Pharmacother. 84, 1937–1944. doi: 10.1016/j.biopha.2016.11.008
Kataoka, K. (2016). The intestinal microbiota and its role in human health and disease. J. Med. Invest. 63, 27–37. doi: 10.2152/jmi.63.27
Kim, S. J., Shin, M. S., Choi, Y. K. (2024). Ameliorative effects of Zingiber officinale Rosc on antibiotic-associated diarrhea and improvement in intestinal function. Molecules 29, 732. doi: 10.3390/molecules29030732
Kim, M. G., Yang, J., Jo, S. K. (2021). Intestinal microbiota and kidney diseases. Kidney Res. Clin. Practice 40, 335–343. doi: 10.23876/j.krcp.21.053
Kuo, Y. R., Lin, C. H., Lin, W. S., Pan, M. H. (2024). L-glutamine substantially improves 5-fluorouracil-induced intestinal mucositis by modulating gut microbiota and maintaining the integrity of the gut barrier in mice. Mol. Nutr. Food Res. 68, e2300704. doi: 10.1002/mnfr.202300704
Lai, Y., Deng, H., Fang, Q., Ma, L., Lei, H., Guo, X., et al. (2023). Water-insoluble polysaccharide extracted from Poria cocos alleviates antibiotic-associated diarrhea based on regulating the gut microbiota in mice. Foods 12, 3080. doi: 10.3390/foods12163080
Lan, T., Tang, T., Li, Y., Duan, Y., Yuan, Q., Liu, W., et al. (2023). FTZ polysaccharides ameliorate kidney injury in diabetic mice by regulating gut-kidney axis. Phytomedicine 118, 154935. doi: 10.1016/j.phymed.2023.154935
Li, Y., Li, X., Tan, Z. (2022). Discussion on “treating the same disease with different therapies” based on the correlation between flora imbalance and diarrhea. Chin. J. Microecol. 34, 857–861. doi: 10.13381/j.cnki.cjm.202207021
Li, K., Liu, H., Wang, M., Zhu, H., Li, C., Zhai, W., et al. (2021). Effect of supplemented Linggui Zhugan decoction on intestinal pathology of rats with renal fibrosis. Lishizhen Med. Materia Med. Res. 32, 1833–1835. doi: 10.3969/j.issn.1008-0805.2021.08.11
Li, X., Peng, X., Qiao, B., Peng, M., Deng, N., Yu, R., et al. (2022). Gut-kidney impairment process of adenine combined with folium sennae-induced diarrhea: association with interactions between Lactobacillus intestinalis, Bacteroides Acidifaciens and Acetic Acid, inflammation, and kidney function. Cells 11, 3261. doi: 10.3390/cells11203261
Li, X., Qiao, B., Wu, Y., Deng, N., Tan, Z. (2023a). Evaluation of toxicological effects of chemical substances by gut microbiota: The example of adenine damage to the kidney and gut. Heliyon 9, e23010. doi: 10.1016/j.heliyon.2023.e23010
Li, X., Qiao, B., Wu, Y., Deng, N., Yuan, J., Tan, Z. (2024). Sishen pill inhibits intestinal inflammation in diarrhea mice via regulating kidney-intestinal bacteria-metabolic pathway. Front. Pharmacol. 15. doi: 10.3389/fphar.2024.1360589
Li, X., Sun, Y., Wang, Q., Kang, H. (2019). Protective effect of Radix Phytolaccae and its components on kidney in rats with doxorubicin-induced nephropathy. Acta Chin. Med. Pharmacol. 49, 12–17. doi: 10.19664/j.cnki.1002-2392.210131
Li, C., Wang, Y., Wang, Y., Yin, J., Yang, S., Liu, Y. D., et al. (2022a). Piceatannol alleviates host inflammation in chronic kidney disease model mice through regulating gut microbiota. Acta Pharm. Sinica 57, 364–374. doi: 10.16438/j.0513-4870.2021-0990
Li, X. P., Wu, H., Xue, G. Z. (2024). An analysis of the interaction between intestinal flora disorder and chronic kidney disease based on the theory of enteric-renal axis. Clin. J. Chin. Med. 16, 137–141. doi: 10.3969/j.issn.1674-7860.2024.07.025
Li, Y., Xia, S., Jiang, X., Feng, C., Gong, S., Ma, J., et al. (2021a). Gut microbiota and diarrhea: An updated review. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.625210
Li, Y. H., Xiang, X. M., Mu, Z. Y., Li, Z. M. (2023). Research progress of integrated traditional Chinese and Western medicine in the treatment of chronic kidney disease based on “gut-kidney axis” theory. Yunnan J. Traditional Chin. Med. Materia Medica 44, 110–115. doi: 10.16254/j.cnki.53-1120/r.2023.05.027
Li, Y., Yuan, Z., Tan, Z. (2021b). Correlation between intestinal flora and traditional Chinese medicine syndromes of diarrhea: A review. Chin. J. Exp. Traditional Med. Formulae 27, 209–217. doi: 10.13422/j.cnki.syfjx.20211698
Li, X., Zhang, C., Hui, H., Tan, Z. (2021c). Effect of Gegenqinlian decoction on intestinal mucosal flora in mice with diarrhea induced by high temperature and humidity treatment. 3 Biotech. 11, 83. doi: 10.1007/s13205-020-02628-0
Li, X., Zhang, C., Tan, Z., Yuan, J. (2021d). Network pharmacology-based analysis of Gegenqinlian decoction regulating intestinal microbial activity for the treatment of diarrhea. Evidence-Based Complementary Altern. Med. 2021, 5520015. doi: 10.1155/2021/5520015
Li, Y. C., Zhang, Y. Z., Yang, Y. F., Chen, L. D., Xu, X. L. (2024). Regulatory effects of couplet medicinals of Atractylodes macrocephala-Aucklandia lappa on gut microbiota and short-chain fatty acid metabolism in the irritable bowel syndrome rat with spleen deficiency and diarrhea. China Pharmacy 35, 304–310. doi: 10.6039/j.issn.1001-0408.2024.03.07
Li, C., Zhou, K., Xiao, N., Peng, M., Tan, Z. (2022b). The effect of Qiweibaizhu powder crude polysaccharide on antibiotic-associated diarrhea mice is associated with restoring intestinal mucosal bacteria. Front. Nutr. 9. doi: 10.3389/fnut.2022.952647
Li, X., Zhu, J., Wu, Y., Tan, Z. (2023b). Correlation between kidney function and intestinal biological characteristics of adenine and Folium Sennae induced diarrhea model in mice. Turkish J. Gastroenterol. 34, 4–12. doi: 10.5152/tjg.2022.211010
Lin, Y., Gao, Y., He, S., Zheng, L., Lin, Y., Li, Y. (2021). The influence of Huosha oral liquid on diamine oxidase, D-lactate, and endotoxin levels in patients with diarrhea-predominant irritable bowel syndrome. Fujian J. Traditional Chin. Med. 52, 18–19. doi: 10.13260/j.cnki.jfjtcm.012239
Liu, J., Gao, L. D., Fu, B., Yang, H. T., Zhang, L., Che, S. Q., et al. (2022). Efficacy and safety of Zicuiyin decoction on diabetic kidney disease: a multicenter, randomized controlled trial. Phytomedicine 100, 154079. doi: 10.1016/j.phymed.2022.154079
Liu, C. S., Liang, X., Wei, X. H., Jin, Z., Chen, F. L., Tang, Q. F., et al. (2019). Gegen Qinlian decoction treats diarrhea in piglets by modulating gut microbiota and short-chain fatty acids. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00825
Liu, M., Ma, J., Xu, J., Huangfu, W., Zhang, Y., Ali, Q., et al. (2024). Fecal microbiota transplantation alleviates intestinal inflammatory diarrhea caused by oxidative stress and pyroptosis via reducing gut microbiota-derived lipopolysaccharides. Int. J. Biol. Macromolecules 261, 129696. doi: 10.1016/j.ijbiomac.2024.129696
Liu, J., Qiao, B., Cai, Y., Tan, Z., Deng, N. (2023). Diarrhea accompanies intestinal inflammation and intestinal mucosal microbiota dysbiosis during fatigue combined with a high-fat diet. BMC Microbiol. 23, 151. doi: 10.1186/s12866-023-02896-9
Lv, X., Zhang, Y., Wang, L., Chen, Y. T., Lu, W. W., Wang, H. C. (2024). Fucoidan modulates gut microbiota alleviating colonic inflammation and renal injury in a mice of chronic kidney disease. Food Fermentation Industries, 1–10. doi: 10.13995/j.cnki.11-1802/ts.038983
Meng, X., Zhang, G., Cao, H., Yu, D., Fang, X., de Vos, W. M., et al. (2020). Gut dysbacteriosis and intestinal disease: mechanism and treatment. J. Appl. Microbiol. 129, 787–805. doi: 10.1111/jam.14661
Miao, X., Wang, Y., Zhao, X., Zhang, Z., Xi, S., Zhao, S. (2023). Effect of Wumeisan on gut lactase activity and microflora diversity of mice with dysbacteriosis diarrhea. Chin. J. Exp. Traditional Med. Formulae 29, 33–42. doi: 10.13422/j.cnki.syfjx.20221906
Parada Venegas, D., de la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10. doi: 10.3389/fimmu.2019.00277
Pushpanathan, P., Mathew, G. S., Selvarajan, S., Seshadri, K. G., Srikanth, P. (2019). Gut microbiota and its mysteries. Indian J. Med. Microbiol. 37, 268–277. doi: 10.4103/ijmm.IJMM_19_373
Qian, X., Fu, L., Zhao, L., Chen, M. (2016). Chinese and Western understanding of the relationship between CKD and intestinal microecology based on the “gut-kidney axis. Hunan J. Traditional Chin. Med. 32, 131–133. doi: 10.16808/j.cnki.issn1003-7705.2016.01.078
Qiao, B., Li, X., Peng, M., Hui, H., Tan, Z. (2023a). Alteration of intestinal mucosal microbiota in mice with Chinese dampness-heat syndrom diarrhea by improper diet combined with high temperature and humidity environments. Front. Cell. Infection Microbiol. 12. doi: 10.3389/fcimb.2022.1096202
Qiao, B., Liu, J., Peng, X., Cai, Y., Peng, M., Li, X., et al. (2023b). Association of short-chain fatty acids with gut microbiota and lipid metabolism in mice with diarrhea induced by high-fat diet in a fatigued state. Mol. Nutr. Food Res. 67, e2300452. doi: 10.1002/mnfr.202300452
Qiao, B., Xiao, N., Deng, N., Tan, Z. (2024). Shenling Baizhu powder attenuates lard diet in a fatigued state-induced diarrhea via targeting microbial metabolites short chain fatty acids-mediated lipid metabolism. 3 Biotech. 14, 203. doi: 10.1007/s13205-024-04045-z
Ramamurthy, T., Kumari, S., Ghosh, A. (2022). Diarrheal disease and gut microbiome. Prog. Mol. Biol. Trans. Sci. 192, 149–177. doi: 10.1016/bs.pmbts.2022.08.002
Shao, H., Zhang, C., Xiao, N., Tan, Z. (2020). Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC Microbiol. 20, 313. doi: 10.1186/s12866-020-01999-x
Shi, H., Yang, J., Li, J. (2022). Pomegranate peel polyphenols interaction with intestinal flora and its metabolic transformation. Xenobiotica 52, 442–452. doi: 10.1080/00498254.2022.2073291
Shu, Y., Hui, Y., Tan, Z. (2022). Advances in correlation between short-chain fatty acids and diarrhea. Chin. J. Infection Control. 21, 937–943. doi: 10.12138/j.issn.1671-9638.20222073
Sun, J., Luo, J. W., Yao, W. J., Luo, X. T., Su, C. L., Wei, Y. H. (2019). Effect of emodin on gut microbiota of rats with acute kidney failure. China J. Chin. Materia Medica 44, 758–764. doi: 10.19540/j.cnki.cjcmm.20181105.002
Tang, Y., Peng, M., Xiao, N., Yang, H. (2020). Effect of Tongxie Yaofang prescription on intestinal microbiota in mice with ganqi-cheng-pi diarrhea. Chin. J. Microecol. 32, 1262–1265 + 1272. doi: 10.13381/j.cnki.cjm.202011004
Ticho, A. L., Malhotra, P., Dudeja, P. K., Gill, R. K., Alrefai, W. A. (2019). Intestinal absorption of bile acids in health and disease. Compr. Physiol. 10, 21–56. doi: 10.1002/cphy.c190007
Wang, J., Luo, H., Lin, X. (2024). Research progress in the treatment of diarrheal irritable bowel syndrome with traditional Chinese medicine classic formulae. Acta Chin. Med. Pharmacol. 52, 112–116. doi: 10.19664/j.cnki.1002-2392.240084
Wang, X., Zeng, Q., Yang, J., Zhan, T., Wang, D., Li, Y., et al. (2024). Yu Renhuan’s experience in the treatment of idiopathic membranous nephropathy from the spleen based on intestinal flora. Beijing J. Traditional Chin. Med. 43, 381–384. doi: 10.16025/j.1674-1307.2024.04.010
Wei, J., Yao, F., Xu, Z., Chang, H., Lu, R. (2018). Clinical observation of Weichang’an pills combined with montmorillonite powder in treatment of acute diarrhea of dyspeptic retention gastroenteric disease. Drugs Clinic 33, 1402–1405. doi: 10.7501/j.issn.1674-5515.2018.06.024
Winston, J. A., Theriot, C. M. (2020). Diversification of host bile acids by members of the gut microbiota. Gut Microbes 11, 158–171. doi: 10.1080/19490976.2019.1674124
Wu, Y., Deng, N., Liu, J., Cai, Y., Yi, X., Tan, Z. (2024). Unlocking the therapeutic potential of Huoxiang Zhengqi san in cold and high humidity-induced diarrhea: insights into intestinal microbiota modulation and digestive enzyme activity. Heliyon 10, e32789. doi: 10.1016/j.heliyon.2024.e32789
Xiao, C., Wang, M., Yang, X., Sun, J., Weng, L., Qiu, Z. (2023). Rice water-fried Atractylodis Rhizoma relieves spleen deficiency diarrhea by regulating the intestinal microbiome. Oxid. Med. Cell. Longevity 2023, 1983616. doi: 10.1155/2023/1983616
Xiao, T., Yu, X., Yang, L., Duan, X. (2023). Study of “Butuyajie” compound on iproving antibiotic-associated diarrhea. Modernization Traditional Chin. Med. Materia Medica-World Sci. Technol. 25, 2743–2751. doi: 10.11842/wst.20220902007
Xie, S., Deng, N., Fang, L., Shen, J., Tan, Z., Cai, Y. (2024a). TMAO is involved in kidney-yang deficiency syndrome diarrhea by mediating the “gut-kidney axis. Heliyon 10, e35461. doi: 10.1016/j.heliyon.2024.e35461
Xie, G., Deng, N., Zheng, T., Peng, X., Zhang, S., Tan, Z. (2022). Total glycosides contribute to the anti-diarrheal effects of Qiwei Baizhu powder via regulating gut microbiota and bile acids. Front. Cell. Infection Microbiol. 12. doi: 10.3389/fcimb.2022.945263
Xie, S., Fang, L., Deng, N., Shen, J., Tan, Z., Peng, X. (2024b). Targeting the gut-kidney axis in diarrhea with kidney-yang deficiency syndrome: the role of Sishen pills in regulating TMAO-mediated inflammatory response. Med. Sci. Monitor 30, e944185. doi: 10.12659/MSM.944185
Xie, J., Ma, X., Zheng, Y., Mao, N., Ren, S., Fan, J. (2022). Panax notoginseng saponins alleviate damage to the intestinal barrier and regulate levels of intestinal microbes in a rat model of chronic kidney disease. Renal Failure 44, 1948–1960. doi: 10.1080/0886022X.2022.2143378
Xie, G., Tan, K., Peng, M., Long, C., Li, D., Tan, Z. (2019). Bacterial diversity in intestinal mucosa of antibiotic-associated diarrhea mice. 3 Biotech. 9, 444. doi: 10.1007/s13205-019-1967-2
Xie, H., Zhang, B., Mukaram, A., Li, N., He, P., Yan, H. (2022). Mechanism of tibetan medicine Shiwuwei Rupeng pills on hyperuricemia nephropathy based on intestinal flora and systematic pharmacology. Chin. Traditional Herbal Drugs 53, 6068–6082. doi: 10.7501/j.issn.0253-2670.2022.19.013
Xu, Y., Cai, Y., Jiang, L., Zhang, Z., Zhou, R., Yang, Y. (2023). Effect of Sisi guben granule on the content of short-chain fatty acids in lBS-D rats based on intestinal flora. J. Liaoning Univ. Traditiona Chin. Med. 25, 18–23. doi: 10.13194/j.issn.1673-842x.2023.02.005
Xu, D., Ma, Y., Niu, R., Zhao, S. (2024). Prevention and mechanisms of walnut meal polyphenol extract on hyperuricemia. Sci. Technol. Food Industry, 1–21. doi: 10.13386/j.issn1002-0306.2023120055
Xu, X., Wang, H., Guo, D., Man, X., Liu, J., Li, J., et al. (2021). Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Renal Failure 43, 1063–1075. doi: 10.1080/0886022X.2021.1944875
Xu, H., Wang, S., Jiang, Y., Wu, J., Chen, L., Ding, Y., et al. (2023). Poria cocos polysaccharide ameliorated antibiotic-associated diarrhea in mice via regulating the homeostasis of the gut microbiota and intestinal mucosal barrier. Int. J. Mol. Sci. 24, 1423. doi: 10.3390/ijms24021423
Xue, H., Mei, C. F., Wang, F. Y., Tang, X. D. (2023). Relationship among Chinese herb polysaccharide (CHP), gut microbiota, and chronic diarrhea and impact of CHP on chronic diarrhea. Food Sci. Nutr. 11, 5837–5855. doi: 10.1002/fsn3.3596
Yang, J., Dong, H., Wang, Y., Jiang, Y., Zhang, W., Lu, Y., et al. (2020). Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int. J. Biol. Macromolecules 163, 442–456. doi: 10.1016/j.ijbiomac.2020.06.153
Yang, L., Zhang, Q., Huang, J., Liu, D., Lan, Y., Yuan, L., et al. (2021). Xianglian pill ameliorates antibiotic-associated diarrhea by restoring intestinal microbiota and attenuating mucosal damage. J. Ethnopharmacol. 264, 113377. doi: 10.1016/j.jep.2020.113377
You, Y., Luo, L., You, Y., Lin, Y., Hu, H., Chen, Y., et al. (2020). Shengmai yin formula modulates the gut microbiota of spleen-deficiency rats. Chin. Med. 15, 114. doi: 10.1186/s13020-020-00394-y
Yu, X. Y., Chang, C., Chen, T. X., Ma., L. C., Chen, X. Y., Cai, W. W. (2024). Study on vaccarin in improving intestinal flora disorder and renal lipid deposition in diabetes nephropathy mice. West China J. Pharm. Sci. 39, 36–42. doi: 10.13375/j.cnki.wcjps.2024.01.008
Yu, L., Huang, C., Yang, W., Ren, Z., Li, L., Cheng, H., et al. (2023). Aqueous cinnamon extract ameliorates bowel dysfunction and enteric 5-HT synthesis in IBS rats. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.1010484
Zhang, D., Cheng, H., Zhang, Y., Zhou, Y., Wu, J., Liu, J., et al. (2023). Ameliorative effect of Aconite aqueous extract on diarrhea is associated with modulation of the gut microbiota and bile acid metabolism. Front. Pharmacol. 14. doi: 10.3389/fphar.2023.1189971
Zhang, N., Liang, T., Jin, Q., Shen, C., Zhang, Y., Jing, P. (2019). Chinese yam (Dioscorea opposita Thunb.) alleviates antibiotic-associated diarrhea, modifies intestinal microbiota, and increases the level of short-chain fatty acids in mice. Food Res. Int. 122, 191–198. doi: 10.1016/j.foodres.2019.04.016
Zhang, Q., Wang, J. (2024). Research progress of intestinal flora and its metabolites in diabetic nephropathy. China Med. 19, 951–955. doi: 10.3760/j.issn.1673-4777.2024.06.033
Zhang, S., Wang, C., Li, Y., Wang, N. (2017). Expert consensus on TCM diagnosis and treatment of diarrhea (2017). J. Traditional Chin. Med. 58, 1256–1260. doi: 10.13288/j.11-2166/r.2017.14.023
Zhang, L., Zhang, W., Nie, J. (2020). Gut microbiota and renal injury. Adv. Exp. Med. Biol. 1238, 93–106. doi: 10.1007/978-981-15-2385-4_7
Zhang, Y., Zhu, X., Fang, C., Zhang, Y. (2023). Clinical efficacy of modified Dahuangmudan decoction in the treatment of early and mid-term chronic kidney disease complicated with hyperuricemia and its effect on intestinal flora and inflammatory factors. World J. Integrated Traditional Western Med. 18, 2038–2043. doi: 10.13935/j.cnki.sjzx.231023
Zhao, H., Chen, C., Zhao, Y., Tang, W. W., Gao, Q., Kong, L. Z., et al. (2021). Effect of polysaccharides from plantaginis semen on renal injury and gut microbiota in rats with membranous nephropathy. Chin. J. Exp. Traditional Med. Formulae 27, 92–99. doi: 10.13422/j.cnki.syfjx.20212101
Zhou, K., Deng, N., Yi, X., Cai, Y., Peng, M., Xiao, N. (2022a). Baohe pill decoction for diarrhea induced by high-fat and high-protein diet is associated with the structure of lactase-producing bacterial community. Front. Cell. Infection Microbiol. 12. doi: 10.3389/fcimb.2022.1004845
Zhou, R., He, D., Xie, J., Zhou, Q., Zeng, H., Li, H., et al. (2021). The synergistic effects of polysaccharides and ginsenosides from American Ginseng (Panax quinquefolius L.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front. Immunol. 12. doi: 10.3389/fimmu.2021.665901
Zhou, R., Huang, Y., Tian, C., Yang, Y., Zhang, Z., He, K. (2023). Coptis chinensis and berberine ameliorate chronic ulcerative colitis: An integrated microbiome-metabolomics study. Am. J. Chin. Med. 51, 2195–2220. doi: 10.1142/S0192415X23500945
Zhou, M., Li, X., Liu, J., Wu, Y., Tan, Z., Deng, N. (2024a). Adenine’s impact on mice’s gut and kidney varies with the dosage administered and relates to intestinal microorganisms and enzyme activities. 3 Biotech. 14, 88. doi: 10.1007/s13205-024-03959-y
Zhou, M., Li, X., Wang, X., Deng, N., Cai, Y., Tan, Z. (2024b). The dysfunction in intestinal microorganisms and enzyme activity as significant contributors to diarrhea with kidney-yang deficiency syndrome. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1324938
Zhou, K., Peng, M., Deng, N., Tan, Z., Xiao, N. (2022b). Lactase bacteria in intestinal mucosa are associated with diarrhea caused by high-fat and high-protein diet. BMC Microbiol. 22, 226. doi: 10.1186/s12866-022-02647-2
Zhou, K., Peng, M., Tan, Z., Xiao, N. (2023a). Diarrhea caused by high-fat and high-protein diet was associated with intestinal lactase-producing bacteria. Turkish J. Gastroenterol. 34, 691–699. doi: 10.5152/tjg.2023.22451
Zhou, M., Yang, L., Zhuo, Y., Li, D., Zhang, L., Cui, L., et al. (2023). Effect of Liangxue Huoxue decoction on intestinal flora and NLRP3/caspase-1/GSDMD signaling pathway in mice model of sepsis-induced acute kidney injury. Chin. Crit. Care Med. 35, 250–255. doi: 10.3760/cma.j.cn121430-20221122-01018
Zhou, K., Yi, X., Tan, Z. J., Peng, M., Xiao, N. (2023b). Baohe pill decoction treats diarrhea induced by high-fat and high-protein diet by regulating lactase-producing bacteria in intestinal mucosa. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1157475
Zhu, J., Li, X., Deng, N., Peng, X., Tan, Z. (2022). Diarrhea with deficiency kidney-yang syndrome caused by adenine combined with folium senna was associated with gut mucosal microbiota. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1007609
Zhu, J., Li, X., Deng, N., Zhou, K., Qiao, B., Li, D., et al. (2023). Intestinal mucosal flora of the intestine-kidney remediation process of diarrhea with deficiency kidney-yang syndrome in Sishen pill treatment: association with interactions between Lactobacillus johnsonii, Ca2+-Mg2+-ATP-ase, and Na+-K+-ATP-ase. Heliyon 9, e16166. doi: 10.1016/j.heliyon.2023.e16166
Keywords: renal-intestinal axis, traditional Chinese medicines, diarrhea, intestinal microbiota, metabolites of intestinal microbiota
Citation: Zhou T, Zhang Y, Li Z, Lu C and Zhao H (2024) Research progress of traditional Chinese medicine on the treatment of diarrhea by regulating intestinal microbiota and its metabolites based on renal-intestinal axis. Front. Cell. Infect. Microbiol. 14:1483550. doi: 10.3389/fcimb.2024.1483550
Received: 20 August 2024; Accepted: 09 September 2024;
Published: 27 September 2024.
Edited by:
Nenqun Xiao, Hunan University of Chinese Medicine, ChinaReviewed by:
Qi Yonghua, Heilongjiang Untversity of Chinese Medicine, ChinaYuan Cheng, Qiqihar Medical University, China
Copyright © 2024 Zhou, Zhang, Li, Lu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunfeng Lu, bHVjaHVuZmVuZ2NoZW5AMTYzLmNvbQ==; Hong Zhao, MDMxNnpoQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Tong Zhou
Tong Zhou Yifan Zhang
Yifan Zhang Zhaoyuan Li
Zhaoyuan Li Chunfeng Lu1,3*
Chunfeng Lu1,3* Hong Zhao
Hong Zhao